Abstract
Chronic kidney disease (CKD) affects 37 million American adults who experience high rates of cardiovascular events and are at risk of kidney failure and mortality. Routine primary care case finding for CKD with estimated glomerular filtration rate (eGFR) and urine albumin-creatinine ratio (uACR) should focus on risk conditions, particularly diabetes, hypertension, and cardiovascular disease, as recommended by clinical practice guidelines. The diagnosis of CKD is associated with many important aspects of care, including patient awareness, patient engagement, and improved implementation of evidence-based interventions. Individualized care that tailors CKD interventions proportional to the adverse outcome risk or the eGFR and uACR heat map is a major challenge for primary CKD care, because the condition is heterogeneous in terms of both the cause and the severity.
The coordinated care approach to CKD management is necessary to deploy best practice in chronic disease management that engages the interdisciplinary team. An integrated system supports the time-constrained primary clinician with CKD registry functions, clinical decision support tools, quality improvement initiatives, and payment model incentives to drive reduction in adverse outcomes and containment of expenditures.
A CKD population health strategy can be built to address primary care education and implementation gaps from the perspectives of testing, detection of disease, interventions, and coordinated system-integrated care. Registry function and data monitoring of the burden of CKD, delivery interventions, and outcomes are key features. Implementation of the Race-free 2021 CKD-(Epidemiology Collaboration) EPI eGFR reporting recommendations by engaging local nephrology, administrative, clinical laboratory, and health equity leaders should help drive the population health design strategy and the data assessment.
Keywords: albuminuria, chronic kidney disease, diabetes, interdisciplinary care, population health, primary care
According to the Centers for Disease Control and Prevention, CKD affects 37 million American adults who experience high rates of cardiovascular events and are at risk of kidney failure.1 Mortality is under-recognized as a competing event versus end-stage kidney disease (ESKD).2 The original definition and stratification of CKD published in 2002 by the US Kidney Disease Outcomes Quality Initiative transformed clinical practice worldwide, promoting adoption of eGFR reporting instead of serum creatinine alone and presenting opportunities for kidney disease recognition and management in the primary care setting.3 The international Kidney Disease: Improving Global Outcomes 2012 Clinical Practice Guideline for CKD Evaluation and Management,4 endorsed in the United States by the Kidney Disease Outcomes Quality Initiative,5 updated the previous work based on epidemiology including more than 1 million patients to describe a cause-glomerular filtration rate-albuminuria (C-G-A) CKD definition and classification to stratify risk based on the eGFR and uACR.
Unfortunately, recent evaluation of US population-level care for individuals with eGFR below 60 ml/min per 1.73 m2 reveals that approximately 40% receive uACR testing,2,6,7 only 12% to 20% have evidence of a CKD diagnosis,6,7 less than 50% have controlled hypertension,6 40% have controlled diabetes,6 29% to 31% use statins to reduce cardiovascular events,6 less than 50% are treated with angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB) drugs,6 and nephrology services are delivered to only approximately 50% of patients with CKD G4 and G5.2 Although these data predominantly represent primary care delivery, nephrology preparation for kidney replacement therapy in the United States needs improvement as reflected among those with ESKD by hemodialysis catheter use in more than 80% of patients at hemodialysis initiation and low rates of both home dialysis and pre-emptive kidney transplantation.2
Kidney health inequities by race and ethnicity are complex with multiple contributors, but these have been documented in the medical literature at least since the 1980s.8 Studies have revealed disparities in health and health care delivery, disproportionately affecting African Americans compared with non-Hispanic White individuals, with almost twice the prevalence of hypertension that causes or contributes to CKD, approximately 3 times the prevalence of ESKD, and less use of patient-centric kidney replacement therapies, home dialysis, and kidney transplantation.2,8 Hispanics, Asians, Hawaiians, Pacific Islanders, and American Indians also have documented kidney health disparities with nuances across the groups.2,8 The reasons for observed disparities are multifactorial, but they may be attributed particularly to social determinants of health.8 As part of American’s reckoning with race and ethnicity in society, unconscious clinician biases and institutionalized racism in health care have been increasingly acknowledged as contributors to kidney health inequities.8, 9, 10
Unfortunately, there are adverse direct effects resulting from the COVID-19 pandemic among Blacks and Hispanic Americans compared with non-Hispanic White individuals, including dramatically higher rates of hospitalization, acute kidney injury, and death.2 Indirect pandemic effects in the uninfected population are reduced clinical encounters, increased gaps in care, lower rates of CKD laboratory monitoring, and fewer medication refills for cardiometabolic CKD risk conditions compared with historical controls.11
There is considerable room for improvement in the care of individuals with CKD by both primary care clinicians and specialists. This brief review will address primary care education and implementation gaps in CKD from the perspectives of testing, detection of disease, interventions for management, interdisciplinary care, and coordinated system-integrated care that is novel only in suggesting incorporation of Race-free 2021 eGFR reporting recommendations and contemporary American health equity momentum in the approach.
Primary Care Implementation of CKD Testing
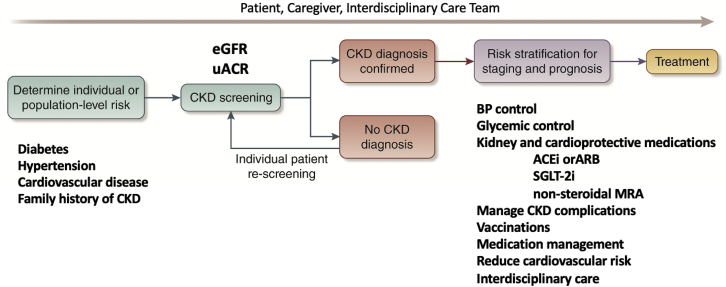
Routine primary care case finding for CKD with eGFR and uACR should focus on risk conditions, particularly; diabetes, hypertension, cardiovascular disease, and a family history of kidney disease, as recommended by clinical practice guidelines from the American Diabetes Association,12 Kidney Disease: Improving Global Outcomes,4 Kidney Disease Outcomes Quality Initiative,5 and other organizations, in contrast to mass or general population screening.13,14 In recent years in the United States, annual uACR testing is approximately 40% for diabetes and less than 10% for hypertension in national data sets from Medicare,2 commercial insurance,2 health systems,15 and clinical laboratories,16 supporting the need for interventions to improve targeted albuminuria testing. There are some challenges for clinicians to order uACR because laboratories do not universally offer the test and reporting formats vary, introducing inconsistencies and complexity in the interpretation of the results. For example, some laboratories only offer the urinary albumin concentration test without offering the ratio. Finally, clinicians are unlikely to order tests that they are not sure how to interpret, suggesting low rates of albuminuria testing may simply reflect an underappreciation in the utility of the results or challenges in the interpretation.17 Testing and recognition of albuminuria will inform the selection of patients who need additional interventions beyond the current ACEi or ARB paradigm, including novel kidney- and cardiovascular-protective therapies, such as sodium-glucose cotransporter-2 inhibitor (SGLT-2i), glucagon-like peptide-1 receptor agonists (GLP1 RA) in type 2 diabetes, and the non-steroidal mineralocorticoid inhibitor (finerenone) in type 2 diabetes.12,17,18 Nephrotic albuminuria or the uACR of approximately >2000 mg/g is important for primary clinicians to recognize as suggesting a glomerular disease cause that can be independent of diabetes or hypertension as a comorbidity,19 requiring nephrology consultation. Primary care education should also review additional diagnostic testing to identify the cause and a possible indication for nephrology consultation for uncertainty on eGFR interpretation.18
Data suggest that clinicians test eGFR much more often than uACR with annual rates of 80% to 90% of the population at risk with diabetes or hypertension.2 The College of American Pathologists international sample revealed that 92% of laboratories reported eGFR in 2019, with most of the laboratories surveyed in North America.20 Laboratory inclusion of eGFR reporting with the ubiquitous sets called the basic and comprehensive metabolic and renal function panels contribute to wider use of the kidney filtration blood test. Until recently, eGFR reporting recommended the use of the 2009 CKD-EPI collaboration eGFRcr equation with and without an African American coefficient which complicates the clinical assessment of eGFR.4,5 The challenges of assigning race in routine practice, the societal concerns on implying a biologic cause to a social construct, and the frought issues of racism in medicine were elegantly reviewed by Darshali Vyas9 in an influential editorial. Use of the 2021 CKD-EPI eGFRcr or eGFRcr-cys addresses these shortcomings.20, 21, 22
Primary Care CKD Detection
The diagnosis of CKD is associated with many important aspects of care, including patient awareness, patient engagement, and improved implementation of evidence-based interventions. Detection of CKD using CKD diagnosis codes remains low in primary care practice, although chart review or natural language processing analysis more accurately reflects clinician diagnosis. The ADD-CKD study of more than 9 thousand US patients with type 2 diabetes managed by 466 primary care clinicians revealed a CKD detection in only 12% of the population with laboratory evidence for the condition.7 Importantly, awareness or patient self-reported CKD was 81.1% with practitioner detection versus 2.6% in the absence of diagnosis.7 Awareness is the first step to enhance patient engagement and self-management. Several studies have revealed a variable but generally positive association between CKD detection and improved implementation of evidenced-based interventions.7,23
Primary Care Interventions Proportional to the CKD Risk
Individualized care that tailors CKD interventions proportional to the adverse outcome risk or the eGFR and uACR heat map is a major challenge for primary CKD care, because the conditions are heterogeneous in terms of cause and severity. CKD is a heterogeneous state, such that people with only slightly low estimated GFR without elevated uACR may have only small management and prognostic implications whereas people with very low estimated GFR and/or severely elevated uACR may be at critical risk of adverse events and require timely interdisciplinary interventions to address the substantial risk for hospitalization, cardiovascular events, kidney failure, and mortality. The controversy regarding the distinction between loss of eGFR with normal aging and CKD among seniors with eGFR 45 to 60 ml/min per 1.73 m2 in the absence of albuminuria (CKD G3aA1) is noteworthy.18 Areas for consideration in this setting would be the potential role of cystatin C testing to help stratify risk with more accurate eGFRcr-cys or 2012 CKD-EPI eGFRcys.8,20,21 Consequences of CKD G3aA1 in seniors include patient medication safety factors, cardiovascular risk, cognitive impairment risks, and risks of major surgery perioperative acute kidney injury, including absence of evidence to support ACEi or ARB and SGLT-2i use for kidney risk indications.4,5,12,18,24
Important interventions for CKD management in primary care include hypertension control targets individualized in a range that should generally be less than 130/80 mm Hg with highly motivated patients at the highest cardiovascular risk offered a <120 mm Hg systolic target and targets 140/90 mm Hg or even higher for patients with or at risk for complications of intensive targets such as hemodynamic acute kidney injury or falls.25,26 Similarly, diabetes control should be individualized in a range with less than 7% as the general recommendation.12,24
Kidney and cardioprotective medications, ACEi or ARB, SGLT-2i, GLP-1 RA, and non-steroidal mineralocorticoid inhibitor (finerenone), should be applied to the population at risk based on recent randomized trials.12,14,24 Cardiovascular thrombotic events, heart failure, and cardiovascular mortality should be emphasized as enriched according to the eGFR and uACR risk stratification.27,28 Thus, reducing cardiovascular risk is important to emphasize with statin-based therapy to reduce acute thrombotic events and particular interventions in heart failure that, in addition to ACEi or ARB and SGLT-2i, may include beta-blocker, neprilysn inhibitor, and diuretic therapies.28
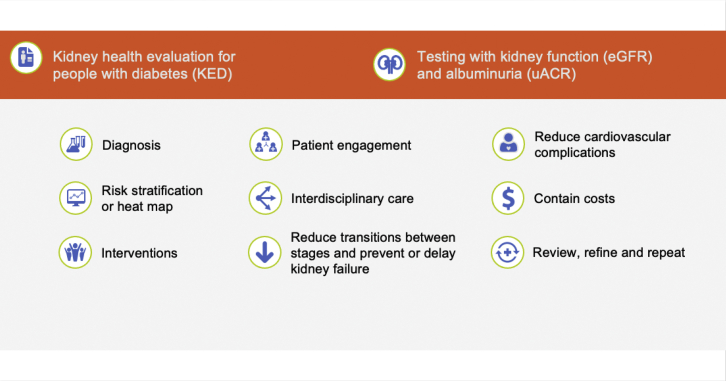
Vaccinations annually for influenza (regular dose or high dose for those 65 years and older) and the pneumococcal vaccination series are evidenced-based not only to reduce infection-associated hospitalization and mortality but also associated with subsequent reductions in cardiovascular hospitalization that may be related to less microinflammation of the endothelium in the vaccinated CKD population.29 Although analogous data are accruing, COVID-19 vaccination is an important contemporary CKD intervention to reduce hospitalization and death risks. Medication management that considers eGFR is important to prevent acute kidney injury, hypoglycemia, and other patient safety risks.4,5 Disseminating distilled clinical practice guideline concepts that would be more readily used in primary care management (Figure 1)14,18,30,31 is important for implementation to link the process of testing with evidenced-based interventions.
Figure 2.
CKD population health summary. CKD, chronic kidney disease.
Figure 1.
Schematic clinical practice guideline concepts adapted to summarize testing, detection, and management of CKD for primary care practitioners.14 ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate; GLP-1 RA, glucagon-like peptide-1 receptor agonists; MRA mineralocorticoid receptor antagonist; SGLT-2i, sodium-glucose cotransporter-2 inhibitor; uACR, urine albumin-creatinine ratio. Adapted from Shlipak MG, Tummalapalli SL, Boulware LE, et al. The case for early identification and intervention of chronic kidney disease: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. 2021;99:34–47.14 Copyright © 2020, Kidney Disease: Improving Global Outcomes (KDIGO). Published by Elsevier Inc. on behalf of the International Society of Nephrology. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
CKD Coordinated Interdisciplinary Care
The coordinated care approach to CKD management is necessary to deploy best practices in chronic disease management that leverages the interdisciplinary team, including, but is not limited to, the primary care practitioner, dietitian, pharmacist, endocrinologist, cardiologist, nephrologist, mental health professional, and social worker.32, 33, 34 An ideal setting is one that includes 2 to more team members in close proximity at a clinical site that patients benefit from the management of their co-morbidities and lifestyle modification through patient engagement and education in an optimized streamlined fashion. The multidisciplinary coordinated approach has revealed promise slower decline in eGFR, enhance blood pressure and diabetes control, increase delivery of CKD interventions, reduce hospitalization, increase arteriovenous fistula use, and increase the proportion of outpatient dialysis initiation that improve morbidity and contain expenditures.34
Medical nutrition therapy (MNT) deserves special emphasis for CKD, as it is widely underused in routine practice.35,36 Contributing factors include low rates of clinician referral, reimbursement limitations, and low access to experienced registered dietitians and diabetes educators.35 This is despite associations with better risk factor control of hypertension and diabetes, attenuation in loss of eGFR, and improvement in outcomes for the patients who initiate dialysis in the year after receiving MNT.35, 36, 37 Comparisons with patients who do not receive MNT require risk adjustment, and the impact is likely to vary.35 Nevertheless, MNT is also an important potential intervention to address health disparities in food security for patients who may reside in food deserts or food swamps where access to healthy dietary choices is limited.
Primary care clinicians also identify barriers to nephrology CKD co-management resulting in a disrupt in the care continuum. Unclear roles and responsibilities, limited communication, and variable access to nephrologists can be overcome by collaboration with a small group of nephrologists, an information exchange focused on selecting nephrologists who communicate effectively and/or use of the same electronic health record as the primary clinician38 and use of an electronic nephrology consult platform in selected cases.39 Systematic education and interventions hold promise to assist the primary clinician to refer selectively, using coordinated care within the interdisciplinary team.
System-Integrated CKD Care
System-integrated care will be key to support primary clinicians in the context of time constraints to accomplish the bold 25% reduction in the incidence of ESKD by 2030 of the Advancing American Kidney Health Initiative Executive Order. CKD Intercept primary care clinician engagement of the National Kidney Foundation includes a Laboratory Engagement Initiative to simplify primary care clinician ordering of kidney tests and a harmonized Kidney Disease: Improving Global Outcomes 2012 reporting scheme for the tests defined by the kidney profile (eGFR and uACR) that have been recognized by the US Choosing Wisely initiative.40,41 Implementation of Race free eGFR reporting as recommended by the National Kidney Foundation–American Society of Nephrology Task Force final report for primary care population health should include collaboration with clinical laboratories, nephrology clinical leaders, and health equity experts to ensure that the 2021 CKD-EPI eGFRcr is reported and interpreted appropriately.20 Systematic efforts facilitate increased, routine, and selected use of cystatin C to confirm eGFR in adults who are at risk for or have CKD, because combining routine kidney filtration markers is more accurate and would support better clinical decisions than either marker alone by using the 2021 CKD-EPI eGFRcr-cys or 2012 CKD-EPI eGFRcys that is also race free.8,20,21 Implementation of systematic validated assessments such as the social deprivation index will assist transitioning primary clinicians away from race and ethnicity as CKD risk conditions to focus alternatively on social determinants of health, primarily and secondarily on genetic ancestry that is a biologic construct.10 For example, APOL-1 genetic testing may be offered to those with or at risk for CKD who voluntarily self-report West African genetic ancestry. Race and ethnicity must continue to be used at a population level in a voluntary, transparent, and culturally sensitive fashion for data collection to evaluate health equity in CKD population health interventions.
The National Kidney Foundation Change Package outlines steps for building a strategy for CKD population health that begins with data collection to identify the impact of CKD in the health system with engagement of leadership that could include clinical, clinical laboratory, data science, administrative, and health equity stakeholders and champions.42 The next steps after convening these are to define the CKD intervention, use data to drive improvement that should inform primary care professional education, and identify gaps in care through short-term quality improvement cycles. Part of the primary care education should include on how to educate and engage the patient population. Outcomes of interest for longer term could include changes in the transitions between CKD G stages, changes in the incident and prevalent ESKD population, impact on cardiovascular complications, and cost implications. An overarching approach monitoring population health data on health equity impact should be emphasized across the stakeholders and leaders.
Quality Improvement
Quality improvement measures and metrics need further development for CKD population health. The National Kidney Foundation also developed the Kidney Health Evaluation for people with diabetes, to ensure that electronic clinical quality measure for eGFR and uACR testing dissemination is being implemented in commercial health insurance plans in the United States by the National Committee for Quality Assurance.43 Refinement of a platform of measures to build on this measure for primary care quality improvement and payment model deployment is ongoing.44
Quality improvement initiatives should focus on the total patient cycle of pre-visit planning, the office visit, and post-visit management. Optimizing patient care for CKD patients includes the following: (i) education of the team members, patient, and caregivers, highlighting the goals of care—importance of checking at least annual uACR and eGFR, and hence, escalation in management after CKD detection; (ii) integrated workflow, for example, electronic health record enhancements, to include registry optimization for patients with CKD, clinical decision support, or other prompts to order the appropriate tests and selected medications, shared decision-making tools, and actionable dashboard for population health management; and (iii) closure of outstanding referral loops and care gaps. An integrated health system albuminuria CKD testing quality initiative study resulted in a 56.1% increase capture of urine albumin in year one and 50.1% increase in 2 years; however, there was no correlated statistical improvement in use of ACEi or ARB in these patients, possibly indicating an opportunity for evaluation of the patient cycle and enhancements in education and operational flow.45
A quality improvement project implementing primary care population health for diabetes and hypertension with interventions based on the eGFR and uACR risk stratification revealed reduced hospitalization, decreased 30-day readmissions, and selected medical per patient per month cost-containment in a commercial health insurance plan’s patient-centered medical home model.46 This quality improvement project is relatively small and has many limitations, including minimal impact on low uACR testing and lack of incorporation of SGLT-2i in the intervention, but it is important because in the short-term, the findings suggest potential cost-effectiveness of CKD quality improvement. An impressive longer-term quality improvement initiative in the Indian Health Service resulted in dramatic 54% reduction of incident ESKD for the type 2 diabetes population.47 Implementing interventions proportionate to the spectrum of CKD risk stratification by eGFR and uACR is a key feature of quality improvement interventions. In addition, population-derived prediction models such as the kidney failure risk equation and novel biomarker panels integrated with the electronic health record have been used to help guide referrals to help inform primary clinicians to select the patient population to offer interdisciplinary care, such as MNT and nephrology services to the patients with the highest risk.48 Finally, the National Kidney Foundation quality improvement CKD change package offers an array of tools that can selectively be incorporated for clinical site or health system integration.42
Payment Models
The Chronic Care Model (CCM) is an effective approach to organize CKD care with examples including navigator-led, nurse-led, pharmacist-led, multidisciplinary specialist-led, and patient centered.49,50 CCM approaches wherein health professionals deliver care according to a structured protocol may improve adherence to treatment targets.49 The navigator-led CCM for CKD G3b and G4 was feasible to implement for 2 years, but it did not reveal improved processes for laboratory testing and nephrology consultation or significantly improved eGFR slope.50 CCM features such as registry function, clinical decision support interventions, and interdisciplinary care have been found to improve processes related to CKD care, but with limited or mixed effects on patient outcomes.49,50 CCM design with payment models revealed CKD outcomes, including reduced hospitalization and reduced dialysis initiation.46,47
The Centers for Medicare and Medicaid Services implemented 4 care models for CKD in January 2022; the Kidney Care First Option and 3 Comprehensive Kidney Care Contracting Graduated, Professional, and Global Model Options assign responsibility to a nephrology practice or a group of health care professionals to care for CKD G4 and G5 and dialysis focusing on delaying CKD progression, managing the transition to dialysis, increasing kidney transplant, and supporting health after kidney transplant to reduce costs and improve the quality of care for patients that will further inform cost-effectiveness of CKD payment models.51 Although these models are limited to the nephrology care setting, the design of the Comprehensive Kidney Care Contracting is based on the Primary Care First and Global and Professional Direct Contracting models. The findings could also serve as a foundation for CKD payment models in primary care, coordinated with nephrology care models. The contractual relationship of the Centers for Medicare and Medicaid Services with Medicare Advantage, and its influence on commercial plans, may also expand the import of the findings.
Summary
A CKD population health strategy (Figure 2) should be built to address primary care education and implementation gaps from the perspectives of testing, detection of disease, interventions, and coordinated system-integrated care. Registry function and data monitoring of the burden of CKD, delivery interventions, and outcomes are key features. Implementation of the Race-free 2021 CKD-EPI eGFR reporting recommendations by engaging local nephrology, administrative, clinical laboratory, and health equity leaders should help drive the population health design strategy and data assessment.
Disclosure
JAV reports receiving consulting fees from Renalytix, PLC, and Boehringer-Ingelheim/Lilly, Inc., for CKD quality improvement and population health interventions in type 2 diabetes. SCB reports receiving consulting fees from Pfizer, Inc. Integrated Delivery Network Advisory Board.
Acknowledgment
Elizabeth Montgomery is acknowledged for the vision and orchestration of the CKD Change Package.
References
- 1.Centers for Disease Control and Prevention Chronic kidney disease surveillance system. http://www.cdc.gov/ckd Accessed February 10, 2022.
- 2.United States Renal Data System. 2021 USRDS annual data report. Epidemiology of Kidney Disease in the United States.: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: 2021.
- 3.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(suppl 2):S1–S266. [PubMed] [Google Scholar]
- 4.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 5.Inker L.A., Astor B.C., Fox C.H., et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63:713–735. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 6.Tummalapalli S.L., Powe N.R., Keyhani S. Trends in quality of care for patients with CKD in the United States. Clin J Am Soc Nephrol. 2019;14:1142–1150. doi: 10.2215/CJN.00060119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szczech L.A., Stewart R.C., Su H.L., et al. Primary care detection of chronic kidney disease in adults with type-2 diabetes: the ADD-CKD Study (awareness, detection and drug therapy in type 2 diabetes and chronic kidney disease) PLoS One. 2014 doi: 10.1371/journal.pone.0110535. 26;9(11):e110535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delgado C., Baweja M., Burrows N.R., et al. Reassessing the inclusion of race in diagnosing kidney diseases: an interim report from the NKF-ASN task force. Am J Kidney Dis. 2021;78:103–115. doi: 10.1053/j.ajkd.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vyas D.A., Eisenstein L.G., Jones D.S. Hidden in plain sight - reconsidering the use of race correction in clinical algorithms. N Engl J Med. 2020;383:874–882. doi: 10.1056/NEJMms2004740. [DOI] [PubMed] [Google Scholar]
- 10.Borrell L.N., Elhawary J.R., Fuentes-Afflick E., et al. Race and genetic ancestry in medicine - a time for reckoning with racism. N Engl J Med. 2021;384:474–480. doi: 10.1056/NEJMms2029562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diamantidis C., Cook D., Westman J., et al. Missing care: the impact of the COVID-19 pandemic on the CKD care. Am J Kidney Dis. 2021 Published online 2021. [Google Scholar]
- 12.American Diabetes Association Professional Practice B Draznin, VR Aroda Chronic kidney disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(Supplement 1):S175–S184. doi: 10.2337/dc22-S011. [DOI] [PubMed] [Google Scholar]
- 13.Tonelli M., Dickinson J.A. Early detection of CKD: implications for low-income, middle-income, and high-income countries. J Am Soc Nephrol. 2020;31:1931–1940. doi: 10.1681/ASN.2020030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shlipak M.G., Tummalapalli S.L., Boulware L.E., et al. The case for early identification and intervention of chronic kidney disease: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. 2021;99:34–47. doi: 10.1016/j.kint.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Stempneiwicz N., Vassalotti J.A., Cuddeback J.K., et al. Chronic kidney disease testing among primary care patients with type 2 diabetes across 24 U.S. health care organizations. Diabetes Care. 2021;44:2000–2009. doi: 10.2337/dc20-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alfego D., Ennis J., Gillespie B., et al. Chronic kidney disease testing among at-risk adults in the U.S. remains low: real-world evidence from a National Laboratory database. Diabetes Care. 2021;44:2025–2032. doi: 10.2337/dc21-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vassalotti J.A., Argyropoulos C. Can community-based albuminuria testing improve care? Kidney Int Rep. 2020;5:392–395. doi: 10.1016/j.ekir.2020.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey A.S., Inker L.A., Coresh J. “Should the definition of CKD be changed to include age-adapted GFR criteria?”: Con: the evaluation and management of CKD, not the definition, should be age-adapted. Kidney Int. 2020;97:37–40. doi: 10.1016/j.kint.2019.08.032. [DOI] [PubMed] [Google Scholar]
- 19.Sumida K., Nadkarni G.N., Grams M.E., et al. Conversion of urine protein–creatinine ratio or urine dipstick protein to urine albumin-creatinine ratio for use in chronic kidney disease screening and prognosis: an individual participant-based meta-analysis. Ann Intern Med. 2020;173:426–435. doi: 10.7326/M20-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller W.G., Kaufman H.W., Levey A.S., et al. National Kidney Foundation Laboratory Engagement Working Group recommendations for implementing the CKD-EPI 2021 race-free equations for estimated glomerular filtration rate: practical guidance for clinical laboratories. Clin Chem. https://doi.org/10.1093/clinchem/hvab27 Published online December 16, 2021. [DOI] [PubMed]
- 21.Inker L.A., Eneanya N.D., Coresh J., et al. New creatinine- and cystatin C–based equations to estimate GFR without race. N Engl J Med. 2021;385:1737–1749. doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delgado C., Baweja M., Crews D.C., et al. A unifying approach for GFR estimation: Recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. Am J Kidney Dis. 2021;79:268–288. doi: 10.1053/j.ajkd.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Allen A.S., Forman J.P., Orav E.J., et al. Primary care management of chronic kidney disease. J Gen Intern Med. 2011;26:386–392. doi: 10.1007/s11606-010-1523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020;98:S1–S115. doi: 10.1016/j.kint.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Kramer H.J., Townsend R.R., Griffin K., et al. KDOQI US commentary on the 2017 ACC/AHA hypertension guideline. Am J Kidney Dis. 2019;73:424–458. doi: 10.1053/j.ajkd.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021;99:S1–S87. doi: 10.1016/j.kint.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Bansal N., Zelnick L., Bhat Z., et al. Burden and outcomes of heart failure hospitalizations in adults with chronic kidney disease. J Am Coll Cardiol. 2019;73:2691–2700. doi: 10.1016/j.jacc.2019.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jankowski J., Floege J., Fliser D., et al. Cardiovascular disease in chronic kidney disease pathophysiological insights and therapeutic options. Circulation. 2021;143:1157–1172. doi: 10.1161/CIRCULATIONAHA.120.050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krueger K.M., Ison M.G., Ghossein C. Practical guide to vaccination in all stages of CKD, including patients treated by dialysis or kidney transplantation. Am J Kidney Dis. 2020;75:417–425. doi: 10.1053/j.ajkd.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 30.Vassalotti J.A., Centor R., Turner B.J., et al. National Kidney Foundation Kidney Disease Outcomes Quality Initiative. Practical approach to detection and management of chronic kidney disease for the primary care clinician. Am J Med. 2016;129:153–162.e7. doi: 10.1016/j.amjmed.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 31.Thavarajah S., Knicely D.H., Choi M.J. CKD for primary care practitioners: can we cut to the chase without too many shortcuts? Am J Kidney Dis. 2016;67:826–829. doi: 10.1053/j.ajkd.2016.02.043. [DOI] [PubMed] [Google Scholar]
- 32.Epping-Jordan J.E., Pruitt S.D., Bengoa R., Wagner E.H. Improving the quality of health care for chronic conditions. Qual Saf Health Care. 2004;13:299–305. doi: 10.1136/qhc.13.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nuño R., Coleman K., Bengoa R., Sauto R. Improving the quality of health care for chronic conditions. Health Policy. 2012;105:55–64. doi: 10.1016/j.healthpol.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Evans J.M., Wheeler S.M., Sati S., et al. Assessing the delivery of coordinated care to patients with advanced chronic kidney disease in Ontario, Canada: a survey of patients and healthcare professionals. Int J Integr Care. 2021;21:30. doi: 10.5334/ijic.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kramer H., Jimenez E.Y., Brommage D., et al. Medical nutrition therapy for patients with non–dialysis-dependent chronic kidney disease: barriers and solutions. J Acad Nutrit Diet. 2018;118:1958–1965. doi: 10.1016/j.jand.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 36.Jimenez E.Y., Kelley K., Schofield M., et al. Medical nutrition therapy access in CKD: a cross-sectional survey of patients and providers. Kidney Med. 2020;3:31–41.e1. doi: 10.1016/j.xkme.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ikizler T.A., Burrowes J.D., Byham-Gray L.D., et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update [published correction appears in Am J Kidney Dis. 2021;77:308] Am J Kidney Dis. 2020;76(3 Suppl 1):S1–S107. doi: 10.1053/j.ajkd.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 38.Greer R.C., Liu Y., Cavanaugh K., et al. Primary care physicians’ perceived barriers to nephrology referral and co-management of patients with CKD: a qualitative study. J Gen Intern Med. 2019;34:1228–1235. doi: 10.1007/s11606-019-04975-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mendu M.L., Waikar S.S., Rao S.K. Kidney disease population health management in the era of accountable care: a conceptual framework for optimizing care across the CKD spectrum. Am J Kidney Dis. 2017;70:122–131. doi: 10.1053/j.ajkd.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 40.Miller W.G., Bachmann L.M., Delanghe J.R., et al. Optimal use of biomarkers for chronic kidney disease. Clin Chem. 2019;65:949–955. doi: 10.1373/clinchem.2018.299073. [DOI] [PubMed] [Google Scholar]
- 41.American Society for Clinical Pathology. http://www.choosingwisely.org/clinician-lists/ascp-serum-creatinine-to-test-for-ckd/
- 42.National Kidney Foundation, Change Package CKD. https://www.ncqa.org/hedis/measures/
- 43.The National Committee for Quality Assurance, HEDISR Technical Specifications, Volume 2, 2022.
- 44.Mendu M.L., Tummalapalli S.L., Lentine K.L., et al. Measuring quality in kidney care: an evaluation of existing quality metrics and approach to facilitating improvements in care delivery. J Am Soc Nephrol. 2020;31:602–614. doi: 10.1681/ASN.2019090869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park K.J., Unitan R.S., Thorp M.L. A quality improvement initiative targeting chronic kidney disease metrics through increased urinary albumin testing. Per M J. 2020;25:1. doi: 10.7812/TPP/20.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vassalotti J.A., DeVinney R., Lukasik S., et al. CKD quality improvement intervention with PCMH integration: health plan results. Am J Manag Care. 2019;25:e326–e333. [PubMed] [Google Scholar]
- 47.Narva A. Population health for CKD and diabetes: lessons from the Indian Health Service. Am J Kidney Dis. 2018;71:407–411. doi: 10.1053/j.ajkd.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tangri N., Grams M.E., Levey A.S., et al. Multinational assessment of accuracy of equations for prediction of kidney failure: a meta-analysis [published correction appears in JAMA. 2016;315:822] JAMA. 2016;315:164–174. doi: 10.1001/jama.2015.18202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nicoll R., Robertson L., Gemmell E., et al. Models of care for chronic kidney disease: a systematic review. Nephrol (Carlton) 2018;23:389–396. doi: 10.1111/nep.13198. [DOI] [PubMed] [Google Scholar]
- 50.Navaneethan S.D., Jolly S.E., Schold J.C., et al. Pragmatic randomized, controlled trial of patient navigators and enhanced personal health records in CKD. Clin J Am Soc Nephrol. 2017;12:1418–1427. doi: 10.2215/CJN.02100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Centers for Medicare and Medicaid Services. https://innovation.cms.gov/innovation-models/kidney-care-choices-kcc-model