Abstract
Nonfuctional pituitary neuroendocrine tumor (NF-PitNET) is highly heterogeneous and generally considered a common intracranial tumor. A series of molecules are involved in NF-PitNET pathogenesis that alter in multiple levels of genome, transcriptome, proteome, and metabolome, and those molecules mutually interact to form dynamically associated molecular-network systems. This article reviewed signaling pathway alterations in NF-PitNET based on the analyses of the genome, transcriptome, proteome, and metabolome, and emphasized signaling pathway network alterations based on the integrative omics, including calcium signaling pathway, cGMP-PKG signaling pathway, mTOR signaling pathway, PI3K/AKT signaling pathway, MAPK (mitogen-activated protein kinase) signaling pathway, oxidative stress response, mitochondrial dysfunction, and cell cycle dysregulation, and those signaling pathway networks are important for NF-PitNET formation and progression. Especially, this review article emphasized the altered signaling pathways and their key molecules related to NF-PitNET invasiveness and aggressiveness that are challenging clinical problems. Furthermore, the currently used medication and potential therapeutic agents that target these important signaling pathway networks are also summarized. These signaling pathway network changes offer important resources for insights into molecular mechanisms, discovery of effective biomarkers, and therapeutic targets for patient stratification, predictive diagnosis, prognostic assessment, and targeted therapy of NF-PitNET.
Keywords: Nonfuctional pituitary neuroendocrine tumor (NF-PitNET), Invasive NF-PitNET, Aggressive NF-PitNET, Multi-omics integration analysis, Calcium signaling pathway, cGMP-PKG signaling pathway, mTOR signaling pathway, PI3K/AKT signaling pathway, MAPK signaling pathway, Oxidative stress response, Mitochondrial dysfunction, Cell cycle dysregulation, Signaling pathway, Molecular network, Biomarker, Therapeutic target, Patient stratification, Predictive diagnosis, Prognostic assessment, Targeted therapy, Predictive preventive personalized medicine (3P medicine; PPPM)
Introduction
Pituitary neuroendocrine tumors (PitNETs) are the second most common primary central nervous system tumors in adults [1]. Among them, non-functional PitNET (NF-PitNET), a PitNET with hormone synthesis but without hormone hypersecretion symptoms, is accounted for 15 ~ 54% [2]. Although NF-PitNET is generally considered a non-malignant tumor, it still significantly decreases patients’ quality of life from multiple aspects [3]. Because of the lack of secondary syndrome from hormone hypersecretion, NF-PitNETs are relatively not easy to be diagnosed at early stage and typically have mass effect symptom with hypopituitarism, headache or visual field defect when they were diagnosed [4, 5]. NF-PitNETs are highly heterogeneous. According to the 2017 World Health Organization classification for tumors in pituitary glands based on adenohypophyseal hormone and transcription factor profile, clinically NF-PitNETs are classified into seven kinds of groups [6]. The high heterogeneity of an NF-PitNET makes it more complex to elucidate the molecular mechanism of formation and progression of NF-PitNET and makes it hard for the common single therapy to achieve an ideal efficacy for NF-PitNET patients [7]. Moreover, more than 40% NF-PitNETs possess invasiveness that is regarded as a malignant potential characteristic of NF-PitNETs [8]. The invasive NF-PITNETs may invade the bone, dura, and cavernous sinuses and can be too infiltrative to be entirely removed during neurosurgery. Patients with invasive NF-PitNETs usually need adjuvant radiotherapy or chemotherapy after neurosurgery, resulting in more complications and poorer prognosis [9]. Nowadays, more and more researchers use omics technologies to analyze genome, transcriptome, proteome, and metabolome of NF-PitNETs to clarify the molecular mechanism of formation and development of NF-PitNETs and find efficient biomarkers and therapeutic targets for NF-PitNET diagnosis and treatment. In addition to single omics analysis, integrative omics analysis, which integrates at least 2 omics technologies, is more widely used in NF-PitNET studies in recent years to meet the requirement of systematic analysis of NF-PitNETs. The molecules at the levels of genome, transcriptome, proteome, and metabolome are mutually regulated and form dynamically associated network in NF-PitNET formation and progression. One molecule change is able to trigger the change of other molecules in the network, it is crucial to study molecular variations at a systematic, multi-omics level [10]. This review explored the current knowledge of single omics and integrative omics of NF-PitNETs and highlighted key signaling pathways and signaling pathway network alterations identified from multi-omics data. Finally, we further probed multi-target pharmaceutical treatment, and biomarkers identified from signaling pathway networks for their important contributions to patient stratification, predictive diagnosis, prognostic assessment, and personalized treatment of NF-PitNETs.
Omics analysis of NF-PitNETs
Genomics analysis of signaling pathway alterations in NF-PitNETs
With the spring up of high-throughput technologies, the application of DNA sequencing technologies, such as next-generation sequencing, genome-level research, is not only used to identify genome sequence but also to expand the space for scientific research such as epigenomics and nucleomics, which paves the way for more accurate molecular medical diagnosis and therapy. With the use of genomic technologies, more and more important signaling pathways affecting NF-PitNET occurrence and progression were identified and deeper analyzed (Table 1). The Ca2+/CaM pathway, which is involved in the activation of tumorgenesis-related PI3K/AKT pathways, was significantly altered with a combination analysis of microarray technology, gene ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG), and protein–protein interaction (PPI) network analysis. The selective inhibitor of Ca2+/calmodulin-dependent protein kinase kinase (CaM-KK), STO-609, exhibited strong inhibiting effects on NF-PitNET growth in experiments in vitro, further verifying the importance of Ca2+/CaM pathway in NF-PitNET formation process and the potential to be a therapeutic target for NF-PitNET [11]. The gene expression profiles related to invasive and non-invasive NF-PitNETs compared to normal pituitaries were mined from the Gene Expression Omnibus (GEO) database and further analyzed with pathway enrichment analysis. Among these differentially expressed genes (DEGs), upregulated genes were mainly enriched in the PI3K-Akt signaling pathway and cysteine biosynthesis/homocysteine degradation (trans-sulfuration) signaling pathway, whereas downregulated genes were significantly associated with chemokine signaling pathway and docosahexaenoate biosynthesis III (mammals) signaling pathway, which clearly demonstrated that the alterations of these pathways might be involved in NF-PitNET invasiveness [12]. Aberrant activation of the Wnt signaling pathway is closely related to tumorigenesis. Secreted frizzled-related proteins (sFRPs) and WIF1 genes both were antagonist genes in the Wnt signaling pathway, and these two genes were decreased in invasive NF-PitNETs, and their low expression showed a significant correlation with tumor invasion. Furthermore, WIF1 promoter was hypermethylated in invasive NF-PitNETs compared to noninvasive NF-PitNETs and miRNA-137 might regulate WIF1 promoter methylation to activate the Wnt signaling pathway and affect NF-PitNET aggressiveness [13]. In addition, protein kinase A in the adenylyl cyclase pathway, key residue (Arg183) in G alpha q in the phospholipase C beta/Ca2+/protein kinase C pathway, and thyrotrophin-releasing hormone (TRH) receptor in the TRH-signaling pathway were studied with valid genomic technologies, respectively. However, for all of them, no mutation was found in NF-PitNETs. It is thereby unlikely that these gene mutations were associated with NF-PitNET etiology, but the mutation possibility of other components in these signaling pathways or other key residues of the same gene cannot be excluded and is still worth further investigating [14–16]. However, all of the omics-level molecules are mutually regulated and form a dynamic system; thus, these reported mutations are nothing but one of the driver factors that cause the alteration of key signaling pathways or molecular networks to affect NF-PitNET occurrence and progression. Therefore, it is necessary for the integration of data from genomics, transcriptomics, proteomics, metabolomics, or other types of omics data to gain a comprehensive vision of NF-PitNETs and find out credible disease management.
Table 1.
Current status of omics studies about signaling pathway alterations in nonfuctional pituitary neuroendocrine tumor (NF-PitNET)
| Omics Type | Method/Tools | Research models | Main signaling pathway alterations | Main result/conclusions | Reference |
|---|---|---|---|---|---|
| Genomics |
Genespring software KEGG analysis |
Microarray data from NF-PitNET and the normal controls |
PI3K-Akt signaling pathway cGMP-PKG signaling pathway |
DEGs and hub genes in NF-PitNET, and their functions and involved key pathways. STO-609 as an inhibitor of CaM-KK might have a therapeutic effect on NF-PitNET by regulating the PI3K-Akt signaling pathway | [11] |
| Tissue microassays | Invasive NF-PitNET compared with non-invasive NF-PitNET | Wnt signaling pathway | Aberrant expression of WIF1 genes might affect the aggressiveness of NF-PitNET. miRNA-137 can regulate the Wnt signaling pathway by increasing promoter methylation of WIF1 to affect NF-PitNET tumorigenesis | [13] | |
| Transcriptomics |
Transcriptomic microarrays analysis KEGG analysis |
Bone-invasive pituitary neuroendocrine tumors [BI-PitNETs) compared with non-bone-invasive pituitary neuroendocrine tumors [NBI-PitNETs) | Inflammatory-related signaling pathways, immune-related signaling pathways, chemokine and-related signaling pathways, and osteoclast differentiation pathway | BI-PitNETs had worse progression-free survival than did NBI-PitNETs. Inflammatory and immune factors play an important role in BI-PitNETs | [17] |
|
In-silico analysis KEGG analysis |
NF-PitNET compared with normal pituitary tissues | Calcium signaling pathway, metabolic pathways, cGMP-PKG signaling pathway, cAMP signaling pathway, TNF signaling pathway, PI3K-Akt signaling pathway, and JAK-STAT signaling pathway | Genes related to calcium metabolism, immune and stem cell were altered in NF-PitNET | [18] | |
|
RNA sequencing Reactome 2016 database |
Invasive NF-PitNET compared with non-invasive NF-PitNET |
Extracellular matrix organization Immune system Integrin cell surface interactions Cytokine signaling in the immune system Innate immune system Assembly of collagen fibrils and other multimeric structures ECM proteoglycans Laminin interactions |
DEGs in invasive NF-PitNET. Local suppression of the immune response and TGF-β signaling can make NF-PitNET prone to invasiveness | [19] | |
|
miRNAs microarray KEGG analysis |
Invasive NF-PitNET compared with non-invasive NF-PitNET |
Prolactin signaling pathways Fatty acid metabolism Endocrine and other factor-regulated calcium reabsorption PPAR signaling pathway |
Differential expressed miRNAs in invasive NF-PitNET, and their functions, and involved key pathways | [20] | |
|
Microarray analyses KEGG analysis |
Gonadotrophin adenoma[GA) compared with normal pituitary tissues |
PI3K-Akt signaling pathway Oxidative phosphorylation cGMP-PKG signaling pathway Calcium signaling pathway JAK-STAT signaling pathway GnRH signaling pathway Prolactin signaling pathway TGF-beta signaling pathway TNF signaling pathway Cytokine-cytokine receptor interaction Fat digestion and absorption |
Differentially expressed lncRNAs and mRNAs in NF-PitNETs. lncRNA-mRNA co-expression network in NF-PitNETs. The signaling pathways alterations that specific mRNAs involved | [21] | |
|
RNA microarray PPI network analysis KEGG analysis |
Fast recurrence NF-PitNET compared with slow recurrence NF-PitNET |
Cell cycle Cytokine-cytokine receptor interaction T cell migration T cell chemotaxis T cell activation TNF signaling pathway Positive regulation of DNA biosynthetic process Adherence junction Regulation of cell–cell adhesion Regulation of protein insertion into mitochondrial membrane involved in an apoptotic signaling pathway ErbB signaling pathway Interferon-γproduction |
Featured lncRNA-mRNA co-expression network related to NF-PitNET recurrence. The signaling pathways alterations that differentially expressed mRNA and lncRNAs involved | [22] | |
| RNA sequencing | GA compared with normal pituitary tissues | mTOR signaling pathway | Specific differentially expressed lncRNA might cause mTOR signaling pathway alteration to affect GA tumorgenesis | [23] | |
|
Transcriptome profiling IPA |
MENX-associated rats GA compared with their normal counterparts |
Cell cycle Cell differentiation/proliferation, development Lipid metabolism |
MENX rat GA model is reliable for human GA pathomechanisms study. The signaling pathway that the dysregulated transcripts of MENX rat GA model mainly enriched on | [24] | |
|
Whole transcriptome sequencing IPA |
Silent subtype III pituitary adenomas[SS-3) and conventional null cell adenomas |
p53 signaling VEGF family ligand-receptor interactions G-protein coupled receptor signaling IL-4 signaling iCOS-iCOSL signaling in T helper Cells Role of NFAT in the regulation of the immune response CD28 signaling in T helper cells PKCh signaling in T lymphocytes Th1 pathway |
DEGs in SS-3 adenomas.SS-3 adenomas actively suppress the immune system | [25] | |
| Proteomics | IPA | Three PitNET proteomic datasets, from NF-PitNET, normal controls, and prolactinoma |
Mitochondrial dysfunction Cell-cycle dysregulation Oxidative stress MAPK-signaling abnormality |
Mitochondrial dysfunction, oxidative stress, cell-cycle dysregulation, and the MAPK-signaling abnormality are significantly associated with NF-PitNET tumorigenesis | [26] |
|
Anti-ubiquitin antibody-based label-free quantitative proteomics method KEGG analysis |
NF-PitNET compared with normal pituitary tissues |
PI3K-AKT signaling pathway ribosome Hippo signaling pathway nucleotide excision repair |
Ubiquitinated proteomic profiling and ubiquitination-involved signaling pathway networks in human NF-PitNET | [27] | |
|
Tandem mass tag [TMT)‐based quantitative proteomics coupled with TiO2 enrichment of phosphopeptides KEGG analysis |
NF-PitNET compared with normal pituitary tissues |
Spliceosome pathway RNA transport pathway Proteoglycans in cancer SNARE interactions in vesicular transport Platelet activation Bacterial invasion of epithelial cells Tight junctions Vascular smooth muscle contraction Protein processing in the endoplasmic reticulum |
Differentially phosphorylated proteins profiling, phosphorylation‐mediated molecular network alterations in NF‐PitNET | [28] | |
|
2DE and MALDI-TOF MS and LC–ESI–MS/MS IPA |
Invasive NF-PitNET compared with non-invasive NF-PitNET |
Mitochondrial dysfunction Oxidative stress Proteolysis abnormality MAPK-signaling abnormality CDK5 signaling abnormality ketogenesis and ketolysis amyloid processing TR/RXR activation |
Proteomic variations and pathway network variations exist between invasive and non-invasive NF-PitNETs | [29] | |
|
2DE and MALDI-TOF PMF and LC–ESI–MS/MS IPA |
Four NF-PitNET subtypes (NF-, LH-, FSH-, and LH/FSH-positive) |
MAPK-signaling abnormality Oxidative stress Mitochondrial dysfunction Cell-cycle dysregulation |
Common and specific DEP profiles and pathway networks among four NF-PitNET subtypes | [30] | |
|
2DE and MALDI-TOF MS IPA |
FSH-positive NF-PitNET |
Mitochondrial dysfunction Oxidative stress Cell-cycle alteration Gluconeogenesis and glycolysis MAPK signaling system Immune response VEGF-signaling TP53-signaling Inflammation signaling pathways |
Functional profile of the proteome of a human FSH-positive NF-PitNET tissue | [31] | |
| TMT-based quantitative proteomics | FSH-positive relative to negative NF-PitNET |
PI3K-Akt signaling pathways ECM-receptor interaction Focal adhesion |
Proteomic variations and the corresponding molecular network alterations in FSH-positive compared to negative NF-PitNET | [32] | |
| Immunohistochemistry | NF-PitNET and somatotrophinomas | pRb/p16/cyclin D1/CDK4 pathway | Components of the pRb/p16/cyclin D1/CDK4 pathway are frequently dysregulated in human NF-PitNET | [33] | |
|
Genome-wide DNA methylation mRNA microarray analysis KEGG analysis |
Invasive NF-PitNET compared with non-invasive NF-PitNET | viral carcinogenesis pathway | DNA methylation alteration in the promoter region and gene expression changes in invasive NF-PitNET | [34] | |
| Epigenomics [methylomic) and transcriptomics |
Human Methylation450 BeadChip PAXgene RNA kit IPA |
Highly proliferative (hpNF-PitNET) and lowly proliferative NF-PitNETs[lpNF-PitNET) |
PPARα/ RXRα signaling pathway Dopamine receptor signaling pathway cAMP-mediated signaling pathway Calcium signaling pathway p38 MAPK signaling pathway EGF and FGF signaling pathway |
Comprehensive multi-omics profiles of CNV, DNA methylation, and gene expression in NF-PitNET, and the pathway networks alteration in hpNF-PitNET that DEGs involved in | [35] |
|
RNA microarray LC–MS/MS IPA |
Invasive NF-PitNET compared with non-invasive NF-PitNET |
Cell cycle Cellular movement, Cellular growth, proliferation Cellular development Cellular dell death and survival Production of NO and ROS in macrophages |
DEGs and DEPs correlated with invasion of NF-PitNET, and the signaling pathway alteration that common differentially expressed molecules involved in | [36] | |
| Transcriptomics and proteomics |
RNA microarray LC–MS/MS IPA |
Invasive pituitary null cell adenomas [PNCAs)compared with noninvasive PNCAs |
IL-6R/JAK2/STAT3/MMP9 signaling pathway Integrin signaling Acute-phase response signaling Leukocyte extravasation signaling Fcγ receptor-mediated phagocytosis in macrophages and monocytes Actin cytoskeleton signaling Production of nitric oxide and reactive Oxygen species in macrophages LXR/RXR activation PPARα/RXRα activation |
DEGs and DEPs patterns and the critical biological signaling pathways involved in the invasion of PNCAs. The overactivation of the IL-6R/JAK2/STAT3/MMP9 pathway is critical for the invasion of PNCAs | [37] |
|
TMT-based quantitative proteomics KEGG analysis |
NF-PitNET and normal pituitary tissues, and their DEGs datasets |
cGMP-PKG pathway Focal adhesion Carbon metabolism Dopaminergic synapse Proteoglycans in cancer Regulation of actin cytoskeleton Biosynthesis of amino acids |
DEGs and DEPs profiling in NF-PitNET, and the key signaling pathways that differentially expressed molecules involved in NF-PitNET | [38] | |
| TMT‐based quantitative proteomics coupled with TiO2 enrichment of phosphopeptides KEGG analysis | NF-PitNET and normal pituitary tissues, and the DEGs datasets from invasive and noninvasive NF-PitNETs |
Cell–cell adhesion GTPase signaling pathway Protein kinase signaling Calcium signaling pathway cGMP–PKG signaling pathway GnRH signaling pathway Inflammatory mediator regulation of TRP channels Fc gamma R-mediated phagocytosis |
Phosphoprotein profiling in NF-PitNET. Phosphorylation-mediated signaling pathway network alteration in NF-PitNET invasiveness | [39] | |
|
Anti-acetyl antibody-based label-free quantitative proteomics method KEGG analysis |
NF-PitNET compared with normal pituitary tissues, and the DEGs datasets from invasive and noninvasive NF-PitNETs |
Oxidative phosphorylation Carbon metabolism Glycolysis / gluconeogenesis |
Acetylomic profiling in human NF-PitNETs and acetylation-mediated signaling pathways alteration related to NF-PitNET invasiveness | [40] | |
|
Meta-analysis coupled with IPA |
Nine NF-PitNET/invasive NF-PitNET omics datasets from transcriptomics, proteomics, phosphoproteomics, and nitroproteomics |
Cell cycle, proliferation and apoptosis-related pathways Mitochondrial dysfunction and energy metabolism -related pathways Cytoskeleton, cell adhesion and movement pathways Angiogenesis, invasion, and metastasis-related pathways Toxin metabolism and oxidative stress-related pathways Protein synthesis, degradation and amino acid metabolism-related pathways Immunity related pathways ER stress-related pathways |
Comprehensive and large-scale pathway network data for NF-PitNET | [10] | |
| Metabolomic and transcriptomic/proteomic |
UPLC-MS KEGG analysis |
Invasive and noninvasive silent corticotroph adenomas [SCAs), and the DEGs datasets from invasive and noninvasive NF-PitNETs |
Glycosylphosphatidylinositol-anchor biosynthesis Glycerophospholipid metabolism Ghosphatidylinositol signaling system Glycerolipid metabolism |
The lipidomic profiling in invasive and noninvasive SCAs | [41] |
|
GC–MS RNA microarray iTRAQ and LC‐MS/MS Enrichment and topology analyses |
Eight pituitary adenoma subtypes and normal pituitary tissues |
Glycolysis / Gluconeogenesis Citrate cycle Glycerolipid metabolism Fatty acid metabolism |
Metabolic pathways changes in pituitary adenomas | [42] |
Transcriptomics analysis of signaling pathway alterations in NF-PitNETs
Transcriptomic technologies, including RNA sequencing and microarray, were extensively used to detect differentially expressed mRNAs, long non-coding RNAs (lncRNAs), and microRNAs (miRNAs) in NF-PitNETs to find out signaling pathways or molecular networks related to tumorgenesis, invasiveness, recurrence, and therapy (Table 1). Patients with bone-invasive PitNETs (BI-PitNETs) were found to have shorter progression-free survival than non-bone-invasive PitNETs (NBI-PitNETs) in the NF-PitNET group. A comprehensive transcriptomics analysis was performed to identify differentially expressed mRNAs, lncRNAs, circRNAs, and miRNAs between BI-PitNETs and NBI-PitNETs and explore key pathway alterations and potential mechanisms for BI-PitNET tumorigenesis. Immune and inflammatory pathways and osteoclast differentiation pathway were altered in BI-PitNET since most differentially expressed mRNAs were enriched in these pathways with GO and KEGG analysis, and other key pathways were also found to involve in BI-PitNET tumorigenesis, including apoptosis and NF-kB signaling pathway by the construction of pathway active network [17]. Research with in silico analysis also reported that genes related to immune and calcium metabolism were altered in NF-PitNETs compared to normal tissues [18]. Another study found that local immune response was attenuated and TGF-β signaling was down-regulated in invasive NF-PitNETs, which might be related to NF-PitNET invasiveness [19]. The miRNAs are single-stranded, non-coding RNAs with approximately 22 nucleotides and function in homologous sequence-dependent gene silencing in cells. A study performed a miRNA microarray analysis to identify differentially expressed miRNAs between invasive and non-invasive NF-PitNETs and explore miRNAs involved in NF-PitNET invasiveness, which revealed that a series of signaling pathways, including endocrine and other factor-regulated calcium reabsorption and fatty acid metabolism, were altered in invasive NF-PitNETs with GO and KEGG analysis of differentially expressed miRNAs [20]. LncRNAs are RNAs that do not code proteins and have more than 200 nucleotides in length. LncRNAs play important roles in many essential biological processes such as tumorgenesis and might be a promising target for diagnosis, therapy, and prognostic assessment in numerous cancers. According to the current understanding regarding lncRNA, the most prominent function of lncRNA appears to regulate mRNA expression so that the co-expression between a lncRNA and a particular mRNA might provide a relatively credible hint to predict the functions of that lncRNA [43]. A study has identified differentially expressed lncRNAs and mRNAs in NF-PitNETs and constructed a lncRNA-mRNA co-expression network to predict the biological function and/or action mechanism of specific lncRNA, the upregulated lncRNAs might be involved in signaling pathway alterations, including oxidative phosphorylation and calcium signaling pathways; and the down-regulated lncRNAs might be involved in signaling pathway alterations, including Jak-STAT signaling pathway and PI3K-AKt signaling pathway [21]. Another study constructed a lncRNA-mRNA co-expression network to reveal the lncRNA related to NF-PitNET recurrence, and these lncRNAs functioned in signaling pathways, including cell cycle, and tumor necrosis factor (TNF) signaling pathway identified with KEGG analysis [22]. Gonadotrophin adenomas (GA) are comprised of 29–35% of NF-PitNETs and often present a larger volume and invasiveness. A co-expression analysis was performed between differentially expressed lncRNAs identified in GAs and differentially expressed mRNAs (extracted with ingenuity pathway analysis) of mTOR (the mammalian target of rapamycin) signaling pathways, which found that some differentially expressed lncRNAs might cause mTOR signaling pathway alterations to affect GA tumorigenesis [23]. Multiple endocrine neoplasia syndrome (MENX)–associated rat GA models were constructed to perform whole transcriptomic analysis for GA molecular mechanism study in humans since remarkable similarities exist between MENX-associated rat GA and their human counterparts [24]. This study found that dysregulated transcripts were mainly enriched in signaling pathways related to cell cycle, cell differentiation/proliferation, development, and lipid metabolism, which demonstrates that these pathways might involve in GA formation [24]. In addition, various studies found that immune-related genes and pathways were altered in NF-PitNETs, particularly in aggressive NF-PitNETs [17–19, 25]. Immunotherapy becomes a promising strategy to deal with refractory NF-PitNETs. In recent years, a novel computational tool, direct data integration, was proposed to combine available microarray datasets of NF-PitNET to find out immune-related genes, which was considered more credible target candidates for NF-PitNET immunotherapy [44]. Transcriptomic technologies have identified much more signaling pathway alterations, and those altered key molecules in those signaling pathways might be target candidates for NF-PitNET therapy, compared to genomic technologies. A transcriptomic dataset is a valuable resource to combine other omic-data for integrative omics analysis of NF-PitNETs to elucidate molecular network alterations for NF-PitNET tumorigenesis and progression and find out effective biomarkers for patient stratification, predictive diagnosis, targeted therapy, and prognostic assessment of NF-PitNET.
Proteomics analysis of signaling pathway alterations in NF-PitNETs
Proteins are the final executor of genetic function in a biological system, whose abnormalities, including the difference in protein abundance, post-translational modifications (PTMs), and dysfunction in protein activity, protein–protein interactions, might alter signaling pathways, protein complex, and metabolism in cells and affect tumorigenesis [45, 46]. With the development and improvement of protein separation and identification technologies, proteomic analysis has identified more and more differentially expressed proteins (DEPs) between different subtypes of NF-PitNETs and controls and revealed corresponding signaling pathways and molecular networks involved in the formation and development of NF-PitNETs [47] (Table 1). Among them, comparative proteomics analysis is the most commonly used approach. A comprehensive pathway network analysis of three NF-PitNET proteomic datasets, including protein-mapping data from an NF-PitNET, comparative proteomics data from NF-PitNETs and prolactinoma relative to control pituitary tissues, and nitroproteomic data from NF-PitNETs and control pituitary tissues, revealed four important signaling pathway alterations, including mitochondrial dysfunction, cell-cycle dysregulation, oxidative stress, and MAPK-signaling abnormality, for NF-PitNET development [26]. Other comparative proteomics analyses between NF-PitNETs and control pituitaries mainly focused on PTM alterations, such as ubiquitination, phosphorylation, and acetylation. A comparative ubiquitinomics analysis found four significantly altered ubiquitination-mediated signaling pathways, including PI3K-AKT signaling pathway, ribosome, hippo signaling pathway, and nucleotide excision repair, to affect tumorigenesis in NF-PitNETs [27]. A comparative phosphoproteomics analysis found nine significantly altered phosphorylation-mediated signaling pathways, including spliceosome pathway, RNA transport pathway, proteoglycan in cancer, SNARE interactions in vesicular transport, platelet activation, bacterial invasion of epithelial cells, tight junction, vascular smooth muscle contraction, and protein processing in the endoplasmic reticulum [28]. A comparative acetylomics analysis found that proteins differentially acetylated in NF-PitNET mainly caused metabolism-related signaling pathway alterations, such as oxidative phosphorylation, citrate cycle, and glycolysis/gluconeogenesis, to affect tumorigenesis [40]. The pathway network analysis of DEP data between invasive and non-invasive NF-PitNETs found that eight signaling pathways were significantly associated with NF-PitNET invasiveness, including mitochondrial dysfunction, oxidative stress, proteolysis abnormality, MAPK-signaling abnormality, CDK5 signaling abnormality, ketogenesis and ketolysis, amyloid processing, and TR/RXR activation [29]. The pathway network analysis of DEPs between four different hormone-expressed subtypes of NF-PitNETs (LH+, FSH+, LH/FSH+, and NF−; NF− means NF-PitNET with negative immunohistochemical stains for ACTH (adrenocorticotropic hormone), GH (growth hormone), FSH (follicle-stimulating hormone), LH (luteinizing hormone), TSH (thyroid-stimulating hormone), and prolactin) and control pituitaries revealed that four signaling pathway systems were commonly altered in each NF-PitNET subtype, including MAPK-signaling abnormality, oxidative stress, mitochondrial dysfunction, and cell-cycle dysregulation [30]. However, these four common pathway systems were not the same among four NF-PitNET subtypes, which mainly reflected in different protein profiles, different expression levels of most protein nodes, and different pathway network profiles [30]. Silent hormone-expressed NF-PitNET subtypes demonstrated a more invasive and aggressive trend [48, 49]. A proteomic analysis identified the protein profiles of a silent hormone-expressed NF-PitNET subtype, FSH+-NF-PitNETs, and these proteins were mainly involved in mitochondrial dysfunction, oxidative stress, cell-cycle alteration, gluconeogenesis and glycolysis, MAPK signaling system, immune response, VEGF-signaling, TP53-signaling, and inflammation signaling pathways [31]. Moreover, a pathway network analysis of DEPs between FSH+-NF-PitNETs and control pituitaries found that three signaling pathway alterations, namely ECM-receptor interaction, focal adhesion, and PI3K-Akt signaling pathways, were significantly associated with tumor invasiveness and aggressiveness [32]. In addition, the pRb/p16/cyclinD1/CDK4 pathway was also altered in NF-PitNET tumorigenesis with the direct detection of the key component expressions (pRb, p16, and cyclin D1) of this pathway [33].
With the concept formation of one gene corresponding to multiple proteoforms, the number of human proteoforms is estimated to be billions in total [50, 51]. However, the achieved proteomic studies of NF-PitNETs mainly focused on the difference in the copy number or abundance of a protein between different characteristics or subtypes of NF-PitNETs compared to control pituitaries, a few studies referred to proteoforms, including PTMs. Proteomic analysis about proteoform alterations in NF-PitNETs is much insufficient, which needs to br extensively carried out for the comprehensive understanding of proteoform alterations and the corresponding pathway-network system alterations at the proteoform level in NF-PitNETs. In addition, the proteome reflects the results of the underlying transcriptome and genome and affects the downstream metabolome to some extent. Thus, the integration of proteomic data with other omics data benefits the in-depth understanding of the significance of proteomic data and overall alterations in pathway-network systems of NF-PitNETs.
Metabolomics analysis of signaling pathway alterations in NF-PitNETs
Metabolomics is a relatively recent entry into the study of omics, which is represented by metabolites, a kind of small molecular chemical entities in cells, biofluids, and tissues [52]. The metabolome is the most downstream stage of the dynamic biological system in humans, and intertwines with the activities of the genome and proteome [53]. However, seldom has metabolomics analysis been performed in NF-PitNETs, and the present metabolomics analysis about NF-PitNETs mainly aimed to find out valuable biomarkers for diagnosis and prognostic assessment [54–56]. Few pathway-network analyses have been performed based on the present metabolomic data, which is a huge gap in the fields of metabolomics analysis of NF-PitNETs, and it is worth more investigating.
Integrative omics analysis of signaling pathway alterations in NF-PitNETs
Each type of omics data mentioned above (“Genomics analysis of signaling pathway alterations in NF-PitNETs,” “Transcriptomics analysis of signaling pathway alterations in NF-PitNETs,” “Proteomics analysis of signaling pathway alterations in NF-PitNETs,” and “Metabolomics analysis of signaling pathway alterations in NF-PitNETs” sections) has typically provided differentially expressed profiles associated with NF-PitNET pathological process. Based on these data, the corresponding pathway-network alterations were identified between NF-PitNETs and controls. However, the information that underlies NF-PitNET formation and progression flows through different omics levels. The analysis of only a single omics type is insufficient to reflect correlation, especially the causative ones among different omics [57]. Integration of the different omics data to analyze NF-PitNETs is promised to conduct a comprehensive insight into its molecular pathogenesis-related pathway-network systems and find out potential causative changes that lead to NF-PitNET formation and progression. The development of high-throughput technologies and abundant omics data enable researchers to integrate multi-omics data for in-depth study. In recent years, more and more integrative omics studies have been performed to analyze NF-PitNETs and found out some significant signaling pathway alterations. These studies focused on the integration of two omics level data, including the integration of epigenomics and transcriptomics, transcriptomics and proteomics, and transcriptomics and metabolomics (Table 1).
Epigenomic (methylomic) and transcriptomic analysis of signaling pathway alterations in NF-PitNETs
DNA methylation plays an important role in the complex, multi-factor epigenetic regulation of gene expressions [58]. Approximately 10% of differentially methylated CpGs were related to gene expression, and generally, the affected genes were involved in various tumorigenesis-related pathways [59]. An integrative analysis of methylomics and transcriptomics between invasive and non-invasive NF-PitNETs found the key genes and pathways that function in tumor invasion. The integrative analysis of differentially methylated genes and DEGs identified 115 genes that altered both in promoter methylation and expression, and among them, 58 genes showed a negative correlation in DNA methylation status vs expression level. KEGG pathway analysis of these 58 genes found that these genes were mainly enriched in the viral carcinogenesis pathway [34]. Moreover, one study used multi-omics approaches to analyze the profile of DNA methylation, copy number variation (CNV), and DEGs between highly proliferative (hpNF-PitNET) and lowly proliferative NF-PitNETs (lpNF-PitNET), which found that loss of methylation occurred in hypermethylated section, and aberrant arm level CNV existed in two samples of hpNF-PitNETs; and in these two samples, chromosomal losses were associated with decreased expressions of DNA methyltransferases, which further altered their global methylation. Methylation in promoter and gene body regions was identified to be involved in gene regulation between DNA methylation and gene expression in all hpNF-PitNETs and lpNF-PitNETs with correlation analysis. Given the epigenetic changes can alter gene expression and affect biological functions, the ingenuity pathway analysis (IPA) of DEGs between hpNF-PitNETs and lpNF-PitNETs found that PPARα/RXRα, cAMP-mediated signaling, calcium signaling, and dopamine receptor signaling were all activated, whereas ERK5 and p38 MAPK signaling were inhibited in hpNF-PitNETs [35]. Other studies that integrated methylomic and transcriptomic profiles focused on the identification of key genes that can be served as biomarkers for NF-PitNET re-growth evaluation, or investigation of the role of DNA methylation in gene expression misregulation of GAs; however, it is a pity that neither of them analyzed the pathway-network alterations that underlie NF-PitNET re-growth or the pathway-network alterations that underlies the misregulation of aberrant DNA methylation to gene expression in GAs [59, 60]. Previous studies revealed that NF-PitNET was the PitNET subtype that was most affected by aberrant DNA methylation [61–63]. Thereby, it is significant to further integrate methylomic and transcriptomic data to investigate pathway-network alterations that operated by aberrant DNA methylation-affected DEGs for in-depth understanding of NF-PitNET molecular pathogenesis. This field is worth more in-depth investigating.
Transcriptomic and proteomic analysis of signaling pathway alterations in NF-PitNETs
The central dogma of molecular biology deems RNA as an intermediate link between DNA and protein, which can read out the genetic information of DNA and direct the translation of proteins [64]. However, large transcriptomic studies have shown that, although up to 80% of the genome is transcribed, only ~ 1% of the genome encodes proteins [65]. A mass of non-coding RNA functions in many biological processes, including endocrine regulation, gene expression regulation, and signal transduction [66]. The single assessment of alterations at the transcriptomic level is insufficient to reflect the alterations at the proteomic level between NF-PitNETs and controls because additional post-transcriptional mechanisms, such as PTMs and alternative splicing, affect the level of a protein presented. Therefore, proteomic analysis is an important complementary technology for transcriptomic analysis to monitor gene expressions. One study preformed transcriptomic analysis with RNA microarray to identify differentially expressed mRNAs and proteomic analysis with liquid chromatography-tandem mass spectrometry (LC–MS/MS) to identify DEPs, between invasive and noninvasive NF-PitNETs; and then integrative analysis of differentially expressed mRNAs and DEPs with IPA analysis discovered significantly altered pathway-network systems and cellular functions with 29 differentially expressed molecules involved in; and these 29 differentially expressed molecules were enriched into 25 significant pathways. Some of these pathways overlapped with each other, and among them, the pathway about production of NO and ROS in macrophages shared the most overlap with other pathways. Based on the 25 pathways, two significant networks related to tumor invasion were also identified, namely (i) cellular movement, cellular growth, proliferation, and cellular development, and (ii) cellular movement, cellular development, and dell death and survival [36].
Pituitary null cell adenoma (PNCA) is an NF-PitNET subtype that originates from pluripotential uncommitted precursor cells and occupies approximately 30% of all NF-PitNETs [37]. Due to the heterogeneities among different NF-PitNET subtypes, the mechanism that underlies PNCA invasion might be different from other subtypes, or the mechanism suggested in the previous study based on data from overall NF-PitNETs. One study performed an integrative transcriptomic and proteomic analysis to investigate the profile of differentially expressed molecules between invasive and noninvasive PNCAs, which found 15 significant signaling pathways involved in PNCA invasion. Among them, eight pathways presented similar trends across the two datasets, one of which is the acute phase response signaling pathway with two molecules (IL-6R and STAT3) enriched. Thereby, the IL-6R/STAT3 cascade was considered to be activated in this signaling pathway in PNCA invasiveness. Furthermore, the disease and biological function analysis in the IPA system was performed to show the downstream effects of these differentially expressed molecules, which also found that IL-6R/STAT3 molecules were related to the migration of cells. The upstream analysis predicted IL-6, the upstream regulator of IL-6R/STAT3, as one of the upstream regulators of these differentially expressed molecules. This study further validated the gene and protein expressions of IL-6R, JAK2, and STAT3 in invasive and noninvasive PNCAs, which found that gene and protein expression of these molecules were increased in the invasive PNCAs. Therefore, IL-6R/JAK2/STAT3 pathway was suggested to be activated in PNCAs and correlated with the invasiveness of PNCA [37].
One study identified the expressed pattern of differential molecules between NF-PitNETs and normal pituitaries from the overlap of transcriptomic and proteomic data, which were obtained from GEO datasets and tandem mass tag (TMT)–based quantitative proteomics analysis, respectively [38]. In total, 52 statistically significant pathways were identified based on these differentially expressed molecules with KEGG pathway analysis, including focal adhesion, platelet activation, cGMP-PKG signaling pathway, carbon metabolism, dopaminergic synapse, proteoglycans in cancer, human cytomegalovirus infection, regulation of actin cytoskeleton, biosynthesis of amino acids, and retrograde endocannabinoid signaling [38]. PTMs, such as nitration, phosphorylation, ubiquitination, and acetylation, are important aspects of a proteome to mediate a large fraction of protein functions and play key roles in many intracellular signaling processes, including controlling enzyme activity, maintaining overall cell structure, and protein turnover and transport. One study identified and quantified phosphoproteins and phosphosites in NF-PitNETs and normal pituitaries with TMT-labeling reagents incorporated with TiO2 enrichment of phosphopeptides and LC–MS/MS, and obtained transcriptomics data between invasive and non-invasive NF-PitNETs from the GEO database. Subsequently, the two datasets were overlapped to investigate phosphorylation-mediated molecular events for NF-PitNET invasive characteristics. KEGG analysis of these overlapped molecules identified phosphorylation-mediated signaling pathway network alterations related to tumor invasiveness, including platelet activation, long-term depression, proteoglycans in cancer, insulin signaling pathway, salivary secretion, gap junction, calcium signaling pathway, estrogen signaling pathway, cGMP-PKG signaling pathway, glucagon signaling pathway, GnRH signaling pathway, vascular smooth muscle contraction, inflammatory mediator regulation of TRP channels, and Fc gamma R–mediated phagocytosis [39].
Another study about acetylated protein profiling of NF-PitNETs was also overlapped with transcriptomics data from invasive and noninvasive NF-PitNETs. KEGG analysis of these overlapped molecules found that they were mainly involved in the metabolism-related signaling pathways, such as carbon metabolism, oxidative phosphorylation, and glycolysis/gluconeogenes, which indicated that the acetylation-mediated signaling pathway network alterations might regulate metabolic reprogram to affect NF-PitNET invasiveness [40].
In addition, one study integrated nine sets of NF-PitNET omics data that previously documented, namely NF-PitNET quantitative transcriptomics datasets, NF-PitNET quantitative proteomics datasets, NF-PitNET mapping protein datasets, NF-PitNET mapping protein nitration datasets, invasive NF-PitNET quantitative transcriptomics datasets, invasive NF-PitNET quantitative proteomics datasets, control mapping protein datasets, control mapping protein nitration datasets, and control mapping phosphorylation datasets, to bring a more comprehensive insight into the molecular-network system that affects NF-PitNET formation and progression by meta-analysis coupled with IPA pathway-network analysis [10]. Based on the nine NF-PitNET omics datasets, a total of 519 statistically significant canonical pathways were identified; and among them, 139 were mined from a least 2 datasets. Among the 139 canonical pathways, 68 were considered that were obviously related to the tumor occurrence and development in direct and indirect ways. Among 68 tumor-related pathways, 54 canonical pathways that were involved in any DEGs or DEPs were further divided into nine canonical-pathway panels based on similar cellular functions and biological processes, including cytoskeleton, cell adhesion and movement pathways, mitochondrial dysfunction and energy metabolism-related pathways, angiogenesis, invasion, and metastasis-related pathways, toxin metabolism and oxidative stress-related pathways, protein synthesis, degradation and amino acid metabolism-related pathways, cell cycle, proliferation and apoptosis-related pathways, immunity-related pathways, ER stress-related pathways, and others. Among them, the expression patterns and phosphorylations of four important molecular-network systems, including mTOR, PI3K/AKT, Wnt, and ERK/MAPK pathway systems, were confirmed to alter in NF-PitNETs with PTMScan experiments [10].
More and more studies have been committed to the integrative analysis of transcriptome and proteome to investigate signaling pathway network alterations affecting NF-PitNET tumorigenesis and progression, but most of them just focus on protein-coding transcriptome and expression level of proteome. In fact, non-coding RNA fields have significantly developed in past decades, and thousands of novel isoforms have been identified. The proteome is even more complex than other omes because of billions of proteoforms from post-translational mechanisms, including PTMs and alternative splicing. Therefore, more attentions are worthy to pay to the protein-noncoding transcriptome and proteoform alterations of NF-PitNETs and their integrative analysis in future researches.
Metabolomic and transcriptomic/proteomic analysis of signaling pathway alterations in NF-PitNETs
The biochemical roles of metabolites are far-reaching. Metabolites can regulate epigenetic mechanisms, modulate PTMs that affect protein activity, and interact with proteins to initiate signaling cascades to facilitate cellular responses, etc. [52, 53, 67]. Lipid metabolism has been reported to substantially reprogram in cancers to meet the needs of increased membrane biogenesis by strong upregulation of lipogenesis [68]. Silent corticotroph adenoma (SCA) is one of the clinically NF-PitNETs without clinical characteristics of Cushing’s syndrome and can be distinguished from other NF-PitNETs only by exhibiting immunopositivity for t-box transcription factor (TPIT) with ACTH immunopositivity or negativity with postoperative pathological examinations. Similar to other NF-PitNETs with hormone immunopositive, SCAs exhibit more aggressively, more frequently invade into surrounding tissues, such as cavernous or sphenoid sinus, and present a higher rate of recurrences than hormone immunonegative NF-PitNETs. To explore the lipid alterations that were associated with SCA invasiveness and the underlying molecular mechanism, one study identified differentially lipidomic profiles between invasive and noninvasive SCAs with ultra-performance liquid chromatography-mass spectrometry (UPLC-MS) [41]. Generally, lipids and genes in the same pathway functioned and were dysregulated together; thus, they further integrated differential lipids and DEGs between invasive and noninvasive SCAs with KEGG pathway analysis to reveal their intrinsic connections. A total of 28 differential lipids and 1114 DEGs were identified between invasive and noninvasive SCAs. Among them, 2 differential lipids and 17 DEGs were found to share four signaling pathways, including glycosylphosphatidylinositol-anchor biosynthesis, glycerophospholipid metabolism, phosphatidylinositol signaling system, and glycerolipid metabolism, and a multiomic functionally connected network was constructed based on these three factors. These key lipids and genes are generally believed to function and dysregulate together [41]. A study integrated the results of metabolic and proteomic analyses in the 8 subtypes of PitNETs with normal pituitary glands as controls, including corticotroph adenomas (silent ACTH and functional ACTH), gonadotroph adenomas, somatotroph adenomas, mammosomatotroph adenomas, lactotroph adenomas, oncocytomas, and null cell adenomas, which revealed that several metabolic pathways changed in these subtypes of PitNETs by the enrichment and topology analyses [42]. However, metabolomics is still rarely applied in NF-PitNET studies. With innovative bioinformatics and analytical technologies developed, in fact, it is now feasible to expand metabolomic analyses to understand the effects of metabolites at the system level. Due to the far-reaching roles of metabolites, metabolomic analysis is worthy to bring into integrative omics studies of NF-PitNETs to aid in finding out key molecules that affect NF-PitNET formation and progression by constructing a systematic molecular network.
Signaling pathways operated in human NF-PitNETs
The integrated omics analysis of NF-PitNETs mentioned above found that several signaling pathways are altered in the NF-PitNET tumorigenesis and progression, which are considered as valuable resources to systematically elucidate the molecular pathogenesis of NF-PitNETs and discover hub-molecules that might become effective biomarkers for diagnosis and therapy of NF-PitNETs. Some of these pathway alterations have been intensively studied in NF-PitNETs or verified as important for tumorigenesis and progression (Table 2).
-
i.
Calcium signaling pathway alteration: This pathway was found to be altered in hpNF-PitNETs compared to lpNF-PitNETs [35]. The phosphorylation alterations of proteins enriched in the calcium signaling pathway can affect NF-PitNET invasive characteristics [39]. Cytosolic calcium ([Ca2+]i) is a pivotal second messenger that directly links to hormone release, which synergizes with the signaling of cyclic adenosine mono-phosphate (cAMP) to control virtually all secretory gland functions [69]. It is reported that pituitary cells appear to proliferate in response to cAMP, leading to tumorigenesis [70]. Some specific peptides, such as hypothalamic peptide and pituitary adenylate cyclase-activating polypeptide, can modulate [Ca2+]i and cAMP formation in NF-PitNETs that suggested their possible modulatory action on tumor growth [71]. An additional potential mechanism by which calcium signaling regulates pituitary cell function is the activation of Ca2+-sensing receptor (CaSR), which induced a significant increase of [Ca2+]i and cAMP [72]. In addition, an assay in vitro was conducted with STO-609, the selective inhibitor of CaM-KK, to verify its anti-NF-PitNET effects, which was found to induce apoptosis of NF-PitNET cells, and inhibit tumor cellular viability, diffusion and migration [11]. Other signaling pathways mined from integrated omics analysis of NF-PitNETs, including the cGMP-PKG signaling pathway and MAPK signaling pathway, have been found to interact with calcium signaling pathway to co-regulate many pathophysiological processes. In gene transcription, Ca2+ acted as an activator to promote its process by recruiting MAPK signaling pathways. In cardiac compensatory hypertrophy, angiotensin II and endothelin that function by Gq and phospholipase C-β to produce inositol-1,4,5-trisphosphate, which could increase Ca2+ and diacylglycerol. This process seems to realize by recruiting the MAPK signaling pathway to stimulate cAMP response element-binding protein [73, 74]. However, the crosstalk of calcium signaling pathway and these two signaling pathways have not been reported in NF-PitNETs so far.
-
ii.
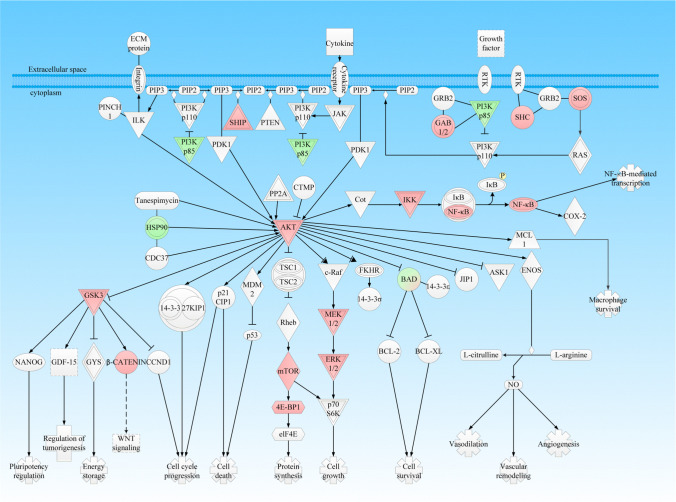
PI3K-Akt signaling pathway alteration: The expression patterns and phosphorylations of PI3K (phosphatidylinositol 3-kinases)-Akt signaling pathway have been confirmed to alter in NF-PitNETs [10] (Fig. 1). The PI3K-Akt signaling pathway is activated by multiple types of cellular stimuli or toxic insults, such as growth factor receptor tyrosine kinases and G-protein-coupled receptors and regulates essential cellular functions such as translation, transcription, growth, proliferation, and survival [75, 76]. Components of this pathway have been extensively studied, which were found to be commonly activated in human cancer [77–79]. The PI3K-AKT signaling pathway also has been reported to alter in NF-PitNETs although it is generally considered a benign tumor. The pAKT, an essential effector of the PI3K-AKT signaling pathway, has been found to present in microvascular areas related to tumor size of PitNETs by immunostaining, which suggested that the pAKT signaling plays a major role in tumor growth and angiogenesis [80]. The somatic mutations and amplifications of the PIK3CA proto-oncogene, which encodes PI3K, have been found to present in NF-PitNETs [81]. In addition, the laminin subunit alpha 2 (LAMA2) gene functions as a tumor suppressor in NF-PitNETs, and overexpression and demethylation of this gene suppressed the invasion of NF-PitNET cells, partially by affecting the PTEN-PI3K-AKT signaling pathway [82]. PI3K signaling pathway has close relations with mTOR signaling pathway and MAPK signaling pathway. It is reported that rapamycin and its analogs induce the MAPK pathway activation in human cancers and this feedback loop depends on an S6K-PI3K-Ras pathway [83]. In prolactinoma, prolactin, and estradiol were found to exert synergistic effects on tumor cell proliferation, and both the protein expressions of estrogen receptor α (ERα) and prolactin receptor (PRLR) increase in bromocriptine-resistant prolactinomas. Further study found that PRL induced the ERα phosphorylation via JAK2-PI3K/Akt-MEK/ERK pathway, while estrogen facilitated PRLR upregulation via pERα, which might be the underlying mechanism that contributes to the bromocriptine resistance for prolactinomas [84]. However, the crosstalk among PI3K signaling pathway, mTOR signaling pathway, and MAPK signaling pathway has not been investigated in NF-PitNETs.
-
iii.
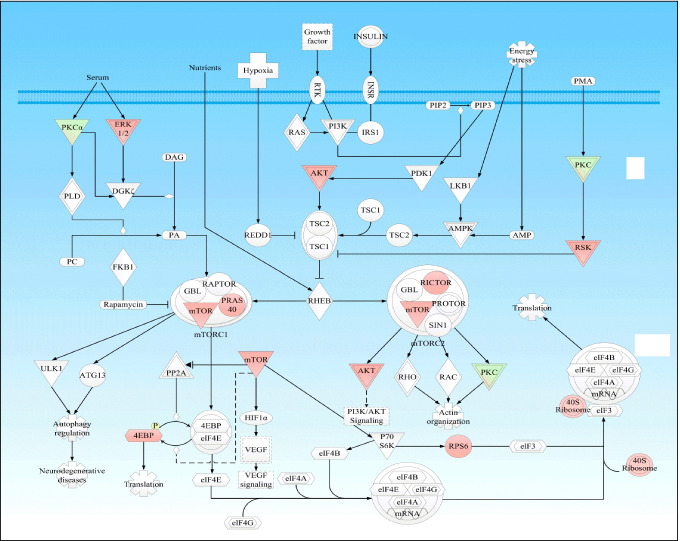
mTOR signaling pathway alteration: The expression patterns and phosphorylations of the mTOR signaling pathway have been confirmed to alter in NF-PitNETs [10] (Fig. 2). The mTOR, a highly conserved serine/threonine protein kinase, is a PI3K/AKT pathway downstream effector. It forms two distinct complexes termed mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). The mTORC1 is sensitive to rapamycin and activated by diverse stimuli, such as nutrients, energies, growth factors, and stress signals, and other essential signaling of pathways, such as MAPK, PI3K, and AMPK, to control cell proliferation, growth, and survival. The mTORC2 is resistant to rapamycin and generally insensitive to nutrients and energy signals. It involves in the regulation of actin cytoskeleton that is related to tumor migration and invasion by activating PKC-α and AKT [85]. Deregulation of multiple components of the mTOR pathway has been reported in many cancers. The mTOR signaling pathway perturbations have also been reported to exist in NF-PitNETs. Components and regulators of the mTOR signaling pathway, such as AKT, p-MEK, and Raf, have been found to present specifically differential expressions (mainly up-regulation) in NP-PitNETs, compared to normal pituitaries [86]. In addition, the expressions of mTOR pathway regulators, mTOR, RICTOR, and RAPTOR, also have been found to be significantly correlated with clinical courses of NF-PitNETs, such as invasion, staging, and tumor growth [87].
-
iv.
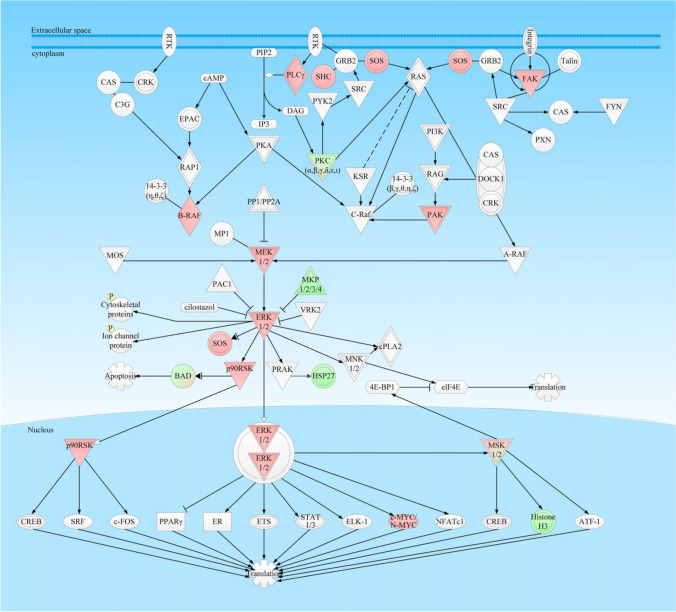
ERK5 and p38 MAPK signaling pathway alterations: This pathway was found to be inhibited in hpNF-PitNETs compared to lpNF-PitNETs, and the expression patterns and phosphorylations of the ERK/MAPK pathway have also been verified to generally alter in NF-PitNETs [35] (Fig. 3). The MAPK signaling system is highly complex and diverse, and both p38 proteins and extracellular signal-related kinases (ERK)-1/2 are the regulated MAPKs, which are activated by specific MAPKKs: MKK3/6 for p38, and MEK1/2 for ERK1/2. However, each MAPKK can be activated by more than one MAPKKK as well. Presumably, each MAPKKK confers responsiveness to different stimuli to exert an effect on different cellular functions, including cell differentiation, proliferation, and migration [88]. Many studies have reported that MAPK signaling pathways extensively involved in tumorigenesis and progression [89–91], including NF-PitNETs. The overexpression of B-Raf mRNA and protein might be a feature of NF-PitNETs, because its overactivity highlights the overactivity of the Ras-B-Raf-MAP kinase pathway to facilitate pituitary tumorigenesis [92]. It has been reported that the crosstalk of the MAPK signaling pathway and PI3k-AKT signaling pathway was involved in pituitary tumorigenesis and progression [93]. For example, a transcription factor and coregulator that are important for pituitary maturation and tumorigenesis, zinc-finger protein (Zac1) lies downstream to both MAPK and PI3K pathways. Its target genes control cell proliferation and hormone synthesis and frequently lose expression in NF-PitNETs [94]. Inhibition of the PI3K pathway by therapeutic drugs, like somatostatin analogs, can upregulate Zac1 expression. Zac1 in fact is an important mediator of the antiproliferative effects of PI3K pathway inhibition, and correlates to outcome in acromegalic patients [95]. In addition, p38 MAPK signaling is also considered an important canonical pathway that participates in oxidative stress response in NF-PitNETs [26].
-
v.
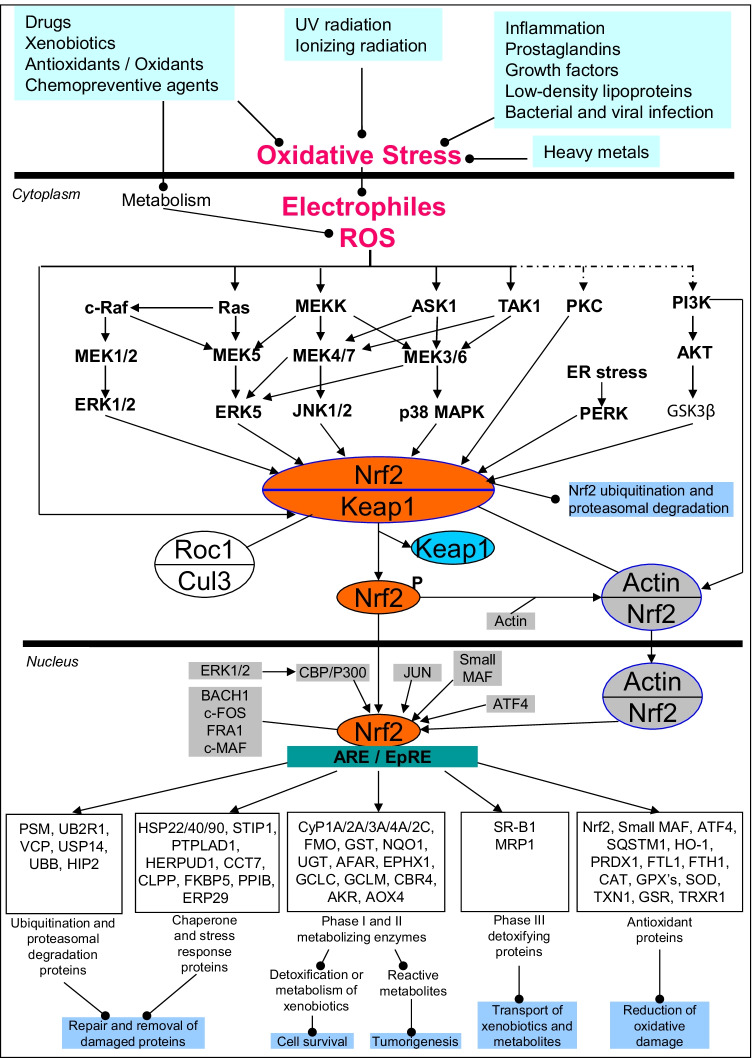
Oxidative stress: Oxidative stress is defined as a relative excess of free-radical/reactive oxygen/nitrogen species (ROS/RNS) when compared with antioxidants, which emphasizes that the balance must be disturbed between the relative abundance of ROS/RNS and antioxidants (Fig. 4). This process is extensively involved in neurodegenerative disease, cardiovascular disease, tumorigenesis, and many other pathologies, including PitNET pathology [96]. Oxidative stress response signaling pathway and the signaling pathways related to the regulation of redox homeostasis, including mitochondrial dysfunction pathway, oxidative phosphorylation pathway, glutathione redox reaction I pathway, superoxide radical degradation pathway, aryl hydrocarbon receptor signaling, glucocorticoid receptor signaling, corticotrophin-releasing hormone signaling, melatonin signaling, methylglyoxal degradation III pathway, and AMPK signaling, have been found to be altered in NF-PitNETs by the integrative analysis of nine sets of documented NF-PitNET omics data, which clearly demonstrated that the imbalance between production and detoxification of free radicals ROS/RNS exist in NF-PitNETs to result in oxidative stress and damage in NF-PitNETs [10]. The toxic peroxynitrite anion (ONOO−) is generated by the reaction of nitric oxide, one of the most important RNS, and superoxide radical, which is able to attack DNAs, RNAs, proteins, and membrane lipids. ONOO− is an important factor that causes protein tyrosine nitration in vivo and alters protein functions [96]. In NF-PitNET, nine nitrotyrosine-containing proteins have been identified, and tyrosine nitration occurs in crucial structural and functional domains, which is able to change protein functions [97]. Recently, a study has shown that signs of oxidative damage, such as ROS levels and signs of antioxidant response; for example, nuclear factor-E2-related factor-2(Nrf2) significantly increases in PitNETs [98]. The transcriptional factor Nrf2 is pivotal to the antioxidant response. DEP and nitroproteomic data have clearly revealed that the Nrf2-mediated oxidative-stress response pathway system is involved in NF-PitNET occurrence and progression [99, 100].
-
vi.
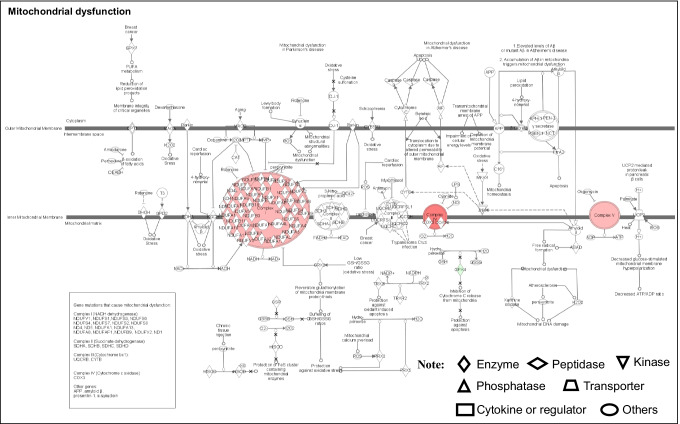
Mitochondrial dysfunction: Mitochondria are subcellular organelles that ubiquitously exist and are responsible for supplying energy to eukaryotic cells; and mitochondria are the key links to oxidative stress, metabolism, cell cycle, cell apoptosis, autophagy, and immunity process [101] (Fig. 5). Thereby, mitochondrial dysfunction is considered a hallmark and plays an important role in many diseases such as cancers, cardiovascular diseases, neurodegenerative diseases, diabetes mellitus, and inflammatory diseases [100, 102]. Recent studies found that increased mitochondrial fission was a pro-tumorigenic phenotype [103]. Also, study about a human PitNET found that mitochondrial dysfunction could be represented as the increased number of mitochondria and mitochondrial morphological change [104, 105]. Many studies have reported that mitochondrial dysfunctions mediate reprogramming energy metabolism, oxidative stress, cell apoptosis dysregulation, and autophagy dysregulation to affect the pituitary tumorigenesis and progression. For mitochondrial dysfunction-mediated energy metabolism reprogram, some mitochondria-associated proteins were found to play a key role in PitNETs, such as oxamate and succinate dehydrogenase [106, 107]. Mitochondrial dysfunction signaling pathway and the signaling pathways related to energy metabolism, including oxidative stress response signaling pathway, oxidative phosphorylation, and AMPK signaling, have been found to be altered in NF-PitNETs with the integrative analysis of nine sets of documented NF-PitNET omics data, which clearly demonstrate that the defective mitochondrial function and energy metabolism alterations exist in NF-PitNETs [10]. Mitochondrial dysfunction-mediated oxidative stress is accompanied by mitochondria swelling during PitNET development, and is associated with augmented biogenesis and increased fusion process [98]. It is reported that mitochondria-mediated ROS- MAPK pathways in PitNETs can be activated by 18beta-glycyrrhetinic acid to induce tumor cells apoptosis, and these activating effects can be attenuated by pretreatment with N-acetyl-L-cysteine, a ROS inhibitor [108]. Mitochondrial dysfunction-mediated apoptosis dysregulation is caused by the change of mitochondrial membrane potential and subsequently internal apoptosis stimulator responses [109]. The imbalanced expressions of apoptosis-related genes/proteins, such as trefoil factor 3 and apoptotic protease-activating factor-1, are able to lead to uncontrolled cell proliferation in PitNETs [110, 111]. Also, targeting mitochondria has an effective impact on PitNET therapy through the apoptosis pathway. Autophagy is a protein degradation system that functions in maintaining homeostasis and inducing apoptosis. Mitophagy is a complex physiological process in that cells selectively eliminate mitochondria through autophagy. Many studies have found that mitophagy and mitochondrial dysfunction are associated with pituitary tumorigenesis and progression [112]. In pituitary GH3 cells, the regulating mechanism of mitophagy has been found to be mediated by Nrf2/PTEN-induced putative kinase protein 1 (PINK1)/E3 ubiquitin ligase Parkin pathway, and the activation of protective protein kinase A signaling pathway can activate the Nrf2/PINK1/Parkin pathway to mediate mitophagy [113]. Moreover, increasing mitophagy and mitochondrial dysfunction might increase chemo-resistance in pituitary GH3 cells [113].
-
vii.
Cell cycle dysregulation: Cell cycle is the basic biological process that regulates cells growth and proliferation, and its dysregulation could cause the uncontrolled growth and proliferation of cells, which results in tumorigenesis [114]. Cell-cycle dysregulation and the signaling pathways related to cell cycle, proliferation and apoptosis, including 14–3-3-mediated signaling, calcium signaling, cardiac β-adrenergic signaling, ERK/MAPK signaling, IGF-1 signaling, mTOR signaling, p53 signaling, PEDF signaling, PI3K/Akt signaling, sonic hedgehog signaling, tec kinase signaling, telomerase signaling, and β-adrenergic signaling, have been found to be altered in NF-PitNETs, which revealed that cell-cycle dysregulation was involved in NF-PitNET tumorigenesis and progression [10]. Numerous cell cycle regulators have been identified to be altered in NF-PitNETs, such as Rb1 and cyclin-dependent inhibitors (CDKIs) (p16, p15, p21, and p27) [115–117]. A recent study further investigated the relationship between the protein expressions of cell cycle regulators, including pituitary adenylate cyclase-activating peptide (PACAP), cyclin D1, vascular endothelial growth factor (VEGF), c-MYC and pituitary tumor transforming gene (PTTG), and patient clinical characteristics (Ki-67, age, regrowth, and tumor size) [118]. Cyclin D1 was positively correlated with Ki-67 and tumor size. The c-MYC was correlated with PTTG nuclear expression, which indicated that PTTG might induce c-MYC expression in PitNETs and c-MYC might have a principal role in early pituitary tumorigenesis [118]. Also, the protein expression of p21 was significantly increased in the regrown NF-PitNETs, which indicated that it was a predictor for residual PitNET progression [119]. However, many studies demonstrated that cell cycle dysregulation in PitNET mainly occurred through the alterations of genes that regulate the G1/S checkpoint, such as CDKIs (p14, p15, p16, p18, p21, and p27), few studies investigated the genes that regulate G2/M checkpoint. Recently a study systematically studied the expressions of the G2/M transition members in NF-PitNETs and found that CDK1 and CDC25A were overexpressed and might have an important role in the pathogenesis of NF-PitNET [120]. Thus, cell cycle dysregulation has currently become one of the research hotspots in the field of NF-PitNET tumorigenesis.
Table 2.
Signaling pathways operated in PitNETs
| Signaling pathway | Molecules involved in the signaling pathway | Molecular change/mechanism | Biological effect | Research models | Reference |
|---|---|---|---|---|---|
| Calcium signaling pathway |
hypothalamic peptide pituitary adenylate cyclase-activating polypeptide |
Modulate [Ca2 +]i and cAMP formation | Modulatory action on NF-PitNETs growth | NF-PitNETs tissues and their cultured cells | [71] |
| Ca2+-sensing receptor | Its activation induces a significant increase of [Ca2+]i and cAMP | Regulation of pituitary cell function | Normal parathyroid, normal pituitaries, and NF-PitNET, and GH secreting adenomas | [72] | |
| CaM-KK | Its inhibition induces apoptosis of NF-PitNET cells, and inhibit tumor cellular viability, diffusion, and migration | Anti-NF-PitNET progression | HP75 cell line, AtT-20 cell line, and GT1-1 cell line | [11] | |
| PI3K-Akt signaling pathway | pAkt | Regulate angiogenesis | Affect NF-PitNETs growth | NF-PitNET, F-PitNET, macroadenomas and microadenomas | [80] |
| PIK3CA proto-oncogene | Somatic mutations and amplifications | Affect NF-PitNETs tumorigenesis | ACTH-secreting microadenomas, GH-secreting macroadenomas, prolactin-secreting macroadenomas, nonfunctioning macroadenomas | [81] | |
| Laminin subunit alpha 2 | Overexpression and demethylation | Suppressed the invasion of NF-PitNETs | NF-PitNETs and GH3 cell line | [82] | |
| ZAC1 | Upregulated ZAC1 through PI3K pathway inhibition by somatostatin analogs | Inhibit tumor cells proliferative activity | GH3 cell line | [95] | |
| mTOR signaling pathway |
AKT p-MEK Raf |
Overexpression and overactivation | Affect NF-PitNETs tumorigenesis and progression | NF-PitNETs and the controls | [86] |
|
mTOR RICTOR RAPTOR |
Overexpression | Affect NF-PitNETs invasion and growth | NF-PitNETs and F-PitNETs | [87] | |
| ERK5 and p38 MAPK signaling pathway | B-Raf | Overexpression and overactivation | Facilitate pituitary tumorigenesis | NF-PitNETs and the controls | [92] |
| ZAC | Reduction or loss of ZAC expression in NF-PitNETs and null-cell adenoma | Affect pituitary growth and differentiation | NF-PitNETs, prolactinoma, GHoma, ACTHoma, thyreotrophinoma, gonadotrophinoma, null-cell adenoma | [94] | |
| Oxidative stress response signaling pathway | ONOO− | Causes protein tyrosine nitration | Alter corrensponding proteins function to affect NF-PitNET tumorigenesis and progression | NF-PitNET | [96, 97] |
|
ROS Nrf-2 |
Increased expression and overactivation during the pituitary tumor growth | Activate oxidative stress response to affect tumor progression | Exogenous estrogen-induced rats pituitary tumors | [98] | |
| Mitochondrial dysfunction signaling pathway | Mitochondria | Mitochondrial enlargement | Adapt to high levels of prolactin secretion and/or ischemia/anoxia microenvironment in PitNET | A prolactin-secreting PitNET from a patient taking oral contraceptives | [104] |
| Mitochondria | Mitochondria enlarged and arranged more densely | Oncocytic adenomas | [105] | ||
| Oxamate | Increase mitochondrial ROS generation | Induce GH3 cell apoptosis | Rat GH3 pituitary adenoma cell line | [106] | |
| Succinate dehydrogenase(SDH) | Germline mutations in SDH | Facilitate PitNET formation | Patients with pheochromocytoma/Paraganglioma and PitNET | [107] | |
| Mitochondria | Mitochondria enlarge to augment biogenesis and increase the fusion process | Cope with oxidative stress and support metabolic shift to make for tumor cell overgrowth | Exogenous estrogen-induced rats pituitary tumors | [98] | |
| 18beta-glycyrrhetinic acid | Activated mitochondria-mediated ROS- MAPK pathways | Induce pituitary tumor cells apoptosis | Rat pituitary adenoma-derived MMQ and GH3 cells | [108] | |
| Trefoil factor 3(TFF3) | TFF3 knockdown downregulate Bcl-2 and caspase-3 precursor proteins to activate mitochondria-induced endogenous apoptosis | Facilitates tumor cells apoptosis | HP75 cell line | [110] | |
| Apoptotic protease-activating factor-1(APAF-1) | Affect intrinsic (mitochondrial) pathway | Facilitates tumor cells apoptosis | F-PitNET and invasive PitNET | [111] | |
| Mitochondria | Mitophagy mediated by PINK1/ E3 ubiquitin ligase Parkin pathway | Affect pituitary tumorigenesis and progression, and increase chemo-resistance | Rat pituitary GH3 cells | [113] | |
| Cell-cycle dysregulation signaling pathway | Rb1 | Methylation of CpG islands in the promoter of Rb1 | Facilitates pituitary tumorigenesis | NF-PitNETs (noninvasive and invasive) and somatotrophinomas(noninvasive and invasive) | [115] |
| p16, p15 | Methylation of CpG islands in the promoter of p16 and p15 | Facilitates pituitary tumorigenesis | NF-PitNETs and F-PitNETs (prolactinomas, GHoma, thyroid-stimulating hormone adenoma) | [116] | |
| p21, p27 | Combined deficiency in p21 and p27 proteins | Associate with pituitary tumorigenesis | Double-knockout mice for p21 and p27 proteins | [117] | |
| PACAP, cyclin D1, VEGF, c-MYC, and PTTG | PTTG induce c-MYC expression | Cyclin D1 positively correlated with pituitary tumor size. c-MYC facilitated early pituitary tumorigenesis | NF-PitNETs, F-PitNETs and GH3 cell line | [118] | |
| p21 | Significantly increased | Regrowthed NF-PitNETs | [119] | ||
| CDK1, CDC25A | Overexpressed | Alter G2/M transition to affect NF-PitNETs pathogenesis | NF-PitNETs | [120] |
Fig. 1.
PI3K-AKT signaling pathway in NF-PitNETs. Red color = upregulation; Green color = downregulation. The gradient color degree represents a slightly different expression tendency of that molecule. Reproduced from Long et al. (2019) [10], copyright permission from Frontiersin publisher open-access publication, copyright 2019
Fig. 2.
mTOR signaling pathway in NF-PitNETs. Red color = upregulation; Green color = downregulation. The gradient color degree represents slightly different expression tendency of that molecule. Modified from Long et al. (2019) [10], copyright permission from Frontiersin publisher open-access publication, copyright 2019
Fig. 3.
ERK-MAPK signaling pathway in NF-PitNETs. Red color = upregulation; Green color = downregulation. The gradient color degree represents slightly different expression tendency of that molecule. Reproduced from Long et al. (2019) [10], copyright permission from Frontiersin publisher open-access publication, copyright 2019
Fig. 4.
NRF2-mediated oxidative-stress response pathway in NF-PitNETs. Reproduced from Zhan, et al. (2021) [99], copyright permission from Frontiersin publisher open-access article, copyright 2021
Fig. 5.
Mitochondrial dysfunctional pathway in NF-PitNETs. Red color = upregulation; Green color = downregulation. The various shapes of nodes represent different functions. A duplicated shape denotes this node contains multiple components. An arrow means the pathway direction. A line with a small circle means a biological result. Reproduced from Zhan et al. (2010) [26], copyright permission from BioMed Central publisher open-access article, copyright 2010
Signaling pathway network-based multi-target pharmaceutical treatment in NF-PitNETs
As described above, multi-omics studies in human NF-PitNETs have demonstrated that several signaling pathways, including calcium signaling pathway, cGMP-PKG signaling pathway, PI3K-Akt signaling pathway, mTOR signaling pathway, MAPK signaling pathway, oxidative stress response signaling pathway, mitochondrial dysfunction signaling pathway, and cell cycle signaling pathway, are important for NF-PitNET pathogenesis, which interregulate and facilitate NF-PitNET tumorigenesis and progression. The roles of these pathways in the treatment of NF-PitNETs are demonstrated by the effects of currently used medications.
Biguanides are well-known medicine commonly prescribed to treat diabetes mellitus, but this drug can convey other beneficial actions, including antitumor effects. A recent study found that biguanides reduced cell viability in all PitNETs, including NF-PitNETs. The effects of biguanides on PitNETs might involve the modulation of both AMP-activated protein kinase signaling pathway-dependent (PI3K/Akt, [Ca2+]i) and independent MAPK mechanisms [121]. A well-known drug used to treat hyperlipidemia/cardiovascular diseases, simvastatin, also was reported to reduce cell viability and/or hormone secretion in all subtypes of PitNETs. This antitumor effect was armed with the modulation of MAPK/PI3K/mTOR pathways and expression levels of key receptors, GHRH-R, ghrelin-R, and Kiss1-R, which regulate pituitary functions [122]. Other drugs include nelfinavir and somatostatin analogs; the former is radiosensitizer for PitNETs, and the latter is the conventional treatment of the major subtypes of PitNETs; and their antitumor effects might attribute to their modulation of PI3K-AKT-mTOR signaling pathway and/or ERK pathway in recent studies [95, 123, 124]. Ketoconazole, initially developed as an antifungal agent, acts as a potent inhibitor of adrenal steroidogenesis and therefore has been used in the treatment of Cushing’s disease. A study further investigated the underlying mechanism of ketoconazole treatment using different pituitary tumoral cell lines, which found a negative relationship between ketoconazole concentration and pituitary cell viability, and ACTH levels decrease in AtT-20 cells after the drug removal. This study has observed that the expressions of apoptosis-related cell death receptors and caspases increased in pituitary cells, and the gene expressions of the cell cycle inhibitors (p27 and p21) were increased in GH3 cells, and the expressions of p21 were increased in aT3.1 cells, which suggested that ketoconazole reduced cell viability in a concentration-dependent way in pituitary tumor cell lines, which is related to the increase of apoptosis- and cell cycle regulation-associated gene expressions [125]. The expressions of epidermal growth factor receptor 2 (HER2)/ERK1/2 signaling were significantly upregulated in PitNETs, and trastuzumab could decrease the expressions of ERK1/2, cyclin D1, and CDK4 as well as the pituitary tumor growth [126]. Further study found that trastuzumab inhibited pituitary tumor growth and modulated HER2/ERK1/2 signaling by blocking HER2 [126]. Many drugs have been found to play their therapeutic roles in PitNETs by targeting mitochondria, including dopamine agonists, melatonin, cyclosporine A, temozolomide and pyrimethamine, T-2 toxin, grifolic acid, and yougui pill [112].
Furthermore, potential novel therapies of PitNETs have been proposed based on putative molecular targets among some of these signaling pathways. All of the components and regulators of the ERK/MAPK signaling pathway are considered to be potential targets for treating PitNETs [127]. Overexpression of epidermal growth factor pathway substrate number 8 (Eps8) and overactivation of Raf, ERK, and MEK in the ERK signaling pathway facilitate cell proliferation and survival in PitNETs [128]. MAPK kinase inhibitor (PD98059) can eliminate the proliferative effects. Silence of Eps8 inhibits cell proliferation as well, which suggested that Eps8 promotes PitNET cell proliferation by enhancing the Raf/MEK/ERK signaling, and it is a potential drug target for PitNET treatment [129]. Also, some components and regulators in PI3K/Akt/mTOR and Raf/MEK/ERK signaling pathways have been recognized to be potential therapeutic targets for NF-PitNETs. PI3K/Akt and ERK signaling pathways are both diverged from the tyrosine kinase receptors IGF-1R and epidermal growth factor receptor (EGFR) [86]. NVP-AEW541, a selective IGF-1R inhibitor, was abrogated IGF-1-induced NF-PitNET cell proliferation and signaling [130]. Although the EGFR expression levels in NF-PitNETs are low according to some studies, EGFR inhibitors might still be effective as it does not always correlate with EGFR expression [131]. Gefitinib, a tyrosine kinase inhibitor (TKI) of EGFR, and lapatinib, a dual TKI of EGFR and HER2, have been studied in human and rat prolactinoma cells, which found they suppressed cell growth [132, 133]. However, for all we knew, no study reported that the EGFR inhibitors affected NF-PitNETs so far. The effects of mTOR inhibitors on NF-PitNETs are complex. mTOR inhibitors, such as everolimus, were found to indeed inhibit pituitary cell proliferation [134], but drug-resistance to NF-PitNETs also existed [135] because of the overactivation of Akt [86]. Moreover, Nrf2 signaling, as the hub of the oxidative stress response, is extensively involved in cancer pathogenesis, and many drugs targeting Nrf2 signaling pathways have been developed and further tested as potential anticancer agents for different cancers. It is thereby considered chemical agents targeting Nrf2 signaling as novel therapeutic strategies for PitNETs. Although no study has investigated Nrf2 signaling as a therapeutic target for PitNETs, it is still strongly believed to be the promising target for new therapeutic strategies for PitNETs [99].
Conclusions
This review summarized current studies about signaling pathways and molecular networks based on different omics analyses in NF-PITNETs, including genomics, transcriptomics, proteomics, metabolomics, and integrative omics. NF-PitNET pathogenesis widely involves the alterations of genome, transcriptome, proteome, and metabolome, and molecules in the different omics levels mutually regulate and form dynamically associated networks. Therefore, the integrative omics-based signaling pathway alterations were emphasized in this review to systematically elucidate the important pathway-network alterations that affected NF-PitNET occurrence and progression, especially invasiveness-related pathway network alterations. Some signaling pathway network alterations were important for NF-PitNET tumorigenesis and development identified with integrative omics analysis, including calcium signaling pathway, cGMP-PKG signaling pathway, mTOR signaling pathway, PI3K/AKT signaling pathway, MAPK signaling pathway, oxidative stress response, mitochondrial dysfunction, and cell cycle dysregulation. Some currently used medicines, such as biguanides, statins, and somatostatin analogs, have an antitumor effect on NF-PitNET targeting the components and/or regulators of these pathways, and some novel agents targeting these pathways are developing and promised to be effective medications for NF-PitNET treatment. In all, multi-omics-based signaling pathways, network alterations pave way for our systematic understanding of NF-PitNET pathogenesis and provide valuable clues for the development of novel effective medication of NF-PitNET to prevent NF-PitNETs from invasion and recurrence.
Outlook and expert recommendations for 3P medicine in NF-PitNET
NF-PitNET is a highly heterogeneous and complex tumor in the hypothalamic-pituitary-target organ axes, which seriously affects the endocrine system and health. Multi-omics is an effective approach to mine the molecular alterations at different levels of genome, transcriptome, proteome, and metabolome and further reveals the signaling pathway network changes in the NF-PitNET biological system. These signaling pathway network changes offer the in-depth understanding of molecular mechanisms, the discovery of effective biomarkers and therapeutic targets/drugs, for patient stratification to simplify highly heterogenous NF-PitNET population for personalized treatment, predictive diagnosis to earlier screen the invasive tumor for early treatment, and prognostic assessment for earlier screening, and treatment to avoid recurrence and drug-resistance. For example, mitochondrial dysfunction is one of important signaling pathway alterations in NF-PitNEts, which is crucial for PPPM strategies such as potential application of “mitochondrial health index” for predictive diagnostics and targeted prevention tailored to the person [136].
This review highlighted the achievement of multi-omics-based signaling pathway network changes in NF-PitNETs. We emphasize strengthening the transitional research of these molecular network data for PPPM clinical practice in NF-PitNETs, especially resolving its invasive and aggressive characteristics of NF-PitNETs.
Acknowledgements
The authors acknowledge the financial support from the Shandong First Medical University Talent Introduction Funds (to X.Z.), Shandong First Medical University High-level Scientific Research Achievement Cultivation Funding Program (to X.Z.), the Shandong Provincial Natural Science Foundation (ZR202103020356/ZR2021MH156 to X.Z.), and the Academic Promotion Program of Shandong First Medical University (2019ZL002).
Author contribution
S.Q. collected and analyzed literature, and wrote the manuscript. C.L. participated in the collection and analysis of literature. X.Z. conceived the concept, designed the manuscript, coordinated, wrote, and critically revised the manuscript, and was responsible for its financial support and the corresponding works. All authors approved the final manuscript.
Funding
This work was supported by the Shandong First Medical University Talent Introduction Funds (to X.Z.), Shandong First Medical University High-level Scientific Research Achievement Cultivation Funding Program (to X.Z.), the Shandong Provincial Natural Science Foundation (ZR202103020356/ ZR2021MH156 to X.Z.), and the Academic Promotion Program of Shandong First Medical University (2019ZL002).
Data Availability
All data and materials are available in the current manuscript.
Code availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, et al. CBTRUS Statistical Report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(Suppl 4):iv1–iv62. doi: 10.1093/neuonc/nov189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molitch ME. Diagnosis and treatment of pituitary adenomas: a review. JAMA. 2017;317:516–524. doi: 10.1001/jama.2016.19699. [DOI] [PubMed] [Google Scholar]
- 3.Andela CD, Lobatto DJ, Pereira AM, van Furth WR, Biermasz NR. How non-functioning pituitary adenomas can affect health-related quality of life: a conceptual model and literature review. Pituitary. 2018;21:208–216. doi: 10.1007/s11102-017-0860-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenman Y, Stern N. Non-functioning pituitary adenomas. Best Pract Res Clin Endocrinol Metab. 2009;23:625–638. doi: 10.1016/j.beem.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Tampourlou M, Fountas A, Ntali G, Karavitaki N. Mortality in patients with non-functioning pituitary adenoma. Pituitary. 2018;21(2):203–207. doi: 10.1007/s11102-018-0863-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mete O, Lopes MB. Overview of the 2017 WHO classification of pituitary tumors. Endocr Pathol. 2017;28:228–243. doi: 10.1007/s12022-017-9498-z. [DOI] [PubMed] [Google Scholar]
- 7.Drummond J, Roncaroli F, Grossman AB, Korbonits M. Clinical and pathological aspects of silent pituitary adenomas. J Clin Endocrinol Metab. 2019;104:2473–2489. doi: 10.1210/jc.2018-00688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheithauer BW, Kovacs KT, Laws ER, Jr, Randall RV. Pathology of invasive pituitary tumors with special reference to functional classification. J Neurosurg. 1986;65:733–744. doi: 10.3171/jns.1986.65.6.0733. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Chen K, Yang Z, Li W, Wang C, Zhang G, et al. Diagnosis of invasive nonfunctional pituitary adenomas by serum extracellular vesicles. Anal Chem. 2019;91:9580–9589. doi: 10.1021/acs.analchem.9b00914. [DOI] [PubMed] [Google Scholar]
- 10.Long Y, Lu M, Cheng T, Zhan X, Zhan X. Multiomics-based signaling pathway network alterations in human non-functional pituitary adenomas. Front Endocrinol (Lausanne). 2019;10:835. doi: 10.3389/fendo.2019.00835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu B, Jiang S, Wang X, Zhong S, Bi Y, Yi D, Liu G, Hu D, Dou G, Chen Y, Wu Y, Dong J. Identification of driver genes and key pathways of non-functional pituitary adenomas predicts the therapeutic effect of STO-609. PLoS One. 2020;15:e0240230. doi: 10.1371/journal.pone.0240230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joshi H, Vastrad B, Vastrad C. Identification of important invasion-related genes in non-functional pituitary adenomas. J Mol Neurosci. 2019;68:565–589. doi: 10.1007/s12031-019-01318-8. [DOI] [PubMed] [Google Scholar]
- 13.Song W, Qian L, Jing G, Jie F, Xiaosong S, Chunhui L, et al. Aberrant expression of the sFRP and WIF1 genes in invasive non-functioning pituitary adenomas. Mol Cell Endocrinol. 2018;474:168–175. doi: 10.1016/j.mce.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Esapa CT, Harris PE. Mutation analysis of protein kinase A catalytic subunit in thyroid adenomas and pituitary tumours. Eur J Endocrinol. 1999;141:409–412. doi: 10.1530/eje.0.1410409. [DOI] [PubMed] [Google Scholar]
- 15.Farrell WE, Talbot JA, Bicknell EJ, Simpson D, Clayton RN. Genomic sequence analysis of a key residue (Arg183) in human G alpha q in invasive non-functional pituitary adenomas. Clin Endocrinol (Oxf). 1997;47:241–244. doi: 10.1046/j.1365-2265.1997.2891088.x. [DOI] [PubMed] [Google Scholar]
- 16.Faccenda E, Melmed S, Bevan JS, Eidne KA. Structure of the thyrotrophin-releasing hormone receptor in human pituitary adenomas. Clin Endocrinol (Oxf). 1996;44:341–347. doi: 10.1046/j.1365-2265.1996.684506.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhu H, Guo J, Shen Y, Dong W, Gao H, Miao Y, et al. Functions and mechanisms of tumor necrosis factor-α and noncoding RNAs in bone-invasive pituitary adenomas. Clin Cancer Res. 2018;24:5757–5766. doi: 10.1158/1078-0432.ccr-18-0472. [DOI] [PubMed] [Google Scholar]
- 18.Taniguchi-Ponciano K, Gomez-Apo E, Chavez-Macias L, Vargas G, Espinosa-Cardenas E, Ramirez-Renteria C, et al. Molecular alterations in non-functioning pituitary adenomas. Cancer Biomark. 2020;28:193–199. doi: 10.3233/cbm-191121. [DOI] [PubMed] [Google Scholar]
- 19.Kim YH, Kim JH. Transcriptome Analysis identifies an attenuated local immune response in invasive nonfunctioning pituitary adenomas. Endocrinol Metab (Seoul). 2019;34:314–322. doi: 10.3803/EnM.2019.34.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu S, Gu Y, Huang Y, Wong TC, Ding H, Liu T, et al. Novel biomarkers for non-functioning invasive pituitary adenomas were identified by using analysis of microRNAs expression profile. Biochem Genet. 2017;55:253–267. doi: 10.1007/s10528-017-9794-9. [DOI] [PubMed] [Google Scholar]
- 21.Xing W, Qi Z. Genome-wide identification of lncRNAs and mRNAs differentially expressed in non-functioning pituitary adenoma and construction of an lncRNA-mRNA co-expression network. Biol Open. 2019;8(1):bio037127. doi: 10.1242/bio.037127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo J, Fang Q, Liu Y, Xie W, Zhang Y, Li C. Identifying critical protein-coding genes and long non-coding RNAs in non-functioning pituitary adenoma recurrence. Oncol Lett. 2021;21:264. doi: 10.3892/ol.2021.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Li C, Wang J, Song G, Zhao Z, Wang H, et al. Genome-wide analysis of differentially expressed lncRNAs and mRNAs in primary gonadotrophin adenomas by RNA-seq. Oncotarget. 2017;8:4585–4606. doi: 10.18632/oncotarget.13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee M, Marinoni I, Irmler M, Psaras T, Honegger JB, Beschorner R, et al. Transcriptome analysis of MENX-associated rat pituitary adenomas identifies novel molecular mechanisms involved in the pathogenesis of human pituitary gonadotroph adenomas. Acta Neuropathol. 2013;126:137–150. doi: 10.1007/s00401-013-1132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson TE, Shen ZJ, Kanchwala M, Xing C, Filatenkov A, Shang P, et al. Aggressive behavior in silent subtype III pituitary adenomas may depend on suppression of local immune response: a whole transcriptome analysis. J Neuropathol Exp Neurol. 2017;76:874–882. doi: 10.1093/jnen/nlx072. [DOI] [PubMed] [Google Scholar]
- 26.Zhan X, Desiderio DM. Signaling pathway networks mined from human pituitary adenoma proteomics data. BMC Med Genomics. 2010;3:13. doi: 10.1186/1755-8794-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian S, Zhan X, Lu M, Li N, Long Y, Li X, et al. Quantitative analysis of ubiquitinated proteins in human pituitary and pituitary adenoma tissues. Front Endocrinol (Lausanne). 2019;10:328. doi: 10.3389/fendo.2019.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Wen S, Li B, Li N, Zhan X. Phosphorylation-mediated molecular pathway changes in human pituitary neuroendocrine tumors identified by quantitative phosphoproteomics. Cells. 2021;10(9):2225. doi: 10.3390/cells10092225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhan X, Desiderio DM, Wang X, Zhan X, Guo T, Li M, et al. Identification of the proteomic variations of invasive relative to non-invasive non-functional pituitary adenomas. Electrophoresis. 2014;35:2184–2194. doi: 10.1002/elps.201300590. [DOI] [PubMed] [Google Scholar]
- 30.Zhan X, Wang X, Long Y, Desiderio DM. Heterogeneity analysis of the proteomes in clinically nonfunctional pituitary adenomas. BMC Med Genomics. 2014;7:69. doi: 10.1186/s12920-014-0069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Guo T, Peng F, Long Y, Mu Y, Yang H, et al. Proteomic and functional profiles of a follicle-stimulating hormone positive human nonfunctional pituitary adenoma. Electrophoresis. 2015;36:1289–1304. doi: 10.1002/elps.201500006. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Cheng T, Lu M, Mu Y, Li B, Li X, et al. TMT-based quantitative proteomics revealed follicle-stimulating hormone (FSH)-related molecular characterizations for potentially prognostic assessment and personalized treatment of FSH-positive non-functional pituitary adenomas. EPMA J. 2019;10:395–414. doi: 10.1007/s13167-019-00187-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simpson DJ, Frost SJ, Bicknell JE, Broome JC, McNicol AM, Clayton RN, et al. Aberrant expression of G(1)/S regulators is a frequent event in sporadic pituitary adenomas. Carcinogenesis. 2001;22:1149–1154. doi: 10.1093/carcin/22.8.1149. [DOI] [PubMed] [Google Scholar]
- 34.Cheng S, Xie W, Miao Y, Guo J, Wang J, Li C, et al. Identification of key genes in invasive clinically non-functioning pituitary adenoma by integrating analysis of DNA methylation and mRNA expression profiles. J Transl Med. 2019;17(1):407. doi: 10.1186/s12967-019-02148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei Z, Zhou C, Li M, Huang R, Deng H, Shen S, et al. Integrated multi-omics profiling of nonfunctioning pituitary adenomas. Pituitary. 2021;24:312–325. doi: 10.1007/s11102-020-01109-0. [DOI] [PubMed] [Google Scholar]
- 36.Yu SY, Hong LC, Feng J, Wu YT, Zhang YZ. Integrative proteomics and transcriptomics identify novel invasive-related biomarkers of non-functioning pituitary adenomas. Tumour Biol. 2016;37:8923–8930. doi: 10.1007/s13277-015-4767-2. [DOI] [PubMed] [Google Scholar]
- 37.Feng J, Yu SY, Li CZ, Li ZY, Zhang YZ. Integrative proteomics and transcriptomics revealed that activation of the IL-6R/JAK2/STAT3/MMP9 signaling pathway is correlated with invasion of pituitary null cell adenomas. Mol Cell Endocrinol. 2016;436:195–203. doi: 10.1016/j.mce.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 38.Cheng T, Wang Y, Lu M, Zhan X, Zhou T, Li B, et al. Quantitative analysis of proteome in non-functional pituitary adenomas: clinical relevance and potential benefits for the patients. Front Endocrinol (Lausanne). 2019;10:854. doi: 10.3389/fendo.2019.00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu D, Li J, Li N, Lu M, Wen S, Zhan X. Integration of quantitative phosphoproteomics and transcriptomics revealed phosphorylation-mediated molecular events as useful tools for a potential patient stratification and personalized treatment of human nonfunctional pituitary adenomas. EPMA J. 2020;11:419–467. doi: 10.1007/s13167-020-00215-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wen S, Li J, Yang J, Li B, Li N, Zhan X. Quantitative acetylomics revealed acetylation-mediated molecular pathway network changes in human nonfunctional pituitary neuroendocrine tumors. Front Endocrinol (Lausanne). 2021;12:753606. doi: 10.3389/fendo.2021.753606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Z, Guo X, Wang W, Gao L, Bao X, Feng M, et al. UPLC-MS/MS-based lipidomic profiles revealed aberrant lipids associated with invasiveness of silent corticotroph adenoma. J Clin Endocrinol Metab. 2021;106:e273–e287. doi: 10.1210/clinem/dgaa708. [DOI] [PubMed] [Google Scholar]
- 42.Feng J, Gao H, Zhang Q, Zhou Y, Li C, Zhao S, et al. Metabolic profiling reveals distinct metabolic alterations in different subtypes of pituitary adenomas and confers therapeutic targets. J Transl Med. 2019;17:291. doi: 10.1186/s12967-019-2042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panda AC, Abdelmohsen K, Gorospe M. SASP regulation by noncoding RNA. Mech Ageing Dev. 2017;168:37–43. doi: 10.1016/j.mad.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Q, Wang Y, Zhang S, Tang J, Li F, Yin J, et al. Biomarker discovery for immunotherapy of pituitary adenomas: enhanced robustness and prediction ability by modern computational tools. Int J Mol Sci. 2019;20(1):151. doi: 10.3390/ijms20010151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monti C, Zilocchi M, Colugnat I, Alberio T. Proteomics turns functional. J Proteomics. 2019;198:36–44. doi: 10.1016/j.jprot.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 46.Karimi P, Shahrokni A, Ranjbar MR. Implementation of proteomics for cancer research: past, present, and future. Asian Pac J Cancer Prev. 2014;15:2433–2438. doi: 10.7314/apjcp.2014.15.6.2433. [DOI] [PubMed] [Google Scholar]
- 47.Li N, Desiderio DM, Zhan X. The use of mass spectrometry in a proteome-centered multiomics study of human pituitary adenomas. Mass Spectrom Rev. 2021 doi: 10.1002/mas.21710. [DOI] [PubMed] [Google Scholar]
- 48.Walsh MT, Couldwell WT. Symptomatic cystic degeneration of a clinically silent corticotroph tumor of the pituitary gland. Skull Base. 2010;20:367–370. doi: 10.1055/s-0030-1253579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou W, Song Y, Xu H, Zhou K, Zhang W, Chen J, et al. In nonfunctional pituitary adenomas, estrogen receptors and slug contribute to development of invasiveness. J Clin Endocrinol Metab. 2011;96:E1237–1245. doi: 10.1210/jc.2010-3040. [DOI] [PubMed] [Google Scholar]
- 50.Hu R, Wang X, Zhan X. Multi-parameter systematic strategies for predictive, preventive and personalised medicine in cancer. EPMA J. 2013;4:2. doi: 10.1186/1878-5085-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhan X, Desiderio DM. Comparative proteomics analysis of human pituitary adenomas: current status and future perspectives. Mass Spectrom Rev. 2005;24:783–813. doi: 10.1002/mas.20039. [DOI] [PubMed] [Google Scholar]
- 52.Rinschen MM, Ivanisevic J. Identification of bioactive metabolites using activity metabolomics. Nat Rev Mol Cell Biol. 2019;20:353–367. doi: 10.1038/s41580-019-0108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson CH, Ivanisevic J, Siuzdak G. Metabolomics: beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol. 2016;17:451–459. doi: 10.1038/nrm.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu C, Zhou Y, Feng J, Zhou S, Li C, Zhao S, et al. Untargeted lipidomics reveals specific lipid abnormalities in nonfunctioning human pituitary adenomas. J Proteome Res. 2020;19:455–463. doi: 10.1021/acs.jproteome.9b00637. [DOI] [PubMed] [Google Scholar]
- 55.Ijare OB, Baskin DS, Pichumani K. Ex Vivo (1)H NMR study of pituitary adenomas to differentiate various immunohistochemical subtypes. Sci Rep. 2019;9:3007. doi: 10.1038/s41598-019-38542-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ijare OB, Holan C, Hebert J, Sharpe MA, Baskin DS, Pichumani K. Elevated levels of circulating betahydroxybutyrate in pituitary tumor patients may differentiate prolactinomas from other immunohistochemical subtypes. Sci Rep. 2020;10:1334. doi: 10.1038/s41598-020-58244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hasin Y, Seldin M, Lusis A. Multi-omics approaches to disease. Genome Biol. 2017;18:83. doi: 10.1186/s13059-017-1215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat Rev Genet. 2007;8:286–298. doi: 10.1038/nrg2005. [DOI] [PubMed] [Google Scholar]
- 59.Kober P, Boresowicz J, Rusetska N, Maksymowicz M. The role of aberrant DNA methylation in misregulation of gene expression in gonadotroph nonfunctioning pituitary tumors. Cancers (Basel). 2019;11(11):1650. doi: 10.3390/cancers11111650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheng S, Li C, Xie W, Miao Y, Guo J, Wang J, et al. Integrated analysis of DNA methylation and mRNA expression profiles to identify key genes involved in the regrowth of clinically non-functioning pituitary adenoma. Aging (Albany NY). 2020;12:2408–2427. doi: 10.18632/aging.102751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duong CV, Emes RD, Wessely F, Yacqub-Usman K, Clayton RN, Farrell WE. Quantitative, genome-wide analysis of the DNA methylome in sporadic pituitary adenomas. Endocr Relat Cancer. 2012;19:805–816. doi: 10.1530/erc-12-0251. [DOI] [PubMed] [Google Scholar]
- 62.Ling C, Pease M, Shi L, Punj V, Shiroishi MS, Commins D, et al. A pilot genome-scale profiling of DNA methylation in sporadic pituitary macroadenomas: association with tumor invasion and histopathological subtype. PLoS One. 2014;9:e96178. doi: 10.1371/journal.pone.0096178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salomon MP, Wang X, Marzese DM, Hsu SC, Nelson N. The epigenomic landscape of pituitary adenomas reveals specific alterations and differentiates among acromegaly, Cushing’s disease and endocrine-inactive subtypes. Clin Cancer Res. 2018;24:4126–4136. doi: 10.1158/1078-0432.ccr-17-2206. [DOI] [PubMed] [Google Scholar]
- 64.Crick F. Central dogma of molecular biology. Nature. 1970;227:561–563. doi: 10.1038/227561a0. [DOI] [PubMed] [Google Scholar]
- 65.Lee H, Zhang Z, Krause HM. Long noncoding RNAs and repetitive elements: Junk or intimate evolutionary partners? Trends Genet. 2019;35:892–902. doi: 10.1016/j.tig.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 66.Anastasiadou E, Jacob LS, Slack FJ. Non-coding RNA networks in cancer. Nat Rev Cancer. 2018;18:5–18. doi: 10.1038/nrc.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li X, Gianoulis TA, Yip KY, Gerstein M, Snyder M. Extensive in vivo metabolite-protein interactions revealed by large-scale systematic analyses. Cell. 2010;143:639–650. doi: 10.1016/j.cell.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheng C, Geng F, Cheng X, Guo D. Lipid metabolism reprogramming and its potential targets in cancer. Cancer Commun (Lond). 2018;38:27. doi: 10.1186/s40880-018-0301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahuja M, Jha A, Maléth J, Park S, Muallem S. cAMP and Ca2+ signaling in secretory epithelia: crosstalk and synergism. Cell Calcium. 2014;55:385–393. doi: 10.1016/j.ceca.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spada A, Lania A, Mantovani S. Cellular abnormalities in pituitary tumors. Metabolism. 1996;45:46–48. doi: 10.1016/s0026-0495(96)90079-7. [DOI] [PubMed] [Google Scholar]
- 71.Lania A, Gil-del-Alamo P, Saccomanno K, Persani L, Faglia G, Spada A. Mechanism of action of pituitary adenylate cyclase-activating polypeptide (PACAP) in human nonfunctioning pituitary tumors. J Neuroendocrinol. 1995;7:695–702. doi: 10.1111/j.1365-2826.1995.tb00811.x. [DOI] [PubMed] [Google Scholar]
- 72.Romoli R, Lania A, Mantovani G, Corbetta S, Persani L, Spada A. Expression of calcium-sensing receptor and characterization of intracellular signaling in human pituitary adenomas. J Clin Endocrinol Metab. 1999;84:2848–2853. doi: 10.1210/jcem.84.8.5922. [DOI] [PubMed] [Google Scholar]
- 73.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 74.Carafoli E, Santella L, Branca D, Brini M. Generation, control, and processing of cellular calcium signals. Crit Rev Biochem Mol Biol. 2001;36:107–260. doi: 10.1080/20014091074183. [DOI] [PubMed] [Google Scholar]
- 75.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 76.Alzahrani AS. PI3K/Akt/mTOR inhibitors in cancer: at the bench and bedside. Semin Cancer Biol. 2019;59:125–132. doi: 10.1016/j.semcancer.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 77.Ediriweera MK, Tennekoon KH, Samarakoon SR. Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer: biological and therapeutic significance. Semin Cancer Biol. 2019;59:147–160. doi: 10.1016/j.semcancer.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 78.Fattahi S, Amjadi-Moheb F, Tabaripour R, Ashrafi GH, Akhavan-Niaki H. PI3K/AKT/mTOR signaling in gastric cancer: epigenetics and beyond. Life Sci. 2020;262:118513. doi: 10.1016/j.lfs.2020.118513. [DOI] [PubMed] [Google Scholar]
- 79.Chamcheu JC, Roy T, Uddin MB, Banang-Mbeumi S, Chamcheu RN, Walker AL, et al. Role and therapeutic targeting of the PI3K/Akt/mTOR signaling pathway in skin cancer: a review of current status and future trends on natural and synthetic agents therapy. Cells. 2019;8:803. doi: 10.3390/cells8080803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Trovato M, Torre ML, Ragonese M, Simone A, Scarfì R, Barresi V, et al. HGF/c-met system targeting PI3K/AKT and STAT3/phosphorylated-STAT3 pathways in pituitary adenomas: an immunohistochemical characterization in view of targeted therapies. Endocrine. 2013;44:735–743. doi: 10.1007/s12020-013-9950-x. [DOI] [PubMed] [Google Scholar]
- 81.Murat CB, Braga PB, Fortes MA, Bronstein MD, Corrêa-Giannella ML, Giorgi RR. Mutation and genomic amplification of the PIK3CA proto-oncogene in pituitary adenomas. Braz J Med Biol Res. 2012;45:851–855. doi: 10.1590/s0100-879x2012007500115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang RQ, Lan YL, Lou JC, Lyu YZ, Hao YC, Su QF, et al. Expression and methylation status of LAMA2 are associated with the invasiveness of nonfunctioning PitNET. Ther Adv Endocrinol Metab. 2019;10:2042018818821296. doi: 10.1177/2042018818821296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/jci34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xiao Z, Yang X, Zhang K, Liu Z, Shao Z, Song C, et al. Estrogen receptor α/prolactin receptor bilateral crosstalk promotes bromocriptine resistance in prolactinomas. Int J Med Sci. 2020;17:3174–3189. doi: 10.7150/ijms.51176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13:1886–1918. doi: 10.3390/ijms13021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rubinfeld H, Shimon I. PI3K/Akt/mTOR and Raf/MEK/ERK signaling pathways perturbations in non-functioning pituitary adenomas. Endocrine. 2012;42:285–291. doi: 10.1007/s12020-012-9682-3. [DOI] [PubMed] [Google Scholar]
- 87.Jia W, Sanders AJ, Jia G, Liu X, Lu R, Jiang WG. Expression of the mTOR pathway regulators in human pituitary adenomas indicates the clinical course. Anticancer Res. 2013;33:3123–3131. [PubMed] [Google Scholar]
- 88.Tanoue T, Nishida E. Docking interactions in the mitogen-activated protein kinase cascades. Pharmacol Ther. 2002;93:193–202. doi: 10.1016/s0163-7258(02)00188-2. [DOI] [PubMed] [Google Scholar]
- 89.Fang JY, Richardson BC. The MAPK signalling pathways and colorectal cancer. Lancet Oncol. 2005;6:322–327. doi: 10.1016/s1470-2045(05)70168-6. [DOI] [PubMed] [Google Scholar]
- 90.Yong HY, Koh MS, Moon A. The p38 MAPK inhibitors for the treatment of inflammatory diseases and cancer. Expert Opin Investig Drugs. 2009;18:1893–1905. doi: 10.1517/13543780903321490. [DOI] [PubMed] [Google Scholar]
- 91.Peng WX, Huang JG, Yang L, Gong AH, Mo YY. Linc-RoR promotes MAPK/ERK signaling and confers estrogen-independent growth of breast cancer. Mol Cancer. 2017;16:161. doi: 10.1186/s12943-017-0727-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ewing I, Pedder-Smith S, Franchi G, Ruscica M, Emery M, Vax V, et al. A mutation and expression analysis of the oncogene BRAF in pituitary adenomas. Clin Endocrinol (Oxf). 2007;66:348–352. doi: 10.1111/j.1365-2265.2006.02735.x. [DOI] [PubMed] [Google Scholar]
- 93.Roof AK, Gutierrez-Hartmann A. Consider the context: Ras/ERK and PI3K/AKT/mTOR signaling outcomes are pituitary cell type-specific. Mol Cell Endocrinol. 2018;463:87–96. doi: 10.1016/j.mce.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 94.Pagotto U, Arzberger T, Theodoropoulou M, Grübler Y, Pantaloni C, Saeger W, et al. The expression of the antiproliferative gene ZAC is lost or highly reduced in nonfunctioning pituitary adenomas. Cancer Res. 2000;60:6794–6799. [PubMed] [Google Scholar]
- 95.Theodoropoulou M, Zhang J, Laupheimer S, Paez-Pereda M, Erneux C, Florio T, et al. Octreotide, a somatostatin analogue, mediates its antiproliferative action in pituitary tumor cells by altering phosphatidylinositol 3-kinase signaling and inducing Zac1 expression. Cancer Res. 2006;66:1576–1582. doi: 10.1158/0008-5472.can-05-1189. [DOI] [PubMed] [Google Scholar]
- 96.Hayes JD, Dinkova-Kostova AT, Tew KD. Oxidative stress in cancer. Cancer Cell. 2020;38:167–197. doi: 10.1016/j.ccell.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhan X, Desiderio DM. Nitroproteins from a human pituitary adenoma tissue discovered with a nitrotyrosine affinity column and tandem mass spectrometry. Anal Biochem. 2006;354:279–289. doi: 10.1016/j.ab.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 98.Sabatino ME, Grondona E, Sosa LDV, Mongi Bragato B, Carreño L, Juarez V, et al. Oxidative stress and mitochondrial adaptive shift during pituitary tumoral growth. Free Radic Biol Med. 2018;120:41–55. doi: 10.1016/j.freeradbiomed.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 99.Zhan X, Li J, Zhou T. Targeting Nrf2-mediated oxidative stress response signaling pathways as new therapeutic strategy for pituitary adenomas. Front Pharmacol. 2021;12:565748. doi: 10.3389/fphar.2021.565748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhan X, Desiderio DM. The use of variations in proteomes to predict, prevent, and personalize treatment for clinically nonfunctional pituitary adenomas. EPMA J. 2010;1:439–459. doi: 10.1007/s13167-010-0028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wallace DC. Mitochondria and cancer. Nat Rev Cancer. 2012;12:685–698. doi: 10.1038/nrc3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Annesley SJ, Fisher PR. Mitochondria in health and disease. Cells. 2019;8(7):680. doi: 10.3390/cells8070680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Srinivasan S, Guha M, Kashina A, Avadhani NG. Mitochondrial dysfunction and mitochondrial dynamics-The cancer connection. Biochim Biophys Acta Bioenerg. 2017;1858:602–614. doi: 10.1016/j.bbabio.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Horoupian DS. Large mitochondria in a pituitary adenoma with hyperprolactinemia. Cancer. 1980;46:537–542. doi: 10.1002/1097-0142(19800801)46:3<537::aid-cncr2820460319>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 105.Saeger W, Lüdecke DK, Buchfelder M, Fahlbusch R, Quabbe HJ, Petersenn S. Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor Registry. Eur J Endocrinol. 2007;156:203–216. doi: 10.1530/eje.1.02326. [DOI] [PubMed] [Google Scholar]
- 106.An J, Zhang Y, He J, Zang Z, Zhou Z, Pei X, et al. Lactate dehydrogenase A promotes the invasion and proliferation of pituitary adenoma. Sci Rep. 2017;7:4734. doi: 10.1038/s41598-017-04366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dénes J, Swords F, Rattenberry E, Stals K, Owens M, Cranston T, et al. Heterogeneous genetic background of the association of pheochromocytoma/paraganglioma and pituitary adenoma: results from a large patient cohort. J Clin Endocrinol Metab. 2015;100:E531–541. doi: 10.1210/jc.2014-3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang D, Wong HK, Feng YB, Zhang ZJ. 18beta-glycyrrhetinic acid induces apoptosis in pituitary adenoma cells via ROS/MAPKs-mediated pathway. J Neurooncol. 2014;116:221–230. doi: 10.1007/s11060-013-1292-2. [DOI] [PubMed] [Google Scholar]
- 109.Xiong S, Mu T, Wang G, Jiang X. Mitochondria-mediated apoptosis in mammals. Protein. Cell. 2014;5:737–749. doi: 10.1007/s13238-014-0089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gao F, Pan S, Liu B, Zhang H. TFF3 knockout in human pituitary adenoma cell HP75 facilitates cell apoptosis via mitochondrial pathway. Int J Clin Exp Pathol. 2015;8:14568–14573. [PMC free article] [PubMed] [Google Scholar]
- 111.Tanase C, Albulescu R, Codrici E, Calenic B, Popescu ID, Mihai S, et al. Decreased expression of APAF-1 and increased expression of cathepsin B in invasive pituitary adenoma. Onco Targets Ther. 2015;8:81–90. doi: 10.2147/ott.s70886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li N, Zhan X. Mitochondrial dysfunction pathway networks and mitochondrial dynamics in the pathogenesis of pituitary adenomas. Front Endocrinol (Lausanne). 2019;10:690. doi: 10.3389/fendo.2019.00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Deyu H, Luqing C, Xianglian L, Pu G, Qirong L, Xu W, et al. Protective mechanisms involving enhanced mitochondrial functions and mitophagy against T-2 toxin-induced toxicities in GH3 cells. Toxicol Lett. 2018;295:41–53. doi: 10.1016/j.toxlet.2018.05.041. [DOI] [PubMed] [Google Scholar]
- 114.Kar S. Unraveling cell-cycle dynamics in cancer. Cell Syst. 2016;2:8–10. doi: 10.1016/j.cels.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 115.Simpson DJ, Hibberts NA, McNicol AM, Clayton RN, Farrell WE. Loss of pRb expression in pituitary adenomas is associated with methylation of the RB1 CpG island. Cancer Res. 2000;60:1211–1216. [PubMed] [Google Scholar]
- 116.Yoshino A, Katayama Y, Ogino A, Watanabe T, Yachi K, Ohta T, et al. Promoter hypermethylation profile of cell cycle regulator genes in pituitary adenomas. J Neurooncol. 2007;83:153–162. doi: 10.1007/s11060-006-9316-9. [DOI] [PubMed] [Google Scholar]
- 117.García-Fernández RA, García-Palencia P, Sánchez M, Gil-Gómez G, Sánchez B, Rollán E, et al. Combined loss of p21(waf1/cip1) and p27(kip1) enhances tumorigenesis in mice. Lab Invest. 2011;91:1634–1642. doi: 10.1038/labinvest.2011.133. [DOI] [PubMed] [Google Scholar]
- 118.Gruppetta M, Formosa R, Falzon S, Ariff Scicluna S, Falzon E, Degeatano J, et al. Expression of cell cycle regulators and biomarkers of proliferation and regrowth in human pituitary adenomas. Pituitary. 2017;20:358–371. doi: 10.1007/s11102-017-0803-0. [DOI] [PubMed] [Google Scholar]
- 119.Matoušek P, Buzrla P. Factors That predict the growth of residual nonfunctional pituitary adenomas: correlations between relapse and cell cycle Markers. Biomed Res Int. 2018;2018:1876290. doi: 10.1155/2018/1876290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Butz H, Németh K, Czenke D, Likó I, Czirják S, Zivkovic V, et al. Systematic investigation of expression of G2/M transition genes reveals CDC25 alteration in nonfunctioning pituitary adenomas. Pathol Oncol Res. 2017;23:633–641. doi: 10.1007/s12253-016-0163-5. [DOI] [PubMed] [Google Scholar]
- 121.Vázquez-Borrego MC, Fuentes-Fayos AC, Herrera-Martínez AD, F LL, Ibáñez-Costa A, Moreno-Moreno P, et al. Biguanides exert antitumoral actions in pituitary tumor cells through AMPK-dependent and -independent mechanisms. J Clin Endocrinol Metab. 2019; 104:3501–3513. 10.1210/jc.2019-00056. [DOI] [PubMed]
- 122.Vázquez-Borrego MC, Fuentes-Fayos AC, Herrera-Martínez AD, Venegas-Moreno E, F LL, Fanciulli A, et al. Statins directly regulate pituitary cell function and exert antitumor effects in pituitary tumors. Neuroendocrinology. 2020; 110:1028–1041. 10.1159/000505923. [DOI] [PubMed]
- 123.Zeng J, See AP, Aziz K, Thiyagarajan S, Salih T, Gajula RP, et al. Nelfinavir induces radiation sensitization in pituitary adenoma cells. Cancer Biol Ther. 2011;12:657–663. doi: 10.4161/cbt.12.7.17172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hubina E, Nanzer AM, Hanson MR, Ciccarelli E, Losa M, Gaia D, et al. Somatostatin analogues stimulate p27 expression and inhibit the MAP kinase pathway in pituitary tumours. Eur J Endocrinol. 2006;155:371–379. doi: 10.1530/eje.1.02213. [DOI] [PubMed] [Google Scholar]
- 125.Guzzo MF, Carvalho LR, Bronstein MD. Ketoconazole treatment decreases the viability of immortalized pituitary cell lines associated with an increased expression of apoptosis-related genes and cell cycle inhibitors. J Neuroendocrinol. 2015;27:616–623. doi: 10.1111/jne.12277. [DOI] [PubMed] [Google Scholar]
- 126.Petiti JP, Sosa LDV, Picech F, Moyano Crespo GD, Arevalo Rojas JZ, Pérez PA, et al. Trastuzumab inhibits pituitary tumor cell growth modulating the TGFB/SMAD2/3 pathway. Endocr Relat Cancer. 2018;25:837–852. doi: 10.1530/erc-18-0067. [DOI] [PubMed] [Google Scholar]
- 127.Lu M, Wang Y, Zhan X. The MAPK pathway-based drug therapeutic targets in pituitary adenomas. Front Endocrinol (Lausanne). 2019;10:330. doi: 10.3389/fendo.2019.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dworakowska D, Wlodek E, Leontiou CA, Igreja S, Cakir M, Teng M, et al. Activation of RAF/MEK/ERK and PI3K/AKT/mTOR pathways in pituitary adenomas and their effects on downstream effectors. Endocr Relat Cancer. 2009;16:1329–1338. doi: 10.1677/erc-09-0101. [DOI] [PubMed] [Google Scholar]
- 129.Xu M, Shorts-Cary L, Knox AJ, Kleinsmidt-DeMasters B, Lillehei K, Wierman ME. Epidermal growth factor receptor pathway substrate 8 is overexpressed in human pituitary tumors: role in proliferation and survival. Endocrinology. 2009;150:2064–2071. doi: 10.1210/en.2008-1265. [DOI] [PubMed] [Google Scholar]
- 130.Rubinfeld H, Kammer A, Cohen O, Gorshtein A, Cohen ZR, Hadani M, et al. IGF1 induces cell proliferation in human pituitary tumors - functional blockade of IGF1 receptor as a novel therapeutic approach in non-functioning tumors. Mol Cell Endocrinol. 2014;390:93–101. doi: 10.1016/j.mce.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 131.Sirotnak FM, Zakowski MF, Miller VA, Scher HI, Kris MG. Efficacy of cytotoxic agents against human tumor xenografts is markedly enhanced by coadministration of ZD1839 (Iressa), an inhibitor of EGFR tyrosine kinase. Clin Cancer Res. 2000;6:4885–4892. [PubMed] [Google Scholar]
- 132.Fukuoka H, Cooper O, Mizutani J, Tong Y, Ren SG, Bannykh S, et al. HER2/ErbB2 receptor signaling in rat and human prolactinoma cells: strategy for targeted prolactinoma therapy. Mol Endocrinol. 2011;25:92–103. doi: 10.1210/me.2010-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vlotides G, Siegel E, Donangelo I, Gutman S, Ren SG, Melmed S. Rat prolactinoma cell growth regulation by epidermal growth factor receptor ligands. Cancer Res. 2008;68:6377–6386. doi: 10.1158/0008-5472.can-08-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zatelli MC, Minoia M, Filieri C, Tagliati F, Buratto M, Ambrosio MR, et al. Effect of everolimus on cell viability in nonfunctioning pituitary adenomas. J Clin Endocrinol Metab. 2010;95:968–976. doi: 10.1210/jc.2009-1641. [DOI] [PubMed] [Google Scholar]
- 135.Cerovac V, Monteserin-Garcia J, Rubinfeld H, Buchfelder M, Losa M, Florio T, et al. The somatostatin analogue octreotide confers sensitivity to rapamycin treatment on pituitary tumor cells. Cancer Res. 2010;70:666–674. doi: 10.1158/0008-5472.can-09-2951. [DOI] [PubMed] [Google Scholar]
- 136.Koklesova L, Samec M, Liskova A, Zhai K, Büsselberg D, Giordano FA, Kubatka P, Golunitschaja O. Mitochondrial impairments in aetiopathology of multifactorial diseases: common origin but individual outcomes in context of 3P medicine. EPMA J. 2021;12(1):1–14. doi: 10.1007/s13167-021-00237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials are available in the current manuscript.
Not applicable.