Abstract
The purpose of the study was to evaluate the effect of local application of vancomycin powder (VP) to prevent surgical site infections (SSIs) after posterior spine surgery.
A comprehensive search of Web of Science, EMBASE, Pubmed, Ovid, and Cochrane Library databases for articles published was performed to collect comparative studies of intrawound vancomycin in posterior spine surgery before March 2021. Two reviewers independently screened eligible articles based on the inclusion and exclusion criteria, assessed the study quality, and extracted the data. Revman 5.4 software was used for data analysis.
A total of 22 articles encompassing 11 555 surgical patients were finally identified for meta-analysis. According to the information provided by the included literature, the combined odds ratio showed that topical use of VP was effective for reducing the incidence of SSIs (P< 0.00001) after posterior spine surgery without affecting its efficacy in the treatment of deep infections (P< 0.00001). However, there is no statistical significance in superficial infections. In a subgroup analysis, VP at a dose of 1, 2, and 0.5–2 g reduced the incidence of spinal SSIs. The result of another subgroup analysis suggested that local application of VP could significantly reduce the risk of SSIs, whether it was administered after posterior cervical surgery or thoracolumbar surgery. Moreover, the percentage of SSIs due to gram-positive germs (P< 0.00001) and MRSA (P< 0.0001) could reduce after intraoperative VP was used, but did not significantly reduce to gram-negative germs.
The local application of VP appears to protect against SSIs, gram-positive germs, and MRSA (methicillin-resistant Staphylococcus aureus) infections after the posterior spinal operation.
Keywords: vancomycin powder, posterior spinal operation, surgical site infections, meta-analysis
Introduction
Surgery site infections (SSIs) is one of the common complications affecting surgical management and patient recovery. Related studies report that the incidence of SSIs is 1–14% (1, 2, 3, 4, 5). The incidence of SSIs is mainly due to the physical condition of the patient and the operation method. Studies have shown that diabetes and posterior spinal surgery are high-risk factors for infection. Comparatively, posterior spinal surgery is a more severe trauma and the posterior vascular supply is poorer, harder to confront bacteria, which leads to the infection rate to be higher than that of anterior surgery. For anterior cervical surgery, the incidence of deep SSIs was 0.4% (6), while for posterior cervical surgery was 0.7% (7). Systemic antibiotics were used before surgery, which was recommended by the evidence-based clinical guideline for prophylactic antibiotics in spinal surgery (8). However, the incidence of SSIs still ranges from 1 to 10%. Once SSIs occur, the impact on patients will be catastrophic, which results in increased hospitalization costs, extended length of hospitalization, and even the need for another operation (1, 9). Hence, further measures to prevent SSIs need to be found.
In the spine SSIs microflora, the most common bacteria is Staphylococcus aureus (45.2%), followed by Staphylococcus epidermidis (31.4%), and methicillin-resistant pathogens (34.3%) of all SSIs (10). In recent years, scholars began to study vancomycin powder (VP) spray, local to lower spine SSIs because of its antimicrobial activity on most of the gram-positive bacteria and cost-effectiveness. In addition, many predecessors have performed a meta-analysis to study the relieving effect of vancomycin in spinal surgery. However, the results of vancomycin in posterior spinal surgery are contradictory. Different surgical approaches have various surgical indications and different infection rates. In posterior surgery, some scholars have found that the application of vancomycin could reduce the occurrence of SSIs and that it is statistically significant. As some conclusions are contrary to it, whether local application of vancomycin can prevent SSIs after posterior spinal surgery remains inconclusive.
In this study, we performed a meta-analysis to answer the following questions: (i) whether local spraying of VP can reduce SSIs in the posterior spine; (ii) whether local application of vancomycin can reduce SSIs in different surgical areas; (iii) which dosage of VP is suitable for posterior spinal operation; (iv) what is the effect of the application of vancomycin on infectious bacteria.
Materials and methods
This meta-analysis was conducted in agreement with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (11). The PRISMA Checklist is provided in the supplemetnary materials (see section on Supplemetnary materials provided at the end of the article). The protocol was registered on PROSPERO (Registration No: CRD42021241661).
Search strategy
All articles published before March 2021 in PubMed, Web of Science, EMBASE, Ovid, and Cochrane Library databases were searched using the terms ‘vancomycin’, ‘posterior’, and ‘spinal or spine’. The search strategy used for the PubMed search were: ‘((‘posterior’[All Fields] OR ‘posteriors’[All Fields]) AND (‘spine’[MeSH Terms] OR ‘spine’[All Fields] OR ‘spines’[All Fields] OR ‘spines’[All Fields] OR (‘spinal’[All Fields] OR ‘spinally’[All Fields] OR ‘spinals’[All Fields]))) AND (‘vancomycin’[MeSH Terms] OR ‘vancomycin’[All Fields] OR ‘vancomycine’[All Fields] OR ‘vancomycins’[All Fields] OR (‘vancomycin’[MeSH Terms] OR ‘vancomycin’[All Fields] OR ‘vancocin’[All Fields] OR ‘vancomycine’[All Fields] OR ‘vancomycin s’[All Fields] OR ‘vancomycins’[All Fields]))’. No language restrictions were applied during the search. We also searched the reference lists of articles retrieved from the electronic search for related articles. In addition, the reference lists of systematic review and meta-analysis articles concerning prophylactic VP and spinal surgery were scanned. Two independent researchers (HL and HZH) performed the searches and made decisions regarding the inclusion and exclusion criteria. When the two researchers had different opinions about the eligibility of a study for inclusion or a consensus was not reached, it was then decided by the senior researcher after a group discussion.
Inclusion criteria: (i) clinical retrospective or prospective studies; (ii) subjects who had undergone open posterior spinal surgery but had no preoperative infection in the spinal region; (iii) intervention measures and intraoperative application of vancomycin in the incision was the main difference between the experimental group and the control group; (iv) outcome index was the rate of SSIs.
Exclusion criteria: (i) case reports, reviews, letters, animal trials, and republished literature; (ii) no control group was established; (iii) non-posterior spinal surgery; (iv) the sample included patients with suspected preoperative spinal tract infection.
Data extraction and study assessment
Two investigators (HZH and HL) independently extracted all the related data from selected studies. Disagreements were resolved by consensus, and a third reviewer addressed the disagreements if investigators were unable to reach a consensus. Data extracted included the first author’s name, year of publication, type of study, sample size, SSIs case number (superficial and deep SSIs), bacterial type of infection, surgical area (cervical spine, thoracolumbar spine), the dosage of VP, and the follow-up time. A simplified version of the Oxford Centre for Evidence-based Medicine (OCEBM) guide (12) was used to evaluate the evidence. The Newcastle–Ottawa scale (NOS) was used to evaluate the literature quality of the retrospective cohort studies (13).
Statistical analysis
The meta-analysis was performed by using RevMan 5.4 software provided by the Cochrane collaboration, and the GRADEpro software was used to examine the following domains: study design, risk of bias, inconsistency, indirectness, and imprecision. First, the heterogeneity among the studies was qualitatively and quantitatively evaluated by Q test and I2 value calculation. When P> 0.1 and I2< 50, the heterogeneity was not significant and the data were combined with a fixed-effect model. When the heterogeneity was significant (P< 0.1 or I2 > 50), the random-effects model merged the data. The odds ratio (OR) and 95% confidence interval (CI) were used to indicate the difference of effects for the data of chloric variables. When OR < 1, it indicated that the event was beneficial to the test group; when P< 0.05, it was suggested that the difference was statistically significant; when P> 0.05, the difference was not statistically significant.
Results
Study characteristics
A total of 284 articles were revealed from PubMed, Web of Science, EMBASE, Ovid, and Cochrane Library databases. Of this, 123 were duplicates and hence excluded. After carefully reading the titles and abstracts of the articles, a further 124 irrelevant articles were excluded and 37 articles were selected. After reading the full text, 15 articles were excluded. Of these 15 deleted studies, one study included combination surgeries using the anterior and posterior approach, 2 studies didn’t report the rate of SSIs, 2 studies didn’t include the control group, 3 studies used different methods to clean the surgical site between the control and intervention group, 5 studies didn’t mention the exact number of the posterior spinal surgery, 3 studies reported the same result, and we included only one study. Finally, a total of 22 studies that followed the inclusion and exclusion criteria were chosen for the present meta-analysis (Fig. 1 ). All these studies are retrospective trials registering a total of 11 555 patients eligible for meta-analysis (14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35). All relevant information mentioned in the included studies is given in Table 1. The quality score of the included studies ranged from 1 to 5 points. All these studies were evaluated by NOS score and were of high-technical quality (Table 2).
Figure 1.
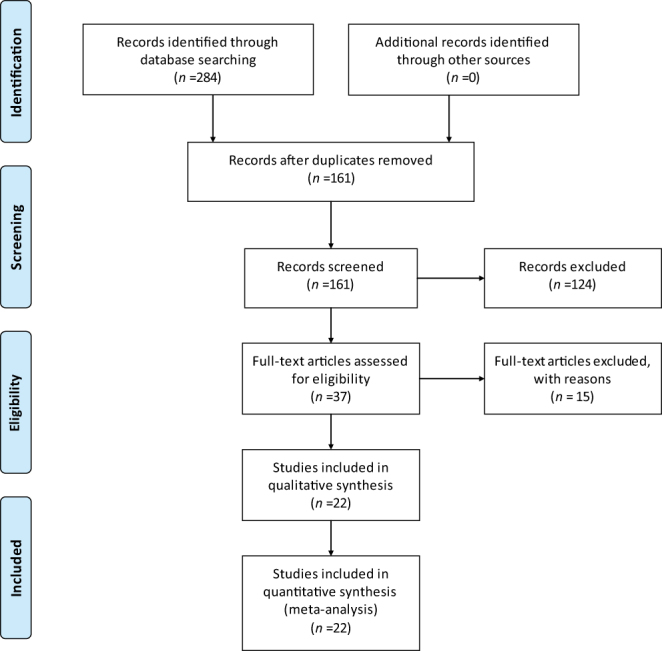
Flow diagram for search and selection of included studies.
Table 1.
Characteristics of the included studies.
| Reference | Design | Sample size | Follow-up (months) |
|---|---|---|---|
| Adhikari et al. (14) | Retrospective | 158 | – |
| Byvaltsev et al. (15) | Retrospective | 214 | – |
| Delgado-López et al. (16) | Retrospective | 300 | – |
| Devin et al. (17) | Retrospective | 2056 | 1 |
| Dewan et al. (18) | Retrospective | 565 | 3 |
| Emohare et al. (19) | Retrospective | 303 | – |
| Garg et al. (20) | Retrospective | 538 | 3 |
| Gun-Ill et al. (21) | Retrospective | 571 | 3 |
| Haimoto et al. (22) | Retrospective | 515 | 6 |
| Heller et al. (23) | Retrospective | 683 | 3 |
| Hill et al. (24) | Retrospective | 300 | 1 |
| Li et al. (25) | Retrospective | 569 | 12 |
| Liu et al. (26) | Retrospective | 334 | – |
| Maajid et al. (27) | Retrospective | 303 | 3 |
| Martin et al. (28) | Retrospective | 306 | 1 |
| Martin et al. (29) | Retrospective | 289 | 1 |
| Oktay et al. (30) | Retrospective | 209 | 3 |
| Pahys et al. (31) | Retrospective | 518 | – |
| Strom et al. (33) (cervical) | Retrospective | 171 | 12 |
| Strom et al. (32) | Retrospective | 253 | 12 |
| Sweet et al. (34) | Retrospective | 1732 | 12 |
| Takeuchi et al. (35) | Retrospective | 668 | 3 |
Table 2.
New Castle–Ottawa Scale ratings. Each asterisk represents one point.
| Reference | Selection | Comparability | Exposure/outcome |
|---|---|---|---|
| Adhikari et al. (14) | **** | – | *** |
| Byvaltsev et al. (15) | **** | – | ** |
| Delgado-López et al. (16) | **** | * | ** |
| Devin et al. (17) | **** | * | ** |
| Dewan et al. (18) | **** | * | *** |
| Emohare et al. (19) | **** | – | ** |
| Garg et al. (20) | **** | ** | *** |
| Gun-Ill et al. (21) | **** | * | ** |
| Haimoto et al. (22) | **** | ** | *** |
| Heller et al. (23) | **** | * | *** |
| Hill et al. (24) | **** | – | ** |
| Li et al. (25) | **** | ** | *** |
| Liu et al. (26) | **** | ** | *** |
| Maajid et al. (27) | **** | ** | *** |
| Martin et al. (28) | **** | ** | ** |
| Martin et al. (29) | **** | ** | ** |
| Oktay et al. (30) | **** | ** | *** |
| Pahys et al. (31) | **** | ** | *** |
| Strom et al. (33) (cervical) | **** | ** | *** |
| Strom et al. (32) | **** | ** | *** |
| Sweet et al. (34) | **** | ** | *** |
| Takeuchi et al. (35) | **** | ** | *** |
Effectiveness of wound spraying with vancomycin powder in the prevention of spinal SSIs
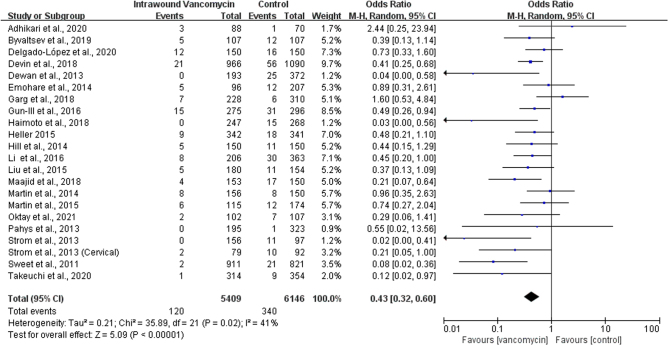
We used 5.4 RevMan software to combine the OR of the results of 22 studies and found significant heterogeneity (P = 0.02, I2 = 41%), and the random-effects model was used to merge the extracted data. Results show that compared with the control group, before closing the wound, the topical use of VP can effectively reduce the posterior spine SSIs, and the result was statistically significant (OR: 0.43, 95% CI: 0.32–0.60; P < 0.00001, Fig. 2). The Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) for this outcome was moderate (O) (Table 3). SSIs are mainly divided into superficial and deep incisional infections in terms of site, and the effect of local application of VP may be different between the two. Some researchers will adjust the spraying according to different situations, such as the patient’s wound size or weight dose. Of course, there are some researchers using the fixed dose of 1 g or 2 g. Additionally, relevant studies have shown that differences in spinal surgery areas may affect the occurrence of SSIs. Therefore, three subgroup analyses were conducted in this paper according to different vancomycin doses, surgical areas, and infected sites.
Figure 2.
Forest plot of comparison: vancomycin vs control, outcome: surgical site infections.
Table 3.
Grading of Recommendations Assessment, Developing, and Evaluation used to assess the systematic review outcomes. Vancomycin was compared to control for patients with posterior spine surgery.
| Outcomes | Anticipated absolute effects, per 1000* | Relative effect, OR (95% CI) | Number of | Certainty of the evidence (GRADE) | ||
|---|---|---|---|---|---|---|
| Risk with control | Risk with vancomycin (95% CI) | Participants | Studies† | |||
| SSIs | 55 | 25 (18–34) | 0.43 (0.32–0.60) | 11 555 | 22 | ⊕⊕⊕◯ Moderate |
| Superficial SSIs | 33 | 36 (19–66) | 1.10(0.58–2.10) | 1112 | 4 | ⊕⊕◯◯ Low |
| Deep SSIs | 35 | 13 (9–20) | 0.37(0.24–0.57) | 4291 | 9 | ⊕⊕⊕◯ Moderate |
| Dose of 1 g | 75 | 27 (20–36) | 0.34(0.25–0.46) | 4590 | 12 | ⊕⊕⊕◯ Moderate |
| Dose of 2 g | 36 | 15 (8–27) | 0.41(0.23–0.74) | 2327 | 3 | ⊕⊕⊕◯ Moderate |
| Dose between 0.5 –and 2 g | 44 | 21 (16–30) | 0.47(0.34–0.66) | 4638 | 7 | ⊕⊕⊕◯ Moderate |
| Cervical | 41 | 18 (8–38) | 0.44(0.20–0.94) | 1071 | 5 | ⊕⊕⊕◯ Moderate |
| Thoracolumbar | 61 | 26 (19–37) | 0.42(0.30–0.59) | 4549 | 10 | ⊕⊕⊕◯ Moderate |
| Infectious bacteria | 61 | 32 (25–43) | 0.51(0.39–0.69) | 4731 | 13 | ⊕⊕◯◯ Low |
| MRSA | 17 | 3 (2–7) | 0.20(0.10–0.43) | 4731 | 13 | ⊕⊕⊕◯ Moderate |
| Gram-positive bacteria | 41 | 15 (10–23) | 0.35(0.23–0.56) | 3591 | 11 | ⊕⊕⊕◯ Moderate |
| Gram-negative bacteria | 15 | 14 (8–26) | 0.98(0.54–1.79) | 2908 | 10 | ⊕⊕◯◯ Low |
*The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
†All studies were observational.
OR, odds ratio.
Effectiveness of wound spraying with vancomycin powder in the prevention of deep and superficial SSIs
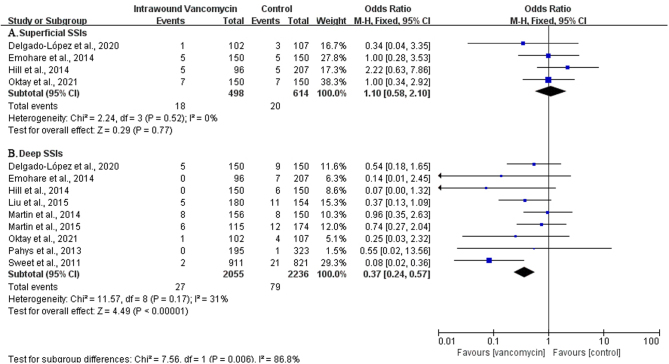
Subgroup analysis of the included studies that explicitly indicated deep and superficial infections was performed, P > 0.1, I2 < 50. The heterogeneity was not significant, and the fixed-effect model was used to combine OR values of the extracted data. The results showed that the local use of VP could play a significant preventive effect in deep infections (OR: 0.37, 95% CI: 0.24–0.57; P< 0.000001, I2 = 31%, moderate GRADE, Fig. 3 and Table 3). However, there was no statistically significant difference in superficial infections between the VP and control groups (OR: 1.10, 95% CI: 0.58–2.10; P = 0.77, I2 = 0%, low GRADE, Fig. 3 and Table 3).
Figure 3.
The subgroup analysis of the surgical site infections (SSIs) in deep and superficial SSIs.
Effectiveness of different doses of vancomycin powder in the prevention of spinal SSIs
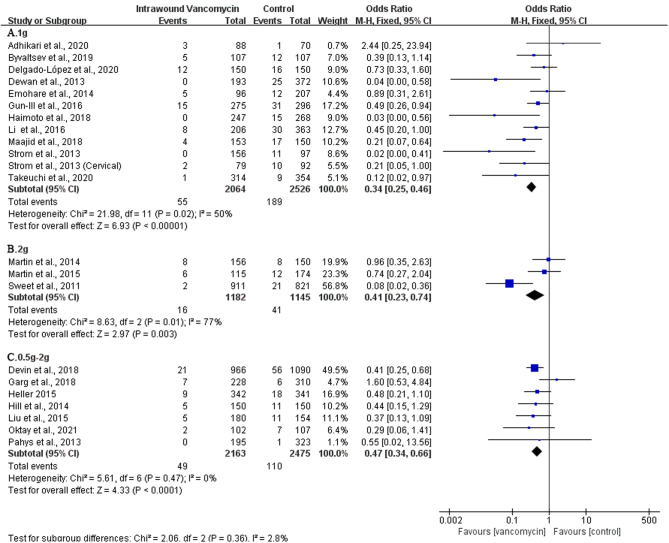
At present, there is no separate report on the dose gradient titer of VP. We can only conduct separate subgroup analysis on the dose of 1g, 2g and 0.5–2 g VP, and the results showed that VP sprayed on the wound, at a dose of 1 (OR: 0.34, 95% CI: 0.25–0.46; P< 0.00001, I2 = 50%, Fig. 4A), 2 (OR: 0.41, 95% CI: 0.23–0.74, P=0.003, I2 = 77%, Fig. 4B), and 0.5–2 g (OR: 0.47; 95% CI: 0.34–0.66; P< 0.0001, I2 = 0%, Fig. 4C), respectively could reduce the occurrence of SSIs after posterior spine surgery. The results had a general meaning, and a fixed quantity of VP has a preventive effect on spinal SSIs, and there was a statistically significant difference in the results. The GRADE for these outcomes was moderate (⊕⊕⊕◯) (Table 3).
Figure 4.
The subgroup analysis of the dosage of vancomycin powder used.
Effectiveness of wound spraying with VP at different surgical areas
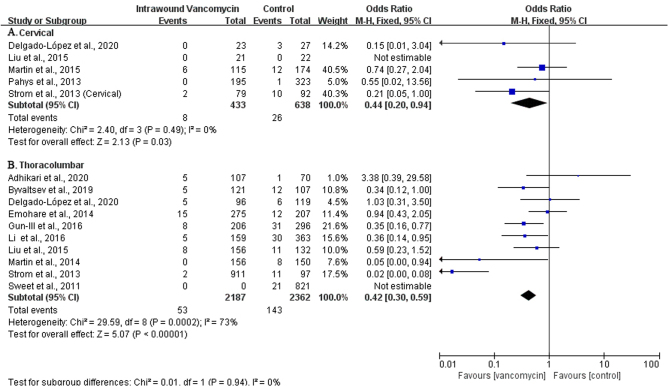
Relevant studies have shown that the difference in spine surgery areas may affect the incidence of SSIs. Therefore, we conducted a subgroup analysis based on the surgery area (cervical spine, thoracic spine, and lumbar spine) as the included studies did not provide enough information about thoracic spine surgery, and as most of the thoracic spine surgery involves the thoracolumbar junction, we analyzed the thoracic and lumbar spine area as a whole. In 22 studies, 1071 patients underwent cervical spine surgery (16, 26, 29, 31, 33), and the incidence of SSIs in the VP group was 1.8% (8/433) and 4.1% (26/638) in the control group. The results showed that the incidence of SSIs had been decreased in the VP group compared to the control group (OR: 0.44; 95% CI: 0.20–0.94; Fig. 5A), which was statistically significant (P< 0.05). Of them, 10 studies included a total of 4549 cases undergoing thoracic and lumbar spine surgery (14, 15, 16, 19, 21, 25, 26, 28, 32, 34), 2187 cases in the experimental group, and 2362 cases in the control group. The results showed that the VP group had reduced infection of the spine surgery site compared with the control group (2.4% vs 6.1%). The difference was statistically significant (OR: 0.42; 95% CI: 0.30–0.59, Fig. 5B). Subgroup analysis shows that local application of vancomycin could reduce the incidence of SSIs in cervical, thoracic, and lumbar surgery. The level of evidence was moderate (⊕⊕⊕◯) (Table 3).
Figure 5.
The subgroup analysis of the use of vancomycin powder in different surgical areas.
The effect of local application of vancomycin on infectious bacteria
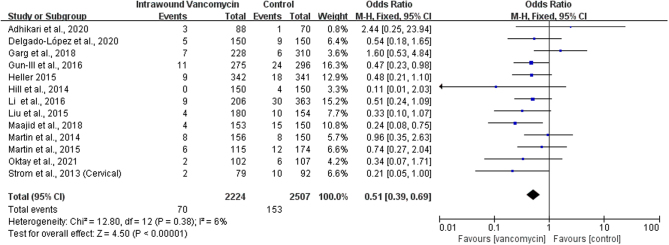
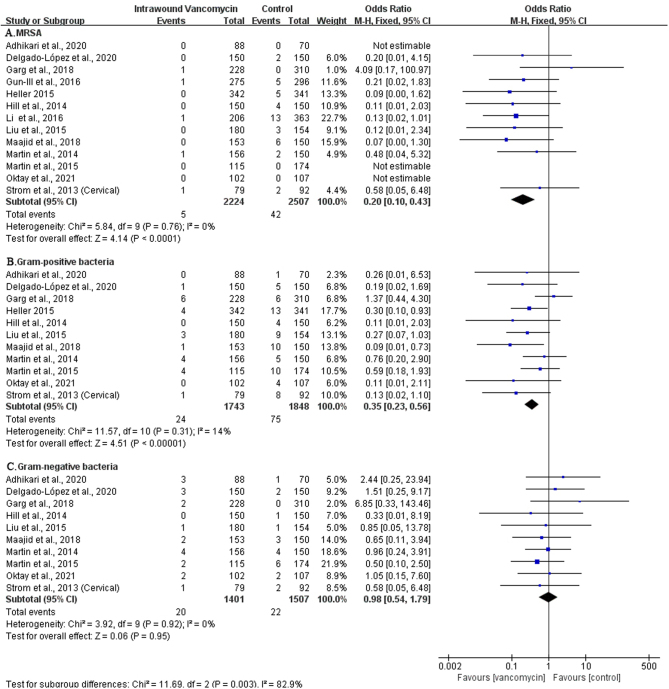
Vancomycin is a glycopeptide antibiotic widely used in the treatment of gram-positive bacterial infections. Studies have shown that vancomycin can reduce the risk of MRSA infections. For this reason, we have analyzed infectious bacteria after the topical use of vancomycin. A total of 13 pieces of literature reported on the types of bacteria infected after topical vancomycin application (14, 16, 20, 21, 23, 24, 25, 26, 27, 28, 29, 30, 33). The results showed that the use of vancomycin could significantly reduce the positive rate of bacterial culture (OR: 0.51; 95% CI: 0.39–0.69; P< 0.00001, I2 = 6%, low GRADE, Fig. 6 and Table 3). We conducted a subgroup analysis of different bacterial types. The results showed that vancomycin could significantly reduce the infection of MRSA (OR: 0.20; 95% CI: 0.10–0.43; P< 0.0001, I2 = 0%, Fig. 7A) and gram-positive bacteria (OR: 0.35; 95% CI: 0.23–0.56; P< 0.00001, I2 = 14%, moderate GRADE, Fig. 7B and Table 3) but was not statistically significant for the prevention of gram-negative bacterial infections (OR: 0.98; 95% CI: 0.54–1.79; P= 0.95, I2 = 0%, low GRADE, Fig. 7C and Table 3).
Figure 6.
The effect of local application of vancomycin on infectious bacteria.
Figure 7.
The subgroup analysis of different bacterial types.
Sensitivity analysis
The sensitivity analysis of the included studies was carried out with the method of one-by-one exclusion, and the OR values of the remaining studies were combined after excluding any study. No single study was found to have a significant impact on the final results.
The funnel plot of bias risk
Figure 8 shows that small sample studies may be the leading cause of bias.
Figure 8.
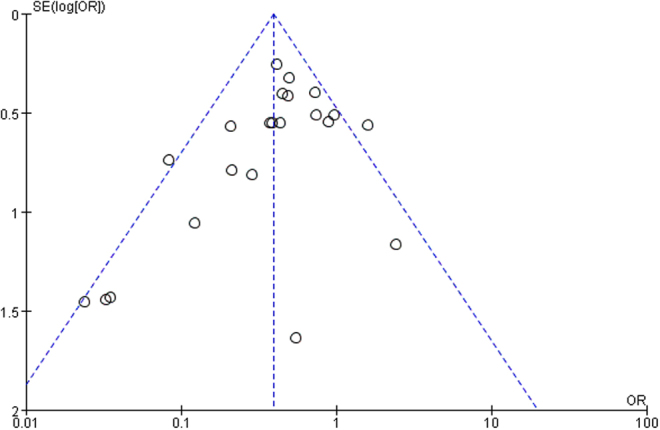
Funnel plot of the included studies in this meta-analysis for the incidence of surgical site infection.
Discussion
SSIs are one of the most common acquired hospital infections (36, 37), and the incidence of spinal SSIs can be as high as 14%. It not only increases the morbidity and mortality of patients (38) but also causes a severe burden to hospitals, patients and their families, and indeed for the whole medical and health system. Spinal SSIs will increase the risk of nerve injury, spinal instability, bone nonunion, deformity, etc. In addition, SSIs treatment needs to increase the application of antibiotics, repeated debridement, revision, and prolonged hospital stay (39, 40, 41). It is reported that the additional medical costs incurred by SSIs in about 500 000 patients in the United States every year can be as high as 10 billion US dollars (42). Studies have shown that about 60% of SSIs can be prevented, and reducing SSIs has become an important goal in improving medical care and health and to improve the quality of work (43). According to the latest guidelines for antibiotic prophylaxis in spinal surgery issued by the Spinal Society of North America (44), prophylactic use of antibiotics such as cephalosporins in spinal surgery can reduce the infection rate of patients at the surgical site because the common pathogens of SSIs are mostly gram-positive bacteria such as cephalosporin-sensitive Staphylococcus aureus and Staphylococcus epidermidis (45).
However, the incidence of spinal SSIs can still reach 0.7–10% with intravenous antibiotic prevention alone, among which, in patients with other diseases (such as diabetes), the incidence of SSIs can get to 2.0–10%, and in patients without these complications, the incidence of SSIs is about 0.7–4.3%.
Vancomycin is a glycopeptide antibiotic widely used in the treatment of gram-positive bacterial infections. Animal experiments have confirmed that spreading VP at the incision can effectively remove Staphylococcus aureus (46), while the toxic, and side effects of local application of VP are low. According to the level obtained from surgical drains, the local concentration of vancomycin has been revealed to range between 263 and 2938 μg/mL on the day of surgery and with a trend down to undetectable levels on postoperative day 4 (34). This generates a concentration nearly 1000-fold higher than the minimum inhibitory concentration for MRSA and coagulase-negative Staphylococcus, thereby reducing the development of resistant bacteria (34, 47). However, intrawound application of VP may select gram-negative infections in spine surgery (48). Since O’Neill and Sweet first reported the use of intraoperative VP at the spinal wound site, it has been supported by the majority of researchers (34, 49). The primary point is that this prophylactic method can decrease healthcare costs, the resources used, improve the quality of life during hospitalization, and reduce postoperative wound infection rates. Additionally, few side effects of intrawound vancomycin used have been revealed during spinal surgeries. However, the randomised control trial (RCT) by Tubaki et al. showed that the local use of vancomycin could not significantly affect the occurrence of spinal SSIs (50). Since then, the discussion about whether local spraying of vancomycin effectively prevents spinal SSIs has not been concluded. There are many studies on the effectiveness of local use of vancomycin in spinal surgery, but no studies focus on posterior spinal surgery. Posterior spinal surgery is a high-risk factor for SSIs (39) and its infection rate is different from other spinal surgery. So in this study, we analyzed whether the local application of vancomycin can prevent SSIs after posterior spinal surgery. Most studies support the conclusion that intrawound VP used in posterior spinal surgery can reduce the postoperative incidence of wound infection without apparent side effects. However, five articles demonstrate no significant difference (14, 16, 20, 28, 29). Hence, it is necessary to assess the efficiency of intraoperative VP in posterior spinal surgery based on the available evidence.
This study combines the latest research results published in recent years, conducted multi-group subgroup analysis according to different surgical areas, vancomycin doses, and infection types, and explored the impact of intraoperative local application of VP on SSIs after posterior spinal surgery in more detail. Data collation and analysis of the 22 included studies found that local spraying of VP can reduce the incidence of SSIs after spinal surgery as a whole (OR: 0.43, P< 0.00001), mainly to reduce the incidence of deep SSIs after posterior spinal surgery. Superficial infections were not statistically significant. In addition, two studies reported that vancomycin causes adverse reactions such as pseudoarthrosis (20, 26). The incidence of adverse reactions with vancomycin was not statistically significant. Studies have found that the difference in SSIs may be related to different spine surgery areas. Therefore, we conducted a subgroup analysis based on the surgery area (cervical spine, thoracolumbar spine) and found that vancomycin can effectively reduce spinal SSIs of thoracolumbar and cervical spine surgery.
In most studies, the dose of vancomycin for topical application is 1g or 2 g. Animal experiments and in vitro experiments have found that topical application of vancomycin can inhibit the activity of osteoblasts, thereby affecting bone healing. When the dose of vancomycin is greater than 3 mg/cm2, it can inhibit the proliferation and migration activity of osteoblasts (51), Codschmidt (52) et al.’s in vitro study on the concentration-dependent effect of vancomycin showed that when the concentration of vancomycin was greater than 4000 µg/mL, it inhibited the growth of fibroblasts. At present, there is no uniform standard for the safety of the dosage of vancomycin. It is often used by surgeons based on clinical experience, and there are not sufficient pharmacokinetic studies. However, according to current research results, the dosage of vancomycin applied locally does not exceed 2 g. Our research results also show that 1g, 2g, and 0.5–2 g vancomycin application can effectively reduce the infection of the posterior spinal incision. A retrospective study on the preventive use of vancomycin in spinal surgery showed that the incidence of SSIs was 6.7%, and the positive rate of gram-negative bacteria in the experimental group was significantly higher than that of the control. Most of them were mixed bacterial infections (53). Our study showed that the topical use of vancomycin could significantly reduce MRSA and gram-positive bacterial infections. However, it was not statistically significant for the prevention of Gram-negative bacterial infections.
Of all the documents included in this study, 15 articles support the intraoperative local application of VP, while the other 5 articles hold different views, and 2 articles did not evaluate the conclusions. The types of surgery and administration methods used in each study are different, and risk factors for the infection are different. Differences in SSIs determination criteria and follow-up time, etc., may be the reasons for disagreements. The primary factors of the included study, such as age, gender, and complications (diabetes, hypertension, obesity, and coronary heart disease), may also cause SSIs. Confounding factors affect the evaluation of the efficacy of vancomycin in reducing SSIs. Although the 15 studies supporting the application of VP have different sample sizes, the types of surgery used are all conventional spinal surgery, and the postoperative follow-up time is generally longer. At the same time, these studies have minimized the differences in other infection risk factors between the experimental and control groups, thereby effectively avoiding the interference of these factors in the research results. Although the different conclusions put forward by the other five studies should have been paid enough attention to, they still exist. The question still needs to be further explored. In addition, the Centers for Disease Control and Prevention defines SSIs as surgery-related infections that occur within 30 days after surgery without internals or within 1 year after surgery with internals (54). Many (24, 28, 29) conducted studies only followed up for 1 month after spinal internal fixation, which is likely to underestimate the incidence of postoperative SSIs. The potential difference between the experimental group and the control group infection rate is also easily ignored and increased heterogeneity.
Limitations
This review carries potential limitations. First of all, the studies we included are retrospective cohort studies, lacking prospective studies and RCT studies. According to the role of Cochrane Collaboration Guidelines, the inclusion of randomized controlled studies in a meta-analysis is most suitable for evaluating the impact of interventions on the disease because randomized controlled trials can eliminate potential biases that affect research results. Therefore, our research results will be affected by bias. Secondly, the included studies lack a unified standard for the definition of SSIs, which could affect the evaluation of SSIs. The sample size included in the study, surgical methods (such as traditional and minimally invasive surgical internal fixation and non-internal fixation), complications, surgical indications, surgical area, and antibiotic application differences will affect the evaluation of vancomycin efficacy and increase the bias risk. Thirdly, the included studies did not fix the potential confounding factors, which led to the limited adaptability of the research results of reducing SSIs of the spine. Therefore, we could not further evaluate the confounding factors that affect SSIs, such as age, BMI, concomitant diseases, smoking, etc. Fourthly, differences in follow-up time will underestimate the incidence of deep SSIs and long-term complications. As there is a lack of uniform standards for the use of VP, it is often based on the experience of surgeons, which could affect the evaluation of the efficacy and safety of vancomycin. Therefore, we need to evaluate further the effect of vancomycin use on infection at the spinal surgery site. To further clarify the impact of topical application of VP on SSIs after spinal surgery, more large-sample, high-quality clinical studies should be conducted in the future, such as randomized controlled trials withstandard medication regimens, unified SSIs determination criteria, and follow-up time limits, etc. At the same time, the pharmacokinetics of VP, drug-resistant bacteria, and the potential complications need to be further studied. Nevertheless, the overall quality of the cohort studies included in the pooled analysis is good, and the level of evidence is moderate, and multicenter double-blind, randomized controlled trials are necessary to confirm these findings.
Conclusion
Local application of VP has become an option for spinal surgeons to reduce SSIs. This study extracted and analyzed relevant literature and found that the local application of VP can effectively reduce the incidence of SSIs in posterior spinal surgery, especially for deep SSIs, but was not statistically significant for superficial SSIs. Local application of vancomycin can provide a high-concentration bactericidal environment with few systemic adverse reactions that is convenient to use, easy to manage, and inexpensive, which can effectively reduce medical costs and resources and improve the quality of life of patients during hospitalization.
Supplementary Material
ICMJE Conflict of Interest Statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding Statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Author contribution statement
All the authors contribute to this study conduction and design. Luo wrote the manuscript. Dr Feng Xue and Dr Zhenghua Hong contributed equally to this work.
References
- 1.Smith JS, Shaffrey CI, Sansur CA, Berven SH, Fu KM, Broadstone PA, Choma TJ, Goytan MJ, Noordeen HH, Knapp DRet al. Rates of infection after spine surgery based on 108,419 procedures: a report from the Scoliosis Research Society Morbidity and Mortality Committee. Spine 201136556–563. ( 10.1097/BRS.0b013e3181eadd41) [DOI] [PubMed] [Google Scholar]
- 2.Abbey DM, Turner DM, Warson JS, Wirt TC, Scalley RD. Treatment of postoperative wound infections following spinal fusion with instrumentation. Journal of Spinal Disorders 19958278–283. ( 10.1097/00002517-199508040-00003) [DOI] [PubMed] [Google Scholar]
- 3.Glassman SD, Dimar JR, Puno RM, Johnson JR. Salvage of instrumental lumbar fusions complicated by surgical wound infection. Spine 1996212163–2169. ( 10.1097/00007632-199609150-00021) [DOI] [PubMed] [Google Scholar]
- 4.Keller RB, Pappas AM. Infection after spinal fusion using internal fixation instrumentation. Orthopedic Clinics of North America 1972399–111. ( 10.1016/S0030-5898(2032182-9) [DOI] [PubMed] [Google Scholar]
- 5.Roberts FJ, Walsh A, Wing P, Dvorak M, Schweigel J. The influence of surveillance methods on surgical wound infection rates in a tertiary care spinal surgery service. Spine 199823366–370. ( 10.1097/00007632-199802010-00016) [DOI] [PubMed] [Google Scholar]
- 6.Guo Q, Zhang M, Wang L, Lu X, Ni B. Deep surgical site infection after anterior decompression and fusion with plate fixation for cervical spondylotic radiculopathy or myelopathy. Clinical Neurology and Neurosurgery 201614113–18. ( 10.1016/j.clineuro.2015.11.003) [DOI] [PubMed] [Google Scholar]
- 7.Fehlings MG, Smith JS, Kopjar B, Arnold PM, Yoon ST, Vaccaro AR, Brodke DS, Janssen ME, Chapman JR, Sasso RCet al. Perioperative and delayed complications associated with the surgical treatment of cervical spondylotic myelopathy based on 302 patients from the AOSpine North America Cervical Spondylotic Myelopathy Study. Journal of Neurosurgery: Spine 201216425–432. ( 10.3171/2012.1.SPINE11467) [DOI] [PubMed] [Google Scholar]
- 8.Watters WC, Baisden J, Bono CM, Heggeness MH, Resnick DK, Shaffer WO, Toton JF. & North American Spine Society. Antibiotic prophylaxis in spine surgery: an evidence-based clinical guideline for the use of prophylactic antibiotics in spine surgery. Spine Journal 20099142–146. ( 10.1016/j.spinee.2008.05.008) [DOI] [PubMed] [Google Scholar]
- 9.Radcliff KE, Neusner AD, Millhouse PW, Harrop JD, Kepler CK, Rasouli MR, Albert TJ, Vaccaro AR. What is new in the diagnosis and prevention of spine surgical site infections. Spine Journal 201515336–347. ( 10.1016/j.spinee.2014.09.022) [DOI] [PubMed] [Google Scholar]
- 10.Abdul-Jabbar A, Berven SH, Hu SS, Chou D, Mummaneni PV, Takemoto S, Ames C, Deviren V, Tay B, Weinstein Pet al. Surgical site infections in spine surgery: identification of microbiologic and surgical characteristics in 239 cases. Spine 201338E1425–E1431. ( 10.1097/BRS.0b013e3182a42a68) [DOI] [PubMed] [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 20096 e1000100. ( 10.1371/journal.pmed.1000100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bob Phillips CB, Sackett D, Badenoch D, Straus S, Haynes B, Dawes M. Oxford Centre for evidence-based medicine: levels of evidence (March 2009), 2009. (available at: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009) [Google Scholar]
- 13.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [cited 2021 Mar 25]. (available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp) [Google Scholar]
- 14.Adhikari P, Nabiyev VN, Bahadir S, Ayhan S, Yuksel S, Palaoglu S, Acaroglu E. Does the application of topical intrawound vancomycin powder affect deep surgical site infection and the responsible organisms after spinal surgery? A retrospective case series with a Historical Control Group. Asian Spine Journal 20201472–78. ( 10.31616/asj.2018.0298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byvaltsev VA, Stepanov IA, Borisov VE, Kalinin AA. Local administration of vancomycin powder in posterior lumbar fusion surgery. Khirurgiia 2019258–64. ( 10.17116/hirurgia201902158) [DOI] [PubMed] [Google Scholar]
- 16.Delgado-López PD, Martín-Alonso J, Martín-Velasco V, Castilla-Díez JM, Galacho-Harriero A, Ortega-Cubero S, Herrero-Gutiérrez AI, Rodríguez-Salazar A. Vancomycin powder for the prevention of surgical site infection in posterior elective spinal surgery. Neurocirugia 20203164–75. ( 10.1016/j.neucir.2019.07.004) [DOI] [PubMed] [Google Scholar]
- 17.Devin CJ, Chotai S, McGirt MJ, Vaccaro AR, Youssef JA, Orndorff DG, Arnold PM, Frempong-Boadu AK, Lieberman IH, Branch Cet al. Intrawound vancomycin decreases the risk of surgical site infection after posterior spine surgery: a multicenter analysis. Spine 20184365–71. ( 10.1097/BRS.0000000000001371) [DOI] [PubMed] [Google Scholar]
- 18.Dewan MC, Godil SS, Zuckerman SL, Mendenhall SK, Shau DN, Parker SL, Devin CJ, McGirt MJ. Comparative effectiveness and cost-benefit analysis of topical vancomycin powder in posterior spinal fusion for spine trauma and degenerative spine disease. Spine Journal 201313 S56. ( 10.1016/j.spinee.2013.07.161) [DOI] [PubMed] [Google Scholar]
- 19.Emohare O, Ledonio CG, Hill BW, Davis RA, Polly Jr DW, Kang MM. Cost savings analysis of intrawound vancomycin powder in posterior spinal surgery. Spine Journal 2014142710–2715. ( 10.1016/j.spinee.2014.03.011) [DOI] [PubMed] [Google Scholar]
- 20.Garg S, Bloch N, Potter M, Quick H, Palmer C, Michael N, O’Donnell C, Erickson M. Topical vancomycin in pediatric spine surgery does not reduce surgical site infection: a retrospective cohort study. Spine Deformity 20186523–528. ( 10.1016/j.jspd.2018.01.010) [DOI] [PubMed] [Google Scholar]
- 21.Lee GI, Bak KH, Chun HJ, Choi KS. Effect of using local intrawound vancomycin powder in addition to intravenous antibiotics in posterior lumbar surgery: midterm result in a single-center study. Korean Journal of Spine 20161347–52. ( 10.14245/kjs.2016.13.2.47) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haimoto S, Schär RT, Nishimura Y, Hara M, Wakabayashi T, Ginsberg HJ. Reduction in surgical site infection with suprafascial intrawound application of vancomycin powder in instrumented posterior spinal fusion: a retrospective case-control study. Journal of Neurosurgery: Spine 201829193–198. ( 10.3171/2017.12.SPINE17997) [DOI] [PubMed] [Google Scholar]
- 23.Heller A, McIff TE, Lai SM, Burton DC. Intrawound vancomycin powder decreases staphylococcal surgical site infections after posterior instrumented spinal arthrodesis. Journal of Spinal Disorders and Techniques 201528E584–E589. ( 10.1097/BSD.0000000000000045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill BW, Emohare O, Song B, Davis R, Kang MM. The use of vancomycin powder reduces surgical reoperation in posterior instrumented and noninstrumented spinal surgery. Acta Neurochirurgica 2014156749–754. ( 10.1007/s00701-014-2022-z) [DOI] [PubMed] [Google Scholar]
- 25.Li X, Qin Y, Wang X. The effect of local application of vancomycin powder after posterior lumbar interbody fusion surgery on prevention of surgical site infection and fusion rate. Chinese Journal of Spine and Spinal Cord 2016261109–1114. ( 10.3969/j.issn.1004-406X.2016.12.10) [DOI] [Google Scholar]
- 26.Liu N, Wood KB, Schwab JH, Cha TD, Puhkan RD, Osler PM, Grottkau BE. Comparison of intrawound vancomycin utility in posterior instrumented spine surgeries between patients with tumor and nontumor patients. Spine 2015401586–1592. ( 10.1097/BRS.0000000000001133) [DOI] [PubMed] [Google Scholar]
- 27.Maajid S, Ali NSenin, Mir AA, Motten T, Dar RA, Dar RA, Bhat MA. Effect of prophylactic intra-operative instillation of vancomycin powder on surgical site infections following spinal surgery: a retrospective case control study. JK Practitioner 20182319–24. [Google Scholar]
- 28.Martin JR, Adogwa O, Brown CR, Bagiey CA, Richardson WJ, Lad SP, Kuchibhatla M, Gottfried ON. Experience with intrawound vancomycin powder for spinal deformity surgery. Spine 201439177–184. ( 10.1097/BRS.0000000000000071) [DOI] [PubMed] [Google Scholar]
- 29.Martin JR, Adogwa O, Brown CR, Kuchibhatla M, Bagley CA, Lad SP, Gottfried ON. Experience with intrawound vancomycin powder for posterior cervical fusion surgery. Journal of Neurosurgery: Spine 20152226–33. ( 10.3171/2014.9.SPINE13826) [DOI] [PubMed] [Google Scholar]
- 30.Oktay K, Özsoy KM, Çetinalp NE, Erman T, Güzel A. Efficacy of prophylactic application of vancomycin powder in preventing surgical site infections after instrumented spinal surgery: a retrospective analysis of patients with high-risk conditions. Acta Orthopaedica et Traumatologica Turcica 20215548–52. ( 10.5152/j.aott.2021.18372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pahys JM, Pahys JR, Cho SK, Kang MM, Zebala LP, Hawasli AH, Sweet FA, Lee DH, Riew KD. Methods to decrease postoperative infections following posterior cervical spine surgery. Journal of Bone and Joint Surgery: American Volume 201395549–554. ( 10.2106/JBJS.K.00756) [DOI] [PubMed] [Google Scholar]
- 32.Strom RG, Pacione D, Kalhorn SP, Frempong-Boadu AK. Lumbar laminectomy and fusion with routine local application of vancomycin powder: decreased infection rate in instrumented and non-instrumented cases. Clinical Neurology and Neurosurgery 20131151766–1769. ( 10.1016/j.clineuro.2013.04.005) [DOI] [PubMed] [Google Scholar]
- 33.Strom RG, Pacione D, Kalhorn SP, Frempong-Boadu AK. Decreased risk of wound infection after posterior cervical fusion with routine local application of vancomycin powder. Spine 201338991–994. ( 10.1097/BRS.0b013e318285b219) [DOI] [PubMed] [Google Scholar]
- 34.Sweet FA, Roh M, Sliva C. Intrawound application of vancomycin for prophylaxis in instrumented thoracolumbar fusions: efficacy, drug levels, and patient outcomes. Spine 2011362084–2088. ( 10.1097/BRS.0b013e3181ff2cb1) [DOI] [PubMed] [Google Scholar]
- 35.Takeuchi H, Oda I, Oshima S, Suzuki M, Fujiya M. Is the administration of vancomycin to operative field effective? Studying from operative wound drainage tube culture. European Journal of Orthopaedic Surgery and Traumatology 202030215–219. ( 10.1007/s00590-019-02579-0) [DOI] [PubMed] [Google Scholar]
- 36.Klevens RM, Edwards JR, Richards Jr CL, Horan TC, Gaynes RP, Pollock DA, Cardo DM. Estimating health care-associated infections and deaths in U.S. Hospitals, 2002. Public Health Reports 2007122160–166. ( 10.1177/003335490712200205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marcus B.Treatment of large, complex, non-healing wounds with cryopreserved amniotic suspension allograft: a case series. Journal of Wound Care 201625 (Supplement 10) S18–S24. ( 10.12968/jowc.2016.25.Sup10.S18) [DOI] [PubMed] [Google Scholar]
- 38.Lieber B, Han B, Strom RG, Mullin J, Frempong-Boadu AK, Agarwal N, Kazemi N, Tabbosha M. Preoperative predictors of spinal infection within the national surgical quality inpatient database. World Neurosurgery 201689517–524. ( 10.1016/j.wneu.2015.12.085) [DOI] [PubMed] [Google Scholar]
- 39.Calderone RR, Garland DE, Capen DA, Oster H. Cost of medical care for postoperative spinal infections. Orthopedic Clinics of North America 199627171–182. ( 10.1016/S0030-5898(2032060-5) [DOI] [PubMed] [Google Scholar]
- 40.Olsen MA, Nepple JJ, Riew KD, Lenke LG, Bridwell KH, Mayfield J, Fraser VJ. Risk factors for surgical site infection following orthopaedic spinal operations. Infection Control and Hospital Epidemiology 200829477–484; discussion 485. ( 10.2106/JBJS.F.01515) [DOI] [PubMed] [Google Scholar]
- 41.Petilon JM, Glassman SD, Dimar JR, Carreon LY. Clinical outcomes after lumbar fusion complicated by deep wound infection: a case-control study. Spine 2012371370–1374. ( 10.1097/BRS.0b013e31824a4d93) [DOI] [PubMed] [Google Scholar]
- 42.van Middendorp JJ, Pull ter Gunne AF, Schuetz M, Habil D, Cohen DB, Hosman AJ, van Laarhoven CJ. A methodological systematic review on surgical site infections following spinal surgery: part 2: prophylactic treatments. Spine 2012372034–2045. ( 10.1097/BRS.0b013e31825f6652) [DOI] [PubMed] [Google Scholar]
- 43.Anderson DJ, Podgorny K, Berríos-Torres S, Bratzler DW, Dellinger EP, Greene L, Nyquist AC, Saiman L, Yokoe DS, Maragakis LLet al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infection Control and Hospital Epidemiology 201435 (Supplement 2) S66–S88. ( 10.1086/676022) [DOI] [PubMed] [Google Scholar]
- 44.Shaffer WO, Baisden JL, Fernand R, Matz PG. & North American Spine Society. An evidence-based clinical guideline for antibiotic prophylaxis in spine surgery. Spine Journal 2013131387–1392. ( 10.1016/j.spinee.2013.06.030) [DOI] [PubMed] [Google Scholar]
- 45.Hidron AI, Edwards JR, Jean P, Horan TC, Sievert DM, Pollock DA, Fridkin SKNational Healthcare Safety Network Team & Participating National Healthcare Safety Network Facilities. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infection Control and Hospital Epidemiology 201329996–1011. [DOI] [PubMed] [Google Scholar]
- 46.Zebala LP, Chuntarapas T, Kelly MP, Talcott M, Greco S, Riew KD. Intrawound vancomycin powder eradicates surgical wound contamination: an in vivo rabbit study. Journal of Bone and Joint Surgery: American Volume 20149646–51. ( 10.2106/JBJS.L.01257) [DOI] [PubMed] [Google Scholar]
- 47.Armaghani SJ, Menge TJ, Lovejoy SA, Mencio GA, Martus JE. Safety of topical vancomycin for pediatric spinal deformity: nontoxic serum levels with supratherapeutic drain levels. Spine 2014391683–1687. ( 10.1097/BRS.0000000000000465) [DOI] [PubMed] [Google Scholar]
- 48.Heller A, Mciff TE, Lai SM, Burton DC. Intrawound vancomycin powder decreases staphylococcal surgical site infections following posterior instrumented spinal arthrodesis. Journal of Spinal Disorders and Techniques 201528E584–E589. ( 10.1097/BSD.0000000000000045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Neill KR, Smith JG, Abtahi AM, Archer KR, Spengler DM, McGirt MJ, Devin CJ. Reduced surgical site infections in patients undergoing posterior spinal stabilization of traumatic injuries using vancomycin powder. Spine Journal 201111641–646. ( 10.1016/j.spinee.2011.04.025) [DOI] [PubMed] [Google Scholar]
- 50.Tubaki VR, Rajasekaran S, Shetty AP. Effects of using intravenous antibiotic only versus local intrawound vancomycin antibiotic powder application in addition to intravenous antibiotics on postoperative infection in spine surgery in 907 patients. Spine 2013382149–2155. ( 10.1097/BRS.0000000000000015) [DOI] [PubMed] [Google Scholar]
- 51.Eder C, Schenk S, Trifinopoulos J, Külekci B, Kienzl M, Schildböck S, Ogon M. Does intrawound application of vancomycin influence bone healing in spinal surgery? European Spine Journal 2016251021–1028. ( 10.1007/s00586-015-3943-9) [DOI] [PubMed] [Google Scholar]
- 52.Goldschmidt E, Rasmussen J, Chabot JD, Gandhoke G, Luzzi E, Merlotti L, Proni R, Loresi M, Hamilton DK, Okonkwo DOet al. The effect of vancomycin powder on human dural fibroblast culture and its implications for dural repair during spine surgery. Journal of Neurosurgery: Spine 201625665–670. ( 10.3171/2016.3.SPINE151491) [DOI] [PubMed] [Google Scholar]
- 53.Ghobrial GM, Thakkar V, Andrews E, Lang M, Chitale A, Oppenlander ME, Maulucci CM, Sharan AD, Heller J, Harrop JSet al. Intraoperative vancomycin use in spinal surgery: single institution experience and microbial trends. Spine 201439550–555. ( 10.1097/BRS.0000000000000241) [DOI] [PubMed] [Google Scholar]
- 54.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. American Journal of Infection Control 19992797–132; quiz 3–4; discussion 96. ( 10.1016/S0196-6553(9970088-X) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a