Abstract
Use of articular antibiotic-eluting cement spacers during two-stage revision arthroplasty for prosthetic joint infection (PJI) is a long-established and proven adjunctive technique during first-stage surgery. Articular spacers come in many forms, either static or dynamic. The authors present an instructional review of current evidence regarding their use.
A total of 45 studies (for spacer use in PJI involving either hip or knee) were analysed for data regarding eradication rate, functional outcomes, mechanical complications and the impact on second-stage surgery. A large number of case series and retrospective cohort studies were retrieved, with only a small number of prospective studies (2).
High levels of infection eradication were commonly reported (>80%). Outcome scores were commonly reported as indicating good-to-excellent function and pain levels. Second-stage procedures were often not required when dynamic spacers were used. Static spacers were associated with more mechanical complications in both the hip and the knee. In the hip, dynamic spacers were more commonly associated with instability compared to static spacers. Consideration should be given to the use of dual-mobility or constrained definitive acetabular components in these cases at second-stage surgery.
The use of antibiotic-eluting polymethylmethacrylate articular spacers in two-stage revision for PJI of hip and knee arthroplasty achieves a high rate of infection eradication. Dynamic spacers may confer a variety of benefits compared to static spacers, with a similar rate of infection eradication.
Keywords: prosthetic joint infection, revision arthroplasty, intra-articular spacer
Introduction
Arthroplasty
Total joint arthroplasty is considered as the definitive treatment for pain and disability ensuing primarily from end-stage osteoarthritis of the hip and the knee (1). Both total hip arthroplasty (THA) and total knee arthroplasty (TKA) are long-demonstrated to confer significant improvements in patient metrics for pain and function (2, 3, 4, 5).
Primary hip and knee arthroplasty have low reported rates of all-cause revision, with recent UK National Joint Registry (NJR) data indicating 10-year revision rates of approximately 2% for a number of commonly used prostheses (6). Nonetheless, prosthetic joint infection (PJI) remains a devastating complication. Of all single- and first-stage revisions (of primary arthroplasty) registered in the NJR since 2003, infection is given as the indication for 9.8 and 16.1% of cases for THA and TKA, respectively (6). PJI can thus be considered as a major cause for revision surgery and has long been reported as such (7, 8, 9). Infection is currently the fourth most common reason for revision following THA and the second most common cause for revision following TKA in the UK (6). Its adverse effects on function, pain and mental wellbeing, as well as its economic impact, are well documented (10, 11, 12). In addition to these factors, patient morbidity and mortality are also grossly affected (13, 14).
Two-stage revision arthroplasty is still considered as the gold standard for surgical treatment of PJI, yet the impact of revision surgery is profound. The inter-stage period of two-stage revision, in particular, has a significant negative impact on mobility and usually confers subsequent dependence on the patient. Other reported significant adverse consequences include burden of systemic antibiotic therapy, change in inter-family dynamics and profound psychological impacts (15).
The use of antibiotic-impregnated/antibiotic-eluting bone cement
The use of antimicrobials in polymethylmethacrylate (PMMA) bone cement intra-operatively since the original use by Buchholz and Engelbrecht (1970) (16) has been well documented and repeatedly validated. Antibiotic-impregnated PMMA, as a means for local delivery of water-soluble agents, has long been used in both primary and revision surgery to reduce PJI rates. Antibiotic-impregnated PMMA enables high local concentrations of antimicrobial agents above minimum bactericidal concentration (MBC) or minimum inhibitory concentration (MIC), while mitigating risks of toxicity from sustained high systemic doses (17). The mechanics of antibiotic elution from cement involve diffusion of water across the polymer. As the antibiotics within the PMMA are water-soluble (typically gentamicin, vancomycin and tobramycin), water diffusing through the polymerised cement matrix carries the antibiotics with it (18). In vivo studies have confirmed high local doses above the MBC/MIC post-operatively but stress the importance of additional concurrent systemic-targeted therapy (19, 20, 21). In vitro studies have shown the greatest concentration of antibiotic elution from PMMA spacers to occur within the first 10–12 days; however, elution can be detected above the MIC level in excess of 80 days when high doses are used. Tobramycin is observed to have better elution characteristics than vancomycin (22, 23). Insall et al. described the classic two-stage revision (knee) arthroplasty procedure in 1983 and set out the universally accepted 6-week post-operative course of targeted antibiotics following revision hip or knee arthroplasty (24). The optimal duration of post-operative antibiotic therapy remains a topic of ongoing debate. Antibiotic therapy during the inter-stage period may continue until the second-stage procedure is completed, with periods of 2–8 weeks of therapy commonly reported. Longer courses of antibiotic therapy (6–8 weeks) are recommended for ‘difficult-to-treat’ (DTT) pathogens and/or in the presence of significant bone or soft tissue compromise; prolonged periods of treatment for over 8 weeks are not recommended, particularly in the presence of articular spacers, as the threshold MIC of elution may be breached, leading to microfilm formation on the spacer itself (25). Other studies have reported similar eradication rates following periods of 6 weeks post-operative antibiotic therapy or less, compared to longer periods following DAIR (Debridement, Antibiotics and Implant Retention) and single-stage revision arthroplasty (26, 27, 28).
The switching from parenteral to oral antibiotic therapy also remains a topic of ongoing discussion. A recent multicentre pragmatic trial has demonstrated non-inferiority of oral antibiotic regimes for complex orthopaedic infection (including PJI), when compared to intravenous therapy (29); however, oral agents with good bone penetration are typically used following an initial period of post-operative parenteral therapy if factors permit, such as known pathogen and sensitivity, healthy overlying soft tissues and falling inflammatory markers (30). The rising incidence of drug-resistant and DTT organisms would suggest caution in early switching to oral therapy, and other modalities such as self-administered out-patient parenteral antimicrobial therapy have been proposed (31).
The use of articular spacers
Articular spacers are an integral component in the surgical management of PJI. The roles of a spacer include reduction of dead space, reduction of intra-/peri-articular haematoma, preservation of soft tissue balance/tension and (PMMA-mediated) local delivery of antibiotics. These biological roles play an important role in the successful eradication of established infection. A spacer may also confer mechanical stability, thus the ability to weightbear through the ipsilateral limb, and in some cases prosthetic articulation. These mechanical roles play an important role in functional outcome and further promote resolution of infection (32, 33, 34, 35).
Methods
The authors searched the MEDLINE database using PubMed from inception to February 2021, restricted to papers published in English. The authors searched for those related to hip and knee arthroplasty by searching the title and abstract fields for the words or phrases ‘hip’, ‘knee’, ‘TKA’, ‘TKR’, ‘THA’, ‘THR’, ‘TJA, ‘TJR’ or ‘arthroplasty*’. This search was restricted to infected and revised cases by combining these with the following terms: ‘infect*’, ‘revision’, ‘revised’, ‘revising’, ‘revise’, ‘pji’, ‘single-stage’, ‘two-stage’, ‘septic’, ‘sepsis’. It was further restricted to the use of articular spacers by combining with a search for the following terms in the title and abstract fields: ‘spacer*’, ‘kiwi’, ‘knee-wi’, ‘CUMAR’, ‘Steinman*’, ‘Hofmann*’, ‘PROSTALAC’, ‘StageOne’, ‘Tecres’, ‘COPAL’ or ‘ANTILOCH’. Any articles which did not deal specifically with the use of articular spacers in the treatment of PJI of the hip and the knee were excluded on full-text search. The references sections of the articles included were interrogated for further relevant publication. Forty-three papers were identified with further 2 articles that were suggested for inclusion on peer-review (36, 37) resulting in 45 studies in total. These studies (and their summarised findings) are listed in Tables 1 and 2. Further studies that informed the discussion around surgical management of PJI are listed in the references.
Table 1.
Use of intra-articular spacers in two-stage revision arthroplasty for infected hip arthroplasty.
| Study | Study type | Cases (n) | Mean follow-up (months) | Case group A | Control group B | Pain/functional outcomes | Impact on second- stage surgery | Mechanical complications (inter-stage) | Eradication rate |
|---|---|---|---|---|---|---|---|---|---|
| Burastero et al. (54) | RC | 71 | 33.2 | Dynamic, preformed hemiarthroplasty & PMMA acetabular component (n = 31) | Dynamic, preformed hemiarthroplasty (n = 40) | – | Group A: significantly quicker second-stage surgery | Group A (6.45%): one dislocation, one spacer fracture. Group B (17.5%): four dislocations, two periprosthetic fractures | NSD |
| D’Angelo et al. (36) | RC | 19 | 40 | Dynamic, handmade hemiarthroplasty (n = 7) | Dynamic, preformed hemiarthroplasty (n = 12) | HHS: Good. Improved post-operatively for both groups | Group B: better preservation of bone stock | Group A (43%): one dislocation, one spacer fracture, one subsidence. Group B (8.3%): one dislocation | 90% NSD |
| D’angelo et al. (37) | CS | 28 | 53 | Dynamic, preformed hemiarthroplasty | HHS: improves significantly post-operatively | – | Three (10.7%) dislocations | 96% | |
| Degen et al. (70) | CS | 33 | 43 | Dynamic, moulded hemiarthroplasty | – | – | – | One (3%) spacer fracture | 85% |
| Faschingbauer et al. (84) | RC | 138 | 2.8 | Dynamic, moulded hemiarthroplasty (n = 93) | Dynamic, handmade hemiarthroplasty (n = 45) | – | – | 27 (19.6%); 12 dislocations (8.7%), 12 spacer fractures (8.7%), 1 periprosthetic fracture (0.7%). No difference between groups | NR |
| Gomez et al. (85) | CS | 178 | 49.1 | Static (n = 139) | Dynamic, moulded hemiarthroplasty (n = 39) | – | – | – | 81.7% |
| Hofmann et al. (60) | CS | 27 | 76 | Dynamic, Hofmann femoral component & PE acetabular component | – | – | ‘Soft tissues… (facilitate) reconstruction’ | Five (18.5%); four dislocations, one periprosthetic fracture | 96% |
| Hsieh et al. (61) | CS | 42 | 55.2 | Dynamic, moulded hemiarthroplasty & PMMA acetabular component | – | Md’A: Good. HHS: Good. WOMAC: Good | ‘Less complicated… the tension and… planes of soft tissues (are) preserved’ | Four (9.5%); two dislocations, two spacer fractures | 95.2% |
| Masri 2007 | CS | 29 | 47 | Dynamic, PROSTALAC | – | HHS: Good | – | – | 89.7% |
| Pattyn et al. (58) | CS | 61 | 36 | Dynamic, preformed hemiarthroplasty | – | HHS: Poor d’A: Poor | – | 11 (18%); 10 dislocations (16.4%), 1 periprosthetic fracture (1.6%) | 96.7% |
| Quayle et al. (88) | CS | 53 | 46.8 | Dynamic, CUMARS | – | – | – | Four dislocations | 88.7% |
| Romano (81) | CS | 183 | 60 | Dynamic, preformed | – | Improved. Overall HHS: Poor | – | 30 dislocations (16.4%) | 94.6% |
| Sabry et al. (87) | RC | 78 | 58.3 | Mixed: Dynamic, preformed hemiarthroplasty (n = 13), injection-mould hemiarthroplasty (n = 14) | Dynamic, CUMARS (n = 51) | No differences. Overall HSS: Poor | – | Seven (9%); six dislocations (7.7%), one spacer fracture (1.3%). No difference between groups | 93.6% |
| Scharfenberger et al. (59) | RC | 23 | n/a | Dynamic, PROSTALAC (n = 23) | (Uninfected) primary THR | HHS: Poor-Fair. WOMAC: Fair (better than pre-THR, worse than control group) | – | Three (12.9%); one dislocation (4.3%), two periprosthetic fractures (8.6%) | NR |
| Takahira 2003 | CS | 9 | 35.7 | Dynamic, handmade | – | JOA: Good | – | – | 88.9% |
| Tikhilov et al. (86) | CS | 217 | 24 | |
Mixed: Static and Dynamic, CUMARS | – | – | – | – | 64.1% NSD |
| Tsung et al. (51) | CS | 76 | 80.4 | Dynamic, CUMARS | – | OHS, HHS & Md’A: ‘pain-free’. Good-excellent function, significant improvement in all scores post-op | – | 14 (18.4%); 8 dislocations (10.5%), 6 periprosthetic fractures (7.9%) | 84.2% |
| Yamamoto et al. (82) | CS | 17 | 38 | Mixed: Dynamic, moulded and handmade hemiarthroplasty | – | Pain: good. ROM: better with moulded | – | Two (11.8%); one dislocation (5.9%), one spacer fracture (5.9%) | 100% |
| Younger et al. (57) | CS | 48 | 43 | Dynamic, PROSTALAC | – | HHS: Poor. Pain: significant improvement | – | Two periprosthetic fractures (4.2%) | 94% |
| Zhang et al. (55) | RC | 36 | 12+ | Dynamic, moulded hemiarthroplasty (n = 23) | Dynamic, CUMARS (n = 13) | HHS: significantly higher with CUMARS | No difference | Group A (33.3%): six dislocations, five spacer fractures, one periprosthetic fracture. Group B (5.6%): one dislocation, one periprosthetic fracture | 94.4% NSD |
CS, case series; HHS, Harris Hip Score; Md’A, Merle d’Aubigne Hip Score; JOA, Japanese Orthopaedic Association Hip Score; NR, none reported; NSD, no significant difference; OHS, Oxford Hip Score; RC, retrospective cohort; ROM, range of motion; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Table 2.
Use of intra-articular spacers in two-stage revision arthroplasty for infected knee arthroplasty.
| Study | Study type | Cases (n) | Mean follow-up (months) | Case group A | Control group B | Group C | Pain/functional outcomes | Impact on second-stage surgery | Mechanical complications (inter-stage) | Eradication rate |
|---|---|---|---|---|---|---|---|---|---|---|
| Chiang et al. (64) | PC | 45 | 40 | Dynamic, moulded (n = 23) | Static, handmade (n = 22) | – | HSS and ROM better with dynamic group | Group B: Increased patella baja and more likely to require extensile approach | Group A: one case of crepitus/pseudolocking (4.3%) | 88.9% NSD |
| Choi et al. (78) | RC | 47 | 58 | Dynamic, mixed, Hoffman-cement/PE articulations (n = 14) | Static, handmade (n = 33) | – | No differences | Grup B: more likely to require extensile approach, more bone loss | – | 68% NSD |
| Classen et al. (91) | CS | 23 | 47 | Dynamic, Hoffman-cement articulation | – | – | – | – | – | 87% |
| Cuckler (92) | CS | 44 | 64.8 | Dynamic, Hoffman-PE articulation | – | – | KSS/ROM: significant improvement inter-stage | No extensile exposures required | – | 97.7% |
| Deboer et al. (90) | RC | 77 | 48 | Dynamic, hybrid moulded PMMA on PE articulation (n = 20) | Dynamic, mixed, preformed/hybrid articulations (n = 57) | – | – | – | 82% NSD | |
| Emerson et al. (89) | RC | 48 | 69.6 | Static, handmade (n = 26) | Dynamic, Hofmann-PE (n = 22) | – | ROM greater for dynamic group | Removal of dynamic components required moderate effort, static spacers associated with more significant bone loss | One subluxed static block (2.1%) | 91.7% NSD |
| Faschingbauer et al. (84) | CS | 133 | n/a | Static, handmade | – | – | – | 14 (10.5%): 12 periprosthetic fractures (9.1%), 1 spacer fracture (0.8%), 1 subluxed patella (0.8%) | 88% | |
| Fehring et al. (75) | RC | 40 | 31 | Static, handmade (n = 25) | Dynamic, moulded (n = 30) | – | HSS & ROM: Good in both groups, no significant difference | Significant bone loss seen static spacers, sometimes requiring augmentation | Subluxation of static spacers seen | 90% NSD |
| Freeman et al. (93) | RC | 76 | 71.2 | Static, handmade (n = 28) | Dynamic, moulded (n = 48) | – | KSS: Group A: fair-good. Group B: good-excellent. No significant differences | – | – | 93.9% NSD |
| Goldman et al. (94) | CS | 64 | <48 | Static, handmade (n = 7) | No spacer (n = 57) | – | – | Easier exposure with spacer | – | NSD |
| Gomez et al. (85) | RC | 326 | 59.7 | Static, handmade (n = 226) | Dynamic, moulded (n = 100) | – | – | – | 10 dislocations (3.1%), 1 fracture (0.3%) | 81.4% |
| Gooding et al. (47) | CS | 115 | 108 | Dynamic, PROSTALAC | – | – | WOMAC, SF-12 (mental) & OKS: significant improvement | – | Two dislocations (1.7%), two spacer fractures (1.7%) | 88% |
| Haleem et al. (95) | CS | 96 | 86.4 | Static | – | – | KSS & ROM: significant improvement | – | – | 85% at 10 years |
| Hart & Jones (96) | CS | 48 | 48.5 | Dynamic, moulded | – | – | ROM: Good | – | Two spacer dislocations (4.2%) | 87.5% |
| Hirakawa et al. (97) | CS | 55 | 61.9 | Static | – | – | – | – | – | 74.5% |
| Hsu et al. (79) | RC | 28 | 68.8 | Static, handmade (n = 7) | Dynamic, moulded (n = 21) | – | KSS & ROM: Significantly better in dynamic group | Group B: Fewer extensile exposures and less bone loss | Group B: one subluxed spacer (3.6%) | 85.7% NSD |
| Jämsen et al. (98) | RC | 34 | 32 | Dynamic, Hoffman-PE articulation (n = 24). | Static, handmade (n = 10). | – | KSS ROM: better in dynamic group. | Group B: More blood loss & longer procedure. | Five subluxed spacers (14.7%). | 88% NSD |
| Johnson et al. (76) | RC | 115 | 27 | Mixed: Dynamic, moulded, preformed, PROSTALAC (n = 34) | Static, handmade (n = 81) | – | KSS & ROM: no significant difference | Group B: More bone loss | (3.5%); four dynamic spacer fractures (11.8%) | 82.6% NSD |
| Juul et al. (99) | CS | 22 | 37.6 | Dynamic, CUMARS | – | – | AKSS & ROM: Good | – | – | 82% |
| Kotwal et al. (100) | CS | 58 | 29.4 | Static, handmade | – | – | – | Bone loss improved by the use of an intramedullary rod | Mechanical complications reduced by the use of intramedullary rod | 83.8% |
| Nahhas 2020 (69) | RT | 49 | 42 | Static, handmade (n = 24) | Dynamic, moulded (n = 25) | –– | Length of stay, KSS & ROM: better in dynamic group | More extensile approaches needed for static group | One spacer fracture (static) | 93.9% |
| Nodzo et al. (101) | RC | 140 | 59 | Dynamic, preformed (n = 58) | Dynamic, moulded (n = 43) | Dynamic, Hofmann-PMMA (n = 39) | – | – | – | 83.6% NSD |
| Park et al. (77) | RC | 36 | 36 | Static, handmade (n = 20) | Dynamic, moulded (n = 16) | – | HSS, KSS & ROM: better in dynamic group. Extensor lag seen in static group | Group A: More bone loss | – | 88.9% NSD |
| Tian et al. (102) | CS | 25 | 64.2 | Dynamic, moulded | – | – | Pain, KSS & ROM: Good-excellent | 13 cases (52%) required extensile exposure | Five dislocations, one spacer fracture | 100% |
| Van Thiel et al. (103) | CS | 60 | 35 | Dynamic, moulded | – | – | KSS & ROM: Good | No bone loss seen | One spacer fracture | 88% |
AKSS, American Knee Society Score; CS, case series; HSS, Hospital of Special Surgery; KSS, Knee Society Score; NSD, no significant difference; OKS, Oxford Knee Score; PC, prospective cohort; RC, retrospective cohort; RT, randomised trial; ROM, range of motion; SF-12, 12-item Short Form Survey; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Aims
The authors present an instructional review of key concepts and current evidence on the use of intra-articular spacers in the staged surgical management of PJI.
Types of articular spacers
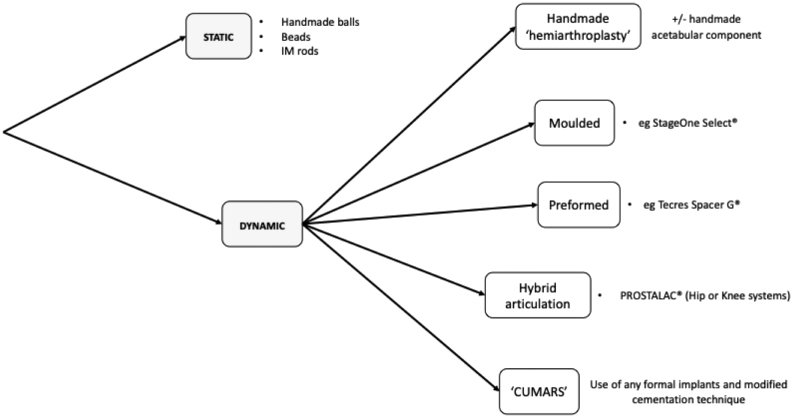
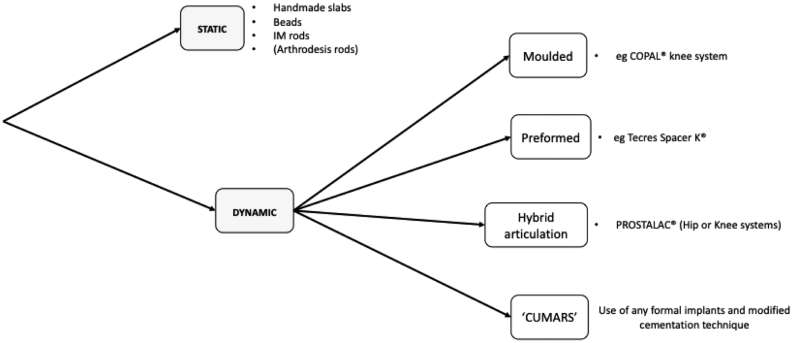
For the purpose of this review article, spacers can be broadly categorised into two mechanical types: static and dynamic. They can be further classified based on how they have been constructed. See Figs 1 and 2 .
Figure 1.
Types of articular spacers used in revision hip arthroplasty for PJI.
Figure 2.
Types of articular spacers used in revision knee arthroplasty for PJI.
Static spacers
Static spacers do not allow functional motion during the inter-stage period. There are no true ‘static’ spacers for the hip; ambulation through the limb and functional pseudarthrosis are typically the case even in the crudest of handmade interpositional PMMA spacers (32). Static spacers in the hip do not confer the same pseudarthrodesis as in the knee.
Dynamic/articulating spacers
As opposed to static spacers, dynamicor articulating spacers allow functional movement throughout the normal plane of motion within the remnant joint, following first-stage revision. They allow weightbearing and improved quality of life but are contraindicated in cases of severe bone loss due to challenges in achieving adequate fixation/stability. In these cases, static spacers are often preferable.
Handmade spacers
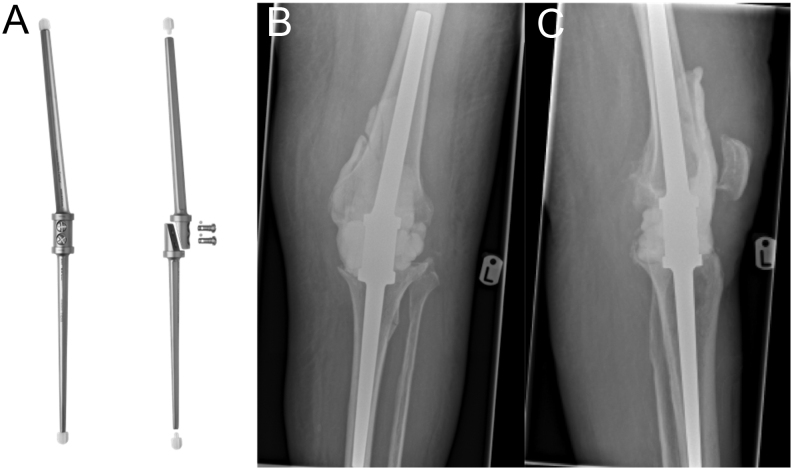
Spacers may be constructed in the surgical field using antibiotic-impregnated PMMA. Common forms of handmade static spacer may include beads, balls, flattened blocks and intramedullary dowels/rods of PMMA, fashioned to fit the local bone defects (27). Handmade spacers enable specific tailoring to accommodate individual defects/anatomy. Handmade dynamic spacers are time-consuming to make and prone to fracture. These are formed around a Steinman pin, K-wire or other such metal ‘endoskeleton’, in order to mitigate the risk of fracture and other mechanical complications (38, 39). In the knee, handmade spacers can also be constructed around arthrodesis intramedullary nails such as the LINK® (LINK Orthopaedics UK Ltd, Edinburgh, UK) arthrodesis nail (Fig. 3).
Figure 3.
Images of the LINK® Endo-model® arthrodesis nail – (from left-to-right) (A) LINK arthrodesis nail; (B and C) Plain film images of LINK® fusion nail with associated (static) cement spacer. Image (A) is reproduced with permission from LINK Orthopaedics UK Ltd. Images (B and C) courtesy of Mr J Palan (co-author).
Moulded spacers
Spacers may also be fashioned using commercially available moulds such as the injection-moulded StageOne® dynamic spacers for hip (Fig. 4) and knee (Zimmer BioMet, Warsaw, IN, USA) or the COPAL® knee moulds (Fig. 5) system (Heraeus Medical GmbH, Wehrheim, Germany). These moulded articulating spacers are quicker to make intra-operatively but confer increased financial cost and sizing options are limited for individual cases.
Figure 4.
StageOne® and StageOne Select® injection mould hip spacers – (from left-to-right) (A) Plain film image of StageOne® dynamic hip spacer (no endoskeleton); (B) StageOne Select® hip spacer injection moulds; (C) StageOne Select® dynamic hip spacer; (D) Plain film image of StageOne Select® dynamic hip spacer in situ. Images (A, B and C) is published with permission from Zimmer Biomet. Image (D) courtesy of Mr S Jain.
Figure 5.

Heraeus COPAL® injection-moulded dynamic knee spacer system. Images published with permission from Heraeus Medical GmbH.
Preformed spacers
Preformed PMMA spacers are also available in various sizes, for both hip and knee surgery, such as the ‘Tecres Spacer G®’ PMMA dynamic hip spacer and ‘Tecres Spacer K®’ dynamic knee spacer (Figs 6 and 7 ) (Tecres SpA, Sommacampagna (VR), Italy). Other available hip spacers exist under the broad description of ‘antibiotic-loaded cement hemiarthroplasty’ (ANTILOCH). Preformed spacers are ready to use directly following removal from the packaging. They are convenient and save further time intra-operatively, but concern has been raised to their relatively low-antibiotic dosage/elution, as well as the limitation on the type of antibiotic used (40, 41). They are also expensive, and sizing options are again limited.
Figure 6.
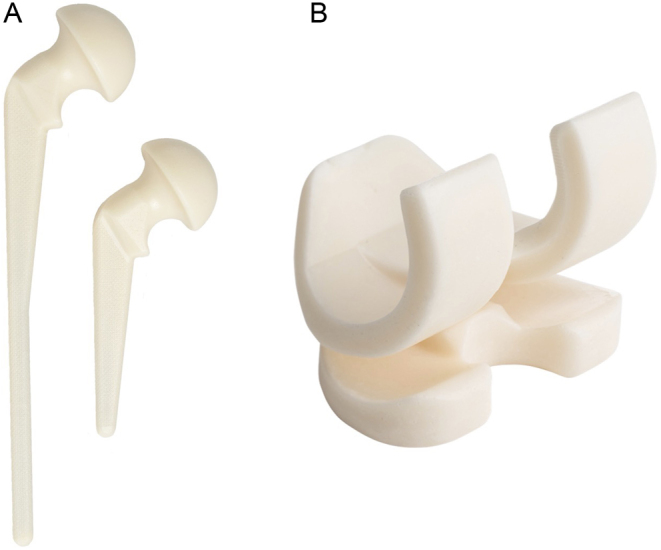
(From left-to-right) (A) Tecres Spacer G®; (B) Tecres Spacer K®. Imagespublished with permission from Summit Medical Ltd.
Figure 7.
(From left-to-right) plain film images of implants in situ – (A) Tecres® Spacer G; (B) Tecres® Spacer K. Images published with permission from Summit Medical Ltd.
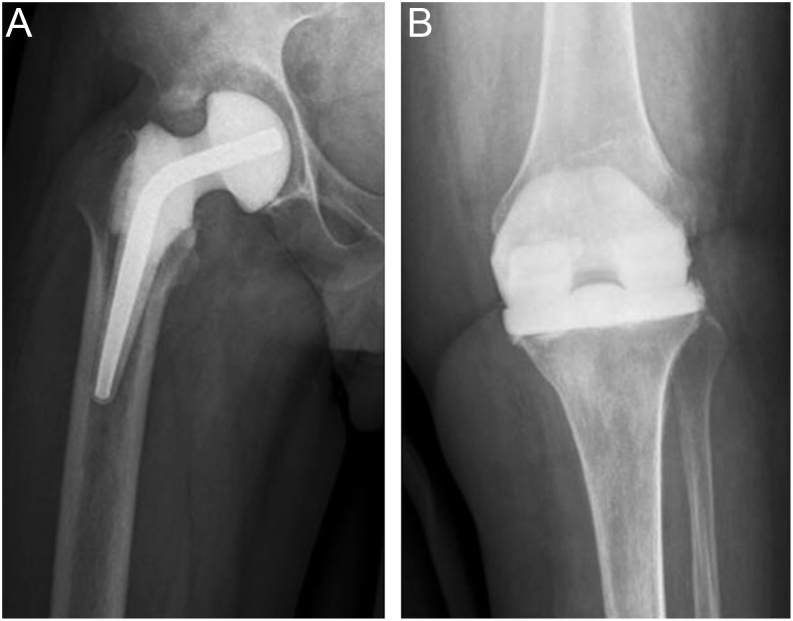
Hybrid PMMA and explant articulations
Hybrid PMMA/biomaterial articulations can be employed; historically, the explanted, autoclaved femoral component has been used on a new polyethylene tibial component (Hofmann technique) (42), although this practice now goes against the advice of both the U.S Food & Drug Administration and the UK’s Medicines and Healthcare products Regulatory Agency (43, 44). Alternatively, components specifically designed for short-term longevity and increased PMMA volume can be used, such as the PROSTALAC® (‘prosthesis of antibiotic-loaded acrylic cement’) hip (and legacy knee) system (Fig. 8) (DePuy Synthes, Warsaw, IN, USA). As such, cement-on-cement, so-called ‘prosthesis-on-polyethylene’ (Hofmann), prosthesis-on-PMMA and metal-on-polyethylene articulations have all been described, and outcomes of these systems have been widely reported in the literature (45, 46, 47, 48, 49). A report of cement-on-polyethylene dynamic spacer in knee surgery by Evans in 2004 described either handmade or moulded PMMA femoral component on a cemented polyethylene tibial component (50). Using processed explanted components is cheap, and good results have been reported, but the technique requires ready access to appropriate equipment and trained personnel in the theatre suite.
Figure 8.
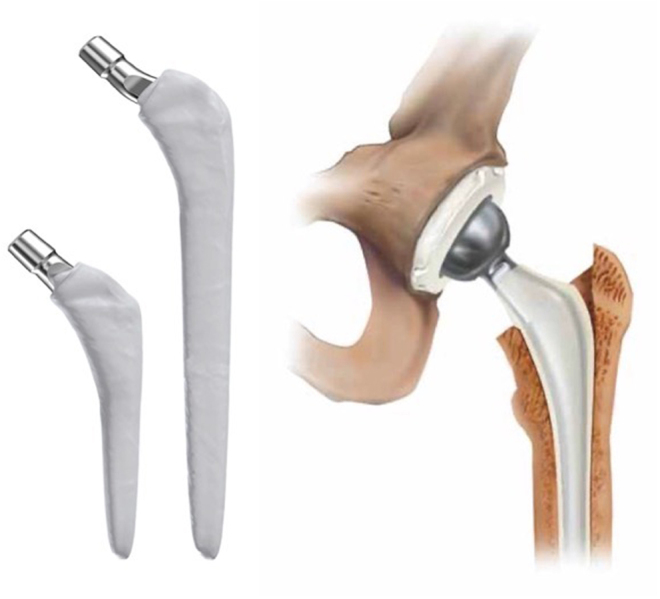
PROSTALAC® articulating hip spacer system. Images published with permission from DePuy Synthes.
Custom-made articulating spacers
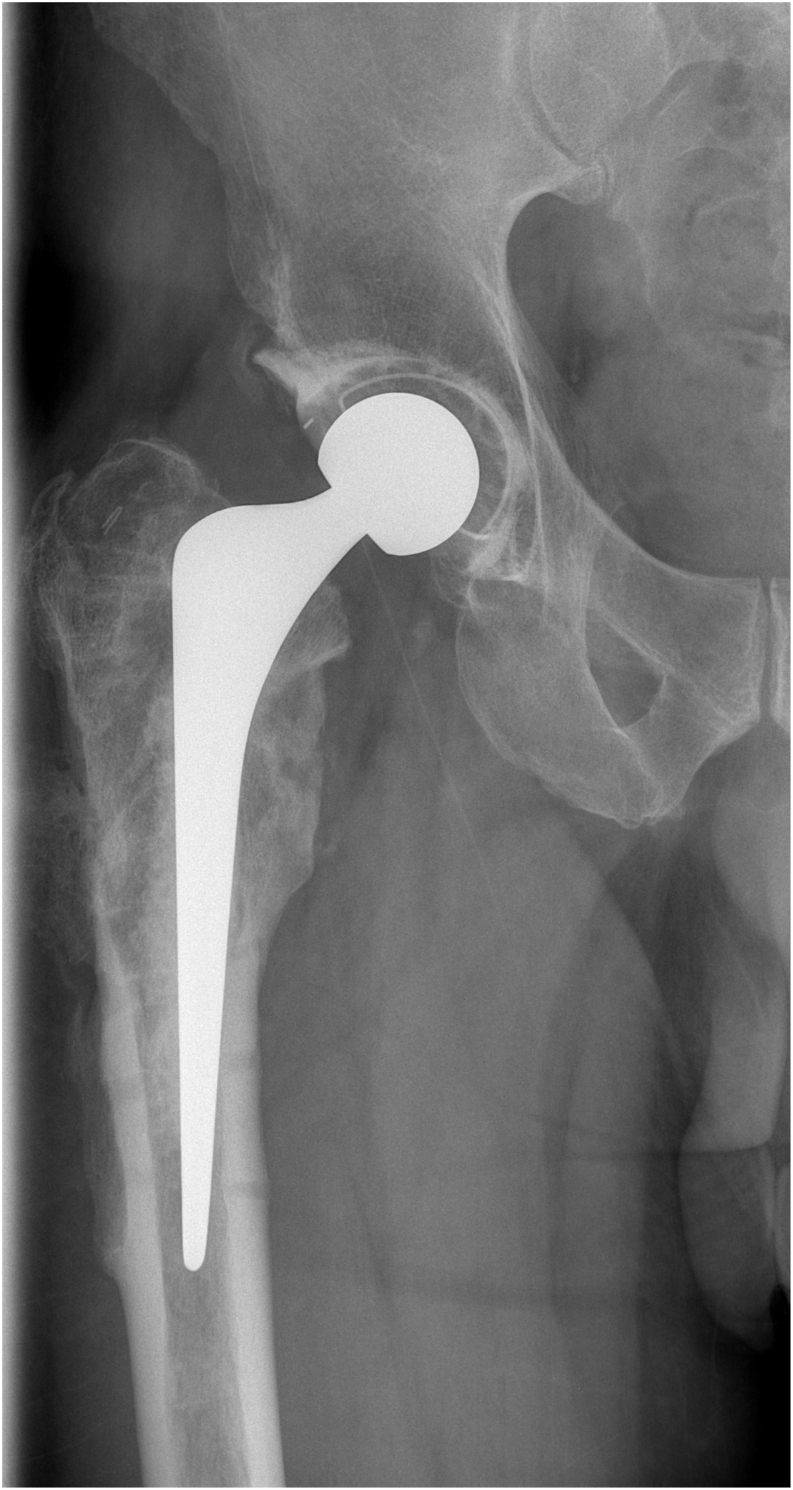
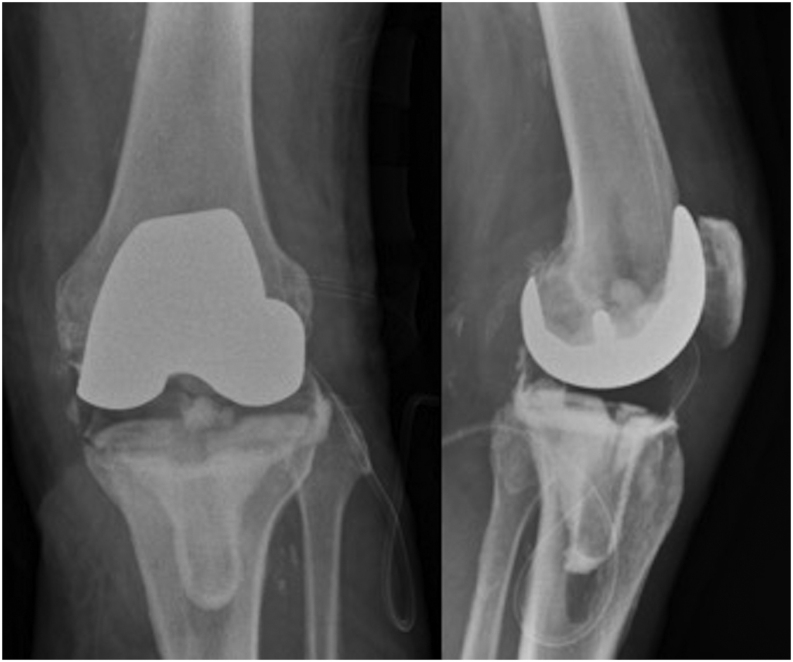
Most recently, use of formal components with modified cementation techniques to facilitate easier removal at second stage has led to the use of ‘custom-made articulating spacers’ (CUMARS) for both hip and knee replacements (Figs 9 and 10) (51, 52). They allow full weightbearing (cement-on-cement bearing surfaces allow only partial weightbearing), normal joint motion and in specific circumstances (e.g. low-demand patients) are able to avoid the need entirely for a second-stage procedure, the so-called 1.5-stage exchange arthroplasty (51, 53). The use of CUMARS has gained popularity amid reports of excellent inter-stage functional performance, simplified second-stage revision surgery and improved biomechanical outcomes (54, 55). Use of CUMARS can lead to excellent outcomes and functional results; however, they can be costly due to the use of ‘formal’/definitive implants as temporary spacers.
Figure 9.
Plain film image of right hip ‘custom-made articulating spacer’ (CUMARS). Image courtesy of Mr J Palan (co-author).
Figure 10.
Plain film images of left knee ‘custom-made articulating spacer’ (CUMARS). Images courtesy of Mr S Jain (co-author).
Comparison of outcomes
Pain and functional outcomes
Hip
Little mention is made in the literature of relative pain levels for different types of hip spacer. Jacobs et al.observed reduced inter-stage hospitalisation with mobile compared to static spacers, although this cannot directly be assumed to be purely due to pain levels (56). Younger et al. associated greater inter-stage patient comfort with PROSTALAC® vs the traditional Girdlestone’s excision arthroplasty with static spacer (57). Pattyn et al. commented that inter-stage comfort was improved for dynamic vs static spacers (58).
Many studies suggested improved functional results from the use of dynamic spacers over static spacers (56, 57, 59, 60). However, these were not reflected in functional outcome scoring. Some reports indicate no discernible difference in measured functional outcomes; however, a literature review by Veltman et al. offered guarded comment owing to heterogeneity of any functional outcome measures used across the literature, with the Harris Hip Score being the most commonly used (33, 36, 37, 40, 57, 61, 62). D’Angelo assessed functional scoring between handmade vs preformed dynamic hip spacers. Although both were significantly improved after insertion of spacers, no difference was seen between groups (1). Inter-stage functional performance following CUMARS has been suggested to be better than other forms of dynamic spacer (55). The use of CUMARS in the hip has even been suggested to be effective enough to preclude the need to progress onto second-stage surgery if the infection is confirmed eradicated and function is deemed adequate (51, 55).
A 2018 international consensus paper reported reduced length-of-stay following first-stage procedure using dynamic spacers; Pattyn et al. also suggested that ease of discharge from hospital was facilitated by use of dynamic spacers (58, 63). No data was offered in support of these statements, however.
Knee
Improved functional outcome scores are reported for use of dynamic spacers (vs static) in revision surgery of the knee (63). In addition to improved outcome scores, some comparative studies also find improved satisfaction and range of motion following dynamic/articulating knee spacers (64). Three systematic reviews found no difference in improvement of Patient-Reported Outcome Measures (PROMs) for both groups but reported an improved post-operative range of motion for the dynamic spacer group (65, 66, 67). A more recent review found no difference in pain but did find an improved Knee Society function score and range of motion with dynamic spacers (68). Selection bias is a criticism of these studies comparing static and dynamic spacers for infected TKA. Static spacers may be performed for more complex cases with greater compromise to bone stock and soft tissue. A recent randomised control trial of 68 patients undergoing two-stage exchange arthroplasty reported comparison between static (handmade) or dynamic StageOne® injection-moulded knee (Zimmer Biomet) spacers (69). Patients in the dynamic group had a significantly better range of motion and higher Knee Society Score (KSS) at follow-up.
Impact on revision surgery
Hip
Many authors offered subjective comment on the perceived benefits of dynamic spacer use with relation to the technical difficulty of second-stage procedures. A consensus paper from 2018 offered the opinion that inter-plane dissection was easier with dynamic spacer use (63). Many authors reported that soft tissue condition, extent of scar tissue formation and ease of dissection was improved at the second stage following the use of a dynamic vs static spacer (33, 56, 57, 58, 60, 61, 70, 71, 72, 73). Despite being based solely on retrospective observation, the body of agreement on this issue is notable despite the heterogeneity of studies included. Increased acetabular bone loss at the second stage following the use of dynamic spacers (articulating with host acetabular bone) has been reported (54), and some authors advise against the use of such spacers in a deficient acetabulum (33). However, García-Oltra et al. assert that bone loss in the acetabulum is largely due to removal of the cup in first-stage surgery; ongoing radiographic development of medial erosion, protrusio acetabuli or superolateral migration due to prolonged use of a preformed dynamic spacer only occurred in those retaining the implant for over 1 year, with full weightbearing (74). Scharfenberger et al. report minimal acetabular bone loss at second-stage procedure following dynamic spacer use (PROSTALAC®), compared to another report of handmade dynamic spacer use ‘similar to a hemiarthroplasty’ in their outcome series (59).
Preservation of residual bone stock and avoidance of disuse osteopenia following the first stage was associated with the use of dynamic spacers by a number of authors (33, 56, 60, 61). This was thought to be due to increased physiological loading of the host bone by partial weightbearing enabled by dynamic spacer use. Lastly, leg length deficiency following second-stage surgery was noted to be improved by the use of dynamic spacers by a number of authors (54, 57, 60, 61).
Knee
Fehring et al. compared static block-type cement spacers in the knee with custom-moulded dynamic cement spacers and found the development of bone loss in 60% of the static spacer group (75). More recent comparative works concur, finding bone loss in 75–100% of patients with static spacers (76, 77). A further systematic review found that patients with dynamic spacers developed significantly less bone loss (67). Although the authors found no difference in the presence of pre-existing bone loss, they did note that static spacer patients tended to have more severe pre-existing femoral bone loss. Dynamic spacers also less frequently require extensile surgical approaches upon second-stage surgery (67, 69, 78, 79).
Instability and other complications
Hip
Recognised complications of dynamic spacers for the hip include dislocation, spacer fracture and periprosthetic fracture. Complication rates are higher in dynamic spacers that articulate against host bone and higher again in ‘bespoke’ or handmade dynamic spacers (36, 63).
Rava et al. suggest an incidence of 8.46% for dislocation in their review of available literature, although this was a combined group of 567 patients across a number of studies with considerable heterogeneity, not least of which was the variety of spacers being used (80). Romano et al. reported a dislocation rate of 16.4% for a series of preformed dynamic hip spacers (81). D’Angelo also reported a dislocation rate of 10.7% in a series of 28 preformed, dynamic hip spacers (Spacer G) (37). The limited head sizing and offset options seen in preformed dynamic spacers may contribute to this relative high dislocation rate (62). Instability is thought to be related to poor spacer design, typically seen more in handmade dynamic spacers, such as short femoral stem and increased head:neck ratio (56). Dislocation rate is thought to be improved in the use of dynamic spacers that articulate against a prosthetic surface, such as the PROSTALAC® system or other CUMARS (54, 71, 72, 73). Relative contraindications to dynamic spacer use have been suggested, including inadequate/defunctioned hip abductors, on-table hip contracture, segmental bone loss affecting the acetabulum or excessive acetabular wear and general poor condition of investing soft tissues and proximal femoral bone stock (56, 63, 72, 73).
Spacer fracture is associated with subsequent instability and local soft tissue damage. Earlier literature reports ongoing incidence associated particularly with handmade/injection-moulded dynamic spacers (3.0–5.9%) (40, 54, 70, 82). Other authors described the incidence of spacer fracture in early cement-on-cement dynamic spacers (3.2–4.8%) (34, 61). Features predisposing to spacer fracture include higher head:neck ratio, short femoral stem with reduced intramedullary anchorage, lack of metal ‘endoskeleton’ (such as re-purposed K-wires, Schanz pins or proprietary stem) (39, 40, 56, 61, 80) and handmade dynamic spacers (40, 82).
Periprosthetic fracture has been reported in a number of case series studies (39, 54, 57, 58, 59, 60, 80). The median incidence of periprosthetic fracture in these reports was 2 (1–3). A higher risk of periprosthetic fracture was associated with increasing bone loss following debridement at first-stage surgery, as per Paprosky classification (58, 73).
Knee
Dynamic spacers rely on soft tissue tensioning for stability. A study of 154 patients with dynamic spacers found that 45% had minor problems of spacer tilting or mediolateral translation, while 12% of spacers were subluxed, fractured or dislocated (45). Lau et al. found that spacer instability was associated with bone loss and a lower KSS (83).
Periprosthetic fracture is also reported in the literature for knee spacers, with one study reporting a 9.1% fracture rate associated with static spacers (83). Mechanical complications with dynamic spacers may be due to preoperative defects in bone stock or soft tissue, surgical technique or a limited selection of prefabricated spacer sizes (63). Another study compared the incidence of mechanical complications (spacer fracture, dislocation or subluxation) in both static and dynamic spacers reporting a 12% rate and a 0% rate, respectively (76). Similarly, a large case series of static spacers reported only one spacer fracture (0.8%) (84). International consensus advises the use of static spacers in patients at higher risk of mechanical complications (63).
Eradication rate
Hip
Almost all reports of successful PJI treatment following two-stage revision hip arthroplasty using an antibiotic-eluting PMMA spacer demonstrate no difference in eradication rates between the use of static and dynamic spacers (33, 56, 57, 58, 61, 62, 82). Furthermore, almost all comparative studies demonstrate no difference in eradication rates between differing varieties of dynamic spacers, such as handmade, preformed, dynamic or CUMARS (36, 40, 58, 60, 62, 85, 86). This is despite some reported concerns of inert prosthetic material (polyethylene) used in CUMARS acting as a substrate for infective recurrence (54, 87). The report by Sabry et al. suggests possible increased rates of treatment failure (infection recurrence) with their CUMARS variant. Considerable interest has persisted in use of CUMARS for two-stage treatment of hip PJI since its initial reporting (51). Subsequent reports have validated the promising initial findings of effectively eradicating PJI, allowing full mobility and full weightbearing through the affected joint. In addition to this, CUMARS uniquely allows the potential for delayed second-stage surgery or even for some patients removes the pressing need for second-stage surgery at all (88).
Some concern has been reported regarding the limitation to antibiotic regimes from preformed dynamic spacers, such as the Spacer G. Lower elution rate/antibiotic dose within preformed dynamic spacers has also been cited as a potential limitation to their efficacy in eradicating infection (54). Furthermore, the option to add additional antibiotics/bespoke regimes is similarly limited in these spacers (58).
Knee
In 2000, Fehring et al. reported outcomes for patients undergoing two-stage revision following infected TKA; they showed no difference in eradication rate between 25 cases with static block-type cement spacers (of varying shapes) and 30 cases with custom-moulded dynamic cement spacers (75). Other smaller comparative studies from the 1980s to early 2000s found similar equivalence (42, 60, 89).
A number of studies have been published more recently, describing the outcomes for either static or dynamic spacers, or comparing both. These vary significantly with respect to the length of follow-up and operative technique. Most systematic reviews of the literature suggest no difference between static and dynamic spacers with respect to eradication rates (65, 66, 68, 85).
One systematic review, utilising wider literature inclusion criteria, did find a better eradication rate for dynamic spacers but noted that a number of factors could have confounded this result, including case complexity, antibiotic choice and method of spacer fabrication (67).
There is little evidence with respect to reinfection rates for specific subtypes of spacer. DeBoer found no difference in outcome when comparing injection-moulded with preformed dynamic spacers, but this study was limited due to its small subgroup sizes (90). Larger studies have been unable to differentiate due to differences in study design and heterogeneous data (66, 67).
Conclusions
Our clinical practice for the use of intra-articular spacers are as follows:
In the hip, we would recommend the use of a dynamic hip spacer wherever possible, as this allows the patient to mobilise more easily and weightbear and reduces the risk of a spacer dislocation. Furthermore, it mitigates against significant leg length shortening on the affected side, which helps reduce the risk of contractures and stiffness in preparation for a second-stage procedure. We advocate the use of a CUMARS as a dynamic spacer, using a cemented, polished, tapered stem and cemented socket (the so-called ‘kiwi’ procedure), both for ‘single-stage’ cases with an infected hip replacement where the bacteria and antibiotic sensitivities have been confirmed and in first-stage revision (of two) for PJI. The use of a CUMARS can be particularly useful for the very frail patient with significant medical co-morbidities where the risks of further surgery are great, as the implanted CUMARS may sometimes (up to 45%) become the definitive reconstruction and avoid the need to proceed to the formal second-stage revision (51).
We would use a static all-cement spacer in patients who have had multiple previous operations for ongoing PJI and/or there is polymicrobial infection including fungal infection or established osteomyelitis. In such cases, reducing the metal presence in the hip and thus reducing the potential for a biofilm may be more beneficial despite the limitations of static spacers.
In the knee, we recommend the use of a CUMARS (dynamic spacer) where possible, using a standard knee cemented femoral component and an all polyethylene tibial component. In patients who have had multiple previous revision surgery, polymicrobial infection or an extensor mechanism deficit, we would recommend using a static spacer. This is either a handmade cement spacer (sometimes around an arthrodesis IM nail) or an injection-moulded spacer.
In summary, the literature on the clinical outcomes of spacers in the management of PJI is very limited and mainly confined to retrospective case series and short-term outcome studies. Many of these studies were often focused more broadly on the outcomes of two-stage revision surgery rather than comparing spacers. It is important that orthopaedic surgeons are familiar with the different types of spacers available for use as part of the strategy for treating patients with PJI.
ICMJE Conflict of Interest Statement
S J declares receiving consultancy fees from Stryker; J P declares receiving lecture fees and grants from B Braun/Medacta. All authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this study.
Funding Statement
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Acknowledgements
The authors are grateful to the following companies for access to, and permissions for, illustrative materials of their products within the body of this review: LINK Orthopaedics UK Ltd, Zimmer Biomet, Heraeus Medical GmbH, Summit Medical Ltd (UK Distributor of Tecres SpA) and DePuy Synthes.
References
- 1.Mandl LA.Determining who should be referred for total hip and knee replacements. Nature Reviews: Rheumatology 20139351–357. ( 10.1038/nrrheum.2013.27) [DOI] [PubMed] [Google Scholar]
- 2.Mancuso CA, Salvati EA, Johanson NA, Peterson MG, Charlson ME. Patients’ expectations and satisfaction with total hip arthroplasty. Journal of Arthroplasty 199712387–396. ( 10.1016/s0883-5403(9790194-7) [DOI] [PubMed] [Google Scholar]
- 3.Finch DJ, Martin BI, Franklin PD, Magder LS, Pellegrini VD. & PEPPER Investigators. Patient-reported outcomes following total hip arthroplasty: a multicenter comparison based on surgical approaches. Journal of Arthroplasty 2020351029, .e3–1035.e3. ( 10.1016/j.arth.2019.10.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callahan CM, Drake BG, Heck DA, Dittus RS. Patient outcomes following tricompartmental total knee replacement. A meta-analysis. JAMA 19942711349–1357. [PubMed] [Google Scholar]
- 5.Klem N-R, Kent P, Smith A, Dowsey M, Fary R, Schütze R, O’Sullivan P, Choong P, Bunzli S. Satisfaction after total knee replacement for osteoarthritis is usually high, but what are we measuring? A systematic review. Osteoarthritis and Cartilage Open 202021000, 32. ( 10.1016/j.ocarto.2020.100032:100032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Joint Registry for England and Wales. NJR 17th annual report. Published online 2020. (available at: https://reports.njrcentre.org.uk/Portals/0/PDFdownloads/NJR%2017th%20Annual%20Report%202020.pdf) [Google Scholar]
- 7.Jafari SM, Coyle C, Mortazavi SMJ, Sharkey PF, Parvizi J. Revision hip arthroplasty: infection is the most common cause of failure. Clinical Orthopaedics and Related Research 20104682046–2051. ( 10.1007/s11999-010-1251-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. Journal of Bone and Joint Surgery: American Volume 200991128–133. ( 10.2106/JBJS.H.00155) [DOI] [PubMed] [Google Scholar]
- 9.Bozic KJ, Kurtz SM, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ. The epidemiology of revision total knee arthroplasty in the United States. Clinical Orthopaedics and Related Research 201046845–51. ( 10.1007/s11999-009-0945-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anis HK, Warren JA, Klika AK, Navale SM, Zhou G, Barsoum WK, Higuera CA, Piuzzi NS. Greater prevalence of mental health conditions in septic revision total knee arthroplasty: a call to action. Journal of Knee Surgery 2020. In press. ( 10.1055/s-0040-1713756) [DOI] [PubMed] [Google Scholar]
- 11.Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. Journal of Arthroplasty 201227 (8 Supplement) 61, .e1–65. ( 10.1016/j.arth.2012.02.022) [DOI] [PubMed] [Google Scholar]
- 12.Lamagni T.Epidemiology and burden of prosthetic joint infections. Journal of Antimicrobial Chemotherapy 201469 (Supplement 1) i5–i10. ( 10.1093/jac/dku247) [DOI] [PubMed] [Google Scholar]
- 13.Shahi A, Tan TL, Chen AF, Maltenfort MG, Parvizi J. In-hospital mortality in patients with periprosthetic joint infection. Journal of Arthroplasty 201732948, .e1–952.e1. ( 10.1016/j.arth.2016.09.027) [DOI] [PubMed] [Google Scholar]
- 14.Zmistowski B, Karam JA, Durinka JB, Casper DS, Parvizi J. Periprosthetic joint infection increases the risk of one-year mortality. Journal of Bone and Joint Surgery: American Volume 2013952177–2184. ( 10.2106/JBJS.L.00789) [DOI] [PubMed] [Google Scholar]
- 15.Moore AJ, Blom AW, Whitehouse MR, Gooberman-Hill R. Deep prosthetic joint infection: a qualitative study of the impact on patients and their experiences of revision surgery. BMJ Open 20155 e009495. ( 10.1136/bmjopen-2015-009495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchholz HW, Engelbrecht H. Depot effects of various antibiotics mixed with palacos resins. Chirurg 197041511–515. [PubMed] [Google Scholar]
- 17.McConoughey SJ, Howlin RP, Wiseman J, Stoodley P, Calhoun JH. Comparing PMMA and calcium sulfate as carriers for the local delivery of antibiotics to infected surgical sites. Journal of Biomedical Materials Research: Part B, Applied Biomaterials 2015103870–877. ( 10.1002/jbm.b.33247) [DOI] [PubMed] [Google Scholar]
- 18.Elson RA, Jephcott AE, McGechie DB, Verettas D. Antibiotic-loaded acrylic cement. Journal of Bone and Joint Surgery: British Volume 197759200–205. ( 10.1302/0301-620X.59B2.873980) [DOI] [PubMed] [Google Scholar]
- 19.Balato G, Ascione T, Rosa D, Pagliano P, Solarino G, Moretti B, Mariconda M. Release of gentamicin from cement spacers in two-stage procedures for hip and knee prosthetic infection: an in vivo pharmacokinetic study with clinical follow-up. Journal of Biological Regulators and Homeostatic Agents 20152963–72. [PubMed] [Google Scholar]
- 20.Anagnostakos K, Meyer C. Antibiotic elution from hip and knee acrylic bone cement spacers: a systematic review. BioMed Research International 201720174657874. ( 10.1155/2017/4657874) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edelstein AI, Okroj KT, Rogers T, Della Valle CJ, Sporer SM. Systemic absorption of antibiotics from antibiotic-loaded cement spacers for the treatment of periprosthetic joint infection. Journal of Arthroplasty 201833835–839. ( 10.1016/j.arth.2017.09.043) [DOI] [PubMed] [Google Scholar]
- 22.Slane J, Gietman B, Squire M. Antibiotic elution from acrylic bone cement loaded with high doses of tobramycin and vancomycin. Journal of Orthopaedic Research 2018361078–1085. ( 10.1002/jor.23722) [DOI] [PubMed] [Google Scholar]
- 23.Stevens CM, Tetsworth KD, Calhoun JH, Mader JT. An articulated antibiotic spacer used for infected total knee arthroplasty: a comparative in vitro elution study of simplex and palacos bone cements. Journal of Orthopaedic Research 20052327–33. ( 10.1016/j.orthres.2004.03.003) [DOI] [PubMed] [Google Scholar]
- 24.Insall JN, Thompson FM, Brause BD. Two-stage reimplantation for the salvage of infected total knee arthroplasty. Journal of Bone and Joint Surgery: American Volume 1983651087–1098. ( 10.2106/00004623-198365080-00008) [DOI] [PubMed] [Google Scholar]
- 25.Izakovicova P, Borens O, Trampuz A. Periprosthetic joint infection: current concepts and outlook. EFORT Open Reviews 20194482–494. ( 10.1302/2058-5241.4.180092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernard L, Legout L, Zürcher-Pfund L, Stern R, Rohner P, Peter R, Assal M, Lew D, Hoffmeyer P, Uçkay I. Six weeks of antibiotic treatment is sufficient following surgery for septic arthroplasty. Journal of Infection 201061125–132. ( 10.1016/j.jinf.2010.05.005) [DOI] [PubMed] [Google Scholar]
- 27.Chaussade H, Uçkay I, Vuagnat A, Druon J, Gras G, Rosset P, Lipsky BA, Bernard L. Antibiotic therapy duration for prosthetic joint infections treated by debridement and implant retention (DAIR): similar long-term remission for 6 weeks as compared to 12 weeks. International Journal of Infectious Diseases 20176337–42. ( 10.1016/j.ijid.2017.08.002) [DOI] [PubMed] [Google Scholar]
- 28.Sandiford NA, McHale A, Citak M, Kendoff D. What is the optimal duration of intravenous antibiotics following single-stage revision total hip arthroplasty for prosthetic joint infection? A systematic review. Hip International 202131286–294. ( 10.1177/1120700020922850) [DOI] [PubMed] [Google Scholar]
- 29.Li HK, Rombach I, Zambellas R, Walker AS, McNally MA, Atkins BL, Lipsky BA, Hughes HC, Bose D, Kümin Met al. Oral versus intravenous antibiotics for bone and joint infection. New England Journal of Medicine 2019380425–436. ( 10.1056/NEJMoa1710926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darley ESR, Bannister GC, Blom AW, Macgowan AP, Jacobson SK, Alfouzan W. Role of early intravenous to oral antibiotic switch therapy in the management of prosthetic hip infection treated with one- or two-stage replacement. Journal of Antimicrobial Chemotherapy 2011662405–2408. ( 10.1093/jac/dkr277) [DOI] [PubMed] [Google Scholar]
- 31.Frieler S, Hanusrichter Y, Bellova P, Geßmann J, Schildhauer TA, Baecker H. Facing multidrug-resistant pathogens in periprosthetic joint infections with self-administered outpatient parenteral antimicrobial therapy – a prospective cohort study. Journal of Orthopaedic Research 202139320–332. ( 10.1002/jor.24906) [DOI] [PubMed] [Google Scholar]
- 32.Mazzucchelli L, Rosso F, Marmotti A, Bonasia DE, Bruzzone M, Rossi R. The use of spacers (static and mobile) in infection knee arthroplasty. Current Reviews in Musculoskeletal Medicine 20158373–382. ( 10.1007/s12178-015-9293-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charette RS, Melnic CM. Two-stage revision arthroplasty for the treatment of prosthetic joint infection. Current Reviews in Musculoskeletal Medicine 201811332–340. ( 10.1007/s12178-018-9495-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anagnostakos K, Fürst O, Kelm J. Antibiotic-impregnated PMMA hip spacers: current status. Acta Orthopaedica 200677628–637. ( 10.1080/17453670610012719) [DOI] [PubMed] [Google Scholar]
- 35.Antoci V, Phillips MJ, Antoci V, Krackow KA. Using an antibiotic-impregnated cement rod-spacer in the treatment of infected total knee arthroplasty. American Journal of Orthopedics 20093831–33. [PubMed] [Google Scholar]
- 36.D’Angelo F, Negri L, Zatti G, Grassi FA. Two-stage revision surgery to treat an infected hip implant. A comparison between a custom-made spacer and a pre-formed one. Chirurgia degli Organi di Movimento 200590271–279. [PubMed] [Google Scholar]
- 37.D’Angelo F, Negri L, Binda T, Zatti G, Cherubino P. The use of a preformed spacer in two-stage revision of infected hip arthroplasties. Musculoskeletal Surgery 201195115–120. ( 10.1007/s12306-011-0128-5) [DOI] [PubMed] [Google Scholar]
- 38.Peng KT, Kuo LT, Hsu WH, Huang TW, Tsai YH. The effect of endoskeleton on antibiotic impregnated cement spacer for treating deep hip infection. BMC Musculoskeletal Disorders 201112 10. ( 10.1186/1471-2474-12-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faschingbauer M, Reichel H, Bieger R, Kappe T. Mechanical complications with one hundred and thirty eight (antibiotic-laden) cement spacers in the treatment of periprosthetic infection after total hip arthroplasty. International Orthopaedics (SICOT) 201539989–994. ( 10.1007/s00264-014-2636-z) [DOI] [PubMed] [Google Scholar]
- 40.Citak M, Masri BA, Springer B, Argenson JN, Kendoff DO. Are preformed articulating spacers superior to surgeon-made articulating spacers in the treatment of PJI in THA? A literature review. Open Orthopaedics Journal 20159255–261. ( 10.2174/1874325001509010255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sculco Pk.Intra-articular hip spacers for infection: fabrication and use. Orthopaedic Proceedings 2019101-B121–121. ( 10.1302/1358-992X.2019.8.121) [DOI] [Google Scholar]
- 42.Hofmann AA, Kane KR, Tkach TK, Plaster RL, Camargo MP. Treatment of infected total knee arthroplasty using an articulating spacer. Clinical Orthopaedics and Related Research 199532145–54. [PubMed] [Google Scholar]
- 43.Medicines and Healthcare products Regulatory Agency (MHRA). Single-Use Medical Devices: Implications and Consequences of Their Reuse. Version 2.4; 2021. [Google Scholar]
- 44.U.S. Food & Drug Administration. Reprocessing of single-use devices. (available at: http://wayback.archive-it.org/7993/20170111015341/http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/ReprocessingofSingle-UseDevices/default.htm). Published 12 March 2013. Accessed 15 June 2021. [Google Scholar]
- 45.Struelens B, Claes S, Bellemans J. Spacer-related problems in two-stage revision knee arthroplasty. Acta Orthopaedica Belgica 201379422–426. [PubMed] [Google Scholar]
- 46.Haddad FS, Masri BA, Campbell D, McGraw RW, Beauchamp CP, Duncan CP. The PROSTALAC functional spacer in two-stage revision for infected knee replacements. Prosthesis of antibiotic-loaded acrylic cement. Journal of Bone and Joint Surgery: British Volume 200082807–812. ( 10.1302/0301-620x.82b6.10486) [DOI] [PubMed] [Google Scholar]
- 47.Gooding CR, Masri BA, Duncan CP, Greidanus NV, Garbuz DS. Durable infection control and function with the PROSTALAC spacer in two-stage revision for infected knee arthroplasty. Clinical Orthopaedics and Related Research 2011469985–993. ( 10.1007/s11999-010-1579-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hofmann AA, Goldberg TD, Tanner AM, Cook TM. Ten-year experience using an articulating antibiotic cement hip spacer for the treatment of chronically infected total hip. Journal of Arthroplasty 200520874–879. ( 10.1016/j.arth.2004.12.055) [DOI] [PubMed] [Google Scholar]
- 49.Biring GS, Kostamo T, Garbuz DS, Masri BA, Duncan CP. Two-stage revision arthroplasty of the hip for infection using an interim articulated Prostalac hip spacer. Journal of Bone and Joint Surgery: British Volume 2009911431–1437. ( 10.1302/0301-620X.91B11.22026) [DOI] [PubMed] [Google Scholar]
- 50.Evans RP.Successful treatment of total hip and knee infection with articulating antibiotic components: a modified treatment method. Clinical Orthopaedics and Related Research 200442737–46. ( 10.1097/01.blo.0000143739.07632.7c) [DOI] [PubMed] [Google Scholar]
- 51.Tsung JD, Rohrsheim JAL, Whitehouse SL, Wilson MJ, Howell JR. Management of periprosthetic joint infection after total hip arthroplasty using a custom made articulating spacer (CUMARS); the Exeter experience. Journal of Arthroplasty 2014291813–1818. ( 10.1016/j.arth.2014.04.013) [DOI] [PubMed] [Google Scholar]
- 52.Marson BA, Walters ST, Bloch BV, Sehat K. Two-stage revision surgery for infected total knee replacements: reasonable function and high success rate with the use of primary knee replacement implants as temporary spacers. European Journal of Orthopaedic Surgery and Traumatology 201828109–115. ( 10.1007/s00590-017-2016-7) [DOI] [PubMed] [Google Scholar]
- 53.Zamora T, Garbuz DS, Greidanus NV, Masri BA. An articulated spacer made of new primary implants in two-stage exchange for infected total knee arthroplasty may provide durable results. Bone and Joint Journal 2020102-B852–860. ( 10.1302/0301-620X.102B7.BJJ-2019-1443.R1) [DOI] [PubMed] [Google Scholar]
- 54.Burastero G, Basso M, Carrega G, Cavagnaro L, Chiarlone F, Salomone C, Papa G, Felli L. Acetabular spacers in 2-stage hip revision: is it worth it? A single-centre retrospective study. Hip International 201727187–192. ( 10.5301/hipint.5000446) [DOI] [PubMed] [Google Scholar]
- 55.Zhang W, Fang X, Shi T, Cai Y, Huang Z, Zhang C, Lin J, Li W. Cemented prosthesis as spacer for two-stage revision of infected hip prostheses: a similar infection remission rate and a lower complication rate. Bone and Joint Research 20209484–492. ( 10.1302/2046-3758.98.BJR-2020-0173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jacobs C, Christensen CP, Berend ME. Static and mobile antibiotic-impregnated cement spacers for the management of prosthetic joint infection. Journal of the American Academy of Orthopaedic Surgeons 200917356–368. ( 10.5435/00124635-200906000-00004) [DOI] [PubMed] [Google Scholar]
- 57.Younger AS, Duncan CP, Masri BA, McGraw RW. The outcome of two-stage arthroplasty using a custom-made interval spacer to treat the infected hip. Journal of Arthroplasty 199712615–623. ( 10.1016/s0883-5403(9790133-9) [DOI] [PubMed] [Google Scholar]
- 58.Pattyn C, De Geest T, Ackerman P, Audenaert E. Preformed gentamicin spacers in two-stage revision hip arthroplasty: functional results and complications. International Orthopaedics 2011351471–1476. ( 10.1007/s00264-010-1172-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scharfenberger A, Clark M, Lavoie G, O’Connor G, Masson E, Beaupre LA. Treatment of an infected total hip replacement with the PROSTALAC system. Part 2: health-related quality of life and function with the PROSTALAC implant in situ. Canadian Journal of Surgery 20075029–33. [PMC free article] [PubMed] [Google Scholar]
- 60.Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clinical Orthopaedics and Related Research 2005430125–131. ( 10.1097/01.blo.0000149241.77924.01) [DOI] [PubMed] [Google Scholar]
- 61.Hsieh PH, Chen LH, Chen CH, Lee MS, Yang WE, Shih CH. Two-stage revision hip arthroplasty for infection with a custom-made, antibiotic-loaded, cement prosthesis as an interim spacer. Journal of Trauma 2004561247–1252. ( 10.1097/01.ta.0000130757.53559.bf) [DOI] [PubMed] [Google Scholar]
- 62.Veltman ES, Moojen DJF, Glehr M, Poolman RW. Similar rate of infection eradication for functional articulating, prefabricated and custom-made spacers in 2-stage revision of the infected total hip: a literature review. Hip International 201626319–326. ( 10.5301/hipint.5000400) [DOI] [PubMed] [Google Scholar]
- 63.Abdel MP, Barreira P, Battenberg A, Berry DJ, Blevins K, Font-Vizcarra L, Frommelt L, Goswami K, Greiner J, Janz Vet al. Hip and knee section, treatment, two-stage exchange spacer-related: Proceedings of International Consensus on Orthopedic Infections. Journal of Arthroplasty 201934S427–S438. ( 10.1016/j.arth.2018.09.027) [DOI] [PubMed] [Google Scholar]
- 64.Chiang ER, Su YP, Chen TH, Chiu FY, Chen WM. Comparison of articulating and static spacers regarding infection with resistant organisms in total knee arthroplasty. Acta Orthopaedica 201182460–464. ( 10.3109/17453674.2011.581266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Voleti PB, Baldwin KD, Lee GC. Use of static or articulating spacers for infection following total knee arthroplasty: a systematic literature review. Journal of Bone and Joint Surgery: American Volume 2013951594–1599. ( 10.2106/JBJS.L.01461) [DOI] [PubMed] [Google Scholar]
- 66.Pivec R, Naziri Q, Issa K, Banerjee S, Mont MA. Systematic review comparing static and articulating spacers used for revision of infected total knee arthroplasty. Journal of Arthroplasty 201429553, .e1–557. ( 10.1016/j.arth.2013.07.041) [DOI] [PubMed] [Google Scholar]
- 67.Guild GN, Wu B, Scuderi GR. Articulating vs. static antibiotic impregnated spacers in revision total knee arthroplasty for sepsis. A systematic review. Journal of Arthroplasty 201429558–563. ( 10.1016/j.arth.2013.08.013) [DOI] [PubMed] [Google Scholar]
- 68.Ding H, Yao J, Chang W, Liu F. Comparison of the efficacy of static versus articular spacers in two-stage revision surgery for the treatment of infection following total knee arthroplasty: a meta-analysis. Journal of Orthopaedic Surgery and Research 201712 151. ( 10.1186/s13018-017-0644-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nahhas CR, Chalmers PN, Parvizi J, Sporer SM, Berend KR, Moric M, Chen AF, Austin MS, Deirmengian GK, Morris MJet al. A randomized trial of static and articulating spacers for the treatment of infection following total knee arthroplasty. Journal of Bone and Joint Surgery: American Volume 2020102778–787. ( 10.2106/JBJS.19.00915) [DOI] [PubMed] [Google Scholar]
- 70.Degen RM, Davey JR, Davey JR, Howard JL, McCalden RW, Naudie DDR. Does a prefabricated gentamicin-impregnated, load-bearing spacer control periprosthetic hip infection? Clinical Orthopaedics and Related Research 20124702724–2729. ( 10.1007/s11999-012-2350-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fehring TK, Odum S, Struble S, Fehring K, Griffin WL, Mason JB. Hip instability in 2-stage reimplantation without an articulating spacer. Journal of Arthroplasty 200722 (Supplement 2) 156–161. ( 10.1016/j.arth.2007.03.028) [DOI] [PubMed] [Google Scholar]
- 72.Sporer SM.Spacer design options and consideration for periprosthetic joint infection. Journal of Arthroplasty 202035S31–S34. ( 10.1016/j.arth.2019.11.007) [DOI] [PubMed] [Google Scholar]
- 73.Wyles CC, Abdel MP. Point/counterpoint: nonarticulating vs articulating spacers for resection arthroplasty of the knee or hip. Journal of Arthroplasty 202035S40–S44. ( 10.1016/j.arth.2019.10.055) [DOI] [PubMed] [Google Scholar]
- 74.García-Oltra E, Bori G, Tomas X, Gallart X, Garcia S, Soriano A. Radiological evaluation of acetabular erosion after antibiotic-impregnated polymethylmethacrylate spacer (Spacer-G). Journal of Arthroplasty 2013281021–1024. ( 10.1016/j.arth.2012.07.013) [DOI] [PubMed] [Google Scholar]
- 75.Fehring TK, Odum S, Calton TF, Mason JB. Articulating versus static spacers in revision total knee arthroplasty for sepsis. The Ranawat Award. Clinical Orthopaedics and Related Research 20003809–16. ( 10.1097/00003086-200011000-00003) [DOI] [PubMed] [Google Scholar]
- 76.Johnson AJ, Sayeed SA, Naziri Q, Khanuja HS, Mont MA. Minimizing dynamic knee spacer complications in infected revision arthroplasty. Clinical Orthopaedics and Related Research 2012470220–227. ( 10.1007/s11999-011-2095-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Park SJ, Song EK, Seon JK, Yoon TR, Park GH. Comparison of static and mobile antibiotic-impregnated cement spacers for the treatment of infected total knee arthroplasty. International Orthopaedics (SICOT) 2010341181–1186. ( 10.1007/s00264-009-0907-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Choi HR, Malchau H, Bedair H. Are prosthetic spacers safe to use in 2-stage treatment for infected total knee arthroplasty? Journal of Arthroplasty 2012271474, .e1–1479.e1. ( 10.1016/j.arth.2012.02.023) [DOI] [PubMed] [Google Scholar]
- 79.Hsu YC, Cheng HC, Ng TP, Chiu KY. Antibiotic-loaded cement articulating spacer for 2-stage reimplantation in infected total knee arthroplasty: a simple and economic method. Journal of Arthroplasty 2007221060–1066. ( 10.1016/j.arth.2007.04.028) [DOI] [PubMed] [Google Scholar]
- 80.Rava A, Bruzzone M, Cottino U, Enrietti E, Rossi R. Hip spacers in two-stage revision for periprosthetic joint infection: a review of literature. Joints 2019756–63. ( 10.1055/s-0039-1697608) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Romanò CL, Romanò D, Albisetti A, Meani E. Preformed antibiotic-loaded cement spacers for two-stage revision of infected total hip arthroplasty. Long-term results. Hip International 201222 (Supplement 8) S46–S53. ( 10.5301/HIP.2012.9570) [DOI] [PubMed] [Google Scholar]
- 82.Yamamoto K, Miyagawa N, Masaoka T, Katori Y, Shishido T, Imakiire A. Clinical effectiveness of antibiotic-impregnated cement spacers for the treatment of infected implants of the hip joint. Journal of Orthopaedic Science 20038823–828. ( 10.1007/s00776-003-0722-y) [DOI] [PubMed] [Google Scholar]
- 83.Lau ACK, Howard JL, Macdonald SJ, Teeter MG, Lanting BA. The effect of subluxation of articulating antibiotic spacers on bone defects and degree of constraint in revision knee arthroplasty. Journal of Arthroplasty 201631199–203. ( 10.1016/j.arth.2015.07.009) [DOI] [PubMed] [Google Scholar]
- 84.Faschingbauer M, Bieger R, Reichel H, Weiner C, Kappe T. Complications associated with 133 static, antibiotic-laden spacers after TKA. Knee Surgery, Sports Traumatology, Arthroscopy 2016243096–3099. ( 10.1007/s00167-015-3646-0) [DOI] [PubMed] [Google Scholar]
- 85.Gomez MM, Tan TL, Manrique J, Deirmengian GK, Parvizi J. The fate of spacers in the treatment of periprosthetic joint infection. Journal of Bone and Joint Surgery: American Volume 2015971495–1502. ( 10.2106/JBJS.N.00958) [DOI] [PubMed] [Google Scholar]
- 86.Tikhilov R, Bozhkova S, Denisov A, Labutin D, Shubnyakov I, Razorenov V, Artyukh V, Klitsenko O. Risk factors and a prognostic model of hip periprosthetic infection recurrence after surgical treatment using articulating and non-articulating spacers. International Orthopaedics (SICOT) 2016401381–1387. ( 10.1007/s00264-015-3072-4) [DOI] [PubMed] [Google Scholar]
- 87.Sabry FY, Szubski CR, Stefancin JJ, Klika AK, Higuera CA, Barsoum WK. Comparison of complications associated with commercially available and custom-made articulating spacers in two-stage total hip arthroplasty revision. Current Orthopaedic Practice 201324406–413. ( 10.1097/BCO.0b013e318297c3fb) [DOI] [Google Scholar]
- 88.Quayle J, Barakat A, Klasan A, Mittal A, Stott P. External validation study of hip peri-prosthetic joint infection with cemented custom-made articulating spacer (CUMARS ). Hip International 2020In press. ( 10.1177/1120700020960669) [DOI] [PubMed] [Google Scholar]
- 89.Emerson RHJ, Muncie M, Tarbox TR, Higgins LL. Comparison of a static with a mobile spacer in total knee infection. Clinical Orthopaedics and Related Research 2002404132–138. ( 10.1097/00003086-200211000-00023) [DOI] [PubMed] [Google Scholar]
- 90.DeBoer DK.Comparison of traditional molded, first-generation premolded, and second-generation premolded antibiotic-loaded polymethylmethacrylate articulating spacers for treatment of chronic prosthetic joint infection of the knee. Journal of Arthroplasty 202035S53–S56. ( 10.1016/j.arth.2019.11.008) [DOI] [PubMed] [Google Scholar]
- 91.Classen T, von Knoch M, Wernsmann M, Landgraeber S, Löer F, Jäger M.Functional Interest of an Articulating Spacer in Two-Stage Infected Total Knee Arthroplasty Revision. Orthopaedics & Traumatology, Surgery & Research: OTSR 2014100409–412. ( 10.1016/j.otsr.2014.01.010) [DOI] [PubMed] [Google Scholar]
- 92.Cuckler J M.The Infected Total Knee: Management Options. The Journal of Arthroplasty 200520 (Supplement 2) 33–36. ( 10.1016/j.arth.2005.03.004) [DOI] [PubMed] [Google Scholar]
- 93.Freeman M G, Fehring T K, Odum S M, Fehring K, Griffin W L, Mason J B.Functional Advantage of Articulating Versus Static Spacers in 2-Stage Revision for Total Knee Arthroplasty Infection. The Journal of Arthroplasty 2007221116–1121. ( 10.1016/j.arth.2007.04.009) [DOI] [PubMed] [Google Scholar]
- 94.Goldman R T, Scuderi G R, Insall J N.2-Stage Reimplantation for Infected Total Knee Replacement. Clinical Orthopaedics and Related Research 1996331118–124. ( 10.1097/00003086-199610000-00016) [DOI] [PubMed] [Google Scholar]
- 95.Haleem A A, Berry D J, Hanssen A D.The Chitranjan Ranawat Award: Mid-Term to Long-Term Followup of Two-Stage Reimplantation for Infected Total Knee Arthroplasty. Clinical Orthopaedics and Related Research 200442835–39. ( 10.1097/01.blo.0000147713.64235.73) [DOI] [PubMed] [Google Scholar]
- 96.Hart W J, Jones R S.Two-Stage Revision of Infected Total Knee Replacements Using Articulating Cement Spacers and Short-Term Antibiotic Therapy. The Journal of Bone and Joint Surgery. 2006881011–1015. ( 10.1302/0301-620X.88B8.17445) [DOI] [PubMed] [Google Scholar]
- 97.Hirakawa K, Stulberg B N, Wilde A H, Bauer T W, Secic M.Results of 2-Stage Reimplantation for Infected Total Knee Arthroplasty. The Journal of Arthroplasty 19981322–28. ( 10.1016/s0883-5403(98)90071-7) [DOI] [PubMed] [Google Scholar]
- 98.Jämsen E, Sheng P, Halonen P, Lehto M U K, Moilanen T, Pajamäki J, Puolakka T, Konttinen Y T.Spacer Prostheses in Two-Stage Revision of Infected Knee Arthroplasty. International Orthopaedics 200630257–261. ( 10.1007/s00264-006-0102-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Juul R, Fabrin J, Poulsen K, Schroder H M.Use of a New Knee Prosthesis as an Articulating Spacer in Two-Stage Revision of Infected Total Knee Arthroplasty. Knee Surgery & Related Research 201628239–244. ( 10.5792/ksrr.2016.28.3.239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kotwal S Y, Farid Y R, Patil S S, Alden K J, Finn H A.Intramedullary Rod and Cement Static Spacer Construct in Chronically Infected Total Knee Arthroplasty. The Journal of Arthroplasty 201227253–259.e4. ( 10.1016/j.arth.2011.04.021) [DOI] [PubMed] [Google Scholar]
- 101.Nodzo S R, Boyle K K, Spiro S, Nocon A A, Miller A O, Westrich G H.Success Rates, Characteristics, and Costs of Articulating Antibiotic Spacers for Total Knee Periprosthetic Joint Infection. The Knee 2017241175–1181. ( 10.1016/j.knee.2017.05.016) [DOI] [PubMed] [Google Scholar]
- 102.Tian M‐q, Yang X‐t Tian, X‐b Sun, Y‐b Duan, Y‐h, Sun L.Short‐term Follow‐up of Antibiotic‐loaded Articulating Cement Spacers in Two‐stage Revision of Infected Total Knee Arthroplasty: A Case Series. Orthopaedic Surgery 201810128–133. ( 10.1111/os.12381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Van Thiel G S, Berend K R, Klein G R, Gordon A C, Lombardi A V, Della Valle C J.Intraoperative Molds to Create an Articulating Spacer for the Infected Knee Arthroplasty. Clinical Orthopaedics and Related Research 2011469994–1001. ( 10.1007/s11999-010-1644-6) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a