Abstract
Background and purpose
Endovascular treatment for acute ischemic stroke has become a recommended treatment option for selected patients after several randomized controlled trials have shown the effectiveness of endovascular treatment. Due to the nature of randomized clinical trials, the generalizability to population based real life settings and the resulting benefits remain difficult to estimate.
Methods
We included 896 consecutive patients treated with intravenous thrombolysis (IVT) within 4.5 h of stroke onset between January 2016 and December 2018, who were treated with additional endovascular treatment according to the new evidence when indicated (new-IVT-cohort). This cohort was compared to 913 intravenous thrombolysis patients treated in the 4.5 h time-window between January 2011 and December 2013 before the era of endovascular treatment (old-IVT-cohort).
Results
In the new-IVT-cohort there were 253 intravenous thrombolysis + endovascular treatment treated patients. The new-IVT-cohort was associated with a better outcome on the modified Rankin Scale at 3 months in univariable ordinal regression (OR 1.27; 95% CI 1.08–1.49). The association remained significant (OR 1.65; 95% CI 1.27–2.14) also after adjustment for following confounding factors: sex, NIHSS, diabetes, atrial fibrillation, hypertension, coronary artery disease, hypercholesterolemia, myocardial infarction, heart failure, history of ischemic stroke, history of TIA, and use of antithrombotic, statins, antihypertensive, anticoagulation treatment, or endovascular treatment (Fig. 1).
Conclusion
We were able to verify the efficacy of endovascular treatment in a real life cohort of intravenous thrombolysis patients even when only 28% of the patients are eligible for endovascular treatment on top of intravenous thrombolysis treatment.
Keywords: Intravenous thrombolysis, Ischemic stroke, Functional outcome, Endovascular treatment
Highlights
-
•
Endovascular treatment is effective in a real-life cohort
-
•
Endovascular treatment leads to better outcomes at 3 months
-
•
Endovascular treatment is safe in a hospital where the treatment is routine
1. Introduction
Recanalization and reperfusion are the mainstay of acute ischemic stroke treatment and can reduce infarct size and reverse neurologic deficits [1]. The most important clinical outcome is functional independence, and prior clinical evidence support the hypothesis that early recanalization is associated with better functional outcome [[2], [3], [4], [5], [6]]. The reported data support the view that the modified Rankin Scale (mRS) is a valuable tool for assessing the impact of stroke treatments [7].
According to our hospital guidelines prior to 2016, endovascular treatment (EVT) was considered as an option only for intravenous thrombolysis (IVT) patients who had occlusion in the proximal anterior intracranial circulation and did not receive a significant clinical response, as judged by the treating physician, to IVT within one hour of follow-up (rescue therapy) or had contraindication for IVT. Prior to 2016, only few patients received EVT as there was no supportive evidence from randomized controlled trials available and our hospital protocol only advised to consider EVT in patients not responding to IVT or when IVT was contra-indicated. In practice, only a few awkward EVT attempts with a long treatmentdelay were made in desperate cases. Some of those treated prior 2016, would not qualify for EVT under the new protocol.
In 2015, a large meta-analysis of five randomized trials showed efficacy of EVT over standard medical care, including IVT if eligible, in patients with acute ischemic stroke due to anterior circulation large vessel occlusion [8]. Already in 2015 EVT became a recommended treatment option for selected acute ischemic stroke patients, but in 2016 the procedure was much more routine. Due to the nature of randomized clinical trials, the generalizability to population based real life settings and the resulting benefits remain difficult to estimate.
We aimed to verify the treatment effect of the recent EVT trials in a real-life cohort of IVT-treated patients by comparing the patient cohorts before and after implementation of routine EVT for IVT-treated patients at a comprehensive stroke center. The indications and the time-window for IVT remained unchanged over the course of the study. Additionally, the admission criterion to our hospital and the demographics of the population remained stable. In this study, the outcome at 3 months on full range of mRS in ischemic stroke patients treated between years 2016–2018 with IVT and directly with additional EVT if suitable, is compared with the outcome of ischemic stroke patients treated between years 2011–2013 with IVT and eventual additional EVT as rescue therapy if patient did not respond to IVT as assessed by the treating physician. In addition, the incidence of symptomatic intracerebral hemorrhage (sICH) and the prevalence of pre-morbid cardiovascular disease are compared between the two patient cohorts.
2. Material and methods
2.1. Patient selection
We performed a retrospective analysis from the Helsinki stroke quality register (HSQR). Every patient admitted to the department of neurology or as a neurologic patient to the emergency department of the Meilahti hospital is included in the HSQR that also contains data on clinical outcome at 3 months. Patients with pre-existing disability, usually a pre-stroke mRS > 2, are preferably primarily referred to regional hospitals. Patients were treated based on our department's written guidelines for acute stroke, which are updated biannually and whenever new scientific evidence becomes available [9]. During the whole study period, IVT (0.9 mg/kg alteplase) was delivered according to recommendations of the American Stroke Association and European Stroke Organization [10,11] within 4.5 h after onset of symptoms in the absence of contraindications. Criteria for IVT-treatment, the referral policy, and the procedure remained unchanged between the cohorts.
Both cohorts consist of consecutive patients treated with IVT within a time window of 4.5 h. In the first cohort (old-IVT-cohort) from January 1, 2011 to December 31, 2013, some of the patients received additional rescue EVT if they did not respond to IVT within one hour. The decision for additional rescue EVT was done by the treating physician based on individual factors, as randomized controlled trial data providing a basis for recommending EVT in guidelines did not exist in the years between 2011 and 2013. In the second cohort (new-IVT-cohort) from January 1, 2016 to December 31, 2018, suitable patients were treated with direct EVT in addition to IVT. We excluded patients who were referred to Meilahti hospital for EVT after having received IVT at the referring hospital. Pre-morbid cardiovascular diseases (atrial fibrillation, coronary artery disease, hypertension, hyperlipidemia, diabetes, heart failure) were recorded.
The Helsinki University hospital granted the research permit for this registry study (HUS/125/2018). As data were collected prospectively as a part of routine clinical care for retrospective analysis, ethical board review was not required at our institution.
2.2. Outcomes
The primary clinical outcome for this retrospective analysis was functional recovery as defined by a 90-day mRS. [12] The outcomes of the cohorts were compared on the full range of scores on the mRS. The secondary clinical outcome was the sICH according to the European Cooperative Acute Stroke Study 2 classification [13].
2.3. Statistical analysis
Quantitative variables are expressed as medians (interquartile range). Categorical variables are expressed as numbers (percentage). For the primary end point, between-group differences were calculated with the chi-square test of proportions (with a two-sided alpha level of 5%). Ninety-five percent confidence intervals (CIs) were calculated for odds ratios (ORs). Data were analyzed using the SPSS Statistics software version 25 (IBM). The proportional-odds assumption was tested and met. Therefore, an ordinal logistic-regression model was used to compare the trial groups across the full range of scores on the mRS, with the effect estimate for an improvement of at least 1 point in the score presented as a common odds ratio with a 95% confidence interval [14].
From the following variables, we selected to the multivariable model the ones, that were associated (p < 0.10) with the mRS at three months in univariable ordinal regression: a history of chronic heart failure, ischemic stroke, diabetes mellitus, coronary artery disease, myocardial infarction, atrial fibrillation, hypertension, the age at stroke onset, sex, total NIHSS at baseline, and being on antithrombotic, statin, antihypertensive, or anticoagulation treatment at admission.
3. Results
A total of 1809 patients were enrolled in this study; 913 of the patients were treated between January 1, 2011 and December 31, 2013 (old-IVT-cohort) and 896 of the patients between January 1, 2016 and December 31, 2018 (new-IVT-cohort). All of these patients were treated with IVT within 4.5 h of symptom onset and either with additional EVT as rescue therapy (89/913 in the old-IVT-cohort) if patient did not respond to IVT and was considered a suitable candidate by the treating physician, or directly with additional EVT if suitable (253/896 in the new-IVT-cohort). In the new-IVT-cohort, 187 patients met the inclusion criteria of the Mr. Clean study, i.e., time to groin puncture <6 h and a proximal anterior circulation occlusion, i.e., M1, ICA or ICA + M1. 52 patients were punctured <6 h but had posterior circulation occlusions (BA 21 patients, PCA 6, VA 1) and distal media occlusions (M2 23 patients, M3 1). 14 patients were punctured >6 h of symptom onset (8 BAO, 1 M1, 2 M2, 1 ICA + M1, 2 ICA). In the old-EVT-cohort there were 19 BAO, 31 M1, 16 M2, 11 ICA + M1, 11 ICA cases, and in 3 cases recanalization after IVT had already occurred.
Baseline characteristics are shown in Table 1. Baseline demographic and clinical characteristics of the two groups were similar, except that there were significant differences between the groups in the incidence history of atrial fibrillation, history of hypertension, history of heart failure, history of hypercholesterolemia, history of ischemic stroke, history of transient ischemic attack, being on antihypertensive treatment, and NIHSS score before IVT. There was no difference in door-to-treatment time between groups.
Table 1.
Baseline characteristics of patients.
| new-IVT-group (n = 896) | old-IVT-group (n = 913) | p | |
|---|---|---|---|
| Age in years, mean (SD) | 67.9 (14.1) | 67.8 (13.2) | 0.84 |
| Sex, men (%) | 514 (57.4%) | 520 (57.0%) | 0.94 |
| NIHSS score, mean (IQR) | 8.4 (3.8–11.8) | 9.3 (4.2–13.0) | <0.01 |
| Door-to-treatment time, min, median (IQR) | 20 (15–30) | 19 (13−33) | 0.32 |
| Onset-to-door time, min, median (IQR) | 84 (55–140) | 90 (63–150) | 0.21 |
| Previosly diagnosed atrial fibrillation, n (%) | 109 (12.2%) | 203 (22.2%) | <0.01 |
| History of hypertension, n (%) | 578 (64.5%) | 508 (55.7%) | <0.01 |
| History of diabetes mellitus, n (%) | 158 (17.6%) | 140 (15.3%) | 0.19 |
| History of hypercholesterolemia, n (%) | 417 (46.5%) | 365 (40.1%) | <0.01 |
| History of chronic heart failure, n (%) | 46 (5.1%) | 69 (7.6%) | 0.03 |
| History of coronary artery disease, n (%) | 163 (18.2%) | 183 (20.0%) | 0.32 |
| History of myocardial infarction, n (%) | 71 (7.9%) | 92 (10.1%) | 0.11 |
| History of ischemic stroke, n (%) | 78 (8.7%) | 105 (11.5%) | 0.05 |
| History of TIA, n (%) | 60 (6.7%) | 85 (9.3%) | 0.04 |
| Admission oral anticoagulation, n (%) | 40 (4.5%) | 59 (6.5%) | 0.06 |
| Admission statin, n (%) | 329 (36.7%) | 304 (33.7%) | 0.19 |
| Admission antithrombotic, n (%) | 287 (32.1%) | 325 (35.7%) | 0.11 |
| Admission antihypertensive, n (%) | 560 (62.5%) | 519 (57.7%) | 0.04 |
SD=Standard deviation. IQR = Interquartile range. IVT = Intravenous thrombolysis. EVT = Endovascular treatment. TIA = Transient ischemic attack. NIHSS=National Institutes of Health Stroke Scale.
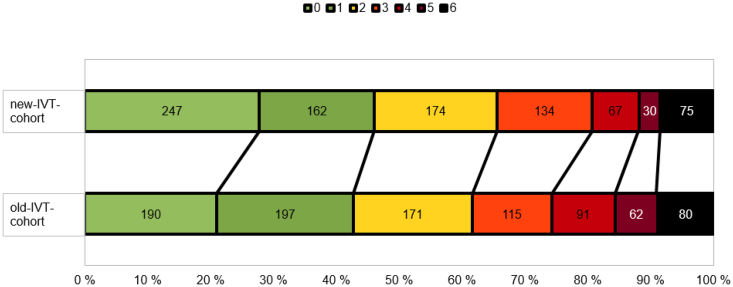
The new-IVT-cohort was associated with a better mRS at 3 months in univariable ordinal regression (OR 1.27; 95% CI 1.08–1.49, Fig. 1).
Fig. 1.
Treatment in the whole (a) intravenous thrombolysis + endovascular treatment (new-IVT-cohort) cohort was significantly associated with better outcome on the modified Rankin Scale at 3 months compared with the old-IVT-cohort (in univariable ordinal regression OR 1.27; 95% CI 1.08–1.49 and in multivariable adjusted ordinal regression OR 1.23; 95% CI 1.04–1.45). Fig. 1b illustrates the EVT treated patients from both cohorts only.
The association of the new-IVT-cohort with better mRS remained significant (OR 1.65; 95% CI 1.27–2.14) also after adjustment for confounding factors: age, sex, NIHSS, diabetes, hypercholesterolemia, atrial fibrillation, coronary artery disease, myocardial infarction, heart failure, hypertension, history of ischemic stroke, history of TIA, and use of antithrombotic, statins, antihypertensive, anticoagulation treatment or EVT (Table 2). In the new-IVT-cohort among EVT-treated patients 48% had a favorable outcome (defined as mRS 0–2 at 3 months). A 3-month mRS data of a few patients were missing (7 patients from the old-IVT-cohort and 5 patients from the new-IVT-cohort).
Table 2.
Risk factor's association with the mRS at three months in univariable ordinal regression.
| OR | p | |
|---|---|---|
| Age at stroke onset, years | 1.03 (1.03–1.04) | <0.01 |
| Sex | 0.88 (0.75–1.04) | 0.14 |
| Total NIHSS score before IVT, points | 1.15 (1.13–1.17) | <0.01 |
| Previously diagnosed atrial fibrillation | 0.55 (0.44–0,68) | <0.01 |
| History of hypertension | 0.72 (0.61–0.85) | <0.01 |
| History of diabetes mellitus | 0.76 (0.61–0.95) | 0.02 |
| History of chronic heart failure | 0.59 (0.43–0.83) | <0.01 |
| History of coronary artery disease | 0.64 (0.52–0.79) | <0.01 |
| History of myocardial infarction | 0.69 (0.52–0.91) | 0.01 |
| History of ischemic stroke | 0.79 (0.61–1.04) | 0.09 |
| Admission oral anticoagulation | 0.50 (0.35–0.72) | <0.01 |
| Admission statin | 0.81 (0.68–0.96) | 0.01 |
| Admission antithrombotic | 0.78 (0.66–0.93) | <0.01 |
| Admission antihypertensive Endovascular treatment |
0.68 (0.58–0.81) 0.46 (0.38–0.57) |
<0.01 <0.01 |
The frequency of sICH was similar in both cohorts (new-IVT-cohort 3% vs. old-IVT-cohort 4%, p = 0.22). The follow-up imaging was missing in one patient in the new-IVT-cohort because he was transported asymptomatically to other hospital before it, and two patients in the old-IVT-cohort died before the follow-up imaging.
4. Discussion
We were able to show, that IVT-treated acute ischemic stroke patients achieved better outcomes for disability and functional independence at 90 days when the suitable patients received a direct EVT compared to EVT as rescue therapy. The number of sICHs did not differ between the two cohorts.
The rates of functional independence that were observed in our new-IVT-cohort are similar to those reported in the meta-analysis of five randomized trials that showed efficacy of EVT over standard medical care, including IVT if eligible, in patients with acute ischemic stroke due to anterior circulation large vessel occlusion [8]. In our new-IVT-cohort the patients who had received EVT in addition to IVT therapy recovered equally well or even better than described in the meta-analysis [8]. In our cohort, 48% of the EVT-treated patients achieved favorable outcome compared to 46% of the meta-analysis patients. We included all EVT patients who had received IVT therapy in our hospital and did not exclude the patients who underwent EVT due to a non-ICA or M1 occlusion. Our hospital guidelines advise the clinician to consider treatment of basilar occlusion or more peripheral occlusions (i.e. M2, PCA, ACA) in selected cases. This inclusion of more peripheral occlusions in the cohort may distort the outcome in a worse direction because there is uncertainty about how peripheral occlusions should be treated and concerns have been raised about the procedural safety of EVT in peripheral occlusions [15].
Another limitation of the study is, that we have not included information on different EVT devices.
Although we named the old-IVT-cohort “old”, we want to point out, that there were no major changes in treatment or patient selection when comparing the new-IVT-cohort and the old-IVT-cohort, except for the implementation of routine EVT as soon as the evidence became available. We chose to leave out the transition time between 2014 and 2015, when the EVT trials were published and the implementation of the new protocol took place. In both cohorts the time-window for anterior circulation IVT was up to 4.5 h and up to 48 h for basilar occlusion. The reasons for the improved outcomes are probably a sum of different factors, of which we should consider on top of the EVT the following: routine EVT service has significantly shortened in-hospital delays for EVT patients; the EVT patients are now selected based on validated criteria, learned from the randomized controlled trials and based on the updated guidelines; the interventional radiologists have gained experience; the available EVT devices have improved over time.
5. Conclusion
For IVT treated patients the implementation of routine EVT, if considered clinically suitable, leads to significantly better outcomes. All stroke code patients should be considered as candidates for both recanalization strategies (IVT and EVT).
Data availability statement
Data openly available in a public repository that issues datasets with DOIs.
Sources of funding
This project has been granted state funding for university-level health research (TYH2019207).
Declarations
Conflicting interests: The Authors declare that there is no conflict of interest.
Funding: This project has been granted state funding for university-level health research. (TYH2019207).
Ethical approval: Not applicable. As data were collected prospectively as a part of routine clinical care for retrospective analysis, ethical board review was not required at our institution.
Informed consent: Not applicable.
Guarantor: KV.
Contributorship: KV and SC researched literature and conceived the study. NMM, GS, MT, and SR involved in patient data collection. KV and SC involved in data analysis. KV wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Declaration of Competing Interest
None.
Acknowledgements
None.
References
- 1.Rabinstein A.A. Treatment of Acute Ischemic Stroke. Contin Minneap Minn. 2017;23(1, Cerebrovascular Disease):62–81. doi: 10.1212/CON.0000000000000420. [DOI] [PubMed] [Google Scholar]
- 2.Endo S., Kuwayama N., Hirashima Y., Akai T., Nishijima M., Takaku A. Results of urgent thrombolysis in patients with major stroke and atherothrombotic occlusion of the cervical internal carotid artery. AJNR Am. J. Neuroradiol. 1998;19(6):1169–1175. [PMC free article] [PubMed] [Google Scholar]
- 3.Christou I., Felberg R.A., Demchuk A.M., et al. Intravenous tissue plasminogen activator and flow improvement in acute ischemic stroke patients with internal carotid artery occlusion. J Neuroimaging Off J Am Soc Neuroimaging. 2002;12(2):119–123. doi: 10.1111/j.1552-6569.2002.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 4.Rabinstein A.A., Wijdicks E.F.M., Nichols D.A. Complete recovery after early intraarterial recombinant tissue plasminogen activator thrombolysis of carotid T occlusion. AJNR Am. J. Neuroradiol. 2002;23(9):1596–1599. [PMC free article] [PubMed] [Google Scholar]
- 5.Zangerle A., Kiechl S., Spiegel M., et al. Recanalization after thrombolysis in stroke patients: predictors and prognostic implications. Neurology. 2007;68(1):39–44. doi: 10.1212/01.wnl.0000250341.38014.d2. [DOI] [PubMed] [Google Scholar]
- 6.Bhatia R., Hill M.D., Shobha N., et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke. 2010;41(10):2254–2258. doi: 10.1161/STROKEAHA.110.592535. [DOI] [PubMed] [Google Scholar]
- 7.Banks J.L., Marotta C.A. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38(3):1091–1096. doi: 10.1161/01.STR.0000258355.23810.c6. [DOI] [PubMed] [Google Scholar]
- 8.Goyal M., Menon B.K., van Zwam W.H., et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet Lond Engl. 2016;387(10029):1723–1731. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 9.Meretoja A., Putaala J., Tatlisumak T., et al. Off-label thrombolysis is not associated with poor outcome in patients with stroke. Stroke. 2010;41(7):1450–1458. doi: 10.1161/STROKEAHA.109.576140. [DOI] [PubMed] [Google Scholar]
- 10.Powers W.J., Rabinstein A.A., Ackerson T., et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46–e110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 11.Wahlgren N., Moreira T., Michel P., et al. Mechanical thrombectomy in acute ischemic stroke: consensus statement by ESO-Karolinska stroke update 2014/2015, supported by ESO, ESMINT, ESNR and EAN. Int J Stroke Off J Int Stroke Soc. 2016;11(1):134–147. doi: 10.1177/1747493015609778. [DOI] [PubMed] [Google Scholar]
- 12.van Swieten J.C., Koudstaal P.J., Visser M.C., Schouten H.J., van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 13.Hacke W., Kaste M., Fieschi C., et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian acute stroke study investigators. Lancet. 1998;352(9136):1245–1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 14.PMW Bath, Gray L.J., Collier T., Pocock S., Carpenter J., Optimising Analysis of Stroke Trials (OAST) Collaboration Can we improve the statistical analysis of stroke trials? Statistical reanalysis of functional outcomes in stroke trials. Stroke. 2007;38(6):1911–1915. doi: 10.1161/STROKEAHA.106.474080. [DOI] [PubMed] [Google Scholar]
- 15.Sarraj A., Sangha N., Hussain M.S., et al. Endovascular therapy for acute ischemic stroke with occlusion of the middle cerebral artery M2 segment. JAMA Neurol. 2016;73(11):1291. doi: 10.1001/jamaneurol.2016.2773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data openly available in a public repository that issues datasets with DOIs.