Summary
Background
Low-density neutrophils (LDN) are increased in several inflammatory diseases and may also play a role in the low-grade chronic inflammation associated with obesity. Here we explored their role in obesity, determined their gene signatures, and assessed the effect of bariatric surgery.
Methods
We compared the number, function, and gene expression profiles of circulating LDN in morbidly obese patients (MOP, n=27; body mass index (BMI) > 40 Kg/m2) and normal-weight controls (NWC, n=20; BMI < 25 Kg/m2) in a case-control study. Additionally, in a prospective longitudinal study, we measured changes in the frequency of LDN after bariatric surgery (n=36) and tested for associations with metabolic and inflammatory parameters.
Findings
LDN and inflammatory markers were significantly increased in MOP compared to NWC. Transcriptome analysis showed increased neutrophil-related gene expression signatures associated with inflammation, neutrophil activation, and immunosuppressive function. However, LDN did not suppress T cells proliferation and produced low levels of reactive oxygen species (ROS). Circulating LDN in MOP significantly decreased after bariatric surgery in parallel with BMI, metabolic syndrome, and inflammatory markers.
Interpretation
Obesity increases LDN displaying an inflammatory gene signature. Our results suggest that LDN may represent a neutrophil subset associated with chronic inflammation, a feature of obesity that has been previously associated with the appearance and progression of co-morbidities. Furthermore, bariatric surgery, as an efficient therapy for severe obesity, reduces LDN in circulation and improves several components of the metabolic syndrome supporting its recognized anti-inflammatory and beneficial metabolic effects.
Funding
This work was supported in part by grants from the National Institutes of Health (NIH; 5P30GM114732-02, P20CA233374 – A. Ochoa and L. Miele), Pennington Biomedical NORC (P30DK072476 – E. Ravussin & LSU-NO Stanley S. Scott Cancer Center and Louisiana Clinical and Translational Science Center (LACaTS; U54-GM104940 – J. Kirwan).
Keywords: Obesity, Bariatric surgery, Low-density neutrophils, LDN, Inflammation
Abbreviations: LDN, low-density neutrophils; BMI, body mass index; MOP, morbidly obese patients; NWC, normal-weight controls; CRP, C-reactive protein; FGF-23, fibroblast growth factor-23; G-CSF, granulocyte colony-stimulating factor; ROS, reactive oxygen species; MetS, metabolic syndrome; T2D, type-2 diabetes; WBC, white blood cells; MDSC, myeloid-derived suppressor cells; PMN, polymorphonuclear neutrophils; HDN, high-density neutrophils; PMN-MDSC, granulocytic or polymorphonuclear MDSC; %WL, percent weight loss; %EBWL, percent excess weight loss; FDR, false discovery rate; DEG, differentially expressed genes; ALPL, alkaline phosphatase
Research in context.
Evidence before this study
Obesity is characterized by chronic low-grade inflammation that has been associated with the pathophysiology of obesity-related co-morbidities. However, the mechanisms underlying this condition and the immune cell mediators remain incompletely understood. Myeloid cells including macrophages, and neutrophils have a critical role in inflammation. Neutrophils can switch phenotypes and display distinctive transcriptional programs and functions depending on the tissue microenvironment. LDN are a subset of neutrophils that are increased in several inflammatory conditions including sepsis, graft vs. host disease, autoimmunity, infectious diseases, and cancer. Therefore, LDN may also play a significant role in the inflammation in obesity and its associated co-morbidities.
Added value of this study
This study demonstrates the increase of LDN with a pro-inflammatory gene signature in patients with severe obesity. LDN decrease following bariatric surgery, in parallel with a decline in inflammatory and metabolic syndrome markers. These findings suggest LDN could contribute to the pathophysiology of obesity and should be further studied to determine their impact on metabolic dysfunction and inflammation in obesity and its role in facilitating the progression of co-morbidities.
Implications of all the available evidence
LDN may represent a novel immunological link to explain how obesity establishes a chronic inflammatory condition that promotes co-morbidities. The molecular and functional characterization of LDN may help determine if LDN serve as a clinical marker to monitor inflammatory status in obesity and response to bariatric surgery. Furthermore, further research is required to determine if LDN have a prognostic value to identify patients with higher risk for developing associated co-morbidities including cardiovascular disease, type-2 diabetes (T2D), and even cancer.
Alt-text: Unlabelled box
Introduction
Obesity is a major risk factor for the development of T2D, hypertension, ischemic stroke, non-alcoholic fatty liver disease, inflammatory arthritis, and at least 13 types of cancer.1,2 Chronic inflammation has been proposed as a common process in the pathophysiology of obesity-associated co-morbidities; however, the mechanisms underpinning low-grade chronic inflammation in obesity are still incompletely understood. Bariatric surgery, an intervention initially intended for weight loss, is known to improve metabolic dysfunction and lower the risk for the development of obesity-related cancers.3 Also, recent studies have shown that bariatric surgery significantly reduces several inflammatory mediators such as cytokines, hormones, and acute phase reactants,4,5 most likely as a result of the resolution of metabolic disturbances.6 However, much less is known about inflammatory cells. Prior research has shown that different stages of adiposity (overweight, obesity, and severe obesity) favor the accumulation of myeloid cells including macrophages,7 and neutrophils.8,9 Obesity alters the cellular architecture of the hematopoietic stem cell compartment which increases the number of early granulocyte and macrophage progenitors,10 including myeloid-derived suppressor cells (MDSC11,12). A balance of pro- and anti-inflammatory functions of these myeloid cells may determine the degree of inflammation. Therefore, understanding the biological role of different inflammatory cells and their gene expression signatures may provide further insight into the mechanisms by which chronic inflammation contributes to health risks in obesity.
Although the effect of obesity in the neutrophilic compartment of myeloid cells is incompletely understood, recently was shown that early weight loss after laparoscopic sleeve gastrectomy was accompanied by a significant reduction in absolute polymorphonuclear neutrophils (PMN) in peripheral blood.13 Given that PMN are a clinical indicator of systemic inflammation, this important finding suggests the main role of neutrophil subsets in the pathophysiology of obesity. In the absence of disease, circulating neutrophils are mainly comprised of mature high-density cells (HDN). Acute or chronic inflammation increases neutrophil heterogeneity,14, 15, 16 primarily characterized by the expansion of circulating LDN, which co-purify with the mononuclear cell fraction when separated over density gradients (≤ 1.077 specific gravity). LDN consist of two subsets, immature LDN resulting from early neutrophil progenitors released from hematopoietic sites, and mature cells derived from HDN that have degranulated in response to certain stimuli. Their functions vary according to physiological and pathological settings.14,15 LDN have shown different functional phenotypes including pro-inflammatory, antigen-presenting, and immunosuppressive functions depending on the disease in which they are found. Pro-inflammatory LDN have been described in autoimmunity and infection,15 while LDN with inducible immunosuppressive activity have been identified in septic shock.17 Granulocytic or polymorphonuclear MDSC (PMN-MDSC), a type of immature LDN, are frequently increased in conditions associated with chronic inflammation.18 In cancer, where PMN-MDSC have been mostly studied, they are characterized by a strong and spontaneous ability to suppress T cell function through several mechanisms including the depletion of arginine by Arginase 1, and the production of ROS.19
Given the important role of LDN in different inflammatory diseases, we tested the hypothesis that LDN were increased in patients with severe obesity as an indicator of inflammation and analyzed the expression of genes to determine pathways indicating their potential function. We also assessed the effect of weight loss through bariatric surgery on LDN in circulation and examined their possible association with markers of metabolic syndrome and inflammation.
Methods
Study cohorts and samples
Eligibility criteria for patients included having severe obesity (BMI > 40 kg/m2) and being candidates for bariatric surgery at Ochsner Medical Center between 2017 and 2020. Participants presenting clinical symptoms of acute or chronic systemic inflammation, cancer, acute infections, tuberculosis, autoimmune disease, or acquired immunodeficiency syndrome (AIDS), as well as patients receiving anti-inflammatory medications (glucocorticoids or non-steroidal anti-inflammatory drugs) were excluded. We powered our study based on a comparison of levels of Myeloid-derived suppressor cells (MDSC) between obese and non-obese groups reported by Bao et al.11 as this was the primary aim of the grants that funded the original research. While doing this initial study, we found significant differences in LDN; therefore, we did not change the power calculations to justify sample size to detect hypotheses related to LDN.
The initial case-control study included 27 MOP and 20 NWC. The demographic distribution (sex, race, age) was similar between both groups, with most individuals being white Caucasian women in their early forties. All MOP were candidates for bariatric surgery, had undergone a 2-weeks pre-operative dietary regimen. The pre-op diet is characterized by low fat, low carbs, high protein (80–120 g protein), and a low-calorie diet (600–800 cal per day) aimed to decrease the size of the liver and abdomen to lower complication rates. Relevant clinical data, such as current treatment for metabolic dysfunction was collected from the patients’ medical records and self-reported by NWC. Some MOP were also on treatment to control hypertension (48.1%), hypertriglyceridemia (11.1%), hypercholesterolemia (7.4%), and/or hyperglycemia (33.3%) using one or more of the following: calcium channel blockers (Amlodipine), beta-blockers (Bystolic, Metoprolol, Bisoprolol, Carvedilol), angiotensin-converting enzyme (ACE) inhibitors (Lisinopril, Enalapril), angiotensin II receptor antagonists (Losartan), diuretics (combination of triamterene and hydrochlorothiazide, Chlorthalidone, Hydrochlorothiazide), fenofibrate, statins (Atorvastatin, Pravastatin), metformin, insulin aspartate, insulin glargine, Humulin, sulfonylureas, canagliflozin, or liraglutide.
The prospective longitudinal study was performed with 36 MOP equally distributed between African Americans and Caucasians, that were evaluated at baseline (time of surgery) and 3- and 6-months post-op. Clinical and biochemical parameters of the metabolic function are shown in detail in Table 1. At baseline, twenty-five MOP (9/36) were having treatment for T2D, 55.5% (20/36) for hypertension, and 8.3% (3/36) for dyslipidemia. The most common type of bariatric surgery performed was sleeve gastrectomy (32/36 patients) followed by Roux-en-Y gastric bypass (RYGB; 4/36 patients). Patient data, clinical information, and blood samples were collected on the day of surgery before receiving anesthesia (baseline), and at 3 and 6 months following bariatric surgery. Omental adipose tissue (± 1 g) was collected from MOP during bariatric surgery and from 6 NWC undergoing elective hernia repair surgical intervention. The demographic and clinical characteristics of all participants are provided in Table 1.
Table 1.
Demographic, BMI, metabolic disorders and biochemical characteristics of study subjects.
| Case-control study |
Prospective longitudinal study |
||||
|---|---|---|---|---|---|
| NWC (N=20) | MOP (N=27) | MOP (N=36) | Post-operative (N=36) |
||
| 3-months | 6-months | ||||
| Gender ratio (Female/male) | 15/5 | 18/9 | 34/2 | ||
| p = 0.75A | |||||
| Race/ethnicity: | 18/36 | ||||
| African American | 1/20 (5%) | 2/27 (7.4%) | |||
| Caucasian | 19/20 (95%) | 25/27 (92.6%) | 18/36 | ||
| p = 1A | |||||
| Age (years); median [25/75- IQR] | 43 [35–49] | 39 [36–45] | 44 [37–51] | ||
| p = 0.66A | |||||
| Weight (pounds); median [25/75- IQR] | 133.1 [119.5–163.3] | 290 [268–332] | 273.5 [249.3–316.3] | 231.5 [215.8–270.8] | 217.5 [202.3–244.8] |
| p < 0.0001A | p < 0.0001B | p < 0.0001C p < 0.001D | |||
| BMI (Kg/m2); median [25/75- IQR] | 22.7 [21.35–25.95] | 47.5 [41.8–51.8] | 46.8 [42.7–50.8] | 39.8 [36.7–43.6] | 37.1 [34.9–40.2] |
| p < 0.0001A | p < 0.0001B | p < 0.0001C p < 0.001D | |||
| Percent of weight loss (%WL); median [25/75- IQR] | 14 [11–16] | 19 [15.3–23] p < 0.0001D | |||
| Percent excess body weight loss (%EBWL); median [25/75- IQR] | 28.8 [24.1–36.1] | 42.6 [32.7–49.6] p < 0.0001D | |||
| Surgery: | 32/36 (88.9%) | ||||
| Sleeve gastrectomy | |||||
| RYGB | 4/36 (11.1%) | ||||
| % Subjects with Metabolic Syndrome*, n/N (%) | 1/12 (8.3%) | 23/27 (85.2%) | 30/36 (83.3%) | 21/35 (60%) | 18/33 (54.5%) |
| p < 0.0001A | |||||
| Subjects pre-diagnosed with T2D, having treatment, and/or having fasting plasma glucose ≥100 mg/dL, n/N (%) | 0/13 (0%) | 12/27 (44.4%) | 15/36 (41.2%) | 8/35 (22.9%) | 9/33 (27.3%) |
| p = 0.004A | |||||
| Subjects pre-diagnosed with hypertension, having treatment, and/or having systolic blood pressure ≥ 130 or diastolic ≥ 85 mmHg, n/N (%) | 3/13 (23%) | 23/27 (85.2%) | 30/36 (83.3%) | 22/36 (61.1%) | 24/36 (66.7%) |
| p = 0.0002A | |||||
| Subjects in treatment for hypercholesterolemia, had total cholesterol ≥ 100 mg/dL, or low levels of HDL-cholesterol, n/N (%) | 8/13 (61.5%) | 23/27 (85.2%) | 27/36 (75%) | 28/35 (80%) | 19/32 (59.4%) |
| p = 0.008A | |||||
| Subjects pre-diagnosed with hypertriglyceridemia, having treatment and/or had triglyceride levels ≥ 150 mg/dL, n/N (%) | 2/13 (16.7%) | 8/27 (29.6%) | 9/36 (25%) | 8/36 (22.2%) | 7/36 (19.4%) |
| Metabolic parameters (median [25/75- IQR]) | |||||
| Fasting Glucose, mg/dL | 85.5 [77.3–91] | 78 [73.8–89] | 78.5 [69.5–103] | 86 [81.3–92] | 85.5 [80–92.8] |
| Insulin, uU/mL | 6.05 [1.4–13.4] | 9.8 [6.5–13.2] | 7.5 [3.7–11.7] | 8.9 [6.6–16.9] | 8.8 [6.4–15.1] |
| p = 0.09A | |||||
| HOMA-B | 90.2 [63.8–148.1] | 146.1 [93.3–191] | 94.3 [59.3–179.6] | 103.5 [87.4–162.8] | 114.5 [89.8–139.7] |
| p = 0.09A | |||||
| HOMA-S | 126.8 [67.25–264.5] | 82.6 [61.95–121.2] | 104.8 [68.3–218.9] | 94.6 [51.7–119.8] | 91 [61.1–123.9] |
| HOMA-IR index | 0.79 [0.4–1.6] | 1.21 [0.8–1.6] | 0.96 [0.5–1.5] | 1.1 [0.84–2.1] | 1.1 [0.8–1.9] |
| Total cholesterol, mg/dL | 197 [170.5–224.3] | 146.5 [115.8–163.3] | 154 [116.5–167.5] | 168 [140.5–200.5] | 175 [149–199] |
| p < 0.0001A | p < 0.01B | p < 0.0001C | |||
| LDL-cholesterol, mg/dL | 108.1 [98.2–137.2] | 83.7 [74.9–104.1] | 87.9 [66.7–108.9] | 105.9 [78.9–121.3] | 105.1 [74–118.4] |
| p = 0.01A | p < 0.01B | p < 0.01C | |||
| HDL-cholesterol, mg/dL | 61 [49.5–74.4] | 37.05 [31.25–44.25] | 42.9 [34–50.6] | 47.1 [41.5–56.5] | 56.9 [45.7–64.5] |
| p < 0.0001A | p < 0.0001C p < 0.01D | ||||
| Triglycerides, mg/dL | 66 [50.5–130.5] | 90 [73.8–114] | 85 [65–106] | 80 [59.5–105.5] | 69 [52–107] |
| p = 0.46A | |||||
| Ratio Cholesterol Total/HDL | 3.2 [2.8–4.2] | 3.8 [3.2–4.8] | 3.3 [2.7–4.2] | 3.5 [3–4.2] | 3.1 [2.8–3.7] |
| p = 0.15A | |||||
| Ratio HDL/LDL | 1 [0–1] | 0 [0–1] | 1 [0–1] | 0 [0–1] | 1 [0–1] |
| p = 0.27A | |||||
| Ratio TG/HDL | 1 [0.25–3] | 2 [2–4] | 2 [1–3] | 1.5 [1–3] | 1 [0–1] |
| p = 0.02A | p < 0.01C | ||||
*Metabolic syndrome thresholds established by the International Diabetes Federation (IDF, consensus 2006) were used to estimate the patients with metabolic syndrome. Those having 3 or more parameters according to the IDF criteria were identified as having MetS. Wilcoxon rank-sum test was used to compare continuous covariates between NWC and MOP for the case-control study. Fisher's exact tests were used to compare distributions of categorical covariates in these groups. Superscript A for p-values comparing pre-op MOP with NWC. Paired Wilcoxon signed-rank tests were used to perform pairwise comparisons of continuous variables (Age, Weight, BMI) and chi-squared tests of independence were used for categorical variables (Gender, Race) for the Prospective longitudinal study. Superscript B and C for p-values comparing 3-months and 6-months, respectively, with the matched pair pre-op patient. Superscript D for p-value comparing 3-months with the matched pair 6-months post-op. Significance level was considered when p ≤ 0•05. NWC indicates Normal Weight Control; MOP, Morbidly Obese Patient; N represents the total number of participants evaluated; n/N, fraction of the population with specific characteristic; BMI, body mass index; RYGB, Roux-en-Y gastric bypass; HOMA-B, beta-cell function; HOMA-S, insulin sensitivity; HOMA-IR, homeostasis model assessment insulin resistance; LDL, low-density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol. Values are shown as n/N (%), median with 25 and 75 interquartile ranges (IQR).
Anthropometric, clinical, and biochemical measurements
Anthropometric measurements: BMI was calculated as weight (kg)/height in meters squared (m2). Postoperative weight loss was determined relative to weight and BMI on the day of surgery. Percentage weight loss (%WL20; and percentage excess BMI loss (%EBWL21;) were estimated as follow: %WL= [(preoperative weight –weight at time of follow-up post-op) / Preoperative weight] x 100, and %EBWL = [preoperative BMI – BMI at time of follow-up]/[preoperative BMI – 25 Kg/m2 as ideal BMI]).
Metabolic Parameters and Metabolic Syndrome (MetS): Blood samples were collected from study subjects using Plastic Serum Tubes, Silicone-Coated tubes (BD Vacutainer, Thermo Fisher Scientific; Waltham, MA, USA) and processed within 2 h of collection. Serum aliquots were prepared and stored at -80°C until used. Metabolic parameters were measured only in fasting serum. Analytes included triglycerides, high-density lipoprotein (HDL)- and low-density lipoprotein (LDL)-cholesterol, glucose, and insulin, which were determined by an automated chemistry analyzer in the Biochemical Core at Pennington Biomedical Research Center of LSU (PBRC). Glucose and insulin measurements were used to calculate Homeostasis Model Assessment Insulin Resistance (HOMA-IR), beta-cell function (HOMA-B), and insulin sensitivity (HOMA-S), using the HOMA Calculator v2·2·3 (Diabetes Trials Unit, University of Oxford http://www.dtu.ox.ac.uk/homa).
MetS was defined using the International Diabetes Foundation criteria shown below (IDF, 2006). Individuals having (i) BMI > 30 kg/m², plus any two or more of the following characteristics: (ii) fasting plasma glucose ≥ 100 mg/dL or previously diagnosed T2D; (iii) triglyceride levels ≥ 150 mg/dL or having treatment for hypertriglyceridemia; (iv) total cholesterol ≥ 200 mg/dL or low levels of HDL-cholesterol (< 40 mg/dL in men and < 50 mg/dL in women) or treatment for dyslipidemia; and (v) previously diagnosed as hypertensive, having treatment for hypertension or having blood pressure > 130/85 mmHg. MetS score was designated from cero (0) to five (5) where 0 indicated an individual having a BMI ≤ 29.9 with metabolic parameters under normal levels accordingly with the IDF criteria.
Inflammatory and obesity markers
Blood samples were collected from the study subjects using vacutainer K2EDTA-containing tubes (BD vacutainer, product code: 366643). Plasma specimens stored at -80°C were used to measure cytokines using a customized multiplex array containing the obesity markers Ghrelin, and Leptin, the inflammatory and insulin resistance marker fibroblast growth factor-23 (FGF-23), and the cytokine granulocyte colony-stimulating factor (G-CSF/CSF-3), which is a major regulator of neutrophilic granulocytes (Thermo Fisher Scientific Inc.; Waltham, MA, USA). The assay was run on Luminex Magpix instrument (MilliporeSigma, Burlington, MA, USA). High-sensitivity C-Reactive Protein (CRP) test was assessed in serum at the Biochemical CORE at PBRC-LSU.
Flow cytometry analysis
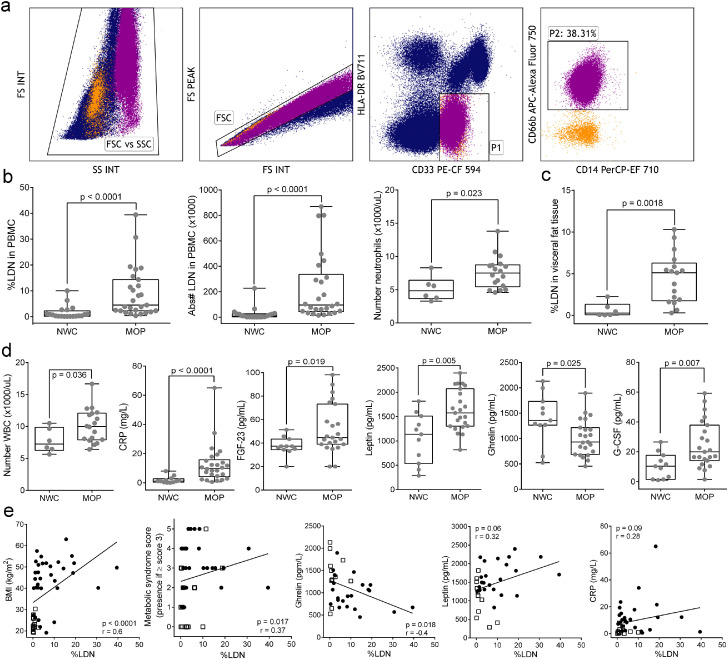
PBMC were isolated by centrifugation over Ficoll-Paque Premium (p = 1.078 g/mL; GE Healthcare, Thermo Fisher Scientific; Waltham, MA) gradient. Multicolor flow cytometry analysis of LDN included fluorochrome-conjugated antibodies for labeling the surface markers CD33-PE-CF594 (BD Biosciences; San Jose, CA; Cat.no: 562492), CD14-PerCP-eFluor 710 (eBioscience, Thermo Fisher Scientific Inc.; Waltham, MA; Cat.no: 46-0149-42), CD66b-APC-AlexaFluor 750 (Beckman Coulter Inc.; Brea, CA; Cat.no: B08756), and HLA-DR-BV711 (BD Biosciences, San Diego, CA, USA; Cat.no: 563696). Tissue infiltrating immune cells were isolated from omental adipose tissue collected at the time of surgery. After removing visible blood vessels and digestion with collagenase II (Sigma-Aldrich Corp. St. Louis, MO; Cat.no: C5138), the single-cell suspension was stained with the previous antibody panel for flow cytometry and CD45-APC (BD Biosciences, San Diego, CA, USA; Cat.no: 46-0149-42). To minimize spectral overlapping, fluorescence-minus-one (FMO) control was used to determine the positive and negative cellular populations needed to establish the multicolor settings. Unstained samples were always included as a negative control to identify autofluorescence and localize the negative populations. All samples were run on a Gallios Flow Cytometer configured with 3 lasers and 8 colors (Beckman Coulter, Brea, CA). All samples were run with the same voltage settings. Data were analyzed using Kaluza software (Beckman Coulter) as is shown in Figure 1a.
Figure 1.
Analysis of LDN and levels of inflammatory and metabolic parameters in circulation. PBMC were isolated from the peripheral blood of all participants by density gradient centrifugation and LDN were analyzed by flow cytometry. (a) Gating strategy to identify LDN from PBMC after plotting FSC-PEAK and FSC-INT for doublet exclusion (FSC gate). LDN were defined as CD33dim and HLA-DRneg (P1) and CD66b+CD14− (P2). (b) Percentage and absolute number of LDN in peripheral blood of MOP (n=27) and NWC (n=20), and absolute counts of polymorphonuclear neutrophils in whole blood cells of NWC (n=6) and MOP (n = 18). (c) Neutrophil infiltration into visceral adipose tissue from NWC undergoing hernia repair (n=6) and MOP (n=17). Minimum to Maximum numbers are indicated in the box and whiskers plot and each dot represents an individual subject. Wilcoxon rank-sum test was used to perform comparisons of the dependent variable distributions within NWC and MOP groups. (d) Levels of CRP (in serum), FGF-23, leptin, ghrelin, and G-CSF (in plasma). (e) Correlation analysis between %LDN and BMI, Metabolic syndrome score, ghrelin, leptin, and CRP. Symbols are as follows: MOP are represented with black circles and NWC with white squares. The results are presented as Spearman's correlation coefficient (r) and p-value. Results were considered significant with a p-value ≤ 0·05. LDN indicates low-density neutrophils; PBMC, peripheral blood mononuclear cells; NWC, Normal-weight controls; MOP, morbidly obese patients; CRP, C-reactive protein; FGF-23, fibroblast growth factor 23; BMI, body mass index; G-CSF, granulocyte colony-stimulating factor; WBC, whole blood cells.
Isolation of LDN and T cells
LDN were isolated by labeling PBMC with anti-CD66b-APC (BD Biosciences; Cat.no: 561650) followed by selection with the Human Positive Selection kit using dextran-coated magnetic particles (Stemcell Technologies, Cambridge, MA; cat.no: 17681). The purity of the LDN ranged from 92%–99% as measured by flow cytometry. CD66b-APC depleted PBMC fraction was further used to isolate CD3+ T cells with a T-cell negative isolation kit (Stemcell Technologies, Cambridge, MA; Cat.no: 17951). Purity ranged between 95% and 99%.
T cell proliferation and suppression assay
T cells were labeled with 1 µM Carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes, Life Technologies; Cat.no: V12883) at 37°C for 10 min, followed by quenching in 10% FBS-RPMI 1640 (Lonza, Cat.no: 12-167F) for an additional 10 min. T cells (1 × 105) were plated in 96-well round-bottom plates bound with affinity-purified antibody goat anti-mouse IgG(H+L) Human Serum Adsorbed (10ug/mL; SeraCare's KPL, Mildford, MA; Cat.no: 5210-0185), soluble anti-CD3 (0·5 µg/mL; Clone OKT3 (Functional Grade); eBioscience; Cat.no: 16-0037-85) and soluble anti-CD28 antibodies (0·25 µg/mL; Clone L293; BD Biosciences; Cat.no: 348040). Autologous LDN (1 × 105) were added in a final volume of 200 uL for a co-culture ratio of 1:1 (T-cells:LDN). Positive and negative controls included T cells alone with or without anti-CD3 and anti-CD28 antibodies, respectively. All cultures were incubated for 72 h at 37°C, 5% CO2 after which proliferation was tested by CFSE fluorescence dilution using a Gallios flow cytometer. Data are expressed as a percent proliferation relative to positive T cells control, calculated as follows: number of proliferating T-cells in co-culture T-cells:LDN x 100 / number of proliferating T cells in positive control represented as T-cells:LDN ratio 1:0.
RNA preparation
Total RNA was extracted from enriched LDN by using the phenol method with Trizol followed by miRNeasy Mini Kit (Qiagen, Germany; Cat.no: 1038703), according to the manufacturer's protocol including an additional step with DNase. RNA concentration was tested by Qubit RNA HS Assay kit in the Qubit fluorometer (Invitrogen, Thermo Fisher Scientific; Cat.no: Q32855) and RNA integrity was assessed using RNA 6000 Pico Kit on the Agilent BioAnalyzer 2100 (Agilent Technologies, Santa Clara, CA. Cat.no: 5067-1513). RNA yield from neutrophils is limited (i.e., 10–20 times less than monocytes), hence, only those samples that showed satisfactory amount and quality (5 NWC and 8 MOP) were used for RNA-Seq.
Transcriptional profile analysis
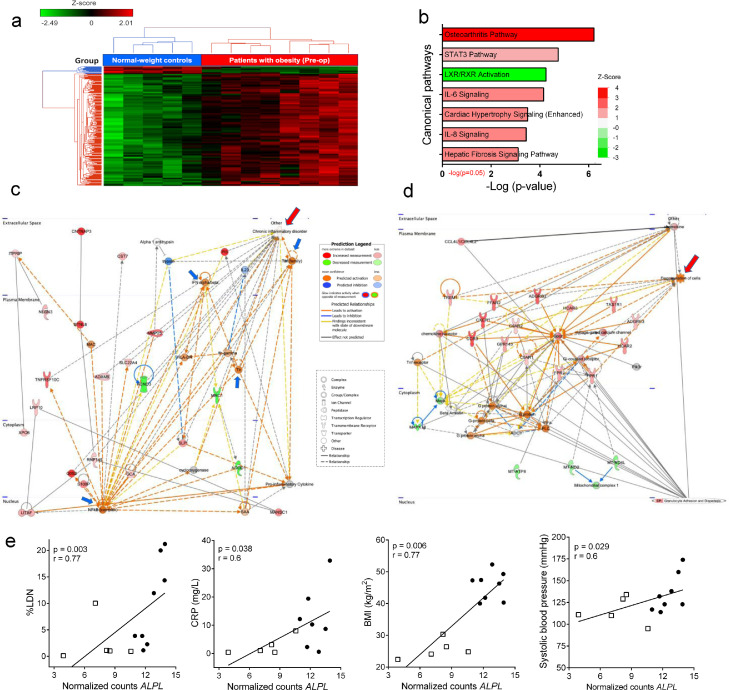
RNA sequencing was done in the Translational Genomics Core (TGC) of the Stanley S. Scott Cancer Center (LSUHSC, New Orleans, LA) as recently described.22 Briefly, paired-end libraries (2 × 75) were prepared (600 ng per sample), validated, and normalized. Isolated RNA was used for whole transcriptome sequencing using the NextSeq 500/550 High Output Kit v2·5, 150 cycles by Illumina (San Diego, CA). NextSeq500 FASTQ output files were uploaded to Partek Flow, contaminants (rDNA, tRNA, mtrDNA) were removed using Bowtie2 (version 2·2·5) and the unaligned reads were aligned to STAR (version 52·5·3a) using the hg19 version of the human genome as a reference. Aligned reads were quantified to the hg19-Ensemble Transcript release 75 and normalized by log2 transformation. Normalized counts were used to determining the differential gene expression between MOP and NWC as assessed by false discovery rate (FDR) correction for multiple hypothesis testing using Gene Specific Analysis (GSA) in Partek Flow. Genes with an FDR ≤ 0·05 and fold change (FC) > 2 were considered differentially expressed between groups. Significantly differentially expressed genes were plotted as heat-map with hierarchical clustering using the embedded algorithm in Partek Flow for hierarchical unsupervised comparison of the samples using Euclidian distance. The values of expression are visualized with colors ranging from red (high expression) through black (intermediate expression) to green (low expression). To determine the distribution of the samples we ran principal component analysis (PCA) in Partek Flow using log2 transformed data. We used the option in which all features are given the same weight in the analysis (mean=0, SD=1) so they contribute equally and not depend on their variance. The variable “Group” (patients vs. controls) was used to identify each sample in the PCA.
Gene interaction and pathway enrichment analysis
We utilized the Ingenuity Pathway Analysis software (IPA version 01-16, QIAGEN Inc., https://www.qiagenbioinformatics.com/products/ingenuitypathway-analysis) to predict gene interactions and biological mechanisms of the functional differences between patients with obesity and normal-weight controls.23 An FDR rate of 10% was used as a cutoff for differentially expressed genes to be considered for analysis (FDR 10%, FDR-unadjusted p < 0·05, n = 390). False discovery rates were considered as a means of multiple-testing correction of p-value estimates. The relative log fold-change values between MOP and NWC groups were used as input for IPA to measure differences among known gene pathways and networks in the Ingenuity Knowledge Base. Quantified enrichment of genes in canonical pathways, molecular and cellular functions, and predicted de novo networks are reported here, based on expected interactions of differentially expressed genes (DEG). All results reported are considered statistically significant (threshold of FDR unadjusted p<0·05), and for de novo networks, scores of 3 or greater indicate with > 99% confidence that the network was not created by random chance.
Statistical analysis
R statistical software (version 3·5·3) and GraphPad Prism v6 were used for statistical analysis. A Wilcoxon rank-sum test was used to compare continuous covariates between NWC and MOP. Fisher's exact tests were used to compare distributions of categorical covariates in these groups. To determine whether continuous variables values change significantly over time in MOP following surgery, a paired Wilcoxon signed rank-test was used to compare at different time points 3-, and 6-months post-op. All the described statistical approaches avoid parametric assumptions of normality to analyze group differences considering that most dependent variables violated normality based on a Shapiro-Wilks test. Continuous values are presented as median with 25 and 75 interquartile ranges (25/75-IQR). Differences in time of nominal variables for MOP were analyzed with McNemar's test. Due to the equal distribution of sex, age and race among the groups, no statistical corrections were made to account for potential confounding. Stratified analysis for the main outcome of interest (%LDN) was performed for sex, race and having treatment for metabolic dysfunction (hypertension, T2D, hypercholesterolemia, hypertriglyceridemia). Significance level was considered when p≤0·05. Correlation analyses were carried out to determine the relationship between %LDN with different parameters. As the raw results did not show a normal distribution, Spearman correlation was performed. The results are presented as Spearman's correlation coefficient (r). Variables that were significantly correlated (p≤0·05) by Spearman correlation were introduced into linear regression models to find the positive or negative association between variables. GraphPad Prism v6 was used for graphing data.
Ethics
The study protocol was approved by the Institutional Review Boards at Louisiana State University Health – New Orleans (IRB #9371, #9838, and #1187) and the Ochsner Clinic Foundation (IRB # 2016.186). The protocol was conducted following the principles of the Declaration of Helsinki. Written informed consent was obtained from each participant before inclusion in the study and enrolled participants were de-identified.
Role of the funding source
None of the funding sources had any role in the study design, collection, analysis, and interpretation of data. The corresponding author held the final responsibility for the decision to submit the paper for publication.
Results
Increased LDN in circulation and infiltrating neutrophilic cells in visceral adipose tissue in patients with morbid obesity
We initially compared the percentage and the absolute number of circulating LDN in MOP (n=27) and NWC (n=20) in the case-control study. As shown in Figure 1b, both percent (median (25/75-interquartile range [25/75-IQR]) = 4.5 [2.28-14.35]) and absolute number (median [25/75-IQR] = 95.3 × 103 [43.7 × 103–336.5 × 103]) of circulating LDN were significantly increased (Wilcoxon rank-sum p<0·0001) in MOP compared to NWC (median [25/75-IQR] = 0.51 [0.17-2.27; 9.4 × 103 [2 × 103–25.9 × 103]). When patients were stratified by gender, the comparison of LDN for men (9 MOP and 5 NWC) and women (18 MOP and 15 NWC) separately showed more statistical significance in women (Wilcoxon rank-sum p<0·0001) than in men (p=0·04 [Wilcoxon rank-sum test]; Supplementary Figure 1a). As certain treatments aimed to treat metabolic dysfunction may have anti-inflammatory properties, we also stratified MOP into those having T2D treatment, receiving anti-hypertensive treatment, or being treated for hypercholesterolemia or hypertriglyceridemia, and analyzed the effect on %LDN. Although there is a lower %LDN in patients undergoing therapy for metabolic dysfunction compared to those not having medications, this is not statistically significant, and both groups of patients showed significantly higher numbers of LDN compared to NWC (Supplementary Figure 1b–1e). Additionally, we also found that PMN counts in white blood cells (WBC) were increased in MOP (n=18) compared to NWC (n=6; p=0.02 [Wilcoxon rank-sum test] Figure 1;d). This finding suggests that the number of LDN could be the result of enhanced demand for the generation and release of PMN from the bone marrow to peripheral blood upon systemic chronic inflammation. Surprisingly, there is no correlation between %LDN and absolute PMN counts (Supplementary Figure 1f), suggesting that the increased subpopulation of LDN is not associated with the accumulation of PMN in WBC and LDN could be specifically expanded in response to the inflammatory obese milieu.
Emerging evidence suggests that neutrophils also accumulate in the visceral adipose tissue of mice on a high-fat diet where they contribute to the development of inflammation and insulin resistance.24,25 We, therefore, tested whether neutrophilic cells were increased in adipose tissue in omental adipose tissue collected during bariatric surgery in 17 MOP, and compared it to omental adipose tissue from 6 NWC undergoing elective hernia repair. Neutrophilic cells were significantly increased in omental adipose tissue from MOP (median [25/75-IQR]=5.12 [1.75-6.28]) as compared to NWC (median [25/75-IQR]=0.28 [0.08–1.33] Figure 1;c). The results suggest that the expansion of LDN and increase total PMN counts in peripheral blood in obesity is accompanied by infiltration of neutrophil cells in the adipose tissue as well.
Circulating markers for inflammation including absolute counts WBC, CRP, fibroblast growth factor-23 (FGF-23), and the hormone leptin, were significantly increased in MOP, while ghrelin was decreased. Similarly, G-CSF a regulator of granulopoiesis,26 survival, proliferation, maturation, and functional activation of the neutrophil lineage27 was also significantly increased in MOP (Figure 1d). As expected, BMI was positively correlated with circulating levels of leptin (r=0·5, p=0·002 [Spearman's rank correlation]), CRP (r=0·56, p=0·0002 [Spearman's rank correlation]), and metabolic syndrome (MetS) score (r=0·59, p=0·0001 [Spearman's rank correlation]). In contrast, BMI was negatively correlated with ghrelin (r=-0·35, =0·036 [Spearman's rank correlation]; Supplementary Figure 2). Furthermore, we aimed to determine potential meaningful clinical associations between the percent LDN and metabolic and inflammatory markers in obesity. The results showed a significant positive correlation between %LDN and BMI (=0·6, p < 0·0001 [Spearman's rank correlation]), and metabolic syndrome score (r=0·45, p=0·005 [Spearman's rank correlation]), and negative correlation with ghrelin (Figure 1e). Surprisingly we failed to find a significant association of %LDN with leptin (r=0·32, p=0·06 [Spearman's rank correlation]), CRP (r=0·28, p=0·09 [Spearman's rank correlation]) and circulating G-CSF (r=0·18, p=0·31 [Spearman's rank correlation]). These results suggest a relationship between obesity and circulating numbers of LDN.
Transcriptome analysis reveals increased inflammatory signaling in LDN from patients with obesity
We also evaluated the transcriptome signatures of purified CD66b+ LDN from 8 MOP and 5 NWC (Demographics from those individuals are described in Table 2) using RNA-Seq analysis. Comparison of the gene expression identified differences in the expression of 5855 RNA transcripts (FDR-unadjusted p ≤ 0·05) with 3788 having a fold change (FC) ≥ 2. After FDR correction (FDR ≤ 0.05), 238 genes were still significantly differentially expressed (FC > 3) between the study groups. Of the 238 genes, 223 were up-regulated and 15 down-regulated in LDN from MOP compared to NWC (Supplementary Table S1). The complete list of the top up- or down-regulated protein-coding differentially expressed genes (DEG) is found in Table 3. Unsupervised hierarchical cluster analysis of the most DEG showed a clear separation between MOP and NWC (Figure 2a), demonstrating that LDN clearly differ transcriptionally between MOP and NWC individuals.
Table 2.
Demographics of study subjects for RNA-Seq analysis
| Case-control study |
||
|---|---|---|
| NWC (N = 5) | MOP (N = 8) | |
| Gender ratio (Female/male) | 3/2 | 5/3 |
| Race/ethnicity: | ||
| African American | 0/5 (0%) | 4/8 (50%) |
| Caucasian | 5/5 (100%) | 4/8 (50%) |
| p = 0.1 | ||
| Age (years); median [25/75- IQR] | 51.5 [33–56.5] | 40.5 [35–45.3] |
| p = 0.44 | ||
| Weight (pounds); median [25/75- IQR] | 158.2 [127.8–181] | 276.5 [251.8–288] |
| p = 0.002 | ||
| BMI (Kg/m2); median [25/75- IQR] | 24.8 [23.3–28.4] | 46.8 [40.7–48.8] |
| p = 0.002 | ||
| % Subjects with Metabolic Syndrome*, n/N (%) | 0/5 (0%) | 7/8 (87.5%) |
| p = 0.005 | ||
| LDN (%) | 1.02 [0.53–5.57] | 7.91 [2.66–18.58] |
| p = 0.02 | ||
| LDN (Absolute counts) | 25200 [5128–126621] | 407950 [103103–535607] |
| p = 0.02 | ||
*Metabolic syndrome thresholds established by the International Diabetes Federation (IDF, consensus 2006) were used to estimate the patients with metabolic syndrome. Those having 3 or more parameters according to the IDF criteria were identified as having MetS. Wilcoxon rank-sum test was used to compare continuous covariates between NWC and MOP. Fisher's exact test was used to compare distributions of categorical covariates in these groups. Significance level was considered when p ≤ 0·05. NWC indicates Normal Weight Control; MOP, Morbidly Obese Patient; N represents the total number of participants evaluated; n/N, fraction of the population with specific characteristic; BMI, body mass index; LDN, low-density neutrophils. Values are shown as n/N (%), median with 25 and 75 interquartile ranges (IQR).
Table 3.
Top 10 Significantly Differentially Expressed Genes (DEG)
| Gene symbol | p-value | FDR | FC | Protein name |
|---|---|---|---|---|
| Up-regulated | ||||
| CCDC64B | < 0.0001 | 0.005 | 18 | BICD Family Like Cargo Adaptor 2 |
| ALPL | < 0.0001 | 0.003 | 17 | Alkaline Phosphatase, Tissue-Nonspecific Isozyme |
| PI3 | < 0.0001 | 0.003 | 16 | Peptidase Inhibitor 3 |
| KAZN | < 0.0001 | 0.003 | 16 | Kazrin |
| G0S2 | < 0.0001 | 0.005 | 15 | G0/G1 switch protein 2 |
| CMTM2 | < 0.0001 | 0.003 | 13 | CKLF Like MARVEL Transmembrane Domain Containing 2 |
| KRT23 | < 0.0001 | 0.003 | 13 | Keratin 23 |
| FCGR3B | < 0.0001 | 0.003 | 13 | Low affinity immunoglobulin gamma Fc region receptor III-B |
| RTDR1 | < 0.0001 | 0.007 | 12 | Rhabdoid Tumor Deletion Region Protein 1 |
| NLRP6 | < 0.0001 | 0.007 | 11 | NLR Family Pyrin Domain Containing 6 |
| Down-regulated | ||||
| GCOM1 | < 0.0001 | 0.043 | -6 | GRINL1A Complex Locus 1 |
| NPIPB9 | < 0.0001 | 0.026 | -6 | Nuclear Pore Complex Interacting Protein Family Member B9 |
| ATP1A4 | < 0.0001 | 0.037 | -6 | ATPase Na+/K+ Transporting Subunit Alpha 4 |
| PLS3 | < 0.0001 | 0.040 | -5 | Plastin 3 |
| IRG1 | < 0.0001 | 0.033 | -5 | Aconitate Decarboxylase 1 |
| FLT3 | < 0.0001 | 0.049 | -4 | Fms Related Tyrosine Kinase 3 |
| MT-ND4L | < 0.0001 | 0.042 | -3 | Mitochondria NADH Dehydrogenase Subunit 4L |
FDR indicates false discovery rate; FC, fold change.
Figure 2.
Differential gene expression between MOP and NWC. (a) Dendrogram and Heat-Map for Unsupervised Hierarchical Clustering in 8 MOP and 5 NWC, based on the 238 DEG. Each column represents a MOP or NWC, and each row represents a single gene. Red and green colors denote increased and decreased gene expression, respectively. Data were z-score normalized. In the color bar above the heatmap, red indicates the 8 patients' group, and blue indicates the 5 individuals from the control group. (b) Top significantly enriched canonical pathways (FDR<0.1, FDR-unadjusted p-value<0·05). The significance of the enrichment is shown on the x-axis, and the bars are colored based on the z-score predicted activation (positive z-score, red) or pathway inhibition (negative z-score, green). (c) Top de-novo network predicted by IPA ‘Cell-To-Cell Signaling and Interaction, Dermatological Diseases and Conditions, Organismal Injury and Abnormalities’ enriched by DEG and their potential interplay (score of 37). The most significant specific disease term highlighted in this network is chronic inflammatory disorder (p=4·98E-06, associated with 19 of 35 genes), denoted with a top-right red arrow. (d) Second de-novo network predicted by IPA indicating enrichment with molecules connected to degranulation of cells, indicated with a top-right red arrow. Nodes represent genes and lines show the relationship between genes. Nodes in red are upregulated in MOP versus NWC, and nodes in green are downregulated. The color intensity represents the relative magnitude of change in gene expression. Orange or blue nodes are predicted to be activated or inhibited genes, respectively, based on the downstream state of other molecules in the network. White nodes have a z-score of 0, indicating neither activation nor inhibition, and those in gray have no predicted activity pattern. Lines are solid or dashed to indicate direct and indirect relationships, respectively. Orange lines indicate a relationship leading to activation, blue lines leads to inhibition, and yellow lines indicate that the downstream state of the genes is inconsistent with the expected relationship. Lines in gray show the interaction but have no predicted effect. Blue arrows are indicating TNF, IFN-alfa/beta, NF-kB and TLR nodes. (e) Correlation between RNA normalized counts of ALPL with %LDN, CRP, BMI, and systolic blood pressure. MOP are represented with black circles and NWC with white squares. The results are presented as Spearman 's correlation coefficient (r) and p-value. Results were considered significant with a p-value ≤ 0.05. MOP indicates morbidly obese patients; NWC. Normal-weight control; DEG, differentially expressed genes; IPA, ingenuity pathway analysis; TNF, tumor necrosis factor; IFN; interferon; NF-kB, nuclear factor kappa B; TLR, toll-like receptor; ALPL, alkaline phosphatase; LDN, low-density neutrophils; CRP, C-reactive protein; BMI, body mass index; FDR, false discovery rates.
To further elucidate possible functional consequences of the transcriptome profile of LDN in obesity, we used the Ingenuity Pathway Analysis (IPA) to compare the DEG (FDR-unadjusted p≤ 0·05; FDR ≤ 10%). The top canonical pathways are shown in Figure 2b and Supplementary Table S2. Pathways showing a predicted positive functional consequence included osteoarthritis, signal transducer and activator of transcription 3 (STAT3), interleukin (IL)-6, and IL-8 (z-score >1) and negative for liver X receptor/retinoid X receptor (LXR/RXR) activation (z-score=-3). The functional enrichment analysis highlighted the overrepresentation of genes that codify CXC-chemokine receptors 1 and 2 (CXCR1 and CXCR2), which are the major chemokine receptors on neutrophils, largely responsible for neutrophil recruitment into specific tissues.28 Other DEGs shared by the top pathways included IL1R1, IL1R2, IL18RAP, MMP9, PTGS2, CEBPB, and CXCL1 (Supplementary Table S2), which are also differentially expressed by activated neutrophils or PMN-MDSC.29,30
IPA was also used to construct de novo networks based on gene interactions. As indicated in Figure 2c-c, the top two enriched networks involved pro-inflammatory neutrophil functions. Specifically, the top de-novo network indicated the activation of pro-inflammatory pathways induced by Toll-like receptor (TLR), NF-κB complex, interferon (IFN), and tumor necrosis factor (TNF) signaling (blue arrows in Figure 2c). Here, the analysis highlighted molecules known to be important in pro-inflammatory neutrophil functions including peptidase inhibitor 3 (PI3), Matrix Metallopeptidase 25 (MMP25), G0/G1 Switch 2 (G0S2,) and S100 proteins, all connected with chronic inflammatory disorders (Figure 2c, red arrow). The second de-novo network predicted by IPA was associated with cellular degranulation (Figure 2d, red arrow) where molecules essential for chemotaxis and neutrophil activation were highlighted, including CXCRs, C5a receptor-1 (C5aR1), and several G protein-coupled receptors such as formylpeptide receptors (FPRs) and free fatty acid receptors (FFARs).
The RNA-Seq-based transcriptome analysis also showed a significant up-regulation (FDR ≤ 5%) in genes associated with neutrophil activation in LDN from MOP, including ALPL, FCGR3B, and CXCR1 (Table 4), suggesting a gene signature of functionally active cells. Interestingly, alkaline phosphatase (ALPL) was recently identified as a neutrophil activation marker in response to obesity31 where it was positively associated with systolic blood pressure as a risk factor for cardiovascular disease (CVD). We found a positive correlation between ALPL mRNA expression and the percent circulating LDN (r=0·78, p=0·003 [Spearman's rank correlation]), serum levels of CRP (r=0·6, p=0·038 [Spearman's rank correlation]), BMI (r=0·77, p=0·0006 [Spearman's rank correlation]) and confirmed the previous reports of its association with systolic blood pressure (r=0·61, p=0·029 [Spearman's rank correlation] Figure 2;e).
Table 4.
Significantly differentially expressed genes involved in activation of neutrophils
| Gene symbol | p-value | FDR | FC |
|---|---|---|---|
| ALPL | 7.81E-07 | 0.003 | 17 |
| FCGR3B | 1.96E-07 | 0.003 | 13 |
| CXCR1 | 1.87E-06 | 0.004 | 9 |
| MME | 9.89E-06 | 0.007 | 9 |
| GPR97 | 2.17E-06 | 0.004 | 9 |
| MMP25 | 2.02E-06 | 0.004 | 8 |
| MGAM | 4.58E-06 | 0.005 | 8 |
| LRG1 | 1.77E-05 | 0.009 | 8 |
| CXCR2 | 1.60E-06 | 0.004 | 7 |
| PLIN5 | 2.02E-05 | 0.010 | 7 |
| CEACAM3 | 2.57E-05 | 0.011 | 6 |
| EGR3 | 9.56E-06 | 0.007 | 6 |
| TNFAIP6 | 9.88E-05 | 0.025 | 5 |
| IL18RAP | 7.92E-05 | 0.021 | 5 |
| MMP9 | 1.52E-05 | 0.008 | 5 |
| FPR2 | 5.36E-06 | 0.005 | 5 |
| CXCL1 | 4.89E-05 | 0.017 | 5 |
| FPR1 | 5.40E-06 | 0.005 | 5 |
| CYSTM1 | 3.51E-04 | 0.044 | 4 |
| HSPA6 | 2.83E-04 | 0.040 | 4 |
| ADAM8 | 1.33E-04 | 0.029 | 4 |
| EMR3 | 2.29E-04 | 0.036 | 4 |
| NCF4 | 2.59E-04 | 0.038 | 3 |
| PADI2 | 2.40E-04 | 0.037 | 3 |
| VNN2 | 2.81E-04 | 0.040 | 3 |
| C5AR1 | 5.92E-05 | 0.018 | 3 |
FDR indicates false discovery rate; FC, fold change.
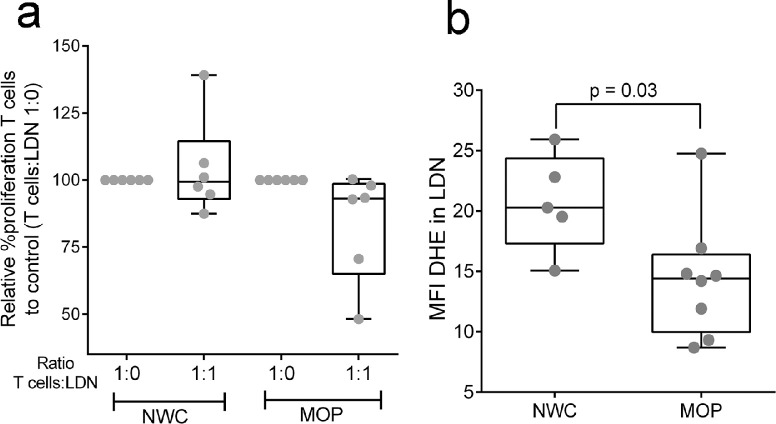
We also explored the potential functional nature of LDN according to their gene profile. Interestingly, a side-by-side comparison of individual genes that were significantly differentially expressed in LDN from MOP and NWC (5855 genes using the cutoff of FDR-unadjusted p<0·05) highlighted genes expressed in immunosuppressive MDSC in cancer.29,32, 33, 34 Significantly up-regulated (FDR-unadjusted p≤0.05; FDR ≤ 5%) genes included metalloproteinases (MMP9/25), NAMPT, HCAR2, PTGS2, TREM1, NOTCH1, CXCR2, C5AR1, IL8, and, in a lesser extent (FDR-unadjusted p ≤ 0.05; FDR ≤ 20%) LYZ, CEBPB, S100A8/A9, DDIT3, STAT5B, CSF1, IL1B, Arg1, IRF1, and STAT3. We, therefore, tested the immunosuppressive capability of LDN from MOP (n=6) and NWC (n=6) by measuring their ability to block T cell proliferation in a co-culture assay. To our surprise, LDN did not significantly inhibit T cell proliferation (Figure 3a), despite expressing genes involved in immunosuppression.
Figure 3.
LDN do not suppress T cell proliferation and display low capacity for ROS production. (a) Test of suppression capacity of LDN. Freshly isolated and CFSE-labeled autologous T cells (1 × 105) stimulated with anti-CD3 and anti-CD28 were co-cultured in a ratio of 1:1 with LDN from NWC (n=6) and MOP (n=6). T cell proliferation was tested after 72h by flow cytometry. Results are expressed as Median, Minimum to Maximum in the box, and whiskers plot. Data are plotted as percent proliferation cells relative to positive T cells control (T cells with anti-CD3 and anti-CD28 stimulating antibodies without LDN [ratio 1:0]). (b) Intracellular ROS in LDN (5 NWC and 8 MOP) as measured by the MFI of fluorescent products of DHE oxidation by flow cytometry. Results are expressed as Median, Minimum to Maximum in the box, and whiskers plot. Wilcoxon rank-sum test was used to perform comparisons of the dependent variable distributions within study groups. Results were considered significant with a p-value≤ 0·05. LDN indicate low-density neutrophils, NWC, normal-weight controls; MOP, morbidly obese patients; MFI, median fluorescence index; DHE, dihydroethidium; ROS, reactive oxygen species.
An additional characteristic of neutrophil function is the production of ROS. Using the same cutoff of FDR-unadjusted p<0·05, we found 31 DEG in LDN related to detoxifying enzymes and ROS production and scavenger proteins. LDN from MOP had a significant up-regulation of superoxide dismutase 2 (SOD2), glutathione peroxidases 2 and 3 (GPX2/3), neutrophil cytosolic factor 4 (NCF4), and significant down-regulation of SOD1, and Cytochrome β Chain (CYBB [NOX2]), among others (Table 5). CYBB is a structural member of NADPH oxidase complex 2 (NOX2) which is the principal enzyme responsible for superoxide generation. As the decreased mRNA expression of CYBB could reflect a decreased production of intracellular ROS, we tested the ability of LDN to produce ROS by measuring the oxidation of DHE by intracellular superoxide. As shown in Figure 3b, LDN from MOP (n=8) have reduced levels of ROS compared to LDN from NWC (n=5). Together, these results suggest that obesity-induced LDN have a gene signature profile of pro-inflammatory cells, with absent oxidative burst capabilities.
Table 5.
Differentially expressed genes related to detoxifying enzymes, reactive oxygen species production and scavenger proteins
| Gene symbol | p-value | FDR | FC |
|---|---|---|---|
| ALOX15 | 0.009404 | 0.178 | 28.50 |
| CYP4F12 | 0.003076 | 0.138 | 5.29 |
| SOD2 | 0.000020 | 0.010 | 4.89 |
| CYP4F3 | 0.000146 | 0.029 | 4.79 |
| CYP2F1 | 0.000616 | 0.061 | 4.68 |
| PTGS2 | 0.000050 | 0.017 | 4.52 |
| CYP26A1 | 0.010522 | 0.184 | 3.75 |
| GPX2 | 0.025164 | 0.198 | 3.56 |
| NCF4 | 0.000259 | 0.038 | 3.43 |
| ALOX5AP | 0.000415 | 0.048 | 2.85 |
| GPX3 | 0.029752 | 0.202 | 2.29 |
| SRXN1 | 0.021324 | 0.195 | 2.26 |
| NQO2 | 0.016323 | 0.192 | 2.18 |
| TXN | 0.028512 | 0.201 | 1.88 |
| NCF1 | 0.031472 | 0.205 | 1.78 |
| ALOX5 | 0.034505 | 0.209 | 1.70 |
| PRDX3 | 0.027021 | 0.201 | -1.68 |
| PRDX1 | 0.028868 | 0.201 | -1.69 |
| CYBB | 0.020038 | 0.195 | -1.72 |
| PTGS1 | 0.038928 | 0.213 | -1.74 |
| PRDX2 | 0.038368 | 0.213 | -1.74 |
| SOD1 | 0.023026 | 0.196 | -1.78 |
| ALOXE3 | 0.024143 | 0.198 | -1.90 |
| GLRX3 | 0.017831 | 0.193 | -1.90 |
| GCLC | 0.013239 | 0.187 | -1.98 |
| CYP4V2 | 0.020294 | 0.195 | -2.05 |
| CYP1B1 | 0.010201 | 0.183 | -2.09 |
| CYP2U1 | 0.020974 | 0.195 | -2.10 |
| TXNRD3 | 0.015726 | 0.191 | -2.34 |
| ALOX12B | 0.013590 | 0.187 | -2.59 |
| ALOX15B | 0.010129 | 0.182 | -3.01 |
FDR indicates false discovery rate; FC, fold change.
Bariatric surgery significantly reduces circulating LDN
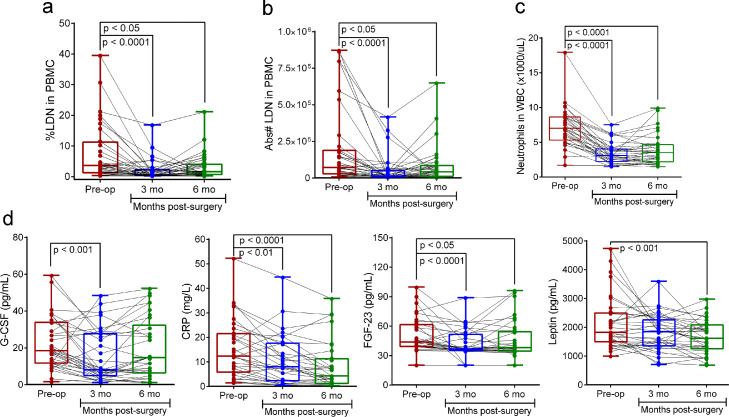
Bariatric surgery is aimed to reduce body mass and improve metabolic dysfunction. As expected, the surgical intervention resulted in a significant improvement of metabolic syndrome and a reduction in BMI (Table 1). As a measure of early postoperative weight loss (0-6 months), the %WL and %EBWL were also calculated. Forty-one percent (15/36) of patients at 3 months and 77.8% (28/36) at 6 months post-op had 15% or more %WL. Seventy-two percent (26/36) of patients at 3 months achieved an excess weight loss (EBWL) ≥ 25% and 47% (17/36) succeeded ≥ 45% EBWL at 6 months (Table 1). As bariatric surgery has shown an important impact on different inflammatory markers,4, 5, 6 we also determined if bariatric surgery affected the accumulation of LDN in circulation as an indicator of improvement of inflammation. For this, we tested the effect of bariatric surgery on LDN in the peripheral blood of MOP in a prospective longitudinal study. Comparison of percentage and absolute numbers of LDN in circulation before (baseline) and after bariatric surgery (3- and 6-months post-op) showed that LDN numbers significantly decline in MOP during the first three months after surgery (Figure 4a and b). Additionally, in agreement with a recent study of patients with obesity undergoing laparoscopic sleeve gastrectomy, bariatric surgery significantly decreased the absolute neutrophil counts in total WBC (Dunn's Posthoc p<0.0001 Figure 4;c). We did not compare differences by surgery type and gender given the small number of individuals who had a Roux-en-Y gastric bypass (4/36) and the few males (2/36) who were enrolled in the study. Additionally, by comparing race, we did not find significant differences in %LDN between African American and Caucasian patients at baseline (Supplementary Figure 3a), and the percentage was significantly reduced in both race groups (p = 0·01 [Dunn's Multiple Comparison Tes]; Supplementary Figure 3b).
Figure 4.
Changes in circulating LDN, total neutrophils, G-CSF, CRP, FGF-23, and leptin after bariatric surgery. (a) Percent LDN, (b) absolute number LDN, (c) Polymorphonuclear neutrophils counts in whole blood cells, and (d) Plasma levels of G-CSF, CRP, FGF-23, and leptin at baseline (Pre-op) and after 3 and 6 months post-bariatric surgery. Median, minimum, and maximum numbers are indicated in the box and whiskers plot and each dot represents an individual participant. Wilcoxon signed-rank tests were used to perform pairwise comparisons of the dependent variable distributions within patients before surgery and at months 3 and 6. Results were considered significant with a p-value≤0·05. LDN indicates low-density neutrophils; PBMC, peripheral blood mononuclear cells; MOP, morbidly obese patients; NWC, normal-weight controls; mo, months.
Similar to the changes in circulating LDN we also found a significant decrease in plasma G-CSF, CRP, FGF-23 levels within the first 3 months after bariatric surgery, and leptin after 6 months post-op (Figure 4d). Despite the simultaneous reductions of these obesity-associated inflammatory and metabolic factors the correlation with changes in the percentage of circulating LDN was only significant for CRP (Supplementary Figure 4a). We also tested the possible association between circulating LDN and changes in metabolic markers associated with obesity. A significant positive correlation was found between the percent LDN with HOMA-S (r=0·21, p=0·033 [Spearman's rank correlation]; Supplementary Figure 4b); however, no correlation was found between % LDN with lipids (triglycerides, cholesterol (total, HDL, and LDL), fasting insulin, insulin resistance (HOMA-IR), MetS score or BMI.
Discussion
The results of this study demonstrate that patients with morbid obesity have an increased number of LDN in circulation that express genes associated with pro-inflammatory, degranulation, and immunosuppressive functions. The results also show that bariatric surgery, an intervention for weight loss and metabolic control, decreases the number of circulating LDN while also restoring metabolic and inflammatory homeostasis. This data together suggests that the increase in LDN during morbid obesity may result as a response to the chronic inflammation induced by a sustained metabolic dysfunction, which is improved by bariatric surgery.
Our study has some limitations that preclude a wider interpretation of the data. Among these is the fact that the obese patients studied had undergone strict pre-operative dietary regimens and pharmacologic intervention to control diabetes, hypertension, and dyslipidemia at the time of collection of the first sample. We recognize the importance of evaluating the inflammatory aspects in patients before starting the pre-operative intervention (6 months before surgery). Therefore, sampling of patients at earlier time points (before initiating the preparatory regimen) or over a longer period may be important to identify specific mechanisms to explain the changes in the number of LDN during obesity. For this, an additional protocol specifically to compare the finding before and after the pre-operative time is awaiting IRB approval for a complementary study. Additionally, the studied population of patients with obesity was under the bariatric surgery program covered by health insurance and treated at a single institution, a not-for-profit medical center (Ochsner Medical Foundation), which may not be representative of socioeconomically disadvantaged patients. Also, although transcriptomic differences were observed between LDN from patients with obesity and controls, the number of analyzed samples is limited. Despite these limitations, collectively our findings indicate that obesity-induced LDN may represent a distinct neutrophil subpopulation displaying different functionalities that may contribute to chronic inflammation in severe obesity.
Different neutrophil subpopulations have been described based on their buoyancy, cell surface maturation markers, molecular profiles, and function.15,35 LDN are found to increase in inflammatory disorders including asthma, sepsis, graft versus host disease, cancer, autoimmunity, and certain infections.14,15 Certain functions may characterize neutrophilic subsets in specific settings. For example, subpopulations of neutrophils with antigen-presenting cells (APC) capabilities are induced by granulocyte-macrophage colony-stimulating factor (GM-CSF) and interferon (IFN)-gamma.36 Highly and spontaneous immunosuppressive LDN are characteristic of cancer and are more commonly referred to as PMN-MDSC.19 On contrary, in systemic lupus erythematosus, LDN display a non-suppressive phenotype,37 which can acquire immunosuppressive activity after a second activating signal.17
The identification of LDN as a neutrophil subset associated with the physiopathology of diseases is limited due to the overlapping surface markers (CD11b+, CD33+, CD66b+, CD15+, and HLA DR-) among the different neutrophilic subpopulations. Due to this limitation, and given the important role of LDN in inflammation, their gene expression profiles have been used as a biomarker to evaluate disease activity, the severity of inflammation, and monitor the response to treatment in autoimmune diseases.38 The transcriptome analysis helped us further characterize the potential function of the LDN and their clinical relevance in obesity. The comparative transcriptomic analysis between LDN from NWC and MOP shows distinctive gene signatures characteristic of activated neutrophils with a pro-inflammatory phenotype in obesity. Furthermore, it supports previous observations where LDN from MOP had an increased expression of the ALPL gene which was identified as a marker of neutrophil activation in response to obesity, and more importantly, appeared to be an indicator of increased risk for cardiovascular disease.31
Interestingly, LDN from MOP also showed a gene signature similar to the immunosuppressive PMN-MDSC found in cancer,29,32, 33, 34 including MMPs, prostaglandin-endoperoxide synthase 2 (PTGS2), S100 proteins, and arginase-1 which are molecules associated with activation and increased immunosuppressive functions in MDSC.39, 40, 41 Surprisingly, however, obesity-induced LDN did not display the immunosuppressive functional properties associated with PMN-MDSC found in cancer patients. It is, therefore, possible that LDN from MOP could represent a subset of myeloid cells that are in an incomplete or “poised” stage of activation which may respond to an additional stimulus that leads to the full activation of inflammatory, oxidative, and immunosuppressive functions. This possibility is important considering that additional signals could derive from infectious agents or malignant cells. It is known that excess weight increases the risk of developing severe infections18,42 and cancer,43,44 suggesting a potential contribution of LDN in developing or exacerbating these co-morbidities.
The concept of pre-activated or “primed” neutrophils has been shown in different inflammatory settings14,17 as well as in healthy individuals.45 Primed neutrophils were described previously as a result of activation by G-CSF and GM-CSF,46, 47, 48 hyperglycemia, or hypercholesterolemia.49,50 Plasma levels of G-CSF were significantly increased in MOP compared to NWC; however, we failed to find associations between G-CSF levels in plasma and numbers of circulating LDN, suggesting that other mechanisms, different from just increased granulopoiesis, might be playing a role in increasing the number of LDN. In addition, we also were unable to demonstrate any correlation between levels of lipids, glucose in serum, and the number of LDN in circulation (data not shown). However, cannot rule out the possibility that G-CSF, hyperglycemia, and dyslipidemia could all contribute to LDN priming in obesity. Additional experimental work will be needed to assess whether obesity-induced LDN are prone to be polarized towards immunosuppressive cells with a vigorous MDSC activity after exposure to a specific local microenvironment. If obesity-induced LDN can become highly immunosuppressive after encountering an additional stimulus such as those derived by malignant cells, it would suggest a biological link between obesity and the increased risk for developing cancer.
The parallel improvement of metabolic dysfunction and decreased inflammatory parameters including the frequency of LDN after bariatric surgery support the remarkable anti-inflammatory effect of the surgically induced weight loss. The functional alterations of LDN after surgery were beyond the scope of our study; however, our findings strongly encourage further evaluation of LDN on response to bariatric surgery and obesity co-morbidities. Furthermore, whether LDN contributes to the risk or severity of obesity-associated co-morbidities including cancer will provide a potential target for therapeutic intervention to reduce this health risk.
Data sharing statement
Raw (FASTQ) and normalized data have been uploaded to the Gene Expression Omnibus (GEO) with the accession number GSE161042. Other data that support the findings of this study are available from the corresponding author upon reasonable request.
Contributors
MDSP, PCR, WSR, and ACO conceived and designed the study. MDSP, JL, YK, RTP, LZ, AAA, and DW performed the methodology and acquired the data. JG performed the RNA sequencing method and JZ, RM, MD compiled RNASeq data and performed bioinformatic analysis. AGCh and MDSP performed statistical analyses. WSR, JBW, JKG, MP, RDA, and LBB, recruited the study participants and provided patients’ samples and clinical data. MDSP and ACO jointly supervised the study. MDSP analyzed and interpreted the data and wrote the manuscript. MDSP, AO, AGCh, and JZ verified the quality and accuracy of sequencing, research, and clinical data. PCR, WSR, LM, and ACO provided intellectual input and edited the manuscript. All authors read and approved the final version of the manuscript.
Declaration of interests
Augusto Ochoa, and Lucio Miele were funded by grants from the National Cancer Institute and the National Institutes of Health. The remaining authors have declared that no conflict of interest exists.
Acknowledgments
We want to acknowledge the work of the Ochsner Biorepository Personnel, the Core Laboratories: Cellular Immunology and Immune Metabolism Core-CIMC, the Translational Genomics Core at the LSU – Cancer Center, Biochemical CORE at Pennington Biomedical Research Center of LSU (PBRC), and LA CaTS Center support. We also thank the patients and controls who participated in this study. This work was supported in part by grants from the National Institutes of Health (NIH; 5P30GM114732-02, P20CA233374-A. Ochoa and L. Miele), Pennington Biomedical NORC (P30DK072476-E. Ravussin & LSU-NO Stanley S. Scott Cancer Center and Louisiana Clinical and Translational Science Center (LACaTS; U54-GM104940-J. Kirwan).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.103910.
Appendix. Supplementary materials
References
- 1.World Cancer Research Fund/American Institute for Cancer Research. Continuous update project expert report 2018. Body Fatness and Weight Gain and the Risk of Cancer. World Cancer Research Fund/American Institute for Cancer Research https://www.wcrf.org/dietandcancer/exposures/body-fatness. [Accessed 20 June, 2021].
- 2.Daien C.I., Sellam J. Obesity and inflammatory arthritis: impact on occurrence, disease characteristics and therapeutic response. RMD Open. 2015;1 doi: 10.1136/rmdopen-2014-000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruno D.S., Berger N.A. Impact of bariatric surgery on cancer risk reduction. Ann Transl Med. 2020;8:S13. doi: 10.21037/atm.2019.09.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freitas W.R., Oliveira L.V.F., Perez E.A., et al. Systemic inflammation in severe obese patients undergoing surgery for obesity and weight-related diseases. Obes Surg. 2018;28:1931–1942. doi: 10.1007/s11695-017-3104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Sousa A.R.T., Freitas W.R., Perez E.A., et al. Surgery for obesity and weight-related diseases changes the inflammatory profile in women with severe obesity: a randomized controlled clinical trial. Obes Surg. 2021;31:5224–5236. doi: 10.1007/s11695-021-05702-5. [DOI] [PubMed] [Google Scholar]
- 6.Villarreal-Calderon J.R., Cuellar-Tamez R., Castillo E.C., Luna-Ceron E., Garcia-Rivas G., Elizondo-Montemayor L. Metabolic shift precedes the resolution of inflammation in a cohort of patients undergoing bariatric and metabolic surgery. Sci Rep. 2021;11:12127. doi: 10.1038/s41598-021-91393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boutens L., Hooiveld G.J., Dhingra S., Cramer R.A., Netea M.G., Stienstra R. Unique metabolic activation of adipose tissue macrophages in obesity promotes inflammatory responses. Diabetologia. 2018;61:942–953. doi: 10.1007/s00125-017-4526-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nijhuis J., Rensen S.S., Slaats Y., van Dielen F.M., Buurman W.A., Greve J.W. Neutrophil activation in morbid obesity, chronic activation of acute inflammation. Obes (Silver Spring) 2009;17:2014–2018. doi: 10.1038/oby.2009.113. [DOI] [PubMed] [Google Scholar]
- 9.Gummlich L. Obesity-induced neutrophil reprogramming. Nat Rev Cancer. 2021;21:412. doi: 10.1038/s41568-021-00372-y. [DOI] [PubMed] [Google Scholar]
- 10.Bowers E., Singer K. Obesity-induced inflammation: the impact of the hematopoietic stem cell niche. JCI Insight. 2021;6 doi: 10.1172/jci.insight.145295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bao Y., Mo J., Ruan L., Li G. Increased monocytic CD14(+)HLADRlow/- myeloid-derived suppressor cells in obesity. Mol Med Rep. 2015;11:2322–2328. doi: 10.3892/mmr.2014.2927. [DOI] [PubMed] [Google Scholar]
- 12.Xia S., Sha H., Yang L., Ji Y., Ostrand-Rosenberg S., Qi L. Gr-1+ CD11b+ myeloid-derived suppressor cells suppress inflammation and promote insulin sensitivity in obesity. J Biol Chem. 2011;286:23591–23599. doi: 10.1074/jbc.M111.237123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lo T., Haridas R.S., Rudge E.J.M., et al. Early changes in immune cell count, metabolism, and function following sleeve gastrectomy: a prospective human study. J Clin Endocrinol Metab. 2021 doi: 10.1210/clinem/dgab673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosales C. Neutrophil: a cell with many roles in inflammation or several cell types? Front Physiol. 2018;9:113. doi: 10.3389/fphys.2018.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deniset J.F., Kubes P. Neutrophil heterogeneity: bona fide subsets or polarization states? J Leukoc Biol. 2018;103:829–838. doi: 10.1002/JLB.3RI0917-361R. [DOI] [PubMed] [Google Scholar]
- 16.Silvestre-Roig C., Fridlender Z.G., Glogauer M., Scapini P. Neutrophil diversity in health and disease. Trends Immunol. 2019;40:565–583. doi: 10.1016/j.it.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aarts C.E.M., Hiemstra I.H., Beguin E.P., et al. Activated neutrophils exert myeloid-derived suppressor cell activity damaging T cells beyond repair. Blood Adv. 2019;3:3562–3574. doi: 10.1182/bloodadvances.2019031609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez-Pino M.D., Dean M.J., Ochoa A.C. Myeloid-derived suppressor cells (MDSC): when good intentions go awry. Cell Immunol. 2021;362 doi: 10.1016/j.cellimm.2021.104302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabrilovich D.I. Myeloid-derived suppressor cells. Cancer Immunol Res. 2017;5:3–8. doi: 10.1158/2326-6066.CIR-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manning S., Pucci A., Carter N.C., et al. Early postoperative weight loss predicts maximal weight loss after sleeve gastrectomy and Roux-en-Y gastric bypass. Surg Endosc. 2015;29:1484–1491. doi: 10.1007/s00464-014-3829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Society for Metabolic and Bariatric Surgery (ASMBS). Metabolic and Bariatric Surgery. https://asmbs.org/resources/metabolic-and-bariatric-surgery. [Accessed 26 Dec, 2021].
- 22.Paredes J., Zabaleta J., Garai J., et al. Immune-related gene expression and cytokine secretion is reduced among African American colon cancer patients. Front Oncol. 2020;10:1498. doi: 10.3389/fonc.2020.01498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kramer A., Green J., Pollard J., Tugendreich S. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics. 2014;30:523–530. doi: 10.1093/bioinformatics/btt703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trim W., Turner J.E., Thompson D. Parallels in immunometabolic adipose tissue dysfunction with ageing and obesity. Front Immunol. 2018;9 doi: 10.3389/fimmu.2018.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watanabe Y., Nagai Y., Honda H., et al. Bidirectional crosstalk between neutrophils and adipocytes promotes adipose tissue inflammation. FASEB J. 2019;33:11821–11835. doi: 10.1096/fj.201900477RR. [DOI] [PubMed] [Google Scholar]
- 26.Demetri G.D., Griffin J.D. Granulocyte colony-stimulating factor and its receptor. Blood. 1991;78:2791–2808. doi: 10.1016/s0955-2235(05)80004-3. [DOI] [PubMed] [Google Scholar]
- 27.Roberts AW. G-CSF: a key regulator of neutrophil production, but that's not all!. Growth Factors 2005;23:33-41. doi: 10.1080/08977190500055836 [DOI] [PubMed]
- 28.Metzemaekers M., Gouwy M., Proost P. Neutrophil chemoattractant receptors in health and disease: double-edged swords. Cell Mol Immunol. 2020;17:433–450. doi: 10.1038/s41423-020-0412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veglia F., Hashimoto A., Dweep H., et al. Analysis of classical neutrophils and polymorphonuclear myeloid-derived suppressor cells in cancer patients and tumor-bearing mice. J Exp Med. 2021;218 doi: 10.1084/jem.20201803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma J., Lam I.K.Y., Lau C.S., Chan V.S.F. Elevated interleukin-18 receptor accessory protein mediates enhancement in reactive oxygen species production in neutrophils of systemic lupus erythematosus patients. Cells. 2021;10 doi: 10.3390/cells10050964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan Y., Choi J.H., Shi H., Zhang L., Su S., Wang X. Discovery and validation of a novel neutrophil activation marker associated with obesity. Sci Rep. 2019;9:3433. doi: 10.1038/s41598-019-39764-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marigo I., Bosio E., Solito S., et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Al-Khami A.A., Zheng L., Del Valle L., et al. Exogenous lipid uptake induces metabolic and functional reprogramming of tumor-associated myeloid-derived suppressor cells. Oncoimmunology. 2017;6 doi: 10.1080/2162402X.2017.1344804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veglia F., Sanseviero E., Gabrilovich D.I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat Rev Immunol. 2021;21:485–498. doi: 10.1038/s41577-020-00490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y., Wang W., Yang F., Xu Y., Feng C., Zhao Y. The regulatory roles of neutrophils in adaptive immunity. Cell Commun Signal CCS. 2019;17:147. doi: 10.1186/s12964-019-0471-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takashima A., Yao Y. Neutrophil plasticity: acquisition of phenotype and functionality of antigen-presenting cell. J Leukoc Biol. 2015;98:489–496. doi: 10.1189/jlb.1MR1014-502R. [DOI] [PubMed] [Google Scholar]
- 37.Rahman S., Sagar D., Hanna R.N., et al. Low-density granulocytes activate T cells and demonstrate a non-suppressive role in systemic lupus erythematosus. Ann Rheum Dis. 2019;78:957–966. doi: 10.1136/annrheumdis-2018-214620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grayson P.C., Carmona-Rivera C., Xu L., et al. Neutrophil-related gene expression and low-density granulocytes associated with disease activity and response to treatment in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 2015;67:1922–1932. doi: 10.1002/art.39153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Umansky V., Blattner C., Gebhardt C., Utikal J. The role of myeloid-derived suppressor cells (MDSC) in cancer progression. Vaccines (Basel) 2016;4 doi: 10.3390/vaccines4040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Haas N., de Koning C., Spilgies L., de Vries I.J., Hato S.V. Improving cancer immunotherapy by targeting the STATe of MDSCs. Oncoimmunology. 2016;5 doi: 10.1080/2162402X.2016.1196312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang W., Xia X., Mao L., Wang S. The CCAAT/enhancer-binding protein family: its roles in MDSC expansion and function. Front Immunol. 2019;10:1804. doi: 10.3389/fimmu.2019.01804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghilotti F., Bellocco R., Ye W., Adami H.O., Trolle Lagerros Y. Obesity and risk of infections: results from men and women in the Swedish national march cohort. Int J Epidemiol. 2019;48:1783–1794. doi: 10.1093/ije/dyz129. [DOI] [PubMed] [Google Scholar]
- 43.Xu Y.X.Z., Mishra S. Obesity-linked cancers: current knowledge, challenges and limitations in mechanistic studies and rodent models. Cancers (Basel) 2018;10 doi: 10.3390/cancers10120523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanchez-Pino M.D., Gilmore L.A., Ochoa A.C., Brown J.C. Obesity-associated myeloid immunosuppressive cells, key players in cancer risk and response to immunotherapy. Obes (Silver Spring) 2021;29:944–953. doi: 10.1002/oby.23108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blanco-Camarillo C., Aleman O.R., Rosales C. Low-density neutrophils in healthy individuals display a mature primed phenotype. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.672520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castellani S., D'Oria S., Diana A., et al. G-CSF and GM-CSF modify neutrophil functions at concentrations found in cystic fibrosis. Sci Rep. 2019;9:12937. doi: 10.1038/s41598-019-49419-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Freeley S.J., Coughlan A.M., Popat R.J., Dunn-Walters D.K., Robson M.G. Granulocyte colony stimulating factor exacerbates antineutrophil cytoplasmic antibody vasculitis. Ann Rheum Dis. 2013;72:1053–1058. doi: 10.1136/annrheumdis-2012-202160. [DOI] [PubMed] [Google Scholar]
- 48.Yousefi S., Mihalache C., Kozlowski E., Schmid I., Simon H.U. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16:1438–1444. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]
- 49.Mazor R., Shurtz-Swirski R., Farah R., et al. Primed polymorphonuclear leukocytes constitute a possible link between inflammation and oxidative stress in hyperlipidemic patients. Atherosclerosis. 2008;197:937–943. doi: 10.1016/j.atherosclerosis.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 50.Wong S.L., Demers M., Martinod K., et al. Diabetesprimes neutrophils to undergo NETosis, which impairs wound healing. Nat Med. 2015;21:815–819. doi: 10.1038/nm.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.