Summary
Primary cilia are hair-like sensory organelles protruding from the surface of most human cells. As cilia are dynamic, several aspects of their biology can only be revealed by real-time analysis in living cells. Here we describe the generation of primary cilia reporter cell lines. Furthermore, we provide a detailed protocol of how to use the reporter cell lines for live-cell imaging microscopy analysis of primary cilia to study their growth as well as intraciliary transport.
For complete details on the use and execution of this protocol, please refer to Bernatik et al. (2020) and Pejskova et al. (2020).
Subject areas: Cell Biology, Cell culture, Microscopy, Molecular Biology
Graphical abstract

Highlights
-
•
Constructing expression vector for preparation of primary cilia reporter cell lines
-
•
Generation of stable cell lines using Flp-InTM T-RExTM and viral transduction
-
•
Analysis of primary cilia growth dynamics in time-lapse live cell imaging
-
•
Imaging and analysis of ciliary transport in living cells
Primary cilia are hair-like sensory organelles protruding from the surface of most human cells. As cilia are dynamic, several aspects of their biology can only be revealed by real-time analysis in living cells. Here we describe the generation of primary cilia reporter cell lines. Furthermore, we provide a detailed protocol of how to use the reporter cell lines for live-cell imaging microscopy analysis of primary cilia to study their growth as well as intraciliary transport.
Before you begin
In this protocol, we describe live-cell imaging analysis of primary cilia in the human cell line hTERT-RPE-1. For this purpose, appropriate ciliary markers, such as a small GTPase ARL13b (Caspary et al., 2007) or an intraflagellar transport (IFT) protein IFT74 (Bhogaraju et al., 2013) are coupled to a fluorescent tag. We begin with a brief outline of a suitable cloning strategy. Subsequently, we describe two approaches for the derivation of cell lines stably expressing mNeonGreen-ARL13b or mNeonGreen-IFT74 cilia reporters. The first strategy is based on Flp-InTM T-RExTM system (developed by Invitrogen / Thermo Fisher Scientific), allowing stable integration of gene of interest (GOI) specifically into a defined locus. The second one utilizes retroviral transduction. If your goal is to prepare a system with two different transgenes, consider using a combination of “Flp-InTM T-RExTM and virus” or “two viruses”. The whole procedure described here is easily adaptable as a “complete pipeline”, from its beginning to the end. Alternatively, only specific parts may be selected, depending on the available tools and the experimental question tested. When planning the experiments, it should be noted that preparation of the reporter cell lines and their validation will require at least several weeks. Importantly, the work involving the production of retroviral particles has to be carried out in a lab suitable for the production of viruses (biosafety level 2 (BSL-2)). The protocol below describes the specific steps using the human cell line hTERT-RPE-1. However, we have also carried out parts of this protocol in HEK293T, NIH3T3, and IMCD3 cells. Before starting, all reagents should be prepared as described below.
Cloning of GOI into expression vectors
Timing: ∼ one week
Cloning of gene of interest (GOI), such as ARL13b or IFT74, into an expression vector suitable for the generation of cilia reporter cell line using either the Flp-InTM T-RExTM system (e.g., pgLAP1_Neo) or retroviral transduction (e.g., Murine stem cell virus pMSCV-N-mNeonGreen-IRES-PURO), is in both cases best achieved by using the Gateway cloning system (Thermo Fisher Scientific). Other cloning methods might also work fine. For the Gateway cloning please follow the manufacturer’s guidelines (www.thermofisher.com). Importantly, one has to consider whether the N- or C-terminal tagging of the selected protein interferes with its function (localization). Therefore, please bear in mind that the vectors mentioned above only allow for the N-terminal tagging.
Note: The mNeonGreen tag offers several advantages over GFP in terms of stability, brightness, and resistance to photobleaching, even when using regular GFP microscopy filters. It has an excitation maximum at 506 nm and an emission maximum at 517 nm (Shaner et al., 2013). However, the use of mNeonGreen is subjected to a license from Allele Biotechnology (Allelebiotechnology.com), while the use of other fluorescent tags, such as GFP or mCherry, is not restricted.
Note: The expression and the correct ciliary localization of the tagged protein of interest should be always verified. Consider using immunofluorescence (IF) microscopy, Western blotting (WB), or ideally both. In the case of both ARL13b and IFT74, we have used their N-terminally tagged variants that displayed subcellular localization identical to their endogenous counterparts. We observed, however, that ARL13b over-expression in hTERT-RPE-1 leads to slightly longer primary cilia if compared to non-transfected cells, in agreement with an earlier report (Larkins et al., 2011). To assess the functionality of the tagged protein, a gold standard is a rescue experiment using knockout (KO) cells (Prasai et al., 2020) or following RNAi-mediated depletion.
Generation of stable cell lines using Flp-InTM T-RExTM system
Timing: typically 3–4 weeks
The following paragraph describes a generation of a reporter cell line expressing a protein of interest from a specific genomic locus under the control of a doxycycline (DOX)-inducible promoter (Figure 1). The Flp-InTM T-RExTM system (www.thermofisher.com) works very well for that purpose. The main advantage of this approach is that the stable cell lines derived from the same parental cells have the GOI integrated in the same site (Ward et al., 2011). This typically leads to a more uniform level of expression across the population, in contrast to random, multiple site integrations of viral vectors. In turn, typically only polyclonal selection of stable Flp-InTM T-RExTM transfectants is required. The main limitation of this system is the need for the Flp-InTM T-RExTM parental cell line. To study ciliogenesis, the hTERT-immortalized retinal pigment epithelial cell line, hTERT-RPE-1 (available from ATCC), is probably the most commonly used cell line. In our experience, this cell line is well suitable for live-cell imaging experiments, as their cilia are typically oriented in the imaging plane. Its Flp-InTM T-RExTM derivate has been recently published (Bernatik et al., 2020; Pejskova et al., 2020). Alternatively, the Flp-InTM T-RExTM parental cell line can be derived from the hTERT-RPE-1 cells or any other cell line suitable to study ciliogenesis (e.g., NIH3T3, IMCD3, etc.) by following these instructions (www.thermofisher.com). It is important to bear in mind that most Flp-InTM T-RExTM – compatible expression vectors (e.g., pgLAP1 (Addgene plasmid #19702) or pgLAP2 (Addgene plasmid #19703)) use hygromycin resistance as a selection marker, which is not suitable for use in hTERT-RPE-1; hygromycin was used for selection of hTERT expressing clones when this cell line was originally established. As PURO resistance (PAC gene) was introduced into the cells on the same plasmid as hygromycin resistance (“PURO resistance”), we highly recommend using expression vectors with neomycin resistance (G418-based selection), such as pgLAP1_Neo (Pejskova et al., 2020), pgLAP2_Neo (Bernatik et al., 2020), or pGFT1.1 (mNeonGreen derivate of pgLAP1_Neo vector).
-
1.
hTERT-RPE-1 Flp-InTM T-RExTM cells are seeded on two or more 6 cm culture dishes (∼45 × 103 cells per cm2). The cells are grown for 1–2 days to ∼80% confluency. This typically gives the best performance in terms of the transfection efficiency and survival.
Note: Prepare one dish per each planned stable cell line, and one additional dish to serve as negative control.
-
2.
Cells are then co-transfected with pgLAP1_Neo/pgLAP2_Neo or derivatives encoding the GOI, and pOG44 plasmid, encoding the Flp recombinase (Ward et al., 2011). Negative control is transfected only with pOG44 plasmid.
Note: We have derived most of our cell lines following transfection using Lipofectamine 3000 (https://tools.thermofisher.com/content/sfs/manuals/lipofectamine3000_protocol.pdf), but other methods might work just as well.
CRITICAL: Due to the low activity of the Flp recombinase in typical cell culture conditions, we recommend the ratio of pgLAP1_Neo-GOI to pOG44 plasmid to be at least 1:29. For optimal performance, we suggest the total amount of transfected DNA per 80% confluent 6 cm culture dish (with surface area ∼21.5 cm2) not to exceed 3 μg (2.9 μg of pOG44: 0.1 μg of pgLAP1_Neo).
-
3.The selection of stably transfected cells should start no earlier than 24 h post-transfection
-
a.Replace the medium with a fresh one supplemented with G418 (typically 400 μg/mL, please see notes below), and blasticidin (10 μg/mL, to keep selection pressure for the integrated pcDNA6/TR plasmid of the T-REx system).
-
b.The medium is initially replaced every 3 days until all cells in the negative control condition die out, then the intervals can be prolonged to 5 days. Note that 1–2 weeks after the selection start most of the cells should die and small colonies of resistant cells should be visible upon visual inspection of the plate. Consider marking the position of the colonies on the bottom of the dish.
-
c.Within additional 5–10 days the colonies of resistant cells are usually sufficiently large (∼ hundreds of tightly packed cells), so can be trypsinized and transferred to a new growth vessel (1 well of 24-well plate or 6-well plate, depending on the total number of cells). Troubleshooting 1
-
a.
Optional: Consider freezing down a backup sample for cryopreservation when passaging the cells.
-
4.During the expansion of the cells
-
a.the expression of the integrated transgene should be verified by WB or IF microscopy.
-
b.Cryopreserve the expanded cells with verified transgene expression.
-
c.Keeping the selection pressure (G418 and blasticidin) after thawing a frozen vial is recommended, but not strictly necessary. Let the cells recover for about 24 h post-thawing before the selection antibiotics are added.
-
a.
Note: Searching the literature will give an estimate of a working dose for a given antibiotic and cell line, respectively (e.g., ∼400 μg/mL G418 for hTERT-RPE-1). As the effectiveness of antibiotics selection depends on several factors, we recommend determining its optimal concentration in your particular cell line and culture conditions before the actual experiment. Generally, at least 3–4 different concentrations are tested to ensure complete killing within 5–7 days.
Note: Always consider including a condition transfected only with pOG44 plasmid to serve as a negative control for the subsequent selection of stable transfectants. We suggest visually inspecting the cells about 4–6 h post-transfection, in case of pronounced signs of post-transfection toxicity, we recommend replacing the medium with a fresh one immediately.
Note: DOX typically induces sufficient expression at dose range 0.01–1 μg/mL within 24–48 h, however, the optimal time window will always reflect the used GOI and the actual experimental question.
Figure 1.
Schematic of the generation of stable cell lines using the Flp-InTM T-RExTM system
Generation of a reporter cell line expressing a protein of interest (in our case IFT74) from a specific genomic locus under the control of a doxycycline (DOX)-inducible promoter. Flp-InTM T-RExTM parental cell line (e.g., hTERT-RPE-1 Flp-InTM T-RExTM) is co-transfected with pgLAP1_Neo/pgLAP2_Neo or derivatives encoding the GOI, and pOG44 plasmid, encoding the Flp recombinase. Due to the low activity of the Flp recombinase in typical cell culture conditions, we recommend the ratio of pgLAP1 Neo-GOI to pOG44 plasmid to be at least 1:29. The selection of stably transfected cells should start no earlier than 24 h post-transfection, by replacing the medium with a fresh one supplemented with G418 and blasticidin (blasticidin resistance comes with the Flp-InTM T-RExTM cassette). The expression of the integrated transgene in the generated reporter cell line is then induced by addition of DOX in the culture medium.
Preparation of viral particles
Timing: 4 days
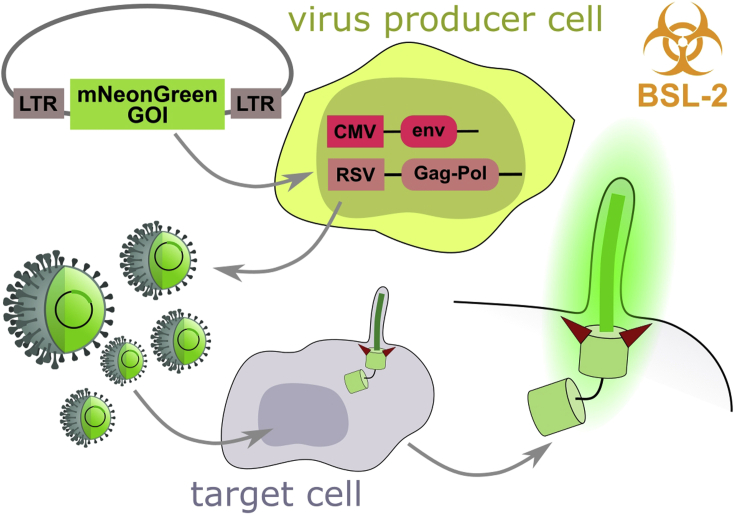
In the following section, we describe the production of viral particles based on the pMSCV retroviral backbone (pMSCV-mNeonGreen vector with GOI of choice (e.g., ARL13b)) (Figure 2). Viral transduction is a fast and efficient way to generate stable transfectants in virtually any cell line. The limitation is typically heterogeneous expression levels of the transgene across the population (multiple integration sites, silencing over time), so we highly recommend enriching the population with the desired expression level by cell sorting or eventually isolating individual clones.
Figure 2.
Schematic of the generation of stable cell lines using viral transduction
Note, that significant part of this procedure needs to be performed in Biosafety level (BSL)-2 lab. pMSCV-mNeonGreen retroviral backbone with the GOI (e.g., ARL13b) is transfected into virus producer cells (here we used Phoenix-Ampho cell line, capable of producing Gag-Pol and envelope protein). Viral particles-enriched medium is subsequently collected and used to infect the target cells (e.g., hTERT-RPE-1) thus generating a stable reporter cell line.
For the production of retroviruses, we have used Phoenix-Ampho cell line, a second-generation retrovirus producer cell line derived from HEK 293T, which is capable of producing Gag-Pol and envelope protein. The viral particles generated by this cell line can infect most mammalian cell lines. Alternatively, Phoenix-Eco producer cell line can be used in situations when Phoenix-Ampho is not optimal (e.g., mouse fibroblasts). Both Phoenix-Ampho and Phoenix-Eco cell lines, derived by the Nolan lab (Swift et al., 1999), can be obtained from ATCC. Other suitable packaging systems might work as well.
The following protocol describes the preparation of 9 mL of viral particles-enriched medium, which is sufficient for subsequent transduction of target cells in 9 wells on a 12-well plate or 4–5 wells of a 6-well plate.
-
5.
Day 1: Seed the production cell line Phoenix-Ampho in HEK complete medium in a 25 cm2 culture flask. We have obtained the best transfection efficiency when transfecting the cells the next day when ∼80% confluent.
-
6.Day 2:
-
a.Before the transfection, carefully change the medium for the Phoenix transfection medium. 3 mL media is typically sufficient to fully cover the surface of the 25 cm2 cell culture flask.
-
b.Prepare a transfection mix to transfect the cells using polyethylenimine (PEI) - mix 300 μL OPTIMEM, 8 μg of pMSCV-mNeonGreen vector with GOI of choice (e.g., ARL13b), and 20 μL PEI solution.
-
c.Briefly vortex and spin down using a tabletop centrifuge (10.000 g, 10 s, RT).
-
d.Following incubation (10 min, RT), add the transfection mixture dropwise to the medium in the flask and mix carefully by gently swaying the flask.
-
a.
IMPORTANT: All the following steps need to be PERFORMED IN THE VIRAL PRODUCTION SUITABLE LAB (BSL-2), see “CRITICAL” section of this part of the protocol.
-
7.
Transfer the cells to viral production suitable lab (equipped with a laminar flow hood, centrifuge, and cell culture CO2 incubator). After ∼3 h replace the medium for a Viral particles production medium (3 mL, pre-heated).
-
8.Day 3:
-
a.We recommend collecting the viral particles-enriched medium every 12 h for 36 h (getting in total 9 mL of virus-containing medium). Keep the falcon tube with the collected viral particles-containing medium at 4°C in between the collection steps.
-
b.Add Viral particles production medium (3 mL, pre-heated) after each collection.
-
a.
-
9.Day 4:
-
a.Pool the collected viral particles-enriched medium.
-
b.Filter the pre-cleared media using 0.45 μm low-binding syringe filters. Troubleshooting 2
-
a.
Optional: If your viral particles-enriched medium has a lot of debris, consider a short spin (3,000 g, 5 min, 4°C) before the filtration step (9b).
Pause Point: The collected viral particles-enriched medium may be used immediately, or stored at −80°C, aliquoted in cryotubes. Try to avoid multiple freezing-thawing cycles.
CRITICAL: Work carefully with the Phoenix-Ampho retrovirus producer cell line, when collecting/replacing the medium. The cells tend to easily detach from the surface of a culture plastic when handled harshly. The cells detach immediately in case a cold medium is used, therefore usage of the preheated medium is essential.
CRITICAL: Perform all the viral particles-related work in an accordingly equipped cell culture lab suitable for viral production (BSL-2). Use appropriate personal protective equipment at all times. Avoid sharing equipment (such as micropipettes) with “non-viral” labs, always use filter tips. All virus-containing waste has to be treated with appropriate disinfectant (e.g., Alcohol-based rapid disinfectant Bacillol), put in a sealed plastic bag, and discarded in a biohazard waste container. The workspace (i.e., laminar flow cell culture hood) needs to be disinfected using appropriate disinfectant and UV light after each use. Take into account that viral particles-related work rules can differ in your institution/country.
Note: We typically employ PEI-based transfection (Longo et al., 2013), to transfect the Phoenix-Ampho cells, step 6b. Other transfection vehicles such as Calcium phosphate or lipid-based reagents are also suitable.
Note: Cells producing large quantities of the virus will typically stop proliferating and change their morphology. Consider monitoring that as a sign the procedure proceeds as expected.
Generation of reporter cell lines using viral transduction
Timing: ∼1 week
This section covers the production of cilia reporter hTERT-RPE-1 cell line using retroviral particles (we use here pMSCV-mNeonGreen-ARL13b retrovirus, please see the previous section). The choice of the appropriate cell line to transduce depends on the design of the planned experiment and the experimental question tested. If you are aiming to prepare a system with two different transgenes, consider using a combination of “Flp-InTM T-RExTM and virus” or “two viruses” (please see the Notes further). Alternatively, any suitable cell line can be used (e.g., hTERT-RPE-1, NIH3T3, IMCD3, MDCKII). Again, all virus-related work needs to be carried out in VIRAL PRODUCTION SUITABLE LAB (BSL-2).
-
10.
Following trypsinization, prepare the appropriate volume of target cell suspension (here we used about 50.000 cells/well of the 12-well plate) and centrifuge (200 g, 4 min) in a 1.5 mL tube.
-
11.
Resuspend the cell pellet in 1 mL of the collected viral particles-enriched medium (either freshly prepared or thawed in a water bath at 37°C, see the previous part of the protocol) with the addition of 5 μg/mL polybrene (1 mg/mL stock) and transfer the cell suspension to the plate.
-
12.
Following the transduction (minimum 4 h, maximum overnight), wash the cells with PBS and add preheated cultivation medium. Longer incubation may improve transduction efficiency. Consider PBS washing and replacing the medium daily, passage the cells when ∼80% confluent. Troubleshooting 3
Note: We recommend replacing the medium at least 5 times and passage the cells at least once (including the change of the culture plastic) before they can be considered free of any remaining virus particles (bear in mind the biosafety rules at your institute may differ). Until then it is advised to work with the cells as if they still contained the virus – so they should be handled only in VIRAL PRODUCTION SUITABLE LAB (BSL2).
-
13.
During the expansion of the transduced cells, the expression of the integrated transgene should be verified by WB or IF microscopy. Cryopreserve the expanded cells with verified transgene expression and correct localization.
Optional: If your vector carries a suitable resistance marker, you may choose to apply selection antibiotics (e.g., G418, Puromycin, etc.) to enrich for the transduced cells. Do not forget to have one extra well as “non-transduced control”, which will be used to assess the selection efficiency. Searching the literature will give an estimate of a working dose for a given antibiotic and cell line, respectively. As several factors are affecting the efficient dose of the antibiotic, we recommend determining the optimal concentration of the antibiotic of choice in your particular cell line and culture conditions before the actual experiment. In the case of G418, at least 3–4 different concentrations should be tested to ensure complete killing within 5–7 days. Note that the pMSCV vector has a selection marker for Puromycin, which is suitable for efficient selection of transductants in e.g., NIH3T3 or IMCD3 cells, but not so much for hTERT-RPE-1 (see before).
Note: Retroviral transduction and Flp-InTM T-RExTM technology can be combined. This is particularly handy in experiments such as live-cell imaging microscopy when a versatile system allowing simultaneous expression of several transgenes (one “marker” and one “tested” gene) is needed (e.g., loss-of-function rescue type of experiment or functional testing of potential dominant-negative effects of a transgene of choice). To give a specific example, we have recently described Flp-InTM T-RExTM hTERT-RPE-1 cells lacking Tau tubulin kinase 2 (TTBK2) (Bernatik et al., 2020), a key regulator of primary cilia assembly (Goetz et al., 2012). Subsequently, pgLAP2_Neo-TTBK2 was used to express FLAG-TTBK2 in a DOX-inducible manner from the Flp-InTM locus. In turn, these cells were transduced by pMSCV-mNeonGreen-ARL13b retrovirus. Inducible expression of FLAG-TTBK2 restored formation of mNeonGreen positive primary cilia, which can be monitored directly in living cells, as described in detail in the following section (see Figure 16 for expected outcome).
Note: It is also possible to transduce the same cell line with two or more pMSCVs with spectrally distinct fluorescent tags. In the case of using multiple pMSCV vectors bearing the same antibiotic resistance gene, the resulting cell line must be sorted for positive cells based on the signal of the fluorescent tags. Using this approach, we successfully generated multiple double-tagged cell lines with the combination of mNeonGreen and mCherry fluorescent tags (unpublished data).
CRITICAL: The population of cells after transduction typically shows a rather heterogeneous expression of a transgene. Moreover, the majority of loci used for the integration by retroviruses are silenced (Mok et al., 2007). Given that, we strongly recommend enriching for cells with the desired level of transgene expression, for instance by fluorescence-activated cell sorting (FACS). Alternatively, patches of cells expressing the transgene can be also isolated from culture plates manually. Once isolated, these cells should be quickly expanded and cryopreserved in sufficient numbers for later use. Sorting out the desired cell population is even more pertinent if no efficient antibiotic selection is available. We emphasize occasional monitoring of the level of expression of the transgene when routinely propagating these cells. If a notable drop in the number of transgene-positive cells is observed (when compared to the starting point) we suggest thawing a low passage vial of these transductants or to re-sort the cell population by FACS to again enrich for cells expressing the transgene at a sufficient level.
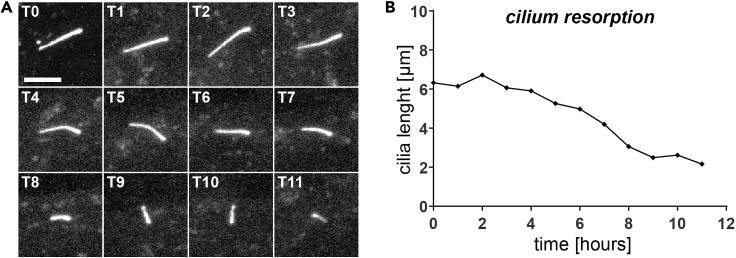
Figure 16.
Outcome of analysis of the time-lapse videos
(A) maximum Z intensity projected representative images of resorbing cilium.
(B) Cilium length (μm) as measured over time. Scale bar=5 μm.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Polyclonal Anti-ARL13B antibody produced in rabbit | Proteintech | Cat# 17711-1 |
| Monoclonal Anti-γ-Tubulin antibody produced in mouse | Sigma-Aldrich/Merck | Cat# T6557 |
| Donkey anti-Rabbit IgG Secondary Antibody, Alexa Fluor 568 | Thermo Fisher Scientific | Cat# A10042 |
| Donkey anti-Mouse Secondary Antibody, Alexa Fluor 647 | Thermo Fisher Scientific | Cat# A-31571 |
| Chemicals, peptides, and recombinant proteins | ||
| Bacillol AF | Hartmann | Cat# 975075 |
| Blasticidin S | Sigma-Aldrich/Merck | Cat# 15205 |
| DMEM, high glucose, GlutaMAX™ Supplement, pyruvate | Gibco/Thermo Fisher Scientific | Cat# 31966021 |
| DMEM/F-12, no glutamine | Gibco/Thermo Fisher Scientific | Cat# 21331020 |
| Doxycycline Hydrochloride, Ready-Made Solution | Sigma-Aldrich/Merck | Cat# D3072-1ML |
| FBS Ultra-low endotoxin | Biosera | Cat# FB-1101/500 |
| FluoroBrite DMEM | Thermo Fisher Thermo Fisher Scientific | Cat# A1896701 |
| G418 | Roche | Cat# 4727878001 |
| L-glutamine | Biosera | Cat# XC-T1715/100 |
| Lipofectamine 3000 | Invitrogen/Thermo Fisher Scientific | Cat# L3000-008 |
| OPTIMEM | Gibco/Thermo Fisher Scientific | Cat# 31985070 |
| Penicillin/Streptomycin | Biosera | Cat# XC-A4122/100 |
| Polybrene | Sigma-Aldrich/Merck | Cat# H9268 |
| Polyethylenimine (PEI) | Sigma-Aldrich/Merck | Cat# 408727 |
| Trypsin-EDTA Solution 0.05% | Serana | Cat# RTL-003-100ML |
| Zeiss Immersol Immersion oil 518 F | Carl Zeiss | Cat# 444960-0000-000 |
| Zeiss Immersol Immersion oil W 2010 | Carl Zeiss | Cat# 444969-0000-000 |
| Experimental models: Cell lines | ||
| hTERT-RPE-1 Flp-In™ T-REx™ (human) | a gift from E. Nigg (University of Basel) | Bernatik et al., 2020 |
| Phoenix-Ampho (human) | ATCC | Cat# CRL-3213 |
| Recombinant DNA | ||
| pGFT1.1 | Derivative of the pgLAP1_Neo with EGFP tag replaced by mNeonGreen. | N/A |
| pgLAP1_Neo | Derivative of the pgLAP1 (a gift from P. Jackson, Stanford University School of Medicine; addgene Plasmid #19702) with Hygromycin resistance gene replaced by G418 (Neomycin) resistance gene for selection of RPE-1 cells | (Bernatik et al., 2020; Pejskova et al., 2020) |
| pgLAP2_Neo | Derivative of the pgLAP2 (a gift from P. Jackson, Stanford University School of Medicine; addgene Plasmid #19703) with Hygromycin resistance gene replaced by G418 (Neomycin) resistance gene for selection of RPE-1 cells | (Bernatik et al., 2020; Pejskova et al., 2020) |
| pMSCV-N-mNG-IRES-PURO | The plasmid MSCV-N-FLAG-HA-IRES-PURO (a gift from W. Harper, Harvard Medical School; addgene plasmid #41033 (Sowa et al., 2009)), was modified by replacing FLAG-HA with mNeonGreen. | (Kiesel et al., 2020) |
| pOG44 | Thermo Fisher Scientific | V600520 |
| Software and algorithms | ||
| Fiji | (Schindelin et al., 2012) | version 2.1.0; https://imagej.net |
| ZEN Black | Carl Zeiss | version 2.3 SP1 |
| ZEN Blue | Carl Zeiss | version 2.6 |
| NIS Elements Advanced Research (Ar) | Nikon | version 5.02 |
| Other | ||
| biosafety level 2 flow box + centrifuge and microscope | N/A | N/A |
| CELLVIEW CELL CULTURE SLIDE, PS, 75/25 MM, | Greiner Bio-One | Cat# 543079 |
| glass bottom dish with 20 mm micro-well | Cellvis | Cat# D35-20-1.5-N |
| μ-Slide 8 well chamber slide | IBidi | ibiTreat; Cat# 80826 |
| table top centrifuge | N/A | N/A |
Materials and equipment
HEK complete medium
| Reagent | Final concentration | Amount |
|---|---|---|
| DMEM | 89% | 445 mL |
| FBS | 10% | 50 mL |
| P/S | 1% | 5 mL |
| Total | n/a | 500 mL |
RPE-1 complete medium
| Reagent | Final concentration | Amount |
|---|---|---|
| DMEM/F-12 | 88% | 440 mL |
| 10% FBS | 10% | 50 mL |
| L-glutamine | 1% | 5 mL |
| P/S | 1% | 5 mL |
| Total | n/a | 500 mL |
Phoenix transfection medium
| Reagent | Final concentration | Amount |
|---|---|---|
| DMEM | 99.50% | 49.75 mL |
| FBS | 0.50% | 0.25 mL |
| Total | n/a | 50 mL |
Imaging medium
| Reagent | Final concentration | Amount |
|---|---|---|
| FluoroBrite DMEM | 98% | 490 mL |
| L-glutamine | 1% | 5 mL |
| P/S | 1% | 5 mL |
| Total | n/a | 500 mL |
Viral particles production medium
| Reagent | Final concentration | Amount |
|---|---|---|
| DMEM | 90% | 45 mL |
| FBS | 10% | 5 mL |
| Total | n/a | 50 mL |
Note: All prepared media can be stored in a fridge (4°C) for several months.
PEI Solution
| Reagent | Final concentration | Amount |
|---|---|---|
| PEI | 2 mg/mL | 80 μL |
| PBS (pH 7.2) | n/a | 40 mL |
| Total | n/a | 80 mL |
Note: Prepared solution can be stored in a fridge (4°C) for several months.
Phoenix transfection mixture
| Reagent | Final concentration | Amount |
|---|---|---|
| OPTIMEM | n/a | 300 μL |
| pMSCV-mNeonGreen with GOI of choice (ARL13b) | n/a | 8 μg |
| PEI Solution | n/a | 20 μL |
| Total | n/a | n/a |
CRITICAL: Antibiotics (such as Penicillin/Streptomycin, G418, etc.) are considered harmful, use appropriate protection when working with them (wear gloves, use cell culture hood).
Step-by-step method details
Analysis of time-lapse videos to study primary cilia dynamics
Timing: 3–4 days
Here we describe in detail the analysis of primary cilia growth/resorption in living cells on the example of mNeonGreen-ARL13b cell line prepared in the previous steps.
-
1.
Day 1: Seed the cells (here we used hTERT-RPE-1 Flp-In T-REx TTBK2 KO cells (Bernatik et al., 2020) with DOX-inducible expression of FLAG-TTBK2 and constitutive mNeonGreen-ARL13b reporter) in a RPE-1 complete medium supplemented with DOX on a 10-well glass-bottom CELLVIEW CELL CULTURE SLIDE, PS, 75/25 MM at a high density (∼25.000–30.000 cells per well).
Note: Typically we use DOX in 1 μg/mL final concentration, please see DOX optimization step in the section “Generation of stable cell lines using Flp-InTM T-RExTM system”.
Note: Other types of chamber slides can also be used here. Throughout this protocol we used CELLVIEW CELL CULTURE SLIDE or IBidi μ-Slide. We recommend choosing CELLVIEW CELL CULTURE SLIDE for long-term experiments such as cilia growth/resorption analysis. Their black chambers offer better protection from light during adjacent well imaging. Moreover, the area of the chamber is smaller and volume of the medium higher, thus decreasing the risk of medium evaporation compared to IBidi μ-Slide.
Note: For the imaging setup using LSM800 (described below) it is advised to use adjacent wells and limit the analysis to 3 conditions (3 wells) maximum to reduce the movement of the slide during imaging. Increasing the number of wells/conditions comes hand in hand with increased time the microscope needs to move between each condition/well and increasing the chance the immersion runs dry.
-
2.
Day 2 (the exact timing may differ in respect to your experimental treatment): Depending on the biological question, this is probably the time to treat the cells as needed (e.g., small molecules, siRNA transfection, etc.).
-
3.Day 3 (the exact timing may differ in respect to your experimental treatment):
-
a.Wash the cells with PBS or serum-free low auto-fluorescence FluoroBrite medium (imaging medium) to remove the remnants of the previous media.
-
b.Add Imaging medium (supplemented with 1 μg/mL of DOX) to the cells to promote ciliogenesis. Add as much medium as possible in the well to prevent its evaporation during the long imaging (we typically use about 300 μL/well).
-
c.Depending on the experimental question either proceed directly to live-cell imaging to monitor the growth of the cilia.
-
d.Or starve the cells for 24 h to promote cilia formation, and before imaging add Imaging medium with 10% FBS (supplemented with 1 μg/mL of DOX, see above) to monitor cilia resorption.
-
a.
Time-lapse live imaging of cilia dynamics acquisition
Given that live cells growing on the surface of the slide are to be imaged in this setup, an inverted microscope system is required. To keep the cells in optimal conditions throughout the whole imaging session (in our case up to 16 h), it is necessary to use a microscope equipped with an environmental chamber, which allows precise control of temperature, humidity, and CO2 levels. The confocal microscope compared to a widefield microscope offers a better signal-to-background noise ratio which might be helpful to extract the specific signal in cells with a higher background signal as seen in our reporter cell line. Inside living tissue, the important events can occur deep within the specimen, further away from the cover glass. Water immersion objectives typically overcome spherical aberrations better than oil immersion objectives, as it better matches the sample refractive index. Due to the increased evaporation it is recommended to use water oil immersion e.g., Zeiss Immersol Immersion oil W 2010 (Carl Zeiss, 444969-0000-000). Moreover, it is very helpful and convenient if the microscope – operating software allows multi-position acquisition with easy position list setup, which will enable to add specific positions of the cells with cilia.
Here we used Inverted (Axio Observer.Z1) laser scanning confocal and epifluorescence microscope LSM800 equipped with an environmental chamber for live-cell imaging (controlled temperature, humidity, CO2). We opted for C-Apochromat 63×/1.20 Korr UV VIS IR water immersion objective. Solid state laser 488 in combination with high-sensitivity GaAsP detector were used to acquire images and the microscope was controlled by ZEN Blue software. Data acquisition was performed in 1024 × 1024 pixel format, with pixel time around 1μs and pixel size 0.10 μm in bidirectional mode with 2× averaging and 8-bit bit depth. Images were taken as Z-stacks thickness 0.6 μm with 0.5 μm slices spacing (see the note below) every 10–15 min for up to 16 h.
Note: Being aware that the optimal Z-slice spacing according to the Nyquist criterion would be lower (0.28 μm) we opted for 0.5 μm slice spacing. This allowed us to keep around 10% overlap between the slices, while significantly reducing the time of acquisition and in turn sample bleaching. Importantly, this setting also allows to use of wider range in the Z-axis, which is particularly relevant for imaging cilia, as they typically spread across the Z-axis. We did not observe any loss of information when using the maximal projection image for the final cilia length analysis.
-
4.
Open the ZEN Blue Software.
-
5.
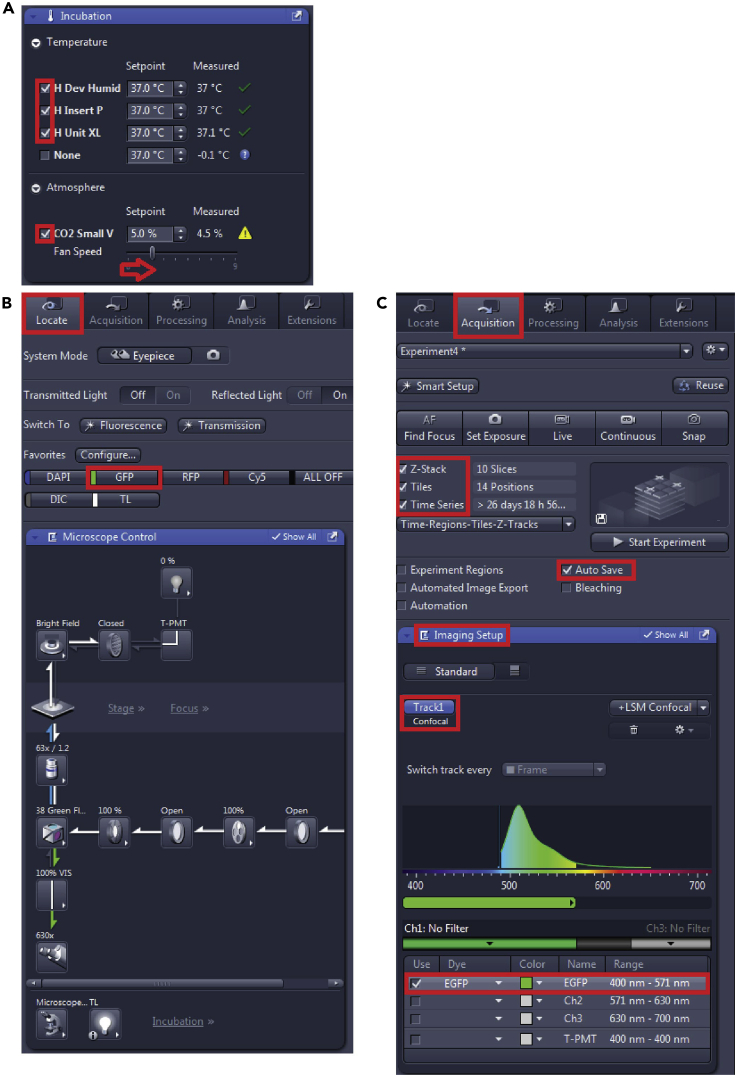
Turn on the temperature and atmosphere control in the “Incubation” window (Figure 3A) at least 20 min before starting the microscope setup for the chamber to reach the set incubation conditions. After sample insertion in the holder the microscope setup will take another 30–40 min, thus the whole system is equilibrated at least for one hour before the actual start of the imaging.
-
6.
Put a drop of the appropriate immersion medium (here we used a Zeiss Immersol Immersion oil W 2010 with a refractive index of water) on the selected objective and insert the slide with the cells in the slide holder, close the chamber with the lid.
-
7.
In the “Locate” tab (Figure 3B) turn on the fluorescence (represented by “GFP” button) and find the focus looking on the specimen through the eyepiece. You can search specifically for a cell with cilium here. Make sure to close the shutter as soon as you find the sample focus, to avoid sample bleaching.
-
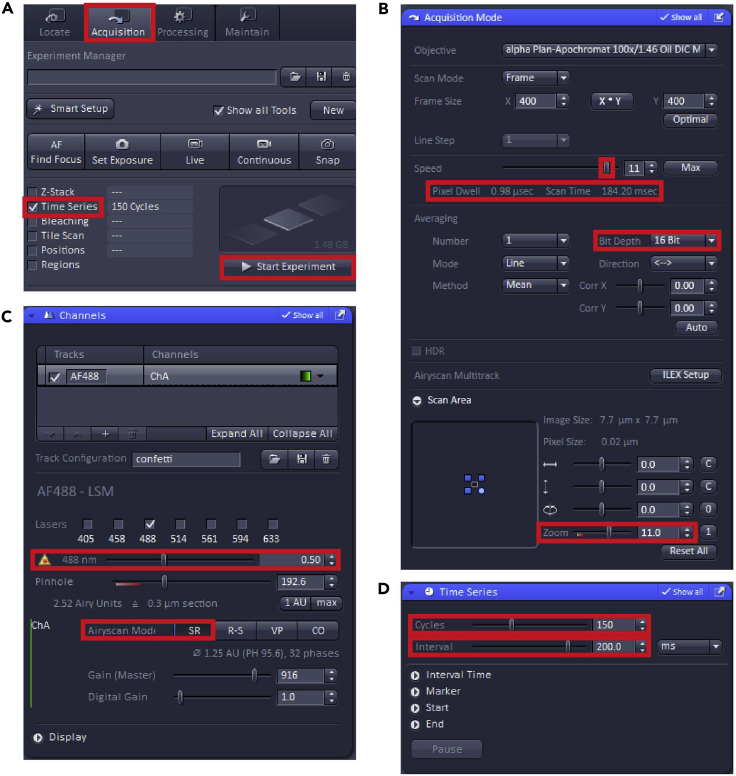
8.
Switch to “Acquisition” tab (Figure 3C), select “Z-stack”, “Tiles” and “Time series” and “Auto Save” to add the particular windows with setup options.
-
9.
In the “Imaging Setup” window (Figure 3C) add “+LSM Confocal”, add “Tracks” in a “Frame” mode, here we only use one channel, thus one Track. Select the “Dye” (here we used the preset EGFP) and the “Color code”.
-
10.
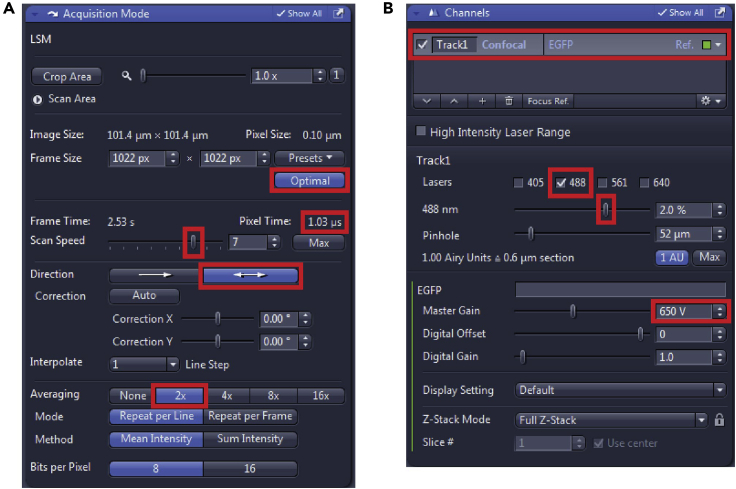
In the “Acquisition Mode” window (Figure 4A) set the “Scan Speed” to get the “Pixel speed” around 1μs. Bidirectional “Direction” of scanning will make the imaging faster and 2× “Averaging” reduces noise and improves image quality.
-
11.
In the “Channels” window (Figure 4B) select one Track at a time, turn on the appropriate laser (here 488 nm for GFP and its derivates such as mNeonGreen), start with the “Master Gain” on ∼650 V, and in the Live mode increase the laser % to see your cells and/or your cilia signal. Keep in mind to leave the laser intensity as low as possible, considering the possible bleaching effect or even cell death over time.
-
12.
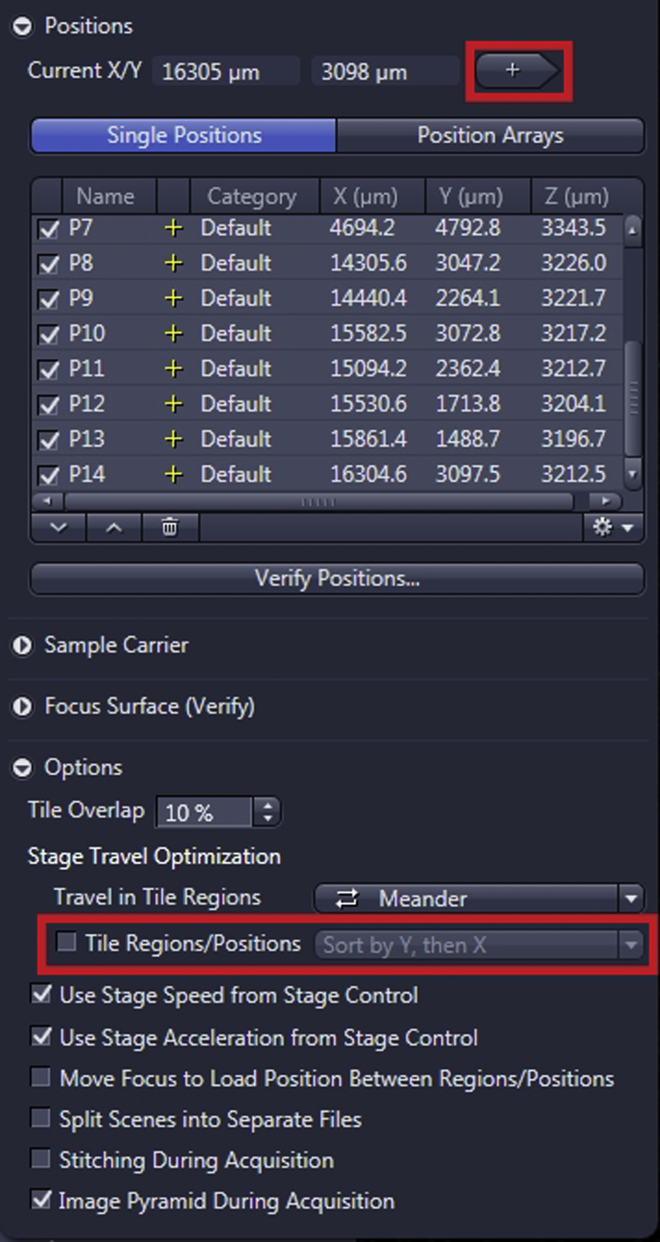
Find the position with the cells of interest either in “Locate” or “Live” mode and add them in “Positions” list in the “Tiles” window by clicking on a “+” button until all positions to be imaged are added to the list. Uncheck the “Tile Regions/Position – Sort by Y then X”, so the positions stay in the order added also for the imaging (Figure 5).
Note: When planning the experiment, keep in mind that only a limited number of positions can be imaged in one experiment, depending on the number of conditions/wells, number of channels, and number of the Z-slices to fit into the selected time between the timepoints. This is also highly dependent on the microscope system of your choice. With our setting with imaging two conditions in one channel, 10–15 Z-slices each and 10–15 min between the steps we used up to 20 positions in total.
-
13.
In the “Definite Focus” window (Figure 6A) in “Live” mode click “Find Surface”, if necessary fine-tune the focus on the center of the cell/cilium and click Store Focus. This creates an offset value, which will be used to set the focus plane in the same distance from the automatically found focus before each timepoint. It is also possible to do this step right at the beginning of the imaging to find the focus plane of the cells and thus eliminating the need for the manual search of the focus plane.
-
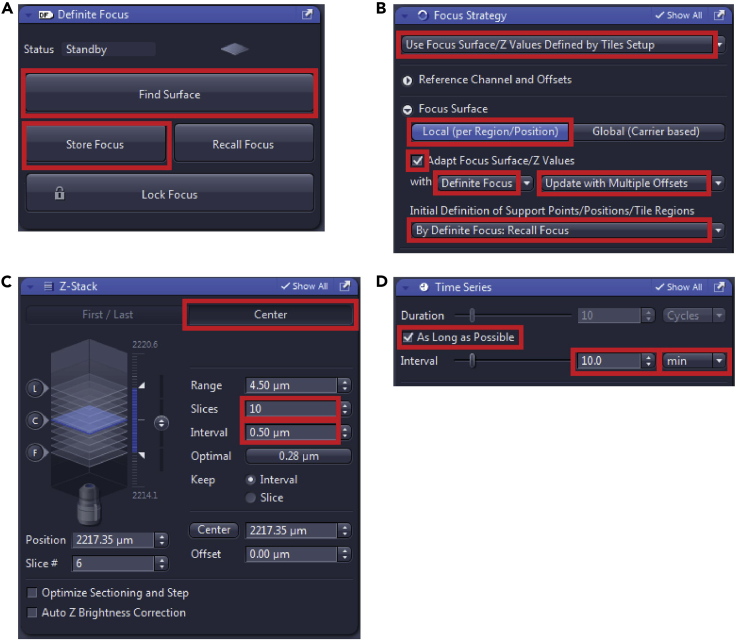
14.In the “Focus Strategy” window (Figure 6B) choose:
-
a.“Use Focus Surface/Z Values Defined by Tiles Setup”.
-
b.In “Focus Surface” choose “Local (per Region/Position)”.
-
c.Check “Adapt Focus Surface/Z values” with “Definite focus”, “Update with Multiple Offsets”.
-
d.In “Initial Definition of Support Points/Positions/Tile Regions” choose “By Definite Focus: Recall Focus”.
-
e.With this setting before each new timepoint, the software will find the surface and update the focus plane, which would help with the loss of focus during the long-term imaging.
-
a.
-
15.
In the “Z-stack” window (Figure 6C) in the “Center” mode set the desired number of slices, and the interval between them, the center position will be taken from the “Tiles setup”, as set in the “Focus Strategy” above.
-
16.
In “Time Series” window (Figure 6D) set “Duration” to “As Long as Possible” so the scanning will continue until manually stopped or set a precise number of cycles. Set an interval between the individual time points and the units – here we used 10 or 15 min.
-
17.
In “AutoSave” window fill in the file name and the saving path.
-
18.
Click “Start Experiment” which will start imaging. Troubleshooting 4, 5, and 6
-
19.For further analysis, the Z-slices can be projected in one layer by Orthogonal maximal projection. This is particularly useful when imaging objects like cilia, which spread through several Z slices. To do so:
-
a.Have your Z-stack ∗.czi file to be processed in the active tab.
-
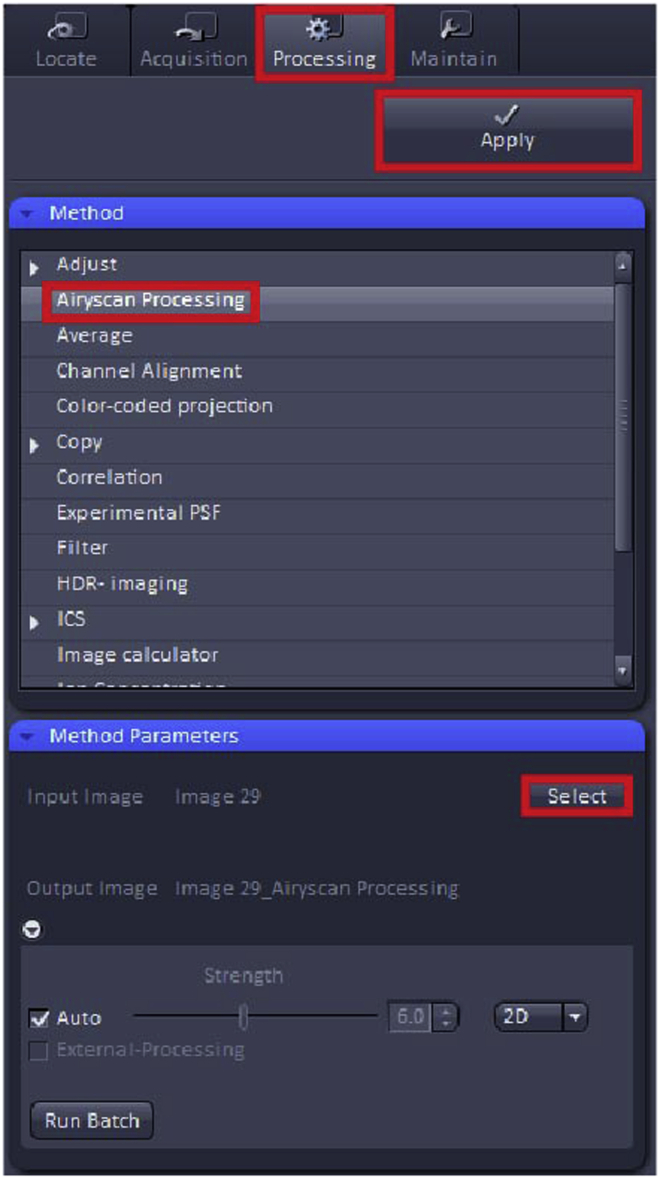
b.Go to “Processing” tab.
-
c.In “Method” find “Orthogonal Projection”.
-
d.Set “Projection Plane” to “Frontal (XY)”, set “Method” to “Maximum” and click “Apply”.
-
e.This will create a maximum intensity projection of all the Z planes into a single XY image.
-
a.
-
20.The resulting file contains all the positions as “Scenes” in one large ∗.czi file. To export individual positions to individual TIFF files:
-
a.Have the ∗.czi file to be processed in the active tab (it can be either the original Z-stack or processed Orthogonal Projection file).
-
b.Go to “Processing” tab.
-
c.In “Method” find OME/TIFF export.
-
d.In the “Method Parameters” unselect “Merge All Scenes” and set the path for the exported TIFF images, click “Apply”.
-
a.
Figure 3.
Setup of the Zeiss LSM800 in ZEN Blue, steps 18, 20–22
(A) Incubation conditions, (B) localizing the cells using the eyepiece and (C) Imaging Setup details.
Figure 4.
Setup of the Zeiss LSM800 in ZEN Blue, steps 23, 24
(A) Details of Acquisition setup and (B) Laser power and Gain setup of Channels.
Figure 5.
Setup of the Zeiss LSM800 in ZEN Blue, step 25
Creating a list of Positions for the subsequent imaging.
Figure 6.
Setup of the Zeiss LSM800 in ZEN Blue, steps 26–29
(A) Definite Focus and Offset setup (B) Focusing strategy details (C) Setup of the Z-stack and (D) Time Series.
Imaging of intraflagellar transport (IFT) in primary cilia of living cells
Timing: ∼4 days (according to the type of treatment)
This section covers live-cell imaging analysis of IFT, namely the measurement of transport frequency and velocity, in primary cilia of hTERT-RPE-1 cells stably expressing mNeonGreen-tagged protein IFT74 (pGFT1.1 - mNeonGreen-IFT74).
-
21.
Day 1: Seed the hTERT-RPE-1 mNeonGreen-IFT74 reporter cell line in RPE-1 complete medium supplemented with DOX (typically 1 μg/mL, please see DOX optimization step in the section “Generation of stable cell lines using Flp-InTM T-RExTM system”) on IBidi ibiTreat μ-Slide 8 well chamber slide (see note after step 14 in the previous section of this protocol) at a high density (∼25.000 cells per well). We used 300 μL of the media per well.
-
22.
Day 2 (the exact timing may differ in respect to your experimental treatment): Depending on the biological question, this is probably the time to treat the cells as needed (e.g., small molecules, siRNA transfection, etc.).
-
23.Day 3 (the exact timing may differ in respect to your experimental treatment):
-
a.Aspirate the medium from the cells grown in μ-Slide 8 well chamber slide.
-
b.Wash the cells with PBS or imaging medium to remove the remnants of the previous media. Aspirate the solution for each well.
-
c.Add Imaging medium (supplemented with 1 μg/mL of DOX, see above) to the cells to promote ciliogenesis.
-
d.After 24 h proceed directly to live-cell imaging to monitor the transport of IFT trains.
-
a.
Note: If your experimental setup is based on the use of small molecule(s), do not forget to add them to the media. If you want to study more rapid response to your intervention, it is probably better to start the treatment just before the imaging (see below).
Time-lapse live imaging of IFT in primary cilia acquisition
As the copy number of each IFT protein in a given train is rather low (Ishikawa and Marshall, 2015), the signal emitted by mNeonGreen-tagged IFT protein per each train is weak. Typically, this could be circumvented by using long exposures to increase the signal-to-noise, but imaging the IFT trains requires taking images at a fast enough rate that the movement of the trains does not cause blurring in the image. Thus, it is necessary to use both fast and sensitive instrumentation. For this purpose, we have employed an inverted Zeiss LSM880 laser scanning confocal microscope mounted on an Axio Observer.7 stand and equipped with an Airyscan detector. We opted for alpha Plan-Apochromat 100×/1.46 oil differential interference contrast M27 immersion objective. Compact light source HXP120V mercury lamp was used for the ocular observation. Images were acquired using Multiline Argon laser 35 mW in combination with a ZEISS Airyscan detector and the microscope was controlled by ZEN Black acquisition software. Data acquisition was performed using the confocal in super resolution mode, with the Airyscan detector, Pixel Dwell Time below 1 μs and Scan Time below 200 ms (depending on scan speed), 16 Bit Depth (8 bit depth can also be used, it could actually help for dim the sample) and Zoom 8–11 (depending on the length of imaged cilia). Images were taken as Time Series taking an image every 200 ms, with 150 images in total. The Airyscan detector allows acquisition with increased resolution and signal-to- noise ratio compared to conventional confocal scanning. A spinning disk confocal microscopy, Total Internal Reflection Fluorescent (TIRF), or “TIRF-like” microscopy (see the section “Imaging IFT using variable-angle epifluorescence microscopy” for details) is also suitable for the IFT imaging due to reduced bleaching of the fluorescence signal and very good signal-to-background noise ratio, in comparison to e.g., standard wide-field microscopy.
Note: Using an oil objective with a higher numerical aperture (NA) will allow for higher resolution imaging, especially in combination with the Airyscan detector (in comparison to the water objective used in the previous section).
-
24.
Before beginning the imaging, prepare the microscope of your choice (here Zeiss LSM 880 with Airyscan detector, operated via ZEN Black acquisition software according to your needs (e.g., preheat the chamber to 37°C and equilibrate it with CO2 (see step 18 in the previous section), switch on argon laser, etc.).
-
25.
Open the ZEN Black acquisition software.
-
26.
Put a drop of the appropriate immersion medium on the selected objective and insert the μ-Slide 8 well coverslips with the cells in the slide holder.
-
27.
In the “Locate” tab turn on the fluorescence (represented by “GFP” button) (Figure 7A) and find the focus with the eyepiece. You can search specifically for a cell with cilium here.
-
28.
In the “Acquisition” tab in “Imaging Setup” window (Figure 7B) add “+LSM Confocal”, add “Tracks” in a “Frame mode”, here we only use one channel, thus one Track. Select the “Dye” and a “Color code”.
-
29.Select the “Acquisition” tab (Figure 8A) and tick “Time Series”. The recommended settings of key parameters of the “Acquisition Mode” window (Figure 8B) to start with is the following:
-
a.Pixel Dwell Time: typically below 1 μs - depending on scan speed,
-
b.Scan Time: typically below 200 ms - depending on scan speed)
-
c.Bit Depth: 16 Bit,
-
d.Zoom: typically 8–11, depending on the length of imaged cilia.
-
a.
Note: For our imaging setup it was sufficient to acquire images in one focus plane in “Airyscan super resolution” module. However, in case the use of Z-stack is desirable the “Fast module” could be used instead of the “Airyscan super resolution” to shorten the time of individual acquisition steps.
-
30.
Set laser line attenuator transmission (for 488 nm: 0.5%) and select the “Airyscan Mode / SR” (superresolution) in “Channels” window (Figure 8C).
-
31.
Set “Cycles” (up to 150) and “Intervals” (to 200 ms) in “Time Series” window (Figure 8D).
-
32.
While being in “Live mode”, adjust focus on primary cilia and select a region of interest with the cilium you want to analyze, click on “Start Experiment” (Figure 8A). Troubleshooting 7
-
33.
Following the time-lapse acquisition, a ∗.czi file is created and saved locally.
-
34.
Go to the “Processing” window and select “Airyscan Processing”, “Select” and then Apply (Figure 9). Airyscan processing is a type of post-acquisition processing, which allows for simultaneous improvement in resolution and signal-to-noise. This generates a processed ∗.czi file with better resolution than the unprocessed ∗.czi file. For illustration of the processed file see Methods Video S1.
Figure 7.
Setup of the Zeiss LSM880 in ZEN Black, steps 40–41
(A) Localizing the cells using the eyepiece and (B) Imaging Setup details.
Figure 8.
Setup of the Zeiss LSM880 in ZEN Black, steps 42–45
Setup details of Acquisition tab (A) Acquisition Mode (B), Channels (C), and Time Series (D).
Figure 9.
Setup of the Zeiss LSM880 in ZEN Black, step 47
Setup details of Airyscan processing.
The four IFT trains distinguished by color are marked as an example in the time-lapse.
Note: For the analysis of IFT74 dynamics in primary cilia we used auto setting of Airyscan processing. For the analysis of molecule movement it is sufficient to use the auto setting when the strength of the filter is calculated for each image separately, which results in different Airyscan processing values. However, when comparing structure sizes or intensities between different samples, the same optimized Airyscan processing setting (with manually adjusted strength of the processing) should be reused in the whole set of images which are to be compared.
-
35.
Repeat the steps 45–47 to obtain a sufficient number of recordings of primary cilia per each condition of your experiment. We recommend measuring at least 20 primary cilia per experimental condition to get sufficiently robust data for the subsequent analysis.
Note: The speed of IFT movement is typically between 0.25 and 1 μm/s (Figure 17C) (Ishikawa and Marshall, 2015). The settings described above allow taking an image every 200 ms, with 150 images in total, which is typically sufficient to detect the movement of IFT trains. We try to minimize the bleaching of IFT trains by setting laser power to minimal levels. Please consider adjusting these parameters according to your needs to get optimal results.
Note: Primary cilia in non-polarized cells (e.g., hTERT-RPE-1) are typically confined in a deep narrow pit created by membrane invagination (Mazo et al., 2016; Sorokin, 1962). While response of such “submerged” primary cilia to clues, such as fluid flow, is limited (Mazo et al., 2016), their imaging and IFT analysis is, in turn, fairly straightforward (as they do not significantly move).
Pause Point: The subsequent computer analysis can be done at any time.
-
36.Here we describe how to assemble obtained images into a kymograph (a very convenient tool to visualize time-lapse data in a single image) and to determine the velocity and frequency of IFT trains. To analyze the obtained data for IFT movement dynamics we suggest using Fiji software (2.1.0 version or newer, no specific plug-ins are required).Note: Commercial image analysis software packages (e.g., MetaMorph) are also suitable.
-
a.Start the Fiji software to open an Airyscan processed ∗.czi file (i.e., drag and drop the file to Fiji toolbar or click on File → Open…) (Methods Video S2, steps 49a–c).Methods Video S2. Step-by-step tutorial for measuring the primary cilium length in Fiji, related to steps 49a–cDownload video file (2.8MB, mp4)
-
b.Click on Image → Adjust → Brightness/Contrast for adjustment of brightness and contrast to create an optimal image display for analysis.
-
c.Right-click on a straight line in the Fiji toolbar and choose Segmented Line. Draw a segmented line from the base to the tip of the primary cilium (left-click to start drawing and adding points, right-click to finish the drawing) and measure the length of the cilium by selecting the panel “Analyze” → “Measure” or keyboard shortcut Ctrl+M. The measured length will be displayed as μm in “Results” window.All steps of time-lapse imaging analysis of IFT transport dynamics using Fiji software can also be found in the tutorial videos (Methods Videos S2 and S3, and Figure 4, Figure 14).
 CRITICAL: Draw the line from base to tip of the primary cilium. To position the cilium correctly, pay attention to the signal difference between the base and the tip (the foci of IFT protein signal at the ciliary base is typically more prominent and also of different shape over the IFT pool in the tip, Figure 10). Alternatively, detection of the cilia orientation can be facilitated by expressing additional markers, such as a basal body protein fused with a red fluorescent tag (e.g., mCherry-TTBK2). In a situation the orientation is not clear, exclude such primary cilium from the analysis.Methods Video S3. Step-by-step tutorial for kymograph creation from the time-lapse data in Fiji, related to steps 49d–51Download video file (15.1MB, mp4)Methods Video S4. Useful functions for kymograph analysis in Fiji, related to step 52 and optional stepsDownload video file (4.1MB, mp4)
CRITICAL: Draw the line from base to tip of the primary cilium. To position the cilium correctly, pay attention to the signal difference between the base and the tip (the foci of IFT protein signal at the ciliary base is typically more prominent and also of different shape over the IFT pool in the tip, Figure 10). Alternatively, detection of the cilia orientation can be facilitated by expressing additional markers, such as a basal body protein fused with a red fluorescent tag (e.g., mCherry-TTBK2). In a situation the orientation is not clear, exclude such primary cilium from the analysis.Methods Video S3. Step-by-step tutorial for kymograph creation from the time-lapse data in Fiji, related to steps 49d–51Download video file (15.1MB, mp4)Methods Video S4. Useful functions for kymograph analysis in Fiji, related to step 52 and optional stepsDownload video file (4.1MB, mp4) -
d.Open the “ROI Manager” in Fiji by selecting “Analyze” → “Tools” → “ROI Manager” (Methods Video S3, steps 49d-51).
-
e.Click on “Analyze” → “Multi Kymograph” → “Multi Kymograph” (with Linewidth 1) to create a kymograph from the time-lapse data.
-
a.
-
37.
Rotate the image 90 degrees by left-clicking on “Image” → “Transform” → “Rotate 90 Degrees Left”.
Optional: You can enlarge the kymograph by pressing the “+” on the keyboard and also adjust brightness and contrast if needed.
Note: Note that the base of the imaged cilium is now at the bottom of the obtained kymograph, while the ciliary tip is at its top (Figure 11A). Consequently, the first image of time-lapse is situated on the very left side of the obtained kymograph, which can in turn be used to track any detected movement of mNeonGreen-IFT74 particles inside the cilium.
Figure 17.
Outcomes of IFT74 transport in primary cilium of living cells analysis
(A–C) Length of the primary cilium (A), number (B) and velocity (C) of the anterograde and retrograde IFT74 trains were determined. Error bars represent mean with SEM.
Figure 14.
Selection of optical configuration, step 63
Highlighted button sets up the high-power illumination configuration. Right-click on the button offers the option “Assign current microscope settings” to update the configuration.
Figure 10.
Primary cilium orientation
IFT signal (green; mNeonGreen) at the base is more distinct and also of a different shape compared to the IFT protein signal at the tip of the primary cilium (PC). Scale bar=1 μm.
Figure 11.
Tracking of movement in kymograph
(A) The kymograph represents the movement of IFT74 particles inside the primary cilium (PC). The primary cilium base and tip are at the bottom and top of the kymograph, respectively. The first image of the time-lapse is located on the left side of the kymograph. The green arrow shows the anterograde transport going from the base to the tip of the cilium while the red arrow indicates the retrograde transport going from the tip to the base of the cilium.
(B) Tracks in the kymograph can be distinguished by color and numbers. Green lines represent the anterograde transport while red lines depict retrograde transport.
(C) The visualized tracks in the kymograph have to be overlaid from ROI manager before you save the image.
-
38.
IFT trains inside cilia use either anterograde or retrograde type of transport (Rosenbaum and Witman, 2002). To track the movement of individual IFT trains, draw straight lines (right-click on a Segmented line in Fiji toolbar and choose Straight Line) through all visible tracks of IFT74 trains in the kymograph and add them to the “ROI manager”.
Note: Note that we track only trajectories clearly distinguishable from the background signal (in turn the drawn lines sometimes do not fully span from the tip to the base).
-
39.
Select the “ROI Manager” panel and click “Measure” (Methods Video S4).
Note: Remember that only the IFT train trajectories tracked in the kymograph in step 49c will be measured. The obtained values represent an angle between each measured trajectory and the horizontal axis, the speed of IFT train is calculated by tan(angle) (Ishikawa and Marshall, 2015). Because the unit of tan(angle) is given in pixel, this can be converted into μm/s by multiplying pixel size and frequency (frames per second), see step 54. The positive angle values represent the anterograde transport while the negative values represent the retrograde transport.
Note: Keep in mind that only IFT trains “passing through” the drawn line (step 49c) will be included in kymograph analysis. Consequently, it may be necessary to draw an additional line to “cover” all IFT particles recorded in the time-lapse Movie.
Optional: To add a color code in the kymograph for better orientation, click on the line or the number in “ROI Manager”, select “Properties” in “ROI Manager”, and change the color (Video 3). In Figure 11B shown as an example, the anterograde transport lines are shown in green while the lines representing the retrograde transport are in red (see for details). The numbering of lines is done automatically, but you can hide the numbers by checking off “Labels” in “ROI Manager” (Methods Video S4).
Optional: To save the kymograph with overlaid track visualization, select “Image” → “Overlay” → “From ROI Manager” (Figure 11C), and save as usual.
CRITICAL: Keep in mind that the position of the signal in the kymograph corresponds to the time when the individual images were recorded. As the time axis goes from left to right, the lines representing detected IFT tracks are also oriented from left to right. In turn, the tracks going from the base of the cilium (bottom of the kymograph) to the tip of the cilium (top of the kymograph) visualize the anterograde transport, while the tracks going from the tip to the base belong to the retrograde transport. The direction of drawing the straight lines in the kymograph is critical for the correct calculation of IFT trains' velocity. Therefore, the individual lines need to be drawn in the proper direction: anterograde from the bottom of the kymograph; retrograde from the top of the kymograph; and in chronological order, i.e., from left to right (Figure 11A).
Pause Point: The subsequent determination of frequency and velocity of measured IFT trains can be done at any time.
Determination of frequency and velocity of IFT trains using mNeonGreen-IFT74:
-
40.
The frequency of trains directly corresponds to the number of green (anterograde) and red (retrograde) lines per used period of time.
-
41.
Calculate the velocity of individual IFT trains:
| Velocity (μm/s) = tan(angle) ∗ pixel size ∗ frames per second |
Note: As mentioned earlier, the determined angle values for retrograde transport will be negative, which in this case reflects the fact the trains are moving in the opposite direction (to the anterograde transport). However, as velocity cannot be negative, simply use absolute values for its calculation in the case of the IFT trains moving via the retrograde route.
Note: The used pixel size and number of frames can be determined in Fiji, click on Image → Properties (Methods Video S4).
Alternative: Imaging IFT transport using variable-angle epifluorescence (VAEM) microscopy
Experiments focused on detailed study of IFT train trajectories require high time resolution and signal-to-noise ratio. If these parameters are critical, wide-field techniques such as TIRF microscopy can be a preferred alternative to confocal microscopy. TIRF microscopy uses critical angle illumination, where incident light is totally internally reflected at the glass-sample interface and only ∼300 nm deep evanescent field penetrates into the sample (Martin-Fernandez et al., 2013). This way, most of the background fluorescence is suppressed and contrast sufficient to image single molecules is achieved in the illuminated part of the cells. Most primary cilia are, unfortunately, located in cells such that they are out of the range of a true TIRF microscopy. However, modifications of the TIRF microscopy, sometimes referred to as “pseudo TIRF”, such as highly inclined and laminated optical sheet (HILO) or variable-angle epifluorescence microscopy (VAEM) can be used to illuminate fluorophores deeper in the sample while still gaining significant improvement in signal-to-noise ratio (Tokunaga et al., 2008) (Figure 12). VAEM illuminates a sample at a sub-critical angle, at which light does not undergo total internal reflection, but enters the sample at a high inclination. It can be set up on any microscope equipped with a TIRF condenser. Here we demonstrate its use to visualize IFT in primary cilia with high time resolution.
Figure 12.
Comparison of epifluorescence microscopy (left) and VAEM (right)
A cilium is marked with an arrow. Note the difference in the background fluorescence signal. Scale bar=10 μm.
We use an inverted widefield microscope Nikon Eclipse Ti-E equipped with a motorized XY stage, Perfect Focus System, H-TIRF module, Nikon CFI Apo TIRF 60× Oil NA 1.4 objective, 488 nm laser diode illumination and Quad Band Filter Set 405/488/561/640 nm (TRF89901, Chroma). Temperature, humidity and CO2 concentration were maintained by module for environmental control (Okolab). Images were recorded by an EMCCD Andor iXon Ultra DU888 camera (Andor Technologies) with 1024 × 1024 sensor format, 13 μm × 13 μm pixel size and 16-bit bit depth, controlled by NIS elements Ar 5.02 software.
-
42.
Prepare ciliated hTERT-RPE-1 cells with mNeonGreen-IFT74 marker as described in previous sections.
-
43.
Switch on the microscope, camera, and 488 nm laser illumination.
-
44.
Install the objective-heating collar and environmental control stage insert, allow at least 20 min for the temperature, CO2 and humidity to stabilize before inserting the sample (see step 18 in the previous section).
-
45.
Start the NIS Elements software.
-
46.
Apply immersion oil to the objective, insert a glass bottom dish with the cells and switch on the “Perfect Focus” system. As the glass/buffer interface has not been detected yet, the “Perfect Focus” button on the microscope will be flashing green.
-
47.
Slowly raise the objective until the immersion oil makes contact with the glass bottom of the dish. Keep raising the objective until the microscope emits a beep and the “Perfect Focus” button stops flashing. This means that the glass/buffer interface has been detected and the “Perfect Focus” is now in control of the objective-sample distance. The focusing knobs on the microscope are no longer active and focus adjustments must be made through the offset control wheel of the “Perfect Focus” system.
-
48.
Switch on 488 nm laser illumination, start “Live” acquisition in NIS Elements, and position the sample to have at least one cilium in the field of view.
-
49.You need to set up one microscope configuration with a low laser power for sample screening and another one with a higher laser power for acquisition. There are three main parameters to set (Figure 13): (a) exposure time, (b) illumination angle, and (c) laser power, and optionally also (d) region of interest. Signal-to-background ratio above 1.5 (typically 1,500 gray value background and 2,250 gray value IFT fluorescence) allows reliable tracking of IFT trains, therefore it should be the minimum target for high laser power acquisition.
-
a.Exposure time will be determined by the experimental question. The minimum time we use is 10 ms. Using shorter exposure times does not improve the time resolution much, but reduces the signal-to-noise ratio significantly. Select an appropriate value in the “DU-888 Settings” window (Figure 13A).
-
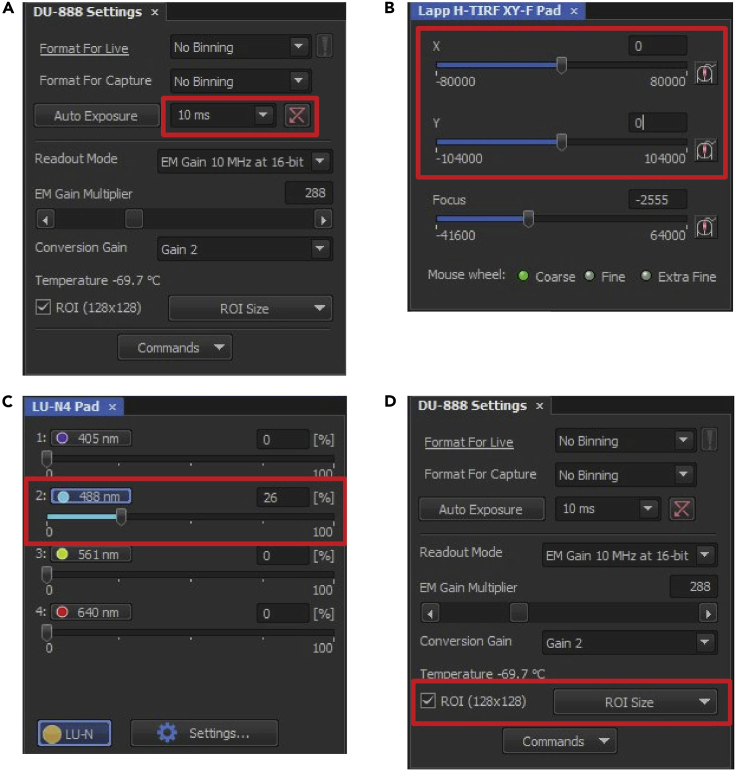
b.Illumination angle needs to be adjusted for each experiment individually. The position of cilia in individual cells varies, which affects their distance from the glass surface. Hence, the optimal illumination angle also varies and needs to be determined empirically. We recommend adjusting the angle each time the sample dish is replaced, or a dish is moved by a significant distance (more than 2 mm). The illumination angle is set by moving the beam position in X and Y in the “Lapp H-TIRF XY-F Pad” window (Figure 13B). When both values are set to 0, the microscope is in epifluorescence mode.Note: To find an optimal illumination angle, first determine the critical angle of a true TIRF illumination. At the critical angle, no cilia will be visible, only the cell regions in direct contact with the glass surface. Subsequently, reduce the angle step-by-step towards the epi-illumination mode until cilia start to be visible. Stop when the signal from the ciliary marker starts to be eclipsed by the fluorescence background. As the cilium in focus is partially bleached at this point, move to a different field of view and verify that the signal-to-background ratio is at its optimal level.Note: The illumination angle will not be consistent across the entire field of view and the optimization and subsequent acquisition should be performed in the same region (usually center) of the field of view.
-
c.We typically use laser power of 50%–75% for no more than 40 s of acquisition. When screening samples the laser power should be set to the lowest level which allows localizing the cilia (e.g., 10%) (Figure 13C).Note: The laser power increases both the signal-to-noise ratio and cell damage significantly and should be set with respect to the intended duration of the acquisition. Several experimental movies should be recorded first to determine if there is significant bleaching of the fluorophores or cell damage (recognizable by IFT trains slowing down) during the recording.
-
d.The full field of view of our camera is 1024 × 1024 pixels (88 μm2 with our optical setup), which can accommodate several cells and cilia.Optional: While imaging multiple cells at once can be an advantage, recording full-frame movies comes with significant time overhead in addition to the selected exposure time and reduces the resulting frame rate. For very fast single cilium acquisitions, check “ROI” box at the “DU-888 Settings” panel (Figure 13D) and using “ROI size / Define ROI” define the region of interest of 128 × 128 pixels (resulting in a 11 × 11 μm field of view with our setup).
-
a.
-
50.Once the desired parameters are set for the screening configuration:
-
a.Create a button (Figure 14) for a new configuration in the OC panel, preferably by duplicating an existing TIRF configuration,
-
b.right-click on it and choose “Assign current microscope settings”.
-
c.Then change the microscope settings to the desired acquisition configuration,
-
d.again create a new button,
-
e.right-click on it and choose “Assign current microscope settings”.
-
a.
-
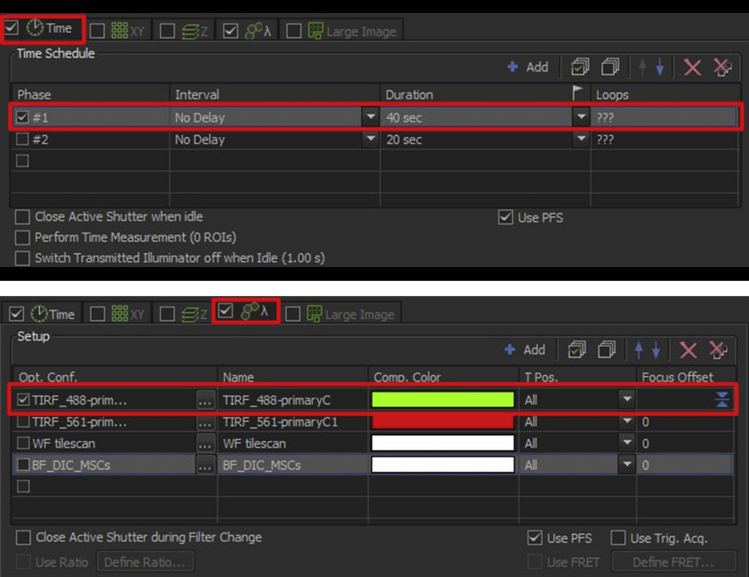
51.Define the experiment flow in the “ND Acquisition” window. For a simple time-lapse experiment, two variables need to be defined: time and optical configuration (Figure 15).
-
a.In the “Time” panel, check “Phase 1” only, set “Interval” to “No Delay” and “Duration” to the desired value.
-
b.In the “Lambda” panel, check the first “Opt. Conf.” option and set it to the optical configuration prepared for acquisition. Set the path for saving movies and appropriate filename base. Software will automatically add incrementing numbers to the filename base if multiple movies are recorded in the same experiment.
-
a.
-
52.
Switch to the low-power screening configuration and start searching for a suitable cilium to image. Once found, move it to the center of the field of view and bring it into focus with the “Perfect Focus” offset control. If you are using the region of interest option, switch on the “ROI” in the “Camera panel” and reposition the cilium to be fully contained in the visible area.
-
53.
Press the “Run now” button in the “ND Acquisition” window. The optical configuration will switch to the high-power illumination configuration and the movie is recorded.
CRITICAL: Time spent on previewing the cell and switching from searching to acquisition should be kept to a minimum to prevent cell damage and photobleaching. It is a good practice to organize the microscope workplace and software layout to have all necessary knobs and buttons at an easy reach.
-
54.
Switch back to the low-power screening configuration and repeat the process with more cilia.
Optional: Review the recorded movie using NIS elements or Fiji to ascertain that the image quality is satisfactory.
Figure 13.
Imaging settings for VAEM, step 62
(A) Exposure time (B) Illumination angle. X and Y sliders set the position of a focused laser beam at the back focal plane of the objective, determining the angle at which light enters the sample. Zero position (shown here) places the beam at the center of the back focal plane, which leads to conventional epifluorescence illumination. Position above 55,000 displaces the beam enough to generate TIRF illumination. (C) Laser intensity setting (D) Region of interest setting.
Figure 15.
Setting up an experiment acquisition, step 64
In the “Time” panel, select one phase with interval option “No delay”. In the “Lambda” panel select one optical configuration and set to the high-power illumination configuration.
Expected outcomes
Analysis of time-lapse videos to study primary cilia dynamics
To assess cilia resorption dynamics using the described protocol, the cells hTERT-RPE-1 Flp-In T-REx TTBK2 KO cells (Bernatik et al., 2020) with DOX-inducible expression of FLAG-TTBK2 and constitutive mNeonGreen-ARL13b reporter, were serum-starved for 24 h, and 10% FBS was added just before the imaging to induce cilium resorption. The primary cilium length was measured using a segmented-line tool for selection of the fluorescence signal of the ciliary marker in maximum Z intensity projected images in Fiji (see step 49c and Methods Video S2). Representative images were acquired every 1 h (Figure 16). Alternatively, starting the experiment right after the serum starvation step will allow to image the cilia growth in time. From the measured lengths, it is also possible to calculate cilia growth/resorption speed, as described e.g., in (Pejskova et al., 2020).
Imaging of IFT transport in primary cilia of living cells
Following the protocol in the section “Imaging of IFT transport in primary cilia of living cells”, we obtained a time-lapse movie (150 images, 1 image per 200 ms), which shows the movement of IFT trains (Methods Video S1).
Based on the kymograph analysis of the time-lapse image sequence, we can determine the length of the primary cilium (see step 49c), frequency (see step 53) of the anterograde and retrograde IFT74 trains, and also their velocity (see step 54). The length of our measured primary cilium is 4.366 μm (Figure 17A). We detected 6 anterograde trains and 5 retrograde trains within 30 s of recording (Figure 17B). The velocities of the individual IFT74 trains were calculated in step 54, giving 0.54 μm/s on average for anterograde transport and 0.32 μm/s on average for retrograde transport (Figure 17C).
Imaging IFT transport using variable-angle epifluorescence (VAEM) microscopy
VAEM microscopy is particularly suited for detailed study of individual IFT trajectories. The high temporal resolution achieved by this technique allows quantifying not only the average velocity of an IFT train, but also any changes in speed, pausing, or collisions (Figure 18).
Figure 18.
Outcome of the variable angle epifluorescence microscopy imaging of intraflagellar transport in the primary cilium of an RPE-1 cell
Total duration of the movie is 40 s, the length of the cilium is 8 μm.
Limitations
We have combined two strategies to generate primary cilia reporter cell lines to study the cilia dynamics, namely the rate of cilia assembly/disassembly as well as the IFT transport, using high time resolution live-cell imaging microscopy. We consider the described protocol sufficiently robust, as it represents a joined effort of three labs where it has been successfully implemented. Nonetheless, certain inherent limitations need to be considered. Some of them have been already discussed in the protocol or are mentioned in the troubleshooting section.
Probably the most critical factor for successful analysis of primary cilia using time-lapse microscopy (and even more so in the case of IFT analysis) is the signal-to-background noise ratio. This parameter is typically affected by several factors, such as properties and position (N-terminal or C-terminal) of the selected fluorescent tag, the level of expression of the cilium reporter in a given cell line, relative enrichment of the reporter in the cilium compartment over the cytoplasm, as well as the abilities and settings of the microscope used for the analysis. Thus, it is important to bear in mind that trade-offs often need to be considered (sufficient signal intensity vs. signal bleaching over time, high speed of imaging vs. phototoxicity, etc.). For example, the laser power increases both the signal and cell damage significantly. Therefore, it should be always set with respect to the planned duration of the acquisition.
Additional point we want to mention relates to an automated image analysis. Various tools have been recently described (Bansal et al., 2021; Hansen et al., 2021; Lauring et al., 2019) and successfully implemented by us as well as others to unbiasedly determine cilia properties (e.g., cilia number, length, signal intensity, etc.) from the acquired images. Similarly, tools for automated kymograph analysis are becoming available (Jakobs et al., 2019; Mangeol et al., 2016) and thus could be used for IFT analysis. We have not tested automated segmentation or automatic kymograph analysis in our IFT analysis experiments. These approaches offer to significantly speed up the process, in contrast to analysis done “manually”. On the other hand, they might struggle with correct detection of the IFT signal in cilia (as the IFT signal in cilia is typically “non-static”, not forming a full line).
Limitation of any reporter-based system, including the one described here, is that a reporter might alter properties of the system. As already mentioned, we and others noted that ARL13b overexpression has positive effect on cilia length. Such factors need to be taken in account when planning your experiments (e.g., by examining possible effect of particular cilia reporter on length/dynamics of cilia in newly established stable cell line). Gene editing to insert a fluorescence reporter into the locus of suitable gene (“endogenous tagging”(Bauer et al., 2016; Cho et al., 2021)) may bypass the “overexpression” problem, but with a risk of insufficient signal strength for planned live-cell imaging experiments.
When implementing this protocol for your needs (especially when you need to adapt it for your particular cell line or protein of choice) always perform small-scale pilot experiments first to determine if there is significant bleaching of the fluorophores or cell damage. A visible slowdown of IFT movements during the acquisition is typically a sign of extensive cell damage by the applied laser illumination.
In general, low expression plasmids are preferred as reporters, as you reduce the chance of getting overexpression artifacts. On the other hand, this may cause problems when trying to get a sufficient signal-to-background noise ratio during the imaging.
Troubleshooting
Problem 1
Generation of stable cell line Flp-InTM T-RExTM gives either no surviving clones following the antibiotics selection or obtained clones show no expression of the used transgene (step 3).
Potential solution
No surviving colony typically indicates too harsh selection pressure. Consider carefully optimizing the effective concentration and time of the selection of antibiotics for your cell line before starting. An outgrowth of “empty” clones (no detectable expression of the transgene) typically indicates toxicity of the used transgene in the given cell line, so there is a negative selection pressure among the stable transfectants. A solution to that is to either use a different transgene or a different cell line, if possible.
Problem 2
Inefficient production of viral particles (low yield) (steps 5–9).
Potential solution
The production of viral particles can be improved by several factors. First, we recommend using the low passage of the Phoenix-Ampho cells. Prolonged cultivation of these cells (>3 weeks) in our hands typically leads to a drop in the virus production efficiency. In addition, prolonged transfection time generally gives higher transfection efficiency and in turn higher virus titer. We also observed that co-transfection with pCL-Ampho plasmid (Naviaux et al., 1996) may further increase the efficiency of virus production. Consider upscaling the protocol (increase the target cell number, amount of viral particle medium, etc. accordingly) to get more transduced cells to start with.
Problem 3
Low efficiency of viral transduction (steps 10–12).
Potential solution
In many situations, simple upscaling of the protocol helps to get a sufficient number of transduced cells for their subsequent isolation by cell sorting. In addition, we use Polybrene (Hexadimethrine bromide) in our protocol, a small, positively charged molecule that binds to cell surfaces and facilitates binding between virus particles and the cellular membrane. As the doses of Polybrene used typically differ between labs/protocols, optimizing the concentration of Polybrene is expected to improve transduction efficiency. There are alternatives to Polybrene such as dextran which may be also considered.
Problem 4
No or only very few cilia were detected in the live-cell imaging experiment (steps 14–16, 34–36, 55).
Potential solution
A low number of cilia seen in live-cell imaging experiments can be caused by several different problems. First, determine whether the problem stems from a suboptimal cilia formation or suboptimal signal detection of the used cilia reporter (cilia are formed, but hard to detect). In the former case, use healthy cells, consider thawing a low passage aliquot of the cells, and check routinely for Mycoplasma. In addition, to promote ciliogenesis, try to optimize the cell density, length of serum starvation period, or consider using small molecules promoting cilia formation (e.g., Cytochalasin D (Bernatik et al., 2021; Kim et al., 2010)). In case the cilia presence is DOX-inducible (i.e., in the case of rescue experiment), we observed that adding DOX already 1–2 days prior to seeding the cells for imaging experiment results in a better rescue effect. If the absence of cilia is rather a detection problem, consider fine-tuning the settings of your imaging system. Bear in mind that boosting your signal often comes with the cost of increased cytotoxicity or photobleaching. See also troubleshooting 5 and 7.
Problem 5
Extensive cytotoxicity in a live-cell imaging experiment (steps 14–16, 29, 34–36, 55).
Potential solution
Consider shortening the starvation period, also always try to keep the laser intensity as low as possible. For long time-lapse experiments, make sure there is a sufficient amount of medium (even its partial evaporation often leads to stress that may affect cell survival). In addition, consider increasing the time between taking individual images during the time-lapse session.
Problem 6
Cells move out of the imaged area during time-lapse recording (see LSM800 microscope parameters in chapter “Analysis of time-lapse videos to study primary cilia dynamics” and steps 14, 24).
Potential solution
Try to increase cell density, as cells tend to migrate and thus can “escape” from the field of view more at lower densities. A further strategy to reduce the impact of cell migration during long time-lapse experiments is to try to increase the imaged area or eventually to use an objective with lower magnification. Using lower laser power helps here as well, as we have often observed that cells tend to escape from the illuminated area when the laser power is set too high.
Problem 7
Weak signal/fast bleaching during measurement of the IFT transport (steps 43, 62c).
Potential solution
A very common problem when analyzing cilia by live-cell imaging approaches, which is not easy to solve and is one of the reasons we have included two protocols describing how to measure IFT transport in mammalian cilia. In general, try to use as low laser power as possible in your experiments. If the problem is at the reporter side, consider to FACS sort a cell population with sufficient expression of your reporter and try to use cells with the low passage as the reporter expression may be silenced in higher passages. If none of that helps, consider using a different reporter and/or cell line for your experiments. Using several copies of the same fluorescent protein for tagging (e.g., triple mNeonGreen) will boost the signal, but bear in mind large tags might affect the function of your tagged protein.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Lukáš Čajánek (cajanek@med.muni.cz).
Materials availability
Unique reagents (i.e., plasmids or cell lines) generated in this study can be requested from the lead contact.
Acknowledgments
This work was supported by grants from the Czech Science Foundation (19-05244S, 21-21612S, and 22-13277S) and the Swiss National Science Foundation (IZ11Z0_166533) to L.C. L.S. was supported by the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska- Curie grant agreement no. 846796 and P.G. by the Charles University project GA UK no. 972120. We acknowledge the core facility CELLIM at Masaryk University and the Imaging Methods Core Facility at BIOCEV, both supported by the Czech-BioImaging large RI project (LM2018129 funded by MEYS CR), for their assistance with obtaining scientific data presented in this article.
Author contributions
L.B. acquired data and prepared figures related to viral transduction and live-cell imaging microscopy. E.M. acquired data and prepared figures related to viral transduction and live-cell imaging microscopy. L.S. acquired data and prepared figures related to live-cell imaging microscopy. O.B. acquired data related to stable cell lines generation. D.V. acquired data related to stable cell lines generation. P.P. and P.G. generated reagents and optimized protocols. L.C. coordinated the study. All authors wrote and edited the manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2022.101199.
Data and code availability
This study did not generate datasets/code.
References
- Bansal R., Engle S.E., Kamba T.K., Brewer K.M., Lewis W.R., Berbari N.F. Artificial intelligence approaches to assessing primary cilia. J. Vis. Exp. 2021:e62521. doi: 10.3791/62521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M., Cubizolles F., Schmidt A., Nigg E.A. Quantitative analysis of human centrosome architecture by targeted proteomics and fluorescence imaging. EMBO J. 2016;35:2152–2166. doi: 10.15252/embj.201694462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernatik O., Paclikova P., Kotrbova A., Bryja V., Cajanek L. Primary cilia formation does not rely on WNT/β-catenin signaling. Front. Cell Dev. Biol. 2021;9:623753. doi: 10.3389/fcell.2021.623753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernatik O., Pejskova P., Vyslouzil D., Hanakova K., Zdrahal Z., Cajanek L. Phosphorylation of multiple proteins involved in ciliogenesis by Tau Tubulin kinase 2. Mol. Biol. Cell. 2020;31:1032–1046. doi: 10.1091/mbc.E19-06-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhogaraju S., Cajanek L., Fort C., Blisnick T., Weber K., Taschner M., Mizuno N., Lamla S., Bastin P., Nigg E.A., Lorentzen E. Molecular basis of tubulin transport within the cilium by IFT74 and IFT81. Science. 2013;341:1009–1012. doi: 10.1126/science.1240985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary T., Larkins C.E., Anderson K.V. The graded response to Sonic Hedgehog depends on cilia architecture. Dev. Cell. 2007;12:767–778. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Cho N.H., Cheveralls K.C., Brunner A.-D., Kim K., Michaelis A.C., Raghavan P., Kobayashi H., Savy L., Li J.Y., Canaj H., et al. OpenCell: proteome-scale endogenous tagging enables the cartography of human cellular organization. 2021. [DOI] [PMC free article] [PubMed]
- Goetz S.C., Liem K.F., Jr., Anderson K.V. The spinocerebellar ataxia-associated gene Tau tubulin kinase 2 controls the initiation of ciliogenesis. Cell. 2012;151:847–858. doi: 10.1016/j.cell.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J.N., Rassmann S., Stüven B., Jurisch-Yaksi N., Wachten D. CiliaQ: a simple, open-source software for automated quantification of ciliary morphology and fluorescence in 2D, 3D, and 4D images. Eur. Phys. J. E Soft Matter. 2021;44:18. doi: 10.1140/epje/s10189-021-00031-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Marshall W.F. Efficient live fluorescence imaging of intraflagellar transport in mammalian primary cilia. Methods Cell Biol. 2015;127:189–201. doi: 10.1016/bs.mcb.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobs M.A., Dimitracopoulos A., Franze K. KymoButler, a deep learning software for automated kymograph analysis. eLife. 2019;8:e42288. doi: 10.7554/eLife.42288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesel P., Alvarez Viar G., Tsoy N., Maraspini R., Gorilak P., Varga V., Honigmann A., Pigino G. The molecular structure of mammalian primary cilia revealed by cryo-electron tomography. Nat. Struct. Mol. Biol. 2020;27:1115–1124. doi: 10.1038/s41594-020-0507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Lee J.E., Heynen-Genel S., Suyama E., Ono K., Lee K., Ideker T., Aza-Blanc P., Gleeson J.G. Functional genomic screen for modulators of ciliogenesis and cilium length. Nature. 2010;464:1048–1051. doi: 10.1038/nature08895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkins C.E., Aviles G.D.G., East M.P., Kahn R.A., Caspary T. Arl13b regulates ciliogenesis and the dynamic localization of Shh signaling proteins. MBoC. 2011;22:4694–4703. doi: 10.1091/mbc.e10-12-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauring M.C., Zhu T., Luo W., Wu W., Yu F., Toomre D. New software for automated cilia detection in cells (ACDC) Cilia. 2019;8:1. doi: 10.1186/s13630-019-0061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo P.A., Kavran J.M., Kim M.-S., Leahy D.J. Transient mammalian cell transfection with polyethylenimine (PEI) Methods Enzymol. 2013;529:227–240. doi: 10.1016/B978-0-12-418687-3.00018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangeol P., Prevo B., Peterman E.J.G. KymographClear and KymographDirect: two tools for the automated quantitative analysis of molecular and cellular dynamics using kymographs. MBoC. 2016;27:1948–1957. doi: 10.1091/mbc.e15-06-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fernandez M., Tynan C., Webb S. A ‘pocket guide’ to total internal reflection fluorescence. J. Microsc. 2013;252:16–22. doi: 10.1111/jmi.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazo G., Soplop N., Wang W.J., Uryu K., Tsou M.B. Spatial control of primary ciliogenesis by subdistal appendages alters sensation-associated properties of cilia. Dev. Cell. 2016;39:424–437. doi: 10.1016/j.devcel.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok H.P., Javed S., Lever A. Stable gene expression occurs from a minority of integrated HIV-1-based vectors: transcriptional silencing is present in the majority. Gene Ther. 2007;14:741–751. doi: 10.1038/sj.gt.3302923. [DOI] [PubMed] [Google Scholar]
- Naviaux R.K., Costanzi E., Haas M., Verma I.M. The pCL vector system: rapid production of helper-free, high-titer, recombinant retroviruses. J. Virol. 1996;70:5701–5705. doi: 10.1128/JVI.70.8.5701-5705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pejskova P., Reilly M.L., Bino L., Bernatik O., Dolanska L., Ganji R.S., Zdrahal Z., Benmerah A., Cajanek L. KIF14 controls ciliogenesis via regulation of Aurora A and is important for Hedgehog signaling. J. Cell Biol. 2020;219:e201904107. doi: 10.1083/jcb.201904107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasai A., Schmidt Cernohorska M., Ruppova K., Niederlova V., Andelova M., Draber P., Stepanek O., Huranova M. The BBSome assembly is spatially controlled by BBS1 and BBS4 in human cells. J. Biol. Chem. 2020;295:14279–14290. doi: 10.1074/jbc.RA120.013905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum J.L., Witman G.B. Intraflagellar transport. Nat. Rev. Mol. Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner N.C., Lambert G.G., Chammas A., Ni Y., Cranfill P.J., Baird M.A., Sell B.R., Allen J.R., Day R.N., Israelsson M., et al. A bright monomeric green fluorescent protein derived from Branchiostoma lanceolatum. Nat. Methods. 2013;10:407–409. doi: 10.1038/nmeth.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin S. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J. Cell Biol. 1962;15:363–377. doi: 10.1083/jcb.15.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowa M.E., Bennett E.J., Gygi S.P., Harper J.W. Defining the human deubiquitinating enzyme interaction landscape. Cell. 2009;138:389–403. doi: 10.1016/j.cell.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift S., Lorens J., Achacoso P., Nolan G.P. Rapid production of retroviruses for efficient gene delivery to mammalian cells using 293T cell–based systems. Curr. Protoc. Immunol. 1999;31:10.17.14–10.17.29. doi: 10.1002/0471142735.im1017cs31. [DOI] [PubMed] [Google Scholar]
- Tokunaga M., Imamoto N., Sakata-Sogawa K. Highly inclined thin illumination enables clear single-molecule imaging in cells. Nat. Methods. 2008;5:159–161. doi: 10.1038/nmeth1171. [DOI] [PubMed] [Google Scholar]
- Ward R.J., Alvarez-Curto E., Milligan G. Using the Flp-InTM T-RexTM system to regulate GPCR expression. Methods Mol. Biol. 2011;746:21–37. doi: 10.1007/978-1-61779-126-0_2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The four IFT trains distinguished by color are marked as an example in the time-lapse.
Data Availability Statement
This study did not generate datasets/code.