Abstract
Stress, as commonplace as it is, is a major environmental risk factor for psychopathology. While this association intuitively, anecdotally, and empirically makes sense, we are still very early in the process of understanding what the neurobiological manifestations of this risk truly are. Seminal work from the past few decades has established structural plasticity in the brain as a potential key mechanism. In this review we discuss evidence linking particularly chronic stress exposure in rodent models to plasticity at the dendrites, like remodeling of dendritic branches and spines, in a range of brain regions. A number of candidate mechanisms that seek to explain how stress influences neuroanatomy at this level have been proposed, utilizing in vivo, ex vivo and in vitro methods. However, a large gap still remains in our knowledge of how such dynamic structural changes ultimately relate to downstream effects such as altered affective and cognitive states relevant for psychopathology. We propose that future work expand our understanding of plasticity of specific stress-related brain circuits and cell-types. We also note that the vast majority of the work has been conducted solely on male rodents. The next big strides in our understanding of the neurobiology of psychopathology will require the inclusion of female subjects, as several studies have suggested both sex divergent and convergent features. By understanding plasticity, we can harness it. The growth of this body of knowledge will inform our efforts to improve the therapeutic options for stress-related psychopathology.
Keywords: Stress, Plasticity, Dendritic remodeling
Abbreviations
- aBNST
anterior bed nucleus of the stria terminalis
- BDNF
brain-derived neurotrophic factor
- BLA
basolateral amygdala
- CORT
corticosterone
- CRH
corticotrophin-releasing hormone
- CSDS
chronic social defeat stress
- CUS
chronic unpredictable stress
- CVS
chronic variable stress
- DG
dentate gyrus
- DRN
dorsal raphe nucleus
- FST
forced swim test
- HPA
hypothalamic-pituitary-adrenal
- HPC
hippocampus
- IL
infralimbic cortex
- LTD
long-term depression
- LTP
long-term potentiation
- MDD
major depressive disorder
- NAc
nucleus accumbens
- PFC
prefrontal cortex
- PL
prelimbic cortex
- PTSD
post-traumatic stress disorder
- SSRI
selective serotonin reuptake inhibitor
1. Introduction
The importance of having a stress response cannot be overstated. Dr Bruce McEwen championed the idea that stress is an allostatic mechanism, a critical form of defense against challenges any organism faces. However, we are also acutely aware that in addition to being survival-promoting, stress can cause significant harm. Both the helpful and harmful consequences of stress can be seen at the level of the brain, all the way from complex affect and cognition down to nanometer-level changes in dendritic spines. Finding the connections between these factors has received a wealth of research attention since the earliest reports that stress in rodents alters neuronal morphology in the 1990s.
Preclinical work, such as rodent models of stress exposure (see Box 1), is necessary for building our understanding of complex human stress-related conditions, such as post-traumatic stress disorder (PTSD), major depressive disorder (MDD), and anxiety disorders. Rodent models allow tighter control of how much and what kind of stress an individual is exposed to, as well as access to tissue collected invasively at specific time points, neither of which is feasible with human subjects. Current methods allow for assessment of both dendritic morphology and behavioral outputs in the same animals, with translational relevance for humans.
Box 1. Summary of chronic stress models referenced in this review.
To probe the effects of stress on the brain, a large variety of rodent models have been developed. Generally, exposure to these protocols alters the animals’ behavior and/or physiology in manners consistent with features of human stress-related psychopathology, such as altered glucocorticoid dynamics, anhedonia, and increased anxiety-like behavior. This box summarizes protocols which are referenced in this review. These protocols, along with many others, are extensively reviewed elsewhere (Ampuero et al., 2015; Lopez and Bagot, 2021; Ménard et al., 2016; Murthy and Gould, 2018).
Restraint stress: The animal's movement is restricted by being enclosed in a tube or small cage, the number of days and amount of time per day can vary.
Immobilization stress: The animal's movement is prevented by enclosure in a plastic cone, the number of days and amount of time per day can vary.
Chronic unpredictable stress (CUS): Animals are exposed to a different, mild stressor every day for a number of weeks. Procedures can vary greatly between labs, and stressors can include e.g. changes in husbandry, temporary changes in water or food availability, cage tilt, and moistened bedding.
Chronic variable stress (CVS): Similar to CUS, but typically with more intense stressors (e.g. tail suspension, inescapable foot shocks), applied once a day for a number of days.
Chronic social defeat stress (CSDS): Male animals are exposed to a larger, more dominant male in a controlled setting, resulting in social subordination when repeated over several days.
Maternal separation: While they are pups, the animals are removed from their nest and mother for a set duration (e.g. 3 h) each day for a number of days. Outcomes are typically measured once the pups reach adulthood.
Escapable vs inescapable shocks: Variations of classic learned helplessness paradigms. Animals are exposed to (cued or uncued) aversive mild electric foot or tail shocks in either an apparatus that they can escape from, or one not allowing escape or cessation of the shock. Pavlovian conditioning can additionally be used to instill (fear conditioning) or extinguish (fear extinction) an association between a neutral stimulus or context with a foot shock. Exposure to this paradigm is also considered an acute stressor.
Alt-text: Box 1
Here, we review the current evidence linking chronic stress exposure to alterations at both the level of dendrites and behavioral outcomes, evaluating both region- and circuit-specific effects. The bulk of the referenced studies were conducted using only male rodents; effects seen in females, sex differences and the importance of including female subjects are also discussed. Finally, we discuss the question of what the role of dendritic remodeling in stress and psychopathology is in the context of allostasis and allostatic load.
2. Brain-wide dendritic plasticity in preclinical stress models
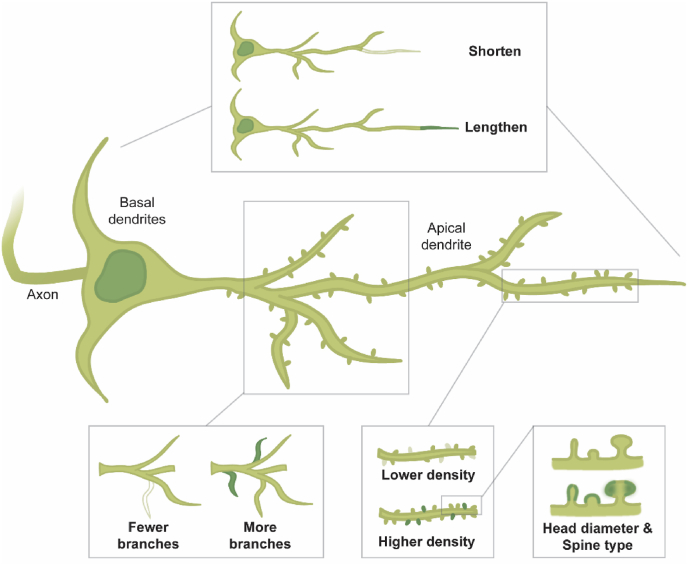
As a form of brain plasticity, dendritic remodeling can take several shapes (Fig. 1). Spines, the anatomically restricted locations of incoming excitatory synaptic inputs, are continuously turned over even in the non-stressed brain. By impacting turnover, stress could thus either increase or decrease spine density with potential downstream effects on synaptic communication (Woolley et al., 1997). Features of the spines themselves, such as shape (thin, stubby or mushroom) and spine head diameter, are also subject to change and can impact synaptic strength (Hotulainen and Hoogenraad, 2010). In addition to density, the total number of spines available on a neuron is affected by dendritic length and the number of branches. These morphological features represent several avenues via which stress could have lasting, yet potentially reversible, effects on brain function.
Fig. 1.
Illustration of different kinds of dendritic remodeling. A schematic of a pyramidal neuron, showing along the apical dendrite how different features of dendrites can display plasticity. The dendrites themselves may become shorter or longer, existing branches may be lost or additional ones gained. Dendritic spines can also be formed or removed to alter spine density. Finally, spines can be characterized into subtypes (typically thin, stubby and mushroom), and their morphology (e.g. head diameter) can change. These features are also plastic on basal dendrites, and on other neuronal types.
Dendritic remodeling can occur either globally, influencing all dendrites in the brain in the same way, or within specific regions. Examinations of spine densities in anatomically distinct stress-associated brain regions in rodents, such as the hippocampus (HPC), prefrontal cortex (PFC) and basolateral amygdala (BLA) have demonstrated that stress effects on spines are regionally dependent. Early work focused on the hippocampal CA3 and indicated that stress exposure was associated with reduced spine density and dendritic length, with numerous replications since the first discoveries (Eiland et al., 2012; Henckens et al., 2015; Lambert et al., 1998; Magariños et al., 1996, 1999; McEwen et al., 1995; McKittrick et al., 2000; Sousa et al., 2000). Vyas et al. (2002) reported dendritic shortening after exposure to chronic restraint stress, used in the aforementioned studies as well, but not after chronic unpredictable stress (CUS). A recovery period after stress and antidepressant treatment have been associated with recovery of dendritic length, although this can occur without improvements in stress-induced anxiety-like behavior (Bessa et al., 2009; Vyas et al., 2004). Social stressors, such as chronic social defeat stress (CSDS), have shown to produce similar effects in the CA1 (Patel et al., 2018).
Similarly, many have reported dendritic atrophy in the PFC after restraint stress and CSDS (Colyn et al., 2019; Cook and Wellman, 2004; Eiland et al., 2012; Goldwater et al., 2009; Henckens et al., 2015; Liston et al., 2006; Luczynski et al., 2015; Radley et al., 2005, 2006). Allowing for a recovery period between stress cessation and sample collection, the proximal but not distal portions of the affected dendrites have been shown to recover, in that their branch lengths resembled unstressed controls (Goldwater et al., 2009; Radley et al., 2005). In the absence of experimental stress, exposure to an enriched environment (which can also restore stress-induced behavioral abnormalities) was sufficient to increase PFC dendritic arborization (Ashokan et al., 2018). Stress-induced atrophy and subsequent recovery reportedly occur primarily in young adult rodents, a period associated with higher capacity for plasticity which wanes in later life (Bloss et al., 2011). Longitudinal two-photon imaging in the frontal association cortex has shown that mushroom spines in particular are eliminated at a higher rate in chronically immobilized mice than controls (Ng et al., 2018). Pre-treatment with acute or chronic ketamine injections protected against this effect. The neurons selected for analysis in these studies are typically pyramidal neurons (based on morphology and location) or of unknown identity. Interestingly, a study examining interneurons in the PFC showed dendritic hypertrophy after immobilization stress (Gilabert-Juan et al., 2013).
Dendritic hypertrophy—e.g. increased dendritic length and branching, as well as higher spine density have been consistently reported in the BLA and nucleus accumbens (NAc) of stress-exposed animals (Bessa et al., 2013; Eiland et al., 2012; Garcia-Keller et al., 2021; Henckens et al., 2015; Hill et al., 2013; Padival et al., 2013a, Padival et al., 2013b; Vyas et al., 2002, 2006). Stress-induced increases in spine density and dendritic length associate with relevant functional changes in BLA neurons, such as increased frequency in firing (Padival et al., 2013a, Padival et al., 2013b; Rosenkranz et al., 2010; Zhang and Rosenkranz, 2012). This BLA effect has been replicated using a variety of stressful protocols, including CSDS (Patel et al., 2018), early-life maternal separation (Koe et al., 2016) and fear conditioning (Heinrichs et al., 2013). The latter two studies also showed remedy of the effects on morphology by exposure to an enriched environment or fear extinction, respectively. Additionally, Cravedi et al. (2021) report reduced head diameter of thin BLA spines following stressful exposure to either predator cues or underwater submersion. In the NAc, a social stressor (CSDS) has been reported to reduce dendritic length, but only in the D1-expressing subtype of medium spiny neurons (Fox et al., 2020). Looking across the brain regions discussed thus far, it is evident that stress effects on dendrites are critically dependent on the region and possibly even cell type within-region. This bidirectionality, along with evidence of reversibility, is a hallmark of plasticity.
Within a given region different neurons have different afferent and efferent connections, and the resulting circuits define the computational capacity of the brain. Stress may affect specific circuits as opposed to whole anatomical regions, which could be relevant for sequelae such as psychopathology. Pathways that have garnered interest so far include subregions of the PFC (infralimbic [IL] and prelimbic [PL] cortices) projecting downstream. For example, in rats exposed to chronic variable stress (CVS), PL neurons in general showed classical atrophy (fewer branch points and lower spine density than controls), but neurons projecting to the anterior bed nucleus of the stria terminalis (aBNST) specifically lost mushroom-shaped spines (Radley et al., 2013). The aBNST outputs, among several targets, to the hypothalamus, with potential influence over the physiological response to stressors. In the BLA, stress-related dendritic lengthening has been reported across the structure, while spine density increases may be specific to some (i.e. ventral HPC projecting) circuits while absent in others (i.e. IL or NAc projecting, (J. Zhang et al., 2019).
Of note, nearly all of the aforementioned studies have been conducted using either only male animals, or the sex of the animals was not reported. Considering the higher prevalence of stress-related psychiatric conditions such as MDD and anxiety disorders in females compared to males (Hasin et al., 2005; Helzer et al., 1987; Kessler, 2007; McLean et al., 2011), this is a significant exclusion (Bangasser and Cuarenta, 2021; McLaughlin et al., 2009; Shansky and Murphy, 2021). A growing body of evidence examining chronic stress and dendrites in both sexes continues to reveal that the effects observed in males can be either absent or occur in the opposite direction in females. While CUS in males affected HPC and NAc dendrites similarly to what was described above, neither of these effects were observed in females (Gaspar et al., 2021). In female mice, peripubertal CUS increased PFC spine density in females mice, while the previously reported decrease was observed in males (Bueno-Fernandez et al., 2021). Restraint stress also has opposing effects on male and female PFC neurons: in females, dendritic length was increased while in males it was decreased (Garrett and Wellman, 2009). In the HPC the finding that restraint induced apical dendrite atrophy in males did not replicate in females, where instead basal dendrites showed reduced branching (Galea et al., 1997). In the BLA, restraint stress did not associate with dendritic hypertrophy in adult females as it did in adult males. On the contrary, female rats showed atrophy in the neighboring lateral amygdala (Blume et al., 2019). If the stressor is applied to younger rats, similar effects can be seen on the dendrites of female and male rats in the PFC, HPC and BLA, suggesting that developmental timing is an important modulator of potential sex differences (Eiland et al., 2012). Interestingly, BLA neurons which were active during an acute exposure to foot shocks showed reduced spine density compared to neurons which were not active, as measured by Arc activity-dependent expression of a fluorescent marker. This effect was observed in both male and female mice (Gruene et al., 2016). Similarly, in the NAc both female and male rats showed increased spine density following an acute restraint stress exposure (Garcia-Keller et al., 2021), suggesting the duration of stress may interact with sex differences.
As evidenced by circuit-specific approaches, sex differences may be quite complex and interactive (Baratta et al., 2019; Shansky et al., 2009, 2010). Projections from the IL to the BLA in female rats have shown an estrogen-dependent stress-induced increase in apical dendrite length and spine density, an effect not observed in male rats despite other IL neurons showing the expected atrophy (Shansky et al., 2009, 2010). A sex difference was also reported for PL neurons projecting to the dorsal raphe nucleus (DRN) following foot shock stress (Baratta et al., 2019). The effect of sex interacted with the amount of control the animals had over this exposure. In males exposed to inescapable foot shocks, PL neurons belonging to this circuit had increased head diameters on mushroom spines compared to non-stressed controls. Meanwhile, female rats showed similarly increased spine diameters but instead of the specificity observed in males, this pattern occurred across spine types, DRN-projecting and non-projecting neurons, and most notably in those exposed to escapable foot shocks. Collectively, studies to date which have included females have unequivocally demonstrated the value of this approach. While many mechanisms likely apply regardless of sex, evidently several do not, with absent or even opposing effects depending on which sex is studied. For a more translational pool of knowledge, including both sexes at the preclinical level is a necessity.
3. Potential mechanisms of plasticity
While understanding the extent and kinds of structural plasticity occurring after stress is important, efforts to uncover the underlying mechanisms of these changes are equally so. Ex vivo and in vitro work has suggested roles for, among others, corticotrophin-releasing hormone (CRH), neuronal nucleoporin proteins (NUP62), dendritic mitochondria, and guanine nucleotide-binding protein subunit (Gαi1/3) in altering spine morphology (Andres et al., 2013; Chen et al., 2013; Jeanneteau et al., 2018; Kinoshita et al., 2014; Marshall et al., 2018; Sui et al., 2018). Circulating hormones, such as estrogens and testosterone, fluctuate and as such can also dynamically alter dendritic morphology (Kovacs et al., 2003; McEwen et al., 2001; Murphy et al., 1998; Shansky et al., 2010; Woolley and McEwen, 1994). Stress influences transcriptomics, and altered availability of mRNAs encoding key synaptic proteins could influence spine morphology (Bagot et al., 2016). Spines are also sites for local translation, enabling rapid adjustment of protein production potentially affected by stress (Thomas et al., 2014).
When it comes to stress, the usual suspect for downstream cellular effects is glucocorticoids. Indeed, a body of work has explored the effects of exogenous glucocorticoid exposure on dendrites. The effects of stress on BLA hypertrophy as well as PFC and HPC hypotrophy have been replicated with injections of corticosterone (CORT), the primary rodent glucocorticoid (Kim et al., 2014; Sousa et al., 2000). Comparing acute and chronic CORT injections, only the former increased BLA dendritic length and decreased open-arm time in the elevated plus maze, an index of anxiety-like behavior (Mitra and Sapolsky, 2008). While the effect of acute CORT is observable even days after the exposure, it has been reported to recover spontaneously over time (Kim et al., 2014). While these studies included morphometric analyses in tissue fixed after extraction, Liston and Gan (2011) tracked the same dendritic segments in vivo throughout chronic CORT administration. They reported that an acute dose increased spine turnover (both formation and elimination), while after a chronic course the effect was restricted to spine elimination. These measurements were carried out in the barrel cortex, possibly explaining the divergent direction of effect compared to the ex vivo studies referenced. These findings suggest CORT may also play a part in naturalistically induced stress-effects, with possibly regionally variable influence. However, as most of the reported stress protocols are chronic in nature while exogenous CORT has the strongest effects acutely, additional factors will be needed to explain this relationship.
Another molecule frequently associated with stress, plasticity and even response to treatments such as antidepressants is brain-derived neurotrophic factor, BDNF (Castrén and Monteggia, 2021). Genetic haploinsufficiency of BDNF exacerbated the aforementioned stress effects on apical dendrites in both the PFC (decreased spine density) and amygdala (increased spine density, (Yu et al., 2012). In the HPC, BDNF haploinsufficiency alone was sufficient to replicate the effects of chronic stress on dendrites (Magariños et al., 2011). Interestingly, BDNF overexpression attenuated the commonly observed hippocampal dendritic atrophy post-stress, but also associated with amygdalar dendritic hypertrophy even in the absence of experimental stress (Govindarajan et al., 2006). Thus, BDNF is an excellent candidate for conveying the effects of stress onto dendritic morphology, and remains of high interest for the research community, particularly as a contributing factor to successful antidepressant responses.
4. Functional relevance of dendritic remodeling for psychiatric phenotypes
One of the major influences of Dr McEwen's thinking on our understanding of stress, plasticity and psychopathology is the distinction between allostasis and allostatic load (McEwen, 1999, 2010; Sterling and Ever, 1990). Allostasis refers to a dynamic physiological change, induced by a change in environmental demands, intended to support the organism prevailing through the environmental change. A stressful encounter requires some physiological adaptations, such as activation of the hypothalamic-pituitary-adrenal (HPA) -axis. The critical features of these events are that they are temporary and reversible when environmental conditions allow homeostasis to be achieved again. Within this framework, dendritic remodeling after stress could reflect such reorganization, as opposed to “damage” (McEwen, 2010). Indeed, Dr McEwen postulated that, at least in the HPC, “damage would be much worse if dendritic remodeling was prevented, due to increased sensitivity to glucocorticoids along with undiminished excitatory input.” (McEwen, 1999, 2010). Subsequent experiments demonstrated that at least in the case where hippocampal dendrites were manipulated to aberrantly increase their arborization, susceptibility to excitotoxic insults was also increased (McCall et al., 2013). Alternatively, dendritic remodeling could be a sign of allostatic overload. When a stressor persists, the allostatic changes may begin to cause “wear and tear” on the body, a framework for conceptualizing how chronic stress can harm health (McEwen, 2001). Empirically, determining which way to view dendritic remodeling is highly challenging.
A key question is: are the detrimental effects of stress on mental health causally linked through an effect on dendrites? Firstly, several of the aforementioned experiments have reported the co-occurrence of dendritic remodeling and behaviors such as impaired performance on a set-shifting task increased anxiety-like behavior (Eiland et al., 2012; Hill et al., 2013; Liston et al., 2006; Mitra and Sapolsky, 2008; Vyas et al., 2006; Zhang et al., 2019). Manipulations of specific dendrites have not been widely possible (discussed below), a step needed to truly probe this causal chain. Secondly, recovery of the affective, social or cognitive phenotypes caused by stress has been reported to co-occur with recovery of the dendritic phenotype. This suggests that it is at least plausible that recovery of dendritic morphology is functionally relevant for behavior as well. Some have reported that an inactive recovery period alone is not sufficient to return BLA dendritic features and anxiety-like behavior to control levels, while hippocampal dendritic morphology does recover at least partially (Sousa et al., 2000; Vyas et al., 2004). Fluoxetine, a selective serotonin re-uptake inhibitor (SSRI), restores stress-induced passive coping on the forced swim test (FST) as well as dendrite length in the HPC but not the PFC, while imipramine, an atypical antidepressant, restored dendritic length in both regions (Bessa et al., 2009). Conversely, others have shown that tianeptine, another atypical antidepressant, reversed and prevented stress-induced HPC dendritic atrophy while fluoxetine did not (Magariños et al., 1999). Another comparison of fluoxetine and imipramine suggests that fluoxetine administration after stress exposure reduced dendrite length in the dentate gyrus (DG), while rats receiving imipramine had longer dendrites in the DG compared to untreated or unstressed controls (Alves et al., 2017). While both antidepressants recovered stress-induced anxiety-like behavior, only imipramine reduced immobility time in the FST. Psilocybin and ketamine, both compounds under investigation for possible antidepressant properties, also recover stress-induced changes in behavior and PFC spine formation (Moda-Sava et al., 2019; Shao et al., 2021). Cortical spine-enhancing properties have also been reported for synthetic psychedelic analog tabernathalog (Lu et al., 2021). These findings suggest a complex relationship between different kinds of pharmacotherapies, spines and behavior, with as-of-yet undetermined causal links.
Another way of probing the functional relevance of stress-related dendritic remodeling is to focus on the aftermath of stress rather than stress exposure. In fact, the estimated percentage of people exposed to traumatic events who go on to develop clinically significant psychopathology is 15.9% (Alisic et al., 2014). We know that there are vast individual differences in the behavioral and molecular responses to stress (Bagot et al., 2016; Etkin et al., 2004; ; Gruene et al., 2015a; Laine et al., 2018). Simply averaging the findings of all stress-exposed individuals in experimental settings risks muddling adaptive and maladaptive morphological changes, as well as mis-assigning an observed finding as one or the other. If dendritic remodeling is important specifically for driving the maladaptive response to stress, we should observe differences between individuals who do exhibit maladaptive responses to stress and individuals who do not. Indeed, experiments factoring in individual differences suggest that such differences do exist. For example, rats that responded to inescapable foot shocks with high levels of freezing showed the aforementioned BLA hypertrophy, while low-freezing rats did not (Neves et al., 2019). Rats either selected for or selective bred for high anxiety-like behavior have shorter PFC dendrites and fewer spines on HPC CA1 neurons than their low-anxiety counterparts even in the absence of experimental stress (Miller et al., 2012; Widman et al., 2019). These individual differences may also be sex-specific, as evidenced by Gruene and colleagues’ (2015b) findings from BLA-projecting IL neurons after fear conditioning. Males exhibiting high levels of freezing behavior had shorter dendrites than males exhibiting low freezing, a difference not observed in high vs low-freezing females. Such individual differences could arise through numerous pre-existing factors, such as genetic background (Hovatta et al., 2005), early maternal care (Millstein and Holmes, 2007) and epigenetics (McEwen, 2017). Considering these findings, experiments stratifying samples by individual differences in key stress response parameters could provide critical insight into the questions raised here.
5. Open questions and conclusions
The growth of the literature on stress-related dendritic plasticity has been steady and encouraging, with pieces of the complex puzzle added continuously. The field is challenged with synthesizing findings from a variety of model systems, species and brain regions to inform a coherent picture. Additionally, novel techniques are needed to assess critical questions. For example, are the stress-induced morphological changes truly causative of stress-induced behavioral changes? There is currently a lack of established techniques available to selectively manipulate subsets of dendrites in desired directions, which would be needed to test these ideas. Emerging tools, such as activated synapse targeting photoactivatable Rac1 (AS-PaRac1), allow the control of synapses along select dendritic segments (Hayashi-Takagi et al., 2015). AS-PaRac1 relies on a genetically engineered construct that is targeted to post-synaptic sites and activated via activity-induced translation. While also functioning as a reporter of activated spines, extended photostimulation can be used to selectively shrink the spines expressing the modified Rac1 protein, with measurable consequences on motor memory. This method has recently been applied to selectively eliminate novel spines induced by post-stress ketamine, which effectively eliminated ketamine's antidepressant effect on rodent behavior (Moda-Sava et al., 2019). Thus, this and similar techniques could soon allow detection and disruption of dendritic spines with stress-induced activity, to probe their relationship with downstream observations.
Given the brain-wide and variable effects of stress, these findings ultimately should be integrated with examinations of other types of plasticity, such as functional synaptic plasticity (LTP/LTD (Leuner and Shors, 2013),), astrocyte morphology (Naskar and Chattarji, 2019), microglia (Gaspar et al., 2021) and myelin plasticity (Laine et al., 2018; Liu et al., 2012). While the complexity of stress-related plasticity is humbling, it also gives hope. Even in the adult brain, especially if aided by therapeutic interventions, plasticity is bidirectional; what can maladapt can also adapt. While we probably cannot “roll back the clock” or “reverse” stress-induced pathology, understanding of these mechanisms can inform us on ways towards resilience and recovery (McEwen, 2017).
CRediT authorship contribution statement
M.A. Laine: Conceptualization, Writing – original draft, preparation. R.M. Shansky: Conceptualization, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgements
We thank Dr Kylie Huckleberry for helpful comments on the manuscript.
References
- Alisic E., Zalta A.K., van Wesel F., Larsen S.E., Hafstad G.S., Hassanpour K., Smid G.E. Rates of post-traumatic stress disorder in trauma-exposed children and adolescents: meta-analysis. Br. J. Psychiatry. 2014;204(Issue 5) doi: 10.1192/bjp.bp.113.131227. [DOI] [PubMed] [Google Scholar]
- Alves N.D., Correia J.S., Patrício P., Mateus-Pinheiro A., Machado-Santos A.R., Loureiro-Campos E., Morais M., Bessa J.M., Sousa N., Pinto L. Adult hippocampal neuroplasticity triggers susceptibility to recurrent depression. Transl. Psychiatry. 2017;7(3) doi: 10.1038/tp.2017.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ampuero E., Luarte A., Santibañez M., Varas-Godoy M., Toledo J., Diaz-Veliz G., Cavada G., Javier Rubio F., Wyneken U. Two chronic stress models based on movement restriction in rats respond selectively to antidepressant drugs: aldolase C as a potential biomarker. Int. J. Neuropsychopharmacol. 2015;18(10) doi: 10.1093/ijnp/pyv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres A.L., Regev L., Phi L., Seese R.R., Chen Y., Gall C.M., Baram T.Z. NMDA receptor activation and calpain contribute to disruption of dendritic spines by the stress neuropeptide CRH. J. Neurosci. 2013;33(43) doi: 10.1523/JNEUROSCI.1445-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashokan A., Lim J.W.H., Hang N., Mitra R. Complex housing causes a robust increase in dendritic complexity and spine density of medial prefrontal cortical neurons. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-25399-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot R.C.C., Cates H.M.M., Purushothaman I., Lorsch Z.S.S., Walker D.M.M., Wang J., Huang X., Schlüter O.M.M., Maze I., Peña C.J.J., Heller E.A.A., Issler O., Wang M., Song W. min, Stein J.L., Liu X., Doyle M.A.A., Scobie K.N.N., Sun H.S.S., Nestler E.J.J. Circuit-wide transcriptional profiling reveals brain region-specific gene networks regulating depression susceptibility. Neuron. 2016;90(5) doi: 10.1016/j.neuron.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Cuarenta A. Sex differences in anxiety and depression: circuits and mechanisms. Nat. Rev. Neurosci. 2021;22(Issue 11) doi: 10.1038/s41583-021-00513-0. [DOI] [PubMed] [Google Scholar]
- Baratta M.v., Gruene T.M., Dolzani S.D., Chun L.E., Maier S.F., Shansky R.M. Controllable stress elicits circuit-specific patterns of prefrontal plasticity in males, but not females. Brain Struct. Funct. 2019;224(5) doi: 10.1007/s00429-019-01875-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessa J.M., Ferreira D., Melo I., Marques F., Cerqueira J.J., Palha J.A., Almeida O.F.X., Sousa N. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol. Psychiatr. 2009;14(8) doi: 10.1038/mp.2008.119. [DOI] [PubMed] [Google Scholar]
- Bessa J.M., Morais M., Marques F., Pinto L., Palha J.A., Almeida O.F.X., Sousa N. Stress-induced anhedonia is associated with hypertrophy of medium spiny neurons of the nucleus accumbens. Transl. Psychiatry. 2013;3 doi: 10.1038/tp.2013.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloss E.B., Janssen W.G., Ohm D.T., Yuk F.J., Wadsworth S., Saardi K.M., McEwen B.S., Morrison J.H. Evidence for reduced experience-dependent dendritic spine plasticity in the aging prefrontal cortex. J. Neurosci. 2011;31(21) doi: 10.1523/JNEUROSCI.0839-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume S.R., Padival M., Urban J.H., Rosenkranz J.A. Disruptive effects of repeated stress on basolateral amygdala neurons and fear behavior across the estrous cycle in rats. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-48683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno-Fernandez C., Perez-Rando M., Alcaide J., Coviello S., Sandi C., Castillo-Gómez E., Nacher J. Long term effects of peripubertal stress on excitatory and inhibitory circuits in the prefrontal cortex of male and female mice. Neurobiol. Stress. 2021;14 doi: 10.1016/j.ynstr.2021.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrén E., Monteggia L.M. Brain-derived neurotrophic factor signaling in depression and antidepressant action. Biol. Psychiatr. 2021;90(Issue 2) doi: 10.1016/j.biopsych.2021.05.008. [DOI] [PubMed] [Google Scholar]
- Chen Y., Kramár E.A., Chen L.Y., Babayan A.H., Andres A.L., Gall C.M., Lynch G., Baram T.Z. Impairment of synaptic plasticity by the stress mediator CRH involves selective destruction of thin dendritic spines via RhoA signaling. Mol. Psychiatr. 2013;18(4) doi: 10.1038/mp.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colyn L., Venzala E., Marco S., Perez-Otaño I., Tordera R.M. Chronic social defeat stress induces sustained synaptic structural changes in the prefrontal cortex and amygdala. Behav. Brain Res. 2019;373 doi: 10.1016/j.bbr.2019.112079. [DOI] [PubMed] [Google Scholar]
- Cook S.C., Wellman C.L. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J. Neurobiol. 2004;60(2):236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Cravedi K.D., May M.D., Abettan J.A., Huckleberry K.A., Trettel S.G., Vuong C.v., Altman D.E., Gauchan S., Shansky R.M., Matson L.M., Sousa J.C., Lowery-Gionta E.G., Moore N.L.T. Response and recovery of endocrine, behavioral, and neuronal morphology outcomes after different traumatic stressor exposures in male rats. Psychoneuroendocrinology. 2021;133 doi: 10.1016/j.psyneuen.2021.105394. [DOI] [PubMed] [Google Scholar]
- Eiland L., Ramroop J., Hill M.N., Manley J., McEwen B.S. Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology. 2012;37(1) doi: 10.1016/j.psyneuen.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Klemenhagen K.C., Dudman J.T., Rogan M.T., Hen R., Kandel E.R., Hirsch J. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44(6) doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Fox M.E., Chandra R., Menken M.S., Larkin E.J., Nam H., Engeln M., Francis T.C., Lobo M.K. Dendritic remodeling of D1 neurons by RhoA/Rho-kinase mediates depression-like behavior. Mol. Psychiatr. 2020;25(5) doi: 10.1038/s41380-018-0211-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea L.A.M., McEwen B.S., Tanapat P., Deak T., Spencer R.L., Dhabhar F.S. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81(3) doi: 10.1016/S0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Garcia-Keller C., Carter J.S., Kruyer A., Kearns A.M., Hopkins J.L., Hodebourg R., Kalivas P.W., Reichel C.M. Behavioral and accumbens synaptic plasticity induced by cues associated with restraint stress. Neuropsychopharmacology. 2021;46(10) doi: 10.1038/s41386-021-01074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett J.E., Wellman C.L. Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience. 2009;162(1) doi: 10.1016/j.neuroscience.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar R., Soares-Cunha C., Domingues A.V., Coimbra B., Baptista F.I., Pinto L., Ambrósio A.F., Rodrigues A.J., Gomes C.A. Resilience to stress and sex-specific remodeling of microglia and neuronal morphology in a rat model of anxiety and anhedonia. Neurobiol. Stress. 2021;14 doi: 10.1016/j.ynstr.2021.100302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilabert-Juan J., Castillo-Gomez E., Guirado R., Moltó M.D., Nacher J. Chronic stress alters inhibitory networks in the medial prefrontal cortex of adult mice. Brain Struct. Funct. 2013;218(6) doi: 10.1007/s00429-012-0479-1. [DOI] [PubMed] [Google Scholar]
- Goldwater D.S., Pavlides C., Hunter R.G., Bloss E.B., Hof P.R., McEwen B.S., Morrison J.H. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 2009;164(2) doi: 10.1016/j.neuroscience.2009.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan A., Shankaranarayana Rao B.S., Nair D., Trinh M., Mawjee N., Tonegawa S., Chattarji S. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc. Natl. Acad. Sci. U.S.A. 2006;103(35) doi: 10.1073/pnas.0605180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruene T., Flick K., Rendall S., Cho J.H., Gray J., Shansky R. Activity-dependent structural plasticity after aversive experiences in amygdala and auditory cortex pyramidal neurons. Neuroscience. 2016;328 doi: 10.1016/j.neuroscience.2016.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruene T., Flick K., Stefano A., Shea S.D., Shansky R.M. Sexually divergent expression of active and passive conditioned fear responses in rats. Elife. 2015;4 doi: 10.7554/elife.11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruene T., Roberts E., Thomas V., Ronzio A., Shansky R.M. Sex-specific neuroanatomical correlates of fear expression in prefrontal-amygdala circuits. Biol. Psychiatr. 2015;78(3) doi: 10.1016/j.biopsych.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin D.S., Goodwin R.D., Stinson F.S., Grant B.F. Archives of General Psychiatry. vol. 62. 2005. Epidemiology of major depressive disorder: results from the national epidemiologic survey on alcoholism and related conditions. 10. [DOI] [PubMed] [Google Scholar]
- Hayashi-Takagi A., Yagishita S., Nakamura M., Shirai F., Wu Y.I., Loshbaugh A.L., Kuhlman B., Hahn K.M., Kasai H. Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature. 2015;525(7569) doi: 10.1038/nature15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs S.C., Leite-Morris K.A., Guy M.D., Goldberg L.R., Young A.J., Kaplan G.B. Dendritic structural plasticity in the basolateral amygdala after fear conditioning and its extinction in mice. Behav. Brain Res. 2013;248 doi: 10.1016/j.bbr.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helzer J.E., Robins L.N., McEvoy L. Post-traumatic stress disorder in the general population. N. Engl. J. Med. 1987;317(26) doi: 10.1056/nejm198712243172604. [DOI] [PubMed] [Google Scholar]
- Henckens M.J.A.G., van der Marel K., van der Toorn A., Pillai A.G., Fernández G., Dijkhuizen R.M., Joëls M. Stress-induced alterations in large-scale functional networks of the rodent brain. Neuroimage. 2015;105 doi: 10.1016/j.neuroimage.2014.10.037. [DOI] [PubMed] [Google Scholar]
- Hill M.N., Kumar S.A., Filipski S.B., Iverson M., Stuhr K.L., Keith J.M., Cravatt B.F., Hillard C.J., Chattarji S., McEwen B.S. Disruption of fatty acid amide hydrolase activity prevents the effects of chronic stress on anxiety and amygdalar microstructure. Mol. Psychiatr. 2013;18(10) doi: 10.1038/mp.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotulainen P., Hoogenraad C.C. Actin in dendritic spines: connecting dynamics to function. JCB (J. Cell Biol.) 2010;189(4) doi: 10.1083/jcb.201003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovatta I., Tennant R.S., Helton R., Marr R.A., Singer O., Redwine J.M., Ellison J.A., Schadt E.E., Verma I.M., Lockhart D.J., Barlow C. Glyoxalase 1 and glutathione reductase 1 regulate anxiety in mice. Nature. 2005;438(7068) doi: 10.1038/nature04250. [DOI] [PubMed] [Google Scholar]
- Jeanneteau F., Barrére C., Vos M., de Vries C.J.M., Rouillard C., Levesque D., Dromard Y., Moisan M.P., Duric V., Franklin T.C., Duman R.S., Lewis D.A., Ginsberg S.D., Arango-Lievano M. The stress-induced transcription factor NR4a1 adjusts mitochondrial function and synapse number in prefrontal cortex. J. Neurosci. 2018;38(6) doi: 10.1523/JNEUROSCI.2793-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C. The global burden of anxiety and mood disorders: putting the European Study of the Epidemiology of Mental Disorders (ESEMeD) findings into perspective. J. Clin. Psychiatr. 2007;68(2) [PMC free article] [PubMed] [Google Scholar]
- Kim H., Yi J.H., Choi K., Hong S., Shin K.S., Kang S.J. Regional differences in acute corticosterone-induced dendritic remodeling in the rat brain and their behavioral consequences. BMC Neurosci. 2014;15 doi: 10.1186/1471-2202-15-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita Y., Hunter R.G., Gray J.D., Mesias R., McEwen B.S., Benson D.L., Kohtz D.S. Role for NUP62 depletion and PYK2 redistribution in dendritic retraction resulting from chronic stress. Proc. Natl. Acad. Sci. U.S.A. 2014;111(45) doi: 10.1073/pnas.1418896111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koe A.S., Ashokan A., Mitra R. Short environmental enrichment in adulthood reverses anxiety and basolateral amygdala hypertrophy induced by maternal separation. Transl. Psychiatry. 2016;6 doi: 10.1038/tp.2015.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs E.G., MacLusky N.J., Leranth C. Effects of testosterone on hippocampal CA1 spine synaptic density in the male rat are inhibited by fimbria/fornix transection. Neuroscience. 2003;122(3) doi: 10.1016/j.neuroscience.2003.08.046. [DOI] [PubMed] [Google Scholar]
- Laine M.A., Trontti K., Misiewicz Z., Sokolowska E., Kulesskaya N., Heikkinen A., Saarnio S., Balcells I., Ameslon P., Greco D., Mattila P., Ellonen P., Paulin L., Auvinen P., Jokitalo E., Hovatta I. Genetic control of myelin plasticity after chronic psychosocial stress. ENeuro. 2018;5(4) doi: 10.1523/ENEURO.0166-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert K.G., Buckelew S.K., Staffiso-Sandoz G., Gaffga S., Carpenter W., Fisher J., Kinsley C.H. Activity-stress induces atrophy of apical dendrites of hippocampal pyramidal neurons in male rats. Physiol. Behav. 1998;65(1) doi: 10.1016/S0031-9384(98)00114-0. [DOI] [PubMed] [Google Scholar]
- Leuner B., Shors T.J. Stress, anxiety, and dendritic spines: what are the connections? Neuroscience. 2013;251 doi: 10.1016/j.neuroscience.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Liston C., Gan W.B. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc. Natl. Acad. Sci. U.S.A. 2011;108(38) doi: 10.1073/pnas.1110444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C., Miller M.M., Goldwater D.S., Radley J.J., Rocher A.B., Hof P.R., Morrison J.H., McEwen B.S. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J. Neurosci. 2006;26(30) doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Dietz K., Deloyht J.M., Pedre X., Kelkar D., Kaur J., Vialou V., Lobo M.K., Dietz D.M., Nestler E.J., Dupree J., Casaccia P. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci. 2012;15(12) doi: 10.1038/nn.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J., Bagot R.C. Defining valid chronic stress models for depression with female rodents. Biol. Psychiatr. 2021;90(Issue 4) doi: 10.1016/j.biopsych.2021.03.010. [DOI] [PubMed] [Google Scholar]
- Lu J., Tjia M., Mullen B., Cao B., Lukasiewicz K., Shah-Morales S., Weiser S., Cameron L.P., Olson D.E., Chen L., Zuo Y. An analog of psychedelics restores functional neural circuits disrupted by unpredictable stress. Mol. Psychiatr. 2021;26(11) doi: 10.1038/s41380-021-01159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczynski P., Moquin L., Gratton A. Chronic stress alters the dendritic morphology of callosal neurons and the acute glutamate stress response in the rat medial prefrontal cortex. Stress. 2015;18(6) doi: 10.3109/10253890.2015.1073256. [DOI] [PubMed] [Google Scholar]
- Magariños A.M., Deslandes A., McEwen B.S. Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. Eur. J. Pharmacol. 1999;371(2–3) doi: 10.1016/S0014-2999(99)00163-6. [DOI] [PubMed] [Google Scholar]
- Magariños A.M., Li C.J., Gal Toth J., Bath K.G., Jing D., Lee F.S., McEwen B.S. Effect of brain-derived neurotrophic factor haploinsufficiency on stress-induced remodeling of hippocampal neurons. Hippocampus. 2011;21(3) doi: 10.1002/hipo.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magariños A.M., McEwen B.S., Flügge G., Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J. Neurosci. 1996;16(10) doi: 10.1523/jneurosci.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J., Zhou X.Z., Chen G., Yang S.Q., Li Y., Wang Y., Zhang Z.Q., Jiang Q., Birnbaumer L., Cao C. Antidepression action of BDNF requires and is mimicked by Gαi1/3 expression in the hippocampus. Proc. Natl. Acad. Sci. U.S.A. 2018;115(15) doi: 10.1073/pnas.1722493115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall T., Weil Z.M., Nacher J., Bloss E.B., el Marouf A., Rutishauser U., McEwen B.S. Depletion of polysialic acid from neural cell adhesion molecule (PSA-NCAM) increases CA3 dendritic arborization and increases vulnerability to excitotoxicity. Exp. Neurol. 2013;241(1) doi: 10.1016/j.expneurol.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. vol. 22. 1999. Stress and hippocampal plasticity. (Annual Review of Neuroscience). [DOI] [Google Scholar]
- McEwen B. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann. N. Y. Acad. Sci. 2001;933 doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- McEwen B. Stress, sex, and neural adaptation to a changing environment: mechanisms of neuronal remodeling. Ann. N. Y. Acad. Sci. 2010;1204(Suppl. 1) doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. Allostasis and the epigenetics of brain and body health over the life course: the brain on stress. JAMA Psychiatr. 2017;74(Issue 6) doi: 10.1001/jamapsychiatry.2017.0270. [DOI] [PubMed] [Google Scholar]
- McEwen B., Akama K., Alves S., Brake W.G., Bulloch K., Lee S., Li C., Yuen G., Milner T.A. Tracking the estrogen receptor in neurons: implications for estrogen-induced synapse formation. Proc. Natl. Acad. Sci. U.S.A. 2001;98(13) doi: 10.1073/pnas.121146898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Albeck D., Cameron H., Chao H.M., Gould E., Hastings N., Kuroda Y., Luine V., Magarinos A.M., McKittrick C.R., Orchinik M., Pavlides C., Vaher P., Watanabe Y., Weiland N. Stress and the brain: a paradoxical role for adrenal steroids. Vitam. Horm. 1995;51(C) doi: 10.1016/S0083-6729(08)61045-6. [DOI] [PubMed] [Google Scholar]
- McKittrick C.R., Magariños A.M., Blanchard D.C., Blanchard R.J., McEwen B.S., Sakai R.R. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000;36(2) doi: 10.1002/(SICI)1098-2396(200005)36:2<85::AID-SYN1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- McLaughlin K.J., Baran S.E., Conrad C.D. Chronic stress- and sex-specific neuromorphological and functional changes in limbic structures. Mol. Neurobiol. 2009;40(2) doi: 10.1007/s12035-009-8079-7. [DOI] [PubMed] [Google Scholar]
- McLean C.P., Asnaani A., Litz B.T., Hofmann S.G. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J. Psychiatr. Res. 2011;45(8) doi: 10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménard C., Hodes G.E., Russo S.J. Pathogenesis of depression: insights from human and rodent studies. Neuroscience. 2016;321 doi: 10.1016/j.neuroscience.2015.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.M., Morrison J.H., McEwen B.S. Basal anxiety-like behavior predicts differences in dendritic morphology in the medial prefrontal cortex in two strains of rats. Behav. Brain Res. 2012;229(1) doi: 10.1016/j.bbr.2012.01.029. [DOI] [PubMed] [Google Scholar]
- Millstein R.A., Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci. Biobehav. Rev. 2007;31(1) doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Mitra R., Sapolsky R.M. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc. Natl. Acad. Sci. U.S.A. 2008;105(14) doi: 10.1073/pnas.0705615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moda-Sava R.N., Murdock M.H., Parekh P.K., Fetcho R.N., Huang B.S., Huynh T.N., Witztum J., Shaver D.C., Rosenthal D.L., Alway E.J., Lopez K., Meng Y., Nellissen L., Grosenick L., Milner T.A., Deisseroth K., Bito H., Kasai H., Liston C. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science. 2019;364(6436) doi: 10.1126/science.aat8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D.D., Cole N.B., Greenberger V., Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J. Neurosci. 1998;18(7) doi: 10.1523/jneurosci.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy S., Gould E. Early life stress in rodents: animal models of illness or resilience? Front. Behav. Neurosci. 2018;12 doi: 10.3389/fnbeh.2018.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naskar S., Chattarji S. Stress elicits contrasting effects on the structure and number of astrocytes in the amygdala versus hippocampus. ENeuro. 2019;6(1) doi: 10.1523/ENEURO.0338-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves L.T., Neves P.F.R., Paz L.V., Zancan M., Milanesi B.B., Lazzari G.Z., da Silva R.B., de Oliveira M.M.B.P., Venturin G.T., Greggio S., da Costa J.C., Rasia-Filho A.A., Mestriner R.G., Xavier L.L. Increases in dendritic spine density in BLA without metabolic changes in a rodent model of PTSD. Brain Struct. Funct. 2019;224(8) doi: 10.1007/s00429-019-01943-4. [DOI] [PubMed] [Google Scholar]
- Ng L.H.L., Huang Y., Han L., Chang R.C.C., Chan Y.S., Lai C.S.W. Ketamine and selective activation of parvalbumin interneurons inhibit stress-induced dendritic spine elimination. Transl. Psychiatry. 2018;8(1) doi: 10.1038/s41398-018-0321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padival M.A., Blume S.R., Rosenkranz J.A. Repeated restraint stress exerts different impact on structure of neurons in the lateral and basal nuclei of the amygdala. Neuroscience. 2013;246 doi: 10.1016/j.neuroscience.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padival M., Quinette D., Amiel Rosenkranz J. Effects of repeated stress on excitatory drive of basal amygdala neurons in vivo. Neuropsychopharmacology. 2013;38(9) doi: 10.1038/npp.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D., Anilkumar S., Chattarji S., Buwalda B. Repeated social stress leads to contrasting patterns of structural plasticity in the amygdala and hippocampus. Behav. Brain Res. 2018;347 doi: 10.1016/j.bbr.2018.03.034. [DOI] [PubMed] [Google Scholar]
- Radley J.J., Anderson R.M., Hamilton B.A., Alcock J.A., Romig-Martin S.A. Chronic stress-induced alterations of dendritic spine subtypes predict functional decrements in an hypothalamo-pituitary-adrenal-inhibitory prefrontal circuit. J. Neurosci. 2013;33(36) doi: 10.1523/JNEUROSCI.0287-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley J.J., Rocher A.B., Janssen W.G.M., Hof P.R., McEwen B.S., Morrison J.H. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp. Neurol. 2005;196(1) doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Radley J.J., Rocher A.B., Miller M., Janssen W.G.M., Liston C., Hof P.R., McEwen B.S., Morrison J.H. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cerebr. Cortex. 2006;16(3) doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Rosenkranz J.A., Venheim E.R., Padival M. Chronic stress causes amygdala hyperexcitability in rodents. Biol. Psychiatr. 2010;67(12) doi: 10.1016/j.biopsych.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky R.M., Hamo C., Hof P.R., Lou W., McEwen B.S., Morrison J.H. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cerebr. Cortex. 2010;20(11) doi: 10.1093/cercor/bhq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky R.M., Hamo C., Hof P.R., McEwen B.S., Morrison J.H. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cerebr. Cortex. 2009;19(10) doi: 10.1093/cercor/bhp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky R.M., Murphy A.Z. Considering sex as a biological variable will require a global shift in science culture. Nat. Neurosci. 2021;24(4) doi: 10.1038/s41593-021-00806-8. [DOI] [PubMed] [Google Scholar]
- Shao L.X., Liao C., Gregg I., Davoudian P.A., Savalia N.K., Delagarza K., Kwan A.C. Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron. 2021;109(16) doi: 10.1016/j.neuron.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N., Lukoyanov N.v., Madeira M.D., Almeida O.F.X., Paula-Barbosa M.M. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97(2) doi: 10.1016/S0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- Sterling, Ever J. Handbook of life stress, cognition and health - allostasis: A new paradigm to explain arousal pathology. Behav. Res. Ther. 1990;28(1) [Google Scholar]
- Sui S., Tian J., Gauba E., Wang Q., Guo L., Du H. Cyclophilin D regulates neuronal activity-induced filopodiagenesis by fine-tuning dendritic mitochondrial calcium dynamics. J. Neurochem. 2018;146(4) doi: 10.1111/jnc.14484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M.G., Pascual M.L., Maschi D., Luchelli L., Boccaccio G.L. Synaptic control of local translation: the plot thickens with new characters. Cell. Mol. Life Sci. 2014;71(Issue 12) doi: 10.1007/s00018-013-1506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A., Jadhav S., Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143(2) doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Vyas A., Mitra R., Shankaranarayana Rao B.S., Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 2002;22(15) doi: 10.1523/jneurosci.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A., Pillai A.G., Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128(4) doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Widman A.J., Cohen J.L., McCoy C.R., Unroe K.A., Glover M.E., Khan A.U., Bredemann T., McMahon L.L., Clinton S.M. Rats bred for high anxiety exhibit distinct fear-related coping behavior, hippocampal physiology, and synaptic plasticity-related gene expression. Hippocampus. 2019;29(10) doi: 10.1002/hipo.23092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley C.S., McEwen B.S. Estradiol regulates hippocampal dendritic spine density via an N-methyl- D-aspartate receptor-dependent mechanism. J. Neurosci. 1994;14(12) doi: 10.1523/jneurosci.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley C.S., Weiland N.G., McEwen B.S., Schwartzkroin P.A. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J. Neurosci. 1997;17(5) doi: 10.1523/jneurosci.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Wang D.D., Wang Y., Liu T., Lee F.S., Chen Z.Y. Variant brain-derived neurotrophic factor Val66met polymorphism alters vulnerability to stress and response to antidepressants. J. Neurosci. 2012;32(12) doi: 10.1523/JNEUROSCI.5048-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.Y., Liu T.H., He Y., Pan H.Q., Zhang W.H., Yin X.P., Tian X.L., Li B.M., Wang X.D., Holmes A., Yuan T.F., Pan B.X. Chronic stress remodels synapses in an amygdala circuit–specific manner. Biol. Psychiatr. 2019;85(3) doi: 10.1016/j.biopsych.2018.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Rosenkranz J.A. Repeated restraint stress increases basolateral amygdala neuronal activity in an age-dependent manner. Neuroscience. 2012;226 doi: 10.1016/j.neuroscience.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]