Summary
Background
HIV-1 pan-resistance refers to a reduced susceptibility to nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, protease inhibitors and integrase strand tranfer inhibitors. Although still anecdotal, its management remains a concern both for affected people living with HIV (PLWH) and for public health.
Methods
We described genotypic resistance testing (GRT) of three PLWH with a documented poor virological response to previous antiretroviral therapies, who started ibalizumab, an anti-CD4 monoclonal antibody, combined with an optimized background therapy. Both historical and most recent GRT on plasma RNA and peripheral blood mononuclear cell DNA were interpreted according to the Stanford HIVDb version 9.0 (last updated on 22 February, 2021). After the switch to a regimen including the monoclonal antibody, HIV-1 RNA has been quantified biweekly (PCR Cobas® HIV-1 test 6800 Systems, Roche Diagnostics). Follow-up was censored at data freezing (16 January, 2021).
Findings
We report findings from heavily treatment-experienced PLWH with a pan-resistant HIV-1 infection, who achieved virological control once introduced injections of ibalizumab, that is free from cross-resistance with all the antiretroviral drugs available and ensures patient adherence due to a close monitoring attributable to the route of administration, combined with recycled enfuvirtide and an optimized background regimen, selected on the basis of an accurate evaluation of resistance mutations.
Interpretation
In these cases, this new approach has revealed to be a turning point in achieving virological control.
Funding
None, this research was supported by internal funding.
Keywords: HIV, Anti-HIV agents, HIV fusion inhibitors, HIV drug resistance, Multiple, Viral load, Ibalizumab, Case report
Research in context.
Evidence before this study
References were identified in PubMed with the search terms “HIV drug resistance”, “HIV-1” and “multi-drug resistance” or, “multi-drug class resistance”, “ibalizumab”, “fostemsavir”, and “enfuvirtide” from 1985 until June, 2021. Only papers published in English were reviewed. The final reference list was generated on the basis of originality and relevance to the broad scope of this paper.
Over the last four decades, while life expectancy of most people living with HIV infection has been significantly improved through highly-active antiretroviral therapy, a minority of people have developed multidrug resistance, and in anecdotal cases pan-resistance, defined as a limited susceptibility to the main four antiretroviral classes. To the best of our knowledge, three cases of pan-resistance to each of the five available antiretroviral classes, including entry inhibitors, have been reported. Pan-resistance represents a life-threatening condition for the individual as well as for public health, owing to the risk of pan-resistant virus transmission from people who do not achieve virological suppression. In this context, taking into account historical genotypes and performing resistance testing when clinically appropriate is highly recommended by international guidelines, with the aim of building an antiretroviral regimen capable of the best achievable control. In the absence of fully active drugs, the development of molecules with innovative mechanisms of action as well as an accurate management of therapeutic tools are crucial for the survival of these people.
Added value of this study
Here we report a series of three people with pan-resistance including reduced susceptibility to entry inhibitors. All of them were previously enrolled in the phase 3 BRIGHTE trial involving subjects with multidrug resistant HIV-1 infection and limited treatment options. After the aforementioned subjects developed protocol-defined virological failure related to non-adherence and associated to a reduced susceptibility to the investigational HIV-1 attachment inhibitor fostemsavir, we proposed a new strategy to achieve virological control. Our approach combines a deep assessment of the historical and current resistance mutations in order to tailor an optimized background therapy with the use of the first monoclonal antibody blocking HIV-1 entry ibalizumab together with the recycling of enfuvirtide.
Implications of all the available evidence
Implications of all the available evidence: The control of the HIV epidemic cannot disregard the management of people with multidrug resistant and pan-resistant infections. Our experience shows that it is feasible to achieve virological control in people living with pan-resistant HIV-1 infection, thus reducing their risk of morbidities, mortality and pan-resistant HIV-1 transmission.
Alt-text: Unlabelled box
Introduction
Antiretroviral therapy (ART) has revolutionized the course of HIV infection, reducing mortality and improving the quality of life for people living with HIV (PLWH).1 However, the emergence of HIV-1 drug resistance has raised concerns because it represents a major determinant of treatment failure.2 For that purpose, international guidelines recommend to define a tailored therapy based on genotypic resistance testing (GRT) in PLWH with virological failure (VF), also considering new drugs, such as ibalizumab (IBA) and fostemsavir, in people with limited options.3,4
Although the prevalence of three and four-class resistance has been estimated about 5–10% in Europe and < 3% in North America with a high rate of morbidity and mortality, HIV-1 pan-resistance is still anecdotal and refers to a limited susceptibility to five antiretroviral classes, including nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors (PIs), and integrase strand transfer inhibitors (InSTIs), suggesting that it could be a challenge for individual management as well as for public health.5, 6, 7, 8 Emerging evidence of HIV-1 resistance has stressed the need to develop drugs with novel mechanisms of action, such as fostemsavir, an attachment inhibitor, lenacapavir, a capsid inhibitor, islatravir, a nucleoside reverse transcriptase translocation inhibitor, and IBA, an anti-CD4+ humanized monoclonal antibody that blocks the entry of HIV-1 by binding to CD4+ cell-surface receptors.
We present a case series where we combined both a deep assessment of the historical and current resistance mutations to tailor an optimized background regimen (OBR) including any drug with a residual antiretroviral activity and a new therapeutic approach based on the use of IBA; it represented an “induction treatment” to get virological control; once this target was achieved, a “maintenance treatment” was proposed, with the main aim to promote good tolerability and adherence.
Methods
Ethics
The treatment here reported received the authorization of the Italian Medicines Agency (AIFA), which supplied ibalizumab by means of the 5% Fund, providing support for the use of orphan drugs for the treatment of rare diseases and potential life-saving drugs, not yet available on the market, for particular and serious diseases. AIFA authorization was then notified to the Ethics Committee of IRCCS San Raffaele Scientific Institute, as recommended for retrospective studies. The research here described was conducted in accordance with the World Medical Association Declaration of Helsinki. Each subject read and subscribed a written informed consent for treatment, collection and use of data or samples, and being included in scientific publications.
Cases
Three PLWH followed at IRCCS San Raffaele Scientific Institute (Milan, Italy) were considered for this retrospective study. At the start of IBA combined with the OBR (baseline), a validated in-house method was used to identify mutations in the reverse transcriptase, protease and integrase genes; HIV-1 RNA was extracted from patients’ plasma samples using QIAamp viral RNA kit (Quiagen, Valencia, CA). Synthesis and amplification of cDNA were performed in a single step by using the commercial SuperScript III One-Step RT-PCR System with Platinum Taq DNA Polymerase (Invitrogen) and specific external primers. Outer and inner primers were designed to amplify overlapping fragments within the pol region in order to obtain genes separately, providing an increaased efficiency of the amplification. We also performed GRT on peripheral blood mononuclear cell (PBMC) HIV-1 DNA, using an ‘‘home-made’’ protocol. HIV-1 DNA was extracted from PBMCs by QIAamp DNA Viral Mini kit, Qiagen); HIV-1 DNA levels were determined by a Taqman real-time quantitative PCR assay result PCR-products were sequenced by using the BigDye terminator v.3.1 cycle sequencing kit (Applied Biosystems), and an automated sequencer (ABI 3130 XL). GRT on plasma HIV-RNA and PBMC HIV-DNA were interpreted according to the Stanford University HIV Drug Resistance Database version 9.0 (hivdb.stanford.edu/hivdb/by-sequences/, last updated on 22 February, 2021).9 We also analyzed previous historical GRT and plasma levels of viro-immunological markers collected from diagnosis to data freezing.
Study medications
IBA was administered intravenously at a 2000 mg loading dose followed by an 800 mg maintenance dose every 14 days. Enfuvirtide (ENF) was injected subcutaneously at a 90 mg twice daily (td) dose until a stable virological control was reached. The OBR consisted of approved antiretrovirals at the recommended dosage, and the regimen was tailored based on GRT-driven susceptibility prediction, clinical and pharmacological history.
Follow-up
Since baseline, HIV-1 RNA had been quantified biweekly or monthly using PCR Cobas® HIV-1 test 6800 Systems, Roche Diagnostics; undetectable viral load was defined as HIV-1 RNA < 50 copies/mL. HIV-1 coreceptor usage was determined as formerly described.10 The follow-up was censored at data freezing (16 January, 2021). HIV-1 RNA and CD4+ cell count collected before and after baseline are reported in Figure 1, Figure 2, Figure 3.
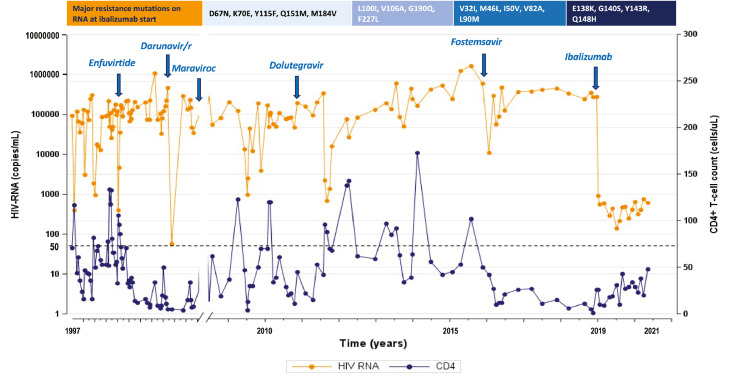
Figure 1.
On the top: major resistance mutations on HIV-RNA at ibalizumab start (according to Stanford HIVDb version 9.0, last updated on 2021-02-22), from the right to the left on NRTI, NNRTI, PI, InSTI. Graphic: HIV-RNA (orange line) and CD4+ T-cell count (blue line) trend represented by all the available values since the first years after HIV-1 infection diagnosis to ibalizumab start (blue arrow on the right), and then monthly until data freezing. Antiretroviral dosages: enfuvirtide 90 mg iv twice daily (td), darunavir/ritonavir 600/100 mg td, maraviroc 300 mg td, dolutegravir 50 mg td, fostemsavir 600 mg td, ibalizumab administered iv at a 2000 mg loading dose followed by 800 mg every 14 days.
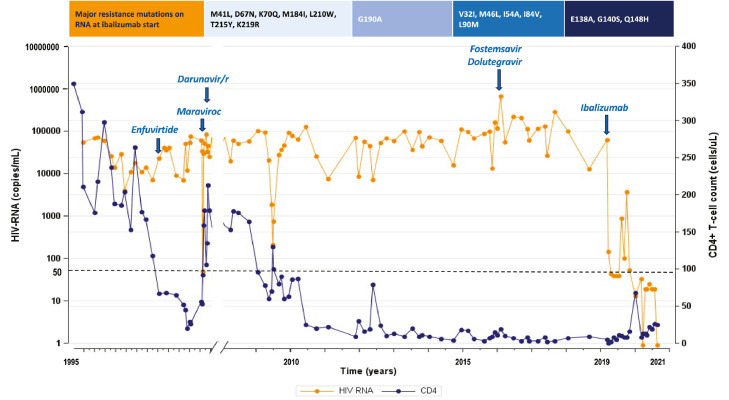
Figure 2.
On the top: major resistance mutations on HIV-RNA at ibalizumab start (according to Stanford HIVDb version 9.0, last updated on 2021-02-22), from the right to the left on NRTI, NNRTI, PI, InSTI. Graphic: HIV-RNA (orange line) and CD4+ T-cell count (blue line) trend represented by all the available values since the first years after HIV-1 infection diagnosis to ibalizumab start (blue arrow on the right), and then monthly until data freezing. Antiretroviral dosages: enfuvirtide 90 mg iv twice daily (td), darunavir/ritonavir 600/100 mg td, maraviroc 300 mg td, dolutegravir 50 mg td, fostemsavir 600 mg td, ibalizumab administered iv at a 2000 mg loading dose followed by 800 mg every 14 days.
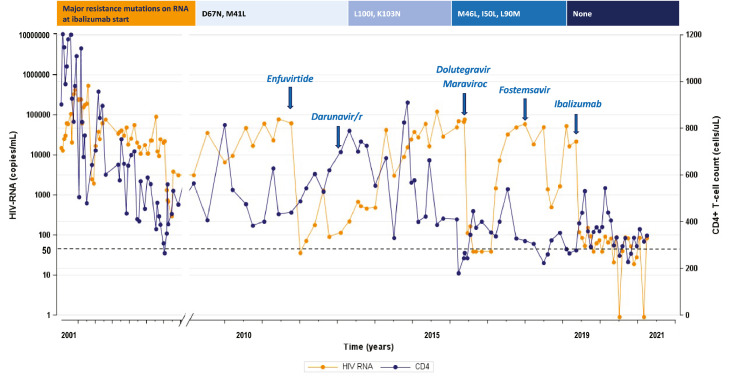
Figure 3.
On the top: major resistance mutations on HIV-RNA at ibalizumab start (according to Stanford HIVDb version 9.0, last updated on 2021-02-22), from the right to the left on NRTI, NNRTI, PI, InSTI. Graphic: HIV-RNA (orange line) and CD4+ T-cell count (blue line) trend represented by all the available values since the first years after HIV-1 infection diagnosis to ibalizumab start (blue arrow on the right), and then monthly until data freezing. Antiretroviral dosages: enfuvirtide 90 mg iv twice daily (td), darunavir/ritonavir 600/100 mg td, maraviroc 300 mg td, dolutegravir 50 mg td, fostemsavir 600 mg td, ibalizumab administered iv at a 2000 mg loading dose followed by 800 mg every 14 days.
Role of funders
This research was supported by internal funding.
Results
Case 1 is a 62-year-old man known for a sexually-transmitted HBV and HIV-1 subtype B co-infection since 1986 and treated with ART from 1990. Opportunistic infections, such as esophageal candidiasis and neurotoxoplasmosis, recurrently occurred due to a poor immune recovery (CD4+ T-cell count < 100 cells/µL). He never maintained a stable virological suppression, partly due to a scarce drug adherence. In 2016, he met elegibility criteria for enrollment into the BRIGHTE trial (NCT02362503) as part of the non-randomised cohort; he started a regimen with fostemsavir (FTR) plus an OBR including atazanavir/cobicistat (ATV/c) 300/150 mg once daily (od) plus maraviroc (MVC). A prior treatment failure under MVC was reported, with R5 tropism at screening. A modest drop in HIV-1 RNA ranging from 294,300 copies/mL at start of FTR+OBR to a nadir of 56,358 copies/mL was described at study week 4, CD4+ count of 12 cells/μL peaked at 27 cells/μL at week 8. Drug accountability indicated non-adherence to FTR+OBR. He met protocol-defined VF (PDVF) criteria at day 172 with emergent gp160 amino acid subsitutions at M426 M/L, and temsavir (TMR) half-maximal inhibitory concentration (IC50) fold change (FC) increased from 0·37 to 17·0 (Table 1, Case 1). Before baseline, GRT both in plasma HIV-RNA and PBMC HIV-DNA were performed, revealing a CXCR4-tropic virus and high-level resistance to all the drugs tested, except for intermediate resistance to darunavir/ritonavir (DRV/r) and potential low-level resistance to tipranavir/r (TPV/r), according to the presence of the major resistance mutations V32I, M46L, I50V, V82AS, L90M (Table 1, Case 1). At baseline, HIV-1 RNA was 275,000 copies/mL and CD4+ 9 cells/µL (0.9%). IBA was associated to DRV/r 600/100 mg td (because of a potential residual activity), recycled ENF 90 mg td, and emtricitabine/tenofovir alafenamide (FTC/TAF) 200/10 mg od, because several studies support the evidence that NRTIs can retain efficacy despite the predominance of variants bearing the M184V/I mutation, according to the hypothesis that maintaining the M184V/I mutation can represent an advantage in terms of viral replication.11, 12, 13, 14 Case 1 showed a viremia ranging between 100 and 600 copies/mL from week 10. At week 34, ENF was stopped as planned, because an improved stable virological control was reached, and dolutegravir (DTG) 50 mg td was added. At week 76, HIV-1 RNA was 948 copies/mL and CD4+ count 30 cells/µL (2·5%).
Table 1.
Case 1-2-3. On the left: major resistance mutations according to Stanford HIVDb version 9.0 (last updated on 2021-02-22) detected on plasma HIV-RNA and peripheral blood mononuclear cell (PBMC) HIV-DNA, cumulative (all the available tests, baseline included) versus baseline (test collected just before ibalizumab plus Optimized Background Therapy starting). NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; InSTI, Integrase strand transfer inhibitor; FPR, false positive rate; PDVF, protocol-defined virological failure; na, not available.
| Class | Sample, time | Major resistance mutations | Drug | Cumulative | Baseline | Plasma HIV-RNA | PBMC HIV-DNA | ||
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | NRTI | Plasma HIV-RNA, historical | D67N, K70E, L74IL, Y115F, Q151M, M184MV, K219EK | ABC | High-Level | High-Level | High-Level | na | RESISTANCE PREDICTION |
| PBMC HIV-DNA, historical | na | FTC | High-Level | High-Level | High-Level | na | |||
| Plasma HIV-RNA, baseline | D67N, K70E, Y115F, Q151M, M184V | 3TC | High-Level | High-Level | High-Level | na | |||
| PBMC HIV-DNA, baseline | na | TDF/TAF | High-Level | High-Level | High-Level | na | |||
| NNRTI | Plasma HIV-RNA, historical | L100IL, G190Q, F227L | DOR | High-Level | High-Level | High-Level | na | ||
| PBMC HIV-DNA, historical | na | EFV | High-Level | High-Level | High-Level | na | |||
| Plasma HIV-RNA, baseline | L100IL, V106AV, G190Q, F227FL | ETR | High-Level | High-Level | High-Level | na | |||
| PBMC HIV-DNA, baseline | na | NVP | High-Level | High-Level | High-Level | na | |||
| RPV | High-Level | High-Level | High-Level | na | |||||
| PI | Plasma HIV-RNA, historical | M46L, I50V, V82AS, L90M | ATV/r | High-Level | High-Level | High-Level | na | ||
| PBMC HIV-DNA, historical | na | DRV/r | Intermediate | Intermediate | Intermediate | na | |||
| Plasma HIV-RNA, baseline | V32I, M46L, I50V, V82A, L90M | LPV/r | High-Level | High-Level | High-Level | na | |||
| PBMC HIV-DNA, baseline | na | TPV/r | Potential Low-Level | Potential Low-Level | Potential Low-Level | na | |||
| InSTI | Plasma HIV-RNA, historical | E138K, G140S, Y143CRHY, Q148H | BIC | High-Level | High-Level | High-Level | High-Level | ||
| PBMC HIV-DNA, historical | E138EK, G140GS, Y143YCHR, Q148QH | DTG | High-Level | High-Level | High-Level | High-Level | |||
| Plasma HIV-RNA, baseline | E138K, G140S, Y143R, Q148H | EVG | High-Level | High-Level | High-Level | High-Level | |||
| PBMC HIV-DNA, baseline | E138EK, G140GS, Y143YCHR, Q148QH | RAL | High-Level | High-Level | High-Level | High-Level | |||
| Entry Inhibitors | Plasma HIV-RNA, historical | CXCR4 (range FPR 0·2-6·7%); gp41: no mutations | MVC | Not effective | Not effective | Not effective | Not effective | ||
| PBMC HIV-DNA, historical | CXCR4 (FPR 1·7%); gp41: no mutations | ||||||||
| Plasma HIV-RNA, baseline | CXCR4 (FPR 1·7%); gp41: na | ENF | Susceptible | na | Susceptible | Susceptible | |||
| PBMC HIV-DNA, baseline | CXCR4 (FPR 1·7%); gp 41: na | ||||||||
| Plasma HIV-RNA, BRIGHTE baseline | no mutations | FTR | Reduced susceptibility to TMRa | na | Reduced susceptibility to TMRa | na | |||
| Plasma HIV-RNA, BRIGHTE PDVF | M426M/L | ||||||||
| Case 2 | NRTI | Plasma HIV-RNA, historical | M41L, D67N, K70Q, M184I, L210W, T215Y, K219R | ABC | High-Level | High-Level | High-Level | High-Level | RESISTANCE PREDICTION |
| PBMC HIV-DNA, historical | M41L, D67N, K70Q, M184I, L210W, T215Y, K219R | FTC | High-Level | High-Level | High-Level | High-Level | |||
| Plasma HIV-RNA, baseline | M41L, D67N, K70Q, M184I, L210W, T215Y, K219R | 3TC | High-Level | High-Level | High-Level | High-Level | |||
| PBMC HIV-DNA, baseline | M41L, D67N, K70Q, M184I, L210W, T215Y, K219R | TDF/TAF | High-Level | High-Level | High-Level | High-Level | |||
| NNRTI | Plasma HIV-RNA, historical | K101KE, Y181V, G190A | DOR | Intermediate | Susceptible | Intermediate | Susceptible | ||
| PBMC HIV-DNA, historical | G190A | EFV | High-Level | Intermediate | High-Level | Intermediate | |||
| Plasma HIV-RNA, baseline | G190A | ETR | High-Level | Potential Low-Level | High-Level | Potential Low-Level | |||
| PBMC HIV-DNA, baseline | G190A | NVP | High-Level | High-Level | High-Level | High-Level | |||
| RPV | High-Level | Low-Level | High-Level | Low-Level | |||||
| PI | Plasma HIV-RNA, historical | V32I, M46L, I54A, I84V, L90M | ATV/r | High-Level | High-Level | High-Level | High-Level | ||
| PBMC HIV-DNA, historical | V32I, M46L, I54A, I84V, L90M | DRV/r | Intermediate | Intermediate | Intermediate | Intermediate | |||
| Plasma HIV-RNA, baseline | V32I, M46L, I54A, I84V, L90M | LPV/r | High-Level | High-Level | High-Level | High-Level | |||
| PBMC HIV-DNA, baseline | V32I, M46L, I54A, I84V, L90M | TPV/r | High-Level | High-Level | High-Level | High-Level | |||
| InSTI | Plasma HIV-RNA, historical | E138A, G140S, Y143H, Q148H | BIC | High-Level | High-Level | High-Level | High-Level | ||
| PBMC HIV-DNA, historical | E138A, G140S, Q148H | DTG | High-Level | High-Level | High-Level | High-Level | |||
| Plasma HIV-RNA, baseline | E138A, G140S, Q148H | EVG | High-Level | High-Level | High-Level | High-Level | |||
| PBMC HIV-DNA, baseline | E138A, G140S, Q148H | RAL | High-Level | High-Level | High-Level | High-Level | |||
| Entry Inhibitors | Plasma HIV-RNA, historical | CXCR4 (range FPR 0·4-2·68%); gp41: G36E, L34M | MVC | Not effective | Not effective | Not effective | Not effective | ||
| PBMC HIV-DNA, historical | CXCR4 (FPR 5·8%); gp41: no mutations | ||||||||
| Plasma HIV-RNA, baseline | CCR5 (FPR 69·8%); gp41: no mutations | ENF | Potential resistance | Susceptible | Potential resistance | Susceptible | |||
| PBMC HIV-DNA, baseline | CXCR4 (FPR 3·1%); gp41: no mutations | ||||||||
| Plasma HIV-RNA, BRIGHTE baseline | no mutations | FTR | Reduced susceptibility to TMRb | na | Reduced susceptibility to TMRb | na | |||
| Plasma HIV-RNA, BRIGHTE PDVF | M426L | ||||||||
| Case 3 | NRTI | Plasma HIV-RNA, historical | M41L, D67N, T69Si, M184IMV, L210W, T215Y, K219EK | ABC | High-Level | Intermediate | High-Level | Intermediate | RESISTANCE PREDICTION |
| PBMC HIV-DNA, historical | M41L, D67N, L210W, T215H, K219E | FTC | High-Level | Susceptible | High-Level | Susceptible | |||
| Plasma HIV-RNA, baseline | M41L, D67N | 3TC | High-Level | Susceptible | High-Level | Susceptible | |||
| PBMC HIV-DNA, baseline | M41L, D67N, L210W, T215H, K219E | TDF/TAF | High-Level | Intermediate | High-Level | Intermediate | |||
| NNRTI | Plasma HIV-RNA, historical | L100IL, K103N | DOR | Intermediate | Intermediate | Intermediate | Intermediate | ||
| PBMC HIV-DNA, historical | L100IL, K103N | EFV | High-Level | High-Level | High-Level | High-Level | |||
| Plasma HIV-RNA, baseline | L100I, K103N | ETR | Intermediate | Intermediate | Intermediate | Intermediate | |||
| PBMC HIV-DNA, baseline | L100I, K103N | NVP | High-Level | High-Level | High-Level | High-Level | |||
| RPV | High-Level | High-Level | High-Level | High-Level | |||||
| PI | Plasma HIV-RNA, historical | M46L, I50V, V82A, L90M | ATV/r | High-Level | High-Level | High-Level | High-Level | ||
| PBMC HIV-DNA, historical | M46L, I50L, V82VA, L90M | DRV/r | Low-Level Resistance | Susceptible | Low-Level | Susceptible | |||
| Plasma HIV-RNA, baseline | M46L, I50L, L90M | LPV/r | High-Level | High-Level | High-Level | High-Level | |||
| PBMC HIV-DNA, baseline | M46L, I50L, V82VA, L90M | TPV/r | Susceptible | Susceptible | Susceptible | Susceptible | |||
| InSTI | Plasma HIV-RNA, historical | Y143CHRY, S147G, N155H | BIC | Intermediate | Intermediate | Intermediate | Intermediate | ||
| PBMC HIV-DNA, historical | S147G, N155H | DTG | Intermediate | Intermediate | Intermediate | Intermediate | |||
| Plasma HIV-RNA, baseline | none | EVG | High-Level | High-Level | High-Level | High-Level | |||
| PBMC HIV-DNA, baseline | S147G, N155H | RAL | High-Level | High-Level | High-Level | High-Level | |||
| Entry Inhibitors | Plasma HIV-RNA, historical | CCR5 (range FPR 50·5-87·8%); gp41: L34M | MVC | Not effective | Not effective | Susceptible | Not effective | ||
| PBMC HIV-DNA, historical | CXCR4 (FPR 1·8%); gp41: L34M | ||||||||
| Plasma HIV-RNA, baseline | CCR5 (FPR 86·2%); gp41: L34M | ENF | Susceptible | Susceptible | Susceptible | Susceptible | |||
| PBMC HIV-DNA, baseline | CXCR4 (FPR 1·8%); gp41: L34M | ||||||||
| Plasma HIV-RNA, BRIGHTE baseline | M426L | FTR | Reduced susceptibility to TMRc | na | Reduced susceptibility to TMRc | na | |||
| Plasma HIV-RNA, BRIGHTE PDVF | M426L |
On the right: resistance prediction according to Stanford HIVDb version 9.0 (last updated on 2021-02-22) for the drugs of common use for each class, distinguished based on time (cumulative plasma + PBMC GRTs versus baseline plasma + PBMC GRTs) and sample (cumulative plasma GRTs versus cumulative PBMC GRTs). ABC, abacavir; FTC, emtricitabine; 3TC, lamivudine; TDF/TAF, tenofovir disoproxil fumarate/tenofovir alafenamide; DOR, doravirine; EFV, efavirenz; ETR, etravirine; NVP, nevirapine; RPV, rilpivirine; ATV/r, atazanavir/ritonavir; DRV/r, darunavir/r; LPV/r, lopinavir/r; TPV/r, tipranavir/r; BIC, bictegravir; DTG, dolutegravir; EVG, elvitegravir; RAL, raltegravir; MRV, maraviroc; ENF, enfuvirtide. (a,b,c) Viral susceptibility to temsavir (TMR) at baseline and after PDVF was reported as fold change (FC) in half-maximal inhibitory concentration (IC50) and were (a) 0·37 and 17; (b) 7·56 and >4669·6; (c) 181 and 288, respectively.
Case 2 is a 56-year-old man with a sexually-transmitted subtype B HIV-1 infection treated since 1995. Over time, he developed a severe lipodystrophy, relapsing skin lesions caused by Molluscum contagiosum, atypical mycobacteriosis, and a life-threatening multi-drug resistant (MDR) Pseudomonas aeruginosa pneumonia. He never achieved stable virological suppression or CD4+ cell recovery, consistently < 100 cells/µL. In 2016, he was enrolled into the non-randomized cohort of the BRIGHTE trial, where investigational FTR was added to an initial OBR of DTG 50 mg td, DRV/r 600/100 mg td and FTC/tenofovir disoproxil fumarate (TDF) 200/300 mg od. Viral load and CD4+ cells did not meaningfully improve (the nadir viral load was 13,354 copies/mL at week 16). Drug accountability indicated non-adherence to FTR+OBR. He met PDVF criteria at week 36 with emergent M426L and IC50 FC increase from 7·56 at baseline to > 4670 (Table 1, Case 2). He was withdrawn from the BRIGHTE study after he developed Cytomegalovirus pneumonia. Baseline GRTs on PBMC HIV-DNA predicted susceptibility to doravirine (DOR), not yet available in our country at that time, low-level resistance to rilpivirine (RPV), potential low-level resistance to etravirine (ETR) and intermediate resistance to efavirenz (EFV), but high-level resistance was predicted on past plasma HIV-RNA to every NNRTI, except for intermediate resistance to DOR (Table 1, Case2). An intermediate resistance to DRV/r was highlighted both in cumulative and baseline plasma HIV-RNA and PBMC HIV-DNA (according to the presence of the major resistance mutations V32I, M46L, I54A, I84V, L90M). High-level resistance to the other available drugs was reported, including CXCR4 tropism. Past GRTs on plasma HIV-RNA documented a G36E and L34M mutation for ENF; viral susceptibility to ENF was not evaluated at baseline. At baseline, he had HIV-1 RNA 62,600 copies/mL and CD4+ 5 cells/µL (0·7%). On the basis of the described resistance prediction, we built an OBR including DRV/r 600/100 mg td, FTC/TAF/RPV 200/25/25 mg od, ENF 90 mg td. Because of the very limited therapeutical options, we added MVC to target residual R5 variants and maximize the antiviral activity of OBR.15 Case 2 viremia reached values permanently < 100 copies/mL from week 4. A transient virological rebound was observed between week 24 and 34 (3690 copies/mL), correlating with ENF interruption because of reduced tolerance. However, upon restarting ENF he achieved complete virological suppression until once again stopped ENF at week 72, due to painful nodules at injection sites. At week 76, HIV-1 RNA was < 50 copies/mL and CD4+ count 25 cells/µL (2·5%).
Case 3 is a 24-year-old male with a mother-to-child transmitted subtype F HIV-1 infection, on ART since 1997 after being diagnosed with Pneumocystis jirovecii pneumonia and disseminated Cytomegalovirus infection. Despite several antiretroviral regimens, he never achieved a stable virological control. In 2016, he met the criteria to be enrolled into the randomised cohort of the BRIGHTE trial, thus he received investigational FTR 600 mg td added to an initial OBR consisting of ATV/r plus DTG td. ENF resulted fully active at the time of enrollment, but the patient refused its use as injectable drug. DTG was the only OBR agent assessed as “fully active”, although previous exposure and a documented raltegravir (RAL) resistance. During BRIGHTE, he reached HIV-1 RNA < 40 copies/mL between study weeks 12 and 36, with increase in CD4 count from 180 to 540 cells/μL. At week 60, he met PDVF criteria. Drug accountability indicated non-adherence to FTR+OBR. Pre-enrollment GRT revealed a polymorphic amino acid replacement on M426L and M434V which was detected also at week 60, while TMR IC50 FC increased from 181 at baseline to 288 at PDVF (Table 1, Case 3). He was discontinued from BRIGHTE at week 156 due to lack of efficacy likely attributed to underlying non-adherence to treatment. Before baseline, plasma HIV-RNA and PBMC HIV-DNA GRT were performed, showing a resistance profile to most of the drugs in each antiretroviral class (Table 1, Case 3). GRTs on PBMC HIV-DNA showed susceptibility to FTC/lamivudine (3TC) and intermediate resistance to TDF/TAF and abacavir (ABC), while high-level resistance resulted from previous plasma HIV-RNA tests. Intermediate resistance to DOR and ETR resulted from all the available tests, as well as low-level resistance to DRV/r and susceptibility to TPV/r. Further, intermediate resistence emerged in all GRTs for DTG and bictegravir (BIC). A CCR5-tropic virus was identified in plasma HIV-RNA, while PBMC HIV-DNA revealed a CXCR4-tropic virus. Only a minor L34M mutation was detected for ENF, described as strongly associated with CXCR4 tropism and a marginal reduction in ENF susceptibility. At baseline, he had HIV-1 RNA 21,966 copies/mL and CD4+ 278 cells/µL (13·6%). We built an OBR which included DTG 50 mg td, MVC 300 mg td, FTC/TAF/RPV od, and ENF 90 mg td. Case 3 reached HIV-1 RNA levels steadily below 200 copies/mL following a single loading dose of IBA, frequently achieving HIV-1 RNA < 50 copies/mL. At week 12, ENF was stopped because of stable viral control, and DRV/r 600/100 mg td was introduced. At week 98, HIV-1 RNA was 84 copies/mL with CD4+ count 341 cells/µL (13·2%).
Discussion
We described three cases of people living with a pan-resistant HIV-1 infection, who reached virological control after introducing IBA and recycling ENF in association with an OBR based on current and historical GRT. All the PLWH had a mild increase of CD4+ cell count once started IBA and none of them has developed opportunistic infections during the observation period; our findings are consistent with data on IBA previously published in the setting of MDR infection.16 IBA has been introduced as a rescue therapy for PLWH with MDR resistance; hence, its cost has been considered affordable if its use is restricted to heavily treatment-experienced (HTE) PLWH who have limited treatment options.17 Moreover, the choice of a directly observed therapy received biweekly in the hospital has certainly allowed reinforcing adherence to oral and subcutaneous ART regularly, even during the SARS-CoV-2 pandemic. In addition, local discomfort due to ENF subcutaneous injection was the only reported adverse event related to OBR and did not affect their compliance.
A personalized OBR strategy was tailored both using historical and current plasma HIV-RNA GRT and also performing HIV-DNA GRT to detect resistance mutations harboured in PBMC. Furthermore, the assessment of resistance detected in HIV-DNA GRT has proved to have a potential role in predicting VF.18,19 Despite the evidence of cumulative resistance to all the antiretroviral classes approved, each of the individual cases achieved undetectable viral load and maintain a significantly improved viral control after failing a prior ART regimen that included FTR, which induced viral suppression in one case. The exhaustion of FTR activity and its causes need to be more investigated. In the BRIGHTE trial, treatment failure was secondary to gp120 substitutions previously associated with a reduced phenotypic susceptibility to TMR, the active FTR-derived compound.20,21 However, a suboptimal adherence may have contributed in failing FTR. It has been recently shown that these amino acid substitutions do not alter the mechanism of action of drugs that interferes with HIV-1 entry, such as IBA or MVC.22 So, the sequential use of different entry inhibitor agents, in particular FTR, a pre-attachment inhibitor that affects gp120, and IBA, a post-attachment inhibitor, has not impaired IBA efficacy. However, mutations in the gp120 glycosylation site have been documented to decrease susceptibility to IBA.23
The approach we describe includes ENF recycling, that has demonstrated to improve survival in PLWH with a MDR infection.11 Other benefits of combining IBA and ENF for a short period are based both on synergistic activity in vitro and lack of cross-resistance between two drugs.24,25 ENF was started together with IBA with the aim to be discontinued when a stable HIV-1 RNA control was achieved, due to high rate of injection site reactions.
In conclusion, it is feasible and effective to use a GRT-driven strategy in clinical practice for people living with a pan-resistant HIV-1 infection. The recycling of ENF associated to a new drug free from cross-resistance with all the antiretrovirals available, including other entry inhibitors, and a close monitoring attributable to a route of administration that has ensured patient adherence, has revealed to be a turning point in achieving virological control in heavily-treated PLWH who have experienced repeated ART failure on any number of prior regimens over a 20–30 year period.
Contributors
All authors have seen and approved the content and have contributed significantly to the work and thus fulfill the criteria for Authorship. AC conceived the study and contributed to writing of the manuscript. DC and CM contributed to conceive and design the study, interpretations, and wrote the manuscript. DC and CM collected and updated data. DC, CM and LG verified data. LG and AP realized the graphics and contributed to the writing of the manuscript. LG, NG, VS, and MF contributed to the interpretation of the results and reviewed the manuscript.
Declaration of interests
DC, CM, LG, AP and MF declare no competing interests. VS has received grants from a Gilead Sciences fellowship program, payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events and support for attending meetings and/or travel from Gilead Sciences and ViiV Healthcare. NG has received consulting fees from Janssen-Cilag and ViiV Healthcare, support for attending meetings and/or travel from ViiV Healthcare and participated on a Data Safety Monitoring Board or Advisory Board for ViiV Healthcare. AC has received consulting fees, honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events, support for attending meetings and/or travel from Merck Sharp & Dohme, Gilead Sciences, Janssen-Cilag, ViiV Healthcare, and Theratecnologies.
Acknowledgments
Acknowledgements
We thank our patients; our nurses A. Bigoloni and G. Annicchiarico; our biologist A. Galli; all the physicians and nurses of Infectious Diseases Day Hospital of IRCCS San Raffaele Scientific Institute involved in the management of biweekly ibalizumab administration; ID specialists from Ospedale Sacco, Milan, and Clinica Pediatrica De Marchi for collecting data; the head of Hospital Pharmacy and her staff; GSK/ViiV Healthcare Team for sharing part of the reported data. Ibalizumab was supported by the Italian Medicines Agency 5% Fund support.
Data sharing statement
All data of the three cases described in the manuscript are present in the paper; deidentified virological and immunological data used in the figures are available to researchers through the public repository “San Raffaele Open Research Data Repository” - ORDR (https://ordr.hsr.it/research-data/; 10.17632/jbwfm2vs7d.1).
References
- 1.Antiretroviral Therapy Cohort Collaboration Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017;4:e349–e356. doi: 10.1016/S2352-3018(17)30066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Godfrey C., Thigpen M.C., Crawford K.W., et al. Global HIV antiretroviral drug resistance: a perspective and report of a national institute of allergy and infectious diseases consultation. J Infect Dis. 2017;216:S798–S800. doi: 10.1093/infdis/jix137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Günthard H.F., Calvez V., Paredes R., et al. Human immunodeficiency virus drug resistance: 2018 recommendations of the international antiviral society-USA panel. Clin Infect Dis. 2019;68:177–187. doi: 10.1093/cid/ciy463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European AIDS Clinical Society Guidelines for treatment of people living with HIV. EACS Guidelines version 10.1, October 2020. https://www.eacsociety.org/files/guidelines-10.1_30032021_1.pdf Accessed 28th June, 2021. [DOI] [PMC free article] [PubMed]
- 5.Judd A., Lodwick R., Noguera-Julian A., et al. Higher rates of triple-class virological failure in perinatally HIV-infected teenagers compared with heterosexually infected young adults in Europe. HIV Med. 2017;18:171–180. doi: 10.1111/hiv.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lombardi F., Giacomelli A., Armenia D., et al. Prevalence and factors associated with HIV-1 multi-drug resistance over the past two decades in the Italian ARCA database. Int J Antimicrob Agents. 2020;28 doi: 10.1016/j.ijantimicag.2020.106252. [DOI] [PubMed] [Google Scholar]
- 7.Galli L., Parisi M.R., Poli A., et al. Burden of disease in PWH harboring a multidrug-resistant virus: data from the PRESTIGIO registry. Open Forum Infect Dis. 2020;7:ofaa456. doi: 10.1093/ofid/ofaa456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puertas M.C., Ploumidis G., Ploumidis M., et al. Pan-resistant HIV-1 emergence in the era of integrase strand-transfer inhibitors: a case report. Lancet Microbe. 2020;1:e130–e135. doi: 10.1016/S2666-5247(20)30006-9. [DOI] [PubMed] [Google Scholar]
- 9.Rhee S.Y., Gonzales M.J., Kantor R., Betts B.J., Ravela J., Shafer RW. Human immunodeficiency virus reverse transcriptase and protease sequence database. Nucl Acids Res. 2003;31:298–303. doi: 10.1093/nar/gkg100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muccini C., Galli L., Spagnuolo V., et al. Brief report: association between low HIV-1 DNA and western blot reactivity to HIV-1 pol in chronically infected individuals. J Acquir Immune Defic Syndr. 2019;82:373–376. doi: 10.1097/QAI.0000000000002147. [DOI] [PubMed] [Google Scholar]
- 11.Cossarini F., Galli L., Sagnelli C., et al. Survival of HIV-1 infected multidrug-resistant patients recycling enfuvirtide after a previous failure. J Acquir Immune Defic Syndr. 2009;51:179–184. doi: 10.1097/QAI.0b013e3181a56f46. [DOI] [PubMed] [Google Scholar]
- 12.Parades R., Sagar M., Marconi V.C., et al. In vivo fitness cost of the M184V mutation in multidrug-resistant human immunodeficiency virus type 1 in the absence of lamivudine. J Virol. 2009;83:2038–2043. doi: 10.1128/JVI.02154-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciaffi L., Koulla-Shiro S., Sawadogo A.B., et al. Boosted protease inhibitor monotherapy versus boosted protease inhibitor plus lamivudine dual therapy as second-line maintenance treatment for HIV-1-infected patients in sub-Saharan Africa (ANRS12 286/MOBIDIP): a multicentre, randomised, parallel, open-label, superiority trial. Lancet HIV. 2017 Sep;4(9):e384–e392. doi: 10.1016/S2352-3018(17)30069-3. [DOI] [PubMed] [Google Scholar]
- 14.Charpentier C., Montes B., Perrier M., Meftah N., Reynes J. HIV-1 DNA ultra-deep sequencing analysis at initiation of the dual therapy dolutegravir + lamivudine in the maintenance DOLULAM pilot study. J Antimicrob Chemother. 2017;72(10):2831–2836. doi: 10.1093/jac/dkx233. Oct 1. [DOI] [PubMed] [Google Scholar]
- 15.Cavarelli M., Mainetti L., Pignataro A.R., et al. Complexity and dynamics of HIV-1 chemokine receptor usage in a multidrug-resistant adolescent. AIDS Res Hum Retrovir. 2014;30(12):1243–1250. doi: 10.1089/aid.2014.0124. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emu B., Fessel J., Schrader S., et al. Phase 3 study of ibalizumab for multidrug-resistant HIV-1. N Engl J Med. 2018;379:645–654. doi: 10.1056/NEJMoa1711460. [DOI] [PubMed] [Google Scholar]
- 17.Millham L.R.I., Scott J.A., Sax P.E., et al. Clinical and economic impact of ibalizumab for people with multidrug-resistant HIV in the United States. J Acquir Immune Defic Syndr. 2020;83:148–156. doi: 10.1097/QAI.0000000000002241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armenia D., Zaccarelli M., Borghi V., et al. Resistance detected in PBMCs predicts virological rebound in HIV-1 suppressed patients switching treatment. J Clin Virol. 2018;104:61–64. doi: 10.1016/j.jcv.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Canetti D., Galli L., Gianotti N., et al. Simplification to high genetic barrier 2-drug regimens in people living with HIV harboring 4-class resistance enrolled in the PRESTIGIO registry. J Acquir Immune Defic Syndr. 2020;84:e24–e28. doi: 10.1097/QAI.0000000000002378. [DOI] [PubMed] [Google Scholar]
- 20.Kozal M., Aberg J., Pialoux G., et al. Fostemsavir in adults with multidrug-resistant HIV-1 infection. N Engl J Med. 2020;382:1232–1243. doi: 10.1056/NEJMoa1902493. [DOI] [PubMed] [Google Scholar]
- 21.Lataillade M., Lalezari J.P., Kozal M., et al. Safety and efficacy of the HIV-1 attachment inhibitor prodrug fostemsavir in heavily treatment-experienced individuals: week 96 results of the phase 3 BRIGHTE study. Lancet HIV. 2020;7:e740–e751. doi: 10.1016/S2352-3018(20)30240-X. [DOI] [PubMed] [Google Scholar]
- 22.Ronald R., Gartland M., Li Z., et al. Clinical evidence for a lack of cross-resistance between temsavir and ibalizumab or maraviroc. AIDS. 2021 doi: 10.1097/QAD.0000000000003097. Oct 7Epub ahead of print. PMID: 34628442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toma J., Weinheimer S.P., Stawiski E., et al. Loss of asparagine-linked glycosylation sites in variable region 5 of human immunodeficiency virus type 1 envelope is associated with resistance to CD4+ antibody ibalizumab. J Virol. 2011;85:3872–3880. doi: 10.1128/JVI.02237-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X.Q., Sorensen M., Fung M., Schooley RT. Synergistic in vitro antiretroviral activity of a humanized monoclonal anti-CD4+ antibody (TNX-355) and enfuvirtide (T-20) Antimicrob Agents Chemother. 2006;50:2231–2233. doi: 10.1128/AAC.00761-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobson J.M., Kuritzkes D.R., Godofsky E., et al. Safety, pharmacokinetics, and antiretroviral activity of multiple doses of ibalizumab (formerly TNX-355), an anti-CD4+ monoclonal antibody, in human immunodeficiency virus type 1-infected adults. Antimicrob Agents Chemother. 2009;53:450–457. doi: 10.1128/AAC.00942-08. [DOI] [PMC free article] [PubMed] [Google Scholar]