Highlights
-
•
Many dual users of e-cigarettes/cigarettes report using e-cigarettes with the ITQ.
-
•
Data was from 3,542 adults (≥18 years) from the PATH cohort (2013–2018).
-
•
Dual users with ITQ were compared with mono cigarette smokers with ITQ.
-
•
Dual users with ITQ were less likely to transition to cessation.
-
•
This needs to be considered when assessing the population impact of e-cigarettes.
Abbreviations: BRR, Balanced repeated replication; ITQ, Intention to quit; PATH, Population Assessment of Tobacco and Health
Keywords: E-cigarettes, Intention to quit, Harm reduction, Longitudinal, Tobacco
Abstract
Many adult dual users of e-cigarettes and cigarettes in the United States report using e-cigarettes with the intention to quit (ITQ) smoking. This study examined transition outcomes among adult dual users of e-cigarettes and cigarettes with the ITQ compared to mono cigarette smokers with ITQ. We conducted a longitudinal analysis of 3,542 adults aged ≥ 18 years with data from Waves 1 and 4 of the United States Population Assessment of Tobacco and Health study (2013–2018) between May 2021 and January 2022. Current dual users (e-cigarettes and cigarettes use on ≥ 20 days in the past month) with the ITQ were compared to current mono cigarette smokers with the ITQ for transition outcomes (cessation, mono e-cigarette, mono cigarette and dual use) three years later. We conducted multinomial logistic regression modeling adjusting for potential confounders and reported the adjusted relative risk ratios (aRRR) with 95% confidence intervals (CI) for the transition outcomes. Approximately 10.7% (7.8–14.3) of dual users with the ITQ (in 2013) reported cessation (no past-month use of any tobacco) three years later, compared to 16.1% (14.6–17.7) of mono cigarette smokers. Dual users were 83% and 79% less likely to transition to cessation (aRRR: 0.17, 95% CI:0.09–0.32) or mono cigarette use (0.21, 0.14–0.32), respectively, compared to mono cigarette smokers. Our findings show that in a real-world scenario, dual e-cigarette and cigarette use may hinder rather than facilitate smoking cessation among those interested in quitting. This needs consideration when assessing the population impact of e-cigarettes and their role in harm reduction.
1. Introduction
Approximately one in six adult cigarette smokers in the United States report using electronic cigarettes (e-cigarettes), many of whom end up as dual users of e-cigarettes and cigarettes ('dual users') (Mirbolouk et al., 2018). One of the main reasons for e-cigarette use among adults in the United States is that e-cigarettes are viewed as a reduced-risk alternative to cigarettes and a cessation aid (Berg et al., 2014, Popova and Ling, 2013). Although e-cigarettes may pose fewer health risks than combustible cigarettes (Polosa et al., 2013) and may assist with smoking cessation (Harrell et al., 2014, Kalkhoran and Glantz, 2016), emerging evidence shows that e-cigarette use is not risk-free (Perrine et al., 2019). For example, in addition to some adverse health consequences (Glantz and Bareham, 2018, Perrine et al., 2019, Wills et al., 2019), e-cigarette use is associated with nicotine addiction as demonstrated by difficulty with smoking cessation (Coleman et al., 2018, Garey et al., 2019), despite intention to quit (ITQ) cigarettes (Robertson et al., 2019).
To date, evidence on the use of e-cigarettes as a cessation or harm reduction tool is conflicting. A prior clinical trial that compared the effectiveness of e-cigarettes to evidence-based cessation treatment found no difference in cessation between the two groups (Bullen et al., 2013). In contrast, a more recent clinical trial found that those who received e-cigarettes had significantly more cessation compared to the group that received nicotine replacement therapy (Hajek et al., 2019). Additionally, a meta-analysis found moderate-certainty evidence that e-cigarettes with nicotine increase quit rates compared to e-cigarettes without nicotine (Hartmann-Boyce et al., 2020). Another aspect of conflicting evidence comes from real-world studies suggesting, for the most part, that e-cigarettes impede rather than encourage cessation (El Dib et al., 2017). However, real-world observational studies are also inconsistent regarding the role of e-cigarettes, mainly because some of the participants included in these studies were not using e-cigarettes with the ITQ (El Dib et al., 2017).
To address this gap, we used data from the Population Assessment of Tobacco and Health (PATH) Study, to assess the prospective associations between current dual use of e-cigarettes and cigarettes with the ITQ compared to current mono use of cigarettes with the ITQ and subsequent transition outcomes (i.e., cessation, mono e-cigarette, mono cigarette and dual use) three years later. We hypothesized that dual users with the ITQ will be less likely to transition to cessation compared to cigarette smokers with the ITQ.
2. Methods
2.1. Study sample
The present study utilized adult data from the public use files of Waves 1 and 4 of the PATH Study, collected three years apart from September 2013 to December 2014 and December 2016 to January 2018 (United States Department of Health and Human Services, 2020). The PATH Study is an ongoing, nationally representative, longitudinal cohort study of United States adults and youth. Recruitment employed a multi-stage stratified probability sampling design that oversampled young adults (aged 18–24 years), adult tobacco users and African Americans (United States Department of Health and Human Services, 2020, Hyland et al., 2017). Audio computer‐assisted self‐interviews were conducted in person with 32,320 adults at Wave 1 and 33,822 adults at Wave 4 to collect baseline and follow-up information with an overall weighted response rate of 73.5% (United States Department of Health and Human Services, 2020). The combination of both datasets had a total of 32,315 participants. A previous study has published on the details of the PATH Study's sampling design and data collection methods (Hyland et al., 2017). The PATH Study received approval from the Westat Institutional Review Board, and this current study was deemed exempt by the Florida International University Institutional Review Board.
2.2. Assessment of tobacco use with the intention to quit
Participants were asked for their use of e-cigarettes and cigarettes on ≥20 days in the past month and their ITQ, and classified as dual users with the ITQ versus mono cigarette users with the ITQ at Wave 1. ITQ was assessed from the question which measures the level of interest in quitting smoking or using tobacco products on a scale from 1 to 10; “Overall, on a scale of 1 to 10 where 1 is not at all interested, how interested are you in quitting [ND FILL 4|tobacco products]? Please enter a number from 1 to 10.” The participants who responded “Not at all interested” to ITQ (i.e., participants with no interest in quitting) were excluded from the current study population . Those who responded “2” and above were treated as those with the intention to quit.
2.3. Assessment of transition outcome
At Wave 4, participants reported their e-cigarette and cigarette use status. Participants were classified as 1) cessation transition if they reported no use of any tobacco product in the past month; 2) mono e-cigarette transition if they reported current use of e-cigarettes only; 3) mono cigarette transition if they reported current use of cigarettes only, and 4) dual use transition if they reported using both e-cigarettes and cigarettes. Members of the mono e-cigarette and mono cigarette transition categories reported no past-month use of other tobacco products.
2.4. Covariates
We included in our models a range of variables measured at Wave 1 identified in the literature (Jackson et al., 2020) as potential confounders in the association between dual use (of e-cigarettes and cigarettes) and cessation. Sociodemographic covariates included age [18–24 (reference), 25–34, 35–44, 44–54, 55 + ]; sex [male and female (reference)], race/ethnicity [Non-Hispanic White (reference), Non-Hispanic Black, Non-Hispanic Other and Hispanic]; education [high school or less (reference), some college and bachelor’s degree or higher]; income [< $25,000 (reference), $25,000-$49,999, ≥$50,000 and not reported]; census region [Northwest (reference), Midwest, South, and West]. Tobacco-related variables were tobacco dependence (assessed on a scale of 1–100) as in previous literature (Strong et al., 2017), quit attempts [0 (reference) versus ≥ 1], and other tobacco use [yes or no (reference)]. Diagnosis of chronic disease was derived from the questions related to if a doctor or other healthcare professional had ever told the participant they had asthma, cancer, chronic bronchitis, chronic obstructive pulmonary disease, congestive heart failure, diabetes or prediabetes, emphysema, high blood pressure, high cholesterol, heart attack or stroke as in prior literature (Kalkhoran et al., 2018). The presence of ≥ 1 chronic disease was considered a diagnosis and compared to no chronic disease (0; reference).
2.5. Statistical analysis
We generated descriptive statistics for participant characteristics at Wave 1. We applied the Chi-square test and one-way analysis of variance (ANOVA) to compare the baseline characteristics of dual users and mono cigarette smokers. For our primary analysis, we used multinomial logistic regression models to analyze the prospective associations between dual use with the ITQ at Wave 1 and the transition outcomes [i.e., 1 = no e-cigarettes, no cigarettes; 2 = yes e-cigarettes, no cigarettes; 3 = no e-cigarettes, yes cigarettes and 4 = yes e-cigarettes, yes cigarettes (reference)] at the three-year follow-up relative to mono cigarette use with the ITQ in Model 1; and adjusted for the covariates measured at Wave 1 in Model 2. We conducted a sensitivity analysis where we included the ITQ variable as a scale in the regression model to adjust for the level of ITQ versus the binary variable.
We conducted all analyses in STATA version 16.1 between May 2021 and January 2022. All analyses were weighted using the Wave 4 single-weights accounting for the PATH Study's sampling design to produce nationally representative estimates. The balanced repeated replication (BRR) method with Fay's adjustment (0.3) was used as recommended in the PATH methodology (United States Department of Health and Human Services, 2020). We excluded participants who had no data on the exposure and outcome of interest from our analysis (N = 28,773), leaving 3,542 participants.
3. Results
The characteristics of the study sample (unweighted numbers) and corresponding population estimates (weighted %), overall, and by tobacco use status at baseline (Wave 1) are shown in Table 1. We observed differences in age, sexual orientation, race/ethnicity, education, census region, other tobacco use, quit attempts, tobacco dependence and ITQ among dual users and mono cigarette smokers (Table 1). A higher proportion of adults aged 18–44 years were dual users compared to mono cigarette smokers [18–24: 14.7 (11.5–18.5) vs. 13.0 (11.8–14.3); 25–34: 27.9 (22.9–33.6) vs. 22.3 (20.5–24.2); 35–44: 22.0 (17.0–27.9) vs. 18.8 (17.1–20.6)]. A larger proportion of dual users reported quit attempts than mono cigarette smokers [87.5% (82.8–91.1) vs. 81.6% (79.5–83.6); p = 0.02] and had higher tobacco dependence scores [Mean (SD): 61.0 (24.7) vs. 52.7 (27.3); p < 0.0001]. Furthermore, 10.7% (95% CI:7.8–14.3) of dual users with the ITQ from Wave 1 reported cessation (no past month use of any tobacco) three years later, compared to 16.1% (95% CI:14.6–17.7) of mono cigarette smokers with the ITQ (Table 1).
Table 1.
Participant characteristics overall and by tobacco use status: PATH Study, 2013–2018.
| Mono cigarette with ITQ, n = 3155 (weighted %, 95% CI) | Dual e-cigarette and cigarette with ITQ, n = 387 (weighted %, 95% CI) | P-value | |
|---|---|---|---|
| Baseline demographic variables | |||
| Age, years | 0.03 | ||
| 18–24 | 13.0 (11.8–14.3) | 14.7 (11.5–18.5) | |
| 25–34 | 22.3 (20.5–24.2) | 27.9 (22.9–33.6) | |
| 35–44 | 18.8 (17.1–20.6) | 22.0 (17.0–27.9) | |
| 45–54 | 21.2 (19.7–22.8) | 16.3 (12.4–21.2) | |
| ≥55 | 24.7 (22.8–26.7) | 19.2 (14.9–24.3) | |
| Sex | 0.95 | ||
| Female | 51.0 (48.9-53.0) | 50.8 (45.7–55.8) | |
| Male | 49.2 (44.2–54.3) | 49.1 (47.1–51.0) | |
| Sexual orientation | 0.013 | ||
| Lesbian/gay/bisexual/other | 6.6 (5.8–7.5) | 10.3 (7.4–7.5) | |
| Heterosexual | 93.4 (92.5–94.2) | 89.7 (85.8–92.6) | |
| Race/ethnicity | <0.0001 | ||
| Non-Hispanic White | 68.0 (66.1–69.8) | 80.5 (75.9–84.4) | |
| Non-Hispanic Black | 13.5 (12.2–14.8) | 5.8 (3.7–9.0) | |
| Non-Hispanic Other | 6.3 (5.3–7.3) | 6.1 (3.8–9.5) | |
| Hispanic | 12.3 (11.3–13.4) | 7.7 (5.4–10.7) | |
| Education | 0.03 | ||
| High school or less | 51.5 (49.5–53.4) | 43.5 (38.4–48.8) | |
| Some college | 37.0 (34.9–39.1) | 41.7 (36.4–47.3) | |
| Bachelor’s degree or higher | 11.6 (10.4–12.9) | 14.8 (11.2–19.2) | |
| Income | 0.37 | ||
| < $25,000 | 43.3 (41.1–45.4) | 39.3 (34.2–44.7) | |
| $25,000–49,999 | 23.4 (21.6–25.3) | 24.2 (19.5–29.5) | |
| ≥ $50,000 | 25.9 (24.0–28.0) | 30.4 (25.1–36.1) | |
| Not reported | 7.4 (6.4–8.5) | 6.2 (3.7–10.2) | |
| Census region | 0.02 | ||
| Northwest | 18.8 (16.9–20.8) | 11.3 (8.5–14.9) | |
| Midwest | 23.6 (21.7–25.7) | 25.5 (20.8–30.8) | |
| South | 39.0 (36.3–41.8) | 45.3 (38.9–51.9) | |
| West | 18.5 (16.6–20.7) | 17.9 (13.4–23.5) | |
| Presence of chronic disease | 0.10 | ||
| No | 49.3 (47.0–51.6) | 49.2 (43.9–54.6) | |
| Yes | 50.8 (48.5–53.0) | 50.8 (45.4–56.1) | |
| Baseline tobacco-related variables | |||
| Other tobacco use | 0.08 | ||
| No | 82.5 (80.9–84.1) | 78.7 (74.0–82.7) | |
| Yes | 17.5 (15.9–19.1) | 21.4 (17.3–26.0) | |
| Quit attempt | 0.02 | ||
| 0 | 18.4 (16.4–20.5) | 12.5 (8.9–17.2) | |
| ≥1 | 81.6 (79.5–83.6) | 87.5 (82.8–91.1) | |
| Tobacco dependence, mean (SD) | 52.7 (27.3) | 61.0 (24.7) | <0.0001 |
| Intention to quit, mean (SD) | 7.6 (2.4) | 8.0 (2.2) | 0.011 |
| Transition outcomes (Wave 4) | <0.0001 | ||
| Cessation | 16.1 (14.6–17.7) | 10.7 (7.8–14.3) | |
| Mono e-cigarette | 2.7 (2.2–3.3) | 11.0 (8.0–14.9) | |
| Mono cigarette | 75.1 (73.0–77.1) | 52.1 (46.5–57.6) | |
| Dual e-cigarette and cigarette | 6.1 (5.2–7.3) | 26.3 (21.8–31.2) | |
Abbreviations: ITQ, Intention to quit; PATH, Population Assessment of Tobacco and Health; SD, Standard deviation.
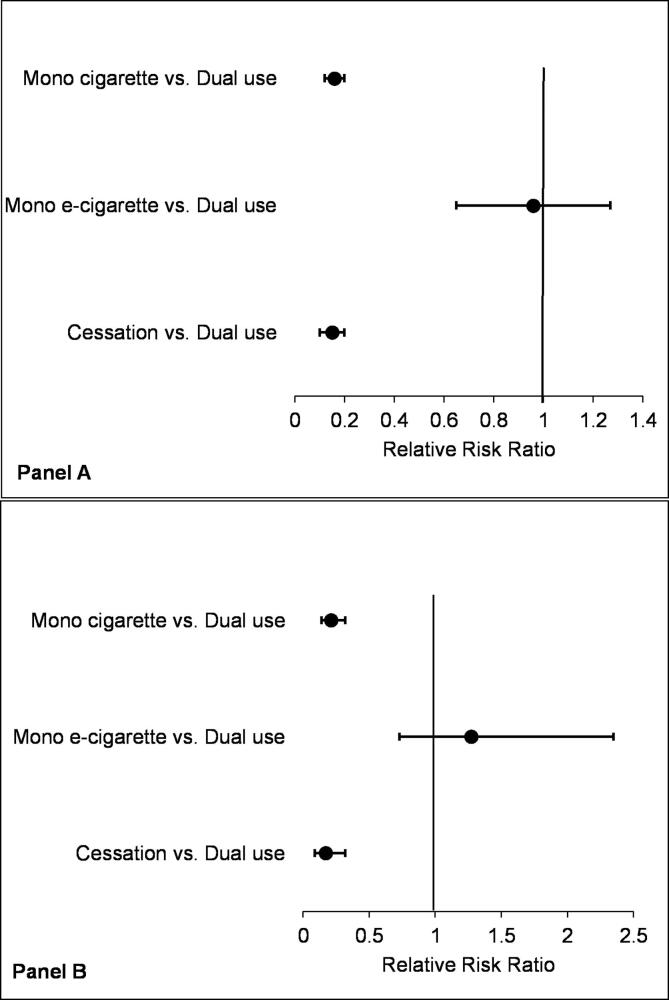
The prospective associations between dual use of e-cigarettes and cigarettes with the ITQ at Wave 1 and the transition outcomes at three-year follow-up are shown in Fig. 1. In the unadjusted regression analysis (panel A; Fig. 1), dual users with ITQ were 85% and 84% less likely to transition to cessation (RRR: 0.15, 95% CI: 0.10–0.24) and mono cigarette use (RRR: 0.16, 95% CI: 0.12–0.24), respectively, compared to mono cigarette smokers with ITQ. The associations were slightly attenuated in the adjusted regression analysis where dual users with the ITQ were 83% and 79% less likely to transition to cessation (aRRR: 0.17, 95% CI:0.09–0.32) and mono cigarette use (aRRR: 0.21, 95% CI:0.14–0.32), respectively, compared to mono cigarette smokers with the ITQ (panel B; Fig. 1). Although not statistically significant, dual users were more likely to transition to mono e-cigarette use (aRRR: 1.31, 95% CI: 0.73–2.35) compared to cigarette smokers with ITQ (panel B; Fig. 1). In the sensitivity analysis, where we included the ITQ variable as a scale in the regression analysis to adjust for the level of intent, the associations previously observed were similar [aRRR: 0.17 95% CI: 0.09–0.31 for cessation; 1.28 (0.72–2.30) for mono e-cigarette use and 0.21 (0.14–0.32) for mono cigarette use] (data not shown).
Fig. 1.
Forest plot showing the associations between dual users of e-cigarettes and cigarettes with the intention to quit and the transition outcomes at 3-year follow-up among adults (≥18 years): Population Assessment of Tobacco and Health Study, 2013–2018. Panel A: Model 1 is unadjusted; Panel B: Model 2 is adjusted for demographic, tobacco-related and presence of chronic disease variables. Dual use with the intention to quit was compared to mono cigarette use with intention to quit.
4. Discussion
By focusing on dual e-cigarette and cigarette users with the intention to quit in a real-world setting, our results show that United States adult dual users were less likely to transition to cessation three years later compared to cigarette smokers. Coming from the largest population-based longitudinal study of tobacco use in the United States, our results highlight the concern that e-cigarettes may hinder rather than facilitate cigarette smoking cessation in a real-world scenario.
Our findings are comparable to previous research conducted among United States adult smokers from the Growth from Knowledge (GfK)'s Panel to assess reasons and outcomes of e-cigarette use among cigarette smokers. They found no evidence that e-cigarettes use helped adult smokers quit after a 1-year follow-up (Weaver et al., 2018). Similarly, a recent study that examined cigarette-only smokers to compare the use of e-cigarettes, non-replacement therapy and non-NRT medication from the PATH Study, found no differences in the cessation rates across groups after a 1-year follow-up (Kaplan et al., 2021). The authors also reported that none of the participants using e-cigarettes with the ITQ became e-cigarette only users; instead, 40% became dual users (Kaplan et al., 2021). In contrast, several prior observational studies examining the relationship between e-cigarette use and cessation found that e-cigarette use was associated with a higher likelihood of successful cessation (Berry et al., 2019, Kalkhoran et al., 2020, Levy et al., 2018). For example, Kalkhoran et al. analyzed data from the PATH Study for two years, with e-cigarette use as the primary exposure among cigarette smokers, and cigarette abstinence as the primary outcome (Kalkhoran et al., 2020). The authors found that daily e-cigarette use was associated with a 77% increased odds of prolonged cigarette smoking abstinence among United States adult cigarette smokers (Kalkhoran et al., 2020). Likewise, Berry et al., using data from the PATH Study after a 1-year follow-up, to examine e-cigarette initiation and cigarette cessation/reduction, found that among current established cigarette smokers who were not current e-cigarette users at baseline, those who began using e-cigarettes daily had about 6 times the odds of reducing by at least 50% their average daily cigarette use compared to e-cigarette non-users (Berry et al., 2019). They also observed that daily e-cigarette users had about 8 folds higher odds of quitting smoking for at least 30 days compared to e-cigarette non-users (Berry et al., 2019). However, unlike our study, their selected groups were only based on patterns of use, without interest in quitting. We believe that including this defining factor is crucial to the assessment of the role of e-cigarettes at the population level, given the current debate about e-cigarettes' potential to offer a new path for adult smokers interested in quitting or harm reduction but cannot achieve that otherwise (Notley et al., 2018).
E-cigarettes have unintended public health consequences among youth that must be taken into consideration. There is a lot of debate in the United States about the increased use of e-cigarettes among youth, particularly due to flavors, as a result the FDA announced restrictions on the sale of flavored e-cigarettes except for menthol and tobacco flavors (Diaz et al., 2021, FDA, 2020), which some argue may be a deterrent to adults using e-cigarettes with the intention to quit cigarette use (Farsalinos and Niaura, 2020). However, population-based studies with representative samples can provide valuable information about balancing the negative and positive effects of e-cigarettes and support the tobacco regulatory authorities with evidence that should be considered when regulating e-cigarettes.
The concern about e-cigarettes' place as a harm reduction tool involves results from clinical trials as well as real world evidence. Findings from a recently updated Cochrane review of randomized trials also suggest that e-cigarettes can help smokers quit with the current level of evidence upgraded to moderate from low in previous editions of the review (Hartmann-Boyce et al., 2020). The authors found that cessation rates at six months or longer were higher among the nicotine e-cigarette group than those that used e-cigarettes without nicotine. The results were more pronounced when the nicotine e-cigarette group was compared to behavioral support only or no support (Hartmann-Boyce et al., 2020). This diverging evidence based on study design is not unexpected and likely reflects the selectiveness of the study sample and delivery of the intervention (Hartmann-Boyce et al., 2020). It seems that in a well monitored and prescribed setting, e-cigarettes may be of help to cigarette smokers, but not when they are marketed freely to consumers. The problem of open marketing of these products is compounded by the massive uptake of e-cigarettes by youth, who are primarily drawn to it for reasons other than harm reduction or cessation (Kong et al., 2015, Tsai et al., 2018). It is also heightened by the additional risk of dual use when real-world use patterns, as our study shows, are not consistent with cessation or harm reduction outcomes.
4.1. Strengths and limitations
The strengths of this study include the prospective design of a sample representing the adult population of the United States with a focus on tobacco use patterns and associated factors. This study comes with limitations as well. While we adjusted for potential sociodemographic and smoking-related confounders, there may be residual confounding of unmeasured variables. Also, our findings only apply to the period between 2013 and 2018. The associations between transition outcomes and product use may fluctuate over time, indicating changes in e-cigarette devices and factors such as changing perceptions for e-cigarettes that could affect people's willingness to act on their ITQ and subsequent outcomes.
5. Conclusions
In this longitudinal, nationally representative cohort study of United States adults, we found that dual users of e-cigarettes and cigarettes with the ITQ were less likely to transition to cessation and mono cigarette transition outcomes three years later. Thus, the dual use of e-cigarettes and cigarettes may hinder rather than facilitate smoking cessation among those interested in quitting. This lies against evidence of e-cigarettes' potential value for cessation and harm reduction found in clinical trials. It suggests that e-cigarettes should be available within a clinical smoking cessation setting rather than as a free consumer product. Further research can help disentangle the nuances of the population-level effect of e-cigarettes compared to clinical randomized trial settings and the role of e-cigarettes in society.
CRediT authorship contribution statement
Olatokunbo Osibogun: Conceptualization, Data curation, Formal analysis, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. Zoran Bursac: Formal analysis, Methodology, Validation, Writing – review & editing. Wasim Maziak: Conceptualization, Formal analysis, Methodology, Project administration, Supervision, Validation, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors thank the investigators, staff and participants of the Population Assessment of Tobacco and Health Study for their valuable contributions.
Funding sources
OO was supported by the NIDA T32DA043449 Grant at the time of conducting this study. ZB is supported by FIU-Research Center in Minority Institution (Grant U54MD012393-01). WM is supported by National Institutes of Health (Grants R01-DA035160, R01-TW010654, R01-DA042477) and the NIDA T32DA043449 Grant. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
References
- Berg C.J., Barr D.B., Stratton E., Escoffery C., Kegler M. Attitudes toward E-cigarettes, reasons for initiating e-cigarette use, and changes in smoking behavior after initiation: a pilot longitudinal study of regular cigarette smokers. Open J. Prev. Med. 2014;04(10):789–800. doi: 10.4236/ojpm.2014.410089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry K.M., Reynolds L.M., Collins J.M., Siegel M.B., Fetterman J.L., Hamburg N.M., Bhatnagar A., Benjamin E.J., Stokes A. E-cigarette initiation and associated changes in smoking cessation and reduction: the population assessment of tobacco and Health Study, 2013–2015. Tob Control. 2019;28:42–49. doi: 10.1136/tobaccocontrol-2017-054108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen C., Howe C., Laugesen M., McRobbie H., Parag V., Williman J., Walker N. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382(9905):1629–1637. doi: 10.1016/S0140-6736(13)61842-5. [DOI] [PubMed] [Google Scholar]
- Coleman, B., Rostron, B., Johnson, S.E., Persoskie, A., Pearson, J., Stanton, C., Choi, K., Anic, G., Goniewicz, M.L., et al., 2018. Transitions in electronic cigarette use among adults in the Population Assessment of Tobacco and Health (PATH) Study, Waves 1 and 2 (2013-2015). Tob Control. [DOI] [PMC free article] [PubMed]
- Diaz M.C., Donovan E.M., Schillo B.A., Vallone D. Menthol e-cigarette sales rise following 2020 FDA guidance. Tob Control. 2021;30(6):700–703. doi: 10.1136/tobaccocontrol-2020-056053. [DOI] [PubMed] [Google Scholar]
- El Dib R., Suzumura E.A., Akl E.A., Gomaa H., Agarwal A., Chang Y., Prasad M., Ashoorion V., Heels-Ansdell D., Maziak W., Guyatt G. Electronic nicotine delivery systems and/or electronic non-nicotine delivery systems for tobacco smoking cessation or reduction: a systematic review and meta-analysis. BMJ Open. 2017;7(2):e012680. doi: 10.1136/bmjopen-2016-012680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K.E., Niaura R. E-cigarettes and smoking cessation in the united states according to frequency of E-cigarette use and quitting duration: analysis of the 2016 and 2017 national health interview surveys. Nicotine Tobacco Res. 2020;22(5):655–662. doi: 10.1093/ntr/ntz025. [DOI] [PubMed] [Google Scholar]
- FDA, 2020. FDA finalizes enforcement policy on unauthorized flavored cartridge-based e-cigarettes that appeal to children, including fruit and mint. FDA NEWS RELEASE. Available at https://www.fda.gov/news-events/press-announcements/fda-finalizes-enforcement-policy-unauthorized-flavored-cartridge-based-e-cigarettes-appeal-children. Accessed January 19, 2022.
- Garey L., Mayorga N.A., Peraza N., Smit T., Nizio P., Otto M.W., Zvolensky M.J. Distinguishing characteristics of E-cigarette users who attempt and fail to quit: dependence, perceptions, and affective vulnerability. J. Stud. Alcohol Drugs. 2019;80(1):134–140. [PubMed] [Google Scholar]
- Glantz S.A., Bareham D.W. E-cigarettes: use, effects on smoking, risks, and policy implications. Ann. Rev. Public Health. 2018;39(1):215–235. doi: 10.1146/annurev-publhealth-040617-013757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek P., Phillips-Waller A., Przulj D., Pesola F., Myers Smith K., Bisal N., Li J., Parrott S., Sasieni P., Dawkins L., Ross L., Goniewicz M., Wu Q.i., McRobbie H.J. A randomized trial of E-cigarettes versus nicotine-replacement therapy. New Engl. J. Med. 2019;380(7):629–637. doi: 10.1056/NEJMoa1808779. [DOI] [PubMed] [Google Scholar]
- Harrell P.T., Simmons V.N., Correa J.B., Padhya T.A., Brandon T.H. Electronic nicotine delivery systems (“e-cigarettes”): review of safety and smoking cessation efficacy. Otolaryngol. Head Neck Surg. 2014;151(3):381–393. doi: 10.1177/0194599814536847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann-Boyce J., McRobbie H., Lindson N., Bullen C., Begh R., Theodoulou A., Notley C., Rigotti N., Turner T., Butler A., Hajek P. Electronic cigarettes for smoking cessation. Cochrane Database Syst. Rev. 2020 doi: 10.1002/14651858.CD010216.pub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland A., Ambrose B.K., Conway K.P., Borek N., Lambert E., Carusi C., Taylor K., Crosse S., Fong G.T., Cummings K.M., Abrams D., Pierce J.P., Sargent J., Messer K., Bansal-Travers M., Niaura R., Vallone D., Hammond D., Hilmi N., Kwan J., Piesse A., Kalton G., Lohr S., Pharris-Ciurej N., Castleman V., Green V.R., Tessman G., Kaufman A., Lawrence C., van Bemmel D.M., Kimmel H.L., Blount B., Yang L., O'Brien B., Tworek C., Alberding D., Hull L.C., Cheng Y.-C., Maklan D., Backinger C.L., Compton W.M. Design and methods of the population assessment of tobacco and health (PATH) study. Tob Control. 2017;26(4):371–378. doi: 10.1136/tobaccocontrol-2016-052934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S.E., Farrow E., Brown J., Shahab L. Is dual use of nicotine products and cigarettes associated with smoking reduction and cessation behaviours? A prospective study in England. BMJ Open. 2020;10(3):e036055. doi: 10.1136/bmjopen-2019-036055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkhoran S., Kruse G.R., Chang Y., Rigotti N.A. Smoking-cessation efforts by US adult smokers with medical comorbidities. Am. J. Med. 2018;131(3):318.e1–318.e8. doi: 10.1016/j.amjmed.2017.09.025. [DOI] [PubMed] [Google Scholar]
- Kalkhoran S., Glantz S.A. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. Lancet Respir. Med. 2016;4(2):116–128. doi: 10.1016/S2213-2600(15)00521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkhoran S., Chang Y., Rigotti N.A. Electronic cigarette use and cigarette abstinence over 2 years among U.S. smokers in the population assessment of tobacco and health study. Nicotine Tob. Res. 2020;22(5):728–733. doi: 10.1093/ntr/ntz114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan B., Galiatsatos P., Breland A., Eissenberg T., Cohen J.E. Effectiveness of ENDS, NRT and medication for smoking cessation among cigarette-only users: a longitudinal analysis of PATH Study wave 3 (2015–2016) and 4 (2016–2017), adult data. Tob Control. 2021 doi: 10.1136/tobaccocontrol-2020-056448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong G., Morean M.E., Cavallo D.A., Camenga D.R., Krishnan-Sarin S. Reasons for electronic cigarette experimentation and discontinuation among adolescents and young adults. Nicotine Tob. Res. 2015;17(7):847–854. doi: 10.1093/ntr/ntu257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy, D.T., Yuan, Z., Luo, Y., Abrams, D.B., 2017. The Relationship of E-Cigarette Use to Cigarette Quit Attempts and Cessation: Insights From a Large, Nationally Representative U.S. Survey, pp. 931-39. [DOI] [PMC free article] [PubMed]
- Mirbolouk M., Charkhchi P., Kianoush S., Uddin S.M.I., Orimoloye O.A., Jaber R., Bhatnagar A., Benjamin E.J., Hall M.E., DeFilippis A.P., Maziak W., Nasir K., Blaha M.J. Prevalence and distribution of E-cigarette use among U.S. adults: behavioral risk factor surveillance system, 2016. Ann. Int. Med. 2018;169(7):429–438. doi: 10.7326/M17-3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notley C., Ward E., Dawkins L., Holland R. The unique contribution of e-cigarettes for tobacco harm reduction in supporting smoking relapse prevention. Harm Reduct. J. 2018;15:31. doi: 10.1186/s12954-018-0237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine C.G., Pickens C.M., Boehmer T.K., King B.A., Jones C.M., DeSisto C.L., Duca L.M., Lekiachvili A., Kenemer B., Shamout M., Landen M.G., Lynfield R., Ghinai I., Heinzerling A., Lewis N., Pray I.W., Tanz L.J., Patel A., Briss P.A., Adjemian J., Amin M., Aponte J., Barry V., Browning D., Cates J., Chandra G., Chang K., Chiang K., Chevinsky J., Delaney A., Dunn A., Evans M., Fields V., Fleischauer A., Garcia M., Green C., Hanchey A., Hartnett K., Hoots B., Islam A., Kaboré C., Krishnasamy V., Lamtahri M., Layden J., Meany-Delman D., Meiman J., Mikosz C., Miller M., Mohamoud Y., Moritz E., Neelam V., Nitschke D., O’Laughlin K., Olson S., Rogers T., Roth N., Salvatore P., Vivolo-Kantor A., Werner A., Wilken J. Characteristics of a multistate outbreak of lung injury associated with E-cigarette use, or vaping - United States, 2019. MMWR. Morb. Mortality Weekly Rep. 2019;68(39):860–864. doi: 10.15585/mmwr.mm6839e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosa R., Rodu B., Caponnetto P., Maglia M., Raciti C. A fresh look at tobacco harm reduction: the case for the electronic cigarette. Harm Reduct. J. 2013;10(1):19. doi: 10.1186/1477-7517-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova L., Ling P.M. Alternative tobacco product use and smoking cessation: a national study. Am. J. Public Health. 2013;103(5):923–930. doi: 10.2105/AJPH.2012.301070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson L., Hoek J., Blank M.L., Richards R., Ling P., Popova L. Dual use of electronic nicotine delivery systems (ENDS) and smoked tobacco: a qualitative analysis. Tob. Control. 2019;28:13–19. doi: 10.1136/tobaccocontrol-2017-054070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong D.R., Pearson J., Ehlke S., Kirchner T., Abrams D., Taylor K., Compton W.M., Conway K.P., Lambert E., Green V.R., Hull L.C., Evans S.E., Cummings K.M., Goniewicz M., Hyland A., Niaura R. Indicators of dependence for different types of tobacco product users: descriptive findings from Wave 1 (2013–2014) of the Population assessment of tobacco and health (PATH) study. Drug Alcohol. Dependence. 2017;178:257–266. doi: 10.1016/j.drugalcdep.2017.05.010. [DOI] [PubMed] [Google Scholar]
- Tsai J., Walton K., Coleman B.N., Sharapova S.R., Johnson S.E., Kennedy S.M., Caraballo R.S. Reasons for electronic cigarette use among middle and high school students - national youth tobacco survey, United States, 2016. MMWR. Morbid. Mortal. Weekly Rep. 2018;67(6):196–200. doi: 10.15585/mmwr.mm6706a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Health and Human Services. National Institutes of Health. National Institute on Drug Abuse, and United States Department of Health and Human Services. Food and Drug Administration. Center for Tobacco Products. Population Assessment of Tobacco and Health (PATH) Study [United States] Public-Use Files. Inter-university Consortium for Political and Social Research [distributor], 2020-10-21. https://doi.org/10.3886/ICPSR36498.v11. Accessed May 2021.
- Weaver, S.R., Huang, J., Pechacek, T.F., Heath, J.W., Ashley, D.L., Eriksen, M.P., 2018. Are electronic nicotine delivery systems helping cigarette smokers quit? Evidence from a prospective cohort study of U.S. adult smokers, 2015-2016. PLoS One 13:e0198047. [DOI] [PMC free article] [PubMed]
- Wills T.A., Pagano I., Williams R.J., Tam E.K. E-cigarette use and respiratory disorder in an adult sample. Drug Alcohol. Depend. 2019;194:363–370. doi: 10.1016/j.drugalcdep.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]