See Clinical Research on Page 526
Acute kidney injury (AKI) during and after hospitalizations for acute decompensated heart failure (ADHF) is a common and established risk factor for adverse clinical outcomes. Although AKI is most often defined by serum creatinine (SCr) values relative to a baseline1 (where specific changes are documented or presumed to have occurred within 7 days), there is increasing understanding that not all AKI is equal. For example, AKI in the context of ADHF can be delineated into various pathophysiological pathways. AKI can be the underlying cause of heart failure (type 3 cardiorenal syndrome), the sequela of ADHF (especially in cardiogenic shock, type 1 cardiorenal syndrome), an unfortunate collateral event from a systemic disease (e.g., sepsis) with detrimental effects on both kidneys and the heart (type 5 cardiorenal syndrome), or some combination of the above. There is also growing recognition that AKI consisting of only small changes in SCr (i.e., stage 1 AKI, meeting only a 0.3 mg/dl increase in SCr) may not be associated with important long-term adverse outcomes in many clinical scenarios. This is especially relevant in ADHF, where acute increases in SCr that occur after initiation of heart failure therapies such as renin-angiotensin system inhibitors and diuretics do not necessary portend adverse outcomes and may signify a therapeutic hemodynamic effect.2
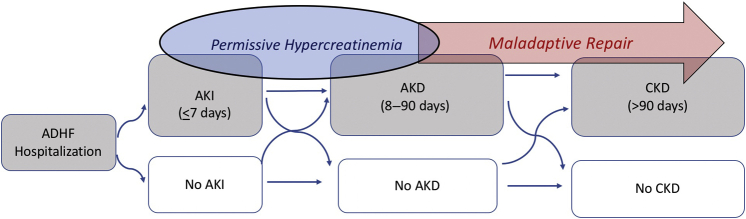
Acute kidney disease (AKD) describes a continuum of kidney dysfunction occurring acutely or subacutely within a 90-day period. AKD can represent nonrecovery from AKI beyond the initial 7-day window, but it may also include subacute and persistent alterations in kidney function without a documented inciting AKI event (Figure 1). AKD was first proposed in the 2012 Kidney Disease: Improving Global Outcomes AKI clinical guidelines, and expert panels have identified the AKD population as one of high research priority.1,3 Despite this, there have been limited studies on the epidemiology of AKD, likely owing to the lack of or short follow-up time of many hospital-based databases and limited pooling of data with community clinics. The concept of AKD is especially relevant in the care of patients with heart failure, as the armament of heart failure medications (with proven mortality benefit) associated with modest changes in SCr has grown. Clinicians are eager to add and uptitrate these medications during and especially in the aftermath of an inciting ADHF episode, and the incidence of AKD may rise as a result.
Figure 1.
Framework for AKD diagnosis after ADHF. ADHF, acute decompensated heart failure; AKD, acute kidney disease; AKI, acute kidney injury; CKD, chronic kidney disease.
In the current issue of the KI Reports, Chen et al.4 explore the incidence, risk factors, and prognosis of AKD after ADHF using comprehensive electronic health data from 7 hospitals in Taiwan. After applying key exclusions, such as patients on maintenance dialysis and those with another obvious insult for AKI (sepsis, urinary obstruction), they identified 7519 adults who were hospitalized for ADHF in a 10-year period, among whom 678 (9%) developed in-hospital AKI and 1592 (21%) developed AKD (defined as persistent kidney dysfunction relative to prehospitalization baseline at or closest to 3 months). The authors then identified key risk factors for AKD, including sex, diabetes, AKI stage, admission SCr level, hemoglobin, albumin, B-type natriuretic peptide, and use of inotropes and high-dose i.v. diuretics. Risk factors for the composite outcome of stage 3 AKD and death were similar except age and admission blood urea nitrogen replaced diabetes and chronic kidney disease as risk factors. In the 5 years of follow-up, AKD was associated with higher risks of all-cause death (hazard ratio 1.32 [95% CI 1.17–1.49]) and major adverse kidney events (hazard ratio 1.30 [95% CI 1.21–1.40]). Finally, the authors developed predictive scoring models with adequate c-statistics for any-stage AKD (0.73 [95% CI 0.71–0.74]) and for composite of stage 3 AKD and death (0.81 [95% CI 0.79–0.82]).
The study is strengthened by its large sample size, comprehensive capture of data including both inpatient and outpatient laboratory values (owing to a large multi-institutional and centralized database), and a clinically important and novel application of AKD, a thus far understudied concept in nephrology and heart failure. Limitations include a lack of external validation of the derived prediction models, especially for patients with heart failure in other countries who may not share the same profile of risk factors as patients in Taiwan. Because SCr values were not systematically collected at 90 days after ADHF admission, AKD as an outcome may be subject to ascertainment bias, as patients with inciting AKI and/or preexisting chronic kidney disease were likely more prone to have follow-up laboratory measurements. Finally, there was limited information regarding important heart failure medications, including renin-angiotensin system inhibitors, sodium-glucose transport protein 2 inhibitors, diuretics, and mineralocorticoid receptor antagonists that are known to cause perturbations in SCr, especially in the critical posthospitalization period during which AKD is ascertained.
A key finding here is only 39% (n = 267) of patients with AKI (n = 678) at the time of ADHF progressed to AKD. Conversely, among the 1592 patients who developed AKD, most (83%, n = 1328) did not have a preceding AKI event. The prediction models created could allow for postdischarge identification of patients with ADHF at risk for AKD—beyond those with an inciting AKI event. This select population of patients with AKD may benefit from earlier and more vigilant clinical and laboratory follow-up and more careful introduction/titration of forementioned medications (renin–angiotensin system inhibitors, sodium-glucose co-transporter 2 inhibitors, mineralocorticoid receptor antagonists) known to confer mortality benefit (and potentially long-term renal benefit) after their ADHF hospitalization.
The concept of “permissive hypercreatinemia”5,6 has grown in popularity and acceptance in the context of heart failure and cardiorenal management. Although the Kidney Disease: Improving Global Outcomes defines AKI and AKD by changes in SCr level, acute changes in SCr level do not necessarily reflect tubular ischemia and damage.7 For example, renin–angiotensin system inhibitors and sodium-glucose transport protein 2 inhibitors may acutely decrease intraglomerular pressure and glomerular filtration rate, leading to increased SCr level, but their long-term use is noted to be renoprotective and cardioprotective.8 Therefore, the “AKI” episodes that occur from the introduction or uptitration of heart failure therapeutics which acutely modulate renal perfusion but offer proven mortality and/or cardiorenal benefits should not be interpreted as signals of tubular and permanent injury and should be permitted with careful monitoring.
Nevertheless, does the concept of permissive hypercreatinemia extend to persistent elevations in SCr level, for example, AKD? For how long and to what extent do we permit the elevated SCr level, especially after acute heart failure? As Chen et al.4 reveal, a subset of AKI clearly leads to AKD and moderate-to-severe AKI (stage 2/3) is a strong risk factor for persistently very elevated creatinine up to 90 days (AKD stage 3). AKD, in turn, strongly predicts major adverse kidney events and death. Exactly when does persistently elevated SCr become clinically worrisome, and how much of the AKI to AKD spectrum in the context of ADHF represents maladaptive kidney repair leading to progressive chronic kidney disease/end-stage kidney disease versus permissive hypercreatinemia? These are questions difficult to answer with retrospective data alone (Figure 1). Further detailed analyses incorporating the longitudinal use of heart failure therapeutics that affect renal perfusion and the duration, severity, and recovery from AKD after ADHF as predictors of major adverse kidney events and mortality may be informative.
Nevertheless, Chen et al.4 identify and characterize AKD in hospitalized patients with ADHF, and the results hint at opportunities for care improvement. Accurately predicting who will develop AKD—especially those without an inciting AKI—with readily available clinical variables is an important, nascent step toward understanding and establishing the metric of care they need. In contrast, a sizable proportion of individuals with AKI who are not at risk for AKD may not need the same level of follow-up care. Until evidence-based quality metrics are established, we should stick with a repertoire of common sense strategies for high-risk patients after ADHF: careful volume status and laboratory assessments, vigilant medication reconciliation, dietary counseling, careful titration of heart failure medications, and removal of aggravating (true) nephrotoxic factors.5,9 Nephrologists should also assume an expert role in managing potential metabolic derangements from heart failure therapies (e.g., hyper/hypokalemia and dysnatremias), along with offering reassurance to patients and team members when small changes in SCr are detected after therapy titrations. Further work is clearly needed to better define the spectrum of pathophysiology of AKD in the setting of ADHF and whether the degree of tubular injury in this context predicts adverse outcomes, beyond AKD itself.
Disclosure
All the authors declared no competing interests.
References
- 1.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 2.McCoy I.E., Chertow G.M. AKI—a relevant safety end point? Am J Kidney Dis. 2020;75:508–512. doi: 10.1053/j.ajkd.2019.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chawla L.S., Bellomo R., Bihorac A., et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13:241–257. doi: 10.1038/nrneph.2017.2. [DOI] [PubMed] [Google Scholar]
- 4.Chen J.-J., Lee T.-H., Kuo G., et al. Acute kidney disease after acute decompensated heart failure. Kidney Int Rep. 2022;7:526–536. doi: 10.1016/j.ekir.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parikh C.R., Coca S.G. “Permissive AKI” with treatment of heart failure. Kidney Int. 2019;96:1066–1068. doi: 10.1016/j.kint.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Meraz-Muñoz A.Y., Weinstein J., Wald R. eGFR decline after SGLT2 inhibitor initiation: the tortoise and the hare reimagined. Kidney360. 2021;2:1042–1047. doi: 10.34067/KID.0001172021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmad T., Jackson K., Rao V.S., et al. Worsening renal function in patients with acute heart failure undergoing aggressive diuresis is not associated with tubular injury [published correction appears in Circulation. 2018;137:e853] Circulation. 2018;137:2016–2028. doi: 10.1161/CIRCULATIONAHA.117.030112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCallum W., Tighiouart H., Ku E., Salem D., Sarnak M.J. Acute declines in estimated glomerular filtration rate on enalapril and mortality and cardiovascular outcomes in patients with heart failure with reduced ejection fraction. Kidney Int. 2019;96:1185–1194. doi: 10.1016/j.kint.2019.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu K.D., Forni L.G., Heung M., et al. Quality of care for acute kidney disease: current knowledge gaps and future directions. Kidney Int Rep. 2020;5:1634–1642. doi: 10.1016/j.ekir.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]