Abstract
Introduction
Acute kidney disease (AKD) represents a continuum of kidney injury for 7 to 90 days after acute kidney injury (AKI). The incidence and prognosis of AKD after acute decompensated heart failure (ADHF) are currently unclear. The aims of this study were to explore the incidence of AKD and the transition from AKI to AKD, to identify risk factors for AKD and develop a prediction model for any-stage AKD, and to evaluate the prognosis of AKD.
Methods
A total of 7519 patients admitted for ADHF between January 1, 2008, and December 31, 2018, from a multi-institutional database were identified. The composite outcomes after ADHF were stage 3 AKD and all-cause death. The prognosis impact of AKD, including major adverse kidney events (MAKEs), all-cause death, and heart failure hospitalization (HFH), during 5 years of follow-up was analyzed.
Results
The overall incidence of AKI and AKD after ADHF was 9% and 21.2%, respectively; 39.4% of the patients diagnosed with having AKI during ADHF subsequently developed AKD whereas 19.4% of the patients without an identified AKI episode subsequently developed AKD. The predictive scoring models revealed C-statistics of 0.726 (95% CI: 0.712–0.740) for any-stage AKD and 0.807 (95% CI: 0.793–0.821) for the composite of stage 3 AKD and death. Finally, AKD was associated with higher risks of all-cause death, MAKE, and HFH during the 5 years of follow-up (P < 0.001).
Conclusion
AKD after ADHF are associated with adverse outcomes. Our model could help in identification of patients at risk for AKD development, especially in those who did not have an index AKI episode.
Keywords: acute decompensated heart failure, acute kidney disease, acute kidney injury
Graphical abstract
See Commentary on Page 378
ADHF is a poor prognostic event in patients with congestive heart failure, with a 1-year death rate of >30% and a high readmission rate.1,2 The condition usually occurs alongside AKI. The incidence of AKI among those admitted for ADHF varies from 9.6% to 43%.3, 4, 5 AKI, as a common complication of ADHF (i.e., acute cardiorenal syndrome type 1 in ADHF), is associated with higher 1-year death and readmission rates.3,4,6 The poor prognostic effect is more significant in those who developed AKI or worse of renal function but without effective decongestion.7
AKD represents a continuum of kidney injury or renal function nonrecovery after initial kidney insult/stress. In addition, the transition from AKI, to AKD, to chronic kidney disease (CKD) reflects a continuum of persistent kidney injury after initial kidney insult.8 The time course for AKD is described as >7 days but within 90 days of initiating AKI. The current AKD definition is based on the consensus of the Acute Disease Quality Initiative 16 Workgroup.8 Therefore, AKD could be considered as a condition that prolonged kidney dysfunction (in the presence or absence of AKI) occurring before a patient meets the 90-day criteria for CKD.8,9 Compared with such research on AKI, studies investigating the incidence and prognostic impact of AKD in patients admitted for ADHF are rare. Some studies evaluating clinical factors related to the development of AKI/CKD or prediction models for patients with ADHF have been published, but few of them have addressed the development of AKD after ADHF.4,10, 11, 12, 13, 14, 15, 16, 17, 18
In this study, we investigated the incidence, clinical factors, and prognostic impact of AKD after ADHF. We also developed a prediction model for AKD after ADHF to facilitate risk stratification and thus promote early AKD identification and intervention.
Methods
Data Source
This was a retrospective cohort study using electronic data from the Chang Gung Research Database (CGRD). The Chang Gung Medical Foundation is the largest medical system in Taiwan, comprising 7 hospitals spanning all of Taiwan. The CGRD is a multi-institutional electronic medical record database that provides more detailed clinical information, such as laboratory results and hemodynamic records, than claims databases and has high overall and disease-specific coverage of Taiwan.19,20
The diseases evaluated in this study were identified using International Classification of Diseases (ICD), Ninth Revision, Clinical Modification diagnostic codes for records before 2015 and ICD, Tenth Revision, Clinical Modification diagnostic codes for those after 2016. The data structure and validation of the CGRD are discussed elsewhere.20, 21, 22 This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital (institutional review board number: 202000915B0). The need for individual consent was waived because personal identification data are not included in the CGRD. This study was conducted according to the STROBE statement (Supplementary Material).
Study Population
Patients who were admitted for ADHF (identified by ICD, Ninth Revision, Clinical Modification : 428 and ICD, Tenth Revision, Clinical Modification: I50 in the hospital primary and secondary diagnosis during hospitalization) between January 1, 2008, and December 31, 2018, and who had sufficient data to determine the presence of AKI and AKD were identified in the CGRD. The use of ICD, Ninth Revision, Clinical Modification: 428 and ICD, Tenth Revision, Clinical Modification: I50 for identified ADHF hospitalized population is verified in other studies23,24 and with positive predictive value >90%.25,26 For patients with multiple ADHF admissions during the study period, the first ADHF admission was used as the index admission. Patients were excluded if they were <18 years old, were diagnosed with having end-stage renal disease and already on maintenance dialysis, or were on extracorporeal membrane oxygenation during index admission. Patients with anticipated cardiac transplantation, who were diagnosed with having sepsis or obstructive uropathy, were exposed to a nephrotoxic agent during admission (including iodine contrast media, a nonsteroidal anti-inflammatory drug, aminoglycosides, or vancomycin), or developed severe AKI requiring dialysis were also excluded (Figure 1a).
Figure 1.
The flowchart for (a) patient selection and (b) the distribution of different AKI and AKD stages. AKD, acute kidney disease; AKI, acute kidney injury; ECMO, extracorporeal membrane oxygenation; ESRD, end-stage renal disease; RRT, renal replacement therapy.
AKI and AKD Definitions
The presence of AKI was determined according to the Kidney Disease: Improving Global Outcomes AKI criteria, which is by comparing a patient’s baseline creatinine levels with their highest creatinine level during the first 7 days of their index admission.27 For the baseline creatinine level, we used the lowest creatinine level in the 3 months before the index admission or, if no creatinine level within 3 months of the index admission was available, the first creatinine level in the same index admission. The first AKI episode in the index admission is identified as index AKI. The presence of AKD was determined based on consensus from the Acute Disease Quality Initiative 16 Workgroup.8 AKD is defined by a condition in which persisted AKI is present ≥7 days after an AKI initiating event. The Acute Disease Quality Initiative workgroup also mentioned that an AKI initiating event can usually be identified but is not required to diagnose AKD.8,9 For AKD staging, the baseline creatinine level was compared with the creatinine level nearest to 90 days after the index admission; AKD stages 1 and 2 were defined as serum creatinine levels 1.5 to 1.9 and 2.0 to 2.9 times baseline, respectively, whereas stage 3 was defined as a serum creatinine level 3.0 times baseline, serum creatinine increase of ≥4.0 mg/dl, or being on renal replacement therapy for 8 to 90 days after the index date. If >1 value was obtained during the 8 to 90 days after the index admission, the presence of AKD was determined based on the creatinine level taken most closely to the 90th day after the index admission.
Potential Predictors (Covariates)
Patients’ clinical characteristics, AKI susceptibility factors,4,10, 11, 12, 13, 14, 15, 16, 17,28, 29, 30 and renal nonrecovery factors for congestive heart failure or critical illness population31, 32, 33, 34, 35 were identified according to previous studies or availability in our data set. The risk factors extracted included age, sex, underlying disease (i.e., diabetes mellitus, hypertension, CKD, liver cirrhosis, and malignancy), heart function assessment by New York Heart Association functional class,36 and left ventricular ejection fraction. Hemodynamic parameters (systolic blood pressure, diastolic blood pressure, and heart rate) at the arrival of emergency room or at the day of admission, first laboratory results during index admission (including hemoglobin [HB]; blood urea nitrogen [BUN]; serum creatinine, albumin, sodium, potassium, proteinuria, and B-type natriuretic peptide [BNP] levels), and related medication prescriptions (outpatient loop diuretics in the previous 3 months and cumulative loop diuretics dosage during ADHF admission, digoxin, inotropes, or dobutamine use during the index admission) were also extracted.
Outcome Definition
There were 2 primary outcomes in this study, which are the following: (i) the development of any stage of AKD and (ii) a composite outcome of stage 3 AKD or all-cause death during the eighth and 90th days after the index admission. The secondary outcomes are all-cause death, MAKE, and HFH from the 91st day to the fifth year after the index admission. MAKE was composed of a new diagnosis of end-stage renal disease requiring long-term renal replacement therapy, new-onset CKD (defined by estimated glomerular filtration rate <60 ml/min per 1.73 m2 according to the Modification of Diet in Renal Disease equation), and all-cause death.
Statistical Analysis
The baseline characteristics of patients with and without AKI or AKD were compared using the independent sample t test for continuous variables and the χ2 test for categorical variables. Univariate logistic regression analysis was used for the initial screening of the possible association between baseline characteristics and the risks of outcomes. Covariates with a significance of <0.2 in the univariate logistic regression analyses were further introduced into a multivariable model with automatic backward elimination. In the multivariable model, continuous parameters (i.e., systolic blood pressure, diastolic blood pressure, heart rate, HB, BUN, creatinine, potassium, and albumin) were categorized based on previous reports or according to clinical definitions.4,10, 11, 12, 13, 14, 15, 16, 17 The models for predicting any-stage AKD, the composite of stage 3 AKD and all-cause death, and all-cause death alone were developed separately.
The clinical and laboratory-based prediction model derived from the multivariable logistic analysis was further transformed into a simplified point system for ease of clinical use.37 The key idea of the simplified point system is to round off the regression coefficients. The first step was to identify a continuous predictor with a wide range of values as the reference variable (i.e., BNP) and then categorize this variable into several clinically meaningful categories and obtain reference values (usually the middle value) for each category of the variable. Predictors other than the reference variable were also categorized accordingly. Finally, the reference value (usually the middle value) of each category of the predictor was calculated according to the value of its regression coefficient relative to that of the reference variable.
The model’s performance was evaluated in several areas, including discrimination, calibration, and internal validation. Its discriminative ability was evaluated using the area under the receiver operating characteristic curve (AUC), in which the SE was calculated using DeLong’s method. Its calibration performance was evaluated using the plot that identifies subgroups as the deciles of fitted (predicted) probabilities. The model was well calibrated when the expected (predicted) and observed probabilities in subgroups were similar. The Hosmer–Lemeshow test was not performed because it is sensitive to large sample sizes. To evaluate the external generalizability of the derived model, an internal validation of the AUC was conducted using 1000 bootstrapped samples.
We further compared the risks of all-cause death and MAKE from the 91st day to the fifth year between the AKD and non-AKD groups by using the univariate Cox proportional hazard model. The risk of HFH between groups during follow-up was compared using the univariate Fine and Gray subdistribution hazard model, which considered all-cause death a competing risk. The assumption of proportional hazard was evaluated using the Schoenfeld residuals method on the long-term outcomes. Finally, patients were divided into 3 equally sized subgroups according to the simplified point system. The risks of all-cause death, MAKE, and HFH across the ordinal risk subgroups were investigated using the aforementioned survival analyses, in which the ordinal risk subgroup was treated as a continuous covariate. All analyses were conducted using R version 4.0.1 (R Development Core Team).
Results
Study Population
The flowchart of patient inclusion is presented in Figure 1a. A total of 11,010 patients admitted for ADHF with sufficient information for AKI and AKD judgment were identified. After exclusion per the abovementioned criteria, 7519 patients admitted for ADHF between January 1, 2008, and December 31, 2018, were eligible for analysis.
Incidence of AKD and Transition From AKI to AKD After ADHF
Of the 7519 patients, 678 (9.0%) developed AKI during the first 7 days (Figure 1a). Of these 678 patients with AKI, 267 (39.4%) developed stage 1, 2, or 3 AKD during follow-up days 8 to 90, with the remaining 411 (60.6%) grouped into stage 0 AKD. Of the 7519 patients, 6841 did not have an identified AKI episode during the first 7 days of hospitalization; of these patients, 1325 (19.4%) developed stage 1, 2, or 3 AKD during follow-up days 8 to 90. Figure 1b reveals the patients’ grouping into different AKI and AKD stages. Those with a higher AKI stage had a higher probability of stage 3 AKD or death than did those with a lower AKI stage. Overall, of the 7519 patients admitted for ADHF, 1592 (21.2%) developed AKD during 8 to 90 days in the follow-up period after first hospitalization day.
Prediction Model for Any-Stage AKD
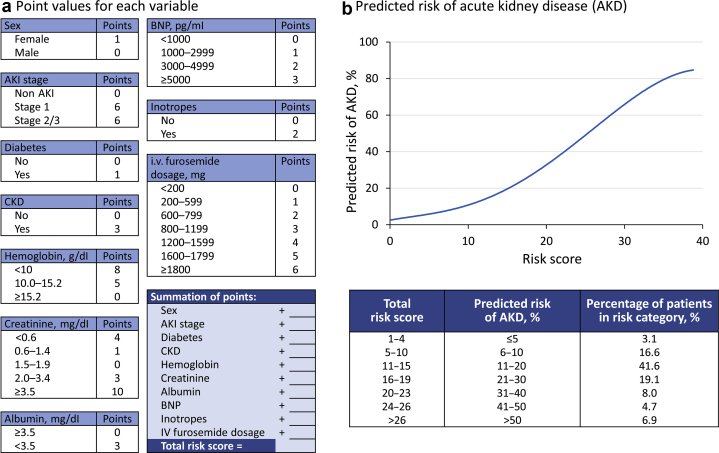
Table 1 and Supplementary Table S1 illustrate the characteristics and relevant clinical risk factors. Clinical factors related to any-stage AKD development after ADHF were identified using univariate logistic regression models (Supplementary Table S2). The multivariable logistic regression model identified the following predictors: female sex, AKI development, AKI severity (non-AKI, mild AKI: stage 1, moderate to severe AKI: stage 2 or 3), comorbidities (diabetes mellitus, CKD), laboratory values (including creatinine, HB, albumin, and BNP), and medication (inotropes and i.v. loop diuretics cumulative dosage) (Supplementary Table S3). The simplified point system was developed based on this model (Table 2). A score of 4 was associated with a 5% probability of AKD development whereas a score of 38 corresponded to an 85% probability of AKD. The AUC of the model was 0.726 (95% CI: 0.712–0.740). The calibration plot revealed a small discrepancy between the predicted and observed probabilities across the decile subgroups, except for the lowest decile subgroup (Supplementary Figure S1). After being corrected for optimism, the AUC was 0.723. The trivial difference between the original and corrected AUCs indicates the acceptable external generalizability of the current model (data not found). The score system and predicted risk of any-stage AKD is summarized in Figure 2a and b.
Table 1.
Baseline characteristics of patients according to the presence or absence of AKI or AKD
| Variables | AKI (n = 678) | No AKI (n = 6841) | P value | AKD (n = 1592) | No AKD/AKD stage 0 (n = 5927) | P value |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, yr | 71.8 ± 14.5 | 71.9 ± 14.4 | 0.768 | 72.6 ± 13.3 | 71.7 ± 14.7 | 0.021 |
| Male | 346 (51.0) | 3821 (55.9) | 0.016 | 790 (49.6) | 3377 (57.0) | <0.001 |
| AKI stage | <0.001 | <0.001 | ||||
| No AKI | 0 (0.0) | 6841 (100.0) | 1325 (83.2) | 5516 (93.1) | ||
| Stage 1 | 410 (60.5) | 0 (0.0) | 135 (8.5) | 275 (4.6) | ||
| Stage 2 | 81 (11.9) | 0 (0.0) | 32 (2.0) | 49 (0.8) | ||
| Stage 3 | 187 (27.6) | 0 (0.0) | 100 (6.3) | 87 (1.5) | ||
| Underlying disease | ||||||
| Diabetes mellitus | 260 (38.3) | 2686 (39.3) | 0.641 | 782 (49.1) | 2164 (36.5) | <0.001 |
| Chronic kidney disease | 363 (53.5) | 3339 (48.8) | 0.019 | 1025 (64.4) | 2677 (45.2) | <0.001 |
| Hypertension | 425 (62.7) | 3976 (58.1) | 0.021 | 1068 (67.1) | 3333 (56.2) | <0.001 |
| Chronic liver disease | 126 (18.6) | 1519 (22.2) | 0.030 | 322 (20.2) | 1323 (22.3) | 0.073 |
| Malignancy | 123 (18.1) | 1318 (19.3) | 0.478 | 316 (19.8) | 1125 (19.0) | 0.435 |
| Heart function | ||||||
| NYHA functional class IV | 143 (21.1) | 995 (14.5) | <0.001 | 306 (19.2) | 832 (14.0) | <0.001 |
| LVEF, % | 48.4 ± 18.1 | 48.2 ± 18.3 | 0.774 | 49.9 ± 17.5 | 47.8 ± 18.5 | <0.001 |
AKD, acute kidney disease; AKI, acute kidney injury; NYHA, New York Heart Association; LVEF, left ventricular ejection fraction.
Table 2.
Simple score function of any AKD (number of patient = 7519)
| Variable | Point | Variables | Point | Total points | Probability of any AKD |
|---|---|---|---|---|---|
| Sex | Albumin, mg/dl | 4 | 0.05 | ||
| Female | 1 | ≥3.5 | 0 | 10 | 0.10 |
| Male | 0 | <3.5 | 3 | 13 | 0.15 |
| AKI stage | BNP, pg/ml | 15 | 0.20 | ||
| Non AKI | 0 | <1000 | 0 | 18 | 0.25 |
| Stage 1 | 6 | 1000–2999 | 1 | 19 | 0.30 |
| Stage 2/3 | 6 | 3000–4999 | 2 | 21 | 0.35 |
| Diabetes mellitus | ≥5000 | 3 | 23 | 0.40 | |
| No | 0 | Inotropes | 24 | 0.45 | |
| Yes | 1 | No | 0 | 26 | 0.50 |
| Chronic kidney disease | Yes | 2 | 27 | 0.55 | |
| No | 0 | i.v. furosemide dosage, mg | 28 | 0.60 | |
| Yes | 3 | <200 | 0 | 30 | 0.65 |
| Hemoglobin, g/dl | 200–590 | 1 | 32 | 0.70 | |
| 8 | 600–790 | 2 | 33 | 0.75 | |
| 10.0–15.2 | 5 | 800–1190 | 3 | 36 | 0.80 |
| ≥15.2 | 0 | 1200–1590 | 4 | 38 | 0.85 |
| Creatinine, mg/dl | 1600–1790 | 5 | |||
| <0.6 | 4 | ≥1800 | 6 | ||
| 0.6–1.4 | 1 | ||||
| 1.5–1.9 | 0 | ||||
| 2.0–3.4 | 3 | ||||
| ≥3.5 | 10 |
AKD, acute kidney disease; AKI, acute kidney injury; BNP, B-type natriuretic peptide.
Figure 2.
Summary of any-stage AKD prediction model. (a) Point values for each variable. (b) Predicted risk for any-stage AKD development. AKD, acute kidney disease; AKI, acute kidney injury; BNP, B-type natriuretic peptide; CKD, chronic kidney disease.
Prediction Model for the Composite of Stage 3 AKD and All-Cause Death
Clinical factors related to stage 3 AKD or death were identified (Supplementary Table S2). The multivariable model identified the following predictors: age, female sex, AKI development, AKI severity (non-AKI, mild AKI: stage 1, moderate to severe AKI: stage 2 or 3), laboratory values (including BUN, creatinine, HB, albumin, and BNP), medication (inotropes and i.v. loop diuretics cumulative dosage), and outpatient loop diuretics prescription (Supplementary Table S3). The clinical prediction model for stage 3 AKD or death was developed based on these risk factors (Table 3). A score of 11 was associated with a 5% probability of stage 3 AKD or death, whereas a score of 40 corresponded to a 95% probability. The AUC was 0.807 (95% CI: 0.793–0.821). The calibration plot revealed a small-to-moderate discrepancy between the predicted and observed probabilities across the decile subgroups (Supplementary Figure S2). After being corrected for optimism, the AUC was 0.724. The trivial difference between the original and corrected AUCs indicates the satisfactory external generalizability of the current model (data not found). The score system and predicted risk of stage 3 AKD is summarized in Figure 3a and b.
Table 3.
Simple score function of the composite of AKD stage 3 and death (number of patient = 7519)
| Variable | Point | Variable | Point | Total points | Probability of outcome | |
|---|---|---|---|---|---|---|
| Age, yr | Albumin, mg/dl | 11 | 0.05 | |||
| <30 | 0 | ≥3.5 | 0 | 15 | 0.10 | |
| 30–49 | 1 | <3.5 | 3 | 17 | 0.15 | |
| 50–79 | 2 | BNP, pg/ml | 19 | 0.20 | ||
| ≥80 | 3 | <1000 | 0 | 20 | 0.25 | |
| Sex | 1000–2499 | 1 | 21 | 0.30 | ||
| Female | 1 | 2500–3999 | 2 | 22 | 0.35 | |
| Male | 0 | 4000–4999 | 3 | 23 | 0.40 | |
| AKI stage | ≥5000 | 4 | 25 | 0.45 | ||
| Non AKI | 0 | Outpatient loop diuretics | 26 | 0.50 | ||
| Stage 1 | 2 | No | 0 | 26 | 0.55 | |
| Stage 2/3 | 3 | Yes | 1 | 27 | 0.60 | |
| Hemoglobin, g/dl | Inotropes | 29 | 0.65 | |||
| <10 | 5 | No | 0 | 30 | 0.70 | |
| 10.0–15.2 | 3 | Yes | 5 | 31 | 0.75 | |
| ≥15.2 | 0 | i.v. furosemide dosage, mg | 32 | 0.80 | ||
| BUN, mg/dl | <400 | 0 | 34 | 0.85 | ||
| ≤24 | 0 | 400–1190 | 1 | 36 | 0.90 | |
| >24 | 3 | 1200–1990 | 2 | 40 | 0.95 | |
| Creatinine, mg/dl | ≥2000 | 3 | ||||
| <0.6 | 2 | |||||
| 0.6–1.4 | 1 | |||||
| 1.5–1.9 | 0 | |||||
| 2.0–3.4 | 3 | |||||
| ≥3.5 | 11 |
AKD, acute kidney disease; AKI, acute kidney injury; BUN, blood urea nitrogen; BNP, B-type natriuretic peptide.
Figure 3.
Summary of stage 3 AKD or mortality prediction model. (a) Point values for each variable. (b) Predicted risk for stage 3 AKD development. AKD, acute kidney disease; AKI, acute kidney injury; BNP, B-type natriuretic peptide; BUN, blood urea nitrogen.
Prediction Model for 90-Day All-Cause Death
Clinical factors related to 90-day death were identified (Supplementary Table S2). The multivariable model identified the following predictors: age, female sex, AKI stage, chronic liver disease, BUN, albumin, BNP, digoxin, outpatient loop diuretics, and inotropes (Supplementary Table S3). The clinical prediction model for death was developed based on these risk factors (Supplementary Table S4). A score of 18 was associated with a 5% probability of death, whereas a score of 28 corresponded to a 30% probability of death. The AUC was 0.746 (95% CI: 0.725–0.768). After being corrected for optimism, the AUC was 0.741. The score system and predicted risk of 90-day death is summarized in Supplementary Figure S3A and S3B.
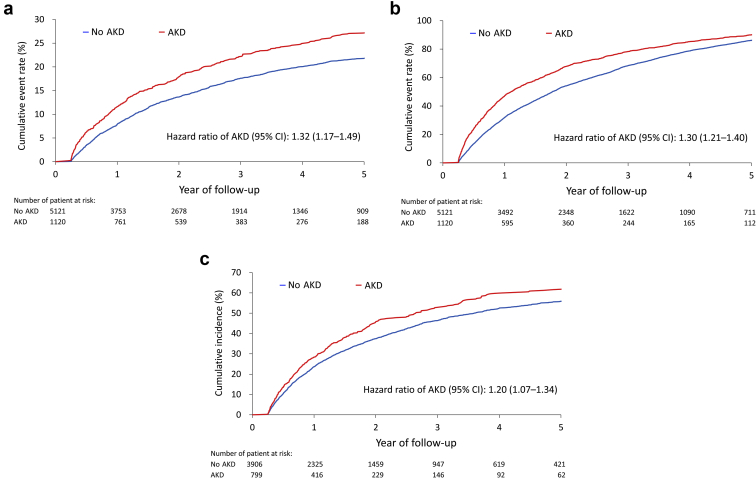
AKD Prediction Scores and the Risk of Long-Term Outcomes
In the 5-year follow-up, the risks of all-cause death, MAKE, and HFH were significantly greater in the patients with AKD than in the patients without AKD (Figure 4a–c). In addition, the correlation of the Schoenfeld residuals was <0.1 for all-cause death, MAKE, and HFH, respectively. These low correlation coefficients suggested that there was no apparent violation of proportional hazard assumption (data not found). After categorizing the patients into tertile subgroups according to their simplified points, the results revealed that patients with higher simplified points tended to have greater risks of all-cause death, MAKE, and HFH in both the any-stage AKD prediction model (Supplementary Figure S4A–C) and the composite prediction model (Supplementary Figure S5A–C).
Figure 4.
The cumulative event rate function of all-cause death (a) and MAKE (b) and cumulative incidence function of HFH (c) of patients stratified by AKD status during 5 years of follow-up. In the analysis of HFH, patients who were readmitted owing to heart failure within 90 days after discharge from the index admission were excluded from the analysis. AKD, acute kidney disease; HFH, heart failure hospitalization; MAKE, major adverse kidney event.
Risk Factors of Long-Term Outcomes
To investigate the potential risk factors of the long-term outcomes (all-cause death, MAKE, and HFH), we conducted multivariable Cox models with backward elimination which included AKD and other variables as the covariates. According to the multivariable Cox model, the following variables were found to be predictors of all-cause death: age, CKD, liver disease, New York Heart Association functional class IV, HB, BUN, sodium, albumin, BNP level, and outpatient loop diuretics. The identified risk factors of long-term MAKE were age, diabetes mellitus, liver disease, cancer, baseline left ventricular ejection fraction, HB, albumin, creatinine, albumin, proteinuria, BNP level, inotropic use, and i.v. furosemide use. Regarding long-term rehospitalization for heart failure, the identified predictors were age, diabetes mellitus, left ventricular ejection fraction, heart rate, BUN, proteinuria, BNP level, outpatient loop diuretics, and i.v. furosemide use (Supplementary Table S5).
Discussion
AKD represents a continuum of kidney injury or renal nonrecovery after AKI. The incidence and prognostic role of AKD after ADHF are rarely discussed. Our study yielded 4 notable results. First, the overall incidence of AKD after ADHF was 21.2%. Second, 39.4% of patients with a diagnosis of AKI during ADHF developed AKD, whereas 19.4% of patients without an initial identified AKI episode developed AKD. Third, the development of AKD was associated with higher risks of mortality and MAKE and a higher incidence of HFH. Fourth, our prediction models identified patients at high risk of any-stage AKD or stage 3 AKD and mortality, with AUROCs of 0.73 and 0.81, respectively.
According to Chen et al.,38 47.6% of patients in the coronary intensive care unit develop AKD and the development of AKD is associated with higher mortality. Both AKI and AKI severity are associated with AKD development.38 According to previous studies, the incidence of AKD in patients without an identified AKI episode is 17% to 37.8%.39, 40, 41, 42 Our study revealed that approximately 40% and 20% of patients with AKI and patients without AKI developed AKD after ADHF, respectively.
Persistent and transient AKI have different outcome effects in patients with AKI.8 The timing of renal function recovery also affects further kidney outcome.43 Cardiorenal syndrome has been used to describe the interrelated derangements of heart–kidney interactions. Five types of cardiorenal syndrome have been proposed, with type 1 cardiorenal syndrome defined by a course in which the acute worsening of cardiac function results in AKI.44 Chronic heart failure may also result in kidney dysfunction, and this process is referred to as type 2 cardiorenal syndrome. According to Brankovic et al.,6 higher renal tubular damage markers entail poor outcomes in the population with stable chronic heart failure. CKD itself is a risk factor for AKI in ADHF and a poor prognostic factor. Our study found that overall 5-year survival, MAKE incidence, and HFH differed significantly between patients with and without an AKD diagnosis.
Clinical models for AKD prediction in patients with sepsis have been published recently. According to Peerapornratana et al.,45 male sex, race, and acute physiology score III are significantly associated with the odds ratio for AKD. Their recorded incidence of AKD after sepsis was 26.9% and their clinical model had an AUROC of 0.71 for AKD prediction. Prediction models for AKI after ADHF with AUROCs ranging from 0.65 to 0.87 have also been published.4,14,16,18 Clinical model-based clinical parameters alone for AKD prediction revealed a predictive ability not inferior to novel biomarkers.45 Furthermore, a post hoc analysis of the FINNAKI study found that adding a urine biomarker to clinical prediction model did not improve AKI discrimination statistically significantly.46 Our prediction model, based on daily practice clinical parameters, yielded an AUROC of 0.73 for AKD prediction and an AUROC of 0.81 for stage 3 AKD and mortality prediction. Our prediction model could be used to identify those who are with high risk for AKD development but without identified index AKI episode after ADHF. Early referral to a nephrologist might be important in these patients.
Our study has several limitations. First, this was a retrospective study. Second, no biomarkers were available and few urine analyses were performed after ADHF for the patients evaluated; thus, we were unable to further group participants into stage 0A, 0B, or 0C AKD. Third, urine output and fluid status were not included in our clinical models. Fourth, lack of external validation from a separate cohort is the major concern regarding to our prediction model. Fifth, potential ascertainment bias should be addressed. We enrolled participants with available creatinine data within 90 days after discharge. It is worthy noted that participants with AKI episode are more likely to receive renal function follow-up after discharge in comparison with those without identified AKI episode. This condition could lead to underestimate the incidence of AKD in patients without identified AKI episode. Furthermore, the method for BNP measurement in our 7 hospitals is the same; however, the cutoff value might be different in other hospitals owing to different measurement methods or assays.
Despite these limitations, this study benefits from its large, multi-institutional sample and is, to our knowledge, the first such study to investigate the incidence and prognostic effect of AKD after ADHF. Our scoring system may serve as a clinical prediction tool for AKD development and correlated long-term patient outcomes. Accordingly, the early identification of patients at high risk of poor long-term prognosis may facilitate early intervention. Despite the known protective role of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker on the population with CKD, recent trials have also revealed a possible beneficial effect of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker on the AKI population47; as such, further randomized controlled trials are needed to evaluate the effect on population with AKD.
Conclusion
AKD after ADHF is associated with a higher mortality rate and higher incidences of adverse kidney events and HFH. We reported that approximately 21% of patients admitted for ADHF developed AKD and a portion of patient without identified AKI episode still developed AKD. Our scoring system is an easy-to-use tool that can effectively predict the risk of AKD after ADHF and thus aid in early AKD diagnosis and intervention.
Disclosure
The authors declared no competing interests.
Acknowledgments
The authors thank Mr. Alfred Hsing-Fen Lin, MS, and Mrs. Bing-Yu Chen, PhD, for their assistance in the statistics analysis. This study is based in part on data from the Chang Gung Research Database provided by Chang Gung Memorial Hospital. The interpretation and conclusions contained herein do not represent the position of Chang Gung Memorial Hospital. This study was supported by grants from Chang Gung Memorial Hospital, Taiwan, (CMRPG5K0141). CHC was supported by the Ministry of Science and Technology (109-2314-B-182A-124).
Data Availability
Data cannot be directly shared publicly because of the regulation of Chang Gung Research Database policy. The data are provided, maintained, and managed by the Chang Gung Medical Foundation. Researchers interested in accessing this data set can submit a formal application to request access (Chang Gung Memorial Hospital, No. 5, Fuxing St., Guishan Dist., Taoyuan City 33305, Taiwan; E-mail: ccling999@gmail.com, +886-03-328120-7721).
Author Contributions
JJC, CHC, and PHC: methodology. GK and CLY: formal analysis. PCF, SWC, and VCCW: data extraction. JJC and THL: writing—original draft preparation. CHC and PHC: writing—review and editing. CHC: project administration. All authors read and approved the final manuscript.
Footnotes
Figure S1. Calibration for any-stage AKD prediction.
Figure S2. Calibration for composite of stage 3 AKD and death prediction.
Figure S3. Summary of prediction model for 90-day death. (A) Point values for each variable. (B) Predicted risk for 90-day death.
Figure S4. The cumulative event rate function of all-cause death (A) and MAKE (B) and cumulative incidence function of HFH (C) of patients stratified by the tertile of the simplified point system for any-stage AKD during 5 years of follow-up.
Figure S5. The cumulative event rate function of all-cause death (A) and MAKE (B) and cumulative incidence function of HFH (C) of patients stratified by the tertile of the simplified point system for the composite of AKD stage 3 and all-cause death during 5 years of follow-up.
Table S1. Detailed characteristics of patients according to the presence or absence of AKI or AKD.
Table S2. Univariate logistic regression analysis of the association between baseline characteristics and risks of outcomes.
Table S3. Multivariable logistic regression analysis with backward elimination.
Table S4. Simple score function of 90-day death.
Table S5. Multivariable Cox regression analysis with backward elimination for the associated factors of long-term outcomes.
STROBE Statement.
Supplementary Material
Figure S1. Calibration for any-stage AKD prediction.
Figure S2. Calibration for composite of stage 3 AKD and death prediction.
Figure S3. Summary of prediction model for 90-day death. (A) Point values for each variable. (B) Predicted risk for 90-day death.
Figure S4. The cumulative event rate function of all-cause death (A) and MAKE (B) and cumulative incidence function of HFH (C) of patients stratified by the tertile of the simplified point system for any-stage AKD during 5 years of follow-up.
Figure S5. The cumulative event rate function of all-cause death (A) and MAKE (B) and cumulative incidence function of HFH (C) of patients stratified by the tertile of the simplified point system for the composite of AKD stage 3 and all-cause death during 5 years of follow-up.
Table S1. Detailed characteristics of patients according to the presence or absence of AKI or AKD.
Table S2. Univariate logistic regression analysis of the association between baseline characteristics and risks of outcomes.
Table S3. Multivariable logistic regression analysis with backward elimination.
Table S4. Simple score function of 90-day death.
Table S5. Multivariable Cox regression analysis with backward elimination for the associated factors of long-term outcomes.
Supplementary Document. STROBE statement—checklist.
STROBE Statement (PDF)
References
- 1.Jong P., Vowinckel E., Liu P.P., Gong Y., Tu J.V. Prognosis and determinants of survival in patients newly hospitalized for heart failure: a population-based study. Arch Intern Med. 2002;162:1689–1694. doi: 10.1001/archinte.162.15.1689. [DOI] [PubMed] [Google Scholar]
- 2.Hernandez A.F., Greiner M.A., Fonarow G.C., et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303:1716–1722. doi: 10.1001/jama.2010.533. [DOI] [PubMed] [Google Scholar]
- 3.Damman K., Jaarsma T., Voors A.A., et al. Both in- and out-hospital worsening of renal function predict outcome in patients with heart failure: results from the Coordinating Study Evaluating Outcome of Advising and Counseling in Heart Failure (COACH) Eur J Heart Fail. 2009;11:847–854. doi: 10.1093/eurjhf/hfp108. [DOI] [PubMed] [Google Scholar]
- 4.Breidthardt T., Socrates T., Noveanu M., et al. Effect and clinical prediction of worsening renal function in acute decompensated heart failure. Am J Cardiol. 2011;107:730–735. doi: 10.1016/j.amjcard.2010.10.056. [DOI] [PubMed] [Google Scholar]
- 5.Bagshaw S.M., Cruz D.N., Aspromonte N., et al. Epidemiology of cardio-renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant. 2010;25:1406–1416. doi: 10.1093/ndt/gfq066. [DOI] [PubMed] [Google Scholar]
- 6.Brankovic M., Akkerhuis K.M., Hoorn E.J., et al. Renal tubular damage and worsening renal function in chronic heart failure: clinical determinants and relation to prognosis (Bio-SHiFT study) Clin Cardiol. 2020;43:630–638. doi: 10.1002/clc.23359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fudim M., Loungani R., Doerfler S.M., et al. Worsening renal function during decongestion among patients hospitalized for heart failure: findings from the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial. Am Heart J. 2018;204:163–173. doi: 10.1016/j.ahj.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 8.Chawla L.S., Bellomo R., Bihorac A., et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13:241–257. doi: 10.1038/nrneph.2017.2. [DOI] [PubMed] [Google Scholar]
- 9.Liu K.D., Forni L.G., Heung M., et al. Quality of care for acute kidney disease: current knowledge gaps and future directions. Kidney Int Rep. 2020;5:1634–1642. doi: 10.1016/j.ekir.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forman D.E., Butler J., Wang Y., et al. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004;43:61–67. doi: 10.1016/j.jacc.2003.07.031. [DOI] [PubMed] [Google Scholar]
- 11.Takaya Y., Yoshihara F., Yokoyama H., et al. Risk stratification of acute kidney injury using the blood urea nitrogen/creatinine ratio in patients with acute decompensated heart failure. Circ J. 2015;79:1520–1525. doi: 10.1253/circj.CJ-14-1360. [DOI] [PubMed] [Google Scholar]
- 12.Takaya Y., Yoshihara F., Yokoyama H., et al. Impact of decreased serum albumin levels on acute kidney injury in patients with acute decompensated heart failure: a potential association of atrial natriuretic peptide. Heart Vessels. 2017;32:932–943. doi: 10.1007/s00380-017-0954-y. [DOI] [PubMed] [Google Scholar]
- 13.Voors A.A., Davison B.A., Felker G.M., et al. Early drop in systolic blood pressure and worsening renal function in acute heart failure: renal results of Pre-RELAX-AHF. Eur J Heart Fail. 2011;13:961–967. doi: 10.1093/eurjhf/hfr060. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y.N., Cheng H., Yue T., Chen Y.P. Derivation and validation of a prediction score for acute kidney injury in patients hospitalized with acute heart failure in a Chinese cohort. Nephrology (Carlton) 2013;18:489–496. doi: 10.1111/nep.12092. [DOI] [PubMed] [Google Scholar]
- 15.Verdiani V., Lastrucci V., Nozzoli C. Worsening renal function in patients hospitalized with acute heart failure: risk factors and prognostic significances. Int J Nephrol. 2010;2011:785974. doi: 10.4061/2011/785974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou L.Z., Yang X.B., Guan Y., et al. Development and validation of a risk score for prediction of acute kidney injury in patients with acute decompensated heart failure: a prospective cohort study in China. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.004035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.James M.T., Pannu N., Hemmelgarn B.R., et al. Derivation and external validation of prediction models for advanced chronic kidney disease following acute kidney injury. JAMA. 2017;318:1787–1797. doi: 10.1001/jama.2017.16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee T.H., Fan P.C., Chen J.J., et al. A validation study comparing existing prediction models of acute kidney injury in patients with acute heart failure. Sci Rep. 2021;11:11213. doi: 10.1038/s41598-021-90756-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai M.S., Lin M.H., Lee C.P., et al. Chang Gung research database: a multi-institutional database consisting of original medical records. Biomed J. 2017;40(5):263–269. doi: 10.1016/j.bj.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shao S.C., Chan Y.Y., Kao Yang Y.H., et al. The Chang Gung Research Database—a multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol Drug Saf. 2019;28:593–600. doi: 10.1002/pds.4713. [DOI] [PubMed] [Google Scholar]
- 21.Chang S.L., Huang Y.L., Lee M.C., et al. Association of varicose veins with incident venous thromboembolism and peripheral artery disease. JAMA. 2018;319:807–817. doi: 10.1001/jama.2018.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin Y.S., Chen T.H., Chi C.C., et al. Different implications of heart failure, ischemic stroke, and mortality between nonvalvular atrial fibrillation and atrial flutter—a view from a national cohort study. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng C.L., Chien H.C., Lee C.H., Lin S.J., Kao Yang Y.H. Validity of in-hospital mortality data among patients with acute myocardial infarction or stroke in National Health Insurance Research Database in Taiwan. Int J Cardiol. 2015;201:96–101. doi: 10.1016/j.ijcard.2015.07.075. [DOI] [PubMed] [Google Scholar]
- 24.Frolova N., Bakal J.A., McAlister F.A., et al. Assessing the use of international classification of diseases—10th revision codes from the emergency department for the identification of acute heart failure. JACC Heart Fail. 2015;3:386–391. doi: 10.1016/j.jchf.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Hsieh C.Y., Su C.C., Shao S.C., et al. Taiwan’s National Health Insurance Research Database: past and future. Clin Epidemiol. 2019;11:349–358. doi: 10.2147/CLEP.S196293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saczynski J.S., Andrade S.E., Harrold L.R., et al. A systematic review of validated methods for identifying heart failure using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):129–140. doi: 10.1002/pds.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Section 2: AKI definition. Kidney Int Suppl (2011) 2012;2:19–36. doi: 10.1038/kisup.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zelt J.G.E., Mielniczuk L.M., Liu P.P., et al. Utility of novel cardiorenal biomarkers in the prediction and early detection of congestive kidney injury following cardiac surgery. J Clin Med. 2018;7:540. doi: 10.3390/jcm7120540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onuigbo M.A.C., Agbasi N., Sengodan M., Rosario K.F. Acute kidney injury in heart failure revisited—the ameliorating impact of “decongestive diuresis” on renal dysfunction in type 1 acute cardiorenal syndrome: accelerated rising pro B naturetic peptide is a predictor of good renal prognosis. J Clin Med. 2017;6:82. doi: 10.3390/jcm6090082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuo G., Chen S.W., Lee C.C., et al. Latent trajectories of fluid balance are associated with outcomes in cardiac and aortic surgery. Ann Thorac Surg. 2020;109:1343–1349. doi: 10.1016/j.athoracsur.2019.09.068. [DOI] [PubMed] [Google Scholar]
- 31.Boulos J., Darawsha W., Abassi Z.A., Azzam Z.S., Aronson D. Treatment patterns of patients with acute heart failure who develop acute kidney injury. ESC Heart Fail. 2019;6:45–52. doi: 10.1002/ehf2.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee B.J., Hsu C.Y., Parikh R., et al. Predicting renal recovery after dialysis—requiring acute kidney injury. Kidney Int Rep. 2019;4:571–581. doi: 10.1016/j.ekir.2019.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panitchote A., Mehkri O., Hastings A., et al. Clinical predictors of renal non-recovery in acute respiratory distress syndrome [published correction appears in BMC Nephrol. 2019;20:286] BMC Nephrol. 2019;20:255. doi: 10.1186/s12882-019-1439-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stads S., Kant K.M., de Jong M.F.C., et al. Predictors of short-term successful discontinuation of continuous renal replacement therapy: results from a prospective multicentre study. BMC Nephrol. 2019;20:129. doi: 10.1186/s12882-019-1327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J.J., Chang C.H., Lee C.C., et al. Proteinuria enhances prediction ability of sequential organ failure assessment score and associated with mortality in coronary care units. Acta Nephrologica. 2017;31:188–196. doi: 10.6221/AN.201712_31(4).0005. [DOI] [Google Scholar]
- 36.Dolgi M., NYHACC. The Criteria Committee of the New York Heart Association . Little Brown & Co.; 1994. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. [Google Scholar]
- 37.Sullivan L.M., Massaro J.M., D’Agostino R.B., Sr. Presentation of multivariate data for clinical use: the Framingham Study risk score functions. Stat Med. 2004;23:1631–1660. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 38.Chen Y.T., Jenq C.C., Hsu C.K., et al. Acute kidney disease and acute kidney injury biomarkers in coronary care unit patients. BMC Nephrol. 2020;21:207. doi: 10.1186/s12882-020-01872-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moledina D.G., Luciano R.L., Kukova L., et al. Kidney biopsy-related complications in hospitalized patients with acute kidney disease. Clin J Am Soc Nephrol. 2018;13:1633–1640. doi: 10.2215/CJN.04910418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chu R., Li C., Wang S., Zou W., Liu G., Yang L. Assessment of KDIGO definitions in patients with histopathologic evidence of acute renal disease. Clin J Am Soc Nephrol. 2014;9:1175–1182. doi: 10.2215/CJN.06150613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.See E.J., Polkinghorne K.R., Toussaint N.D., Bailey M., Johnson D.W., Bellomo R. Epidemiology and outcomes of acute kidney diseases: a comparative analysis. Am J Nephrol. 2021;52:342–350. doi: 10.1159/000515231. [DOI] [PubMed] [Google Scholar]
- 42.Chang C.H., Chen S.W., Chen J.J., et al. Incidence and transition of acute kidney injury, acute kidney disease to chronic kidney disease after acute type A aortic dissection surgery. J Clin Med. 2021;10:4769. doi: 10.3390/jcm10204769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siew E.D., Abdel-Kader K., Perkins A.M., et al. Timing of recovery from moderate to severe AKI and the risk for future loss of kidney function. Am J Kidney Dis. 2020;75:204–213. doi: 10.1053/j.ajkd.2019.05.031. [DOI] [PubMed] [Google Scholar]
- 44.Ronco C., Bellasi A., Di Lullo L. Cardiorenal syndrome: an overview. Adv Chronic Kidney Dis. 2018;25:382–390. doi: 10.1053/j.ackd.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 45.Peerapornratana S., Priyanka P., Wang S., et al. Sepsis-associated acute kidney disease. Kidney Int Rep. 2020;5:839–850. doi: 10.1016/j.ekir.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Törnblom S., Nisula S., Petäjä L., et al. Urine NGAL as a biomarker for septic AKI: a critical appraisal of clinical utility—data from the observational FINNAKI study. Ann Intensive Care. 2020;10:51. doi: 10.1186/s13613-020-00667-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gayat E., Hollinger A., Cariou A., et al. Impact of angiotensin-converting enzyme inhibitors or receptor blockers on post-ICU discharge outcome in patients with acute kidney injury. Intensive Care Med. 2018;44:598–605. doi: 10.1007/s00134-018-5160-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data cannot be directly shared publicly because of the regulation of Chang Gung Research Database policy. The data are provided, maintained, and managed by the Chang Gung Medical Foundation. Researchers interested in accessing this data set can submit a formal application to request access (Chang Gung Memorial Hospital, No. 5, Fuxing St., Guishan Dist., Taoyuan City 33305, Taiwan; E-mail: ccling999@gmail.com, +886-03-328120-7721).