Abstract
The present study was performed to know the effects of chronic lead exposure on serum lipids, lipoproteins, and liver enzymes in a cohort study among of lead mine workers. We followed of 200 Iranian workers for 3- years (2018–2020), 100 of them with known occupational exposure to lead thorough their work in lead mine while the others 100 were with no such exposure. Blood lead level (BLL), serum lipids, lipoproteins, and liver enzymes of the exposure group for 3- years were measured and compared with those attained in the non-exposed workers. The BLL levels of the lead-mine workers were higher than with recommended level and the non-exposed group (24.15 and 6.35 µg/dL, respectively). The findings indicated a positive and significant relationship between BLL and lactate dehydrogenase, aspartate transaminase, alkaline phosphatase, alanine transaminase, and bilirubin levels (P < 0.01). Also while we found a negative and significant correlation between BLL and triglyceride, total protein, albumin, and globulin levels (P < 0.01). This report depicted that chronic lead exposure is a risk factor for hematological, liver, and cardiovascular diseases. Despite the fact that the level of liver function parameters was in the normal range, the results of 3- years follow-up show a significant relationship between BLL and alteration of biochemical parameters levels.
Abbreviations: Pb, Plumbum; BLL, Blood lead level; : TG, Triglyceride; : HDL, High density lipoprotein; : LDL, Low density lipoprotein; : Ch, Cholesterol; : LDH, Lactate dehydrogenase; : AST, Aspartate transaminase; : ALK, Alkaline phosphatase; : ALT, Alanine transaminase; : BUN, Urea nitrogen; BEI, Biological exposure index; ACGIH, American conference of governmental industrial hygienists
Keywords: Lead Poisoning, Occupational Exposure, Liver Function Test; Biomarkers; Mines
Graphical Abstract
Highlights
-
•
Occupational exposure to lead can cause alter liver enzymes.
-
•
LDH, AST, ALK, ALT, and bilirubin are increased due to chronic exposure to lead.
-
•
Compare with non- exposure group, exposure group had a lower level of TG.
-
•
Total protein, albumin, and globulin are decreased due to chronic exposure to lead.
-
•
Occupational exposure to lead had no significant effect on BUN levels.
1. Introduction
Lead is a high-consumption mineral metal and exposure to lead (Pb: Plumbum) compounds is mainly due to human activities [1]. Although lead toxicity has been considered since ancient times, it is still an important environmental and occupational health problem [2].
Oral and inhalation are the main absorption routs of the inorganic Pb compounds (40% from the respiratory tract and approximately 5–10% from the gastrointestinal tract). Lead after absorption into the bloodstream is distributed in several organs particularly to the kidney and liver, which then may be accumulated in the bones and cause hurt to the various organs including the liver, central and/or peripheral nervous system, heart, immune system, kidneys, and male gonads [2], [3]. Chronic exposure may lead to irreversible functional and morphological changes in the liver and renal [4], [5].
Lead can disrupt the normal function of cations and essential enzymes throughout the body's cells, especially calcium; beside Pb poisoning is mostly accompanied by multi-systemic symptoms and signs. Autopsy researches on the humans exposed to Pb showed that the liver is the most important reservoir of lead among soft tissues of the human body (approximately 33%) [2], [6].
Various researchers have demonstrated the effects of lead exposure on liver function alteration, but still, the relationship between lead exposure and lipid metabolism is argumentative [7]. Assi et.al have reported that chronic occupational exposure to Pb not only can cause dyslipidemia, hormone depression, and hypercholesterolemia, but also increase the risk of atherosclerosis [8]. Also, Alya and Kshirsagar have stated that Pb exposure can impair the detoxification of xenobiotics and alter the levels of lipoprotein, serum lipid profile, and tryptophan metabolism [7], [9]. Hand-off course and previous studies have illustrated that chronic exposure to Pb can result in dyslipidemia, hypercholesterolemia, thereby increasing the risk of atherosclerosis, as well as levels of serotonin and 5- hydroxy indole acetic acid in the brain [8], [10]. The affinity of Pb to bind to electron donor groups (such as proteins, glutathione, and sulfhydryl group) disrupts various enzymatic processes [11]. In this way, Pb can weaken the function of the antioxidant defense system, produce reactive oxygen species, interfere with some essential elements of the body, damage or destroy cell membranes and inhibit antioxidant enzymes (dependent on sulfhydryl group) [12].
Mining is one of the hardest and most harmful occupations posing many health risks to miners. The emission of heavy metals caused by mining and smelting in lead- zinc mines pollutes air, water, and soil, which can have adverse effects on the health of miners and residents around mines [13]. The operation process in lead-zinc mines, involving underground mining, ore mining, transport, crushing, lab examination, grinding, roughing, and smelting, can cause emission of various heavy metals especially the lead (Pb). In Iran, sixty-eight types of minerals including Pb mine are found and this country has the largest reserves of lead and zinc in Asia and the world (approximately 222 million tons) [14]. It should be noted that moreover occupational exposure to Pb, lifestyle factors can also cause changes in liver function tests; therefore that drinking coffee decreases serum albumin, total protein, and aspartate aminotransferases levels [15], [16], [17]. Moreover; obesity and heavy alcohol consumption increase the level of lipoproteins, and even cigarette smoking leads to increased serum alkaline phosphatase and γ-glutamyl transferase levels and decreases serum albumin and proteins levels [18], [19], [20].
Although it is well documented in previous literature, but is need to know of the scenario of blood lead level (BLL) and its liver effects on lead-mine workers. To the best of our knowledge, there is no report for the assessment of liver function parameters and alteration of biochemical parameters levels due to the long time occupational exposure to Pb among the mineworkers. This is the first cohort study to provide knowledge of the associations between chronic occupational exposure to lead and its biochemical effects on the serum lipid profiles, lipoproteins, and liver enzymes during 3- years among the workers of the Middle East largest lead- mine (Iran).
2. Materials and methods
2.1. Study population
This prospective occupational cohort study was performed in the lead mine complex in Iran from 2018 to 2020. Before data and biological specimen collection, all the subjects were well informed about the objectives of the study and health risks of the Pb exposure and its toxicity. This cohort study started with a 2018 self-reporting questionnaire that investigated the personal, occupational, and medical history of the subjects. These factors included age, weight, alcohol intake, tobacco smoke, consumption herbal supplements, employment duration, and average daily exposure time average to lead sources. The recruitment and selection of study subjects were based on their medical history. Those who had systemic disease, or were on medication for other reasons, were excluded from this study. The sample size for this study was calculated to be 100 subjects for each group (N: 200), based on considering α = 0.05 and β = 0.05, 25% drop out samples, and %24.7 prevalence of high ALT as reported in Kshirsagar study [9]. Finally, 200 healthy male workers were grouped into two sub-cohorts of exposed (N = 100) and non-exposed (N = 100). It should be noted that the food and habits dietary intake of all the entire subjects were the normal states. The consent was taken from the participants of the two groups. Liver function tests were performed annually from 2018 to 2020 in both groups.
2.2. Ethic
Written informed consent to workers and for publication of the results was obtained from the workers in this study. They were also assured of confidentiality and all the biological samples and questionnaires were kept anonymous. The present study was reviewed and approved by the Institutional Review Board and ethics committee of Larestan University of Medical Sciences (Ethical code: IR.LARUMS.REC.1400.019).
All experiments were performed in accordance with the General Medical Council guidelines. Informed consents were obtained from human participants of this study. Before collecting blood samples, the workers the requested to sign the consent form.
2.3. Sampling and laboratory procedures
In the morning (at 7–8 a.m. on Saturday as the first day of the weekly work shift), 10 mL of fasting venous blood from the basilica vein was obtained from the subjects before the commencement of the work. Of the blood sample, 2 mL was used for blood lead measurement, and the remaining blood for the biochemical analyses. In order to collect blood samples serum, vacuum tubes contained K3-EDTA (Greiner- Germany) and plain tubes were used. Also, all the tests were repeated twice by the same device and operator.
Determination of blood lead level (BLL) was performed by means of a graphite furnace atomic absorption spectrophotometer (900 T, Perkin Elmer). The lead levels in the blood samples were accurately measured from as little as 20 µL of blood at a wavelength of 283.3 nm.
Triglyceride (TG), high density lipoprotein (HDL), low density lipoprotein (LDL), cholesterol, lactate dehydrogenase (LDH), aspartate transaminase (AST), alkaline phosphatase (ALK), alanine transaminase (ALT), total serum protein, bilirubin, globulin, albumin, and blood urea nitrogen (BUN) levels were measured by an semi-automated biochemistry analyzer (Roche – Hitachi MODULAR Analytics, Japan) using the Roche kits.
The ALT and AST were determined using the UV-kinetic method by reagent obtained from Roche Ldt. Serum total bilirubin was measure based on the Jendrassik method [21]. Determination of serum total proteins (TP) was performed using the Roche Kit (Roche – Hitachi MODULAR Analytics, Japan) based on the Biuret method. Serum albumin was measured by the BCG method, which is based on the bonds of the serum albumin with the tetrabromocresol sulfonephthalein green at pH of 4.2 and production of a blue green colored solution (detected at 600 nm). Also, the serum globulins and Albumin /Globulin ratio was estimated by using serum albumin and TP values. The serum ALK was measured according to the King Armstrong method using the Roche diagnostics kit (Roche – Hitachi MODULAR Analytics, Japan). The BUN was measured by colorimetric method via Spectrophotometry UV-Vis (Lambda 950. Perkin Elmer) set at 520 nm.
2.4. Statistical analysis
To analyze the collected data, we used appropriate descriptive and analytical statistics such as mean, standard deviation, Mann-Whitney U test, Kolmogorov Smirnov, Friedman test and chi-square test. In order to compare quantitative variables, we first examined the data for normal distribution, for this purpose; Kolmogorov Smirnov test was used. The results showed that except for a few cases, most variables did not follow the normal distribution, thus, non-parametric tests were used to analyze the collected data. Mann Whitney U test was used when comparison of demographic variable, BLL and liver function tests of both groups. Fried man test was used to compare of the trend of average change BLL and liver function tests of healthy over three years. Chi-square test was also used when comparison of qualitative variable ratio of tobacco smoking in two groups. Data analysis was performed using SPSS software version 19.0 and maximum alpha error of 0.05 was considered as acceptable cut-point for significance.
3. Results
3.1. Demographic and biochemical data
The demographic and biochemical parameters of the exposure and non-exposure groups are also depict in in Table 1. Mann-Whitney nonparametric test was used to compare the demographic variables between the exposure and the non-exposure groups. The results of this test showed that there was a significant difference in the mean of daily exposure in the exposure and non-exposure groups (P = 0.00), which is logical because the exposure in the control group was zero. Based on chi-square test, the proportion of smokers in the exposure groups was slightly higher than that of the non-exposure groups, but this difference was not significant (P = 0.058).
Table 1.
Comparison of various demographic and biochemical parameters between the exposure and the non-exposure groups.
| Parameters |
Exposure (N = 100) |
Non-exposure (N = 100) |
p-Value |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | |||
| Age (yr) | 33.76 | 4.8 | 23–46 | 33.46 | 5.3 | 21–45 | 0.80 | |
| BMI | 23.19 | 1.5 | 19–28 | 23.56 | 1.8 | 19–27 | 0.22 | |
| Alcohol Intake (%) | Yes | 3 (3%) | 2 (2%) | 0.21 | ||||
| No | 97 (97%) | 98 (98%) | ||||||
| Herbal supplement (%) | Yes | 1 (1%) | 4 (4%) | 0.033 | ||||
| No | 99 (99%) | 96 (96%) | ||||||
| Tobacco smoke (%) | Yes | 22 (22%) | 9 (9%) | 0.058 | ||||
| No | 88 (88%) | 91 (91%) | ||||||
| Work experience (year) | 8.64 | 4.5 | 3–22 | 7.66 | 3.8 | 1–18 | 0.17 | |
| Daily exposure time (hour) | 6.42 | 3.8 | 1–13 | 0.00 | 0.0 | 0 | 0.0 | |
| Blood Lead level (µg/dL) | 24.15 | 8.3 | 9–36 | 6.34 | 1.83 | 3–9 | 0.001 | |
| Triglyceride (mg/dL) | 91.21 | 41.3 | 33–182 | 103.12 | 12.3 | 68–142 | 0.032 | |
| Low density lipoprotein | 89.30 | 22.8 | 50–166 | 76.23 | 11.3 | 50–100 | 0.001 | |
| High density lipoprotein (mg/dL) | 74.17 | 14.2 | 68–110 | 91.25 | 6.8 | 65–108 | 0.001 | |
| Cholesterol (mg/dL) | 163.47 | 30.6 | 115–209 | 167.45 | 8.9 | 118–189 | 0.058 | |
| Lactate dehydrogenase (U/L) | 227.1 | 41.9 | 160–291 | 160.7 | 11.6 | 140–183 | 0.001 | |
| Aspartate transaminase (U/L) | 153.08 | 52.7 | 65–236 | 125.34 | 4.9 | 113–134 | 0.005 | |
| Alanine transaminase (U/L) | 38.53 | 8.5 | 23–51 | 29.31 | 2.5 | 24–35 | 0.001 | |
| Protein (mg/dL) | 7.03 | 0.7 | 5.8–8.32 | 7.46 | 0.6 | 6.9–8.25 | 0.001 | |
| Bilirubin (mg/dL) | 1.17 | 0.62 | 0.8–2.21 | 0.82 | 0.08 | 0.63–96 | 0.001 | |
| Globulin (gr/dL) | 3.09 | 0.27 | 2.7–3.7 | 3.23 | 0.05 | 3.1–3.3 | 0.001 | |
| Albumin (gr/dL) | 3.75 | 0.6 | 2.9–4.8 | 4.04 | 0.36 | 3.6–4.3 | 0.001 | |
| Blood urea nitrogen | 14.82 | 3.41 | 7.4–20.5 | 14.63 | 3.35 | 7.4–21.5 | 0.071 | |
BMI: Body mass index; SD: Standard deviation; U: Unit.
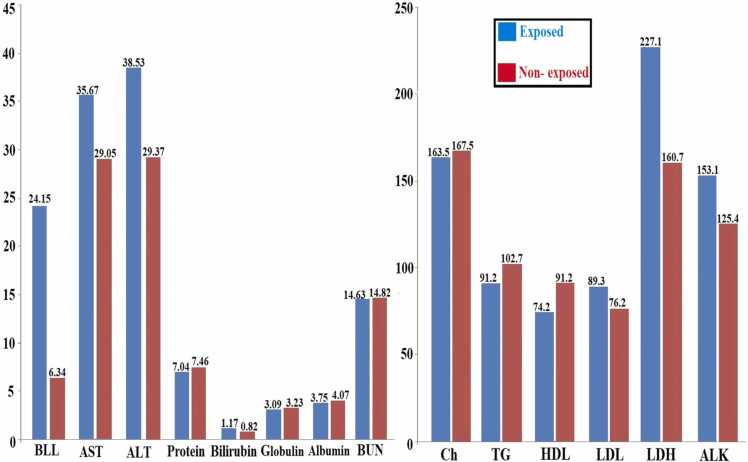
The three-years average of BLL and biochemical parameters among exposed and Non- exposed workers presented in Fig. 1.
Fig. 1.
Three- years average level of BLL and liver function parameters in exposed and non- exposed groups.
3.2. Comparison BLL and biochemical parameters between groups
The means lead levels in the blood of the exposed workers was higher than the non-exposure worker (24.15 and 6.34 µg/dL, respectively). The changes in blood lead level, serum lipid profiles, lipoproteins and liver enzymes in exposure and non-exposure groups during 3-years follow-up are shown in Table 2. The mean of BLL and biochemical parameters of the two groups by different years was compared based on Mann-Whitney U test. The results showed that the mean of the BLL in the exposure groups in all years (2018–2020) was much higher than this scale in the non-exposure groups and this difference was statistically significant (P < 0.001).
Table 2.
Comparison of changes in blood lead levels and biochemical parameters in exposed and non-exposed groups.
| Parameters | Mean ± SD | Normal range | Years |
Pa | |||
|---|---|---|---|---|---|---|---|
| Y18 | Y19 | Y20 | Pb | ||||
| BLL (µg/dL) | Exposure Group | 20 | 24.16 ± 8.36 | 24.05 ± 8.17 | 24.24 ± 8.25 | 0.402 | < 0.001 |
| Non Exposure Group | 6.05 ± 1.93 | 6.48 ± 1.86 | 6.51 ± 1.64 | 0.001 | |||
| Pc | – | < 0.001 | < 0.001 | < 0.001 | – | ||
| TG (mg/dL) | Exposure Group | 30–200 | 90.7 ± 41.2 | 91.5 ± 41.2 | 91.3 ± 41.3 | 0.854 | 0.013 |
| Non Exposure Group | 102 ± 12.3 | 104 ± 12.3 | 102 ± 12.3 | 0.066 | |||
| P | – | 0.013 | 0.007 | 0.007 | – | ||
| LDL (mg/dL) | Exposure Group | 100–130 | 89.99 ± 24.50 | 90.02 ± 23.64 | 87.90 ± 21.87 | 0.253 | < 0.001 |
| Non Exposure Group | 75.15 ± 10.68 | 77.79 ± 11.05 | 75.77 ± 12.20 | 0.195 | |||
| P | – | < 0.001 | < 0.001 | < 0.001 | – | ||
| HDL (mg/dL) | Exposure Group | Up of 60 | 73.05 ± 14.6 | 74.45 ± 14.32 | 75.02 ± 13.61 | 0.018 | < 0.001 |
| Non Exposure Group | 92.18 ± 6.34 | 89.76 ± 7.95 | 91.82 ± 6.02 | < 0.001 | |||
| P | – | < 0.001 | < 0.001 | < 0.001 | – | ||
| Cholesterol (mg/dL) | Exposure Group | 110–200 | 163.04 ± 31.7 | 164.47 ± 30.22 | 162.92 ± 29.06 | 0.186 | 0.228 |
| Non Exposure Group | 167.33 ± 7.35 | 167.43 ± 8.39 | 167.6 ± 9.71 | 0.350 | |||
| P | – | 0.620 | 0.432 | 0.279 | – | ||
| Ch/HDL | Exposure Group | < 4 | 2.26 ± 0.39 | 2.24 ± 0.35 | 2.19 ± 0.33 | 0.616 | < 0.001 |
| Non Exposure Group | 1.82 ± 0.17 | 1.88 ± 0.19 | 1.83 ± 0.17 | 0.028 | |||
| P | – | < 0.001 | < 0.001 | < 0.001 | – | ||
| LDH (U/L) | Exposure Group | 140–280 | 223.07 ± 40.7 | 226.99 ± 41.51 | 231.25 ± 42.84 | < 0.001 | < 0.001 |
| Non Exposure Group | 159.85 ± 12.6 | 159.92 ± 10.1 | 162.32 ± 12.0 | 0.017 | |||
| P | – | < 0.001 | < 0.001 | < 0.001 | – | ||
| AST (U/L) | Exposure Group | 8–38 | 35.17 ± 10.15 | 35.79 ± 9.36 | 36.07 ± 9.67 | 0.224 | < 0.001 |
| Non Exposure Group | 28.72 ± 2.68 | 28.73 ± 2.32 | 29.71 ± 2.15 | 0.032 | |||
| P | – | < 0.001 | < 0.001 | < 0.001 | – | ||
| ALK (U/L) | Exposure Group | 30–140 | 152.85 ± 52.53 | 153.56 ± 51.70 | 152.85 ± 53.76 | 0.168 | < 0.001 |
| Non Exposure Group | 124.57 ± 5.02 | 125.1 ± 5.34 | 126.35 ± 4.56 | 0.026 | |||
| P | – | 0.004 | 0.005 | < 0.001 | – | ||
| ALT (U/L) | Exposure Group | 10–55 | 38.6 ± 8.42 | 38.57 ± 8.66 | 38.44 ± 8.43 | 0.831 | < 0.001 |
| Non Exposure Group | 29.02 ± 2.7 | 29.05 ± 2.65 | 29.86 ± 2.36 | 0.117 | |||
| P | – | < 0.001 | < 0.001 | < 0.001 | – | ||
| Protein (mg/dL) | Exposure Group | 6.6–8.3 | 7.038 ± 0.698 | 7.031 ± 0.7 | 7.041 ± 0.68 | 0.829 | < 0.001 |
| Non Exposure Group | 7.5 ± 0.249 | 7.31 ± 1.33 | 7.58 ± 0.28 | 0.090 | |||
| P | – | < 0.001 | < 0.001 | < 0.001 | – | ||
| Bilirubin (mg/dL) | Exposure Group | 0.5–1 | 1.12 ± 0.24 | 1.23 ± 0.88 | 1.17 ± 0.25 | < 0.001 | < 0.001 |
| Non Exposure Group | 0.81 ± 0.085 | 0.83 ± 0.081 | 0.82 ± 0.089 | 0.119 | |||
| P | – | < 0.001 | < 0.001 | < 0.001 | – | ||
| Globulin (gr/dL) | Exposure Group | 2.8–3.2 | 3.13 ± 0.28 | 3.10 ± 0.28 | 3.05 ± 0.3 | < 0.001 | < 0.001 |
| Non Exposure Group | 3.23 ± 0.047 | 3.22 ± 0.051 | 3.24 ± 0.063 | 0.223 | |||
| P | – | < 0.001 | < 0.001 | < 0.001 | – | ||
| Albumin (gr/dL) | Exposure Group | 3–5 | 3.77 ± 0.5 | 3.76 ± 0.5 | 3.73 ± 0.8 | < 0.001 | < 0.001 |
| Non Exposure Group | 4.01 ± 0.17 | 4.12 ± 0.78 | 4 ± 0.14 | 0.951 | |||
| P | – | < 0.001 | < 0.001 | < 0.001 | – | ||
| A/G | Exposure Group | 1.5–2 | 1.19 ± 0.088 | 1.19 ± 0.093 | 1.23 ± 0.2 | 0.014 | 0.005 |
| Non Exposure Group | 1.24 ± 0.058 | 1.27 ± 0.24 | 1.23 ± 0.048 | 0.350 | |||
| P | – | < 0.001 | < 0.001 | < 0.001 | – | ||
| BUN | Exposure Group | 8–24 | 14.3 ± 3.29 | 15.26 ± 3.5 | 14.91 ± 3.37 | < 0.001 | 0.575 |
| Non Exposure Group | 14.57 ± 3.27 | 14.46 ± 3.46 | 14.87 ± 3.22 | 0.236 | |||
| P | – | 0.510 | 0.100 | 0.846 | – | ||
: Based on compare the trend of annual changes by two groups. Significant: P < 0.05.
: Based on comparing the trend of annual changes in each group;
: Based on comparisons between the two groups by year;
3.3. BLL and serum lipid profile
The findings indicated that the serum TG level of both groups was within the normal range, but the level of this parameter in the exposure group was significantly lower than that of the non-exposure (P < 0.05). Also the TG level in the exposed workers did not change significantly during 3- years follow-up (See Table 2).
The results showed that the LDL levels in the individuals with lead exposure were higher than those in the non-exposure subjects (P < 0.001) however; the HDL levels in the exposure group were lower than those in the non-exposure group (P < 0.001). But also, the means of the LDL and HDL levels for the both groups were within the normal range. Furthermore, the LDL levels of the exposure subjects decreased during 3- years follow-up; this is while, and the HDL level increased over the period (Table 2). The cholesterol level of the both groups was within the normal range, and no significant difference was observed between the exposed and non-exposure groups (P > 0.05). Also, the cholesterol level among worker with occupational lead exposure did not change significantly during the three years (See Table 2). Moreover, the cholesterol/ HDL ratio of the worker exposure group was significantly higher than that of the non-exposure subjects (P < 0.001). Though cholesterol/ HDL ratio in the two groups was in the normal range; this ratio increased in the exposed group during the three years follow-up.
3.4. BLL and serum liver enzyme's
The results indicated that the serum LDH, AST, ALK, ALT, and bilirubin of the exposure subjects were significant higher than those of the non-exposure group (P < 0.01), even though, these parameters in the both groups were within the normal range (See Table 2). As shown in Table 2, the serum LDH, AST increased in the exposed group during the three years, while the serum ALT decreased during the three years. Also, the ALK and bilirubin values did not have significant change during in the period follow-up.
3.5. BLL and serum lipoproteins
Following investigation of lipoproteins parameters, the results showed that total serum protein, albumin, globulin and albumin/ globulin levels were lower in the exposed group than those in the non-exposed group (P < 0.002), and these parameters had a decreasing trend during the three years follow-up. On comparison of the exposed with the non-exposed subjects, no significant difference was found in term BUN value and this parameter in both groups within in the normal range.
As shown in Table 3, the trend of changes of the HDL, LDH, bilirubin, globulin, albumin, albumin/ globulin ratio, and BUN levels during three years follow- up in the exposure group was significant. Table 3 presents the Spearman correlation coefficient between the BLL and biochemical parameters.
Table 3.
Comparison of the trend and correlation between average changes BLL and biochemical parameters of exposure group over three years (p-Value and Spearman R-value).
| Parameters | Years |
||||
|---|---|---|---|---|---|
| Y17 | Y18 | Y19 | Mean | ||
| TG | R | -0.749 | -0.763 | -0.718 | -0.743a |
| p-Value | 0.001 | 0.001 | 0.001 | – | |
| LDL | R | -0.012 | 0.121 | 0.178 | 0.095 |
| p-Value | 0.990 | 0.142 | 0.283 | – | |
| HDL | R | 0.089 | 0.421 | 0.213 | 0.241 |
| p-Value | 0.243 | 0.616 | 0.112 | – | |
| Cholesterol | R | 0.039 | 0.221 | 0.105 | 0.121 |
| p-Value | 0.644 | 0.223 | 0.208 | – | |
| Ch/HDL | R | -0.124 | 0.067 | 0.012 | 0.026 |
| p- Value | 0.304 | 0.70 | 0.969 | – | |
| LDH | R | 0.693 | 0.759 | 0. 773 | 0.741b |
| p-Value | 0.001 | 0.001 | 0.001 | – | |
| AST | R | 0.752 | 0.793 | 0.784 | 0.776b |
| p-Value | 0.001 | 0.001 | 0.001 | – | |
| ALK | R | 0.762 | 0.735 | 0.753 | 0.750b |
| p-Value | 0.001 | 0.001 | 0.001 | – | |
| ALT | R | 0.731 | 0.673 | 0.718 | 0.707b |
| p-Value | 0.001 | 0.001 | 0.001 | – | |
| Protein | R | -0.805 | -0.812 | -0.831 | -0.816a |
| p-Value | 0.001 | 0.001 | 0.001 | – | |
| Bilirubin | R | 0.795 | 0.792 | 0.805 | 0.797b |
| p-Value | 0.001 | 0.001 | 0.001 | – | |
| Globulin | R | -0.725 | -0.695 | -0.715 | -0.711a |
| p-Value | 0.001 | 0.001 | 0.001 | – | |
| Albumin | R | -0.781 | -0.731 | -0.712 | -0.741a |
| p-Value | 0.001 | 0.001 | 0.001 | – | |
| A/G | R | -0.705 | -0.728 | -0.683 | -0.705a |
| p-Value | 0.001 | 0.001 | 0.001 | – | |
| BUN | R | 0.163 | 0.129 | -0.157 | 0.149 |
| p-Value | 0.164 | 0.150 | 0.880 | – | |
Correlation coefficient negative and significant. Significant: P < 0.05.
Correlation coefficient positive and significant;
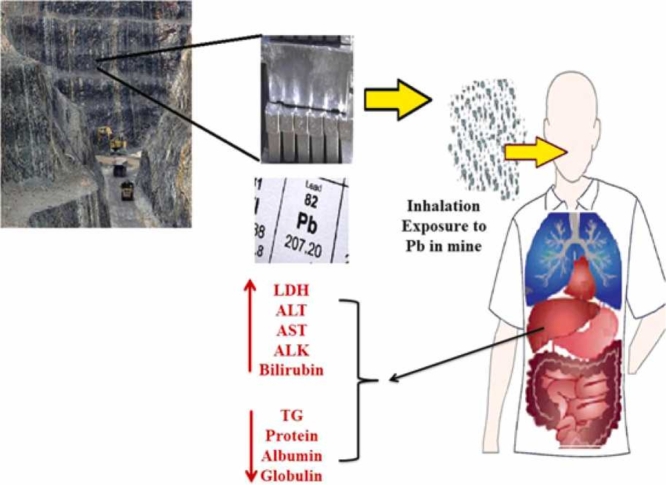
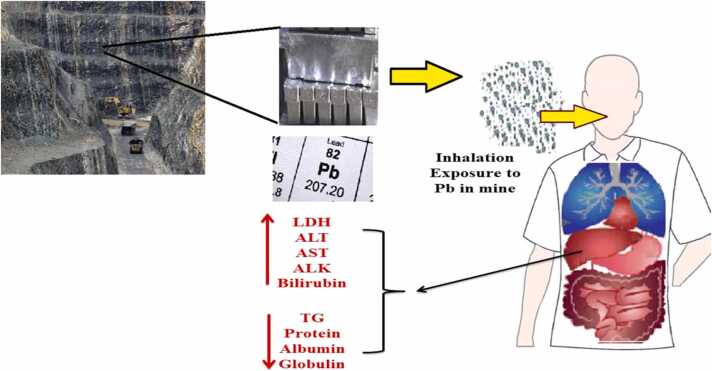
Fig. 2 illustrates the abstract of changes in the lipid profiles, lipoproteins, and liver enzymes among the studied lead mineworkers.
Fig. 2.
Schematic abstract of changes of the biochemical parameters by occupational chronic exposure to Lead.
4. Discussion
This prospective occupational cohort study evaluated changes in some biochemical parameters due to occupational exposure to lead among the lead- mine workers. The blood lead level and biochemical parameters including TG, LDL, HDL, cholesterol, cholesterol/ HDL, LDH, AST, ALK, ALT, total protein, bilirubin, globulin, albumin, albumin/ globulin, and BUN in the exposed and non-exposed subjects were followed during 2018–2020.
The BLL during the three years in the lead- mine workers exceeded the Biological exposure index (BEI) 2021 for BLL recommended by the American Conference of Governmental Industrial Hygienists (ACGIH) (20 µg/dL) [22], which is much higher than that in the non-exposure subjects (P < 0.001), showing that the lead-miners were significantly occupational exposure to Pb and its health risks [23], [24]. Various operations at the mine studied including the extraction, crushing, roughing, purification, lab examination, and transportation of lead ore caused the emission of lead-containing dust into the inhaled air of miners. Despite the provision of control measures and the use of personal protective equipment (mouth mask), the BLL in the exposed workers was still higher than the BEI and control subjects. In addition to respiratory exposure, contact of lead-containing dust with the oral mucosa of miners in the workplace can cause gastrointestinal exposure to lead [2], [25].
TG and HDL levels of the exposed group were lower than those of the non-exposed group, while the LDL levels of the exposed subjects were higher than those of the non-exposed group. There was a negative and significant correlation between the BLL and TG level of the lead miners, however, a significant correlation was not found between BLL and cholesterol, LDL and HDL levels. Previous studies have acknowledged that chronic exposure to lead (battery workers) has no significant effect on cholesterol levels and the age of workers can interference with the TG, LDL, LDH, and cholesterol levels [9], [26].
AST, ALK, ALT, LDH, total protein, bilirubin, and albumin can be used for the comprehensive assessment of the liver function. We found that the levels of serum LDH, AST, ALK, ALT, and bilirubin level increased significantly (P < 0.001) in the lead- mine workers as compared with the non-exposed subjects, while serum protein in the exposure group was lower than that in the non-exposure group and a negative and significant correlation between BLL and total protein was observed (P < 0.001, r = −0.816).
The increased in level of LDH in the worker population might have been resulted from damage to the skeletal or cardiovascular system [27], [28]. Chronic lead exposure can cause slowing intermediary metabolism, which leads to an increase in LDH level. However, chronic exposure to Pb can result in elevated serum ALP and LDH levels due to liver-kidney damages. Furthermore, chronic exposure to lead can interfere with heme synthesis, then cause hemolysis and release hemoglobin containing more LDH, and ultimately increases serum LDH levels [29].
Pb increases the susceptibility of cells to oxidative attack by changing membrane integrity and fatty acid composition that causes the alteration of membrane integrity and fatty acid composition and is associated with the increase in malondialdehyde (MDA) level in the liver [30], [31]. Pb, interact directly with DNA and DNA replication resulting in DNA damage or as a consequence of increased production of reactive oxygen species [32].
Lead can accumulate in the liver tissues and have toxic effect through per-oxidative damage to liver cell membranes and then increase serum ALT and AST levels. Previous studies have shown that long-term occupational exposure to Pb causes an increased level of serum transaminase enzymes [33], [34]. ALT (P < 0.00, r = 0.707) and AST (P < 0.00, r = 0.776) levels during three years of follow-up in the exposed lead mineworkers significantly increased as compared with the non-exposure workers, which illustrates the occurrence of hepatocellular injury. Previous studies have suggested that elevated serum AST and ALK levels may be due to mitochondrial degradation after the destruction of liver cells [35], [36].
Bones contain high levels of the ALK, which chronic exposure to lead can cause damage to bone structure (replacement of lead with bone calcium) and then leads to elevated serum ALK levels. A direct and significant correlation between BLL and serum ALK level in the exposed group (P < 0.005, r = 0.750) confirms this hypothesis [27]. Moreover, ALK is usually present in the walls of the biliary ducts. An increase in serum ALK level may indicate hepatobiliary or hepatocellular damages [37]. Liver damage can lead to impaired transport functions of the biliary tree ducts or of the hepatocytes, which ultimately increases serum ALK levels [38]. Lead can cause interference and breakdown of cell membranes. On the other hand, phosphate is known as an intracellular anion that increases serum ALK levels when the cell membrane is damaged or destroyed. Thus, chronic exposure to lead can lead to elevated serum ALK levels [39], [40].
A high BLL level results in morphological changes and hemolysis of red blood cells (RBC), which can cause an increase in the serum bilirubin levels. In the present study, the results indicated a positive and significant relationship between the BLL level and bilirubin level of the exposed group (P < 0.001, r = 0.797), which is consistent with results of similar previous studies [24], [37], [41].
The serum total protein reflects the important changes the liver function and is an indirect assessment of protein status [37]. It was found that an increase in BLL, decreased the levels of total protein (P < 0.00, r = −0.816), globulin (P < 0.001, r = −0.711), albumin (P < 0.001, r = −0.741) and subsequently decreased the albumin/ globulin ratio (P < 0.0, r = −0.705). The increased BLL can leads to decreases in the synthesis of serum proteins reported in various earlier studies [9], [37], [42]. Experimental animal studies show that chronic exposure to lead can inhibit globulin synthesis in animal bone marrow, therefore, serum protein levels can be used to detect liver damages due to occupational exposure to lead [9], [43], [44].
The previous studies approve of the strong susceptibility of antioxidant enzymes in the liver to Pb toxicity [32]. Pb has a high affinity for sulfhydryl groups in enzymes of the antioxidant defense system, such as catalase, glutathione peroxidase, glucose-6-phosphate dehydrogenase dismutase, and also superoxide, which, inhibit their activity. Moreover, Pb also inhibits enzymes glutathione peroxidase, glutathione reductase, and glutathione S-transferase that are important for the preservation of glutathione levels [45], [46], [47].
The results of the present study demonstrated that occupational exposure to lead had no significant effect on BUN levels. These observations suggest that the BUN levels may not be a suitable indicator to evaluate renal injuries due to chronic occupational exposure to lead, because the clinical manifestations do not become apparent until more than 50% of the nephrons were destroyed [48]. So, BUN level may not be a sufficiently sensitive indicator for the diagnosis of lead-induced renal impairment, which is consistent with the results of previous similar studies [24], [39], [48].
In conclusion, the average BLL levels of the lead mine workers during the 3 years of follow-up was higher than the recommended level and therefore it may lead to health risks associated with Pb. Although the level of biochemical parameters were in the normal range, their changes were statistically significant based on the blood lead level. Our results indicated that chronic exposure to lead can alter some serum liver enzymes, lipid profile, and lipoproteins levels, although there was no evidence of major hepatic impairment in lead miners. The study can be helpful in raising awareness of alteration in liver functions due to occupational exposure to lead. It is suggested that in future research, in order to investigate the effects of chronic occupational exposure to lead in mine, the lead concentration in inhaled air of workers' should be measured as a complement to the results of biological monitoring.
Financial support
The authors would like to thanks the Vice-chancellor for Research and Technology of Larestan University of Medical Sciences, Iran for financial support.
CRediT authorship contribution statement
Conceive and design the study was done by AF, and RR. Drafting of the manuscript was done by AR and AP. Critical revision of the manuscript for important intellectual content was done by SR and AF. Statistical analysis was also done by AA. SR and RR conceived and supervised entire study and edited the manuscript. All authors approved final version of manuscript.
Authors Contributions
Conceive and design the study was done by AF, and RR. Drafting of the manuscript was done by AR and AP. Critical revision of the manuscript for important intellectual content was done by SR and AF. Statistical analysis was also done by AA. SR and RR conceived and supervised entire study and edited the manuscript. All authors approved final version of manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Dr. Aristidis Tsatsakis
References
- 1.Ilychova S.A., Zaridze D.G. Cancer mortality among female and male workers occupationally exposed to inorganic lead in the printing industry. Occup. Environ. Med. 2012;69:87–92. doi: 10.1136/oem.2011.065201. [DOI] [PubMed] [Google Scholar]
- 2.Flora G., Gupta D., Tiwari A. Toxicity of lead: a review with recent updates. Interdiscip. Toxicol. 2012;5:47–58. doi: 10.2478/v10102-012-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carocci A., Catalano A., Lauria G., Sinicropi M.S., Genchi G. Reviews of Environmental Contamination and Toxicology. Springer; 2016. Lead toxicity, antioxidant defense and environment; pp. 45–67. [DOI] [PubMed] [Google Scholar]
- 4.Wron´ska-Nofer T., Pisarska A., Trzcinka-Ochocka M., Hałatek T., Stetkiewicz J., Braziewicz J., Nofer J.-R., Waąsowicz W. Scintigraphic assessment of renal function in steel plant workers occupationally exposed to lead. J. Occup. Health. 2015;57:91–99. doi: 10.1539/joh.14-0115-OA. [DOI] [PubMed] [Google Scholar]
- 5.Himani R.Kumar, Ansari J.A., Mahdi A.A., Sharma D., Karunanand B., Datta S.K. Blood lead levels in occupationally exposed workers involved in battery factories of Delhi-NCR region: effect on vitamin D and calcium metabolism. Ind. J. Clin. Biochem. 2020;35:80–87. doi: 10.1007/s12291-018-0797-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wani A.L., Ara A., Usmani J.A. Lead toxicity: a review. Interdiscip. Toxicol. 2015;8:55–64. doi: 10.1515/intox-2015-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alya A., Ines D.B., Montassar L., Najoua G., Saloua E.F. Oxidative stress, biochemical alterations, and hyperlipidemia in female rats induced by lead chronic toxicity during puberty and post puberty periods. Iran J. Basic Med. Sci. 2015;18:1034–1043. [PMC free article] [PubMed] [Google Scholar]
- 8.Assi M.A., Hezmee M.N.M., Haron A.W., Sabri M.Y.M., Rajion M.A. The detrimental effects of lead on human and animal health. Vet. World. 2016;9:660–671. doi: 10.14202/vetworld.2016.660-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kshirsagar M., Patil J., Patil A., Ghanwat G., Sontakke A., Ayachit R. Biochemical effects of lead exposure and toxicity on battery manufacturing workers of Western Maharashtra (India): with respect to liver and kidney function tests. Al Ameen J. Med. Sci. 2015;8:107–114. [Google Scholar]
- 10.Ono A., Horiguchi H. Reassessment of the threshold of the blood lead level to increase urinary δ-aminolevulinic acid based on their relationship in recent lead workers in Japan. J. Occup. Health. 2021;63 doi: 10.1002/1348-9585.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mudipalli A. Lead hepatotoxicity and potential health effects. Indian J Med Res. 2007;126:518–527. [PubMed] [Google Scholar]
- 12.Omidi F., Jafaryan H., Patimar R., Harsij M., Paknejad H. Biochemical biomarkers of skin mucus in Neogobius melanostomus for assessing lead pollution in the Gulf of Gorgan (Iran) Toxicol. Rep. 2020;7:109–117. doi: 10.1016/j.toxrep.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S.-M., Suh J., Oh S., Son J., Hyun C.-U., Park H.-D., Shin S.-H., Choi Y. Assessing and prioritizing environmental hazards associated with abandoned mines in Gangwon-do, South Korea: the total mine hazards index. Environ. Earth Sci. 2016;75:369. [Google Scholar]
- 14.T.J. Brown, T. Bide, S. Hannis, N. Idoine, L. Hetherington, R. Shaw, A. Walters, P. Lusty, R. Kendall, World mineral production 2004–2008, British Geological Survey, Nottingham, UK, 2010.
- 15.Bravi F., Bosetti C., Tavani A., Vecchia C.L. Coffee drinking and hepatocellular carcinoma: an update. Hepatology. 2009;50:1317–1318. doi: 10.1002/hep.23272. [DOI] [PubMed] [Google Scholar]
- 16.Davies S.C., Fowler T., Watson J., Livermore D.M., Walker D. Annual report of the chief medical officer: infection and the rise of antimicrobial resistance. Lancet. 2013;381:1606–1609. doi: 10.1016/S0140-6736(13)60604-2. [DOI] [PubMed] [Google Scholar]
- 17.Roderick P. Liver function tests: defining what's normal. BMJ. 2004;328:987. doi: 10.1136/bmj.38055.451968.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breitling L.P., Raum E., Müller H., Rothenbacher D., Brenner H. Synergism between smoking and alcohol consumption with respect to serum gamma-glutamyltransferase. Hepatology. 2009;49:802–808. doi: 10.1002/hep.22727. [DOI] [PubMed] [Google Scholar]
- 19.Jang E.S., Jeong S.-H., Hwang S.H., Kim H.Y., Ahn S.Y., Lee J., Lee S.H., Park Y.S., Hwang J.H., Kim J.-W., Kim N., Lee D.H. Effects of coffee, smoking, and alcohol on liver function tests: a comprehensive cross-sectional study. BMC Gastroenterol. 2012;12:145. doi: 10.1186/1471-230X-12-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wannamethee S.G., Shaper A.G. Cigarette smoking and serum liver enzymes: the role of alcohol and inflammation. Ann. Clin. Biochem. 2010;47:321–326. doi: 10.1258/acb.2010.009303. [DOI] [PubMed] [Google Scholar]
- 21.Varely H., Gowenlock A.H., Bell M. Williain Heinemann Medical Book Ltd; London: 1980. Serum Bilirubin and its determination in Practical Clinical Biochemistry. [Google Scholar]
- 22.ACGIH, TLVs and BEIs: threshold limit values for chemical substances and physical agents biological exposure indices 2019, in, 2019.
- 23.Agyemang V., Acquaye J., Asante K.-P., Olayemi E. Blood lead levels among’At Risk’occupational groups and blood donors living in a mining area in Ghana. Br J Haematol. 2019;185:199–200. [Google Scholar]
- 24.Rahimpoor R., Rostami M., Assari M.J., Mirzaei A., Zare M.R. Evaluation of blood lead levels and their effects on hematological parameters and renal function in iranian lead mine workers. Health Scope. 2020;9 [Google Scholar]
- 25.Fujimura Y., Araki S., Murata K., Sakai T. Assessment of peripheral, central and autonomic nervous system functions in two lead smelters with high blood lead concentrations: a follow-up study. J. Occup. Health. 1998;40:9–15. [Google Scholar]
- 26.Ghiasvand M., Aghakhani K., Salimi A., Kumar R. Ischemic heart disease risk factors in lead exposed workers: research study. J. Occup. Med. Toxicol. 2013;8:11. doi: 10.1186/1745-6673-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Can S., Bağci C., Ozaslan M., Bozkurt A.I., Cengiz B., Çakmak E.A., Kocabaş R., Karadağ E., Tarakçioğlu M. Occupational lead exposure effect on liver functions and biochemical parameters. Acta Physiol. Hung. 2008;95:395–403. doi: 10.1556/APhysiol.95.2008.4.6. [DOI] [PubMed] [Google Scholar]
- 28.Henry J.B. W.B. Saunders Company; Philadelphia: 2001. Clinical Diagnosis and Management by Laboratory Methods. [Google Scholar]
- 29.Burtis C.A., Bruns D.E. W.B. Saunders Company; Philadelphia: 2014. Tietz Fundamentals of Clinical Chemistry and Molecular Diagnostics-e-Book. [Google Scholar]
- 30.Kasperczyk S., Dobrakowski M., Kasperczyk A., Machnik G., Birkner E. Effect of N-acetylcysteine administration on the expression and activities of antioxidant enzymes and the malondialdehyde level in the blood of lead-exposed workers. Environ. Toxicol. Pharmacol. 2014;37:638–647. doi: 10.1016/j.etap.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 31.Malekirad A.A., Oryan S., Fani A., Babapor V., Hashemi M., Baeeri M., Bayrami Z., Abdollahi M. Study on clinical and biochemical toxicity biomarkers in a zinc-lead mine workers. Toxicol. Ind. Health. 2010;26:331–337. doi: 10.1177/0748233710365697. [DOI] [PubMed] [Google Scholar]
- 32.Matović V., Buha A., Ðukić-Ćosić D., Bulat Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem. Toxicol. 2015;78:130–140. doi: 10.1016/j.fct.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 33.Mohammed S.M. Hematological, biochemical and blood lead level profile among gasoline exposed station workers in Sulaimaniya city. ARO Sci. J. Koya Univ. 2016;2:6–11. [Google Scholar]
- 34.Rahimi Moghadam S., Afshari M., Ganjali A., Moosazadeh M. Effect of occupational exposure to petrol and gasoline components on liver and renal biochemical parameters among gas station attendants, a review and meta-analysis. Rev. Environ. Health. 2020;35:517–530. doi: 10.1515/reveh-2019-0107. [DOI] [PubMed] [Google Scholar]
- 35.Kasperczyk A., Dziwisz M., Ostałowska A., Świętochowska E., Birkner E. Function of the liver and bile ducts in humans exposed to lead. Hum. Exp. Toxicol. 2013;32:787–796. doi: 10.1177/0960327112468177. [DOI] [PubMed] [Google Scholar]
- 36.Liu C.-M., Ma J.-Q., Sun Y.-Z. Protective role of puerarin on lead-induced alterations of the hepatic glutathione antioxidant system and hyperlipidemia in rats. Food Chem. Toxicol. 2011;49:3119–3127. doi: 10.1016/j.fct.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 37.Dongre N.N., Suryakar A., Patil A.J., Rathi D. Occupational lead exposure in automobile workers in North Karnataka (India): effect on liver and kidney functions. Al Ameen J. Med. Sci. 2010;3:284–292. [Google Scholar]
- 38.Plaa G.L., Hewitt W.R. CRC Press; USA: 1998. Toxicology of the Liver. [Google Scholar]
- 39.Dioka C.E., Orisakwe O.E., Adeniyi F.A.A., Meludu S.C. Liver and renal function tests in artisans occupationally exposed to lead in Mechanic Village in Nnewi, Nigeria. Int. J. Environ. Res. Public Health. 2004;1:21–25. doi: 10.3390/ijerph2004010021. [DOI] [PubMed] [Google Scholar]
- 40.Rendón-Ramírez A.-L., Maldonado-Vega M., Quintanar-Escorza M.-A., Hernández G., Arévalo-Rivas B.-I., Zentella-Dehesa A., Calderón-Salinas J.-V. Effect of vitamin E and C supplementation on oxidative damage and total antioxidant capacity in lead-exposed workers. Environ. Toxicol. Pharmacol. 2014;37:45–54. doi: 10.1016/j.etap.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 41.Nakhaee S., Amirabadizadeh A., Brent J., Mehrpour O. Impact of chronic lead exposure on liver and kidney function and haematologic parameters. Basic Clin. Pharmacol. Toxicol. 2019;124:621–628. doi: 10.1111/bcpt.13179. [DOI] [PubMed] [Google Scholar]
- 42.Patil A.J., Bhagwat V., Patil J., Dongre N., Ambekar J., Das K. Occupational lead exposure in Battery Manufacturing workers, Silver Jewellery workers and spray painters of Western Maharashtra (India): effect of liver and kidney functions. J. Basic Clin. Physiol. Pharmacol. 2007;18:63–80. doi: 10.1515/jbcpp.2007.18.2.87. [DOI] [PubMed] [Google Scholar]
- 43.Dresner D.L., Ibrahim N.G., Mascarenhas B.R., Levers R.D. Modulation of bone marrow heme and protein synthesis by trace elements. Environ. Res. 1982;28:55–66. doi: 10.1016/0013-9351(82)90153-0. [DOI] [PubMed] [Google Scholar]
- 44.Prashanth L., Kattapagari K., Chitturi R., Baddam V., Prasad L. A review on role of essential trace elements in health and disease. J. Dr. NTR Univ. Health Sci. 2015;4:75–85. [Google Scholar]
- 45.Mitra P., Sharma S., Purohit P., Sharma P. Clinical and molecular aspects of lead toxicity: an update. Crit. Rev. Clin. Lab. Sci. 2017;54:506–528. doi: 10.1080/10408363.2017.1408562. [DOI] [PubMed] [Google Scholar]
- 46.Abdou H.M., Hassan M.A. Protective role of omega-3 polyunsaturated fatty acid against lead acetate-induced toxicity in liver and kidney of female rats. Biomed. Res. Int. 2014;2014 doi: 10.1155/2014/435857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu C.-M., Ma J.-Q., Sun Y.-Z. Puerarin protects the rat liver against oxidative stress-mediated DNA damage and apoptosis induced by lead. Exp. Toxicol. Pathol. 2012;64:575–582. doi: 10.1016/j.etp.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 48.Goodman D.S. VanNostrand Reinhold; USA: New York: 1985. Industrial Toxicology-safety and Health Application in Workplace:nephrotoxicity: Toxic Efforts in Kidneys. [Google Scholar]