Abstract
This study aimed to determine whether the innate immune system in the proventriculus of broiler chicks responds to lipopolysaccharide (LPS) and whether this response is affected by Newcastle disease and infectious bronchitis (ND/IB) vaccination. Chicks were divided into 4 groups: nonvaccinated and injected with PBS or LPS (V-L- and V-L+), and vaccinated and injected with PBS or LPS (V+L- and V+L+). Vaccination was performed on d 1, and LPS was intraperitoneally injected on d 11 of age. The gene expression and protein levels of immune molecules, including toll-like receptors (TLRs), antimicrobial peptides, interleukin-1β (IL-1B), and immunoglobulin A (IgA) in the proventriculus and serum were analyzed. The results showed that the expression levels of TLR21 were higher in vaccinated (V+L-) group than in nonvaccinated (V-L-) group. Gene expression levels of avian β-defensin (AvBDs) and cathelicidin1 (Cath1) were not different among the 4 groups. However, the results of LC/MS analysis showed that the levels of AvBD2, 6, and 7 significantly increased after the LPS challenge in nonvaccinated and vaccinated chicks; the levels were higher in V-L+ and V+L+ than in V-L- and V+L-, respectively. Immunohistochemistry analysis revealed the localization of AvBD1 protein in the epithelial cells of the surface glands and AvBD2 and CATH1 in the heterophil-like cells in the lamina propria of surface glands. Although IL-1B gene expression and protein concentration in the proventriculus tissues were not different among the 4 groups, serum IL-1B levels were upregulated by LPS in both the nonvaccinated and vaccinated groups (V-L- vs. V-L+, V+L- vs. V+L+). Moreover, IgA levels in the proventriculus and serum were not affected by vaccination or LPS challenge. Taken together, we conclude that LPS derived from gram-negative bacteria upregulates the innate immune system, including antimicrobial peptide synthesis in the proventriculus. ND/IB vaccination may not significantly affect antimicrobial peptide synthesis in response to LPS; however, TLR21 expression is upregulated by that vaccination. The antimicrobial peptides synthesized in the proventriculus probably prevent pathogenic microbes from entering the intestine.
Key words: chick proventriculus, innate immune system, vaccination, lipopolysaccharide
INTRODUCTION
Prevention of infections by pathogenic gut microbes is one of the most important concerns for poultry production. The gut mucosal tissues are protected from infection by both innate and adaptive immune systems. In chickens, B cells were detected in the circulation on d 6 post-hatching (Lawrence et al., 1981), whereas endogenous immunoglobulin A (IgA) in the intestine was almost undetectable before 14 d of age (Lammers et al., 2010). Thus, chicken B cells may require a few weeks post-hatching to develop into immunoglobulin-synthesizing cells. Maternal IgY is catabolized during the initial 14 d post-hatching (Grindstaff et al., 2003). Antimicrobial peptides, including avian β-defensins (AvBDs) and cathelicidins (CATHs), play roles in the innate immune system. Previous studies have reported that AvBDs are expressed in the intestines of embryonic and neonatal chicks, and their expression decreases from d 4 post-hatching (Crhanova et al., 2011; Terada et al., 2018). Thus, the innate immune function of the gut defense system is expected to be strengthened during the early phase of life in chicks with immature adaptive immune systems.
The avian stomach consists of the proventriculus and gizzard (Klasing, 1999). In the proventriculus, gastric juice containing gastric acid and pepsin is secreted, whereas in the gizzard, the luminal contents are mechanically ground and digested by the gastric juice. Germicidal activity in the proventriculus is important to reduce the pathogenic microbes in the luminal contents before they enter the intestine. Although gastric acid is known to exert germicidal activity, the innate immune function responsible for regulating the luminal microbes in the proventriculus remains to be studied.
Toll-like receptors (TLRs) recognize microbe-associated molecular patterns (MAMPs) leading to the expression of immune factors, including cytokines and antimicrobial factors that play important roles in the defense against infection (St Paulet al., 2013; Yoshimura, 2015). TLR2 and TLR4 recognize the peptidoglycans and lipopolysaccharides (LPS) of gram-positive and gram-negative bacteria, respectively. TLR5 recognizes bacterial flagellin, whereas TLR15, a unique TLR expressed in chickens, recognizes the non-secreted, heat-stable component of bacteria in addition to secreted virulence-associated fungal and bacterial proteases. TLR3 and TLR7 recognize dsRNA and ssRNA viruses, respectively, whereas TLR21 recognizes unmethylated CpG-DNA of microbes. Fourteen AvBDs (AvBD1–14) and 4 cathelicidins (CATH1–3 and CATH-B1) with broad-spectrum antimicrobial activity have been identified (Hong et al., 2012; Wang et al., 2020). Regulation of the expression of proinflammatory cytokines and antimicrobial peptides by TLR ligands and pathogenic microbes is reported in the chicken oviduct and other organs (Cuperus et al., 2013; Abdel-Mageed et al., 2014; Kamimura et al., 2017; Terada et al., 2020b). Mohammed et al., who examined the effects of LPS and probiotics on the gene expression of antimicrobial peptides in the chick gastrointestinal tract, reported that the expression of AvBD6 and 12 under LPS stimulation was enhanced by probiotics in the proventriculus (Mohammed and Radey, 2021), whereas CATHs expression was not affected (Mohammed et al., 2016). However, the details of innate immune system in the proventriculus and its response to MAMPs remain to be examined by further studies including the protein level analysis.
The adaptive immune response to pathogens is modulated by vaccination, owing to the formation of specific memory lymphocytes. Recent studies have suggested that innate immune training (innate immune memory) may also be possible in many organisms, including invertebrates (Kurtz, 2005) and mammals (Netea et al., 2011; Morimoto et al., 2012; van der Meer et al., 2015; de Bree et al., 2018; Netea et al., 2020). Vaccination with Bacillus Calmette-Guérin (BCG) enhances specific adaptive immune activity as well as innate immunity through the activation of macrophages, monocytes, and natural killer cells (Kleinnijenhuis et al., 2014; Netea et al., 2016). Nonspecific innate immune responses to pathogenic agents, which are unrelated to the vaccine antigen, are increased after BCG vaccination (Kleinnijenhuis et al., 2012), and this trained immunity leads to increased cytokine production in response to unrelated pathogens (Kleinnijenhuis et al., 2014). We reported that routine multiple vaccinations, including vaccines for Marek's disease (MD), avian infectious bronchitis virus (IB), Newcastle disease and IB virus (ND/IB), and infectious bursal disease, caused the increase in the expression of TLR2 and 21 and the decrease in the AvBDs expression in the ovary of 21-day-old chicks (Kang et al., 2019). Meanwhile, our recent study showed that ND/IB vaccination upregulated the expression of TLR7 and 21 among 3 TLRs (TLR3, 7, and 21), and MD vaccination modulated the expression of AvBDs in the kidney of 3-day-old chicks (Shimizu et al., 2020). Thus, we hypothesize that training of the innate immunities by vaccination may be possible in the different organs in chicks as reported in mammals. If the innate immune response in the chick gut is strengthened by vaccination, it may lead to a new strategy for enhancing the gut innate immunodefense system. However, it remains unknown whether vaccination modulates the innate immunity in chick guts, including the proventriculus.
The aim of this study was to determine whether the innate immune system in the proventriculus of broiler chicks responds to LPS and whether this response is affected by ND/IB vaccination. To examine it, chicks were inoculated with or without ND/IB vaccine at day-old and injected with or without LPS at 11-day-old of age. Then the gene expression and protein levels of innate immune molecules in the proventriculus tissue, including TLRs, antimicrobial peptides, interleukin 1-β (IL-1B), and IgA were compared between LPS-injected and noninjected groups with or without ND/IB vaccination. The concentration of IL-1B and IgA in the serum was also examined to confirm that injected LPS was absorbed to induce the synthesis of them. The LPS challenge was performed because many pathogenic microbes infecting the gut include gram-negative bacteria such as Salmonella bacteria and Campylobacter. The ND/IB vaccine was expected to affect the innate immunity because our previous study showed that the expression of two of three TLRs was upregulated by that vaccine in the chick kidney (Shimizu et al., 2020).
MATERIALS AND METHODS
Experimental Design
Day-old female broiler chicks were inoculated with or without mixed live ND/IB vaccines, and then intraperitoneally injected with LPS or PBS at d 11 of age. Accordingly, the chicks were divided into 4 groups: nonvaccinated and injected with PBS (V-L-), nonvaccinated and injected with LPS (V-L+), vaccinated and injected with PBS (V+L-), and vaccinated and injected with LPS (V+L+). The experimental trials to analyze the gene and protein expression of immune molecules were performed twice under the same conditions using 5 chicks in each group per trial, and the data of the 2 trials were finally pooled into one (n = 10 in each group). Immunohistochemistry was performed to investigate the localization of antimicrobial peptides in chicks from the 4 groups in one trial (n = 5 in each group).
Experimental Birds and Vaccine Inoculation
Broiler female chicks (Chunky) were obtained by incubating fertilized eggs purchased from a local hatchery (Fukuda Breeder, Okayama, Japan), and their sex was determined by feather sexing. Day-old female chicks were inoculated, through a nasal drip, with mixed ND/IB vaccines (Poulvac COMBI, Kyoritsu Seiyaku Co., Tokyo, Japan; containing ND virus B1 strain and IB virus H120 strain) or with sterile PBS instead of the vaccine. They were reared in brooding rooms (1 chick group in 1 room) with electric heaters under lighting conditions of 23 h light and 1 h dark with free access to a commercial starter diet (Nichiwa Sangyo Co. Ltd., Hyogo, Japan) and water. At d 11 of age, chicks in the V-L+ and V+L+ groups were intraperitoneally (at the caudal part of the abdomen) injected with LPS at a dose of 250 μg/ kg of BW, and those in V-L- and V+L- groups were injected with PBS at a volume of 1 mL/kg of BW. The LPS stock solution was prepared by dissolving LPS from S. minnesota (Wako Pure Chemical Industries, Osaka, Japan) in PBS at a concentration of 250 μg/mL. Five hours after LPS injection, the chicks were euthanized using carbon dioxide, and the proventriculus tissues and blood in the heart atrium were collected. The wall of the proventriculus, including the mucosa and smooth muscle layers, was collected at the middle part along the longitudinal axis, and frozen on solid carbon dioxide or placed in RNA later (Thermo Fisher Scientific, Inc., Waltham, MA). They were stored at −80°C until further analysis of the protein concentration and gene expression levels of immune molecules. Blood samples were coagulated and centrifuged at 1,400 × g to obtain serum, which was stored at −30°C until enzyme-linked immunosorbent assay (ELISA) was performed to detect the expression levels of IL-1B and IgA. Some of the proventriculus tissues were fixed in 10% (v/v) formalin in PBS and used for immunohistochemistry. This study was approved by the Hiroshima University Animal Research Committee (No. C15-16).
Quantitative Real-Time PCR Analysis for Immune Molecules
-
(1)
RNA isolation and cDNA preparation: RNA was extracted from the proventriculus tissues using Sepasol RNA I Super (Nacalai Tesque, Inc. Kyoto, Japan), according to the manufacturer's instructions. The extracted total RNA samples were dissolved in TE buffer (10 mM Tris–HCl, pH 8.0, 1 mM EDTA) and stored at −80°C until use. The concentration of total RNA in each sample was measured using a NanoDrop Lite (Thermo Fisher Scientific). The samples were treated with RQ1 RNase-free DNase (Promega Co., Madison, WI) on a programmable thermal controller (PTC-100; MJ Research, Waltham, MA) according to the manufacturer's instructions. Subsequently, RNA samples were reverse-transcribed using Rever-Tra Ace (Toyobo Co., Ltd., Osaka, Japan) according to the manufacturer's instructions. Reverse transcription was performed at 42°C for 30 min, followed by heat inactivation at 99°C for 5 min using a programmable thermal controller. The obtained cDNA samples were stored at −80°C until use.
-
(2)
Real-time PCR analysis: Real-time PCR was performed using the AriaMix Real-time PCR System (Agilent Technologies Japan, Ltd., Tokyo, Japan). The reaction mixture (10 μL) consisted of 1 μL cDNA, 1 × Brilliant III SYBR Green QPCR Mix (Agilent Technologies Japan, Ltd.), 0.25 μM of each primer, and water. The primer sequences used in this study are listed in Table 1. The expression levels of TLR2-1, 3, 4, 5, 7, 15, and 21 were examined as receptors that recognize bacterial and viral molecular patterns. In addition, the gene expression levels of AvBD1, 2, 6, 7, and cathelicidin1 (Cath1) were analyzed as their products were detectable in the liquid chromatography–mass spectrometry (LC/MS) analysis, and the PCR amplification products showed relatively high densities. The expression of IL-1B was analyzed because it is one of the proinflammatory cytokines that may induce the innate immune response following LPS challenge (Sonoda et al., 2013). The amplification protocol was 50 cycles at 95°C for 5 s and 55°C (AvBD1), 60°C (TLR2-1, 3, 4, 5, 7, 15, and 21; and RPS17), 62°C (AvBD2, 6, and 7; Cath1), or 63°C (IL-1B) for 10 s each. To calculate the relative levels of gene expression in each sample, real-time PCR data were analyzed using the 2-ΔΔCT method and expression levels of the target gene were normalized against the RPS17 housekeeping gene expression (Livak and Schmittgen, 2001).
Table 1.
Primer sequences of TLRs, cytokines, AvBDs, and RPS17 for PCR.
| Target genes | Sequences 5’-3’ | Accession No. |
|---|---|---|
| TLR2-1 | F: CTGCAACGGTCATCTCAGCTA R: CCGGGGGAATGAAGTCCAAA |
NM_204278 |
| TLR3 | F: TCAGTACATTTGTAACACCCCGCC R: GGCGTCATAATCAAACACTCC |
NM001011691 |
| TLR4 | F: AGTCTGAAATTGCTGAGCTCAAAT R: GCGACGTTAAGCCATGGAAG |
NM_001020693 |
| TLR5 | F: CCACATCTGACTTCTGCCTTT R: TGCACATGTTTTCTCCTAGGT |
NM_001024586 |
| TLR7 | F: CCTGACCCTGACTATTAACCATT R: CGTAAAGTAGCAGGAAGACCC |
NM_001011688 |
| TLR15 | F: GTTCTCTCTCCCAGTGGGTGAAATAGC R: GTGGTTCATTGGTTGTTTTTAGGAC |
NM_001037835 |
| TLR21 | F: TGCCCCTCCCACTGCTGTCCACT R: AAAGGTGCCTTGACATCCT |
NM_001030558 |
| IL-1β | F: GTGAGGCTCAACATTGCGCTGTA R: TGTCCAGGCGGTAGAAGATGAAG |
NM_204524 |
| AvBD1 | F: GATCCTCCCAGGCTCTAGGAAG R: GCCCCATATTCTTTTGC |
NM_204993.1 |
| AvBD2 | F: GTTCTGTAAAGGAGGGTCCTGCCAC R: ACTCTACAACACAAAACATATTGC |
NM_204992 |
| AvBD6 | F: GATCCTTTACCTGCTGCTGTCT : TCCTCACACAGCAAGATTTTAGTC |
NM_001001193 |
| AvBD7 | F: ACCTGCTGCTGTCTGTCCTC R: TGCACAGCAAGAGCCTATTC |
NM 001001194.1 |
| CATH1 | F: GCTGTGGACTCCTACAACCAAC R: GGAGTCCACGCAGGTGACATC |
NM_001001605.3 |
| RPS17 | F: AAGCTGCAGGAGGAGGAGAGG R: GGTTGGACAGGCTGCCGAAGT |
NM_204217 |
Abbreviations: F, forward; R, reverse.
Quantitative LC/MS Analysis for AvBDs and Cath1 Protein in the Proventriculus
Approximately 100 mg of proventriculus tissue was cut into small fragments, and 1,500 μL of extraction buffer (70% acetonitrile and 0.05% trifluoroacetic acid) was added per 100 mg of tissue. The samples were crushed on Tissue Lyser II (QIAGEN, Hilden, Germany) using 1-mm zirconia beads at 25 Hz for 2 min and this crushing step were repeated again. The samples were heated at 80°C for 3 min to inactivate the proteases. Subsequently, the samples were centrifuged at 4°C for 10 min at 6,000 × g, and the supernatant was collected. Protein concentration was quantified using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Twenty micrograms of protein was aliquoted and dissolved in 100 mM triethylammonium bicarbonate, followed by the addition of an internal standard peptide at a final concentration of 66 ng/mL. Dithiothreitol was added at a final concentration of 5 mM, and the sample was heated at 37°C for 30 min. Next, iodoacetamide was added at a final concentration of 16.5 mM and the sample was heated at 37°C for 30 min followed by the addition of 1 μg of trypsin (Trypsin/Lys-C Mix, Mass Spec Grade, Promega) to digest the sample proteins at 37°C overnight. Trifluoroacetic acid was added at a final concentration of 0.5% to stop the digestion. The samples were desalted using GL-Tip SDB (GL Science Inc., Tokyo, Japan) and dried by blowing nitrogen and then redissolved in 35 μL of 0.1% formic acid for LC/MS analysis. One microliter of the sample was injected into a DIONEX Ultimate 3000 RSLCnano Pump (Thermo Fisher Scientific) and separated on an Acclaim PepMap 100 (75 μm × 15 cm; Thermo Fisher Scientific) at a flow rate of 350 nL/min. Mobile phase A was water with 0.1% (v/v) formic acid, and mobile phase B was methanol. Separation was started with 5% mobile phase B, which was increased to 55% within 30 min and to 98% within 10 min, and then kept constant at 98% for 5.0 min and at 5% for 14.5 min. The LC eluent was introduced into a Xevo TQ-S (Waters, Milford, MA) for multiple reaction monitoring (MRM) using positive ion mode with electrospray ionization. Final MS parameters were as follows: Nanoflow gas flow, 0.20 Bar; collision-gas flow, 0.25 mL/min; capillary voltage, 1.50 kV; and source temperature, 80°C. Quantification was performed by MRM of the doubly protonated precursor molecular ions [M + 2H]2+ and the related product ions. The MRM transition list of AvBDs and CATH1 is shown in Table 2. Chromatograms and mass spectral data were acquired and processed using the MassLynx (version 4.1) software (Waters, MA). The quantitative concentration value was calculated by dividing the integrated peak area of the target molecule by that of the internal standard. The relative values from the mean of the V-L- group were calculated.
Table 2.
Multiple reaction monitoring transition of LC/MS for avian β-defensins (AvBDs) and cathelicidin 1 (CATH1).
| Analyte | Sequence | Q1 (m/z) | Q3 (m/z) | Dwell time (msec) | Collision energy (V) |
|---|---|---|---|---|---|
| AvBD1 | SGFCAFLK | 465.2311 | 638.3330 | 66 | 15 |
| AvBD2 | VGSCFGFR | 462.2185 | 773.3399 | 66 | 15 |
| AvBD6 | GVCIPGPCR | 508.2442 | 586.2766 | 66 | 15 |
| AvBD7 | NGICFPGICR | 597.2813 | 909.4070 | 66 | 19 |
| CATH1 | TVIAGYNLYR | 585.3193 | 856.4312 | 66 | 19 |
Immunohistochemistry for Antimicrobial Peptides in the Proventriculus Tissue
The proventriculus tissues were fixed in 10% (v/v) formalin in PBS and processed to obtain paraffin sections (4 μm in thickness). After deparaffinization, they were autoclaved with 0.1 M citric acid (pH 6.0) for antigen retrieval using 2100Retriever (Aptum Biologics Ltd., Hants, UK). After cooling and washing with PBS (5 min × 3 times), the sections were incubated with 1% (w/v) blocking reagent (Roche Co., Basel, Switzerland) for 30 min to block the nonspecific binding of antibodies, followed by incubation with antibodies against AvBD1, AvBD2, or CATH1 (all of them were diluted at 20 μg/mL in PBS) at 4°C overnight. The sections were then washed with PBS (5 min × 3 times) and incubated with the biotin-conjugated anti-rabbit IgG (1:200) and avidin-biotin-peroxidase complex (1:50) for 1 h each using the VECTASTAIN ABC Kit (Vector Laboratories, Inc., Burlingame, CA). The immunoreaction products were visualized using a reaction mixture of 0.02% (w/v) 3,3′-diaminobenzidine-4HCl and 0.05% (v/v) H2O2. The sections were then counterstained with hematoxylin and covered after dehydration. Negative control staining was performed to confirm the specificity of immunostaining by replacing the primary antibodies with normal rabbit IgG (Santa Cruz Biotechnology, Inc., Dallas, TX).
The antibodies against AvBD1, AvBD2, and CATH1 were used in our previous studies involving immunohistochemistry and western blot analysis (Elhamouly et al., 2018; Elhamouly et al., 2019; Shimizu et al., 2020; Terada et al., 2020a). Antisera against AvBD1, AvBD2, and CATH1 was raised by immunization of rabbits with keyhole limpet hemocyanin-conjugated synthetic peptide (anti-AvBD1 was obtained from Operon Biotechnologies K.K., Tokyo Japan; anti-AvBD2 was obtained from Medical & Biological Laboratories, Nagoya, Japan; and CATH1 was obtained from Sigma-Aldrich Japan K.K., Tokyo, Japan). The sequence of the synthetic AvBD1 peptide was a mixture of GRKSDCFRKSGF and SLTLISGKCSRFYL, whereas the synthetic AvBD2 peptide was CPSHLIKVGS, and the synthetic CATH1 peptide was C-C6-YRAIKKK. The antibodies in the serum were purified using an affinity column (HiTrapTM NHS-activated HP, GE Healthcare Japan, Tokyo, Japan) conjugated with each synthetic peptide, as per manufacturer's instructions.
ELISA Analysis for IL-IB and IgA in the Serum and Proventriculus Tissue
The concentrations of IL-1B and IgA in the serum and the proventriculus tissues were examined using ELISA kits for IL-1B (Product No. SEA563Ga; Cloud-Clone Co, Katy, TX) and chicken IgA (Bethyl Laboratories, Inc., Montgomery, TX). Serum samples were diluted 100 times using PBS. The proventriculus tissue samples were homogenized in PBS (10 times the tissue) using a Polytron homogenizer (Polytron PT1200ci, Kinematica AG, Switzerland), followed by centrifugation at 13,000 × g. The supernatants were collected and their protein concentrations were measured using a NanoDrop Lite Spectrophotometer (Thermo Fisher Scientific). Subsequently, the supernatants were diluted 100 times with PBS for the ELISA analysis. IL-1B and IgA levels were measured according to the manufacturer's instructions.
Statistical Analysis
The significance of differences in gene expression of TLRs between vaccinated and nonvaccinated groups without LPS challenge (V-L- vs. V+L-) was determined by t test. The significance of differences in the gene expression levels and protein amounts of immune molecules among V-L-, V-L+, V+L-, and V+L+ was determined using the Kruskal-Wallis test followed by the Steel-Dwass test. The differences were considered significant at P < 0.05.
RESULTS
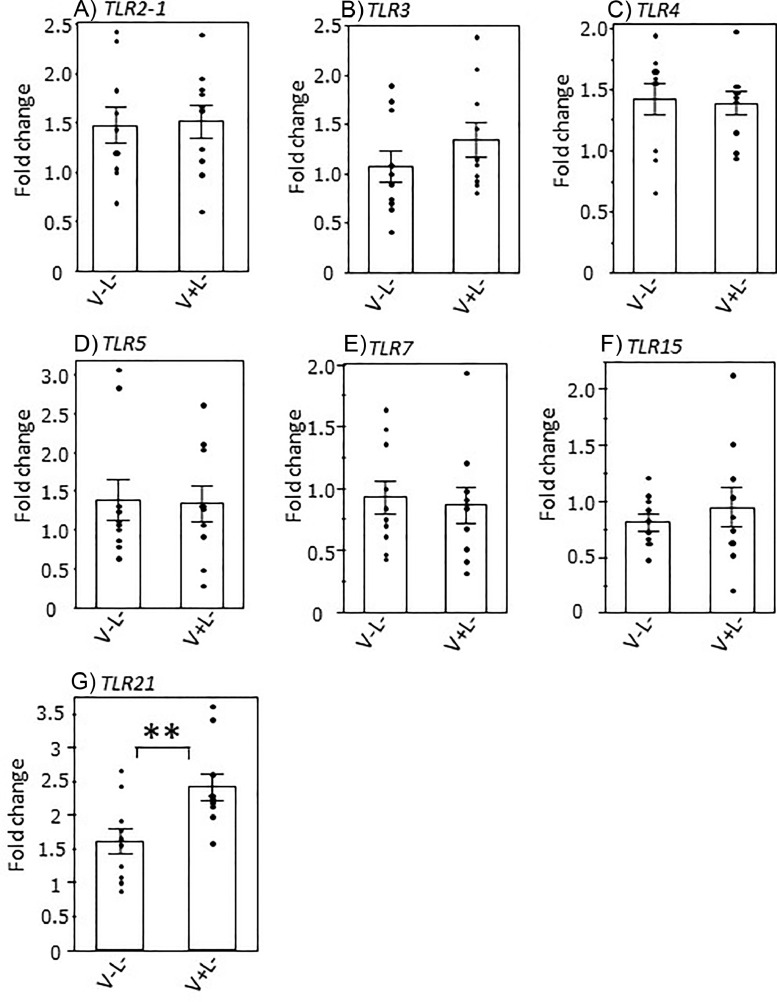
Figure 1 shows the effects of ND/IB vaccination on gene expression levels of TLRs in the proventriculus without LPS challenge. The expression levels of TLR2-1, 3, 4, 5, 7, 15, and 21 were determined in the proventriculus tissues of vaccinated and nonvaccinated chicks. The expression level of TLR21 was significantly higher in the vaccinated (V+L-) group than in the nonvaccinated (V-L-) group.
Figure 1.
Effects of Newcastle disease and infectious bronchitis (ND/IB) vaccination on the toll-like receptor (TLR) expression in the chick proventriculus. Day-old chicks were administered phosphate-buffered saline or ND/IB vaccine, and the proventriculus tissues were collected without lipopolysaccharide challenge (V-L- and V+L- groups, respectively) on d 11 post-hatching. The dots indicate the values for each individual. Bars represent mean ± SEM (n = 10). Asterisks indicate significant differences between the nonvaccinated and vaccinated groups (**P < 0.01, n = 10).
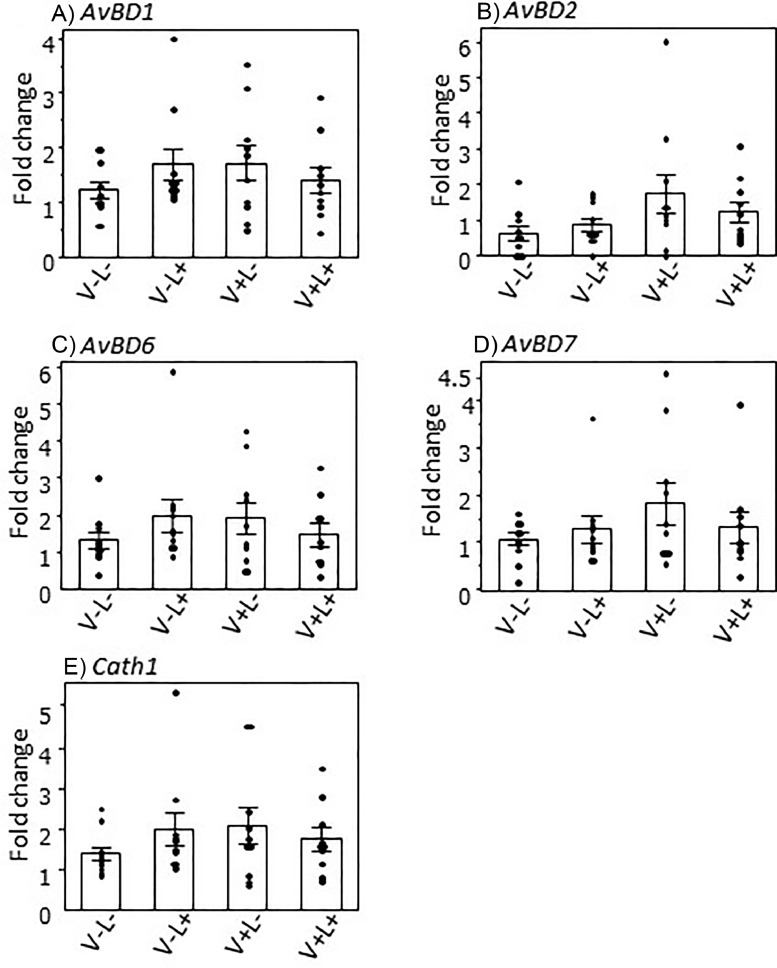
The effects of LPS on the expression levels of AvBDs and Cath1 in the proventriculus of vaccinated and nonvaccinated chicks are shown in Figure 2. The expression levels of AvBD1, 2, 6, 7, and Cath1 did not show significant differences among the four groups with or without vaccination and LPS challenge, namely V-L-, V-L+, and V+L-, and V+L+ groups.
Figure 2.
Effects of lipopolysaccharide (LPS) challenge on the gene expression of avian β-defensins (AvBDs) and cathelicidin 1 (Cath1) in the chick proventriculus with or without Newcastle disease and infectious bronchitis (ND/IB) vaccination. Day-old broiler chicks were inoculated with or without ND/IB vaccines, and then intraperitoneally injected with PBS or LPS 5 h before the examination at d 11 of age. Four experimental chick groups were designed: non-vaccinated and injected with PBS (V-L-), nonvaccinated and injected with LPS (V-L+), vaccinated and injected with PBS (V+L-), and vaccinated and injected with LPS (V+L+). Dots represent the values for each individual. Bars represent mean ± SEM (n = 10).
Figure 3 shows the effects of LPS challenge on the relative levels of AvBDs and CATH1 proteins in the proventriculus of vaccinated and nonvaccinated chicks. The levels of AvBD2, 6, and 7 were significantly increased by LPS challenge in both nonvaccinated and vaccinated chicks; namely the levels were significantly higher in V-L+ and V+L+ groups than in V-L- and V+L- groups (Figures 3B–3D). The levels of AvBD2, 6 and 7 were also greater in V-L+ than V+L- and in V+L+ than V-L- (Figures 3B–3D). These findings suggest that the levels of AvBD2, 6, and 7 were higher in the LPS-challenged groups regardless of the vaccination. Differences in the levels of AvBDs between nonvaccinated and vaccinated groups challenged with or without LPS, namely V-L- vs. V+L-, and V-L+ vs. V+L+, were not significant (P > 0.05; Figures 3B–3D). Furthermore, two-way analysis of variance results did not show significant interaction effects of vaccination and LPS challenge for any AvBDs and CATHs (data not shown). In addition, the levels of AvBD1 and CATH1 did not differ among the four groups (V-L-, V-L+, V+L-, and V+L+) treated with or without vaccines and LPS (Figures 3A and 3E).
Figure 3.
Effects of lipopolysaccharide (LPS) challenge on the levels of avian β-defensins (AvBDs) and cathelicidin 1 (CATH1) in the chick proventriculus with or without Newcastle disease and infectious bronchitis (ND/IB) vaccination. Day-old broiler chicks were inoculated with or without ND/IB vaccines, and then intraperitoneally injected with or without lipopolysaccharide 5 h before the examination at d 11 of age. The levels of AvBDs and CATH1 in the proventriculus were analyzed using liquid chromatography–mass spectrometry (LC/MS). See Figure 2 for the distribution of experimental chick groups V-L-, V-L+, V+L-, and V+L+. Individual values are indicated by dots. Bars represent mean ± SEM (n = 10). The asterisks indicate significant differences between the groups (*P < 0.05, **P < 0.05; Kruskal Wallis and Steel-Dwass tests).
Figure 4 shows the immunolocalization of AvBD1, AvBD2, and CATH1 in the proventriculus tissues of a chick that received ND/IB vaccination, followed by LPS challenge (V+L+). In the surface layer, mucosal folds were well developed, and surface glands showing tubule structures were formed at the bottom of the folds. Heterophils were localized throughout the lamina propria, and lymphocytes were assembled to form lymph nodule-like structures. The AvBD1 immunopositive signal was identified in the epithelial cells of the surface glands (Figure 4C). The AvBD2-positive signal was found in the cells assembled to form groups in the lamina propria (Figure 4D). CATH1-positive cells were localized throughout the lamina propria tissues (Figure 4E). Although the localization profiles of AvBD2 and CATH1 cells were not the same, they were localized in the tissues where heterophils were found (Figure 4B). Similar localization of AvBD1, AvBD2, and CATH1 cells was also found in the V-L-, V-L+, and V+L- groups. Negative control staining using normal rabbit IgG in place of the first antibody showed no staining (Figure 4E).
Figure 4.
Immunolocalization of avian β-defensins (AvBDs) and cathelicidin 1 (CATH1) in the proventriculus of a chick treated with Newcastle disease and infectious bronchitis (ND/IB) vaccine and lipopolysaccharide (LPS). (A and B) Sections of proventriculus mucosa under low and high magnifications, respectively. In the surface layer, mucosal folds (F) are formed, and surface glands (Sg) showing tubular structures are formed at the bottom of the folds. Formation of lymphocyte clusters can be seen. Note that the heterophils are localized in the whole lamina propria (arrows) and a part of them form small groups. (C) AvBD1 immunostaining. Note the AvBD1 immunopositive signals in the epithelial cells of surface glands (arrows). (D) AvBD2 immunostaining. Note that the AvBD2-positive cells are assembled to form groups (arrows). (E) CATH1 immunostaining. Note that the CATH1-positive cells are localized throughout the lamina propria (arrows). Abbreviations: Dg, deep glands; L, lumen; Lp, lamina propria; Ly, lymphocyte accumulation. Scale bars = 50 μm.
The effects of LPS challenge on the gene expression and protein concentration of IL-1B in the proventriculus and serum of nonvaccinated and vaccinated chicks are shown in Figure 5. IL-1B gene expression and protein concentration in the proventriculus tissues did not differ among the 4 groups with or without vaccination and LPS challenge (V-L-, V-L+, V+L-, and V+L+), as shown in Figure 5A and B. The IL-1B concentration in the serum was significantly upregulated by LPS in both the nonvaccinated and vaccinated groups (V-L- vs. V-L+, V+L- vs. V+L+; Figure 5C). Serum IL-1B levels were also higher in the LPS-challenged group regardless of vaccination (V-L- vs. V+L+, V-L+ vs. V+L-; Figure 5C).
Figure 5.
Effects of lipopolysaccharide (LPS) on the gene expression and concentration of interleukin 1β (IL-1B) in the proventriculus tissue and serum of chicks inoculated with or without Newcastle disease/infectious bronchitis (ND/IB) vaccine. Day-old broiler chicks were inoculated with or without ND/IB vaccine and were intraperitoneally injected with PBS or LPS 5 h before the examination at d 11 of age. IL-1B protein concentration was examined using enzyme-linked immunosorbent assay, and gene expression was analyzed using quantitative RT-PCR. See Figure 2 for the distribution of experimental chick groups V-L-, V-L+, V+L-, and V+L+. Individual values are indicated by dots. Bars represent mean ± SEM (n = 10). The asterisks indicate significant differences between the groups (*P < 0.05, **P < 0.01; Kruskal Wallis and Steel-Dwass tests).
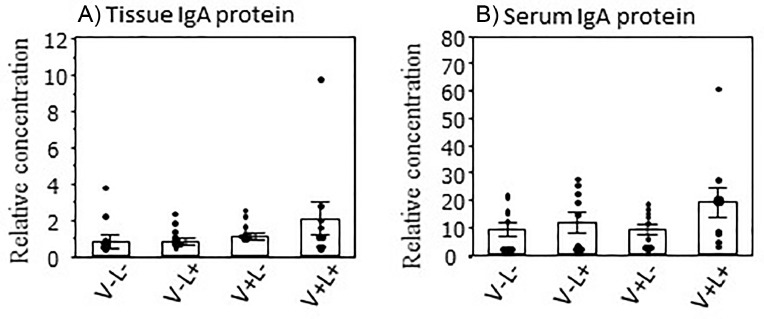
The effects of LPS challenge on IgA concentration in the proventriculus and serum of nonvaccinated and vaccinated chicks are shown in Figure 6. The IgA concentrations in both the proventriculus tissue and serum did not show any differences among the four groups treated with or without the vaccination and LPS challenge (V-L-, V-L+, V+L-, and V+L+; Figures 6A and 6B).
Figure 6.
Effects of Newcastle disease and infectious bronchitis (ND/IB) vaccination and lipopolysaccharide (LPS) on the IgA concentration in the proventriculus tissue and serum. Day-old broiler chicks were inoculated with or without ND/IB vaccines, followed by intraperitoneal injection with PBS or LPS 5 h before the examination at d 11 of age. IgA concentration was examined using enzyme-linked immunosorbent assay. See Figure 2 for the distribution of experimental chick groups V-L-, V-L+, V+L-, and V+L+. Individual values are indicated by dots. Bars represent mean ± SEM (n = 10).
DISCUSSION
We examined whether the innate immune system in the proventriculus responded to LPS challenge and whether the response was modulated by ND/IB vaccination. This study is the first to demonstrate the presence of AvBDs and CATH1 peptide molecules in the proventriculus identified by LC/MS, supporting previous reports that showed their gene expression (Achanta et al., 2012; Mohammed et al., 2016; Lyu et al., 2020). From the immunohistochemistry results, we suggest that the epithelial cells of the surface glands are responsible for AvBD1 synthesis. Heterophils may be responsible for the synthesis of AvBD2 and CATH1 because AvBD2-positive cells and CATH1-positive cells were localized in the lamina propria tissues where heterophils were identified. However, because the localization profiles of AvBD2-positive cells were different from those of CATH1-positive cells, there may be a difference in their ability to synthesize these antimicrobial peptides among the heterophils.
The expression of TLR21 was higher in the proventriculus of chicks administered with the ND/IB vaccine (V+L-) than in nonvaccinated chicks (V-L-). Recently, we reported that ND/IB vaccination increased the expression of TLR7 and TLR21 in chick kidneys (Shimizu et al., 2020). Thus, it is likely that the inoculation of chicks with ND/IB vaccine upregulates the expression of TLR21 that recognize unmethylated CpG-DNA of DNA virus and bacteria in both the proventriculus and kidney. Studies in mammals have also suggested that vaccination upregulates innate immune functions; for instance, in BCG-vaccinated mice, inflammatory cytokine production after LPS challenge was significantly higher, and the function of circulating monocytes was enhanced with increase in the expression of TLR4 (Kleinnijenhuis et al., 2012, 2014). The current results showed that a higher expression of TLR21 in the proventriculus was kept at least 11 d after ND/IB vaccination. However, in the chick kidney, the increase in the TLR7 and 21 expressions by that vaccination was found at 3 d, but not at 10 d, postinoculation (Shimizu et al., 2020). Although it remains unknown why the higher expression of TLR21 was kept longer time in the proventriculus, we assume that more TLR expressing cells that are sensitive to ND/IB vaccine existed in the proventriculus than in the kidney.
We identified the gene expression and protein molecules of AvBDs and CATH1 using RT-qPCR and LC/MS analysis, suggesting that these antimicrobial peptides are synthesized in the chick proventriculus. To maintain gut health, it is important to eliminate pathogenic microbes in the stomach before they enter the intestine. Many of the pathogenic microbes are gram-negative bacteria, such as Campylobacter and Salmonella bacteria, which contain LPS. In our in vivo studies, LPS injection in laying hens upregulated the gene expression of AvBDs in the theca of ovarian follicles and vaginal mucosa (Subedi et al., 2007; Mageed et al., 2008; Yoshimura, 2015). In the current study, the gene expression of these antimicrobial peptides in the proventriculus was not different between LPS-challenged and nonchallenged groups in nonvaccinated (V-L- vs. V-L+) and vaccinated chicks (V+L- vs. V+L+). However, LC/MS analysis showed that the levels of AvBD2, 6, and 7 were increased by LPS in the proventriculus of both vaccinated and nonvaccinated chicks. This result suggests that LPS stimulates the synthesis of these AvBDs in the proventriculus infected by gram-negative bacteria. However, the exact reason for the lack of difference in gene expression levels between the LPS-challenged and nonchallenged groups is unknown. Mohammed and Radey reported that the gene expression of AvBD6 and 12 in the proventriculus under stimulation with LPS was higher in probiotics-fed chicks than control (Mohammed and Radey, 2021), suggesting their expression could be changed by LPS and probiotics. Thus, we assume that although the gene expression of AvBDs might be elevated after LPS challenge, it returned to a level similar to that in the nonchallenged group by 5 h in the current study. In contrast, we found no significant difference in the gene expression and protein levels of AvBDs and CATH1 between the ND/IB-vaccinated and nonvaccinated groups (V-L- vs. V+L-, and V-L+ vs. V+L+). Thus, ND/IB vaccination may not significantly affect AvBD and CATH1 synthesis functions in the proventriculus in the presence or absence of LPS.
Although the gene expression and protein levels of IL-1B in the proventriculus tissue were not affected by vaccination and LPS, the IL-1B levels in the serum were increased by LPS in non-vaccinated (V-L- vs. V-L+) and vaccinated (V+L- vs. V+L+) chicks. This result confirms that intraperitoneally injected LPS is absorbed in the blood circulation and stimulated the IL-1B producing cells in the body. We have previously reported that not only LPS but also IL-1B stimulates AvBD gene expression in the chicken oviduct (Sonoda et al., 2013; Yoshimura, 2015). Thus, it is possible that the LPS and also IL-1B in the serum may play a role in the induction of AvBD synthesis in the proventriculus. However, IL-1B synthesis and its secretion into the serum in response to LPS are likely not affected by ND/IB vaccination.
The current results showed that the IgA levels in the proventriculus tissue and serum were not different among the 4 groups of chicks treated with or without ND/IB vaccine and LPS. There are two possibilities for the origin of the IgA; the first one is maternal IgA, and the second one is IgA synthesized by the chick plasma cells. Vaccinations generally stimulate the synthesis of antibodies, including IgA. Induction of IgA synthesis by LPS has been reported in the intestines of mammals (Maes et al., 2007; Shibata et al., 2018). Thus, ND/IB vaccination and LPS may stimulate IgA synthesis. However, since it takes a few weeks post-hatching for the maturation of the lymphoid system in chicks (Lammers et al., 2010), it is assumed that the IgA level was not affected by vaccination and LPS in the 11-day-old chicks whose lymphoid system might not have been well developed in this study. In mature chicks, IgA, in combination with antimicrobial peptides, may play a role in the defense against mucosal infection by pathogenic agents.
Taken together, the results of the current study show that the innate immune system, consisting of TLRs, antimicrobial peptides, and IL-1B, is formed in the chick proventriculus. We suggest that LPS challenge increases the concentrations of AvBD2, 6, and 7 peptides in the proventriculus. The ND/IB vaccination may not affect their synthesis in response to LPS, one of the reasons for which may be because the expression of TLR4 was not affected by the vaccination. However, the ability to recognize unmethylated CpG-DNA in the proventriculus may be enhanced by the ND/IB vaccination since the expression of TLR21 was upregulated. Future studies are necessary to develop the vaccines that are useful to enhance the expression of innate immune members including TLRs. In the current study, we conclude that LPS derived from gram-negative bacteria modulates the innate immune system, including antimicrobial peptide synthesis in the proventriculus, probably to prevent pathogenic microbes from entering the intestine.
ACKNOWLEDGMENTS
This work was supported by a Grant-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science (No. 17H03904) to YY. This work was part of a joint research project between Hiroshima University and the Bioscience Research Laboratory, Sumitomo Chemical Co., Ltd.
Author contributions: YY designed the study, contributed to the animal treatments, histological studies, and writing of the manuscript. HK contributed to the LC/MS analysis. KT and YT worked on manuscript preparation, and TN and NI worked on RT-qPCR and ELISA analyses, respectively. All authors contributed to the discussion and preparation of the manuscript.
DISCLOSURES
The authors declare that they have no conflict of interest.
REFERENCES
- Abdel-Mageed A.M., Isobe N., Yoshimura Y. Effects of different TLR ligands on the expression of proinflammatory cytokines and avian beta-defensins in the uterine and vaginal tissues of laying hens. Vet. Immunol. Immunopathol. 2014;162:132–141. doi: 10.1016/j.vetimm.2014.10.013. [DOI] [PubMed] [Google Scholar]
- Achanta M., Sunkara L., Dai G., Bommineni Y., Jiang W., Zhang G. Tissue expression and developmental regulation of chicken cathelicidin antimicrobial peptides. J. Anim. Sci. Biotechnol. 2012;3:15. doi: 10.1186/2049-1891-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crhanova M., Hradecka H., Faldynova M., Matulova M., Havlickova H., Sisak F., Rychlik I. Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica Serovar Enteritidis infection. Infect. Immun. 2011;79:2755–2763. doi: 10.1128/IAI.01375-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuperus T., Coorens M., van Dijk A., Haagsman H. Avian host defense peptides. Develop. Comp. Immunol. 2013;41:352–369. doi: 10.1016/j.dci.2013.04.019. [DOI] [PubMed] [Google Scholar]
- de Bree L., Koeken V., Joosten L., Aaby P., Benn C., van Crevel R., Netea M. Non-specific effects of vaccines: current evidence and potential implications. Semin. Immunol. 2018;39:35–43. doi: 10.1016/j.smim.2018.06.002. [DOI] [PubMed] [Google Scholar]
- Elhamouly M., Nii T., Isobe N., Yoshimura Y. Age-related modulation of the isthmic and uterine mucosal innate immune defense system in laying hens. Poult. Sci. 2019;98:3022–3028. doi: 10.3382/ps/pez118. [DOI] [PubMed] [Google Scholar]
- Elhamouly M., Terada T., Nii T., Isobe N., Yoshimura Y. Innate antiviral immune response against infectious bronchitis virus and involvement of prostaglandin E2 in the uterine mucosa of laying hens. Theriogenology. 2018;110:122–129. doi: 10.1016/j.theriogenology.2017.12.047. [DOI] [PubMed] [Google Scholar]
- Grindstaff J.L., Brodie E.D., Ketterson E.D. Immune function across generations: integrating mechanism and evolutionary process in maternal antibody transmission. Proc. Biol. Sci. 2003;270:2309–2319. doi: 10.1098/rspb.2003.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y., Song W., Lee S., Lillehoj H. Differential gene expression profiles of beta-defensins in the crop, intestine, and spleen using a necrotic enteritis model in 2 commercial broiler chicken lines. Poult. Sci. 2012;91:1081–1088. doi: 10.3382/ps.2011-01948. [DOI] [PubMed] [Google Scholar]
- Kamimura T., Isobe N., Yoshimura Y. Effects of inhibitors of transcription factors, nuclear factor-kappa B and activator protein 1, on the expression of proinflammatory cytokines and chemokines induced by stimulation with Toll-like receptor ligands in hen vaginal cells. Poult. Sci. 2017;96:723–730. doi: 10.3382/ps/pew366. [DOI] [PubMed] [Google Scholar]
- Kang Y., Nii T., Isobe N., Yoshimura Y. Effects of the routine multiple vaccinations on the expression of innate immune molecules and induction of histone modification in ovarian cells of layer chicks. Poult. Sci. 2019;98:5127–5136. doi: 10.3382/ps/pez214. [DOI] [PubMed] [Google Scholar]
- Klasing K. Avian gastrointestinal anatomy and physiology. Semin. Avian Exotic Pet Med. 1999;8:42–50. [Google Scholar]
- Kleinnijenhuis J., Quintin J., Preijers F., Joosten L., Ifrim D., Saeed S., Jacobs C., van Loenhout J., de Jong D., Stunnenberg H., Xavier R., van der Meer J., van Crevel R., Netea M. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. U. S. A. 2012;109:17537–17542. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinnijenhuis J., Quintin J., Preijers F., Joosten L., Jacobs C., Xavier R., van der Meer J., van Crevel R., Netea M. BCG-induced trained immunity in NK cells: role for non-specific protection to infection. Clin. Immunol. 2014;155:213–219. doi: 10.1016/j.clim.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz J. Specific memory within innate immune systems. Trends Immunol. 2005;26:186–192. doi: 10.1016/j.it.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Lammers A., Wieland W.H., Kruijt L., Jansma A., Straetemans T., Schots A., den Hartog G., Parmentier H.K. Successive immunoglobulin and cytokine expression in the small intestine of juvenile chicken. Dev. Comp. Immunol. 2010;34:1254–1262. doi: 10.1016/j.dci.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Lawrence E.C., Arnaud-Battandier F., Grayson J., Koski I.R., Dooley N.J., Muchmore A.V., Blaese R.M. Ontogeny of humoral immune function in normal chickens: a comparison of immunoglobulin-secreting cells in bone marrow, spleen, lungs and intestine. Clin. Exp. Immunol. 1981;43:450–457. [PMC free article] [PubMed] [Google Scholar]
- Livak K., Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lyu W., Zhang L., Gong Y., Wen X., Xiao Y., Yang H. Developmental and tissue patterns of the basal expression of chicken avian. Biomed. Res. Int. 2020;2020 doi: 10.1155/2020/2567861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M., Mihaylova I., Leunis J.C. Increased serum IgA and IgM against LPS of enterobacteria in chronic fatigue syndrome (CFS): indication for the involvement of gram-negative enterobacteria in the etiology of CFS and for the presence of an increased gut-intestinal permeability. J. Affect. Disord. 2007;99:237–240. doi: 10.1016/j.jad.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Mageed A.M.A., Isobe N., Yoshimura Y. Expression of avian beta-defensins in the oviduct and effects of lipopolysaccharide on their expression in the vagina of hens. Poult. Sci. 2008;87:979–984. doi: 10.3382/ps.2007-00283. [DOI] [PubMed] [Google Scholar]
- Mohammed E., Isobe N., Yoshimura Y. Effects of probiotics on the expression of cathelicidins in response to stimulation by Salmonella minnesota lipopolysaccharides in the proventriculus and cecum of broiler chicks. J. Poult. Sci. 2016;53:298–304. doi: 10.2141/jpsa.0160064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed E.S.I., Radey R. Immunomodulation of antimicrobial peptides expression in the gastrointestinal tract by probiotics in response to stimulation by Salmonella minnesota lipopolysaccharides. Probiotics Antimicrob. Proteins. 2021;13:1157–1172. doi: 10.1007/s12602-021-09746-y. [DOI] [PubMed] [Google Scholar]
- Morimoto K., Kanda N., Shinde S., Isobe N. Effect of enterotoxigenic Escherichia coli vaccine on innate immune function of bovine mammary gland infused with lipopolysaccharide. J. Dairy Sci. 2012;95:5067–5074. doi: 10.3168/jds.2012-5498. [DOI] [PubMed] [Google Scholar]
- Netea M., Joosten L., Latz E., Mills K., Natoli G., Stunnenberg H., O'Neill L., Xavier R. Trained immunity: a program of innate immune memory in health and disease. Science. 2016;352 doi: 10.1126/science.aaf1098. (6284):aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea M., Quintin J., van der Meer J. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9:355–361. doi: 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Netea M.G., Domínguez-Andrés J., Barreiro L.B., Chavakis T., Divangahi M., Fuchs E., Joosten L.A.B., van der Meer J.W.M., Mhlanga M.M., Mulder W.J.M., Riksen N.P., Schlitzer A., Schultze J.L., Stabell Benn C., Sun J.C., Xavier R.J., Latz E. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020;20:375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata N., Kunisawa J., Hosomi K., Fujimoto Y., Mizote K., Kitayama N., Shimoyama A., Mimuro H., Sato S., Kishishita N., Ishii K.J., Fukase K., Kiyono H. Lymphoid tissue-resident Alcaligenes LPS induces IgA production without excessive inflammatory responses via weak TLR4 agonist activity. Mucosal Immunol. 2018;11:693–702. doi: 10.1038/mi.2017.103. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Nii T., Isobe N., Yoshimura Y. Effects of avian infectious bronchitis with Newcastle disease and Marek's disease vaccinations on the expression of toll-like receptors and avian β-defensins in the kidneys of broiler chicks. Poult. Sci. 2020;99:7092–7100. doi: 10.1016/j.psj.2020.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda Y., Mageed A.M.A., Isobe N., Yoshimura Y. Induction of avian beta-defensins by CpG oligodeoxynucleotides and proinflammatory cytokines in hen vaginal cells in vitro. Reproduction. 2013;145:621–631. doi: 10.1530/REP-12-0518. [DOI] [PubMed] [Google Scholar]
- St Paul M., Brisbin J., Abdul-Careem M., Sharif S. Immunostimulatory properties of Toll-like receptor ligands in chickens. Vet. Immunol. Immunopathol. 2013;152:191–199. doi: 10.1016/j.vetimm.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Subedi K., Isobe N., Nishibori M., Yoshimura Y. Changes in the expression of gallinacins, antimicrobial peptides, in ovarian follicles during follicular growth and in response to lipopolysaccharide in laying hens (Gallus domesticus) Reproduction. 2007;133:127–133. doi: 10.1530/REP-06-0083. [DOI] [PubMed] [Google Scholar]
- Terada T., Nii T., Isobe N., Yoshimura Y. Changes in the expression of avian beta-defensins (AvBDs) and proinflammatory cytokines and localization of AvBD2 in the intestine of broiler embryos and chicks during growth. J. Poult. Sci. 2018;55:280–287. doi: 10.2141/jpsa.0180022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada T., Nii T., Isobe N., Yoshimura Y. Effect of antibiotic treatment on microbial composition and expression of antimicrobial peptides and cytokines in the chick cecum. Poult. Sci. 2020;99:3385–3392. doi: 10.1016/j.psj.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada T., Nii T., Isobe N., Yoshimura Y. Effects of Toll-like receptor ligands on the expression of proinflammatory cytokines and avian beta-defensins in cultured chick intestine. J. Poult. Sci. 2020;57:210–222. doi: 10.2141/jpsa.0190086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer J., Joosten L., Riksen N., Netea M. Trained immunity: a smart way to enhance innate immune defence. Mol. Immunol. 2015;68:40–44. doi: 10.1016/j.molimm.2015.06.019. [DOI] [PubMed] [Google Scholar]
- Wang Y., Wang M., Shan A., Feng X. Avian host defense cathelicidins: structure, expression, biological functions, and potential therapeutic applications. Poult. Sci. 2020;99:6434–6445. doi: 10.1016/j.psj.2020.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura Y. Avian beta-defensins expression for the innate immune system in hen reproductive organs. Poult. Sci. 2015;94:804–809. doi: 10.3382/ps/peu021. [DOI] [PubMed] [Google Scholar]