Abstract
Background
The COVID-19 infection has impacted pregnancy outcomes; however, few studies have assessed the association between haematological parameters and virus-related pregnancy and neonatal outcomes. We hypothesised differences in routine haematology indices in pregnant and non-pregnant COVID-19 patients as well as COVID-19-negative pregnant subjects and observed neonatal outcomes in all pregnant populations. Further, we tested if pattern identification in the COVID-19 pregnant population would facilitate prediction of neonates with a poor Apgar score.
Methods
We tested our hypothesis in 327 patients (111 COVID-19-positive pregnant females, 169 COVID-19-negative pregnant females and 47 COVID-19-positive non-pregnant females) in whom standard routine laboratory indices were collected on admission.
Results
Pregnant COVID-19-positive patients exhibited higher WBC, neutrophil, monocyte counts as well as neutrophil/lymphocyte and neutrophil/eosinophil ratio compared to non-pregnant COVID-19-positive patients (p = 0.00001, p = 0.0023, p = 0.00002, p = 0.0402, p = 0.0161, p = 0.0352, respectively). Preterm delivery was more prevalent in COVID-19-positive pregnant patients accompanied with a significantly lower birth weight (2894.37 (± 67.50) g compared with 3194.16 (± 50.61) g, p = 0.02) in COVID-19-negative pregnant patients. The COVID-19-Induced Immunity Response (CIIR) was defined as (WBC × neutrophil) / eosinophil; Apgar scores were significantly and inversely correlated with the CIIR index (r =—0.162).
Interpretation
Pregnancy appears to give rise to an increased immune response to COVID-19 which appears to protect the mother, however may give rise to complications during labour as well as neonatal concerns. CIIR is a simple metric that predicts neonatal distress to aid clinicians in determining the prognosis of COVID-19 and help provide early intensive intervention to reduce complications.
Keywords: COVID-19, Pregnancy, Biomarkers, Perinatal health
Introduction
Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) is a ribonucleic acid (RNA) respiratory virus responsible for the Coronavirus disease 2019 (COVID-19) pandemic. COVID-19 presentation ranges from asymptomatic, through to mild influenza-like symptoms to multiple organ failure and death [1, 2]. The rapid spread of the virus overwhelmed healthcare systems worldwide, consequently vulnerable and high-risk populations including pregnant women were identified to optimise their management [3]. Pregnant women are particularly prone to respiratory pathogens, like SARS-CoV-2, owing to physiological changes during pregnancy such as increased oxygen requirements and diaphragm elevation making pregnant women susceptible to hypoxia [4].
Pregnant women are considered one of the most unique groups owing to the infection affecting the mothers and their neonates. There is accumulating evidence on pregnant women with COVID-19 suggesting whilst pregnant women are not at an increased risk of morbidity, there is an increased risk for intensive care provision, need for intubation and neonatal distress [5–7]. Studies suggest risk of acute infections is higher in the late stages of pregnancy [8, 9] with an increased incidence of caesarean delivery due to obstetric indications. Furthermore, foetal distress has also been noted in several studies [6, 10, 11].
Hospitals across the world have been collecting data prospectively since patients with COVID-19 first presented, looking for patterns in clinical findings and routine laboratory markers that may predict risk of a poor health outcome in a variety of patient groups. Haematological blood parameters and indices including differential white blood cell count, plasma platelets concentration, platelets and red blood cells distribution width, neutrophil/lymphocyte ratio (NLR), neutrophil/basophil ratio (NBR), neutrophils/ eosinophils (NER) ratio are part of the well-established parameters of inflammatory responses used as simple and reliable prognostic indicators used guide interventions [12–15]. A meta-analysis observed COVID-19-positive pregnant patients to have elevated neutrophils (71.4%; 95% CI 38.5–90.9), elevated CRP (67.7%; 95% 50.6–81.1), and low haemoglobin (57.3%; 95% CI 26.0–87.8) as well as a preterm birth rate of 34.2%, and caesarean section rate of 82.7% [16]. However, few studies have assessed the association between the haematological parameters in COVID-19-positive pregnant patients and virus-related pregnancy and neonatal outcomes [17].
This study aimed to analyse the haematological laboratory parameters, clinical manifestations, maternal and perinatal outcomes in COVID-19 infected pregnant women and compare with the non-pregnant population of reproductive age. We hypothesised that certain routine haematological indices may be altered in those pregnant patients with COVID-19 in whom neonates may consequently be adversely affected, and that pattern identification would facilitate prediction of those at high risk of severe disease and foetal distress.
Methods
Study design and participants
This retrospective cohort study included all women (pregnant and not pregnant) with a confirmed diagnosis of COVID-19 as well as a cohort of randomly selected group of pregnant women without COVID-19 infection admitted between 3rd of February 2020 and the 31st of March 2021 at a single centre National Health Trust in the UK.
Data from 380 participants were initially screened for study inclusion, of which 53 individuals were excluded due to incomplete of plasma biomarkers analysis. All patient results were collected within 3 days of COVID-19 diagnosis and, for COVID-19-negative participants, samples were collected during pregnancy booking bloods (less than 10 weeks and again at 24 weeks) following the UK antenatal care national guidelines [18].
The remaining 327 participants were included in the final analysis and classified into four groups. Group A: COVID-19-negative pregnant females (111 participants); Group B: group A data after COVID-19 infection (111 participants); Group C: COVID-19-negative pregnant females (169 participants) and Group D: COVID-19-positive non-pregnant females (47 participants of reproductive age) (Fig. 1).
Fig. 1.
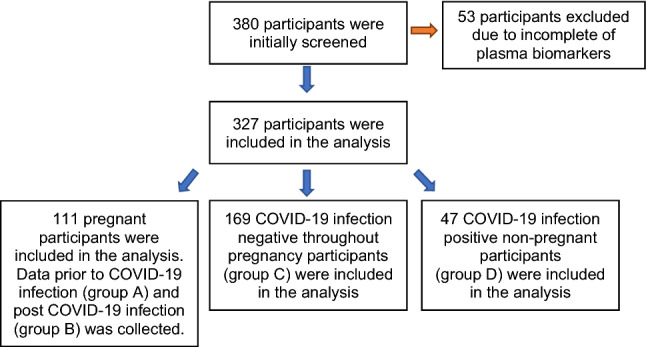
Study participant’s selection process and grouping for analysis
Clinical data, laboratory tests, pregnancy outcomes, and foetus outcomes were collected from the hospital’s electronic medical records. Study inclusion criteria were defined as pregnant women who acquired COVID-19 infection during their pregnancy confirmed by a real-time polymerase chain reaction (RT-PCR) (repeated twice) and age-matched non-pregnant COVID-19-positive individuals. Individuals diagnosed with any haematological pathologies defined as anaemia, blood cancers and haemorrhagic conditions or any condition or medications that can suppress bone marrow function were excluded from the study. Results from patients with incomplete medical records were excluded from the final analysis.
The used treatment strategy for the enrolled patients followed the recommended National Health Service (NHS, UK) published COVID-19 management protocols. The study was sponsored by the research and development committee of the Trust (IRAS number 289571) and had ethical approval as a part of our ongoing COVID-19 study of hospital patients. This study was designed and conducted in accordance with the tenets of the Declaration of Helsinki.
Laboratory procedures
Patients were identified as COVID-19 positive by Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) from throat/nose swabs on a ROCHE COBAS™ analyser (Roche Ltd, Basel, Switzerland). Nasopharyngeal or oropharyngeal samples were collected from patients for the detection of SARS CoV-2 RNA. The Xpert® Xpress SARS-CoV-2 (Cepheid Ltd) real-time RT-PCR assay was performed to achieve qualitative detection of SARS-CoV-2 RNA.
A Sysmex™-XN (Sysmex LTD, Tokyo, Japan) automated haematology analyser was used for routine complete blood count analysis which included haemoglobin, platelets, white blood cells (WBCs), neutrophils, lymphocytes, monocytes, eosinophils, basophils, neutrophils /lymphocytes, neutrophils /eosinophils and finally WBCs* neutrophils /eosinophils termed the CIIR factor. This novel factor was developed to amplify differences within the patient cohort and then observe critical differences between patient groups that may otherwise be lost by analysing biomarker on their own.
Sample size and statistical analysis
As the study design was multifactorial in nature, it was calculated that a sample size of n = 327 is sufficient to provide 95% power at an alpha level of 0.05. All analyses were performed using Statistica® software (version 13.3, StatSoft Inc., Tusla, OK, USA). Distributions of continuous variables were determined by the Shapiro–Wilks test. In cases where the normality of the data could not be confirmed, appropriate data transformations were made, or non-parametric statistical alternatives were used, and categorical variables are expressed as percentages. Univariate associations were determined using Pearson’s (normally distributed data) or Spearman’s method (non-normally distributed data), and forward stepwise regression analyses were performed to test the influence of measured clinical outcomes and the circulatory biomarkers. Differences between groups were subsequently assessed using one-way ANOVA or ANCOVA, as appropriate, followed by Tukey’s post hoc analysis. Comparisons between pregnant and non-pregnant groups were measured using the χ2 or Fisher exact test for categorical variables, whereas the t-test or Mann–Whitney U test was used for continuous variables. p < 0.05 was considered statistically significant.
Results
Clinical characteristics
General demographics of the study population are presented in Table 1. There were no significant differences in age, systolic blood pressure, diastolic blood pressure, pulse rate and respiratory rate between all the study groups (all p > 0.05). The average age of pregnant patients was 30 years compared with 35.6 years for non-pregnant patients. Of the infected pregnant group, 109 developed mild to moderate respiratory symptoms, and two were admitted to the intensive care unit (ICU). Specifically, one of these patients developed type-1 respiratory failure requiring intubation and extra-corporeal mechanical ventilation (ECMO) and underwent a prolonged recovery period after developing pulmonary fibrosis. She delivered a healthy baby via an emergency caesarean section. The second mother admitted to ICU following a normal vaginal delivery. However, she died of multi-organ failure due to acute fatty liver which was not related to the COVID-19 infection. In both these patients admitted to the ICU, babies survived. Finally, two stillbirths were recorded from the whole cohort of COVID-19 patients. In the first case, documented cause of death was a combination of vascular malperfusion and COVID-19-related placentitis causing premature placental separation. In the second case, there was no documented cause of death; however, foetal and maternal vascular malperfusion may be linked with umbilical cord hypercoiling and stricture as well as prolonged meconium exposure.
Table 1.
Demographic and clinical observations findings of patients on admission
| Pregnant | Non-pregnant | p value | |||
|---|---|---|---|---|---|
| (Group A) Prior to COVID-19 infection (111 patient) |
(Group B) Post COVID-19 infection (111 patient) |
(Group C) COVID-19 Negative throughout pregnancy (169 patient) |
(Group D) COVID-19-Positive patients (47 patient) |
||
| Age | 30 (0.56) | 29.42 (0.61) | 29.3 (0.45) | 35.61 (1.63) | 0.80111 |
| SBP | 118.55 (15.67) | 119.95 (16.50) | 119.10 (17.34) | 119.06 (16.27) | 0.65833 |
| DBP | 72.56 (9.63) | 75.34 (10.50) | 74.65 (8.80) | 76.12 (11.20) | 0.94454 |
| HR | 90.52 (15.44) | 92.71 (17.24) | 91.85 (19.06) | 93.32 (17.24) | 0.32467 |
| RR | 16.02 (6. 80) | 15.90 (6.28) | 14.90 (5.88) | 16.82 (6.28) | 0.87247 |
| O2 Saturation | 98% | 99% | 97% | 99% | 0.21457 |
Data are presented as mean (standard deviation), p values were calculated by a one-way ANOVA or ANCOVA, as appropriate, followed by Tukey’s post hoc analysis. Comparisons between pregnant and non-pregnant groups were measured using the χ2 or Fisher exact test for categorical variables, whereas the t-test or Mann–Whitney U test was used for continuous variables
SBP systolic blood pressure, DBP diastolic blood pressure, HR heart rate, RR respiratory rate, O2 saturation oxygen saturation
*Significant p values are indicated where p < 0.05 was considered significant
Haematological findings
Peripheral blood analysis showed statistically lower haemoglobin, platelets, lymphocytes, and eosinophils counts in pregnant women post COVID-19 infection compared to their results before acquiring the infection (p = 0.00002, p = 0.00182, p = 0.02672, p = 0.00172, respectively) (Table 2). On the other hand, WBCs, neutrophils and monocytes counts were significantly higher in pregnant women after COVID-19 infection than before the infection (p = 0.00001, p = 0.00001, p = 0.00046, respectively). Similarly, other inflammatory blood cell parameters including neutrophils/lymphocytes (NLR) and neutrophils/ eosinophils ratios (NER) were significantly higher (p = 0.00001 and p = 0.00001, respectively) in pregnant women after acquiring the infection compared to their normal pregnancy baseline results. Additionally, the COVID-19-induced immunity response (CIIR) index represented as WBCs × (neutrophils/eosinophils) was higher in COVID-19-positive pregnant women after the infection compared to before infection (p = 0.00001). There were no statistically significant differences in any haematological blood cell parameters between pregnant women before the COVID-19 infection and pregnant women who did not obtain the infection during their pregnancy (all p > 0.05).
Table 2.
Haematological findings of the study population
| Pregnancy status | Pregnant | Pregnant |
p value (A vs B) |
Pregnant |
p value (A vs B vs C) |
Post hoc analysis | Non-pregnant |
p value (B vs D) |
|---|---|---|---|---|---|---|---|---|
| COVID-19 status |
(Group A) Prior to COVID-19 infection |
(Group B) Post COVID-19 infection |
(Group C) COVID-19 Negative throughout pregnancy |
(Group D) COVID-19-Positive patients |
||||
| Number of participants | 111 | 111 | - | 169 | 47 | – | ||
|
Haemoglobin (115–160 g/L) |
122.98 (1.32) | 114.40 (1.22) | 0.00002* | 122.21 (0.98) | 0.0001* | A = C > B | 119.15 (3.55) | 0.8839 |
|
Platelets (150–450 × 109/L) |
285.67 (6.91) | 251.71 (6.43) | 0.00182* | 282.12 (5.20) | .03144* | A = C > B | 273.92 (18.74) | 0.8181 |
|
WBCs (4–11 × 109/L) |
8.72 (0.31) | 11.022 (0.29) | 0.00001* | 8.89 (0.24) | 0.001* | A = C < B | 5.93 (0.85) | 0.00001* |
|
Neutrophil (1.7–7.5 × 109/L) |
6.03 (0.28) | 8.51 (0.26) | 0.00001* | 6.04 (0.22) | 0.001* | A = C < B | 4.008 (0.78) | 0.0023* |
|
Lymphocytes (1–4 × 109/L) |
1.95 (0.06) | 1.72 (0.056) | 0.02672* | 2.032 (0.045) | 0.0349* | A = C > B | 1.47 (0.16) | 0.62849 |
|
Monocytes (0.2*80 × 109/L) |
0.57 (0.023) | 0.70 (0.02) | 0.00046* | 0.60 (0.02) | 0.0001* | A = C < B | 0.38 (0.062) | 0.00002* |
|
Eosinophils (> 0.5 × 109/L) |
0.15 (0.017) | 0.07 (0.015) | 0.00172* | 0.17 (0.013) | 0.004* | A = C > B | 0.058 (0.046) | 0.9999 |
|
Basophils (> 0.1 × 109/L) |
0.031 (0.0018) | 0.03 (0.002) | 0.71904 | 0.035 (0.001) | 0.861 | A = B = C | 0.018 (0.005) | 0.5174 |
| Neut/Lymph | 3.32 (0.27) | 5.91 (0.25) | 0.00001* | 3.13 (0.21) | 0.0001* | A = C < B | 3.90 (0.72) | 0.0402* |
| Neut/Eso | 76.65 (15.38) | 225.9 (15.71) | 0.00001* | 84.76 (11.53) | 0.0203* | A = C < B | 126.72 (49.97) | 0.2307 |
|
WBCs*Neut/Eos (CIIR) |
650.92 (219.91) | 2568.95 (224.69) | 0.00001* | 765.97 (164.88) | 0.0001* | A = C < B | 802.29 (714.48) | 0.0352* |
Data are presented as means (standard deviation), p values were calculated by one-way ANOVA or ANCOVA, as appropriate, followed by Tukey’s post hoc analysis
WBCs white blood cells, Neut/Lymph Neutrophil/Lymphocytes, Neut/Baso Neutrophil/ Basophils, Neut/Eso Neutrophil/ Eosinophils
*Significant p values are indicated where p < 0.05 was considered significant
Non-pregnant COVID-19-positive patients exhibited lower WBCs, neutrophils, monocyte counts and neutrophil/lymphocyte ratio, WBCs*neutrophil/ eosinophil ratios compared to pregnant COVID-19-positive females (p = 0.00001, p = 0.0023, p = 0.00002, p = 0.0402, p = 0.0352, respectively). Furthermore, no significant difference was found between basophils counts among all the study groups (all p > 0.05).
Delivery and neonatal outcomes
Of the COVID-19-positive group, 73% delivered by vaginal delivery and 27% by caesarean section compared to 66% and 34% in the COVID-19-negative group (p = 0.067) (Table 3). Patients who acquired the infection in the third trimester of pregnancy delivered 4.41 (± 1.90) days preterm compared to 24 (± 10.094) days in females who acquired the infection in the second trimester (p = 0.044). Indications for early delivery were not reported; however, preterm birth was prevalent in the COVID-19 patients regardless of the severity of the disease. We did not collect these data for COVID-19-negative patients. Data on pregnancy complications from COVID-19 are limited; however, evidence suggests that complications are more common in patients who acquired the infection in the third trimester compared to the second trimester. Among the assessed pregnancies, women affected by COVID-19 in the third trimester showed incidences of maternal haemorrhages (three patients), gestational hypertension (four patients) and preeclampsia (one patient). Pregnancy complications were not observed in the pregnancies affected by COVID-19 infection in the second trimester.
Table 3.
Delivery and neonate outcomes up to 5 min of birth
| COVID-19-positive pregnancy (111 patients) |
COVID-19-negative pregnancy (169 patients) |
p value | |
|---|---|---|---|
| Vaginal delivery | 56 (73%) | 111 (66%) | |
| Spontaneous | 37 (50%) | 93 (55%) | |
| Induced | 19 (27%) | 18 (11%) | 0.067 |
| C-Section | 18 (27%) | 58 (34%) | |
| Early delivery (days) | – | ||
| 3rd Trimester | − 4.41 (1.90) | – | |
| 2nd Trimester | − 24 (10.094) | – | |
| Birth Weight (g) | 2894.37 (67.50) | 3194.16 (50.61) | 0.02379* |
| Apgar scale | |||
| 01 | 7.66 (0.21) | 8.90 (0.16) | 0.00741* |
| 05 | 8.4 (0.16) | 8.88 (0.12) | 0.02016* |
Data are presented as mean (standard deviation), p values were calculated by a t-test, (−) represents data not obtained
*Significant p values are indicated where p < 0.05 was considered significant
Pregnant participants in all the groups delivered live-born neonates with two stillborn babies in the COVID-19-positive group. In the 280 born babies, no cases of vertical transmission were reported, and no sets of twins were delivered. The average weight of the babies in the COVID-positive group was significantly lower than the COVID-19-negative group (2894.37 (± 67.50) g compared with 3194.16 (± 50.61) g, p = 0.02379) (Table 3).
Using the Apgar risk score to evaluate the newborn babies’ health after 1 and 5 min, neonates of COVID-19-negative mothers showed higher Apgar scores compared to that of COVID-19-positive mothers (p = 0.00741 and p = 0.02016, respectively). Twenty-one babies in the COVID-19-positive group scored six or less in the Apgar risk score. The Apgar risk scores (5 min) were significantly inversely correlated with the COVID-19-induced immunity response factor (r = − 0.162, Fig. 2).
Fig. 2.
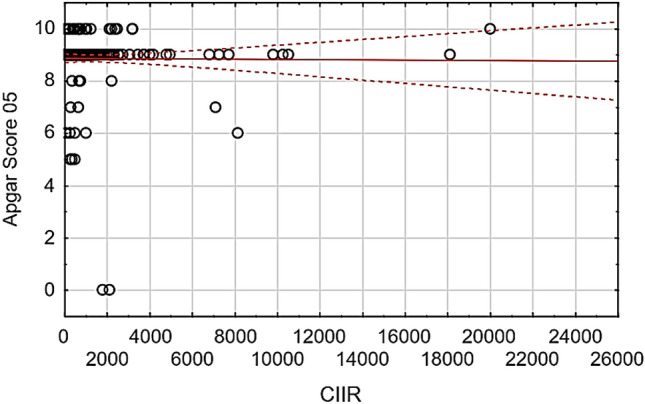
Correlation between the Apgar Score after five minutes and the COVID-19-induced immunity response factor. Univariate associations were determined Spearman’s method (non-normally distributed data), and forward stepwise regression analyses were performed to test the influence of measured clinical outcomes and the circulatory biomarkers
Discussion
The results of this cohort study provide several important insights of the impact of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) on pregnancy, the impact of pregnancy on the course of the disease and the implications of the virus on pregnancy outcomes observed from a single centre in the West Midlands, UK. Pregnancy caused an increased immune response to COVID-19; however, infection led to an increase in preterm delivery, a decrease in neonatal birth weight and an increase in neonatal distress defined by the Apgar score. We suggest the use of a novel COVID-19 Induced Immunity Response (CIIR) index as a practical indicator for neonatal distress.
Pregnancy is often considered a partial immunosuppressive state [19]; hence, the interaction of specific pathogens with the foetal/placental unit and the maternal response depending on pregnancy gestation should be studied to optimise prophylaxis or therapy. Similar to previous findings, our study found leucocytosis and lymphopenia are prevalent in COVID-19-positive women compared to non-pregnant and COVID-19-negative individuals along with neutrophilia [4]. However, in addition to this, our study observed a more pronounced immune response in pregnant COVID-19 patients compared with non-pregnant. In more severe cases, we noticed uncontrolled inflammatory and immune responses characterised by high neutrophil/lymphocyte (NLR) and neutrophil/eosinophil (NER) ratios and progression to pregnancy complications such as haemorrhage, hypertension and preeclampsia was positively associated with increased NLR. Furthermore, we tested a novel index termed COVID-19-induced immunity response factor (CIIR, defined as (WBC × neutrophil)/ eosinophil) and compared to non-pregnant COVID-19-positive women, to their pregnant counterparts who showed a higher CIIR. Eosinophil count decreased more significantly than any other white cell fraction indicating its consumption/activity in the fight against the infection whilst the WBCs yield the total power of the immune response against COVID-19 infection. This immune response power, CIIR, was significantly enhanced in pregnant patients to protect the growing embryo. This suggests pregnancy resulted in an increased immune response perhaps to protect the mother. It has previously been suggested that there is an increased emphasis on infection prevention during pregnancy and that maternal immunity strives to decrease inflammatory events so as not to expose the foetus to potentially dangerous inflammatory signals [19].
Despite the increase in immune response observed by the laboratory markers, no significant difference in clinical observations was noted regardless of gestational age. The increased levels of circulating progesterone and oestrogen during pregnancy increase the tidal volume of the lung, arterial partial oxygen saturation, and minute ventilation, which helps the lungs to be more flexible compromising any added stresses [20]. The immunomodulatory properties of the progesterone positively impact many immune pathways, including immune-mediated injuries [21]. Furthermore, during pregnancy, the circulating levels of interleukin-1 receptor antagonist and tumour necrosis factor-α (TNF) receptor increase while the plasma levels of interleukin-1β and TNF‐α decrease, which adds to the physiological protective response against the virus [22]. Adding to this, as it was reported that the COVID-19 virus activates innate and adaptive immune responses, pregnant women could be protected by pregnancy-associated immunomodulation [23]. This further supports our earlier suggestion of pregnancy inducing a hyper-protective state for the mother.
Considering the pro-inflammatory state accompanying pregnancy in the first and third trimesters, this study found higher pregnancy-associated complications in individuals who acquired the infection in the third trimester of pregnancy compared to the second trimester [24]. Nonetheless women gave birth through both vaginal and caesarean section delivery with the percentage of women delivering by caesarean section similar in the COVID-19-positive and -negative females. This indeed reflects the clinical stability of the COVID-19-positive cases with no evidence for any potential respiratory complications during labour to promote an elective caesarean decision. In our study, patients with SARS-CoV-2 had a higher incidence of preterm delivery, especially individuals who acquired the infection in the second trimester. Our results support the observations during early pandemic case series and reports describing preterm deliveries and early ruptures of the membranes in COVID-19-infected women [6, 11, 25]. One suggested explanation of this is the well-known link between the activation of the inflammatory pathways resulting in inflammation of the placenta, termed acute chorioamnionitis and the premature rupture of the membranes and preterm deliveries [26, 27]. In our opinion, the activation of these inflammatory pathways (macrophages and IL-6) in COVID-19 infections and the evidence from other studies highlighting Interleukins and cytokines as markers of preterm delivery in normal pregnancies [28–31] are a case for further evaluation of this association in COVID-19-positive pregnant women.
Our study observed lower birth weights and Apgar scores following COVID-19 maternal infection. Since an increase in preterm delivery was observed in COVID-19-positive females, it is expected to see a reduction in neonatal birth weight. Premature babies are at increased risk of sustaining a range of short and long term complications of prematurity [32, 33]. The Apgar score is not intended for prediction of outcome beyond the immediate postnatal period; however, as low scores correlate with prenatal and perinatal issues, many studies have examined the relationship between low scores (< 7), duration of low scores and subsequent respiratory distress [34], neurologic disability and poor cognitive function [35, 36]. Importantly, the CIIR was inversely correlated with the neonates Apgar risk score five minutes after birth suggesting this marker may be of use in predicting neonates that will require intensive care. Currently, there is growing evidence supporting the COVID-19-induced intrauterine inflammation can cause placental histopathological changes and adverse obstetric and neonatal events, including maternal and foetal vascular malperfusion, infarction, chorioamnionitis and umbilical arteritis [37, 38]. This involvement of the placenta in COVID-19 infection and its consequent complications can explain our findings and support our recommendation to use the (CIIR) ratio as an early indicator of COVID-19-induced maternal and foetal complications.
Overall, in COVID-19-infected pregnant patients, we observed 1.8% were admitted to ICU and 0.9% died, furthermore, 1.8% of neonates died. We did not find COVID-19 listed as a cause of death; however, infection may have precipitated the outcome. A study collating routine clinical data (in select cases, samples for research and development) from a network of over 300 NHS hospitals across the UK between March 2020 and February 2021 observed, of symptomatic pregnant women hospitalised with COVID-19, 10% received critical care and 1% died [39]. Furthermore, findings from a study collating data from UK and USA registries of pregnancies with COVID-19 infection observed the proportions of pregnancies affected by stillbirth was not higher than historical and contemporaneous UK and USA data. They also observed maternal death was uncommon; however, the rate was higher than expected based on UK and USA population data, owing to under ascertainment of patients affected by mild or asymptomatic infection [40]. A systematic review considering neonatal outcomes associated with COVID-19-infected pregnancies found the incidence of preterm births, low birth weight, C-section, neonatal ICU admission appear higher than the general population [41]. Thus, it seems that infection with COVID-19 does not lead to overwhelming maternal complications or increased mortality in the pregnant patient, although admission to ICU is variable. Furthermore, infection could lead to neonatal complications further highlighting the potential benefits of our proposed CIIR score to predict which neonates need intensive care.
Our study has limitations. First, due to the retrospective study design, not all laboratory tests were carried out or recorded in all patients, therefore, their role might be underestimated in predicting in-hospital outcomes. Second, lack of information regarding drug treatment might have also affected the clinical outcomes in some patients. Further, information of any treatment for chronic conditions was not collected in this study and could have affected clinical outcomes. Third, interpretation of our findings might be limited by the sample size. However, by including all pregnant COVID-19-positive patient across the trust, we believe our study population is representative of cases diagnosed and treated in West Birmingham. Finally, serological data, asymptomatic or overlooked COVID-19 subjects were missing from the study and we did not observe any pregnant women infected with COVID-19 in the first trimester.
SARS-CoV-2 is a complex disease; understanding its impact on both the mother and the foetus is crucial to protect both from adverse effects. Our study describes a range of haematological blood parameters and clinical findings that can help early detection of patients at risk of developing maternal and foetal complications. Timely reporting of pregnancy status, exposure time, symptoms, clinical presentation, and laboratory abnormalities are critical in developing appropriate evaluation and management plans for pregnant patients with COVID-19 infection. The authors of this study would recommend routine evaluation of the inflammatory blood cell parameters and CIIR ratio in assessing COVID-19-positive pregnant patients to help predict maternal and neonatal complications. These data can help improve the management of COVID-19-infected pregnant patients and their neonates whilst building clinical guidance for treating COVID-19 during pregnancy.
Acknowledgements
The authors would like to thank Martin Cammies, for his kind assistance in gathering data on Pathology Telepath IT system, thanks Adela Kinsella and Samantha Pell for acquiring maternity data from Badger net IT system as well as Kuldeep Singh for his assistance in data processing.
Author contributions
FW, MM and SS designed the study. SS collected the data. HS analysed the data. HS, MM, SS and FW drafted the manuscript. SA and KW critically revised the manuscript. All authors gave final approval for the version to be published.
Funding
No funds, grants, or other support was received.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics approval
This study was granted ethical approval by the Integrated Research Approval System (289571) and sponsored by research and development committee of the Trust site (20Haem60).
Consent to participate
This is a retrospective study.
Consent for publication
Not applicable.
Footnotes
M. Marwah and H. Shokr have contributed an equal amount to this work
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet Elsevier. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Transmission of SARS-CoV-2: implications for infection prevention precautions [Internet]. [cited 2021 Aug 1]. Available from: https://www.who.int/publications/i/item/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations
- 3.Krouse HJ. COVID-19 and the Widening Gap in Health Inequity: 10.1177/0194599820926463 [Internet]. SAGE PublicationsSage CA: Los Angeles, CA; 2020 [cited 2021 Jul 27];163:65–6. Available from: https://journals.sagepub.com/doi/full/10.1177/0194599820926463 [DOI] [PubMed]
- 4.Liu D, Li L, Wu X, Zheng D, Wang J, Yang L, et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. 10.2214/AJR2023072 [Internet]. American Roentgen Ray Society; 2020 [cited 2021 Jul 26];215:127–32. Available from: www.ajronline.org [DOI] [PubMed]
- 5.Zambrano LD. Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status — United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep [Internet]. Centers for Disease Control MMWR Office; 2020 [cited 2021 Aug 1];69:1641–7. Available from: https://www.cdc.gov/mmwr/volumes/69/wr/mm6944e3.htm [DOI] [PMC free article] [PubMed]
- 6.Zhu H, Wang L, Fang C, Peng S, Zhang L, Chang G, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr [Internet]. AME Publications; 2020 [cited 2021 Jul 25];9:51. Available from: /pmc/articles/PMC7036645/ [DOI] [PMC free article] [PubMed]
- 7.Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet [Internet]. Elsevier; 2020 [cited 2021 Aug 1];395:809–15. http://www.thelancet.com/article/S0140673620303603/fulltext [DOI] [PMC free article] [PubMed]
- 8.Knight M, Bunch K, Vousden N, Morris E, Simpson N, Gale C, et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ [Internet]. British Medical Journal Publishing Group; 2020 [cited 2021 Aug 1];369. https://www.bmj.com/content/369/bmj.m2107 [DOI] [PMC free article] [PubMed]
- 9.E M, D E, RM V, P O, E M. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol [Internet]. Ultrasound Obstet Gynecol; 2020 [cited 2021 Aug 1];55:586–92. https://pubmed.ncbi.nlm.nih.gov/32180292/ [DOI] [PubMed]
- 10.Breslin N, Baptiste C, Gyamfi-Bannerman C, Miller R, Martinez R, Bernstein K, et al. Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM [Internet]. Elsevier; 2020 [cited 2021 Aug 1];2:100118. http://www.ajogmfm.org/article/S2589933320300483/fulltext [DOI] [PMC free article] [PubMed]
- 11.Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet Elsevier. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao D, Zhou F, Luo L, al. et. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet Haematol. 2020;7:e671–8. [DOI] [PMC free article] [PubMed]
- 13.Ciccullo A, Borghetti A, Verme LZD, Tosoni A, Lombardi F, Garcovich M, et al. Neutrophil-to-lymphocyte ratio and clinical outcome in COVID-19: a report from the Italian front line. Int J Antimicrob Agents [Internet]. Elsevier; 2020 [cited 2021 Jul 27];56:106017. Available from: /pmc/articles/PMC7211594/ [DOI] [PMC free article] [PubMed]
- 14.Dahlawi H. Changes in haematological parameters among covid-19 patients. Int J Curr Res Rev. Radiance Research Academy; 2020;12:2–4.
- 15.Bawiskar N, Andhale A, Acharya S, Kumar S, Shukla S. Haematological manifestations of COVID 19 and their prognostic significance-a cross-sectional study. Int J Res Pharm Sci [Internet]. J. K. Welfare and Pharmascope Foundation; 2020 [cited 2021 Jul 27];11:918–22. https://covid19.elsevierpure.com/en/publications/haematological-manifestations-of-covid-19-and-their-prognostic-si
- 16.Zhang C, Chu H, Pei YV, Zhang J. Laboratory Effects of COVID-19 Infection in Pregnant Women and Their Newborns: A Systematic Review and Meta-Analysis. Front Glob Women’s Heal. Frontiers; 2021;0:17. [DOI] [PMC free article] [PubMed]
- 17.Pirkle CM. Evidence based care for pregnant women with covid-19. BMJ [Internet]. British Medical Journal Publishing Group; 2020 [cited 2021 Jul 27];370. https://www.bmj.com/content/370/bmj.m3510 [DOI] [PubMed]
- 18.Overview | Antenatal care | Guidance | NICE [Internet]. [cited 2022 Jan 27]. Available from: https://www.nice.org.uk/guidance/ng201
- 19.Mor G, Cardenas I. REVIEW ARTICLE: The Immune System in Pregnancy: A Unique Complexity. Am J Reprod Immunol [Internet]. John Wiley & Sons, Ltd; 2010 [cited 2021 Jul 22];63:425–33. https://onlinelibrary.wiley.com/doi/full/10.1111/j.1600-0897.2010.00836.x [DOI] [PMC free article] [PubMed]
- 20.LoMauro A, Aliverti A. Respiratory physiology of pregnancy. Breathe [Internet]. European Respiratory Society; 2015 [cited 2021 Jul 21];11:297–301. https://breathe.ersjournals.com/content/11/4/297 [DOI] [PMC free article] [PubMed]
- 21.Mauvais-Jarvis F, Klein SL, Levin ER. Estradiol, Progesterone, Immunomodulation, and COVID-19 Outcomes. Endocrinology [Internet]. Oxford Academic; 2020 [cited 2021 Aug 3];161:1–8. https://academic.oup.com/endo/article/161/9/bqaa127/5879027 [DOI] [PMC free article] [PubMed]
- 22.Bouchghoul H, Vigoureux S. Do pregnant women have protective immunity against COVID‐19? Bjog [Internet]. Wiley-Blackwell; 2020 [cited 2021 Jul 22];127:1298–9. /pmc/articles/PMC7361529/ [DOI] [PMC free article] [PubMed]
- 23.Hegde UC. Immunomodulation of the mother during pregnancy. Med Hypotheses Churchill Livingstone. 1991;35:159–164. doi: 10.1016/0306-9877(91)90042-W. [DOI] [PubMed] [Google Scholar]
- 24.Karimi-Zarchi M, Neamatzadeh H, Dastgheib SA, Abbasi H, Mirjalili SR, Behforouz A, et al. Vertical Transmission of Coronavirus Disease 19 (COVID-19) from Infected Pregnant Mothers to Neonates: A Review. 10.1080/1551381520201747120 [Internet]. Taylor & Francis; 2020 [cited 2021 Jul 22];39:246–50. https://www.tandfonline.com/doi/abs/10.1080/15513815.2020.1747120 [DOI] [PMC free article] [PubMed]
- 25.L Z, Y J, M W, BH C, XC Z, J L, et al. [Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province]. Zhonghua Fu Chan Ke Za Zhi [Internet]. NLM (Medline); 2020 [cited 2021 Jul 25];55:166–71. https://europepmc.org/article/med/32145714 [DOI] [PubMed]
- 26.Alexander JM, Cox SM. Clinical course of premature rupture of the membranes. Semin Perinatol. W.B. Saunders; 1996;20:369–74. [DOI] [PubMed]
- 27.Lannon SMR, Vanderhoeven JP, Eschenbach DA, Gravett MG, Waldorf KMA. Synergy and Interactions Among Biological Pathways Leading to Preterm Premature Rupture of Membranes: 10.1177/1933719114534535 [Internet]. SAGE PublicationsSage CA: Los Angeles, CA; 2014 [cited 2021 Jul 25];21:1215–27. Available from: https://journals.sagepub.com/doi/full/10.1177/1933719114534535?casa_token=MpFbP72lL8IAAAAA%3AM_jgm15PVR19GG8KWITumh-TryrzOyAALBuRDpBLEKiLFzOAoPzBcyScwsXh-Fx4QMngH_GYUJM [DOI] [PMC free article] [PubMed]
- 28.Yoneda S, Shiozaki A, Yoneda N, Shima T, Ito M, Yamanaka M, et al. Prediction of exact delivery time in patients with preterm labor and intact membranes at admission by amniotic fluid interleukin-8 level and preterm labor index. J Obstet Gynaecol Res. 2011;37:861–866. doi: 10.1111/j.1447-0756.2010.01453.x. [DOI] [PubMed] [Google Scholar]
- 29.Dudley DJ, Hunter C, Mitchell MD, Varner MW. Clinical value of amniotic fluid interleukin-6 determinations in the management of preterm labour. BJOG An Int J Obstet Gynaecol. 1994;101:592–597. doi: 10.1111/j.1471-0528.1994.tb13649.x. [DOI] [PubMed] [Google Scholar]
- 30.Jacobsson B, Mattsby-Baltzer I, Andersch B, Bokström H, Holst RM, Wennerholm UB, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women in preterm labor. Acta Obstet Gynecol Scand. 2003;82:120–128. doi: 10.1034/j.1600-0412.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 31.ROMERO R, YOON BH, KENNEY JS, GOMEZ R, ALLISON AC, SEHGAL PB. Amniotic Fluid Interleukin-6 Determinations Are of Diagnostic and Prognostic Value in Preterm Labor. Am J Reprod Immunol [Internet]. John Wiley & Sons, Ltd; 1993 [cited 2021 Jul 25];30:167–83. https://onlinelibrary.wiley.com/doi/full/10.1111/j.1600-0897.1993.tb00618.x [DOI] [PubMed]
- 32.ERIC - EJ938197 - New Perspectives on Premature Infants and Their Parents, Zero to Three (J), 2003-Nov [Internet]. [cited 2021 Aug 1]. https://eric.ed.gov/?id=EJ938197
- 33.Irving RJ, Belton NR, Elton RA, Walker BR. Adult cardiovascular risk factors in premature babies. Lancet Elsevier. 2000;355:2135–2136. doi: 10.1016/S0140-6736(00)02384-9. [DOI] [PubMed] [Google Scholar]
- 34.Wennergren M, Krantz M, Hjalmarson O, Karls-Son K. Wennergren et al, Low Apgar score and respiratory disorders in the newborn 153 j. Permat. Med. Low Apgar score as a risk factor for respiratory disturbances in the 15(1987)153 newborn infant. [DOI] [PubMed]
- 35.Ehrenstein V, Pedersen L, Grijota M, Nielsen GL, Rothman KJ, Sørensen HT. Association of Apgar score at five minutes with long-term neurologic disability and cognitive function in a prevalence study of Danish conscripts. BMC Pregnancy Childbirth 2009 91 [Internet]. BioMed Central; 2009 [cited 2021 Aug 1];9:1–7. Available from: https://bmcpregnancychildbirth.biomedcentral.com/articles/10.1186/1471-2393-9-14 [DOI] [PMC free article] [PubMed]
- 36.Stuart A, Otterblad Olausson P, Källen K. Apgar scores at 5 minutes after birth in relation to school performance at 16 years of age. Obstet Gynecol [Internet]. 2011 [cited 2021 Aug 1];118:201–8. https://journals.lww.com/greenjournal/Fulltext/2011/08000/Apgar_Scores_at_5_Minutes_After_Birth_in_Relation.2.aspx [DOI] [PubMed]
- 37.Prochaska E, Jang M, Burd I. COVID-19 in pregnancy: Placental and neonatal involvement. Am J Reprod Immunol [Internet]. John Wiley & Sons, Ltd; 2020 [cited 2021 Jul 26];84:e13306. https://onlinelibrary.wiley.com/doi/full/10.1111/aji.13306 [DOI] [PMC free article] [PubMed]
- 38.Shanes ED, Mithal LB, Otero S, Azad HA, Miller ES, Goldstein JA. Placental Pathology in COVID-19. Am J Clin Pathol [Internet]. Oxford Academic; 2020 [cited 2021 Jul 26];154:23–32. https://academic.oup.com/ajcp/article/154/1/23/5842018 [DOI] [PMC free article] [PubMed]
- 39.UKOSS/ISARIC/CO-CIN: Females in Hospital with SARS-CoV-2 infection, the association with pregnancy and pregnancy outcomes, 25 March 2021 - GOV.UK [Internet]. [cited 2021 Dec 20]. https://www.gov.uk/government/publications/ukossisaricco-cin-females-in-hospital-with-sars-cov-2-infection-the-association-with-pregnancy-and-pregnancy-outcomes-25-march-2021
- 40.Mullins E, Hudak ML, Banerjee J, Getzlaff T, Townson J, Barnette K, et al. Pregnancy and neonatal outcomes of COVID-19: coreporting of common outcomes from PAN-COVID and AAP-SONPM registries. Ultrasound Obstet Gynecol [Internet]. John Wiley & Sons, Ltd; 2021 [cited 2021 Dec 20];57:573–81. https://onlinelibrary.wiley.com/doi/full/10.1002/uog.23619 [DOI] [PMC free article] [PubMed]
- 41.Smith V, Seo D, Warty R, Payne O, Salih M, Chin KL, et al. Maternal and neonatal outcomes associated with COVID-19 infection: A systematic review. PLoS One [Internet]. Public Library of Science; 2020 [cited 2021 Dec 20];15:e0234187. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0234187 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
Not applicable.


