Abstract
This perspective piece on head-up tilt table testing is part of a series on autonomic function testing. The tilt table can be a useful diagnostic test, but methodologies vary, and the results are sometimes misinterpreted. The intent here is not to review comprehensively the utility of various tilt table testing protocols but to convey a number of general points that may give perspective and have practical clinical value, based on an understanding of autonomic physiology and our long clinical and research experience in the evaluation of autonomic disorders. The goals of tilt table testing are to assess orthostatic hypotension (OH), chronic orthostatic intolerance (COI), and unexplained syncope. The testing is useful for distinguishing neurogenic from non-neurogenic OH, identifying failure of the sympathetic noradrenergic system in autonomic neuropathies and ganglionopathies, and assessing baroreflex-sympathoneural function in α-synucleinopathies. For COI the testing can provide objective data related to the patient’s symptoms, diagnose postural tachycardia syndrome (POTS), and distinguish POTS from other causes of tachycardia. Provocative tilt table testing can help understand bases for recurrent transient loss of consciousness in patients with syncope, distinguish autonomic mediated syncope from psychogenic pseudosyncope, and separate syncope-related convulsion from epileptic seizures. For each of these purposes the goals, formats, endpoints, and clinical utility are different. As for any autonomic test, tilt table findings must be interpreted in the context of the patient’s clinical presentation.
Keywords: orthostatic intolerance, orthostatic hypotension, tilt table testing, syncope, vasovagal syncope, postural orthostatic tachycardia syndrome, autonomic nervous system diseases
Introduction
The ability to rise to one’s feet and engage in activities while upright is a defining characteristic of humanity. Many explanations have been proposed for the evolution of upright posture in humans [1]. Three relevant aspects seem well established: (a) this development occurred relatively recently in evolutionary time; (b) the force of gravity poses a challenge in terms of maintenance of adequate delivery of blood to the brain during orthostasis, especially given the concurrent evolution of a large brain in humans; and (c) the sympathetic noradrenergic system is the body’s main effector for redistributing regional blood flows during standing up.
Unlike other challenges (e.g., meal ingestion, traumatic blood loss, exposure to altered environmental temperature) that have been in operation over millions of years of mammalian evolution and for which there are several autonomic effectors available to meet those challanges, when it comes to our ability to maintain blood flow to the brain during orthostasis, the sympathetic noradrenergic system stands alone.
Muscle pumping is effective but only temporarily so, and activation of neuroendocrine systems such as the renin-angiotensin-aldosterone system takes place over the course of many minutes. This is why symptoms of orthostatic intolerance such as lightheadedness, fatigue, visual obscuration, cognitive inefficiency, nuchal headache, and palpitations, or signs such as orthostatic hypotension and syncope are cardinal manifestations of sympathetic noradrenergic failure.
As noted previously in this series on autonomic testing, the first step in evaluating the patient with orthostatic symptoms or transient loss of consciousness is a careful history and physical examination, including orthostatic blood pressure measurements [2,3]. In selected patients, a further key component of the clinical evaluation of cardiovascular autonomic function is head-up tilt table testing. Changing the body axis with respect to gravity under controlled conditions while assessing changes in blood pressure (BP) and heart rate (HR) provides important information about the dynamic functions of the sympathetic noradrenergic and parasympathetic cholinergic components of the autonomic nervous system in reflexive response to orthostatic stress.
The body rises and blood falls
Upon assuming the upright posture, the force of gravity redistributes the blood volume downward. Within 2 to 3 minutes, 500–800 mL of blood (approximately 10% of total blood volume and 25% of thoracic blood volume) is rapidly displaced to the lower body, especially the splanchnic and pelvic organs and proximal legs [4]. With prolonged standing, plasma volume declines further due to transcapillary diffusion [5,6]. Of these changes, the most important indicator of orthostatic stress is the amount of thoracic blood volume redistribution [4].
In healthy persons, the compensatory reflexive responses to standing are quite effective. The unloading of carotid and cardiopulmonary baroreceptors drives sympathetic noradrenergic outflows, which increase peripheral vasoconstrictor tone and cardiac rate and inotropic state. Concurrent activation of abdominal and lower extremity muscles in rhythmic cycles of contraction and relaxation, the “skeletal muscle pump,” produces an unconscious swaying motion of the body during standing and becomes more active when the person shifts weight from leg to leg or walks, squeezes capacitance vessels, and promotes the return of venous blood to the heart [4,7]. Neuroendocrine responses such as increased release of arginine vasopressin, decreased secretion of atrial natriuretic peptide [8], and activation of the renin-angiotensin-aldosterone system also contribute.
In disorders of orthostatic tolerance, including orthostatic hypotension (OH), its subset neurogenic OH, syncope, and postural tachycardia syndrome (POTS) and other forms of chronic orthostatic intolerance (COI), these compensatory responses fail or are insufficient. The diagnostic need to reproduce orthostatic pathophysiology in a controlled, monitored setting has led to the clinical and experimental use of the tilt table test.
Historical perspective
That exsanguination can lead to collapse, prostration, and death has been known since the dawn of humanity. Recognition that not only the loss but also the redistribution of blood volume can impair health and consciousness emerged incrementally over time as perceptive physicians observed syncopal phenomena in their patients and physiologists investigated the cardiovascular system in new ways.
William Harvey was the first to demonstrate the circulation of the blood. He concluded in 1628, “that blood in the animal body moves around in a circle continuously, and that the action or function of the heart is to accomplish this by pumping” [9]. Half a century before Isaac Newton published his law of universal gravitation in Principia, Harvey commented that blood falls “by its weight into areas lower down” [9]. He also noted that arteries dilate synchronously with cardiac pulsations [9] but was unaware of the role of the nervous system in constricting blood vessels and modulating peripheral vascular resistance. Harvey thought that blood “concentrates toward the interior, as drops of water spilled on a table tend to run together,” but he also recognized the role of the muscle pump, as blood moves “from the tiny veins to the intermediate branches and then to the larger veins because of the movements of the extremities and the compression of muscles” [9].
Friedrich Hoffmann in 1740 described several cases of collapse upon standing, which he viewed as being caused by stagnation of humoral motions [10]. Pierre Piorry brought further attention to influence of gravity on the circulation in 1830, noting that, “The arterial, venous, and capillary circulations are in part under the laws of gravitation, especially in feeble subjects” [11]. Summoned to evaluate a patient who had lost consciousness, he found the pulse to be feeble, the face pale, and the respirations stertorous. Refusing the request to bleed the patient, as suggested by his friends who had supported him in a sitting posture for 15 minutes, Piorry laid him down horizontally. “Immediately his eyes opened, respiration was accelerated, the colour came back to the face and in three minutes all the unfavourable symptoms had disappeared” [12].
Leonard Hill expanded the circulatory model in 1895, commenting that the “influence of the force of gravity on the circulation is a question of very obvious importance, yet it is one curiously neglected by physiologists” [12]. Hill constructed “an animal holder which could be swung round a horizontal axis” for the purpose of studying the hemodynamic effects of feet-down versus feet-up posture after dividing or stimulating the vagus, splanchnic nerves, and spinal cord [12]. He contributed the concept that blood flow varies with vascular constriction, such that reduction of peripheral vascular resistance impaired blood flow to the head [13]. Hill was a contemporary of John Newport Langley, who at that time was investigating the role of sympathetic nerves in vasomotor phenomena and introduced the term “autonomic nervous system” [14]. Following his 1891 finding that stimulation of sympathetic nerves caused flushing or pallor in the feet of cats [15], Langley proposed that peripheral sympathetic nerves synapse onto vascular smooth muscle [16].
Probably the first investigator to employ a tilting board to study hemodynamic changes in humans was Egon Helmreich, who in 1923 measured the heart rates of healthy children who were placed on a broad board, secured at the shoulders, pelvis, and feet, and tilted incrementally to 20°, 40°, 60°, and 90° [17]. In 1930, Abby Turner, Isabel Newton, and Florence Haynes employed a padded tilting board equipped with adjustable head, foot, and shoulder rests, to study HR and BP responses in young women tilted to 60° or 90° for 15 minutes. They found that HR increased as pulse pressure narrowed and that the response was less apparent if subjects were given abdominal pressure support [18].
Kenny and colleagues in 1986 introduced the technique of passive head-up tilting for investigating unexplained syncope [19], and since then it has been routinely used in clinical practice.
Poor reproducibility of tilting protocols as well as the long time (typically 20–60 minutes), required to evoke syncope, and the inability to reproduce syncope consistently in susceptible patients prompted a search for methodologies having greater sensitivity and specificity. Bearn and colleagues combined head-up tilt with hemorrhage [20], but the use of medical bloodletting declined in the late 19th century, and this testing idea did not catch on, nor did the application of venous occlusion cuffs to sequester blood in the lower extremities [20].
Another approach initially described by Michael McCally and colleagues [21] and developed further by Roger Hainsworth [22,23] is to intensify orthostatic stress by combining head-up tilt with a lower body negative pressure device. Lower body negative pressure is a technique derived from space medicine research to simulate the negative G forces that cause blood to move to the lower extremities, causing greyout, blackout, and loss of consciousness in pilots.
A number of investigators have intensified the stimulus of head-up tilting by use of provocative pharmacologic agents. Soma Weiss, Robert Wilkins, and Florence Haynes in 1937 were the first to administer the vasodilator sodium nitrite during tilt table testing to induce vasomotor collapse and, in a controlled manner, provoke vasovagal syncope [24]. Beginning in the mid-1980s, Menashe Waxman and colleagues were the first to publish on intravenous infusions of the ß2-adrenoreceptor agonist and vasodilator isoproterenol to supplement head-up tilting of patients with unexplained syncope [25]. As syncope is often preceded by a stimulus that arouses the sympathetic nervous system, by elevated catecholamine levels, or by increased heart rate, the rationale was to identify those predisposed to a vasodepressor reaction. Antonio Raviele and colleagues in 1994 were the first to publish on the use of nitroglycerin infusions during head-up tilting in the evaluation of unexplained syncope [26]. Nitroglycerin is converted by mitochondrial aldehyde dehydrogenase to nitric acid, a potent venodilator. The following year they reported comparable results using sublingual nitroglycerin [27].
In each of these studies utilizing a provocative pharmacologic agent, 6% to 24% of healthy control subjects without a prior history of loss of consciousness, some of whom were physically robust and athletic, developed vasomotor collapse and syncope during head-up tilt [18,24–27].
The clinical significance of false positive tilt table tests is a matter of ongoing debate, and the following anecdote drives home the issue. This is the story of the Reggie Lewis case.
The Reggie Lewis case
Reggie Lewis was a star basketball player for the Boston Celtics. On April 29, 1993, during game 1 of the Eastern Conference First Round of the NBA Playoffs, he collapsed on the basketball court. He came back later and finished with 17 points. YouTube videos of the episode are available at https://www.youtube.com/watch?v=AxO8koWxZMQ.
Lewis was subsequently evaluated by a 12-member cardiology “dream team” at the New England Baptist Hospital. They thought he had a form of cardiomyopathy and recommended that Lewis cease playing. Needless to say, millions of dollars were at stake.
Lewis went for a second opinion by another cardiologist, who concluded that Lewis had “athlete’s heart” and neurocardiogenic syncope—benign conditions—and could resume playing. That assessment became one of the most widely publicized and second-guessed opinions in the annals of medicine. According to a New York Times article, a key procedure that led to this opinion was a tilt table test. During head-up tilting at 60 degrees from horizontal, Lewis reported the same lightheadedness that he had experienced before collapsing on the Celtics’ NBA court.
The tilt table testing yielded either false-positive results or findings that, though valid, were unrelated to his eventual outcome. Before Lewis ever played another NBA game, while shooting hoops at Brandeis University on July 27, 1993 he collapsed again—and died. He was autopsied and found to have an abnormal, enlarged, extensively scarred heart, but the exact cause of death was never made public. His death was attributed variously to hypertrophic cardiomyopathy, a viral myocarditis, or even cocaine cardiotoxicity. A lawsuit filed against the cardiologist resulted in a mistrial.
Indications for testing
The goals of the head-up tilt table test are to assess OH, COI (including POTS), and unexplained syncope. With regard to OH, the testing in conjunction with other autonomic tests is useful for distinguishing neurogenic from non-neurogenic OH, identifying failure of the sympathetic noradrenergic system in autonomic neuropathies and ganglionopathies, and assessing baroreflex-sympathoneural function in alpha-synucleinopathies such as Parkinson disease, multiple system atrophy, pure autonomic failure, and dementia with Lewy bodies (Table 1). For COI the testing can provide objective data related to the patient’s symptoms, diagnose POTS, and distinguish POTS from other causes of tachycardia. Provocative tilt table testing can help understand bases for recurrent transient loss of consciousness in patients with syncope, distinguish neutrally-mediated syncope (which includes neurocardiogenic syncope and reflex syncope) from psychogenic pseudosyncope, and separate syncope-related myoclonic jerks from epileptic seizures.
Table 1.
Indications for Tilt Table Testing
|
For each of these purposes the goals, formats, and endpoints are different. For instance, in a patient with a history of recurrent syncope, the goal is to provoke syncope under controlled conditions, and so the duration of tilting is up to many minutes. In patients referred for orthostatic intolerance or fainting in the setting of evidence of central neurodegeneration, the goal is not to provoke syncope but to assess hemodynamic changes in response to decreased venous return to the heart, and the duration of testing is much shorter.
Active standing vs. passive tilt
Whereas measuring BP at the bedside supine and standing remains an indispensable component of the clinical autonomic assessment [3], the added value of tilt table testing consists in the ability to assess hemodynamic responses to orthostatic stress apart from activation of the large skeletal muscles needed to support the weight of the body. The tilt table also allows for hemodynamic monitoring for longer periods of time than some patients feel comfortable standing.
It is important to understand that the physiological effects of passive head-up tilt differ from those of active standing. In healthy subjects, active standing compared to passive head-up tilt causes a transient but greater reduction of BP, a larger increase in HR, a greater decrease in total peripheral resistance, and a greater increase in cardiac output during the first 30 seconds [28]. Tanaka and colleagues found that in young healthy subjects the maximal reduction in mean BP occurred approximately 10 sec after standing and was −39 ± 10 vs. −16 ± 7 mmHg on the tilt table, whereas HR increased by 35 ± 8 vs. 12 ± 7 beats/min [28]. Hemodynamic studies have shown that the initial drop in BP on active standing is driven by systemic vasodilatation caused by activation of cardiopulmonary baroreflexes as a reflex response to the rapid return of blood to the heart resulting from contraction of abdominal and lower extremity muscles that compress sphlanchnic and muscular vessels [28,29]. Wieling and colleagues detected a 50% increase in cardiac output, maximal at 5 seconds, and a 36% fall in total peripheral vascular resistance reaching its nadir at 8 seconds, with values returning to baseline within 30 seconds [29].
This initial fall in BP during active standing, which is typically not seen on tilt table testing, may explain some cases of falling or syncope that occur immediately upon standing [30,31]. A normal tilt table test does not rule this out. In a prospective comparison of head-up tilt table testing versus active standing in 290 patients seen in a geriatric clinic, the prevalence of OH during head-up tilting compared to active standing (measured at the third minute) was 19% vs. 37%, but the frequency of recurrent falls was higher in the group with OH by head-up tilt (36.4% vs. 21.7%, P=0.004) [32].
Active standing is associated also with a greater transient rise in HR than passive head-up tilt. In a study of young healthy subjects, Borst and colleagues found that active standing evoked an immediate bimodal increase in HR, reaching its first peak of 27 beats/min above baseline at 3 sec, its second peak of 30 beats/min above baseline at 12 sec, and returning to baseline by 20 sec. As the first peak in HR is almost identical to that evoked by handgrip exercise, and it is abolished by atropine but unaffected by propranolol, the mechanism of this rapid cardioacceleration most likely indicates a central command with reflex withdrawal of vagal control [44,45]. The secondary HR peak, which is also abolished by atropine, reflects the unloading of carotid and cardiopulmonary baroreceptors [33].
Head-up tilt, by contrast, did not evoke these first or second peaks in HR during the first 20 sec, but both active standing and head-up tilt induced a third peak consisting of a gradual and continued increase in HR after 30 sec. This gradual increase in HR was attributed to the unloading and partial reloading of carotid sinus and cardiopulmonary receptors, muscle receptors, and increased plasma catecholamine levels [33]. The magnitude and time course of the HR response to standing was independent of the level of physical training [33].
The relevance of these differences in acute responses to head-up tilting and active standing may depend on the purpose of the testing. If an abnormal increase in HR or decrease in BP corresponding to the patient’s symptoms is detected on active standing, a tilt table test may not be needed unless evaluating the hemodynamic response to prolonged orthostatic stress is also needed, for example, in the patient with syncope.
Not all tilt table testing is the same
Each academic center or medical practice has its own way of conducting tilt-table testing. There are common ingredients, but many practical details likely to influence the outcome rarely are published or even individually charted. This section considers some of these details.
It is worth bearing in mind that consistency and reproducibility are important, but the effective clinician must be able to adapt the testing to maximize the likelihood of obtaining useful information.
Goals:
There are different goals of tilt table testing. The goal for each individual should be decided on in advance. Symptoms reported during the tilting can be helpful if they are typical of what the patient is experiencing in daily life and the clinician is able to correlate these with BP and HR values. Reproduction of symptoms may be important for validating the patient’s complaint, but this is of little diagnostic value compared to objective physiological or neurochemical data. Later we discuss the quite different parameters for identifying and quantifying orthostatic hypotension (OH, including immediate and delayed OH), diagnosing postural tachycardia syndrome (POTS), and provoking neurally mediated hypotension (NMH) or neurally mediated syncope (which includes vasovagal syncope).
Context:
Head-up tilt table testing should be done, not as an isolated test, but in the context of a complete clinical evaluation including the all-important autonomic history and physical examination. Combining tilt table testing with other autonomic tests, especially beat-to-beat blood pressure and heart rate responses to the Valsalva maneuver, is highly desirable as it is indispensable to distinguishing noradrenergic impairment. Although not invariably needed or available in all laboratory settings, additional ancillary tests such as systemic hemodynamic measurements and serial blood sampling for plasma levels of catechols can be informative.
Time of day:
There are diurnal rhythms in autonomic outflows. Patients with neurogenic OH often have lower BP and are more symptomatic in the morning than later in the day. Patients with syncope also have more frequent symptoms and have reduced tolerance to orthostatic challenge in the morning [35–37]. Accordingly, patients with COI have significantly higher tachycardia in the morning hours [38]. Healthy individuals also have relatively reduced orthostatic tolerance in the morning [38,39]. By contrast, the physiologic responses in initial OH and delayed OH appear to be independent of diurnal rhythms [40].
Room temperature:
It is well known that people are more likely to faint in a warm than cool environment; however, there is no research literature on the relationship between room temperature and hemodynamic responses or the likelihood of positive tilt table test results. We recommend that the temperature of the room be within a comfortable range (22–24 °C) that is not so cold as to induce shivering or so warm as to evoke sweating under resting conditions.
Meals:
Patients are instructed to eat no more than a light meal before coming to the testing room, abstain from alcohol or caffeine, and for at least two hours prior to the testing to fast or take only clear liquids. These general instructions suffice for most clinical settings. Quantified specifications of calories and volume may be appropriate in certain research settings. What is important for clinicians to recognize is that patients with alpha-synucleinopathies or postprandial hypotension may have more pronounced OH during orthostatic challenge after a high-carbohydrate meal [41–43]. The potential influences of a meal have not been studied formally in patients with syncope. Additionally, because of the possibility of acute vomiting, the patient should not be tested shortly after eating a meal.
Psychological milieu:
Whereas the alerting sound of alarms or pagers, cold or hot air from ventilation ducts, and room brightness might affect the test results but have not been systematically studied, anecdotal experience suggests that all of these stimuli can influence autonomic responses. The autonomic testing room should be quiet and isolated from noise, although a white noise generator may be useful to mask nearby sounds. The patient should feel relaxed and, to the degree possible, should not be in pain. The patient should be encouraged to empty the bladder before the setup for the testing. A minimum number of staff should be present so that the patient feels at ease.
Prescribed medications:
Appropriate management of prescribed medications (and dietary supplements) in preparation for tilt table testing could itself be a topic for a substantial review. There is no substitute for in-depth knowledge by the clinician about autonomic pharmacology, diagnoses being entertained, and hypothesized pathophysiologic mechanisms. Questions to be considered include which drugs should be continued, which held, and for how long. Consideration should be given to the potential for rebound phenomena (e.g., from beta-adrenoceptor agonists, alpha-adrenoceptor antagonists, clonidine, benzodiazepines, serotonin/norepinephrine reuptake inhibitors) and the timing of anticipated peak rebound. When a patient is referred for tilt table testing, it should be decided beforehand who will monitor risks and take responsibility for adverse events. The potential influence of medications should be considered when interpreting tilt table findings.
Continuous vs. intermittent monitoring:
BP and HR are assessed frequently and continuously throughout the procedure. The state of the art of tilt table testing is brachial sphygmomanometry by arm cuff combined with automated, continuous tracking of beat-to-beat BP by a finger cuff device. The latter is non-invasive and has largely supplanted intra-arterial BP recording for obtaining beat-to-beat BP measurement in clinical settings. Brachial sphygmomanometry yields accurate BP values, although systolic BP may be overestimated if there is substantial arterial stiffening. Continuous finger BP recording can detect rapid trends or oscillations in BP. Finger BP should be calibrated against brachial sphygmomanometry at baseline. In patients with digital vasoconstriction, the finger BP may be substantially below the brachial BP or may not even be recordable. In this setting the hand may be warmed passively; however, if this is done the potential artifactual effects should be considered.
Position before head-up tilt testing:
The patient should rest in the supine posture for at least 10 minutes [44,45] to establish a stable baseline.
Arm position:
If the brachial blood pressure is being monitored, or if a finger BP system is used that does not incorporate a height correction unit, then for all postures BP measurements should be taken at the vertical level of the heart via an adjustable arm rest extended laterally. This may not be necessary if a finger BP device is used that incorporates a height correction unit. The patient should not have the arm extended without support, because this introduces the possibility of effects of isometric exercise on the measurement.
Physical parameters:
The tilt table must be structurally strong enough to support the weight of the patient safely. At the end of the table there typically is a foot plate on which the patient’s feet rest during upright positioning. This helps to keep the patient securely on the table but might facilitate muscle pumping that could affect the results. Adjustable straps used to secure the patient should secure but not constrict the patient’s legs. The table should be padded to help the patient relax. A pillow placed behind the patient’s head minimizes discomfort; however, the pillow should be removed when the head of the table is tilted up, because the pillow may fall.
A method that eliminates muscle pumping is to suspend the patient in a harness in which the feet do not touch the ground. This “parachute tilt” method is seldom implemented, as it does not permit gradual or incremental changes in the tilt angle, and the device can be uncomfortable.
Velocity of tilting:
It has been suggested that the tilt table be raised slowly from the horizontal plane [46]. Different motorized tilt tables have different rates of changing the tilt angle, and some are adjusted manually. The rate of change in angle is rarely recorded and has not been studied. A slow velocity of tilting has been shown to cause less activation of muscle sympathetic nerve activity [47] and decreases the tendency to tense anti-gravity muscles. A slow angular velocity is also less likely to stimulate velocity-dependent otolith afferents and cause vertigo.
Angle of tilting:
In choosing the angle of head-up tilt, the goal is to expose the patient to adequate gravitational stress while minimizing skeletal muscle contractions. At increased angles, the responses of muscle sympathetic nerve activity, heart rate, stroke volume, and cardiac output to orthostatic stress are stronger [48]. A reasonable compromise is to tilt the patient to an angle of between 60° to 80°. An optimum angle in our experience is 70° (Figure 1). Angles of 60°, 70°, and 80° expose the patient to 87%, 94%, and 98% of the gravitational exposure of a 90° angle. An angle of 90° would be equivalent to active standing, which differs physiologically, as discussed above.
Figure 1.
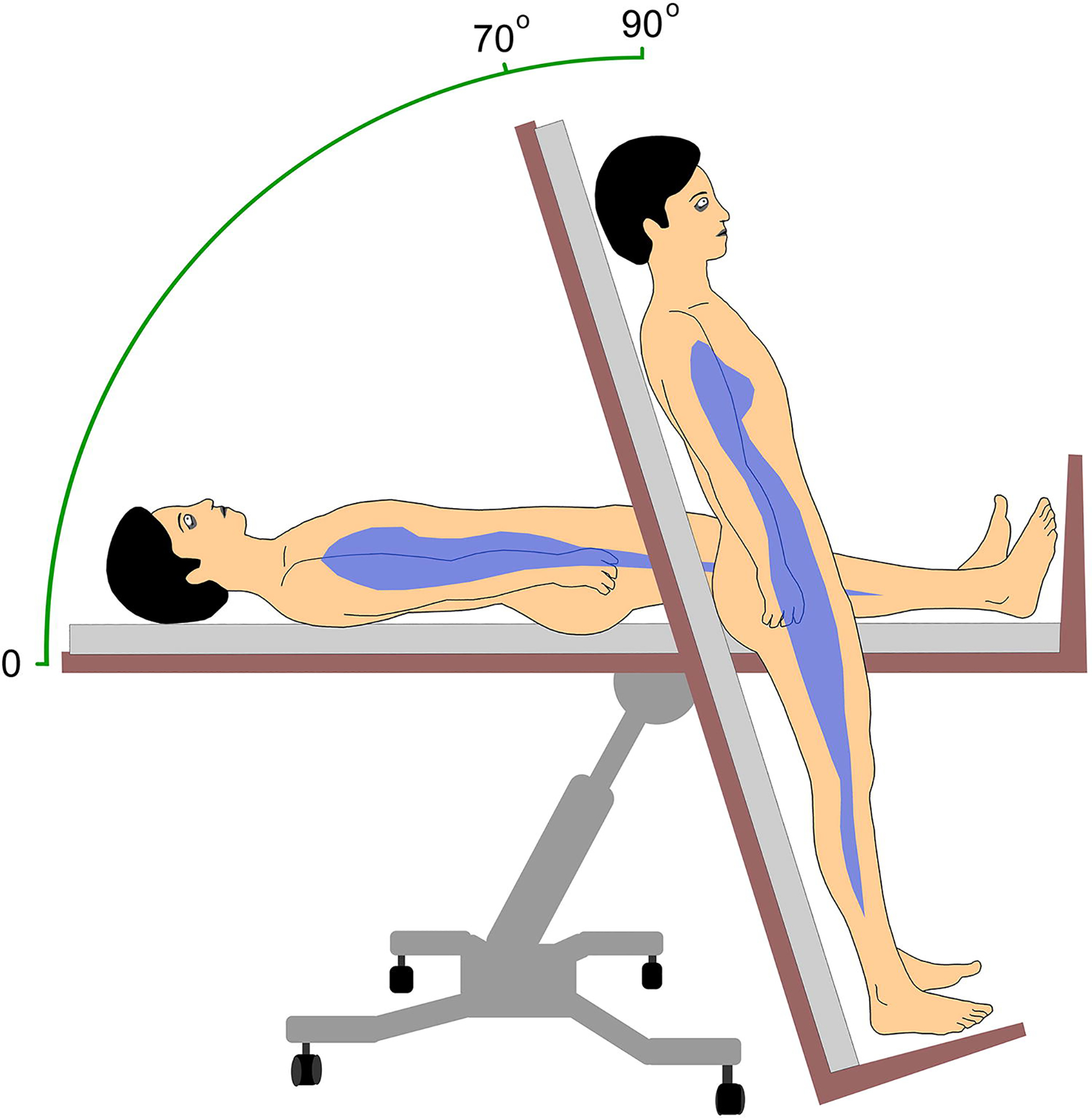
Schematic diagram of the head-up tilt table test. The blue shape represents an approximation of the distribution of the central venous blood volume, which at a baseline angle of 0° is liberally present in the thorax. At at an angle of 70° the force of gravity shifts much of the thoracic venous blood volume downward into the pelvis and proximal lower extremities. A tilt angle of 70° results in a gravitational force of 0.94 G, which approximates the vertical angle but does fully engage the leg muscles to remain upright.
Duration of tilting:
The duration of head-up tilting depends on the clinical question being addressed—from 5 minutes in a patient referred for OH to 45 or even 60 minutes in a patient referred for syncope.
Endpoint of tilting:
By consensus of autonomics experts, OH is “a sustained reduction of systolic blood pressure of at least 20 mmHg or diastolic blood pressure of 10 mmHg within 3 min of standing or head-up tilt to at least 60° on a tilt table” [46]. For head-up tilt testing to diagnose OH the consensus definition seems straightforward. There are some aspects, however, that bear comment. Briefly, the consensus definition involves compromises due to differences in practices among autonomics centers.
-
1
The qualifier “sustained” may seem puzzling since the duration of observation is specified to be just 3 minutes. What the experts have in mind is that in many apparently healthy people BP falls rapidly as soon as they stand up from lying down, but the fall in BP is transient, recovering within 30 seconds. On the other hand, patients with neurogenic OH can have such a rapid, severe fall in BP that it is unsafe to keep the patient upright for a longer period of time. The consensus definition is a practical compromise that recognizes that a rapid fall in BP that does not recover within 3 minutes is usually a positive finding.
-
2
The consensus definition does not specify the angle or duration of posture before the person stands up or is tilted. Going from supine to standing is not the same as going from seated to standing, and the baseline BP is affected by how long the patient has been at rest. This also is a compromise, because autonomic centers differ in their methods of obtaining orthostatic vital signs. It seems intuitively obvious that in patients with failure of the sympathetic noradrenergic system the extent of fall in BP between lying down and standing is greater than the fall between being sitting and standing.
In our opinion, before the baseline BP is measured, the patient undergoing a tilt table test should be supine (with head on pillow) for at least 10 minutes. During this time, the observer can list all the medications and dietary supplements that the patient has taken within the past 24 hours and when they were taken. The location of the measurement, the time of day, and when and what the patient last ate should be noted (the latter because of the possibility of post-prandial hypotension). When measuring orthostatic vital signs in the outpatient clinic, a supine rest time of 10 minutes is not always practical, and a time of 2 minutes is a reasonable compromise. The time supine can be extended by performing other parts of the physical examination supine and then measuring BP supine and standing.
-
3
The word “or” in “reduction of systolic blood pressure of at least 20 mmHg or diastolic blood pressure of 10 mmHg” allows for a diagnosis of OH if only the systolic or only the diastolic BP drops. This is another compromise. In our opinion the orthostatic fall in systolic BP is the most important indicator of OH. An abnormal decrease in diastolic BP without an abnormal decrease in systolic BP is rare in patients with orthostatic disorders [49], and systolic BP changes correlate more closely than diastolic BP changes with orthostatic intolerance [50]. Diastolic BP, although having a greater contribution to the mean arterial pressure, is less useful because it is more difficult to measure precisely, as the demarcation between Korotkov sounds IV and V can be barely audible.
-
4
The choice of “standing or head-up tilt to at least 60°” limits the standardization and generalizability of the definition of OH. As previously discussed, the physiology and hemodynamic responses of active standing and passive head-up tilt are not identical. Going from supine to standing, seated to standing, horizontal to 60°, or horizontal to 90° are very different procedures, and measurements that count as OH in one situation might differ and not count in another. This is yet another compromise. Many practitioners do not have have access to a tilt table. Among those who do, some tilt the patient to a full head-up position—i.e., the patient is made to stand upright—and others tilt the patient to 60° or another angle less than 90°.
Ancillary testing:
A variety of ancillary tests can provide important supplementary information and are typically used at referral centers or in research settings (Figure 2). Finger cuff BP systems may include built-in programs for continuous tracking of cardiac stroke volume and thereby of cardiac output and total peripheral resistance. Via an indwelling intravenous catheter, serial blood samples can be drawn to track plasma levels of catecholamines and so detect and quantify sympathoadrenal imbalance, where plasma epinephrine responses increase more than plasma norepinephrine responses prior to tilt-induced neurally mediated hypotension or syncope (Figure 2) [51]. When people faint, they sweat [52], and this can be tracked by monitoring skin electrical conductance (Figure 2). It is commonly thought that syncope can result from hyperventilation-induced cerebral vasoconstriction. This can be assessed by monitoring respiration using a simple impedance device or monitoring exhaled carbon dioxide. Skin temperature monitoring, laser-Doppler flowmetry, or forearm blood flow by impedance plethysmography may also be considered. Transcranial Doppler-ultrasound recording can detect changes in cerebral blood flow velocity, but this measurement is technically challenging during changes of posture and has been used as a research tool [53]. Finally, video recording has substantial potential for tracking relevant changes such as patient speech and postural adjustments, pallor, and eye position and movements but has not yet been incorporated in routine clinical practice.
Figure 2.
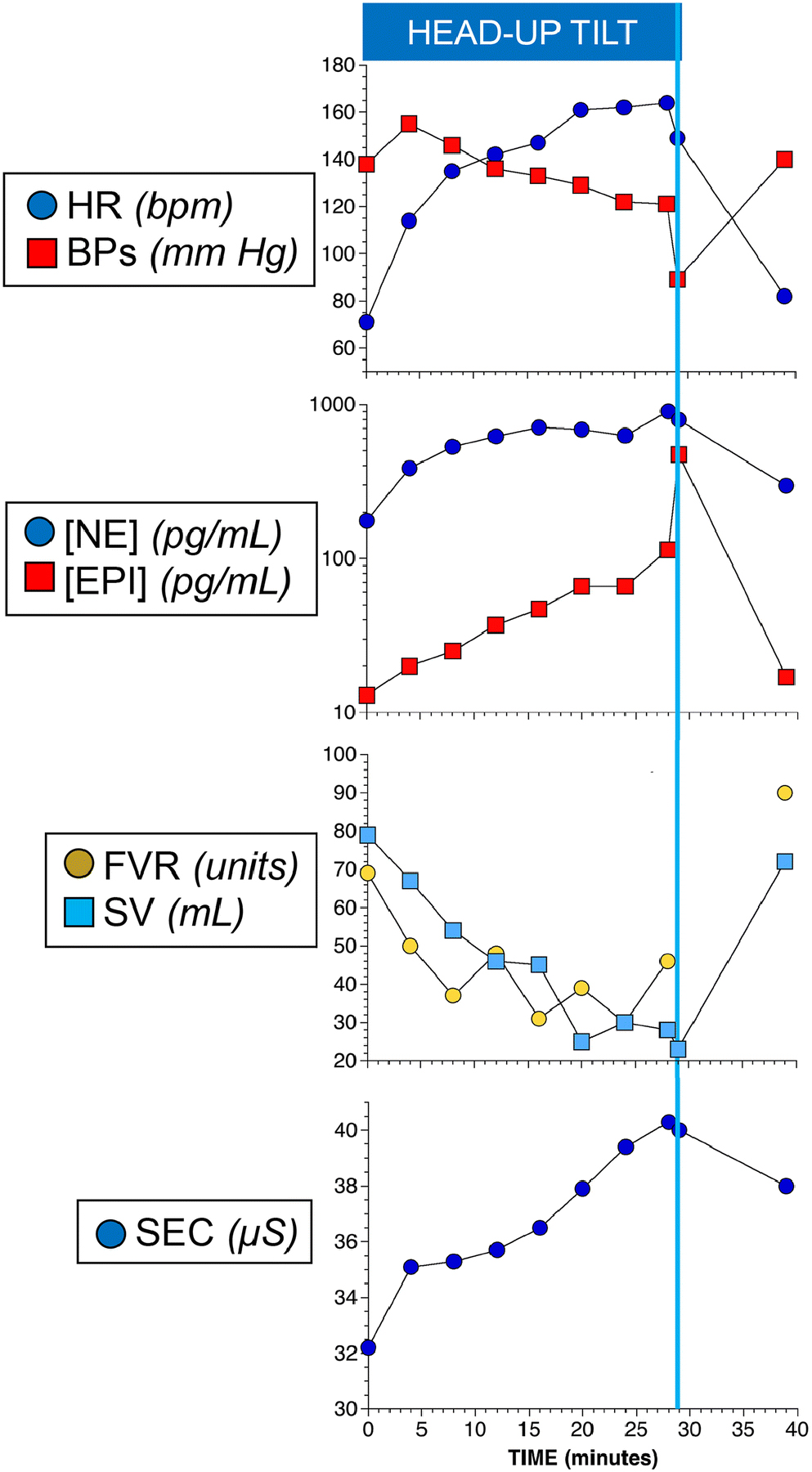
Values for physiological and neurochemical variables during and at 10 minutes of supine recovery after head-up tilt table testing in a patient evaluated for orthostatic intolerance. Abbreviations: HR=heart rate; BPs=systolic blood pressure; NE=plasma norepinephrine concentration; EPI=plasma epinephrine concentration; FVR=forearm vascular resistance; SV=stroke volume; SEC=skin electrical conductance. There is substantial orthostatic tachycardia. BPs declines slowly progressively during tilting and then suddenly falls (neurally mediated hypotension, NMH). Plasma EPI increases to a proportionately greater extent than does plasma NE (sympthoadrenal imbalance). FVR decreases during the tilting (normally FVR increases), and SV decreases progressively to a low value. SEC increases progressively prior to NMH. The patient was studied at the National Institutes of Health Clinical Center after having given written informed consent to participate in a research protocol approved by the Institutional Review Board of the National Institute of Neurological Disorders and Stroke.
Head-up tilt in the diagnostic evaluation of orthostatic hypotension
The use of the tilt table in diagnosing OH is usually straightforward. The diagnosis does not require symptoms; in fact, it is not unusual for a patient with chronic neurogenic OH to report no specific symptoms despite a profound drop in BP. Upon reaching the head-up posture, BP typically falls rapidly, remains low relative to baseline, and does not recover until the horizontal posture is restored [54]. Systolic values are more informative than diastolic values [49]. A tilt test of 5 minutes duration is usually sufficient to detect OH.
Moreover, head-up tilt is useful in distinguishing neurogenic OH from other, less serious causes of OH, such as venous pooling or dehydration from inadequate fluid intake or excessive fluid loss. A minority of patients with OH will have neurogenic OH, which is a cardinal manifestation of sympathetic neurocirculatory failure [55] and is caused by deficient reflexive release of norepinephrine. Two features are helpful diagnostically. First, in most patients with neurogenic OH, the fall in BP tends to be larger (≥30 mmHg systolic), and the HR response is blunted [56]. A prospective study of 423 patients, 378 of whom had neurogenic OH associated with degenerative alpha-synucleinopathies, found that neurogenic OH could be reliably distinguished from other causes of OH when the ΔHR/ΔSBP ratio was <0.5 beats/min per mmHg [57]. However, in our experience the HR change is not always sufficient to distinguish neurogenic OH, particularly in patients with early autonomic failure, who are taking β-blockers, or have a cardiac pacemaker. In these cases a second parameter is useful, namely, in neurogenic OH beat-to-beat BP responses to the Valsalva maneuver are always impaired [58].
Head-up tilt table testing may identify OH variants. One such variant is initial OH, defined as an immediate transient BP decrease of >40 mmHg systolic or >20 mmHg diastolic with symptoms of cerebral hypoperfusion occurring within 5–15 sec of assuming the upright posture [31]. As previously discussed, initial OH is typically seen and best evaluated during active standing. Patients who have this typically do not exhibit abnormal findings during head-up tilt [46], but occasionally it is seen.
Another variant is delayed OH, defined as a fall in BP that fulfills the consensus criteria for OH except that it occurs after 3 minutes [46]. In a follow-up study, 10 years later half had progressed to nOH, and nearly a third phenoconverted to an α-synucleinopathy [59]. Its temporal profile (Figure 3) tends to be gradual in comparison to that of the vasodepressor response of syncope [54].
Figure 3.
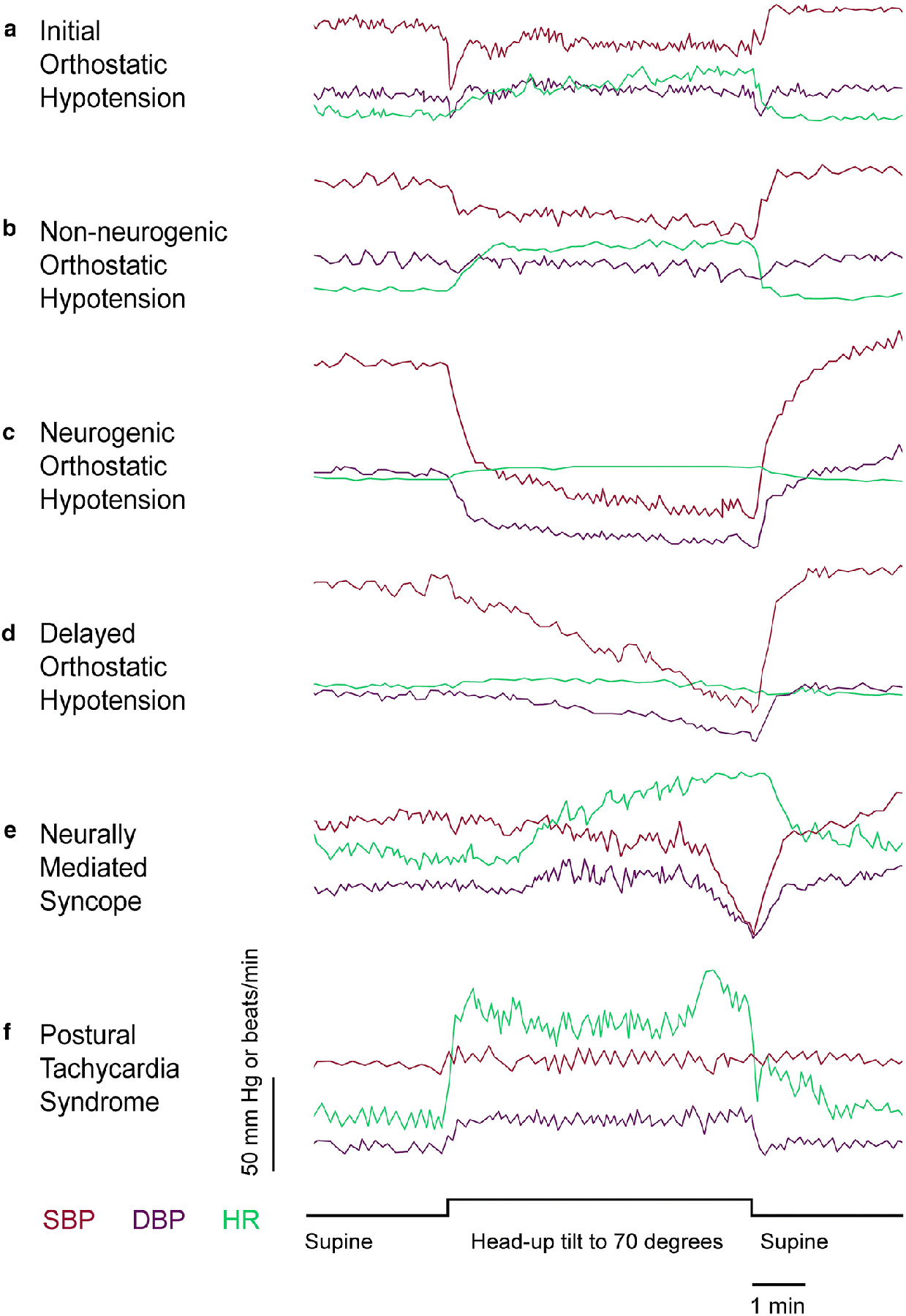
Representative continuous beat-to-beat BP and HR recordings in selected patients demonstrating common abnormal profiles on passive head-up tilt table testing. (A) In initial OH (seen more typically during active standing), a transient drop in BP of >40 mmHg occurs within 5–15 sec and recovers within one minute. (B) In non-neurogenic OH, there is a sustained decrease in systolic BP of >20 mmHg. In most cases, unless the patient is taking a beta blocker or has a pacemaker, compensatory tachycardia occurs. (C) In neurogenic OH, there is a larger sustained decrease in systolic BP, typically >30 mmHg, and in most cases compensatory tachycardia is absent. Supine hypertension may be present. A diagnosis of neurogenic OH should be confirmed by detecting inadequate BP responses to the Valsalva maneuver. (D) In delayed OH, the BP does not reach the threshold for OH within the first 3 minutes of head-up tilt, but a further gradual decrease in BP occurs, reaching the criterion for OH beyond 3 minutes of standing or head-up tilt. (E) In syncope caused by neurally mediated hypotension, a delayed decrease in BP occurs more suddenly than that of delayed OH. This profile is typical of a vasodepressor response and is accompanied by relative bradycardia. Note the preceding tachycardia (which in this patient qualifies for POTS) and oscillations in BP indicating the sympathoadrenal imbalance typical of the presyncopal prodrome. At the moment of loss of consciousness, the table was returned to the horizontal position and the patient regained consciousness and baseline BP. (F) In POTS, the HR sharply rises during the first 30 sec of head-up tilt and remains elevated as long as the patient is in the upright position. OH does not occur. Oscillations in beat-to-beat BP and HR may be seen while in the head-up position.
Tilt table testing in the diagnosis of OH is recommended by the American Autonomic Society, the American Academy of Neurology, and the European Federation of Neurological Sciences [46,60].
Head-up tilt in the diagnostic evaluation of orthostatic intolerance
The use of the tilt table in diagnosing COI and POTS requires more interpretation. Patients in the head-up position may report symptoms of dizziness, weakness, warmth, dyspnea, nausea, or headache – whether they have corresponding hemodynamic abnormalities or not. The hemodynamic manifestations of orthostatic intolerance range from subtle to overt. POTS is defined by a sustained heart rate increment of ≥30 beats/min (≥40 beats/min for patients <20 years of age) within 10 min of standing or head-up tilt. By definition, the tachycardia cannot occur in response to OH [46], although POTS patients can have progressive declines in BP followed by sudden neurally mediated hypotension (NMH). Figure 3 shows an example of this phenomenon.
The criterion of sustained tachycardia is important. As normative data for HR during head-up tilt are based on time-averaged HR, momentary spikes in HR exceeding 30 bpm when the mean HR is within normal limits would not qualify for a diagnosis of POTS [61]. Another pattern that may be seen is a temporal profile of initial tachycardia that settles down into the normal range while the patient is still in the head-up posture (Figure 3). Transient tachycardia at the beginning of head-up tilt and then subsides is more typical of the tachycardia that occurs during the physiology of active standing or that might be triggered by anxiety in response to the test or stimulation of the vestibular system by the rotation of the table.
The potential influence of concurrent medications or provocative agents administered during the test should not be overlooked. Numerous medications can increase or decrease the HR at baseline as well as influence the magnitude of HR change during head-up tilt. Isoproterenol, a non-selective β-adrenoceptor agonist agonist, increases the HR by as much as 30 beats/min at doses used as a provocative agent to induce neurally mediated syncope. Similarly, other stimulant drugs, norepinephrine transporter inhibitors, and anticholinergic drugs that elevate the heart rate should be withheld during testing if a diagnosis of POTS is being considered [62]. POTS should not be diagnosed when medications explain the tachycardia seen on head-up tilt. Analogously, one would not diagnose essential hypertension after giving a pressor drug or a sleep disorder after giving a hypnotic.
With or without tachycardia, patients with symptomatic COI will often exhibit fluctuations in beat-to-beat BP in the head-up posture. In some cases these oscillations are synchronous with respiratory sinus arrhythmia. In other cases they represent Mayer waves reflecting oscillations in baroreceptor or chemoreceptor function. When prominent, they may indicate a susceptibility to vasomotor instability.
Tilt table testing in the diagnosis of orthostatic intolerance is a Class IIb recommendation by the Heart Rhythm Society [63].
Head-up tilt in the diagnostic evaluation of syncope
The use of the tilt table in diagnosing syncope may be thought of as a “deep-dish” application. Tilt table testing has been studied more extensively for the diagnosis of syncope than for any other condition [45,63–66]. The reasons for this are that the clinical evaluation of syncope can be challenging, often eluding diagnosis despite extensive and costly cardiac tests, and when syncope occurs without warning or is recurrent, its associated morbidity and potential mortality are nontrivial [62]. Syncope is also common, occurring in at least 40% of people at least once during their lifetimes [67,68].
Syncope has long been thought of as being either a vasodepressor phenomenon caused by withdrawal of sympathetic vasoconstriction, or a cardioinhibitory phenomenon caused by a drop in cardiac output. Many patients will exhibit some combination of these. The pathophysiology of syncope is complex and remains incompletely understood.
An attractive but disproven model was the Bezold-Jarisch reflex, which explained syncope as a reflex response elicited by forceful contractions of the left ventricle under conditions of decreased cardiac filling, the so-called “empty heart syndrome.” However, there is no convincing evidence that cardiac filling decreases substantially prior to tilt-evoked syncope [69]. Additionally, cardiac transplant patients who lack afferent or efferent nervous connections to the heart can have spontaneous and evoked syncope [70,71]. Another perspective considers vasodilatation from the withdrawal of sympathetic vasopressor tone to be the final common pathway leading to syncope, but electrophysiologic studies challenge this model as well. Recordings of muscle sympathetic nerve activity have shown persistent activity during syncope, indicating that full withdrawal of baroreflex-mediated skeletal muscle vascular tone is not an obligatory prerequisite for syncope [72–75]. Among the additional factors that have been suggested to play a role in syncope are the vasodilator nitric oxide [76] and hypocapneic cerebral vasoconstriction [77].
Ultimately, whereas syncope is manifested by cardiovascular changes, the preponderance of evidence points to its origin in the brain. In recognition of this, the terminology “neurally mediated syncope,” “neurally mediated hypotension,” or “neurocardiogenic syncope” is often used synonymously with “vasovagal syncope.” Piorry recognized this nearly two centuries ago, writing: “Syncope is produced by moral impressions, odours, the sight of a disagreeable object, all which can act only on the brain or organs of sense” [11]. Mental states such as anxiety, fear, emotional distress, or the sight of blood are well-recognized triggers in otherwise healthy persons [64,67,78]. Studies of medical students, for example, have found that approximately one-third had a prior history of syncope [79], and 12% had near or actual syncope during their first exposure to the operating theater [80]. An intriguing clue to this brain-heart phenomenon is right insular atrophy detected by volumetric MRI studies in patients with neurally mediated syncope on head-up tilt [81]. The insular cortex is intimately involved in interoceptive awareness, visceral states associated with emotional experience, and autonomic regulation. If syncope originates in the brain, then a stimulus, such as the tilt table, that stresses the circulatory system may not succeed in reproducing the phenomenon in every case.
A characteristic prodrome precedes the loss of consciousness that occurs in syncope. Beat-to-beat monitoring of BP and HR during head-up tilt typically shows a build-up of tachycardia with progressive narrowing of pulse pressure lasting several minutes. The tachycardia may rise to the level of POTS (Figure 3); in fact, POTS and syncope sometimes coexist in the same patient [82]. During the prodrome the patient may report symptoms of lightheadedness, warmth or coldness, nausea, impaired mental focus, muffled hearing, visual graying or blackening, tunnel vision, palpitations, dyspnea, clammy skin, fecal urgency, or “coat hanger” distribution nuchal aching. The autonomic physical examination continues during the tilt table test. Physical signs may include facial pallor, declining level of consciousness with reduced speech output, pupillary dilatation, increased rate of respiration, yawning, sighing, sweating, lower extremity erythema, or increased peristalsis [64,66,83]. When these symptoms and signs occur, syncope is imminent, and the patient should not be left unattended.
This prodrome is followed by a drop in BP over 1–3 minutes and a more rapid drop in HR over less than a minute (Figures 2, 3). The hypotension rarely occurs immediately on tilt-up, as it typically does in neurogenic OH, but after many minutes. Once systolic BP decreases to 50–60 mmHg or lower for 7 sec, loss of consciousness occurs [66]. When loss of consciousness occurs, the tilt table should be returned promptly to the horizontal position. Leaving the unconscious patient supported for a long time in the upright posture, particularly if carotid stenosis is present, places the patient at risk for complications of cerebral hypoperfusion such as a secondary seizure. Very rarely, watershed ischemic cerebral infarctions can occur in patients with syncope who are restrained in a seated position [84], although to our knowledge this has not been reported in the context of tilt table testing.
When head-up tilt results in syncope or near syncope, as can occur in healthy individuals as well as those with a history of transient loss of consciousness, it establishes a predisposition but alone does not prove that syncope is the cause of the patient’s particular symptoms. Tilt table testing in patients with syncope has an estimated diagnostic sensitivity of 25% to 75% and specificity of 90% to 100% [44,83]. The reproducibility on repeat testing of an initial positive syncopal response is 31% to 92%, whereas the reproducibility of an initial negative study is 85% to 94% [85]. The tilt table test is useful in distinguishing between convulsive syncope and epilepsy, between syncope and psychogenic pseudosyncope, and when the history is limited or the cause of transient loss of consciousness remains unclear. The tilt table is not useful, however, in predicting the response to specific medical treatments [63], one reason being its inconsistent reproducibility. Tilt table testing in the diagnosis of syncope is a Class IIa recommendation by the Heart Rhythm Society [63].
Pharmacologic provocation
The use of pharmacologic agents to provoke syncope on the tilt table is an innovation that has proven to be problematic. Isoproterenol and nitroglycerin have been the agents most commonly used with the goal of increasing the diagnostic yield of syncope on the tilt table [44]. Adenosine and clomipramine have also been used [86,87].
Two sentinel studies initiated the use of isoproterenol during tilt table testing to identify patients predisposed to neurally mediated syncope. Waxman and colleagues administered isoproterenol in intravenous boluses of 2–8 μg to 48 patients, ages 17 to 74 years, with or without a history of syncope, who were tilted to 60° for 5 to 15 minutes. The sensitivity and specificity for predicting syncope were 73% and 85%, respectively [25]. The same year Almquist and colleagues administered isoproterenol in doses of 1–5 μg/min to 24 patients, ages 14 to 80 years, all of whom had had at least three episodes of unexplained syncope or presyncope, who were tilted to 80° for up to 10 minutes. In patients with syncope, the sensitivity and specificity for tilt table testing alone were 27% and 96%, but with the addition of isoproterenol were 82% and 88 percent, respectively [88].
Subsequently Shen and colleagues compared passive head-up tilting to 70° for 45 minutes in 111 patients with a history of syncope and 23 control subjects without a history of syncope who were randomized to receive either isoproterenol infused at 0.05 μg/min or no drug. In the patients with a history of syncope, passive head-up tilt produced syncope in 32% and isoproterenol during head-up tilt produced syncope in 56% of patients (P=0.002). In the normal control subjects, passive head-up tilt produced syncope in 9% and isoproterenol during head-up tilt produced syncope in 17% of subjects (P=0.002) [44]. Across studies, isoproterenol increases the diagnostic sensitivity of head-up tilt to approximately 60% to 85%, while the diagnostic specificity as compared to passive head-up tilt is reduced to 35% to 83% [44,45,83].
Isoproterenol infusion is not without potential risks, especially in patients with structural heart disease, and enthusiasm for its use has waned following reports of supraventricular arrhythmias, variant angina pectoris, and ventricular fibrillation during tilt table testing [89,90]. A potential confounding factor is the placement of an intravenous catheter, as intravascular instrumentation itself is a stimulus that can provoke syncope [91].
Sublingual nitroglycerin as a provocative agent has the advantage, particularly in children, of avoiding the need for intravenous catheterization. In the diagnosis of syncope, nitroglycerin affords a sensitivity of approximately 50% to 80% and a specificity of 85% to 95% [27, 90,92]. Comparison studiees of nitroglycerin during head-up tilt have shown 12% more frequent positive responses in comparison to isoproterenol and favorable tolerability [93,94].
Noting consistent reports of decreased diagnostic specificity with the use of pharmacologic provocative agents, it may be unclear in individual patients whether syncope observed on the tilt table is pathophysiologic or iatrogenic. The Heart Rhythm Society consensus guidelines, while drawing attention to decreased specificity, recommend neither for nor against the use of these agents [63]. In our opinion, these agents should not be used in routine diagnostic tilt table testing because of the high rates of false positives.
False positive tilt studies
How one should interpret syncope or near syncope that occurs during head-up tilt table testing in the patient without a history of loss consciousness is a question that arises in clinical practice. (This question may not come up for practitioners who perform tilt testing only on patients with a history of transient loss of consciousness.) Much depends on the context. If the patient is acutely dehydrated, hypotension or tachycardia during head-up tilt may simply reflect the patient’s current intravascular volume status.
A positive tilt table test without pharmacologic provocation probably indicates a physiologic predisposition to syncope, whether the patient has a history of fainting or not. This assumes the test was performed under standard conditions, as it is known from aviation medicine that anyone can faint under sufficient intensity or duration of gravitational stress. Physiologic predispositions come in degrees, and whereas a positive test might reflect an increased probability of subsequent fainting, not everyone with a preposition is destined to faint in ordinary life. There is the temptation in clinical practice to point to a positive test result as a way to explain enigmatic symptoms. However, a test alone does not establish a diagnosis. The tilt table findings must be combined with other clinical information. It is also important not to label a healthy patient who has never fainted with a diagnosis of syncope on the basis of a tilt table test.
A positive tilt table test with pharmacologic provocation may also indicate a physiologic predisposition to syncope, but with less degree of certainty. Further, as the Reggie Lewis case exemplifies, a positive tilt table test does not exclude alternative or additional diagnoses.
Tilt table artifacts
Not all variations in BP and HR during tilt table testing indicate autonomic pathology. Valid assessment of hemodynamic changes requires that testing be conducted under controlled conditions and interpreted by a clinician knowledgeable about autonomic physiology.
A diagnosis of OH, for example, requires a valid baseline from which to evaluate changes in the upright posture. Prior to tilting, the patient should remain at rest in a recumbent position for at least 10 minutes. Factors that can elevate baseline BP include pain, anxiety, and pressor drugs. Without a sufficient time of rest, if baseline elevation of BP is not allowed to reach equilibrium, a falsely positive determination of OH may be made.
While the patient is positioned head-up, it is important to maintain the arm where BP is being measured at the same height as the heart. If the arm is allowed to descend, the hydrostatic gradient between the heart and the measuring device will result in a falsely elevated BP of 0.8 mmHg for each cm of vertical displacement [95]. Mechanical properties of arteries, which increase in stiffness at higher pressures, also affect the accuracy of BP meaurement [96].
Artifactual drops in BP frequently occur when the finger or wrist device loses contact with the peripheral pulse. This can happen if the patient moves the arm slightly or when the patient’s position on the table shifts during the transition to a vertical orientation. These drops are typically abrupt and may exhibit a stuttering pattern of inconsistent pulse detection. In other situations they may taper off if the pulse pressure narrows or as a result of finger vasoconstriction [61]. Finger cuff measurements should be confirmed by sphygmomanometry in the opposite arm. Oscillations or fluctuations in BP during head-up tilt may indicate orthostatic intolerance, but they may also occur in response to limb tensing, coughing, or hyperkinetic movement disorders. Tremors are typically much higher in frequency than sympathoneural BP variations [61].
Conclusion
Tilt table testing is an invaluable component of the clinical assessment of the autonomic nervous system and is particularly useful in the evaluation of disorders that impair orthostatic tolerance. With or without the use of a tilt table, an expertly obtained history is indispensable to the accurate diagnosis of syncope and orthostatic disorders [2,83,97].
Abbreviations:
- ANS
autonomic nervous system
- BP
blood pressure
- COI
chronic orthostatic intolerance
- EPI
epinephrine
- FVR
forearm vascular resistance
- HR
heart rate
- HUT
head-up tilt
- NE
norepinephrine
- NET
cell membrane norepinephrine transporter
- NMH
neurally mediated hypotension
- nOH
neurogenic orthostatic hypotension
- OH
orthostatic hypotension
- POTS
postural tachycardia syndrome
- SNS
sympathetic noradrenergic system
- SV
cardiac stroke volume
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Niemitz C (2010) The evolution of the upright posture and gait – a review and a new synthesis. Naturwissenschaften 97:241–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein DS, Cheshire WP (2017) The autonomic medical history. Clin Auton Res 27:223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheshire WP, Goldstein DS (2018) The physical examination as a window into autonomic disorders. Clin Auton Res 28:23–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith JJ, Porth CM, Erickson M (1994) Hemodynamic response to the upright posture. J Clin Pharmacol 34:375–386 [DOI] [PubMed] [Google Scholar]
- 5.Waterfield RL (1931) The effects of posture on the circulating blood volume. J Physiol 72:110–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson WO, Thompson PK, Dailey ME (1928) The effect of posture upon the composition and volume of the blood in man. J Clin Invest 5:573–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borst C, van Brederode JF, Wieling W, van Montfrans GA, Dunning AJ (1984) Mechanisms of initial blood pressure resposne to postural change. Clin Sci (Lond) 67:321–327 [DOI] [PubMed] [Google Scholar]
- 8.Nilsson D, Sutton R, Tas W, Burri P, Melander O, Fedorowski A (2015) Orthostatic changes in hemodynamics and cardiovascular biomarkers in dysautonomic patients. PLoS One 10(6):e0128962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harvey W (1628) Exercitatio Anatomica de Motu Cordis et Sanguinis in Animalibus. Leake CD, trans., Fifth Edition, Springfield, IL: Charles C. Thomas, 1970 [Google Scholar]
- 10.Hoffman F (1740) De situ erecto in morbis periculosis valde noxio. Opera Omnia Physico-Medica 6:169–173 [Google Scholar]
- 11.Piorry PA (1830) Du procédé operatoire, &c. Of the operative process to be followed in the examination of organs by mediate percussion, and a collection of memoirs on physiology, pathology, and diagnosis. Edinburgh Med Surg J 43:417–427 [Google Scholar]
- 12.Hill L (1895) The influence of the force of gravity on the circulation of the blood. J Physiol 18:15–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill L, Barnard H (1897) The influence of the force of gravity on the circulation. J Physiol 21:323–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langley JN (1898) On the union of cranial autonomic (visceral) fibres with nerve cells of the superior cervical ganglion. J Physiol 23:240–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langley JN (1891) Note on the connection with nerve-cells of the vaso-motor nerves for the feet. J Physiol 12:375–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langley JN (1923) The vascular dilatation caused by the sympathetic and the course of vaso-motor nerves. J Physiol 58:70–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helmreich E (1923) Statische und dynamische Pulsacceleration. Z ges exp Med 36:226–235 [Google Scholar]
- 18.Turner AH, Newton MI, Haynes FW (1930) The circulatory reaction to gravity in healthy young women. Am J Physiol 94:507–520 [Google Scholar]
- 19.Kenny RA, Ingram A, Bayliss J, Sutton R (1986) Head-up tilt: a useful test for investigating unexplained syncope. Lancet 1(8494):1352–1355 [DOI] [PubMed] [Google Scholar]
- 20.Bearn AG, Billing B, Edholm OG, Sherlock S (1951) Hepatic blood flow and carbohydrate changes in man during fainting. J Physiol 115:442–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCally M, Piemme TE, Murray RH (1966) Tilt table responses of human subjects following application of lower body negative pressure. Aerosp Med 37:1247–1249 [PubMed] [Google Scholar]
- 22.El Bedawi KM, Hainsworth R (1994) Combined head-up tilt and lower body suction: a test of orthostatic tolernace. Clin Auton Res 43:41–47 [DOI] [PubMed] [Google Scholar]
- 23.Protheroe CL, Ravensbergen HR, Inskip JA, Claydon VE (2013) Tilt testing with combined lower body negative pressure: a “gold standard” for measuring orthostatic tolerance. J Vis Exp (73), e4315, doi: 10.3791/4315 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss S, Wilkins RW, Haynes FW (1937) The nature of circulatory collapse induced by sodium nitrite. J Clin Invest 16:73–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waxman MB, Yao L, Cameron DA, Wald RW, Roseman J (1989) Isoproterenol induction of vasodepressor-type reaction in vasodepressor-prone persons. Am J Cardiol 63:58–65 [DOI] [PubMed] [Google Scholar]
- 26.Raviele A, Gasparini G, Di Pede F, Menozzi C, Brignole M, Dinelli M, Alboni P, Piccolo E (1994) Nitroglycerin infusion during upright tilt: a new test for the diagnosis of vasovagal syncope. Am Heart J 127:103–111 [DOI] [PubMed] [Google Scholar]
- 27.Raviele A, Menozzi C, Brignole M, Gasparini G, Alboni P, Musso G, Lolli G, Oddone D, Dinelli M, Mureddu R (1995) Value of head-up tilt testing potentiated with sublingual nitroglycerin to assess the origin of unexplained syncope. Am J Cardiol 76:287–272 [DOI] [PubMed] [Google Scholar]
- 28.Tanaka H, Sjöberg BJ, Thulesius O (1996) Cardiac output and blood pressure during active and passive standing. Clin Physiol 16:157–170 [DOI] [PubMed] [Google Scholar]
- 29.Sprangers RL, Wesseling KH, Imholz AL, Imholz BP, Wieling W (1985) Initial blood pressure fall on stand up and exercise explained by changes in total peripheral resistance. J Appl Physiol 70:523–530 [DOI] [PubMed] [Google Scholar]
- 30.Wieling W, Harms MP, Kortz RA, Linzer M (2001) Initial orthostatic hypotension as a cause of recurrent syncope: a case report. Clin Auton Res 11:269–270 [DOI] [PubMed] [Google Scholar]
- 31.Wieling W, Krediet CT, van Dijk N, Linzer M, Tschakovsky ME (2007) Initial orthostatic hypotension: review of a forgotten condition. Clin Sci (Lond) 112:157–165 [DOI] [PubMed] [Google Scholar]
- 32.Aydin AE, Soysal P, Isik AT (2017) Which is preferable for orthostatic hypotension diagnosis in older adults: active standing trest or head-up tilt table test? Clin Interv Aging 12:207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borst C, Wieling W, van Brederode JF, Hond A, de Rijk LG, Dunning AJ (1982) Mechanisms of initial heart rate response to postural change. Am J Physiol 243:H676–681 [DOI] [PubMed] [Google Scholar]
- 34.Ewing DJ, Hume L, Campbell IW, Murray A, Neilson JM, Clarke BF (1980) Autonomic mechanisms in the initial heart rate response to standing. J Appl Physiol Respir Environ Exerc Physiol 59:809–814 [DOI] [PubMed] [Google Scholar]
- 35.Lewis NC, Atkinson G, Lucas SJ, Grant EJ, Jones H, Tzeng YC, Horsman H, Ainslie PN (2010) Diurnal variation in time to presyncope and associated circulatory changes during a controlled orthostatic challenge. Am J Physiol Regul Integr Comp Physiol 299:R55–R61 [DOI] [PubMed] [Google Scholar]
- 36.Zoghi M, Duygu H, Gungor H, Nalbantgil S, Ozerkan F, Akilli A, Akin M (2008) Circadian and infradian rhythms of vasovagal syncope in young and middle-aged subjects. Pacing Clin Electrophysiol 31:1581–1584 [DOI] [PubMed] [Google Scholar]
- 37.Mineda Y, Sumiyoshi M, Tokano T, Yasuda M, Nakazato K, Nakazato Y, Nakata Y, Yamaguchi H (2000) Circadian variation of vasovagal syncope. J Cardiovasc Electrophysiol 11:1078–1080 [DOI] [PubMed] [Google Scholar]
- 38.Brewster JA, Garland EM, Biaggioni I, Black BK, Ling JF, Shibao CA, Robertson D, Raj SR (2012) Diurnal variability in orthostatic tachycardia: implications for the postural tachycardia syndrome. Clin Sci (Lond) 122:25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis NC, Ainslie PN, Atkinson G, Jones H, Grant EJ, Lucas SJ (2012) The effect of time-of-day and sympathetic α1-blockade on orthostatic tolerance. Chronobiol Int 29:882–890 [DOI] [PubMed] [Google Scholar]
- 40.Lewis NC, Atkinson G, Lucas SJ, Grant EJ, Jones H, Tzeng YC, Horsman H, Ainslie PN (2011) Is there diurnal variation in initial and delayed orthostatic hypotension during standing and head-up tilt? Chronobiol Int 28:135–145 [DOI] [PubMed] [Google Scholar]
- 41.Maurer MS, Karmally W, Rivadeneira H, Parides MK, Bloomfield DM (2000) Upright posture and postprandial hypotension in elderly persons. Ann Intern Med 133:533–536 [DOI] [PubMed] [Google Scholar]
- 42.Teramoto S, Akishita M, Fukuchi Y, Toba K, Ouchi Y (1997) Assessment of autonomic nervous function in elderly subjects with or without postprandial hypotension. Hypertens Res 20:257–261 [DOI] [PubMed] [Google Scholar]
- 43.Thomaides T, Bleasdale-Barr K, Chaudhuri KR, Pavitt D, Marsden CD, Mathias CJ (1993) Cardiovascular and hormonal responses to liquid food challenge in idiopathic Parkinson’s disease, multiple system atrophy, and pure autonomic failure. Neurology 43:900–904 [DOI] [PubMed] [Google Scholar]
- 44.Shen W-K, Jahangir A, Beinborn D, Lohse CM, Hodge DO, Rea RF, Hammill SC (1999) Utility of a single-stage isoproterenol tilt table test in adults. J Amm Coll Cardiol 33:985–990 [DOI] [PubMed] [Google Scholar]
- 45.Saal DP, Thijs RD, van Dijk JG (2016) Tilt table testing in neurology and clinical neurophysiology. Clin Neurophysiol 127:1022–1030 [DOI] [PubMed] [Google Scholar]
- 46.Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch EE, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz I, Schondorf R, Stewart JM, van Dijk JG (2011) Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 21:69–72 [DOI] [PubMed] [Google Scholar]
- 47.Kamiya A, Kawada T, Shimizu S, Iwase S, Sugimachi M, Mano T (2009) Slow head-up tilt causes lower activation of muscle sympathetic nerve activity: loading speed dependence of orthostatic sympathetic activation in humans. Am J Physiol Heart Circ Physiol 297:H53–H58 [DOI] [PubMed] [Google Scholar]
- 48.Shamsuzzaman AS, Sugiyama Y, Kamiya A, et al. (1998) Head-up suspension in humans: effects on sympathetic vasomotor activity and cardiovascular responses. J Appl Physiol 84:1513–1519 [DOI] [PubMed] [Google Scholar]
- 49.Fedorowski A, Hamrefors V, Sutton R, van Dijk JG, Freeman R, Lenders JW, Wieling W (2017) Do we need to evaluate diastolic blood pressure in patients with suspected orthostatic hypotension? Clin Auton Res 27:167–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romero-Ortuno R, Cogan L, Fan CW, Kenny RA (2010) Intolerance to initial orthostasis relates to systolic BP changes in elders. Clin Auton Res 20:39–45 [DOI] [PubMed] [Google Scholar]
- 51.Goldstein DS, Holmes C, Frank SM, Naqibuddin M, Dendi R, Snader S, Calkins H (2003) Sympathoadrenal imbalance before neurocardiogenic syncope. Am J Cardiol 91:53–58 [DOI] [PubMed] [Google Scholar]
- 52.Heyer GL, Harvey RA, Islam MP (2018) Signs of autonomic arousal precede tilt-induced psychogenic nonsyncopal collapse amon g youth. Epilepsy Behav 86:166–172 [DOI] [PubMed] [Google Scholar]
- 53.Norcliffe-Kaufmann L, Galindo-Mendez B, Garcia-Guarniz AL, Villarreal-Vitorica E, Novak V (2018) Transcranial Doppler in autonomic testing: standards and clinical applications. Clin Auton Res 28:187–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheshire WP (2017) Clinical classification of orthostatic hypotensions. Clin Auton Res 27:133–135 [DOI] [PubMed] [Google Scholar]
- 55.Goldstein DS (2009) Neurogenic orthostatic hypotension: a pathophysiological approach. Circulation 119:139–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheshire WP (2018) Chemical pharmacotherapy for the treatment of orthostatic hypotension. Expert Opin Pharmacother doi: 10.1080/14656566.2018.1543404 [DOI] [PubMed] [Google Scholar]
- 57.Norcliffe-Kaufmann L, Kaufmann H, Palma JA, Shibao CA, Biaggioni I, Peltier AC, Singer W, Low PA, Goldstein DS, Gibbons CH, Freeman R, Robertson D (2018) Orthostatic heart rate changes in patients with autonomic failure caused by neurodegenerative synucleinopathies. Ann Neurol 83:522–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vogel ER, Sandroni P, Low PA (2005) Blood pressure recovery from Valsalva maneuver in patients with autonomic failure. Neurology 65:1533–1537 [DOI] [PubMed] [Google Scholar]
- 59.Gibbons CH, Freeman R (2015) Clinical implications of delayed orthostatic hypotension: a 10-year follow-up study. Neurology 85:1362–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lahrmann H, Cortelli P, Hilz M, Mathias CJ, Struhal W, Tassinari M (2006) EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol 13:930–936 [DOI] [PubMed] [Google Scholar]
- 61.Cheshire WP (2019) Artifact in autonomic neurophysiology studies. In: Tatum WO, editor, Atlas of Artifacts in Clinical Neurophysiology. New York: Springer, pp. 237–258 [Google Scholar]
- 62.Cheshire WP (2016) Stimulant medication and postural orthostatic tachycardia syndrome: a tale of two cases. Clin Auton Res 26:229–233 [DOI] [PubMed] [Google Scholar]
- 63.Sheldon RS, Grubb BP, Olshansky B, Shen W-K, Calkins H, Brignole M, Raj SR, Krahn AD, Morillo CA, Stewart JM, Sutton R, Sandroni P, Friday KJ, Hachul DT, Cohen MI, Lau DH, Mayuga KA, Moak JP, Sandhu RK, Kanjwal K (2015) 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 12:e41–e63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grubb BP (2005) Neurocardiogenic syncope. New Engl J Med 352:1004–1010 [DOI] [PubMed] [Google Scholar]
- 65.Moya A, Sutton R, Ammirati F, Blanc J-J, Brignole M, Dahm JB, Deharo J-C, Gajek J, Gjesdal K, Krahn A, Massin M, Pepi M, Pezawas T, Granell RR, Sarasin F, Ungar A, van Dijk JG, Walma EP, Wieling W, et al. (2009) Guidelines for the diagnosis and management of syncope. The Task Force for the Diagnosis and Management of Syncope of the European Society of Cardiology (ESC). Eur Heart J 30:2631–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wieling W, Thijs RD, van Dijk N, Wilde AA, Benditt DG, van Dijk JG (2009) Symptoms and signs of syncope: a review of the link between physiology and clinical clues. Brain 132:2630–2642 [DOI] [PubMed] [Google Scholar]
- 67.Ganzeboom KS, Mairuhu G, Reitsma JB, Linzer M, Wieling W, van Dijk N (2006) Lifetime cumulative incidence of syncope in the general population: a study of 549 Dutch subjects aged 35–60 years. J Cardiovasc Electrophysiol 17:1172–1176 [DOI] [PubMed] [Google Scholar]
- 68.Solbiati M, Casazza G, Dipaola F, Rusconi AM, Cernuschi G, Barbic F, Montano N, Sheldon RS, Furlan R, Costantino G (2015) Syncope recurrence and mortality: a systematic review. Europace 17:300–308 [DOI] [PubMed] [Google Scholar]
- 69.Novak V, Honos G, Schondorf R (1996) Is the heart “empty” at syncope? J Auton Nerv Syst 60:83–92 [DOI] [PubMed] [Google Scholar]
- 70.Scherrer U, Vissing S, Morgan BJ, Hanson P, Victor RG (1990) Vasovagal syncope after infusion of a vasodilator in a heart transplant recipient. New Engl J Med 322:602–604 [DOI] [PubMed] [Google Scholar]
- 71.Mitro P, Hijová E (2006) Myocardial contractility and cardiac filling measured by impedance cardiography in patient with nitroglycerine-induced vasovagal syncope. PACE 29:1–8 [DOI] [PubMed] [Google Scholar]
- 72.Vaddadi G, Esler MD, Dawood T, Lambert E (2010) Persistence of muscle sympathetic nerve activity during vasovagal syncope. Eur Heart J 31:2027–2033 [DOI] [PubMed] [Google Scholar]
- 73.Cooke WH, Rickards CA, Ryan KL, Kuusela TA, Convertino VA (2009) Muscle sympathetic nerve activity during intense lower body negative pressure to presyncope in humans. J Physiol 587:4987–4999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fu Q, Levine BD (2014) Pathophysiology of neurally mediated syncope: role of cardiac output and total peripheral resistance. Auton Neurosci 184;24–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fu Q, Verheyden B, Wieling W, Levine BD (2012) Cardiac output and sympathetic vasocontrictor responses during upright tilt to presyncope in healthy humans. J Physiol 590:1839–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stewart JM, Sutton R, Kothari ML, Goetz AM, Visintainer P, Medow MS (2017) Nitric oxide synthase inhibition restores orthostatic tolerance in young vasovagal syncope patients. Heart 103:1711–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Norcliffe-Kaufmann L, Kaufmann H, Hainsworth R (2007) enhanced vascular responses to hypocapnia in neurally mediated syncope. Ann Neurol 63:288–294. [DOI] [PubMed] [Google Scholar]
- 78.Engel GL (1978) Psychologic stress, vasodepressor (vasovagal) syncope, and sudden death. Ann Intern Med 89:403–412 [DOI] [PubMed] [Google Scholar]
- 79.Ganzeboom KS, Colman N, Reitsma JB, Shen WK, Wieling W (2003) Prevalence and triggers of syncope in medical students. Am J Cardiol 91:1006–1008 [DOI] [PubMed] [Google Scholar]
- 80.Jamjoom AAB, Nikkar-Esfahani A, Fitzgerald JEF (2009) Operating theatre related syncope in medical students: a cross sectional study. BMC Medical Education 9:14. doi: 10.1186/1472-6920-9-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim JB, Suh S-I, Seo W-K, Koh S-B, Kim JH (2014) Right insular atrophy in neurocardiogenic syncope: a volumetric MRI study. AJNR Am J Neuroradiol 34:113–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Diehl RR, Linden D, Chalkiadaki A, Diehl A (1999) Cerebrovascular mechanisms in neurocardiogenic syncope with and without postural tachycardia syndrome. J Auton Nerv Syst 76:159–166 [DOI] [PubMed] [Google Scholar]
- 83.Cheshire WP (2017) Syncope. Continuum 23:335–358 [DOI] [PubMed] [Google Scholar]
- 84.Finsterer J, Stöllberger C (2016) Neurological complications of cardiac disease (heart brain disorders). Minerva Med 107:14–25 [PubMed] [Google Scholar]
- 85.Brignole M, Alboni P, Benditt DG, Bergfeldt L, Blanc JJ, Bloch Thomsen PE, van Dijk JG, Fitzpatrick A, Hohnloser S, Janousek J, Kapoor W, Kenny RA, Kulakowski P, Masotti G, Moya A, Raviele A, Sutton R, Theodorakis G, Ungar A, Wieling W; Task Force on Syncope, European Society of Cardiology (2004) Guidelines on management (diagnosis and treatment) of syncope—update 2004. Europace 6:467–537 [DOI] [PubMed] [Google Scholar]
- 86.Mittal S, Stein KM, Markowitz SM, Iwai S, Guttigoli A, Lerman BB (2004) Single-stage adenosine tilt testing in patients with unexplained syncope. J Cardiovasc Electrophysiol 15:637–640 [DOI] [PubMed] [Google Scholar]
- 87.Theodorakis GN, Markianos M, Zarvalis E, Livanis EG, Flevari P, Kremastinos DT (2000) Provocation of neurocardiogenic syncope by clomipramine administration during the head-up tilt test in vasovagal syndrome. J Am Coll Cardiol 36:174–178 [DOI] [PubMed] [Google Scholar]
- 88.Almquist A, Goldenberg IF, Milstein S, Chen M-Y, chen X, Hansen R, Cornick CC, Benditt DG (1989) Provocation of bradycardia and hypotension by isoproterenol and upright posture in patients with unexplained syncope. N Engl J Med 320:346–351 [DOI] [PubMed] [Google Scholar]
- 89.Leman RB, Clarke E, Gillette P (1999) Significant complications can occur with ischemic heart disease and tilt table testing. PACE 22:675–677 [DOI] [PubMed] [Google Scholar]
- 90.Aerts AJ, Dendale P (2003) Nitrate stimulated tilt table testing: a review of the literature. Pacing Clin Electrophysiol 26:1528–1537 [DOI] [PubMed] [Google Scholar]
- 91.Stevens PM (1966) Cardiovascular dynamics during orthostatis and the influence of intravascular instrumentation. Am J Cardiol 17:211–218 [DOI] [PubMed] [Google Scholar]
- 92.Aerts AJ, Dendale P, Block P, Dassen WR (2005) Reproducibility of nitrate-stimulated tilt testing in patients with suspected vasovagal syncope and a healthy control group. Am Heart J 150:251–256 [DOI] [PubMed] [Google Scholar]
- 93.Delépine S, Prunier F, Lefthériotis G, Dupuis J, Vielle B, Geslin P, Victor J (2002) Comparison between isoproterenol and nitroglycerin sensitized head-upright tilt in patients with unexplained syncope and negative or positive passive head-up tilt response. Am J Cardiol 90:488–491 [DOI] [PubMed] [Google Scholar]
- 94.Prabhu MA, Pillai V, Shenthar J (2017) Comparison of Efficacy, Pattern of Response, Occurrence of Arrhythmias, and the Tolerability of Nitroglycerine and Isoprenaline as Provocative Drugs During Head-Up Tilt Test. Heart Lung Circ 26:586–592 [DOI] [PubMed] [Google Scholar]
- 95.Seymour RS, Hargens AR, Pedley TJ (1993) The heart works against gravity. Am J Physiol 265:R715–720 [DOI] [PubMed] [Google Scholar]
- 96.Gavish B, Gavish L (2011) Blood pressure variation in response to changing arm cuff height cannot be explained solely by the hydrostatic effect. J Hypertens 29:2099–2104 [DOI] [PubMed] [Google Scholar]
- 97.Wieling W, van Dijk N, de Lange FJ, Olde Nordkamp LRA, Thijs RD, van Dijk JG, Linzer M, Sutton R (2015) History taking as a diagnostic test in patients with syncope: developing expertise in syncope. Eur Heart J 36:277–280 [DOI] [PubMed] [Google Scholar]


