Abstract
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death in the United States. The precise role of bacterial infection in the course and pathogenesis of COPD has been a source of controversy for decades. Chronic bacterial colonization of the lower airways contributes to airway inflammation; more research is needed to test the hypothesis that this bacterial colonization accelerates the progressive decline in lung function seen in COPD (the vicious circle hypothesis). The course of COPD is characterized by intermittent exacerbations of the disease. Studies of samples obtained by bronchoscopy with the protected specimen brush, analysis of the human immune response with appropriate immunoassays, and antibiotic trials reveal that approximately half of exacerbations are caused by bacteria. Nontypeable Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae are the most common causes of exacerbations, while Chlamydia pneumoniae causes a small proportion. The role of Haemophilus parainfluenzae and gram-negative bacilli remains to be established. Recent progress in studies of the molecular mechanisms of pathogenesis of infection in the human respiratory tract and in vaccine development guided by such studies promises to lead to novel ways to treat and prevent bacterial infections in COPD.
It is estimated that in 1995, 16.4 million people in the United States suffered from chronic obstructive pulmonary disease (COPD) (226). COPD is also the fourth most common cause of death in the United States (14). Both the prevalence of and mortality from this disease have been increasing worldwide (118, 226, 330). COPD is defined physiologically by the presence of irreversible or partially reversible airway obstruction in patients with chronic bronchitis and/or emphysema (14). Chronic bronchitis is defined clinically by the presence of cough with sputum production for most days of at least 3 months a year for 2 consecutive years (200). Other causes of chronic cough need to be excluded. Emphysema is defined pathologically as permanent dilation of the airspaces distal to the terminal bronchioles, accompanied by destruction of the alveolar septa in the absence of fibrosis (292). More than 80% of COPD cases encountered in the Western world are related to tobacco smoke exposure. Occupational exposures and alpha-1 antitrypsin deficiency are uncommon precedents for the development of COPD (177, 234).
Several potential contributions of bacterial infection to the etiology, pathogenesis, and clinical course of COPD can be identified (219). However, the precise role of bacterial infection in COPD has been a source of controversy for several decades (175, 296, 307). Opinion regarding the contribution of bacteria to the pathogenesis of COPD has ranged from the idea that it has a preeminent role (along with mucus hypersecretion) as embodied in the British hypothesis in the 1950s and 1960s, to the idea that it is a mere epiphenomenon in the 1970s and 1980s (200, 296, 307). In the last decade, new research techniques have become available, and traditionally noninfectious diseases such as peptic ulcer have been shown to be of infectious origin (240). This has renewed interest in the area of bacteria and COPD, and these new research methodologies should lead to a precise delineation of the contribution of bacterial infection to this disease.
Five potential pathways by which bacteria could contribute to the course and pathogenesis of COPD can be identified. (i) Childhood lower respiratory tract infection impairs lung growth, reflected in smaller lung volumes in adulthood. (ii) Bacteria cause a substantial proportion of acute exacerbations of chronic bronchitis which cause considerable morbidity and mortality. (iii) Chronic colonization of the lower respiratory tract by bacterial pathogens amplifies the chronic inflammatory response present in COPD and leads to progressive airway obstruction (vicious circle hypothesis). (iv) Bacterial pathogens invade and persist in respiratory tissues, alter the host response to cigarette smoke, or induce a chronic inflammatory response and thus contribute to the pathogenesis of COPD. (v) Bacterial antigens in the lower airway induce hypersensitivity that enhances airway hyperreactivity and induces eosinophilic inflammation. Evidence supporting these roles will be discussed in this review, with an emphasis on information gained from newer research techniques in the last decade. The second part of this review will discuss each of the major pathogens, with emphasis on recent developments related specifically to infections in COPD.
POTENTIAL ROLES OF BACTERIAL INFECTION IN COPD
Childhood Lower Respiratory Tract Infection and Adult Lung Function
Four recent studies have reported lung function (measured by spirometry) in cohorts of adult patients for whom reliable information was available regarding the incidence of lower respiratory tract infection (bronchitis, pneumonia, or whooping cough) in childhood (<14 years of age) (Table 1) (26, 152, 279, 280). These studies have consistently shown a lower forced expiratory volume in 1 s (FEV1) and often a lower forced vital capacity among adults who experienced childhood lower respiratory tract infection compared to others in the cohort who did not experience such infection (26, 152, 279, 280). FEV1 and forced vital capacity are widely used tests of pulmonary function. This association is seen after controlling for confounding factors such as tobacco exposure. The magnitude of this defect in FEV1 has varied among the studies but tends to be greater in older cohorts. The extent of decrease in FEV1 is unlikely to cause symptomatic pulmonary disease per se but could make the individual susceptible to the effects of additional injurious agents, such as tobacco smoke, and environmental or occupational exposure to air-borne pollutants. The defect in lung function is not airway obstruction, as the FEV1/FVC ratio is preserved. Instead, it is consistent with “smaller lungs”, suggesting impaired lung growth.
TABLE 1.
Association of childhood lower respiratory tract infection with lung function in adults
| Study | No. in study | Lower respiratory tract infection history | Age at followup (yr) | Effect on FEV1 |
|---|---|---|---|---|
| Barker et al. (26) | 639 (all male) | Bronchitis or pneumonia in first yr | 59–67 | Lowered by 200 ml |
| Shaheen et al. (279) | 618 | Pneumonia or bronchitis in first 2 yr | 67–74 | Lowered by 650 ml in males with pneumonia |
| Johnston et al. (152) | 1,392 | Pneumonia or whooping cough in first 7 yr | 34–35 | Lowered by 102 ml with pneumonia |
| Shaheen et al. (280) | 239 | Pneumonia in first 14 yr | 57.6 ± 4.3 | Lowered by 390 ml |
| Bronchitis in first 14 yr | Lowered by 130 ml |
Although the association between childhood lower respiratory tract infection and impaired lung function in adulthood is now well established, there is ongoing debate whether this association reflects a cause-effect relationship. Such a relationship could be explained by damage caused to a vulnerable lung undergoing rapid postnatal growth and maturation by the infectious process. If this were the case, then the effect of the infection on lung function should be seen only in the first 2 years of life, the major period of postnatal lung growth, but not in later childhood (3 to 14 years). However, this has not been a consistent observation in the studies to date (26, 152, 279, 280). An alternative explanation for the observed association between childhood lower respiratory tract infection and impaired lung function in adulthood is that an undetermined genetic factor predisposes these individuals to lower respiratory tract infections in childhood as well as a lower FEV1 in adulthood. This explanation implies that impaired lung growth antedates the respiratory tract infection, with the infectious episode a result of the vulnerability of smaller lungs to infection in childhood.
The etiology of childhood lower respiratory tract infection was not established in these studies, and therefore whether the impact of viral infection differs from that of bacterial infection is not known. Though it is likely that a substantial proportion of these childhood infections were viral, bacterial infection, especially with Streptococcus pneumoniae and Haemophilus influenzae, is a common cause of severe pneumonia in children (333). The impact of childhood bacterial lower respiratory tract infection on the prevalence of COPD is likely to be greater in developing countries, where these infections are common and are often inadequately treated.
Bacterial Pathogens as a Cause of Acute Exacerbations of COPD
Bacteria are isolated from sputum in 40 to 60% of acute exacerbations of COPD (274). Table 2 shows the sputum bacteriology obtained in 14 clinical trials of antibiotics in acute exacerbation published in the last 4 years (12, 19, 53–55, 71, 74, 127, 170–172, 251, 278, 348). Variation in the relative incidence of specific pathogens is seen and may relate to patient inclusion criteria and sputum culture techniques. The three predominant bacterial species isolated are nontypeable Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae. Other infrequently isolated potential pathogens are Haemophilus parainfluenzae, Staphylococcus aureus, Pseudomonas aeruginosa, and members of the family Enterobacteriaceae.
TABLE 2.
Bacterial pathogens isolated from sputum in recent studies of acute exacerbation of chronic bronchitis
| Study | No. of patients | No. of culture positive | No. of bacterial isolates | % of bacterial isolatesa
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Haemophilus influenzae | Moraxella catarrhalis | Streptococcus pneumoniae | Staphylococcus aureus | Pseudomonas aeruginosa | Haemophilus parainfluenzae | Enterobacteriaceae | ||||
| Allegra et al. (12) | 728 | 298 | 375 | 28 | 11 | 26 | 5 | 11 | —b | 15 |
| Anzueto et al. (19) | 2,180 | 673 | 777 | 13 | 18 | 7 | 17c | 4 | 15 | 18 |
| Chodosh et al. (55) | 376 | 234 | 274 | 36 | 20 | 14 | 1 | 5 | 4 | 7 |
| Chodosh et al. (54) | 307 | 208 | 253 | 25 | 21 | 10 | 4 | 3 | 8 | 15 |
| Chodosh et al. (53) | 624 | 290 | 379 | 18 | 21 | 7 | 20c | DNd | 6 | DN |
| Davies et al. (71) | 140 | 124 | 146 | 50 | 17 | 21 | 1 | 8 | — | 3 |
| DeAbate et al. (74) | 798 | 647 | 835 | 18 | 9 | 8 | 5 | 4 | 32 | 8 |
| Habib et al. (127) | 373 | 192 | 181 | 25 | 14 | 8 | 7 | 13 | 12 | 19 |
| Langan et al. (170) | 684 | 192 | 211 | 34 | 4 | 12 | 9 | 5 | 11 | 5 |
| Langan et al. (172) | 802 | 400 | 513 | 36 | 12 | 11 | 3 | DN | 27 | DN |
| Langan et al. (171) | 656 | 478 | 542 | 41 | 19 | 23 | 1 | 3 | 6 | DN |
| Read et al. (251) | 364 | 103 | 128 | 46 | 9 | 9 | 8 | 5 | 3 | 15 |
| Shah et al. (278) | 832 | 547 | 577 | 36 | 16 | 18 | 3 | 8 | 2 | 5 |
| Wilson et al. (348) | 750 | 287 | 342 | 31 | 15 | 25 | 5 | 1 | 5 | 5 |
The values given for each bacterial pathogen represent the percentage of total isolates in each study.
—, not reported.
Increased incidence probably related to sputum processing in centralized laboratories.
DN, details not available in the article to calculate percentage of isolates.
Whether isolation from sputum of a potential pathogen represents infection of the lower airway causing the exacerbation episode has been a controversial issue for several decades. In the 1950s and 1960s, the British hypothesis of the pathogenesis of COPD included as major contributors recurrent bacterial infection and mucus hypersecretion (200). With the realization of tobacco smoke exposure as the primary pathogenic mechanism of COPD and the emergence of evidence that mucus hypersecretion and worsening airway obstruction were independent, the British hypothesis fell into disfavor (28, 102, 145). Several longitudinal cohort studies in the 1960s and 1970s demonstrated that the incidence of bacterial isolation from sputum during exacerbations of COPD was not different from the incidence during stable COPD (123, 196). These studies also failed to demonstrate a higher bacterial titer in sputum during acute exacerbation than during stable COPD (123). Serological studies conducted in the same time period that compared serum antibody titers to airway bacterial pathogens such as nontypeable H. influenzae in COPD patients with those in controls arrived at confusing and contradictory conclusions (reviewed in reference 219). Results of placebo-controlled antibiotic trials in acute exacerbations have also been inconsistent, demonstrating either small or no benefit with antibiotic therapy (17, 269).
A common interpretation of these observations has been that isolation of bacteria from sputum during exacerbations represents chronic colonization, an innocent bystander role for the bacteria (95, 228, 296, 307). Alternative explanations for the contradictory observations from serological studies and antibiotic therapy should be considered (148, 219). Serological studies of antibodies to bacterial pathogens in exacerbations yielded confusing and contradictory results for the following reasons. (i) Laboratory strains were often used as the antigen instead of homologous patient strains. Thus, the strain variation in the surface antigens of the bacterial pathogens that has only recently been understood was not taken into account in these studies. (ii) The immunological methods employed in these studies often were not specific for antibodies to surface-exposed epitopes on the bacteria. A bacterial pathogen presents several hundred antigenic epitopes to a host, many of which are non-surface exposed and therefore potentially irrelevant to host defense. Furthermore, several of these epitopes are cross-reactive among bacterial species. A protective immune response that develops after an infection may be limited to a few epitopes on the bacterial surface. Detecting this response among the multitude of irrelevant, non-surface-exposed antigen-antibody interactions requires immunological assays that are specific for antibodies that bind to surface-exposed epitopes on the bacteria. Such assays include radioimmunoprecipitation assays, flow cytometry assays with whole bacterial cells as an antigen, and assays that measure functional (bactericidal or opsonophagocytic) antibodies. Immunoblots and whole bacterial immunodots that were used in previous serological studies do not have such specificity (115). (iii) Not all studies used true preinfection sera for comparison with the convalescent-phase serum. Instead, acute-phase serum taken at the time that the patient presents with symptoms was used. The symptoms of an acute exacerbation have often been present for several days, and therefore a serological response to the strain may be missed if an acute-phase serum is substituted for a preinfection serum.
Trials showing no benefit with antibiotics in acute exacerbations also have several potential explanations. (i) An exacerbation is a mucosal infection, and the use of antibiotics in other mucosal infections such as otitis media and sinusitis is also not associated with dramatic efficacy over placebo (345). This does not imply that mucosal infections are nonbacterial. (ii) The expected benefits from antibiotics in a mucosal infection are primarily a more rapid resolution of symptoms and prevention of complications. Unfortunately, most studies of antibiotics in acute exacerbations have not measured the speed of resolution of symptoms. Instead, the endpoint has been whether the treatment was successful at 3 weeks after the onset of the exacerbation. The systemic immune-inflammatory response would be expected to resolve a large proportion of bacterial exacerbations in this time period, disguising any potential effect of antibiotics (17). (iii) Many studies include patients with mild impairment of lung function who are likely to have a low rate of complications, making a difference from the placebo group prone to type 2 error. In other words, the study populations could have contained too few individuals with potential for benefit for a benefit to be observed. (iv) Exacerbations are nonbacterial in 50% of patients, with no expected benefit from antibiotics, again predisposing studies to a type 2 error. (v) Antibiotic resistance in some of these pathogens that may be compounded by lack of penetration into the bronchial tissues and fluids of some of the antibiotics is likely to diminish the effect of antibiotics in exacerbations.
In the last decade, several investigators have reexamined the issue of whether bacteria cause acute exacerbations of COPD using either new diagnostic modalities or new research techniques, including bronchoscopic sampling of the lower respiratory tract (96, 208, 239, 294), immune response to bacterial pathogens in exacerbations (49, 222, 355), molecular epidemiology of bacterial pathogens (116, 220, 270, 271, 288, 289, 290), and airway inflammation measurement and correlation with bacteriology (161, 293). These methods provide new data which contribute to a more rigorous evaluation of the etiology of exacerbations.
Bronchoscopic sampling of lower respiratory tract in exacerbations of COPD.
An attractive approach to understanding the role of bacterial infection in exacerbated COPD is sampling of distal airway secretions for quantitative culture by protected specimen brush or by bronchoalveolar lavage (BAL) to determine bacterial concentrations in the distal airways. Such an approach has contributed tremendously to our understanding of nosocomial pneumonia (50). Samples obtained from the distal airways with these techniques have low levels of contamination by upper respiratory tract secretions. Bacterial concentrations above certain thresholds on quantitative culture have been found to correlate with tissue infection in patients with pneumonia (50). Four studies that have used this method in acute exacerbations have been published, and all have consistently shown significant bacterial infection of the distal airways in approximately 50% of patients experiencing an exacerbation (Table 3) (96, 208, 239, 294). The bacterial species isolated in these studies represent the same spectrum of pathogens commonly isolated from sputum cultures of patients with acute exacerbation.
TABLE 3.
Bronchoscopic studies in acute exacerbations of COPD
| Study | Subjects | Diagnostic methods | % of subjects with bacterial pathogen present | No. of isolates
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H. influenzae | M. catarrhalis | S. pneumoniae | H. parainfluenzae | P. aeruginosa | Other
|
|||||
| Gram negative | Gram positive | |||||||||
| Fagon et al. (96) | 50 ICU patients on ventilator | Protected specimen brush | 50a | 6 | 3 | 7 | 11 | 3 | 5 | 9 |
| Monso et al. (208) | 29 outpatients | Protected specimen brush | 51.7 | 10 | 2 | 3 | 2 | |||
| Soler et al. (294) | 50 ICU patients on ventilatorb | Protected specimen brush, BAL, endotracheal aspirate | 52 | 11 | 4 | 4 | 9 | 6 | ||
| Pela et al. (239) | 40 outpatients | Protected specimen brush | 52.5 | 1 | 2 | 10 | 1 | 1 | 7 | |
A positive culture was defined as ≥102 CFU/ml instead of the usual ≥103 CFU/ml.
Twenty-one patients had antimicrobial therapy in the 24 h prior to admission to the intensive care unit (ICU).
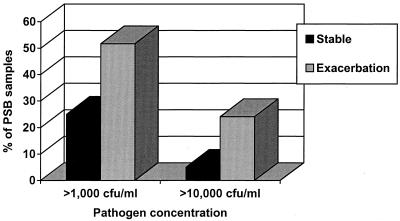
The study done by Monso et al. is especially informative, as it included a control group of 29 patients with stable COPD (208). They demonstrated that exacerbation was associated twice as often with distal airway infection at ≥103 CFU of pathogenic bacteria per ml and four times as often with ≥104 CFU/ml (P < 0.05 for both comparisons) (Fig. 1). Soler et al. examined a more severely ill population of 50 patients who were placed on mechanical ventilation for an acute exacerbation and obtained lower airway secretions for culture by bronchoscopy with a protected specimen brush and BAL and tracheobronchial aspirates (294). Although 21 of their 50 patients had received antibiotics before the samples were obtained, bacterial infection was demonstrated in 21 of 50 (42%) patients and infection with a virus or atypical pathogen was found in 5 (10%) patients. The distribution of specific bacterial pathogens isolated in their study is remarkable for a large proportion of Pseudomonas aeruginosa and other gram-negative bacilli (14 of 50, 28%). Recently, two studies using sputum cultures have also demonstrated an increasing frequency of isolation of these groups of pathogens in exacerbations of severe COPD (90, 201). Whether this is due to environmental factors (such as antibiotic selection pressure or exposure to hospital flora from frequent exacerbations) or is related to a greater degree of host immune compromise is not clear.
FIG. 1.
Culture results of bronchoscopic samples obtained from patients with stable COPD and those with acute exacerbation. The number of positive samples at both pathogen concentrations was significantly greater (P < 0.05) in the exacerbation group. Data taken from the work of Monso et al. (208). PSB, protected specimen brush; CFU/ml, CFU of pathogenic bacteria per milliliter of epithelial lining fluid.
The consistent results of these four studies and the greater rate of isolation of pathogenic bacteria in exacerbated than in stable COPD in the Monso study supports the pathogenic role of bacteria in a proportion of acute exacerbations of this chronic disease.
Immune responses to bacterial pathogens.
Older studies of immune response to nontypeable H. influenzae in COPD had several limitations, as discussed above (219). Recently, we and other investigators have explored the immune response to bacterial pathogens in acute exacerbations of COPD with methods that avoid the pitfalls of earlier studies. These studies are discussed in detail later. These studies have demonstrated the development of specific immune response to infecting strains of nontypeable H. influenzae and M. catarrhalis and support the role of bacterial infection in acute exacerbations of COPD. Similar evidence with other bacterial species (see Table 2) would help us better define their role in acute exacerbations.
Molecular epidemiology of bacterial pathogens.
Strains of a bacterial species can differ considerably in their surface antigenic structure. The “bacterial load” model of bacterial infection in COPD assumes that an increase in the titer of a bacterial species in the airway is responsible for the transition from stable COPD to exacerbation of COPD (346). Studies of bacterial titers in the sputum of patients with COPD have not supported this model (123). This model does not take into account the genetic diversity within the bacterial species, including alterations in surface antigenic structure. An alternative model is that infection with a bacterial strain with an antigenic structure new to the host leads to an immune and inflammatory response that presents clinically as an acute exacerbation. Longitudinal studies of patients with COPD in combination with molecular typing of the strains will allow investigators to test this model.
Airway inflammation and correlation with bacteriology.
Bacterial infection of the lower airways during an acute exacerbation should be associated with neutrophilic inflammation as is seen in other mucosal sites such as the middle ear and sinuses. One would therefore expect airway inflammation in bacterial exacerbations to be associated with significantly greater neutrophilic inflammation than a nonbacterial exacerbation. Therefore, sputum culture results should correlate with measures of airway inflammation in acute exacerbations. Data from our laboratory support this hypothesis, with pathogen-positive acute exacerbations having substantially increased measures of airway inflammation in expectorated sputum compared to pathogen-negative exacerbations (276). Furthermore, Stickley et al. showed that increased purulence is associated with recovery of a bacterial pathogen at the time of exacerbation, suggesting that purulence is a marker for bacterial exacerbation (305a).
Overall, the weight of evidence indicates that bacterial pathogens cause approximately 40 to 50% of acute exacerbations of COPD. Further studies with sophisticated immunological assays, molecular epidemiology, and measurement of airway inflammation should refine our understanding of the pathogenesis of bacterial exacerbation and the mechanisms of protection and recurrence.
Vicious Circle Hypothesis
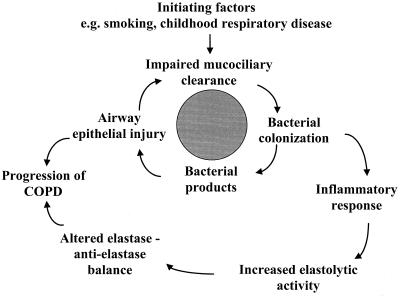
Tobacco smoking cannot be the sole factor responsible for the pathogenesis of COPD, as only a small proportion (15%) of smokers develop chronic bronchitis and an even smaller proportion go on to develop obstructive airway disease (COPD). In the absence of underlying lung disease, the tracheobronchial tree is sterile. In patients with COPD, the tracheobronchial tree is chronically colonized with potential respiratory pathogens, predominantly nontypeable H. influenzae, S. pneumoniae, and M. catarrhalis (124, 173, 208, 358). Several years ago, we proposed a vicious circle hypothesis to explain how chronic bacterial colonization of the lower airways in patients with COPD can perpetuate inflammation and contribute to progression of the disease (Fig. 2) (63, 219). A similar mechanism is believed to contribute to the pathogenesis of lung disease in individuals with cystic fibrosis. Substantial supporting evidence for this hypothesis in COPD, both in vitro and in vivo, has now accumulated and is discussed below.
FIG. 2.
Diagrammatic representation of the vicious circle hypothesis.
Central to the vicious circle hypothesis is the notion that once bacterial pathogens have gained a foothold in the lower respiratory tract from impaired mucociliary clearance due to tobacco smoking, the bacteria persist by further impairing mucociliary clearance (Fig. 2). This impairment of mucociliary clearance can be due to enhanced mucus secretion, disruption of normal ciliary activity, and airway epithelial injury. Experimental evidence demonstrates that respiratory tract pathogens and their products can cause all of these effects in vitro.
Bacterial infection and chronic mucus hypersecretion.
Adler et al. examined the effect of cell-free filtrates of broth cultures of nontypeable H. influenzae, S. pneumoniae, and P. aeruginosa on the secretion of mucous glycoproteins by explanted guinea pig airway tissue (1). Seven of 28 (25%) strains of nontypeable H. influenzae, 10 to 26 (34%) strains of S. pneumoniae, and 12 of 18 (66%) strains of P. aeruginosa stimulated mucin secretion. This stimulation was a true secretory effect and not passive release of preformed intracellular macromolecules due to cellular damage, as ultrastructural assessment (by light, transmission, and scanning electron microscopy) demonstrated an absence of cytotoxicity. The Pseudomonas stimulatory products were proteases of 60 to 100 kDa. The Haemophilus and pneumococcal stimulatory exoproducts were 50 to 300 kDa in size and did not possess proteolytic activity.
Bacterial infection and mucociliary clearance.
The tracheobronchial ciliary escalator is of paramount importance in maintaining sterility of the lower respiratory tract by transporting bacteria trapped in mucus towards the pharynx (347). Disruption of this ciliary activity is therefore likely to be important in the establishment of chronic colonization in the tracheobronchial tree. Wilson et al. measured by photometry the effect of cell-free supernatants of nontypeable H. influenzae, P. aeruginosa, and S. aureus on ciliary beat frequency of strips of human nasal ciliary epithelium (349). Rapid inhibition of ciliary beat frequency was seen with nontypeable H. influenzae and P. aeruginosa but not with S. aureus. On direct examination, ciliary dyskinesia and ciliostasis were seen. Human neutrophil elastase inhibits ciliary activity and damages respiratory epithelium (15). Bacterial products in the airways may be a potent stimulus for neutrophil migration into the airways, and elastase released from these neutrophils can act synergistically with bacterial products and cause further inhibition of tracheobronchial ciliary function.
Bacterial infection and airway epithelial injury.
An important component of the vicious circle hypothesis is the potentially damaging effects of bacteria and bacterial products on airway epithelial lining cells. Such epithelial injury in the large airways would contribute to bacterial persistence and in the small airways could contribute to the respiratory bronchiolitis that causes progressive airway obstruction (67, 107). In an in vitro tissue culture model of nasal turbinate epithelium, Read et al. have demonstrated that nontypeable H. influenzae causes airway epithelial injury (252). They studied these epithelia after 30 min, 14 h, and 24 h of incubation with a nontypeable H. influenzae strain. At 30 min, the airway epithelium and cilia were intact and the bacteria were associated with the overlying mucus layer. At 14 h, patchy injury developed to the airway epithelium, with bacterial cells now associating with these damaged epithelial cells but not with intact epithelium. At 24 h, detached epithelial cells with adherent bacteria were seen.
The studies discussed above demonstrate that bacteria that colonize and infect the lower respiratory tract in COPD are capable of fostering an environment in the tracheobronchial tree in which they can persist, supporting the central tenet of the vicious circle hypothesis (Fig. 2). Recently, more attention is being directed towards another portion of the vicious circle, the effects of the chronic inflammatory response occasioned by bacterial products on the elastase-antielastase balance in the lung. If bacterial products in the tracheobronchial tree cause neutrophil influx and degranulation in the airways and lung parenchyma, they could contribute to the chronic inflammation, parenchymal lung damage, and progressive small airway obstruction seen in COPD (15, 140, 235).
Bacterial infection and airway inflammation.
The presence of bacteria in the lower airways in patients with stable COPD has been labeled colonization. However, this bacterial presence is definitely abnormal and is not confined to the large airways. Bacteria have been shown to extend to the peripheral airways by cultures of bronchoscopic protected specimen brushings and BAL (208, 358). Even during colonization, bacteria in these airways are in a constant state of turnover, releasing extracellular products, undergoing lysis with release of a variety of proteins, lipooligosaccharide (LOS), and peptidoglycan (121). LOS is a potent inflammatory stimulus; in fact, repeated instillation of LOS can lead to the development of emphysema in hamsters (306). It is therefore quite likely that this colonization is actually a low-grade smoldering infection that induces chronic airway inflammation. In the large airways such inflammation would contribute to mucus production, and in the small airways it could contribute to respiratory bronchiolitis and progressive airway obstruction (79, 310). Recent data that support this hypothesis include in vitro experiments with LOS of nontypeable H. influenzae and a bronchoscopic study that demonstrates that bacterial colonization may be an independent stimulus to airway inflammation in patients with stable COPD, as described below. Furthermore, Hill et al. recently demonstrated an association between bacterial numbers and markers of airway inflammation in stable chronic bronchitis (141a).
Khair et al. incubated explant cultures of human bronchial epithelium with LOS from nontypeable H. influenzae at 10 and 100 μg/ml (161). Epithelial cell permeability, intracellular adhesion molecule-1 (ICAM-1) expression, and release of interleukin-6 (IL-6), IL-8, and tumor necrosis factor alpha (TNF-α) into the culture medium were measured. IL-6 and TNF-α secretion and ICAM-1 expression by the bronchial epithelial cells were significantly increased only by the higher concentration of LOS (100 μg/ml), while IL-8 expression was stimulated by LOS at both 10 and 100 μg/ml. The levels of inflammatory mediators attained in the culture medium were adequate to increase neutrophil chemotaxis and adherence in vitro. There was no increase in epithelial cell permeability.
In a recent study, Soler et al. compared the levels of several cytokines, including IL-1β, IL-6, IL-8, IL-10, and TNF-α, in BAL fluid obtained from 52 patients with stable COPD, 18 smokers, and 8 nonsmoking healthy controls (293). Among the smokers said to be without COPD, nine (50%) actually had chronic bronchitis, which confounds some of their findings, as discussed below. Bacterial colonization of the distal airways was determined by quantitative cultures of BAL fluid (≥103 CFU/ml was significant) and of protected specimen brush (≥102 CFU/ml was significant) samples from the distal airways. Pathogens isolated were classified into potential pathogenic microbes (PPM) and non-PPM. The PPM included nontypeable H. influenzae, S. pneumoniae, M. catarrhalis, S. aureus, P. aeruginosa, and gram-negative enteric bacteria. None of the healthy controls had PPM isolated in significant concentrations, compared to 42% of the smokers and 32% of the patients with COPD. Isolation of a significant number of PPM in the BAL was associated with significantly more polymorphonuclear cells and TNF-α compared to BAL which did not have PPM. A trend to higher IL-8 levels in the BAL was also seen. Isolation of non-PPM in significant amounts was not associated with an increase in BAL cytokines or airway neutrophilia. This study demonstrates that bacterial colonization of the lower respiratory tract with PPM occurs not only in patients with established COPD, but also in smokers who may have chronic bronchitis but do not have significant airway obstruction. Therefore, bacterial colonization of the lower airways appears to be an early phenomenon in the course of the disease. Furthermore, bacterial colonization is an independent stimulus for inflammation in the distal airways and therefore may contribute to the progression of COPD. This is analogous to young patients with cystic fibrosis in remission, who are chronically colonized in the distal airways mostly with P. aeruginosa, but also with nontypeable H. influenzae and S. aureus. This colonization is also associated with an active inflammatory process in the distal airways (164).
It is becoming increasingly apparent that the presence of bacteria in the lower airways in patients with COPD, even when they are clinically stable, is not innocuous. Nontypeable H. influenzae has the ability in vitro to disrupt ciliary motility, induce mucus hypersecretion, and damage the airway epithelium. These effects parallel the effects of tobacco smoke and contribute to the persistence of nontypeable H. influenzae in the lower airways of smokers. Nontypeable H. influenzae persistence in the lower airways appears to stimulate airway epithelium to produce proinflammatory cytokines, especially those that promote neutrophil chemotaxis, and therefore leads to additional airway inflammation. Whether this additional airway inflammation contributes to symptoms and progression of airway obstruction in COPD is unknown but should be a fertile area of investigation.
Chronic Bacterial Infection of Respiratory Tissues
Nontypeable H. influenzae has always been regarded as an extracellular pathogen that infects the airway lumen in COPD. Recently, invasion of the upper and lower respiratory tract tissues by this pathogen has been demonstrated. Whether COPD is associated with chronic Chlamydia pneumoniae infection of the respiratory tract has also recently been investigated. These studies used newer detection techniques with greater sensitivity than bacterial culture for determining the presence of bacterial organisms in tissue and made interesting and somewhat surprising observations.
Intracellular and intercellular invasion of H. influenzae.
Nontypeable H. influenzae is present in the lumen of the respiratory tract, binds with specificity to mucin, and adheres to the surface of respiratory epithelial cells (see below). More recently, research from several groups has shown that the organism's niche in the human respiratory tract is not limited to adherence to the surface of epithelial cells. Several lines of investigation involving in vitro and in vivo studies have established that nontypeable H. influenzae invades beyond the surface of the respiratory epithelium.
Studies utilizing cultures of human epithelial cells have revealed that a small percentage of adherent nontypeable H. influenzae enter epithelial cells in a process that involves actin filaments and microtubules (303). Organ culture studies utilizing lung epithelial cells on permeable supports revealed clusters of H. influenzae bacterial cells between cells, indicating that bacteria penetrated by paracytosis or passage between cells (326). Bacteria passed through confluent layers of epithelial cells without affecting the permeability or viability of the cell layer. Nontypeable H. influenzae which penetrate the epithelial cell layer in this model system are protected from the bactericidal activity of several antibiotics and antibody-mediated bactericidal activity (325). In assays employing primary human airway cultures. Ketterer et al. (160) showed that nontypeable H. influenzae adhered to and entered exclusively nonciliated cells in the population. The surface of infected cells showed evidence of cytoskeletal rearrangements, manifested by microvilli and lamellipodia extending toward bacteria, indicating that bacteria were entering epithelial cells by the process of macropinocytosis (160).
In addition to the these elegant in vitro studies, investigators from two centers have performed in vivo studies which confirm that nontypeable H. influenzae penetrate the mucosal surface during colonization of the human respiratory tract. In situ hybridization and selective cultures revealed that viable nontypeable H. influenzae are present in macrophagelike cells in the adenoids of children (104, 105). In a second approach to investigating whether nontypeable H. influenzae is present in intracellular or intercellular locations in the human respiratory tract, Moller et al. (207) obtained lung explants from patients undergoing lung transplant. H. influenzae was diffusely present in the epithelium, the submucosa of the bronchi, the bronchioles, the interstitium, and the alveolar epithelium, as determined by in situ hybridization and PCR.
In summary, these observations indicate that when nontypeable H. influenzae colonizes the human respiratory tract, the bacterium is present in several locations, including in the lumen of the respiratory tract, adhering to mucosal epithelial cells, in the interstitium of the submucosa, and within cells of the respiratory tract. Bacteria in tissues are protected from antibiotics and bactericidal antibodies and may act as reservoirs of infection (325). Tissue infection by nontypeable H. influenzae could also contribute to the pathogenesis of COPD directly or indirectly. Chronic low-grade infection could directly induce a chronic inflammatory response in the parenchyma and the airways of the lung that could be additive or synergistic to the inflammatory effects of tobacco smoke. Indirectly, such an infection could enhance the damaging effects of tobacco smoke on respiratory tissues. On the other hand, it is possible that this tissue infection is simply a marker of compromised local immunity. Whether tissue infection by nontypeable H. influenzae is seen in early COPD and the effect of this infection in tissue models need to be investigated.
Chronic Chlamydia pneumoniae infection in COPD.
C. pneumoniae is an obligate intracellular atypical bacterial pathogen. Acute C. pneumoniae infection can cause bronchitis, pneumonia, and acute exacerbations of COPD (see below). Chronic infection with C. pneumoniae is being actively investigated as a cause of several systemic diseases, especially coronary artery disease (178). Von Hertzen et al. studied whether the incidence of chronic C. pneumoniae infection is increased in COPD (332). The presence of chronic C. pneumoniae infection was determined by three different methods: serum antibodies to C. pneumoniae (immunoglobulin G [IgG] and IgA and circulating immune complexes), sputum IgA antibodies to C. pneumoniae, and PCR of sputum for C. pneumoniae DNA. Two of the three methods had to yield positive results for the same patient to demonstrate a chronic C. pneumoniae infection. The incidence of chronic C. pneumoniae infection (as defined above) was 71% in patients with severe COPD, 46% in mild to moderate COPD, and 0% in the control group. Whether this chronic infection contributes to the pathogenesis of COPD as discussed above or is a reflection of compromised local immunity warrants further investigation.
Hypersensitivity to Bacterial Antigens
Allergic bronchopulmonary aspergillosis is an infectious disease with predominantly allergic manifestations mediated by a Th2-type immune response and characterized by IgE and eosinophil predominance (158). Inefficient removal of bacteria from the lower respiratory tract is characteristic of chronic bronchitis, resulting in prolonged contact between the airway lymphoid tissue and bacterial antigens. This could lead to the emergence of IgE antibodies to bacterial antigens, which could induce eosinophil infiltration and mast cell degranulation on repeated exposures to the bacterial antigens. An increased number of eosinophils is characteristic of airway inflammation in most patients with COPD, and tissue and airway lumen eosinophilia becomes more prominent during exacerbations (268). Furthermore, a small subgroup of patients with COPD have an eosinophilic bronchitis that is responsive to steroids (132).
The ability of bacterial pathogens to induce histamine release, hypersensitivity, and IgE-mediated inflammation has been investigated sporadically. Mast cells release histamine by non-IgE-mediated and IgE-mediated mechanisms. Clementsen et al. exposed mast cells obtained by BAL from the airways of patients with chronic bronchitis and normal individuals by BAL to Formalin-killed suspensions of nontypeable H. influenzae, S. pneumoniae, M. catarrhalis, and S. aureus. Nontypeable H. influenzae and S. aureus induced non-IgE-mediated and enhanced IgE-mediated histamine release (61). The enhancement of IgE-mediated histamine release appears to be mediated by the endotoxin of nontypeable H. influenzae (62). Histamine increases bronchial epithelium permeability, stimulates mucus secretion, and induces bronchoconstriction.
Patients with acute exacerbations of chronic bronchitis have had basophil-bound IgE and serum IgE to homologous strains of nontypeable H. influenzae and S. pneumoniae isolated from sputum with the acute exacerbation (162). In another study in asthmatics, 29% of patients had serum IgE antibodies to nontypeable H. influenzae and/or S. pneumoniae (238). This sensitization to bacterial antigens may contribute to the bronchoconstriction and airway inflammation seen with acute exacerbations of COPD.
These observations regarding histamine release and IgE to bacterial antigens suggest that bacterial pathogens, either directly or indirectly via a Th2-type immune response, could contribute to the eosinophilia, airway hyperreactivity, and bronchoconstriction seen in patients with COPD. Further investigation in this area is warranted, especially in the group of COPD patients with eosinophilic bronchitis (132).
BACTERIAL PATHOGENS
Nontypeable Haemophilus influenzae
Dynamics of colonization and molecular epidemiology.
Nontypeable H. influenzae strains are common inhabitants of the human upper respiratory tract, being present in up to three-fourths of healthy adults. When serial cultures are performed, the organism can be recovered from the sputum of virtually all patients with chronic bronchitis. Adults with chronic bronchitis are colonized in the lower airways with nontypeable H. influenzae and other bacteria (45, 173). Colonization with nontypeable H. influenzae is a dynamic process, with new strains being acquired and replacing old strains periodically (271). Multiple strains frequently colonize the respiratory tract simultaneously in the setting of chronic bronchitis (116, 220).
The development of typing systems for nontypeable H. influenzae has led to important information about the epidemiology of respiratory tract colonization and infection. Earlier studies with outer membrane protein (OMP) subtyping and restriction endonuclease analysis were important in beginning to understand epidemiology and pathogenesis (23, 116, 216). The development and application of more powerful typing systems have further characterized the epidemiology of respiratory tract colonization and also elucidated genetic relationships among nontypeable H. influenzae strains (224, 243, 270, 289). Typing systems for nontypeable H. influenzae and the basis of strain differentiation for each are listed in Table 4.
TABLE 4.
Typing systems for nontypeable Haemophilus influenzae
| Typing system | Basis of strain differentiation | References |
|---|---|---|
| OMP subtyping | Molecular mass differences of OMPs | 23, 116, 206, 216 |
| Restriction endonuclease analysis | Molecular size of small fragments of genomic DNA restricted with frequently cutting restriction enzymes | 43, 93, 116, 271 |
| Electrophoretic typing | Electrophoretic mobility of isoforms of metabolic enzymes | 224, 243, 244 |
| RAPD and REP-PCRa | DNA fingerprints of PCR-amplified genomic DNA using various primers | 32, 153, 154, 206, 289, 319 |
| Ribotyping and long PCR ribotyping | Restriction enzyme patterns of ribosomal DNA | 288–290 |
| Pulsed field gel electrophoresis | Molecular size of large fragments of genomic DNA restricted with infrequently cutting restriction enzymes | 270 |
RAPD, randomly amplified polymorphic DNA; REP, repetitive extragenic palindrome.
Studies in which prospectively collected strains are subjected to genomic typing will reveal important data about colonization patterns. For example, it will be important to know how long individual strains of nontypeable H. influenzae colonize the respiratory tract of adults with COPD. Such studies will reveal whether acquisition of a new strain predicts the occurrence of an exacerbation. Application of strain typing to analysis of colonization and exacerbation patterns will begin to reveal whether protective immune responses to individual strains occur. Such information will be important in more precisely defining the role of nontypeable H. influenzae in causing exacerbations and designing immunization strategies as vaccines are developed.
Mechanisms of adherence.
(i) Mucin binding.
The first step in the pathogenesis of infection by nontypeable H. influenzae is colonization of the respiratory tract. Since the human respiratory mucosa is covered with mucus, bacteria initially encounter mucus in the respiratory tract. The mucus gel is a complex mixture of secreted molecules, cells, and debris, including mucins, which are high-molecular-weight glycoproteins with O-glycoside-linked carbohydrate side chains. Mucins bind bacteria and therefore likely influence bacterial adhesion to the epithelium. Mucin-bacterium interactions may serve as a host defense mechanism facilitating removal of bacteria from the respiratory tract by the mucociliary elevator. Alternatively, binding of bacteria to mucin may represent the initial step in bacterial adherence to the epithelium and colonization of the respiratory tract.
Analysis of the interaction of nontypeable H. influenzae with purified human nasopharyngeal mucin reveals a specific interaction between mucin and the bacterium (72, 165). Binding of mucin is mediated by OMPs P2, P5, and a third as yet unidentified OMP (253, 254). Furthermore, it appears that a protein-oligosaccharide interaction is responsible for binding, because asialo-mucin does not bind to nontypeable H. influenzae OMPs (254). Elucidating the molecular interaction of mucin with nontypeable H. influenzae will be important in understanding mechanisms of pathogenesis and may lead to the development of strategies to prevent colonization and infection.
(ii) Adherence to respiratory mucosa.
Research in the past decade has witnessed the identification and characterization of multiple adhesins expressed by nontypeable H. influenzae (300, 301) (Table 5). Teleologically, the expression of multiple adhesin molecules and the ability to modulate expression of these adhesins support the notion that adherence to the respiratory tract is critical for survival of the bacterium.
TABLE 5.
Adhesins of nontypeable Haemophilus influenzae
| Adhesin | Approx. molecular mass (kDa) | % of strainsa | References |
|---|---|---|---|
| Pili | 27 | ∼33 | 109, 110, 195, 322, 323 |
| HMW1 and HMW2 | 125 | 70–80 | 20, 21, 24, 230, 304 |
| Hia (H. influenzae adhesin) | 115 | 20–30 | 25 |
| Hap (Haemophilus adhesin and penetration) | 155 (110)b | 100 | 302 |
| OMP P5 (fimbrin) | 35 | 100 | 231, 284 |
| OapA (opacity-associated protein A) | 47 | 100 | 246, 341 |
Percentage of strains which are capable of expressing the adhesin.
The gene product is 155 kDa, and the processed protein is 110 kDa.
Like many gram-negative bacteria, some strains of nontypeable H. influenzae express pili, which mediate adherence to mucosal cells (110, 156). The pili of H. influenzae are hairlike projections composed of polymeric helical structures with a distal-tip adhesin. The gene cluster responsible for the biogenesis of pili contains five genes: hifA, encodes the major structural protein, hifB encodes a periplasmic chaperone, hifC encodes an outer membrane usher, and hifD and hifE encode minor protein subunits and participate in the biogenesis of pili (323). Phase variation of pilus expression is mediated by slipped-strand mispairing in the promoter site of hifA and hifB (322). Examination of clinical isolates from children has revealed that only one third of such isolates contain the pilus gene cluster and are capable of expressing pili (300). The proportion of piliated strains of nontypeable H. influenzae recovered from adults with chronic bronchitis has not been rigorously studied.
The observation that nonpiliated strains of nontypeable H. influenzae are capable of adhering to cultured human epithelial cells suggested the presence of nonpilus adhesins. Barenkamp and coworkers identified the high-molecular-weight surface proteins HMW1 and HMW2 and isolated the genes which encode the proteins (22). Analysis of the sequences revealed similarity with Bordetella pertussis filamentous hemagglutinin, a known adhesin molecule. Construction of isogenic mutants which lack HMW1 and HMW2 and expression of the recombinant proteins in Escherichia coli clearly established these proteins as adhesins of H. influenzae (304). Approximately 70 to 80% of nontypeable H. influenzae strains express HMW1 and HMW2 (305).
Strains which lack HMW1 and HMW2 are capable of adherence to epithelial cells in vitro, suggesting the presence of additional adhesin molecules. A gene which encodes another adhesin was identified in and isolated from a nontypeable strain which lacked the genes which encode HMW1 and HMW2 (25). The gene has been named hia and encodes a protein of ∼115 kDa. Analysis of an isogenic mutant and expression of recombinant Hia in E. coli have established that Hia is an adhesin for nontypeable H influenzae. Hia has sequence homology with Hsf of H. influenzae type b strains and also demonstrates binding characteristics similar to Hsf (300).
Another gene which encodes a 155-kDa nonpilus adhesin was described by St. Geme et al. (139, 302). Hap is present in all strains and is involved in adherence and invasion of cultured human epithelial cells. Hap shows significant homology to serine-type IgA1 proteases of H. influenzae and Neisseria species but is distinct from IgA1 protease. Like IgA1 protease, Hap is synthesized as a larger preprotein that contains a prokaryotic signal sequence which facilitates transport to the periplasm. The carboxy terminus then inserts into the outer membrane and forms a pore through which the remainder of the protein passes. The Hap protein cleaves a 110-kDa fragment, which is released, and the remaining 45-kDa fragment remains associated with the membrane (138, 247). The precise role of the Hap protein in pathogenesis is still unclear, but one intriguing possibility is that the proteolytic activity is important once the bacterium is intracellular (300).
Strains of nontypeable H. influenzae recovered from children with otitis media express a nonhemagglutinating surface appendage which has been called a fimbria by Sirakova et al. (231, 284). This ∼36-kDa protein is OMP P5, which is an OMP A-like protein (210). Disruption of the gene results in reduced adherence to human oropharyngeal cells and alteration in the ability to cause otitis media in chinchillas. Immunization of chinchillas with OMP P5 is protective in animals challenged with the homologous strain (284).
OapA (opacity-associated protein A) is responsible for the transparent-colony phenotype of H. influenzae and is required for efficient colonization of the nasopharynx in an infant rat model of H. influenzae carriage (341). More recently, OapA has been identified as an adhesin which mediates adherence of nontypeable H. influenzae to Chang epithelial cells (246). The protein is present in all strains of H. influenzae examined thus far.
The adherence of nontypeable H. influenzae to the human respiratory tract mucosal surface is the result of a complex interaction of bacterial adhesins and host molecules. Several adhesins have been identified, and their precise roles in the pathogenesis of infection of humans remain to be defined. Elucidating the mechanisms of the interactions between adhesins and host, the antigenic structure of the molecules involved, the relative importance of the various adhesins, the ability of the bacterium to modulate expression of adhesins, and the conditions under which specific adhesins are expressed will be important in understanding the molecular mechanisms of pathogenesis. Such observations may lead directly to developing novel strategies to prevent colonization or infection by nontypeable H. influenzae in the setting of chronic bronchitis.
(iii) Intracellular and intercellular invasion.
Nontypeable H. influenzae is present in the lumen of the respiratory tract, binds with specificity to mucin, and adheres to the surface of respiratory epithelial cells. More recently, research from several groups has shown that the organism's niche in the human respiratory tract is not limited to adherence to the surface of epithelial cells. Several lines of investigation involving in vitro and in vivo studies have established that nontypeable H. influenzae invades beyond the surface of the respiratory epithelium. These studies were discussed previously.
Iron uptake.
Bacteria require a source of iron for several metabolic processes. In the human host, most iron is present intracellularly in heme-containing compounds or bound to ferritin. Most extracellular iron is bound to transferrin or lactoferrin. The level of free iron is below the level necessary to support bacterial growth. Bacteria have developed mechanisms to acquire iron for growth in the human host.
Iron acquisition by H. influenzae is a complex process which involves several components. Iron is acquired from transferrin by transferrin-binding proteins in the outer membrane, and subsequent transport of iron from the periplasmic space to the cytoplasm is dependent on the hitA, hitB, and hitC genes. Molecules which are involved in iron uptake are summarized in Table 6.
TABLE 6.
Proteins involved in uptake of iron and heme by Haemophilus influenzaea
| Protein | Gene | Molecular mass (kDa) | Location | Function | References |
|---|---|---|---|---|---|
| Tbp1 | tbpA | 95 | Outer membrane | Transferrin transport | 111, 112, 179 |
| Tbp2 | tbpB | 68–85 | Outer membrane | Transferrin binding | 111, 112, 179 |
| FbpA | hitA | 36 | Periplasmic space | Iron binding | 272 |
| HitB | hitB | 51 | Cytoplasmic membrane | Permease | 272 |
| HitC | hitC | 40 | Cytoplasmic membrane | Energy transduction | 272 |
| HxuA | hxuA | 100 | Secreted | Heme/hemopexin binding | 65, 66 |
| HxuB | hxuB | 60 | Outer membrane | Release of HxuA | 66 |
| HxuC | hxuC | 78 | Outer membrane | Heme transport | 66 |
| 57-kDa protein | ? | 57 | Outer membrane | Hemopexin binding | 350, 351 |
| HbpA | hbpA | 61 | ? | Heme binding | 131 |
| HgpA | hgpA | 120 | Outer membrane | Hemoglobin/haptoglobin binding | 150 |
| HgpB | hgpB | 115 | ? | Hemoglobin/haptoglobin binding | 257 |
| HhuA | hhuA | 115 | Outer membrane | Hemoglobin/haptoglobin binding | 185 |
| P4 | hel | 30 | Outer membrane | Heme transport | 256 |
Adapted from reference 249 with permission of the publisher.
Since H. influenzae lacks the enzymes necessary to convert δ-aminolevulinic acid to protoporphyrin IX, the organism requires heme for growth. Indeed, the requirement for heme is used in the clinical microbiology laboratory to confirm the identity of a clinical isolate as H. influenzae. Several molecules are involved in the uptake of heme, and these are summarized in Table 6.
A comprehensive discussion of the mechanisms of iron and heme uptake is beyond the scope of this review. Suffice it to say that these mechanisms are the focus of intense investigation and are important from the perspective of understanding the pathogenesis of H. influenzae infection. Furthermore, proteins involved in iron uptake are the subject of study as potential vaccine antigens, and the observation that these proteins are transcribed and expressed in vivo further supports their potential as vaccines (142, 344).
Antigenic variation of surface proteins.
(i) Antigenic heterogeneity.
Nontypeable H. influenzae expresses six to eight major proteins in its outer membrane. Studies in the 1980s demonstrated that a high degree of variability in the molecular weights of these outer membrane proteins existed among strains of nontypeable H. influenzae (23, 216). OMP P2, which coustitutes approximately half of the protein content of the outer membrane, shows a particularly high degree of size variability among strains (216). P2 is the major porin protein of H. influenzae, allowing small hydrophilic molecules to pass through the outer membrane (318). Analysis of the sequence of the gene which encodes P2 revealed that portions of the protein which are buried within the outer membrane are relatively conserved among strains but that several of the eight loops which are exposed on the bacterial surface show a high degree of sequence variability among strains (31, 84, 283). Since antibodies to P2 elicit strain-specific protection (117, 157, 312), these observations suggested that antigenic heterogeneity of the major surface protein plays a role in the ability of nontypeable H. influenzae to cause recurrent respiratory tract infections in humans.
(ii) Point mutations under immune selective pressure.
Analysis of OMP patterns from strains of nontypeable H. influenzae recovered prospectively from patients with chronic bronchitis reveals a high degree of turnover of strains, with frequent infection by new strains in some patients and persistent infection by the same strain in other patients (116). Among the strains which show persistence in the respiratory tract, variants with changes in the molecular weight of P2 but identical DNA fingerprints have been observed (116, 117). To determine the mechanism of this antigenic drift, Duim et al. (85) studied the sequences of the genes encoding P2 in these variants. The antigenic drift resulted from single-base changes in the P2 gene, all generating amino acid changes in surface-exposed loops of the P2 protein (85). Similar single-base changes were observed in the P2 gene from variants selected in subcutaneous cages implanted in rabbits and from a variant which survived antibody-mediated killing in vitro (85, 86, 329). All of the point mutations in the P2 gene were nonsynonymous, since they resulted in amino acid changes. Since all of the substitutions resulted in amino acid changes, these mutations produced a selective advantage for the bacterium. These observations strongly suggested that the accumulation of point mutations under immune selective pressure resulted in antigenic drift of surface-exposed regions of a major OMP. This mechanism of evading an immune response by the host could allow persistent H. influenzae infection in COPD.
(iii) Horizontal transfer of genes.
Recent studies in an Aboriginal community in the Northern Territory of Australia reveal another mechanism by which nontypeable H. influenzae alters its P2 molecule to evade host defenses. Rural Aboriginal children are heavily colonized by nontypeable H. influenzae in the nasopharynx at an early age (174). Ribotyping of prospectively recovered isolates has revealed that the children are colonized by multiple strains of H. influenzae simultaneously and that strains are acquired and cleared frequently, resulting in a high rate of turnover (288). By determining the sequences of P2 genes from selected strains, Smith-Vaughn et al. (291) demonstrated the presence of identical P2 genes in strains with different genetic backgrounds. In view of the wide diversity of P2 gene sequences, the authors concluded that horizontal transfer of the P2 gene occurred among strains. The presence in the human respiratory tract of simultaneous, multiple strains of a bacterium which is competent for DNA uptake provides a powerful mechanism for the bacterium to alter expression of surface molecules. This phenomenon is likely to occur in other settings in which multiple strains of nontypeable H. influenzae colonize the respiratory tract, such as cystic fibrosis (206) and chronic bronchitis (220).
Antigenic variation of LOS.
(i) Structure.
Endotoxin, or lipopolysaccharide, is the major glycolipid in the outer membrane of gram-negative bacteria. Endotoxin is essential to the integrity and functioning of the bacterial cell wall. Nonenteric gram-negative mucosal pathogens, including H. influenzae, express an endotoxin molecule which lacks the long, repeating polysaccharide side chains which are typical of lipopolysaccharide of enteric gram-negative bacteria such as E. coli and Salmonella spp. Therefore, the endotoxin of H. influenzae is more accurately called LOS.
LOS is involved in several stages in the pathogenesis of infection, including colonization of the respiratory tract and cytotoxic injury to target tissues. The importance of LOS in pathogenesis has generated considerable interest in studies of the biosynthesis and structure of the molecule. Such studies are complicated because it is necessary to study tertiary gene products of genes which are turned on and off at high frequencies. Nevertheless, considerable new information about LOS biosynthesis and structure has been obtained in the past decade.
LOS contains a membrane-anchoring lipid A portion. This part of the molecule is responsible for its endotoxin like properties, including mitogenicity, pyrogenicity, platelet aggregation, cytokine activation, and adjuvant activity. Lipid A is linked by a single 2-keto-3-deoxyoctulosonic acid molecule to a heterogeneous oligosaccharide composed of glucose, galactose, and heptose. Marked intrastrain and interstrain variation in the size of LOS is observed in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). This variation is a result of differences in the quantity and assembly of the neutral sugars, particularly galactose (340).
Surface determinants which are essential for the organism at one stage of colonization or infection may be unnecessary or even detrimental at a later stage of infection. Bacteria have evolved adaptive mechanisms for phenotypic variation of surface molecules, including LOS (262).
The LOS of H. influenzae shows considerable structural heterogeneity both between strains and within a clonal population derived from a single strain. This heterogeneity occurs as a result of several mechanisms. LOS is the end product of a complex biosynthetic process, and some variation occurs as a result of factors which influence the interaction of enzymes, regulatory proteins, and substrates (262). Such factors may determine the number of phosphate substitutions, anomeric linkages, saccharide branching chains, and other structural modifications, so that LOS is expressed as a family of molecules on the bacterial surface. A variety of environmental factors influence LOS structure as well. Such factors include exposure to serum; exposure to mixtures of glucose, lactate, urea, and bicarbonate in vitro; alteration of growth rate; and cystine limitation.
(ii) Phase variation.
Another mechanism by which H. influenzae alters its LOS is phase variation, which is the ability to regulate the expression of molecules by turning on and off the expression of selected genes. The LOS of H. influenzae demonstrates phase variation, which occurs through a mechanism known as slipped-strand mispairing (141). The lic locus, which is responsible for synthesis of oligosaccharide structures, contains open reading frames which are preceded by multiple tandem repeats of the tetramer 5′-CAAT-3′. Alterations in the number of repeats through the nonrecombinational mechanism of slipped-strand mispairing shift upstream initiation codons into or out of frame, creating a translational switch and resulting in phase variation (141). Multiple oligosaccharide structures undergo phase variation in a complex pattern. Some genes vary independently, and some vary in a coordinate fashion with other genes. As a result, the bacterium has the ability to display a varied array of LOS structures on its surface. This ability enables H. influenzae to adapt to its environment in the various stages of colonization and infection.
(iii) Molecular mimicry of host tissue.
The LOS of many strains of H. influenzae contain a terminal digalactoside, Gal-α-(1-4)-β-Gal, which is also present in human glycosphingolipids in the urinary tract, intestinal epithelium, and erythrocytes (187). The mimicry of host tissue may be an adaptive mechanism which promotes bacterial survival in the respiratory tract of the host.
The LOS components which resemble moieties in human tissue can be altered by the addition of sialic acid both in vitro and in vivo (187). Indeed, many strains of H. influenzae contain sialylated LOS (188). The oligosaccharide portion of sialylated LOS may also resemble sialylated oligosaccharides present in human glycosphingolipids. Sialylated LOS may play a variety of potential roles in the pathogenesis of colonization and infection by H. influenzae (187). These include antirecognition of bacterial surface antigens by the host, downregulation of opsonophagocytosis by bacteria, since bacteria with sialylated LOS are more resistant to phagocytosis, decreased adherence of bacteria to host cells or to other bacteria, intracellular survival of bacteria, and alteration of bacterial or host cell signaling pathways.
In summary, H. influenzae has an enormous capacity to alter the expression of its LOS by a variety of mechanisms. The mechanisms which have evolved illustrate some of the adaptive potential of surface bacterial determinants and their role in colonization and infection of the human respiratory tract.
Immune response.
(i) Interpreting the literature.
Human antibody responses to nontypeable H. influenzae in patients with COPD have been studied for decades. Two elements of the experimental design of such studies are critical in interpreting this literature: (i) the importance of using the homologous infecting isolate as the source of antigen in the immunoassays, and (ii) the importance of using immunoassays which detect antibodies to epitopes which are exposed on the surface of the intact bacterium.
Evidence is mounting that most immune responses following infection by nontypeable H. influenzae are strain specific (see below). Therefore, studies with laboratory isolates rather than homologous clinical isolates need to be reinterpreted in this context.
(ii) Strain-specific immune responses.
OMP P2 is strongly immunogenic in experimental animals and humans (117, 213, 298, 354). Analysis of monoclonal antibodies to P2 which were generated by immunizing mice with whole bacterial cells revealed that most antibodies were directed toward a single surface-exposed loop on the P2 protein (125, 126). All of these antibodies were highly specific for the immunizing strain. This observation suggested that nontypeable H. influenzae expresses an immunodominant, strain-specific epitope on the bacterial surface.
To test the hypothesis that the expression of strain-specific and immunodominant epitopes on the bacterial surface induces a strain-specific immune response, mice and rabbits were challenged with whole cells of a strain of nontypeable H. influenzae (354). Analysis of the antibody response with immunoblot, bactericidal, and immunoprecipitation assays revealed a prominent antibody response almost exclusively to a single surface-exposed loop of the P2 molecule (354). These observations, along with studies involving P2 in longitudinally collected isolates from adults with COPD (84–86, 117), support the notion that the surface-exposed loops of the P2 protein are under intense immune selective pressure. The expression of strain-specific, immunodominant epitopes represents a mechanism by which the bacterium induces antibodies which will protect against recurrent infection by the homologous strain but will not protect against infection by heterologous strains.
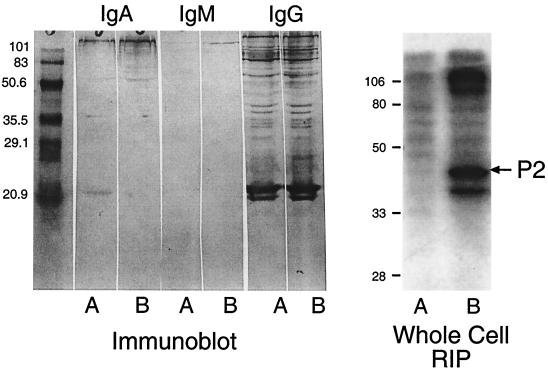
To determine whether a similar phenomenon occurs in humans, serum from two adults with exacerbations of COPD due to nontypeable H. influenzae were characterized (355). Both patients developed new bactericidal antibodies to their infecting strain. Immunoblot assays with homologous strains revealed antibodies to many antigens, with minimal differences between pre-and postexacerbation sera (Fig. 3). This observation illustrates the second critical element in the design of experiments to characterize the immune response to nontypeable H. influenzae. Immunoassays which detect antibodies to epitopes which are present on the bacterial surface will measure the potentially relevant antibody responses.
FIG. 3.
Immunoblot assay (left panel) and whole-cell radioimmunoprecipitation (RIP) assay (right panel) with preexacerbation serum (lanes A) and postexacerbation serum (lanes B) from an adult with COPD who experienced an exacerbation due to nontypeable H. influenzae. Assays were performed with the homologous infecting strain. The positions of molecular mass standards are noted (in kilodaltons) to the left of each panel. Note that immunoblot assays detect antibodies to many bands with minimal difference between pre- and postexacerbation sera. By contrast, whole-cell radioimmunoprecipitation assays with the same sera show the development of new antibodies to P2 (arrow) and higher-molecular-mass proteins following the exacerbation.
(iii) Antibodies to surface-exposed epitopes.
Figure 3 shows that serum from a patient who experienced an exacerbation due to nontypeable H. influenzae had antibodies to many antigens before the exacerbation and in spite of developing new bactericidal antibodies, had no new detectable antibodies by immunoblot assay. This observation is consistent with that of Groeneveld et al. (115), who showed that patients with abundant antibodies to H. influenzae in serum and sputum still experienced infection. Immunoblot assays detect antibodies to many epitopes on OMPs, including those which are buried within the outer membrane and not available for binding on the intact bacterium. Antibodies which bind to epitopes which are not on the bacterial surface are not likely to be protective. The OMPs of H. influenzae share cross-reactive epitopes with the OMPs of many gram-negative bacteria. Most of the cross-reactive epitopes are buried in the membrane and not on the bacterial surface. In order to detect the meaningful and potentially protective immune response, immunoassays which specifically detect antibodies to epitopes on the bacterial surface should be used. Such assays include whole-cell radioimmunoprecipitation assays, flow cytometry, and functional assays such bactericidal and opsonophagocytosis assays. Subjecting the serum in Fig. 3 to whole-cell radioimmunoprecipitation revealed that the patient developed new antibodies to P2 and to higher-molecular-mass proteins, an observation which was missed by immunoblot assay. Adsorption studies further established that new bactericidal antibodies were directed at strain-specific epitopes on the P2 protein (355).
In summary, the literature on the human immune response to nontypeable H. influenzae must be interpreted with caution. Recent research with improved study design reveals that adults with COPD make strain-specific antibody responses to surface-exposed epitopes following infection with nontypeable H. influenzae (222, 355).
Prospects for vaccines.
In view of the morbidity, mortality, and health care costs associated with bacterial infection in chronic bronchitis, there is interest in developing vaccines to prevent bacterial infections in this population. A large number of investigators in academia and industry are conducting research in pursuit of vaccines to prevent infections caused by nontypeable H. influenzae.
(i) Correlates of protection.
Identifying a protective immune response is a critical step in developing a vaccine to prevent any infection. It may be possible to generate immune responses to a variety of bacterial antigens, but the key question is whether that immune response will be effective in preventing infection in the target population. Clinical trials are necessary to establish the efficacy of a vaccine. However, in characterizing and evaluating potential vaccine antigens prior to clinical trials, in vitro and in vivo assays are useful in predicting which antigens are most likely to generate protective immune responses.
Animal models of infection have been widely used in testing vaccine antigens for a variety of infectious agents. Several models have been useful in evaluating vaccine antigens for nontypeable H. influenzae. These include the chinchilla model of otitis media (21, 76, 114) and various pulmonary and nasopharyngeal clearance models in the mouse and rat (130, 144, 166, 167, 204, 335, 352). The ability of an antigen to generate a protective immune response in an animal model is used as a rationale to proceed with further testing of a potential vaccine antigen. However, it is worth noting that the correlation between protection in animal models and protection in humans for nontypeable H. influenzae has not been established in any animal model to date.
The best correlate of protection for infection by nontypeable H. influenzae appears to be a bactericidal antibody response. The presence of serum bactericidal antibody is associated with protection from otitis media due to nontypeable H. influenzae in children (91, 282). In view of this observation, the ability of an antigen to generate bactericidal antibodies is used as a second strategy for identifying potential vaccine antigens.
(ii) Vaccine strategies.
Nontypeable H. influenzae causes mucosal infections. Therefore, a mucosal immune response may be the most effective in preventing infections (106). One avenue of investigation is the development of technologies for the mucosal delivery of vaccine antigens to generate a mucosal immune response (167, 169, 335, 336). This exciting and potentially fruitful approach is being pursued by several groups of investigators.
While the induction of mucosal immune responses to prevent infections caused by nontypeable H. influenzae is rational, whether such an immune response will be protective remains to be seen. As noted above, the best correlate of protection from otitis media (also a mucosal infection) is the presence of serum bactericidal antibody to the infecting strain. Furthermore, conjugate vaccines for H. influenzae type b infections are administered systemically and are also effective in reducing or eliminating colonization of the respiratory tract by type b strains. Therefore, systemically administered vaccines for preventing infections due to nontypeable H. influenzae are also a viable approach. Whether systemic vaccines, mucosal vaccines, or a combination will be most effective awaits further study.
Vaccines to prevent infections caused by nontypeable H. influenzae in patients with chronic bronchitis will also have application in preventing otitis media in infants and children. The three most common bacterial causes of otitis media and exacerbations of chronic bronchitis are S. pneumoniae, nontypeable H. influenzae, and M. catarrhalis. As a result, vaccine formulations to be tested in humans may include combinations of vaccine antigens from these three organisms.
(iii) Vaccine antigens.
A vaccine should be capable of generating an immune response which is effective in preventing infection by all or most strains of a species in the target population. A vaccine antigen should have several characteristics. (i) It should be conserved among strains so that the immune response is effective against many strains. This characteristic is especially important for nontypeable H influenzae in view of the extensive antigenic heterogeneity observed in surface antigens. (ii) The vaccine antigen should be located on the bacterial cell surface so that protective antibodies can bind to the intact bacterium. (iii) The vaccine should generate an immune response which is protective from infection in the target population. (iv) The vaccine antigen must be immunogenic in the target population.
Several surface proteins of nontypeable H. influenzae have the characteristics of potential vaccine antigens and are therefore generating substantial interest as vaccines. Several integral OMPs are being investigated, including P4, P5, P6, protein D, OMP26, D15, iron-regulated proteins, and others (11, 101, 113, 144, 150, 168, 179, 180, 185, 214, 215, 284, 295, 344). In addition, the adhesin molecules discussed earlier in this review are also under consideration as potential vaccine antigens (Table 5). Antibodies (particularly mucosal antibodies) to adhesins may be capable of blocking adherence of the organism to the respiratory mucosa. Finally, there is interest in using detoxified LOS as a vaccine antigen (120, 122). Research in the next decade promises substantial progress in the challenge of developing vaccines for nontypeable H. influenzae.
(iv) Clinical trials with killed whole-cell oral vaccine.
Placebo-controlled clinical trials with a vaccine formulation consisting of Formalin-killed whole bacteria of a nontypeable H. influenzae strain have been conducted in Australia and Papua New Guinea (58, 59, 176, 308). The vaccine produced a statistically significant reduction in the frequency of exacerbations compared to placebo-treated controls. The apparent protective effect was present for 1 year. Although a specific mucosal antibody response is observed following vaccination (60), no clear correlation between clinical protection and either salivary antibody to H. influenzae or colonization with H. influenzae was observed (58). The mechanism of the protective effect is not known at this time. These provocative studies may provide important information about mechanisms of protection in the human respiratory tract and support the concept that vaccines offer potential in reducing bacterial infections in COPD.
Moraxella catarrhalis
M. catarrhalis as a cause of exacerbations of COPD.
Nontypeable H. influenzae and S. pneumoniae have long been recognized as causes of purulent exacerbations of COPD. More recently, M. catarrhalis has been implicated as an etiologic agent in such exacerbations. The recognition of M. catarrhalis as a human lower respiratory tract pathogen has been delayed for several reasons. The organism colonizes the upper respiratory tract of children and adults in the absence of clinical signs of infection. Another factor accounting for the difficulty in recognizing the role of this organism as a human pathogen is the observation that the colony morphology of M. catarrhalis is difficult to distinguish from that of commensal Neisseria species, which are part of the normal upper respiratory tract flora. Finally, M. catarrhalis causes noninvasive infections, so is rarely recovered in blood or pleural fluid cultures.
Four principal lines of evidence implicate M. catarrhalis in this setting, and these have been reviewed recently (56, 211, 212). (i) Using strict criteria to evaluate the quality of sputum samples, a subset of patients with exacerbations of COPD have sputum smears which show a predominance of gram-negative diplococci on Gram strain and nearly pure cultures of M. catarrhalis (49, 198, 227, 299, 331). (ii) Pure cultures of M. catarrhalis have been obtained in transtracheal aspirates from patients experiencing exacerbations and pneumonia (10, 81, 128, 229, 343). (iii) Clinical improvement is seen in patients with M. catarrhalis infections following specific antibiotic therapy. Many penicillins are not active against M. catarrhalis because most strains produce β-lactamase. Patients with β-lactamase-positive strains who fail therapy with β-lactam antibiotics show clinical improvement following administration of an antibiotic active against M. catarrhalis (198, 227). (iv) Patients with chronic bronchitis who experience exacerbations associated with clinical and laboratory evidence of M. catarrhalis infection develop a new bactericidal antibody response to the homologous strain (49). The observation of a specific immune response to the organism following clinical infection provided evidence that the bacterium caused the infection.
Taken together, these lines of evidence indicate that M. catarrhalis causes exacerbations of COPD. It is difficult to estimate the proportion of exacerbations which are due to M. catarrhalis. However, one study performed in a Veterans Administration facility found that 30% of exacerbations were caused by M. catarrhalis (328). In our prospective study of patients with COPD, M. catarrhalis is the second most common cause of exacerbations after nontypeable H. influenzae (unpublished observations).
Typing systems.
Initial studies involving the analysis of banding patterns of OMPs by SDS-PAGE revealed that the molecular masses of OMPs were quite similar among strains of M. catarrhalis (27). This observation precluded the use of OMP patterns for strain differentiation of M. catarrhalis. The sole currently available serotyping system for M. catarrhalis is based on antigenic differences in LOS among strains (248, 320). This system distinguishes three LOS types. The method is limited by the small number of serotypes and the observation that 60% of clinical isolates belong to a single serotype. Additional work on the antigenic structure of LOS will be important to determine whether serotyping based on LOS structure will be feasible.
The application of DNA-based molecular typing systems to epidemiologically well-defined strains of M. catarrhalis has been useful in studying nosocomial outbreaks and is providing important observations about the epidemiology of colonization and infection by M. catarrhalis. Table 7 summarizes typing methods for M. catarrhalis.
TABLE 7.
Typing methods for Moraxella catarrhalis
| Category and methods | Assay | Basis of strain differentiation | References |
|---|---|---|---|
| Phenotypic | |||
| SDS-PAGE and immunoblot assay | Immunoblot of bacterial lysates with normal human serum | Antigenic and molecular weight differences of cellular proteins | 197, 209, 259 |
| Esterase electrophoretic polymorphism | Detection of specific esterases by electrophoresis | Relative electrophoretic mobility of esterases | 77, 241 |
| LOS typing | Inhibition ELISA with typing antisera | Antigenic differences in LOS | 320 |
| Genotypic | |||
| Restriction enzyme analysis of genomic DNA | |||
| Agarose gel electrophoresis | Electrophoretic separation of small fragments of DNA | DNA sequence differences detected by restriction enzymes | 77, 79, 94, 147, 197, 209, 236, 237, 259 |
| Pulsed-field gel electrophoresis | Electrophoretic separation of large fragments of DNA | DNA sequence differences detected by restriction enzymes | 159, 163, 190, 261, 334 |
| DNA probe | Southern blot probed with labeled random genomic fragments | DNA sequence differences in selected regions | 30 |
| Ribotyping | Southern blot probed with labeled rRNA | DNA sequence differences detected by restriction enzymes and hybridization with rRNA | 44, 77 |
| Randomly amplified polymorphic DNA analysis | Electrophoretic separation of DNA fragments amplified by PCR from genomic DNA | DNA sequence differences detected by PCR amplification with random primers | 334 |
Epidemiology of colonization.
M. catarrhalis is recovered exclusively from humans. No animal or environmental reservoirs for the organism have been identified. The bacterium colonizes mainly the respiratory tract, although it is rarely recovered from the genitourinary tract (80, 286). A strong relationship exists between rate of colonization and age in a population. Upper respiratory tract colonization with M. catarrhalis is common throughout infancy (16, 94, 184, 321, 324). For example, in one study involving monthly nasopharyngeal cultures taken from healthy infants from birth, 78% of infants were colonized at some time during the first 2 years of life (94). The prevalence of colonization with M. catarrhalis decreases with age, so that approximately 1 to 5% of healthy adults are colonized with M. catarrhalis (88, 155, 242, 321).
Several studies have surveyed the results of cultures of sputum samples and examined the clinical status of the patients from whom M. catarrhalis was recovered (41, 242, 287). These studies show that sputum samples which grow M. catarrhalis are more likely to be recovered from patients with chronic lung diseases than from healthy adults. These and other studies suggest that adults with chronic lung diseases are colonized with M. catarrhalis at a higher rate than are healthy adults. However, this observation has not been studied rigorously.
Two studies have examined the dynamics of respiratory tract colonization by subjecting isolates of M. catarrhalis recovered prospectively to molecular typing (94, 163). One study involved healthy children, while the other involved adults with bronchiectasis. Both studies showed that strains of M. catarrhalis are eliminated and acquired frequently, indicating that colonization with M. catarrhalis is a dynamic process. The dynamics of colonization of the respiratory tract in COPD is an important area of investigation. Such information is important in elucidating the precise role of M. catarrhalis in causing exacerbations, understanding the role of the immune response in clearing strains from the respiratory tract, devising a rational approach to antibiotic therapy, and designing an effective vaccine strategy.
Surface antigens.
(i) Iron-regulated proteins.
M. catarrhalis can utilize human transferrin and lactoferrin as sources of iron in the absence of siderophore production (46). Work in the last few years has led to the identification and characterization of transferrin- and lactoferrrin-binding proteins (Table 8). The arrangement of the genes encoding transferrin-binding proteins in M. catarrhalis differs from the arrangement in other organisms. However, overall, M. catarrhalis iron transport appears to function similarly to that of other mucosal pathogens, such as H. influenzae and the pathogenic Neisseria species.
TABLE 8.
Proteins involved in uptake of iron by Moraxella catarrhalis
| Protein | Gene | Molecular mass (kDa) | Function | References |
|---|---|---|---|---|
| TbpA | tbpA | 115–120 | Transferrin transport | 183 |
| TbpB (OMP B1) | tbpB | 80–84 | Binds transferrin | 183, 192, 225, 258, 275, 356 |
| CopB (OMP B2) | copB | 80 | Under study | 2, 5, 134, 136, 277 |
| LbpA | lbpA | 110 | Binds lactoferrin | 39, 40, 83 |
| LbpB | lbpB | 98 | Binds lactoferrin | 39, 40, 83, 356 |
(ii) OMPs.
OMP patterns observed by SDS-PAGE show a high degree of similarity among strains from diverse clinical and geographic sources (27). Over the past decade, several OMPs have been characterized in some detail (Table 9).
TABLE 9.
OMPs of Moraxella catarrhalis
| OMP | Molecular mass (kDa) | Function | References |
|---|---|---|---|
| UspA1 (HMW OMP) | 88 (oligomer)a | Putative adhesin | 3, 4, 51, 52, 64, 135, 199 |
| UspA2 (HMW OMP) | 62 (oligomer)a | Involved in serum resistance | 3, 4, 51, 52, 64, 135, 199 |
| 200-kDa protein | 200 | Hemagglutination? | 98, 99 |
| OMP CD | 46b | Porin | 146, 217, 218, 273, 353 |
| OMP E | 50 | Unknown | 36, 37 |
Molecular mass varies among strains.
OMP CD runs aberrantly (apparent molecular mass, ∼60 kDa) in SDS-PAGE.
UspA (ubiquitous surface protein A, also called HMW-OMP) migrates as an oligomer in SDS-PAGE. UspA is encoded by two genes, uspA1 and uspA2, which encode proteins with deduced molecular masses of 88 and 62 kDa, respectively. Sequence analysis reveals that UspA1 and UspA2 both have an internal segment of 140 amino acids with 93% identity. Furthermore, several different and repetitive amino acid motifs are present in the two proteins (64). UspA1 is an adhesin, and UspA2 is involved in serum resistance (3, 199). The UspA proteins have generated considerable interest as vaccine antigens.
M. catarrhalis expresses a trypsin-sensitive, heat-modifiable hemagglutinin (100). Fitzgerald et al. (98) report that strains of M. catarrhalis which contain a 200-kDa protein agglutinate human erythrocytes, whereas strains which lack the 200-kDa protein do not. The authors further report that strains of M. catarrhalis recovered from adults with signs of clinical infection are more likely to hemagglutinate than strains recovered from patients who are colonized and lack clinical signs of infection (100). They suggest that hemagglutination may be a marker of pathogenicity.
OMP CD is a heat-modifiable protein which migrates aberrantly in SDS-PAGE (217, 273). The deduced amino acid sequence reveals a protein of 46 kDa, whereas the protein migrates at an apparent molecular mass of 60 kDa (218). This aberrant migration is due to a proline-rich region in the central part of the protein. OMP CD is highly conserved among strains and shows homology with OprF, a porin in Pseudomonas species (218). OMP CD is abundantly expressed on the bacterial surface (273). The nucleotide sequence encoding OMP CD in strains of M. catarrhalis which persist in the human respiratory tract is stable, indicating that OMP CD does not appear to change under immune selective pressure in vivo (146). OMP CD binds specifically to mucin purified from the human respiratory tract (254).
OMP E is another heat-modifiable protein which is abundantly expressed on the bacterial surface (36, 37). OMP E is a 50-kDa protein which is highly conserved among strains of M. catarrhalis. The function of OMP E is not yet known; it has borderline homology with FadL of E. coli, a protein which is involved in fatty acid transport.
(iii) LOS.
The outer membrane of M. catarrhalis contains LOS which lacks the long O-polysaccharide side chains present in enteric lipopolysaccharide and is thus similar in structure to the LOS of other nonenteric gram-negative bacteria such as Haemophilus and Neisseria spp. (143). In addition, the LOS of M. catarrhalis has at least one epitope in common with the LOS of other nonenteric gram-negative bacteria (47).
The lipid A portion of the LOS molecule of M. catarrhalis is similar in structure to the lipid A of other gram-negative bacteria and is responsible for the profound biological effects of LOS (143, 151, 191). Antigenic differences in the LOS among strains of M. catarrhalis reside in the oligosaccharide portion of the molecule (87, 103). The observation that 95% of strains belong to just three LOS serotypes indicates that less antigenic heterogeneity is present in the LOS of M. catarrhalis than in that of other nonenteric gram-negative bacteria such as Haemophilus and Neisseria spp., whose LOS show enormous antigenic heterogeneity among strains.
(iv) Pili.
Many gram-negative species express pili (also called fimbriae), which mediate adherence to host cells. Strains of M. catarrhalis recovered from the human respiratory tract express pili, as observed by electron microscopy (6–9, 189, 260). Several lines of evidence indicate that some strains express type 4 pili (189). Little else is known about the role of pili in colonization, and this is an area which deserves study.
Immune response.
A large number of studies have been performed to characterize the serological response to M. catarrhalis, and these have been reviewed recently by Christensen (56). This review will consider observations and issues related specifically to the human immune response to M. catarrhalis in COPD.
Chapman et al. (49) demonstrated that adults with COPD develop new bactericidal antibodies to their homologous isolates of M. catarrhalis following lower respiratory tract infection. This observation is important in defining M. catarrhalis as a pathogen and also emphasizes the importance of using immunoassays which detect antibodies to surface-exposed epitopes to elucidate a potentially protective human immune response to M. catarrhalis. Whether this immune response following infection with M. catarrhalis is strain- specific, as observed with nontypeable H. influenzae, remains to be determined.
Little information is available regarding antibody responses to individual antigens of M. catarrhalis in patients with COPD. The human antibody response in patients with COPD to OMP CD and OMP E, which are highly conserved surface proteins, is quite variable among individuals (37, 217). The majority of patients who experienced exacerbations due to M. catarrhalis had detectable serum IgG to the antigens, but none developed new antibodies to OMPs CD and E following infection. Analysis of the mucosal antibody response revealed that IgA was the predominant immunoglobulin to OMPs CD and E in sputum supernatants (37, 217). Antibodies to surface-exposed epitopes on OMP B1 are observed in the serum of patients with bronchiectasis (275). The antibody response to OMP B1 in patients with COPD has not yet been rigorously evaluated.
The immune response to M. catarrhalis in COPD deserves further study. Since M. catarrhalis causes predominantly mucosal infections, characterization of the mucosal immune response will be important. Identifying the nature of a protective immune response to M. catarrhalis will be important as well. As discussed in regard to studies of the immune response to nontypeable H. influenzae, attention should be paid to determining whether infection with M. catarrhalis induces immune responses to strain-specific or antigenically conserved antigens in patients with COPD. In addition, careful attention should be paid to using immunoassays which are capable of detecting antibodies to epitopes which are available on the bacterial surface, because such antibodies are most likely to be associated with a protective immune response.
Prospects for vaccines.
The recognition of the importance of M. catarrhalis as a cause of lower respiratory tract infection in patients with COPD and the role of the bacterium as a cause of otitis media in children has stimulated research which has led to much progress in identifying potential vaccine antigens. Several OMPs have been the focus of study as potential vaccine antigens. The ideal OMP would contain abundantly expressed surface-exposed epitopes in all phases of growth, be highly conserved among strains, be expressed in vivo, and generate a protective immune response in the population that is susceptible to infection with M. catarrhalis. The OMPs listed in Tables 8 and 9 have several of these characteristics to a greater or lesser extent and are the focus of intense investigation. In addition, a detoxified LOS molecule has been studied as a potential vaccine antigen (119).
In spite of substantial progress in identifying antigens with vaccine potential, an important factor which is limiting the development of vaccines for M. catarrhalis is the lack of information about what constitutes a protective immune response to M. catarrhalis. A number of studies have demonstrated immune responses to M. catarrhalis in humans and animal models; however, none of the immune responses identified thus far are clearly associated with protection from infection.
Considerable effort has been devoted to developing a useful animal model for M. catarrhalis. Identifying such an animal model has been difficult. Table 10 lists three animal model systems which have been used to study M. catarrhalis. While animal models have yielded important information, particularly in assessing potential vaccine antigens, each of the proposed models has significant limitations. M. catarrhalis is an exclusively human pathogen and does not cause natural disease in animals. The immune response to M. catarrhalis in humans may differ from that observed in animals. Furthermore, none of the animal models developed thus far is a true model of infection. Rather, they measure the rate of clearance of the bacterium from the animal. The mouse pulmonary clearance model has been the most widely used model to assess potential vaccine antigens.
TABLE 10.
Animal models of Moraxella catarrhalis infection
| Model | Animal | Route and inoculum (CFU) | Outcome measurement | Observations | References |
|---|---|---|---|---|---|
| Pulmonary clearance | Mouse | Lung (1 × 105–5 × 105) | Rate of clearance of bacteria from lungs | M. catarrhalis cleared from lungs by 24 h | 134, 136, 186, 232, 317, 327 |
| Otitis media | Chinchilla | Intrabulbar (3 × 108) | Signs of otitis media and clearance of bacteria from middle ear | M. catarrhalis cleared from middle ear by 5 days | 57, 82 |
| Systemic infection | SCID mouse | Intranasal, intraperitoneal, or intravenous (102–107) | Clinical and postmortem findings | M. catarrhalis not recovered from blood | 133 |
Another approach to identifying a correlate of protection is in vitro assays of functional immune responses. For example, the presence of serum bactericidal antibodies is associated with protection from otitis media due to nontypeable H. influenzae (91, 282). The identification of such a correlate of protection has guided vaccine development. UspA, OMP CD, and OMP B1 of M. catarrhalis are targets of bactericidal antibodies (51, 225, 353). Whether bactericidal antibodies to M. catarrhalis are associated with protection awaits further study.
Streptococcus pneumoniae
Streptococcus pneumoniae has been studied extensively since its first isolation in 1881. Indeed, some of the most important discoveries in microbiology and infectious diseases resulted from research on the pneumococcus. These landmark discoveries include identification of DNA as genetic material, the association of capsular polysaccharide with bacterial virulence, the role of bacterial capsule in resistance to phagocytosis, the concept of type-specific immunity to bacterial infection, and the use of polysaccharide antigens as vaccines (13, 219). S. pneumoniae is the most common cause of community-acquired pneumonia, and the organism is an important cause of invasive infections in adults. The frequency of invasive human infection by the pneumococcus and the serious nature of many of these infections have demanded the attention of investigators, who have studied the epidemiology and pathogenesis of and immunity to pneumococcal infection.
In contrast to invasive pneumococcal infections, the role of S. pneumoniae in respiratory tract infections in COPD has received far less attention for several reasons. As discussed previously in this review, a reliable method to distinguish between colonization and clinical infection in individual patients does not currently exist. Most pneumococcal infections in the setting of COPD are not associated with bacteremia, so the presence of the bacterium in a normally sterile body fluid cannot be used as a diagnostic tool. Furthermore, the pneumoccus is frequently recovered from the sputum of patients with chronic bronchitis even in the absence of clinical infection; therefore, sputum cultures will not reveal the etiology of an exacerbation. These difficulties in defining infection, along with the association of S. pneumoniae with less fulminant infections in COPD, account for the relative lack of information on the role of the pneumococcus in this clinical setting.
A review of work on invasive infections caused by S. pneumoniae is beyond the scope of this review. Selected aspects of epidemiology, pathogenesis, and pneumococcal vaccines as they relate to infection in adults with COPD will be considered.
Epidemiology of colonization.
The rate of respiratory tract colonization by S. pneumoniae varies with age. Prevalence studies reveal that 20 to 40% of healthy children and 10 to 20% of healthy adults are colonized at any one time (108, 137, 221). Several longitudinal studies have established that colonization of the upper airway with S. pneumoniae is common in infants and children (74, 89, 92, 181, 255, 285). Most children are colonized with the pneumococcus at some time during the first 2 years of life. Surprisingly little information is available from longitudinal studies of adults with COPD. Cultures of expectorated sputum from adults experiencing exacerbations of COPD reveal that S. pneumoniae is isolated from 7 to 26% of such samples (Table 2). More information is needed on the dynamics of colonization of patients with COPD, including the frequency of simultaneous colonization by multiple strains, the duration of colonization of individual strains, the rate of turnover of strains, and the distribution of capsular serotypes in patients with COPD. Such information will help to guide vaccination strategies.
Pathogenesis.
(i) Virulence factors and surface antigens.
The polysaccharide capsule of S. pneumoniae is the major virulence factor for the organism. Encapsulated strains are 105 times more virulent than isogenic mutants lacking capsule (316, 338, 339). Capsule accounts for resistance to phagocytosis and survival in the bloodstream, and antibodies to capsular polysaccharide are protective from infection. Ninety serotypes based on structural differences in capsular polysaccharide have been identified.
Cell wall polysaccharide is a major surface antigen which has a common structure among all serotypes. Its structure consists of teichoic acid which contains phosphoryl choline. Enzyme-linked immunosorbent assays (ELISAs) to measure antibodies to type-specific polysaccharide sometimes detect antibodies to cell wall polysaccharide, so caution must be used in interpreting the results of these assays (223).
Cell wall and cell wall polysaccharide induce inflammation. The cell wall consists of peptidoglycan, which is a single macromolecule consisting of a variety of distinct glycopeptides. These cell wall fragments have potent biological effects and play a major role in the inflammation observed in pneumococcal infection (48, 313, 315). The lower airways of adults with COPD are colonized by pneumococci, which are continuously dividing and shedding cell wall fragments into the airways. These bacterial antigens may contribute to the airway inflammation observed in COPD.
Pneumolysin is a thiol-activated toxin which has a variety of toxic effects on different cell types (250, 266). Pneumolysin-negative mutants are less virulent than their parental strains, and antibodies are protective in animal models (34, 35, 314). Autolysin lyses pneumococci upon release of pneumolysin by the bacterium (35). Neuraminidase may facilitate attachment to epithelial cells by cleaving sialic acid from the host glycolipids and gangliosides (311). PspA is a surface protein which is required for full virulence of the pneumococcus, and antibodies to PspA are protective in a mouse model (42). S. pneumoniae expresses IgA1 protease, which may facilitate colonization by cleaving secretory IgA in the respiratory tract (337). However, elucidation of the precise role of IgA1 protease in pathogenesis awaits further work.
(ii) Adherence.
S. pneumoniae binds to respiratory tract epithelial cells to colonize the human respiratory tract. Work in the past decade has identified several adhesins and has begun to elucidate some of the molecular mechanisms of adherence of pneumococci to cells in the respiratory tract.
Pneumococcal isolates undergo spontaneous phase variation between opaque and transparent colony morphologies. These differences in colony morphology correlate with rates of autolysis and appear to be relevant to virulence, particularly in adherence to host cells. Transparent variants adhere more readily to host cells and are able to colonize the nasopharynx of mice more efficiently than opaque variants (69, 342). The mechanism of enhanced colonization appears to involve increased adherence to GlcNAc and platelet-activating factor receptors on host cells (69).
Airway epithelial cells express receptors which show different specificities for the transparent and opaque variants. Activation of host cells by cytokines alters expression of receptors. For example, IL-1 activates epithelial cells, and TNF-α activates endothelial cells to express platelet-activating factor, which is a receptor for the pneumococcus (68). Such mechanisms likely contribute to the outcome of pneumococcal infections.
Several adhesins have been identified on the pneumococcal surface. PsaA is a 37-kDa protein which mediates attachment to type II pneumocytes and is involved in colonization of the respiratory tract in mice (33, 73). CbdA is a choline-binding protein of 75 kDa which is an adhesin and a determinant of virulence (264). A pyruvate oxidase, encoded by spxB, is important in the expression of adhesins, since a mutation in spxB leads to downregulation of multiple adhesive properties of S. pneumoniae (297). Although pneumolysin is a cytoplasmic protein released only after autolysis, it may play an indirect role in adherence by directly damaging epithelial surfaces (250). In vitro studies confirm reduced adherence to epithelial cells in pneumolysin-deficient mutants (266). However, pneumolysin does not appear to be a major determinant in nasopharyngeal colonization in mice (266).
The molecular mechanisms of adherence of S. pneumoniae and induction of inflammation by pneumococcal antigens are areas of research which will lead to novel methods of treatment and prevention of pneumococcal infection.
Pneumococcal vaccine.
Anticapsular antibody provides the greatest degree of protection from invasive infection, and this observation has guided vaccine development for the pneumococcus. The Centers for Disease Control and the American College of Physicians recommend that all patients with COPD receive the 23-valent capsular polysaccharide vaccine. While this approach is rational, a critical review of the literature discloses no convincing evidence that immunization of adults with COPD reduces the incidence of pneumococcal infection in COPD (reviewed in reference 219). No new trials which would change that conclusion have been published (97, 233).
The capsular polysaccharide vaccine is not uniformly immunogenic in the elderly, and this observation likely accounts for the variable efficacy of the vaccine in this population (182, 263, 265, 267). Since COPD is a disease of the elderly, immune responses in this population are of particular relevance with regard to COPD. It has been estimated that the vaccine has an efficacy of 50 to 75% (149). Recently developed conjugate vaccines in which pneumococcal capsular polysaccharides are coupled to protein carriers hold promise as being more immunogenic. Some early trials in infants and children have revealed good immunogenicity and reduction in nasopharyngeal colonization by the pneumococcus (18, 70, 193, 194, 357). The ability of the vaccines to eradicate colonization may be especially useful in patients with COPD, since colonization likely contributes to airway inflammation and exacerbations are mucosal infections. Unfortunately, early studies with conjugate vaccines in the elderly suggest that they may not offer a major advantage in older populations (245, 281). Further study of pneumococcal conjugate vaccines in patients with COPD is clearly warranted.
Antibodies to capsular polysaccharide of S. pneumoniae are generally measured by ELISA. Recent efforts have been directed toward improving the specificity and standardizing the quantitation of capsule-specific antibodies by ELISA. Furthermore, since serum levels of antibodies to capsule do not always correlate with protection, recent work has focused on functional antibody responses, particularly in the elderly (149, 263). Romero-Steiner et al. (263) showed that functional immune responses, measured by in vitro killing assays, after pneumococcal vaccination were significantly lower in elderly adults than in young adults. Interestingly, functional antibody responses did not correlate closely with antibody levels measured by ELISA but did show correlation with antibody avidity. In view of these observations, it is important to critically assess indicators of vaccine protection by correlating clinical outcomes with antibody levels, functional activity, and antibody avidity to have an accurate surrogate for protection against pneumococcal infection (149). In addition, it will be important to assess the efficacy of pneumococcal vaccines specifically in patients with COPD, since protection against recurrent exacerbations and colonization by the pneumococcus may be different from protection from invasive infection, which has been the most common measure of efficacy of the vaccines in trials to date.
Haemophilus parainfluenzae
H. parainfluenzae is present as part of the normal upper respiratory tract flora in humans. Therefore, the bacterium is frequently recovered from expectorated sputum from adults with COPD (309). The presence of H. parainfluenzae in the sputum of 2 to 27% of patients experiencing exacerbations (Table 2) has raised the question of whether H. parainfluenzae causes exacerbations of COPD. The presence of the organism in sputum during an exacerbation does not establish etiology. In order to address this question, one must examine the type of evidence which has established that H. influenzae and M. catarrhalis cause exacerbations.
Bronchoscopy with the protected specimen brush performed during exacerbations has been used as a method to establish the etiology of some exacerbations in four recent studies (96, 208, 239, 294) (Table 3). Bacteria were present in 50 to 72% of patients with exacerbations. In one study (96), 25% of the samples contained H. parainfluenzae, while in the other three studies, H. parainfluenzae was not recovered at all (208, 239, 294). The discrepancy is difficult to explain, but these studies do not provide convincing evidence one way or the other for H. parainfluenzae's being a common cause of exacerbations.
Documenting an immune response to a putative pathogen is another approach to establishing etiology. Mitchell and Hill (202) recently studied the immune response to H. parainfluenzae in three patients with chronic bronchitis and showed that these patients had higher titers of antibodies to H. parainfluenzae than healthy controls. Essentially no other work on the immune response to H. parainfluenzae in COPD has been published. This is an important area of research to pursue.
Overall, there is simply not enough evidence at this time to state with any degree of confidence the role, if any, that H. parainfluenzae plays as a pathogen in COPD.
Chlamydia pneumoniae
C. pneumoniae is an obligate intracellular human pathogen which causes acute infections of the upper and lower respiratory tract, including pharyngitis, sinusitis, bronchitis, and community-acquired pneumonia (129). Chronic infection with C. pneumoniae occurs in patients with COPD, as discussed above. Studies of C. pneumoniae in COPD are complicated by several observations. (i) The organism is difficult to cultivate and detect directly in the respiratory tract. (ii) Coinfection with other bacteria is common. (iii) Variability among authors exists in interpretation of the results of serological assays. (iv) By early adulthood, at least half of the population worldwide is serologically positive for C. pneumoniae (129). (v) Smoking is associated with increased levels of serum antibodies to C. pneumoniae in patients with and without COPD. (vi) Serological conversion occurs even in the absence of symptoms.
C. pneumoniae likely causes a small proportion of acute exacerbations of COPD, an observation based on serological evidence. Table 11 summarizes four studies which used serological criteria to establish the presence of acute C. pneumoniae infection in adults experiencing acute exacerbations of COPD. Although geographic variation may occur, the best estimates are that 5 to 10% of exacerbations of COPD are associated with C. pneumoniae.
TABLE 11.
Chlamydia pneumoniae as a cause of acute exacerbations of COPD
| Reference | Country | No. of exacerbations studied | % caused by C. pneumoniae |
|---|---|---|---|
| Blasi et al. (38) | Italy | 142 | 4 |
| Mogulkac et al. (205) | Turkey | 49 | 16 (sole etiology), 6 (other agents) |
| Miyashita et al. (203) | Japan | 77 | 7.8 |
| Beaty et al. (29) | United States | 44 | 5 |
Gram-Negative Bacilli
Samples collected from the lower airways with the protected specimen brush contain gram-negative bacilli, including Pseudomonas aeruginosa, E. coli, and Proteus mirabilis, in a small proportion of patients experiencing acute exacerbations of COPD (96, 208, 239, 294) (Table 3). These bacteria are isolated most often in the setting of severe exacerbations in patients with advanced COPD. The presence of these bacteria in the lower airways could reflect selective pressure by repeated exposure to antibiotics, a greater degree of compromise of lung defenses, or a combination of these factors. Defining the role of gram-negative bacilli in acute exacerbations is important in understanding the pathogenesis of infection and also in guiding a rational approach to antimicrobial therapy. Analysis of immune responses to these putative pathogens following exacerbations is a fruitful avenue of research to lead to a more precise definition of the role of these gram-negative bacilli in acute exacerbations of COPD.
CONCLUSIONS AND FUTURE DIRECTIONS
Research in the past decade has produced exciting new observations on the important role of bacteria in COPD. Better techniques to sample the lower airways, advances in techniques to study molecular mechanisms of microbial pathogenesis, and powerful methods to study host inflammatory responses provide opportunities to more precisely elucidate the role of bacteria in the pathogenesis and course of COPD. Emphasis should be placed on defining the contribution of bacteria in the lower airways to airway inflammation which is a hallmark of COPD. Identifying the microbial antigens responsible will allow investigators to design novel interventions to test the hypothesis that bacterial colonization of the lower airways accelerates progression of the disease. Developing reliable and widely applicable methods to define the etiology of exacerbations will be important in devising strategies to better treat and prevent exacerbations. Finally, the development of vaccines to prevent infections caused by nontypeable H. influenzae, M. catarrhalis, and S. pneumoniae would have a major impact on the course of COPD.
ACKNOWLEDGMENTS
This work was supported by the Department of Veterans Affairs and by NIH grants AI19641 and AI28304.
We thank Alan Lesse and Adeline Thurston for expertise in preparing the manuscript.
REFERENCES
- 1.Adler K B, Hendley D D, Davis G S. Bacteria associated with obstructive pulmonary disease elaborate extracellular products that stimulate mucin secretion by explants of guinea pig airways. Am J Pathol. 1986;125:501–514. [PMC free article] [PubMed] [Google Scholar]
- 2.Aebi C, Cope L D, Latimer J L, Thomas S E, Slaughter C A, McCracken G H, Jr, Hansen E J. Mapping a protective epitope of the CopB outer membrane protein of Moraxella catarrhalis. Infect Immun. 1998;66:540–548. doi: 10.1128/iai.66.2.540-548.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aebi C, LaFontaine E R, Cope L D, Latimer J L, Lumbley S L, McCracken G H, Jr, Hansen E J. Phenotypic effect of isogenic uspA1 and uspA2 mutations on Moraxella catarrhalis 035E. Infect Immun. 1998;66:3113–3119. doi: 10.1128/iai.66.7.3113-3119.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aebi C, Maciver I, Latimer J L, Cope L D, Stevens M K, Thomas S E, McCracken G H, Jr, Hansen E J. A protective epitope of Moraxella catarrhalis is encoded by two different genes. Infect Immun. 1997;65:4367–4377. doi: 10.1128/iai.65.11.4367-4377.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aebi C, Stone B, Beucher M, Cope L D, Maciver I, Thomas S E, McCracken G H, Jr, Sparling P F, Hansen E J. Expression of the CopB outer membrane protein by Moraxella catarrhalis is regulated by iron and affects iron acquisition from transferrin and lactoferrin. Infect Immun. 1996;64:2024–2030. doi: 10.1128/iai.64.6.2024-2030.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed K. Fimbriae of Branhamella catarrhalis as possible mediators of adherence to pharyngeal epithelial cells. APMIS. 1992;100:1066–1072. doi: 10.1111/j.1699-0463.1992.tb04042.x. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed K, Masaki H, Dai T C, Ichinose A, Utsunomiya Y, Tao M, Nagatake T, Matsumoto K. Expression of fimbriae and host response in Branhamella catarrhalis respiratory infections. Microbiol Immunol. 1994;38:767–771. doi: 10.1111/j.1348-0421.1994.tb01855.x. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed K, Rikitomi N, Matsumoto K. Fimbriation, hemagglutination and adherence properties of fresh clinical isolates of Branhamella catarrhalis. Microbiol Immunol. 1992;36:1009–1017. doi: 10.1111/j.1348-0421.1992.tb02105.x. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed K, Rikitomi N, Nagatake T, Matsumoto K. Electron microscopic observation of Branhamella catarrhalis. Microbiol Immunol. 1990;34:967–975. doi: 10.1111/j.1348-0421.1990.tb01518.x. [DOI] [PubMed] [Google Scholar]
- 10.Aitken J M, Thornley P E. Isolation of Branhamella catarrhalis from sputum and tracheal aspirate. J Clin Microbiol. 1983;18:1262–1263. doi: 10.1128/jcm.18.5.1262-1263.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akkoyunlu M, Janson H, Ruan M, Forsgren A. Biological activity of serum antibodies to nonacylated form of lipoprotein D of Haemophilus influenzae. Infect Immun. 1996;64:4586–4592. doi: 10.1128/iai.64.11.4586-4592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allegra L, Konietzko N, Leophonte P, Hosie J, Pauwels R, Guyen J N, Petitpretz P. Comparative safety and efficacy of sparfloxacin in the treatment of acute exacerbations of chronic obstructive pulmonary disease: a double-blind, randomised, parallel, multicentre study. J Antimicrob Chemother. 1996;37:93–104. doi: 10.1093/jac/37.suppl_a.93. [DOI] [PubMed] [Google Scholar]
- 13.Alonso De Velasco E, Verheul A F M, Verhoef J, Snippe H. Streptococcus pneumoniae: virulence factors, pathogenesis, and vaccines. Microbiol Rev. 1995;59:591–603. doi: 10.1128/mr.59.4.591-603.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995;152:S77–S120. [PubMed] [Google Scholar]
- 15.Amitani R, Wilson R, Rutman A, Read R, Ward C, Burnett D, Stockley R A, Cole P J. Effects of human neutrophil elastase and Pseudomonas aeruginosa proteinases on human respiratory epithelium. Am J Respir Cell Mol Biol. 1991;4:26–32. doi: 10.1165/ajrcmb/4.1.26. [DOI] [PubMed] [Google Scholar]
- 16.Aniansson G, Alm B, Andersson B, Larsson P, Nylen O, Peterson H, Rigner P, Svanborg M, Svanborg C. Nasopharyngeal colonization during the first year of life. J Infect Dis. 1992;165(S1):S38–S42. doi: 10.1093/infdis/165-supplement_1-s38. [DOI] [PubMed] [Google Scholar]
- 17.Anthonisen N R, Manfreda J, Warren C P W, Hershfield E S, Harding G K M, Nelson N A. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196–204. doi: 10.7326/0003-4819-106-2-196. [DOI] [PubMed] [Google Scholar]
- 18.Anttila M, Eskola J, Ahman H, Kayhty H. Avidity of IgG for Streptococcus pneumoniae type 6B and 23F polysaccharides in infants primed with pneumococcal conjugates and boosted with polysaccharide or conjugate vaccines. J Infect Dis. 1998;177:1614–1621. doi: 10.1086/515298. [DOI] [PubMed] [Google Scholar]
- 19.Anzueto A, Niederman M S, Tillotson G S Bronchitis Study Group. Etiology, susceptibility, and treatment of acute bacterial exacerbations of complicated chronic bronchitis in the primary care setting: ciprofloxacin 750 mg BID vs clarithromycin 500 mg BID. Clin Ther. 1998;20:885–900. doi: 10.1016/s0149-2918(98)80071-4. [DOI] [PubMed] [Google Scholar]
- 20.Bakaletz L O, Barenkamp S J. Localization of high-molecular-weight adhesion proteins of nontypeable Haemophilus influenzae by immunoelectron microscopy. Infect Immun. 1994;62:4460–4468. doi: 10.1128/iai.62.10.4460-4468.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barenkamp S J. Immunization with high-molecular-weight adhesion proteins of nontypeable Haemophilus influenzae modifies experimental otitis media in chinchillas. Infect Immun. 1996;64:1246–1251. doi: 10.1128/iai.64.4.1246-1251.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barenkamp S J, Leininger E. Cloning, expression, and DNA sequence analysis of the genes encoding nontypeable Haemophilus influenzae high-molecular-weight surface-exposed proteins related to filamentous hemagglutinin of Bordetella pertussis. Infect Immun. 1992;60:1302–1313. doi: 10.1128/iai.60.4.1302-1313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barenkamp S J, Munson R S, Jr, Granoff D M. Outer membrane protein and biotype analysis of pathogenic nontypable Haemophilus influenzae. Infect Immun. 1982;36:535–540. doi: 10.1128/iai.36.2.535-540.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barenkamp S J, St. Geme J W., III Genes encoding high-molecular-weight adhesion proteins of nontypeable Haemophilus influenzae are part of gene clusters. Infect Immun. 1994;62:3320–3328. doi: 10.1128/iai.62.8.3320-3328.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barenkamp S J, St. Geme J W., III Identification of a second family of high-molecular-weight adhesion proteins expressed by non-typable Haemophilus infleunzae. Mol Microbiol. 1996;19:1215–1223. doi: 10.1111/j.1365-2958.1996.tb02467.x. [DOI] [PubMed] [Google Scholar]
- 26.Barker D J P, Godfrey K M, Fall C, Osmond C, Winter P D, Shaheen S O. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. Br Med J. 1991;303:671–675. doi: 10.1136/bmj.303.6804.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartos L C, Murphy T F. Comparison of the outer membrane proteins of 50 strains of Branhamella catarrhalis. J Infect Dis. 1988;158:761–765. doi: 10.1093/infdis/158.4.761. [DOI] [PubMed] [Google Scholar]
- 28.Bates D V. The fate of the chronic bronchitic: a report of the ten-year follow-up in the Canadian Department of Veteran's Affairs coordinated study of chronic bronchitis. Am Rev Respir Dis. 1998;108:1043–1065. doi: 10.1164/arrd.1973.108.5.1043. [DOI] [PubMed] [Google Scholar]
- 29.Beaty C D, Grayston J T, Wang S-P, Kuo C-C, Reto C S, Martin T R. Chlamydia pneumoniae, strain TWAR, infection in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1991;144:1408–1410. doi: 10.1164/ajrccm/144.6.1408. [DOI] [PubMed] [Google Scholar]
- 30.Beaulieu D, Scriver S, Bergeron M G, Low D E, Parr T R, Jr, Patterson J E, Matlow A, Roy P H. Epidemiological typing of Moraxella catarrhalis by using DNA probes. J Clin Microbiol. 1993;31:736–739. doi: 10.1128/jcm.31.3.736-739.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bell J, Grass S, Jeanteur D, Munson R S., Jr Diversity of the P2 protein among nontypeable Haemophilus influenzae isolates. Infect Immun. 1994;62:2639–2643. doi: 10.1128/iai.62.6.2639-2643.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernstein J M, Dryja D, Yuskiw N, Murphy T F. Analysis of isolates recovered from multiple sites of the nasopharynx of children colonized by nontypeable Haemophilus influenzae. Eur J Clin Microbiol Infect Dis. 1997;16:750–753. doi: 10.1007/BF01709258. [DOI] [PubMed] [Google Scholar]
- 33.Berry A M, Paton J C. Sequence heterogeneity of PsaA, a 37-kilodalton putative adhesin essential for virulence of Streptococcus pneumoniae. Infect Immun. 1996;64:5255. doi: 10.1128/iai.64.12.5255-5262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berry A M, Paton J C. Additive attenuation of virulence of Streptococcus pneumoniae by mutation of the genes encoding pneumolysin and other putative pneumococcal virulence proteins. Infect Immun. 2000;68:133–140. doi: 10.1128/iai.68.1.133-140.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berry A M, Paton J C, Hansman D. Effect of insertional inactivation of the genes encoding pneumolysin and autolysin on the virulence of Streptococcus pneumoniae type 3. Microb Pathog. 1992;12:87–93. doi: 10.1016/0882-4010(92)90111-z. [DOI] [PubMed] [Google Scholar]
- 36.Bhushan R, Craigie R, Murphy T F. Molecular cloning and characterization of outer membrane protein E of Moraxella (Branhamella) catarrhalis. J Bacteriol. 1994;176:6636–6643. doi: 10.1128/jb.176.21.6636-6643.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhushan R, Kirkham C, Sethi S, Murphy T F. Antigenic characterization and analysis of the human immune response to outer membrane protein E of Branhamella catarrhalis. Infect Immun. 1997;65:2668–2675. doi: 10.1128/iai.65.7.2668-2675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blasi F, Legnani D, Lombardo V M, Negretto G G, Magliano E, Pozzoli R, Chiodo F, Fasoli A, Allegra L. Chlamydia pneumoniae infection in acute exacerbations of COPD. Eur Respir J. 1993;6:19–22. [PubMed] [Google Scholar]
- 39.Bonnah R A, Wong H, Loosmore S M, Schryvers A B. Characterization of Moraxella (Branhamella) catarrhalis lbpB, lpbA, and lactoferrin receptor orf3 isogenic mutants. Infect Immun. 1999;67:1517–1520. doi: 10.1128/iai.67.3.1517-1520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonnah R A, Yu R-H, Wong H, Schryvers A B. Biochemical and immunological properties of lactoferrin binding proteins from Moraxella (Branhamella) catarrhalis. Microb Pathog. 1998;24:89–100. doi: 10.1006/mpat.1997.0173. [DOI] [PubMed] [Google Scholar]
- 41.Boyle F M, Georghiou P R, Tilse M H, McCormack J G. Branhamella (Moraxella) catarrhalis: pathogenic significance in respiratory infections. Med J Aust. 1991;154:592–596. doi: 10.5694/j.1326-5377.1991.tb121219.x. [DOI] [PubMed] [Google Scholar]
- 42.Briles D E, Tart R C, Swiatlo E, Dillard J P, Smith P, Benton K A, Ralph B A, Brooks-Walter A, Crain M J, Hollingshead S K, McDaniel L S. Pneumococcal diversity: considerations for new vaccine strategies with emphasis on pneumococcal surface protein A (PspA) Clin Microbiol Rev. 1998;11:645–657. doi: 10.1128/cmr.11.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruce K D, Jordens J Z. Characterization of noncapsulate Haemophilus influenzae by whole-cell polypeptide profiles, restriction endonuclease analysis, and rRNA gene restriction patterns. J Clin Microbiol. 1991;29:291–296. doi: 10.1128/jcm.29.2.291-296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brygge K, Sorensen C H, Colding H, Ejlertsen T, Hojbjerg T, Bruun B. Ribotyping of strains of Moraxella (Branhamella) catarrhalis cultured from the nasopharynx and middle ear of children with otitis media. Acta Otolaryngol (Stockholm) 1998;118:381–385. doi: 10.1080/00016489850183476. [DOI] [PubMed] [Google Scholar]
- 45.Cabello H, Torres A, Celis R, El-Ebiary M, Puig de la Bellacasa J, Xaubet A, Gonzalez J, Agusti C, Soler N. Bacterial colonization of distal airways in healthy subjects and chronic lung disease: a bronchoscopic study. Eur Respir J. 1997;10:1137–1144. doi: 10.1183/09031936.97.10051137. [DOI] [PubMed] [Google Scholar]
- 46.Campagnari A A, Shanks K L, Dyer D W. Growth of Moraxella catarrhalis with human transferrin and lactoferrin: expression of iron-repressible proteins without siderophore production. Infect Immun. 1994;62:4909–4914. doi: 10.1128/iai.62.11.4909-4914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campagnari A A, Spinola S M, Lesse A J, Abu Kwaik Y, Mandrell R E, Apicella M A. Lipooligosaccharide epitopes shared among gram-negative non-enteric mucosal pathogens. Microb Pathog. 1990;8:353–362. doi: 10.1016/0882-4010(90)90094-7. [DOI] [PubMed] [Google Scholar]
- 48.Carlsen B D, Kawana M, Kawana C, Tomasz A, Giebink G S. Role of the bacterial cell wall in middle ear inflammation caused by Streptococcus pneumoniae. Infect Immun. 1992;60:2850–2854. doi: 10.1128/iai.60.7.2850-2854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chapman A J, Musher D M, Jonsson S, Clarridge J E, Wallace R J. Development of bactericidal antibody during Branhamella catarrhalis infection. J Infect Dis. 1985;151:878–882. doi: 10.1093/infdis/151.5.878. [DOI] [PubMed] [Google Scholar]
- 50.Chastre J, Fagon J-Y, Bornet-Lecso M, Calvat S, Dombret M-C, Khani R A, Basset F, Gibert C. Evaluation of bronchoscopic techniques for the diagnosis of nosocomial pneumonia. Am J Respir Crit Care Med. 1995;152:231–240. doi: 10.1164/ajrccm.152.1.7599829. [DOI] [PubMed] [Google Scholar]
- 51.Chen D, Barniak V, van der Meid K R, McMichael J C. The levels and bactericidal capacity of antibodies directed against the UspA1 and UspA2 outer membrane proteins of Moraxella (Branhamella) catarrhalis in adults and children. Infect Immun. 1999;67:1310–1316. doi: 10.1128/iai.67.3.1310-1316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen D, McMichael J C, van der Meid K R, Hahn D, Mininni T, Cowell J, Eldridge J. Evaluation of purified UspA from Moraxella catarrhalis as a vaccine in a murine model after active immunization. Infect Immun. 1996;64:1900–1905. doi: 10.1128/iai.64.6.1900-1905.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chodosh S, Lakshminarayan S, Swarz H, Breisch S. Efficacy and safety of a 10-day course of 400 or 600 milligrams of grepafloxacin once daily for treatment of acute bacterial exacerbations of chronic bronchitis: comparison with a 10-day course of 500 milligrams of ciprofloxacin twice daily. Antimicrob Agents Chemother. 1998;42:114–120. doi: 10.1128/aac.42.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chodosh S, McCarty J, Farkas S, Drehobl M, Tosiello R, Shan M, Aneiro L, Kowalsky S Bronchitis Study Group. Randomized, double-blind study of ciprofloxacin and cefuroxime axetil for treatment of acute bacterial exacerbations of chronic bronchitis. Clin Infect Dis. 1998;27:722–729. doi: 10.1086/514930. [DOI] [PubMed] [Google Scholar]
- 55.Chodosh S, Schreurs J M, Siami G, Barkman H W, Jr, Anzueto A, Shan M, Moesker H, Stack T, Kowalsky S Bronchitis Study Group. Efficacy of oral ciprofloxacin vs clarithromycin for treatment of acute bacterial exacerbations of chronic bronchitis. Clin Infect Dis. 1998;27:730–738. doi: 10.1086/514934. [DOI] [PubMed] [Google Scholar]
- 56.Christensen J J. Moraxella (Branhamella) catarrhalis: clinical, microbiological and immunological features in lower respiratory tract infections. APMIS. 1999;107(Suppl.):1–36. [PubMed] [Google Scholar]
- 57.Chung M-H, Enrique R, Lim D J, DeMaria T F. Moraxella (Branhamella) catarrhalis-induced experimental otitis media in the chinchilla. Acta Otolaryngol. 1994;114:415–422. doi: 10.3109/00016489409126080. [DOI] [PubMed] [Google Scholar]
- 58.Clancy R, Cripps A, Murree-Allen K, Yeung S, Engel M. Oral immunisation with killed Haemophilus influenzae for protection against acute bronchitis in chronic obstructive lung disease. Lancet. 1985;ii:1395–1397. doi: 10.1016/s0140-6736(85)92559-0. [DOI] [PubMed] [Google Scholar]
- 59.Clancy R L, Cripps A W, Gebski V. Protection against recurrent acute bronchitis after oral immunization with killed Haemophilus influenzae. Med J Aust. 1990;152:413–416. doi: 10.5694/j.1326-5377.1990.tb125268.x. [DOI] [PubMed] [Google Scholar]
- 60.Clancy R L, Cripps A W, Husband A J, Buckley D. Specific immune response in the respiratory tract after administration of an oral polyvalent bacterial vaccine. Infect Immun. 1983;39:491–496. doi: 10.1128/iai.39.2.491-496.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clementsen P, Larsen F O, Milman N, Skov P S, Norn S. Haemophilus influenzae release histamine and enhance histamine release from human bronchoalveolar cells. APMIS. 1995;103:806–812. [PubMed] [Google Scholar]
- 62.Clementsen P, Milman N, Kilian M, Fomsgaard A, Baek L, Norn S. Endotoxin from Haemophilus influenzae enhances IgE-mediated and non-immunological histamine release. Allergy. 1990;45:10–17. doi: 10.1111/j.1398-9995.1990.tb01078.x. [DOI] [PubMed] [Google Scholar]
- 63.Cole P. Host-microbe relationships in chronic respiratory infection. Respiration. 1989;55(S1):5–8. doi: 10.1159/000195745. [DOI] [PubMed] [Google Scholar]
- 64.Cope L D, LaFontaine E R, Slaughter C A, Hasemann C A, Jr, Aebi C, Henderson F W, McCracken G H, Jr, Hansen E J. Characterization of the Moraxella catarrhalis uspA1 and uspA2 genes and their encoded products. J Bacteriol. 1999;181:4026–4034. doi: 10.1128/jb.181.13.4026-4034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cope L D, Thomas S E, Latimer J L, Slaughter C A, Muller-Eberhard U, Hansen E J. The 100 kDa haem:haemopexin-binding protein of Haemophilus influenzae: structure and localization. Mol Microbiol. 1994;13:863–873. doi: 10.1111/j.1365-2958.1994.tb00478.x. [DOI] [PubMed] [Google Scholar]
- 66.Cope L D, Yogev R, Muller-Eberhard U, Hansen E J. A gene cluster involved in the utilization of both free heme and heme: hemopexin by Haemophilus influenzae type b. J Bacteriol. 1995;177:2644–2653. doi: 10.1128/jb.177.10.2644-2653.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cosio M, Gheezo H, Hogg J C, Corbin R, Loveland M, Dosman J, Macklem P T. The relations between structural changes in small airways and pulmonary-function tests. N Engl J Med. 1977;298:1277–1281. doi: 10.1056/NEJM197806082982303. [DOI] [PubMed] [Google Scholar]
- 68.Cundell D R, Gerard N P, Gerard C, Idanpaan-Helkklla I, Tuomanen E I. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 69.Cundell D R, Weiser J N, Shen J, Young A, Tuomanen E I. Relationship between colonial morphology and adherence of Streptococcus pneumoniae. Infect Immun. 1995;63:757–761. doi: 10.1128/iai.63.3.757-761.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dagan R, Melamed R, Muallem M, Piglansky L, Greenberg D, Abramson O, Mendelman P M, Bohidar N, Yagupsky P. Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate pneumococcal vaccine. J Infect Dis. 1996;174:1271–1278. doi: 10.1093/infdis/174.6.1271. [DOI] [PubMed] [Google Scholar]
- 71.Davies B I, Maesen F P V. Clinical effectiveness of levofloxacin in patients with acute purulent exacerbations of chronic bronchitis: the relationship with in vitro activity. J Antimicrob Chemother. 1999;43:83–90. doi: 10.1093/jac/43.suppl_3.83. [DOI] [PubMed] [Google Scholar]
- 72.Davies J, Carlstedt I, Nilsson A-K, Hakansson A, Sabharwal H, van Alphen L, van Ham M, Svanborg C. Binding of Haemophilus influenzae to purified mucins from the human respiratory tract. Infect Immun. 1995;63:2485–2492. doi: 10.1128/iai.63.7.2485-2492.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De B K, Sampson J S, Ades E W, Huebner R C, Jue D L, Johnson S E, Espina M, Stinson A R, Briles D E, Carlone G M. Purification and characterization of Streptococcus pneumoniae palmitoylated pneumococcal surface adhesin A expressed in Escherichia coli. Vaccine. 2000;18:1811–1921. doi: 10.1016/s0264-410x(99)00481-8. [DOI] [PubMed] [Google Scholar]
- 74.DeAbate C A, Henry D, Bensch G, Jubran A, Chodosh S, Harper L, Tipping D, Talbot G H Sparfloxacin Multicenter ABECB Study Group. Sparfloxacin vs ofloxacin in the treatment of acute bacterial exacerbations of chronic bronchitis. Chest. 1998;114:120–130. doi: 10.1378/chest.114.1.120. [DOI] [PubMed] [Google Scholar]
- 75.De Lencastre H, Kristinsson K G, Brito-Avo A, Sanches I S, Sa-Leao R, Saldanha J, Sigvaldadottir E, Karlsson S, Oliveira D, Mato R, de Sousa M A, Tomasz A. Carriage of respiratory tract pathogens and molecular epidemiology of Streptococcus pneumoniae colonization in healthy children attending day care centers in Lisbon, Portugal. Microb Drug Resist. 1999;5:19–29. doi: 10.1089/mdr.1999.5.19. [DOI] [PubMed] [Google Scholar]
- 76.DeMaria T F, Murwin D M, Leake E R. Immunization with outer membrane protein P6 from nontypeable Haemophilus influenzae induces bactericidal antibody and affords protection in the chinchilla model of otitis media. Infect Immun. 1996;64:5187–5192. doi: 10.1128/iai.64.12.5187-5192.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Denamur E, Picard-Pasquier N, Mura C, Picard B, Orfila J, Krishnamoorthy R. Comparison of molecular epidemiological tools for Branhamella catarrhalis typing. Res Microbiol. 1991;142:585–589. doi: 10.1016/0923-2508(91)90191-c. [DOI] [PubMed] [Google Scholar]
- 78.Dickinson D P, Loos B G, Dryja D M, Bernstein J M. Restriction fragment mapping of Branhamella catarrhalis: a new tool for studying the epidemiology of this middle ear pathogen. J Infect Dis. 1988;158:205–208. doi: 10.1093/infdis/158.1.205. [DOI] [PubMed] [Google Scholar]
- 79.Di Stefano A, Capelli A, Lusuardi M, Balbo P, Vecchio C, Maestrelli P, Mapp C E, Fabbri L M, Donner C F, Saetta M. Severity of airflow limitation is associated with severity of airway inflammation in smokers. Am J Respir Crit Care Med. 1998;158:1277–1285. doi: 10.1164/ajrccm.158.4.9802078. [DOI] [PubMed] [Google Scholar]
- 80.Doern G V, Gantz N M. Isolation of Branhamella (Neisseria) catarrhalis from men with urethritis. Sex Transm Dis. 1982;9:202–204. doi: 10.1097/00007435-198210000-00008. [DOI] [PubMed] [Google Scholar]
- 81.Doern G V, Miller M J, Winn R E. Branhamella (Neisseria) catarrhalis systemic disease in humans. Arch Intern Med. 1981;141:1690–1692. [PubMed] [Google Scholar]
- 82.Doyle W J. Animal models of otitis media: other pathogens. Pediatr Infect Dis J. 1989;8:S45–S47. [PubMed] [Google Scholar]
- 83.Du R-P, Wang Q, Yang Y-P, Schryvers A B, Chong P, Klein M H, Loosmore S M. Cloning and expression of the Moraxella catarrhalis lactoferrin receptor genes. Infect Immun. 1998;66:3656–3665. doi: 10.1128/iai.66.8.3656-3665.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Duim B, Dankert J, Jansen H M, van Alphen L. Genetic analysis of the diversity in outer membrane protein P2 of non-encapsulated Haemophilus influenzae. Microb Pathog. 1993;14:451–462. doi: 10.1006/mpat.1993.1044. [DOI] [PubMed] [Google Scholar]
- 85.Duim B, van Alphen L, Eijk P, Jansen H M, Dankert J. Antigenic drift of non-encapsulated Haemophilus influenzae major outer membrane protein P2 in patients with chronic bronchitis is caused by point mutations. Mol Microbiol. 1994;11:1181–1189. doi: 10.1111/j.1365-2958.1994.tb00394.x. [DOI] [PubMed] [Google Scholar]
- 86.Duim B, Vogel L, Puijk W, Jansen H M, Meloen R H, Dankert J, van Alphen L. Fine mapping of outer membrane protein P2 antigenic sites which vary during persistent infection by Haemophilus influenzae. Infect Immun. 1996;64:4673–4679. doi: 10.1128/iai.64.11.4673-4679.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Edebrink P, Jansson P-E, Rahman M M, Widmalm G, Holme T, Rahman M, Weintraub A. Structural studies of the O-polysaccharide from the lipopolysaccharide of Moraxella (Branhamella) catarrhalis serotype A (strain ATCC 25238) Carbohydr Res. 1994;257:269–284. doi: 10.1016/0008-6215(94)80040-5. [DOI] [PubMed] [Google Scholar]
- 88.Ejlertsen T, Thisted E, Ebbesen F, Olesen B, Renneberg J. Branhamella catarrhalis in children and adults: a study of prevalence, time of colonisation, and association with upper and lower respiratory tract infections. J Infect. 1994;29:23–31. doi: 10.1016/s0163-4453(94)94979-4. [DOI] [PubMed] [Google Scholar]
- 89.Ekdahl K, Ahlinder I, Hansson H B, Melander E, Molstad S, Soderstrom M, Persson K. Duration of nasopharyngeal carriage of penicillin-resistant Streptococcus pneumoniae: experiences from the South Swedish Pneumococcal Intervention Project. Clin Infect Dis. 1997;25:1113–1117. doi: 10.1086/516103. [DOI] [PubMed] [Google Scholar]
- 90.Eller J, Ede A, Schaberg T, Niederman M S, Mauch H, Lode H. Infective exacerbations of chronic bronchitis: relation between bacteriologic etiology and lung function. Chest. 1998;113:1542–1548. doi: 10.1378/chest.113.6.1542. [DOI] [PubMed] [Google Scholar]
- 91.Faden H, Bernstein J, Brodsky L, Stanievich J, Krystofik D, Shuff C, Hong J J, Ogra P L. Otitis media in children. I. The systemic immune response to nontypable Haemophilus influenzae. J Infect Dis. 1989;160:999–1004. doi: 10.1093/infdis/160.6.999. [DOI] [PubMed] [Google Scholar]
- 92.Faden H, Duffy L, Wasielewski R, Wolf J, Krystofik D, Tung Y Tonawanda/Williamsville Pediatrics. Relationship between nasopharyngeal colonization and the development of otitis media in children. J Infect Dis. 1997;175:1440–1445. doi: 10.1086/516477. [DOI] [PubMed] [Google Scholar]
- 93.Faden H, Duffy L, Williams A, Krystofik D A, Wolf J Tonawanda/Williamsville Pediatrics. Epidemiology of nasopharyngeal colonization with nontypeable Haemophilus influenzae in the first 2 years of life. J Infect Dis. 1995;172:132–135. doi: 10.1093/infdis/172.1.132. [DOI] [PubMed] [Google Scholar]
- 94.Faden H, Harabuchi Y, Hong J J Tonawanda/Williamsville Pediatrics. Epidemiology of Moraxella catarrhalis in children during the first 2 years of life: relationship to otitis media. J Infect Dis. 1994;169:1312–1317. doi: 10.1093/infdis/169.6.1312. [DOI] [PubMed] [Google Scholar]
- 95.Fagon J-Y, Chastre J. Severe exacerbations of COPD patients: the role of pulmonary infections. Semin Respir Infect. 1996;11:109–118. [PubMed] [Google Scholar]
- 96.Fagon J-Y, Chastre J, Trouillet J-L, Domart Y, Dombret M-C, Bornet M, Gibert C. Characterization of distal bronchial microflora during acute exacerbation of chronic bronchitis. Am Rev Respir Dis. 1990;142:1004–1008. doi: 10.1164/ajrccm/142.5.1004. [DOI] [PubMed] [Google Scholar]
- 97.Fine M J, Smith M A, Carson C A, Meffe F, Sankey S S, Weissfeld L A, Detsky A S, Kapoor W N. Efficacy of pneumococcal vaccination in adults. A meta-analysis of randomized controlled trials. Arch Intern Med. 1994;154:2666–2677. doi: 10.1001/archinte.1994.00420230051007. [DOI] [PubMed] [Google Scholar]
- 98.Fitzgerald M, Mulcahy R, Murphy S, Keane C, Coakley D, Scott T. A 200 kDa protein is associated with haemagglutinating isolates of Moraxella (Branhamella) catarrhalis. FEMS Immunol Med Microbiol. 1997;18:209–216. doi: 10.1111/j.1574-695X.1997.tb01047.x. [DOI] [PubMed] [Google Scholar]
- 99.Fitzgerald M, Mulcahy R, Murphy S, Keane C, Coakley D, Scott T. Transmission electron microscopy studies of Moraxella (Branhamella) catarrhalis. FEMS Immunol Med Microbiol. 1999;23:57–66. doi: 10.1111/j.1574-695X.1999.tb01717.x. [DOI] [PubMed] [Google Scholar]
- 100.Fitzgerald M, Murphy S, Mulcahy R, Keane C, Coakley D, Scott T. Tissue culture adherence and haemagglutination characteristics of Moraxella (Branhamella) catarrhalis. FEMS Immunol Med Microbiol. 1999;24:105–114. doi: 10.1111/j.1574-695X.1999.tb01271.x. [DOI] [PubMed] [Google Scholar]
- 101.Flack F S, Loosmore S, Chong P, Thomas W R. The sequencing of the 80-kDa D15 protective surface antigen of Haemophilus influenzae. Gene. 1995;156:97–99. doi: 10.1016/0378-1119(95)00049-c. [DOI] [PubMed] [Google Scholar]
- 102.Fletcher F, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1:1645–1648. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fomsgaard J S, Fomsgaard A, Hoiby N, Bruun B, Galanos C. Comparative immunochemistry of lipopolysaccharides from Branhamella catarrhalis strains. Infect Immun. 1991;59:3346–3349. doi: 10.1128/iai.59.9.3346-3349.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Forsgren J, Samuelson A, Ahlin A, Jonasson J, Rynnel-Dagoo B, Lindberg A. Haemophilus influenzae resides and multiplies intracellularly in human adenoid tissue as demonstrated by in situ hybridization and bacterial viability assay. Infect Immun. 1994;62:673–679. doi: 10.1128/iai.62.2.673-679.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Forsgren J, Samuelson A, Borrelli S, Christensson B, Jonasson J, Lindberg A A. Persistence of nontypeable Haemophilus influenzae in adenoid macrophages: a putative colonization mechanism. Acta Oto-Laryngol. 1996;116:766–773. doi: 10.3109/00016489609137922. [DOI] [PubMed] [Google Scholar]
- 106.Foxwell A R, Kyd J M, Cripps A W. Nontypeable Haemophilus influenzae: pathogenesis and prevention. Microbiol Mol Biol Rev. 1998;62:294–308. doi: 10.1128/mmbr.62.2.294-308.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gelb A F, Schein M, Kuei J, Tashkin D P, Muller N L, Hogg J C, Epstein J D, Zamel N. Limited contribution of emphysema in advanced chronic obstructive pulmonary disease. Am Rev Respir Dis. 1993;147:1157–1161. doi: 10.1164/ajrccm/147.5.1157. [DOI] [PubMed] [Google Scholar]
- 108.Ghaffar F, Friedland I R, McCracken G H., Jr Dynamics of nasopharyngeal colonization by Streptococcus pneumoniae. Pediatr Infect Dis. 1999;18:638–646. doi: 10.1097/00006454-199907000-00016. [DOI] [PubMed] [Google Scholar]
- 109.Gilsdorf J R, Marrs C F, McCrea K W, Forney L J. Cloning, expression, and sequence analysis of the Haemophilus influenzae type b strain M43p+ pilin gene. Infect Immun. 1990;58:1065–1072. doi: 10.1128/iai.58.4.1065-1072.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gilsdorf J R, McCrea K W, Marrs C F. Role of pili in Haemophilus influenzae adherence and colonization. Infect Immun. 1997;65:2997–3002. doi: 10.1128/iai.65.8.2997-3002.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gray-Owen S D, Loosmore S, Schryvers A B. Identification and characterization of genes encoding the human transferrin-binding proteins from Haemophilus influenzae. Infect Immun. 1995;63:1201–1210. doi: 10.1128/iai.63.4.1201-1210.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gray-Owen S D, Schryvers A B. Characterization of transferrin binding proteins 1 and 2 in invasive type b and nontypeable strains of Haemophilus influenzae. Infect Immun. 1995;63:3809–3815. doi: 10.1128/iai.63.10.3809-3815.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Green B A, Farley J E, Quinn-Dey T, Deich R A, Zlotnick G W. The e (P4) outer membrane protein of Haemophilus influenzae: biologic activity of anti-e serum and cloning and sequencing of the structural gene. Infect Immun. 1991;59:3191–3198. doi: 10.1128/iai.59.9.3191-3198.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Green B A, Vazquez M E, Zlotnick G W, Quigley-Reape G, Swarts J D, Green I, Cowell J L, Bluestone C D, Doyle W J. Evaluation of mixtures of purified Haemophilus influenzae outer membrane proteins in protection against challenge with nontypeable H. influenzae in the chinchilla otitis media model. Infect Immun. 1993;61:1950–1957. doi: 10.1128/iai.61.5.1950-1957.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Groeneveld K, Eijk P P, van Alphen L, Jansen H M, Zanen H C. Haemophilus influenzae infections in patients with chronic obstructive pulmonary disease despite specific antibodies in serum and sputum. Am Rev Respir Dis. 1990;141:1316–1321. doi: 10.1164/ajrccm/141.5_Pt_1.1316. [DOI] [PubMed] [Google Scholar]
- 116.Groeneveld K, van Alphen L, Eijk P P, Jansen H M, Zanen H C. Changes in outer membrane proteins of nontypable Haemophilus influenzae in patients with chronic obstructive pulmonary disease. J Infect Dis. 1988;158:360–365. doi: 10.1093/infdis/158.2.360. [DOI] [PubMed] [Google Scholar]
- 117.Groeneveld K, van Alphen L, Voorter C, Eijk P P, Jansen H M, Zanen H C. Antigenic drift of Haemophilus influenzae in patients with chronic obstructive pulmonary disease. Infect Immun. 1989;57:3038–3044. doi: 10.1128/iai.57.10.3038-3044.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gross C P, Anderson G F, Powe N R. The relation between funding by the National Institutes of Health and the burden of disease. N Engl J Med. 1999;340:1881. doi: 10.1056/NEJM199906173402406. [DOI] [PubMed] [Google Scholar]
- 119.Gu X-X, Chen J, Barenkamp S J, Robbins J B, Tsai C-M, Lim D J, Battey J. Synthesis and characterization of lipooligosaccharide-based conjugates as vaccine candidates for Moraxella (Branhamella) catarrhalis. Infect Immun. 1998;66:1891–1897. doi: 10.1128/iai.66.5.1891-1897.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gu X-X, Sun J, Jin S, Barenkamp S J, Lim D J, Robbins J B, Battey J. Detoxified lipooligosaccharide from nontypeable Haemophilus influenzae conjugated to proteins confers protection against otitis media in chinchillas. Infect Immun. 1997;65:4488–4493. doi: 10.1128/iai.65.11.4488-4493.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gu X-X, Tsai C-M, Apicella M A, Lim D J. Quantitation and biological properties of released and cell-bound lipooligosacchrides from nontypeable Haemophilus influenzae. Infect Immun. 1995;63:4115–4120. doi: 10.1128/iai.63.10.4115-4120.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gu X-X, Tsai C-M, Ueyama T, Barenkamp S J, Robbins J B, Lim D J. Synthesis, characterization, and immunologic properties of detoxified lipooligosaccharide from nontypeable Haemophilus influenzae conjugated to proteins. Infect Immun. 1996;64:4047–4053. doi: 10.1128/iai.64.10.4047-4053.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gump D W, Phillips C A, Forsyth B R, McIntosh F K, Lamborn K R, Stouch W H. Role of infection in chronic bronchitis. Am Rev Respir Dis. 1976;113:465–473. doi: 10.1164/arrd.1976.113.4.465. [DOI] [PubMed] [Google Scholar]
- 124.Haas H, Morris J F, Samson S, Kilbourn J P, Kim P J. Bacterial flora of the respiratory tract in chronic bronchitis: comparison of transtracheal, fiberbronchoscopic, and oropharyngeal sampling methods. Am Rev Respir Dis. 1977;116:41–47. doi: 10.1164/arrd.1977.116.1.41. [DOI] [PubMed] [Google Scholar]
- 125.Haase E M, Campagnari A A, Sarwar J, Shero M, Wirth M, Cumming C U, Murphy T F. Strain-specific and immunodominant surface epitopes of the P2 porin protein of nontypeable Haemophilus influenzae. Infect Immun. 1991;59:1278–1284. doi: 10.1128/iai.59.4.1278-1284.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Haase E M, Yi K, Morse G D, Murphy T F. Mapping of bactericidal epitopes on the P2 porin protein of nontypeable Haemophilus influenzae. Infect Immun. 1994;62:3712–3722. doi: 10.1128/iai.62.9.3712-3722.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Habib M P, Gentry L O, Rodriguez-Gomez G, Morowitz W, Polak E, Rae J K, Morgan N S, Williams R R. Multicenter, randomized study comparing efficacy and safety of oral levofloxacin and cefaclor in treatment of acute bacterial exacerbations of chronic bronchitis. Infect Dis Clin Pract. 1998;7:101–109. [Google Scholar]
- 128.Hager H, Verghese A, Alvarez S, Berk S L. Branhamella catarrhalis respiratory infections. Rev Infect Dis. 1987;9:1140–1149. doi: 10.1093/clinids/9.6.1140. [DOI] [PubMed] [Google Scholar]
- 129.Hahn D L. Chlamydia pneumoniae, asthma, and COPD: what is the evidence? Ann Allergy Asthma Immunol. 1999;83:271–292. doi: 10.1016/S1081-1206(10)62666-X. [DOI] [PubMed] [Google Scholar]
- 130.Hansen E J, Hart D A, McGhee J L, Toews G B. Immune enhancement of pulmonary clearance of nontypable Haemophilus influenzae. Infect Immun. 1988;56:182–190. doi: 10.1128/iai.56.1.182-190.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hanson M S, Slaughter C, Hansen E J. The hbpA gene of Haemophilus influenzae type b encodes a heme-binding lipoprotein conserved among heme-dependent Haemophilus species. Infect Immun. 1992;60:2257–2266. doi: 10.1128/iai.60.6.2257-2266.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hargreave F E, Leich R. Induced sputum, eosinophilic bronchitis, and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:S53–S57. doi: 10.1164/ajrccm.160.supplement_1.14. [DOI] [PubMed] [Google Scholar]
- 133.Harkness R E, Guimond M-J, McBey B-A, Klein M H, Percy D H, Croy B A. Branhamella catarrhalis pathogenesis in SCID and SCID/beige mice. APMIS. 1993;101:805–810. [PubMed] [Google Scholar]
- 134.Helminen M E, Maciver I, Latimer J L, Cope L D, McCracken G H, Jr, Hansen E J. A major outer membrane protein of Moraxella catarrhalis is a target for antibodies that enhance pulmonary clearance of the pathogen in an animal model. Infect Immun. 1993;61:2003–2010. doi: 10.1128/iai.61.5.2003-2010.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Helminen M E, Maciver I, Latimer J L, Klesney-Tait J, Cope L D, Paris M, McCracken G H, Jr, Hansen E J. A large, antigenically conserved protein on the surface of Moraxella catarrhalis is a target for protective antibodies. J Infect Dis. 1994;170:867–872. doi: 10.1093/infdis/170.4.867. [DOI] [PubMed] [Google Scholar]
- 136.Helminen M E, Maciver I, Paris M, Latimer J L, Lumbley S L, Cope L D, McCracken G H, Jr, Hansen E J. A mutation affecting expression of a major outer membrane protein of Moraxella catarrhalis alters serum resistance and survival in vivo. J Infect Dis. 1993;168:1194–1201. doi: 10.1093/infdis/168.5.1194. [DOI] [PubMed] [Google Scholar]
- 137.Hendley J O, Sande M A, Stewart P M, Gwaltney J M J. Spread of Streptococcus pneumoniae in families. I. Carriage rates and distribution of types. J Infect Dis. 1975;132:55–61. doi: 10.1093/infdis/132.1.55. [DOI] [PubMed] [Google Scholar]
- 138.Hendrixson D R, de la Morena M L, Stathopoulos C, St. Geme J W. Structural determinants of processing and secretion of the Haemophilus influenzae Hap protein. Mol Microbiol. 1997;26:505–518. doi: 10.1046/j.1365-2958.1997.5921965.x. [DOI] [PubMed] [Google Scholar]
- 139.Hendrixson D R, St. Geme J W., III The Haemophilus influenzae Hap serine protease promotes adherence and microcolony formation, potentiated by a soluble host protein. Mol Cell. 1998;2:841–850. doi: 10.1016/s1097-2765(00)80298-1. [DOI] [PubMed] [Google Scholar]
- 140.Hiemstra P S, van Wetering S, Stolk J. Neutrophil serine proteinases and defensins in chronic obstructive pulmonary disease: effects on pulmonary epithelium. Eur Respir J. 1998;12:1200–1208. doi: 10.1183/09031936.98.12051200. [DOI] [PubMed] [Google Scholar]
- 141.High N J, Jennings M P, Moxon E R. Tandem repeats of the tetramer 5′-CAAT-3′ present in lic2A are required for phase variation but not lipopolysaccharide biosynthesis in Haemophilus influenzae. Mol Microbiol. 1996;20:165–174. doi: 10.1111/j.1365-2958.1996.tb02498.x. [DOI] [PubMed] [Google Scholar]
- 141a.Hill A T, Campbell E J, Hill S L, Bayley D L, Stockley R A. Association between airway bacterial load and markers of airway inflammation in patients with stable chronic bronchitis. Am J Med. 2000;109:288–295. doi: 10.1016/s0002-9343(00)00507-6. [DOI] [PubMed] [Google Scholar]
- 142.Holland J, Langford P R, Towner K J, Williams P. Evidence for in vivo expression of transferrin-binding proteins in Haemophilus influenzae type b. Infect Immun. 1992;60:2986–2991. doi: 10.1128/iai.60.7.2986-2991.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Holme T, Rahman M, Jansson P-E, Widmalm G. The lipopolysaccharide of Moraxella catarrhalis. Structural relationships and antigenic properties. Eur J Biochem. 1999;265:524–529. doi: 10.1046/j.1432-1327.1999.00731.x. [DOI] [PubMed] [Google Scholar]
- 144.Hotomi M, Saito T, Yamanaka N. Specific mucosal immunity and enhanced nasopharyngeal clearance of nontypeable Haemophilus influenzae after intranasal immunization with outer membrane protein P6 and cholera toxin. Vaccine. 1998;16:1950–1956. doi: 10.1016/s0264-410x(98)00122-4. [DOI] [PubMed] [Google Scholar]
- 145.Howard P. Evolution of the ventilatory capacity in chronic bronchitis. BMJ. 1967;3:392–395. doi: 10.1136/bmj.3.5562.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hsiao C B, Sethi S, Murphy T F. Outer membrane protein CD of Branhamella catarrhalis: sequence conservation in strains recovered from the human respiratory tract. Microb Pathog. 1995;19:215–225. doi: 10.1016/s0882-4010(95)90272-4. [DOI] [PubMed] [Google Scholar]
- 147.Ikram R B, Nixon M, Aitken J, Wells E. A prospective study of isolation of Moraxella catarrhalis in a hospital during the winter months. J Hosp Infect. 1993;25:7–14. doi: 10.1016/0195-6701(93)90004-j. [DOI] [PubMed] [Google Scholar]
- 148.Isada C M. Pro: antibiotics for chronic bronchitis with exacerbations. Semin Respir Infect. 1993;8:243–253. [PubMed] [Google Scholar]
- 149.Janoff E N, Rubins J B. Editorial response: predicting protection against encapsulated pathogens. Clin Infect Dis. 1999;29:289–291. doi: 10.1086/520201. [DOI] [PubMed] [Google Scholar]
- 150.Jin H, Ren Z, Pozsgay J M, Elkins C, Whitby P W, Morton D J, Stull T L. Cloning of a DNA fragment encoding a heme-repressible hemoglobin-binding outer membrane protein from Haemophilus influenzae. Infect Immun. 1996;64:3134–3141. doi: 10.1128/iai.64.8.3134-3141.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Johnson K G, McDonald I J, Perry M B. Studies on the cellular and free lipopolysaccharides from Branhamella catarrhalis. Can J Microbiol. 1976;22:460–467. doi: 10.1139/m76-072. [DOI] [PubMed] [Google Scholar]
- 152.Johnston I D A, Strachan D P, Anderson H R. Effect of pneumonia and whooping cough in childhood on adult lung function. N Engl J Med. 1998;338:581–587. doi: 10.1056/NEJM199802263380904. [DOI] [PubMed] [Google Scholar]
- 153.Jordens J Z. Characterisation of non-capsulate Haemophilus influenzae by repetitive extragenic palindromic (REP)-PCR. J Med Microbiol. 1998;47:1031–1034. doi: 10.1099/00222615-47-11-1031. [DOI] [PubMed] [Google Scholar]
- 154.Jordens J Z, Leaves N I, Anderson E C, Slack M P E. Polymerase chain reaction-based strain characterization of noncapsulate Haemophilus influenzae. J Clin Microbiol. 1993;31:2981–2987. doi: 10.1128/jcm.31.11.2981-2987.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jousimies-Somer H R, Savolainen S, Ylikoski J S. Comparison of the nasal bacterial floras in two groups of healthy subjects and in patients with acute maxillary sinusitis. J Clin Microbiol. 1989;27:2736–2743. doi: 10.1128/jcm.27.12.2736-2743.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kar S, To S C M, Brinton C C., Jr Cloning and expression in Escherichia coli of LKP pilus genes from a nontypeable Haemophilus influenzae strain. Infect Immun. 1990;58:903–908. doi: 10.1128/iai.58.4.903-908.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Karasic R B, Trumpp C E, Gnehm H, Rice P A, Pelton S I. Modification of otitis media in chinchillas rechallenged with nontypable Haemophilus influenzae and serological response to outer membrane antigens. J Infect Dis. 1985;151:273–279. doi: 10.1093/infdis/151.2.273. [DOI] [PubMed] [Google Scholar]
- 158.Kauffman H F, Tomee J F C, van der Werf T S, de Monchy J G R, Koeter G K. Review of fungus-induced asthmatic reactions. Am J Respir Crit Care Med. 1995;151:2109–2116. doi: 10.1164/ajrccm.151.6.7767565. [DOI] [PubMed] [Google Scholar]
- 159.Kawakami Y, Ueno I, Katsuyama T, Furihata K, Matsumoto H. Restriction fragment length polymorphism (RFLP) of genomic DNA of Moraxella (Branhamella) catarrhalis isolates in a hospital. Microbiol Immunol. 1994;38:891–895. doi: 10.1111/j.1348-0421.1994.tb02142.x. [DOI] [PubMed] [Google Scholar]
- 160.Ketterer M R, Shao J Q, Hornick D B, Buscher B, Bandi V K, Apicella M A. Infection of primary human bronchial epithelial cells by Haemophilus influenzae: macropinocytosis as a mechanism of airway epithelial cell entry. Infect Immun. 1999;67:4161–4170. doi: 10.1128/iai.67.8.4161-4170.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Khair O A, Davies R J, Devalia J L. Bacterial-induced release of inflammatory mediators by bronchial epithelial cells. Eur Respir J. 1996;9:1913–1922. doi: 10.1183/09031936.96.09091913. [DOI] [PubMed] [Google Scholar]
- 162.Kjaergard L L, Larsen F O, Norn S, Clementsen P, Skov P S, Permin H. Basophil-bound IgE and serum IgE directed against Haemophilus influenzae and Streptococcus pneumoniae in patients with chronic bronchitis during acute exacerbations. APMIS. 1996;104:61–67. [PubMed] [Google Scholar]
- 163.Klingman K L, Pye A, Murphy T F, Hill S L. Dynamics of respiratory tract colonization by Moraxella (Branhamella) catarrhalis in bronchiectasis. Am J Respir Crit Care Med. 1995;152:1072–1078. doi: 10.1164/ajrccm.152.3.7663786. [DOI] [PubMed] [Google Scholar]
- 164.Konstan M W, Hilliard K A, Norvell T M, Berger M. Bronchoalveolar lavage findings in cystic fibrosis patients with stable, clinically mild lung disease suggest ongoing infection and inflammation. Am J Respir Crit Care Med. 1994;150:448–454. doi: 10.1164/ajrccm.150.2.8049828. [DOI] [PubMed] [Google Scholar]
- 165.Kubiet M, Ramphal R. Adhesion of nontypeable Haemophilus influenzae from blood and sputum to human tracheobronchial mucins and lactoferrin. Infect Immun. 1995;63:899–902. doi: 10.1128/iai.63.3.899-902.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kurono Y, Shigemi H, Kodama S, Mogi G. Effects of oral and systemic immunization on nasopharyngeal clearance of nontypeable Haemophilus influenzae in BALB/c mice. Laryngoscope. 1996;106:614–618. doi: 10.1097/00005537-199605000-00018. [DOI] [PubMed] [Google Scholar]
- 167.Kyd J M, Cripps A W. Modulation of antigen-specific T and B cell responses influence bacterial clearance of non-typeable Haemophilus influenzae from the lung in a rat model. Vaccine. 1996;14:1471–1478. doi: 10.1016/s0264-410x(96)00034-5. [DOI] [PubMed] [Google Scholar]
- 168.Kyd J M, Cripps A W. Potential of a novel protein, OMP26, from nontypeable Haemophilus influenzae to enhance pulmonary clearance in a rat model. InfectImmun. 1998;66:2272–2278. doi: 10.1128/iai.66.5.2272-2278.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kyd J M, Dunkley M L, Cripps A W. Enhanced respiratory clearance of nontypeable Haemophilus influenzae following mucosal immunization with P6 in a rat model. Infect Immun. 1995;63:2931–2940. doi: 10.1128/iai.63.8.2931-2940.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Langan C, Clecner B, Cazzola C M, Brambilla C, Holmes C, Staley H. Short-course cefuroxime axetil therapy in the treatment of acute exacerbations of chronic bronchitis. Int J Clin Pract. 1998;52:289–297. [PubMed] [Google Scholar]
- 171.Langan C E, Cranfield R, Breisch S, Pettit R. Randomized, double-blind study of grepafloxacin versus amoxycllin in patients with acute bacterial exacerbations of chronic bronchitis. J Antimicrob Chemother. 1997;40:63–72. doi: 10.1093/jac/40.suppl_1.63. [DOI] [PubMed] [Google Scholar]
- 172.Langan C E, Zuck P, Vogel F, McIvor A, Pierzchala W, Smakal M, Staley H, Marr C. Randomized, double-blind study of short-course (5 day) grepafloxacin versus 10 day clarithromycin in patients with acute bacterial exacerbations of chronic bronchitis. J Antimicrob Chemother. 1999;44:515–523. doi: 10.1093/jac/44.4.515. [DOI] [PubMed] [Google Scholar]
- 173.Laurenzi G A, Potter R T, Kass E H. Bacteriologic flora of the lower respiratory tract. N Engl J Med. 1961;265:1273–1278. doi: 10.1056/NEJM196112282652601. [DOI] [PubMed] [Google Scholar]
- 174.Leach A J, Boswell J B, Asche V, Nienhuys T G, Mathews J D. Bacterial colonization of the nasopharynx predicts very early onset and persistence of otitis media in Australian Aboriginal infants. Pediatr Infect Dis J. 1994;13:983–989. doi: 10.1097/00006454-199411000-00009. [DOI] [PubMed] [Google Scholar]
- 175.Leeder S R. Role of infection in the cause and course of chronic bronchitis and emphysema. J Infect Dis. 1975;131:731–742. doi: 10.1093/infdis/131.6.731. [DOI] [PubMed] [Google Scholar]
- 176.Lehmann D, Coakley K J, Coakley C A, Spooner V, Montgomery J M, Michael A, Riley I D, Smith T, Clancy R L, Cripps A W, Alpers M P. Reduction in the incidence of acute bronchitis by an oral Haemophilus influenzae vaccine in patients with chronic bronchitis in the highlands of Papua New Guinea. Am Rev Respir Dis. 1991;144:324–330. doi: 10.1164/ajrccm/144.2.324. [DOI] [PubMed] [Google Scholar]
- 177.Liberman J, Winter B, Sastre A. Alpha 1-antitrypsin Pi-types in 965 COPD patients. Chest. 1986;89:370–373. doi: 10.1378/chest.89.3.370. [DOI] [PubMed] [Google Scholar]
- 178.Linnanmaki E, Leinonen M, Mattila K, Nieminen M S, Valtonen V, Saikku P. Chlamydia pneumoniae—specific circulating immune complexes in patients with chronic coronary heart disease. Circulation. 1993;87:1130–1134. doi: 10.1161/01.cir.87.4.1130. [DOI] [PubMed] [Google Scholar]
- 179.Loosmore S M, Yang Y-P, Coleman D C, Shortreed J M, England D M, Harkness R E, Chong P S C, Klein M H. Cloning and expression of the Haemophilus influenzae transferrin receptor genes. Mol Microbiol. 1996;19:575–586. doi: 10.1046/j.1365-2958.1996.406943.x. [DOI] [PubMed] [Google Scholar]
- 180.Loosmore S M, Yang Y-P, Oomen R, Shortreed J M, Coleman D C, Klein M H. The Haemophilus influenzae HtrA protein is a protective antigen. Infect Immun. 1998;66:899–906. doi: 10.1128/iai.66.3.899-906.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lopez B, Vazquez C F, Fenoll A, Gutierrez J, Fidalgo C, Caicoya M, Mendez F J. Epidemiological study of Streptococcus pneumoniae carriers in healthy primary-school children. Eur J Clin Microbiol Infect Dis. 1999;18:771–776. doi: 10.1007/s100960050399. [DOI] [PubMed] [Google Scholar]
- 182.Lottenbach K R, Mink C M, Barenkamp S J, Anderson E L, Homan S M, Powers D C. Age-associated differences in immunoglobulin G1 (IgG1) and IgG2 subclass antibodies to pneumococcal polysaccharides following vaccination. Infect Immun. 1999;67:4935–4938. doi: 10.1128/iai.67.9.4935-4938.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Luke N R, Campagnari A A. Construction and characterization of Moraxella catarrhalis mutants defective in expression of transferrin receptors. InfectImmun. 1999;67:5815–5819. doi: 10.1128/iai.67.11.5815-5819.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lundgren K, Ingvarsson L. Acute otitis media in Sweden. Role of Branhamella catarrhalis and the rationale for choice of antimicrobial therapy. Drugs. 1986;31:125–131. doi: 10.2165/00003495-198600313-00028. [DOI] [PubMed] [Google Scholar]
- 185.Maciver I, Latimer J L, Liem H H, Muller-Eberhard U, Hrkal Z, Hansen E J. Identification of an outer membrane protein involved in utilization of hemoglobin-haptoglobin complexes by nontypeable Haemophilus influenzae. Infect Immun. 1996;64:3703–3712. doi: 10.1128/iai.64.9.3703-3712.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Maciver I, Unhanand M, McCracken G H, Jr, Hansen E J. Effect of immunization on pulmonary clearance of Moraxella catarrhalis in an animal model. J Infect Dis. 1993;168:469–472. doi: 10.1093/infdis/168.2.469. [DOI] [PubMed] [Google Scholar]
- 187.Mandrell R E, Apicella M A. Lipo-oligosaccharides (LOS) of mucosal pathogens: molecular mimicry and host-modification of LOS. Immunobiology. 1993;187:382–402. doi: 10.1016/S0171-2985(11)80352-9. [DOI] [PubMed] [Google Scholar]
- 188.Mandrell R E, McLaughlin R, Abu Kwaik Y, Lesse A, Yamasaki R, Gibson B, Spinola S M, Apicella M A. Lipooligosaccharides (LOS) of some Haemophilus species mimic human glycosphingolipids, and some LOS are sialylated. Infect Immun. 1992;60:1322–1328. doi: 10.1128/iai.60.4.1322-1328.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Marrs C F, Weir S. Pili (fimbriae) of Branhamella species. Am J Med. 1990;88(5A):36S–45S. doi: 10.1016/0002-9343(90)90260-k. [DOI] [PubMed] [Google Scholar]
- 190.Martinez G, Ahmed K, Zheng C H, Watanabe K, Oishi K, Nagatake T. DNA restriction patterns produced by pulsed-field gel electrophoresis in Moraxella catarrhalis isolated from different geographical areas. Epidemiol Infect. 1999;122:417–422. doi: 10.1017/s0950268899002381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Masoud H, Perry M B, Richards J C. Characterization of the lipopolysaccharide of Moraxella catarrhalis: structural analysis of the lipid A from M. catarrhalis serotype A lipopolysaccharide. Eur J Biochem. 1994;220:209–216. doi: 10.1111/j.1432-1033.1994.tb18616.x. [DOI] [PubMed] [Google Scholar]
- 192.Mathers K E, Goldblatt D, Aebi C, Yu R, Schryvers A B, Hansen E J. Characterization of an outer membrane protein of Moraxella catarrhalis. FEMS Immunol Med Microbiol. 1997;19:231–236. doi: 10.1111/j.1574-695X.1997.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 193.Mbelle N, Huebner R E, Wasas A D, Kimura A, Chang I, Klugman K P. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. J Infect Dis. 1999;180:1171–1176. doi: 10.1086/315009. [DOI] [PubMed] [Google Scholar]
- 194.Mbelle N, Huebner R E, Wasas A D, Kimura A, Chang I, Klugman K P. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. J Infect Dis. 1999;180:1171–1176. doi: 10.1086/315009. [DOI] [PubMed] [Google Scholar]
- 195.McCrea K W, Watson W J, Gilsdorf J R, Marrs C F. Identification of hifD and hifE in the pilus gene cluster of Haemophilus influenzae type b strain Eagan. Infect Immun. 1994;62:4922–4928. doi: 10.1128/iai.62.11.4922-4928.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.McHardy V U, Inglis J M, Calder M A, Crofton J W. A study of infective and other factors in exacerbations of chronic bronchitis. Br J Dis Chest. 1980;74:228–238. doi: 10.1016/0007-0971(80)90048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.McKenzie H, Morgan M G, Jordens J Z, Enright M C, Bain M. Characterisation of hospital isolates of Moraxella (Branhamella) catarrhalis by SDS-PAGE of whole-cell proteins, immunoblotting and restriction-endonuclease analysis. J Med Microbiol. 1992;37:70–76. doi: 10.1099/00222615-37-1-70. [DOI] [PubMed] [Google Scholar]
- 198.McLeod D T, Ahmad F, Capewell S, Croughan M J, Calder M A, Seaton A. Increase in bronchopulmonary infection due to Branhamella catarrhalis. Br Med J. 1986;292:1103–1105. doi: 10.1136/bmj.292.6528.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.McMichael J C, Fiske M J, Fredenburg R A, Chakravarti D N, van der Meid K R, Barniak V, Caplain J, Bortell E, Baker S, Arumugham R, Chen D. Isolation and characterization of two proteins from Moraxella catarrhalis that bear a common epitope. Infect Immun. 1998;66:4374–4381. doi: 10.1128/iai.66.9.4374-4381.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Medical Research Council. Definitions and classification of chronic bronchitis for clinical and epidemiological purposes: a report to the Medical Research Council by their committee on the aetiology of chronic bronchitis. Lancet. 1965;i:775–779. [PubMed] [Google Scholar]
- 201.Miravitlles M, Espinosa C, Fernandez-Laso E, Martos J A, Maldonado J A, Gallego M Study Group of Bacterial Infection in COPD. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Chest. 1999;116:40–46. doi: 10.1378/chest.116.1.40. [DOI] [PubMed] [Google Scholar]
- 202.Mitchell J L, Hill S L. Immune response to Haemophilus parainfluenzae in patients with chronic obstructive lung disease. Clin Diagn Lab Immunol. 2000;7:25–30. doi: 10.1128/cdli.7.1.25-30.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Miyashita N, Niki Y, Nakajima M, Kawane H, Matsushima T. Chlamydia pneumoniae infection in patients with diffuse panbronchiolitis and COPD. Chest. 1998;114:969–971. doi: 10.1378/chest.114.4.969. [DOI] [PubMed] [Google Scholar]
- 204.Miyazaki S, Nunoya T, Matsumoto T, Tateda K, Yamaguchi K. New murine model of bronchopneumonia due to cell-bound Haemophilus influenzae. J Infect Dis. 1997;175:205–209. doi: 10.1093/infdis/175.1.205. [DOI] [PubMed] [Google Scholar]
- 205.Mogulkoc N, Karakurt S, Isalska B, Bayindir Y, Celikel T, Korten V, Colpan N. Acute purulent exacerbation of chronic obstructive pulmonary disease and Chlamydia pneumoniae infection. Am J Respir Crit Care Med. 1999;160:349–353. doi: 10.1164/ajrccm.160.1.9809041. [DOI] [PubMed] [Google Scholar]
- 206.Moller L V M, Regelink A G, Grasselier H, Dankert-Roelse J E, Dankert J, van Alphen L. Multiple Haemophilus influenzae strains and strain variants coexist in the respiratory tract of patients with cystic fibrosis. J Infect Dis. 1995;172:1388–1392. doi: 10.1093/infdis/172.5.1388. [DOI] [PubMed] [Google Scholar]
- 207.Moller L V M, Timens W, van der Bij W, Kooi K, de Wever B, Dankert J, van Alphen L. Haemophilus influenzae in lung explants of patients with end-stage pulmonary disease. Am J Respir Crit Care Med. 1998;157:950–956. doi: 10.1164/ajrccm.157.3.9707010. [DOI] [PubMed] [Google Scholar]
- 208.Monso E, Ruiz J, Rosell A, Manterola J, Fiz J, Morera J, Ausina V. Bacterial infection in chronic obstructive pulmonary disease. A study of stable and exacerbated outpatients using the protected specimen brush. Am J Respir Crit Care Med. 1995;152:1316–1320. doi: 10.1164/ajrccm.152.4.7551388. [DOI] [PubMed] [Google Scholar]
- 209.Morgan M G, McKenzie H, Enright M C, Bain M, Emmanuel F X S. Use of molecular methods to characterize Moraxella catarrhalis strains in a suspected outbreak of nosocomial infection. Eur J Clin Microbiol Infect Dis. 1992;11:305–312. doi: 10.1007/BF01962069. [DOI] [PubMed] [Google Scholar]
- 210.Munson R S, Jr, Grass S, West R. Molecular cloning and sequence of the gene for outer membrane protein P5 of Haemophilus influenzae. Infect Immun. 1993;61:4017–4020. doi: 10.1128/iai.61.9.4017-4020.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Murphy T F. Branhamella catarrhalis: epidemiology, surface antigenic structure, and immune response. Microbiol Rev. 1996;60:267–279. doi: 10.1128/mr.60.2.267-279.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Murphy T F. Branhamella catarrhalis: epidemiological and clinical aspects of a human respiratory tract pathogen. Thorax. 1998;53:124–128. doi: 10.1136/thx.53.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Murphy T F, Bartos L C. Human bactericidal antibody response to outer membrane protein P2 of nontypeable Haemophilus influenzae. Infect Immun. 1988;56:2673–2679. doi: 10.1128/iai.56.10.2673-2679.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Murphy T F, Bartos L C, Campagnari A A, Nelson M B, Apicella M A. Antigenic characterization of the P6 protein of nontypable Haemophilus influenzae. Infect Immun. 1986;54:774–779. doi: 10.1128/iai.54.3.774-779.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Murphy T F, Bartos L C, Rice P A, Nelson M B, Dudas K C, Apicella M A. Identification of a 16,600-dalton outer membrane protein on nontypable Haemophilus influenzae as a target for human serum bactericidal antibody. J Clin Investig. 1986;78:1020–1027. doi: 10.1172/JCI112656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Murphy T F, Dudas K C, Mylotte J M, Apicella M A. A subtyping system for nontypable Haemophilus influenzae based on outer-membrane proteins. J Infect Dis. 1983;147:838–846. doi: 10.1093/infdis/147.5.838. [DOI] [PubMed] [Google Scholar]
- 217.Murphy T F, Kirkham C, Denardin E, Sethi S. Analysis of antigenic structure and human immune response to outer membrane protein CD of Moraxella catarrhalis. Infect Immun. 1999;67:4578–4585. doi: 10.1128/iai.67.9.4578-4585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Murphy T F, Kirkham C, Lesse A J. The major heat-modifiable outer membrane protein CD is highly conserved among strains of Branhamella catarrhalis. Mol Microbiol. 1993;10:87–98. doi: 10.1111/j.1365-2958.1993.tb00906.x. [DOI] [PubMed] [Google Scholar]
- 219.Murphy T F, Sethi S. Bacterial infection in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1992;146:1067–1083. doi: 10.1164/ajrccm/146.4.1067. [DOI] [PubMed] [Google Scholar]
- 220.Murphy T F, Sethi S, Klingman K L, Brueggemann A B, Doern G V. Simultaneous respiratory tract colonization by multiple strains of nontypeable Haemophilus influenzae in chronic obstructive pulmonary disease: implications for antibiotic therapy. J Infect Dis. 1999;180:404–409. doi: 10.1086/314870. [DOI] [PubMed] [Google Scholar]
- 221.Musher D M. Streptococcus pneumoniae. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. Philadelphia, Pa: Churchill Livingstone; 2000. p. 2128. [Google Scholar]
- 222.Musher D M, Kubitschek K R, Crennan J, Baughn R E. Pneumonia and acute febrile tracheobronchitis due to Haemophilus influenzae. Ann Intern Med. 1983;99:444–450. doi: 10.7326/0003-4819-99-4-444. [DOI] [PubMed] [Google Scholar]
- 223.Musher D M, Luchi M J, Watson D A, Hamilton R, Baughn R E. Pneumococcal polysaccharide vaccine in young adults and older bronchitics: determination of IgG responses by ELISA and the effect of adsorption of serum with non-type-specific cell wall polysaccharide. J Infect Dis. 1990;161:728–735. doi: 10.1093/infdis/161.4.728. [DOI] [PubMed] [Google Scholar]
- 224.Musser J M, Barenkamp S J, Granoff D M, Selander R K. Genetic relationships of serologically nontypable and serotype b strains of Haemophilus influenzae. Infect Immun. 1986;52:183–191. doi: 10.1128/iai.52.1.183-191.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Myers L E, Yang Y-P, Du R-P, Wang Q, Harkness R E, Schryvers A B, Klein M H, Loosmore S M. The transferrin binding protein B of Moraxella catarrhalis elicits bactericidal antibodies and is a potential vaccine antigen. Infect Immun. 1998;66:4183–4192. doi: 10.1128/iai.66.9.4183-4192.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.National Heart, Lung and Blood Institute. Morbidity and mortality chartbook on cardiovascular, lung and blood diseases. Bethesda, Md: National Institutes of Health; 1998. [Google Scholar]
- 227.Nicotra B, Rivera M, Luman J I, Wallace R J. Branhamella catarrhalis as a lower respiratory tract pathogen in patients with chronic lung disease. Arch Intern Med. 1986;146:890–893. [PubMed] [Google Scholar]
- 228.Nicotra M B, Kronenberg R S. Con: antibiotic use in exacerbations of chronic bronchitis. Semin Respir Infect. 1993;8:254–258. [PubMed] [Google Scholar]
- 229.Ninane G, Joly J, Kraytman M. Bronchopulmonary infection due to Branhamella catarrhalis: 11 cases assessed by transtracheal puncture. Br Med J. 1978;1:276–278. doi: 10.1136/bmj.1.6108.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Noel G J, Barenkamp S J, St. Geme III J W, Haining W N, Mosser D M. High-molecular-weight surface-exposed proteins of Haemophilus influenzae mediate binding to macrophages. J Infect Dis. 1994;169:425–429. doi: 10.1093/infdis/169.2.425. [DOI] [PubMed] [Google Scholar]
- 231.Novotny L A, Jurcisek J A, Pichichero M E, Bakaletz L O. Epitope mapping of the outer membrane protein P5-homologous fimbrin adhesin of nontypeable Haemophilus influenzae. Infect Immun. 2000;68:2119–2128. doi: 10.1128/iai.68.4.2119-2128.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Onofrio J M, Shulkin A N, Heidbrink P J, Toews G B, Pierce A K. Pulmonary clearance and phagocytic cell response to normal pharyngeal flora. Am Rev Respir Dis. 1981;123:222–225. doi: 10.1164/arrd.1981.123.2.222. [DOI] [PubMed] [Google Scholar]
- 233.Ortqvist A, Hedlund J, Burman L-A, Elbel E, Hofer M, Leinonen M, Lindblad I, Sundeloff B, Kalin M Swedish Pneumococcal Vaccination Study Group. Randomised trial of 23-valent pneumococcal capsular polysaccharide vaccine in prevention of pneumonia in middle-aged and elderly people. Lancet. 1998;351:399. doi: 10.1016/s0140-6736(97)07358-3. [DOI] [PubMed] [Google Scholar]
- 234.Oxman A D, Muir D C F, Shannon H S, Stock S R, Hnizdo E, Lange H J. Occupational dust exposure and chronic obstructive pulmonary disease. Am Rev Respir Dis. 1993;148:38–48. doi: 10.1164/ajrccm/148.1.38. [DOI] [PubMed] [Google Scholar]
- 235.Pardo A, Selman M. Proteinase-antiproteinase imbalance in the pathogenesis of emphysema: the role of metalloproteinases in lung damage. Histol Histopathol. 1999;14:227–233. doi: 10.14670/HH-14.227. [DOI] [PubMed] [Google Scholar]
- 236.Patterson J E, Patterson T F, Farrel P, Hierholzer W J, Jr, Zervos M J. Evaluation of restriction endonuclease analysis as an epidemiologic typing system for Branhamella catarrhalis. J Clin Microbiol. 1989;27:944–946. doi: 10.1128/jcm.27.5.944-946.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Patterson T F, Patterson J E, Masecar B L, Barden G E, Hierholzer W J, Jr, Zervos M J. A nosocomial outbreak of Branhamella catarrhalis confirmed by restriction endonuclease analysis. J Infect Dis. 1988;157:996–1001. doi: 10.1093/infdis/157.5.996. [DOI] [PubMed] [Google Scholar]
- 238.Pauwels R, Verschraegen G, van der Straeten M. IgE antibodies to bacteria in patients with bronchial asthma. Allergy. 1980;157:665–669. doi: 10.1111/j.1398-9995.1980.tb02019.x. [DOI] [PubMed] [Google Scholar]
- 239.Pela R, Marchesani F, Agostinelli C, Staccioli D, Cecarini L, Bassotti C, Sanguinetti C M. Airways microbial flora in COPD patients in stable clinical conditions and during exacerbations: a bronchoscopic investigation. Monaldi Arch Chest Dis. 1998;53:262–267. [PubMed] [Google Scholar]
- 240.Peterson W L. Helicobacter pylori and peptic ulcer disease. N Engl J Med. 1991;324:1043–1048. doi: 10.1056/NEJM199104113241507. [DOI] [PubMed] [Google Scholar]
- 241.Picard B, Goullet P, Denamur E, Suerdmondt G. Esterase electrophoresis: a molecular tool for studying the epidemiology of Branhamella catarrhalis nosocomial infection. Epidemiol Infect. 1989;103:547–554. doi: 10.1017/s0950268800030946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Pollard J A, Wallace R J, Jr, Nash D R, Luman J I, Wilson R W. Incidence of Branhamella catarrhalis in the sputa of patients with chronic lung disease. Drugs. 1986;31:103–108. doi: 10.2165/00003495-198600313-00022. [DOI] [PubMed] [Google Scholar]
- 243.Porras O, Caugant D A, Gray B, Lagergard T, Levin B R, Svanborg-Eden C. Difference in structure between type b and nontypable Haemophilus influenzae populations. Infect Immun. 1986;53:79–89. doi: 10.1128/iai.53.1.79-89.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Porras O, Caugant D A, Lagergard T, Svanborg-Eden C. Application of multilocus enzyme gel electrophoresis to Haemophilus influenzae. Infect Immun. 1986;53:71–78. doi: 10.1128/iai.53.1.71-78.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Powers D C, Anderson E L, Lottenbach K, Mink C M. Reactogenicity and immunogenicity of a protein-conjugated pneumococcal oligosaccharide vaccine in older adults. J Infect Dis. 1996;173:1014–1018. doi: 10.1093/infdis/173.4.1014. [DOI] [PubMed] [Google Scholar]
- 246.Prasadarao N V, Lysenko E, Wass C A, Kim K S, Weiser J N. Opacity-associated protein A contributes to the binding of Haemophilus influenzae to Chang epithelial cells. Infect Immun. 1999;67:4153–4160. doi: 10.1128/iai.67.8.4153-4160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Qiu J, Hendrixson D R, Baker E N, Murphy T F, St. Geme III J W, Plaut A G. Human milk lactoferrin inactivates two putative colonization factors expressed by Haemophilus influenzae. Proc Natl Acad Sci USA. 1998;95:12641–12646. doi: 10.1073/pnas.95.21.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Rahman M, Holme T. Antibody response in rabbits to serotype-specific determinants in lipopolysaccharides from Moraxella catarrhalis. J Med Microbiol. 1996;44:348–354. doi: 10.1099/00222615-44-5-348. [DOI] [PubMed] [Google Scholar]
- 249.Rao V K, Krasan G P, Hendrixson D R, Dawid S, St. Geme J W., III Molecular determinants of the pathogenesis of disease due to non-typable Haemophilus influenzae. FEMS Microbiol Rev. 1999;23:99–129. doi: 10.1111/j.1574-6976.1999.tb00393.x. [DOI] [PubMed] [Google Scholar]
- 250.Rayner C F J, Jackson A D, Rutman A, Dewar A, Mitchell T J, Andrew P W, Cole P J, Wilson R. Interaction of pneumolysin-sufficient and -deficient isogenic variants of Streptococcus pneumoniae with human respiratory mucosa. Infect Immun. 1995;63:442–447. doi: 10.1128/iai.63.2.442-447.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Read R C, Kuss A, Berrisoul F, Torres A, Kubin R. The efficacy and safety of a new ciprofloxacin suspension compared with co-amoxiclav tablets in the treatment of acute exacerbations of chronic bronchitis. Respir Med. 1999;93:252–261. doi: 10.1016/s0954-6111(99)90021-5. [DOI] [PubMed] [Google Scholar]
- 252.Read R C, Wilson R, Rutman A, Lund V, Odd H C, Brain A P R, Jeffery P K, Cole P J. Interaction of nontypable Haemophilus influenzae with human respiratory mucosa in vitro. J Infect Dis. 1991;163:549–558. doi: 10.1093/infdis/163.3.549. [DOI] [PubMed] [Google Scholar]
- 253.Reddy M S, Bernstein J M, Murphy T F, Faden H S. Binding between outer membrane proteins of nontypeable Haemophilus influenzae and human nasopharyngeal mucin. Infect Immun. 1996;64:1477–1479. doi: 10.1128/iai.64.4.1477-1479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Reddy M S, Murphy T F, Faden H S, Bernstein J M. Middle ear mucin glycoprotein: purification and interaction with nontypable Haemophilus influenzae and Moraxella catarrhalis. Otolaryngol Head Neck Surg. 1997;116:175–189. doi: 10.1016/S0194-59989770321-8. [DOI] [PubMed] [Google Scholar]
- 255.Reichler M R, Allphin A A, Breiman R F, Schreiber J R, Arnold J E, McDougal L K, Facklam R R, Boxerbaum B, May D, Walton R O, Jacobs M R. The spread of multiply resistant Streptococcus pneumoniae at a day care center in Ohio. J Infect Dis. 1992;166:1346–1353. doi: 10.1093/infdis/166.6.1346. [DOI] [PubMed] [Google Scholar]
- 256.Reidl J, Mekalanos J J. Lipoprotein e(P4) is essential for hemin uptake by Haemophilus influenzae. J Exp Med. 1996;183:621–629. doi: 10.1084/jem.183.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ren Z, Jin H, Morton D J, Stull T L. hgpB, a gene encoding a second Haemophilus influenzae hemoglobin- and hemoglobin-haptoglobin-binding protein. Infect Immun. 1998;66:4733–4741. doi: 10.1128/iai.66.10.4733-4741.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Retzer M D, Yu R-H, Schryvers A B. Identification of sequences in human transferrin that bind to the bacterial receptor protein, transferrin-binding protein B. Mol Microbiol. 1999;32:111–121. doi: 10.1046/j.1365-2958.1999.01331.x. [DOI] [PubMed] [Google Scholar]
- 259.Richards S J, Greening A P, Enright M C, Morgan M G, McKenzie H. Outbreak of Moraxella catarrhalis in a respiratory unit. Thorax. 1993;48:91–92. doi: 10.1136/thx.48.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Rikitomi N, Andersson B, Matsumoto K, Lindstedt R, Svanborg C. Mechanism of adherence of Moraxella (Branhamella) catarrhalis. Scand J Infect Dis. 1991;23:559–567. doi: 10.3109/00365549109105178. [DOI] [PubMed] [Google Scholar]
- 261.Robert M C, Pang Y, Spencer R C, Winstanley T G, Brown B A, Wallace R J., Jr Tetracycline resistance in Moraxella (Branhamella) catarrhalis: demonstration of two clonal outbreaks by using pulsed-field gel electrophoresis. Antimicrob Agents Chemother. 1991;35:2453–2455. doi: 10.1128/aac.35.11.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Roche R J, Moxon E R. Phenotypic variation of carbohydrate surface antigens and the pathogenesis of Haemophilus influenzae infections. Trends Microbiol. 1995;3:304–309. doi: 10.1016/s0966-842x(00)88959-3. [DOI] [PubMed] [Google Scholar]
- 263.Romero-Steiner S, Musher D M, Centron M S, Pais L B, Groover J E, Fiore A E, Plikaytis B D, Carlone G M. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin Infect Dis. 1999;29:281–288. doi: 10.1086/520200. [DOI] [PubMed] [Google Scholar]
- 264.Rosenow C, Ryan P, Weiser J N, Johnson S, Fontan P, Ortqvist A, Masure H R. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol Microbiol. 1997;25:819–829. doi: 10.1111/j.1365-2958.1997.mmi494.x. [DOI] [PubMed] [Google Scholar]
- 265.Rubins J B, Alter M, Loch J, Janoff E N. Determination of antibody responses of elderly adults to all 23 capsular polysaccharides after pneumococcal vaccination. Infect Immun. 1999;67:5979–5984. doi: 10.1128/iai.67.11.5979-5984.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Rubins J B, Paddock A H, Charboneau D, Berry A M, Paton J C, Janoff E N. Pneumolysin in pneumococcal adherence and colonization. Microb Pathog. 1998;25:337–342. doi: 10.1006/mpat.1998.0239. [DOI] [PubMed] [Google Scholar]
- 267.Rubins J B, Puri A K G, Loch J, Charboneau D, MacDonald R, Opstad N, Janoff E N. Magnitude, duration, quality, and function of pneumococcal vaccine responses in elderly adults. J Infect Dis. 1998;178:431–440. doi: 10.1086/515644. [DOI] [PubMed] [Google Scholar]
- 268.Saetta M, Di Stefano A, Maestrelli P, Turato G, Ruggieri M P, Roggeri A, Calcagni P, Mapp C E, Ciaccia A, Fabbri L M. Airway eosinophilia in chronic bronchitis during exacerbations. Am J Respir Crit Care Med. 1995;150:1646–1652. doi: 10.1164/ajrccm.150.6.7952628. [DOI] [PubMed] [Google Scholar]
- 269.Saint S, Bent S, Vittinghoff E, Grady D. Antibiotics in chronic obstructive pulmonary disease exacerbations: a meta-analysis. JAMA. 1995;273:957–960. [PubMed] [Google Scholar]
- 270.Saito M, Umeda A, Yoshida S-I. Subtyping of Haemophilus influenzae strains by pulsed-field gel electrophoresis. J Clin Microbiol. 1999;37:2142–2147. doi: 10.1128/jcm.37.7.2142-2147.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Samuelson A, Freijd A, Jonasson J, Lindberg A A. Turnover of nonencapsulated Haemophilus influenzae in the nasopharynges of otitis-prone children. J Clin Microbiol. 1995;33:2027–2031. doi: 10.1128/jcm.33.8.2027-2031.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Sanders J D, Cope L D, Hansen E J. Identification of a locus involved in the utilization of iron by Haemophilus influenzae. Infect Immun. 1994;62:4515–4525. doi: 10.1128/iai.62.10.4515-4525.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Sarwar J, Campagnari A A, Kirkham C, Murphy T F. Characterization of an antigenically conserved heat-modifiable major outer membrane protein of Branhamella catarrhalis. Infect Immun. 1992;60:804–809. doi: 10.1128/iai.60.3.804-809.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Sethi S. Etiology and management of infections in chronic obstructive pulmonary disease. Clin Pulmon Med. 1999;6:327–332. [Google Scholar]
- 275.Sethi S, Hill S L, Murphy T F. Serum antibodies to outer membrane proteins of Moraxella (Branhamella) catarrhalis in patients with bronchiectasis: identification of OMP B1 as an important antigen. Infect Immun. 1995;63:1516–1520. doi: 10.1128/iai.63.4.1516-1520.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Sethi S, Muscarella K, Evans N, Klingman K L, Grant B J B, Murphy T F. Airway inflammation and etiology of acute exacerbations of chronic bronchitis. Chest. 2000;118:1557–1565. doi: 10.1378/chest.118.6.1557. [DOI] [PubMed] [Google Scholar]
- 277.Sethi S, Surface J M, Murphy T F. Antigenic heterogeneity and molecular analysis of CopB of Moraxella (Branhamella) catarrhalis. Infect Immun. 1997;65:3666–3671. doi: 10.1128/iai.65.9.3666-3671.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Shah P M, Maesen F P V, Dolmann A, Vetter N, Fiss E, Wesch R. Levofloxacin versus cefuroxime axetil in the treatment of acute exacerbation of chronic bronchitis: results of a randomized, double-blind study. J Antimicrob Chemother. 1999;43:529–539. doi: 10.1093/jac/43.4.529. [DOI] [PubMed] [Google Scholar]
- 279.Shaheen S O, Barker D J P, Shiell A W, Crocker F J, Wield G A, Holgate S T. The relationship between pneumonia in early childhood and impaired lung function in late adult life. Am J Respir Crit Care Med. 1994;149:616–619. doi: 10.1164/ajrccm.149.3.8118627. [DOI] [PubMed] [Google Scholar]
- 280.Shaheen S O, Sterne J A C, Tucker J S, Florey C d V. Birth weight, childhood lower respiratory tract infection, and adult lung function. Thorax. 1998;53:549–553. doi: 10.1136/thx.53.7.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Shelly M A, Jacoby H, Riley G J, Graves B T, Pichichero M, Treanor J J. Comparison of pneumococcal polysaccharide and CRM197 conjugated pneumococcal oligosaccharide vaccines in young and elderly adults. Infect Immun. 1997;65:242–247. doi: 10.1128/iai.65.1.242-247.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Shurin P A, Pelton S I, Tazer I B, Kasper D L. Bactericidal antibody and susceptibility to otitis media caused by nontypeable strains of Haemophilus influenzae. J Pediatr. 1980;97:364–369. doi: 10.1016/s0022-3476(80)80182-x. [DOI] [PubMed] [Google Scholar]
- 283.Sikkema D J, Murphy T F. Molecular analysis of the P2 porin protein of nontypeable Haemophilus influenzae. Infect Immun. 1992;60:5204–5211. doi: 10.1128/iai.60.12.5204-5211.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Sirakova T, Kolattukudy P E, Murwin D, Billy J, Leake E, Lim D, DeMaria T, Bakaletz L. Role of fimbriae expressed by nontypeable Haemophilus influenzae in pathogenesis of and protection against otitis media and relatedness of the fimbrin subunit to outer membrane protein A. Infect Immun. 1994;62:2002–2020. doi: 10.1128/iai.62.5.2002-2020.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Sluijter M, Faden H, de Groot R, Lemmens N, Goessens W H F, van Belkum A, Hermans P W M. Molecular characterization of pneumococcal nasopharynx isolates collected from children during their first 2 years of life. J Clin Microbiol. 1998;36:2248–2253. doi: 10.1128/jcm.36.8.2248-2253.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Smith G L. Branhamella catarrhalis infection imitating gonorrhea in a man. NEJM. 1987;316:1277. [PubMed] [Google Scholar]
- 287.Smith J M B, Lockwood B M. A 2-year survey of Branhamella catarrhalis in a general hospital. J Hosp Infect. 1986;7:277–282. doi: 10.1016/0195-6701(86)90078-2. [DOI] [PubMed] [Google Scholar]
- 288.Smith-Vaughan H C, Leach A J, Shelby-James T M, Kemp K, Kemp D J, Mathews J D. Carriage of multiple ribotypes of non-encapsulated Haemophilus influenzae in Aboriginal infants with otitis media. Epidemiol Infect. 1996;116:177–183. doi: 10.1017/s0950268800052419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Smith-Vaughan H C, Sriprakash K S, Leach A J, Mathews J D, Kemp D J. Low genetic diversity of Haemophilus influenzae type b compared to nonencapsulated H. influenzae in a population in which H. influenzae is highly endemic. Infect Immun. 1998;66:3403–3409. doi: 10.1128/iai.66.7.3403-3409.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Smith-Vaughan H C, Sriprakash K S, Mathews J D, Kemp D J. Long PCR-ribotyping of nontypeable Haemophilus influenzae. J Clin Microbiol. 1995;33:1192–1195. doi: 10.1128/jcm.33.5.1192-1195.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Smith-Vaughan H C, Sriprakash K S, Mathews J D, Kemp D J. Nonencapsulated Haemophilus influenzae in aboriginal infants with otitis media: prolonged carriage of P2 porin variants and evidence for horizontal P2 gene transfer. Infect Immun. 1997;65:1468–1474. doi: 10.1128/iai.65.4.1468-1474.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Snider G L, Kleinerman J, Thurlbeck W M, Bengali Z K. The definition of emphysema: report of a National Heart, Lung and Blood Institute Division of Lung Diseases workshop. Am Rev Respir Dis. 1985;132:182–185. doi: 10.1164/arrd.1985.132.1.182. [DOI] [PubMed] [Google Scholar]
- 293.Soler N, Ewig S, Torres A, Filella X, Gonzalez J, Zaubet A. Airway inflammation and bronchial microbial patterns in patients with stable chronic obstructive pulmonary disease. Eur Respir J. 1999;14:1015–1022. doi: 10.1183/09031936.99.14510159. [DOI] [PubMed] [Google Scholar]
- 294.Soler N, Torres A, Ewig S, Gonzalez J, Celis R, El-Ebiary M, Hernandez C, Rodriguez-Roisin R. Bronchial microbial patterns in severe exacerbations of chronic obstructive pulmonary disease (COPD) requiring mechanical ventilation. Am J Respir Crit Care Med. 1998;157:1498–1505. doi: 10.1164/ajrccm.157.5.9711044. [DOI] [PubMed] [Google Scholar]
- 295.Song X-M, Forsgren A, Janson H. The gene encoding protein D (hpd) is highly conserved among Haemophilus influenzae type b and nontypeable strains. Infect Immun. 1995;63:696–699. doi: 10.1128/iai.63.2.696-699.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Speizer F E, Tager I B. Epidemiology of chronic mucus hypersecretion and obstructive airways disease. Epidemiol Rev. 1979;1:124–142. doi: 10.1093/oxfordjournals.epirev.a036206. [DOI] [PubMed] [Google Scholar]
- 297.Spellerberg B, Cundell D R, Sandros J, Pearce B J, Idanpaan-Heikkila I, Rosenow C, Masure H R. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. Mol Microbiol. 1996;19:803–813. doi: 10.1046/j.1365-2958.1996.425954.x. [DOI] [PubMed] [Google Scholar]
- 298.Srikumar R, Chin A C, Vachon V, Richardson C D, Ratcliffe M J H, Saarinen L, Kayhty H, Makela P H, Coulton J W. Monoclonal antibodies specific to porin of Haemophilus influenzae type b: localization of their cognate epitopes and tests of their biological activities. Mol Microbiol. 1992;6:665–676. doi: 10.1111/j.1365-2958.1992.tb01514.x. [DOI] [PubMed] [Google Scholar]
- 299.Srinivasan G, Raff M J, Templeton W C, Givens S J, Graves R C, Melo J C. Branhamella catarrhalis pneumonia. Report of two cases and review of the literature. Am Rev Respir Dis. 1981;123:553–555. doi: 10.1164/arrd.1981.123.5.553. [DOI] [PubMed] [Google Scholar]
- 300.St. Geme J W., III Insights into the mechanism of respiratory tract colonization by nontypable Haemophilus influenzae. Pediatr Infect Dis J. 1997;16:931–935. doi: 10.1097/00006454-199710000-00005. [DOI] [PubMed] [Google Scholar]
- 301.St. Geme J W, III, Cutter D. Influence of pili, fibrils, and capsule on in vitro adherence by Haemophilus influenzae type b. Mol Microbiol. 1996;21:21–31. doi: 10.1046/j.1365-2958.1996.6241331.x. [DOI] [PubMed] [Google Scholar]
- 302.St. Geme J W, III, de la Morena M L, Falkow S. A Haemophilus influenzae IgA protease-like protein promotes intimate interaction with human epithelial cells. Mol Microbiol. 1994;14:217–233. doi: 10.1111/j.1365-2958.1994.tb01283.x. [DOI] [PubMed] [Google Scholar]
- 303.St. Geme J W, III, Falkow S. Haemophilus influenzae adheres to and enters cultured human epithelial cells. Infect Immun. 1990;58:4036–4044. doi: 10.1128/iai.58.12.4036-4044.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.St. Geme J W, III, Falkow S, Barenkamp S J. High-molecular-weight proteins of nontypable Haemophilus influenzae mediate attachment to human epithelial cells. Proc Natl Acad Sci USA. 1993;90:2875–2879. doi: 10.1073/pnas.90.7.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.St. Geme J W, III, Kumar V V, Cutter D, Barenkamp S J. Prevalence and distribution of the hmw and hia genes and the HMW and Hia adhesins among genetically diverse strains of nontypeable Haemophilus influenzae. Infect Immun. 1998;66:364–368. doi: 10.1128/iai.66.1.364-368.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305a.Stockley R A, O'Brien C, Pye A, Hill S L. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest. 2000;117:1638–1645. doi: 10.1378/chest.117.6.1638. [DOI] [PubMed] [Google Scholar]
- 306.Stolk J, Rudolphus A, Davies P, Osinga D, Dukman J H, Agarwal L, Keenan K P, Fletcher D, Kramps J A. Induction of emphysema and bronchial mucus cell hyperplasia by intratracheal instillation of lipopolysaccharide in the hamster. J Pathol. 1992;167:349–356. doi: 10.1002/path.1711670314. [DOI] [PubMed] [Google Scholar]
- 307.Tager I, Speizer F E. Role of infection in chronic bronchitis. N Engl J Med. 1975;292:563–571. doi: 10.1056/NEJM197503132921105. [DOI] [PubMed] [Google Scholar]
- 308.Tandon M K, Gebski V. A controlled trial of a killed Haemophilus influenzae vaccine for prevention of acute exacerbations of chronic bronchitis. Aust NZ J Med. 1991;21:427–432. doi: 10.1111/j.1445-5994.1991.tb01346.x. [DOI] [PubMed] [Google Scholar]
- 309.Taylor D C, Cripps A W, Clancy R L, Murree-Allen K, Hensley M J, Saunders N A, Sutherland D C. Biotypes of Haemophilus parainfluenzae from the respiratory secretions in chronic bronchitis. J Med Microbiol. 1992;36:279–282. doi: 10.1099/00222615-36-4-279. [DOI] [PubMed] [Google Scholar]
- 310.Thompson A B, Daughton D, Robbins R A, Ghafouri M A, Oehlerking M, Rennard S I. Intraluminal airway inflammation in chronic bronchitis: characterization and correlation with clinical parameters. Am Rev Respir Dis. 1989;140:1527–1537. doi: 10.1164/ajrccm/140.6.1527. [DOI] [PubMed] [Google Scholar]
- 311.Tong H H, McIver M A, Fisher L M, DeMaria T F. Effect of lacto-N-neotetraose, asialoganglioside-GM1 and neuraminidase on adherence of otitis media-associated serotypes of Streptococcus pneumoniae to chinchilla tracheal epithelium. Microb Pathog. 1999;26:111–119. doi: 10.1006/mpat.1998.0257. [DOI] [PubMed] [Google Scholar]
- 312.Troelstra A, Vogel L, van Alphen L, Eijk P, Jansen H, Dankert J. Opsonic antibodies to outer membrane protein P2 of nonencapsulated Haemophilus influenzae are strain specific. Infect Immun. 1994;62:779–784. doi: 10.1128/iai.62.3.779-784.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Tuomanen E, Liu H, Hengstler B, Zak O, Tomasz A. The induction of meningeal inflammation by components of the pneumococcal cell wall. J Infect Dis. 1985;151:859–868. doi: 10.1093/infdis/151.5.859. [DOI] [PubMed] [Google Scholar]
- 314.Tuomanen E, Pollack H, Parkinson A, Davidson M, Facklam R, Zak O. Microbiological and clinical significance of a new property of defective lysis in clinical strains of pneumococci. J Infect Dis. 1988;158:36–43. doi: 10.1093/infdis/158.1.36. [DOI] [PubMed] [Google Scholar]
- 315.Tuomanen E, Rich R, Zak O. Induction of pulmonary inflammation by components of the pneumococcal cell surface. Am Rev Respir Dis. 1987;135:869–874. doi: 10.1164/arrd.1987.135.4.869. [DOI] [PubMed] [Google Scholar]
- 316.Tuomanen E I, Austrian R, Masure H R. Pathogenesis of pneumococcal infection. NEJM. 1995;332:1280–1284. doi: 10.1056/NEJM199505113321907. [DOI] [PubMed] [Google Scholar]
- 317.Unhanand M, Maciver I, Ramilo O, Arencibia-Mireles O, Argyle J C, McCracken G H, Jr, Hansen E J. Pulmonary clearance of Moraxella catarrhalis in an animal model. J Infect Dis. 1992;165:644–650. doi: 10.1093/infdis/165.4.644. [DOI] [PubMed] [Google Scholar]
- 318.Vachon V, Kristjanson D N, Coulton J W. Outer membrane porin protein of Haemophilus influenzae type b pore size and subunit structure. Can J Microbiol. 1988;34:134–140. doi: 10.1139/m88-027. [DOI] [PubMed] [Google Scholar]
- 319.Van Belkum A, Duim B, Regelink A, Moller L, Quint W, van Alphen L. Genomic DNA fingerprinting of clinical Haemophilus influenzae isolates by polymerase chain reaction amplification: comparison with major outer-membrane protein and restriction fragment length polymorphism. J Med Microbiol. 1994;41:63–68. doi: 10.1099/00222615-41-1-63. [DOI] [PubMed] [Google Scholar]
- 320.Vaneechoutte M, Verschraegen G, Claeys G, Van Den Abeele A M. Serological typing of Branhamella catarrhalis strains on the basis of lipopolysaccharide antigens. J Clin Microbiol. 1990;28:182–187. doi: 10.1128/jcm.28.2.182-187.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Vaneechoutte M, Verschraegen G, Claeys G, Weise B, Van Den Abeele A M. Respiratory tract carrier rates of Moraxella (Branhamella) catarrhalis in adults and children and interpretation of the isolation of M. catarrhalis from sputum. J Clin Microbiol. 1990;28:2674–2680. doi: 10.1128/jcm.28.12.2674-2680.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Van Ham S M, van Alphen L, Mooi F R, van Putten J P M. Phase variation of H. influenzae fimbriae: transcriptional control of two divergent genes through a variable combined promoter region. Cell. 1993;73:1187–1196. doi: 10.1016/0092-8674(93)90647-9. [DOI] [PubMed] [Google Scholar]
- 323.Van Ham S M, van Alphen L, Mooi F R, van Putten J P M. The fimbrial gene cluster of Haemophilus influenzae type b. Mol Microbiol. 1994;13:673–684. doi: 10.1111/j.1365-2958.1994.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 324.Van Hare G F, Shurin P A, Marchant C D, Cartelli N A, Johnson C E, Fulton D, Carlin S, Kim C H. Acute otitis media caused by Branhamella catarrhalis: biology and therapy. Rev Infect Dis. 1987;9:16–27. doi: 10.1093/clinids/9.1.16. [DOI] [PubMed] [Google Scholar]
- 325.van Schilfgaarde M, Eijk P, Regelink A, van Ulsen P, Everts V, Dankert J, van Alphen L. Haemophilus influenzae localized in epithelial cell layers is shielded from antibiotics and antibody-mediated bactericidal activity. Microb Pathog. 1999;26:249–262. doi: 10.1006/mpat.1998.0269. [DOI] [PubMed] [Google Scholar]
- 326.van Schilfgaarde M, van Alphen L, Eijk P, Everts V, Dankert J. Paracytosis of Haemophilus influenzae through cell layers of NCI-H292 lung epithelial cells. Infect Immun. 1995;63:4729–4737. doi: 10.1128/iai.63.12.4729-4737.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Verghese A, Berro E, Berro J, Franzus B W. Pulmonary clearance and phagocytic cell response in a murine model of Branhamella catarrhalis infection. J Infect Dis. 1990;162:1189–1192. doi: 10.1093/infdis/162.5.1189. [DOI] [PubMed] [Google Scholar]
- 328.Verghese A, Roberson D, Kalbfleisch J H, Sarubbi F. Randomized comparative study of cefixime versus cephalexin in acute bacterial exacerbations of chronic bronchitis. Antimicrob Agents Chemother. 1990;34:1041–1044. doi: 10.1128/aac.34.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Vogel L, Duim B, Geluk F, Eijk P, Jansen H, Dankert J, van Alphen L. Immune selection for antigenic drift of major outer membrane protein P2 of Haemophilus influenzae during persistence in subcutaneous tissue cages in rabbits. Infect Immun. 1996;64:980–986. doi: 10.1128/iai.64.3.980-986.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Vollmer W M, Osborne M L, Buist A S. 20-year trends in the prevalence of asthma and chronic airflow obstruction in an HMO. Am J Respir Crit Care Med. 1998;157:1079–1084. doi: 10.1164/ajrccm.157.4.9704140. [DOI] [PubMed] [Google Scholar]
- 331.von Graevenitz A, Rathbone R R. Branhamella catarrhalis in respiratory secretions: clinical correlation in 16 cases. South Med J. 1981;74:1095–1096. doi: 10.1097/00007611-198109000-00021. [DOI] [PubMed] [Google Scholar]
- 332.von Hertzen L, Alakarppa H, Koskinen R, Liippo K, Surcel H-M, Leinonen M, Saikku P. Chlamydia pneumoniae infection in patients with chronic obstructive pulmonary disease. Epidemiol Infect. 1997;118:155–164. doi: 10.1017/s095026889600725x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Vuori E, Peltola H, Kallio M J T, Leinonen M, Hedman K, Study Group SE-TU. Etiology of pneumonia and other common childhood infections requiring hospitalization and parenteral antimicrobial therapy. Clin Infect Dis. 1998;27:566–572. doi: 10.1086/514697. [DOI] [PubMed] [Google Scholar]
- 334.Vu-Thien H, Dulot C, Moissenet D, Fauroux B, Garbarg-Chenon A. Comparison of randomly amplified polymorphic DNA analysis and pulsed-field gel electrophoresis for typing of Moraxella catarrhalis strains. J Clin Microbiol. 1999;37:450–452. doi: 10.1128/jcm.37.2.450-452.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Wallace F J, Clancy R L, Cripps A W. An animal model demonstration of enhanced clearance of nontypable Haemophilus influenzae from the respiratory tract after antigen stimulation of gut-associated lymphoid tissue. Am Rev Respir Dis. 1989;140:311–316. doi: 10.1164/ajrccm/140.2.311. [DOI] [PubMed] [Google Scholar]
- 336.Wallace F J, Witt C S, Clancy R L, Cripps A W. Protection against non-typable Haemophilus influenzae following sensitization of gut associated lymphoid tissue: role of specific antibody and phagocytes. Immunol Cell Biol. 1995;73:258–265. doi: 10.1038/icb.1995.42. [DOI] [PubMed] [Google Scholar]
- 337.Wani J H, Gilbert J V, Plaut A G, Weiser J N. Identification, cloning, and sequencing of the immunoglobulin A1 protease gene of Streptococcus pneumoniae. Infect Immun. 1996;64:3967–3974. doi: 10.1128/iai.64.10.3967-3974.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Watson D A, Musher D M. Interruption of capsule production in Streptococcus pneumoniae serotype 3 by insertion of transposon Tn916. Infect Immun. 1990;58:3135–3138. doi: 10.1128/iai.58.9.3135-3138.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Watson D A, Musher D M, Verhoef J. Pneumococcal virulence factors and host immune responses to them. Eur J Clin Microbiol Infect Dis. 1995;14:479–490. doi: 10.1007/BF02113425. [DOI] [PubMed] [Google Scholar]
- 340.Weiser J N. The oligosaccharide of Haemophilus influenzae. Microb Pathog. 1992;13:335–342. doi: 10.1016/0882-4010(92)90077-2. [DOI] [PubMed] [Google Scholar]
- 341.Weiser J N, Chong S T H, Greenberg D, Fong W. Identification and characterization of a cell envelope protein of Haemophilus influenzae contributing to phase variation in colony opacity and nasopharyngeal colonization. Mol Microbiol. 1995;17:555–564. doi: 10.1111/j.1365-2958.1995.mmi_17030555.x. [DOI] [PubMed] [Google Scholar]
- 342.Weiser J N, Markiewicz Z, Tuomanen E I, Wani J H. Relationship between phase variation in colony morphology, intrastrain variation in cell wall physiology, and nasopharyngeal colonization by Streptococcus pneumoniae. Infect Immun. 1996;64:2240–2245. doi: 10.1128/iai.64.6.2240-2245.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.West M, Berk S L, Smith J K. Branhamella catarrhalis pneumonia. South Med J. 1982;75:1021–1023. doi: 10.1097/00007611-198208000-00029. [DOI] [PubMed] [Google Scholar]
- 344.Whitby P W, Sim K E, Morton D J, Patel J A, Stull T L. Transcription of genes encoding iron and heme acquisition proteins of Haemophilus influenzae during acute otitis media. Infect Immun. 1997;65:4696–4700. doi: 10.1128/iai.65.11.4696-4700.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Williams R L, Chalmers T C, Stange K C, Chalmers F T, Bowlin S J. Use of antibiotics in preventing recurrent acute otitis media and in treating otitis media with effusion. JAMA. 1993;270:1344–1351. [PubMed] [Google Scholar]
- 346.Wilson R. The role of infection in COPD. Chest. 1998;113:242S–248S. doi: 10.1378/chest.113.4_supplement.242s. [DOI] [PubMed] [Google Scholar]
- 347.Wilson R, Cole P. The effect of bacterial products on ciliary functions. Am Rev Respir Dis. 1988;138:S49–S53. doi: 10.1164/ajrccm/138.6_Pt_2.S49. [DOI] [PubMed] [Google Scholar]
- 348.Wilson R, Kubin R, Ballin I, Deppermann K-M, Bassaris H P, Leophonte P, Schreurs J M, Torres A, Sommerauer B. Five day moxifloxacin therapy compared with 7 day clarithromycin therapy for the treatment of acute exacerbations of chronic bronchitis. J Antimicrob Chemother. 1999;44:501–513. doi: 10.1093/jac/44.4.501. [DOI] [PubMed] [Google Scholar]
- 349.Wilson R, Roberts D, Cole P. Effect of bacterial products on human ciliary function in vitro. Thorax. 1984;40:125–131. doi: 10.1136/thx.40.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Wong J C Y, Holland J, Parsons T, Smith A, Williams P. Identification and characterization of an iron-regulated hemopexin receptor in Haemophilus influenzae type b. Infect Immun. 1994;62:48–59. doi: 10.1128/iai.62.1.48-59.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Wong J C Y, Patel R, Kendall D, Whitby P W, Smith A, Holland J, Williams P. Affinity, conservation, and surface exposure of hemopexin-binding proteins in Haemophilus influenzae. Infect Immun. 1995;63:2327–2333. doi: 10.1128/iai.63.6.2327-2333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Yang Y-P, Munson R S, Jr, Grass S, Chong P, Harkness R E, Gisonni L, James O, Kwok Y, Klein M H. Effect of lipid modification on the physicochemical, structural, antigenic and immunoprotective properties of Haemophilus influenzae outer membrane protein P6. Vaccine. 1998;15:976–987. doi: 10.1016/s0264-410x(96)00296-4. [DOI] [PubMed] [Google Scholar]
- 353.Yang Y-P, Myers L E, McGuinness U, Chong P, Kwok Y, Klein M H, Harkness R E. The major outer membrane protein, CD, extracted from Moraxella (Branhamella) catarrhalis is a potential vaccine antigen that induces bactericidal antibodies. FEMS Immunol Med Microbiol. 1997;17:187–199. doi: 10.1111/j.1574-695X.1997.tb01012.x. [DOI] [PubMed] [Google Scholar]
- 354.Yi K, Murphy T F. Importance of an immunodominant surface-exposed loop on outer membrane protein P2 of nontypeable Haemophilus influenzae. Infect Immun. 1997;65:150–155. doi: 10.1128/iai.65.1.150-155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Yi K, Sethi S, Murphy T F. Human immune response to nontypeable Haemophilus influenzae in chronic bronchitis. J Infect Dis. 1997;176:1247–1252. doi: 10.1086/514119. [DOI] [PubMed] [Google Scholar]
- 356.Yu R-H, Bonnah R A, Ainsworth S, Schryvers A B. Analysis of the immunological responses to transferrin and lactoferrin receptor proteins from Moraxella catarrhalis. Infect Immun. 1999;67:3793–3799. doi: 10.1128/iai.67.8.3793-3799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Yu X, Gray B, Chang S, Ward J I, Edwards K M, Nahm M H. Immunity to cross-reactive serotypes induced by pneumococcal conjugate vaccines in infants. J Infect Dis. 1999;180:1569–1576. doi: 10.1086/315096. [DOI] [PubMed] [Google Scholar]
- 358.Zalacain R, Sobradillo V, Amilibia J, Barron J, Achotegui V, Pijoan J I, Llorente J L. Predisposing factors to bacterial colonization in chronic obstructive pulmonary disease. Eur Respir J. 1999;13:343–348. doi: 10.1034/j.1399-3003.1999.13b21.x. [DOI] [PubMed] [Google Scholar]