Abstract
Background:
Peripheral nerve stimulation (PNS) has been increasingly used to manage acute and chronic pain. However, the level of clinical evidence to support its use is not clear.
Objectives:
To assess the clinical evidence of PNS in the treatment of acute or chronic pain.
Study Design:
A systematic review of the efficacy and safety of PNS in managing acute or chronic pain.
Methods:
Data sources were PubMed, Cochrane Library, Scopus, CINAHL Plus, Google Scholar, and reference lists. The literature search was performed up to December 2019. Study selection included randomized trials, observational studies, and case reports of PNS in acute or chronic pain. Data extraction and methodological quality assessment were performed utilizing Cochrane review methodologic quality assessment and Interventional Pain Management Techniques–Quality Appraisal of Reliability and Risk of Bias Assessment (IPM-QRB) and Interventional Pain Management Techniques–Quality Appraisal of Reliability and Risk of Bias Assessment for Nonrandomized Studies (IPM-QRBNR). The evidence was summarized utilizing principles of best evidence synthesis on a scale of 1 to 5. Data syntheses: 227 studies met inclusion criteria and were included in qualitative synthesis.
Results:
Evidence synthesis based on randomized controlled trials (RCTs) and observational studies showed Level I and II evidence of PNS in chronic migraine headache; Level II evidence in cluster headache, postamputation pain, chronic pelvic pain, chronic low back and lower extremity pain; and Level IV evidence in peripheral neuropathic pain, and postsurgical pain. Peripheral field stimulation has Level II evidence in chronic low back pain, and Level IV evidence in cranial pain.
Limitations:
Lack of high-quality RCTs. Meta-analysis was not possible due to wide variations in experimental design, research protocol, and heterogeneity of study population.
Conclusions:
The findings of this systematic review suggest that PNS may be effective in managing chronic headaches, postamputation pain, chronic pelvic pain, and chronic low back and lower extremity pain, with variable levels of evidence in favor of this technique.
Keywords: Acute pain, chronic pain, neuromodulation, peripheral nerve stimulation
Peripheral nerve stimulation (PNS) has been actively investigated and increasingly used in clinical practice to treat chronic pain of different origin (1,2). PNS, in a broad sense, may include transcutaneous and percutaneous nerve stimulation. Although most percutaneous PNS studies utilized electrodes designed for spinal cord stimulation (SCS) or deep brain stimulation, a new generation of devices has recently been developed that allows for external pulse generators to transmit impulses wirelessly to the implanted electrode, produced by StimWave, Bioness, and SPR Therapeutics (3). Here we systematically reviewed preclinical studies on the mechanisms of action and clinical evidence of percutaneous PNS in acute and chronic pain management. The goal is to facilitate data-driven clinical decision-making and evidence-based best practices, and to identify gaps for further investigation of PNS as standard of care for specific clinical applications. From anatomic perspective, the dorsal root ganglia (DRG) are in fact part of the peripheral nervous system. However, for regulatory and other reasons, DRG stimulation is generally accepted as a form of SCS and is therefore not covered in this review.
METHODS
Eligibility Criteria
Randomized controlled trials (RCTs), prospective or retrospective observational studies, case series or case reports on PNS or peripheral nerve field stimulation (PNFS) in patients with acute and chronic pain were all included.
Data Sources
We conducted a systematic literature search in PubMed, Scopus, CINAHL Plus, Google Scholar, and the Cochrane library for reports of PNS for pain management up to December of 2019.
Search Strategy and Data Collection Process
The search term included “peripheral nerve stimulation,” “peripheral nerve neuromodulation,” “peripheral neurostimulation,” “trigeminal,” “supraorbital,” “infraorbital,” “occipital,” “headache,” “migraine disorders,” “migraine,” “hemicrania continua,” “paroxysmal hemicranias,” “sinusitis,” “trigeminal autonomic cephalalgia,” “trigeminal neuralgia,” “neuropathy,” “limb,” “torso,” “trauma,” “CRPS,” “amputation,” “surgical,” “postoperative,” and “peripheral nerve field stimulation.” The following is an example of the query that was performed for the PubMed database: (“peripheral nerve stimulation”[MeSH Terms]) OR (“peripheral neuromodulation”[Mesh Terms]) AND (“pain”[All Fields]) OR (“amputation”[Mesh Terms]). Prospective RCTs and meta-analysis were given preference. Well-designed nonrandomized studies were preferred to observational and case serial studies. If there was an overlap on the same topic, the most recent report was selected. A further manual search was done to exclude irrelevant articles by screening the titles and the abstracts. The remained abstracts were reviewed, and full-article analyzed. The stepwise compliance of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist was used (Fig. 1, PRISMA flow diagram).
Fig. 1.
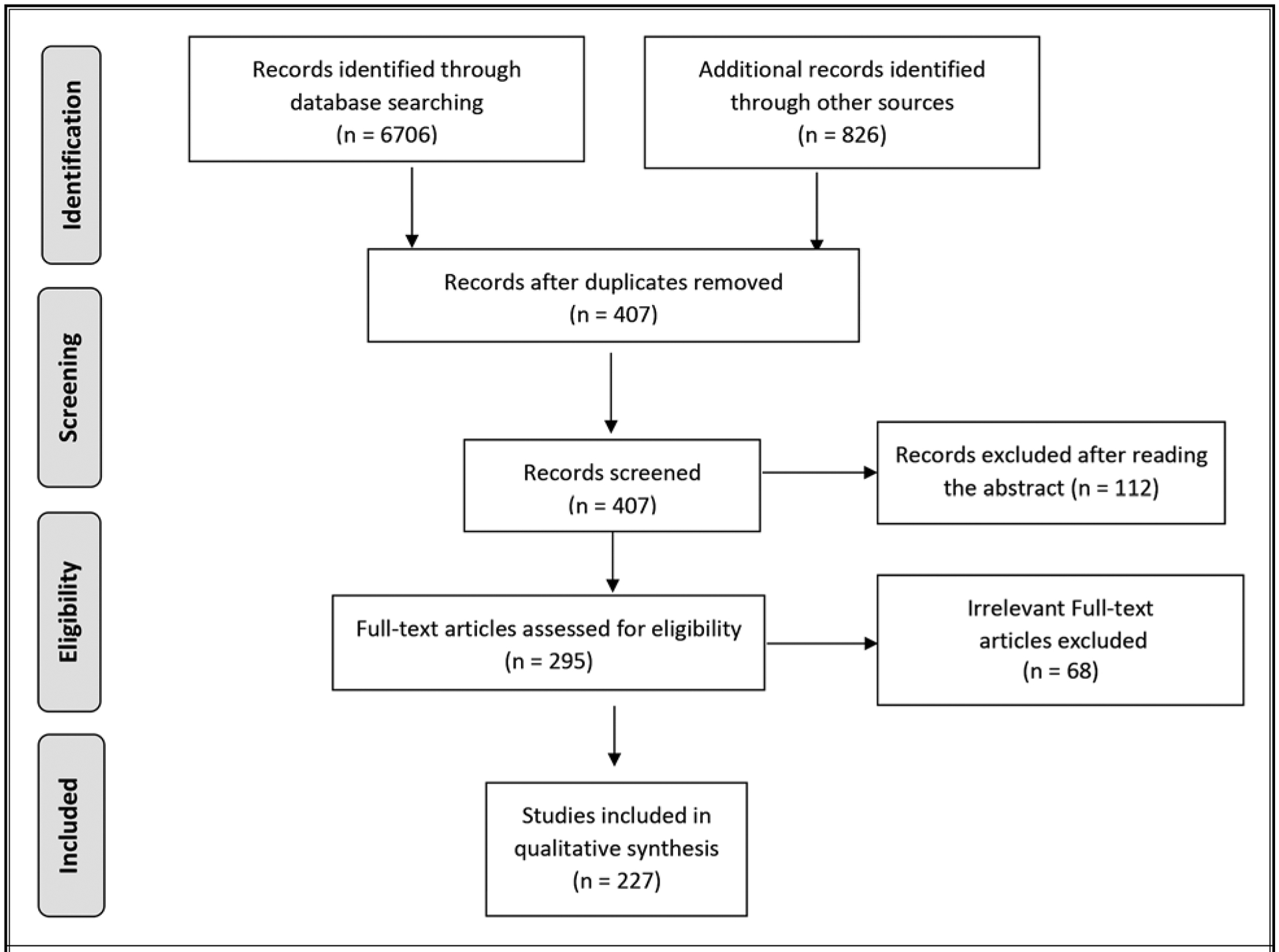
PNS in pain management: PRISMA flow diagram.
Data Syntheses and Analyses
Data syntheses and analyses were performed with assessment of risk of bias or quality of individual studies, outcomes assessment, and qualitative analysis. The quality of each individual article used in this analysis was assessed by Cochrane review criteria (4) and Interventional Pain Management Techniques–Quality Appraisal of Reliability and Risk of Bias Assessment (IPM–QRB) for randomized trials (5). Methodologic quality assessment was performed by 2 authors (JX and ZS) independently in an unblinded, standardized manner. Reviewers performed their methodological quality assessment so as to prevent any discrepancies. If discrepancies occurred, a third reviewer performed an assessment, and a consensus was reached. For the outcome analysis, either 20% improvement from the baseline pain score or a change of at least 20 points on a 101-point pain scale of 0 to 100 was considered clinically significant. For functional status improvement the change was 20% or more of disability scores.
Analysis of Evidence
The analysis of the evidence was performed based on best-evidence synthesis and was modified and collated using multiple available criteria, including the Cochrane Review criteria and US Preventive Task Force (USPSTF) criteria as illustrated in Table 1 (6). The analysis was conducted using 5 levels of evidence ranging from strong to opinion- or consensus-based. The results of best evidence were analyzed by at least 2 of the review authors independently. Any disagreements between reviewers were resolved by a third author and consensus.
Table 1.
Qualitative modified approach to grading of evidence.
| Level I | Strong | Evidence obtained from multiple relevant high-quality RCTs |
| Level II | Moderate | Evidence obtained from at least one relevant high-quality RCT or multiple relevant moderate- or low-quality RCTs |
| Level III | Fair | Evidence obtained from at least one relevant moderate- or low-quality RCT with multiple relevant observational studies or Evidence obtained from at least one relevant high-quality nonrandomized trial or observational study with multiple moderate- or low-quality observational studies |
| Level IV | Limited | Evidence obtained from multiple moderate- or low-quality relevant observational studies |
| Level V | Consensus based | Opinion or consensus of large group of clinicians and/or scientists |
Adapted from Manchikanti L, Falco FJE, Benyamin RM, Kaye AD, Boswell MV, Hirsch JA. A modified approach to grading of evidence. Pain Physician 2014; 17:E319-E325 (6).
RESULTS
Preclinical Studies and Proposed Mechanisms of Action
Our understanding of the mechanism of action behind peripheral nerve stimulators is still growing. Currently, the most cited hypothesis remains the gate control theory first described by Melzack and Wall (7) in 1965. This theory, which proposes that activation of large diameter sensory fibers inhibits transmission of small diameter, nociceptive afferents in the spinal cord dorsal horn, has been supported by studies that have shown that stimulation of non-nociceptive Aβ fibers with PNS results in suppression of nociceptive processing in healthy volunteers (8).
There have been a large number of translational studies on the mechanism of PNS. Although PNS typically refers to stimulation of a specific nerve or nerve trunk, most of these studies have been done with stimulation at the distal terminals of sensory nerves with transcutaneous electrical nerve stimulation (TENS). Several spinal mechanisms of PNS have been identified. TENS has been found to increase the release of gamma-aminobutyric acid, as well as decrease concentrations of glutamate and aspartate through δ-opioidergic-mediated blockade in the spinal cord (9,10). Supraspinal mechanisms of PNS have also been identified. In rat models, TENS was found to activate the descending noradrenergic, serotonergic, muscarinic, and dopamine systems in the spinal cord (11–13). Dorsal horn cell activity decreases during TENS stimulation. There is also evidence that TENS reduces central sensitization and hyperalgesia (14,15). In 2008, Desantana et al (16) showed that in arthritic rats, the application of mixed- and alternating-frequency TENS significantly reduced mechanical hyperalgesia associated with joint inflammation as measured by paw withdrawal. TENS has further been shown to activate opioid receptors both in the spinal cord and in the rostral ventral medulla and the periaqueductal gray (17,18).
Besides TENS, other forms of PNS such as subcutaneous electrical stimulation (SQS) and electroacupuncture (EA) have also been studied (19–21). Chen et al (22) showed that low-frequency stimulation to the median nerve through acupuncture needles led to an opioid-independent analgesic mechanism mediated by orexin 1 receptor-initiated 2- arachidonoylglycerol signaling in the ventrolateral periaqueductal gray. Vera-Portocarrero et al (19) compared TENS to SQS in 2 different rodent models, one modeling inflammatory pain and the other modeling neuropathic pain. SQS was defined as electrical stimulation delivered through electrodes placed in the subcutaneous space rather than electrical stimulation through electrodes placed on the skin. This study showed that although SQS led to antihypersensitivity effects in both inflammatory and neuropathic pain models, TENS did not reveal significant benefit in the neuropathic pain model. This suggests that SQS and TENS may act through different mechanisms. Wang et al (23) found that the use of brief electrical impulses applied to the sciatic nerve through EA changed discharge frequencies of hippocampal CA1 pain-related neurons that likely related to its mechanism of pain relief.
Finally, there have been various preclinical studies regarding the concept of low-frequency electrical stimulation in accelerating axon growth and nerve regeneration, which may also serve as a mechanism of pain relief secondary to PNS (24–26). Overall, these studies highlight that both spinal and supraspinal mechanisms are involved in PNS. Further studies are needed to fully understand the contribution of these mechanisms, as well as new mechanisms to the pain relief from PNS.
PNS for Headaches
Migraine Headaches
A randomized multicenter trial by Dodick et al (27) tested the efficacy of occipital nerve stimulation in treating migraine headaches. They found that occipital nerve stimulation significantly improved headache-related pain and disability (27). Mekhail et al (28) performed a single-center trial that included 20 patients who were implanted with an occipital nerve stimulation system randomized to active or control group for 12 weeks and then received open-label treatment for an additional 40 weeks. They reported efficacy of occipital nerve stimulation in treating headache (28). Saper et al (29) conducted a multicenter, randomized, blinded, controlled feasibility study that compared the efficacy of occipital nerve stimulation versus medication management for treating chronic migraine and showed better efficacy of occipital nerve stimulation as compared with medication management (29). Serra and Marchioretto (30) enrolled patients who responded to an occipital nerve stimulation trial for treating chronic migraine. The patients were then randomized to stimulation-on and stimulation-off groups, and then groups crossed over after 1 month. The study showed the modality to be safe and effective (30). These results are consistent with a randomized controlled multicenter study in which a neurostimulation device was implanted close to the occipital nerves and randomized 2:1 to active (n = 105) or sham (n = 52) stimulation. The study showed that PNS of the occipital nerves reduced pain and disability (31).
Cluster Headaches
A randomized, sham-controlled study of 32 patients was performed to evaluate the use of sphenopalatine ganglion (SPG) stimulation for the acute treatment of chronic cluster headache. The study showed that SPG stimulation is both safe and effective for the acute treatment of cluster headache (32). A multicenter, sham-controlled study testing an implantable on-demand SPG stimulator showed that SPG stimulation is an effective and safe modality in treating chronic cluster headache (33).
In summary, there is Level I evidence to support the use of PNS to treat migraine headaches, and Level II evidence for cluster headaches. PNS should be considered as an option for migraine and cluster headache when other noninvasive measures fail.
PNS for Limb Pain
Peripheral Neuropathic Pain
In one of the first published trials, Campbell and Long (34) reported that 6 out of 8 patients with upper extremity peripheral neuropathy due to traumatic injuries had good to excellent response to PNS, whereas only 3 out of 15 patients with sciatic injury (n = 15 out of 23) obtained partial pain relief from PNS at 9- to 17-month follow-up. Subsequent case series showed similar results. Law et al (35) reported 62% of patients with posttraumatic neuropathy only use the stimulator for pain relief during 9 to 88 months follow-up. In another 19 patients with posttraumatic neuropathy, Waisbrod et al (36) reported that 58% of patients (n = 19) obtained complete pain relief, and another 21% patients obtained sufficient pain relief, enough to discontinue pain medications. A retrospective study (n = 46) with a follow-up period of 3 to 16 years showed that 78% of patients reported good (defined as ≥ 50% pain relief) and 22% reported poor (defined as < 50% pain relief) results (37). In an open trial, Stevanato et al (38) implanted quadripolar PNS electrode in 7 patients with posttraumatic neuropathy of the brachial plexus. All patients reported an average Numeric Rating Scale (NRS-11) pain reduction of 76% and 71% at 6 and 12 months, respectively. There were no significant adverse effects. These PNS were implanted by neurosurgeons employing open procedures.
Huntoon and Burgher (39) and Narouze et al (40) were among the first to report ultrasound-assisted through-the-needle PNS placement. Huntoon and Burgher (39) implanted 7 peripheral nerve stimulators in 6 patients. The probable etiology of the peripheral neuropathy was trauma. Isolated single major peripheral nerve neuropathy was confirmed by more than 80% pain relief with ultrasound-guided block of the target nerve. The standard 8-contact percutaneous electrode was deployed through a standard 14-gauge epidural needle. The electrode was manipulated to be perpendicular to the nerve with the middle contacts in closest proximity to the target nerve. Six out of the 7 PNS systems had more than 50% pain reduction at 8 to 14 months follow-up, and 3 permanent systems produced more than 80% pain relief. There was one infection in one patient. In the Narouze et al case report (40), in addition to an electrode placed longitudinally to the femoral nerve, a horizontally across the femoral nerve electrode was placed to cover the below-knee pain. The patient continued to be pain free at 20 month follow-up. A recent study found that an ultrasound-guided PNS trial is feasible in screening for permanent PNS implantation (41). An interesting finding of this study is that patients with longer duration of pain prior to the trial tend to have poor response to the PNS treatment.
In a prospective, randomized, double-blind, crossover study, Deer et al (42) investigated the efficacy and safety of a wireless PNS device (StimRouter) designed by Bioness, Inc. Ninety-four patients with severe intractable chronic pain (> 3 months) of peripheral nerve origin associated with posttraumatic/postsurgical neuralgia were implanted and then randomized to the treatment (received therapeutic stimulation and stable dosing of pain medications, 45 patients) or the control group (received no therapeutic stimulation and a stable dosing of pain medication, 49 patients). At 3 months following the implant, the “responder” (defined as at least a 30% decrease in pain without an increase in pain medicine use) rate in the treatment group (38%) was statistically significantly higher than that in the control group (10%). The treatment group achieved a mean pain reduction of 27.2% from baseline compared with a 2.3% reduction in the control group (P < 0.0001). Crossover to the treatment group was offered to the control group at 90 days follow-up. Thirty out of the 45 patients in the control group chose to crossover to the treatment group. During the partial crossover period, 30% (9/30) of patients were classified as responders. The treatment group also had significantly better improvement than the control group in secondary outcomes (e.g., worst pain score, Brief Pain Inventory [BPI] score, quality of life (QOL), global impression of degree of change, and patient satisfaction, etc.) measured at 3 months. No significant difference between patients with limb pain and patients with trunk pain was reported. No serious adverse events were reported throughout the trial and with follow-up to 1 year. All device-related adverse events were minor and self-limiting. The authors concluded that the novel PNS device is a safe and effective treatment strategy to address neuropathic pain of peripheral nerve origin.
To summarize, there is only one high-quality study (Level II evidence) that demonstrated efficacy of PNS in patients with peripheral neuropathic pain secondary to trauma or surgery; others are case reports (Level IV evidence). PNS can be considered in this patient population when the pain is refractory to other more conservative treatments.
Complex Regional Pain Syndrome
Hassenbusch et al (43) reported a prospective, consecutive series using PNS to manage pain in patients with complex regional pain syndrome (CRPS) symptoms limited to one major peripheral nerve. Thirty-two patients were tested, and 30 of them obtained 50% or more pain reduction and thus received permanent surgical implant of a plate-type electrode. Patients were followed for 2 to 4 years and interviewed by a third-party. Nineteen (63%) patients experienced good or fair relief with Visual Analog Scale (VAS) pain score reduced from 8.3 ± 0.3 to 3.5 ± 0.4. Six (20%) patients were able to return to work. Cooney (44) also reported that PNS improved pain, sleep, and psychological sense of well-being in patients with CRPS in the upper extremity. Both studies pointed out that effectiveness of PNS is better in those patients with symptoms mainly associated with one major peripheral nerve. Recent case reports also showed the usefulness of PNS in patients with CRPS (45,46).
The majority of studies of PNS on CRPS are case series or reports. The quality of these studies is limited, providing Level IV evidence for PNS in the management of CRPS. Further studies of high quality are needed.
Postamputation Pain
After amputation, up to 90% of patients may develop chronic postamputation pain (PAP), including residual limb pain and phantom limb pain (47). Pharmacologic therapies are often inadequate to treat PAP. PNS has been used to effectively treat PAP (48–51). Rauck et al (49) was among the first to report using PNS in 16 patients with PAP for a range of 0.2 to 33 years since amputation. The percutaneous leads were placed at 0.5 to 3.0 cm away from the target nerves under ultrasound guidance. Fourteen patients responded to the stimulation on the initial in-clinic testing. Nine of them finished the 2 week home trial and 4 weeks follow-up after the end of the trial. Clinically significant relief was reported in mean daily worst pain reported (n = 9), average residual (n = 7) and phantom (n = 7) limb pain, residual (n = 6) and phantom (n = 7) limb pain interference, and Pain Disability Index (n = 9). In a recent multicenter, double-blinded, randomized, placebo-controlled trial, Gilmore et al (50) reported 28 patients with chronic PAP who underwent ultrasound-guided percutaneous PNS or placebo (sham) stimulation for 4 weeks. The placebo group then crossed over and all patients received PNS for 4 additional weeks. More patients in the PNS group (58% vs. 14% as compared with placebo group during week 1–4; 67% vs. 14% at week 8) had greater than 50% pain reduction. Functional improvement also occurred more in PNS than placebo group (80% vs. 15%). Four of 5 PNS patients reported 50% or greater pain reduction at 12-month follow-up. The authors concluded that percutaneous PNS may provide long-lasting pain relief in patients with chronic PAP. Lead fracture was not reported during treatment but occurred in 15% of patients on lead removal.
These traditional PNS modalities cause paresthesia during stimulation. Kilgore and Bhadra (52) introduced a high-frequency (HF-10 kHz) alternating current nerve block, which was then used to deliver paresthesia-free stimulation via a surgically implanted peripheral nerve cuff electrode (53). HF-10 nerve stimulation causes a complete depolarizing nerve block that is similar to that provided by local anesthetics. If a patient is responsive to the trial local anesthetic block, implantation is done by exposing and wrapping the target nerve with a cuff lead via open surgery. The cuff is secured around the nerve with nonabsorbable sutures (not too tightly) after an impedance check. Soin et al (54) reported HF-10 PNS for PAP in a pilot study. Ten patients with chronic and severe lower extremity residual limb pain or phantom limb pain were enrolled after they obtained significant pain reduction with local nerve block. Seven patients were implanted with a cuff electrode wrapped around the sciatic or tibial nerve. HF-10 PNS resulted in an average pain reduction of 75% at the 3 month primary end point, and the treatment efficacy sustained through the follow-up period of up to 12 months. Pain medication use and interference of pain on functions were also significantly reduced. No significant adverse effects were observed. A multicenter pivotal study is ongoing with planned 180 patients and estimated primary completion date is September 2021 (https://clinicaltrials.gov/ct2/show/NCT02221934).
In summary, a few high-quality studies of PNS provide Level II evidence for PAP, although the sample size of these studies was small. Larger sample size studies are warranted to confirm the efficacy of PNS in PAP. Nevertheless, because PAP is usually difficult to treat, PNS should be considered in the treatment algorithm.
Shoulder Pain
Shoulder pain is an important medical and socioeconomic problem in Western society with a 1 year prevalence of 4.7% to 46.7% (55). PNS has been applied to treat shoulder pain that has failed other treatments. Yu et al (56) first tried PNS on a 58-year-old stroke survivor with chronic poststroke shoulder pain. A microstimulator was placed near the axillary nerve within the quadrilateral space and delivered up to 6 hours of stimulation daily over 12 weeks. The shoulder pain was decreased from 8/10 before treatment to 4/10 after treatment, and decreased further to 3/10 at 3 month follow-up. Passive range of motion and motor function also were improved after PNS stimulation. However, the changes in sensation, shoulder subluxation, activities, and QOL were not observed (56). Since then, several case reports demonstrated the effects of PNS on hemiplegic shoulder pain (HSP), chronic subacromial impingement syndrome, and adhesive shoulder capsulitis (57–59). The only RCT of PNS on chronic HSP (60) reports that 25 patients with chronic HSP were randomized to receive a 3 week treatment of single-lead PNS (n = 13) or usual care (UC, n = 12). The primary outcome measured was BPI-SF3 (BPI-Short Form item 3), which was measured at base line and follow-ups. There was a significantly greater pain reduction in the PNS group compared with the UC group after treatment. The mean severity rating at baseline was 7.5 (± 0.7) and 7.6 (± 0.7) for the PNS and UC groups, respectively, which dropped to a 3.2 (± 0.7) and 6.1 (± 0.8), respectively, at 10 weeks, and remained a 3.0 (± 0.7) and 6.1 (± 0.8), respectively, at 16 weeks. Pairwise comparisons revealed significant differences between groups of 2.9 (95% confidence interval [CI], 0.8–5.0) at 10 weeks, and of 3.1 (95% CI, 1.0–5.2) at 16 weeks. Both PNS and UC were associated with significant improvements in pain interference and physical health-related QOL. A retained electrode fragment owing to fracturing of the tip of the electrode during explant is the major adverse event found in this study. This RCT provides evidence that a single-lead, 3 week PNS is an efficacious and safe treatment for the reduction of chronic HSP.
In summary, the effects of PNS on shoulder pain management was observed with Level II evidence. Most of the related studies were from certain groups. Additional clinical trials conducted from different centers are necessary to explore its efficacy. Future studies should determine the indication, mechanism of action, optimal stimulation delivery, and long-term effectiveness.
PNS for Torso Pain
Thoracic Postherpetic Neuralgia
There are one million new cases of acute herpetic zoster every year in the United States (61), and approximately 10% to 15% of patients develop postherpetic neuralgia (PHN) with a persistent or intermittent pain, most commonly in thoracic, cervical, or ophthalmic dermatomes (62). The treatment options consist of pharmaceutical management, TENS, behavioral therapy, nerve blocks, and neuromodulation. SCS and PNS always are considered as last resort treatments for patients who have failed other options. PNS is an option in cases not suitable for SCS, and it has been reported to produce sustained paresthesia in difficult-to-treat regions of the body. Yakovlev and Peterson (63) first applied PNS for thoracic PHN treatment in 2007. Several other case reports also endorsed the good pain relief from PNS for PHN patients (64). Rossi et al (65) reported a multicenter prospective nonrandomized study in 2016 to treat neuropathic pain with a mini-invasive approach on 76 patients. Among them, 21 patients had PHN. NRS-11 and Neuropathic Pain Scale (NPS) decreased significantly after PNS, and the reduction remained constant over time to 6 months follow-up (65).
There remains a scarcity of published evidence and a lack of high-quality study to recommend for clinical application of PNS on thoracic postherpetic neuralgia. Although technically feasible and theoretically attractive, additional clinical trials are necessary to demonstrate its efficacy.
Inguinal/Genital/Pelvic Pain
Pelvic and urogenital pain syndromes include chronic pelvic pain/chronic prostatitis, bladder-pain syndrome, groin/inguinal pain, and genital pain, affecting both men and women (66). Organs occupying the pelvis include the urinary bladder and the uterus in their empty states, the rectum, vagina, and distal parts of the male reproductive system. Both the visceral and somatic nerves innervate structures within the pelvis and are involved in pain regulation. All of these characteristics make pelvic and urogenital pain management challenging. Despite a range of conservative and pharmacologic options, there remains a group of patients who are resistant to pharmacologic interventions. This patient group is usually considered for neuromodulation, particularly if they have shown short-term responsiveness to nerve blocks.
Sacral neuromodulation (SNM) uses electrical stimulation to modulate the pathophysiological response of the bladder and other pelvic viscera. The InterStim device (Medtronic, Minneapolis, MN) was first approved by the US Food and Drug Administration (FDA) to treat urgency urinary incontinence. Later it was approved for urinary urgency frequency syndrome and non obstructive urinary retention, and finally for fecal incontinence. However, SNM has not been approved for treatment of chronic pelvic pain by the FDA. In recent years, several studies have assessed the effectiveness of SNM in the treatment of various pelvic pain. Peters (67) treated 22 patients with refractory interstitial cystitis (IC) with SNM. He found the SNM not only improved urinary frequency/urgency and incontinence, but also improved pelvic pain in 65% of patients and vaginal pain in 54% patients (67). Similarly, in a study by Comiter (68), 17 patients with IC underwent permanent sacral nerve stimulator implantation. At an average of 14 months follow-up, average pain scores decreased from 5.8 to 1.6 points (P < 0.01) (68). Peters (67) later did an RCT to compare SNM with pudendal nerve stimulation (PdNS) for IC (69). Twenty-two patients had a tined lead placed at S3 and a second electrode implanted at the pudendal nerve. Each lead was tested for 7 days. At 6 months after implantation, the 10 cm VAS scores for pain decreased by 49% for SNM (7.9–4.0) and 29% for PdNS (4.5–3.2). This is the first blinded study to compare SNM versus PdNS, and the overall reduction in symptoms was 59% for PdN-Sand 44% for SNM (P < 0.05). Gajewski and Al-Zahrani (70) performed SNM in 44 patients with bladder pain syndrome and observed good long-term success in 72% of the patients at a median 61.5 months (standard deviation ± 27.7) follow-up (70). In the Martellucci et al (71) study, 27 patients with nonorganic or noninfective pelvic pain without recognizable cause, in which symptoms lasted for at least 6 months, were enrolled and underwent SNM. Among these patients, 18 patients (66.5%) reported a history of previous pelvic surgery. The mean VAS score was decreased from 8.1 (range, 6–8) preoperatively to 2.1 ± 1.2 at 6 month follow-up (P < 0.0001), 2.1 ± 1.1 at 12 months (16 patients), 2.0 ± 1.2 at 24 months (13 patients), 2.3 ± 1.4 at 36 months (9 patients), 2.1 ± 1.5 at 48 months (5 patients), and 1.9 ± 1.3 at 60 months (3 patients) (71). Aboseif et al (72) performed permanent SNM on 64 patients with refractory pelvic floor dysfunction. Fifty-one patients (80%) had 50% or greater improvement in their presenting symptoms and QOL after the procedure, with a mean follow-up of 24 months. Patients with chronic pelvic pain showed a decrease in the severity of pain from a score of 5.8 to 3.7. However, this was not statistically significant.
The management of functional anorectal pain remains a challenge, and SNM treatment was addressed in several studies. In a prospective study by Rongqing et al (73), a total of 120 patients received temporary SNM at the S3 nerve root (2 Hz, 1.50 mA, 0.10 ms). Of these, 75 patients were pain free, 41 improved, and 4 had an ineffective outcome. The total effectiveness rate was 96.7%, and the median VAS score reduced from 8 to 3 one year after treatment. Patients also had significant improvement on anal maximum contraction pressure and anal rest pressure (73). Similarly, Falletto et al (74) reported that in 12 patients with idiopathic anal pain, VAS score significantly improved from 8.2 ± 1.7 to 2.2 ± 1.3 (P < 0.001) and 36 Item Short Form Health Survey (SF-36) physical component scores increased from 26.27 ± 5.65 to 38.95 ± 9.08 (P < 0.02) after sacral nerve stimulation during a mean follow-up of 15 months. Govaert et al (75) described a single-center experience with permanent SNM for the treatment of chronic functional anorectal pain in 9 patients. Median pain score decreased from 8.0 (6.0–9.0) to 1.0 (0–2.0) after the treatment, and all patients experienced lasting improvement during the follow-up until 24 months (75). In this report, the complications were discussed. Pain at the implantation site appears to be one of the main complications, which occurred in up to 39% of all patients with implanted SNM. Infection rates for SNM were approximately 5%. Most of the infections were minor and responded to antibiotics treatment.
Several studies have demonstrated effectiveness of SNM on chronic pelvic pain. However, there are still approximately 10% to 25% of patients who fail to respond to SNM (76). In 1989, Schmidt (77) described for the first time a puncture technique to target the pudendal nerve, and pudendal nerve modulation became an alternative treatment. Pudendal nerve modulation was performed uni- and bilaterally in a pilot series of 20 patients with chronic pelvic pain. After 4 weeks of treatment, mean pelvic pain intensity decreased significantly from 85 to 40 mm (P = 0.018) (78). Percutaneously placed tibial nerve stimulation (PTNS) was used for the treatment of chronic pelvic pain. In a prospective multicenter trial, PTNS was evaluated in 33 patients with chronic pelvic pain (79). The electrode was placed between the posterior margin of the tibia and the soleus muscle tendon. After 12 weeks’ of treatment, VAS score was decreased more than 50% in 21% of patients and more than 25% in 18% of patients. SF-36 and total pain rate intensity (McGill Pain Questionnaire (SF-MPQ)) were significantly improved in all patients at 12 weeks follow-up. Istek et al (80) performed an RCT to investigate the long-term effects of PTNS on chronic pelvic pain. Thirty-three women with chronic pelvic pain were randomized into PTNS or control groups. PTNS group received a weekly PTNS in 30 minute sessions for 12 weeks, whereas the control group received no stimulation. The pelvic pain intensity-Visual Analog Scale (PPI-VAS) was significantly improved at 6 months, whereas no change was observed in the control group. There was significant improvement in all domains of short-form McGill Pain Questionnaire (SF-MPQ) and SF-36 in the PTNS group with continuing effects at 6 months, whereas no significant change was observed in the control group. One limitation of this study is the lack of homogeneity between the 2 groups despite randomization regarding the age of the patients and the baseline PPI-VAS results. The other limitation of this study is that the control group did not receive any placebo or sham stimulation (80).
In summary, there are still limited high-quality data regarding PNS use in managing inguinal/genital/pelvic pain. The complexity of pelvic and urogenital pain makes a powered and well-designed RCT trial challenge. With a favorable safety profile and the advancement of PNS systems, additional clinical trials are necessary to explore its efficacy, indications, and appropriate nerve targets in pelvic and urogenital pain management.
Lower Back Pain
Chronic low back pain, including failed back surgery syndrome (FBSS), can be debilitating and difficult to treat. Patients refractory to medications and/or other conservative treatments or procedures may consider neuromodulation. SCS is the most common form of neuromodulation used in managing chronic low back pain (1). However, practitioners also report difficulties with achieving adequate pain control over the long term for all patients, especially those experiencing chronic low back pain as a result of surgery (81). Verrills et al (82) report a case series of 14 patients diagnosed as chronic lower back pain or FBSS. Those patients failed conservative treatments and a variety of procedures, including sacroiliac joint injections, medial branch blocks, zygapophysial joint injections, hip examinations under local anesthetic, radiofrequency neurotomies, discographies, and nucleoplasties. PNS significantly decreased the pain levels with an average reduction of 3.77 VAS points. Eleven patients (85%) reported successful outcomes and an average pain reduction of 4.18 points. Pain relief was highly correlated with reduced analgesia and patient satisfaction. This study suggests that PNS may be effective in reducing pain and should be considered as a treatment option for patients with chronic low back pain and FBSS that have failed to respond to alternative treatments. Ultrasound guidance is a useful technique to assist with electrode placement at the most appropriate depth beneath the skin during the PNS placement (83).
Eldabe et al (84) performed the first RCT comparing PNS with optimized medical management (OMM), which also is the largest RCT of PNS for the treatment of the low back pain due to FBSS. A total of 116 patients were recruited from 21 centers and randomized (1:1) to PNS+OMM or OMM alone groups. The patients in the PNS group were implanted with a neurostimulator, and up to 2 subcutaneous percutaneous cylindrical leads were placed in the area of pain. In total, 116 patients were randomized: 56 in the PNS+OMM group and 60 in the OMM alone group. The responder rate (>50% reduction in back pain intensity) at 9 months in the PNS+OMM group was 33.9% (n = 19; 95% CI, [21.5–46.3%]) compared with 1.7% (n = 1; 95% CI, [0.0–4.9%]) in the OMM alone group. The difference between arms in the intention-to-treat analysis is statistically significant (Fisher exact test; P < 0.0001). PNS also significantly reduced mean back pain scores, whereas in the OMM group, scores remained stable over the time during follow-up. Their results indicate that the addition of PNS to OMM is more effective than OMM alone in relieving low back pain at up to 9 months. These findings support the results of a number of earlier uncontrolled case series (84).
Recently, Cohen et al (85) treated 9 low back pain patients with PNS using a unique, coiled, fine wire lead. Percutaneous PNS improved patients’ function, as reflected by clinically and statistically significant reductions in pain, disability, and pain interference. There was a reduction of analgesic medication usage by all patients taking analgesic medications at baseline. More than 83% of patients experienced at least a 50% reduction in opioid and non-opioid analgesic medication usage, which continued long-term up to 7 months.
PNS has also been applied to therapy-refractory sacroiliac joint pain (SIJ) pain, and the long-term effects were analyzed in a case series of 16 patients (86). Sixteen consecutive patients were treated with PNS and followed for 4 years in 3 patients, 3 years in 6 patients, 2 years in 1 patient, 12 months in 4 patients, and 6 months in 1 patient. Patients reported a significant pain reduction from 8.8 to 1.6 (VAS) at 1 year (P < 0.001), and 13 of 14 patients (92.9%) rated the therapy as effective. At 2-year follow-up, average pain score was 1.9 (P < 0.001), and 9 of 10 patients (90.0%) considered the treatment a success. At 3 year follow-up, 8 of 9 patients (88.9%) were satisfied with the treatment results, reporting an average VAS score of 2.0 (P < 0.005). At 4 years, 2 of 3 patients were satisfied with the treatment results.
In summary, most of the current studies observed that PNS provided clinically significant pain reduction in low back pain patients with minimal adverse events. However, the evidence is limited to Level II or III. There is a clear need for further, better quality research into its efficacy. Future trials also should be designed with the type of low back pain clearly reported, and the technique of PNS placement well described.
PNFS
PNFS builds on PNS to include areas that have an expanse of coverage for pain beyond a single nerve distribution. Although PNS is focal and discrete, the strength of PNFS is to cover a wide ranging area. PNFS has been utilized and reported for cranial pain, axial cervicothoracic, thoracic, and lumbar indications. Studies are presented in the literature highlighting sole use of PNFS and combination therapy. A confounding factor in combination therapies includes the concomitant effects of PNFS and the additional modality (SCS, PNS, or TENS). The following studies (87–97) highlight that PNFS does show promise and value to stakeholders; however, rigorous studies must be devised, executed and results analyzed to glean better insights for clinical practice.
Craniofacial Pain
A potential indication for PNFS is chronic headache pain (87), and one study involved evaluating this technology in nonmalignant, nontrigeminal nerve cases. In this study, 83 consecutive patients underwent PNFS targeting the nerve regions including occipital and supraorbital and infraorbital nerves, which best corresponded with their area of head pain. Sixty patients reported a successful trial and underwent a subsequent implant of the PNFS system. An average pain reduction of 4.8 points was observed (preimplant 7.4 ± 1.6; follow-up 2.6 ± 2.1 [P ≤ 0.001]). Of the 60 patients, 41 reported greater than 50% pain relief. Medication use was reduced in 83% of patients who were previously taking analgesics or prophylactic medications. Similarly, reductions in degree of disability and depression also were observed. Ten surgical revisions were required due to hardware failure and lead migration without long-term complications. A significant limitation of this study was that patients with headache were not stratified based on etiology of headache; that is, whether the patient had occipital neuralgia, migraine headache, or any other source of pain, and separating diagnoses and analyzing efficacy was not performed.
Trigeminal PNFS has been evaluated in a retrospective study (88). Patients were followed for 15 months after implant with 73% of patients demonstrating improvement. As is common in neuromodulation in the head and neck, this study was complicated by a high revision rate.
PNFS for craniofacial pain is an important tool in the treatment options interventionalists have for headache and facial pain. Discrete indications should be evaluated for PNFS analgesic benefit. The benefit of the technology is that a wider field of coverage is achieved with lead placement. Studies thus far demonstrate Level III evidence and a high revision rate with implantation; clearly hardware and technical approaches must be improved prior to standard of care status.
Back Pain
A prospective observational study from Europe (89) in which PFNS was used for patients with chronic low back pain highlights this combination therapy. Although patients had a benefit in medication decrease, QOL, and Oswestry Disability Index (ODI) for a 6-month follow-up, a significant limitation of this study is the commingling of therapies. Additionally, there was no discrete control group along with the open-label design of this study, which imparts bias. An important result, however, is that the authors pointed out that the greater area of coverage afforded with PFNS may be a factor in determining efficacy of treatment. The greater the area of coverage, the more effective the treatment.
An additional study evaluated patients with a single therapy and had good results on pain reduction and reduction of anxiety and depression, highlighting a positive balance on the affective toll of chronic pain (90). A recent retrospective study evaluated the benefit and predictive value of TENS for PNFS (91). The latter was found to be superior in providing analgesia and QOL as measured via NRS-11, 5-level EQ-5D version (EQ-5D-5L), ODI, Arrhythmia-Specific questionnaire in Tachycardia and Arrhythmia (ASTA), and the Client Satisfaction Questionnaire (CSQ-8). TENS was not found to be predictive in determining subsequent benefit with PNFS in patients with chronic low back pain. However, in a systematic review (92), 7 PNFS/PNS studies were highlighted with conflicting results. In 5 studies, patients had significant improvement in chronic pain, with 2 studies showing no improvement. Furthermore, teasing out PNFS from PNS, which are combined in the analysis of this review, leads to challenges in formulating conclusions. A multicenter RCT of 52 patients in the Dutch literature (93) also highlights hybrid therapy of SCS and PNFS. The modalities were evaluated with the addition of PNFS found to add cost efficacy and improved Quality of Life Years (QALY).
An innovative, multisite RCT evaluated PNFS in patients with localized chronic intractable back pain (94). The unique aspect was a 2-phase approach with randomization of programming during the initial trial phase. During phase I, patients rotated through 4 stimulation groups (minimal, subthreshold, low frequency, and standard stimulation). If a 50% reduction in pain was achieved during any of the 3 active stimulation groups (responder), the patient proceeded to phase II, which began with implant of the permanent system and lasted 52 weeks. Of the enrolled patients, 32 were implanted with a trial system and 30 completed phase I. During phase I, there were significant differences in mean VAS scores between minimal stimulation and subthreshold stimulation (P = 0.003), low frequency stimulation (P < 0.001), and standard stimulation (P < 0.001). Twenty-four patients were classified as responders to the therapy, and 23 patients received permanent system placement. Significant differences in VAS scores were observed between baseline and all follow-up visits during phase II (P < 0.001). The results support safety and effectiveness of PNFS in the management of chronic, localized back pain.
An additional study evaluating chronic back pain is a prospective case–control study (95). A total of 26 patients were evaluated with 50% of patients going from trial to implant. Patients were followed for 24 months and were noted to have a decrease in analgesic use, improved VAS, ODI, and QOL. Concerns with this study include a relatively small sample size, with 50% of patients not responding and proceeding to implant.
Thoracic pain from various causes has been evaluated with PNFS (96). A prospective study, albeit with only 20 patients, evaluated PNFS as a therapeutic option for patients with chronic pain. Contact leads (8 in number) were utilized for the PNFS for the greater coverage area required. The trial to permanent rate was 65% to 70% in this study. Implants from Medtronic, St. Jude, and Boston Scientific were utilized in the study. As a result of a 12 month follow-up, NRS-11 decreased from 7.75 preoperatively to 2.25 postoperatively.
PNFS for back pain is an indication that may gain significant traction as a viable option for a difficult problem to treat. Studies with a larger number of patients are required for demonstrating definitive validity of therapy. Additionally, studies must differentiate sources of back pain to stratify etiology and response to treatment. These results will lead to further support for highlighting PNFS for back pain as a standard of care.
Other Neuropathic Pain Conditions
Neuropathic pain of various etiologies (PHN, FBSS, postthoracotomy pain, and atypical facial or trigeminal pain) has been studied in a prospective case series in the evaluation of 22 patients (97). Although the number of patients was low, the reduction in the VAS scores of these patients decreased by 5.50 points, decreasing from 8.86 preoperatively to 3.36 in postoperative evaluation. These patients also reduced their analgesic drug use after PNFS. No early or long-term complications were observed. Thus PNFS can be considered an effective and safe option to treat carefully selected, drug-resistant and chronic neuropathic pain patients.
PNS for Postoperative Pain
The first report of management. Using leads designed for SCS (Medtronic 1 × 8 compact lead), the percutaneous peripheral nerve stimulators (sciatic pPNS) was first reported in a case series of 2 soldiers suffering from combat-related lower extremity neuropathic pain of fewer than 5 months by Kent et al (98). Significant improvement in their pain along with decreased opioid usage and improved functionality were observed. Prompted by the efficacy of pPNS in chronic pain treatment and attempts to spare both opioids and other medications, pPNS is recently evolving into acute pain management. With a specially designed lead for pPNS (MicroLead; SPR Therapeutics, Cleveland, OH), femoral and/or sciatic pPNS was placed in 5 patients by Ilfeld et al (99) postsurgically (ranging from 6–97 days) to provide analgesia following total knee arthroplasty (TKA). Following 1 hour of a single episode of electrical stimulation, pain decreased an average of 63% at rest, with 4 of 5 patients having relief of greater than 50%. During passive and active knee flexion, pain decreased an average of 14% and 50%, respectively. The same group, using the same protocol and pPNS system, studied the same patient population of 5 patients within 60 days post-TKA, and with consistently favorable outcomes (100). This second series emphasized the clinical feasibility, effectiveness, and possibly lower risk of infection related to pPNS, and noted that the pain relief was comparable in degree to the adductor canal block.
To further explore the applicability of pPNS in controlling acute postoperative pain following TKA, Ilfeld et al (101) conducted a prospective study of 7 patients, in which both sciatic and femoral pPNS were placed preoperatively (within 7 days before surgery). Immediately prior to surgery, as the standard of care, all patients received a preoperative single-injection adductor canal block with 0.5% ropivacaine of 20 mL. Within 20 hours after TKA surgery, the continuous ambulatory pPNS was activated for up to 6 weeks as a part of multimodal analgesia. No falls, motor block, or lead infections were reported. This study suggests that the preoperative placement of pPNS for TKA is technically feasible and safe. Analgesia might be provided while gross sensory and motor function were maintained during stimulation of pPNS.
Thereafter the same group published 3 more proofs-of-concept, randomized, controlled, partial-crossover studies of pPNS in ambulatory surgery (102–104). A similar protocol design was used to study 3 different ambulatory surgical populations. The pPNS was placed in proximity to the targeted nerve within 1 week before surgery. A preoperative continuous peripheral nerve catheter was additionally placed (but not initiated) as the part of standard of care. In the immediate postoperative period, patients received 5 minutes of either stimulation or sham in a randomized, double-masked fashion followed by a 5 minute crossover period, and then continuous stimulation on an outpatient basis until lead removal on postoperative days 14 to 28. To provide postdischarge analgesia, patients were instructed to first increase the stimulation level on their pulse generators, then take oral opioids, and initiate the single shot or the perineural infusion (which was removed within 3 days of surgery) as the rescue analgesia if others fail.
The first study investigated the sciatic pPNS in 7 patients undergoing primary, unilateral hallux valgus osteotomy (bunionectomy) (103). This study demonstrated that surgical pain reduction was associated well with true stimulation but not sham treatment. Additionally, pain scores gradually decreased to an average of 52% of baseline during the subsequent 30 minutes of stimulation. The rescue popliteal sciatic nerve catheter was initiated in 3 patients (43%) during postoperative days 0 to 3. Overall, resting and dynamic pain scores (NRS-11) averaged less than 1, and opioid use averaged less than 1 tablet daily during active stimulation. Furthermore, a “carryover” effect following pPNS was observed so that patients continued to receive a variable duration and degree of analgesia following electrical current discontinuation. However, of the leads in the popliteal fossa region, one lead dislodged, 2 fractured during use, and one fractured during intentional withdrawal.
The second study initially researched the suprascapular pPNS in 16 patients undergoing rotator cuff repairs. Unfortunately, the first 2 patients with suprascapular pPNS did not experience any appreciable postoperative analgesia. Subsequently, the interscalene brachial plexus pPNS at the level of root and/or trunk was studied for the rest of the 14 patients, among them, 3 withdrew before data collection (102). During the first 40 minutes of active stimulation in the postsurgical recovery phase, no improvement in their pain scores was appreciated. Therefore a rescue interscalene brachial plexus single shot was initiated in 7 of 11 patients before the discharge. However, during postoperative days 1 to 14, the median pain score on NRS-11 was 1 or less, and opioid consumption averaged less than oxycodone 5 mg a day. Significant rates of lead dislodgement (2/13) and fractures (4/13) were reported.
The third study looked into femoral pPNS in 10 patients undergoing anterior cruciate ligament reconstruction (104). There was no appreciable pain benefit in those randomized to active stimulation or those in the sham group during the initial 5 minute treatment period. Therefore a rescue continuous adductor canal nerve block was activated in a majority of patients (8/10) in addition to stimulation during postoperative days 1 to 3. Afterward, the median resting and dynamic pain scores remained or were less than 1.5 on NRS-11, respectively. There were 3 early removals of lead and one broken lead.
In summary, there are still little data regarding pPNS use in managing acute surgical pain. Although preoperative placement of sciatic, femoral, or interscalene pPNS are technically feasible and theoretically attractive, the clinical analgesia value of pPNS following ambulatory/orthopedic surgeries is yet to establish. With a favorable safety profile, minimal motor impairment, and the advancement of PNS systems, additional clinical trials are necessary to explore its efficacy, indications, and appropriate peripheral nerve targets in acute pain management.
Socioeconomic Benefit of PNS
Novak and Mackinnon (105) contacted 17 patients who had peripheral nerve stimulators implanted for at least 5 months by the same surgeon via a telephone survey. Prior to implantation, 12 of these 17 patients were not working. Two patients were still employed prior to implantation. Three patients were already retired at the time of implantation. Following implantation, 50% of the patients (n = 6) who were unable to work prior to implantation returned to work. In the study conducted by Strege et al (106), 24 patients were followed for 12 to 120 months (mean 32 months) after peripheral nerve stimulator implantation. Follow-up was completed either via telephone interviews or direct questioning during clinic visits. Eight of the patients reported returning to useful employment following implantation, but not necessarily the same job they had prior to implantation. Specifically, only 4 out of the 8 patients held the same job they had prior to implantation. The other 16 patients reported that they experienced meaningful pain reduction and were able to increase their activities. However, these patients did not return to work following implantation. Out of the 41 patients followed in these 2 studies, 14 patients who were unemployed prior to implantation were able to return to work; 2 patients continued to work before and after implantation; 22 patients were unable to return to work; and 3 patients were already retired and out of the workforce prior to implantation. Overall, the percentage of patients who were able to work and therefore contribute to the socioeconomic benefit of PNS was approximately 42%. Other aspects of socioeconomic impact, such as health care expenditure, have not been investigated.
Complications of PNS
Complications can be biological or hardware-related. Biological complications include infections, hematoma or seroma, pain, dural puncture, and nerve damage (107). Hardware-related complications include lead-related complications (such as lead migration, fracture, and disconnection) and implantable pulse generator (IPG)-related complications, including battery life, battery position, and recharging difficulties.
Traditionally PNS for the treatment of chronic pain has been used mainly with devices developed for SCS applications. However, with newer devices designed specifically for PNS, the complications reported appear to be somewhat different from those with traditional SCS. Specifically, lead fracture seems to be more common with the fine PNS electrodes.
One of the reasons that the PNS approach has had a history of high complication rates may be due to the anatomy around the targeted peripheral nerves. The surrounding tissues vary significantly from the epidural spinal space for which the traditional devices were designed. However, the morbidity associated with the PNS approach is minor despite the high complication rate.
With PNS, the leads are placed directly next to the peripheral nerves. In 2 different respective analyses, lead migration rates ranged from 2% to 13% when the leads were sutured to deep fascia. Only 2.1% of patients required surgical revision (90,107,108). In a prospective study of thoracic pain treated with PNS, lead migration was reported in 2 out of 20 patients, both of which were resolved via lead repositioning (109). Lead migration also depends on the anatomic location and is particularly common in the head and neck. For example, it ranged from 10% to 24% in reports of RCTs using cylindrical leads for occipital nerve stimulation (110). As expected, higher rates (up to 100%) were seen in case series with longer follow-up (110). Lead malfunction has been reported to be as high as 5% (107). We were unable to find specific reporting on battery replacement owing to failure or depletion prior to the expected date with regard to peripheral nerve stimulators. There was one notable case of battery migration per Verrills et al (90) in 2011.
The most prevalent biological complications with spinal cord stimulator devices are pain related to device components. However, with regard to PNS studies, we were only able to find one study that resulted in pain at the IPG site, which ultimately led to removal (96). A major complication of stimulation devices is also wound infection both superficial and deep, as well as wound breakdown. The percentage of infection rates have been reported as high as 1% to 6% (90,107,108). Although some of these were able to be treated with antibiotic therapy, others required explantation ultimately (109). Skin erosion or hardware failure has been reported to be up to 7%. There are many reasons for device removal, including infection, failure of therapy, and persistent pain over hardware sites. Verrills et al (90) reported device removal due to hardware failure in 2% of cases. Although neurologic injury is a significant concern with SCS, there are no reports of complications related to neurologic damage specifically for PNS.
In summary, PNS therapies are safe and reversible therapies, which may result in a range of minor complications. Hardware-related complications are more common than biological complications. Serious adverse events such as neurologic damage are rare or unreported.
CONCLUSIONS
PNS has the potential to deliver focused stimulation to the target nerve that innervates the painful region. Clinical use of PNS in the past was compromised by invasiveness of the procedure, migration/dislodgement and/or fracture of the leads. Technological advancement in the last 2 decades has made PNS an attractive treatment modality for selective patients with specific chronic refractory neuropathic pain conditions. There are moderate to strong evidence (Level II or I, Tables 1 and 2) for the use of PNS in chronic migraine, cluster headache, lower extremity PAP, chronic pelvic pain, chronic lower back pain and pain in the lower extremity, and chronic shoulder pain. Percutaneous PNS for other indications, including acute postoperative pain, has low level of evidence based on available literature. Rigorously designed RCTs are warranted to further validate the use of percutaneous PNS for most indications in pain management.
Table 2.
PNS in pain management: characteristics of included studies.
| Pain Syndrome and Reference | Level of Evidence | Type of the Study | N (size) | Major Characteristics (design, interventions, follow-up period, outcomes, and limitations, etc.) | Adverse Effects |
|---|---|---|---|---|---|
| Chronic migraine | |||||
| Dodick et al, 2015 (27) | I | Long-term results from a randomized, multicenter, double-blinded, controlled study | 157 | Randomized, double-blind, controlled study. Patients received occipital nerve stimulators for treating migraine headache. Patients were randomized to active stimulation or no stimulations. Patients were followed up for 52 weeks. Outcomes measured were VAS, MIDAS, and PAD. Intervention group received better relief in all variables. | = total of 183 device/procedure-related AEs. |
| Saper et al, 2011 (29) | I | Prospective, multicenter, randomized, blinded, and placebo-controlled | 110 | This was a multicenter control placebo trial, patients were randomized into 3 groups adjustable stimulation, preset stimulation, and medical management. Patients were followed up for 3 months. Outcomes measured included changes in headache days, pain, duration, the Profile of Moods States (POMS), MIDAS, SF-36, functional disability, and patient satisfaction scores. | Fifty-six ADEs occurred in 36 of the 51 patients implanted. |
| Mekhail et al, 2017 (28) | II | Randomized, double-blind, controlled, single-center experience | 20 | Randomized, double-blind, controlled study, 20 patients were enrolled, patients received occipital nerve stimulator and were in randomized stimulation and no stimulation groups. Outcomes included VAS, MIDAS, and headache diary. Patients were followed up until 52 weeks postimplant. Intervention group reported better improvement in all outcomes as compared with the control group. | Eight patients reported 8 AEs: stimulation related (1), hardware related (4), and biological (3). |
| Serra et al, 2012 (30) | II | Prospective, randomized, crossover study | 34 | Prospective, randomized, crossover study. Patients received occipital nerve stimulators and randomized to stimulation on or off. Outcomes included VAS, MIDAS, SF-36, and medication intake. Intervention group showed improvement in all outcomes as compared with the control group. | A total of 5 AEs occurred: 2 infections and 3 lead migrations. |
| Cluster headache | |||||
| Schoenen et al, 2013 (33) | II | Randomized, sham-controlled trial | 32 | This was a randomized, sham-controlled trial. Patients used SPG stimulator at 15, 30, 60, and 90 minutes after implant. Outcomes measured were pain severity, headache attack frequency, HIT-6, PCS, MCS, and SF-36v2. Intervention group showed improvement in all outcomes as compared with the sham group. | Five SAEs occurred, and most patients (81%) experienced transient, mild/moderate loss of sensation within distinct maxillary nerve regions; 65% of events resolved within 3 months. |
| Peripheral neuropathic pain | |||||
| Campbell and Long 1976 (34) | IV | Case series | 33 | Eight patients had excellent and 7 had intermediate results. Excellent results were seen in patients with peripheral nerve trauma. Follow-up with an average of 12 months. | One infection, one noninfectious tissue reaction, and one considerable tenderness in the area of the receiver. |
| Law et al, 1980 (35) | IV | Case series | 22 | Thirteen patients (62%) experienced pain reduction for an average of 25 months. Six patients (29%) had no useful pain relief. | No mortality nor permanent morbidity reported. |
| Waisbrod et al, 1985 (36) | IV | Case series | 19 | In 19 patients with chronic pain from traumatic peripheral neuropathy, 11 (58%) had complete pain relief and 4 (21%) had sufficient relief to discontinue pain medications with an average follow-up of 11.5 months. | Not reported. |
| Eisenberg et al, 2004 (37) | IV | Retrospective | 46 | Thirty-six patients (78%) had good (referring as 50% or more pain relief) results with isolated painful neuropathies. The follow-up period was 3–16 years. | Two wound infection, one skin necrosis, 2 electrode migration. |
| Stevanato et al, 2014 (38) | IV | Case series | 7 | Intractable chronic isolated peripheral nerve pain from posttraumatic brachial plexus lesions. All patients had good (defined as 50%−74% pain reduction) results at 6 and 12 month follow-up. | No complications occurred in any of the patients. |
| Huntoon and Burgher, 2009 (39) | IV | Retrospective case series | 8 | Ultrasound-guided PNS for upper and lower extremity neuropathic pain. Six of 8 patients had a successful trial and underwent permanent PNS implant. Five of these had > 50% pain relief with 2–14 months follow-up. | Infection in one case that led to explantation. |
| Narouze et al, 2009 (40) | IV | Case report | 1 | Ultrasound-guided PNS for intractable femoral neuropathy. Pain free at 20 months follow-up, but weakness did not improve. | Not reported. |
| Deer et al, 2016 (42) | II | RCT | 94 | Ninety-four patients were implanted and then randomized to the treatment (45) or the control (49) groups. At 3 months, 38% treatment patients vs. 10% control patients had significant (> 30%) pain reduction. Treatment patients had a mean pain reduction of 27.2% from baseline vs. 2.3% in control patients. During crossover, treatment significantly improved pain. Treatment also improved the QOL and satisfaction. | At 1 year, no serious AEs related to the device. All device-related AEs were minor and self-limiting. |
| Reddy et al, 2017 (41) | IV | Retrospective case series | 17 | Ultrasound-guided StimuCath implant for peripheral neuropathy due to various reasons. Mean follow-up 3 years; 10 patients with good pain relief. | One infection. Four explants. |
| CRPS | |||||
| Hassenbusch et al, 1996 (43) | IV | Case series | 32 | Thirty (94%) patients underwent permanent implants. Follow-up for 2–4 years. Nineteen (63%) of 30 patients experienced long-term good or fair relief. Six (20%) of the 30 patients returned to part-time or full-time work. | Eight required revision. |
| Jeon et al, 2009 (45) | IV | Case report | 1 | CRPS II, median nerve stimulation. Mechanical pain decreased but not trophic and vasomotor symptoms. | Not reported. |
| Herschkowitz et al, 2019 (46) | IV | Case report | 1 | CRPS I. Two StimWave electrodes under intraoperative electrophysiological and ultrasound guidance along radial and median nerves. High-frequency stimulation provided good pain relief at 1 year follow-up. | Not reported. |
| PAP | |||||
| Rauck et al, 2012 (48) | IV | Case report | 1 | PNS placed >1 cm away from the femoral nerve in a patient with severe RLP for 33 years following a below-knee amputation. Pain reduced by > 60% during the 2 week trial. | No AEs. |
| Rauck et al, 2014 (49) | IV | Case series | 16 | Fourteen patients underwent a 2-week home trial of ultrasound-guided PNS placed 0.5–3 cm away from the sciatic and/or femoral nerves. Nine responders reported good reduction in both PLP and RLP. | Temporary discomfort (an anticipated adverse effect) in 4 patients without tissue damage. |
| Gilmore et al, 2019 (50) | II | Multicenter, randomized, placebo-controlled trial | 28 | Lower extremity PAP. PNS (placed 0.5–3 cm away from the sciatic and/or femoral nerves) or placebo for 4 weeks then the placebo group crossed over and all patients received PNS for an additional 4 weeks. Some 58% of patients who received PNS had ≥ 50% reductions in average PAP and pain interference. | Twenty-two study-related events in 46% (13 of 28) of patients including skin irritation or redness at the lead exit site (7), adhesive return electrode pad site (3) or bandage site (4), pain owing to implantation or stimulation (5). No lead fracture during treatment but 15% (5 of 34) lead fractured on removal. No fragment-related sequelae. |
| Cohen et al, 2019 (51) | II | Narrative review | 24 | A total of 18/24 (75%) patients reported substantial (≥ 50%) clinically significant relief of both RLP and PLP during the stimulation period. | Mild discomfort, irritation at the lead exit site, lead fracture (7.5% across 267 leads). |
| Soin et al, 2015 (53) | IV | Case series | 10 | Cuff electrode wrapped around the sciatic or tibial nerves for severe RLP and PLP. Seven patients who received the high-frequency stimulation treatment had 75% pain reduction for up to 12 months. | Dislodgement and loss of function for one electrode in one patient. |
| Thoracic postherpetic neuralgia | |||||
| Rossi et al, 2016 (65) | IV | Prospective nonrandomized study | 21 | Percutaneous electrical nerve stimulation decreased NRS-11 and NPS significantly after 60 minutes and the reduction remained constant over time at follow-up on 6 months. | Contralateral dysesthesia. |
| Inguinal/genital/pelvic pain | |||||
| Heinze et al, 2015 (78) | IV | Case series | 20 | After 4 weeks of bilateral pudendal neuromodulation, mean pelvic pain intensity decreased statistically significantly from 85–40 mm (P = 0.018). | N/A |
| Falletto et al, 2009 (74) | IV | Prospective nonrandomized study | 12 | VAS pain scores of chronic idiopathic anal pain had significantly improved from 8.2 ±1.7 to 2.2 ± 1.3 (P < 0.001). SF-36 physical component scores increased from 26.27 ± 5.65 to 38.95 ± 9.08 (P < 0.02) after sacral nerve stimulation during a mean follow-up of 15 months. | Infection of the site. |
| van Balken et al, 2003 (79) | IV | Prospective nonrandomized study | 33 | After percutaneous tibial nerve stimulation on chronic pelvic pain, VAS decreased > 50% in 21% of patients and > 25% in 18% of patients; VAS < 3 in 21% of patients; SF-36 and total pain rate intensity (McGill) are significantly improved in all patients at 12 weeks follow-up. | N/A |
| Istek et al, 2014 (80) | II | RCT | 33 | Percutaneous tibial nerve stimulation significantly improved chronic PPI-VAS at 6 months, whereas no change was observed in the control group. There was significant improvement in all domains of short-form McGill Pain Questionnaire (SF-MPQ) and SF-36 in the PTNS group with continuing effects at 6 months, whereas no significant change was observed in the control group. | Slight pain and mild ecchymosis. |
| Martellucci et al, 2012 (71) | IV | Case series | 27 | After sacral nerve modulation on chronic pelvic pain, the mean VAS was decreased from 8.1 (range, 6–8) to 2.1±1.2 at 6-month follow-up (P < 0.001), to 2 ± 1.2 at 24 months, to 2.3 ± 1.4 at 36 months, to 2.1 ± 1.5 at 48 months, and to 1.9 ± 1.3 at 60 months. | N/A |
| Aboseif et al, 2002 (72) | IV | Prospective nonrandomized study | 41 | Patients with chronic pelvic pain treated with sacral nerve stimulation showed a decrease in the severity of pain from a score of 5.8–3.7. However, this was not statistically significant. | Seroma, infection, lead migration. |
| Govaert et al, 2010 (75) | IV | Case series | 9 | Chronic anorectal pain score decreased from 8.0 (6.0–9.0) to 1.0 (0–2.0) after SNM. All patients experienced a lasting improvement during the follow-up until 24 months. Global perceived effect in successful patient was 1 (completely recovered) in one patient and 2 (much improved) in 3 patients. | Pain at the implantation site, infection. |
| Rongqing et al, 2019 (73) | IV | Case series | 120 | The total effectiveness rate of anorectal pain was 96.7% 1 year after sacral nerve stimulation. There was a significant reduction in the median VAS score from 8–3. | N/A |
| Gajewski and Al-Zahrani, 2011 (70) | IV | Case series | 78 | Permanent SNM was performed in patients with bladder pain syndrome (44 out of 78) who showed at least 50% improvement in their symptoms with a temporary peripheral nerve evaluation test. Median follow-up was 61.5 months (standard deviation ± 27.7). Good long-term success was seen in 72% of the patients. | Pain at the site. |
| Peters et al, 2007 (69) | II | RCT | 22 | Twenty-two patients with IC had a tined lead placed at S3 and a second electrode implanted at the pudendal nerve. Each lead was tested for 7 days. At 6 months after implantation, the 10-cm VAS scores for pain decreased by 49% for sacral (7.9–4.0) and 29% for pudendal (4.5–3.2). | Seroma. |
| Peters, 2002 (67) | IV | Prospective nonrandomized study | 22 | After the placement of a permanent sacral nerve stimulator, the pelvic pain moderately improved in 35% of patients and markedly improved in 30% of patients. | N/A |
| Comiter, 2003 (68) | IV | Prospective nonrandomized study | 17 | Seventeen patients with IC underwent permanent sacral nerve stimulator implantation. At an average of 14 months follow-up, average pain decreased from 5.8–1.6 points on a scale of 0–10 (P < 0.01). | N/A |
| Lower back pain | |||||
| Cohen et al, 2019 (85) | IV | Case series | 9 | PNS provides clinically and statistically significant reductions in lower back pain, disability, and pain interference. Patients also experienced reductions in opioid and non-opioid analgesic medication usage and reported improvements in QOL with treatment. | Mild skin irritation. |
| Eldabe et al, 2019 (84) | II | RCT | 116 | SQS plus OMM (SQS+OMM arm) is more effective than OMM alone in relieving low back pain at up to 9 months. | Back pain and pain in extremity. |
| Guentchev et al, 2017 (86) | IV | Case series | 12 | SIJ PNS reduce ODI from 57%−32% and VAS from 9–2.1 after 2 weeks; after 6 months, the therapy was rated as effective in 7/8 patients reporting at that period. Twelve months after stimulation, 6/7 patients considered their treatment as a success. | N/A |
| Verrills et al, 2009 (82) | IV | Prospective nonrandomized study | 14 | Eleven patients (85%) reported successful outcomes and an average pain reduction of 4.18 points after percutaneous PNS. Pain relief was highly correlated with reduced analgesia and patient satisfaction. | No |
| Burgher et al, 2012 (83) | IV | Retrospective case series | 16 | Average (mean) patient-reported percentage of pain relief at last follow-up was 45% (range 20%−80%) after PNS on lower back pain at average 4.5 months (range 2–9 months) follow-up. One patient was noted to have reduced daily opioid intake. | Lead migration. |
| Shoulder pain | |||||
| Wilson et al, 2014 (60) | II | RCT | 25 | Twenty-five patients with chronic shoulder pain after stroke were randomized to receive a 3-week treatment of single-lead PNS (n = 13) or UC (n = 12). There was a significantly greater reduction in pain for the PNS group compared with UC group, with significant differences at 6 and 12 weeks after treatment. | Retained electrode fragment owing to fracturing of the tip of the electrode during explantation. |
| Postsurgical pain | |||||
| Ilfeld et al, 2019 (101) | IV | Case series of feasibility study | 7 | Preoperatively, patients undergoing TKA received both sciatic and femoral of pPNS. Within 20 hours after TKA, the pPNS was activated as part of multimodal analgesia up to a total of 6 weeks. In 6 of 7 patients (86%), the average of daily VAS pain scores across the first 2 weeks were < 4 of 10. In 4 out of 7 patients (57%), opioid use was ceased within the first week. Gross sensory/motor function was maintained during stimulation. | No complications. |
| Ilfeld et al, 2018 (103) | IV | Proof-of-concept, randomized, controlled, partial-crossover study | 7 | Preoperatively, patients undergoing hallux valgus osteotomy received sciatic pPNS placement between the subgluteal region and bifurcation as the primary regimen, and the popliteal sciatic nerve catheter as the rescue analgesia. Immediate postoperatively, the patients received 5 minutes of either stimulation or sham in a randomized, double-masked fashion followed by a 5 minute crossover period, and then continuous stimulation until lead removal on postoperative days 14–28. Optimal analgesia with decreased opioid requirements was obtained, and a “carryover” effect was observed following electrical current discontinuation. | One lead dislodged, 2 fractured during use, and one fractured during intentional withdrawal. |
| Ilfeld et al, 2019 (102) | IV | Proof-of-concept, randomized, controlled, partial-crossover study | 16 | Preoperatively, in patients undergoing rotator cuff repair, suprascapular pPNS was placed in 2 patients who did not experience any appreciable analgesia. Subsequently, the interscalene brachial plexus pPNS was implanted in the 14 patients for the same procedure. Three patients withdrew prior to data collection. Immediate postoperatively, the patients received 5 minutes of either stimulation or sham in a randomized, double-masked fashion followed by a 5 minute crossover period, and then continuous stimulation until lead removal postoperative days 14–28. Analgesia immediately following surgery does not appear to be as potent as local anesthetic-based peripheral nerve blocks. However, pPNS may provide analgesia and decrease opioid requirements in the days following surgeries. | Two leads dislodged during use and, 4 fractured on withdrawal. |
| Ilfeld et al, 2019 (104) | IV | Proof-of-concept, randomized, controlled, partial-crossover study | 10 | Preoperatively, patients undergoing anterior cruciate ligament reconstruction received femoral pPNS placement as the primary regimen, and adductor canal nerve catheter as the rescue analgesia. Immediate postoperatively, the patients received 5 minutes of either stimulation or sham in a randomized, double-masked fashion followed by a 5 minute crossover period, and then continuous active stimulation until lead removal postoperative day 14–28. Reduction in pain and opioid requirements were obtained, although the continuous adductor canal nerve catheter was activated in most patients (80%) for rescue analgesia (in addition to stimulation) during postoperative days 1–3. | Early removal of lead in 3 cases; broken lead in one case. |
| Peripheral field stimulation | |||||
| Kloimstein et al, 2014 (89) | III | Prospective, observational | 105 | Patients evaluated at multiple centers in Austria and Switzerland. SCS/PNFS hybrid and PNFS alone studied. Seventy-four patients completed the entire study for 6 months at follow-up. Reduction of medication, QOL, SF-12, ODI, and BDI were evaluated. | Infection, loss of stimulation, skin irritation of implant. |
| Verrills et al, 2011 (90) | IV | Consecutive study | 100 | One hundred patients evaluated with ODI, anxiety scale, depression. Follow-up was 8.1 months on average. Pain reduction averaged 4.2. | No long-term complications. |
| Hofmeister et al, 2020 (92) | I | Systematic review | Systematic review of neuromodulatory technologies. For PNFS, 5 studies demonstrated symptomatic benefit in patients, 2 did not show improvements. | None reported. | |
| McRoberts et al, 2013 (94) | II | Multisite RCT | 30 | Multisite study evaluating PNFS in patients with chronic low back pain. Patients had 1 of 4 stimulation patterns with responders evaluated after implant for VAS, pain index, SF-36, QOL, and pain coverage. Patient satisfaction and analgesic were requirements studied. | Loss of therapy and lead migration were most common AEs. |
| Verrills et al, 2014 (87) | IV | Case control | 60 | This study evaluated PNFS for chronic headache conditions. NRS-11, analgesic use, Zung depression scale, Neck Disability Index, and patient satisfaction were studied. | None reported. |
| Ellis et al, 2015 (88) | IV | Retrospective review | 35 | Review of patients undergoing PFNS for trigeminal pain. Patients followed up to 15 months with 73% of patients demonstrating improvement. High revision rate. | Revision. |
| Mitchell et al, 2016 (96) | IV | Prospective case series | 20 | Twenty patients with chronic thoracic pain responded to PNFS trial. Implant with 8 contact leads. NRS-11 decreased from 7.75 preoperatively to 2.25 postoperative. Patients evaluated up to 12 months follow-up. | Pain at generator site, recurrent infection. |
| Schwarm et al, 2019 (91) | IV | Retrospective review | 26 | NRS-11, EQ-5D-5L, ODI, ASTS, CSQ-8 was assessed in patients implanted with PNFS with chronic low back pain. Select patients trialed with TENS, which had no predictive value for efficacy of PFNS. Follow-up was for 6 and 12 months. Statistically significant decrease in pain from 8.5–5.2. Also, patients demonstrated a reduction in analgesic medication and QOL improvement. | None reported. |
| D’Ammando et al, 2016 (97) | IV | Prospective case series | 22 | Twenty-two patients evaluated who suffered from neuropathic pain. Some 59% of patients had VAS reduction of 5.50 (ranged from 8.86 preoperative to 3.36 postoperative). | None. |
| van Gorp et al, 2020 (93) | II | Multicenter RCT | 52 | Cost effectiveness of a Dutch multicenter RCT on the effectiveness of hybrid SCS and PNFS. PNFS was found to be adding cost effectivity and improved Quality of Life Years (QALY). | None reported. |
| Ishak et al, 2018 (95) | IV | Prospective case control | 26 | Consecutive patients with 13 patients going from trial to implant with chronic low back pain. Decreased analgesics, VAS, ODI, and QOL. Patients followed for 24 months and maintained benefits. Limitation includes relatively small sample size. | Intolerance, migration, deep discharge. |
Abbreviations: ADEs, adverse device-related events; ASTS, actual mood state scale; BDI, Becks Depression Inventory; CSQ, Client Satisfaction Questionnaire; MCS, mental component summary scores of SF-36v2; MIDAS, migraine disability assessment; N/A, not available; ODI, Oswestry Disability Index; PAD, Zung Pain and Distress; PCS, physical component summary scores of SF-36v2; SAEs, device- or procedure-related serious adverse events; SF-12, Short Form-12 item Health survey; SPG, sphenopalatine ganglion
Disclaimer:
Jijun Xu is supported by the MENTR program at Cleveland Clinic Lerner Research Institute, and a National Institutes of Health grant K08CA228039.
Footnotes
Conflict of interest: Krishnan V. Chakravarthy is a founder of Douleur Therapeutics, Newrom Biomedical, and a consultant for Abbott, Medtronic, Boston Scientific, Medincell, Omnia Medical, and Bioness. He has stock options in Nalu Medical, Higgs Boson Health, and Oska Wellness. Alaa Abd-Elsayed is a consultant for Medtronic and Avanos. Paul Christo is a consultant for Daiichi Sankyo and GlaxoSmithKline..
REFERENCES
- 1.Mobbs RJ, Nair S, Blum P. Peripheral nerve stimulation for the treatment of chronic pain. J Clin Neurosci 2007; 14:216–221; discussion 222–213. [DOI] [PubMed] [Google Scholar]
- 2.Henderson JM. Peripheral nerve stimulation for chronic pain. Curr Pain Headache Rep 2008; 12:28–31. [DOI] [PubMed] [Google Scholar]
- 3.Nayak R, Banik RK. Current innovations in peripheral nerve stimulation. Pain Res Treat 2018:2018: 9091216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furlan AD, Malmivaara A, Chou R, et al. 2015 Updated method guideline for systematic reviews in the cochrane back and neck group. Spine (Phila Pa 1976) 2015; 40:1660–1673. [DOI] [PubMed] [Google Scholar]
- 5.Manchikanti L, Hirsch JA, Cohen SP, et al. Assessment of methodologic quality of randomized trials of interventional techniques: Development of an interventional pain management specific instrument. Pain Physician 2014; 17:E263–E290. [PubMed] [Google Scholar]
- 6.Manchikanti L, Falco FJ, Benyamin RM, et al. A modified approach to grading of evidence. Pain Physician 2014; 17:E319–E325. [PubMed] [Google Scholar]
- 7.Melzack R, Wall PD. Pain mechanisms: A new theory. Science 1965; 150:971–979. [DOI] [PubMed] [Google Scholar]
- 8.Ellrich J, Lamp S. Peripheral nerve stimulation inhibits nociceptive processing: An electrophysiological study in healthy volunteers. Neuromodulation 2005; 8:225–232. [DOI] [PubMed] [Google Scholar]
- 9.Maeda Y, Lisi TL, Vance CG, et al. Release of GABA and activation of GABA(A) in the spinal cord mediates the effects of TENS in rats. Brain Res 2007; 1136:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sluka KA, Vance CG, Lisi TL. High-frequency, but not low-frequency, transcutaneous electrical nerve stimulation reduces aspartate and glutamate release in the spinal cord dorsal horn. J Neurochem 2005; 95:1794–1801. [DOI] [PubMed] [Google Scholar]
- 11.Men DS, Matsui Y. Activation of descending noradrenergic system by peripheral nerve stimulation. Brain Res Bull 1994; 34:177–182. [DOI] [PubMed] [Google Scholar]
- 12.Men DS, Matsui Y. Peripheral nerve stimulation increases serotonin and dopamine metabolites in rat spinal cord. Brain Res Bull 1994; 33:625–632. [DOI] [PubMed] [Google Scholar]
- 13.Radhakrishnan R, Sluka KA. Spinal muscarinic receptors are activated during low or high frequency TENS-induced antihyperalgesia in rats. Neuropharmacology 2003; 45:1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garrison DW, Foreman RD. Decreased activity of spontaneous and noxiously evoked dorsal horn cells during transcutaneous electrical nerve stimulation (TENS). Pain 1994; 58:309–315. [DOI] [PubMed] [Google Scholar]
- 15.Ma YT, Sluka KA. Reduction in inflammation-induced sensitization of dorsal horn neurons by transcutaneous electrical nerve stimulation in anesthetized rats. Exp Brain Res 2001; 137:94–102. [DOI] [PubMed] [Google Scholar]
- 16.Desantana JM, Santana-Filho VJ, Sluka KA. Modulation between high- and low-frequency transcutaneous electric nerve stimulation delays the development of analgesic tolerance in arthritic rats. Arch Phys Med Rehabil 2008; 89:754–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeSantana JM, Da Silva LF, De Resende MA, et al. Transcutaneous electrical nerve stimulation at both high and low frequencies activates ventrolateral periaqueductal grey to decrease mechanical hyperalgesia in arthritic rats. Neuroscience 2009; 163:1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalra A, Urban MO, Sluka KA. Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical nerve stimulation (TENS). J Pharmacol Exp Ther 2001; 298:257–263. [PubMed] [Google Scholar]
- 19.Vera-Portocarrero LP, Cordero T, Billstrom T, et al. Differential effects of subcutaneous electrical stimulation (SQS) and transcutaneous electrical nerve stimulation (TENS) in rodent models of chronic neuropathic or inflammatory pain. Neuromodulation 2013; 16:328–335; discussion 335. [DOI] [PubMed] [Google Scholar]
- 20.Lao L, Zhang RX, Zhang G, et al. A parametric study of electroacupuncture on persistent hyperalgesia and Fos protein expression in rats. Brain Res 2004; 1020:18–29. [DOI] [PubMed] [Google Scholar]
- 21.Takagi J, Sawada T, Yonehara N. A possible involvement of monoaminergic and opioidergic systems in the analgesia induced by electro-acupuncture in rabbits. Jpn J Pharmacol 1996; 70:73–80. [DOI] [PubMed] [Google Scholar]
- 22.Chen YH, Lee HJ, Lee MT, et al. Median nerve stimulation induces analgesia via orexin-initiated endocannabinoid disinhibition in the periaqueductal gray. Proc Natl Acad Sci U S A 2018; 115:E10720–E10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang JY, Chen R, Chen SP, et al. Electroacupuncture reduces the effects of acute noxious stimulation on the electrical activity of pain-related neurons in the hippocampus of control and neuropathic pain rats. Neural Plast 2016; 2016:6521026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vivo M, Puigdemasa A, Casals L, et al. Immediate electrical stimulation enhances regeneration and reinnervation and modulates spinal plastic changes after sciatic nerve injury and repair. Exp Neurol 2008; 211:180–193. [DOI] [PubMed] [Google Scholar]
- 25.Haastert-Talini K, Schmitte R, Korte N, et al. Electrical stimulation accelerates axonal and functional peripheral nerve regeneration across long gaps. J Neurotrauma 2011; 28:661–674. [DOI] [PubMed] [Google Scholar]
- 26.Asensio-Pinilla E, Udina E, Jaramillo J, et al. Electrical stimulation combined with exercise increase axonal regeneration after peripheral nerve injury. Exp Neurol 2009; 219:258–265. [DOI] [PubMed] [Google Scholar]
- 27.Dodick DW, Silberstein SD, Reed KL, et al. Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: Long-term results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia 2015; 35:344–358. [DOI] [PubMed] [Google Scholar]
- 28.Mekhail NA, Estemalik E, Azer G, et al. Safety and efficacy of occipital nerves stimulation for the treatment of chronic migraines: Randomized, double-blind, controlled single-center experience. Pain Pract 2017; 17:669–677. [DOI] [PubMed] [Google Scholar]
- 29.Saper JR, Dodick DW, Silberstein SD, et al. Occipital nerve stimulation for the treatment of intractable chronic migraine headache: ONSTIM feasibility study. Cephalalgia 2011; 31:271–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serra G, Marchioretto F. Occipital nerve stimulation for chronic migraine: A randomized trial. Pain Physician 2012; 15:245–253. [PubMed] [Google Scholar]
- 31.Silberstein SD, Dodick DW, Saper J, et al. Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: Results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia 2012; 32:1165–1179. [DOI] [PubMed] [Google Scholar]
- 32.Lainez MJ, Puche M, Garcia A, et al. Sphenopalatine ganglion stimulation for the treatment of cluster headache. Ther Adv Neurol Disord 2014; 7:162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schoenen J, Jensen RH, Lanteri-Minet M, et al. Stimulation of the sphenopalatine ganglion (SPG) for cluster headache treatment. Pathway CH-1: A randomized, sham-controlled study. Cephalalgia 2013; 33:816–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campbell JN, Long DM. Peripheral nerve stimulation in the treatment of intractable pain. J Neurosurg 1976; 45:692–699. [DOI] [PubMed] [Google Scholar]
- 35.Law JD, Swett J, Kirsch WM. Retrospective analysis of 22 patients with chronic pain treated by peripheral nerve stimulation. J Neurosurg 1980; 52:482–485. [DOI] [PubMed] [Google Scholar]
- 36.Waisbrod H, Panhans C, Hansen D, et al. Direct nerve stimulation for painful peripheral neuropathies. J Bone Joint Surg Br 1985; 67:470–472. [DOI] [PubMed] [Google Scholar]
- 37.Eisenberg E, Waisbrod H, Gerbershagen HU. Long-term peripheral nerve stimulation for painful nerve injuries. Clin J Pain 2004; 20:143–146. [DOI] [PubMed] [Google Scholar]
- 38.Stevanato G, Devigili G, Eleopra R, et al. Chronic post-traumatic neuropathic pain of brachial plexus and upper limb: A new technique of peripheral nerve stimulation. Neurosurg Rev 2014; 37:473–479; discussion 479–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huntoon MA, Burgher AH. Ultrasound-guided permanent implantation of peripheral nerve stimulation (PNS) system for neuropathic pain of the extremities: Original cases and outcomes. Pain Med 2009; 10:1369–1377. [DOI] [PubMed] [Google Scholar]
- 40.Narouze SN, Zakari A, Vydyanathan A. Ultrasound-guided placement of a permanent percutaneous femoral nerve stimulator leads for the treatment of intractable femoral neuropathy. Pain Physician 2009; 12:E305–E308. [PubMed] [Google Scholar]
- 41.Reddy CG, Flouty OE, Holland MT, et al. Novel technique for trialing peripheral nerve stimulation: Ultrasonography-guided StimuCath trial. Neurosurg Focus 2017; 42:E5. [DOI] [PubMed] [Google Scholar]
- 42.Deer T, Pope J, Benyamin R, et al. Prospective, multicenter, randomized, double-blinded, partial crossover study to assess the safety and efficacy of the novel neuromodulation system in the treatment of patients with chronic pain of peripheral nerve origin. Neuromodulation 2016; 19:91–100. [DOI] [PubMed] [Google Scholar]
- 43.Hassenbusch SJ, Stanton-Hicks M, Schoppa D, et al. Long-term results of peripheral nerve stimulation for reflex sympathetic dystrophy. J Neurosurg 1996; 84:415–423. [DOI] [PubMed] [Google Scholar]
- 44.Cooney WP. Electrical stimulation and the treatment of complex regional pain syndromes of the upper extremity. Hand Clin 1997; 13:519–526. [PubMed] [Google Scholar]
- 45.Jeon IC, Kim MS, Kim SH. Median nerve stimulation in a patient with complex regional pain syndrome type II. J Korean Neurosurg Soc 2009; 46:273–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herschkowitz D, Kubias J. A case report of wireless peripheral nerve stimulation for complex regional pain syndrome type-I of the upper extremity: 1 year follow up. Scand J Pain 2019; 19:829–835. [DOI] [PubMed] [Google Scholar]
- 47.Hsu E, Cohen SP. Postamputation pain: Epidemiology, mechanisms, and treatment. J Pain Res 2013; 6:121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rauck RL, Kapural L, Cohen SP, et al. Peripheral nerve stimulation for the treatment of postamputation pain – A case report. Pain Pract 2012; 12:649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rauck RL, Cohen SP, Gilmore CA, et al. Treatment of post-amputation pain with peripheral nerve stimulation. Neuromodulation 2014; 17:188–197. [DOI] [PubMed] [Google Scholar]
- 50.Gilmore C, Ilfeld B, Rosenow J, et al. Percutaneous peripheral nerve stimulation for the treatment of chronic neuropathic postamputation pain: A multicenter, randomized, placebo-controlled trial. Reg Anesth Pain Med 2019; 44:637–645. [DOI] [PubMed] [Google Scholar]
- 51.Cohen SP, Gilmore CA, Rauck RL, et al. Percutaneous peripheral nerve stimulation for the treatment of chronic pain following amputation. Mil Med 2019; 184:e267–e274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kilgore KL, Bhadra N. Nerve conduction block utilising high-frequency alternating current. Med Biol Eng Comput 2004; 42:394–406. [DOI] [PubMed] [Google Scholar]
- 53.Soin A, Fang ZP, Velasco J. Peripheral neuromodulation to treat postamputation pain. Prog Neurol Surg 2015; 29:158–167. [DOI] [PubMed] [Google Scholar]
- 54.Soin A, Shah NS, Fang ZP. High-frequency electrical nerve block for postamputation pain: A pilot study. Neuromodulation 2015; 18:197–205; discussion 205–196. [DOI] [PubMed] [Google Scholar]
- 55.Luime JJ, Koes BW, Hendriksen IJ, et al. Prevalence and incidence of shoulder pain in the general population; A systematic review. Scand J Rheumatol 2004; 33:73–81. [DOI] [PubMed] [Google Scholar]
- 56.Yu DT, Friedman AS, Rosenfeld EL. Electrical stimulation for treating chronic poststroke shoulder pain using a fully implanted microstimulator with internal battery. Am J Phys Med Rehabil 2010; 89:423–428. [DOI] [PubMed] [Google Scholar]
- 57.Wilson RD, Bennett ME, Lechman TE, et al. Single-lead percutaneous peripheral nerve stimulation for the treatment of hemiplegic shoulder pain: A case report. Arch Phys Med Rehabil 2011; 92:837–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson RD, Harris MA, Bennett ME, et al. Single-lead percutaneous peripheral nerve stimulation for the treatment of shoulder pain from subacromial impingement syndrome. PM R 2012; 4:624–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elahi F, Reddy CG. Neuromodulation of the suprascapular nerve. Pain Physician 2014; 17:E769–E773. [PubMed] [Google Scholar]
- 60.Wilson RD, Gunzler DD, Bennett ME, et al. Peripheral nerve stimulation compared with usual care for pain relief of hemiplegic shoulder pain: A randomized controlled trial. Am J Phys Med Rehabil 2014; 93:17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Insinga RP, Itzler RF, Pellissier JM, et al. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med 2005; 20:748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Niv D, Maltsman-Tseikhin A, Lang E. Postherpetic neuralgia: What do we know and where are we heading? Pain Physician 2004; 7:239–247. [PubMed] [Google Scholar]
- 63.Yakovlev AE, Peterson AT. Peripheral nerve stimulation in treatment of intractable postherpetic neuralgia. Neuromodulation 2007; 10:373–375. [DOI] [PubMed] [Google Scholar]
- 64.Kurklinsky S, Palmer SC, Arroliga MJ, et al. Neuromodulation in postherpetic neuralgia: Case reports and review of the literature. Pain Med 2018; 19:1237–1244. [DOI] [PubMed] [Google Scholar]
- 65.Rossi M, DeCarolis G, Liberatoscioli G, et al. A novel mini-invasive approach to the treatment of neuropathic pain: The PENS Study. Pain Physician 2016; 19:E121–E128. [PubMed] [Google Scholar]
- 66.Marcelissen T, Jacobs R, van Kerrebroeck P, et al. Sacral neuromodulation as a treatment for chronic pelvic pain. J Urol 2011; 186:387–393. [DOI] [PubMed] [Google Scholar]
- 67.Peters KM. Neuromodulation for the treatment of refractory interstitial cystitis. Rev Urol 2002; 4(Suppl 1):S36–S43. [PMC free article] [PubMed] [Google Scholar]
- 68.Comiter CV. Sacral neuromodulation for the symptomatic treatment of refractory interstitial cystitis: A prospective study. J Urol 2003; 169:1369–1373. [DOI] [PubMed] [Google Scholar]
- 69.Peters KM, Feber KM, Bennett RC. A prospective, single-blind, randomized crossover trial of sacral vs pudendal nerve stimulation for interstitial cystitis. BJU Int 2007; 100:835–839. [DOI] [PubMed] [Google Scholar]
- 70.Gajewski JB, Al-Zahrani AA. The long-term efficacy of sacral neuromodulation in the management of intractable cases of bladder pain syndrome: 14 years of experience in one centre. BJU Int 2011; 107:1258–1264. [DOI] [PubMed] [Google Scholar]
- 71.Martellucci J, Naldini G, Del Popolo G, et al. Sacral nerve modulation in the treatment of chronic pain after pelvic surgery. Colorectal Dis 2012; 14:502–507. [DOI] [PubMed] [Google Scholar]
- 72.Aboseif S, Tamaddon K, Chalfin S, et al. Sacral neuromodulation as an effective treatment for refractory pelvic floor dysfunction. Urology 2002; 60:52–56. [DOI] [PubMed] [Google Scholar]
- 73.Rongqing G, Yafei W, Zhimin W, et al. Treatment outcome of acute sacral nerve stimulation in functional anorectal pain. Pain Pract 2019; 19:390–396. [DOI] [PubMed] [Google Scholar]
- 74.Falletto E, Masin A, Lolli P, et al. Is sacral nerve stimulation an effective treatment for chronic idiopathic anal pain? Dis Colon Rectum 2009; 52:456–462. [DOI] [PubMed] [Google Scholar]
- 75.Govaert B, Melenhorst J, van Kleef M, et al. Sacral neuromodulation for the treatment of chronic functional anorectal pain: A single center experience. Pain Pract 2010; 10:49–53. [DOI] [PubMed] [Google Scholar]
- 76.Bosch JL. An update on sacral neuromodulation: Where do we stand with this in the management of lower urinary tract dysfunction in 2010? BJU Int 2010; 106:1432–1442. [DOI] [PubMed] [Google Scholar]
- 77.Schmidt RA. Technique of pudendal nerve localization for block or stimulation. J Urol 1989; 142:1528–1531. [DOI] [PubMed] [Google Scholar]
- 78.Heinze K, Hoermann R, Fritsch H, et al. Comparative pilot study of implantation techniques for pudendal neuromodulation: Technical and clinical outcome in first 20 patients with chronic pelvic pain. World J Urol 2015; 33:289–294. [DOI] [PubMed] [Google Scholar]
- 79.van Balken MR, Vandoninck V, Messelink BJ, et al. Percutaneous tibial nerve stimulation as neuromodulative treatment of chronic pelvic pain. Eur Urol 2003; 43:158–163; discussion 163. [DOI] [PubMed] [Google Scholar]
- 80.Istek A, Gungor Ugurlucan F, Yasa C, et al. Randomized trial of long-term effects of percutaneous tibial nerve stimulation on chronic pelvic pain. Arch Gynecol Obstet 2014; 290:291–298. [DOI] [PubMed] [Google Scholar]
- 81.Paicius RM, Bernstein CA, Lempert-Cohen C. Peripheral nerve field stimulation for the treatment of chronic low back pain: Preliminary results of long-term follow-up: A case series. Neuromodulation 2007; 10:279–290. [DOI] [PubMed] [Google Scholar]
- 82.Verrills P, Mitchell B, Vivian D, et al. Peripheral nerve stimulation: A treatment for chronic low back pain and failed back surgery syndrome? Neuromodulation 2009; 12:68–75. [DOI] [PubMed] [Google Scholar]
- 83.Burgher AH, Huntoon MA, Turley TW, et al. Subcutaneous peripheral nerve stimulation with inter-lead stimulation for axial neck and low back pain: Case series and review of the literature. Neuromodulation 2012; 15:100–106; discussion 106–107. [DOI] [PubMed] [Google Scholar]
- 84.Eldabe SS, Taylor RS, Goossens S, et al. A randomized controlled trial of subcutaneous nerve stimulation for back pain due to failed back surgery syndrome: The SubQStim Study. Neuromodulation 2019; 22:519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cohen S, Gilmore C, Kapural L, et al. Percutaneous peripheral nerve stimulation for pain reduction and improvements in functional outcomes in chronic low back pain. Mil Med 2019; 184:537–541. [DOI] [PubMed] [Google Scholar]
- 86.Guentchev M, Preuss C, Rink R, et al. Long-term reduction of sacroiliac joint pain with peripheral nerve stimulation. Oper Neurosurg (Hagerstown) 2017; 13:634–639. [DOI] [PubMed] [Google Scholar]
- 87.Verrills P, Rose R, Mitchell B, et al. Peripheral nerve field stimulation for chronic headache: 60 cases and long-term follow-up. Neuromodulation 2014; 17:54–59. [DOI] [PubMed] [Google Scholar]
- 88.Ellis JA, Mejia Munne JC, Winfree CJ. Trigeminal branch stimulation for the treatment of intractable craniofacial pain. J Neurosurg 2015; 123:283–288. [DOI] [PubMed] [Google Scholar]
- 89.Kloimstein H, Likar R, Kern M, et al. Peripheral nerve field stimulation (PNFS) in chronic low back pain: A prospective multicenter study. Neuromodulation 2014; 17:180–187. [DOI] [PubMed] [Google Scholar]
- 90.Verrills P, Vivian D, Mitchell B, et al. Peripheral nerve field stimulation for chronic pain: 100 cases and review of the literature. Pain Med 2011; 12:1395–1405. [DOI] [PubMed] [Google Scholar]
- 91.Schwarm FP, Stein M, Uhl E, et al. A retrospective analysis of 25 cases with peripheral nerve field stimulation for chronic low back pain and the predictive value of transcutaneous electrical nerve stimulation for patient selection. Neuromodulation 2019; 22:607–614. [DOI] [PubMed] [Google Scholar]
- 92.Hofmeister M, Memedovich A, Brown S, et al. Effectiveness of neurostimulation technologies for the management of chronic pain: A systematic review. Neuromodulation 2020; 23:150–157. [DOI] [PubMed] [Google Scholar]
- 93.van Gorp E, Adang EMM, Gultuna I, et al. Cost-effectiveness analysis of peripheral nerve field stimulation as add-on therapy to spinal cord stimulation in the treatment of chronic low back pain in failed back surgery syndrome patients. Neuromodulation 2020; 23:639–645. [DOI] [PubMed] [Google Scholar]
- 94.McRoberts WP, Wolkowitz R, Meyer DJ, et al. Peripheral nerve field stimulation for the management of localized chronic intractable back pain: Results from a randomized controlled study. Neuromodulation 2013; 16:565–574; discussion 574–565. [DOI] [PubMed] [Google Scholar]
- 95.Ishak B, Campos B, Brunn H, et al. Feasibility, safety, and efficacy of subcutaneous peripheral nerve field stimulation for the treatment of refractory low back pain: A two-year single-center study. Neuroscience 2018; 387:38–47. [DOI] [PubMed] [Google Scholar]
- 96.Mitchell B, Verrills P, Vivian D, et al. Peripheral nerve field stimulation therapy for patients with thoracic pain: A prospective study. Neuromodulation 2016; 19:752–759. [DOI] [PubMed] [Google Scholar]
- 97.D’Ammando A, Messina G, Franzini A, et al. Peripheral nerve field stimulation for chronic neuropathic pain: A single institution experience. Acta Neurochir (Wien) 2016; 158:767–772. [DOI] [PubMed] [Google Scholar]
- 98.Kent M, Upp J, Spevak C, et al. Ultrasound-guided peripheral nerve stimulator placement in two soldiers with acute battlefield neuropathic pain. Anesth Analg 2012; 114:875–878. [DOI] [PubMed] [Google Scholar]
- 99.Ilfeld BM, Grant SA, Gilmore CA, et al. Neurostimulation for postsurgical analgesia: A novel system enabling ultrasound-guided percutaneous peripheral nerve stimulation. Pain Pract 2017; 17:892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ilfeld BM, Gilmore CA, Grant SA, et al. Ultrasound-guided percutaneous peripheral nerve stimulation for analgesia following total knee arthroplasty: A prospective feasibility study. J Orthop Surg Res 2017; 12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ilfeld BM, Ball ST, Gabriel RA, et al. A feasibility study of percutaneous peripheral nerve stimulation for the treatment of postoperative pain following total knee arthroplasty. Neuromodulation 2019; 22:653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ilfeld BM, Finneran JJ, Gabriel RA, et al. Ultrasound-guided percutaneous peripheral nerve stimulation: Neuromodulation of the suprascapular nerve and brachial plexus for postoperative analgesia following ambulatory rotator cuff repair. A proof-of-concept study. Reg Anesth Pain Med 2019; 44:310–318. [DOI] [PubMed] [Google Scholar]
- 103.Ilfeld BM, Gabriel RA, Said ET, et al. Ultrasound-guided percutaneous peripheral nerve stimulation: Neuromodulation of the sciatic nerve for postoperative analgesia following ambulatory foot surgery, a proof-of-concept study. Reg Anesth Pain Med 2018; 43:580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ilfeld BM, Said ET, Finneran JJ, et al. Ultrasound-guided percutaneous peripheral nerve stimulation: Neuromodulation of the femoral nerve for postoperative analgesia following ambulatory anterior cruciate ligament reconstruction: A proof of concept study. Neuromodulation 2019; 22:621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Novak CB, Mackinnon SE. Outcome following implantation of a peripheral nerve stimulator in patients with chronic nerve pain. Plast Reconstr Surg 2000; 105:1967–1972. [DOI] [PubMed] [Google Scholar]
- 106.Strege DW, Cooney WP, Wood MB, et al. Chronic peripheral nerve pain treated with direct electrical nerve stimulation. J Hand Surg Am 1994; 19:931–939. [DOI] [PubMed] [Google Scholar]
- 107.Eldabe S, Buchser E, Duarte RV. Complications of spinal cord stimulation and peripheral nerve stimulation techniques: A review of the literature. Pain Med 2016; 17:325–336. [DOI] [PubMed] [Google Scholar]
- 108.Sator-Katzenschlager S, Fiala K, Kress HG, et al. Subcutaneous target stimulation (STS) in chronic noncancer pain: A nationwide retrospective study. Pain Pract 2010; 10:279–286. [DOI] [PubMed] [Google Scholar]
- 109.Gazelka HM, Freeman ED, Hooten WM, et al. Incidence of clinically significant percutaneous spinal cord stimulator lead migration. Neuromodulation 2015; 18:123–125; discussion 125. [DOI] [PubMed] [Google Scholar]
- 110.Chen YF, Bramley G, Unwin G, et al. Occipital nerve stimulation for chronic migraine – A systematic review and meta-analysis. PLoS One 2015; 10:e0116786. [DOI] [PMC free article] [PubMed] [Google Scholar]


