Abstract
Measurement of beat-to-beat blood pressure and heart rate responses to the Valsalva maneuver is the basis for a highly informative autonomic function test. Whereas in the past this measurement required intra-arterial cannulation, the development of finger cuff devices that acquire arterial pressure waveforms indistinguishable from those recorded intra-arterially has made it possible to obtain accurate measurements noninvasively. In a patient with orthostatic hypotension, the pattern of blood pressure responses during and after release of the maneuver can identify a neurogenic basis—sympathetic neurocirculatory failure. The quantifiable change in cardiac interbeat interval per unit change in systolic pressure during the maneuver can identify baroreflex-cardiovagal failure.
Keywords: Autonomic, Sympathetic, Parasympathetic, Valsalva, Blood Pressure
INTRODUCTION
Despite its apparent simplicity, the Valsalva maneuver is the basis for one of the most important clinical physiological tests for autonomic failure. The test requires the use of a method to measure blood pressure and heart rate continuously (beat-to-beat).
This is a limited review and does not cover areas such as confounding variables, technical aspects, and normative data. The goal here is mainly didactic.
Historical Perspective
In his De aure humana tractatus, published in Latin in 1704 (Figure 1), Antonio Valsalva (1666–1723) described the maneuver that now bears his name. Valsalva devised the maneuver not to track reflexive blood pressure responses to decreased cardiac filling but to clear material from the middle ear. According to one English translation given by Jellinek [18], Valsalva wrote, “… if with occluded mouth and nostrils air is compressed inwardly, this action will extrude sanies from the middle ear [and is] a remedial exercise, to be repeated, [and will lead to] extrusion of praeter-natural cerebral matter either via the wound, via the nostrils, via the mouth, or via the auditory meatus … with great benefit …”
Figure 1:

Text from Valsalva’s De aure humana tractatus.
From this translation one might infer that the modern meaning of the Valsalva maneuver, attempting to exhale against a resistance, is the opposite of the what Valsalva described. Thus, the 1911 edition of the Encyclopedia Britannica contains the following entry: “By a forcible expiration, the oral and nasal cavities being closed, air may be driven into the tympanum, while a forcible inspiration (Valsalva’s experiment) will draw air from that cavity. In the first case, the membrana tympani will bulge outwards, in the second case inwards…” [21].
On the other hand, Derbes and Kerr [6] translated the same passage as follows, “…if, I say, he should attempt to compress the air within, after having closed the mouth and nostrils; the exudate usually is pushed forth thereby copiously into the auditory meatus; so that I am accustomed to recommend nothing more rapid in cleansing an ulcer of this kind, or remedy more useful for the patient than a fairly frequent repetition of such an effort.” And an editorial in the Journal of the American Medical Association used the following translation: “Thus (in order to offer one of many proofs) if someone would instill a medicinal fluid into the tympanic cavity or in the area of an ulcer or in the outer portion of the auditory meatus and if now, with mouth and nose closed, an attempt is made to compress the air, fluid would flow copiously from the auditory meatus. I recommend this for a prompt evacuation of a suppurative lesion since this may be remedial for the illness which might not occur by itself” [34].
There is some controversy about priority with respect to the first description of the maneuver that bears Valsalva’s name. Ambroise Pare (1510–1590) and Leonard of Bertipaglia (ca. 1380–1463) have also been mentioned.
Effects of the Valsalva maneuver on the cardiovascular system were not reported until about 150 years later. In 1851, Ernst Heinrich Weber, a founder of the field of perceptual psychology, demonstrated—in himself—a dramatic weakening of the pulse, loss of consciousness, and convulsion after straining against a closed glottis and compression of the chest [18]. A few years later Donders noted the typical increase in heart rate during the maneuver [19]. There is a long history of interpreting the heart rate changes associated with the maneuver in healthy individuals and patients with various cardiovascular disorders [8]. This has been quantified by the heart rate ratio—the maximum heart rate during the maneuver divided by the lowest heart rate within 30 seconds of the peak heart rate.
In the early to mid-20th century Sharpey-Schafer reported systematic studies about effects of the Valsalva maneuver on continuously recorded intra-arterial blood pressure in the normal and failing heart [30]. In the 1960s Appenzeller described effects of cerebrovascular disease on blood pressure responses to the maneuver [1] and findings in acute pandysautonomia [3] and Parkinson’s disease [2].
In the late 1980s non-invasive means to measure blood pressure for each pulse wave were introduced, enabling assessment of sympathetic neurocirculatory function by tracking the beat-to-beat blood pressure responses to the Valsalva maneuver [17]. Finger cuff devices such as the Finapres™ and NexFin™ photoplethysmography technologies measure blood pressure indirectly via infrared light transmission. Light at this wavelength is primarily absorbed by hemoglobin. Monitoring fluctuations in the intensity of the transmitted infrared light through the finger provides information about the cross-sectional area that is occupied by blood. The blood pressure is calculated from the dynamic changes in the volume of the blood.
For non-invasive detection of sympathetic neurocirculatory failure by the characteristic abnormalities in Phase II and Phase IV, the use of the finger cuff infrared and radial artery tonometric methods was validated by comparison with simultaneously recorded intra-arterial blood pressure [13]. Its validity and reliability having been established, the finger cuff method is now used widely to assess baroreflex-sympathoneural and baroreflex-cardiovagal responses.
The Four Phases of the Blood Pressure Responses to the Valsalva Maneuver
When a person blows against a resistance for several seconds and then relaxes, the continuously recorded blood pressure changes in a characteristic way. Four phases of this change have been distinguished [14] (Figure 2).
Figure 2: Four Phases of the heart rate and blood pressure responses to the Valsalva maneuver.
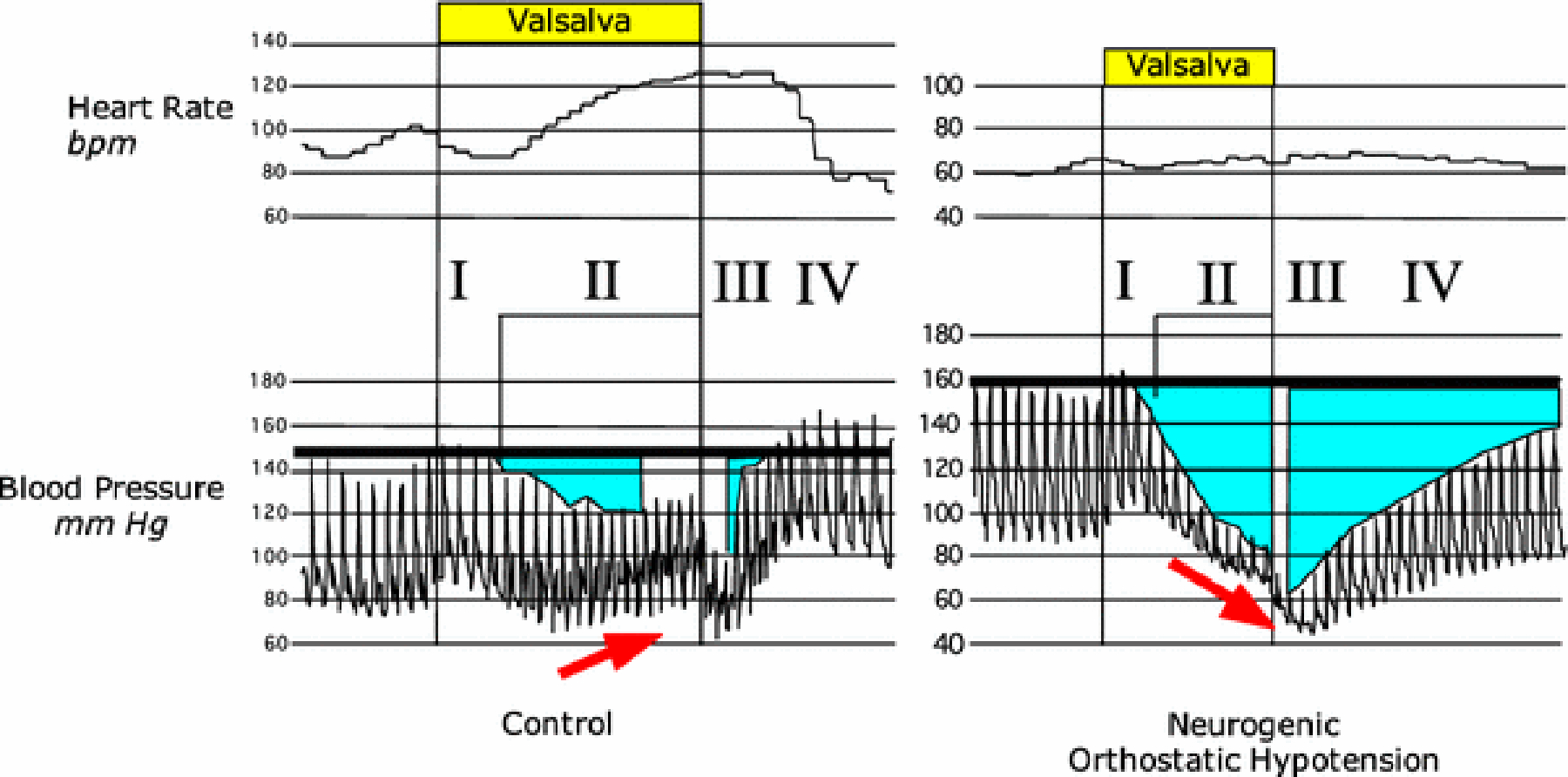
Areas in aqua illustrate increased areas under the baseline blood pressure in Phase II and Phase IV in neurogenic orthostatic hypotension.
In the first phase (Phase I), the moment the person begins to exhale against resistance, intra-thoracic pressure increases suddenly, and the arterial blood pressure increases briefly. One way to conceptualize the mechanism for the Phase I increase in pressure is that the aortic blood is being forced out of the chest and down the arteries to the arms. This is a mechanical effect on intravascular distribution that has nothing to do with neural reflexes.
As the straining continues, the increased intrathoracic pressure impedes the return of venous blood into the thorax. Reduced cardiac filling leads to a decrease in stroke volume, and soon afterward systemic arterial pressure decreases (Phase II). These changes release the sympathetic noradrenergic systemic from restraint by the low- and high-pressure baroreceptors, and sympathetic outflows increase reflexively. Norepinephrine is released from post-ganglionic sympathetic nerve terminals in the heart and blood vessel walls, the norepinephrine binds to adrenoceptors, the arterioles constrict, total peripheral resistance to blood flow increases, and near the end of Phase II the blood pressure increases from its nadir, despite the fall in cardiac output.
One way to think of the reflexive changes in blood pressure during Phase II of the Valsalva maneuver is to consider the water pressure in a garden hose. If you were to turn down the faucet, the pressure in the hose would decrease. You could bring the pressure back up by tightening the nozzle.
In Phase III the person relaxes. Momentarily the blood pressure falls as intrathoracic pressure returns to baseline. This is like a mirror image of the changes in Phase I—a mechanical effect independent of reflexes.
Finally, in Phase IV, since there is no longer an impediment to venous return to the heart, cardiac filling increases, and the heart ejects the blood into the reflexively constricted vasculature. This is like your turning up the faucet of the garden hose, so that the rate of filling of water into the hose is back to where it was originally—but the nozzle is still tightened. The pressure overshoots. This is what happens normally to the blood pressure in Phase IV of the Valsalva maneuver. The blood pressure increases rapidly and overshoots the baseline pressure. Because of the overshoot and consequent arterial baroreflex stimulation, sympathetic noradrenergic outflows are restrained, and the blood pressure soon decreases to the baseline value.
Teleologically, if the brain “wanted” to maintain its blood flow in response to a fall in arterial blood pressure, one way to do so would be to increase the heart rate. This is what happens normally in Phase II. As the pressure falls below baseline, the heart rate increases reflexively due to restraint of parasympathetic cardiovagal outflow. Thus, blockade of muscarinic cholinergic receptors by atropine markedly attenuates the increase in heart rate during Phase II of the Valsalva maneuver, despite a larger decrease in blood pressure [12].
In most forms of autonomic failure, the abnormalities of blood pressure and heart rate responses to the Valsalva maneuver occur together; however, there are exceptions. For instance, in dopamine-β-hydroxylase deficiency, in which there is an inability to synthesize norepinephrine, the blood pressure responses to the Valsalva maneuver are abnormal, but the heart rate responses are normal [27]. Similar findings would be expected in an individual with a high thoracic spinal cord transection [16].
Baroreflex-Cardiovagal Function
In 1969, Smyth, Sleight, and Pickering introduced what came to be called the “Oxford method” for quantifying baroreflex-cardiovagal sensitivity [33]. Blood pressure and cardiac interbeat interval are tracked after bolus intravenous injection of a vasoconstrictor that does not directly affect heart rate. Baroreflex sensitivity is defined as the slope of the relationship between the interbeat interval (in milliseconds) vs. systolic blood pressure (in mmHg). The scatterplot is based on the interbeat interval after a 1-beat delay, which approximates the timing of the reflex. Originally, angiotensin was the vasoconstrictor, but because of tachyphylaxis with repeated injections of angiotensin, this was changed to phenylephrine. By this technique, Bristow et al. described decreased baroreflex sensitivity as a function of high blood pressure [4].
In the early 1980s Goldstein compared 6 different techniques to assess baroreflex-cardiovagal function, including the relationship between cardiac interbeat interval and systolic blood pressure during and after release of the Valsalva maneuver [11]. The average intercorrelation among these measures (r = 0.36) was statistically significant but suggested that variance in one measure accounted for an average of about 13% of the variance in the others measures. Standard deviations across subjects were often as large as the mean, indicating important inter-individual variability as well. These findings demonstrated that baroreflex-cardiovagal sensitivity varies widely among subjects and that different techniques for measuring baroreflex sensitivity probably measure different aspects of baroreflex function.
The Valsalva heart rate ratio is of limited value and is largely obsolete, because it does not take into account the dependence of the heart rate response on the blood pressure response.
Baroreflex-Sympathoneural Function
In a patient with baroreflex-sympathoneural failure—whether because of decreased ability to modulate afferent traffic from arterial baroreceptors (e.g., from carotid arteriosclerosis preventing pressure-related distortion of carotid sinus baroreceptors), dys-coordination of input-output relationships among brainstem centers (Figure 3, e.g., from multiple system atrophy), interference with ganglionic neurotransmission (e.g., from autoimmune autonomic ganglionopathy), loss of sympathetic noradrenergic neurons (e.g., from pure autonomic failure), lack of releasable stores of norepinephrine (e.g., dopamine-β-hydroxylase deficiency), or decreased adrenoceptor-mediated responses (e.g., α-adrenoceptor blockade), the same abnormal pattern of blood pressure occurs in Phase IV (Figure 2). Blood pressure increases slowly from the minimum value in Phase III, and there is no pressure overshoot. This pattern of abnormalities can be more sensitive than measurement of orthostatic blood pressure in detecting sympathetic neurocirculatory failure [28].
Figure 3: Diagram of brainstem pathways mediating baroreflxes.
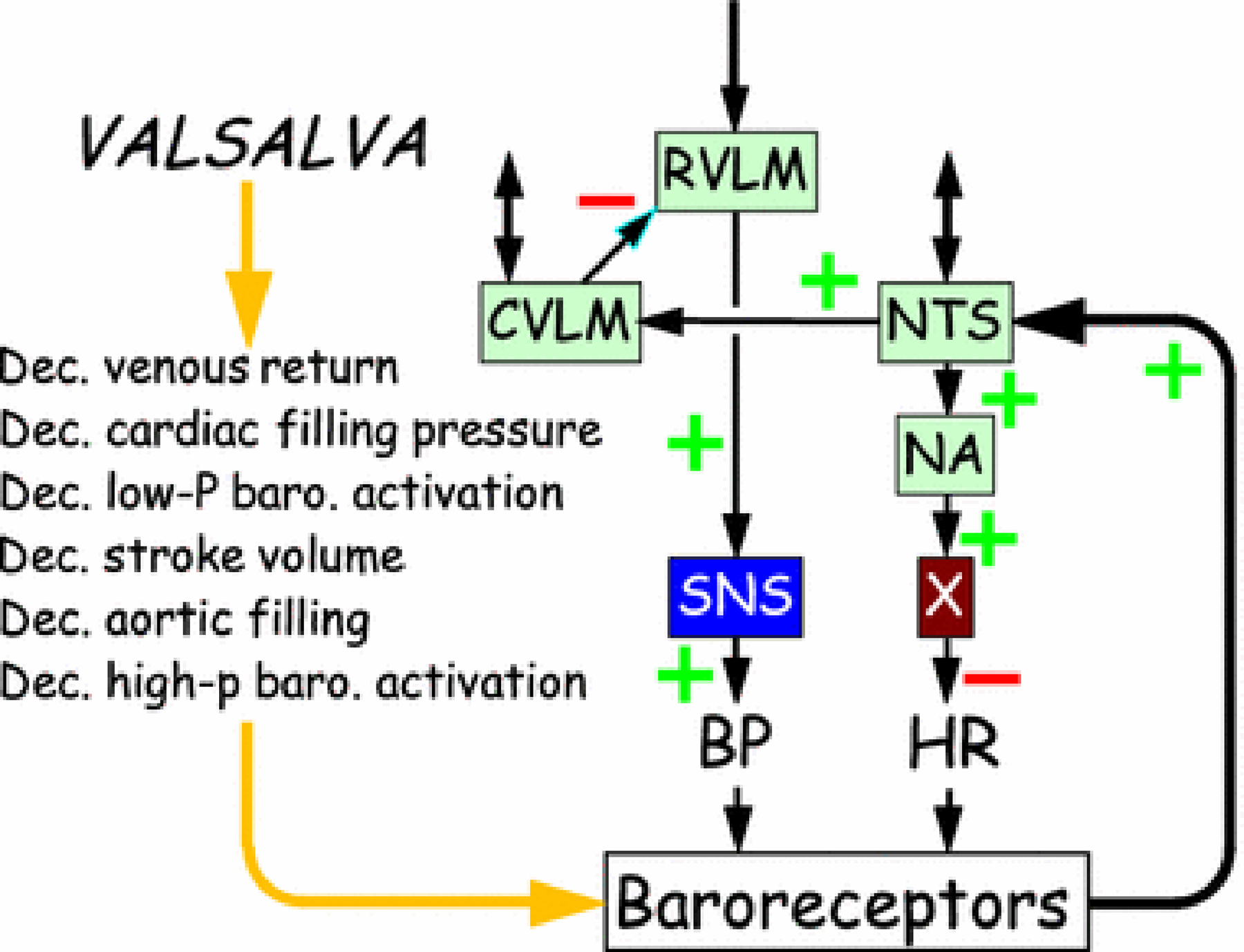
Abbreviations: BP=blood pressure; CVLM=caudal ventrolateral medulla; Dec.=decreased; hi-P=high pressure; HR=heart rate; lo-P=low pressure; NTS=nucleus of the solitary tract; NA=nucleus ambiguus; SNS=sympathetic noradrenergic system; X=vagus (the tenth cranial nerve). The baroreflex arcs involve negative feedback loops, indicated by an odd number of (−) signs across the stations in the loop.
Conversely, if a patient had an abnormal blood pressure pattern in both Phase II (progressive fall in pressure without recovery) and Phase IV (delayed return of pressure to baseline and no overshoot), this would provide evidence for baroreflex-sympathoneural failure.
Compared to quantitative assessment of baroreflex-cardiovagal function by the decrease in interbeat interval per unit decrease in systolic blood pressure during Phase II of the Valsalva maneuver, which was introduced almost a half century ago [25], quantitative methods to assess baroreflex-sympathoneural function by physiological responses to the Valsalva maneuver are more recent. As noted above, baroreflex-sympathoneural failure manifests qualitatively by progressive fall in pressure during Phase II, slow return of blood pressure toward baseline in Phase IV, and no pressure overshoot in Phase IV.
Investigators at the Mayo Clinic introduced a few techniques to accomplish this. Using intravenous pharmacologic tools, Sandroni et al. distinguished separate physiologic components of the sympathoneural response to the Valsalva maneuver. Whereas α-adrenergic blockade with phentolamine blocked or markedly reduced the blood pressure recovery during late Phase II and increased the blood pressure rise during Phase IV, β-adrenergic blockade with propranolol attenuated the Phase IV blood pressure overshoot. On this basis they concluded that the late Phase II response is dependent mostly on peripheral α-adrenergic innervation and Phase IV is dependent mostly on cardiac β-adrenergic innervation [28]. Schrezenmeier et al. validated an index of “adrenergic baroreflex sensitivity” based on the pressure recovery time in Phase IV and the preceding decrease in pressure during Phase II [29]. The most sensitive method is to measure the pressure recovery time (PRT), defined as the time for the blood pressure to return to baseline during Phase IV [36]. Because the extent of variability of the PRT depends on the value for PRT, the log of the PRT may be more appropriate for comparisons with normal. Novak preferred the difference in blood pressure between baseline and the end of Phase II [23]. Rahman et al. applied the trapezoid method to calculate “baroreflex areas” corresponding to the areas under the curve from baseline pressure in Phases II and IV (Figure 2 [25]. For a single overall measure they proposed the log of the sum of the two areas [26].
Although quantitative measures of baroreflex-sympathoneural function have been published, for clinical purposes a qualitative description of the blood pressure trends at the end of Phase II and in Phase III-IV seems sufficient, along with noting the PRT.
The “Square Wave” Response
In some people, instead of blood pressure decreasing to below baseline during Phase II of the Valsalva maneuver, the pressure fails to decrease or can even increase. The Phase III fall in pressure is still present, but because blood pressure did not drop during Phase II – the stimulus for activation of sympathetic noradrenergic outflow – there is no Phase IV overshoot in pressure. This has been called the “square wave response” or the “flat top response” because of the pattern of the pressure during and after the maneuver resembling a mathematical square wave sign [35].
The square wave response occurs in situations where there is increased cardiac filling coupled with increased sympathetic noradrenergic outflow. The most well-known example is congestive heart failure [30]. In healthy people, infusion of norepinephrine increases cardiac filling and changes the shape of the blood pressure pattern to the square wave phenomenon [24]. We noted this in a healthy subject who performed the Valsalva maneuver and had intra-arterial blood pressure recorded continuously, under resting conditions and after rapid infusion of 2 liters of ice-cold saline via a centralized IV catheter to induce mild core hypothermia [9]. The infusion, which increased central venous pressure, resulted in profound, generalized sympathetic noradrenergic activation [10]. As shown in Figure 4, the infusion of cold saline produced the square wave phenomenon.
Figure 4: The square wave response evoked by central IV infusion of cold saline.
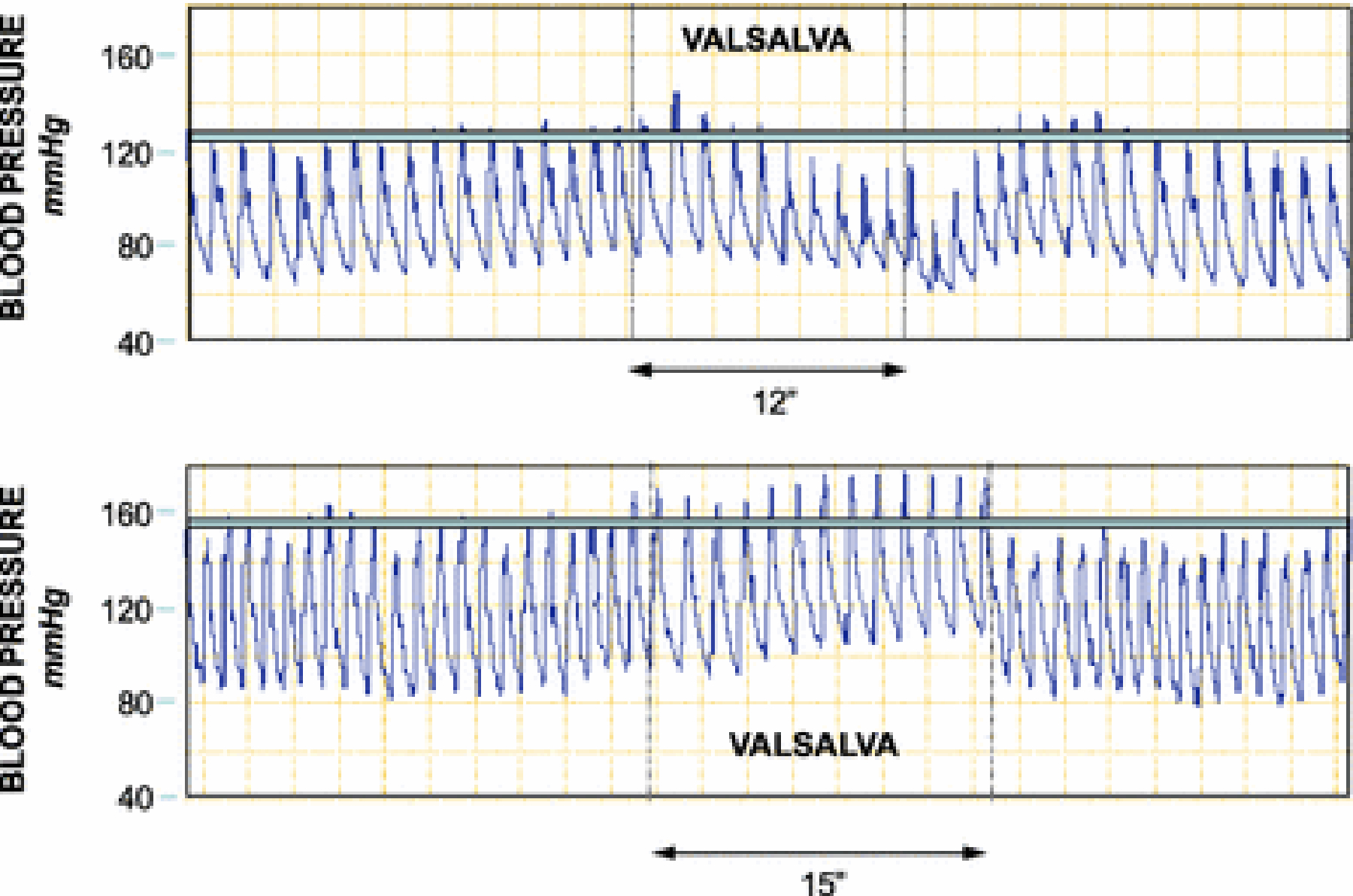
The top panel shows intra-arterial pressure associated with the Valsalva maneuver at baseline, and the bottom panel shows the pressure associated with the Valsalva maneuver in the same subject near the end of a 2-liter infusion of ice-cold saline over about 20 minutes via a centralized large-bore catheter. Note that at baseline, during the maneuver blood pressure decreases below baseline, whereas with cold saline infusion the blood pressure failes to decrease below baseline—the square wave response (flat-top response).
Parameters of the Valsalva Maneuver in Autonomic Function Testing
Different centers use different methods of measuring beat-to-beat hemodynamic responses to the Valsalva maneuver as part of autonomic function testing. The duration varies from 10–20 seconds, and the intensity varies from 30–40 mmHg; or else the subject strains against a closed glottis. The subject usually is supine with head on pillow, although arterial baroreflex gain is not particularly affected by upright posture [7]. If a square wave response is noted, the subject may be tilted head-up to 20 degrees from horizontal to decrease cardiac filling, resulting in a greater fall in blood pressure during Phase II at the expense of decreased specificity of the test due to postural activation of sympathetic outflow [19, 35].
Concurrent Measurements
In order to verify that the subject has delivered the target positive pressure for the targeted duration, it is highly advisable to record the delivered pressure.
Performance of the Valsalva maneuver is associated with a transient wave of sweating, which can be detected by tracking skin electrical conductance. This can be worthwhile, because the absence of sweating during the Valsalva may indicate deficiency of the sympathetic cholinergic system. In other words, tracking beat-to-beat blood pressure, heart rate, and skin electrical conductance can provide information about the status of three components of the autonomic nervous system simultaneously—sympathetic noradrenergic, parasympathetic cholinergic, and sympathetic cholinergic. There have been no studies on the validity and clinical utility of measuring skin electrical conductance during the performance of the Valsalva maneuver; however, this is a well accepted measure of dynamic changes in sweat production, which is an autonomically-mediated response.
Relatively recent advances include recording of skeletal muscle sympathetic nerve activity by peroneal microneurography [5] and functional magnetic resonance imaging during the Valsalva maneuver [15]. These are two of several research findings that require technologies not available at most autonomic testing centers.
Effects of Aging or Bed Rest
Many cross-sectional studies have shown that baroreflex-cardiovagal gain decreases as a function of subject age. The smaller literature about aging effects on baroreflex-cardiovagal gain as assessed by interbeat interval and systolic blood pressure responses to the Valsalva maneuver fit with this inference. Most of this literature refers to data from Phase IV [22]. Based on Phase II data, baroreflex-cardiovagal gain has been reported to be weakly negatively correlated with subject age [11], but at least one report found no aging-related decline in baroreflex-cardiovagal gain based on Phase II data [20]. There are no longitudinal studies on this topic.
In using the magnitude of fall in systolic blood pressure during Phase II and the Phase IV overshoot to gauge sympathetic neurocirculatory function, one should bear in mind that the former is related positively and the latter negatively to normal aging [31]. This means that compared groups in clinical research reports should be of similar age.
Prolonged bed rest has been reported to augment the fall in pressure in Phase II and to increase the Phase IV pressure overshoot [32].
A Closing Anecdote
The paternal grandfather of the second author of this paper, having ruptured both tympanic membranes in 1918 during a grenade explosion in the trenches in World War I, from his hospital bed wrote about his personal experience with the Valsalva maneuver:
I just discovered last night that I can breathe through my ears! And I can shut my mouth, take a long breath, hold my nose, lean back my head, and blow out the two candles on each side of the head of my bed with my ears! It is the best parlour stunt going, and when I get home, the only work I am going to do is to hire myself out to blow out the candles on birthday cakes at parties, and I feel sure I shall get rich at it.
(Text of a letter from James Webb Cheshire (1890–1980) to Mrs. Joseph Blount Cheshire, from Base Hospital No. 18, American Expeditionary Force, France, March 3, 1918)
Forcibly exhaling did not seem to have caused symptomatic hypotension in his case, despite prolonged bed rest [32], because he refused the order to be discharged home and, though injured, returned to combat on the Hindenburg line.
Figure 5 shows still images from a YouTube video of a person blowing up a balloon through his ear. The video can be accessed at https://www.youtube.com/watch?v=qf1VA22s92E.
Figure 5: Still images from a YouTube video clip of a man blowing up a balloon through his ear.

The video can be accessed at https://www.youtube.com/watch?v=qf1VA22s92E. Permission to reproduce these images was obtained from Diagonal View (www.diagonal-view.com).
SYNOPSIS.
Beat-to-beat Blood Pressure and Heart Rate Responses to the Valsalva Maneuver
Measuring beat-to-beat blood pressure and heart rate responses to the Valsalva maneuver is a valuable autonomic function test, which can establish whether orthostatic hypotension is neurogenic and can be used to assess baroreflex-cardiovagal function.
Financial support:
The research reported here was supported by the Division of Intramural Research, NINDS, NIH.
Abbreviations:
- AAG
autoimmune autonomic ganglionopathy
- ANS
autonomic nervous system
- COI
chronic orthostatic intolerance
- NE
norepinephrine
- MSA
multiple system atrophy
- OH
orthostatic hypotension
- OI
orthostatic intolerance
- PAF
pure autonomic failure
- PNS
parasympathetic nervous system
- POTS
postural tachycardia syndrome
- SAS
sympathetic adrenergic system
- SCS
sympathetic cholinergic system
- SEC
skin electrical conductance
- SNS
sympathetic noradrenergic system
REFERENCES
- 1.Appenzeller O, Descarries L (1964) Circulatory Reflexes in Patients with Cerebrovascular Disease. N Engl J Med 271:820–823 [DOI] [PubMed] [Google Scholar]
- 2.Appenzeller O, Goss JE (1971) Autonomic deficits in Parkinson’s syndrome. Arch. Neurol 24:50–57 [DOI] [PubMed] [Google Scholar]
- 3.Appenzeller O, Kornfeld M (1973) Acute pandysautonomia. Clinical and morphologic study. Arch Neurol 29:334–339 [DOI] [PubMed] [Google Scholar]
- 4.Bristow JD, Honour J, Pickering GW, Sleight P, Smyth HS (1969) Diminished baroreflex sensitivity in high blood pressure. Circulation 39:48–54 [DOI] [PubMed] [Google Scholar]
- 5.Delius W, Hagbarth KE, Hongell A, Wallin BG (1972) Manoeuvres affecting sympathetic outflow in human muscle nerves. Acta Physiol. Scand 84:82–94 [DOI] [PubMed] [Google Scholar]
- 6.Derbes VJ, Kerr A Jr. (1955) Valsalva’s maneuver and Weber’s experiment. N Engl J Med 253:822–823 [DOI] [PubMed] [Google Scholar]
- 7.Eckberg DL, Abboud FM, Mark AL (1976) Modulation of carotid baroreflex responsiveness in man: effects of posture and propranolol. J Appl Physiol 41:383–387 [DOI] [PubMed] [Google Scholar]
- 8.Elisberg EI (1963) Heart rate response to the Valsalva maneuver as a test of circulatory integrity. JAMA 186:200–205 [DOI] [PubMed] [Google Scholar]
- 9.Frank SM, Satitpunwaycha P, Bruce SR, Herscovitch P, Goldstein DS (2003) Increased myocardial perfusion and sympathoadrenal activation during mild core hypothermia in awake humans. Clin. Sci 104:503–508 [DOI] [PubMed] [Google Scholar]
- 10.Goldstein DS, Frank SM (2001) The wisdom of the body revisited: The adrenomedullary response to mild core hypothermia in humans. Endocrine Regul. 35:3–7 [PubMed] [Google Scholar]
- 11.Goldstein DS, Horwitz D, Keiser HR (1982) Comparison of techniques for measuring baroreflex sensitivity in man. Circulation 66:432–439 [DOI] [PubMed] [Google Scholar]
- 12.Goldstein DS, Keiser HR (1984) Pressor and depressor responses after cholinergic blockade in humans. Am. Heart J 107:974–979 [DOI] [PubMed] [Google Scholar]
- 13.Goldstein DS, Tack C (2000) Non-invasive detection of sympathetic neurocirculatory failure. Clin. Auton. Res 10:285–291 [DOI] [PubMed] [Google Scholar]
- 14.Hamilton WF, Woodbury RA, Harper HT Jr. (1936) Physiologic relationships between intrathoracic, intraspinal and arterial pressures. JAMA 107:853–856 [Google Scholar]
- 15.Henderson LA, Macey PM, Macey KE, Frysinger RC, Woo MA, Harper RK, Alger JR, Yan-Go FL, Harper RM (2002) Brain responses associated with the Valsalva maneuver revealed by functional magnetic resonance imaging. J Neurophysiol 88:3477–3486 [DOI] [PubMed] [Google Scholar]
- 16.Houtman S, Oeseburg B, Hopman MT (1999) Non-invasive assessment of autonomic nervous system integrity in able-bodied and spinal cord-injured individuals. Clin Auton Res 9:115–122 [DOI] [PubMed] [Google Scholar]
- 17.Imholz BP, van Montfrans GA, Settels JJ, van der Hoeven GM, Karemaker JM, Wieling W (1988) Continuous non-invasive blood pressure monitoring: reliability of Finapres device during the Valsalva manoeuvre. Cardiovasc. Res 22:390–397 [DOI] [PubMed] [Google Scholar]
- 18.Jellinek EH (2006) The Valsalva manoeuvre and Antonio Valsalva (1666–1723). J R Soc Med 99:448–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Looga R (2005) The Valsalva manoeuvre--cardiovascular effects and performance technique: a critical review. Respir. Physiol. Neurobiol 147:39–49 [DOI] [PubMed] [Google Scholar]
- 20.Matsukawa T, Sugiyama Y, Watanabe T, Kobayashi F, Mano T (1998) Baroreflex control of muscle sympathetic nerve activity is attenuated in the elderly. J. Auton. Nerv. Syst 73:182–185 [DOI] [PubMed] [Google Scholar]
- 21.McKendrick JG (1910) Hearing. In: The Encyclopaedia Britannica The Encyclopaedia Britannica Company, New York, NY, p 123a [Google Scholar]
- 22.Monahan KD, Dinenno FA, Seals DR, Clevenger CM, Desouza CA, Tanaka H (2001) Age-associated changes in cardiovagal baroreflex sensitivity are related to central arterial compliance. Am. J. Physiol 281:H284–289 [DOI] [PubMed] [Google Scholar]
- 23.Novak P (2011) Assessment of sympathetic index from the Valsalva maneuver. Neurology 76:2010–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Neill A, Cudkowicz L (1965) Effect of L-norepinephrine on right atrial pressure and the “square wave response” to the Valsalva maneuver. Am. Heart J 69:220–228 [DOI] [PubMed] [Google Scholar]
- 25.Pickering TG, Sleight P (1969) Quantitative index of baroreflex activity in normal and hypertensive subjects using Valsalva’s manoeuvre. Br. Heart J 31:392. [PubMed] [Google Scholar]
- 26.Rahman F, Goldstein DS (2014) Quantitative indices of baroreflex-sympathoneural function. Clin. Auton. Res 24:103–110 [DOI] [PubMed] [Google Scholar]
- 27.Robertson D, Haile V, Perry SE, Robertson RM, Phillips JA 3rd, Biaggioni I (1991) Dopamine beta-hydroxylase deficiency. A genetic disorder of cardiovascular regulation. Hypertension 18:1–8 [DOI] [PubMed] [Google Scholar]
- 28.Sandroni P, Benarroch EE, Low PA (1991) Pharmacological dissection of components of the Valsalva maneuver in adrenergic failure. J Appl Physiol (1985) 71:1563–1567 [DOI] [PubMed] [Google Scholar]
- 29.Schrezenmaier C, Singer W, Swift NM, Sletten D, Tanabe J, Low PA (2007) Adrenergic and vagal baroreflex sensitivity in autonomic failure. Arch. Neurol 64:381–386 [DOI] [PubMed] [Google Scholar]
- 30.Sharpey-Schafer EP (1955) Effects of Valsalva’s manoeuvre on the normal and failing circulation. Br Med J 1:693–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimada K, Kitazumi T, Ogura H, Sadakane N, Ozawa T (1986) Effects of age and blood pressure on the cardiovascular responses to the Valsalva maneuver. J. Am. Geriatr. Soc 34:431–434 [DOI] [PubMed] [Google Scholar]
- 32.Shoemaker JK, Hogeman CS, Sinoway LI (2003) Sympathetic responses to Valsalva’s manoeuvre following bed rest. Can. J. Appl. Physiol 28:342–355 [DOI] [PubMed] [Google Scholar]
- 33.Smyth HS, Sleight P, Pickering GW (1969) Reflex regulation of arterial pressure during sleep in man: quantitative method of assessing baroreflex sensitivity. Circ. Res 24:109–121 [DOI] [PubMed] [Google Scholar]
- 34.Vaisrub S (1974) Editorial: The Valsalva maneuver in diabetic neuropathy. JAMA 228:1151. [PubMed] [Google Scholar]
- 35.Vogel ER, Corfits JL, Sandroni P, Sletten DM, Benarroch EE, Fealey RD, Suarez GA, Gehrking TL, Gehrking JA, Low PA (2008) Effect of position on valsalva maneuver: supine versus 20 degree position. J. Clin. Neurophysiol 25:313–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vogel ER, Sandroni P, Low PA (2005) Blood pressure recovery from Valsalva maneuver in patients with autonomic failure. Neurology 65:1533–1537 [DOI] [PubMed] [Google Scholar]


