Abstract
BACKGROUND
To examine the associations between urbanization and hypertension, stage II hypertension, and hypertension control.
METHODS
Data on 16,360 US adults aged 18 years or older from the 2013–2018 National Health and Nutrition Examination Survey (NHANES) were used to estimate the prevalence of hypertension (blood pressure (BP) ≥130/80 mm Hg or use of medication for hypertension), stage II hypertension (BP ≥140/90 mm Hg), and hypertension control (BP <130/80 mm Hg among hypertensives) by urbanization, classified by levels of metropolitan statistical areas as large MSAs (population ≥1,000,000), medium to small MSAs (population 50,000–999,999), and non-MSAs (population <50,000).
RESULTS
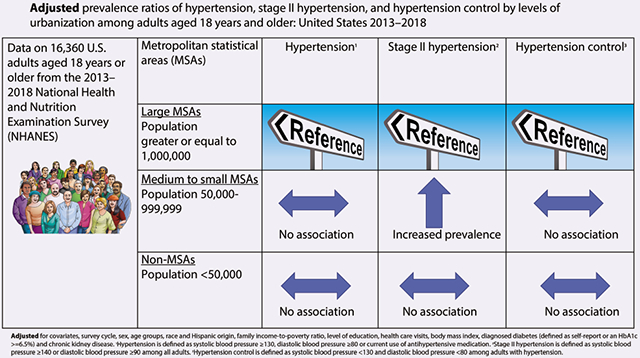
All prevalence ratios (PRs) were compared with large MSAs and adjusted for demographics and risk factors. The PRs of hypertension were 1.07 (95% confidence interval (CI) = 0.99–1.14) for adults residing in medium to small MSAs and 1.06 (95% CI = 0.99–1.13) for adults residing in non-MSAs. For stage II hypertension, the PRs were higher for adults residing in medium to small MSAs 1.21 (95% CI = 1.06–1.36) but not for adults residing in non-MSAs 1.06 (95% CI = 0.88–1.29). For hypertension control, the PRs were 0.96 (95% CI = 0.91–1.01) for adults residing in medium to small MSAs and 1.00 (95% CI = 0.93–1.06) for adults residing in non-MSAs.
CONCLUSIONS
Among US adults, urbanization was associated with stage II hypertension.
Keywords: blood pressure, hypertension, hypertension stage II, hypertension control, NHANES, urbanization
Graphical Abstract
Hypertension is a well-known risk factor for cardiovascular disease. In 2015, it was the number one reason for disability-adjusted life years globally.1 In the United States, data from the National Health and Nutrition Examination Survey (NHANES) showed the age-adjusted prevalence of hypertension (blood pressure (BP) ≥130/80 or currently taking medication to lower BP) decreased from 47.0% in 1999–2000 to 41.7% in 2013–2014, and then increased to 45.4% in 2017–2018. While men followed a similar pattern, women did not change from during the same timeframe.2 Additionally, Muntner et al. used NHANES data from 10 survey cycles to examine trends in hypertension control spanning from 1999 to 2018. The findings showed that there was an increase from 1999–2000 to 2007–2008. The results also showed the estimated proportion of controlled hypertension plateaued in 2013–2014 at 53.8% and then declined to 43.7% in 2017–2018.3
In 2018, coronary heart disease (CHD) was the leading cause (42.1%) of deaths associated with cardiovascular disease in the United States.4 The physiological link between CHD and hypertension is well established, specifically high BP brings about changes in the structure of the left ventricle resulting in left ventricle remodeling.5,6 In a meta-analysis, Yildiz et al. showed there was more than a 2-fold increase in odds of developing left ventricular hypertrophy with the presence of hypertension.5 Factors, such as demographics, health care utilization, and health risk factors are all known to be associated with CHD.7,8 However, an additional factor associated with CHD is place of residence and geographic variation.9-14 Indeed, the role of urbanicity and geographic variation on the incidence of CHD mortality and morbidity has been recently stressed by the National Heart, Lung, and Blood Institute workshop report that recommended including geographical considerations in cardiovascular research.15
Although urbanization is a major risk factor for CHD, few reports have described its association with hypertension in the United States. The Behavioral Risk Factor Surveillance System (BRFSS), a nationwide survey that collects self-reported data, has been used to examine the association between urbanization and hypertension and reported hypertension to be 5% more prevalent in rural areas compared with urban/metropolitan areas.16,17 In contrast to BRFSS, the NHANES objectively measures BP on participants as part of the medical examination component of the survey. However, there have been only 2 reports of using the NHANES BP measurements (NHANES Epidemiologic Follow-Up Study (1971–1984) and NHANES III (1988–1994)) to determine hypertension and its association with urbanization.18,19
Using measured BP data from the 2013–2018 NHANES, the purpose of this analysis is to explore the association between hypertension, stage II hypertension, and hypertension control and urbanization with more recent data.
METHODS
Survey description
The NHANES, conducted by the National Center for Health Statistics (NCHS), is a cross-sectional, nationally representative survey of the US civilian noninstitutionalized population. The survey uses a complex, stratified, multistage probability cluster-sampling design.20 Participants are interviewed at their homes to obtain information on health history and health behaviors. Subsequently, they undergo a physical examination at a mobile examination center (MEC).21 Written informed consent is obtained from all adult participants, and the NCHS Research Ethics Review Board approved the NHANES protocol.
Sample
Between 2013–2018 NHANES identified 30,043 individuals aged 18 years and older from screened households who were eligible to participate in the survey. Specifically, the response rates for the different survey cycles were as follows: 64% in 2013–2014 (screened = 9,216; examined = 5,924); 59% in 2015–2016 (screened = 9,800; examined = 5,735); and 50% in 2017–2018 (screened = 11,027; examined = 5,533). Overall, 17,192 individuals were examined between 2013 and 2018. Of those examined, 832 persons were excluded for the following reasons: 190 were pregnant and 642 had missing systolic and diastolic BP data. These exclusions resulted in a final analytic sample of 16,360 participants aged 18 years and older.
Outcome variables
All BP readings were obtained using a Bauman true gravity mercury wall model with standard Bauman cuffs and took place during a single examination visit. Appropriate BP cuff sizes were selected based on the measurement of the participant’s mid-arm circumference. After a 3-minute rest and establishing maximum inflation levels, the participants had their systolic and diastolic BPs (onset of Korotkoff phase 1 [K1] sound and fading of Korotkoff phase 5 [K5] sound) measured; BP measurements were taken 30 seconds apart. The average of up to 3 brachial systolic and diastolic BP readings was used as the participant’s systolic and diastolic BP values. For participants with only 1 reading (n = 53 or 0.32%), that single reading was used. For more details see the procedure manual.22
A participant was defined as having hypertension if at least one of the following conditions was satisfied: systolic BP of 130 mm Hg or greater, diastolic BP of 80 mm Hg or greater, or the participant reported currently taking medication for high BP.2,23 Stage II hypertension was defined as systolic BP of 140 mm Hg or greater, or diastolic BP of 90 mm Hg or greater among the whole adult sample. Hypertension control was defined as systolic BP <130 mm Hg and diastolic BP <80 mm Hg among those with hypertension.23
Independent variable
Urbanization
NCHS developed a 6-level urban–rural classification scheme based on the county of residence.24 Data on county of residence and the county exam location in NHANES can only be obtained through the NCHS Research Data Center or other federal research data center (https://www.cdc.gov/rdc/). The scheme delineated 4 metropolitan and 2 nonmetropolitan classifications for the survey years analyzed.24 The metropolitan classifications included: (i) large central metropolitan or counties in metropolitan statistical areas (MSAs) with a population of 1 million or more that contain all or part of the area’s principal city; (ii) large fringe metropolitan or counties in MSAs with a population of 1 million or more that surrounded the large central metropolitan counties; (iii) medium metropolitan or counties in MSAs with a population of 250,000–999,999; and (iv) small metropolitan or counties in MSAs with a population of <250,000. Nonmetropolitan, or the most rural areas classification, included: (i) micropolitan or counties in MSAs, which contained an urban cluster with 2,500–49,999 population; and (ii) noncore, or nonmetropolitan counties that did not qualify as micropolitan.
To increase the sample size and the degrees of freedom for the subgroup analysis, we condensed the 6-level NCHS scheme into 3 categories: large MSAs (combined large central and fringe metropolitan counties, for a population of 1 million or more); medium to small MSAs (combined medium and small metropolitan counties, for a population <1 million); and non-MSAs (combined micropolitan and noncore counties, for a population of <50,000) as done by previous urbanization studies using NHANES data.25
Covariates
Demographics
Age was categorized as 18–39, 40–59, 60–79, and 80 years and over. Data on race and Hispanic origin, which is self-reported in NHANES, were categorized as non-Hispanic white, non-Hispanic black, non-Hispanic Asian, Hispanic, and other/multi-racial. Individuals reporting “other/multi-racial” were included in the overall analyses but not reported separately due to small numbers among this group.
Income
Family income-to-poverty ratio is the ratio of a family’s income to its appropriate poverty guidelines as established by the U.S. Department of Health and Human Services.26 Four categories of approximately equal numbers of participants in each category were used for these analyses: <1.00, 1.00 to <2.00, 2.00 to <4.00, and ≥4.00. Larger family income-to-poverty ratios indicate higher income, adjusted for the size of a family.
Education level
Education level was self-reported based on response to the question, “What is the highest grade or level of school you have completed or the highest degree you have received?”27 Response categories included: high school or less, more than high school or some college, and college graduate.
Risk factors
Health care utilization
Frequency of visits to a health care provider was self-reported based on the answer to the home interview question, “During the past 12 months, how many times have you seen a doctor or other health care professional about your health at a doctor’s office, a clinic, a hospital emergency room, at home, or some other place?”27 The frequencies of visits ranged from: 0–1, 2–3, and 4 or more.
Several biophysiological risk factors are associated with hypertension and hypertension control and were included in this analysis as covariates; among them are body mass index (BMI), chronic kidney disease, and diabetes.4,28 During the physical examination in the MEC, standardized measurements of weight and height were obtained.27 BMI was calculated as weight over height in meters squared (kg/m2), and was categorized using criteria established by the National Institutes of Health28 as underweight (<18.5), normal weight (18.5–24.9), overweight (25.0–29.9), Class I obesity (30.0–34.9), Class II obesity (35.0–39.9) and Class III obesity (≥40).29 Underweight was included in the overall analysis but was not reported separately due to small numbers among this group.
Diagnosed diabetes was defined based on participant self-reported of ever having been told by a doctor or health care provider that he/she has diabetes (see suggested citation) or a glycated hemoglobin ≥6.5%.30
Blood and urine samples were collected in the MEC. Chronic kidney disease was defined based on the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation estimated glomerular filtration rate and the presence of albuminuria (urine creatinine to albumin ratio). Chronic kidney disease was defined as an estimated glomerular filtration rate serum creatinine <60 ml/min per 1.73 m2 or urine albumin-to-creatinine ratio >30 mg/g.31
Statistical analysis
All statistical analyses were performed using survey procedures in SAS 9.4 for Windows (SAS Institute, Cary, NC) and SAS callable SUDAAN 11.0 software (Research Triangle Institute, Research Triangle Park, NC). All estimates were weighted using the MEC sample weights and incorporated sampling design information; the sample weights accounted for the unequal probabilities of selection resulting from the complex sample design, survey nonresponse, and the planned oversampling of selected population subgroups. The calculated variance estimates accounted for the complex survey design by using Taylor series linearization.
Prevalence of hypertension, stage II hypertension, and hypertension control was calculated by selected covariates. Following the 2017 NHANES analytic guidelines, the 95% confidence intervals (CIs) of prevalence estimates were calculated using the Korn and Graubard method.32 Effective sample size, absolute and relative 95% CI width, and degrees of freedom were evaluated to determine the reliability of prevalence estimates according to the NCHS Data Standards for Proportions.33 A Satterthwaite-adjusted Wald chi-square test was used to test for independence of each variable and urbanization.34 Pairwise comparisons of prevalence of hypertension, stage II hypertension, and hypertension control between the subcategories were tested using a linear contrast. Linear and quadratic trends were tested for survey years, age groups, and BMI using orthogonal polynomial contrasts which tested whether the linear and quadratic effects were zero (SUDAAN’s proc descript procedure with polynomial option).35 Prevalence of hypertension and stage II hypertension was age adjusted by the direct method to the 2000 US census population, using age groups 18–39, 40–59, 60–79, and 80 years and older.36
In addition, following the recommendations of Crim et al.,37 we calculated age-adjusted prevalence of hypertension control adjusted to the subpopulation of individuals who had hypertension in NHANES 2007–2016 using age groups 18–39, 40–59, 60–79, and 80 and older. This adjustment was done because hypertension is more prevalent among older individuals and using a standard age adjustment to the US population will over-represent the younger age groupings relative to the subpopulation of individuals with hypertension.37
One univariate and 2 multivariate log-linear regression models were used to estimate the unadjusted and adjusted prevalence ratios (PRs) of hypertension, stage II hypertension, and hypertension control by urbanization categories. The univariate models included urbanization as a predictor. The first multivariate model was adjusted for the nonmodifiable demographic confounding variables including age groups, sex, and race and Hispanic origin. The second multivariate models were adjusted for more confounding covariates (survey cycle, sex, age groups, race and Hispanic origin, family income, education, health care visits, BMI, diabetes, and chronic kidney disease) in addition to age groups, sex, and race and Hispanic origin. Unadjusted and adjusted PRs with a 95% CI not including 1.0 were considered statistically significant. We derived PRs in this paper to avoid overestimating the strength of the association of the prevalence odds ratio when the prevalence is high.38
RESULTS
Sample characteristics
No statistical differences were found for sex or age group; however, all other covariates were associated with urbanization (Table 1). Non-Hispanic white adults were less likely to live in large MSAs (42.9%) compared with Hispanic (60.5%), non-Hispanic black (62.1%), and non-Hispanic Asian (72.5%) adults. Among adults with the lowest income-to-poverty ratio (family income-to-poverty ratio <1), 45% lived in large MSAs and 19.9% lived non-MSAs. Among adults with the highest family income-to-poverty ratio (≥4), 56.6% lived in large MSAs and 9.2% lived in non-MSAs. College graduates were more likely to live in large MSAs (60.7%) compared with adults with high school or less education (42.9%) and adults with more than high school or some college education (46.3%). About 50.8% adults with 0–1 health care visits in past year lived in large MSAs while 48.1% adults with 4 or more health care visits in past year lived in large MSAs. Among adults categorized as having a normal BMI, 54.7% lived in large MSAs and 11.5% lived in non-MSAs, whereas adults categorized as having Class III obesity (BMI >40), 39.0% lived in large MSAs and 21.2% lived in non-MSAs. Adults diagnosed with diabetes or having chronic kidney disease were less likely to live in large MSAs (44.6% and 46.1%, respectively) compared with those who were not diagnosed with diabetes or having chronic kidney disease (49.9% and 49.7%).
Table 1.
Sample characteristics of adults aged 18 years and older by levels of metropolitan statistical areas (MSAs): United States, 2013–2018
| Large MSAs (n = 8,884) | Medium to small MSAs (n = 5,277) | Non-MSAsa (n = 2,199) | |||
|---|---|---|---|---|---|
| n e | Percent (95% CI) | P valueb | |||
| Sex | 0.41 | ||||
| Male | 7,981 | 48.9 (38.9–58.9) | 37.1 (25.3–50.0) | 14.1 (7.6–23.0) | |
| Female | 8,379 | 49.8 (39.8–59.9) | 36.0 (24.5–48.8) | 14.2 (7.6–23.4) | |
| Age | 0.16 | ||||
| 18–39 years | 5,775 | 50.6 (40.1–61.1) | 36.9 (25.1–50.0) | 12.5 (6.7–20.5) | |
| 40–59 years | 5,175 | 49.8 (40.2–59.5) | 35.7 (24.3–48.5) | 14.4 (7.4–24.3) | |
| 60–79 years | 4,414 | 47.6 (36.9–58.3) | 36.7 (24.6–50.1) | 15.8 (8.5–25.9) | |
| 80 years and older | 996 | 44.6 (33.7–55.9) | 38.1 (24.8–52.8) | 17.3 (9.1–28.7) | |
| Race and Hispanic originc | <0.001 | ||||
| Non-Hispanic white (NHW) | 5,984 | 42.9 (32.0–54.3) | 40.5 (26.8–55.3) | 16.6 (8.2–28.5) | |
| Hispanic | 4,169 | 60.5 (48.6–71.6) | 28.4 (17.8–41.2) | 11.1 (5.0–20.5)d | |
| Non-Hispanic black (NHB) | 3,543 | 62.1 (49.3–73.8) | 27.3 (17.0–39.7) | 10.7 (3.9–22.1)d | |
| Non-Hispanic Asian (NHA) | 2,011 | 72.5 (58.4–83.9) | 26.0 (14.5–40.4) | 1.6 (0.6–3.2)d | |
| Family income-to-povertyb ratio | <0.001 | ||||
| <1 | 3,199 | 45.0 (35.5–54.8) | 35.0 (23.4–48.1) | 19.9 (11.2–31.5) | |
| 1 to <2 | 3,969 | 43.0 (32.8–53.5) | 37.8 (25.5–51.5) | 19.2 (10.6–30.6) | |
| 2 to <4 | 3,911 | 45.4 (35.5–55.7) | 39.9 (27.0–53.8) | 14.7 (7.5–24.8) | |
| 4 or more | 3,616 | 56.6 (44.7–67.9) | 34.2 (22.4–47.6) | 9.2 (4.6–16.1) | |
| Level of educationb | <0.001 | ||||
| High school or less | 7,508 | 42.9 (33.7–52.5) | 38.0 (25.9–51.3) | 19.1 (10.2–31.1) | |
| More than high school or some college | 4,990 | 46.3 (36.5–56.4) | 38.3 (26.1–51.8) | 15.4 (8.5–24.8) | |
| College graduate | 3,847 | 60.7 (48.5–71.9) | 32.7 (21.2–46.0) | 6.6 (3.2–11.9) | |
| Health care visits in past yearb | <0.05 | ||||
| 0–1 | 5,642 | 50.8 (41.1–60.5) | 36.4 (25.1–49.1) | 12.7 (6.8–21.1) | |
| 2–3 | 4,759 | 49.4 (39.0–59.9) | 36.6 (24.8–49.6) | 14.0 (7.4–23.3) | |
| 4 or more | 5,932 | 48.1 (37.8–58.5) | 36.4 (24.5–49.7) | 15.5 (8.2–25.7) | |
| Body mass index (BMI, kg/m2)b | <0.001 | ||||
| Normal (18.5–24.9) | 4,447 | 54.7 (44.5–64.6) | 33.8 (22.5–46.7) | 11.5 (5.8–19.8) | |
| Overweight (25.0–29.9) | 5,107 | 51.7 (41.5–61.8) | 34.1 (23.1–46.6) | 14.2 (7.7–23.2) | |
| Class I obesity (30.0–34.9) | 3,402 | 44.8 (34.6–55.3) | 40.4 (28.0–53.7) | 14.8 (7.8–24.7) | |
| Class II obesity (35.0–39.9) | 1,635 | 46.0 (35.4–56.9) | 39.3 (26.6–53.0) | 14.7 (7.7–24.6) | |
| Class III obesity (≥40) | 1,295 | 39.0 (29.3–49.5) | 39.8 (26.2–54.6) | 21.2 (11.7–33.6) | |
| *Diagnosed Diabetesb | <0.01 | ||||
| Yes | 2,660 | 44.6 (35.0–54.4) | 36.9 (24.7–50.4) | 18.6 (10.0–30.1) | |
| No | 12,983 | 49.9 (39.9–60.0) | 36.5 (25.0–49.3) | 13.6 (7.3–22.3) | |
| Chronic kidney diseaseb | <0.01 | ||||
| Yes | 2,713 | 46.1 (36.8–55.6) | 36.1 (24.6–49.0) | 17.8 (10.0–28.2) | |
| No | 12,673 | 49.7 (39.5–59.8) | 36.7 (25.1–49.6) | 13.6 (7.3–22.5) | |
Degrees of freedom F < 8 (degrees of freedom = 7).
P value is from Satterthwaite-adjusted Wald chi-square test of independence.20
Sample sizes not adding up to total of 100% since results on non-Hispanic and other groups not shown.
The proportion does not meet NCHS presentation standard for proportions (relative width of Korn and Graubard 95% confidence interval >130%).
Unweighted sample size.
Diagnosed Diabetes = defined as self-report or an HbA1c ≥6.5%.
Source: 2013–2018 National Health and Nutrition Examination Survey.
The prevalence of hypertension, stage II hypertension, and hypertension control by urbanization
Hypertension prevalence increased (P < 0.05 compared with large MSAs) with decreasing level of urbanization, from 41.1% (95% CI = 39.2%–43.0%) in large MSAs, 45.4% (95% CI = 42.3%–48.5%) in medium to small MSAs, and 48.6% (95% CI = 44.4%–52.8%) in non-MSAs (Table 2). Stage II hypertension prevalence was 14.1% (95% CI = 12.7%–15.6%) in large MSAs, 17.1% (95% CI = 15.2%–19.2%) in medium or small MSAs (P < 0.01 compared with large MSAs), and 16.4% (95% CI = 12.0%–21.7%) in non-MSAs which were similar to the prevalence of large MSAs (P = 0.22). The prevalence of controlled hypertension was similar between large MSAs (21.0%, 95% CI = 18.6%–23.5%) and medium to small MSAs (20.6%, 95% CI = 17.9%–23.5%), but was higher in non-MSAs (25.9%, 95% CI = 21.3%–31.0%) compared with the reference large MSAs (P < 0.05) (Table 3).
Table 2.
Age-adjusted prevalence of hypertension and stage II hypertension among adults 18 years of age and older: United States 2013–2018
| Hypertension |
Stage II hypertension |
||||
|---|---|---|---|---|---|
| n e | Prevalence (95% CI) | P valuea | Prevalence (95% CI) | P valuea | |
| Overalld | 16,360 | 43.7 (42.4–45.0) | 15.5 (14.5–16.6) | ||
| Survey yearsd | |||||
| 2013–2014 | 5,657 | 41.8 (39.8, 43.8) | <0.05, for linear trend | 13.7 (11.7, 15.8) | <0.01, for linear trend |
| 2015–2016 | 5,504 | 43.7 (41.2, 46.3) | 15.2 (13.5, 17.0) | ||
| 2017–2018 | 5,199 | 45.6 (42.5, 48.7) | 17.7 (15.4, 20.2) | ||
| Urbanizationd | |||||
| Large MSAs | 8,884 | 41.1 (39.2, 43.0) | Refb | 14.1 (12.7, 15.6) | Refb |
| Medium to small MSAs | 5,277 | 45.4 (42.3, 48.5) | <0.01 | 17.1 (15.2, 19.2) | <0.01 |
| Non MSAs | 2,199 | 48.6 (44.4, 52.8)c | <0.001 | 16.4 (12.0, 21.7)3 | 0.22 |
| Demographicsd | |||||
| Sex | |||||
| Male | 7,981 | 47.9 (46.0–49.7) | Refb | 17.0 (15.5–18.6) | Refb |
| Female | 8,379 | 39.3 (37.8–40.8) | <0.001 | 13.9 (13.0–14.9) | <0.001 |
| Age | |||||
| 18–39 years | 5,775 | 20.4 (18.8, 22.0) | <0.001, for linear trend | 5.1 (4.4, 5.8) | <0.01, for linear trend |
| 40–59 years | 5,175 | 51.6 (49.3, 54.0) | 17.0 (15.1, 18.9) | ||
| 60–79 years | 4,414 | 72.6 (70.0, 75.1) | 29.3 (27.3, 31.4) | ||
| 80 years and older | 996 | 84.1 (81.7, 86.3) | 47.4 (43.2, 51.5) | ||
| Race and Hispanic origind | |||||
| Non-Hispanic white | 5,984 | 42.1 (40.2, 44.1) | Refb | 14.1 (12.5, 15.7) | Refb |
| Hispanic | 4,169 | 42.4 (40.9, 43.9) | 0.81 | 16.4 (14.9, 17.9) | <0.05 |
| Non-Hispanic black | 3,543 | 55.1 (53.3, 57.0) | <0.001 | 23.9 (22.2, 25.5) | <0.001 |
| Non-Hispanic Asian | 2,011 | 42.7 (40.1, 45.3) | 0.70 | 16.5 (14.8, 18.3) | <0.05 |
| Family income-to-poverty ratiod | |||||
| <1 | 3,199 | 45.9 (43.6, 48.2) | Refb | 18.9 (16.4, 21.5) | Refb |
| 1 to <2 | 3,969 | 46.7 (44.0, 49.5) | 0.58 | 17.2 (15.4, 19.1) | 0.20 |
| 2 to <4 | 3,911 | 45.2 (43.0, 47.3) | 0.58 | 15.1 (13.7, 16.6) | <0.01 |
| 4 or more | 3,616 | 40.4 (37.9, 43.0) | <0.001 | 13.4 (11.5, 15.5) | <0.001 |
| Level of educationd | |||||
| High school or less | 7,508 | 46.3 (44.7, 47.9) | Refb | 17.8 (16.1, 19.6) | Refb |
| More than high school or some college | 4,990 | 47.0 (44.6, 49.4) | 0.63 | 15.9 (14.5, 17.3) | 0.56 |
| College graduate | 3,847 | 37.2 (34.9, 39.6) | <0.001 | 12.4 (10.9, 14.1) | <0.001 |
| Health care utilizationd | |||||
| Health care visits in past year | |||||
| 0–1 | 5,642 | 39.2 (36.7, 41.7) | Refb | 17.1 (15.6, 18.7) | Refb |
| 2–3 | 4,759 | 42.6 (40.4, 44.8) | <0.05 | 14.3 (12.9, 15.9) | <0.01 |
| 4 or more | 5,932 | 47.6 (45.5, 49.7) | <0.001 | 15.5 (13.9, 17.2) | 0.11 |
| Risk factors | |||||
| Body mass index (BMI, kg/m2)d | |||||
| Normal (18.5–24.9) | 4,447 | 30.8 (28.4, 33.3) | <0.001, for linear trend | 11.9 (10.4, 13.6) | <0.001, for linear trend |
| Overweight (25.0–29.9) | 5,107 | 40.3 (38.6, 42.1) | 14.3 (12.7, 15.9) | ||
| Class I obesity (30.0–34.9) | 3,402 | 50.0 (47.9, 52.1) | 15.4 (13.8, 17.2) | ||
| Class II obesity (35.0–39.9) | 1,635 | 57.5 (53.7, 61.2) | 21.4 (18.1, 25.0) | ||
| Class III obesity (≥40) | 1,295 | 66.5 (63.0, 69.9) | 25.0 (22.1, 28.2) | ||
| *Diagnosed Diabetesd | |||||
| Yes | 2,660 | 67.2 (64.4, 70.0) | Refb | 23.2 (21.2, 25.3) | Refb |
| No | 12,983 | 41.1 (39.6, 42.6) | <0.001 | 14.6 (13.4, 15.9) | <0.001 |
| Chronic kidney diseased | |||||
| Yes | 2,713 | 57.8 (55.1, 60.6) | Refb | 25.7 (23.5, 28.0) | Refb |
| No | 12,673 | 41.5 (39.9, 43.1) | <0.001 | 13.7 (12.6, 15.0) | <0.001 |
Notes: Hypertension is defined as systolic blood pressure ≥130, diastolic blood pressure ≥80 or current use of antihypertensive medication. Stage 2 hypertension is defined as systolic blood pressure ≥140 or diastolic blood pressure ≥90. Abbreviations: CI, confidence interval; MSAs, metropolitan statistical areas.
P value for trend test or for pairwise comparison.
The reference group for pairwise comparison.
Degrees of freedom <8 (degrees of freedom = 7).
Age-adjusted to the U.S. Census 2000 population using age groups 18–39, 40–59, 60–79, 80 years and older with weights 0.4203, 0.3572, 0.1776, and 0.0449.
Unweighted sample size.
Diagnosed Diabetes = defined as self-report or an HbA1c ≥6.5%.
Source: 2013–2018 National Health and Nutrition Examination Survey.
Table 3.
Age-adjusted prevalence of controlled hypertension among adults 18 years of age and older with hypertension: United States 2013–2018
| n e | Age-adjusted % Controlled (95% CI) |
P valuea | |
|---|---|---|---|
| Overalld | 8,229 | 21.7 (20.0–23.4) | |
| Survey yearsd | |||
| 2013–2014 | 2,630 | 24.8 (21.6, 28.1) | <0.01, for linear trend |
| 2015–2016 | 2,755 | 21.7 (18.1, 25.7) | |
| 2017–2018 | 2,844 | 18.9 (16.4, 21.5) | |
| Urbanizationd | |||
| Large MSAs | 4,280 | 21.0 (18.6, 23.5) | Refb |
| Medium to small MSAs | 2,736 | 20.6 (17.9, 23.5) | 0.80 |
| Non MSAs | 1,213 | 25.9 (21.3, 31.0)c | <0.05 |
| Demographics | |||
| Sexd | |||
| Male | 4,260 | 20.4 (18.5–22.5) | Refb |
| Female | 3,969 | 23.8 (21.6–26.1) | <0.01 |
| Age | |||
| 18–39 years | 1,173 | 7.0 (5.2, 9.2) | <0.001, for linear trend |
| 40–59 years | 2,796 | 20.9 (17.9, 24.1) | |
| 60–79 years | 3,424 | 29.6 (26.9, 32.5) | |
| 80 years and older | 836 | 21.7 (17.7, 26.1) | |
| Race and Hispanic origind | |||
| Non-Hispanic white | 3,025 | 22.9 (20.5, 25.3) | Refb |
| Hispanic | 1,878 | 17.4 (15.2, 19.8) | <0.01 |
| Non-Hispanic black | 2,139 | 19.5 (17.6, 21.5) | <0.05 |
| Non-Hispanic Asian | 881 | 14.3 (11.9, 17.0) | <0.001 |
| Family income-to-poverty ratiod | |||
| <1 | 1,510 | 21.5 (18.6, 24.7) | Refb |
| 1 to <2 | 2,096 | 20.9 (18.2, 23.7) | 0.74 |
| 2 to <4 | 1,997 | 22.2 (19.4, 25.3) | 0.71 |
| 4 or more | 1,741 | 22.8 (19.4, 26.4) | 0.52 |
| Level of educationd | |||
| High school or less | 3,963 | 20.8 (18.9, 22.9) | Refb |
| More than high school or some college | 2,525 | 24.1 (21.5, 26.8) | <0.01 |
| College graduate | 1,730 | 20.0 (17.1, 23.1) | 0.61 |
| Health care utilization | |||
| Health care visits in the past yeard | |||
| 0–1 | 2,148 | 8.3 (6.9, 9.9) | Refb |
| 2–3 | 2,392 | 23.6 (20.6, 26.8) | <0.001 |
| 4 or more | 3,675 | 27.9 (25.7, 30.1) | <0.001 |
| Risk factors | |||
| Body mass index (BMI, kg/m2)d | |||
| Normal (18.5–24.9) | 1,573 | 14.5 (12.1, 17.2) | <0.001, for quadratic trend |
| Overweight (25.0–29.9) | 2,558 | 21.5 (18.5, 24.8) | |
| Class I obesity (30.0–34.9) | 1,962 | 24.6 (21.8, 27.5) | |
| Class II obesity (35.0–39.9) | 1,035 | 24.9 (20.8, 29.2) | |
| Class III obesity (≥40) | 900 | 22.4 (18.5, 26.7) | |
| *Diagnosed Diabetesd | |||
| Yes | 2,077 | 29.9 (26.9, 33.1) | Refb |
| No | 5,804 | 19.8 (17.7, 22.0) | <0.001 |
| Chronic kidney diseased | |||
| Yes | 2,077 | 24.5 (22.2, 26.9) | Refb |
| No | 5,634 | 21.0 (18.9, 23.3) | <0.001 |
Note: Hypertension control is defined as systolic blood pressure <130 and diastolic blood pressure <80 among adults with hypertension. Abbreviations: CI, confidence interval; MSAs, metropolitan statistical areas.
P value for trend test or for pairwise comparison.
The reference group for pairwise comparison.
Degrees of freedom <8 (degrees of freedom = 7).
Age-adjusted to subpopulation of individuals who had hypertension in NHANES 2007–2016 using age groups 18–39, 40–59, 60–79, 80 years and older with weights 0.1661, 0.4061, 0.3446, and 0.0832.
Unweighted sample size.
Diagnosed Diabetes = defined as self-report or an HbA1c ≥6.5%.
Source: 2013–2018 National Health and Nutrition Examination Survey.
Unadjusted and adjusted PRs
Model 1 shows, when compared with large MSAs, the unadjusted PR for hypertension was higher among adults residing in medium to small MSAs (unadjusted PR = 1.12, 95% CI = 1.04–1.12) and non-MSAs (unadjusted PR = 1.24, 95% CI = 1.15–1.33) (Table 4). Similarly, the unadjusted PR for stage II hypertension was higher among adults residing in medium to small MSAs compared with large MSAs (unadjusted PR = 1.24, 95% CI = 1.08–1.41) and non-MSAs (unadjusted PR = 1.28, 95% CI = 1.02–1.59). Hypertension control was higher among adults residing in non-MSAs (unadjusted PR = 1.24, 95% CI = 1.01–1.53) but did not differ among adults residing in medium to small MSAs (unadjusted PR = 0.99, 95% CI = 0.84–1.52) when compared with large MSAs.
Table 4.
Unadjusted and adjusted prevalence ratios of hypertension, stage II hypertension, and hypertension control by levels of urbanization among adults aged 18 years and older: United States 2013–2018
| Prevalence ratio (95% CI) for the outcomes |
||||
|---|---|---|---|---|
| Models | Urbanization | Hypertensiona | Stage II hypertensionb |
Hypertension controlc |
| Model 1 (unadjusted) | Large MSAs | Reference | Reference | Reference |
| Medium to small MSAs | 1.12 (1.04–1.12) | 1.24 (1.08–1.41) | 0.99 (0.84–1.52) | |
| Non-MSAs | 1.24 (1.15–1.33) | 1.28 (1.02–1.59) | 1.24 (1.01–1.53) | |
| Model 2 (adjusted for age, sex, race, and Hispanic origin) | Large MSAs | Reference | Reference | Reference |
| Medium to small MSAs | 1.13 (1.05–1.20) | 1.28 (1.13–1.45) | 0.93 (0.88–0.98) | |
| Non-MSAs | 1.19 (1.11–1.28) | 1.25 (1.01–1.55) | 0.93 (0.86–1.00) | |
| Model 3 (adjusted, full model) | Large MSAs | Reference | Reference | Reference |
| Medium to small MSAs | 1.07 (0.99–1.14) | 1.21 (1.06–1.36) | 0.96 (0.91–1.01) | |
| Non-MSAs | 1.06 (0.99–1.13) | 1.06 (0.88–1.29) | 1.00 (0.93–1.06) | |
Note: Model 3: adjusted for covariates, survey cycle, sex, age groups, race and Hispanic origin, family income-to-poverty ratio, level of education, health care visits, body mass index, diagnosed diabetes (defined as self-report or an HbA1c ≥6.5%), and chronic kidney disease. Abbreviations: CI, confidence interval; MSAs, metropolitan statistical areas.
Hypertension is defined as systolic blood pressure ≥130, diastolic blood pressure ≥80 or current use of antihypertensive medication.
Stage II hypertension is defined as systolic blood pressure ≥140 or diastolic blood pressure ≥90 among all adults.
Hypertension control is defined as systolic blood pressure <130 and diastolic blood pressure <80 among adults with hypertension.
Source: 2013–2018 National Health and Nutrition Examination Survey.
Model 2 shows that, after adjusting for age groups, sex, and race and Hispanic origin, the adjusted PR was similar to those of the unadjusted PRs for hypertension (adjusted PR = 1.13, 95% CI = 1.05–1.20 for medium to small MSAs; adjusted PR = 1.19, 95% CI = 1.11–1.28 for non-MSAs) and stage II hypertension (adjusted PR = 1.28, 95% CI = 1.13–1.45 for medium to small MSAs; adjusted PR = 1.25, 95% CI = 1.01–1.55 for non-MSAs). While for hypertension control, the associations changed. Specifically, compared with large MSAs, the adjusted PR for hypertension control became lower for adults residing for medium to small MSAs (adjusted PR = 0.93, 95% CI = 0.88–0.98) and was no longer different for adults residing in non-MSAs (adjusted PR = 0.93, 95% CI = 0.86–1.00).
Model 3 shows the PRs after adjusting for all confounding variables including survey cycle, age group, gender, race and Hispanic origin, family income-to-poverty ratio, level of education, health care visits, BMI, diagnosed diabetes, and chronic kidney disease. For hypertension and stage II hypertension, the PRs decreased, and the associations were attenuated. Specifically, compared with large MSAs, the adjusted PRs for hypertension there were no longer different for adults residing in medium to small MSAs (adjusted PR = 1.07, 95% CI = 0.99–1.14) nor in non-MSAs (adjusted PR = 1.06, 95% CI = 0.99–1.13). As for stage II hypertension, compared with large MSAs, the adjusted PR was higher for adults residing in medium to small MSAs (adjusted PR = 1.21, 95% CI = 1.06–1.36) but not different for adults residing in non-MSAs (adjusted PR = 1.06, 95% CI = 0.88–1.29). As for hypertension control, after adjusting for confounding covariates and compared with large MSAs, the adjusted PRs did not differ among adults residing in medium to small MSAs (adjusted PR = 0.96, 95% CI = 0.91–1.01) and in non-MSAs (adjusted PR = 1.00, 95% CI = 0.93–1.06).
DISCUSSION
Our results showed disparities in the prevalence of hypertension between the urban and rural areas in the United States. This study provides the most recent data on hypertension and urbanization from a large, nationally representative sample of adults based on objective BP measurements using standardized procedures. Additionally, NHANES collects data on clinical risk factors for hypertension which were also assessed using standardized physical and laboratory methods, thus enabling a comprehensive analysis with a broad range of covariates.
Two recent studies have examined the association between hypertension (BP ≥140/90 or currently taking medications for hypertension) and geography in the United States using self-reported hypertension diagnosis.16,17 Both studies found that age-adjusted prevalence of self-reported hypertension was higher in rural areas when compared with urban areas. Using 2013 BRFSS, researchers reported age-adjusted prevalence of self-reported hypertension of 32.6% in metropolitan counties and 38.1% in nonmetropolitan counties and found that these differences persisted after accounting for county economic status.16 This study also reported a small but note-worthy increase in hypertension between metropolitan and nonmetropolitan counties after adjustment for other factors. Another study, using the 2017 BRFSS, examined the association between urbanization and hypertension overall and by region and reported an age-adjusted prevalence of self-reported hypertension of 28.5% in large central metro areas and 34.1% in noncore (most rural) areas.17
Data from the 2014–2016 National Ambulatory Medical Care Survey (NAMCS), a nationally representative survey of visits to nonfederal, office-based physicians, showed a similar pattern of visits to physicians for hypertension by urbanization. Among adults aged 18 years and older, the percentage of visits among patients with diagnosed hypertension was 34.2% in large metro suburban areas, 37.9% for individuals who resided in small–medium metro areas, and 40.1% for individuals who resided in rural areas.39 Of note, it is important to consider that visits to nonfederal, office-based physicians may be confounded by access to health care. Although the reported estimates were age adjusted, these estimates were not adjusted for additional covariates. Our age-adjusted prevalence of hypertension, based on measured BP, showed a similar relationship between levels of urbanization and hypertension prevalence, although with higher prevalence estimates (large MSAs 41.1%, medium to small MSAs 45.4%, and non-MSAs 48.6%), but this association was attenuated in the multivariable analysis.
Only 2 US studies using NHANES data have examined the association between hypertension determined by measured BP values and geography. A study using the NHANES I Epidemiological Follow-Up Study (1971–1984) data, reported that the age-adjusted relative odds ratio of hypertension prevalence between 1971 and 1984 were not associated with region or with urbanization level.18 In another study, NHANES III (1988–1994) to assess the prevalence of hypertension by region and urbanization.19 Their analysis showed that region (south vs. non-south) rather than level of urbanization was associated with hypertension. Residence in southern regions of the United States were associated with higher prevalence of hypertension among participants aged 40–59 years, specifically among non-Hispanic white men, non-Hispanic black men, and women.19 These 2 studies are different from our study in 2 ways, 1 study associated hypertension with regions, and both cited studies are dated so no comparison is possible.
For hypertension and hypertension control, we found no differences across urbanization after all covariates were included in the third regression model. The differences of hypertension and hypertension control across urbanization without covariates adjustment may be explained by the differences of confounding factors across urbanization (i.e., the differences in race/ethnicity, poverty ratio, and other confounding variables shown in Table 1). After adjusting covariates, the stage II hypertension was associated with medium to small MSAs.
More studies are needed focusing on these geographical locations. One possibility of incorporating both regionality and urbanization is leveraging electronic health records as was done using in the Yale New Haven Health System.40 This approach opens the possibility of using concurrently both measured BP and geographical locations. Lastly, the US government has also identified this issue in health and human services 2020 Rural Action Plan that aims to “improve the health outcomes of people in rural communities with cardiovascular disease.”41
The findings in this report are subject to limitations. NHANES sampling design is not meant to provide individual-level urbanization prevalence estimates. Despite our attempt to increase the sample size and the degrees of freedom by combining the NCHS metropolitan classification into 3 categories, medium to small MSAs or non-MSAs characteristically have a smaller sample size and fewer degrees of freedom which resulted in some estimates and standard errors that did not meet statistical reliability criteria.24 It is possible that other factors not accounted for in this analysis such as occupational status, neighborhoods, and access to health care may explain this association between stage II hypertension and urbanization and thus when not considered we may miss the wider construct that reflects more complex association that impact health outcome.9 Additionally, we did not examine in the multivariable logistic model the effect of behavioral covariates associated with hypertension such as smoking or physical activity. Although these behaviors when modified may attenuate the risk for hypertension.23 We focused in this analysis solely on biophysiological risk factors.
The adjusted PRs of stage II hypertension were higher in medium to small MSAs compared with large MSAs. These analyses support the importance of including urbanization level as an additional demographic variable when estimating hypertension prevalence. Studies utilizing a multivariable framework provide a better understanding of the association of urbanization levels and BP.
ACKNOWLEDGMENTS
We would like to thank Michele Chiappa, B.A., Peraton Corporation for her editorial support. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the US Public Health Service.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Curfman G, Bauchner H, Greenland P. Treatment and control of hypertension in 2020: the need for substantial improvement. JAMA 2020; 324:1166–1167. [DOI] [PubMed] [Google Scholar]
- 2.Ostchega Y, Fryar CD, Nwankwo T, Nguyen DT. Hypertension Prevalence Among Adults Aged 18 and Over: United States, 2017–2018. NCHS Data Brief, no. 364. National Center for Health Statistics: Hyattsville, MD, 2020. [PubMed] [Google Scholar]
- 3.Muntner P, Hardy ST, Fine LJ, Jaeger BC, Wozniak G, Levitan EB, Colantonio LD. Trends in blood pressure control among US adults with hypertension, 1999–2000 to 2017–2018. JAMA 2020; 324:1190–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Heart Association. 2021. Heart Disease and Stroke Statistics Update Fact Sheet At-a-Glance. 2021 Statistics Update – At-a-Glance Statistics. [Google Scholar]
- 5.Yildiz Y, Afşin Oktay A, Stewart MH, Milani RV, Ventura HO, Lavie CJ. Left ventricular hypertrophy and hypertension. Prog Cardiovasc Dis 2020; 63:10–21. [DOI] [PubMed] [Google Scholar]
- 6.Brandes RP. Endothelial dysfunction and hypertension. Hypertension 2014; 64:924–928. [DOI] [PubMed] [Google Scholar]
- 7.Hamad R, Penko J, Kazi DS, Coxson P, Guzman D, Wei PC, Mason A, Wang EA, Goldman L, Fiscella K, Bibbins-Domingo K. Association of low socioeconomic status with premature coronary heart disease in US adults. JAMA Cardiol 2020; 5:899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Mestral C, Stringhini S. Socioeconomic status and cardiovascular disease: an update. Curr Cardiol Rep 2017; 19:115. [DOI] [PubMed] [Google Scholar]
- 9.Mensah GA, Goff DC, Gibbons GH. Cardiovascular mortality differences place matters. JAMA 2017; 317(19):1955–1957. doi: 10.1001/jama.2017.4168. [DOI] [PubMed] [Google Scholar]
- 10.O’Connor A, Wellenius G. Rural-urban disparities in the prevalence of diabetes and coronary heart disease. Public Health 2012; 126:813–820. [DOI] [PubMed] [Google Scholar]
- 11.Murray CJ, Kulkarni SC, Michaud C, Tomijima N, Bulzacchelli MT, Iandiorio TJ, Ezzati M. Eight Americas: investigating mortality disparities across races, counties, and race-counties in the United States. PLoS Med 2006; 3:e260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roth GA, Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, Morozoff C, Naghavi M, Mokdad AH, Murray CJL. Trends and patterns of geographic variation in cardiovascular mortality among US counties, 1980–2014. JAMA 2017; 317:1976–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marmot M, Friel S, Bell R, Houweling TA, Taylor S; Commission on Social Determinants of Health. Closing the gap in a generation: health equity through action on the social determinants of health. Lancet 2008; 372:1661–1669. [DOI] [PubMed] [Google Scholar]
- 14.Diez Roux AV, Stein Merkin S, Arnett D, Chambless L, Massing M, Nieto J, Sorlie P, Szklo M, Tyroler HA, Watson RL. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med 2001; 345(2):99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 15.Mensah GA, Cooper RS, Siega-Riz AM, Cooper LA, Smith JD, Brown CH, Westfall JM, Ofili EO, Price LN, Arteaga S, Green Parker MC, Nelson CR, Newsome BJ, Redmond N, Roper RA, Beech BM, Brooks JL, Furr-Holden D, Gebreab SY, Giles WH, James RS, Lewis TT, Mokdad AH, Moore KD, Ravenell JE, Richmond A, Schoenberg NE, Sims M, Singh GK, Sumner AE, Treviño RP, Watson KS, Avilés-Santa ML, Reis JP, Pratt CA, Engelgau MM, Goff DC Jr, Pérez-Stable EJ. Reducing cardiovascular disparities through community-engaged implementation research: a National Heart, Lung, and Blood Institute workshop report. Circ Res 2018; 122:213–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw KM, Theis KA, Self-Brown S, Roblin DW, Barker L. Chronic disease disparities by county economic status and metropolitan classification, behavioral risk factor surveillance system, 2013. Prev Chronic Dis 2016; 13:160088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samanic CM, Barbour KE, Liu Y, Wang Y, Fang J, Lu H, Schieb L, Greenlund KJ. Prevalence of self-reported hypertension and antihypertensive medication use by county and rural-urban classification—United States, 2017. MMWR Morb Mortal Wkly Rep 2020; 69(18):533–539. doi: 10.15585/mmwr.mm6918a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillum RF, Mussolino ME, Madans JH. Relation between region of residence in the United States and hypertension incidence—the NHANES I epidemiologic follow-up study. J Natl Med Assoc 2004; 96:625–634. [PMC free article] [PubMed] [Google Scholar]
- 19.Obisesan TO, Vargas CM, Gillum RF. Geographic variation in stroke risk in the United States. Region, urbanization, and hypertension in the Third National Health and Nutrition Examination Survey. Stroke 2000; 31:19–25. [DOI] [PubMed] [Google Scholar]
- 20.NHANES Survey Methods and Analytic Guidelines. cdc.gov. Accessed 5 March 2021. [Google Scholar]
- 21.Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National Health and Nutrition Examination Survey: plan and operations, 1999–2010. Vital Health Stat 2013; (56):1–37. [PubMed] [Google Scholar]
- 22.National Health and Nutrition Examination Survey (NHANES). Physician Examination Procedures Manual. cdc.gov. Accessed 5 March 2021. [Google Scholar]
- 23.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/AphA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary. J Am Coll Cardiol 2018; 71(19):e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 24.National Center for Health Statistics. NCHS Urban-Rural Classification Scheme for Counties. https://www.cdc.gov/nchs/data_access/urban_rural.htm. Accessed 5 March 2021.
- 25.Hales CM, Fryar CD, Carroll MD, Freedman DS, Aoki Y, Ogden CL. Differences in obesity prevalence by demographic characteristics and urbanization level among adults in the United States, 2013–2016. JAMA 2018; 319:2419–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Department of Health and Human Services. Poverty Guidelines, Research, and Measurement. Poverty Research ∣ ASPE. hhs.gov. Accessed 5 March 2021. [Google Scholar]
- 27.Centers for Disease Control and Prevention, National Center for Health Statistics, National Health and Nutrition Examination Survey (NHANES). NHANES Questionnaires, Datasets, and Related Documentation. cdc.gov. Accessed 5 March 2021. [Google Scholar]
- 28.Ostchega Y, Hughes JP, Wright JD, McDowell MA, Louis T. Are demographic characteristics, health care access and utilization, and comorbid conditions associated with hypertension among US adults? Am J Hypertens 2008; 21:159–165. [DOI] [PubMed] [Google Scholar]
- 29.National Heart, Lung, and Blood Institute. Managing Overweight and Obesity in Adults: Systematic Evidence Reviews from the Obesity Expert Panel. https://www.nhlbi.nih.gov/sites/default/files/media/docs/obesity-evidence-review.pdf. 2013. Accessed 5 March 2021.
- 30.American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes 2019. Diabetes Care 2019; 42(Suppl 1):S13–S28. [DOI] [PubMed] [Google Scholar]
- 31.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korn EL, Graubard BI. Confidence intervals for proportions with small expected number of positive counts estimated from survey data. Surv Methodol 1998; 23:193–201. [Google Scholar]
- 33.National Center for Health Statistics. National Center for Health Statistics Data Presentation Standards for Proportions. NCHS Vital and Health Statistics, Series 2, Number 175. cdc.gov. 2017. Accessed 5 March 2021. [PubMed] [Google Scholar]
- 34.Rao JNK, Scott AJ. The analysis of categorical data from complex sample surveys: chi-squared tests for goodness of fit and independence in two-way tables. J Am Stat Assoc 1981; 76:374, 221–230. [Google Scholar]
- 35.Research Triangle Institute. SUDAAN Language Manual, Release 11. Research Triangle Institute: Research Triangle Park, NC, 2012. [Google Scholar]
- 36.Klein RJ, Schoenborn CA. Age Adjustment Using the 2000 Projected U.S. Population. Healthy People 2010 Statistical Notes, no. 20. National Center for Health Statistics: Hyattsville, MD, 2001. [PubMed] [Google Scholar]
- 37.Crim MT, Yoon SS, Ortiz E, Wall HK, Schober S, Gillespie C, Sorlie P, Keenan N, Labarthe D, Hong Y. National surveillance definitions for hypertension prevalence and control among adults. Circ Cardiovasc Qual Outcomes 2012; 5:343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tamhane AR, Westfall AO, Burkholder GA, Cutter GR. Prevalence odds ratio versus prevalence ratio: choice comes with consequences. Stat Med 2016; 35:5730–5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis D, Rui P. Urban-Rural Differences in Visits to Office-Based Physicians by Adults with Hypertension: United States, 2014–2016. National Health Statistics Reports, no 147. National Center for Health Statistics: Hyattsville, MD, 2020. [PubMed] [Google Scholar]
- 40.Lu Y, Huang C, Mahajan S, Wade L. Schulz WL, Nasir K, Erica S, Spatz ES, Krumholz HM. Leveraging the electronic health records for population health: a case study of patients with markedly elevated blood pressure. J Am Heart Assoc 2020; 9:e015033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.US Department of Health and Human Services. Rural Action Plan. Department of Health and Human Services. hhs.gov. 2020. Accessed 5 March 2021. [Google Scholar]


