Abstract
Importance:
Cardiovascular disease is the leading cause of death in the US. The burden of cardiovascular disease morbidity and mortality disproportionately affects racial/ethnic minority groups, who now compose almost 40% of the US population in aggregate. As part of the 2010 American Heart Association (AHA) Strategic Impact Goal, the AHA established 7 cardiovascular health (CVH) metrics (also known as Life’s Simple 7) with the goal to improve the CVH of all individuals in the US by 20% by 2020. National estimates of CVH are important to track and monitor at the population level but may mask important differences across and within racial/ethnic minority groups. It is critical to understand how CVH may differ between racial/ethnic minority groups and consider how these differences in CVH may contribute to disparities in cardiovascular disease burden and overall longevity.
Observations:
This narrative review summarizes the available literature on individual CVH metrics and composite CVH scores across different race/ethnic minority groups (specifically Hispanic/Latino, Asian, and non-Hispanic Black individuals) in the US. Disparities in CVH persist among racial/ethnic groups, but key gaps in knowledge exist, in part, owing to underrepresentation of these racial/ethnic groups in research or misrepresentation of CVH because of aggregation of race/ethnicity subgroups. A comprehensive, multilevel approach is needed to target health equity and should include (1) access to high-quality health care, (2) community-engaged approaches to adapt disruptive health care delivery innovations, (3) equitable economic investment in the social and built environment, and (4) increasing funding for research in racial/ethnic minority populations.
Conclusions and relevance:
Significant differences in CVH exist within racial/ethnic groups. Given the rapid growth of diverse, minority populations in the US, focused investigation is needed to identify strategies to optimize CVH. Opportunities exist to address inequities in CVH and to successfully achieve both the interim (AHA 2024) and longer-term (AHA 2030) Impact Goals in the coming years.
Introduction
Cardiovascular disease (CVD), including heart disease and stroke, remains the most common cause of death in the US.1 Aiming to reduce mortality associated with CVD, the American Heart Association (AHA) defined the cardiovascular health (CVH) construct in its 2010 Strategic Impact Goal,2 which integrates modifiable CVD risk factors into a composite score. This construct was designed to characterize CVH in individuals and populations, monitor trends over time, and provide a framework for risk factor prevention and modification.2 The ideal CVH profile formed the cornerstone of the AHA’s 2020 Strategic Impact Goal, which included an aim to improve CVH by 20% in the US population by 2020 and transformed the focus from an emphasis on disease to health promotion.2 The 7 CVH metrics include body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), diet quality, physical activity, smoking status, blood pressure level, fasting blood glucose level, and total cholesterol level, which are often termed Life’s Simple 7. These metrics are graded as poor, intermediate, or ideal (eTable in the Supplement) and are combined to obtain a composite CVH score ranging from 0 to 14 or 0 to 7 for dichotomous categorization into number of ideal factors.
Extensive observational evidence has since demonstrated that higher CVH in younger and middle age is associated not only with lower lifetime risks for CVD,3–6 but also lower non-CVD mortality7; lower risks for cancer and other chronic diseases of aging,8 substantially longer healthy longevity, and compression of morbidity6,9; enhanced quality of life10; better cognitive function11,12; and lower health care costs throughout the life span.13,14 Likewise, interventions that preserve higher CVH and promote improvement in CVH in adulthood are associated with favorable health outcomes.15,16 CVH trajectories from childhood to midlife (ages 8 to 55 years) are associated with subclinical CVD,17 and CVH measured in late adolescence to young adulthood is associated with long-term CVD outcomes and all-cause mortality throughout 3 decades of follow-up.18 Thus, the CVH construct is an effective measure for monitoring population-level and individual-level health, predicting risk for adverse outcomes, and serving as a useful surrogate outcome for research in younger individuals.
Significant disparities in CVH metrics remain persistent in the US across self-identified racial and ethnic groups, leading to disparities in health-adjusted life expectancy and overall longevity.19 These disparities in CVH have also contributed to the disproportionate burden of disease experienced by racial/ethnic minority groups during the COVID-19 pandemic.20 Among 7868 patients hospitalized with COVID-19 in the AHA COVID-19 Cardiovascular Disease Registry, Black patients and Hispanic/Latino patients were a mean of 9 and 12 years younger, respectively, but accounted for 53% of all deaths.21 The COVID-19 pandemic has highlighted long-standing disparities in health outcomes among racial/ethnic minority groups and emphasizes the urgent need for strategies to improve health equity.
To improve CVH in all US individuals, it is important to first understand CVH among the growing population of racial/ethnic minority groups in the US (Figure 122). Historically, minority groups including Hispanic/Latino and Asian populations in the US have been underrepresented in studies of CVH in the US. When these groups are included, data are commonly aggregated (eg, Hispanic/Latino) or focus on 1 subgroup (eg, Mexican), which may misrepresent subgroups within these populations. For example, the National Health and Nutrition Examination Survey (NHANES), a nationally representative survey of the US population, most reliably identifies CVH among non-Hispanic White, non-Hispanic Black, and Mexican men in the US (eFigure 1 in the Supplement) and women (eFigure 2 in the Supplement). Only since 2011 were Asian individuals separately identified. In analyses of trends of CVH among non-Hispanic White, non-Hispanic Black, and Mexican populations in the US, there has been little change in individual CVH metrics or composite CVH.19,23,24
Figure 1. Census Population Distribution and Projections (in 100 000s) by Race/Ethnicity Group in the United States, 2010-2050.
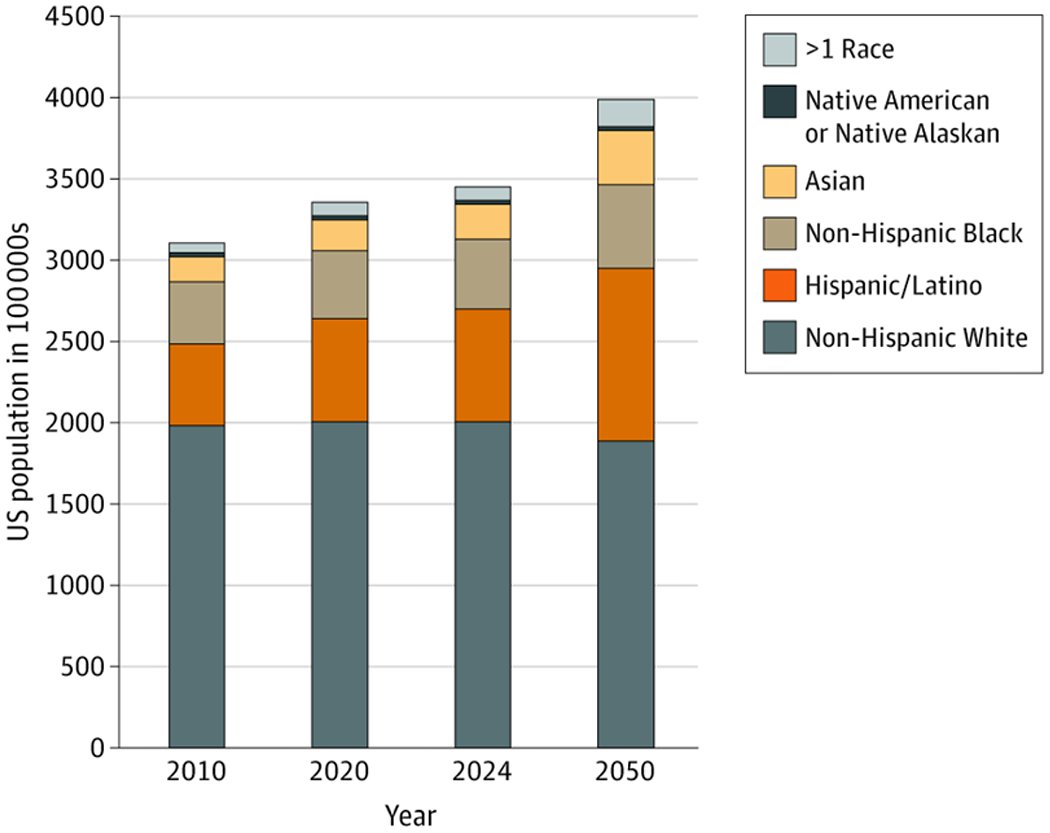
There is a growing population of racial/ethnic minority groups in the US that is projected to increase further by 2050. Data are accessed from the US Centers for Disease Control and Prevention Wide-Ranging Online Data for Epidemiologic Research.22 Data are not available on disaggregated Hispanic/Latino and Asian subgroups in the US.
The AHA recently defined short-term (2024) and longer-term (2030) Impact Goals focusing on achieving health equity. The 2024 Impact Goal commits AHA to being a champion for health equity by “advanc[ing] cardiovascular health for all, including identifying and removing barriers to health care access and quality.”25 The longer-term 2030 goal aims to “equitably increase healthy life expectancy from 66 to at least 68 years by 2030” in the US.26 To inform strategies to achieve these goals, we conducted a narrative review to summarize and synthesize existing literature on CVH within Hispanic, Asian, and non-Hispanic Black adults in the US to identify key gaps in knowledge and opportunities to reduce inequities.
CVH Among Hispanic/Latino Individuals and Communities in the US
Hispanic/Latino individuals are the largest race/ethnic minority group by population in the US, currently composing approximately 17% of the US population and expected to grow to about 30% by 2050.27 Among Puerto Rican, Mexican, and Cuban individuals in the US, CVD accounts for 1139, 868, and 841 years of potential life lost per 100 000, respectively.28 Despite their population size, CVH data remain limited, especially among Hispanic/Latino subgroups.
The National Health Interview Survey (NHIS) and NHANES provide estimates of CVH within the US population but commonly assess Hispanic/Latino populations in aggregate (NHIS) or identify Mexican individuals (the largest Hispanic/Latino subgroup) as a surrogate for all Hispanic/Latino individuals (NHANES). NHIS and NHANES data from 2009 to 2013 reveal that as a group, Hispanic/Latino individuals had a lower prevalence of ideal glucose levels (diabetes prevalence: 14.0% vs 6.0%) and ideal BMI (obesity prevalence: 39.9% vs 32.4%) compared with non-Hispanic White individuals in the US. More Hispanic/Latino individuals were nonsmokers (13.5%) compared with non-Hispanic White individuals in the US (23.8%). Although both groups reported a similar prevalence of poor lipid levels and poor blood pressure levels, Hispanic/Latino individuals were less likely to have controlled blood pressure levels (32.3% vs 45.6%).29 Mexican and non-Hispanic White adults in NHANES had similar CVH scores overall, but Mexican women had a significantly lower total CVH score (7.7 [95% CI, 7.4-8.0] of 14) compared with non-Hispanic White women (8.4 [95% CI, 8.1-8.7] of 14) in the US.19
While these NHIS and NHANES statistics are informative, the Hispanic/Latino population is heterogeneous (Figure 2A30). The diversity of immigration patterns and cultural factors influencing CVH behaviors (eg, diet and physical activity) among Hispanic/Latino subgroups limit generalization of results from aggregate Hispanic/Latino samples to individual Hispanic/Latino subgroups. The Hispanic Community Health Study/Study of Latinos (HCHS/SOL) is the largest available prospective cohort study of Hispanic/Latino individuals and identified participants by their self-identified subgroup identity: Mexican, Puerto Rican, Central American, Cuban, or South American.31 Within HCHS/SOL, fewer than 1% of participants had ideal criteria for all 7 CVH metrics. Overall, 76.5% had ideal smoking status, 65.2% had ideal physical activity, 64.4% had ideal fasting blood glucose level, 50.6% had ideal total cholesterol level, 46.5% had ideal blood pressure level, 22.3% had an ideal BMI, and only 1.7% had an ideal diet (Table32–35). Women had a higher prevalence of ideal diet, smoking status, blood pressure level, and fasting blood glucose level compared with men. Mexican and South American participants had a higher prevalence of ideal blood pressure level. Mexican participants in the US had the highest prevalence of an ideal diet. Dominican and Puerto Rican participants in the US had higher prevalence of ideal total cholesterol levels. South American participants had the highest prevalence of ideal fasting blood glucose level and ideal BMI. Aside from Cuban participants in the US, who had the lowest prevalence of ideal physical activity, there was no significant difference in ideal physical activity among the other subgroups. Puerto Rican and Cuban participants in the US had the lowest prevalence of participants meeting ideal smoking criteria, although more than three-quarters of participants were nonsmokers.36
Figure 2. Distribution of Disaggregated Hispanic/Latino and Asian Subgroups in the United States, 2015-2019.
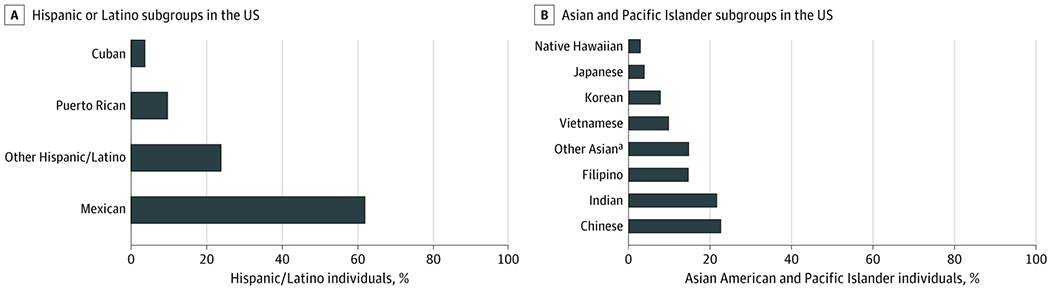
Hispanic/Latino and Asian individuals in the US represent a diverse, heterogeneous group of cultures that can and should be further disaggregated in census reporting, research studies, and interventions. Data are accessed from the American Community Survey 5-year estimates (2015-2019).30
aThe other Asian category includes individuals who self-reported as Asian ancestry other than Chinese, Indian, Filipino, Vietnamese, Korean, or Japanese.
Table.
Percentage of Ideal Cardiovascular Health Among Different Race/Ethnic Subgroups
| Race/ethnic subgroup | Cohort | Sample size. No. | Age range, y | Male/female, % | Ideal level. % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diet | Physical activity | Smoking | Blood pressure | BMI | Cholesterol | Fasting blood glucose | |||||
| Hispanic/Latino adults | |||||||||||
| Total | HCHS/SOL31 | 15 825 | 18-74 | 47.8/52.2 | 1.7 | 65.2 | 76.5 | 46.5 | 22.3 | 50.6 | 64.4 |
| Mexican | HCHS/SOL31 | 6472 | 18-74 | 46.8/53.2 | 3.0 | 67.9 | 81.1 | 52.5 | 20.8 | 50.1 | 63.1 |
| Puerto Rican | HCHS/SOL31 | 2728 | 18-74 | 50.6/49.4 | 0.6 | 68.1 | 63.3 | 42.4 | 20.4 | 54.4 | 62.6 |
| Cuban | HCHS/SOL31 | 2348 | 18-74 | 52.3/47.8 | 0.9 | 55.7 | 70.0 | 40.3 | 25.8 | 47.6 | 66.0 |
| Dominican | HCHS/SOL31 | 1472 | 18-74 | 39.6/60.5 | 1.2 | 64.6 | 86.9 | 41.2 | 20.8 | 55.5 | 67.3 |
| Central American | HCHS/SOL31 | 1732 | 18-74 | 47.3/52.8 | 1.2 | 68.7 | 83.5 | 46.1 | 22.6 | 47.5 | 65.1 |
| South American | HCHS/SOL31 | 1072 | 18-74 | 45.5/54.5 | 2.2 | 68.5 | 84.4 | 53.9 | 27.8 | 47.1 | 70.5 |
| Asian adults | |||||||||||
| Total | NHANES (2011-2016)32 | 1486 | >20 (mean, 44.5) | 47.6/52.4 | 5.0 | 39.0 | 90.4 | 44.0 | 55.9 | 55.1 | 61.4 |
| South Asians | MASALA33 | 875 | 40-84 | 53.0/47.0 | 2.4 | 65.0 | 83.1 | 33.0 | 44.0 | 40.0 | 56.0 |
| Chinese | MESA34 | 658 | 45-85 (mean, 63) | 46.8/53.2 | 10.8 | 56.1 | 75.8 | 41.5 | 64.0 | 53.3 | 70.5 |
| Non-Hispanic Black adults | |||||||||||
| Total | Jackson Heart Study35 | 5301 | 35-84 | 63.5/36.5 | 0.9 | 19.3 | 88.0 | 17.8 | 13.7 | 45.2 | 45.3 |
| Non-Hispanic White adults | |||||||||||
| Total | NHANES (2011-2016)32 | 5278 | >20 (mean, 49.9) | 49.4/50.6 | 2.7 | 40.9 | 78.5 | 40.1 | 29.7 | 54.1 | 73.5 |
Abbreviations: BMI, body mass index; HCHS/SOL, Hispanic Community Health Study/ Study on Latinos; MASALA, Mediators of Atherosclerosis in South Asians Living in America; MESA, Multi-Ethnic Study of Atherosclerosis; NHANES, National Health and Nutrition Examination Survey 2015-2016.
The variability of CVH metrics within Hispanic/Latino subgroups in the HCHS/SOL indicates that evaluation of CVH in aggregated Hispanic/Latino populations masks important differences. Several groups have studied interventions to promote CVH among Hispanic/Latino adults, including programs focusing on physical activity,37 obesity,38 and other CVH factors. Future work should investigate modifiable reasons for CVH differences between Hispanic/Latino subgroups to identify, adapt, and tailor potential interventions to optimize CVH in this rapidly growing population.
CVH Among Asian Individuals and Communities in the US
Asian individuals are the third largest race/ethnic minority group in the US, comprising approximately 20 million people (about 5% of the population) and projected to increase to 34 million by 2050.39 The 6 largest Asian subgroups in the US are South Asian (including Asian Indian, Pakistani, Bangladeshi, Sri Lankan, and Nepali), Chinese, Filipino, Korean, Japanese, and Vietnamese individuals, which account for more than 90% of Asian-identifying individuals in the US (Figure 2B30). Similar to Hispanic populations, most surveillance and clinical research studies do not disaggregate Asian individuals in the US. However, CVH and CVD likely vary across Asian subgroups. For example, Asian Indian and Vietnamese men in the US lose approximately 17 to 18 years of potential life to ischemic heart disease (compared with 14 years in non-Hispanic White men). Filipina, Korean, and Vietnamese women in the US lose approximately 16 to 17 years of life to cerebrovascular disease (compared with 11 years in non-Hispanic White women).40
In NHANES 2011-2016, Asian individuals in the US overall had a higher prevalence of nonsmokers (90.4% vs 78.5%), ideal body weight (BMI <25; 55.9% vs 29.7%), and ideal blood pressure level (44.0% vs 40.1%) compared with non-Hispanic White adults, with no difference in cholesterol levels or physical activity.32 Notably, prior research suggests that standard BMI categories may not be appropriate for individuals of Asian ancestry because their risk for cardiometabolic diseases increases at lower BMI compared with other groups.41 Accounting for Asian-specific BMI ranges (ie, “normal” BMI is 18.5-22.9) revealed that Asian individuals in the US in aggregate still have a higher prevalence of a “normal” BMI (34.2% vs 29.7%) compared with non-Hispanic White individuals in the US.32 Asian adults in the US also have a higher proportion with ideal CVH (8.7% vs 5.9%) and lower proportion with poor CVH (26.3% vs 33.5%) compared with non-Hispanic White adults.32 Additionally, Asian adults in the US were less likely to have an ideal level of fasting blood glucose compared with non-Hispanic White adults (61.4% vs 73.5%).32
There are several examples demonstrating differences in CVH factors between Asian subgroups. Of 875 participants in the Mediators of Atherosclerosis in South Asians Living in America (MASALA) study,33 no participant had ideal criteria for all 7 CVH metrics, while only 20% had ideal criteria for 5 of 7 CVH metrics. Among South Asian individuals in MASALA (compared with the contemporaneous general US adult population in NHANES), 83% did not currently smoke (compared with 74%), 65% had ideal physical activity (compared with 41%), 44% had an ideal BMI (compared with 25%), 40% had ideal cholesterol level (compared with 34%), 33% had ideal blood pressure level (compared with 34%), and 2% had an ideal diet (compared with <1%).42,43 The Multi-Ethnic Study of Atherosclerosis (MESA) included Chinese participants, of whom 5.6% were current smokers, 15.7% had diabetes, and 36.1% had hypertension at baseline.34
The MASALA study demonstrated that South Asian individuals have a higher prevalence of diabetes (23%) compared with groups in the MESA cohort (6% in non-Hispanic White, 18% in non-Hispanic Black, 17% in Hispanic/Latino, 13% in Chinese adults in the US).33 A study of Asian individuals in a large northern California health care system identified that Filipino individuals had the highest prevalence of hypertension (56%), while Chinese individuals had the lowest (34%); Korean individuals had the highest smoking prevalence (8%), while South Asian individuals had the lowest (3%).44
These examples are not exhaustive. CVH statistics likely vary by region in the US, and Pacific Islander populations are frequently not separately identified, but these findings demonstrate the importance of disaggregating Asian individuals in the US in clinical and national surveillance data to better understand CVH within this heterogenous population in the US. Further, the variation in CVH metrics across Asian subgroups in the US suggests that CVH promotion may benefit from cultural adaptation of evidence-based interventions for specific Asian subgroups in the US experiencing disproportionate burden of CVD.45 For instance, the South Asian Heart Lifestyle Intervention (SAHELI) trial46 randomizes South Asian adults to an intervention consisting of a series of culturally tailored classes focused on physical activity, diet, weight, and stress management, vs control. The SAHELI pilot study found that this community-based behavior intervention promoted weight loss (−1.5 kg; P = .04) and reduced hemoglobin A1c level (−0.43%; P < .01) at 6 months; the expanded randomized clinical trial is currently in progress.46
CVH Among Non-Hispanic Black Individuals and Communities
Non-Hispanic Black individuals are the second largest race/ethnic minority group in the US, composing about 13% of the population.47 Despite efforts and promote CVH in non-Hispanic Black adults, this group has a higher burden of premature CVD mortality compared with other race/ethnic groups. In 2018, Black men had an age-adjusted mortality rate from CVD of 260 per 100 000, compared with 206 per 100 000 in White men. In Black women, age-adjusted mortality rate from CVD was 162 per 100 000, compared with 127 per 100 000 in White women. In terms of population-level years of potential life lost in 2018, CVD accounted for 1602 and 1210 years of potential life lost among non-Hispanic Black men and women (compared with 1205 and 744 in non-Hispanic White men and women), respectively.48
One prominent study that reports CVH in non-Hispanic Black adults is the Jackson Heart Study,35 a prospective cohort of non-Hispanic Black adults in Jackson, Mississippi. In this cohort, only 3.2% of participants had 5 or more ideal CVH metrics, and no participant had ideal criteria for all 7 CVH metrics. Itemized by metric, 88% had ideal smoking status, 45% had ideal blood glucose level, 45% had ideal cholesterol level, 19% had ideal physical activity, 18% had ideal blood pressure level, 14% had an ideal BMI, and less than 1% had an ideal diet.35 Throughout the duration of the study, the proportion of participants with ideal criteria for cholesterol level, fasting blood glucose level, and BMI has declined. Participants with ideal levels of physical activity improved from 18% to 25%. The prevalence of participants with ideal blood pressure did not change.49
Not only is prevalence of ideal blood pressure lower in non-Hispanic Black compared with non-Hispanic White adults, but rates of hypertension control are also lower (48% non-Hispanic Black vs 56% non-Hispanic White adults) despite greater hypertension awareness and treatment rates in non-Hispanic Black adults in several cohorts (eg, Multi-Ethnic Study of Atherosclerosis, Coronary Artery Risk Development in Young Adults, Atherosclerosis Risk in Communities).50–52 Despite similar prevalence of diagnosed hyperlipidemia in non-Hispanic Black and non-Hispanic White adults in the Reasons for Geographic and Racial Differences in Stroke study cohorts,53,54 non-Hispanic Black adults have lower odds of cholesterol control compared with non-Hispanic White adults (odds ratio, 0.67; 95% CI, 0.58-0.77).54
Several studies have investigated community-based interventions to improve CVH in non-Hispanic Black adults. One program demonstrated that blood pressure counseling and pharmacist-led antihypertensive prescribing at barbershops improved blood pressure control among non-Hispanic Black adults by approximately 8.8% (P = .04).55,56 Another trial evaluating the effectiveness of church-based counseling on lifestyle change and motivational interviewing revealed that systolic blood pressure level decreased by 5.8 mm Hg compared with participants in the control arm (P = .03).57 These studies suggest that culturally adapted, community-engaged interventions may help improve CVH beyond only blood pressure among non-Hispanic Black individuals. Acknowledging that the non-Hispanic Black population is also heterogenous, future efforts should also focus on differences in health status among Black subgroups, including those from African countries.
Gaps in Knowledge
Optimization and maintenance of high CVH in all individuals in the US appears to be eminently achievable. Previous data demonstrated that 60% of young adults who follow 5 healthy lifestyles have high CVH in middle age, compared with just 3% of those following no healthy lifestyles.58 However, significant gaps in knowledge remain with regard to determinants of CVH within Hispanic/Latino, Asian, and non-Hispanic Black adults in the US. Much of the existing data are limited by either underrepresentation of these groups in research or misrepresentation of the variability of CVH status owing to aggregation of diverse subgroups into broad race/ethnic categories, which hampers efforts to achieve health equity. Several groups not identified in this review also warrant attention and further investigation, such as Pacific Islander, Native Hawaiian, Native American, Native Alaskan, Middle Eastern, and North African populations.
Acknowledging that race and ethnicity are social constructs and the CVH differences described are not intended to imply that race/ethnicity is a biological determinant, disparities in CVH between segments of the US population are likely largely driven by social determinants of health, including structural and systemic barriers to optimal health (eg, racism).59–61 However, the social and structural determinants relevant to individual subgroups remain understudied. Social determinants of health are broadly defined by the World Health Organization as “the circumstances in which people are born, grow, live, work, and age.”62 Exposures to adverse social determinants of health have been associated with disparities in health outcomes ranging from CVD to COVID-19.63 Yet, progress in screening for and addressing these disparities in clinical and population settings has been slow.64 For example, individuals with higher incomes and higher level of education have experienced a greater decline in smoking prevalence, while the prevalence of diabetes has increased in populations with lower income and education between 1971 and 2002; additionally, lack of health insurance coverage is associated with a lower likelihood of having ideal CVH.65
Beyond the need to characterize unique determinants of CVH among the diverse subgroups of the US population, identifying effective interventions to address these multilevel social determinants of CVH remains largely unaddressed. Equitably optimizing CVH will require identification and adaptation of multilevel evidence-based health promotion strategies unique to racial/ethnic minority subgroups followed by community translation and dissemination. Shifting the focus of prevention interventions to earlier in the life course to maintain CVH starting in childhood or even earlier may yield the greatest long-term population benefit. One proposal to target CVH has focused on a bold new goal of 50 × 50 × 50 to target a prevalence of optimal CVH in 50% or more of the population, at age 50 years, by 2050 or sooner.66 These strategies and goals will be particularly critical amidst the ongoing COVID-19 pandemic, during which health status has declined across the US population. For these high-priority evidence gaps, targeted funding investment is warranted, yet recent funding trends suggest considerable opportunity for improvement. For instance, National Institutes of Health funding for research in Asian American and Pacific Islander individuals composed only 0.18% of the total budget since 2000.67
Future Steps to Target Equity in CVH
The diversity of the US population necessitates equally diverse strategies for maintenance of CVH to address the factors and behaviors that lead to the morbidity and mortality associated with CVD in the US. A comprehensive agenda must target all determinants of CVH and the unique CVH profiles of individual groups and communities to achieve health equity. Such an agenda may be informed by the following multicomponent action plan (Figure 3), accounting for the epidemiology of CVH in various US communities, which is based on evidence of successful CVH promotion and disease prevention strategies:
Figure 3. A Multipronged Framework for Targeting Equity in Cardiovascular Health in the United States.
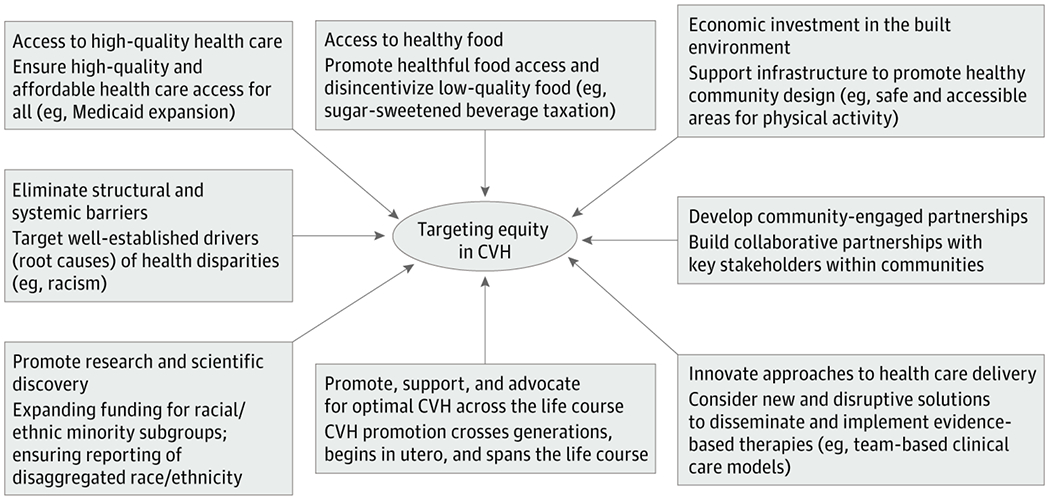
A comprehensive agenda, as proposed here, must target all determinants of cardiovascular health (CVH), and the unique CVH profiles of individual groups and communities, to achieve health equity.
Develop, advocate for, and implement multilevel policy interventions that promote CVH starting early in life and are adapted for the life course, such as ensuring access to healthful food while disincentivizing consumption of low-quality foods; equitable access to built environments that support physical activity; reducing financial barriers to medication adherence; and adoption of Medicaid expansion to enhance access to clinical care.
Promote a team-based clinical care model that leverages the expertise of nonphysician health care professionals including pharmacists, advanced practice clinicians, and social workers to integrate clinical and social delivery of culturally appropriate care to support CVH.
Seek and build partnerships between health care systems, clinicians, researchers, and community-based stakeholders to adapt, implement, and disseminate CVH maintenance promotion programs for at-risk communities. Such partnerships will enhance identification of community-specific social determinants, in particular where systemic and structural racism, low health literacy, and socioeconomic disadvantage affect health.
Ensure that future research endeavors include more, and better characterized, racial/ethnic groups to truly represent the growing diverse population. Funding should be aimed at increasing representation of these groups within clinical and population studies. Researchers should seek to understand local population demographics to ensure their participant sample represents the population in which the study is being conducted. Future research endeavors should collect disaggregated racial/ethnic group self-identification. Although disaggregated groups may be too small for reliable statistical comparison, disaggregated data should still be published in supplemental material, which will support and inform future research.68
Conclusions
Given the rapidly diversifying US population, differences and disparities that exist between racial/ethnic groups must be addressed to achieve the goals of promoting and maintaining CVH, increasing health-adjusted life expectancy, and equitably reducing CVD mortality for all.
Supplementary Material
Funding/Support:
This study was supported by the National Institutes of Health/National Heart, Lung, and Blood Institute (grants KL2TR001424, P30AG059988, P30DK092939) and the American Heart Association (grant 19TPA34890060) to Dr Khan. Research reported in this publication was supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences (grant KL2TR001424; Dr Khan).
Role of the Funder/Sponsor:
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of Interest Disclosures:
Drs Lloyd-Jones and Khan report grants from the National Institutes of Health and American Heart Association during the conduct of the study. No other disclosures were reported.
Footnotes
Publisher's Disclaimer: Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Heron M Deaths: leading causes for 2015. Natl Vital Stat Rep. 2017;66(5):1–76. [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Hong Y, Labarthe D, et al. ; American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 3.Berry JD, Dyer A, Cai X, et al. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366(4): 321–329. doi: 10.1056/NEJMoa1012848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huffman MD, Berry JD, Ning H, et al. Lifetime risk for heart failure among white and black Americans: cardiovascular lifetime risk pooling project. J Am Coll Cardiol. 2013;61(14):1510–1517. doi: 10.1016/j.jacc.2013.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113(6):791–798. doi: 10.1161/CIRCULATIONAHA.105.548206 [DOI] [PubMed] [Google Scholar]
- 6.Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR, Lloyd-Jones DM. Lifetime risk and years lived free of total cardiovascular disease. JAMA. 2012;308 (17):1795–1801. doi: 10.1001/jama.2012.14312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lloyd-Jones DM, Dyer AR, Wang R, Daviglus ML, Greenland P. Risk factor burden in middle age and lifetime risks for cardiovascular and non-cardiovascular death (Chicago Heart Association Detection Project in Industry). Am J Cardiol. 2007;99(4):535–540. doi: 10.1016/j.amjcard.2006.09.099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lloyd-Jones DM. Improving the cardiovascular health of the US population. JAMA. 2012;307(12): 1314–1316. doi: 10.1001/jama.2012.361 [DOI] [PubMed] [Google Scholar]
- 9.Khan SS, Ning H, Wilkins JT, et al. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol. 2018;3(4):280–287. doi: 10.1001/jamacardio.2018.0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daviglus ML, Liu K, Pirzada A, et al. Favorable cardiovascular risk profile in middle age and health-related quality of life in older age. Arch Intern Med. 2003;163(20):2460–2468. doi: 10.1001/archinte.163.20.2460 [DOI] [PubMed] [Google Scholar]
- 11.Bancks MP, Allen NB, Dubey P, et al. Cardiovascular health in young adulthood and structural brain MRI in midlife: the CARDIA study. Neurology. 2017;89(7):680–686. doi: 10.1212/WNL.0000000000004222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reis JP, Loria CM, Launer LJ, et al. Cardiovascular health through young adulthood and cognitive functioning in midlife. Ann Neurol. 2013;73(2):170–179. doi: 10.1002/ana.23836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daviglus ML, Liu K, Greenland P, et al. Benefit of a favorable cardiovascular risk-factor profile in middle age with respect to Medicare costs. N Engl J Med. 1998;339(16):1122–1129. doi: 10.1056/NEJM199810153391606 [DOI] [PubMed] [Google Scholar]
- 14.Daviglus ML, Liu K, Pirzada A, et al. Cardiovascular risk profile earlier in life and Medicare costs in the last year of life. Arch Intern Med. 2005;165(9):1028–1034. doi: 10.1001/archinte.165.9.1028 [DOI] [PubMed] [Google Scholar]
- 15.Spring B, Moller AC, Colangelo LA, et al. Healthy lifestyle change and subclinical atherosclerosis in young adults: Coronary Artery Risk Development in Young Adults (CARDIA) study. Circulation. 2014; 130(1):10–17. doi: 10.1161/CIRCULATIONAHA.113.005445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaye B, Tajeu GS, Vasan RS, et al. Association of changes in cardiovascular health metrics and risk of subsequent cardiovascular disease and mortality. J Am Heart Assoc. 2020;9(19):e017458. doi: 10.1161/JAHA.120.017458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen NB, Krefman AE, Labarthe D, et al. Cardiovascular health trajectories from childhood through middle age and their association with subclinical atherosclerosis. JAMA Cardiol. 2020;5 (5):557–566. doi: 10.1001/jamacardio.2020.0140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perak AM, Ning H, Khan SS, et al. Associations of late adolescent or young adult cardiovascular health with premature cardiovascular disease and mortality. J Am Coll Cardiol. 2020;76(23):2695–2707. doi: 10.1016/j.jacc.2020.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pool LR, Ning H, Lloyd-Jones DM, Allen NB. Trends in racial/ethnic disparities in cardiovascular health among US adults from 1999-2012. J Am Heart Assoc. 2017;6(9):e006027. doi: 10.1161/JAHA.117.006027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yancy CW. COVID-19 and African Americans. JAMA. 2020;323(19):1891–1892. doi: 10.1001/jama.2020.6548 [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez F, Solomon N, de Lemos JA, et al. Racial and ethnic differences in presentation and outcomes for patients hospitalized with COVID-19: findings from the American Heart Association’s COVID-19 cardiovascular disease registry. Circulation. 2020. doi: 10.1161/CIRCULATIONAHA.120.052278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.US Centers for Disease Control and Prevention. Population changes: national birth, death, migration projections: United States by year, age, gender, and race for years 2014-2060. Accessed April 13, 2021. https://wonder.cdc.gov/population.html
- 23.Holland AT, Palaniappan LP. Problems with the collection and interpretation of Asian-American health data: omission, aggregation, and extrapolation. Ann Epidemiol. 2012;22(6):397–405. doi: 10.1016/j.annepidem.2012.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paulose-Ram R, Burt V, Broitman L, Ahluwalia N. Overview of Asian American data collection, release, and analysis: National Health and Nutrition Examination Survey 2011-2018. Am J Public Health. 2017;107(6):916–921. doi: 10.2105/AJPH.2017.303815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Heart Association. 2024 Health equity impact goal. Accessed April 13, 2021. https://www.heart.org/en/about-us/2024-health-equity-impact-goal
- 26.Angell SY, McConnell MV, Anderson CAM, et al. The American Heart Association 2030 impact goal: a presidential advisory from the American Heart Association. Circulation. 2020;141(9):e120–e138. doi: 10.1161/CIR.0000000000000758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez CJ, Allison M, Daviglus ML, et al. ; American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Cardiovascular and Stroke Nursing. Status of cardiovascular disease and stroke in Hispanics/Latinos in the United States: a science advisory from the American Heart Association. Circulation. 2014;130(7):593–625. doi: 10.1161/CIR.0000000000000071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manjunath L, Hu J, Palaniappan L, Rodriguez F. Years of potential life lost from cardiovascular disease among Hispanics. Ethn Dis. 2019;29(3):477–484. doi: 10.18865/ed.29.3.477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dominguez K, Penman-Aguilar A, Chang MH, et al. ; Centers for Disease Control and Prevention (CDC). Vital signs: leading causes of death, prevalence of diseases and risk factors, and use of health services among Hispanics in the United States: 2009-2013. MMWR Morb Mortal Wkly Rep. 2015;64(17):469–478. [PMC free article] [PubMed] [Google Scholar]
- 30.United States Census Bureau. Asian American data links. Updated March 18, 2020. Accessed April 13, 2021. https://www.census.gov/about/partners/cic/resources/data-links/asian.html
- 31.Daviglus ML, Pirzada A, Talavera GA. Cardiovascular disease risk factors in the Hispanic/Latino population: lessons from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Prog Cardiovasc Dis. 2014;57(3):230–236. doi: 10.1016/j.pcad.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 32.Fang J, Zhang Z, Ayala C, Thompson-Paul AM, Loustalot F. Cardiovascular health among Non-Hispanic Asian Americans: NHANES, 2011-2016. J Am Heart Assoc. 2019;8(13):e011324. doi: 10.1161/JAHA.118.011324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanaya AM, Herrington D, Vittinghoff E, et al. Understanding the high prevalence of diabetes in U.S. South Asians compared with four racial/ethnic groups: the MASALA and MESA studies. Diabetes Care. 2014;37(6):1621–1628. doi: 10.2337/dc13-2656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2005;111(10):1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B [DOI] [PubMed] [Google Scholar]
- 35.Ommerborn MJ, Blackshear CT, Hickson DA, et al. Ideal cardiovascular health and incident cardiovascular events: the Jackson Heart Study. Am J Prev Med. 2016;51(4):502–506. doi: 10.1016/j.amepre.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.González HM, Tarraf W, Rodríguez CJ, et al. Cardiovascular health among diverse Hispanics/Latinos: Hispanic Community Health Study/Study of Latinos (HCHS/SOL) results. Am Heart J. 2016;176:134–144. doi: 10.1016/j.ahj.2016.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loya JC. Systematic review of physical activity interventions in Hispanic adults. Hisp Health Care Int. 2018;16(4):174–188. doi: 10.1177/1540415318809427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez LG, Arredondo EM, Elder JP, Barquera S, Nagle B, Holub CK. Evidence-based obesity treatment interventions for Latino adults in the U.S.: a systematic review. Am J Prev Med. 2013;44 (5):550–560. doi: 10.1016/j.amepre.2013.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palaniappan LP, Araneta MR, Assimes TL, et al. ; American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Nutrition, Physical Activity, and Metabolism; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing; Council on Cardiovascular Nursing. Call to action: cardiovascular disease in Asian Americans: a science advisory from the American Heart Association. Circulation. 2010;122 (12):1242–1252. doi: 10.1161/CIR.0b013e3181f22af4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iyer DG, Shah NS, Hastings KG, et al. Years of potential life lost because of cardiovascular disease in Asian-American subgroups, 2003-2012. J Am Heart Assoc. 2019;8(7):e010744. doi: 10.1161/JAHA.118.010744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Consultation WE; WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. doi: 10.1016/S0140-6736(03)15268-3 [DOI] [PubMed] [Google Scholar]
- 42.Talegawkar SA, Jin Y, Kandula NR, Kanaya AM. Cardiovascular health metrics among South Asian adults in the United States: prevalence and associations with subclinical atherosclerosis. Prev Med. 2017;96:79–84. doi: 10.1016/j.ypmed.2016.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mozaffarian D, Benjamin EJ, Go AS, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015; 131(4):e29–e322. doi: 10.1161/CIR.0000000000000152 [DOI] [PubMed] [Google Scholar]
- 44.Gordon NP, Lin TY, Rau J, Lo JC. Aggregation of Asian-American subgroups masks meaningful differences in health and health risks among Asian ethnicities: an electronic health record based cohort study. BMC Public Health. 2019;19(1):1551. doi: 10.1186/s12889-019-7683-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chapman J, Qureshi N, Kai J. Effectiveness of physical activity and dietary interventions in South Asian populations: a systematic review. Br J Gen Pract. 2013;63(607):e104–e114. doi: 10.3399/bjgp13X663064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kandula NR, Dave S, De Chavez PJ, et al. Translating a heart disease lifestyle intervention into the community: the South Asian Heart Lifestyle Intervention (SAHELI) study; a randomized control trial. BMC Public Health. 2015;15:1064. doi: 10.1186/s12889-015-2401-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carnethon MR, Pu J, Howard G, et al. ; American Heart Association Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; and Stroke Council. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136 (21):e393–e423. doi: 10.1161/CIR.0000000000000534 [DOI] [PubMed] [Google Scholar]
- 48.Shah NS, Molsberry R, Rana JS, et al. Heterogeneous trends in burden of heart disease mortality by subtypes in the United States, 1999-2018: observational analysis of vital statistics. BMJ. 2020;370:m2688. doi: 10.1136/bmj.m2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Djoussé L, Petrone AB, Blackshear C, et al. Prevalence and changes over time of ideal cardiovascular health metrics among African-Americans: the Jackson Heart Study. Prev Med. 2015;74:111–116. doi: 10.1016/j.ypmed.2015.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carson AP, Howard G, Burke GL, Shea S, Levitan EB, Muntner P. Ethnic differences in hypertension incidence among middle-aged and older adults: the multi-ethnic study of atherosclerosis. Hypertension. 2011;57(6):1101–1107. doi: 10.1161/HYPERTENSIONAHA.110.168005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gu Q, Burt VL, Dillon CF, Yoon S. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: the National Health And Nutrition Examination Survey, 2001 to 2010. Circulation. 2012;126(17):2105–2114. doi: 10.1161/CIRCULATIONAHA.112.096156 [DOI] [PubMed] [Google Scholar]
- 52.Howard G, Prineas R, Moy C, et al. Racial and geographic differences in awareness, treatment, and control of hypertension: the REasons for Geographic and Racial Differences in Stroke study. Stroke. 2006;37(5):1171–1178. doi: 10.1161/01.STR.0000217222.09978.ce [DOI] [PubMed] [Google Scholar]
- 53.Goff DC Jr, Bertoni AG, Kramer H, et al. Dyslipidemia prevalence, treatment, and control in the Multi-Ethnic Study of Atherosclerosis (MESA): gender, ethnicity, and coronary artery calcium. Circulation. 2006;113(5):647–656. doi: 10.1161/CIRCULATIONAHA.105.552737 [DOI] [PubMed] [Google Scholar]
- 54.Zweifler RM, McClure LA, Howard VJ, et al. Racial and geographic differences in prevalence, awareness, treatment and control of dyslipidemia: the Reasons For Geographic and Racial Differences in Stroke (REGARDS) study. Neuroepidemiology. 2011;37(1):39–44. doi: 10.1159/000328258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Victor RG, Lynch K, Li N, et al. A cluster-randomized trial of blood-pressure reduction in black barbershops. N Engl J Med. 2018; 378(14):1291–1301. doi: 10.1056/NEJMoa1717250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Victor RG, Ravenell JE, Freeman A, et al. Effectiveness of a barber-based intervention for improving hypertension control in black men: the BARBER-1 study: a cluster randomized trial. Arch Intern Med. 2011;171(4):342–350. doi: 10.1001/archinternmed.2010.390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schoenthaler AM, Lancaster KJ, Chaplin W, Butler M, Forsyth J, Ogedegbe G. Cluster Randomized clinical trial of FAITH (Faith-Based Approaches in the Treatment of Hypertension) in Blacks. Circ Cardiovasc Qual Outcomes. 2018;11(10): e004691. doi: 10.1161/CIRCOUTCOMES.118.004691 [DOI] [PubMed] [Google Scholar]
- 58.Liu K, Daviglus ML, Loria CM, et al. Healthy lifestyle through young adulthood and the presence of low cardiovascular disease risk profile in middle age: the Coronary Artery Risk Development in (Young) Adults (CARDIA) study. Circulation. 2012; 125(8):996–1004. doi: 10.1161/CIRCULATIONAHA.111.060681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Churchwell K, Elkind MSV, Benjamin RM, et al. ; American Heart Association. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American Heart Association. Circulation. 2020;142 (24):e454–e468. doi: 10.1161/CIR.0000000000000936 [DOI] [PubMed] [Google Scholar]
- 60.Havranek EP, Mujahid MS, Barr DA, et al. ; American Heart Association Council on Quality of Care and Outcomes Research, Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing, Council on Lifestyle and Cardiometabolic Health, and Stroke Council. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2015;132(9):873–898. doi: 10.1161/CIR.0000000000000228 [DOI] [PubMed] [Google Scholar]
- 61.Goff DC Jr, Buxton DB, Pearson GD, et al. ; Writing Group for the Division of Cardiovascular Sciences’ Strategic Vision Implementation Plan. Implementing the National Heart, Lung, and Blood Institute’s strategic vision in the Division of Cardiovascular Sciences. Circ Res. 2019;124(4):491–497. doi: 10.1161/CIRCRESAHA.118.314338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.World Health Organization. Closing the Gap in a Generation: Health Equity Through Action on the Social Determinants of Health—Final Report of the Commission on Social Determinants of Health. Published August 27, 2008. Accessed April 12, 2021. https://www.who.int/publications/i/item/WHO-IER-CSDH-08.1
- 63.Karmakar M, Lantz PM, Tipirneni R. Association of social and demographic factors with COVID-19 incidence and death rates in the US. JAMA Netw Open. 2021;4(1):e2036462. doi: 10.1001/jamanetworkopen.2020.36462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davidson KW, McGinn T. Screening for social determinants of health: the known and unknown. JAMA. Published online August 29, 2019. doi: 10.1001/jama.2019.10915 [DOI] [PubMed] [Google Scholar]
- 65.Kanjilal S, Gregg EW, Cheng YJ, et al. Socioeconomic status and trends in disparities in 4 major risk factors for cardiovascular disease among US adults, 1971-2002. Arch Intern Med. 2006;166(21):2348–2355. doi: 10.1001/archinte.166.21.2348 [DOI] [PubMed] [Google Scholar]
- 66.Labarthe D, Lloyd-Jones DM. 50×50×50: Cardiovascular health and the cardiovascular disease endgame. Circulation. 2018;138(10):968–970. doi: 10.1161/CIRCULATIONAHA.118.035985 [DOI] [PubMed] [Google Scholar]
- 67.Ðoàn LN, Takata Y, Sakuma KK, Irvin VL. Trends in clinical research including Asian American, Native Hawaiian, and Pacific Islander participants funded by the US National Institutes of Health, 1992 to 2018. JAMA Netw Open. 2019;2(7):e197432.doi: 10.1001/jamanetworkopen.2019.7432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shah NS, Kandula NR. Addressing Asian American misrepresentation and underrepresentation in research. Ethn Dis. 2020; 30(3):513–516. doi: 10.18865/ed.30.3.513 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


