Abstract
Various neurological complications have been described in COVID-19 patients, especially Guillain–Barre syndrome (GBS). The underlying mechanisms on the association between SARS-CoV-2 infection and GBS remain unclear, but several hypotheses have been proposed. It seems that post-SARS-CoV-2 GBS shares many characteristics with classic post-infectious GBS; however, it may occur in sedated and intubated patients hospitalized in the intensive care unit for SARS-CoV-2 acute respiratory distress syndrome, which presents challenges in the diagnosis and treatment of GBS. In this study, we describe three cases of post-SARS-CoV-2 GBS that were hospitalized in the intensive care unit.
Keywords: Guillain–Barre syndrome, Acute inflammatory demyelinating polyneuropathy, COVID-19, SARS-CoV-2, Intensive care unit, Miller Fischer syndrome
Abbreviations: AIDP, Acute inflammatory demyelinating polyradiculoneuropathy; ARDS, Acute respiratory distress syndrome; CIP, Critical illness polyneuropathy; CMV, Cytomegalovirus; CSF, Cerebrospinal fluid; EBV, Epstein Barr virus; EMG, Electromyography; GBS, Guillain–Barré syndrome; HLA, Human leukocyte antigen; ICU, Intensive care unit
1. Introduction
Since December 2019, the COVID-19 pandemic quickly spread all over the world, causing an increase in the number of hospitalizations and deaths due to acute respiratory distress syndrome (ARDS) (Hasan et al., 2020). However, as we have learned more about SARS-CoV-2, it has become clear that COVID-19 is responsible for a multitude of systemic complications, including neurological complications. Various studies have described a temporal relationship between COVID-19 and Guillain–Barre syndrome (GBS) (Meppiel et al., 2021; Shehata et al., 2021), but no causal relationship has been proven till date. GBS is an acute inflammatory demyelinating polyradiculoneuropathy (AIDP) that includes other acute inflammatory polyradiculoneuropathies, such as Miller Fisher, and often occurs post infection (Sejvar et al., 2011). The relationship between GBS and Campylobacter jejuni, Zika virus, or influenza virus has been previously described (Loshaj-Shala et al., 2018; Leonhard et al., 2021; Grisanti et al., 2021). The diagnosis of post-COVID-19 GBS could be difficult, especially when patients are hospitalized in the intensive care unit (ICU), sedated, and intubated (Finsterer and Scorza, 2021a) or suffering from critical illness polyneuropathy (CIP) (Finsterer and Scorza, 2021b). To better describe GBS in the ICU, we analyzed the characteristics of three patients infected by SARS-CoV-2 and diagnosed with GBS in the ICU in our center.
2. Material and methods
Between March 1, 2020, and May 31, 2021, we identified all COVID-19 patients in the ICU in our French tertiary care center with a diagnosis of GBS that met the Brighton criteria. We started collecting data in March 2020 at the beginning of the pandemic in France. We collected data from clinical examinations (motor and sensitive testing, deep tendon reflexes, and cranial nerve examination), routine blood chemistry analyses, cerebrospinal fluid (CSF) analyses (including cell count, protein, glucose, standard bacterial culture, and search of specific anti-ganglioside antibodies [the tested antibodies were anti-GM1, anti-GM2, anti-GM3, anti-GD1a, anti-GD2, anti-GD3, anti-GT1a, anti-GT1b, anti-GQ1b and anti-GM4]), electromyography (EMG) with measurements for motor and sensory nerve conduction, and brain imaging. The criteria by Hadden et al. (1998) were used to define demyelination on EMG. Thus, the primary demyelinating form was defined as a motor conduction velocity inferior to 90% of the lower limit of normal (LLN), a distal motor latency superior to 110% of the upper limit of normal (ULN), a compound muscle action potential amplitude after proximal stimulation/compound muscle action potential amplitude after distal stimulation (dCMAP) ratio inferior to 0.5, dCMAP superior or equal to 20% of the LLN, and a F-response latency inferior to 120% of the ULN. We collected the data during the patients' follow-ups at weeks 4, 8, and 12. The study was approved by the Ethical Committee of Medicine Odontology and Pharmacy Faculties and Hospitals (University Hospital of Strasbourg) N° CE-2020-32.
3. Results
Among the 294 adult patients with confirmed SARS-CoV-2 infection hospitalized in the ICU in our center between March 1, 2020, and May 31, 2021, three were diagnosed with GBS (1.02%): Two had classical GBS and one had a Miller Fisher syndrome with involvement of the peripheral nerves. Their demographic characteristics, clinical data, and results of investigations are shown in Table 1 . The mean age of the three patients was 66 years (range, 53–78 years). None of them had other neurological comorbidities. All patients required invasive mechanical ventilation, of which two required it due to respiratory failure caused by ARDS and they did not present any neurological symptoms at the beginning of respiratory failure when they were still conscious. One of the patients required invasive mechanical ventilation due to neuromuscular deficiency and respiratory failure caused by GBS. This patient did not present any respiratory symptoms before the beginning of tetraparesia and no damage was observed on chest tomography. Lumbar puncture and EMG were performed in all patients, and the results were compatible with those of GBS (Table 1). EMG was performed 2 days after the onset of neurological symptoms in patient 1 and approximately 15 days after the onset of neurological symptoms in patients 2 and 3. Hence, a diagnosis of GBS was made in the ICU for the three patients. In addition, anti-ganglioside antibodies were found in one patient. Serological test results for other infections that could trigger GBS (C. jejuni, Mycoplasma pneumoniae, EBV, CMV, HBV, HCV, HEV, and HIV) were negative, except anti-CMV IgM and anti-VCA IgM in one patient and anti-M. pneumoniae IgG in one patient. The serology data are missing for one patient. There was no clinical argument for these infections. For this study, we arrived at a diagnosis of GBS when the Brighton criteria were fulfilled (Fokke et al., 2014; Shahrizaila et al., 2021) (Table 2 ). One patient had neurological symptoms before sedation, which presented 20 days after the first COVID-19 symptoms when the patient was not yet hospitalized. Two patients were sedated before the first neurological symptoms appeared; the delay of neurological symptom onset could not be precisely defined (approximately 14 days after the onset of COVID-19 symptoms). All patients were sedated with sufentanil and midazolam for 5 to 10 days. All patients had a certain or highly probable GBS diagnosis following the Brighton criteria (level 1 or 2). All patients were treated by intravenous immunoglobulin (IVIg; 400 mg/kg/day) for 5 consecutive days, and two of them received two courses of IVIg treatment with the same protocol due to the severity of the neurologic impairment. The three patients had a favorable outcome complete recovery (n = 1) or with negligible to moderate sequela (n = 2), albeit with a slow recovery, as evidenced by the length of stay in the ICU (mean, 51.7 days; range, 41–73 days).
Table 1.
Patients' characteristics.
| Patient | 1 | 2 | 3 |
|---|---|---|---|
| Age | 53 y | 68 y | 78 y |
| Sex | Male | Female | Female |
| Comorbidity | BPPV, asthma | Localized scleroderma (morphea) | Atrial fibrillation, hypothyroidism, depressive disorder, herniated disc |
| Early symptoms of COVID-19 | Fever, myalgia, abdominal pain, emesis | Asthenia, cough, dyspnea | Asthenia, cough |
| COVID-19 diagnosis | Positive RT-PCR COVID on nasopharyngeal swab | Positive RT-PCR COVID on nasopharyngeal swab | Positive RT-PCR COVID on nasopharyngeal swab |
| Time from COVID-19 symptom onset to hospitalization | 19 days | 10 days | 6 days |
| Mechanical ventilation | Yes | Yes | Yes |
| Severity of COVID-19 | Mild illness (non-respiratory symptoms) |
Critical illness (Respiratory failure with invasive mechanical ventilation) |
Critical illness (Respiratory failure with invasive mechanical ventilation) |
| Time from intensive care unit admission to GBS diagnosis | 3 days | 22 days | 18 days |
| GBS subtypes | AIDP | Miller Fisher syndrome with involvement of the peripheral nerves | AIDP |
| Neurological symptoms | Painful tetraparesis Sensory impairment |
External ophthalmoplegia Flaccid tetraplegia |
Flaccid tetraplegia Facial diplegia |
| Neurological examination | Areflexia in lower limbs Hyporeflexia in upper limbs |
Diffuse areflexia Divergent strabismus |
Diffuse areflexia |
| Findings on CSF analysis | High protein level (1.7 g/L), 2 cells/mm3 | Normal protein level (0.23 g/L), 2 cells/mm3 | High protein level (3.31 g/L), 2 cells/mm3 |
| Anti-ganglioside antibodies (Immunodot Generic Assay; ref. 5003; Eurobio) | anti-GD3 IgG anti-GT1a IgG anti-GQ1b IgG |
Negative | Negative |
| Brain MRI | Not done | Not done | Leukoencephalopathy; cortical/subcortical brain atrophy |
| Electromyography | Decreased motor conduction velocity in left tibial nerve, increased distal motor latency in left median and right tibial nerves, increased F-response latency in right tibial nerve, and decreased sensitive conduction velocity in left ulnar nerve | Increased distal motor latency in right ulnar nerve and no F-response latency in right ulnar nerve | Decreased motor conduction velocity in left tibial and left ulnar nerves and increased distal motor latency in left and right ulnar nerves and left and right tibial nerves |
| Neurotoxic drugs before diagnosis | No | Hydroxychloroquine Atazanavir/ritonavir |
No |
| Treatment of GBS | Immunoglobulins (two courses) | Immunoglobulins (one course) | Immunoglobulins (two courses) |
| Specific COVID-19 therapies | No | Hydroxychloroquine Azithromycin Atazanavir/ritonavir |
Dexamethasone |
| Differential diagnosis | |||
|
Serology negative | No data available | Serology negative |
|
anti-EBNA IgG negative | No data available | anti-EBNA IgG negative |
| anti-VCA IgG negative | anti-VCA IgG negative | ||
| anti-VCA IgM positive | anti-VCA IgM negative | ||
| PCR negative | |||
|
IgG positive | No data available | IgG negative |
| IgM positive | IgM negative | ||
|
Anti-Hbs positive | No data available | Anti-Hbs positive |
| Ag Hbs and Anti-Hbc negative | Ag Hbs negative | ||
|
Anti-VHC negative | No data available | Anti-Hbc negative |
|
anti-VHE IgM negative | No data available | Anti-VHC negative |
|
Serology negative | No data available | anti-VHE IgM negative |
|
No data available | No data available | Serology negative |
| Clinical outcome | Sensory impairment of sole, balance disorder | Complete recovery | Weakness in lower limbs, balance disorder |
| Length of stay in intensive care unit | 41 days | 41 days | 73 days |
| Length of stay in hospital | 97 days | 79 days | 156 days |
BPPV, benign paroxysmal positional vertigo; GBS, Guillain–Barre syndrome; CSF, cerebral spinal fluid; MRI, magnetic resonance imaging; RT-PCR, reverse transcriptase-polymerase chain reaction; AIDP, acute inflammatory demyelinating polyneuropathy; EBV, Epstein-Barr virus; CMV, Cytomegalovirus; HBV, Hepatitis B virus; HCV: Hepatitis C virus: HEV, Hepatitis E virus; HIV: Human immunodeficiency virus; M. pneumoniae: M. pneumoniae.
Table 2.
Brighton criteria.
| Diagnostic criteria |
Level of diagnostic certainty |
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Bilateral and flaccid weakness of limbs | + | + | + | ± |
| Decreased or absent deep tendon reflexes in weak limbs | + | + | + | ± |
| Monophasic course and time between onset-nadir 12 h to 28 days | + | + | + | ± |
| CSF count cell <50/μl | + | +* | − | ± |
| CSF protein concentration > normal value | + | ± | − | ± |
| NCS findings consistent with one of the subtypes of GBS | + | ±* | − | ± |
| Absence of alternative diagnosis for weakness | + | + | + | + |
+, present; −, absent; ±, present or absent; GBS, Guillain-Barre syndrome; NCS, nerve conduction studies.
*If CSF is not collected or results not available, nerve electrophysiology results must be consistent with the diagnosis of Guillain-Barré syndrome. Level 1 is the highest level of diagnostic certainty, level 4 is the lowest level of diagnostic certainty. From Fokke et al., 2014, “Diagnosis of Guillain-Barré syndrome and validation of Brighton criteria”.
4. Discussion
We report three cases of GBS in the ICU that were diagnosed according to the clinical, biological, and electrophysical criteria during their hospitalization for COVID-19. The pathophysiology of GBS post-SARS-CoV-2 infection remains unclear and debated. Some studies have suggested the possibility of an inflammatory response targeting the nervous system, demonstrated by an increased level of pro-inflammatory markers in CSF such as IL-6, IL-8, or TNF-α (Gigli et al., 2020; Manganotti et al., 2021). Araújo et al. have proposed the mechanism of direct viral toxicity on the nervous system, highlighting the positivity of the reverse transcriptase-polymerase chain reaction COVID in the CSF of one patient (Araújo et al., 2021). Other authors have considered molecular mimicry (Siracusa et al., 2021), already largely described with other post-infectious GBS like C. jejuni, which have been linked with the detection of anti-ganglioside antibodies in the CSF, as we found in one patient (Loshaj-Shala et al., 2018). Finally, some authors have suggested the eventuality of a genetic predisposition by alleles of human leukocyte antigen (HLA) (Gigli et al., 2020). Our three patients were over 50 years old; however, younger cases have been described in the literature, including pediatric cases (Curtis et al., 2021). Two patients had a critical COVID-19 illness (respiratory failure requiring mechanical ventilation), and one patient had a mild COVID-19 illness (non-respiratory symptoms without oxygen supplementation). However, the relationship between COVID-19 severity and GBS is unclear. Gigli et al. described a paucisymptomatic patient infected by SARS-CoV-2 who developed GBS (Gigli et al., 2020). Some studies have suggested a higher frequency of the demyelinating form of GBS (AIDP) when it occurs after COVID-19 (Palaiodimou et al., 2021; Sheikh et al., 2021). In our study, two patients had a AIDP and one patient had a Miller Fisher syndrome with involvement of the peripheral nerves. Our study only considered sedated and intubated patients hospitalized in the ICU, which made it harder to confirm the diagnosis of GBS since the patients cannot express any muscular weaknesses or sensory impairments. However, in one patient, the first GBS symptoms started before ICU admission, which is why lumbar puncture has to be done as soon as possible when a patient presents with neurological symptoms. In two patients, the first GBS symptoms started in the ICU after they were sedated and treated with specific COVID-19 therapy. As it was not possible to perform motor examinations for these patients, the main argument to consider regarding the diagnosis of GBS was the difficulty in weaning from mechanical ventilation and the absence of clinical response to the modification of the respiratory rate imposed with the ventilator. Given the inability to perform a complete neurological examination on a sedated patient, it is essential to consider and eliminate all differential diagnosis prior to diagnosing GBS (Finsterer and Scorza, 2021b). The first condition to rule out is CIP, which can be clinically and electromyographically difficult to distinguish from GBS, especially with the axonal variant (Finsterer and Scorza, 2021b; Zhang et al., 2014). We excluded CIP due to the delay between ICU admission and diagnosis, which was considered too short for patient 1 (3 days), clinical examination (external ophthalmoplegia for patient 2, which is not compatible with CIP), CSF analyses (cytoalbuminologic dissociation for two patients and presence of anti-ganglioside antibodies for patient 1) and electrophysiological data. CIP usually occurs due to axonal damage (Lacomis, 2013), and all three patients had the primary demyelinating form according to Hadden's criteria. Second, we excluded all toxic neuropathy, which is a classical differential diagnosis of GBS and can be frequently found in patients under polymedication in the ICU. Only one patient was administered neurotoxic drugs before the onset of the first neurological symptom, but the exposure time to this drug seems too short to be responsible for neuropathy (7 days of treatment with hydroxychloroquine and 5 days of treatment with atazanavir/ritonavir). One potential explanation for a causal relationship between COVID-19 and GBS is the negativity of the infection's serologies that can trigger GBS. One patient had positive anti-VCA IgM but negative EBV PCR, which excludes primary EBV infection. This patient had doubtful anti-CMV IgM (0.7) (negativity threshold inferior to 0.7; positivity threshold superior to 1). Since CMV serology was performed on the second day of IgIV, we could not interpret the IgG results. It is likely that the positivity of CMV IgM reflects viral reactivation or polyclonal stimulation, especially since the patient did not present any clinical argument for CMV infection. Patient 3 had positive anti-M. pneumoniae IgG but negative IgM, which evoked an old infection.
The Brighton criteria were developed and validated by studies (Fokke et al., 2014) to help clinicians standardize the diagnosis of GBS. GBS is a therapeutic urgency, particularly when patients are hospitalized in the ICU, for whom the rehabilitation is often longer and more challenging (Dhar et al., 2008) due to prolonged bed rest and intensive care complications being added to GBS. In our study, the mean duration of ICU stay was 51.7 days (range, 41–73 days), and the duration of hospitalization was 97, 79, and 156 days for patients 1, 2, and 3, respectively, which reflects the severity of clinical presentations and the latency of rehabilitation. During the COVID-19 crisis, free hospital beds are rare and diagnosis should be rapid to reduce the duration of ICU stay. Two patients received two courses of IVIg. There is no consensus about the efficacy of the second 5-day course of IVIg for patients with severe neurological impairment. Although some studies have reported significant improvement after the second course (e.g., Hadden et al., 1998), recent studies have refuted this result and showed no significant difference between first and second courses of IVIg (Walgaard et al., 2021).
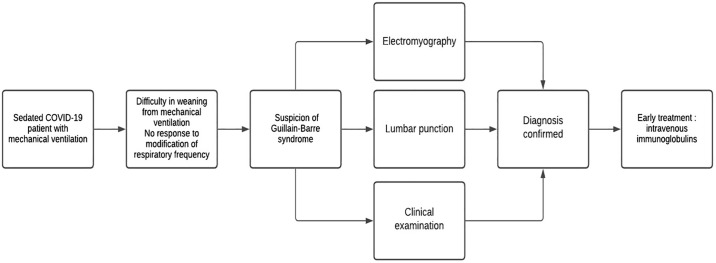
Our study has several limitations. The first is due to its retrospective and monocentric character. Second, the use of the Brighton criteria as a diagnostic method in our study was questionable because the clinical criteria evaluation was only performed after the initiation of specific treatment of GBS by IVIg and neurological examination was performed on sedated patients, which could introduce a potential bias in the study. Regardless, our study has provided a new viewpoint about post-COVID-19 GBS patients and highlights the necessity to consider GBS in the setting of new-onset neurological symptoms in the ICU and the difficulties related to arriving at this diagnosis. Diagnosing GBS in the ICU remains difficult, especially when patients are sedated. Thus, clinicians should aim to diagnose GBS before ventilation weaning becomes difficult for COVID-19 patients. In addition, lumbar puncture should be performed as soon as possible and EMG should be performed to reduce the delay before beginning specific treatment. To help clinicians manage post-COVID-19 GBS, we propose the algorithm presented in Fig. 1 .
Fig. 1.
Algorythm proposal for the management of a clinical suspicion of Guillain-Barre syndrome in SARS-CoV-2 patients under mechanical ventilation.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Funding acknowledgment
None.
Declaration of Competing Interest
None.
Acknowledgment
The authors would like to thank Enago (www.enago.com) for the English language review.
References
- Araújo N.M., Ferreira L.C., Dantas D.P., Silva D.S., Dos Santos C.A., Cipolotti R., Martins-Filho P.R. First report of SARS-CoV-2 detection in cerebrospinal fluid in a child with Guillain–Barre syndrome. Pediatr. Infect. Dis. J. 2021;40:e274–e276. doi: 10.1097/INF.0000000000003146. [DOI] [PubMed] [Google Scholar]
- Curtis M., Bhumbra S., Felker M.V., Jordan B.L., Kim J., Weber M., Friedman M.L. Guillain–Barre syndrome in a child with COVID-19 infection. Pediatrics. 2021;147 doi: 10.1542/peds.2020-015115. [DOI] [PubMed] [Google Scholar]
- Dhar R., Stitt L., Hahn A.F. The morbidity and outcome of patients with Guillain-Barre syndrome admitted to the intensive care unit. J. Neurol. Sci. 2008;264:121–128. doi: 10.1016/j.jns.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Finsterer J., Scorza F.A. Guillain-Barre syndrome in 220 patients with COVID-19. Egypt J. Neurol. Psychiatr. Neurosurg. 2021;57:55. doi: 10.1186/s41983-021-00310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterer J., Scorza F.A. Consider differentials before diagnosing SARS-CoV-2 associated Guillain-Barre syndrome. J. Med. Virol. 2021 doi: 10.1002/jmv.27084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fokke C., van den Berg B., Drenthen J., Walgaard C., van Doorn P.A., Jacobs B.C. Diagnosis of Guillain-Barre syndrome and validation of Brighton criteria. Brain. 2014;137:33–43. doi: 10.1093/brain/awt285. [DOI] [PubMed] [Google Scholar]
- Gigli G.L., Vogrig A., Nilo A., Fabris M., Biasotto A., Curcio F., Miotti V., Tascini C., Valente M. HLA and immunological features of SARS-CoV-2-induced Guillain-Barre syndrome. Neurol. Sci. 2020;41:3391–3394. doi: 10.1007/s10072-020-04787-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisanti S.G., Franciotta D., Garnero M., Zuppa A., Massa F., Mobilia E.M., Pesce G., Schenone A., Benedetti L. A case series of parainfectious Guillain-Barre syndrome linked to influenza A (H1N1) virus infection. J. Neuroimmunol. 2021;357 doi: 10.1016/j.jneuroim.2021.577605. [DOI] [PubMed] [Google Scholar]
- Hadden R.D., Cornblath D.R., Hughes R.A., Zielasek J., Hartung H.P., Toyka K.V., Swan A.V. Electrophysiological classification of Guillain-Barre syndrome: clinical associations and outcome. Plasma exchange/Sandoglobulin Guillain-Barré syndrome trial group. Ann. Neurol. 1998;44:780–788. doi: 10.1002/ana.410440512. [DOI] [PubMed] [Google Scholar]
- Hasan S.S., Capstick T., Ahmed R., Kow C.S., Mazhar F., Merchant H.A., Zaidi S.T.R. Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: a systematic review and meta-analysis. Exp. Rev. Respir. Med. 2020;14:1149–1163. doi: 10.1080/17476348.2020.1804365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacomis D. Electrophysiology of neuromuscular disorders in critical illness. Muscle Nerve. 2013;47:452–463. doi: 10.1002/mus.23615. [DOI] [PubMed] [Google Scholar]
- Leonhard S.E., Halstead S., Lant S.B., de Albuquerque M.F.P.M., de Brito C.A.A., de Albuquerque L.B.B., Ellul M.A., de França R.F.O., Gourlay D., Griffiths M.J., de Henriques-Souza A.M.M., de Machado M.Í.M., Medialdea-Carrera R., Mehta R., da Melo R.P., Mesquita S.D., Moreira Á.J.P., Pena L.J., Santos M.L., Turtle L., Solomon T., Willison H.J., Jacobs B.C., Ferreira M.L.B. Guillain-Barre syndrome during the Zika virus outbreak in Northeast Brazil: an observational cohort study. J. Neurol. Sci. 2021;420 doi: 10.1016/j.jns.2020.117272. [DOI] [PubMed] [Google Scholar]
- Loshaj-Shala A., Colzani M., Brezovska K., Poceva Panovska A., Suturkova L., Beretta G. Immunoproteomic identification of antigenic candidate Campylobacter jejuni and human peripheral nerve proteins involved in Guillain-Barre syndrome. J. Neuroimmunol. 2018;317:77–83. doi: 10.1016/j.jneuroim.2018.01.006. [DOI] [PubMed] [Google Scholar]
- Manganotti P., Bellavita G., Tommasini V., Acunto D., Fabris M., Cecotti L., Furlanis G., Sartori A., Bonzi L., Buoite Stella A., Pesavento V. Cerebrospinal fluid and serum interleukins 6 and 8 during the acute and recovery phase in COVID-19 neuropathy patients. J. Med. Virol. 2021 doi: 10.1002/jmv.27061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meppiel E., Peiffer-Smadja N., Maury A., Bekri I., Delorme C., Desestret V., Gorza L., Hautecloque-Raysz G., Landre S., Lannuzel A., Moulin S., Perrin P., Petitgas P., SellaI F., Wang A., Tattevin P., de Broucker T., contributors to the NeuroCOVID registry Neurologic manifestations associated with COVID-19: a multicentre registry. Clin. Microbiol. Infect. 2021;27:458–466. doi: 10.1016/j.cmi.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaiodimou L., Stefanou M.-I., Katsanos A.H., Fragkou P.C., Papadopoulou M., Moschovos C., Michopoulos I., Kokotis P., Bakirtzis C., Naska A., Vassilakopoulos T.I., Chroni E., Tsiodras S., Tsivgoulis G. Prevalence, clinical characteristics and outcomes of Guillain-Barre syndrome spectrum associated with COVID-19: a systematic review and meta-analysis. Eur. J. Neurol. 2021 doi: 10.1111/ene.14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejvar J.J., Kohl K.S., Gidudu J., Amato A., Bakshi N., Baxter R., Burwen D.R., Cornblath D.R., Cleerbout J., Edwards K.M., Heininger U., Hughes R., Khuri-Bulos N., Korinthenberg R., Law B.J., Munro U., Maltezou H.C., Nell P., Oleske J., Sparks R., Velentgas P., Vermeer P., Wiznitzer M., Brighton Collaboration GBS Working Group Guillain-Barre syndrome and fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2011;29:599–612. doi: 10.1016/j.vaccine.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Shahrizaila N., Lehmann H.C., Kuwabara S. Guillain-Barre syndrome. Lancet. 2021;397:1214–1228. doi: 10.1016/S0140-6736(21)00517-1. [DOI] [PubMed] [Google Scholar]
- Shehata G.A., Lord K.C., Grudzinski M.C., Elsayed M., Abdelnaby R., Elshabrawy H.A. Neurological complications of COVID-19: underlying mechanisms and management. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22084081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh A.B., Chourasia P.K., Javed N., Chourasia M.K., Suriya S.S., Upadhyay S., Ijaz F., Pal S., Moghimi N., Shekhar R. Association of Guillain-Barre syndrome with COVID-19 infection: an updated systematic review. J. Neuroimmunol. 2021;355 doi: 10.1016/j.jneuroim.2021.577577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siracusa L., Cascio A., Giordano S., Medaglia A.A., Restivo G.A., Pirrone I., Saia G.F., Collura F., Colomba C. Neurological complications in pediatric patients with SARS-CoV-2 infection: a systematic review of the literature. Ital. J. Pediatr. 2021;47:123. doi: 10.1186/s13052-021-01066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walgaard C., Jacobs B.C., Lingsma H.F., Steyerberg E.W., van den Berg B., Doets A.Y., Leonhard S.E., Verboon C., Huizinga R., Drenthen J., Arends S., Budde I.K., Kleyweg R.P., Kuitwaard K., van der Meulen M.F.G., Samijn J.P.A., Vermeij F.H., Kuks J.B.M., van Dijk G.W., Wirtz P.W., Eftimov F., van der Kooi A.J., Garssen M.P.J., Gijsbers C.J., de Rijk M.C., Visser L.H., Blom R.J., Linssen W.H.J.P., van der Kooi E.L., Verschuuren J.J.G.M., van Koningsveld R., Dieks R.J.G., Gilhuis H.J., Jellema K., van der Ree T.C., Bienfait H.M.E., Faber C.G., Lovenich H., van Engelen B.G.M., Groen R.J., Merkies I.S.J., van Oosten B.W., van der Pol W.L., van der Meulen W.D.M., Badrising U.A., Stevens M., Breukelman A.-J.J., Zwetsloot C.P., van der Graaff M.M., Wohlgemuth M., Hughes R.A.C., Cornblath D.R., van Doorn P.A., Dutch GBS Study Group Second intravenous immunoglobulin dose in patients with Guillain-Barre syndrome with poor prognosis (SID-GBS): a double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2021;20:275–283. doi: 10.1016/S1474-4422(20)30494-4. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wu L., Ni F., Ji W., Zhou C., Wu J. Critical illness polyneuropathy and myopathy: a systematic review. Neural Regen. Res. 2014;9:101. doi: 10.4103/1673-5374.125337. [DOI] [PMC free article] [PubMed] [Google Scholar]