Highlights
-
•
The national seroprevalence of anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies was 28.4%.
-
•
A geographic variation in seroprevalence of anti-SARS-CoV-2 antibodies was identified within Senegal.
-
•
The prevalence of SARS-CoV-2 seropositive people was similar between those who were symptomatic and those who were asymptomatic.
-
•
No correlation was found between SARS-CoV-2 and Plasmodium seroreactivity.
KEYWORDS: SARS-CoV-2, Serology, ELISA, IgG and IgM, Seroprevalence, Population-based survey, Senegal
Abstract
Objectives
A nationwide cross-sectional epidemiological survey was conducted to capture the true extent of coronavirus disease 2019 (COVID-19) exposure in Senegal.
Methods
Multi-stage random cluster sampling of households was performed between October and November 2020, at the end of the first wave of COVID-19 transmission. Anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies were screened using three distinct ELISA assays. Adjusted prevalence rates for the survey design were calculated for each test separately, and thereafter combined. Crude and adjusted prevalence rates based on test performance were estimated to assess the seroprevalence. As some samples were collected in high malaria endemic areas, the relationship between SARS-CoV-2 seroreactivity and antimalarial humoral immunity was also investigated.
Results
Of the 1463 participants included in this study, 58.8% were female and 41.2% were male; their mean age was 29.2 years (range 0.20–84.8.0 years). The national seroprevalence was estimated at 28.4% (95% confidence interval 26.1–30.8%). There was substantial regional variability. All age groups were impacted, and the prevalence of SARS-CoV-2 was comparable in the symptomatic and asymptomatic groups. An estimated 4 744 392 (95% confidence interval 4 360 164–5 145 327) were potentially infected with SARS-CoV-2 in Senegal, while 16 089 COVID-19 RT-PCR laboratory-confirmed cases were reported by the national surveillance. No correlation was found between SARS-CoV-2 and Plasmodium seroreactivity.
Conclusions
These results provide a better estimate of SARS-CoV-2 dissemination in the Senegalese population. Preventive and control measures need to be reinforced in the country and especially in the south border regions.
Research in context.
Evidence before this study
Coronavirus disease 2019 (COVID-19), first identified in December 2019 in Wuhan, China, is an acute respiratory infectious disease caused by a newly discovered coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Globalization, urbanization, and environmental changes have greatly accelerated this pandemic emergence and spread, generating an unprecedented global pandemic. Almost all countries and territories around the world (with the exception of 22 island states/territories, including Greenland, Tuvalu, Tonga, Tokelau, and Saint Helena) have been affected. By June 6, 2021, SARS-CoV-2 had infected more than 172 million people worldwide and had claimed the lives of more than 3.7 million people, with the European and American regions being the most affected and accounting for nearly 80% of all cases and deaths. The African region has reported around 3.5 million cases and 87 731 deaths since the first case of COVID-19 was declared on February 15, 2020 in Egypt. With only 2% of reported cases and 3% of recorded deaths, the African region has surprisingly been less impacted than the rest of the world. Although low testing rates have impacted the true extent of COVID-19 prevalence in Africa, there are no tangible indications that a large number of COVID-19 cases and deaths have been missed.
The first case in Senegal was declared on March 2, 2020. Despite the early implementation of prevention and control measures, the virus has gradually spread in the population. As of June 6, 2021, 41 581 confirmed cases and 1144 deaths had been reported. The control strategy in Senegal is largely based on a molecular testing system. As COVID-19 vaccines are being slowly implemented in Africa and in Senegal, it has become crucial to better evaluate the actual level of population exposure to SARS-CoV-2 through seroprevalence studies.
Added value of this study
In Africa, several seroprevalence surveys have already been conducted in specific population groups and targeted areas. The present study is the first cross-sectional population-based study conducted in all administrative regions of an African country. The study results show that the national surveillance system, mostly based on passive detection of symptomatic cases of COVID-19, only captured a tiny part of the virus transmission within the population. With an estimated national seroprevalence of 28.4%, this study describes a significant disparity between regions. Alongside the expected high seroprevalence in regions such as Dakar (44%, 11 016 confirmed cases) and Thiès (24.3%, 1974 confirmed cases), the study data showed a strong and silent circulation of the virus in regions such as Ziguinchor (56.7%), Sédhiou (48.0%), Kaolack (33.1%), and Kaffrine (26.9%). This latter region recorded only 20 COVID-19 confirmed cases during the survey period. All age groups were impacted and the prevalence of SARS-CoV-2 seropositive people was similar between symptomatic (27.8%) and asymptomatic (28.1%) people.
Implications of all the available evidence
This first African nationwide sero-epidemiological survey reveals that even if the COVID-19 outcomes have been less severe among African populations, the spread of SARS-CoV-2 among the population has been, to date, heterogeneous, extended, and silent. Therefore, African countries must not relax their prevention and surveillance efforts (including repeated cross-sectional serological surveys), as the emergence of several variants that transmit more easily, thus contributing to a more rapid spread, may cause a more severe pathology, specifically among the young population, leading to overloading of fragile healthcare systems.
Alt-text: Unlabelled box
1. Introduction
Classified as a global pandemic on March 11, 2020, coronavirus disease 2019 (COVID-19) is present in almost all countries and territories of the world, with more than 158 million confirmed cases and 3.2 million deaths reported by May 10, 2021 (Johns Hopkins, n.d., World health Organization 2021). While European and American regions continue to account for nearly 80% of all cases and deaths, the African region has been surprisingly less impacted, with only 2% (3 320 786) of reported cases and 3% (83 650) of recorded deaths. Among African countries, South Africa has been the most affected (with >1.5 million confirmed cases and 54 700 deaths), followed by Ethiopia and Nigeria (Lukman et al., 2020; World Health Organization, 2020).
Worldwide reported cases have been based on molecular diagnostic assays (real-time reverse transcription PCR, qRT-PCR) performed on nasopharyngeal or oropharyngeal swabs, and these have mainly been done for symptomatic carriers or their contacts; these people represent only the tip of the COVID-19 transmission iceberg. Indeed, the number of laboratory-confirmed cases does not fully reflect the true extent of infection. Several epidemiological studies have estimated that the proportion of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-infected people who are asymptomatic or paucisymptomatic is between 20% and 50% (Al-Qahtani et al., 2021; Buitrago-Garcia et al., 2020; Syangtan et al., 2021). In Africa, COVID-19 statistics are largely underestimated, in part because of the limited laboratory testing capacity and changing health authority strategies, but also because of the stigmatization associated with this new pathology and fear of attending health facilities. Consequently, studies measuring the prevalence of antibodies directed against SARS-CoV-2 proteins are important to strengthen pandemic surveillance and to guide timely public health strategies. This is needed especially in the context of emerging new variants of concern, the complexity of COVID-19 clinical presentations, and the slow rollout of an effective vaccine in Africa.
Most available serological studies have been conducted in European or American countries (Lai et al., 2020, Serological evidence of human infection with SARS-CoV-2: a systematic review and meta-analysis - The Lancet Global Health 2021). In Africa, a limited number of seroprevalence surveys have been conducted in specific population groups, including blood donors, healthcare workers, and COVID-19 exposed populations or the general population in some targeted districts/states (Chibwana et al., 2020; Etyang et al., 2021; Halatoko et al., 2020; Kempen et al., 2020; Milleliri et al., 2021; Mulenga et al., 2021; Uyoga et al., 2021,Wiens et al., 2021). However, it appears that, to date, no population-based studies at a country level have been performed in Africa.
Since the first COVID-19 confirmed case reported in Senegal on March 2, 2020, the country has implemented a range of lockdown measures aimed at spatial and physical distancing (Dia et al., 2020). However, the virus gradually spread to all regions, with a recorded 41 713 COVID-19 confirmed cases and up to 1148 deaths on June 7, 2021 (Sitrep 98 : Coronavirus 2021).
Senegal faced the first wave of COVID-19 between March and September 2020, with a peak of transmission recorded in June–July. Surveillance associated with COVID-19 has mostly focused on symptomatic patients and their close contacts. To better understand the epidemiological characteristics of the COVID-19 outbreak in Senegal, a population-based sero-epidemiological survey was conducted in all regions with the aims of assessing the level of population exposure to SARS-CoV-2 and guiding public health interventions. In this study, the seroprevalence results were also compared with the reported COVID-19 confirmed case rates within the same population during the same period.
2. Methods
2.1. Study design and sampling
Senegal is located in West Africa, is bordered by Mauritania, Mali, Guinea, and Guinea-Bissau, and surrounds The Gambia. Senegal has around 16.7 million inhabitants, a quarter of whom live in the Dakar region (0.3% of the territory). The country is administratively divided into 14 regions.
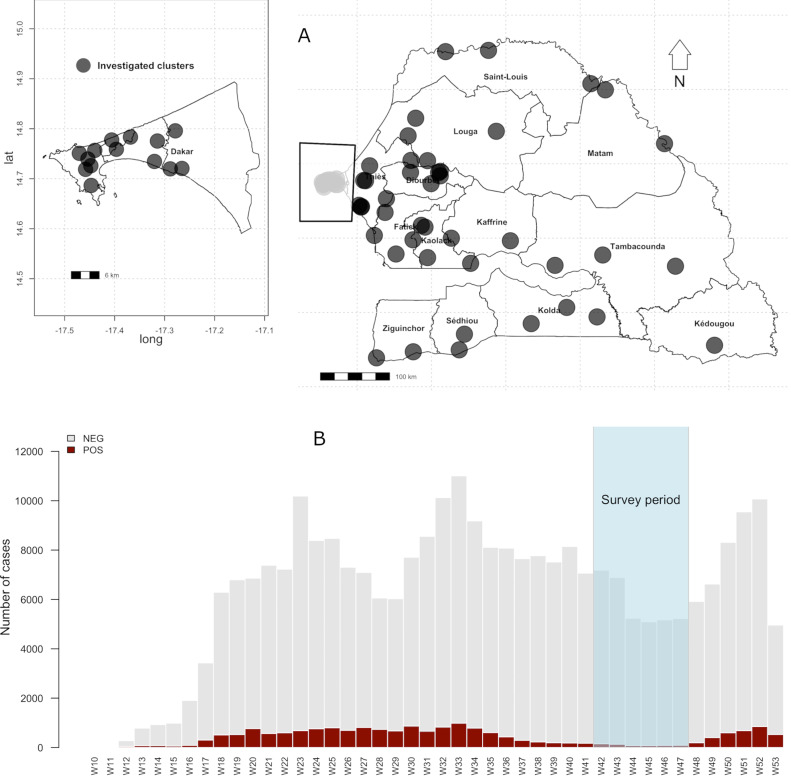
To evaluate the seroprevalence of anti-SARS-CoV-2 antibodies, a cross-sectional sero-epidemiological survey at the national level was conducted in all regions of Senegal at the end of the first COVID-19 transmission wave (from October 25 to November 26, 2020; Figure 1. A multi-stage cluster sampling was applied to randomly select 70 clusters, provided by the National Agency for Statistics and Demography (ANSD). In the first stage, areas were selected across Senegal by identifying the number of clusters by region according to the population size and using sampling proportional to the probability by size (PPS). In the second step, systematic random sampling to select 10 households in each selected area chosen was performed. Finally, for each selected household, a simple random sampling was done to systematically enroll two people over 5 years old and one child under 5 years old. The geographical localizations of the investigated clusters are shown in Figure 1. The study was designed following the World Health Organization (WHO) protocol for COVID-19 population-based sero-epidemiological studies (World Health Organization, 2020).
Figure 2.
Mapping of clusters investigated and the Senegal COVID-19 epidemic curve from March 2 to December 31, 2020 (Data sourced from the Ministry of Health).
All field workers were trained in protocol procedures, how to complete the questionnaires, and sample collection procedures under COVID-19 biosafety guidelines.
2.2. Ethical considerations
All randomly selected individuals who fulfilled the study inclusion criteria were invited to participate. All participants consented to participate in the study. For those younger than 18 years of age, a legal representative provided informed consent. The study was approved by the Senegalese National Ethics Committee for Research in Health (reference number N°0176/MSAS/DPRS/CNERS, 10 October 2020).
2.3. Sample size calculation
The sample size needed to estimate prevalence in all regions was assessed to be 1215 individuals (with a minimum sample size of 1395 and a maximum sample size of 2100), based on a design effect of 3 to account for intra-household clustering of cases, with an absolute error of ±5%, a power of 90%, and a seroprevalence rate of 50%. The sample size calculation took into account a 10% non-response rate and 5% non-valid samples.
2.4. Blood sampling and data collection
For each participant, a whole blood sample was collected into a dry vacutainer tube by standard venipuncture technique. Blood samples were immediately stored at 4°C and quickly directed to Institut Pasteur de Dakar within 24–72 hours, according to the routing conditions required for potentially infectious samples. Tracking procedures were established to ensure temperature-controlled delivery to Institut Pasteur de Dakar. After a centrifugation step, serum samples were collected, aliquoted, and stored at −20°C until use. Sociodemographic information such as age, sex, region, place of residence, occupation, education level, contact with COVID-19 confirmed cases, and history of symptoms during the 6 months prior to the survey, were also collected.
2.5. COVID-19 serological assays (ELISA)
The antibodies specifically directed against SARS-CoV-2 proteins were detected by ELISA. In this study, three CE-marked, indirect semi-quantitative commercially available ELISA kits were used: (1) the Omega Diagnostics COVID-19 IgG ELISA Kit (Mologic Ltd and Omega Diagnostics Ltd, Cambridgeshire, UK; Ref. ODL150/10), which detects IgG directed against the nucleocapsid (NP) and spike (subunit S2) proteins (Staines et al., 2021); (2) the ID Screen SARS-CoV-2-N IgG Indirect ELISA (IDVet; Innovative Diagnostics, France; Ref. SARSCOV2S), which detects IgG directed against only the SARS-CoV-2 NP (Krüttgen et al., 2021); (3) the Wantai SARS-CoV-2 Ab ELISA (Beijing Wantai Biological Pharmacy Enterprise, Beijing, China; Ref. WS-1096) recommended by the WHO for sero-epidemiological studies, which detects total antibodies (including IgM and IgG) binding the SARS-CoV-2 spike protein receptor-binding domain (S1/RBD) (GeurtsvanKessel et al., 2020). The main characteristics of each immunoassay are depicted in Supplementary Material Table S8. Serum samples were analyzed in duplicate according to the recommendations of the suppliers.
The diagnostic performance (sensitivity and specificity) of each ELISA assay was re-evaluated with a panel of 40 archived, well-documented serum or plasma samples, and the data obtained were used to adjust the seroprevalence. According to the performance obtained, in order to limit the rate of false IgG-positive results, after an evaluation of IgG directed against virus proteins using the OMEGA assay, all positive and doubtful samples were validated using the IDVet kit. In parallel, the Wantai kit was used to capture the whole antibody (including IgG and IgM) reactivity to SARS-CoV-2. The flowchart used to analyze the serum samples is described in Supplementary Material Figure S1.
2.6. Plasmodium falciparum antigen preparation and ELISA assay
Cross-reactivity on SARS-CoV-2 serological tests usually validated in high-income countries with pre-pandemic samples taken in malaria endemic areas have been reported by several studies. Therefore, cross-reactivity was explored in the study samples following the technique described below.
Crude schizont antigens from the P. falciparum 0703 field-adapted strain were prepared from in vitro continuous culture on O+ erythrocytes in RPMI medium containing 0.5% AlbuMAX. Schizont stage parasites were harvested and lysed in three volumes of sterile distilled water and stored in aliquots in liquid nitrogen. The ELISA assay was performed as described previously by Diop et al. and detailed in Supplementary Material Methods (Diop et al., 2015). Finite mixture models, assuming two underlying distributions of ‘negative’ (unexposed) and ‘positive’ (exposed) individuals, were created from log-transformed Median Fluorescence Intensity (MFI) values to determine the seropositivity cut-off. Finite mixture models were fit with the flexmix package in R version 3.5.1 (Comprehensive R Archive Network, Vienna, Austria).
2.7. Statistical analysis
Crude and standardized seroprevalence rates were calculated with the 95% confidence interval (CI). The 95% CI for seroprevalence were estimated using the Clopper–Pearson method. Weighted prevalence estimates were assessed using 2020 population data from ANSD and direct standardization on the observed seroprevalence and population weights by age andsex. For combined seroprevalence results, samples were counted as positive if they were positive with one of the two tests and negative if they were negative with both tests. The performance of combined tests was calculated using the performance of both tests (Weinstein et al., 2005). A multiple regression analysis was conducted. Statistical significance was assumed at a p-value <0.05. All statistical analyses were done with R version 4.0.4. The spatial distribution of seroprevalence was mapped using mapplots R package.
3. Results
3.1. Population characteristics
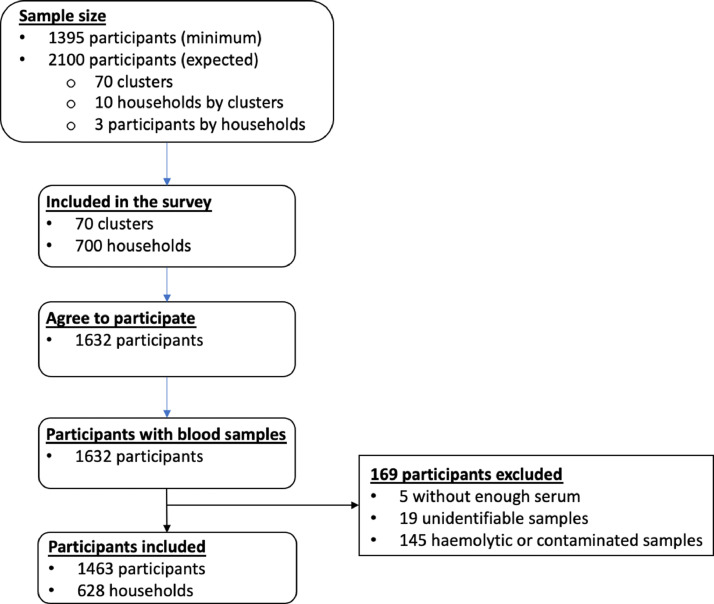
All of the Senegalese administrative regions were investigated. Among the 70 clusters initially targeted, only 66 were included in the study. One cluster in Sédhiou, in the south of the country, did not agree to participate in the study, and blood samples from three remote clusters in the Diourbel region were not delivered in compliance with the laboratory requirements. Of the 700 randomly selected households, 628 (89.7%) for which the head of the household consented to participate were included in the study. Hemolytic and under-filled blood samples were excluded from the serological analysis. Overall, 1632 people were enrolled, among whom 1463 (89.4%) with complete sociodemographic data and an eligible blood sample were included in the serological analysis (Figure 2).
Figure 1.
Flowchart of participant enrolment in the anti-SARS-CoV-2 antibody national seroprevalence study in Senegal.
This cross-sectional household survey was initiated just after the first wave of COVID-19 transmission (March to October 2020) (Figure 1). The mean age of the participants was 29.2 years, ranging from 0.20 to 84.8 years. There were significantly more female participants than male participants (58.8% vs 41.2%; P < 0.001). During the survey, a high number of people (n = 958; 67%) declared having a history of symptoms compatible with COVID-19 during the 2–3 months prior to the survey. Fever (73.8%), headaches (32.4%), and cough (30.0%) were the most often described COVID-19-related symptoms (Table 1). Anosmia and/or ageusia was only reported by 2.8% of the participants.
Table 1.
Main characteristics of the study population
| Overall (N = 1463) | |
|---|---|
| Age (years) | |
| Missing (n) | 17 |
| Mean (SD) | 29.20 (20.64) |
| Range | 0.20–84.8 |
| Age group (years), n (%) | |
| Missing (n) | 17 |
| 0–5 | 283 (19.6%) |
| >5–15 | 179 (12.4%) |
| >15–30 | 337 (23.3%) |
| >30–45 | 297 (20.5%) |
| >45–60 | 233 (16.1%) |
| >60 | 117 (8.1%) |
| Sex, n (%) | |
| Female | 860 (58.8%) |
| Male | 603 (41.2%) |
| Occupation, n (%) | |
| Missing | 281 |
| Traders | 81 (6.9%) |
| Schoolchild – student | 230 (19.5%) |
| Housewife | 325 (27.5%) |
| Workers | 65 (5.5%) |
| Jobless | 364 (30.8%) |
| Others | 117 (9.9%) |
| Level of education, n (%) | |
| Missing | 52 |
| None | 615 (43.6%) |
| Primary school | 251 (17.8%) |
| Secondary school | 298 (21.1%) |
| Koranic school | 247 (17.5%) |
| Region, n (%) | |
| Dakar | 302 (20.6%) |
| Diourbel | 121 (8.3%) |
| Fatick | 76 (5.2%) |
| Kaffrine | 80 (5.5%) |
| Kaolack | 116 (7.9%) |
| Kedougou | 21 (1.4%) |
| Kolda | 60 (4.1%) |
| Louga | 119 (8.1%) |
| Matam | 45 (3.1%) |
| Saint-Louis | 94 (6.4%) |
| Sédhiou | 57 (3.9%) |
| Tambacounda | 112 (7.7%) |
| Thiès | 210 (14.4%) |
| Ziguinchor | 50 (3.4%) |
| COVID-19 symptomatica, n (%) | |
| Missing | 41 |
| No | 464 (32.6%) |
| Yes | 958 (67.4%) |
| COVID-19 symptoms, n (%) | |
| Fever | 707 (73.8%) |
| Headache | 311 (32.4%) |
| Cough | 286 (30.0%) |
| Rhinorrhea | 187 (19.5%) |
| Fatigue | 148 (15.4%) |
| Myalgia | 72 (7.5%) |
| Sore throat | 34 (3.5%) |
| Diarrhea | 33 (3.4%) |
| Taste or smell lost | 27 (2.8%) |
SD, standard deviation.
History of symptoms compatible with COVID-19 less than 6 months before the survey.
3.2. Seroprevalence of anti-SARS-CoV-2 antibody
The prevalence data were weighted according to the age and sex distribution of the general population and adjusted according to the performance of each immunoassay. In accordance with the serological analysis strategy, seroprevalence data were given first using the results obtained with the OMEGA/IDVet ELISA (Supplementary Material Tables S3 and S4) and Wantai ELISA (Supplementary MaterialTables S5 and S6) separately, and subsequently after a combination of both results (Tables 2 and 3). At the country level, the overall seroprevalence was estimated at 22.5% (95% CI 20.4–24.7%) with the OMEGA/IDVet ELISA targeting IgG directed against SARS-CoV-2 proteins, and at 28.1% (95% CI 25.8–30.5%) with the Wantai ELISA measuring both IgG and IgM against the SARS-CoV-2 S1/RBD proteins. Combining the two approaches, OMEGA/IDVet and Wantai ELISA results, the global seroprevalence was estimated at 28.4% (95% CI 26.1–30.8%). SARS-CoV-2 combined seroprevalence data are presented below (Supplementary Material Figure S2).
Table 2.
Seroprevalence of anti-SARS-CoV-2 IgG per age group and sex: combined results obtained with OMEGA/IDVet and Wantai ELISA
| All samples | Seropositive samples | Crude seroprevalence |
Senegal population (2020) | Standardized seroprevalence |
Adjusted seroprevalence |
||||
|---|---|---|---|---|---|---|---|---|---|
| % | (95% CI) | % | (95% CI) | % | (95% CI) | ||||
| Age group (years) | |||||||||
| 0–5 | 283 | 45 | 15.9 | 11.8–20.7 | 3 224 935 | 15.9 | 11.8–20.7 | 16.1 | 12.2–21.1 |
| >5–15 | 179 | 44 | 24.6 | 18.5–31.6 | 4 096 096 | 24.6 | 18.5–31.6 | 24.9 | 19–32.2 |
| >15–30 | 337 | 124 | 36.8 | 31.6–42.2 | 4 423 193 | 36.5 | 31.3–41.9 | 37.3 | 32.2–42.8 |
| >30–45 | 297 | 81 | 27.3 | 22.3–32.7 | 2 687 369 | 29.9 | 24.8–35.5 | 27.6 | 22.6–33.1 |
| >45–60 | 233 | 70 | 30.0 | 24.2–36.4 | 1 418 953 | 30.0 | 24.2–36.4 | 30.4 | 24.6–36.8 |
| >60 | 117 | 29 | 24.8 | 17.3–33.6 | 855 062 | 24.9 | 17.3–33.6 | 25.1 | 17.3–33.6 |
| Sex | |||||||||
| Female | 850 | 235 | 27.6 | 24.7–30.8 | 8 391 358 | 27.6 | 24.7–30.8 | 28.0 | 25–31.2 |
| Male | 596 | 158 | 26.5 | 23–30.2 | 8 314 250 | 27.2 | 23.6–30.9 | 26.9 | 23.3–30.6 |
CI, confidence interval.
Table 3.
Seroprevalence of anti-SARS-CoV-2 IgG per region: combined results obtained with OMEGA/IDVet and Wantai ELISA
| Region | All samples (n) | Seropositive samples (n) | Crude seroprevalence |
Adjusted seroprevalence |
Population per region | Estimated COVID-19 infection | Confirmed COVID-19 cases | Ratio of reported cases to estimated infection | ||
|---|---|---|---|---|---|---|---|---|---|---|
| % | (95% CI) | % | (95% CI) | |||||||
| Dakar | 295 | 128 | 43.4 | 37.7–49.3 | 44.0 | 38.3–49.9 | 3 835 011 | 1 687 405 | 11 016 | 1:153 |
| Diourbel | 117 | 22 | 18.8 | 12.2–27.1 | 19.0 | 12.2–27.1 | 1 859 503 | 353 306 | 754 | 1:469 |
| Fatick | 75 | 13 | 17.3 | 9.6–27.8 | 17.5 | 9.6–27.8 | 900 800 | 157 640 | 325 | 1:485 |
| Kaffrine | 79 | 21 | 26.6 | 17.3–37.7 | 26.9 | 17.3–37.7 | 728 951 | 196 088 | 20 | 1:9804 |
| Kaolack | 113 | 37 | 32.7 | 24.2–42.2 | 33.1 | 24.2–42.2 | 1 191 577 | 394 412 | 291 | 1:1355 |
| Kedougou | 21 | 4 | 19.0 | 5.4–41.9 | 19.2 | 05.4–41.9 | 190 509 | 36 578 | 219 | 1:167 |
| Kolda | 52 | 12 | 23.1 | 12.5–36.8 | 23.4 | 12.5–36.8 | 822 003 | 192 349 | 216 | 1:891 |
| Louga | 118 | 13 | 11.0 | 6–18.1 | 11.1 | 6–18.1 | 1 061 612 | 117 839 | 75 | 1:1571 |
| Matam | 45 | 5 | 11.1 | 3.7–24.1 | 11.2 | 3.7–24.1 | 732 863 | 82 081 | 51 | 1:1609 |
| Saint-Louis | 94 | 15 | 16.0 | 9.2–25 | 16.2 | 9.2–25 | 1 091 735 | 176 861 | 319 | 1:554 |
| Sédhiou | 57 | 27 | 47.4 | 34–61 | 48.0 | 34–61 | 572 101 | 274 608 | 151 | 1:1819 |
| Tambacounda | 106 | 25 | 23.6 | 15.9–32.8 | 23.9 | 15.9–32.8 | 872 155 | 208 445 | 111 | 1:1878 |
| Thiès | 200 | 48 | 24.0 | 18.3–30.5 | 24.3 | 18.7–31.1 | 2 162 833 | 525 568 | 1974 | 1:266 |
| Ziguinchor | 50 | 28 | 56.0 | 41.3–70 | 56.7 | 41.3–70 | 683 955 | 387 802 | 567 | 1:684 |
| National | 1422 | 398 | 27.9 | 25.7– 30.4 | 28.4 | 26.1–30.8 | 16 705 608 | 4 744 393 | 16 089 | 1:295 |
CI, confidence interval.
SARS-CoV-2 transmission occurred in all age groups investigated, with the 15–30 years group showing the highest seroprevalence at 37.3% (95% CI 32.2–42.8%) (Table 2). The lowest weighted prevalence of 16.1% (95 CI 12.2–21.1%) was observed in children under 5 years old. Adjusted prevalence by sex was similar for female and male participants: 28.0% (95% CI 25.0–31.2%) and 26.9% (95% CI 23.3–30.6%), respectively (Table 2).
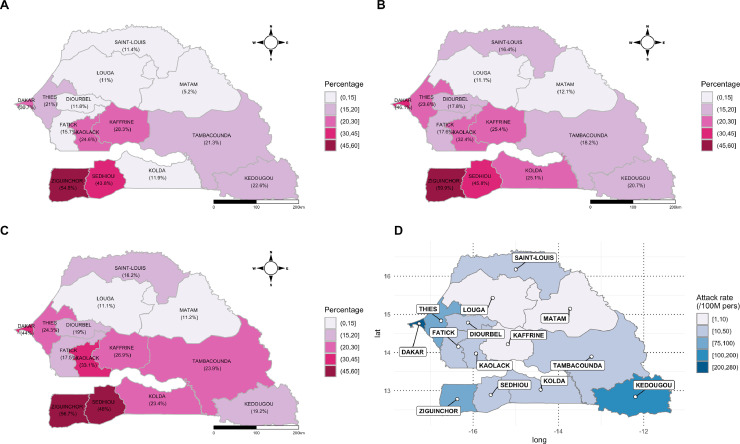
Regarding SARS-CoV-2 seroprevalence at the regional level, substantial variability was observed (Table 3, Figure 3). As expected, a high seroprevalence was recorded for the regions of Dakar, with 44.0% (95% CI 38.3–49.9%) of people harboring antibodies against SARS-CoV-2. Interestingly, two south border regions displayed the highest seroprevalence: Ziguinchor with 56.7% (95% CI 41.3–70.0%), followed by Sédhiou with 48.0% (95% CI 34.0–61.0%). The two regions located in the center north of Senegal, Louga and Matam, presented the lowest COVID-19 prevalence rates of 11.1% (95% CI 6.0–18.1%) and 11.2% (95% CI 3.7–24.1%), respectively. Of note, in Kaffrine, only 20 qRT-PCR confirmed cases were recorded during the survey period, while the seroprevalence was up to 26.9% (95% CI 17.3–37.7%), which was similar to that found in Thiès (24.0%, 95% CI 18.7–31.1%), the second most impacted region (after Dakar) with 1974 COVID-19 qRT-PCR confirmed cases.
Figure 3.
Mapping of the seroprevalence data obtained by ELISA (panels A, B, and C) and COVID-19 attack rate cases at the time of the household sero-survey (panel D).
Of the 958 (67.4%) participants who reported a history of symptoms compatible with COVID-19, 28.1% (n = 269) were COVID-19 seropositive. The prevalence of anti-SARS-CoV-2 antibodies among participants without documented COVID-19 symptomatology history (n = 464) was comparable (27.8%, n = 169). The seroprevalence of antibodies to SARS-CoV-2 among people reporting a history of COVID-19-related symptoms was variable: 27.0% (191 of 707) for fever, 31.1% (97 of 311) for headache, 24.8% (71 of 286) for cough, 29.2% (21 of 72) for myalgia, and 40.7% (11 of 27) among participants with anosmia and/or ageusia. No significant correlation was observed between reported COVID-19 symptoms and the serostatus (Supplementary Material Table S7).
3.3. Risk factors associated with COVID-19 transmission
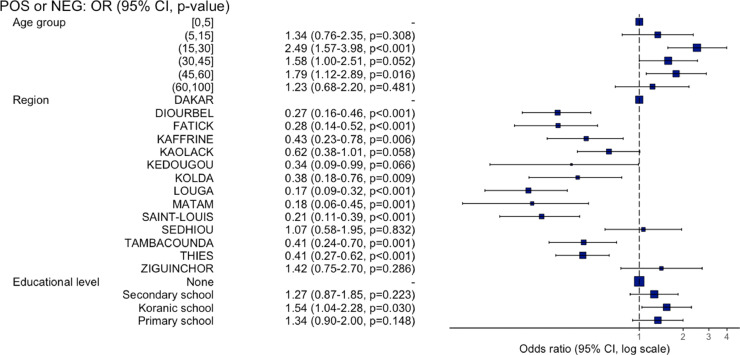
Participants aged 15–30 years (odds ratio (OR) 2.49, 95% CI 1.57–3.98; P < 0.001) and 45–60 years (OR 1.79, 95% CI 1.12–2.89; P = 0.016) were around two times more infected than children aged 0–5 years. No association was observed between SARS-CoV-2 seroprevalence and sex. The Dakar region was more at risk than all of the other regions except Sédhiou (OR 1.07; P = 0.832) and Ziguinchor (OR 1.42; P = 0.286) (Supplementary Material Table S1, Figure 4). No association was observed between COVID-19 seropositivity and occupation. Interestingly, a significant association was identified between SARS-CoV-2 seroprevalence and attendance at Koranic schools (OR 1.54; P = 0.030).
Figure 4.
Association between SARS-CoV-2 prevalence and sociodemographic factors.
3.4. Estimation of the national prevalence of COVID-19 in Senegal
Seroprevalence estimate results highlighted a strong discrepancy with the reported prevalence of COVID-19 based on RT-PCR-confirmed COVID-19 cases (Table 3). Indeed, the analyses estimated that 4 744 392 cases of SARS-CoV-2 infection (95% CI 4 360 164–5 145 327) potentially occurred, while only 16 089 cases were detected in the country during the survey period, with important variability between regions. At the national level, the ratio of reported cases to estimated infections was 1:295 and ranged between regions from 1:153 in Dakar to 1:9804 in Kaffrine.
3.5. Relationship between SARS-CoV-2 seropositivity and malaria seropositivity
To explore the impact of malaria seropositivity on immune reactivity to the SARS-CoV-2 proteins used in the serological tests, the immune reactivity (IgG) against a crude antigen extract of P. falciparum and SARS-CoV-2 ELISA on 863 randomly selected samples were also analyzed. Overall, 541 participants (62.7%) had IgG against P. falciparum antigens. The proportion of individuals seropositive for SARS-CoV-2 by OMEGA/IDVet ELISA screening was similar between individuals with and without immune reactivity towards P. falciparum antigen, i.e., 60.9% (142 of 233) and 59.7% (203 of 340), respectively. Similarly, SARS-CoV-2 seropositivity did not differ significantly between subjects with (26.2%, 142 of 541) and without (28.2%, 91 of 322) P. falciparum antibodies (P = 0.833). Comparable results were obtained with the Wantai COVID-19 total Ig immunoassay (Supplementary MaterialTable S2).
4. Discussion
This study appears to be the first COVID-19 cross-sectional, sero-epidemiological survey to be performed at a national level in Africa since the beginning of the pandemic. The survey was conducted at the end of the first wave of the COVID-19 epidemic and before the roll-out of COVID-19 vaccines in Senegal.
Several studies, including the present study, have reported cross-reactivity on SARS-CoV-2 serological tests usually validated in high-income countries with pre-pandemic samples taken in malaria endemic areas (Emmerich et al., 2021; Lapidus et al., 2021; Steinhardt et al., 2021). This cross-reactivity was detected either on SARS-CoV-2 nucleocapsid (N) or spike (S) proteins and thus complicated the use of any of the homemade or already available serological tests. They may be due to the high exposure of African populations to multiple pathogens, including one of the most prevalent parasites, Plasmodium. The present study results suggest no evidence of cross-reactivity interference between malaria and SARS-CoV-2 ELISA immunoassays using the double screening strategy with OMEGA/IDVet and Wantai ELISA assays(Supplementary Material Table S2).
The adjusted national prevalence of anti-SARS-CoV-2 IgG/IgM was estimated at 28.4%, with a great disparity between regions. Despite some areas of transmission like Dakar, Ziguinchor, and Sédhiou presenting high seroprevalence rates (>40%), the global seroprevalence suggests that a vast majority (around two in three) of Senegalese people still remained susceptible to SARS-CoV-2 infection. Indeed, it is estimated that about 4 744 392 cases of SARS-CoV-2 infection potentially occurred among the 16.7 million population. With the emergence of variants of concern in December 2020, these data may explain the rebound observed following the survey period, with a second wave that presented a higher amplitude in terms of the number of cases (41 416 compared to 16 089) and deaths (333 compare to 1139) in comparison to the first wave, as herd immunity was not reached.
The highest prevalence was observed in the regions of Ziguinchor and Sédhiou, with seroprevalence of 56.7% (574 confirmed cases) and 48.0% (151 confirmed cases), respectively. These regions are located in the south-western part of the country, close to the borders with the neighboring countries of Guinea-Bissau and Gambia. These high seroprevalence data do not mirror the surveillance data recorded at the time of the survey. Several factors could explain these discrepancies, including (1) the lack of molecular testing at the beginning of the COVID-19 pandemic in these remote areas (on-site laboratory testing was put in place only in August 2020), (2) an airport in Ziguinchor (a tourist region) facilitating imported cases before the closure of air traffic, and (3) the difficulty controlling inter-regional and cross-border trade. Louga and Matam, with a seroprevalence of around 11%, seemed to be less impacted by the COVID-19 epidemic. After the Tambacounda region, these rural regions are the two largest regions in the country, with low population densities of around 20–30 inhabitants/km2. These sociodemographic characteristics could explain the lower circulation of the virus. Kaffrine, one of the main commercial crossroads of the country, recorded a high seroprevalence (26.9%) with regard to the low number of COVID-19 confirmed cases (n = 20) registered. High population movement with a lack of on-site laboratory facilities for molecular testing may greatly have contributed to this underestimation of the extent of the COVID-19 burden. This underestimation can be explained by the large number of asymptomatic individuals or those with limited symptoms, not requiring medical consultation.
Other studies performed in Africa over the same period or at the beginning of 2021 have reported similar results, i.e. Juba, South-Sudan (22.6% in August–September) and neighboring Mali (54.7% in December to January 2021), although these studies involved only a part of the population or a targeted population (Sagara et al., 2021, Wiens et al., 2021). Altogether, this demonstrates an important circulation of SARS-CoV-2 in Senegal and in general in Africa. The slow SARS-CoV-2 transmission was not mirrored by the data obtained through the surveillance system, highlighting the need to assess the true exposure of COVID-19.
Given the scale of the pandemic, the Senegalese authorities quickly implemented several measures to limit the spread of the virus, such as social distancing, lockdown of markets, schools, and universities, masking in public places and public transport, hand washing, contact tracing and testing, isolation, and treatment of SARS-CoV-2-positive persons in health centers, among others. It is difficult to assess how much and how strictly these measures were followed by the population between March and July 2020. However, transmission was not stopped and social living conditions in Africa remain favorable to airborne transmissible viruses. This study clearly demonstrated the invisible and extended spread of the virus in the population regardless of age, sex, or level of education. The results are corroborated by other studies showing that the rate of infection with SARS-CoV-2 was higher than the rate announced by the surveillance systems (Sagara et al., 2021; Sykes et al., 2021). The hidden face of the progression of the virus was enhanced by the low number of molecular tests performed and a smaller proportion of severe cases of the disease as compared to European and American regions.
The severity of COVID-19 disease may have been tempered by one of the demographic features of the African population, i.e. the youth of the Senegalese population (mean age 19 years), a more robust non-specific immunity gained by multiple and abundant exposures to pathogens that protect against the severe form of COVID-19, and the absence of self-reporting of mild symptoms compatible with COVID-19 during the first wave. Indeed, a high proportion of participants (67.2%) reported having a history of COVID-19-related symptoms during the 2–3 months prior to the survey (corresponding to the peak of transmission in June–July). However, in the absence of more severe symptoms, none of them made a voluntary laboratory diagnosis of COVID-19. This observation highlights the complexity of attributing these non-specific symptoms to COVID-19, the stigmatization around this pathology, and also the fear of attending health facilities or being diagnosed as SARS-CoV-2-positive. In addition to forgetting the symptoms, all of these aspects may explain the fact that, in the present study, the seroprevalence of anti-SARS-CoV-2 antibodies was comparable between symptomatic and asymptomatic individuals.
There were more female participants than male participants; however, no significant difference in seroprevalence data was observed between the two groups. The most affected age group was 15–30 years, which is the most active age group, followed by the 45–60 years group, when compared to the under-5 years age group. The household contact mode seems to be the main transmission mode, as the <5 years age group and the >60 years age group were also impacted. No significant difference in the level of infection by occupation was noted. This could be explained by the fact that all occupations were at the same risk level, with a significant community-based transmission. Interestingly, a significant association was identified between SARS-CoV-2 seroprevalence and attendance at Koranic schools (OR 1.54, 95% CI 1.04–2.28; P = 0.030). This could reflect that schools in general (including Koranic schools) are places of favorable transmission due to the regrouping of individuals of all age groups without necessarily the required respect for social distancing measures.
This study is the first population-based representative national survey to estimate the number of SARS-CoV-2 infections in Senegal and in West Africa. The study provides an estimate of the current level of transmission of the disease, and points out the impact of community-based COVID-19 transmission and the cross-regional or cross-border transmission increased by population movement. These data also demonstrate the need to strengthen decentralized laboratories equipped with a capacity for molecular biology analysis of SARS-CoV-2 infection in all regions, in order to improve monitoring. With the emergence of SARS-CoV-2 variants, regular population-based national sero-epidemiological studies will be mandatory to guide prevention, control, and vaccination strategies. A larger number of clusters in some regions would provide a better estimate of the prevalence.
Acknowledgments
Acknowledgements
This work was supported by the US Centers for Disease Control and Prevention (CDC), the Senegalese Ministry of Health, the Senegalese National Agency for Statistics and Demography (ANSD), the WHO Unity Program, and the COVID-19 Task-force of the International Pasteur Institute Network (IPIN, REPAIR project). ANSD also provided the census information necessary for the random selection of households. Beijing Wantai SARS-CoV-2 Ab ELISA kits were a generous gift of the WHO Unity Program. We express our gratitude to the population of the regions and districts investigated and especially to the children, parents, and guardians who participated in the study. We also thank all of the people who facilitated the survey, heads of regions and districts, local administration authorities, local health authorities from the Ministry of Health, and community health workers (especially the Badiène-Gox). We also thank the survey teams. Additionally, we would like to thank Dr Thomas Poiret and Dr Amy K. Bei for their review and feedback on the writing of the manuscript. The findings and conclusions in this article are those of the authors and do not necessarily reflect the position of the Senegalese Ministry of Health or the US Centers for Disease Control and Prevention (CDC).
Declarations
Data sharing: De-identified participant data used for this analysis can be requested from Institut Pasteur de Dakar. Interested researchers must submit a research proposal for consideration by the study investigators. If approved, the requestor must sign a data use agreement. Additionally, the study protocol is available upon request. All data requests should be directed to the corresponding author.
Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Funding: US Centers for Disease Control and Prevention (CDC). The study funder was involved in the study design, data collection, data analysis, data interpretation, writing of the manuscript, or the decision to submit the manuscript for publication.
Conflict of interest: We declare no competing interests.
Author contributions
OusF, CT, AAS, JLR, JT, OF, CL, BD, MN, CTD, OP, and IVW conceived and designed the study. CT, SN, MB, AT, MD, MarD, IMK, JPD and JLR co-coordinated the survey and data collection. ON, OF, RF, AAM, IVW, BaD, MDia, and CTD performed the serological analysis. CT, IVW, and MD accessed and verified the underlying data. CT, CL, MarD, TW, MW, ON, MST, and IVW analyzed the data. CT, IVW, JLR, and BD wrote the manuscript. All authors contributed to the data interpretation, critically reviewed the first draft and approved the final version of the manuscript, and agreed to be accountable for the work. All authors had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijregi.2022.02.007.
Appendix. Supplementary materials
References
- Al-Qahtani M, AlAli S, AbdulRahman A, Salman Alsayyad A, Otoom S, Atkin SL. The prevalence of asymptomatic and symptomatic COVID-19 in a cohort of quarantined subjects. International Journal of Infectious Diseases. 2021;102:285–288. doi: 10.1016/j.ijid.2020.10.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buitrago-Garcia D, Egli-Gany D, Counotte MJ, Hossmann S, Imeri H, Ipekci AM, et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: A living systematic review and meta-analysis. PLOS Medicine. 2020;17 doi: 10.1371/journal.pmed.1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibwana MG, Jere KC, Kamn'gona R, Mandolo J, Katunga-Phiri V, Tembo D, et al. High SARS-CoV-2 seroprevalence in health care workers but relatively low numbers of deaths in urban Malawi. MedRxiv. 2020 doi: 10.1101/2020.07.30.20164970. 2020.07.30.20164970. [DOI] [Google Scholar]
- Dia N, Lakh NA, Diagne MM, Mbaye KD, Taieb F, Fall NM, et al. COVID-19 Outbreak, Senegal, 2020. Emerg Infect Dis. 2020;26:2771–2773. doi: 10.3201/eid2611.202615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diop F, Diop G, Niang M, Diouf B, Ndiaye D, Richard V, et al. The value of local malaria strains for serological studies: local strains versus Palo Alto reference strain. Malaria Journal. 2015;14:229. doi: 10.1186/s12936-015-0734-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerich P, Murawski C, Ehmen C, von Possel R, Pekarek N, Oestereich L, et al. Limited specificity of commercially available SARS-CoV-2 IgG ELISAs in serum samples of African origin. Trop Med Int Health. 2021;26:621–631. doi: 10.1111/tmi.13569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etyang AO, Lucinde R, Karanja H, Kalu C, Mugo D, Nyagwange J, et al. Seroprevalence of Antibodies to SARS-CoV-2 among Health Care Workers in Kenya. Clin Infect Dis. 2021:ciab346. doi: 10.1093/cid/ciab346. [DOI] [Google Scholar]
- GeurtsvanKessel CH, Okba NMA, Igloi Z, Bogers S, Embregts CWE, Laksono BM, et al. An evaluation of COVID-19 serological assays informs future diagnostics and exposure assessment. Nat Commun. 2020;11:1–5. doi: 10.1038/s41467-020-17317-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halatoko WA, Konu YR, Gbeasor-Komlanvi FA, Sadio AJ, Tchankoni MK, Komlanvi KS, et al. Prevalence of SARS-CoV-2 among high-risk populations in Lomé (Togo) in 2020. PLOS ONE. 2020;15 doi: 10.1371/journal.pone.0242124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempen JH, Abashawl A, Suga HK, Difabachew MN, Kempen CJ, Debele MT, et al. SARS-CoV-2 Serosurvey in Addis Ababa, Ethiopia. The American Journal of Tropical Medicine and Hygiene. 2020;103:2022–2023. doi: 10.4269/ajtmh.20-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüttgen A, Cornelissen CG, Dreher M, Hornef MW, Imöhl M, Kleines M. Determination of SARS-CoV-2 antibodies with assays from Diasorin, Roche and IDvet. J Virol Methods. 2021;287 doi: 10.1016/j.jviromet.2020.113978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C-C, Wang J-H, Hsueh P-R. Population-based seroprevalence surveys of anti-SARS-CoV-2 antibody: An up-to-date review. Int J Infect Dis. 2020;101:314–322. doi: 10.1016/j.ijid.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidus S, Liu F, Casanovas-Massana A, Dai Y, Huck JD, Lucas C, et al. Plasmodium infection induces cross-reactive antibodies to carbohydrate epitopes on the SARS-CoV-2 Spike protein. MedRxiv. 2021 doi: 10.1101/2021.05.10.21256855. 2021.05.10.21256855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukman AF, Rauf RI, Abiodun O, Oludoun O, Ayinde K, Ogundokun RO. COVID-19 prevalence estimation: Four most affected African countries. Infect Dis Model. 2020;5:827–838. doi: 10.1016/j.idm.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milleliri JM, Coulibaly D, Nyobe B, Rey J-L, Lamontagne F, Hocqueloux L, et al. SARS-CoV-2 Infection in Ivory Coast: A Serosurveillance Survey among Gold Mine Workers. Am J Trop Med Hyg. 2021 doi: 10.4269/ajtmh.21-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulenga LB, Hines JZ, Fwoloshi S, Chirwa L, Siwingwa M, Yingst S, et al. Prevalence of SARS-CoV-2 in six districts in Zambia in July, 2020: a cross-sectional cluster sample survey. The Lancet Global Health. 2021;9:e773–e781. doi: 10.1016/S2214-109X(21)00053-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagara I, Woodford J, Kone M, Assadou MH, Katile A, Attaher O, et al. Rapidly increasing SARS-CoV-2 seroprevalence and limited clinical disease in three Malian communities: a prospective cohort study. MedRxiv. 2021 doi: 10.1101/2021.04.26.21256016. 2021.04.26.21256016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19 Map. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html (accessed November 28, 2021)
- Serological evidence of human infection with SARS-CoV-2: a systematic review and meta-analysis - The Lancet Global Health. n.d. https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(21)00026-7/fulltext (accessed October 14, 2021). [DOI] [PMC free article] [PubMed]
- Sitrep 98 : Coronavirus: Riposte à l’épidémie du nouveau Coronavirus COVID-19, Sénégal. Rapport de Situation N°98 du 07 Juin 2021 | MINISTÈRE DE LA SANTÉ ET DE L'ACTION SOCIALE. n.d. https://www.sante.gouv.sn/activites/sitrep-98-coronavirus-riposte-%C3%A0-l%C3%A9pid%C3%A9mie-du-nouveau-coronavirus-covid-19-s%C3%A9n%C3%A9gal-rapport (accessed October 14, 2021).
- Staines HM, Kirwan DE, Clark DJ, Adams ER, Augustin Y, Byrne RL, et al. IgG Seroconversion and Pathophysiology in Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Emerging Infectious Diseases journal - CDC. 2021;27(1) doi: 10.3201/eid2701.203074. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt LC, Ige F, Iriemenam NC, Greby SM, Hamada Y, Uwandu M, et al. Cross-Reactivity of Two SARS-CoV-2 Serological Assays in a Setting Where Malaria Is Endemic. J Clin Microbiol. 2021;59 doi: 10.1128/JCM.00514-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syangtan G, Bista S, Dawadi P, Rayamajhee B, Shrestha LB, Tuladhar R, et al. Asymptomatic SARS-CoV-2 Carriers: A Systematic Review and Meta-Analysis. Front Public Health. 2021;8 doi: 10.3389/fpubh.2020.587374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes W, Mhlanga L, Swanevelder R, Glatt TN, Grebe E, Coleman C, et al. Prevalence of anti-SARS-CoV-2 antibodies among blood donors in Northern Cape, KwaZulu-Natal, Eastern Cape, and Free State provinces of South Africa in January 2021. Res Sq. 2021 doi: 10.21203/rs.3.rs-233375/v1. rs.3.rs-233375. [DOI] [Google Scholar]
- Uyoga S, Adetifa IMO, Karanja HK, Nyagwange J, Tuju J, Wanjiku P, et al. Seroprevalence of anti–SARS-CoV-2 IgG antibodies in Kenyan blood donors. Science. 2021;371:79–82. doi: 10.1126/science.abe1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein S, Obuchowski NA, Lieber ML. Clinical Evaluation of Diagnostic Tests. American Journal of Roentgenology. 2005;184:14–19. doi: 10.2214/ajr.184.1.01840014. [DOI] [PubMed] [Google Scholar]
- Wiens KE, Mawien PN, Rumunu J, Slater D, Jones FK, Moheed S, et al. Seroprevalence of Severe Acute Respiratory Syndrome Coronavirus 2 IgG in Juba, South Sudan, 2020. Emerging Infectious Diseases journal - CDC n.d. 2021;27(6) doi: 10.3201/eid2706.210568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2020. Population-based age-stratified seroepidemiological investigation protocol for coronavirus 2019 (COVID-19) infection, 26 May 2020. [Google Scholar]
- Weekly epidemiological update on COVID-19, https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---15-march-2022 (accessed November 30, 2021), 2021
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.