Abstract
The specter of bioterrorism has captured the attention of government and military officials, scientists, and the general public. Compared to other sectors of the population, clinical microbiologists are more directly impacted by concerns about bioterrorism. This review focuses on the role envisioned for clinical laboratories in response to a bioterrorist event. The microbiology and clinical aspects of the biological agents thought to be the most likely tools of bioterrorists are presented. The historical background of the problem of bioterrorism and an overview of current U.S. preparedness planning, with an emphasis on the roles of health care professionals, are also included.
While the recent growing awareness of the threat of biological weapons affects all segments of the population, the impact on clinical microbiologists is more direct. If a bioterrorist event occurred, the average clinical microbiology laboratory could be instrumental in helping to detect and identify the biological weapon that was used and in alerting authorities. Although Bacillus anthracis and smallpox have received a great deal of publicity as potential biological weapons, the term biological agent applies to a diverse group of microorganisms as well as toxins of microorganisms, plants, and animals. Bioterrorism is more specifically defined as the use of biological agents to inflict disease and/or death on humans, animals or plants. Thus, crops and livestock as well as human populations are considered possible bioterrorist targets.
Bioterrorist acts could have political, religious, idealogical, or criminal motivation and could conceivably be planned by groups or a single individual or be part of state-sponsored terrorist activities.
The biological agents thought to be the most likely weapons of bioterrorists include B. anthracis (anthrax), Francisella tularensis (tularemia), Yersinia pestis (plague), variola virus (smallpox), agents of viral hemorrhagic fevers, and botulism toxin. Brucella spp. (brucellosis) were recently removed from the list of most likely agents but remain a possible agent along with Vibrio cholerae (cholera), Burkholderia pseudomallei (glanders), Coxiella burnetti (Q fever), agents of viral encephalitis, staphylococcal enterotoxin, ricin, and mycotoxins (31; S. R. Perry, Centers for Disease Control and Prevention [CDC] and Association of Public Health Laboratories-sponsored course “Response to Bioterrorism: The Role of the Clinical Laboratory,” Boston, Mass. 1999). While some biological agents would harm only the population exposed (e.g., botulism toxin), infectious agents producing contagious disease (e.g., smallpox) could disseminate through susceptible populations unaffected directly by the initial bioterrorist event. The reader is reminded that chemical weapons (e.g., the nerve gas sarin) have been used in terrorist attacks and, although they serve as a complement to the arsenal of possible terrorist weapons, will not be considered in this review.
How would a biological agent, used with intent to harm a civilian population, be delivered to its target? First, the agent must be “weaponized,” or produced in sufficient quantity and in a form that would be relatively stable and easily disseminated. The attack itself would be either overt (announced) or covert. The prospect of a covert attack is most disturbing because the event itself might be completely unnoticed. In the case of covertly disseminated infectious agents, there might be no realization that an attack had occurred until numerous victims fell ill and their common illness had been diagnosed. The obvious and age-old method of contamination of food or water supplies is considered a plausible modern route for dissemination of some biological agents. While percutaneous administration, of certain toxins for example, represents another mode of dissemination, this method would be extremely challenging if the target were a sizeable population (23).
The most efficient method of delivering biological agents is thought to be the air-borne route, with agents dispersed in aerosols. Wide dissemination of infectious agents and even toxins can be achieved with this method. Low-cost, easily obtainable equipment (as employed in the agricultural industry) can be used to produce aerosols with particle sizes of 1 to 10 μm (23). Under ideal conditions these particles may remain suspended for hours and are sufficiently small to make their way into the distal bronchioles and terminal alveoli after inhalation. Aerosols can be delivered from stationary point sources, aircraft, boats, or missiles. The meteorological skills of the bioterrorist would be put to the test in such an event, since wind speeds that are too high can disperse an aerosol, destroying its effectiveness. Miscalculation of wind direction could result in a missed target or worse (at least for the bioterrorist), self-contamination with the agent being dispersed (21, 23). The far-reaching consequences of air-borne dispersal of the biological agents B. anthracis and smallpox virus on a civilian population are addressed in the hypothetical scenarios of Ingelsby (29), O'Toole (39), and others (4, 6).
The idea of mounting some form of defensive activity against possible bioterrorist actions is appealing because although bioterrorist acts are expected to be infrequent, they are regarded as “low-probability, high-consequence events” (49). The news media have encouraged and highlighted public concerns about bioterrorism (16, 38). Public feelings about bioterrorism are reminiscent of the widespread concern about nuclear weapons that arose in the post-World War II Cold War era. Fear of nuclear, chemical, and biological weapons of mass destruction is well founded and perhaps reflects an innate human revulsion, based on moral and ethical grounds, against the use of weapons producing such catastrophic suffering in fellow humans, even those construed as enemies (15, 46). The duck and cover exercises of 1950s schoolchildren may have produced a sense of being able to defend ourselves against a nuclear attack, but it's difficult to imagine a simple duck-and-cover defensive strategy against biological weapons.
The potential of a bioterrorist attack has ebbed into the consciousness of the American people. International terrorism against the United States has mainly targeted sites abroad and was directed against U.S. military installations and embassies. The bombing of the World Trade Center in New York marked the introduction of such activity to U.S. territory, and there are indications that terrorists could use biological agents in the future. President Clinton stated, “This is not a cause for panic. It is a cause for serious, deliberate, disciplined, long-term concern” (44). The U.S. Department of Health and Human Services increased the budget to prepare for bioterrorism in the fiscal year 2000 to $230,000,000. The funds support an antibioterrorism initiative with the goal of preparing the nation to better defend itself against a potential attack with biological agents.
This review will trace the origins of biological weapons and describe current perceptions of the threat proposed by the use of such weapons. Developing ideas on effective defensive strategies against bioterrorist attacks will be discussed, including the perceived roles of clinical microbiologists and clinical microbiology laboratories. The clinical and microbiological aspects of the agents thought most likely to be used by bioterrorists will be described. Finally, proposals for the steps that need to be taken in order to further defensive preparedness will be examined.
HISTORY OF BIOLOGICAL WEAPONS
Events prior to the 20th Century
The concept of biological agents as weapons is hardly a novel idea. History offers examples, tempered by the existing levels of scientific knowledge about infectious diseases, of the use of biological agents for inflicting harm upon enemies. Long before the germ theory of disease was advanced, humans associated disease with foul odors; contagion was thought to be spread by “miasmas,” or bad vapors (21). Existing evidence suggests that ancient civilizations (the Greeks, Romans, and Persians) attempted to pollute the drinking-water supplies of their enemies by contamination with foul-smelling dead animals. Human cadavers were added to the well-polluting arsenal by Barbarossa in 12th century Italy. Poisoning enemy drinking-water supplies with dead animals was still employed in the 19th century during the Civil War in the United States (40).
During medieval times, warriors pressed their catapults into service for spreading pestilence to their enemies. One example of this practice occurred during the 14th century siege of Kaffa, a seaport city in what is now the Ukraine. Tatar forces attacking Kaffa catapulted deceased plague victims from among their own ranks into the besieged city in order to spread disease and hasten a victory. A plague outbreak was documented in Kaffa, and it has been hypothesized that the fleeing citizens (as well as rats) of Kaffa who escaped via ship to various Mediterranean ports aided in the development of the second plague pandemic in the mid-1300s. Christopher and colleagues (13), however, point out that there is little epidemiological evidence to support the apparently successful biological warfare activities of the Tatars. Fleas serving as plague vectors desert cadavers in favor of living hosts, and the seemingly successful plague epidemic caused by the Tatars may have in reality been a natural outbreak of disease in the besieged city of Kaffa.
The concept of inanimate fomites as vehicles for spreading disease to enemies was chronicled in the 18th century. In 1763, Sir Jeffrey Amherst, the commander of British troops in North America, was concerned about activities of Native Americans along the western frontier (extending from Pennsylvania to Detroit) who were unsympathetic to the British. When he learned that smallpox had broken out among British troops at Fort Pitt, he suggested that the disease could be used as a biological weapon against the Native Americans. The plan was to pass along blankets or handkerchiefs used by the British smallpox victims to the hostile Native Americans. An epidemic of smallpox did occur among these Native American tribes, but it cannot be assumed that the outbreak resulted from biological warfare activities of the British. The Native Americans were immunologically naive as far as smallpox was concerned and had many opportunities to contract the disease in other contacts with European settlers. Historical evidence also suggests that the French used smallpox as a weapon against Native Americans during this era (13, 40).
All of the above activities, which may be considered early attempts at biological warfare, occurred before the germ theory of disease was formulated and widely accepted. While the activities of early microbiologists in the late 19th century introduced the concept of microorganisms as agents of infectious disease and provided a foundation for the science of microbiology, their work also ushered in a new phase in biological weapons development. Pathogenic microorganisms could now be isolated and grown in quantity in pure cultures on laboratory media. While these newly acquired skills supported research designed to help us understand, prevent, and eradicate disease, they were also available for nefarious purposes.
Developments during the Era of the World Wars
The development of biological weapons became much more focused in the 20th century. During World War I, Germany was thought to have employed the agents of cholera and plague against humans and anthrax and glanders against livestock (13). While use of biological weapons was minimal during World War I, the world was able to witness the effects of chemical weapons on military personnel. Reaction to the use of these terrible weapons led to the formulation of the Geneva Protocol (Protocol for the Prohibition of the Use in War of Asphyxiating, Poisonous or Other Gases, and of Bacteriological Methods of Warfare). This 1925 treaty banned the use of chemical as well as biological weapons in war, but did not seek to limit or regulate the development or production of such weapons. Although the agreement expressed an anti-biological and chemical weapons spirit, it did little to prevent the further development of biological weapons (13).
In the period between World Wars I and II, a number of countries, including the USSR, Japan, and the United Kingdom, stepped up their biological warfare research programs. The Japanese effort was notable, with a number of military units engaged in offensive biological weapons research until the end of World War II. One of the most notorious of these, Unit 731, was headed by Army physician-microbiologist Ishii Shiro from its inception in 1937 until 1941. Unit 731, the second such unit that Ishii had commanded, was located in Japanese-occupied Manchuria. At the height of its operations, the unit's staff of 3,000 was quartered in 150 buildings at Ping Fan. Unit 731 personnel also oversaw at least five satellite operations, each with its own staff of 300 to 500. This biological warfare unit and others like it were responsible for extensive research and development, using both animal and imprisoned human subjects (usually criminals or political dissidents). It is estimated that during 13 years of Japanese biological warfare research in Manchuria and China, 10,000 unwilling human “subjects” lost their lives (24). An extensive menu of bacterial, viral, and rickettsial diseases was investigated during the Japanese effort in the 1930s and early 1940s. The Japanese also conducted at least a dozen field tests in Manchuria and China. These tests included the contamination of water and food supplies, aerial spraying, and the dropping of small bombs containing plague-infected fleas. Outbreaks of plague, cholera, and typhus were attributed to these activities (24).
The biological warfare activities of other countries during the 1930s and 1940s were minimal compared to those of the Japanese. The German effort was mostly defensive, aimed at developing vaccines and antimicrobial drugs. Their work did, however, include the use of concentration camp prisoners as experimental subjects. Allied forces produced anthrax bombs that were tested on Gruinard Island off the coast of Scotland. The island remained heavily contaminated until the 1980s, when successful decontamination was accomplished using seawater and formaldehyde (13). The United States began its offensive biological weapons program in 1942. Research and production facilities were built and testing sites were identified, but the detection of widespread contamination problems in the production process (tests were performed with the nonpathogenic B. anthracis simulant Bacillus subtilis var. globigii) dampened enthusiasm for large-scale production. The United States would wait until the 1950s to begin a more extensive program (13).
Events in the Post-World War II Era
After World War II, the U.S. military established research and development (Fort Detrick) and testing sites for investigation of a number of possible biological agents. Testing involved the use of animals and human volunteers. The Army also carried out unannounced tests with simulants on American civilians. One of the best-described of these tests involved the release of Serratia marcescens in San Francisco in the early 1950s (13).
Both the U.S. Army and the Air Force worked on biological weapon projects until 1969, the year of President Nixon's biological weapons disarmament declaration. Christopher J. Davis (19) comments that this decision “conveyed the impression that biological weapons were uncontrollable and that the U.S. program had not been successful in producing usable weapons (when in fact the opposite was true).” This decision was followed in 1972 by the Biological and Toxic Weapon Convention, after which Western governments stopped biological weapons development and withdrew workers and funding from the projects.
The subsequent era during the Cold War can be characterized by “nuclear blindness … a vision defined by the attitude that nuclear power is such a threat that nothing else counts” (19). During this time, the Soviet Union as well as Iraq independently developed their successful biological weapons programs. In 1972, Washington and Moscow had agreed by treaty to give up biological weapons, but the Soviet Union started a clandestine program which reaches beyond our imagination in its scientific, technological, and production capacity.
Lenin had recognized the strategic value of biological agents, and experimental work had already been carried out in the late 1920s (19). As in the United States, the modern industrial build-up of a competent biological weapon industry started in the post-World War II era. Of particular interest is a research and manufacturing organization that was created between 1973 and 1974 under the name Biopreparat. The bioarchipelago operated by Biopreparat comprised a chain of 52 sites that provided extensive research and development and production capability for bioweapons, existing behind a screen of civilian biotechnology research. It is estimated that the civilian sector's activity at no time exceeded 15% of Biopreparat's total activity. The organization was headed (and still is) by a general and scientist, Yuri T. Kalinin. “General Kalinin has headed Biopreparat since its inception in 1973, and Western officials say he is the focal point of concern among American and British analysis about whether Moscow has fully given up research into germ warfare, and that [then] acting President Vladimir V. Putin will decide” (New York Times, 25 January 2000). Biopreparat was controlled by the Ministry of Defense and constituted an important part of the military industrial complex. During the past 25 years, 50,000 (or perhaps even 60,000) people worked there, many of them highly trained scientists and technicians. Their productivity during this time span should not be underestimated.
Since the Kremlin had cosigned the 1972 Biological Weapons Convention, Biopreparat had to operate in higher secrecy than its nuclear weapons counterpart and conceal its clandestine and illegal activities behind a front of civilian pharmaceutical and biotechnical research and production. With the support of the Academy of Sciences of the USSR, Biopreparat applied biotechnological methods for genetic engineering to augment the ability of agents to express toxins, increase virulence, acquire resistance to antibiotics, or improve their survival rate during storage and aerosolization. The ingenuity of Biopreparat created a variety of different agents for specific strategic situations and geographic or climatic demands, such as strains resistant to degradation by heat, light, cold, UV, and ionizing radiation (19). The germs were adapted to specific dissemination systems, including cruise missiles. The new creations also featured chimeras, or combination organisms (allegedly, a hybrid between the smallpox and Ebola viruses was created). Biopreparat production levels were impressive. The Soviet Union stored more than 30 metric tons of B. anthracis spores and more than 20 metric tons of smallpox virus. Even the Marburg virus was part of the arsenal. The Soviet Union had the ability to make strategic attacks using plague or smallpox to target enemy population centers (19). In 1979, the citizens of Sverdlovsk became civilian casualties of the Soviet Union's biological weapons manufacturing program. Sverdlovsk was located downwind of what was described as a government microbiology “facility” when it became the site of one of the largest outbreaks of anthrax ever recorded. At the time, Soviet authorities attributed the outbreak to consumption of contaminated meat, but over a decade later it was finally publicly admitted that the outbreak had been caused by the unintentional release of a cloud of anthrax spores as a result of the malfunctioning of filters at the facility (35).
When the Russian confederation succeeded the Soviet Union in 1992, Biopreparat survived, and Western intelligence suspected a continuation of the offensive biological weapons program. This fact was confirmed by a senior defector who was a former deputy director of Biopreparat, Ken Alibek, the man in charge of operations. His revelations described a gruesome picture of this elaborate death factory. In 1999, Dr. Alibek published a book entitled Biohazard: The Chilling True Story of the Largest Covert Biological Weapons Program, Told from the Inside by the Man Who Ran It (2). According to Alibek, the former Soviet Union's warfare program included the aiming of SS-18 intercontinental ballistic missiles armed with anthrax and other agents at New York, Los Angeles, Seattle, and Chicago in 1988, as ordered by then-President Gorbachev. Alibek's account also describes the creation of multiply resistant anthrax strains, unsuccessful attempts at weaponizing the AIDS virus, the use of the agent of glanders in germ warfare in Afghanistan, and other Biopreparat activities (New York Times, 5 April 1999). Independent of Biopreparat, the Ministry of Defense operated its own biological weapons program, with production sites for the agents of plague, tularemia, glanders, anthrax, smallpox, and Venezuelan equine encephalomyelitis.
The U.S. government has in the recent past voiced concerns that, since the dissolution of the Soviet Union, unpaid scientists, impoverished guards, and lack of security could render Russia's arsenals vulnerable. A 10 December 1999 New York Times report stated: “The Clinton administration has made impressive strides in preventing former Soviet scientists from working for rogue states and terrorists seeking unconventional weapons, but it should spend much more to achieve that aim, a Washington research group said today.” The Pentagon had allocated several billion dollars to help the former Soviet states secure and dismantle their weapons of mass destruction and keep the scientists peacefully employed. There was concern that “scientists who need to feed their families will find it difficult to withstand the prosperity that proliferations offer …” However, American aid to turn Russian biological warfare centers to peaceful use has remained a very controversial political effort. On 8 January 2000, the New York Times reported the 25th anniversary of the founding of Vector, the state research center for virology and biotechnology in Koltsovo, Novosibirsk, once the Soviet Union's largest laboratory for developing viral weapons. This installation did not appear on maps and functioned as “the crown jewel of the germ warfare empire.” The article states that “some American intelligence analysis and congressional staff members remain wary of Vector's research, and fearful that Russia may still be conducting secret germ warfare work at closed military sites and institutes.” The transformation of these laboratories into collaborative partners for Western scientific research and biotechnology has not yet been fully achieved. “Enmeshed in Soviet-style secrecy and nationalism, hardliners in Russia have resisted scientific exchanges and Vector's growing ties to the West, American officials and Russian scientists say” (New York Times, 8 January 2000).
Containing and securing the Russian arsenal of biological weapons with American financial support may perhaps be “nurturing a snake.” A 25 January 2000 New York Times report underscores this suspicion: “Some of the American money awarded to support Russia's civilian biological research was secretly shifted to Biopreparat, a shadowy organization, that once directed the Soviet Union's germ warfare program, several Russian scientists say.” In the center of these allegations is Biopreparat, where General Yuri Kalinin still exerts his bureaucratic powers over the institutes and employees, diverting funds for dubious purposes. General Kalinin has meanwhile assumed the role of Dr. Kalinin and, as Biopreparat's director has allegedly diverted funds coming from National Aeronautics and Space Administration grants intended for biological research in space to his organization. In the eyes of many analysts, Biopreparat seems not yet to have achieved the status of a legitimate player in the international scientific arena.
Looking at the global scenario of countries with an active interest in biological agents suitable for offensive purposes, the Clinton administration states that at least 12 nations have acquired or are trying to acquire germ weapons (New York Times, 5 February 2000); among these are rogue states like North Korea. Since 1974, Iraq has been very successful in building a major biological weapons industrial conglomerate, with state-of-the-art research and development and production sites. These factories produced large quantities of botulinum toxin and B. anthracis and Clostridium perfringens spores. Ricin toxin, anticrop agents, and camelpox virus were also among the agents investigated as part of Iraq's biological warfare strategy. A report by Christopher J. Davis (19) summarizes that “a rationale based on a possession of a multi-potent arsenal having lethal, incapacitating, oncogenic, ethnic, economic, terror, and variable time-onset capabilities” stands behind this concept. The United Nations Special Commission believed that, from 1992 to 1995, Iraq was able to preserve biological weapons capability, that the true scope of the program remains unknown, and that Iraq has not abandoned its biological weapons program (19).
Bioterrorist events have occurred in recent history both in the United States and abroad. In 1984, the salad bars at two restaurants in the Dalles, Oregon, were contaminated with Salmonella by followers of Bhagwan Shree Rajneesh. The perpetrators of this bioterrorist action were attempting to sicken citizens and prevent them from voting in an upcoming election. Members of the Rajneeshee commune were concerned that without their intervention, the results of the election might lead to land use decisions that would restrict their planned development of a world headquarters for their sect (48). In the late 1990s, a number of anthrax threats were made in the United States, but all were hoaxes (36). One of the most frightening recent terrorist attacks involved release of the nerve gas sarin in the Tokyo subway system in 1995. Aum Shinrikyo, the cult responsible for killing 12 people and injuring approximately 3,800 in the sarin attack, has also attempted to develop botulinum toxin, anthrax, cholera, and Q fever for bioterrorist use (37).
PLANS FOR RESPONSE TO BIOTERRORIST EVENTS
Recent Efforts to Establish Preparedness
The United States is currently developing response plans for dealing with possible bioterrorist events. Efforts started in earnest in 1995 with the issuing of Presidential Decision Directive 39, U.S. Policy on Counterterrorism (PPD-39). This directive defined the responsibilities of numerous federal agencies in the event of a bioterrorist attack. PPD-62 and PPD-63, both classified documents, addressed this issue in greater detail. PPD-39 identifies the Federal Bureau of Investigation (FBI) as being in charge of immediate crisis management and criminal investigation. The Federal Emergency Management Agency (FEMA) is given the lead role in consequence management (assistance in the aftermath of an attack). The FBI and FEMA will be supported by other federal agencies, such as the departments of Defense, Energy, Agriculture, Transportation, and Health and Human Services and the Environmental Protection Agency (49).
Ongoing activities by federal agencies to ensure preparedness include research programs for development of devices that can detect and identify biological and chemical agents. Programs have also been developed for the training of first responders (firefighters, police, and emergency medical personnel), the training of clinical laboratory personnel (see below), and the establishment of National Guard Rapid Assessment and Initial Detection Teams, which will act as standby units stationed throughout the country. The Marine Corps and the Army have also created their own specialized rapid-response units (27).
The CDC has established a Bioterrorism Preparedness and Response Program that addresses public health response to bioterrorist actions (www.bt.cdc.gov). The components for a comprehensive public health response to bioterrorism identified by the CDC include detection (disease surveillance), rapid laboratory diagnosis of biological agents, epidemiologic investigation, communication (between local, state, and federal public health authorities), preparedness planning, and readiness assessment. The CDC maintains emergency response capability at all times [phone number (770) 488-7100]. In addition, the CDC has been charged with developing and maintaining the National Pharmaceutical Stockpile Program in cooperation with the Department of Health and Human Services Office of Emergency Preparedness (32). The CDC has also aided the Association for Professionals in Infection Control and Epidemiology (APIC) in their effort to devise a template preparedness plan that can be adapted for use by health care facilities (22). This document, “Bioterrorism Readiness Plan: A Template for Healthcare Facilities,” is available on the web sites of both APIC (www.APIC.org) and the CDC (www.CDC.gov/ncidod/hip). The CDC's program for developing a nationwide network of hospital-based and public health microbiology laboratories to facilitate detection of bioterrorist events is described below.
Role of the Clinical Microbiology Laboratory
Clinical microbiology laboratories could play a key role in the detection and identification of biological agents likely to be used in bioterrorist events. In the immediate aftermath of a covert attack, specimens containing critical biological agents might be submitted to the laboratory for routine processing before the presence of the agent was even suspected. Would the average clinical microbiologist recognize agents likely to be used by bioterrorists? What are the risks of handling these agents in an average clinical laboratory? What protocols should laboratory supervisory personnel follow for notification of public health and law enforcement authorities in such cases?
The CDC, working with state public health authorities, is developing a nationwide plan for bioterrorism preparedness for clinical microbiology laboratories with various capabilities. Participation of non-public health laboratories in this Laboratory Response Network (LRN) is voluntary, and public health authorities are attempting to ease the burden of any additional work involved by providing training and technical advice to participating organizations. During the summer of 1999, the CDC and local public health and FBI personnel presented seminars in multiple U.S. cities on the role of the clinical laboratory in response to bioterrorism. After this series of presentations, state public health authorities organized additional training sessions within their own jurisdictions in order to reach additional clinical microbiologists. The sessions provided an overview of bioterrorism and the aims of the LRN along with information on presumptive identification methods for critical biological agents to be used in the average clinical laboratory and how and when to ship such agents or potentially infected specimens to reference labs. Some of this information is summarized below, but readers are strongly urged to contact their local public health authorities or the CDC for more details about this program. The CDC maintains a bioterrorism internet site (www.bt.cdc.gov), and information on response plans and biological agents can be accessed via this site, along with downloadable Powerpoint files of slides shown at the presentations delivered across the nation in 1999 (click on Learning Resources).
In the LRN for bioterrorism, laboratories are classified into one of four levels depending on their testing facilities and abilities. Level A laboratories are represented by the majority of clinical microbiology laboratories that culture and identify routinely isolated pathogens. These laboratories would perform a small number of simple rule-out tests on suspected isolates and, depending on test results, refer those organisms to a higher-level laboratory for further testing. Thus, the role of the level A laboratory is summarized as rule out or refer. Level B laboratories in the LRN are represented by many public health laboratories and should contain biosafety level (BSL) 3 facilities. Level B laboratory activities include tests for rapid presumptive identification (e.g., with fluorescent antibody reagents), confirmatory identification, and antimicrobial susceptibility testing. Critical biological agents would be referred from level B laboratories (rule in and refer) to level C facilities (BSL 3), which have the capacity for nucleic acid amplification testing, molecular typing, and toxin testing. Level C laboratories (rule in and refer) would include certain public health and other laboratories that can perform strain-typing procedures. Critical biological agents would finally be referred to level D laboratories, which are BSL 4 facilities (maximum containment “hot labs,” like the facility at the CDC). The role of these laboratories is archiving critical biological agents and the performance of other specialized tests, such as culture or molecular identification of highly dangerous viral agents that require BSL 4 facilities.
Level A laboratories are the largest component in the LRN, and their characteristics are presented here in more detail. Level A laboratories must practice BSL 2 procedures and have a class II certified biological safety cabinet. The CDC-National Institutes of Health NIH publication Biosafety in Microbiological and Biomedical Laboratories (12) contains details of biosafety level practices and classification of biological safety cabinets. The fourth edition of this useful guide can be obtained from the CDC and is also available on the Internet (www.cdc.gov). Briefly, BSL 2 laboratories follow BSL 1 practices (basic safety procedures such as the prohibition of eating or drinking in the laboratory and handwashing before leaving the laboratory) and a number of other protocols summarized here. BSL 2 laboratories are directed by competent scientists, access to the laboratory is controlled, and proper biohazard signage is in place. Personnel employed in a BSL 2 laboratory must be specifically trained in the handling of pathogenic agents and the appropriate use of personal protective equipment (lab coats, gloves, and face shields). Policies for the proper disposal of sharps and other infectious waste must be in place and documented in a biosafety manual. Standard laboratory practices and procedures are designed to minimize production of infectious aerosols. Level A laboratories in the LRN must also have a certified class II biological safety cabinet (characterized by HEPA filtering of intake and exhaust air). Most full-service hospital or commercial clinical microbiology laboratories fulfill these requirements.
Level A laboratories participating in the LRN will be prepared to recognize the basic gram stain and growth characteristics of critical bacterial agents and perform simple rule-out tests like the ones found in the Microbiology and Clinical Aspects of Possible Agents of Bioterrorism section of this review. Some critical biological agents (B. anthracis, Y. pestis, and specimens containing botulism toxins) can be safely handled using BSL 2 practices. BSL 2 to 3 is recommended for F. tularensis and Brucella spp. (12), meaning that BSL 3 is recommended if larger quantities of these agents are to be cultured and manipulated. In a level A laboratory, gram stains and simple rule-out tests on these highly infectious agents should be performed in a biological safety cabinet, never on the open bench. If rule-out tests suggest a critical biological agent, the level A laboratory will refer the isolate to a higher-level laboratory within the LRN for confirmatory testing. Specimens suspected of harboring certain critical biological agents (viral agents) would not be processed in a level A laboratory but immediately referred to a higher-level laboratory after consultation. Level A laboratories must therefore also be prepared to contact the nearest level B facility (usually a public health laboratory) and properly package and ship infectious agents.
Shipping regulations are strict, numerous, and subject to change and may vary among commercial shipping companies. Generally, commercially available shipping containers that meet current regulations should be used along with proper labels and documentation. These containers are combination packaging, consisting of a primary container (e.g., a tube containing the isolate or specimen), secondary packaging (rigid and leak-proof), and outer packaging (often a sufficiently strong cardboard box). LRN level A laboratories can get detailed information and training on proper shipping from their local public health authorities, manufacturers of approved shipping containers, and in reference 12. Level A laboratories should also be aware of chain-of-custody procedures used in their institution, since they may be called upon to handle or store materials that are classified as forensic evidence. Local public health authorities and local branches of the FBI can offer advice on these procedures.
The developing LRN is envisioned as a highly interconnected (facilitated by the Internet) network of communicating laboratories that will be able to support laboratory response to a bioterrorist event. The LRN is visualized as a pyramidal structure. Level A laboratories provide the base of the LRN, referring agents to increasingly smaller numbers of laboratories at higher levels. The system will function as intended only if there is wide participation at level A. Interlaboratory communication will also facilitate epidemiological data collection for infrequently occurring natural infections. Such a network will clearly benefit public health efforts in general as well as providing a plan for response to bioterrorist events (28, 34). Complete information on voluntary participation in the LRN as a level A laboratory and details of suggested level A laboratory procedures can be obtained from local public health authorities.
MICROBIOLOGY AND CLINICAL ASPECTS OF POSSIBLE AGENTS OF BIOTERRORISM
In addition to the agents described below, other organisms and toxins have also been considered potential biological weapons. The CDC has not yet issued recommendations for specimen processing and presumptive identification for these agents, since they are considered less likely to be used by terrorists than the agents already discussed. Recommendations for dealing with these agents may be formulated as the CDC's bioterrorism preparedness program moves forward.
Bacillus anthracis
Bacillus anthracis ranks high on the list of potential agents for bioterrorist attacks. The agent was isolated and characterized by Robert Koch, who described its cultural and morphological characteristics in detail in 1867. It was this organism that fulfilled Koch's postulates for the first time. B. anthracis forms large gram-positive rods (1 to 1.5 μm by 4 to 10 μm). Cells of this facultative anaerobe are nonmotile, encapsulated, and arranged in chains. Capsule formation is usually evident in smears from infected tissues, but not when the organism is grown on most common laboratory culture media. The bacterium forms oval subterminal spores that do not cause swelling of the sporangium (Fig. 1). Spores are formed only in the presence of oxygen. B. anthracis grows well on sheep blood agar plates at 35°C, forming nonhemolytic colonies 2 to 5 mm in diameter after 24 h of incubation. The colonies are flat or slightly convex and round with irregular edges, and curly tailing edges may be observed. B. anthracis colonies have a ground-glass appearance and a sticky consistency when manipulated with a loop (33).
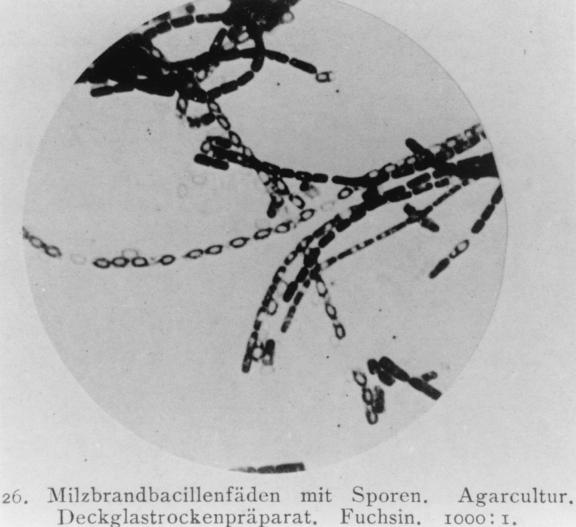
FIG. 1.
Chains of B. anthracis with spores. Agar culture. Dry coverslip preparation. Stained with fuchsin. Magnification, ×1,000. Reproduced from reference 23a with permission of the publisher.
Depending on the type of infection, B. anthracis may be isolated from cutaneous lesions, respiratory specimens, stool or other gastrointestinal specimens, cerebrospinal fluid, and blood cultures. B. anthracis can be isolated and presumptively identified safely in the average clinical microbiology laboratory by following BSL 2 practices (BSL 2 is characteristic of routine activities in average full-service clinical microbiology laboratories). Vaccination is not required for minimal handling of this organism, but laboratory coats and gloves, face shields if necessary, non-aerosol-generating techniques, and handwashing are recommended. Presumptive identification criteria include characteristic Gram stain, colony morphology, ability to sporulate in air, and motility. Table 1 summarizes information on specimen type, processing, and culture for B. anthracis and other possible agents of bioterrorism. Table 2 describes protocols for presumptive identification, and Table 3 lists some differentiating characteristics of B. anthracis and similar Bacillus species. Isolates presumptively identified as B. anthracis should be immediately referred to the nearest public health laboratory for confirmation. (33; H. T. Holmes, CDC- and Association of Public Health Laboratories-sponsored course “Response to Bioterrorism: The Role of the Clinical Laboratory,” Boston, Mass. 1999).
TABLE 1.
Processing of specimens suspected to contain selected biological agentsa
| Agent and BSL | Specimens | Specimen processing |
|---|---|---|
| B. anthracis (BSL 2) | (Sputum, blood (inhalation); stool, blood (gastrointestinal); vesicular fluid, swabs (cutaneous inoculation) | Sputum: three routine media (e.g., SBA, CA, MAC) Blood: routine methods Stool: routine methods plus PEA Cutaneous: three routine media (e.g., SBA, CA, MAC) Incubate at 35 to 37°C under ambient conditions, examine at 18 to 24 h |
| F. tularensis (BSL 2 for specimen processing, BSL 3 for culture manipulation)b | Blood, sputum, bronchial washings (pneumonic); lymph nodes, wounds (cutaneous inoculation) | Specimens other than blood: SBA, CA, MAC; add BCYE, CHA, or CHAB if available, incubate agar media for 24 to 72 h at 35°C |
| Blood: routine methods. Broth media such as thioglycolate or BHI, even when supplemented with 1.0% Isovitalex, support only poor growth of F. tularensis | ||
| Brucella spp. (BSL 2 for specimen processing, BSL 3 for culture manipulation)b | Blood, bone marrow, spleen, liver, abscess material Serum for serological diagnosis: acute-phase serum should be shipped immediately followed by convalescent-phase serum sample collected 21 to 28 days later | Blood or bone marrow cultures: routine blood culture methods and media. Some current automated systems can detect most Brucella isolates within 7 days, but extended incubation (at least 21 days) with weekly blind subcultures is recommended. Incubate blind subculture for at least 7 days Tissue or wound specimens: SBA, CA, MAC |
| Incubate all cultures at 35°C in 5% CO2 and examine daily for at least 7 days | ||
| Serum (10 to 12 ml) should be stored and shipped frozen or preserved by addition of 10.0 ml of 1.0% merthiolate to each 1.0 ml of serum | ||
| Y. pestis (BSL 2) | Blood, sputum, bronchial washings (pneumonic plague); spleen, liver, bubo aspirates | Routine culture media and protocols (routine blood culture, SBA, CA, MAC) Prepare smears for Wayson or DFA staining.c |
| Y. pestis grows well on SBA; growth on non-blood-supplemented media such as BHI agar, TSA, or nutrient agar is slower than on SBA; growth on media incubated at 35°C is slower than growth at 28°C or room temperature | ||
| Variola virus (BSL 4)d; Notify public health authorities immediately if smallpox is suspected | Vesicular fluid from lesions, scabs or scrapings, biopsies | If smallpox is suspected, contact the CDC's Poxvirus section at (404) 639-2184 for approval to ship specimens and to make shipping arrangements. The ideal specimen is vesicular fluid from a single lesion collected as a droplet on a single slide and allowed to dry; each slide is then placed into a separate, nonbreakable holder. |
| Tissue and scrapings are placed into leak-proof containers that can withstand freezing. | ||
| Store specimens at 4°C for up to 6 h, but for longer storage-transport times, store specimens frozen at −20 to −70°C | ||
| VHF agents (BSL 4)e; notify public health authorities immediately if VHF is suspected | Serological diagnosis: acute-phase serum, 5 to12 ml (minimum, 5 ml); also collect convalescent-phase serum 21 days later or post mortem heart blood Immunohistochemical testing: tissue in paraffin blocks; PCR testing: biopsy tissue, buffy coat, clot material | If VHF is suspected, consult the CDC at (404) 639-1115 to arrange for sending specimens Serum should be shipped cold or on dry ice in a plastic tube Paraffinized tissue should be shipped at room temperature Specimens for PCR testing should be at least 1 cm3 in size and should be shipped with dry ice |
| Botulism toxin (BSL 2); handle specimens in a class II biological safety cabinet with gloves, lab coats, and face shield if necessary | Serum (10 to 12 ml) Vomitus/gastric contents (20 ml) Stool (10 to 50 g) Food samples (10 to 50 g) Tissue or wound anaerobic swab | Contact local state health department epidemiology office when botulism is suspected Wound specimens are shipped at room temperature, but all other specimen types should be held and shipped at 4°C |
Material in this table was summarized from information that can be accessed through the CDC's websites http://www.bt.cdc.gov/bioagents.asp and http://www.bt.cdc.gov/roleofclinlab.asp. Abbreviations: SBA, sheep blood agar; CA, chocolate agar; MAC, MacConkey agar; PEA, phenyl ethyl alcohol agar; CHA, cystine heart agar; CHAB, cystine heart agar supplemented with 9% heated (chocolatized) sheep red blood cells; DFA, direct fluorescent antibody; BHI, brain heart infusion; TSA, Trypticase soy agar; VHF, viral hemorrhagic fever; CSF, cerebrospinal fluid.
Handle culture materials in a class II biological safety cabinet with gloves and lab coats. Avoid aerosol production.
These staining procedures may currently not be routinely performed in level A laboratories. Procedural details can be found in the Y. pestis section, accessible at http://www.bt.cdc.gov/bioagents.asp. Level A laboratories can prepare smears of specimens for shipment to a reference laboratory, where these staining techniques may be performed.
Laboratory hazards include ingestion, parenteral inoculation, and exposure of mucous membranes or broken skin to aerosols or infectious droplets.
Laboratory hazards include parenteral inoculation and exposure of mucous membranes or broken skin to aerosols or infectious droplets.
TABLE 2.
Criteria for presumptive identification of selected biological agentsa
| Agent (BSL) | Stain(s) | Presumptive identification |
|---|---|---|
| B. anthracis (BSL 2) | Gram stain: large (1.0 to 1.5 by 3 to 5 μm) gram-positive rods Spores present in clinical specimens only if specimens have been exposed to ambient atmosphere. Cells grown on agar media form oval, central to subterminal spores that do not swell the sporangium. Capsules may be observed in cells present in clinical specimens, but not in cells cultured on routinely used agar media. India ink stain may be used for observation of capsules in blood or CSF | Colony morphology on SBA: nonhemolytic, flat or slightly convex with irregular edges, ground-glass appearance. Comma-shaped projections often observed. Colonies are 2 to 5 mm after 18 to 24 h of incubation at 35°C. Growth on SBA has a tenacious consistency. No growth on MAC Nonmotile: use motility test medium and examine after 18 to 24 h of incubation at 35 to 37°C. Wet mounts can also be prepared from fresh broth cultures or by suspending growth from a 12- to 20-h-old colony in sterile water. |
| F. tularensis (BSL 2 for specimen processing, BSL 3 for culture manipulation)b | Gram stain: tiny (0.2 to 0.5 by 0.7 to 1.0 μm), poorly staining pleomorphic gram-negative rods/coccobacilli | Handle cultures in a class II biological safety cabinet with gloves, lab coats. Colony morphology: on SBA, F. tularensis grows poorly, forming nonhemolytic, gray-white opaque colonies 1 to 2 mm in diameter after 48 h of incubation at 35°C. On CA, colonies are 1 to 2 mm in diameter after 48 h of incubation, blue-white to gray, flat, entire, smooth, and shiny. On CHA and CHAB, colonies are 2 to 4 mm in diameter after 48 h of incubation and greenish-white. No growth on MAC Growth is poor when media are incubated at 28°C. Poor growth occurs in broth media. Growth in thioglycolate broth characteristically appears as a dense band near the top of the medium that later diffuses throughout the medium |
| Brucella spp. (BSL 2 for specimen processing, BSL 3 for culture manipulation)b | Tiny, faintly staining coccobacilli (0.5 to 0.7 by 0.6 to 1.5 μm) | Handle cultures in a class II biological safety cabinet with gloves, lab coats. Colony morphology on SBA: small (0.5 to 1.0 mm), convex, glistening, nonhemolytic, nonpigmented colonies appear after 2 to 3 days of incubation at 35°C in 5% CO2 Some strains may grow slowly on MAC. Oxidase positive (0.5% tetramethyl-p-phenylendiamine reagent) Urea hydrolysis positive (Christensen's urea agar incubated at35°C). B. suis and B. canis are usually positive within 5 min of incubation, but other Brucella strains may require overnight incubation before a positive reaction is evident. |
| Y. pestis (BSL 2) | Gram stain: gram-negative rods (1.0 by 0.5 μm) that may exhibit bipolar staining | Colony morphology on SBA after 48 h of incubation at 35°C: gray-white to slightly yellow opaque colonies 1 to 2 mm in diameter. After 48 to 72 h of incubation, colonies develop a fried-egg appearance which becomes more pronounced as the colonies age. Colonies may also have a hammered-copper shiny appearance. Little or no hemolysis is observed. MAC: small lactose-negative colonies after 24 h of incubation at 35°C. Growth in BHI broth: inoculated broths are incubated at 28 and 35°C without agitation for 24 to 48 h. Growth is clumped or flocculent and more rapid at lower incubation temperatures. |
See Table 1, footnote a.
Handle culture materials in a class II biological safety cabinet with gloves and lab coats. Avoid aerosol production.
TABLE 3.
Differentiating features of B. anthracis and similar Bacillus species
| Bacillus speciesa | Resultb
|
||
|---|---|---|---|
| Beta-hemolysis | Motility | Capsulec | |
| B. anthracis | − | − | + |
| B. cereus var. mycoides | − | V | − |
| B. cereus | + | + | − |
| B. thuringiensis | + | + | − |
| B. megaterium | − | V | − |
B. anthracis, B. cereus var.mycoides, B. cereus, and B. thuringiensis are members of the B. cereus group (Bacillus morphology group 1). Species in this group have similar colonial morphology and produce ellipsoidal spores that do not swell the sporangium.
+, positive; −, negative; V, variable. Data are based on information in reference 33.
Capsules are usually only seen in smears of infected material, not in smears made from growth on most commonly used laboratory media.
Depending on the site of the infection, anthrax cases have very different clinical manifestations. In cutaneous anthrax, spores are introduced into the skin. Germination occurs within hours, and vegetative cells produce anthrax toxin. A red macule develops at the site of inoculation. This lesion subsequently develops into a papular-vesicular stage, followed by ulceration, with a blackened necrotic eschar surrounded by brawny edema. Characteristically, the lesion is painless. A regional lymphadenitis is commonly seen in these patients. Spontaneous healing occurs in 80 to 90% of untreated cases. When bacteremia develops, it leads to high fever and death (47).
In inhalation anthrax (wool-sorter's disease), the spores (less than 5 μm in diameter) are aerosolized and enter the alveoli of the lungs. There the spores are phagocytized by alveolar macrophages and begin to germinate; some spores germinate in the mediastinal lymph nodes. The result is hemorrhagic mediastinitis and massive B. anthracis bacteremia, accompanied by secondary pneumonia. Meningitis may also occur as a complication of B. anthracis bacteremia. The early symptoms of an airway infection with B. anthracis spores resemble those of a severe viral respiratory disease during the first 1 to 3 days. In the acute phase, symptoms include fever, dyspnea, stridor, and hypoxia accompanied by hypotension. Death can occur within 24 h of the primary phase of infection. In some cases patients present very quickly with fulminant disease. The chest X-ray is very characteristic of this manifestation (symmetrical mediastinal widening as a consequence of hemorrhagic mediastinitis). There is no person-to-person spread of inhalation anthrax, the form of disease most likely to be contracted as the result of a bioterrorist event (47).
Gastrointestinal anthrax is contracted via the ingestion of contaminated meat that is not thoroughly cooked. Oropharyngeal infection or intestinal infection follows. The symptoms are variable and include fever, vomiting, abdominal pain, bloody diarrhea, ascites, and hemoconcentration. In the oropharyngeal form of anthrax, the symptoms are fever, dysphagia, painful lymphadenitis, toxemia, respiratory distress, and a primary tonsilitis. B. anthracis bacteremia can develop in all three forms of human anthrax and is seen in nearly all fatal cases. Cutaneous anthrax is the most frequent form of disease (95%), followed by inhalation anthrax (5%). Gastrointestinal anthrax is extremely rare and seen in less than 1% of all clinical cases. Penicillin is the drug of choice for treatment of anthrax (47), although concerns about the creation of resistant B. anthracis strains by bioterrorists (2) make the use of other antibiotics seem prudent. Erythromycin, ciprofloxacin, and vancomycin are also active against this organism. Treatment may also include passive immunization with anthrax antitoxin, which is not commercially available in the United States (47).
The virulence factors of B. anthracis consist of toxin and an antiphagocytic capsular polypeptide containing d-glutamic acid (47). The genes for these virulence factors are located on separate plasmids. The anthrax toxin is composed of three proteins, PA (protective antigen), EF (edema factor), and LF (lethal factor). All three components have been purified and characterized, and their structural genes have been sequenced. The PA fragment can attach to specific receptors on the host cell surface. Part of the molecule is cleaved by a cellular protease, producing a PA fragment that functions as a specific receptor for EF or LF. The toxin is then taken into the host cell via a process referred to as receptor-mediated endocytosis (47).
The innate resistance of B. anthracis spores is remarkable, and they may survive in dry soil for years (47). Anthrax is enzootic in many parts of the world, and historical accounts have documented outbreaks of the disease in livestock and humans. Herbivores can become infected with anthrax by grazing in pastures that are contaminated with spores. The animals develop bacteremia and contaminate the environment with vegetative organisms, which can subsequently sporulate and persist in the soil for up to 40 years. Animal carcasses are highly infectious. Biting flies can become vectors for the spread of anthrax. Contact with animals (butchering, skinning, or exposure to hides or wool), contact with flies, and consumption of contaminated meat are risk factors for infection in humans. The incidence of inhalation anthrax (wool-sorter's disease) has been considerably reduced by decontamination procedures for wool and goat hair. The active vaccination of workers as well as livestock is an other valuable preventive measure (47).
Live (attenuated) vaccines containing spores similar to the one developed by Louis Pasteur are used in many countries to immunize herbivores. These preparations are also used for humans in Russia. Pasteur's spore vaccine consisted of live attenuated bacilli produced by prolonged incubation at 42°C, which leads to a partial loss of the plasmid that encodes anthrax toxin. In the United States, a sterile protein-based human anthrax vaccine that was licensed in 1970 has been mandated for use in all U.S. military personnel. Vaccination of monkeys with two doses of this vaccine given 2 weeks apart completely protected against infection from an aerosol anthrax challenge administered at 8 and 38 weeks after vaccination. At 100 weeks after vaccination, the vaccine was observed to be 88% effective. U.S. vaccine supplies are, however, limited and U.S. production capacity is modest. The vaccination regimen comprises six subcutaneous injections on days 0, 14, and 28 plus boosters after 6, 12, and 18 months. The vaccine is not very well tolerated, and vaccinees complain about local and systemic side effects (23, 47).
Francisella tularensis
Francisella tularensis forms small (0.2 by 0.2 to 0.7 μm) pleomorphic gram-negative coccobacilli. The organism is a fastidious nonmotile, non-spore-forming strict aerobe that is catalase positive and oxidase and urease negative. Other species or biogroups of Francisella may cause human infection, but F. tularensis appears to be the most virulent group in the genus. F. tularensis forms small, greenish, opalescent colonies on cystine heart agar after 48 h of incubation at 35°C. Smaller gray-white colonies appear on sheep blood agar, chocolate agar, or Thayer-Martin medium at 48 h. Increased concentrations of CO2 in the growth atmosphere may stimulate growth. Buffered charcoal yeast extract (BCYE) agar will also support the growth of Francisella strains and can serve as a widely available substitute for cystine heart agar. Francisella colonies growing on BCYE will not, however, develop the characteristic greenish sheen that is observed on cystine heart agar (H. T. Holmes, CDC- and Association of Public Health Laboratories-sponsored course “Response to Bioterrorism, the Role of the Clinical Laboratory,” Boston, Mass. 1999). Specimens suspected of harboring F. tularensis should be processed using BSL 2 practices. If Francisella-like colonies are cultured, any manipulation of growth should be carried out in a biological safety cabinet (51). Tables 1 and 2 summarize specimen-processing methods for and tests for presumptive identification of F. tularensis. Tests helpful for differentiating this organism from similar gram-negative coccobacilli are summarized in Table 4. Suspected F. tularensis isolates (based on the Gram stain and growth characteristics described above) should be sent to a reference laboratory for confirmatory identification. This practice is recommended not only because of the unfamiliarity of most clinical microbiologists with this relatively infrequent isolate, but also because of the danger of laboratory-acquired infection with this virulent and highly infectious pathogen.
TABLE 4.
Differentiating characteristics of gram-negative bacteria that are or resemble critical biological agentsa
| Organism | Gram stainb | Resultc
|
||||
|---|---|---|---|---|---|---|
| SBA | MAC | Oxidase | Urease | Motility | ||
| Brucella spp.d | CB | + | V | +/− | + | − |
| Psychrobacter phyenylpyruvicusd | CB | + | + | + | + | − |
| Oligella ureolytica | CB | + | V | + | + | V |
| Bordetella bronchiseptica | CB, R | + | + | + | + | + |
| Actinobacillus spp. | CB | + | V | +/− | +/− | − |
| Haemophilus influenzae | CB | − | − | V | V | − |
| Haemophilus aphrophilus | CB, R | + | −/+ | −/+ | − | − |
| Francisella tularensis | CB | +/−e | − | − | − | − |
| Yersinia pestis | R | + | + | − | − | − |
| Acinetobacter spp. | CB | + | + | − | V | −/+ |
The names of possible agents of bioterroism are in bold type. Information in this table was complied from references 43, 45, and 51 and information accessible through the CDC's bioterrorism preparedness site (www.bt.cdc.gov).
CB, coccobacilli; R, rods. SBA, growth on sheep blood agar; MAC, growth on MacConkey agar.
+, positive; −, negative; +/−, most strains positive; −/+, most strains negative; V, variable reactions.
Brucella spp. cells stain faintly in the Gram stain, while the phenotypically similar Psychrobacter phenylpyruvicus (formerly Moraxella phenylpyruvica) exhibits more intense staining.
F. tularensis may be recovered initially on blood agar but cannot be successfully subcultured on this medium.
The natural reservoirs of F. tularensis include lagomorphs, rodents, and other animals. Humans can become infected after direct animal contact (often due to hunting, dressing, and consuming infected animals) or via insect vectors such as ticks, biting flies, and mosquitoes (17). The clinical manifestations of tularemia in human hosts, which occurs primarily in the northern hemisphere, are related to the route of exposure to the organism (cutaneous inoculation, inhalation, or ingestion). Thus, infection with F. tularensis may be classified as ulceroglandular, glandular, oculoglandular, pharyngeal, typhoidal, or pneumonic, although more than one set of symptoms may be present in a given patient. The pneumonic form of tularemia (the most likely form to be contracted as the result of a bioterrorist event) is characterized by symptoms of an atypical pneumonia, including cough, little or no sputum production, pleuritic chest pain, and fever (17).
Tularemia may be diagnosed by isolation of the organism from blood, pleural fluid, sputum, lymph nodes, wounds, or gastric aspirates, depending on the form of infection. Diagnosis is often made on the basis of serological tests, but cross-reactions of anti-Francisella antibodies with Brucella spp., Proteus OX19, and Yersinia spp. have been documented (17). The aminoglycosides streptomycin and gentamicin are the drugs of choice for treatment of tularemia. Tetracycline and chloramphenicol treatment is associated with relapse, due to the bacteriostatic nature of these agents against F. tularensis. Effective vaccines against tularemia are still under development (17).
Brucella spp.
Isolates of Brucella form small, faintly staining gram-negative cocci or short rods (0.5 to 0.7 by 0.6 to 1.5 μm). Members of this genus typically behave as slow-growing, fastidious organisms on primary isolation and are nonmotile, non-spore-forming strict aerobes that are catalase positive and usually oxidase and urease positive. Brucella spp. produce small, nonhemolytic, convex, glistening colonies on sheep blood agar that are visible only after 48 to 72 h of incubation at 35°C (45). BCYE medium or selective BCYE agar will also support the growth of Brucella strains (41). Increased CO2 is required for growth by many isolates. DNA-DNA hybridization studies performed in the 1980s suggested that Brucella was a monospecific genus, but isolates from human infection are still classified into groups using the species names Brucella abortus, Brucella melitensis, Brucella suis, and Brucella canis, which reflect the animal species from which strains of the species are likely to be isolated (cattle, goats, pigs, and dogs, respectively.) These groups can be differentiated on the basis of phenotypic traits, such as sensitivity to various dyes, H2S production, and phage susceptibility (45).
While traditional recommendations for recovery of Brucella spp. from blood called for extended incubation and periodic blind subcultures, recent evidence suggests that 95% of positive cultures will be detected by some automated blood culture systems within a 7-day incubation period without the need for blind subcultures (53). Best recovery of isolates from brucellosis patients is seen with specimens of blood and bone marrow, although other specimen types may also yield the organism. Specimens containing Brucella can be safely handled using BSL 2 protocols, but suspected Brucella isolates should be sent to a reference laboratory for identification, since this organism is the most commonly reported agent of laboratory-associated infection. As with Francisella, any suspected Brucella isolates encountered in the clinical laboratory should be handled in a biological safety cabinet (45). Tables 1 and 2 contain information for specimen processing and presumptive identification of Brucella spp. Table 4 summarizes differential characteristics for Brucella spp. and similar organisms. Brucella isolates may be misidentified by commercially available identification systems, often as Psychrobacter phenylpyruvicus (formerly Moraxella phenylpyruvica) on the basis of phenotypic traits (5, 7). When encountered in the clinical laboratory, isolates exhibiting Gram stains, growth characteristics on agar, and oxidase and urease activities characteristic of Brucella spp. should be sent to a reference laboratory for confirmatory identification.
Brucellosis is a zoonotic disease contracted by humans as a result of direct or indirect contact with animals that are infected (usually chronically) with Brucella. Infection can be established via cutaneous, respiratory, or gastrointestinal routes. Symptoms of brucellosis are fairly nonspecific, and the onset of illness may be acute or insidious. As a result of the systemic nature of brucellosis, almost any organ in the body may become infected. Chronic brucellosis cases are usually due to persistent infective foci (e.g., in bone, liver, spleen, or kidneys). Diagnosis of brucellosis can be made by culture of the organism from infected specimens or by serological studies. False-positive serological tests may result from cross-reactions with antibodies to Yersinia, Vibrio cholerae, or F. tularensis (54).
Yersinia pestis
As a member of the family Enterobacteriaceae, Y. pestis is an oxidase-negative facultative aerobe. Like all members of the genus Yersinia, Y. pestis has an optimum growth temperature of 25 to 28°C. Y. pestis is nonmotile, in contrast to other yersiniae, which express motility at lower temperatures but not at 37°C. Y. pestis forms rod-shaped cells (0.5 to 0.8 μm by 1 to 3 μm) that have characteristic bipolar staining, producing a “closed safety pin” appearance of individual cells. Methylene blue, Giemsa, Wright, and Wayson stains are recommended for visualizing this trait, which is best observed in direct smears of infected specimens. Y. pestis isolates can be recovered on blood and MacConkey agars and cefsulodin-irgasan-novobiocin media with reduced (4 μg/ml) cefsulodin (1). Unlike most other enterics, Y. pestis forms small pinpoint colonies on agar medium after 24 h of incubation at 35°C. The colonies increase in size after a second 24 h of incubation and appear as lactose negative on MacConkey agar. Colonies may assume a fried-egg appearance after additional incubation. Y. pestis isolates incubated for 48 h at 25°C produce negative reactions for metabolism of citrate, ornithine, sucrose, rhamnose, cellobiose, sorbose, and fucose. The majority of strains metabolize melibiose under these incubation conditions. The organism is Voges-Proskauer, indole, and urease negative and ferments glucose without production of gas (1). Specimen-processing protocols and tests for presumptive identification of Y. pestis isolates are summarized in Tables 1 and 2. Tests that differentiate Y. pestis from other gram-negative bacteria are displayed in Table 4.
In cases of plague, Y. pestis is likely to be isolated from blood, sputum, or lymph node aspirates. Bacteremia is characteristically intermittent, and multiple blood cultures are more sensitive than a single blood culture for isolation of the organism. Y. pestis can often be viewed in smears of infected material, as noted above, and reference laboratories employ a more specific fluorescent-antibody stain for capsular antigen. Cultures of Y. pestis should be handled using BSL 2 procedures, with special attention (as with any infectious agent) to minimization of procedures that may create aerosols. Although Y. pestis is included in the databases of a number of commercially available identification systems, their true accuracy for identification of this organism has not been assessed. Any suspected Y. pestis isolates should be forwarded to a state public health laboratory or the CDC for identification (1).
Y. pestis is the agent of plague, a zoonotic disease of rodents and other animals that is usually transmitted to humans via fleabites. This route of infection results in the bubonic form of plague, characterized by the sudden onset of fever and malaise and the painful form of lymphadenitis referred to as a bubo. Patients in the early stages of plague also suffer from intermittent bacteremia. Purpuric lesions may develop during the systemic stages of infection. These lesions can become necrotic and gangrenous and likely explain the term black death as a descriptor of plague. The term septicemic plague describes fulminant infection without the presence of a characteristic bubo. Pneumonic plague occurs as a result of hematogenous spread of plague bacilli from a bubo to the lungs or after inhalation of organisms, the most likely route of infection as a result of a bioterrorist event. The pneumonic form of the disease is highly contagious and can be spread from person to person via air-borne droplets. Administration of streptomycin or tetracycline early in the course of the disease is an effective treatment, which can reduce mortality from approximately 50% in untreated plague cases to about 5%. A single case of plague due to a multiply resistant strain (resistant to streptomycin, tetracycline, choramphenicol, and sulfonamide) was noted in Madagascar in the mid-1990s (11), serving as a reminder that such naturally occurring strains could conceivably be pressed into service by bioterrorists with a minimum of microbiological knowledge. A plague vaccine, formerly produced by only one manufacturer in the United States, has been discontinued (11).
Variola Virus
The smallpox (variola) virus is the largest of the animal viruses. The virus particles are brick-shaped to ovoid and measure approximately 300 by 200 by 100 μm. Morphologically, the virus is indistinguishable from the less pathogenic, closely related vaccinia virus, which is one of the best-investigated human viruses. The variola virus contains double-stranded DNA and has a complex structure. Two lipoprotein membrane layers surround the dumbbell-shaped nucleoid. The nucleoid is embedded in an ellipsoid body, forming the thick center of the virion. A double membrane surrounds the virus particle. The variola virus is highly contagious and very virulent, with a case fatality rate of 30% in unvaccinated persons (18, 42). Although the extreme biohazards involved in working with the smallpox virus have limited research on this organism, when the World Health Organization (WHO) decided to destroy the virus, laboratories in the United States and former Soviet Union sequenced its genome (9).
Poxviruses are divided into four different groups. Group 1 comprises variola, vaccinia, cowpox, ectromelia, rabbitpox, and monkeypox viruses. The variola virus exists as one of two strains: variola major causing severe smallpox and variola minor, causing mild smallpox or alastrim. These two strains are immunologically indistinguishable. Vaccination against smallpox is performed with the vaccinia virus, which has many antigenic structures in common with the smallpox agent. The vaccinia virus does not exist in nature and is considered a laboratory artifact. It was used worldwide in a live vaccine against smallpox and served as a laboratory model for the poxviruses. The origin of the vaccinia virus remains unclear. It is different from Jenner's cowpox virus and may be a mutant of the variola and alastrim viruses (18, 42).
At least seven distinct major variola virus antigens can be recognized by immunodiffusion techniques, and 17 polypeptide chains can be identified. Hemagglutinating, complement-fixing, and neutralizing antibodies may be produced in reponse to antigens of the smallpox virus. Neutralizing antibodies are directed against two antigens in the surface membrane of the virus particle. Complement-fixing antibodies react with a family antigen common to each subgroup of the unclassified poxviruses. The hemagglutinin reacts with erythrocytes of 50% of chickens (18, 42).
Smallpox virus is a BSL 4 organism, and consequently the average clinical laboratory is not equipped to provide a definitive diagnosis of smallpox. Clinical laboratories could, however, be called upon to handle infectious specimens in a bioterrorist event involving this pathogen. The smallpox virus and its antigens are very stable, and specimens of blood, scrapings from skin lesions, saliva, pustular fluid, and crusts can be transported and stored for short periods without refrigeration. The primary routes of infection for laboratory personnel handling such specimens are ingestion, aerosol exposure of mucous membranes, and parenteral inoculation. Extreme caution should be taken to avoid these types of exposure. Table 1 summarizes specimen-handling protocols in suspected cases of smallpox. If smallpox is suspected, laboratorians should contact the CDC and their local public health authorities for guidance (18, 20, 26, 42). Material from smallpox patients (dried fluid and crusts) containing virus remains infectious at room temperature for approximately 1 year despite the complex structure and membrane envelope of the virus particles. The infectivity of the virus is maintained at 4°C for several months and at −20 to −70°C for years. The decontamination of patients' clothes, laundry, and furniture is important. The relative resistance of the virus poses some practical problems. Dilute phenol and many common disinfectants are not completely efficient. Inactivation can be achieved using apolar lipophilic solvents (chloroform) and quaternary ammonia compounds. Heating for 10 min at 60°C and autoclaving both destroy the viability of the smallpox and vaccinia viruses (18, 20, 42).
In laboratories equipped to handle smallpox specimens, a presumptive diagnosis can be made with Giemsa-stained smears of material from skin lesions in which Guanieri inclusion bodies may be seen. Electron microscope studies can reveal viral particles with typical Orthopoxvirus morphology. Blood or material from skin lesions inoculated in chorioallantoic membranes of 12- to 14-day-old chicken embryos will result in the formation of pox lesions within 2 to 3 days. Morphology allows the differentiation between pox caused by smallpox virus and vaccinia virus. The smallpox virus can be propagated in many human tissue cultures and cells from a variety of animals. Human embryonic cell lines and monkey kidney cells are most frequently used for this purpose. Cytopathic effect develops within 5 to 8 days, with eosinophilic cytoplasmic inclusions (Guarnieri bodies) evident after 48 h.
Hemadsorption with susceptible chicken erythrocytes is an early detection method for infection with smallpox virus. Blood, vesicle fluid, pustule fluid, and saline extracts from crusts or scrapings contain soluble antigen in certain stages of disease. These antigens can be detected via complement fixation, immunofluorescence, and Ouchterlony techniques. Serological diagnosis can be made by observation of a fourfold rise in titer in complement fixation or hemagglutination inhibition tests. Serologic response is variable in partially immune patients, who may present clinically with variola sine eruptione (without skin rash). Table 5 summarizes information on the usefulness of traditional laboratory testing (performed only in laboratories capable of handling smallpox) for smallpox diagnosis during various stages of the disease. The CDC and the U.S. Army Medical Research Institute for Infectious Diseases have established a PCR test for the demonstration of smallpox virus for rapid diagnosis (18, 20, 26, 42).
TABLE 5.
Diagnosis of variola by laboratory testsa
| Stage | Material used | Microscopic examination (1 h) | Detection or isolation of of virusb (1 to 3 days) | Antigen detectionc (3 to 24 h) | Detection of antibodiesd (3 h to 3 days) |
|---|---|---|---|---|---|
| Preeruptive | Blood | ± | ± | − | |
| Maculopapular | Blood | ± | ± | ± | |
| Skin lesions | + | + | + | ||
| Saliva | + | ||||
| Vesicular | Blood | ± | + | ||
| Skin lesions | + | + | + | ||
| Pustular | Blood | + | |||
| Pustular fluid | ± | + | + | ||
| Crusting | Blood | + | |||
| Crusts | − | + | + |
The time required for completion of each test is given. Results: +, usually positive; −, usually negative; ±, positive or negative.
Culture on chorioallantoic membrane of chicken embryos or in tissue culture.
Complement fixation, agar gel precipitation, or immunofluorescence.
Hemagglutination inhibition, complement fixation, or neutralization. Antibodies may appear earlier in previously vaccinated patients. +, rise in antibody titer.
The usual route of infection is through inhalation of droplets containing infectious virus particles. The virus enters the upper respiratory tract, where it multiplies in mucosal cells and regional lymph nodes. A transient viremia spreads the virus to the internal organs (liver, spleen, and lungs) and the skin. The subsequent multiplication with high-virus yields leads to a second viremia that marks the end of the incubation period and the beginning of the toxemic phase. Clinically, this phase is characterized by prodromal rashes and symptoms, including a fever of about 104°F (40°C), headaches, aches and pains, malaise, and prostration. The incubation period lasts 12 to 14 days, and skin eruption follows within 3 to 4 days. The rash starts on the tongue and roof of the mouth and spreads to the face, forearms, and hands (patients are highly infectious at this point). Subsequently, the rash spreads over the arms, legs, and trunk. The virus-shedding skin lesions go through synchronous stages, macular, papular, vesicular, and pustular, during the second week of the disease. The pustules can become hemorrhagic or confluent. In the repair stage, scabs form and the lesions turn into scars. As a complication, internal organs can show fatty degeneration and focal necrosis. In biopsies of a skin lesion or scrapings from pustules, eosinophilic cytoplasmic inclusion bodies can be found, surrounded by a clear halo. The changes are observed in skin and mucous membranes infected with variola and vaccinia viruses. For the differential diagnosis, the exclusion of chickenpox is important. In chickenpox, the trunk has more lesions than the face, while smallpox affects the face more than the trunk. In smallpox, the pustules appear more simultaneously and can cover palms and plantar areas. Following natural infection, neutralizing antibodies persist for at least 20 years and may provide lifelong protection. Reinfection can result in mild clinical cases (variola sine eruptione). While humoral immunity can offer protection from infection, cell-mediated immunity is critical for successful recovery, as in many viral infections (18, 20, 26, 42).
Humans are the only natural host of smallpox virus, and infection is spread from person to person. During the incubation period and prodromal stage, virus is difficult to detect. However, it is abundant in lesions during the active disease. The first avenue of transmission is from lesions in the upper respiratory tract, where the virus replicates in the mucosa. Respiratory droplets may be transferred to other hosts and can also contaminate eating and drinking utensils. Skin lesions are a source of contagion for fomites (bed linens, clothing, utensils, and books). Air-borne transmission has been demonstrated in hospitals without efficient isolation facilities, as illustrated in the Meschede outbreak in Germany in 1969 (26). The spread of infection in an institution can only be successfully contained with negative-pressure isolation facilities that are separate from the institution's ventilation and air-conditioning systems. There are few options for treatment of infected patients. Leukocyte transfer from immune persons and methisazone have been used to treat vaccinia gangrenosa, and hyperimmune globulin has shown variable success against generalized vaccinia virus infections. Although the use of antiviral agents for treatment of smallpox is currently under investigation, no proven antiviral treatment exists at this time (18).
Successful measures for the control of smallpox were set forth in the recommendations by the American Public Health Association in 1960 (18, 52) and have been adopted by the U.S. Armed Forces (8) and the U.S. Public Health Service. All measures to contain an outbreak of smallpox and prevent the spread of the disease depend on recognition of the disease. During a general outbreak, the diagnosis can be relatively easily established by the clinical presentation and epidemiological evidence. In early cases, especially in those with partial immunity and atypical clinical manifestations, laboratory diagnosis is indispensable. Preventive measures include primary vaccination of infants and revaccination of children upon entry into school and upon exposure to high risk of infection. All cases of infection are to be reported to the public health authority and isolated in the hospital until all crusts have disappeared. All oral and nasal discharges and articles associated with the patient should be disinfected by burning, high-pressure steam, or boiling. All persons having contact with infected patients should be vaccinated or revaccinated and kept under surveillance for 16 days from time of last contact. Any rise in temperature during surveillance indicates the need for prompt isolation until smallpox can be excluded. The immediate source case must be sought assiduously. Recent cases of adult chickenpox and patients with hemorrhagic or pustular lesions of the skin not diagnosed as having smallpox should be carefully reviewed for errors in diagnosis.
In epidemic smallpox, the recommendations for isolation of cases, vaccination, and surveillance of contacts described above should be followed. The public should be made aware of the situation immediately in order to facilitate control of the epidemic. Mass immunization of the affected community is indicated when there is evidence of spread of the outbreak. On the international level, the WHO and adjacent countries should be notified of the existence of epidemic smallpox, and travel and transport should be limited.
An early method of immunization against smallpox called variolation was empirically practiced in China, Persia, and Turkey and employed material from old crusts for inoculation. Edward Jenner, in 1796, used cowpox virus for smallpox vaccination based on the clinical observation that milkmaids who had been previously infected with cowpox were spared from smallpox infection even during epidemics. Two years after Jenner's publication, Lady Wortley Montague, the wife of the British ambassador to Turkey, brought the Turkish vaccination procedure employing material from the crusts of alastrim patients back to England (42).
Strains of the vaccinia virus were used for immunization in many countries for nearly two centuries. Today's vaccine strain of vaccinia virus has many antigenic structures in common with smallpox virus and is distinctly different from the cowpox virus. It is thought that this strain may have originated as a cowpox virus and evolved during laboratory passage, or it may have been unknowingly replaced by an attenuated variola virus. Vaccination consists of administering live attenuated virus intradermally by scarification. Standard methods of vaccine production used animal skin for replication of the vaccinia virus. Scrapings from lesions were then treated with 1% phenol to kill bacteria and stabilized with 40% glycerol before purification processing. Frozen vaccines have a shelf life of about 3 months. More modern methods of vaccine preparation, beginning in the 1970s, used chicken chorioallantoic membranes or primate cell tissue cultures for virus propagation. A vaccine consisting of purified soluble antigens was also developed (18, 20, 42).
With the standard vaccine, protective immunity develops within 7 to 10 days postvaccination, and postexposure vaccination is successful if performed within 3 to 4 days after exposure. Vaccination at 5 days postexposure may mitigate the disease. Protective immunity persists for 3 to 7 years. Reimmunization every 3 years usually results in uninterrupted protection. Since breakthrough infections of mild smallpox have been documented within 1 year of successful vaccination, revaccination is recommended in cases of exposure. For health care workers with potential exposure, yearly revaccination is recommended. Experience with the standard vaccine suggests that it is relatively safe, but some complications have been noted. Skin complications (eczema vaccinatum) may occur in immunocompromised patients or patients with eczema. Other possible complications include allergic reactions at the vaccination site, vaccinia gangrenosa, eye infections (due to virus spread from the vaccination site), postvaccinal encephalitis, intrauterine vaccinia (vaccination of pregnant patients is contraindicated), and viremia. Modern tissue culture vaccines exhibit fewer side effects and are easier to standardize (18, 20, 42, 50). Currently, all vaccine production sites in the United States have been dismantled, and only about 7 million doses of outdated vaccine are held in storage by a single manufacturer. It is estimated that 50 to 100 million doses exist worldwide. In view of the potentially catastrophic consequences of a bioterrorist attack with smallpox we will have to carefully consider the risks and benefits of the reinstitution of vaccination. Certainly tissue culture vaccines or even newer biotechnological approaches to tailor an effective and safe smallpox vaccine would influence the decision in a more favorable way (26).
Although the WHO advised the destruction of all stocks of variola major and variola minor virus by June 1999, President Clinton decided to postpone the final destruction of stocks in the United States. The WHO then delayed the decision until 2002 in order to allow more research on the smallpox virus. Proponents of preservation of smallpox stocks point out that the variola virus is an old wayfarer of mankind and the genome represents an irreplaceable evolutionary genetic heritage. Preservation is also justified by the unique host specificity of the variola virus and its as yet unidentified virulence genes. The existence of variola virus-encoded proteins which might alter host immunity and regulatory function has been proposed, and study of these proteins may prove useful in the elucidation of human defense mechanisms. Proponents of destruction of smallpox virus stocks note that the genomes of reference strains have been sequenced, and monkeypox virus (which is 90% homologous to variola virus) could serve as a surrogate in variola virus research. Proponents of smallpox virus destruction also note that variola virus represents a genuine threat as a biological weapon, and all stocks should therefore be destroyed (25).
Agents of Viral Hemorrhagic Fevers
A diverse group of viruses are capable of causing viral hemorrhagic fever syndrome. These include RNA viruses that are members of the Filoviridae (Ebola and Marburg viruses), Arenaviridae (Lassa fever, Argentine or Junin, Bolivian or Machupo, Venezuelan or Guanarito, and Brazilian or Sabia hemorrhagic fever viruses), Bunyaviridae (hantavirus, Rift Valley fever, and Congo-Crimean hemorrhagic fever viruses), and Flaviviridae (yellow fever and dengue fever viruses). Humans are exposed to these agents by contact with infected animals or via arthropod vectors. The infections caused by this group of viral agents are characterized by vascular damage and altered vascular permeability. Symptoms commonly include fever and myalgias, prostration, hemorrhages in mucous membranes, and shock. Viral hemorrhagic fevers cause high morbidity, and in many cases high mortality rates are observed. Treatment consists mostly of supportive measures, although the antiviral agent ribavirin seems to be useful for treatment of infection with certain agents such as Lassa fever virus, Junin, Bolivian, and Congo-Crimean hemorrhagic fever viruses, and Rift Valley fever virus (23). Only a handful of reference laboratories (usually BSL 4 facilities) are equipped to diagnose agents of viral hemorrhagic fevers by culture or nonculture techniques, which include serological, immunohistological, and nucleic acid amplification methods. Contact precautions should be observed for patients with suspected viral hemorrhagic fever, and specimens obtained from such cases should be handled with care. Table 1 summarizes specimen-processing protocols for specimens from suspected cases of viral hemorrhagic fever. Public health authorities should be contacted for guidance if such agents are suspected (23).
Botulism Toxin
Clostridium botulinum strains and some isolates of clostridia classified as Clostridium butyricum, Clostridium baratii, and Clostridium argentinense produce a family of seven immunologically distinct potent neurotoxins, designated A through G. Types A, B, E, and F are the usual agents of botulism in humans and cause the clinical syndromes identified as food-borne botulism (an intoxication), wound botulism (infection and toxin production), and botulism caused by toxin production after clostridial colonization of the intestines of infants (infant botulism) or older children and adults. Botulinum toxins spread through the bloodstream and exert their effects at neuromuscular junctions, where they inhibit the release of acetylcholine. The classical presentation of botulism is acute flaccid paralysis that begins in the head and descends symmetrically. When breathing becomes impaired, patients with botulism should be treated with respiratory support. Treatment with antitoxin should also be instituted as soon as the diagnosis is made. Clinicians suspecting botulism should notify the CDC immediately. The CDC provides antitoxin and epidemiological and diagnostic services for botulism cases (3).
The average clinical laboratory is not involved in the diagnosis of botulism but may be called upon to refer specimens to a public health laboratory with facilities for diagnosing botulism. Since botulism toxins are extremely potent, such specimens should be handled with care, employing a biological safety cabinet, disposable gloves, lab coat, and, if needed, face shield. A summary of specimen types useful for diagnosis of botulism is found in Table 1. At referral laboratories capable of diagnosing botulism, serum specimens are tested for toxin, as are other specimens, such as vomitus, stool, or tissue debrided from infected wounds. Some specimens may also be cultured for C. botulinum. Any isolates are identified and then tested for toxogenicity. While the symptoms, diagnosis, and treatment of food-borne botulism have been well described, less is known about intoxication resulting from inhalation of toxin. Evidence suggests that toxin acquired via an aerosol is less likely to be detected in serum or stool specimens. Nevertheless, the clinical microbiology laboratory could be called upon to process and refer a variety of specimen types in a suspected bioterrorist event involving aerosolized toxin. However, administration of botulinum antitoxin appears to be extremely effective for treatment of botulism acquired in this manner if antitoxin is given before clinical symptoms become apparent (23).
Table 6 summarizes some of the characteristics of the agents discussed above that relate to their use as bioterrorist weapons.
TABLE 6.
Summary of characteristics of selected bioterrorism agents
| Agent | Incubation period | Person-to-person spread | Morbidiity/mortality if untreated | Diagnosisa |
|---|---|---|---|---|
| B. anthracis | 1–5 days | No | High/high | Culture, serology |
| Y. pestis | 2–3 days | Yes | High/high | Culture, serology |
| F. tularensis | 2–10 days | No | High/low | Culture, serology |
| Brucella spp. | 5 days–2 months | No | High/low | Culture, serology |
| Botulinum toxins | 1–5 days | No | High/high | ELISA or mouse inoculation for toxin detection |
| Variola virus | 7–17 days | Yes | High/high | Detection via ELISA, PCR, or virus isolation |
| Viral hemorrhagic fever agents | 4 days–3 weeks | No | High/high | PCR, ELISA, serology, virus isolation |
ELISA, enzyme-linked immunosorbent assay.
BIOTERRORISM: ARE WE READY?
Alternative Views on Preparedness
While government employees and civilians in the clinical diagnostic and health care fields are mobilizing preparedness plans, it should be remembered that there are alternative views on what to do about the threat of bioterrorism. Leonard Cole stresses that emphasis on the ethical and moral reasons against the use of biological (and chemical and nuclear) weapons, along with stronger treaties, verification regimens, and surveillance, are the best methods for preventing the use of such weapons (15). Cohen and colleagues (14) draw a parallel between the current emphasis on defense against biological agents and the civil defense programs promoted by the government during the Cold War era, when the most feared agents of mass destruction were nuclear weapons. Cohen and colleagues note that health professionals in that era recognized the delusional nature of attempting to survive a nuclear attack and instead advocated pursuing nuclear disarmament and the root causes of conflict (poverty, hunger, and lack of health care) as a long-term solution to the problem. The authors state that since the likelihood of deployment of biological agents is small, resources would be better spent on mechanisms that would improve the standard of living and health care for all. These efforts would lead to a healthier population with increased resistance to infection and would also diminish the causes of terrorism (14). In spite of these views, the U.S. government is currently strongly committed to developing extensive biological weapon defense plans for both military personnel and the civilian population.
Challenges for the Medical and Scientific Communities
Louis Pasteur's (1822–1895) statement, “Chance favors only the prepared mind,” can be directed at the medical community, which together with the public health service has to assume a leading role in the nation's response to bioterrorism, a response that must be founded in fact and science. Bioterrorism is an event in a civil setting that is equivalent to an epidemic in a medical scenario. This differentiation results in a direct challenge to the medical community and charges us with the responsibility of initiating preparedness in laboratory and clinical diagnostics for infectious agents potentially used in bioterrorism, epidemiological surveillance, collaboration between medicine and public health in response to bioterrorist activity, training of personnel for this specialized task (first responders), preparation of regional stockpiles of vaccines, supplies, and drugs, immunization of first responders, research and development of new rapid test methods, investigation of therapeutic drugs and protective, safe immunization methods, and investigation of the need for specialized hospital centers with specially trained personnel to handle bioterrorist attack victims.
The medical professions must not only assume this role, but also participate actively in the discussion and planning strategies in order to influence formulation of the policies that will govern response to a bioterrorist event. These policies must integrate the various activities and interests of federal and local government agencies, law enforcement, fire departments, and other agencies involved in reaction to a massive bioterrorist attack. The efficiency of this novel interdisciplinary cooperation will be crucial to contain and prevent such events. These measures do not chase a fictitious foe. We live in an era of globalization and growing risk of dangerous epidemics of infectious diseases. The perils of the contagion are looming in new epidemics as well as in bioterrorism.
In the 21st century we are likely to continue to see the emergence of new pathogenic organisms and the spread of existing organisms to new demographic areas and hosts. The possibility of massive epidemic outbreaks due to bioterrorism also exists. In spite of these potential events, the Public Health Service in the United States has been in a gradual decline during the last decades. The rebuilding of the public health service and a new coordination of the medical infrastructure together with the antibioterrorism initiatives discussed in this review will strengthen the nation's ability to respond with vigor and efficiency against an attack or an introduced epidemic (10). President Clinton remarked in a speech given at the National Academy of Sciences in 1999: “These cutting edge efforts will address not only the threat of weapons of mass destruction, but also the equally serious danger of emerging infectious diseases. So we will benefit, even if we are successful in avoiding these attacks.”
For the scientific community, the challenge reaches beyond these pragmatic issues. Our beliefs of today can well be our errors of tomorrow. We will have to rethink the reciprocal relationship between host and microbe, because both had and have a distinct effect on each other's evolution. What do we know about the evolutionary effects on our immune system if it is no longer exposed to antigens of Mycobacterium tuberculosis, some of the most immunogenic substances known? Wrong philosophies can lead to wrong conclusions and subsequently transform into false paradigms, as we have learned from the overambitious eradication plan for tuberculosis in this country. We can expect a tremendous boost for vaccinology from these pressing questions.
Potential for Bioterrorism Really Exists
The world center of knowledge for biological warfare agents was and probably still is the former Soviet Union. In a recent article, the New York Times (26 January 2000) reports, under the headline “U.S. Aid is Diverted to Germ Warfare, Russians Scientists Say,” that funds coming from the United States were shifted to Biopreparat, a shadowy organization that once directed the Soviet Union's germ warfare program, according to several Russian scientists. Due to security leaks, the agency has become a potential source of knowledge and supplies for international and rogue-state terrorists. The threat of bioterrorism is real. Donna Shalala, Secretary of Health and Human Services, said in 1999: “We need to replace complacency with a new sense of urgency” (44). The first National Symposium on Medical and Public Health Response to Bioterrorism, held 16–17 February 1999, in Washington, D.C., was groundbreaking but also marked a watershed to hand the problem back to the medical profession, which is destined and challenged to lead with expertise in the defense against bioterrorism.
CONCLUSIONS
At this point in the discussion of the complex issue of bioterrorism, we are in a position to raise more questions than answers. However, our intent is to contribute to a fruitful dialogue which will stimulate the process to generate answers that will be the building blocks for our preparedness and the nation's defense against the threat of bioterrorism. The threat of bioterrorism is real and looming before us. The involvement of clinical microbiologists and other health care professionals in preparing for a bioterrorist act should extend beyond the institution of protocols and plans to be followed in the wake of such an event. To be prepared in a responsive and responsible way, we need a paradigm of scenario planning in which we look at a range of possibilities and countermeasures rather than construct a linear and reactive strategic plan. This demands that, in our collective imagination, we move the previously unthinkable into the realm of possibility in order to develop a realistic response strategy. We will have to integrate many conflicting issues and satisfy conflicting needs through compromises that seek to find the second-best solutions. This will still be smarter than having no solutions at all. The answer is to reconsider the present rather than to prophesy the future based on vague assumptions. The time has come to get prepared and develop an integrated policy.
REFERENCES
- 1.Aleksic S, Bockemuhl J. Yersinia and other Enterobacteriaceae. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington. D.C.: American Society for Microbiology; 1999. pp. 483–496. [Google Scholar]
- 2.Alibek K, Handelman S. Biohazard: the chilling true story of the largest covert biological weapons program in the world. New York, N.Y: Random House; 1999. [Google Scholar]
- 3.Allen S D, Emery C L, Siders J A. Clostridium. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: American Society for Microbiology; 1999. pp. 654–671. [Google Scholar]
- 4.Bardi J. Aftermath of a hypothetical smallpox disaster. Emerg Infect Dis. 1999;5:547–551. doi: 10.3201/eid0504.990417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barham W B, Church P, Brown J E, Paparello S. Misidentification of Brucella species with use of rapid bacterial identification systems. Clin Infect Dis. 1993;17:1068–1069. doi: 10.1093/clinids/17.6.1068. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett J G. Applying lessons learned from anthrax case history to other scenarios. Emerg Infect Dis. 1999;5:561–563. doi: 10.3201/eid0504.990420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batchelor B I, Brindle R J, Gilks G F, Selkon J B. Biochemical and mis-identification of Brucella melitensis and subsequent laboratory-acquired infections. J Hosp Infect. 1992;22:159–162. doi: 10.1016/0195-6701(92)90100-z. [DOI] [PubMed] [Google Scholar]
- 8.Batten P J. Present status of smallpox vaccination: a review. US Armed Forces Med J. 1960;11:876–883. [PubMed] [Google Scholar]
- 9.Breman J G, Henderson D A. Poxvirus dilemmas—monkeypox, smallpox, and biologic terrorism. N Engl J Med. 1998;339:556–559. doi: 10.1056/NEJM199808203390811. [DOI] [PubMed] [Google Scholar]
- 10.Bryan J L, Fields H F. An ounce of prevention is worth a pound of cure—shoring up the public health infrastructure to respond to bioterrorist attacks. Am J Infect Control. 1999;27:465–467. doi: 10.1016/s0196-6553(99)70021-0. [DOI] [PubMed] [Google Scholar]
- 11.Butler T. Yersinia species, including plague. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 5th ed. Philadelphia, Pa: Churchill Livingstone; 2000. pp. 2406–2414. [Google Scholar]
- 12.Centers for Disease Control and Prevention and National Institutes of Health. Biosafety in microbiological and biomedical laboratories. 4th ed. Washington, D.C.: U.S. Department of Health and Human Services, Public Health Service; 1999. [Google Scholar]
- 13.Christopher G W, Cieslak T J, Pavlin J A, Eitzen E M., Jr Biological warfare: a historical perspective. JAMA. 1997;278:412–417. [PubMed] [Google Scholar]
- 14.Cohen H W, Gould R M, Sidel V W. Bioterrorism initiatives: public health in reverse? Am J Public Health. 1999;89:1629–1631. doi: 10.2105/ajph.89.11.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole L A. The specter of biological weapons. Sci Am. 1996;275:60–65. doi: 10.1038/scientificamerican1296-60. [DOI] [PubMed] [Google Scholar]
- 16.Cole L A. Risks of publicity about bioterrorism: anthrax hoaxes and hype. Am J Infect Control. 1999;27:470–473. doi: 10.1016/s0196-6553(99)70023-4. [DOI] [PubMed] [Google Scholar]
- 17.Cross J T, Jr, Penn R L. Francisella tularensis (tularemia) In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 5th ed. Philadelphia, Pa: Churchill Livingstone; 2000. pp. 2393–2402. [Google Scholar]
- 18.Davis B D, Dulbecco R, Eisen H N, Ginsberg H S, Wood W G, Jr, McCarty M. Microbiology. 2nd ed. Hagerstown, Md: Harper & Row, Publishers, Inc.; 1973. Poxviruses; pp. 1258–1277. [Google Scholar]
- 19.Davis C J. Nuclear blindness: an overview of the biological weapons programs of the former Soviet Union and Iraq. Emerg Infect Dis. 1999;5:509–512. doi: 10.3201/eid0504.990408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Downie A W. The poxvirus group. In: Horsfall F L Jr, Tamm I, editors. Viral and rickettsial infections of man. 4th ed. Philadelphia, Pa: J. B. Lippincott Company; 1965. pp. 932–964. [Google Scholar]
- 21.Eickhoff T C. Airborne disease, including chemical and biological warfare. Am J Epidemiol. 1996;144:S39–S46. doi: 10.1093/aje/144.supplement_8.s39. [DOI] [PubMed] [Google Scholar]
- 22.English J F. Overview of bioterrorism readiness plan: a template for health care facilities. Am J Infect Control. 1999;27:468–469. doi: 10.1016/s0196-6553(99)70022-2. [DOI] [PubMed] [Google Scholar]
- 23.Franz D R, Jahrling P B, Friedlander A M, McClain D J, Hoover D L, Bryne W R, Pavlin J A, Christopher G W, Eitzen E M. Clinical recognition and management of patients exposed to biological warfare agents. JAMA. 1997;278:399–411. doi: 10.1001/jama.278.5.399. [DOI] [PubMed] [Google Scholar]
- 23a.Günther C. Einfuhrung in das Studium der Bakteriologie mit besonderer Berucksichtigung der mikroskopishen Technik. Stuttgart, Germany: Georg Thieme; 1890. [Google Scholar]
- 24.Harris S. Japanese biological warfare research on humans: a case study on microbiology and ethics. Ann NY Acad Sci. 1992;666:21–52. doi: 10.1111/j.1749-6632.1992.tb38021.x. [DOI] [PubMed] [Google Scholar]
- 25.Henderson D A. Bioterrorism as a public health threat. Emerg Infect Dis. 1998;4:448–492. doi: 10.3201/eid0403.980340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henderson D A. Smallpox: clinical and epidemiological features. Emerg Infect Dis. 1999;5:537–539. doi: 10.3201/eid0504.990415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henderson D A. The looming threat of bioterrorism. Science. 1999;283:1279–1282. doi: 10.1126/science.283.5406.1279. [DOI] [PubMed] [Google Scholar]
- 28.Hughes J M. The emerging threat of bioterrorism. Emerg Infect Dis. 1999;5:494–5. doi: 10.3201/eid0504.990403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inglesby T V. Anthrax: a possible case history. Emerg Infect Dis. 1999;5:556–560. doi: 10.3201/eid0504.990419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaufmann A F, Meltzer M I, Schmid G P. The economic impact of a bioterrorist attack: are prevention and postattack intervention programs justifiable? Emerg Infect Dis. 1997;3:83–94. doi: 10.3201/eid0302.970201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kortepeter M G, Parker G W. Potential biological weapons threats. Emerg Infect Dis. 1999;5:523–527. doi: 10.3201/eid0504.990411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lillibridge S R, Bell A J, Roman R S. Centers for Disease Control and Prevention bioterrorism preparedness and response. Am J Infect Control. 1999;27:463–464. doi: 10.1016/s0196-6553(99)70020-9. [DOI] [PubMed] [Google Scholar]
- 33.Logan N A, Turnbull P C B. Bacillus and recently derived genera. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: American Society for Microbiology; 1999. pp. 357–369. [Google Scholar]
- 34.McDade J E. Addressing the potential threat of bioterrorism—value added to an improved public health infrastructure. Emerg Infect Dis. 1999;5:591–592. doi: 10.3201/eid0504.990428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meselson M, Guillemin J, Hugh-Jones M, Langmuir A, Popova I, Shelokov A, Yampolskaya O. The Sverdlovsk anthrax outbreak of 1979. Science. 1994;266:1202–1208. doi: 10.1126/science.7973702. [DOI] [PubMed] [Google Scholar]
- 36.Moran G J. Update on emerging infections from the Centers for Disease Control and Prevention: bioterrorism alleging use of anthrax and interim guidelines for management—United States, 1998. Ann Emerg Med. 1999;34:229–232. doi: 10.1016/s0196-0644(99)70237-4. [DOI] [PubMed] [Google Scholar]
- 37.Olson K B. Aum Shinrikyo: once and future threat? Emerg Infect Dis. 1999;5:513–516. doi: 10.3201/eid0504.990409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osterholm M T. Bioterrorism: media hype or real potential nightmare? Am J Infect Control. 1999;27:461–462. doi: 10.1016/s0196-6553(99)70019-2. [DOI] [PubMed] [Google Scholar]
- 39.O'Toole T. Smallpox: an attack scenario. Emerg Infect Dis. 1999;5:540–546. doi: 10.3201/eid0504.990416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poupard J A, Miller L A. History of biological warfare: catapults to capsomeres. Ann NY Acad Sci. 1992;666:9–20. doi: 10.1111/j.1749-6632.1992.tb38020.x. [DOI] [PubMed] [Google Scholar]
- 41.Raad I, Rand K, Gaskins D. Buffered charcoal-yeast extract medium for the isolation of brucellae. J Clin Microbiol. 1990;28:1671–1672. doi: 10.1128/jcm.28.7.1671-1672.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rhodes A J, van Rooyen E E. Textbook of virology. Baltimore, Md: The Williams and Wilkins Company; 1968. DNA-containing viruses; pp. 299–344. [Google Scholar]
- 43.Schreckenberger P C, vonGraevenitz A. Acinetobacter, Achromobacter, Alcaliegenes, Moraxella, Methylobacterium, and other nonfermentative gram-negative rods. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: American Society for Microbiology; 1999. pp. 539–560. [Google Scholar]
- 44.Shalala D E. Bioterrorism: how prepared are we? Emerg Infect Dis. 1999;5:492–493. doi: 10.3201/eid0504.990402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shapiro D S, Wong J D. Brucella. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington. D.C.: American Society for Microbiology; 1999. pp. 625–631. [Google Scholar]
- 46.Stern J. The prospect of domestic bioterrorism. Emerg Infect Dis. 1999;5:517–522. doi: 10.3201/eid0504.990410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swartz M N. Aerobic spore-forming bacilli. In: Davis B D, Dulbecco R, Eisen H N, Ginsberg H S, editors. Microbiology. 4th ed. Philadelphia, Pa: J. B. Lippincott Company; 1990. pp. 625–631. [Google Scholar]
- 48.Torok T J, Tauxe R V, Wise R P, Livengood J R, Sokolow R, Mauvais S, Birkness K A, Skeels M R, Horan J M, Foster L R. A large community outbreak of salmonellosis caused by intentional contamination of restaurant salad bars. JAMA. 1997;278:389–395. doi: 10.1001/jama.1997.03550050051033. [DOI] [PubMed] [Google Scholar]
- 49.Tucker J B. National health and medical services response to incidents of chemical and biological terrorism. JAMA. 1997;278:362–368. [PubMed] [Google Scholar]
- 50.Wiktor T J, Plotkin S A, Koprowski H. Development and clinical trials of the new human rabies vaccine of tissue culture (human diploid cell) origin. Dev Biol Stand. 1978;40:3–9. [PubMed] [Google Scholar]
- 51.Wong J D, Shapiro D S. Francisella. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington. D.C.: American Society for Microbiology; 1999. pp. 647–651. [Google Scholar]
- 52.World Health Organization Expert Committee on Smallpox. 1964. First report. WHO Tech. Rep. Ser. 283. [PubMed]
- 53.Yagupsky P. Detection of brucellae in blood cultures. J Clin Microbiol. 1999;37:3437–3442. doi: 10.1128/jcm.37.11.3437-3442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Young E J. Brucella species. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 5th ed. Philadelphia, Pa: Churchill Livingstone; 2000. pp. 2386–2393. [Google Scholar]