Abstract
Objectives
To examine the state of B-cell immunity 6 months after the second vaccination against SARS-CoV-2 in comparison to the state observed 2 weeks after vaccination.
Methods
Sera of 439 participants, whose immune responses to two doses of an mRNA-based vaccine (BNT162b2 or mRNA-1273) were previously characterized, was examined for anti-S1 IgG and IgA, anti-NCP IgG and neutralizing antibodies (nAb), and antinuclear antibodies (ANA).
Results
Levels of all examined markers decreased significantly from 2 weeks to 6 months after second vaccination (anti-S1 IgG: 3744 ± 2571.4 vs. 253 ± 144 binding antibody units (BAU)/mL; anti-S1 IgA: 12 ± 0 vs. 1.98 ± 1.75 optical density (OD) ratio; nAb: 100% ± 0% vs. 82% ± 19.3%), the vast majority of participants retaining reactive levels of anti-S1 IgG (436/439) and anti-S1 IgA (334/439) at 6 months. Immune responses were stronger for mRNA-1273 compared with BNT162b2 (anti-S1 IgG: 429 ± 289 vs. 243 ± 143 BAU/mL; anti-S1 IgA: 5.38 ± 3.91 vs. 1.89 ± 1.53 OD ratio; nAb: 90.5% ± 12.6% vs. 81% ± 19.3%). There was no meaningful influence of sex and age on the examined markers. There was a strong correlation between anti-S1 IgG and the surrogate neutralization assay (rho = 0.91, p <0.0001), but not for for IgA and the surrogate neutralization assay (rho = 0.52, p <0.0001). There was a ceiling effect for the association between anti-S1 IgG titres and the inhibition of binding between S1 and ACE2. ANA prevalence was unchanged from 2 weeks to 6 months after the second vaccination (87/498 vs. 77/435), as were the median ANA titres (1:160 vs. 1:160).
Discussion
Although the clinical consequences of decreasing anti-SARS-CoV-2 antibody titres cannot be estimated with certainty, a lowered degree of clinical protection against SARS-CoV-2 is possible. Persistently stronger responses to mRNA-1273 suggest that it might confer greater protection than BNT162b2, even 6 months after the second vaccination. Neither examined vaccinations induced ANA within the examined time frame.
Keywords: BNT162b2, COVID-19, Immune response, mRNA-1273, SARS-CoV-2, Vaccination
Introduction
Vaccination against SARS-CoV-2 has been rolled out on a worldwide basis since its approval by the CDC and the European Medicines Agency (EMA; among others) in late 2020. Since then, multiple studies have been conducted on the immune system's response to different vaccines, as well as the resulting clinical efficacy [[1], [2], [3], [4], [5], [6]]. We, among others, found that vaccination with BNT162b2 or mRNA-1273 leads to high titres of specific IgG antibodies against the spike protein of SARS-CoV-2, with stronger reactions for mRNA-1273 [7]. In the short term, there was no induction of antinuclear antibodies (ANA) as a correlate for autoimmunity caused by the vaccination. Contrary to findings by others [8,9], we could not find a pronounced influence of age on B- and T-cell responses 2 weeks after the second dose of the vaccine.
There is evidence for the waning of antibody titres over time after vaccination against SARS-CoV-2 [10]. In the current study, we examined whether we could find a similar waning of antibodies against SARS-CoV-2 for our cohort 6 months after the second vaccination, and if so, how great was the reduction of different markers of B-cell immunity against SARS-CoV-2. We also examined associations between B-cell immunity and age, sex, and the vaccine received (BNT162b2 vs. mRNA-1273), as well as correlations between the different markers. Lastly, we examined the possible induction of ANA by the vaccine. We hypothesized the following:
-
1.
Levels of markers of B-cell immunity after vaccination against SARS-CoV-2 would decrease significantly.
-
2.
Reactions would remain stronger for recipients of mRNA-1273 compared to those of BNT162b2.
-
3.
There would be no relevant influence of sex and age (within the examined age span) on the measured immune responses.
-
4.
There would be no induction of ANA 6 months after the second vaccination.
Methods
Study population
All 531 participants of the previous study were asked to participate in a follow-up examination of their B-cell immunity against SARS-CoV-2. The initial recruitment of this cohort and vaccination program they underwent are detailed elsewhere [7]. In short, all participants were health care professionals from a German university hospital who received either BNT1662b2 or mRNA-1273 with a 5-week interval between doses.
All participants provided written informed consent for this study. The study was approved by the University of Kiel institutional review board (AZ: D642/20). The study was conducted in accordance with the Declaration of Helsinki [11].
Anti-SARS-CoV-2 antibodies
Antibodies of the classes IgG and IgA against the S1 subunit of the spike protein of SARS-CoV-2 (anti-S1) as well as IgG against the nucleocapsid protein of SARS-CoV-2 (anti-NCP) were measured using the respective ELISA test kits by EUROIMMUN (Lübeck, Germany). S1 was chosen as the target antigen to monitor the response to the vaccination, as vaccination induces the production of S1 within the body as an immunogenic target. NCP was chosen to identify possible SARS-CoV-2 infection within the cohort, as anti-NCP is only present after infection with SARS-CoV-2 and not after vaccination. More information on the assays can be found in the supplement.
Neutralizing antibodies
Neutralizing antibodies were examined via a surrogate neutralization assay (SNA) (NeutraLISA; EUROIMMUN) according to the manufacturer's instructions. In brief, this is a competitive ELISA in which anti-S1 antibodies within the examined serum compete with ACE2 contained in a buffer for binding at S1 bound to a solid phase. The amount of bound ACE2 that can be detected via enzymatic reaction after a washing step is inversely proportional to the level of inhibition of SARS-CoV-2 achieved via anti-SARS-CoV-2 antibodies. More information on the SNA can be found in the supplement.
ANA
ANA were examined via indirect immunofluorescence testing (IIF) on HEp-2 cells (EUROIMMUN). All ANA IIFs were evaluated by the same experienced laboratory professional, minimizing interobserver variance.
Statistical analysis
Differences in continuous variables between groups were examined via analyses of variance (ANOVAs), with a Benjamini-Yekutieli correction in case of multiple comparisons [12]. Post hoc testing was performed via Tukey's honest significant differences. Differences between two groups were tested via the Student t-test. Differences in the distribution of categorically scaled variables between groups was tested via Pearson's χ2 test. For associations between two continuous variables, correlations using Spearman's rho were calculated. Statistical significance was assumed for p <0.05. Average values with a measure of dispersion are reported as median and median absolute deviation, unless otherwise stated. All statistical analyses were performed using the open-source software for statistical computing and graphics, R (v4.1.0), with the integrated development environment RStudio (v1.4.1717) [13] (see Table 1 ).
Table 1.
Average values (median ± median absolute deviation) for all markersa
| Group | 14 d after second dose |
6 mo after second dose |
||||
|---|---|---|---|---|---|---|
| Anti-S1 IgA (OD ratio) | Anti-S1 IgG (BAU/mL) | Neutral. antibodies (%) | Anti-S1 IgA (OD ratio) | Anti-S1 IgG (BAU/mL) | Neutral. antibodies (%) | |
| Whole cohort | 12 ± 0 | 3744 ± 2571.4 | 100 ± 0 | 1.98 ± 1.75 | 253 ± 144 | 82 ± 19.3 |
| Women | 12 ± 0 | 3830.4 ± 2585.7 | 100 ± 0 | 1.85 ± 1.61 | 246 ± 138 | 81 ± 19.3 |
| Men | 12 ± 0 | 3564.8 ± 2495.5 | 100 ± 0 | 2.35 ± 1.79 | 280 ± 160 | 84 ± 16.3 |
| Previously infected | 12 ± 0 | 3323.2 ± 517.1 | 100 ± 0 | 7.46 ± 3.77 | 298 ± 26.6 | 98 ± 3 |
| BNT162b2 | 12 ± 0 | 3654.4 ± 2571.4 | 100 ± 0 | 1.89 ± 1.53 | 243 ± 143 | 81 ± 19.3 |
| mRNA-1273 | 12 ± 0 | 4926.4 ± 3598.6 | 100 ± 0 | 5.38 ± 3.91 | 429 ± 289 | 90.5 ± 12.6 |
Results measured at 6 months after the second vaccination for the whole cohort and the following subgroups: women, men, participants previously infected with SARS-CoV-2, participants who received BNT162b2, and participants who received mRNA-1273. Shown are data for the time points 2 weeks after the second vaccination (data already published elsewhere) and 6 months after the second vaccination (data newly accumulated).
Results
Study population and sample characteristics
Of the 531 participants who donated a serum sample during the previous study, 439 (82.7%) did so again during the current study. Of these, 322 (73.3%) were female and 403 (91.8%) received BNT162b2. Their median age was 45 ± 14.8 (range: 20–66) years. There was no statistically significant difference in age between the two sexes (df = 1, F = 3.729, p = 0.054) or between recipients of the different vaccines (df = 2, F = 2.379, p = 0.124). Pearson's χ2 test revealed no statistically significantly different distribution of sexes between recipients of the two vaccines (χ2 = 0.444, df = 1, p = 0.505).
Samples were donated at a median of 181 (±1.48) days after the second dose of the vaccine against SARS-CoV-2.
Seven participants had a known history of COVID-19, five of them before the administration of the first dose, one who tested positive (via PCR) between doses, and one who tested positive (via PCR) after having received both doses of the vaccine.
Anti-SARS-CoV-2 antibodies
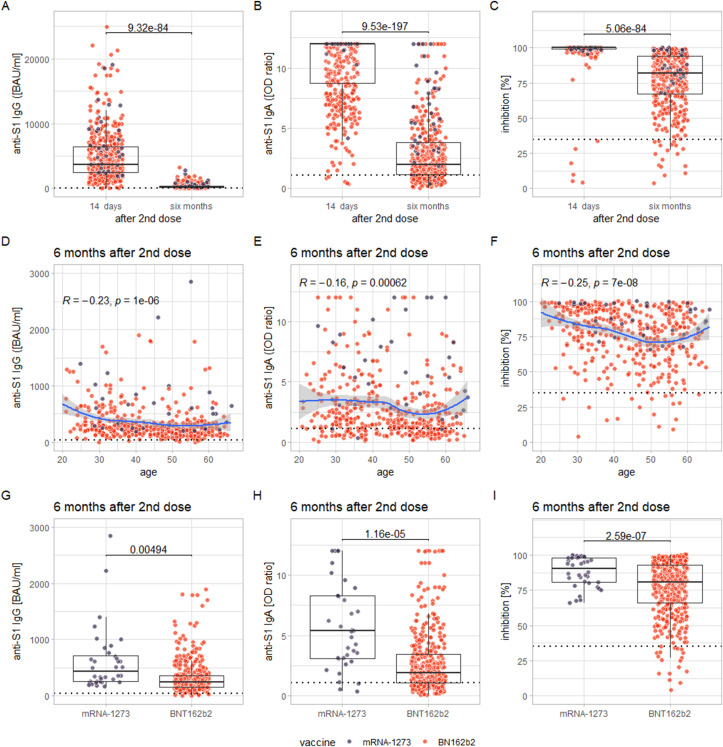
Levels of all examined markers decreased significantly from 14 days to 6 months after the second vaccination (all p <0.0001, see Fig. 1 (A-C)). Two-way ANOVAs with the factors sex and vaccine revealed a statistically significant main effect of the vaccine administered for all markers (with higher levels for recipients of mRNA-1273; all p <0.001, see Fig. 1(G-I), but no significant main effect of sex or interaction effect of sex and the vaccine administered (all p >0.1; see Fig. S1. Notably, only three participants, who exhibited reactive anti-S1 IgG titres 2 weeks after the second dose, showed levels of anti-S1 IgG below the cut-off for reactivity. However, their anti-S1 IgG titres 2 weeks after the second dose were already below the first percentile of the cohort. Of 439 participants, 334 (76.1%) were anti-S1 IgA reactive after 6 months.
Fig. 1.
(A–C) The decrease in measured levels from 14 days to 6 months after the second dose of the vaccine for anti-S1 IgG (A), anti-S1 IgA (B), and inhibition via SNA (C). (D–F) The correlation of these markers at 6 months after the second dose with age (the blue line representing the conditional smoothed mean, calculated via locally estimated scatterplot smoothing (LOESS), with a grey 95% confidence band around it). (G–I) The comparison of levels of these markers between recipients of mRNA-1273 and BNT162b2 at 6 months after the second dose. The individual measurements, plotted as dots, are colour coded according to the vaccination received (lilac: mRNA-1273; orange: BNT162b2).
Correlations between all examined markers and age were of small to negligible effect size (albeit statistically significant: anti-S1 IgG and age: rho = –0.23, p <0.0001; anti-S1 IgA and age: rho = –0.16, p <0.001; inhibition via SNA and age: rho = –0.25, p <0.0001; see Fig. 1(D-F). Effect sizes were not significantly greater when these correlations were examined separately for recipients of the two different vaccines, for which a significant main effect on the levels of examined markers was found Figure S1.
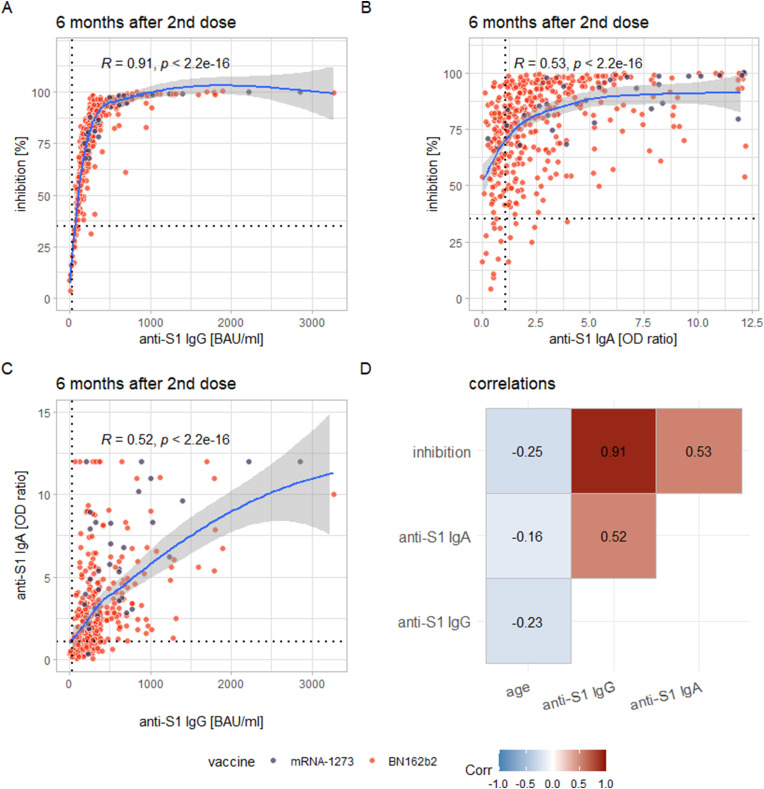
Among the examined markers, there was a strong correlation between anti-S1 IgG and inhibition via SNA (rho = 0.91, p <0.0001) and correlations of medium effect size between anti-S1 IgA and inhibition via SNA (rho = 0.53, p <0.0001) and between anti-S1 IgG and anti-S1 IgA (rho = 0.52, p <0.0001; Fig. 2 ).
Fig. 2.
(A–C) The correlations found between inhibition (via SNA) and anti-S1-IgG (A), inhibition and anti-S1 IgA (B), and anti-S1 IgA and anti-S1 IgG (C). The blue lines again represent the smoothed means (via LOESS) with a 95% confidence band. (D) A correlation matrix for all examined markers of immunity against SARS-CoV-2 as well as age. The individual measurements, plotted as dots, are colour coded according to the vaccination received (lilac: mRNA-1273; orange: BNT162b2).
Of the 439 participants of the current study, seven tested positive for anti-NCP IgG. Of these seven, one had a known history of COVID-19, five were deemed false positives, and for one a possible asymptomatic infection could not be ruled out. Four participants with a history of COVID-19 who were anti-NCP positive in the previous study were negative in the current study, and two participants never developed anti-NCP-IgG at any time point despite a history of COVID-19. More detailed data of all previously infected participants, as well as all participants who tested anti-NCP IgG positive after 6 months, can be found in the supplement.
ANA
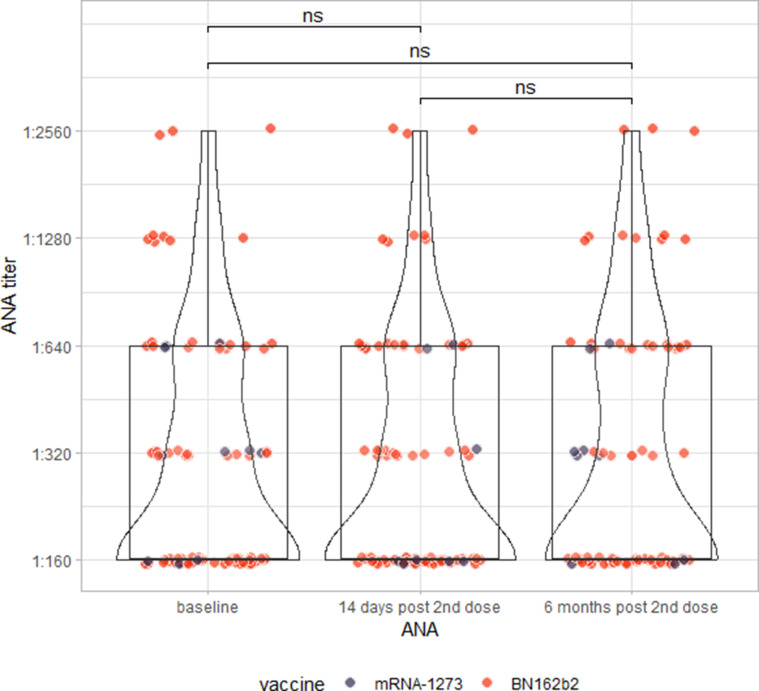
ANA testing via indirect immunofluorescence testing revealed that 6 months after the administration of the second dose of the vaccine, there was no change in qualitative ANA results (McNemar's χ2 = 2.5574, df = 3, p = 0.465), nor in the semiquantitative titres of those individuals who tested ANA positive at any time point (Wilcoxon signed-rank test: V = 46, p = 0.606; Fig. S2).
Discussion
In the current study, we found that levels of markers of B-cell immunity against SARS-CoV-2, such as anti-S1 IgG and IgA, as well as in vitro inhibition via SNA decrease significantly over the course of 6 months since the administration of the second dose of the vaccine against SARS-CoV-2.
We again found that recipients of mRNA-1273 had significantly higher titres of all examined markers compared to recipients of BNT162b2. This difference, found by others as well [9,14], manifests as early as 14 days after the first dose of the vaccination [7] and apparently persists long term, despite the overall decrease in antibody levels. The fact that vaccination with mRNA-1273 leads to higher levels of all examined markers than vaccination with BNT162b2 is most likely explained by the greater amount of mRNA contained in the former (100 μg) compared to the latter (30 μg) [2,3], although it is surprising that this difference is persistent. Given that higher levels of anti-S1 IgG convey a higher degree and longer duration of protection from SARS-CoV-2, mRNA-1273 may be preferable to BNT162b2 in this particular respect.
Nearly all participants still exhibited anti-S1 IgG reactive titres 6 months after the second dose of the vaccine. Furthermore, those participants who did not exhibit reactivity at 6 months after the second dose already exhibited low levels of anti-S1 IgG 2 weeks after the second dose. Therefore, the exact consequences of waning titres for protection against SARS-CoV-2 are difficult to estimate, especially in the absence of a clearly established cut-off value for anti-S1 IgG above which protection can be assumed.
Notably, anti-S1 IgG correlates strongly with inhibition via SNA, but anti-S1 IgA does not (the correlation of medium effect size between anti-S1 IgA and the SNA is likely explained by a correlation of the same effect size between anti-S1 IgA and IgG). This is surprising because in theory, anti-S1 IgA competes with ACE2 for binding at the S1 bound on the solid phase to the same extent as anti-S1 IgG. Our findings, however, suggest that, at least in vitro, serum anti-S1 contributes markedly less to the neutralization of SARS-CoV-2 compared to anti-S1 IgG. On the other hand, it is also surprising that a high proportion of participants (76.1%) still exhibit anti-S1 IgA 6 months after the second dose of the vaccine, as it is usually considered an early marker of humoral immunity against SARS-CoV-2 with a tendency to wane faster than anti-S1 IgG [15]. Anti-S1 IgA may remain an important part of the mucosal first line of defence against SARS-CoV-2 even in the long term, which might explain its persistence in serum.
The correlation between anti-S1 IgG and the SNA reveals another insight: Levels of inhibition sharply increase with increasing anti-S1 IgG only until anti-S1 IgG titres of about 350 binding antibody units (BAU)/mL. After this point a ceiling effect is reached, with inhibition levels reaching nearly 100%. As a consequence of the assay's design, apparently all S1 on the solid phase is bound by anti-S1, which, probably through a combination of higher affinity and concentration, vastly outcompetes the ACE2 in the buffer for binding at S1. On one hand, this demonstrates the limitations of this SNA; on the other hand, as the SNA was modelled after actual plaque reduction neutralization tests [16], it is conceivable that a similar constellation of anti-S1, S1 and ACE2 is reached in vivo at titres of 350 to 400 BAU/mL. The resulting hypothesis that titres above these limits might confer a relatively certain degree of immunity is highly speculative and would have to be tested in a clinical setting.
Almost all participants who had developed anti-NCP IgG in the course of the previous study as a result of a previous infection with SARS-CoV-2 returned a negative result during the current study, suggesting that anti-NCP IgG had waned in the meantime. On the other hand, the majority of positive anti-NCP IgG results were likely false positives. This is most likely explained by the low prevalence of COVID-19 in our cohort and the resulting low pre-test probability. In a low-prevalence population such as our own, even a test with excellent sensitivity and specificity can have a low positive predictive value, leading to a relatively high proportion of false positives. In our experience, previously infected individuals are most reliably identified by the particular dynamics of their antibody response to vaccination, with significantly higher titres of anti-S1 IgG after the first dose of the vaccination. Oligo- or asymptomatic cases of COVID-19 that may lack development of anti-NCP IgG (as was the case for one participant in our cohort) are difficult to detect serologically and may go undetected without PCR testing.
Finally, we did not find any induction of ANA by vaccination against SARS-CoV-2 6 months after the second dose, nor did we see increases in ANA titres in participants with a pre-existing ANA. Therefore, we could not establish any link between SARS-CoV-2 vaccination and autoimmunity (as detected via ANA).
Our study had several limitations. There were obvious imbalances within the cohort, with females and recipients of BNT162b2 being overrepresented. However, because the distribution of age groups between the sexes and the distribution of age groups and sexes between the recipients of the different vaccines was not significantly different, it is fair to assume that the effects we found (e.g. stronger reactions for mRNA-1273) are representative and not caused by skewed distributions. Also, there was no routine PCR testing for SARS-CoV-2 within the cohort. Oligo- or asymptomatic cases of SARS-CoV-2 without development of anti-NCP IgG during or after the vaccination program might therefore have been overlooked. Furthermore, although ANA testing via IIF is a relatively broad screening for autoantibodies within serum, it is possible that the induction of autoantibodies not covered by this assay may have gone unnoticed.
In conclusion, we found that markers of B-cell immunity against SARS-CoV-2 decrease significantly within 6 months after the second vaccination against SARS-CoV-2, with little to no discernible influence of age or sex in our cohort. Despite this, recipients of mRNA-1273 still exhibit higher levels of all examined markers than recipients of BNT162b2 at this time point, continuing a phenomenon that can already be detected after the first dose of the vaccination. At least in vitro, anti-S1 IgG seems to contribute significantly more to the inhibition of binding between S1 and ACE2 than serum anti-S1 IgA. Lastly, we did not see any induction of autoimmunity (as examined via ANA IIF) via vaccination against SARS-CoV-2 6 months after the second dose.
Transparency declaration
RM declares support from the EUROIMMUN AG for attending the 15th Dresden Symposium on autoantibodies 2021. KS, VH, DZ, and CK currently are employees of EUROIMMUN AG. They furthermore declare a planned patent for a method and reagents for the detection of an immune response to SARS-CoV-2. All other coauthors have no conflict of interest to declare.
No external funding was received for this study.
Author contributions
RM, DP, SS, K-P W, BS, FL, and RJ designed the study. RM, JD, SE, JR and SG contributed to recruitment of participants. The described vaccinations were performed under the direction of SG. The assays were performed by RM, DP, KS, VH, DZ, and CK. Data analysis was performed by RM, KS, VH, and DZ. The manuscript was written by RM, with support from all authors. All authors have read and approved the final version of the manuscript.
Acknowledgements
The authors want to express their gratitude towards all colleagues and coworkers who have made this study possible. Special thanks to Mrs. Taylor Legorreta, Mrs. Ingrid Ascha, Mrs. Franziska Peters, Mrs. Dorothea Bachmann, Mrs. Sarah Schultz, Mrs. Silke Lipke-Reinke, Mrs. Astrid Messall, Mrs. Kathrin Johannsen, Mrs. Michaela Seidel, Mrs. Alexandra Jurat, Mrs. Grazyna Wardzinski, Mrs. Sarah Kuckertz, Mrs. Sabine Arp, Mrs. Jana Eicke-Metzenthin, and Mrs. Gesa Schreyer, without whom this project would not have been possible.
Editor: L. Kaiser
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2022.02.028.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fig. S1.
References
- 1.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson M.G., Burgess J.L., Naleway A.L., Tyner H.L., Yoon S.K., Meece J., et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers - eight U.S. locations, December 2020-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:495–500. doi: 10.15585/mmwr.mm7013e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daniel W., Nivet M., Warner J., Podolsky D.K. Early evidence of the effect of SARS-CoV-2 vaccine at one medical center. N Engl J Med. 2021;384:1962–1963. doi: 10.1056/NEJMc2102153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Markewitz R., Pauli D., Dargvainiene J., Steinhagen K., Engel S., Herbst V., et al. The temporal course of T- and B-cell-responses to vaccination with BNT162b2 and mRNA-1273. Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2021.09.006. S1198-743X(21)00496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collier D.A., Ferreira I.A.T.M., Kotagiri P., Datir R.P., Lim E.Y., Touizer E., et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596:417–422. doi: 10.1038/s41586-021-03739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richards N.E., Keshavarz B., Workman L.J., Nelson M.R., Platts-Mills T.A.E., Wilson J.M. Comparison of SARS-CoV-2 antibody response by age among recipients of the BNT162b2 vs the mRNA-1273 vaccine. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.24331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 12.Benjamini Y., Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29:1165–1188. [Google Scholar]
- 13.R Core Team. R . 2020. A language and environment for statistical computing. Vienna, Austria.https://www.R-project.org [Internet]; Available from: [Google Scholar]
- 14.Steensels D., Pierlet N., Penders J., Mesotten D., Heylen L. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA. 2021;326:1533–1535. doi: 10.1001/jama.2021.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sterlin D., Mathian A., Miyara M., Mohr A., Anna F., Claër L., et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med. 2021;13:eabd2223. doi: 10.1126/scitranslmed.abd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.