Abstract
Objectives
Severe COVID-19 is associated with an imbalanced immune response. We hypothesized that patients with enhanced inflammation, as demonstrated by increased levels of certain inflammatory biomarkers, would benefit from interleukin-6 blockage.
Methods
Patients hospitalized with COVID-19, hypoxemia, and at least two of four markedly elevated markers of inflammation (interleukin-6, C-reactive protein, ferritin, and/or D-dimer) were randomized for tocilizumab (TCZ) plus standard of care (SoC) or SoC alone. The primary endpoint was clinical status at day 28 assessed using a seven-category ordinal scale, and the secondary endpoints included intensive care unit admission, respiratory support, and duration of hospital admission.
Results
Clinical status at day 28 was significantly better in patients who received TCZ in addition to SoC compared with those who received SoC alone (p = 0.037). By then, 93% of patients who received TCZ (n = 53 of 57) and 86% of control patients (n = 25 of 29) had been discharged from the hospital. In addition, 47% of TCZ patients (n = 27 of 57) and 24% of control patients (n = 7 of 29) had resumed normal daily activities. The median length of hospitalization was 9 days (interquartile range, 7–12) in the TCZ group and 12 days (interquartile range, 9–15) in the control group (p = 0.014).
Discussion
In patients hospitalized with COVID-19, hypoxemia, and elevated inflammation markers, administration of TCZ in addition to SoC was associated with significantly better clinical recovery by day 28 and a shorter hospitalization compared with SoC alone.
Keywords: COVID-19, Hospitalization, Interleukin-6, SARS-CoV-2, Tocilizumab
Introduction
Severe COVID-19 is associated with an imbalanced host response [1]. Indeed, high serum levels of inflammation markers interleukin (IL) 6, C-reactive protein (CRP), and ferritin correlate with disease severity [[2], [3], [4]]. Severe COVID-19 may be complicated by thromboembolic or immunothrombotic events [[5], [6], [7]], and elevated levels of D-dimer are associated with increased mortality rates [8]. SARS-CoV-2 infection induces immune dysfunction, widespread endothelial injury, and complement-associated coagulopathy in severe cases [9].
Tocilizumab (TCZ), an IL-6 receptor antagonist, has been found effective in the treatment of severe SARS-CoV-2 infection by reducing the likelihood of progression to the composite outcome of mechanical ventilation or death and, when administered within 24 hours of intensive care unit (ICU) admission, by improving outcomes including survival [10,11]. In a meta-analysis, administration of an IL-6 blocker was associated with reduced mortality 28 days after randomization without increased risk of secondary infection [12].
We hypothesized that IL-6 blockage would be beneficial in a subset of patients with enhanced inflammation, as indicated by increased levels of the markers IL-6, CRP, ferritin, and D-dimer.
Methods
Study design
We conducted a prospective, randomized, single-centre, open-label study on the effect of TCZ and standard of care (SoC) versus SoC alone (2:1) in patients hospitalized with COVID-19, respiratory insufficiency, and systemic inflammation fulfilling certain laboratory criteria (COVIDSTORM study–COVID-19: Salvage Tocilizumab as a Rescue Measure, NCT04577534).
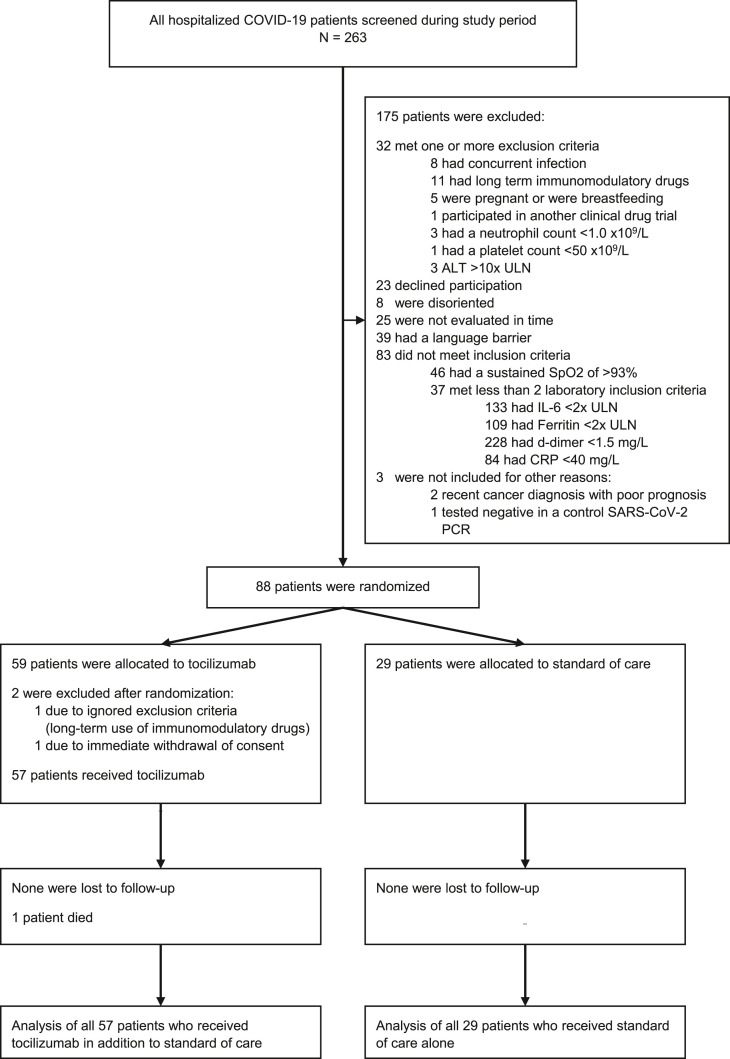
At the request of the REACT working group, an unplanned interim analysis was performed when data of 39 patients were ready to report for a WHO meta-analysis [12]. As the inclusion criteria and mortality rates of our study differed substantially from other studies, our trial was continued even when evidence of mortality benefit in patients receiving TCZ appeared from other trials. The study was prematurely halted owing to 1 month without new patients eligible for inclusion as incidence declined rapidly in June 2021. By then, 263 hospitalized patients were screened, and 88 of these patients were randomized (Fig. 1 ).
Fig. 1.
Consolidated Standard of Reporting Trial flow chart of all screened patients. All patients admitted for COVID-19 were screened for eligibility. Excluded patients may have been excluded for several reasons. Eighty-eight patients were randomized, of whom 59 were allocated to the TCZ and 29 to the SoC group. Two patients in the TCZ group were excluded, one because of immediate withdrawal of informed consent and the other because of an ignored exclusion criterion. Data of all 57 patients who received TCZ and all 29 patients who were allocated to the SoC group were analyzed. ALT: alanine transaminase; CRP: C-reactive protein; IL-6: interleukin 6; SoC: standard of care; TCZ: tocilizumab; ULN: upper limit normal.
Participants
The study was conducted at the Turku University Hospital in South-West Finland from August 12, 2020 to June 16, 2021. Day 1 was the day of randomization. The eligibility criteria included hospitalization due to COVID-19 and hypoxemia. A markedly increased level of at least two of four biomarkers of inflammation was a prerequisite for inclusion in the study. All inclusion and exclusion criteria are presented in Table 1 .
Table 1.
Inclusion and exclusion criteria in COVIDSTORM study
| Inclusion criteria | Exclusion criteria |
|---|---|
|
- Previous severe allergic reaction to monoclonal antibody therapy - Concurrent infection (confirmed or probable) other than COVID-19 - Imminent and inevitable progression to death within 24 hours, irrespective of provision of treatments - Long-term immunomodulatory drugs, including corticosteroids equivalent to >15 mg/d of methylprednisolone - Pregnant or breastfeeding - Participating in other clinical drug trials - Neutrophil count <1.0 × 109/L - Platelet count <50 × 109/L - Alanine transaminase >350 IU/L in women or >500 IU/L in men (10 × ULN) |
ULN: upper limit of normal.
Interventions
At our hospital, SoC did not include antivirals (e.g. remdesivir) or hydroxychloroquine or other experimental treatments, but could include subcutaneous low-molecular weight heparin and glucocorticoids. Patients assigned to the intervention arm received a single infusion of TCZ over 60 minutes immediately after randomization (day 1). Doses were dependent on body weight (400 mg for <60 kg, 600 mg for 60–90 kg, and 800 mg for >90 kg).
Outcomes
The primary endpoint was clinical status at day 28 assessed using a seven-category ordinal scale, where 1 = at home, normal daily activities; 2 = at home, assistance needed; 3 = hospitalized, no supplemental oxygen; 4 = hospitalized (non-ICU), receiving supplemental oxygen; 5 = in ICU, no invasive mechanical ventilation (IMV); 6 = in ICU receiving IMV and/or extracorporeal membrane oxygenation; and 7 = dead. The differentiation between clinical outcome categories one and two was assessed by comprehensive interview and included resumption of professional and nonprofessional routine activities, including ways of commuting. Patients assigned to clinical outcome categories three through seven were still hospitalized, and their categories were determined by the treatment they were receiving on day 28.
The secondary endpoints were incidence of IMV, duration of respiratory support other than supplemental oxygen, incidence and duration of ICU stay, mortality rate on day 28, time to hospital discharge or ready for discharge (as evidenced by normal body temperature, respiratory rate, and stable oxygen saturation on ambient air), and duration of supplemental oxygen treatment.
Sample size
At the onset of this study, no data on TCZ in COVID-19 were yet available. Therefore, sample size calculation was based on expert opinion on how the seven-category clinical status might be distributed after 28 days. Based on a Wilcoxon rank-sum test scenario, among 84 patients (56 for TCZ and 28 for SoC group) the following proportions were assumed for clinical statuses one through seven: 70%, 10%, 0%, 5%, 5%, 5%, and 5% for the TCZ group and 40%, 15%, 0%, 10%, 5%, 10%, and 20% for the SoC group. A statistical power of 80% and significance level of 0.05 (two-tailed) was chosen. The calculations were performed with SAS, version 9.4. Due to the great amount of uncertainty, the chosen sample size was 60 patients for the TCZ group and 30 for the SoC group.
Randomization
Randomization was performed with random permuted blocks in a 2:1 ratio using a block size of six and programmed by a biostatistician with SAS, version 9.4, for Windows. The randomization file was imported to REDCap, and the investigator randomized patients in REDCap after confirming their eligibility for inclusion.
Statistical methods
The primary outcome (clinical status scored 1–7 at 28 days) and length of hospital stay were compared between the groups with a Wilcoxon rank sum test. Duration of hospitalization and ICU stay, length of oxygen supplementation, and IMV treatment were compared between the groups with the Wilcoxon rank sum test. Proportions (ICU, IMV, and noninvasive ventilation) were compared using the Fisher exact test. P-values <0.05 (two-tailed) were considered statistically significant. CRP values (log transformed) were analyzed for the first 7 days from randomization by linear mixed models for repeated measurements. The model included group, time as categorical within factor, and group by time interaction.
Statistical reporting was performed using SAS software, version 9.4 of the SAS System for Windows (SAS Institute Inc., Cary, NC).
Laboratory procedures
The blood samples for biomarker-level analysis were collected on admission and during the first few days in hospital. IL-6 was available for analysis only during weekdays and CRP, ferritin, and D-dimer on any day. Therefore, up to a 72-hour difference was allowed between baseline IL-6 measurement and study day 1. All other inflammation markers were taken on the day of randomization. Serum CRP, plasma ferritin, and D-dimer levels were analyzed at the department of chemistry according to standard procedures, and plasma IL-6 levels were analyzed using a commercial sandwich enzyme immunoassay (Human Interleukin-6 ELISA, BioVendor, Czech Republic) at the department of clinical microbiology of the Turku University Hospital per the manufacturer's instructions. The detection limit was 3.8 ng/L, and values > 5.9 ng/L were considered elevated.
Ethical statement
This study was conducted in accordance with Good Clinical Practice and the Declaration of Helsinki. All randomized study patients signed informed consent for randomization. The study was approved by National Committee on Medical Research Ethics (TUKIJA), Dnro 68/06.00.01/2020 (June 9, 2020) and Hospital District of Southwestern Finland, research approval number T124/2020.
Results
Study population and clinical characteristics at baseline
The Consolidated Standard of Reporting Trial flowchart is presented in Fig. 1. The median National Early Warning Score at randomization was 6, with 1.2% of patients (n = 1 of 86) receiving IMV at randomization. In addition, 5.3% of patients in the TCZ group (n = 3 of 57) and 6.9% of patients in the SoC group (n = 2 of 29) did not receive supplemental oxygen or any other respiratory support at randomization. All patients in the SoC group and 91% of patients in the TCZ group (n = 52 of 57) were receiving glucocorticoids (almost all oral dexamethasone 6 mg daily) at randomization. None were fully vaccinated and only two were partially vaccinated by the time of randomization. There were no clinically meaningful differences in the baseline data between the groups (Table 2 ).
Table 2.
Baseline characteristics of study patients
| Characteristic | Tocilizumab group (n = 57) | Standard of care group (n = 29) | Total (N = 86) |
|---|---|---|---|
| Male sex, n (%) | 34 (59.6) | 14 (48.3) | 48 (55.8) |
| Age (y) | |||
| Mean ± SD | 58.4 ± 14.1 | 58.8 ± 13.7 | 58.5 ± 13.9 |
| Age distribution, n (%) | |||
| 18–64 | 38 (66.7) | 18 (62.1) | 56 (65.1) |
| 65–84 | 18 (31.6) | 11 (37.9) | 29 (33.7) |
| ≥85 | 1 (1.75) | 0 (0) | 1 (1.2) |
| Body mass index (kg/m2), mean ± SD | 33.15 ± 6.4 | 32.8 ± 13.7 | 33.02 ± 6.9 |
| Illness severity by NEWS | |||
| Patients with NEWS assessed, n (%) | 51 (89.5) | 28 (96.6) | 79 (91.9) |
| NEWS, median (range) | 6 (1–12) | 6 (1–9) | |
| NEWS, mean ± SD | 5.9 ± 2.4 | 6 ± 2 | |
| IL-6 (normal range <5.9 ng/L) | |||
| Patients with IL-6 available at randomization, n (%) | 52 (91.2) | 27 (93.1) | 79 (91.8) |
| IL-6 (ng/L), mean ± SD | 73 ± 124 | 53 ± 58 | |
| IL-6 (ng/L), median (range) | 44 (3.75–775) | 34 (3.75–206) | |
| Patients with IL-6 at least 2 × ULN, n (% of tested) | 43 (82.7) | 25 (92.6) | 68 (86.1) |
| CRP at randomization (normal range <11 mg/L) | |||
| Patients with CRP available at randomization, n (%) | 57 (100) | 29 (100) | 86 (100) |
| CRP (mg/L), mean ± SD | 91 ± 55 | 87 ± 49 | |
| CRP (mg/L), median (range) | 84 (5–215) | 97 (7–190) | |
| Patients with CRP >40 mg/L, n (%) | 47 (82.5) | 22 (75.9) | 69 (80.2) |
| Ferritin (normal range in men <400 μg/L; women <150 μg/L) | |||
| Patients with ferritin available at randomization, n (%) | 57 (100) | 28 (96.5) | 85 (98.8) |
| Ferritin (μg/L), mean ± SD | 1036 ± 881 | 1067 ± 837 | |
| Ferritin (μg/L), median (range) | 829 (18–4199) | 924 (89–3652) | |
| Patients with ferritin at least 2 × ULN, n (% of tested) | 45 (78.9) | 22 (78.6) | 67 (78.8) |
| D-dimer (normal range <0.5 mg/L) | |||
| Patients with D-dimer available at randomization, n (%) | 57 (100) | 28 (96.6) | 85 (98.8) |
| D-dimer (mg/L), mean ± SD | 0.5 ± 0.4 | 0.5 ± 0.6 | |
| D-dimer (mg/L), median (range) | 0.3 (0.2–2.3) | 0.25 (0.2–2.5) | |
| Patients with D-dimer >1.5 mg/L, n (% of tested) | 2 (3.5) | 2 (7.1) | 4 (4.7) |
| Treatments at randomization, n (%) of patients | |||
| Low-flow (≤15 L/min) oxygen treatment | 38 (67) | 21 (72) | |
| High-flow (>15 L/min) oxygen treatment | 12 (21) | 4 (14) | |
| Noninvasive ventilation | 4 (7) | 0 (0) | |
| Invasive mechanical ventilation | 0 (0) | 1 (3.4) | 1 (1.2) |
| Glucocorticoid treatment at randomization | 52 (91) | 29 (100) | 81 (94) |
| Frequency of reported symptoms at time of admission, n (%) | |||
| Fever | 54 (94.7) | 24 (82.8) | 78 (90.8) |
| Cough | 38 (66.7) | 20 (69.0) | 58 (67.44) |
| Shortness of breath | 41 (71.9) | 22 (75.9) | 63 (73.2) |
| Chest pain | 12 (21.1) | 3 (10.3) | 15 (17.4) |
| Myalgia | 11 (19.3) | 10 (34.5) | 21 (24.4) |
| Headache | 14 (24.6) | 8 (27.6) | 22 (25.6) |
| Loss of smell and/or taste | 4 (7.0) | 3 (10.3) | 7 (8.1) |
| Fatigue | 44 (77.2) | 22 (75.9) | 66 (76.7) |
| Other neurological symptoms | 7 (12.3) | 3 (10.3) | 10 (11.6) |
| Diarrhoea | 15 (26.3) | 6 (20.7) | 21 (24.4) |
| Vomiting | 8 (14.0) | 6 (20.7) | 14 (16.3) |
| Time from onset of symptoms | |||
| Time from onset of symptoms (d), mean | 10.6 | 10.9 | |
| Time from onset of symptoms (d), median (range) | 10 (4–18) | 10 (4–18) | |
| Underlying conditions and comorbidities, n (%) | |||
| ≥1 diagnosis | 47 (82.5) | 24 (82.7) | 71 (82.5) |
| Obesity (body mass index ≥30 kg/m2) | 34 (60.7) | 20 (69.0) | 54 (63.5) |
| Diabetes mellitus | 15 (26.3) | 6 (20.7) | 21 (24.4) |
| Hypertension | 22 (38.6) | 10 (34.5) | 32 (37.2) |
| Atherosclerosis | 7 (12.3) | 2 (6.9) | 9 (10.7) |
| Chronic heart failure | 4 (7.0) | 1 (3.5) | 5 (5.81) |
| Asthma | 9 (15.8) | 3 (10.3) | 12 (14.0) |
| Chronic obstructive pulmonary disease | 2 (3.5) | 1 (3.5) | 3 (3.5) |
| Obstructive sleep apnoea | 9 (15.8) | 8 (27.6) | 17 (19.77) |
| Malignancy (treated or untreated) | 6 (10.5) | 4 (13.8) | 10 (11.6) |
| Patient independent on daily activities | 56 (98.2) | 28 (96.5) | 84 (97.7) |
| Preexisting do-not-resuscitate order in place | 5 (8.8) | 5 (17.2) | 10 (11.6) |
CRP, C-reactive protein; IL-6, interleukin 6; NEWS, National Early Warning Score; SD: standard deviation.
Primary endpoint
Clinical status on day 28, as measured by the seven-category scale, was significantly better in patients who received TCZ in comparison with those who received SoC (p = 0.037). By then, 93% of patients in the TCZ group (n = 53 of 57) and 86% in the SoC group (n = 25 of 29) had been discharged from the hospital. Moreover, 47% of patients in the TCZ group (n = 27 of 57) and 24% of patients in the SoC group (n = 7 of 29) had resumed normal daily activities.
One patient in the TCZ group and none in the SoC group had died. A 79-year-old patient with atrial fibrillation and type 2 diabetes as underlying conditions had a do-not-resuscitate decision before randomization according to her own request. She died on day 27 without evidence of secondary infections or thromboembolic complications. An autopsy was not performed.
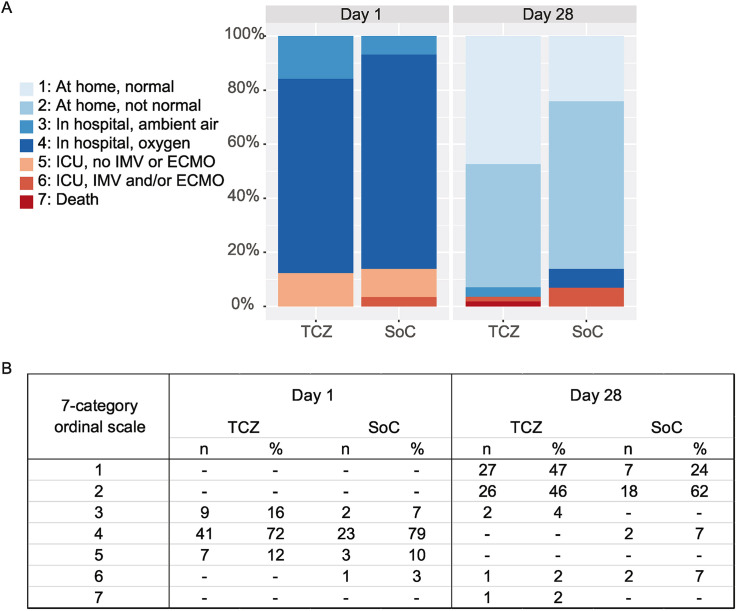
The primary endpoint is presented in Fig. 2 .
Fig. 2.
Clinical outcome. (A and B) Clinical status of patients as assessed on a seven-category ordinal scale at randomization (day 1) and day 28. At randomization, all patients were hospitalized, and seven patients in the TCZ group (12.3%) and four patients in the SoC group (13.8%) were in the intensive care unit. On day 28, 53 patients in the TCZ group (93.0%) and 25 patients in the SoC group (86.2%) were discharged to home. On day 28, one patient in the TCZ group (1.8%) and no patients in the SoC group had died. CIs of the primary endpoint for each category in the TCZ and SoC groups are as follows: 1: 0.35–0.60, 2: 0.33–0.58, 3: 0.01–0.12, 6: 0.003–0.09, and 7: 0.003–0.09, and 1: 0.12–0.42, 2: 0.44–0.77, 4: 0.02–0.22, and 6: 0.02–0.22, respectively. CIs of hospital stays are 8 to 11 in the TCZ group and 10 to 15 in the SoC group. Categories on the ordinal scale were as follows: 1: at home, normal daily activities; 2: at home, assistance needed; 3: hospitalized, no supplemental oxygen; 4: hospitalized, receiving supplemental oxygen; 5: in ICU, no IMV or ECMO; 6: in ICU, receiving IMV; and 7: dead. ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; IMV, invasive mechanical ventilation; SoC, standard of care; TCZ, tocilizumab.
Secondary endpoints
The median duration of hospital admission was 9 days (interquartile range, 7–12 days) for patients receiving TCZ and 12 days (interquartile range, 9–15 days) for the control group (p = 0.014). Incidence of ICU treatment was 8.0% (n = 4 of 50) in the TCZ group and 16.0 % (n = 4 of 25) in the SoC group (p = 0.43), and the median duration of ICU stay was 6 days (interquartile range, 4–12 days) and 5 days (interquartile range, 3.5–24 days), respectively. The incidence of IMV treatment was 8.8% (n = 5 of 57) in the TCZ group and 10.8% (n = 3 of 28) in the SoC group (not significant). None of the patients received extracorporeal membrane oxygenation or renal replacement therapy.
The median duration of respiratory support other than supplemental oxygen alone during the treatment period at the Turku University Hospital among all patients (n = 86) was 5 days (interquartile range, 4–10 days) in the TCZ group and 4 days (interquartile range, 3–7 days) in the SoC group. The median duration of any oxygen supply was 6 days (interquartile range, 3–8 days) in the TCZ group and 6.5 days (interquartile range, 3.5–10 days) in the SoC group during admission. In addition, 24% of patients (n = 21 of 86) were referred to a local hospital or health centre for continuation of inpatient treatment. For these patients, data on supplemental oxygen were only available from the primary centre. Secondary outcomes are summarized in Table 3 .
Table 3.
Secondary endpoints in study patients during hospitalization (Turku University Hospital, Finland)
| Tocilizumab group (n = 57) | Standard-of-care group (n = 29) | p-value | |
|---|---|---|---|
| Hospitalization (d), median (interquartile range) | 9 (7–12) | 12 (9–15) | 0.014 |
| Oxygen supplementation (d), median (interquartile range) | 6 (3–8) | 6.5 (3.5–10) | 0.54 |
| Incidence of treatment in ICU in patients not in ICU at baseline, n (%) | 4 of 50 (8.0) | 4 of 25 (16.0) | 0.43 |
| Duration of ICU stay (d), median (interquartile range)a | 6 (4–12) n = 11 (19.3%) | 5 (3.5–24) n = 8 (27.6%) | 0.54 |
| Incidence of IMV in patients not on IMV at baseline, n (%) | 5 of 57 (8.8) | 3 of 28 (10.7) | 1.0 |
| Duration of IMVb (d), median (interquartile range) | 11 (10–19) n = 5 | 20.5 (10–29.5) n = 4 | 0.42 |
| Death at day 28, n (%) | 1 (1.8) | 0 (0) | N/A |
ICU, intensive care unit; IMV, invasive mechanical ventilation; N/A, not applicable.
Calculated only among patients admitted to ICU.
Calculated only among patients who were intubated.
Other exploratory data
Levels of inflammatory markers were routinely monitored for clinical purposes in all patients. In all patients receiving TCZ, CRP levels decreased rapidly and steadily over 1 week's time and were significantly lower compared with the control group from day 3 onward (Fig. 3 and S1 A and B). This was not observed for IL-6, ferritin, or D-dimer (data not shown).
Fig. 3.
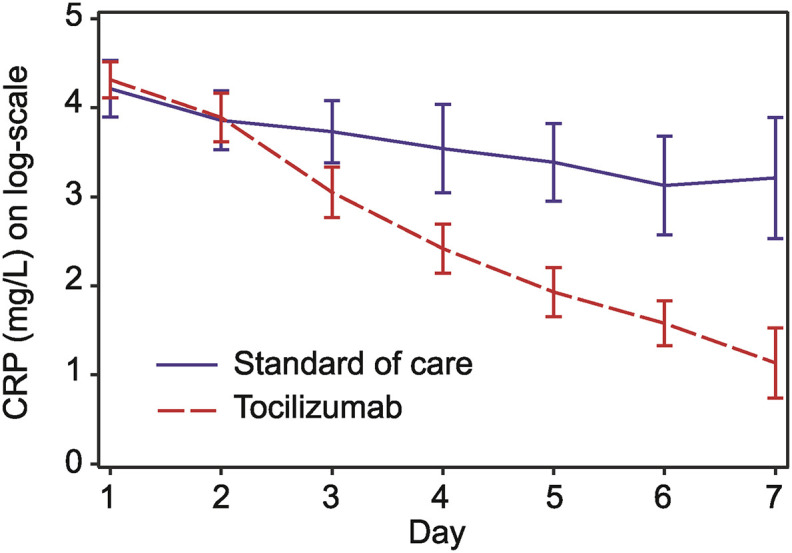
C-reactive protein (CRP) in the first week after randomization. CRP values (log transformed) during the first 7 days from randomization by linear mixed models for repeated measurements. The model included group, time as categorical within factor, and group by time interaction.
Safety data
Severe adverse events were recorded in three patients. One patient in the SoC group had a probable secondary bacterial infection during ICU stay, and one patient in TCZ group had bacteraemia on day 24. One patient in the TCZ group died, as noted earlier.
Discussion
In our randomized study, patients receiving TCZ in addition to SoC displayed a significantly better clinical recovery by day 28 compared with patients receiving SoC alone. Furthermore, administration of TCZ was associated with shorter hospitalization. CRP values decreased rapidly and consistently after administration of TCZ.
Previous randomized trials indicate that TCZ improves survival, reduces incidence of IMV, and may lead to shorter admission for patients hospitalized with COVID-19 [[12], [13], [14]]. In the WHO meta-analysis, TCZ was associated with a significant reduction in mortality (summary OR: 0.83; 95% CI, 0.74–0.92), which was even stronger in patients receiving corticosteroids (summary OR: 0.77; 95% CI, 0.68–0.87) [12]. In another meta-analysis of randomized studies on TCZ use, the 1-month mortality rate was 24.5% in the TCZ group and 29.1% in the control group [15]. Importantly, administration of TCZ was not associated with a higher risk of secondary infections or other adverse events [12,15,16].
In the COVACTA trial, which had nearly identical primary study endpoints as our study, clinical status at day 28 was similar between the TCZ and placebo groups [13]. However, symptom duration before inclusion and levels of CRP and IL-6 varied much more than in our study [13]. The more homogenous selection of patients might explain why a clinical benefit was detected in the primary endpoint of our study.
The overall mortality rate was 32.8% (n = 1350 of 4116) in the RECOVERY trial and 19.6% (n = 86 of 438) in the COVACTA trial [13,14]. With an overall mortality of 1.2% (n = 1 of 86), our trial differs from other published TCZ trials. Glucocorticoids were used in 94% of patients in our study (n = 81 of 86), and the corresponding number was 82% (n = 3385 of 4116) in the RECOVERY trial and 22% (n = 98 of 438) in the COVACTA trial. The differences in patient selection and concomitant glucocorticoids may explain the substantial differences in mortality, which also may be related to the low case-fatality rate in Finland. In South-West Finland, the case-fatality rate was 0.5% (n = 50 of 9946) by August 28, 2021 (approximately a third of that in the United States), the ICU admission rate was 15.0% among hospitalized patients, and mortality among ICU patients was 9.0% (unpublished data). The influence of vaccination was marginal, because none of the patients were fully vaccinated and 2.3% (n = 2 of 86) were partially vaccinated against COVID-19.
A study using a different set of inflammation markers did not observe any clinical benefit of IL-6 blockage, but the study was not comparable to ours in terms of severity of COVID-19 [17]. As far as we are aware, no other TCZ trials have used a set of inflammation markers as inclusion criteria. In our, albeit relatively small, study, biomarker-guided administration of TCZ is associated with significant clinical outcome benefits. In the RECOVERY trial, CRP >75 mg/L was among the inclusion criteria, next to low oxygen saturation <92% [14]. An exploratory analysis of the data from the COVACTA trial showed that in the subgroup of patients with elevated levels of ferritin, TCZ decreased the probability of death, mechanical ventilation, and worsening clinical status at day 28 compared with placebo [18]. Together with these findings, our results suggest that laboratory markers of inflammation may help direct TCZ to patients who would benefit the most. For definite conclusions, a noninferiority study of TCZ versus SoC should be performed to investigate whether TCZ can safely be omitted in patients with respiratory insufficiency but low levels of laboratory markers of inflammation. More studies are needed to optimize the set of biomarkers and their levels to select patients who are the most eligible for treatment with TCZ.
The administration of glucocorticoids is now one of the cornerstones of inflammation-suppressing treatment of severe COVID-19; thus, the results from early studies with IL-6 blockers without glucocorticoid treatment should be interpreted with caution.
Our study has several limitations. Compromised health care services and shortness of clinical staff was anticipated early in the pandemic; thus, we decided to perform this study without placebo or blinding, despite the risk of bias. Of the screened patients, only 33% were included. The high rate of screening failure was partly caused by the screening procedure, because all patients hospitalized with COVID-19 were screened for eligibility in the study. Language barrier was a frequent reason for exclusion, because the COVID-19-pandemic disproportionally affected ethnic minorities [19,20]. For our study, informed consent could only be acquired in Finnish, Swedish, or English. The underrepresentation of ethnic minorities is of concern, because they are overrepresented in disease demographics. For future studies, availability of a range of translations of study information should be accounted for, especially when migrant populations are at risk.
The COVID-19 pandemic has proved to be highly dynamic, influenced not only by viral factors, such as the introduction of new variants, but also by the introduction of vaccines, new drugs, and nonpharmaceutical interventions, which each may affect risk factors, risk populations, and clinical outcome differently. Our study contributes to the mounting evidence supporting a role for IL-6 blockage in patients hospitalized for COVID-19, but the role of established treatments should be questioned as vaccination programs roll out, new drugs become available, and new viral variants appear.
In conclusion, our study shows that addition of TCZ to SoC in hospitalized patients is associated with improved clinical recovery by day 28 and shorter hospitalization. Our study differs from others in the selection of patients eligible for treatment with TCZ based on a combination of laboratory markers of inflammation.
Transparency declaration
N. Broman reports receiving funding from University of Turku. T. Feuth reports receiving compensation for a lecture outside the submitted work from GlaxoSmithKline. U. Hohenthal reports receiving compensation for serving on the advisory board outside the submitted work from GlaxoSmithKline and congress travel from Grifols Nordic, MSD-Finland, and Pfizer. P. Jalava-Karvinen reports receiving compensation for serving on the advisory board outside the submitted from GlaxoSmithKline and MSD-Finland; travel grants from Gilead, MSD, and Pfizer; and stock ownership of Orion. H. Marttila reports receiving compensations for lectures, travel grants, or advisory boards outside the submitted work from MSD Finland, Pfizer, Immunodiagnostic, and Roche Diagnostics. J. Oksi reports receiving compensations for lectures or advisory boards outside the submitted work from Biocodex, Gilead, GlaxoSmithKline, MSD-Finland, Orion, and Roche. All other authors declare no conflicts of interest. No external funding was received for this study or article.
Author contributions
Conceptualization and design of the study: NB, TF, TV, EL, and JO. Methodology: NB, TF, TV, EL, and JO. Acquisition of the data and care of the patients: NB, TF, MV, UH, TH, PJK, HM, MN, and JO. Analysis and interpretation of data: NB, TF, TV, MV, EL, and JO. Software and validation: NB, TF, and EL. Drafting of the original version: NB, TF, EL, and JO. Drafting, critical revising, and editing: all authors. Visualization: NB, TF, EL, and JO. Supervision: TV and JO.
Acknowledgements
The authors are grateful to all patients for their collaboration. The authors thank all clinical and nursing staff who cared for the patients. The authors also thank Henri Jegoroff for advice regarding the RedCap system.
Editor: M. Paul
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2022.02.027.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Blanco-Melo D., Nilsson-Payant B.E., Liu W., Uhl S., Hoagland D., Møller R., et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045.e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broman N., Rantasarkka K., Feuth T., Valtonen M., Waris M., Hohenthal U., et al. IL-6 and other biomarkers as predictors of severity in COVID-19. Ann Med. 2021;53:410–412. doi: 10.1080/07853890.2020.1840621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stringer D., Braude P., Myint P.K., Evans L., Collins J.T., Verduri A., et al. The role of C-reactive protein as a prognostic marker in COVID-19. Int J Epidemiol. 2021;50:420–429. doi: 10.1093/ije/dyab012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gandini O., Criniti A., Ballesio L., Giglio S., Galardo G., Gianni W., et al. Serum ferritin is an independent risk factor for acute respiratory distress syndrome in COVID-19. J Infect. 2020;81:979–997. doi: 10.1016/j.jinf.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicolai L., Leunig A., Brambs S., Kaiser R., Joppich M., Hoffknecht M.L., et al. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020;142:1176–1189. doi: 10.1161/CIRCULATIONAHA.120.048488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L., Yan X., Fan Q., Liu H., Liu X., Liu Z., et al. D-dimer levels on admission to predict in-hospital mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perico L., Benigni A., Casiraghi F., Ng L.F.P., Renia L., Remuzzi G. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat Rev Nephrol. 2020;17:1–19. doi: 10.1038/s41581-020-00357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.REMAP-CAP Investigators. Gordon A.C., Mouncey P.R., Al-Beidh F., Rowan K.M., Nichol A.D., et al. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salama C., Han J., Yau L., Reiss W.G., Kramer B., Neidhart J.D., et al. Tocilizumab in patients hospitalized with COVID-19 pneumonia. N Engl J Med. 2021;384:20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Shankar-Hari M., Vale C.L., Godolphin P.J., Fisher D., Higgins J.P.T., Spiga F., et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA. 2021;326:499–518. doi: 10.1001/jama.2021.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosas I.O., Bräu N., Waters M., Go R.C., Hunter B.D., Bhagani S., et al. Tocilizumab in hospitalized patients with severe COVID-19 pneumonia. N Engl J Med. 2021;384:1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.RECOVERY Collaborative Group Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klopfenstein T., Gendrin V., Gerazime A., Conrozier T., Balblanc J.C., Royer P.Y., et al. Systematic review and subgroup meta-analysis of randomized trials to determine tocilizumab's place in COVID-19 pneumonia. Infect Dis Ther. 2021;10:1195–1213. doi: 10.1007/s40121-021-00488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tleyjeh I.M., Kashour Z., Damlaj M., Riaz M., Tlayjeh H., Altannir M., et al. Efficacy and safety of tocilizumab in COVID-19 patients: a living systematic review and meta-analysis. Clin Microbiol Infect. 2021;27:215–227. doi: 10.1016/j.cmi.2020.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Declercq J., Van Damme K.F.A., De Leeuw E., Maes B., Bosteels C., Tavernier S.J., et al. Effect of anti-interleukin drugs in patients with COVID-19 and signs of cytokine release syndrome (COV-AID): a factorial, randomised, controlled trial. Lancet Respir Med. 2021;9:1427–1438. doi: 10.1016/S2213-2600(21)00377-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tom J., Bao M., Tsai L., Qamra A., Summers D., Carrasco-Triguero M., et al. Prognostic and predictive biomarkers in patients with coronavirus disease 2019 treated with tocilizumab in a randomized controlled trial. Crit Care Med. 2022;50:398–409. doi: 10.1097/CCM.0000000000005229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans M.K. COVID's color line–Infectious disease, inequity, and racial justice. N Engl J Med. 2020;383:408–410. doi: 10.1056/NEJMp2019445. [DOI] [PubMed] [Google Scholar]
- 20.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.