Abstract
We aimed to determine the risk of incident cancer in autoimmune hepatitis (AIH) compared with the general population and siblings. AIH was defined by the presence of a medical diagnosis of AIH and results of examination of a liver biopsy specimen in a nationwide Swedish population-based cohort study. We identified 5,268 adults with AIH diagnosed during 1969–2016 and 22,996 matched, general population, reference individuals and 4,170 sibling comparators. Using Cox regression, hazard ratios were determined for any incident cancer, and subtypes were determined from the Swedish Cancer Register. During follow-up, a cancer diagnosis was made in 1,119 individuals with AIH (17.2 per 1,000 person-years) and 4,450 reference individuals (12.0 per 1,000 person-years). This corresponded to a hazard ratio of 1.53 (95% confidence interval: 1.42, 1.66). Cancer risk was highest in those with cirrhosis. There was a 29.18-fold increased risk of hepatocellular carcinoma (HCC) (95% confidence interval: 17.52, 48.61). The annual incidence risk of HCC in individuals with AIH who had cirrhosis was 1.1% per year. AIH was also linked to nonmelanoma skin cancer (hazard ratio (HR) = 2.69) and lymphoma (HR = 1.89). Sibling analyses yielded similar risk estimates for any cancer (HR = 1.84) and HCC (HR = 23.10). AIH is associated with an increased risk of any cancer, in particular, HCC and extrahepatic malignancies. The highest risk for cancer, especially HCC, is in patients with cirrhosis.
Keywords: cirrhosis, hepatitis, hepatocellular carcinoma, histopathology, malignancy, population-based
Abbreviations:
- AIH
autoimmune hepatitis
- CI
confidence interval
- ESPRESSO
Epidemiology Strengthened by Histopathology Reports in Sweden
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- ICD
International Classification of Disease
- PBC
primary biliary cholangitis
- PSC
primary sclerosing cholangitis
Autoimmune hepatitis (AIH) is a chronic inflammatory liver disease that can progress to cirrhosis and liver failure (1, 2). Chronic inflammation and immune dysregulation characteristic of autoimmune diseases are known to have protumorigenic effects (3, 4). However, the risks of both incident hepatic and extrahepatic cancers in individuals with AIH with and without cirrhosis remain unclear (5–11) due to small sample size, limitation to tertiary referral centers, and lack of comparison groups.
In this study, we aimed to define the risk of incident cancer in individuals with AIH compared with the general population and siblings using a large, nationwide, population-based cohort with liver histopathology report data.
METHODS
Histopathology report data from the Epidemiology Strengthened by Histopathology Reports in Sweden (ESPRESSO) Cohort from 1965 through 2016 were obtained from all 28 pathology departments in Sweden (12). Through a unique personal identity number assigned to all Swedish residents, histopathology report data were linked to clinical data in the Swedish National Registers (13).
Individuals with AIH
Individuals were included if they were adults at least 18 years old and had a diagnosis of AIH, defined as the presence of an International Classification of Disease (ICD) code for AIH and liver histopathology report data between January 1, 1969, and December 31, 2016 (Figure 1, Web Table1 (available at https://doi.org/10.1093/aje/kwab119)). Inpatient and hospital-based outpatient diagnoses (available from 2001 onward) were included. Liver biopsy could occur before or after ICD coding, and the date of AIH diagnosis in this study was defined as the second of either liver biopsy date or ICD diagnosis date. Using the presence of ICD codes for AIH and liver biopsy, we identified 9,926 individuals (Figure 1).
Figure 1.
Flow chart of identified individuals with autoimmune hepatitis and their matched general-population reference individuals, Sweden 1969–2016. AIH, autoimmune hepatitis; CHF, congestive heart failure; NAFLD, nonalcoholic fatty liver disease.
To further reduce AIH misclassification, individuals were excluded if they had an ICD diagnosis for other chronic liver diseases (Figure 1, Web Table1). Individuals were also excluded if they had a liver transplant before or at the time of liver biopsy, had not lived in or had formally emigrated from Sweden in the last 5 years, had less than 3 months of follow-up, or had data irregularities (Figure 1). To ensure that incident cases of cancer were identified, individuals with any cancer codes at or before the start of follow-up were excluded. On the basis of these criteria, 4,660 individuals with an initial AIH diagnosis were excluded (Figure 1).
To further assess our diagnostic criteria, we reviewed 100 random histopathology free-text reports of individuals who met our criteria for AIH from the full study period. Of the 100 reports, 96 had sufficient information to be evaluated. Of these 96, 92 had a histopathology report consistent with AIH, resulting in a 96% positive predictive value (95% confidence interval (CI): 89, 99). We also determined that 82 of 100 random individuals in our cohort had at least 2 ICD codes for AIH (82%; 95% CI: 72, 88).
Population reference individuals and siblings
Five general-population reference individuals were matched to each individual with AIH according to age, sex, county, and calendar year. The same exclusion criteria for individuals with AIH were applied to reference individuals (Figure 1). In sensitivity analyses, all available full siblings were used as comparators and the same exclusion criteria were applied.
Histopathology report data
Histopathology report data were available for each individual in the ESPRESSO cohort. As mutually exclusive categories, stage F4 fibrosis or cirrhosis, stage F1–F3 fibrosis, inflammation without fibrosis, and other histopathologically determined stages were defined using Systemized Nomenclature of Medicine codes that use the Batts-Ludwig criteria (Web Table 2) (14). Liver necrosis was not mutually exclusive (Web Table 2). Few individuals had data from a repeated liver biopsy; thus, these data were not used.
Outcomes
The primary outcome was defined as any incident cancer determined from the Cancer Register (Web Table 3) (15). If a patient had multiple cancers, then the first cancer was counted. Any cancer was divided into the following subgroups as secondary outcomes: any hepatobiliary cancer, hepatocellular carcinoma (HCC), biliary cancers, extrahepatic cancer, extrahepatic solid-organ cancers, and extrahepatic hematologic cancers (Web Table 3). Biliary cancers included intra- and extrahepatic cholangiocarcinoma, cancers of the ampulla of Vater, and unspecified biliary tract cancers. Specific subtypes of extrahepatic solid organ cancers were further examined on the basis of their high frequency or known association with AIH, including all gastrointestinal organs (including esophageal, stomach, small intestine, colon, rectal, anal, and pancreatic cancers), colon, nonmelanoma skin, melanoma, breast, and lung cancers (8, 11). Specific subtypes of extrahepatic hematologic cancers studied included lymphoma, leukemia, and multiple myeloma. Secondary outcomes also included cancer-related death and HCC-related death determined from the Cause of Death Register (16).
Statistical analyses
Main analyses.
Kaplan-Meier failure curves with the first incidence of cancer, cancer-related death, and HCC-related death were determined. The incidence rate, 10- and 20-year cumulative incidences, and absolute-risk differences were calculated and reported with a 95% confidence interval. We also determined, by fibrosis status, the incident risk per year (annual incidence) of HCC in individuals with AIH.
In the main analyses and subanalyses, we first performed Cox regression adjusted for age, sex, county, and calendar period to determine hazard ratios of cancer outcomes in individuals with AIH compared with reference individuals. We then additionally adjusted for education, country of birth, family history of cancer, cardiovascular disease, diabetes, end-stage renal disease, other autoimmune disease, and alcohol-use disorder after AIH diagnosis, and all hazard ratios reported in the Results section are from this additionally adjusted Cox regression. Covariates met the proportional hazards assumption. See Web Table 4 for covariate ICD codes.
In all main analyses, follow-up was started at 3 months after diagnosis date, and individuals were censored if the cancer outcome of interest developed, or they died, emigrated, or reached the last follow-up date of December 31, 2016. We excluded patients with no follow-up to less than 3 months of follow-up to reduce misclassification and avoid liver biopsies performed at the time of cancer diagnosis or autopsy. If a reference individual or sibling received an ICD code for AIH and a liver biopsy, they were censored and recategorized as individuals with AIH. Statistical significance was defined as 95% confidence intervals for risk estimates that did not include 1.0. SAS, version 9.4 (SAS Institute Inc.) statistical software was used to perform all analyses.
Subanalyses.
In prespecified subanalyses, we stratified patients by follow-up time, sex, age at diagnosis, year of diagnosis, country of birth, and education (17). We also stratified patients by fibrosis status, portal hypertension, acute or subacute liver failure, overlap syndromes with primary biliary cholangitis (PBC) or primary sclerosing cholangitis (PSC), family history of cancer in first-degree relatives (aged ≤50 and > 50 years), and comorbid conditions including cardiovascular disease, diabetes, end-stage renal disease, and other autoimmune diseases (Web Table 4); these analyses are key to the study of AIH. We also repeated stratified analyses within a cohort limited exclusively to persons with AIH.
Sensitivity analyses
We performed several sensitivity analyses for any cancer. First, we repeated Cox regression analyses after including the first 3 months of follow-up. Second, we repeated analyses in which patients with PSC or PBC were excluded from the cohort at baseline. Third, we repeated analyses in which liver transplantation was also censored (18). Fourth, we restricted analyses to individuals diagnosed with AIH from 1997 through 2016 to coincide with more specific ICD codes for AIH and viral hepatitis. Fifth, to avoid the contribution of differential follow-up in later years of diagnosis and different age groups and to increase comparability, we restricted analyses to 5-year follow-up in calendar-period and age-group subanalyses. Sixth, we restricted the time between AIH ICD code and liver biopsy to less than 1 year to confirm that liver biopsy was performed as part of AIH diagnosis. Seventh, because of concerns that HCC may be underreported in the Cancer Register (19), we repeated analyses on the risk of HCC by defining HCC from both the National Patient Register and Cancer Register. Eighth, we repeated analyses in which we additionally defined cirrhosis using ICD codes as a time-varying variable to recategorize patients without cirrhosis at time of diagnosis as having cirrhosis if they acquired an ICD code for cirrhosis during follow-up. Ninth, we compared AIH diagnosed when a patient was a hospital inpatient versus when a patient was an outpatient from 2002 onward after hospital-based outpatient diagnoses were available. Tenth, we determined baseline characteristics of individuals with AIH with sibling comparators and repeated Cox regression analyses to account for intrafamilial confounding due to genetics and environmental and socioeconomic conditions that tend to be shared within families.
Ethics
The ESPRESSO cohort was reviewed by the Stockholm Ethics Board (no. 2014/1287–31/4) approved on August 27, 2014.
RESULTS
The final cohort consisted of 5,268 individuals with AIH and 22,996 general-population reference individuals (Figure 1). The mean age of individuals with AIH was 52.3 years and 59.1% (n = 3,115) were female (Table 1). The median follow-up time was 9.3 (interquartile range, 4.0–20.2) years, with a follow-up time of at least 10 years in 47.5% of patients (n = 2,504). The median time between ICD coding and liver biopsy was 0.2 (range, 0.0–2.5) years. Examination of liver biopsy specimens revealed 13.9% of persons with AIH had cirrhosis (n = 733), 21.1% had fibrosis (n = 1,110), 35.0% had inflammation without fibrosis (n = 1,842), and 30.0% (n = 1,583) had other pathology findings; 2.4% had hepatic necrosis (n = 129) (Table 1).
Table 1.
Characteristics of Patients With Autoimmune Hepatitis and Matched General-Population Reference Individuals, Sweden 1969–2016
| Group | Individuals With AIH ( n = 5,268) | Reference Individuals ( n = 22,996) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | Mean (SD) | Median (IQR) | Range | No. | % | Mean (SD) | Median (IQR) | Range | |
| Follow-up | 12.4 (9.9) | 9.3 (4.0, 20.2) | 0.3–41.6 | 16.2 (10.7) | 15.0 (6.3, 25.1) | 0.3–46.2 | ||||
| 3–11 months | 5,268 | 100 | 22,996 | 100 | ||||||
| 1.0–4.9 years | 4,918 | 93.4 | 22,351 | 97.2 | ||||||
| 5.0–9.9 years | 3,631 | 68.9 | 18,428 | 80.1 | ||||||
| ≥10.0 years | 2,504 | 47.5 | 14,273 | 62.1 | ||||||
| Sex | ||||||||||
| Female | 3,115 | 59.1 | 13,278 | 57.7 | ||||||
| Male | 2,153 | 40.9 | 9,718 | 42.3 | ||||||
| Age, years | 52.3 (16.3) | 53.8 (40.0, 65.3) | 18.1–90.6 | 51.7 (16.1) | 52.9 (39.6, 64.4) | 18.0–90.9 | ||||
| 18.0–29.9 | 594 | 11.3 | 2,702 | 11.7 | ||||||
| 30.0–39.9 | 722 | 13.7 | 3,242 | 14.1 | ||||||
| 40.0–49.9 | 909 | 17.3 | 4,146 | 18.0 | ||||||
| 50.0–59.9 | 1,103 | 20.9 | 4,896 | 21.3 | ||||||
| 60.0–69.9 | 1,150 | 21.8 | 4,884 | 21.2 | ||||||
| ≥70.0 | 790 | 15.0 | 3,126 | 13.6 | ||||||
| Year of diagnosis | ||||||||||
| 1969–1986 | 1,831 | 34.8 | 8,494 | 36.9 | ||||||
| 1987–2001 | 1,751 | 33.2 | 7,703 | 33.5 | ||||||
| 2002–2016 | 1,686 | 32.0 | 6,799 | 29.6 | ||||||
| Time to register-based definition of AIHa | 2.7 (5.2) | 0.2 (0.0, 2.5) | 0.0–45.5 | |||||||
| Country of birth | ||||||||||
| Nordic | 4,962 | 94.2 | 21,570 | 93.8 | ||||||
| Other | 304 | 5.8 | 1,424 | 6.2 | ||||||
| Missing | 2 | 0.0 | 2 | 0.0 | ||||||
| Level of education, years | ||||||||||
| ≤9 | 1,646 | 31.2 | 7,911 | 34.4 | ||||||
| 10–12 | 1,923 | 36.5 | 8,430 | 36.7 | ||||||
| >12 | 1,042 | 19.8 | 5,003 | 21.8 | ||||||
| Missing | 657 | 12.5 | 1,652 | 7.2 | ||||||
| Level of education, yearsb | ||||||||||
| ≤9 | 1,679 | 31.9 | 7,938 | 34.5 | ||||||
| 10–12 | 1,932 | 36.7 | 8,450 | 36.7 | ||||||
| >12 | 1,050 | 19.9 | 5,010 | 21.8 | ||||||
| Missing | 607 | 11.5 | 1,598 | 6.9 | ||||||
| Pathology findings | ||||||||||
| Cirrhosis | 733 | 13.9 | ||||||||
| Fibrosis | 1,110 | 21.1 | ||||||||
| Inflammation, no fibrosis | 1,842 | 35.0 | ||||||||
| Other | 1,583 | 30.0 | ||||||||
| Necrosis | 129 | 2.4 | ||||||||
| Severity of liver disease | ||||||||||
| Portal hypertension | 263 | 5.0 | ||||||||
| Acute/subacute liver failure | 139 | 2.6 | ||||||||
| AUD during follow-up | 493 | 9.4 | 572 | 2.5 | ||||||
| Overlap syndromes | ||||||||||
| PSC | 84 | 1.6 | ||||||||
| PBC | 201 | 3.8 | ||||||||
| Family history of cancer in first-degree relative | ||||||||||
| Overall | 1,221 | 23.2 | 5,130 | 22.3 | ||||||
| Age ≤50 years | 590 | 11.2 | 2,596 | 11.3 | ||||||
| Age >50 years | 631 | 12.0 | 2,534 | 11.0 | ||||||
| Comorbid conditions | ||||||||||
| Cardiovascular disease | 680 | 12.9 | 1,201 | 5.2 | ||||||
| Diabetes | 519 | 9.9 | 427 | 1.9 | ||||||
| End-stage renal disease | 13 | 0.2 | 16 | 0.1 | ||||||
| Other autoimmune diseases | 751 | 14.3 | 319 | 1.4 | ||||||
| Liver transplant during follow-up | 199 | 3.8 | 4 | 0.0 | ||||||
Abbreviations: AIH, autoimmune hepatitis; AUD, alcohol use disorder; IQR, interquartile range; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; SD, standard deviation.
a Time in years between first AIH diagnosis and biopsy procedure.
b Highest level of education of parents used when data were missing.
Any cancer
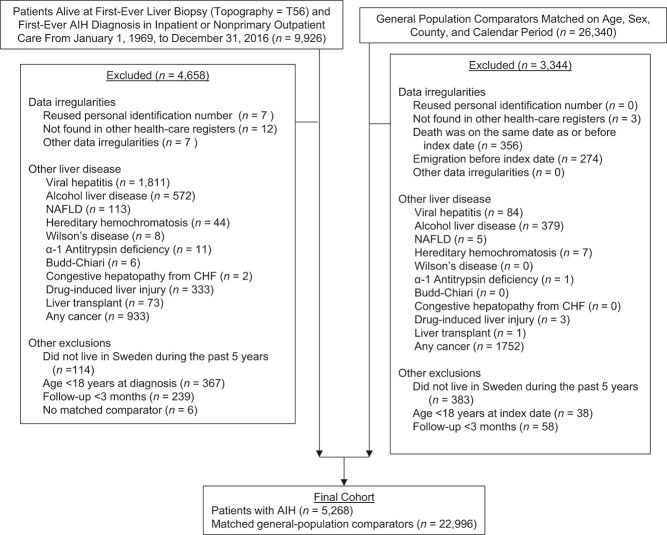
Overall, there were 1,119 cancer events (i.e., first episode of cancer) in individuals with AIH (cancer incidence rate, 17.2 per 1,000 person-years; 95% CI: 16.2, 18.2) and 4,450 cancer events in reference individuals (cancer incidence rate, 12.0 per 1,000 person-years; 95% CI: 11.6, 12.3) (Table 2). The 10-year cumulative incidence of any cancer in individuals with AIH was 13.6% (95% CI: 12.5, 14.7) and was 9.1% (95% CI: 8.7, 9.5) for reference individuals (Table 3). The overall hazard ratio for any cancer was 1.53 (95% CI: 1.42, 1.66), and this risk of any cancer was elevated early in follow-up and remained elevated beyond 10 years of follow-up (Table 2, Figure 2).
Table 2.
Risk of Cancer in Patients With Autoimmune Hepatitis and Matched General-Population Reference Individuals, Sweden 1969–2016
| Total | Events | Incidence Rate Per 1,000 PY | 95% CI | 95% CI | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AIH | Comparators | AIH | Comparators | AIH | 95% CI | Comparators | 95% CI | HR a | HR b | |||||||
| Outcome | No. | % | No. | % | No. | % | No. | % | ||||||||
| Any cancer, time | ||||||||||||||||
| Overall (≥3 months) | 5,268 | 100 | 22,996 | 100 | 1,119 | 21.2 | 4,450 | 19.4 | 17.2 | 16.2, 18.2 | 12.0 | 11.6, 12.3 | 1.65 | 1.54, 1.78 | 1.53 | 1.42, 1.66 |
| 3–11 months | 5,268 | 100 | 22,996 | 100 | 77 | 1.5 | 154 | 0.7 | 15.0 | 11.7, 18.4 | 6.8 | 5.7, 7.8 | 2.17 | 1.64, 2.87 | 1.89 | 1.35, 2.65 |
| 1.0–4.9 years | 4,918 | 93.4 | 22,351 | 97.2 | 251 | 5.1 | 754 | 3.4 | 14.7 | 12.9, 16.5 | 9.2 | 8.6, 9.9 | 1.64 | 1.41, 1.90 | 1.47 | 1.25, 1.74 |
| 5.0–9.9 years | 3,631 | 68.9 | 18,428 | 80.1 | 215 | 5.9 | 829 | 4.5%) | 14.2 | 12.3, 16.1 | 10.2 | 9.5, 10.9 | 1.58 | 1.34, 1.86 | 1.49 | 1.25, 1.78 |
| ≥10.0 years | 2,504 | 47.5 | 14,273 | 62.1 | 576 | 23.0 | 2,713 | 19.0 | 20.7 | 19.0, 22.4 | 14.6 | 14.0, 15.1 | 1.63 | 1.47, 1.81 | 1.54 | 1.38, 1.72 |
| Any hepatobiliary cancer, time | ||||||||||||||||
| Overall (≥3 months) | 5,268 | 100 | 22,996 | 100 | 195 | 3.7 | 94 | 0.4 | 2.8 | 2.4, 3.2 | 0.2 | 0.2, 0.3 | 13.84 | 10.24, 18.72 | 12.39 | 8.78, 17.48 |
| 3–11 months | 5,268 | 100 | 22,996 | 100 | 25 | 0.5 | 1 | 0.0 | 4.9 | 3.0, 6.8 | 0.0 | 0.0, 0.1 | 103.60 | 14.00, 766.33 | ||
| 1.0–4.9 years | 4,955 | 94.1 | 22,483 | 97.8 | 45 | 0.9 | 15 | 0.1 | 2.6 | 1.8, 3.3 | 0.2 | 0.1, 0.3 | 14.40 | 7.75, 26.77 | 16.51 | 6.40, 42.57 |
| 5.0–9.9 years | 3,761 | 71.4 | 19,001 | 82.6 | 33 | 0.9 | 24 | 0.1 | 2.1 | 1.4, 2.8 | 0.3 | 0.2, 0.4 | 10.31 | 5.39, 19.69 | 9.68 | 4.45, 21.09 |
| ≥10.0 years | 2,670 | 50.7 | 15,046 | 65.4 | 92 | 3.4 | 54 | 0.4 | 2.9 | 2.3, 3.5 | 0.3 | 0.2, 0.3 | 12.07 | 7.87, 18.50 | 12.36 | 7.61, 20.06 |
| HCC, time | ||||||||||||||||
| Overall (≥3 months) | 5,268 | 100 | 22,996 | 100 | 178 | 3.4 | 41 | 0.2 | 2.5 | 2.2, 2.9 | 0.1 | 0.1, 0.1 | 32.01 | 20.52, 49.93 | 29.18 | 17.52, 48.61 |
| 3–11 months | 5,268 | 100 | 22,996 | 100 | 24 | 0.5 | 0 | 0.0 | 4.7 | 2.8, 6.5 | 0 | |||||
| 1.0–4.9 years | 4,955 | 94.1 | 22,483 | 97.8 | 43 | 0.9 | 5 | 0.0 | 2.5 | 1.7, 3.2 | 0.1 | 0.0, 0.1 | 45.82 | 16.42, 127.85 | 35.10 | 9.71, 126.78 |
| 5.0–9.9 years | 3,762 | 71.4 | 19,002 | 82.6 | 30 | 0.8 | 6 | 0.0 | 1.9 | 1.2, 2.6 | 0.1 | 0.0, 0.1 | 61.62 | 14.68, 258.57 | 182.71 | 14.94, 2234.03 |
| ≥10.0 years | 2,673 | 50.7 | 15,048 | 65.4 | 81 | 3.0 | 30 | 0.2 | 2.6 | 2.0, 3.1 | 0.1 | 0.1, 0.2 | 18.80 | 10.95, 32.26 | 21.40 | 11.19, 40.95 |
| Biliary cancers, time | ||||||||||||||||
| Overall (≥3 months) | 5,268 | 100 | 22,996 | 100 | 17 | 0.3 | 53 | 0.2 | 0.2 | 0.1, 0.4 | 0.1 | 0.1, 0.2 | 1.88 | 1.04, 3.41 | 1.39 | 0.67, 2.87 |
| 3–11 months | 5,268 | 100 | 22,996 | 100 | 1 | 0.0 | 1 | 0.0 | 0.2 | 0.0, 0.6 | 0.0 | 0.0, 0.1 | 4.47 | 0.28, 71.81 | ||
| 1.0–4.9 years | 4,965 | 94.2 | 22,483 | 97.8 | 2 | 0.0 | 10 | 0.0 | 0.1 | 0.0, 0.3 | 0.1 | 0.0, 0.2 | 0.80 | 0.17, 3.76 | ||
| 5.0–9.9 years | 3,772 | 71.6 | 19,001 | 82.6 | 3 | 0.1 | 18 | 0.1 | 0.2 | 0.0, 0.4 | 0.2 | 0.1, 0.3 | 1.05 | 0.29, 3.81 | 0.32 | 0.05, 2.23 |
| ≥10.0 years | 2,675 | 50.8 | 15,046 | 65.4 | 11 | 0.4 | 24 | 0.2 | 0.3 | 0.1, 0.6 | 0.1 | 0.1, 0.2 | 3.21 | 1.40, 7.37 | 3.34 | 1.19, 9.35 |
| Extrahepatic cancer, time | ||||||||||||||||
| Overall (≥3 months) | 5,268 | 100 | 22,996 | 100 | 926 | 17.6 | 4,357 | 18.9 | 14.2 | 13.2, 15.1 | 11.7 | 11.4, 12.1 | 1.38 | 1.27, 1.49 | 1.30 | 1.19, 1.41 |
| 3–11 months | 5,268 | 100 | 22,996 | 100 | 52 | 1.0 | 153 | 0.7 | 10.1 | 7.4, 12.9 | 6.7 | 5.7. 7.8 | 1.46 | 1.06, 2.01 | 1.34 | 0.92, 1.94 |
| 1.0–4.9 years | 4,928 | 93.5 | 22,351 | 97.2 | 207 | 4.2 | 739 | 3.3 | 12.1 | 10.5, 13.8 | 9.0 | 8.4, 9.7 | 1.37 | 1.17, 1.61 | 1.25 | 1.05, 1.49 |
| 5.0–9.9 years | 3,642 | 69.1 | 18,429 | 80.1 | 182 | 5.0 | 805 | 4.4 | 12.0 | 10.2, 13.7 | 9.9 | 9.2, 10.6 | 1.34 | 1.13, 1.60 | 1.28 | 1.06, 1.54 |
| ≥10.0 years | 2,510 | 47.6 | 14,274 | 62.1 | 485 | 19.3 | 2,660 | 18.6 | 17.3 | 15.8–18.8 | 14.3 | 13.8, 14.8 | 1.39 | 1.24, 1.55 | 1.32 | 1.18, 1.48 |
| Extrahepatic solid organ cancer, time | ||||||||||||||||
| Overall (≥3 months) | 5,268 | 100 | 22,996 | 100 | 859 | 16.3 | 4,057 | 17.6 | 13.1 | 12.2, 14.0 | 10.9 | 10.5, 11.2 | 1.36 | 1.25, 1.47 | 1.28 | 1.17, 1.39 |
| 3–11 months | 5,268 | 100 | 22,996 | 100 | 46 | 0. 9 | 147 | 0.6 | 9.0 | 6.4, 11.6 | 6.5 | 5.4, 7.5 | 1.35 | 0.96, 1.88 | 1.20 | 0.81, 1.78 |
| 1.0–4.9 years | 4,931 | 93.6 | 22,356 | 97.2 | 186 | 3.8 | 691 | 3.1 | 10.9 | 9.3, 12.4 | 8.5 | 7.8, 9.1 | 1.30 | 1.10, 1.54 | 1.19 | 0.99, 1.43 |
| 5.0–9.9 years | 3,654 | 69.4 | 18,462 | 80.3 | 173 | 4.7 | 753 | 4.1 | 11.4 | 9.7, 13.0 | 9.3 | 8.6, 9.9 | 1.33 | 1.11. 1.58 | 1.26 | 1.04–1.52 |
| ≥10.0 years | 2,519 | 47.8 | 14,308 | 62.2 | 454 | 18.0 | 2,466 | 17.2 | 16.1 | 14.6, 17.6 | 13.2 | 12.7, 13.7 | 1.40 | 1.25, 1.57 | 1.33 | 1.18, 1.50 |
| GI cancer | 5,268 | 100 | 22,996 | 100 | 174 | 3.3 | 797 | 3.5 | 2.5 | 2.1, 2.9 | 2.0 | 1.9, 2.2 | 1.48 | 1.24, 1.76 | 1.25 | 1.03, 1.53 |
| Colon cancer | 5,268 | 100 | 22,996 | 100 | 74 | 1.4 | 352 | 1.5 | 1.1 | 0.8, 1.3 | 0.9 | 0.8, 1.0 | 1.42 | 1.08, 1.86 | 1.22 | 0.91, 1.65 |
| Nonmelanoma skin cancer | 5,268 | 100 | 22,996 | 100 | 160 | 3.0 | 464 | 2.0 | 2.3 | 2.0, 2.7 | 1.2 | 1.1, 1.3 | 2.74 | 2.22, 3.38 | 2.69 | 2.15, 3.37 |
| Melanoma | 5,268 | 100 | 22,996 | 100 | 44 | 0.8 | 207 | 0.9 | 0.6 | 0.4, 0.8 | 0.5 | 0.5, 0.6 | 1.43 | 1.01, 2.03 | 1.60 | 1.09, 2.35 |
| Lung cancer | 5,268 | 100 | 22,996 | 100 | 54 | 1.0 | 301 | 1.3 | 0.8 | 0.6, 1.0 | 0.8 | 0.7, 0.8 | 1.06 | 0.78, 1.44 | 0.92 | 0.65, 1.30 |
| Breast cancer | 5,268 | 100 | 22,996 | 100 | 98 | 1.9 | 514 | 2.2 | 1.4 | 1.1, 1.7 | 1.3 | 1.2, 1.4 | 1.02 | 0.82, 1.28 | 1.05 | 0.83, 1.33 |
| Extrahepatic hematologic cancer, time | ||||||||||||||||
| Overall (≥3 months) | 5,268 | 100 | 22,996 | 100 | 67 | 1.3 | 302 | 1.3 | 1.0 | 0.7, 1.2 | 0.8 | 0.7, 0.8 | 1.65 | 1.23, 2.20 | 1.57 | 1.14, 2.14 |
| 3–11 months | 5,268 | 100 | 22,996 | 100 | 6 | 0.1 | 6 | 0.0 | 1.2 | 0.2, 2.1 | 0.3 | 0.1, 0.5 | 4.21 | 1.34, 13.18 | 5.62 | 1.02, 30.89 |
| 1.0–4.9 years | 4,962 | 94.2 | 22,478 | 97.7 | 21 | 0.4 | 48 | 0.2 | 1.2 | 0.7, 1.7 | 0.6 | 0.4, 0.7 | 2.40 | 1.40, 4.11 | 2.34 | 1.23, 4.48 |
| 5.0–9.9 years | 3,761 | 71.4 | 18,968 | 2.5 | 9 | 0.2 | 53 | 0.3 | 0.6 | 0.2, 0.9 | 0.6 | 0.5, 0.8 | 1.48 | 0.69, 3.18 | 1.56 | 0.62, 3.91 |
| ≥10.0 years | 2,669 | 50.7 | 15,014 | 65.3 | 31 | 1.2 | 195 | 1.3 | 1.0 | 0.6, 1.3 | 0.9 | 0.8, 1.1 | 1.25 | 0.83, 1.90 | 1.21 | 0.78, 1.87 |
| Lymphoma | 5,268 | 100 | 22,996 | 100 | 40 | 0.8 | 149 | 0.6 | 0.6 | 0.4, 0.7 | 0.4 | 0.3, 0.4 | 1.91 | 1.30, 2.80 | 1.89 | 1.25, 2.86 |
| Leukemia | 5,268 | 100 | 22,996 | 100 | 19 | 0.4 | 93 | 0.4 | 0.3 | 0.1, 0.4 | 0.2 | 0.2, 0.3 | 1.59 | 0.92, 2.72 | 1.25 | 0.66, 2.36 |
| Multiple myeloma | 5,268 | 100 | 22,996 | 100 | 7 | 0.1 | 50 | 0.2 | 0.1 | 0.0, 0.2 | 0.1 | 0.1, 0.2 | 1.09 | 0.47, 2.52 | 1.22 | 0.48, 3.10 |
Abbreviations: AIH, autoimmune hepatitis; CI, confidence interval; GI, gastrointestinal; HCC, hepatocellular carcinoma; HR, hazard ratio; PY, person-years.
a Conditioned on age, sex, county, and calendar period.
b Conditioned on age, sex, county, and calendar period, and further adjusted for education, country of birth, family history of cancer, baseline medical comorbid conditions (cardiovascular disease, diabetes, end-stage renal disease, and other autoimmune disease), and time-dependent adjustment for alcohol use disorder.
Table 3.
Cumulative Incidence at 10 Years of Follow-up of Any Cancer, Any Hepatobiliary Cancer, Hepatocellular Carcinoma, Biliary Cancer, and Any Extrahepatic Cancer for Patients With Autoimmune Hepatitis and Matched General-Population Reference Individuals, Sweden 1969–2016
| Group | 10-Year Cumulative Incidence | 20-Year Cumulative Incidence | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AIH, % | 95% CI | Comparators, % | 95% CI | Unadjusted Risk Difference, % | 95% CI | AIH, % | 95% CI | Comparators, % | 95% CI | Unadjusted Risk Difference, % | 95% CI | |
| Any cancer | 13.6 | 12.5, 14.7 | 9.1 | 8.7, 9.5 | 4.5 | 4.0, 5.0 | 28.6 | 26.9, 30.3 | 19.9 | 19.3, 20.6 | 8.6 | 8.0, 9.3 |
| Any hepatobiliary cancer | 2.5 | 2.1, 3.1 | 0.2 | 0.2, 0.3 | 2.3 | 2.1, 2.5 | 5.3 | 4.6, 6.3 | 0.4 | 0.3, 0.6 | 4.9 | 4.6, 5.2 |
| HCC | 2.4 | 1.9, 2.9 | 0.1 | 0.0, 0.1 | 2.3 | 2.1, 2.5 | 4.8 | 4.1, 5.7 | 0.2 | 0.1, 0.3 | 4.6 | 4.3, 4.9 |
| Biliary cancers | 0.2 | 0.1, 0.4 | 0.2 | 0.1, 0.2 | 0.0 | 0.0, 0.1 | 0.5 | 0.3, 0.9 | 0.3 | 0.2, 0.3 | 0.3 | 0.2, 0.4 |
| Extrahepatic cancer | 11.2 | 10.3, 12.3 | 8.9 | 8.5, 9.3 | 2.4 | 1.9, 2.9 | 24.2 | 22.6, 25.9 | 19.5 | 18.9, 20.2 | 4.7 | 4.0, 5.3 |
| Extrahepatic solid-organ cancer | 10.4 | 9.5, 11.4 | 8.3 | 7.9, 8.7 | 2.1 | 1.6, 2.5 | 22.7 | 21.1, 24.3 | 18.2 | 17.6, 18.8 | 4.5 | 3.8, 5.1 |
| Extrahepatic hematologic cancer | 0.9 | 0.6, 1.2 | 0.6 | 0.5, 0.7 | 0.3 | 0.2, 0.5 | 1.8 | 1.4, 2.4 | 1.5 | 1.3, 1.7 | 0.3 | 0.1, 0.5 |
Abbreviations: AIH, autoimmune hepatitis; CI, confidence interval; HCC, hepatocellular carcinoma.
Figure 2.
Kaplan-Meier failure curves of time to any cancer in individuals with autoimmune hepatitis and in matched general-population reference individuals, Sweden 1969–2016. AIH, autoimmune hepatitis.
Women and men with AIH had a similar risk of any cancer (Table 4). Analyses revealed a particularly high risk in the youngest age group (18–29 years; HR = 2.60; 95% CI: 1.89, 3.59). As expected, this age group had the longest median time to cancer (Web Figure 1, Web Table 5). Risks of any cancer were similar across all calendar periods and also independent of education (Table 4).
Table 4.
Risk of Any Cancer in Patients With Autoimmune Hepatitis and Matched General-Population Reference Individuals, Sweden 1969–2016
| Total | Events | Incidence Rate Per 1,000 PY | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AIH | Comparators | AIH | Comparators | |||||||||||||
| Group | No. | % | No. | % | No. | % | No. | % | AIH | 95% CI | Comparators | 95% CI | HR a | 95% CI | HR b | 95% CI |
| Overall (≥3 months) | 5,268 | 100 | 22,996 | 100 | 1,119 | 21.2 | 4,450 | 19.4 | 17.2 | 16.2, 18.2 | 12.0 | 11.6, 12.3 | 1.65 | 1.54, 1.78 | 1.53 | 1.42, 1.66 |
| Sex | ||||||||||||||||
| Female | 3,115 | 59.1 | 13,278 | 57.7 | 611 | 19.6 | 2,352 | 17.7 | 17.1 | 15.7, 18.4 | 11.8 | 11.4, 12.3 | 1.57 | 1.43, 1.73 | 1.52 | 1.37, 1.69 |
| Male | 2,153 | 40.9 | 9,718 | 42.3 | 508 | 23.6 | 2,098 | 21.6 | 17.3 | 15.8, 18.8 | 12.1 | 11.6, 12.7 | 1.76 | 1.58, 1.97 | 1.54 | 1.37, 1.74 |
| Age at diagnosis, years | ||||||||||||||||
| 18.0–29.9 | 594 | 11.3 | 2,702 | 11.7 | 80 | 13.5 | 165 | 6.1 | 8.3 | 6.5, 10.1 | 3.3 | 2.8, 3.8 | 2.44 | 1.85, 3.23 | 2.60 | 1.89, 3.59 |
| 30.0–39.9 | 722 | 13.7 | 3,242 | 14.1 | 120 | 16.6 | 361 | 11.1 | 9.6 | 7.9, 11.4 | 5.3 | 4.8, 5.9 | 2.11 | 1.68, 2.66 | 1.82 | 1.41, 2.34 |
| 40.0–49.9 | 909 | 17.3 | 4,146 | 18.0 | 229 | 25.2 | 871 | 21.0 | 15.7 | 13.6, 17.7 | 10.4 | 9.8, 11.1 | 1.69 | 1.44, 1.98 | 1.49 | 1.25, 1.78 |
| 50.0–59.9 | 1,103 | 20.9 | 4,896 | 21.3 | 265 | 24.0 | 1,238 | 25.3 | 18.8 | 16.5, 21.0 | 15.0 | 14.1, 15.8 | 1.43 | 1.24, 1.66 | 1.29 | 1.10, 1.51 |
| 60.0–69.9 | 1,150 | 21.8 | 4,884 | 21.2 | 273 | 23.7 | 1,211 | 24.8 | 27.4 | 24.2, 30.7 | 19.8 | 18.7, 20.9 | 1.48 | 1.28, 1.72 | 1.41 | 1.20, 1.65 |
| ≥70.0 | 790 | 15.0 | 3,126 | 13.6 | 152 | 19.2 | 604 | 19.3 | 34.4 | 28.9, 39.9 | 22.5 | 20.7, 24.3 | 1.80 | 1.46, 2.21 | 1.76 | 1.41, 2.20 |
| Age at diagnosis, restricting follow-up to 5 years, years | ||||||||||||||||
| 18.0–29.9 | 594 | 11.3 | 2,702 | 11.7 | 14 | 2.4 | 35 | 1.3 | 5.2 | 2.5, 7.9 | 2.8 | 1.9, 3.7 | 1.71 | 0.92, 3.19 | 1.49 | 0.65, 3.43 |
| 30.0–39.9 | 722 | 13.7 | 3,242 | 14.1 | 14 | 1.9 | 35 | 1.1 | 4.2 | 2.0, 6.4 | 2.3 | 1.5, 3.1 | 1.94 | 1.03, 3.64 | 1.10 | 0.47, 2.54 |
| 40.0–49.9 | 909 | 17.3 | 4,146 | 18.0 | 31 | 3.4 | 65 | 1.6 | 7.5 | 4.9, 10.2 | 3.3 | 2.5, 4.1 | 2.12 | 1.37, 3.28 | 1.07 | 0.58, 1.95 |
| 50.0–59.9 | 1,103 | 20.9 | 4,896 | 21.3 | 57 | 5.2 | 175 | 3.6 | 11.9 | 8.8, 15.0 | 7.8 | 6.6, 8.9 | 1.42 | 1.05, 1.93 | 1.05 | 0.73, 1.50 |
| 60.0–69.9 | 1,150 | 21.8 | 4,884 | 21.2 | 113 | 9.8 | 328 | 6.7 | 24.8 | 20.2, 29.4 | 15.1 | 13.5, 16.8 | 1.62 | 1.30, 2.03 | 1.51 | 1.17, 1.95 |
| ≥70.0 | 790 | 15.0 | 3,126 | 13.6 | 99 | 12.5 | 270 | 8.6 | 36.6 | 29.4, 43.8 | 20.8 | 18.3, 23.3 | 2.01 | 1.56, 2.58 | 2.04 | 1.55, 2.70 |
| Year of diagnosis | ||||||||||||||||
| 1969–1986 | 1,831 | 34.8 | 8,494 | 36.9 | 520 | 28.4 | 2,404 | 28.3 | 17.5 | 16.0,19.0 | 12.8 | 12.3, 13.3 | 1.58 | 1.42, 1.76 | 1.38 | 1.22, 1.55 |
| 1987–2001 | 1,751 | 33.2 | 7,703 | 33.5 | 441 | 25.2 | 1,611 | 20.9 | 16.9 | 15.3, 18.4 | 11.2 | 10.7, 11.8 | 1.76 | 1.57, 1.98 | 1.70 | 1.50, 1.92 |
| 2002–2016 | 1,686 | 32.0 | 6,799 | 29.6 | 158 | 9.4 | 435 | 6.4 | 17.1 | 14.4, 19.7 | 10.8 | 9.7, 11.8 | 1.60 | 1.33, 1.94 | 1.56 | 1.27, 1.92 |
| 1997–2016 | 1,871 | 35.5 | 7,572 | 32.9 | 198 | 10.6 | 564 | 7.4 | 17.1 | 14.7, 19.5 | 10.8 | 9.9, 11.7 | 1.68 | 1.41, 1.99 | 1.63 | 1.36, 1.97 |
| Year of diagnosis, restricting follow-up to 5 years | ||||||||||||||||
| 1969–1986 | 1,831 | 34.8 | 8,494 | 36.9 | 121 | 6.6 | 337 | 4.0 | 15.1 | 12.4, 17.8 | 8.3 | 7.4, 9.2 | 1.93 | 1.55, 2.40 | 1.53 | 1.18, 1.99 |
| 1987–2001 | 1,751 | 33.2 | 7,703 | 33.5 | 109 | 6.2 | 299 | 3.9 | 13.9 | 11.3, 16.6 | 8.1 | 7.2, 9.0 | 1.76 | 1.40, 2.21 | 1.72 | 1.33, 2.22 |
| 2002–2012 | 1,154 | 21.9 | 4,675 | 20.3 | 78 | 6.8 | 230 | 4.9 | 15.1 | 11.7, 18.4 | 10.4 | 9.1, 11.7 | 1.47 | 1.13, 1.92 | 1.33 | 0.99, 1.79 |
| 1997–2012 | 1,339 | 25.4 | 5,448 | 23.7 | 178 | 13.3 | 522 | 9.6 | 17.1 | 14.6, 19.6 | 11.0 | 10.1, 12.0 | 1.67 | 1.39, 1.99 | 1.66 | 1.36, 2.02 |
| Country of birth | ||||||||||||||||
| Nordic | 4,962 | 94.2 | 21,570 | 93.8 | 1,067 | 21.5 | 4,255 | 19.7 | 17.3 | 16.3, 18.4 | 12.1 | 11.7, 12.4 | 1.68 | 1.55, 1.81 | 1.55 | 1.43, 1.68 |
| Other | 304 | 5.8 | 1,424 | 6.2 | 52 | 17.1 | 195 | 13.7 | 14.7 | 10.7, 18.6 | 10.4 | 9.0, 11.9 | 2.96 | 1.03, 8.52 | 4.05 | 0.97, 16.97 |
| Education, years | ||||||||||||||||
| ≤9 | 1,679 | 31.9 | 7,938 | 34.5 | 391 | 23.3 | 1,875 | 23.6 | 17.2 | 15.5, 18.9 | 13.4 | 12.8, 14.0 | 1.51 | 1.30, 1.75 | 1.48 | 1.26, 1.73 |
| 10–12 | 1,932 | 36.7 | 8,450 | 36.7 | 396 | 20.5 | 1,441 | 17.1 | 14.6 | 13.2, 16.0 | 10.3 | 9.8, 10.8 | 1.80 | 1.53, 2.12 | 1.73 | 1.45, 2.07 |
| >12 | 1,050 | 19.9 | 5,010 | 21.8 | 193 | 18.4 | 748 | 14.9 | 15.5 | 13.3, 17.7 | 9.4 | 8.7, 10.1 | 1.71 | 1.32, 2.21 | 1.66 | 1.24, 2.23 |
| Missing data | 607 | 11.5 | 1,598 | 6.9 | 139 | 22.9 | 386 | 24.2 | 49.5 | 41.2, 57.7 | 31.5 | 28.4, 34.7 | 1.83 | 1.35, 2.48 | 1.86 | 1.31, 2.62 |
| Pathology findings | ||||||||||||||||
| Cirrhosis | 733 | 13.9 | 3,203 | 13.9 | 190 | 25.9 | 703 | 21.9 | 28.5 | 24.4, 32.5 | 13.8 | 12.8, 14.8 | 2.87 | 2.37, 3.48 | 2.78 | 2.25, 3.43 |
| Fibrosis | 1,110 | 21.1 | 4,622 | 20.1 | 170 | 15.3 | 502 | 10.9 | 19.0 | 16.2, 21.9 | 10.6 | 9.7, 11.6 | 1.97 | 1.63, 2.38 | 1.79 | 1.44, 2.21 |
| Inflammation, no fibrosis | 1,842 | 35.0 | 8,053 | 35.0 | 375 | 20.4 | 1,550 | 19.2 | 15.4 | 13.9, 17.0 | 11.4 | 10.9, 12.0 | 1.52 | 1.34, 1.72 | 1.47 | 1.29, 1.68 |
| Other | 1,583 | 30.0 | 7,118 | 31.0 | 384 | 24.3 | 1,695 | 23.8 | 15.2 | 13.7, 16.7 | 12.3 | 11.7, 12.9 | 1.37 | 1.21, 1.54 | 1.23 | 1.08, 1.40 |
| Necrosis | 129 | 2.4 | 557 | 2.4 | 26 | 20.2 | 104 | 18.7 | 15.3 | 9.4, 21.2 | 11.3 | 9.2, 13.5 | 1.36 | 0.85, 2.17 | 1.35 | 0.79, 2.31 |
| Severity of liver disease | ||||||||||||||||
| Portal hypertension | 275 | 5.2 | 1,178 | 5.1 | 58 | 21.1 | 241 | 20.5 | 32.4 | 23.3, 39.4 | 14.6 | 12.8, 16.5 | 3.22 | 2.26, 4.58 | 2.99 | 1.99, 4.50 |
| Acute/subacute liver failure | 139 | 2.6 | 629 | 2.7 | 39 | 28.1 | 110 | 17.5 | 22.5 | 15.4, 29.5 | 10.2 | 8.3, 12.1 | 3.03 | 1.99, 4.62 | 2.42 | 1.47, 3.97 |
| Overlap syndromes | ||||||||||||||||
| PSC | 84 | 1.6 | 362 | 1.6 | 17 | 20.2 | 29 | 8.0 | 27.8 | 14.6, 41.0 | 7.5 | 4.8, 10.2 | 5.10 | 2.46, 10.59 | 1.33 | 0.03, 60.01 |
| PBC | 201 | 3.8 | 815 | 3.5 | 32 | 15.9 | 122 | 15.0 | 22.7 | 14.8, 30.5 | 14.9 | 12.3, 17.5 | 1.80 | 1.17, 2.77 | 1.78 | 1.12, 2.82 |
| Family history of cancer in first-degree relative | ||||||||||||||||
| Overall | 1,221 | 23.2 | 5,130 | 22.3 | 228 | 18.7 | 726 | 14.2 | 16.1 | 14.0, 18.2 | 10.1 | 9.4, 10.8 | 1.61 | 1.30, 1.99 | 1.51 | 1.20, 1.90 |
| Age ≤50 years | 590 | 11.2 | 2,596 | 11.3 | 118 | 20.0 | 366 | 14.1 | 12.2 | 10.0, 14.4 | 7.5 | 6.7, 8.2 | 1.58 | 1.15, 2.18 | 1.51 | 1.07, 2.14 |
| Age >50 years | 631 | 12.0 | 2,534 | 11.0 | 110 | 17.4 | 360 | 14.2 | 24.5 | 19.9, 29.1 | 15.7 | 14.0, 17.3 | 1.68 | 1.26, 2.24 | 1.59 | 1.17, 2.18 |
| Comorbid conditions | ||||||||||||||||
| Cardiovascular disease | 680 | 12.9 | 1,201 | 5.2 | 131 | 19.3 | 190 | 15.8 | 29.3 | 24.3, 34.3 | 19.6 | 16.8, 22.4 | 2.27 | 1.24, 4.15 | 2.58 | 1.26, 5.30 |
| Diabetes | 519 | 9.9 | 427 | 1.9 | 117 | 22.5 | 48 | 11.2 | 30.0 | 24.6, 35.5 | 15.8 | 11.3, 20.2 | 1.83 | 0.66, 5.06 | 7.86 | 0.48, 128.64 |
| End-stage renal disease | 13 | 0.2 | 16 | 0.1 | 5 | 38.5 | 1 | 6.3 | 52.8 | 6.5, 99.1 | 10.6 | 0.0, 31.4 | ||||
| Other autoimmune diseases | 751 | 14.3 | 319 | 1.4 | 115 | 15.3 | 26 | 8.2 | 17.2 | 14.0, 20.3 | 10.6 | 6.5, 14.7 | 1.09 | 0.27, 4.39 | ||
Abbreviations: AIH, autoimmune hepatitis; CI, confidence interval; HR, hazard ratio; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis.
a Conditioned on age, sex, county, and calendar period.
b Conditioned on age, sex, county, and calendar period, and further adjusted for education, country of birth, family history of cancer, baseline medical comorbid conditions (cardiovascular disease, diabetes, end-stage renal disease, and other autoimmune disease), and time-dependent adjustment for alcohol use disorder.
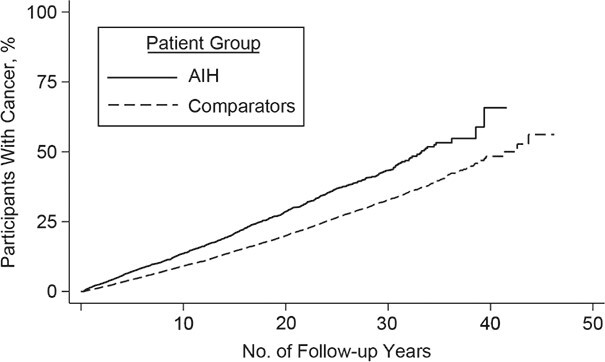
The highest risks of any cancer were in individuals with AIH whose liver biopsy specimens revealed cirrhosis (HR = 2.78; 95% CI: 2.25, 3.43), portal hypertension (HR = 2.99; 95% CI: 1.99, 4.50), and acute or subacute liver failure (HR = 2.42; 95% CI: 1.47, 3.97) (Table 4). Individuals with AIH who had fibrosis, inflammation without fibrosis, and necrosis had elevated relative risks of cancer that were comparable to the overall cancer risk in all individuals with AIH (Table 4, Figure 3). Individuals with AIH and concurrent PBC (n = 201) had an increased risk of any cancer, but those with concurrent PSC (n = 84) or other autoimmune diseases (n = 751) did not (Table 4).
Figure 3.
Kaplan-Meier failure curves of time to any cancer in individuals with autoimmune hepatitis by histopathology-finding group and in matched general-population reference individuals, Sweden 1969–2016. A) Cirrhosis, fibrosis, and other histopathology; B) cirrhosis; C) fibrosis; D) other histopathology. AIH, autoimmune hepatitis.
Individuals with AIH who had cirrhosis (HR = 1.89; 95% CI: 1.57, 2.27) and fibrosis (HR = 1.31; 95% CI: 1.06, 1.62) had increased risk of cancer compared with individuals with AIH who had other histopathology, but there was no increased risk in individuals with AIH who had inflammation but no fibrosis (HR = 1.07; 95% CI: 0.92, 1.24) (Web Table 6).
Any hepatobiliary cancer
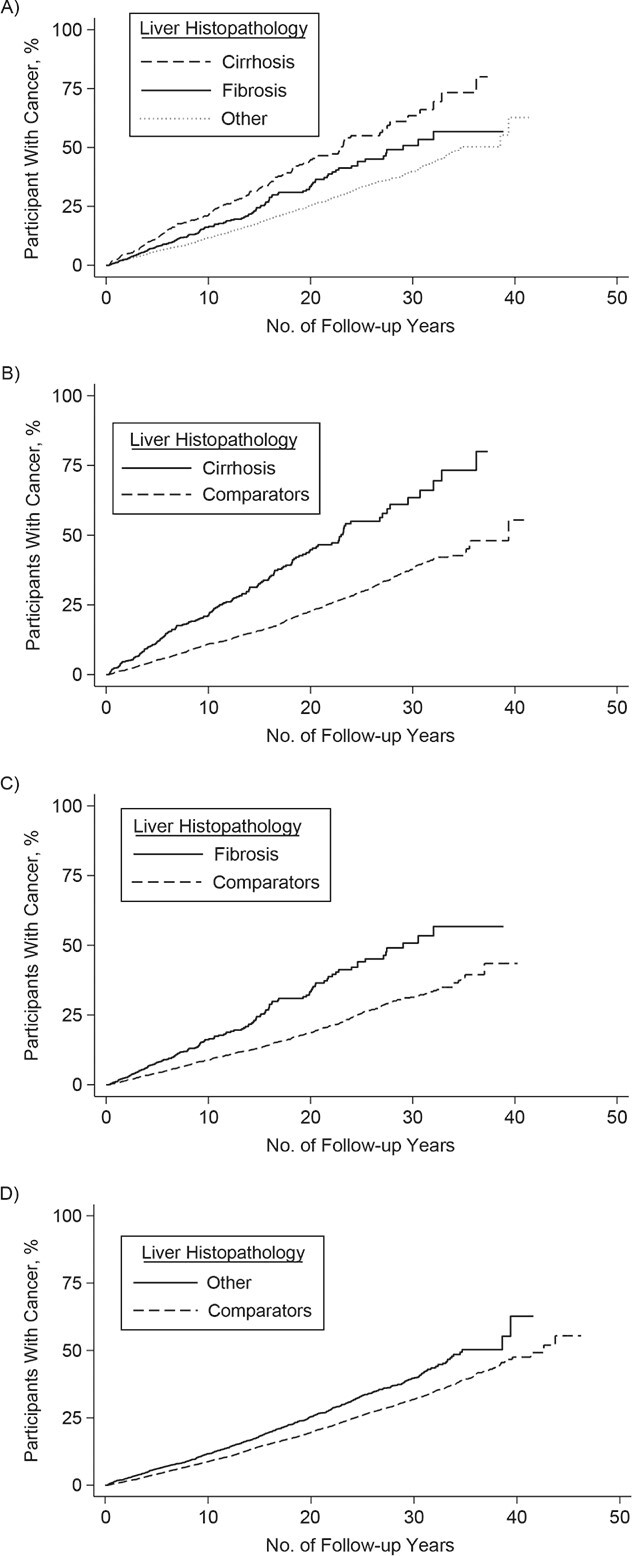
The incidence rate of any hepatobiliary cancer was 2.8 per 1,000 person-years (95% CI: 2.4, 3.2) in individuals with AIH and 0.2 per 1,000 person-years (95% CI: 0.2, 0.3) in reference individuals (Table 2). Overall, individuals with AIH were at a 12.39 (95% CI: 8.78, 17.48) times higher risk of any hepatobiliary cancer, compared with reference individuals (Table 2, Figure 4).
Figure 4.
Kaplan-Meier failure curves of time to hepatobiliary cancer in individuals with autoimmune hepatitis and in matched general-population reference individuals, Sweden 1969–2016. A) Hepatobiliary cancer B) hepatocellular cancer C) biliary cancer. AIH, autoimmune hepatitis.
Hepatocellular carcinoma
The incidence rates of HCC were 2.5 per 1,000 person-years (95% CI: 2.2, 2.9) for individuals with AIH and 0.1 per 1,000 person-years (95% CI: 0.1, 0.1) for reference individuals (Table 2). The 10-year cumulative incidence for HCC was 2.4% (95% CI: 1.9, 2.9) in those with AIH and 0.1% (95% CI: 0.1, 0.1) in reference individuals (Table 3). Individuals with AIH had a 29.18-fold increased risk of HCC (95% CI: 17.52, 48.61), and this elevated risk remained beyond 10 years of follow-up (Table 2, Figure 4).
Risks of HCC were similar in men and women (Table 5) but high in younger individuals with AIH aged 18–29 years at diagnosis. Hazard ratios were highest among individuals with AIH with cirrhosis, but this risk was also elevated in those with fibrosis and those with inflammation without fibrosis (Table 5). The incidence rates of HCC in individuals with AIH and cirrhosis, fibrosis, and inflammation without fibrosis were 8.1 (95% CI: 6.0, 10.2), 2.2 (95% CI: 1.3, 3.2), and 2.1 (95% CI: 1.5, 2.6) per 1,000 person-years, respectively (Tables 5 and 6). The annual incidence risk per year for HCC in individuals with cirrhosis with AIH was 1.1% per year.
Table 5.
Risk of Hepatocellular Carcinoma in Patients With Autoimmune Hepatitis and Matched General-Population Reference Individuals, Sweden 1969–2016
| Total | Events | Incidence Rate Per 1,000 PY | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AIH | Comparators | AIH | Comparators | AIH | 95% CI | Comparators | 95% CI | |||||||||
| Group | No. | % | No. | % | No. | % | No. | % | HR a | 95% CI | HR b | 95% CI | ||||
| Overall (≥3 months) | 5,268 | 100 | 22,996 | 100 | 178 | 3.4 | 41 | 0.2 | 2.5 | 2.2, 2.9 | 0.1 | 0.1, 0.1 | 32.01 | 20.52, 49.93 | 29.18 | 17.52, 48.61 |
| Sex | ||||||||||||||||
| Female | 3,115 | 59.1 | 13,278 | 57.7 | 73 | 2.3 | 15 | 0.1 | 1.9 | 1.5, 2.3 | 0.1 | 0.0, 0.1 | 31.89 | 15.91, 63.89 | 37.67 | 16.17, 87.74 |
| Male | 2,153 | 40.9 | 9,718 | 42.3 | 105 | 4.9 | 26 | 0.3 | 3.4 | 2.7, 4.0 | 0.1 | 0.1, 0.2 | 32.10 | 18.00, 57.24 | 31.79 | 15.12, 66.83 |
| Age at diagnosis, years | ||||||||||||||||
| 18.0–29.9 | 594 | 11.3 | 2,702 | 11.7 | 8 | 1.3 | 0 | 0.0 | 0.8 | 0.2, 1.3 | 0.0 | 0.0, 0.0 | ||||
| 30.0–39.9 | 722 | 13.7 | 3,242 | 14.1 | 28 | 3.9 | 3 | 0.1 | 2.1 | 1.4, 2.9 | 0.0 | 0.0, 0.1 | 38.57 | 11.68, 127.37 | 185.16 | 6.82, 5024.3 |
| 40.0–49.9 | 909 | 17.3 | 4,146 | 18.0 | 29 | 3.2 | 9 | 0.2 | 1.8 | 1.2, 2.5 | 0.1 | 0.0, 0.2 | 28.72 | 10.05, 82.06 | 58.18 | 11.13, 304.11 |
| 50.0–59.9 | 1,103 | 20.9 | 4,896 | 21.3 | 34 | 3.1 | 11 | 0.2 | 2.2 | 1.5, 2.9 | 0.1 | 0.0, 0.2 | 31.62 | 11.16, 89.60 | 70.95 | 8.09, 622.60 |
| 60.0–69.9 | 1,150 | 21.8 | 4,884 | 21.2 | 49 | 4.3 | 12 | 0.2 | 4.5 | 3.2, 5.8 | 0.2 | 0.1, 0.3 | 23.21 | 10.93, 49.33 | 22.34 | 8.97, 55.64 |
| ≥70.0 | 790 | 15.0 | 3,126 | 13.6 | 30 | 3.8 | 6 | 0.2 | 6.4 | 4.1, 8.6 | 0.2 | 0.0, 0.4 | 36.33 | 11.03, 119.66 | 28.32 | 6.52, 122.97 |
| Age at diagnosis, restricting follow-up to 5 years, years | ||||||||||||||||
| 18.0–29.9 | 594 | 11.3 | 2,702 | 11.7 | 1 | 0.2 | 0 | 0.0 | 0.4 | 0.0, 1.1 | 0.0 | 0.0, 0.0 | ||||
| 30.0–39.9 | 722 | 13.7 | 3,242 | 14.1 | 5 | 0.7 | 0 | 0.0 | 1.5 | 0.2, 2.8 | 0.0 | 0.0, 0.0 | ||||
| 40.0–49.9 | 909 | 17.3 | 4,146 | 18.0 | 7 | 0.8 | 0 | 0.0 | 1.7 | 0.4, 2.9 | 0.0 | 0.0, 0.0 | ||||
| 50.0–59.9 | 1,103 | 20.9 | 4,896 | 21.3 | 6 | 0.5 | 1 | 0.0 | 1.2 | 0.2, 2.2 | 0.0 | 0.0, 0.1 | 23.68 | 2.82, 199.14 | ||
| 60.0–69.9 | 1,150 | 21.8 | 4,884 | 21.2 | 21 | 1.8 | 1 | 0.0 | 4.5 | 2.6, 6.4 | 0.0 | 0.0, 0.1 | ||||
| ≥70.0 | 790 | 15.0 | 3,126 | 13.6 | 27 | 3.4 | 3 | 0.1 | 9.7 | 6.0, 13.3 | 0.2 | 0.0, 0.5 | 34.58 | 10.45, 114.38 | 26.12 | 6.12, 111.45 |
| Year of diagnosis | ||||||||||||||||
| 1969–1986 | 1,831 | 34.8 | 8,494 | 36.9 | 83 | 4.5 | 23 | 0.3 | 2.6 | 2.0, 3.1 | 0.1 | 0.1, 0.2 | 29.15 | 15.49, 54.83 | 23.09 | 11.33, 47.08 |
| 1987–2001 | 1,751 | 33.2 | 7,703 | 33.5 | 76 | 4.3 | 16 | 0.2 | 2.7 | 2.1, 3.3 | 0.1 | 0.1, 0.2 | 31.25 | 16.13, 60.54 | 51.29 | 18.72, 140.58 |
| 2002–2016 | 1,686 | 32.0 | 6,799 | 29.6 | 19 | 1.1 | 2 | 0.0 | 2.0 | 1.1, 2.9 | 0.0 | 0.0, 0.1 | 70.96 | 9.46, 532.03 | ||
| 1997–2016 | 1,871 | 35.5 | 7,572 | 32.9 | 26 | 1.4 | 5 | 0.1 | 2.1 | 1.3, 3.0 | 0.1 | 0.0, 0.2 | 32.31 | 9.74, 107.23 | 78.41 | 8.05, 763.76 |
| Year of diagnosis, restricting follow-up to 5 years | ||||||||||||||||
| 1969–1986 | 1,831 | 34.8 | 8,494 | 36.9 | 29 | 1.6 | 2 | 0.0 | 3.6 | 2.3, 4.9 | 0.0 | 0.0 0.1 | 125.52 | 17.08, 922.16 | 45.68 | 5.41, 386.00 |
| 1987–2001 | 1,751 | 33.2 | 7,703 | 33.5 | 26 | 1.5 | 3 | 0.0 | 3.3 | 2.0, 4.5 | 0.1 | 0.0, 0.2 | 37.49 | 11.32, 124.16 | 67.07 | 6.79, 662.91 |
| 2002–2012 | 1,154 | 21.9 | 4,675 | 20.3 | 10 | 0.9%) | 0 | 0.0 | 1.9 | 0.7, 3.1 | 0.0 | 0.0, 0.0 | ||||
| 1997–2012 | 1,339 | 25.4 | 5,448 | 23.7 | 24 | 1.8 | 5 | 0.1 | 2.2 | 1.3, 3.1 | 0.1 | 0.0, 0.2 | 30.25 | 9.06, 100.94 | 91.49 | 7.38, 1134.14 |
| Country of birth | ||||||||||||||||
| Nordic | 4,962 | 94.2 | 21,570 | 93.8 | 162 | 3.3 | 40 | 0.2 | 2.4 | 2.1, 2.8 | 0.1 | 0.1, 0.1 | 28.90 | 18.30, 45.64 | 25.98 | 15.49, 43.59 |
| Other | 304 | 5.8 | 1,424 | 6.2 | 16 | 5.3 | 1 | 0.1 | 4.3 | 2.2, 6.5 | 0.1 | 0.0, 0.1 | ||||
| Education, years | ||||||||||||||||
| ≤9 | 1,679 | 31.9 | 7,938 | 34.5 | 52 | 3.1 | 21 | 0.3 | 2.1 | 1.5, 2.7 | 0.1 | 0.1, 0.2 | 24.38 | 8.66, 68.63 | 27.42 | 8.22, 91.47 |
| 10–11 | 1,932 | 36.7 | 8,450 | 36.7 | 54 | 2.8 | 13 | 0.2 | 1.9 | 1.4, 2.4 | 0.1 | 0.0, 0.1 | 46.11 | 11.131, 90.98 | 87.97 | 9.47, 816.95 |
| >12 | 1,050 | 19.9 | 5,010 | 21.8 | 34 | 3.2 | 5 | 0.1 | 2.5 | 1.7, 3.4 | 0.1 | 0.0, 0.1 | ||||
| Missing data | 607 | 11.5 | 1,598 | 6.9 | 38 | 6.3 | 2 | 0.1 | 12.7 | 8.7, 16.8 | 0.2 | 0.0, 0.4 | 63.55 | 8.50, 475.26 | 58.15 | 6.99, 483.73 |
| Pathology findings | ||||||||||||||||
| Cirrhosis | 733 | 13.9 | 3,203 | 13.9 | 59 | 8.0 | 5 | 0.2 | 8.1 | 6.0, 10.2 | 0.1 | 0.0, 0.2 | 249.59 | 34.56, 1802.6 | ||
| Fibrosis | 1,110 | 21.1 | 4,622 | 20.1 | 21 | 1.9 | 3 | 0.1 | 2.2 | 1.3, 3.2 | 0.1 | 0.0, 0.1 | 39.86 | 9.30, 170.91 | 65.17 | 4.01, 1059.82 |
| Inflammation, no fibrosis | 1,842 | 35.0 | 8,053 | 35.0 | 54 | 2.9 | 9 | 0.1 | 2.1 | 1.5–2.6 | 0.1 | 0.0–0.1 | 41.95 | 16.73, 105.23 | 121.88 | 19.88, 747.41 |
| Other | 1,583 | 30.0 | 7,118 | 31.0 | 44 | 2.8 | 24 | 0.3 | 1.6 | 1.1, 2.1 | 0.2 | 0.1, 0.2 | 11.93 | 6.51, 21.89 | 14.66 | 6.61, 32.55 |
| Necrosis | 129 | 2.4 | 557 | 2.4 | 2 | 1.6 | 0 | 1.1 | 0.0, 2.6 | 0.0 | 0.0, 0.0 | |||||
| Severity of liver disease | ||||||||||||||||
| Portal hypertension | 263 | 5.0 | 1,130 | 4.9 | 17 | 6.5 | 3 | 0.3 | 8.7 | 4.5, 12.8 | 0.2 | 0.0, 0.4 | 75.43 | 10.02, 567.70 | ||
| Acute/subacute liver failure | 139 | 2.6 | 629 | 2.7 | 9 | 6.5 | 0 | 4.8 | 1.7, 7.9 | 0.0 | 0.0, 0.0 | |||||
| Overlap syndromes | ||||||||||||||||
| PSC | 84 | 1.6 | 362 | 1.6 | 6 | 7.1 | 0 | 9.2 | 1.8, 16.6 | 0.0 | 0.0, 0.0 | |||||
| PBC | 201 | 3.8 | 815 | 3.5 | 8 | 4.0 | 1 | 0.1 | 5.2 | 1.6, 8.8 | 0.1 | 0.0, 0.3 | ||||
| Family history of cancer in 1st degree relative | ||||||||||||||||
| Overall | 1,221 | 23.2 | 5,130 | 22.3 | 35 | 2.9 | 4 | 0.1 | 2.3 | 1.6, 3.1 | 0.1 | 0.0, 0.1 | ||||
| Age ≤50 years | 590 | 11.2 | 2,596 | 11.3 | 20 | 3.4 | 1 | 0.0 | 2.0 | 1.1, 2.8 | 0.0 | 0.0, 0.1 | ||||
| Age >50 years | 631 | 12.0 | 2,534 | 11.0 | 15 | 2.4 | 3 | 0.1 | 3.1 | 1.5, 4.7 | 0.1 | 0.0, 0.3 | ||||
| Comorbid conditions | ||||||||||||||||
| Cardiovascular disease | 680 | 12.9 | 1,201 | 5.2 | 28 | 4.1 | 1 | 0.1 | 5.7 | 3.6, 7.8 | 0.1 | 0.0, 0.3 | ||||
| Diabetes | 519 | 9.9 | 427 | 1.9 | 23 | 4.4 | 0 | 5.4 | 3.2, 7.5 | 0.0 | 0.0, 0.0 | |||||
| End-stage renal disease | 13 | 0.2 | 16 | 0.1 | 0 | 0 | 0.0 | 0.0, 0.0 | 0.0 | 0.0, 0.0 | ||||||
| Other autoimmune diseases | 751 | 14.3 | 319 | 1.4 | 13 | 1.7 | 0 | 1.8 | 0.8, 2.8 | 0.0 | 0.0, 0.0 | |||||
Abbreviations: AIH, autoimmune hepatitis; CI, confidence interval; HR, hazard ratio; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; PY, person-years.
a Conditioned on age, sex, county, and calendar period.
b Conditioned on age, sex, county, and calendar period, and further adjusted for education, country of birth, family history of cancer, baseline medical comorbid conditions (cardiovascular disease, diabetes, end-stage renal disease, and other autoimmune disease), and time-dependent adjustment for alcohol use disorder.
Table 6.
Cumulative Incidence at 10 Years of Follow-up for Hepatocellular Carcinoma and Extrahepatic Cancer for Patients With Autoimmune Hepatitis and Matched General-Population Reference Individuals, Sweden 1969–2016
| Group | 10-Year Cumulative Incidence for HCC | 10-Year Cumulative Incidence for Extrahepatic Cancer | ||||||
|---|---|---|---|---|---|---|---|---|
| AIH, % | 95% CI | Comparators, % | 95% CI | AIH, % | 95% CI | Comparators, % | 95% CI | |
| Overall | 2.4 | 1.9, 2.9 | 0.1 | 0.0, 0.1 | 11.2 | 10.3, 12.3 | 8.9 | 8.5, 9.3 |
| Age at diagnosis, years | ||||||||
| 18.0–29.9 | 0.8 | 0.3, 2.2 | 0.0 | 0.0, 0.0 | 4.8 | 3.2, 7.2 | 2.5 | 1.9, 3.2 |
| 30.0–39.9 | 1.1 | 0.5, 2.3 | 0.0 | 0.0, 0.0 | 2.9 | 1.8, 4.6 | 2.3 | 1.8, 3.0 |
| 40.0–49.9 | 1.4 | 0.8, 2.6 | 0.0 | 0.0, 0.0 | 6.6 | 5.0, 8.6 | 4.4 | 3.7, 5.1 |
| 50.0–59.9 | 1.9 | 1.1, 3.1 | 0.1 | 0.0, 0.2 | 11.4 | 9.4, 13.7 | 8.6 | 7.7, 9.5 |
| 60.0–69.9 | 3.8 | 2.7, 5.5 | 0.1 | 0.0, 0.3 | 20.1 | 17.3, 23.3 | 15.7 | 14.6, 16.9 |
| ≥70.0 | 5.1 | 3.5, 7.5 | 0.2 | 0.1, 0.6 | 22.9 | 18.9, 27.7 | 19.9 | 18.2, 21.7 |
| Pathology findings | ||||||||
| Cirrhosis | 7.5 | 5.5, 10.2 | 0.1 | 0.0, 0.4 | 14.4 | 11.5, 18.0 | 10.7 | 9.6, 12.0 |
| Fibrosis | 1.8 | 1.0, 3.3 | 0.0 | 0.0, 0.3 | 14.2 | 11.7, 17.3 | 8.6 | 7.7, 9.7 |
| Inflammation, no fibrosis | 1.6 | 1.1, 2.4 | 0.0 | 0.0, 0.1 | 10.0 | 8.5, 11.7 | 8.8 | 8.1, 9.5 |
| Other | 1.6 | 1.1, 2.5 | 0.1 | 0.0, 0.2 | 10.0 | 8.5, 11.7 | 8.2 | 7.6, 8.9 |
| Necrosis | 1.9 | 0.5, 7.4 | 0.0 | 0.0, 0.0 | 12.3 | 6.9, 21.3 | 7.5 | 5.4, 10.5 |
| Severity of liver disease | ||||||||
| Portal hypertension | 8.4 | 5.0, 14.1 | 0.3 | 0.1, 1.1 | 16.1 | 10.8, 23.7 | 12.9 | 10.9, 15.3 |
| Acute/subacute liver failure | 5.3 | 2.4, 11.6 | 0.0 | 0.0, 0.0 | 13.4 | 8.1, 21.8 | 7.8 | 5.8, 10.5 |
| Overlap syndromes | ||||||||
| PSC | 5.5 | 1.7, 16.7 | 0.0 | 0.0, 0.0 | 11.1 | 4.2, 27.5 | 5.8 | 3.4, 9.8 |
| PBC | 6.2 | 2.7, 13.9 | 0.0 | 0.0, 0.0 | 15.1 | 9.7, 23.2 | 13.9 | 11.1, 17.3 |
Abbreviations: AIH, autoimmune hepatitis; CI, confidence interval; HCC, hepatocellular carcinoma; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis.
Biliary cancer
The incidence rates of biliary cancers were 0.2 per 1,000 person-years (95% CI: 0.1, 0.4) for individuals with AIH and 0.1 per 1,000 person-years (95% CI: 0.1, 0.2) for reference individuals (Table 2). Overall, individuals with AIH were not at increased risk of biliary cancers, but we found an increased risk beyond 10 years of follow-up (HR = 3.34; 95% CI: 1.19, 9.35) (Table 2, Figure 4).
Extrahepatic cancer
The incidence rates of extrahepatic cancer were 14.2 per 1,000 person-years (95% CI: 13.2, 15.1) and 11.7 per 1,000 person-years (95% CI: 11.4, 12.1) in persons with AIH and reference individuals, respectively (Table 2). Overall, individuals with AIH were at a 1.30 times higher risk of extrahepatic cancer, compared with reference individuals (95% CI: 1.19, 1.41), and this risk was stable beyond 10 years of follow-up (Table 2, Figure 5). The 10-year cumulative incidences of extrahepatic cancer in individuals with AIH with cirrhosis (14.4%; 95% CI: 11.5, 18.0) and fibrosis (14.2%; 95% CI: 11.7, 17.3) were higher than those of the reference individuals (Table 6).
Figure 5.
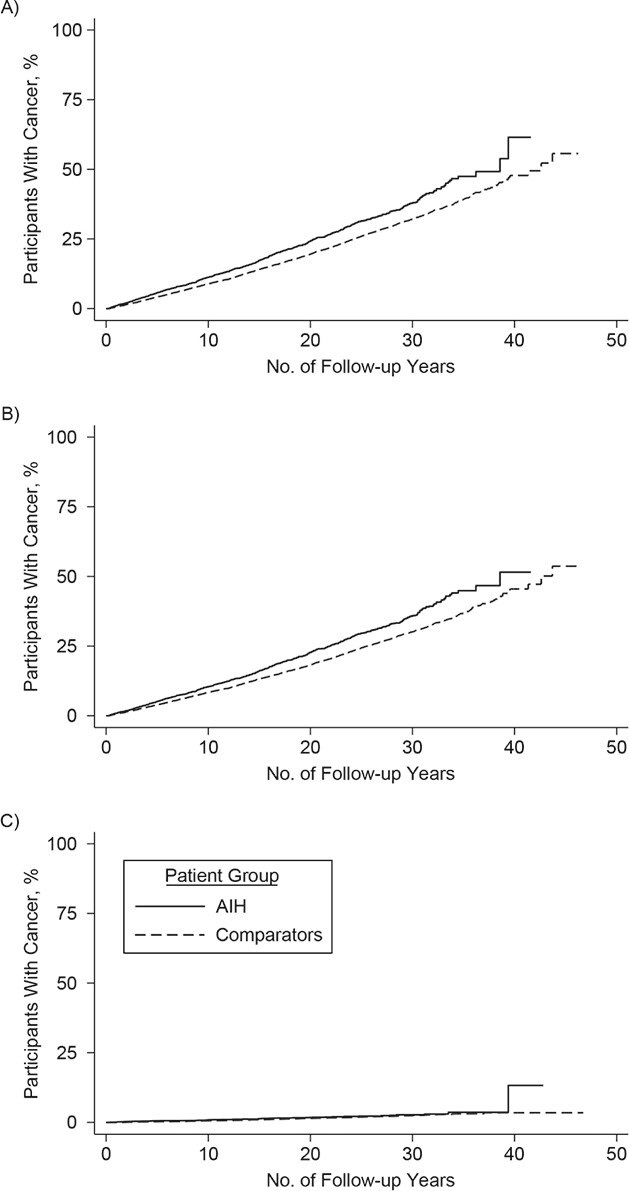
Kaplan-Meier failure curves of time to extrahepatic cancer in individuals with autoimmune hepatitis and in matched general-population reference individuals, Sweden 1969–2016. A) Extrahepatic cancer; B) extrahepatic solid-organ cancer; C) extrahepatic hematologic cancer. AIH, autoimmune hepatitis.
AIH was associated with increased risks of both extrahepatic solid and hematologic cancers (Table 2, Figure 5). The hazard ratio for nonmelanoma skin cancer was 2.69 (95% CI: 2.15, 3.37) and for lymphoma was 1.89 (95% CI: 1.25, 2.86).
Cancer-related death
The overall incidence rate of any cancer-related death in individuals with AIH was 2.5 per 1,000 person-years (95% CI: 2.1, 2.9) versus 0.8 per 1,000 person-years (95% CI: 0.7, 0.9) in reference individuals. This corresponded to a hazard ratio of 4.34 (95% CI: 3.28, 5.73). This risk was the highest in the first year of follow-up (HR = 29.16; 95% CI: 9.37, 90.71) and then stabilized to a hazard ratio of 3.19 (95% CI: 2.09, 4.88) beyond 10 years of follow-up (Web Table 7, Web Figure 2).
The overall incidence rate per 1,000 person-years of HCC-caused death was 0.7 (95% CI: 0.5, 0.9) in individuals with AIH and 0.0 (95% CI: 0.0, 0.0) in reference individuals. This was equal to a hazard ratio of 142.44 (95% CI: 12.93, 1569.58) (Web Table 7, Web Figure 2).
Sensitivity analyses
When including events from no follow-up to less than 3 months of follow-up, cancer risks were slightly elevated compared with our main analyses (Web Table 8). After excluding PSC and PBC at baseline, all cancer risks, especially hepatobiliary cancer risk, were similar to the main analyses (Web Table 9).
Adding censoring for liver transplant (HR = 1.48; 95% CI: 1.37, 1.61), restricting the study period to 1997–2016 (HR = 1.63; 95% CI: 1.36, 1.97), and restricting the time between ICD coding and liver biopsy to less than 1 year (HR = 1.42; 95% CI: 1.30, 1.56) did not affect the risk estimates (Web Table 10, Table 4). When restricting follow-up to 5 years, the hazard ratio for each ICD era only changed marginally (Table 4). Defining HCC through both the National Patient Register and Cancer Register did not influence the hazard ratio (HR = 28.41 (95% CI: 18.35, 43.99) compared with HR = 29.18 in our main analysis results reported in Table 2) (Web Table 10).
When recategorizing patients without cirrhosis at diagnosis as having cirrhosis if they received a cirrhosis ICD code during follow-up, the risks of any cancer were similar to main analyses (Web Table 11). Finally, the risk of any cancer in persons diagnosed with AIH while they were an inpatient (HR = 1.40, 95% CI: 0.92, 2.13) was similar to those diagnosed when they were an outpatient (HR = 1.57, 95% CI: 1.22, 2.03).
Sibling analyses
Compared with 4,170 siblings, the 2,185 individuals with AIH remained at increased risk of any cancer (HR = 1.84; 95% CI: 1.55, 2.18) and HCC (HR = 31.22; 95% CI: 7.30, 133.46) (Web Tables 12–13, Web Figure 3).
DISCUSSION
In this nationwide, population-based cohort of more than 5,000 individuals with AIH, we found that individuals with AIH had an increased risk of incident cancer compared with the general population and the risk persists beyond 10 years of follow-up. In particular, individuals with AIH had increased risks for HCC and extrahepatic cancers such as lymphoma and nonmelanoma skin cancer. Most notably, cancer risk was even higher in individuals with cirrhosis, likely driven by HCC.
We report an overall risk of any cancer in individuals with AIH (HR = 1.53) that is both similar to (8, 20) but also less than what was reported in previous studies (7, 11). Differing results from prior studies likely are due to smaller sample sizes, tertiary care centers that likely represent sicker patients, and potential AIH misclassification because of a lack of biopsy data. Our population-based design represents a wider range of disease severity sampled from both outpatient clinics and all tiers of hospitals. In addition, prior studies included follow-up periods ranging from 0 to less than 3 months, which could overestimate cancer risks given that we show a disproportionately high prevalence of cancer in the first 3 months of follow-up. To our knowledge, this is also the first study to use sibling comparators to reduce intrafamilial confounding; our results further confirm an increased risk of cancer in individuals with AIH.
Our reported incidence rates of HCC in all individuals with AIH and those with cirrhosis are slightly lower than pooled results from a meta-analysis (5) that comprised smaller, single-center studies with patients who likely were sicker than those in our study (6, 9, 21, 22) and smaller population-based studies that did not have complete liver biopsy data (7, 8, 10, 11, 23). We report a 1.1% HCC incidence risk per year in individuals with AIH who had cirrhosis, which is near but still below the recommended 1.5% per year cutoff for cost-effective HCC surveillance (1, 2, 24).
We also show that the risk of extrahepatic cancers, such as nonmelanoma skin cancer and lymphoma, was elevated in individuals with AIH compared with reference individuals (7, 8, 20, 25, 26), whereas the risks of common cancers such as colon, lung, and breast were not elevated. In subanalyses, we show that the 10-year cumulative incidence of extrahepatic cancer was increased in individuals with cirrhosis and fibrosis, suggesting that liver scarring may be tumorigenic beyond the liver (23), possibly due to increased systemic inflammation (27, 28) and gut dysbiosis (29) due to fibrosis and cirrhosis, but more studies are needed.
This study has several strengths. To our knowledge, this study is the largest, nationwide, population-based study in which the most individuals with AIH were identified, compared with any other study, by requiring the presence of a liver biopsy specimen for AIH diagnosis and thereby reducing misclassification, given that guidelines require liver biopsy (2, 30). Biopsy specimen–verified fibrosis status for each person with AIH in the ESPRESSO cohort and the large number of incident cancers allowed for key subanalyses by fibrosis status adjusted for confounders and for the calculation of precise incidence rates by fibrosis status in HCC that were not possible before, because of underpowered studies (5, 6, 9, 10). Because we only can comment on fibrosis status at diagnosis, risk estimates were similar to those of the main analyses when we reclassified persons without cirrhosis at diagnosis as having cirrhosis if they acquired a cirrhosis ICD code. In addition, defining outcomes using the Cancer Register and Death Register enabled us to obtain nearly 100% follow-up on all patients (15, 16). To increase sensitivity for HCC, we also defined HCC through both the cancer and patient registers, which resulted in similar hazard ratios, further suggesting high validity of our main analyses. As opposed to earlier studies, we excluded cancer at the start of follow-up, allowing us to calculate incidence. To improve the robustness of estimation of cancer risk, we excluded follow-up periods of 0 to less than 3 months to reduce the misclassification of biopsy specimens obtained at the time of cancer diagnoses, autopsy, or severe illness that overestimates risks. Finally, using siblings as comparators did not change our risk estimates, suggesting that intrafamilial confounding is unlikely to confound the association between AIH and cancer.
We also recognize some limitations. We did not have access to biological samples, patient charts, and laboratory tests to verify AIH diagnosis or track AIH disease activity. We report a 96% positive predictive value of having a histopathology report consistent with AIH and show a high proportion (82%) of individuals having 2 AIH ICD codes, which indicate high accuracy of our AIH criteria. To coincide with scoring systems for AIH diagnosis, ICD-10 codes more specific to AIH and viral hepatitis, and the inclusion of hospital-based outpatient data, we performed sensitivity analyses from 1997 onward and from 2002 onward, which resulted in similar cancer risk compared with older periods and the main analyses. When also excluding PSC and PBC from the cohort, censoring at liver transplant, and limiting time between ICD coding and biopsy, the risks of cancer were also similar to those determined in the main analyses. Of the 30% of individuals with other histopathology changes, the 2 most common features were steatosis, supported by prior evidence that 17%–30% of individuals with AIH have steatosis (1). Normal morphology was the third most common, but we did not have access to patient charts and could not determine whether these were remission biopsy procedures. We cannot rule out selection bias due to differences in biopsy practices or avoidance of liver biopsy in high-risk individuals. We adjusted for a higher prevalence of comorbid conditions in individuals with AIH, and the positive association between AIH and cancer still persisted, suggesting that the increased risk of any cancer in individuals with AIH is not confounded by comorbid conditions. Finally, there is a potential for residual confounding because the national registers do not include race/ethnicity, smoking, and body mass index data (31, 32).
In conclusion, this appears to be the first large, nationwide, population-based study to provide robust data on the risks of incident cancer in AIH, allowing physicians to appropriately counsel and monitor patients, especially those with cirrhosis. Although the HCC risk per year in individuals with cirrhosis is less than the recommended cost-effective cutoff, ongoing HCC surveillance is still warranted in those individuals, given their increased risk, and studies are needed to determine AIH-specific cutoffs and surveillance intervals.
Supplementary Material
ACKNOWLEDGMENTS
Author Affiliations: Center for Liver Disease and Transplantation, Division of Digestive and Liver Diseases, Columbia University Irving Medical Center, New York, New York, United States (Rajani Sharma, Elizabeth C. Verna); Division of Digestive and Liver Diseases, Department of Medicine, Columbia University College of Physicians and Surgeons, New York, New York, United States (Rajani Sharma, Elizabeth C. Verna, Jonas F. Ludvigsson, Peter H. R. Green); Transplant Hepatology, Division of Gastroenterology, Massachusetts General Hospital, Boston, Massachusetts, United States (Tracey G. Simon); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (Jonas Söderling, Jonas F. Ludvigsson); Unit of Hepatology, Center for Digestive Diseases, Karolinska University Hospital, and Unit of Clinical Epidemiology, Department of Medicine, Solna, Karolinska Institutet, Stockholm, Sweden (Hannes Hagström); Celiac Disease Center, Department of Medicine, Columbia University Irving Medical Center, New York, New York, United States (Peter H. R. Green); Department of Pediatrics, Örebro University Hospital, Örebro, Sweden (Jonas F. Ludvigsson); and Division of Epidemiology and Public Health, School of Medicine, and University of Nottingham, Nottingham, United Kingdom (Jonas F. Ludvigsson).
This work was funded by the Celiac Disease Center at Columbia University Medical Center (to P.H.R.G.) and The Karolinska Institutet (J.F.L.). H.H. was supported by grants from Stockholm County Council (a clinical postdoctoral appointment). T.S. is supported by the National Institutes of Health (grant K23 DK122104).
We thank Dr. Lorna Dove and Dr. Jean Emond for their contributions to this study.
Data are not available due to ethical restrictions.
None of the funding organizations had any role in the design and conduct of the study; in the collection, management, and analysis of the data; or in the preparation, review, and approval of the manuscript.
Conflicts of interest: JF.L. coordinates a study unrelated to the present study on behalf of the Swedish IBD Quality Register. That study has received funding from Janssen. H.H. has received research funding from Intercept, AstraZeneca. and Gilead. H.H. has served as a scientific board advisor for Bristol Myers Squibb and Gilead. None of these is deemed relevant to the present study. T.G.S. reports grants to Massachusetts General Hospital from Amgen; T.G.S. has previously served as a consultant to Aetion for work unrelated to this manuscript. E.C.V. is on the Gilead advisory board and receives Salix research support. This study did not receive any support from these organizations and these organizations did not have a role in the design and conduct of the study. Authors not named here have disclosed no conflicts of interest.
REFERENCES
- 1. Mack CL, Adams D, Assis DN, et al. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American Association for the Study of Liver Diseases. Hepatology. 2020;72(2):671–722. [DOI] [PubMed] [Google Scholar]
- 2. European Association for the Study of the Liver . EASL clinical practice guidelines: autoimmune hepatitis. J Hepatol. 2015;63(4):971–1004. [DOI] [PubMed] [Google Scholar]
- 3. Moss SF, Blaser MJ. Mechanisms of disease: inflammation and the origins of cancer. Nat Clin Pract Oncol. 2005;2(2):90–97. [DOI] [PubMed] [Google Scholar]
- 4. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tansel A, Katz LH, el-Serag HB, et al. Incidence and determinants of hepatocellular carcinoma in autoimmune hepatitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2017;15(8):1207–1217.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yeoman AD, al-Chalabi T, Karani JB, et al. Evaluation of risk factors in the development of hepatocellular carcinoma in autoimmune hepatitis: implications for follow-up and screening. Hepatology. 2008;48(3):863–870. [DOI] [PubMed] [Google Scholar]
- 7. Danielsson Borssén Å, Almer S, Prytz H, et al. Hepatocellular and extrahepatic cancer in patients with autoimmune hepatitis--a long-term follow-up study in 634 Swedish patients. Scand J Gastroenterol. 2015;50(2):217–223. [DOI] [PubMed] [Google Scholar]
- 8. Werner M, Almer S, Prytz H, et al. Hepatic and extrahepatic malignancies in autoimmune hepatitis. A long-term follow-up in 473 Swedish patients. J Hepatol. 2009;50(2):388–393. [DOI] [PubMed] [Google Scholar]
- 9. Hoeroldt B, McFarlane E, Dube A, et al. Long-term outcomes of patients with autoimmune hepatitis managed at a nontransplant center. Gastroenterology. 2011;140(7):1980–1989. [DOI] [PubMed] [Google Scholar]
- 10. Grønbæk L, Vilstrup H, Jepsen P. Autoimmune hepatitis in Denmark: incidence, prevalence, prognosis, and causes of death. A nationwide registry-based cohort study. J Hepatol. 2014;60(3):612–617. [DOI] [PubMed] [Google Scholar]
- 11. Ngu JH, Gearry RB, Frampton CM, et al. Mortality and the risk of malignancy in autoimmune liver diseases: a population-based study in Canterbury, New Zealand. Hepatology. 2012;55(2):522–529. [DOI] [PubMed] [Google Scholar]
- 12. Ludvigsson JF, Lashkariani M. Cohort profile: ESPRESSO (Epidemiology Strengthened by histoPathology Reports in Sweden). Clin Epidemiol. 2019;11:101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, et al. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19(12):1409–1417. [DOI] [PubMed] [Google Scholar]
- 15. Barlow L, Westergren K, Holmberg L, et al. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol. 2009;48(1):27–33. [DOI] [PubMed] [Google Scholar]
- 16. Brooke HL, Talbäck M, Hörnblad J, et al. The Swedish Cause of Death Register. Eur J Epidemiol. 2017;32(9):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ludvigsson JF, Svedberg P, Olén O, et al. The Longitudinal Integrated Database for Health Insurance and Labour Market Studies (LISA) and its use in medical research. Eur J Epidemiol. 2019;34(4):423–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Åberg F, Pukkala E, Höckerstedt K, et al. Risk of malignant neoplasms after liver transplantation: a population-based study. Liver Transpl. 2008;14(10):1428–1436. [DOI] [PubMed] [Google Scholar]
- 19. Törner A, Stokkeland K, Svensson Å, et al. The underreporting of hepatocellular carcinoma to the cancer register and a log-linear model to estimate a more correct incidence. Hepatology. 2017;65(3):885–892. [DOI] [PubMed] [Google Scholar]
- 20. Wang KK, Czaja AJ, Beaver SJ, et al. Extrahepatic malignancy following long-term immunosuppressive therapy of severe hepatitis B surface antigen-negative chronic active hepatitis. Hepatology. 1989;10(1):39–43. [DOI] [PubMed] [Google Scholar]
- 21. Montano-Loza AJ, Carpenter HA, Czaja AJ. Predictive factors for hepatocellular carcinoma in type 1 autoimmune hepatitis. Am J Gastroenterol. 2008;103(8):1944–1951. [DOI] [PubMed] [Google Scholar]
- 22. Wong RJ, Gish R, Frederick T, et al. Development of hepatocellular carcinoma in autoimmune hepatitis patients: a case series. Dig Dis Sci. 2011;56(2):578–585. [DOI] [PubMed] [Google Scholar]
- 23. Sørensen HT, Friis S, Olsen JH, et al. Risk of liver and other types of cancer in patients with cirrhosis: a nationwide cohort study in Denmark. Hepatology. 1998;28(4):921–925. [DOI] [PubMed] [Google Scholar]
- 24. Sarasin FP, Giostra E, Hadengue A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class A cirrhosis. Am J Med. 1996;101(4):422–434. [DOI] [PubMed] [Google Scholar]
- 25. Leung J, Dowling L, Obadan I, et al. Risk of non-melanoma skin cancer in autoimmune hepatitis. Dig Dis Sci. 2010;55(11):3218–3223. [DOI] [PubMed] [Google Scholar]
- 26. Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch Intern Med. 2005;165(20):2337–2344. [DOI] [PubMed] [Google Scholar]
- 27. Lin C-Y, Tsai I-F, Ho Y-P, et al. Endotoxemia contributes to the immune paralysis in patients with cirrhosis. J Hepatol. 2007;46(5):816–826. [DOI] [PubMed] [Google Scholar]
- 28. Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis. 2008;28(1):26–42. [DOI] [PubMed] [Google Scholar]
- 29. Bajaj JS, Heuman DM, Hylemon PB, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60(5):940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Manns MP, Lohse AW, Vergani D. Autoimmune hepatitis--update 2015. J Hepatol. 2015;62(1 Suppl):S100–S111. [DOI] [PubMed] [Google Scholar]
- 31. Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. [DOI] [PubMed] [Google Scholar]
- 32. Gandini S, Botteri E, Iodice S, et al. Tobacco smoking and cancer: a meta-analysis. Int J Cancer. 2008;122(1):155–164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.