Abstract
Poxviruses continue to pose a major threat to human health. Monkeypox is endemic in central Africa, and the discontinuation of the vaccination (with vaccinia virus) has rendered most humans vulnerable to variola virus, the etiologic agent of smallpox, should this virus be used in biological warfare or terrorism. However, a large variety of compounds have been described that are potent inhibitors of vaccinia virus replication and could be expected to be active against other poxviruses as well. These compounds could be grouped in different classes: (i) IMP dehydrogenase inhibitors (e.g., EICAR); (ii) SAH hydrolase inhibitors (e.g., 5′-noraristeromycin, 3-deazaneplanocin A, and various neplanocin A derivatives); (iii) OMP decarboxylase inhibitors (e.g., pyrazofurin) and CTP synthetase inhibitors (e.g., cyclopentenyl cytosine); (iv) thymidylate synthase inhibitors (e.g., 5-substituted 2′-deoxyuridines); (v) nucleoside analogues that are targeted at viral DNA synthesis (e.g., Ara-A); (vi) acyclic nucleoside phosphonates [e.g., (S)-HPMPA and (S)-HPMPC (cidofovir)]; and (vii) polyanionic substances (e.g., polyacrylic acid). All these compounds could be considered potential candidate drugs for the therapy and prophylaxis of poxvirus infections at large. Some of these compounds, in particular polyacrylic acid and cidofovir, were found to generate, on single-dose administration, a long-lasting protective efficacy against vaccinia virus infection in vivo. Cidofovir, which has been approved for the treatment of cytomegalovirus retinitis in immunocompromised patients, was also found to protect mice, again when given as a single dose, against a lethal aerosolized or intranasal cowpox virus challenge. In a biological warfare scenario, it would be advantageous to be able to use a single treatment for an individual exposed to an aerosolized poxvirus. Cidofovir thus holds great promise for treating human smallpox, monkeypox, and other poxvirus infections. Anecdotal experience points to the efficacy of cidofovir in the treatment of the poxvirus infections molluscum contagiosum and orf (ecthyma contagiosum) in immunosuppressed patients.
It has been more than 22 years since the last case of smallpox was confirmed and 20 years since the World Health Organization declared that the global eradication of smallpox had been achieved. The subsequent discontinuation of vaccination against smallpox (with vaccinia virus [VV] vaccine) has rendered most humans vulnerable to smallpox infection. The recent outbreak of monkeypox (against which the smallpox vaccine is protective) in the Democratic Republic of the Congo (formerly Zaire) and the genuine threat that variola virus, the etiological agent of smallpox, might be used as a biological weapon in warfare or terrorism (14, 60) have instigated the search for control measures to prevent or treat smallpox, monkeypox, and poxvirus infections in general.
Fittingly, the search for antiviral agents started with the poxviruses as target, when, over 50 years ago, the thiosemicarbazones, introduced by Domagk et al. (53) as tuberculostatic (antituberculous) agents, were also found to be active against VV (57). This work was continued by Bauer (5) and culminated in the demonstration by Bauer et al. (8) in 1963 that the thiosemicarbazone derivative methisazone (Marboran, N-methylisatin 3-thiosemicarbazone) was effective in the prophylaxis of smallpox. In several trials during Indian epidemics, methisazone proved its value by reducing the attack rate by 75 to 95%. Promising results have also been reported in the treatment of the complications of smallpox vaccination, vaccinia gangrenosa and eczema vaccinatum (55). As the principal side effect of methisazone, vomiting was noted, which, although severe, was considered a justifiable risk in the face of threatened smallpox (55). Needless to say, the drug was no substitute for vaccination, and with the imminent eradication of smallpox due to the successful implementation of the smallpox vaccine, the prophylactic or therapeutic usefulness of methisazone against smallpox was not further pursued.
Rifampin, which is still among the most effective tuberculostatic drugs, represents another example of an antibacterial agent that was found to interfere with the growth of poxviruses (i.e., VV) as well (108). Rifampin apparently inhibits the cleavage of a protein precursor that otherwise is cleaved into smaller polypeptides at the time of assembly (69). However, high concentrations of rifampin are needed to inhibit viral growth (100 μg/ml), which makes its clinical use in the treatment of poxvirus infections unlikely (79).
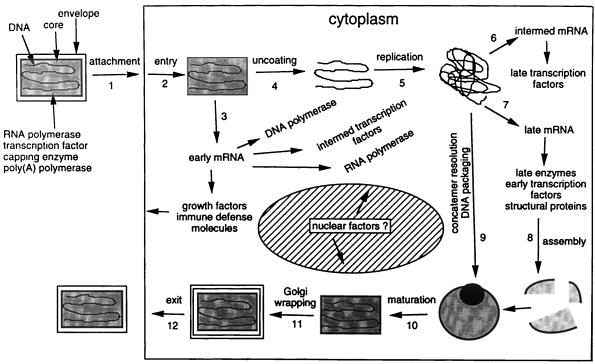
Poxviruses, including VV and variola virus, are characterized by a large linear double-stranded DNA genome (130 to 300 kb) packaged in a large virion (∼350 by 270 nm) and a cytoplasmic site of replication (Fig. 1) (78). Assembly of VV particles begins with the formation of intracellular mature virions, which then acquire additional membranes to form multiple membrane-wrapped, intracellular enveloped virions that fuse with the cell plasma membrane to form cell-associated enveloped virions, which are finally released from the cell as extracellular enveloped virions (reviewed in reference 64). The large genome, the concomitant large number of viral genes, the cytoplasmic site of replication, and the fastidious production process signal the possibility of chemotherapeutic intervention at a multitude of targets in the replicative cycle of poxviruses.
FIG. 1.
Poxvirus life cycle. Reprinted from reference 78 with permission of the publisher.
For more than 25 years, since we started the search for a selective antiviral chemotherapy (30), VV has been included in the panel of viruses that were evaluated for their susceptibility to a large variety of different classes of compounds. This search has yielded a wealth of substances, lead compounds, and approaches that proved effective against VV and therefore could be further pursued for the chemoprophylaxis and chemotherapy of poxvirus infections at large. Most of the compounds that have been identified as anti-VV agents are nucleoside analogues (28) that fall within the following categories: IMP dehydrogenase inhibitors (e.g., ribavirin), S-adenosylhomocysteine (AdoHcy, SAH) hydrolase inhibitors (e.g., neplanocin A), OMP decarboxylase inhibitors (e.g., pyrazofurin), CTP synthetase inhibitors (e.g., Ce-Cyd), thymidylate synthase inhibitors (dThd analogues), acyclic nucleoside phosphonates (e.g., cidofovir), and other nucleoside derivatives targeted at viral DNA synthesis.
IN VITRO EFFICACY OF DIFFERENT CLASSES OF COMPOUNDS AGAINST POXVIRUS INFECTIONS
IMP Dehydrogenase Inhibitors
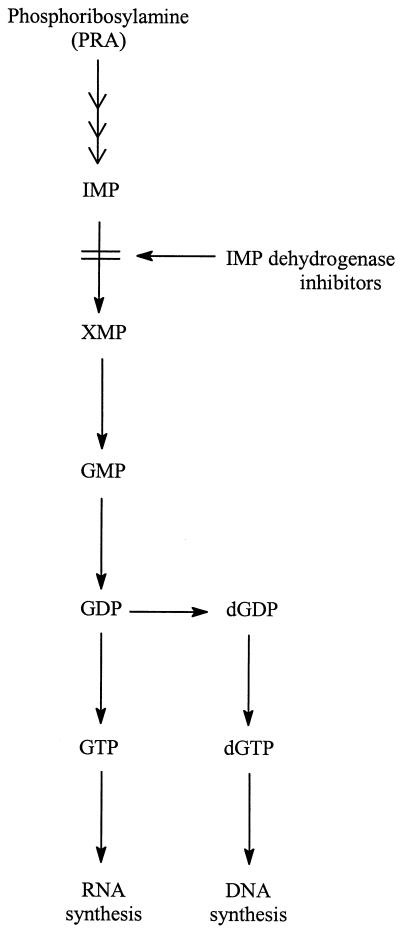
Ribavirin (Virazole; 1-β-d-ribofuranosyl-1,2-4-triazole-3-carboxamide) (Fig. 2) was the first nucleoside analogue shown to be active against a broad variety of both RNA and DNA viruses, including VV (102). This broad-spectrum antiviral activity has been attributed to the inhibition of IMP dehydrogenase by ribavirin 5′-monophosphate (107). IMP dehydrogenase converts IMP to XMP, a crucial step in the biosynthesis of the purine mononucleotides, i.e., GTP and dGTP (Fig. 3). Thus, inhibition of IMP dehydrogenase may be expected to lead to a depletion of both GTP and dGTP pools and hence to inhibition of both RNA and DNA synthesis. Reversal of the inhibition of VV growth by ribavirin upon addition of guanosine (68) is compatible with an action of ribavirin 5′-monophosphate targeted at IMP dehydrogenase.
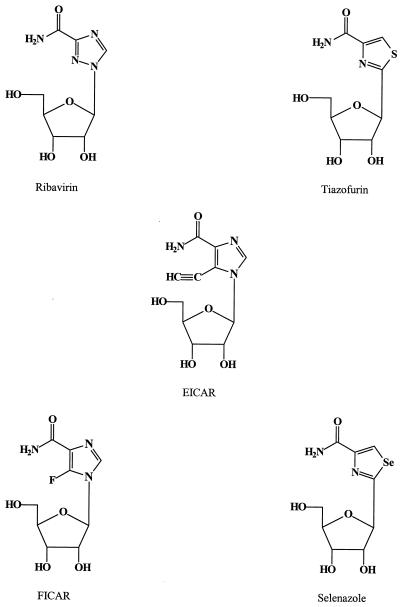
FIG. 2.
IMP dehydrogenase inhibitors.
FIG. 3.
Mechanism of action of IMP dehydrogenase inhibitors (ribavirin 5′-monophosphate and EICAR 5′-monophosphate).
In addition to ribavirin, several structurally related nucleoside analogues exhibit a similar antiviral activity spectrum, namely, FICAR (34), EICAR (50), tiazofurin (70), and selenazole (70), (Fig. 2). These compounds may, akin to ribavirin, be assumed to interact with IMP dehydrogenase, and, indeed, EICAR (5-ethynyl-1-β-d-ribofuranosylimidazole-4-carboxamide) 5′-monophosphate has proved to be a potent inhibitor of IMP dehydrogenase in intact cells (3, 4). Among the IMP dehydrogenase inhibitors, EICAR holds the greatest potential as an antipoxvirus agent (Table 1): its 50% inhibitory concentration (IC50) required to inhibit VV replication was 0.2 μg/ml, while it was not toxic to the host cell (primary rabbit kidney) monolayers, as assessed by microscopic inspection of cell morphology, at the highest concentration tested (400 μg/ml).
TABLE 1.
Comparative potency of different classes of compounds against VV
| Class and compound | Cell culturea | IC50b (μg/ml) | Reference(s) |
|---|---|---|---|
| IMP dehydrogenase inhibitors | |||
| Ribavirin | PRK | 4–20 | 34, 50 |
| FICAR | PRK | 100 | 34 |
| EICAR | PRK | 0.2 | 50 |
| Tiazofurin | Vero | 45 | 70 |
| Selenazole | Vero | 3 | 70 |
| SAH hydrolase inhibitors | |||
| (S)-DHPA | PRK | 10–20 | 35 |
| (RS)-AHPA | PRK | 3–30 | 41 |
| c3 Ado | PRK | 7 | 39 |
| C-c3 Ado | PRK | 0.8 | 39 |
| Neplanocin C | PRK | 0.1 | 24 |
| Neplanocin A | PRK | 0.03 | 24 |
| 3-Deazaneplanocin A | PRK | 0.07 | 47 |
| 3-Deazaneplanocin A | Vero | 0.03–0.3 | 109 |
| DHCeA | PRK | 0.7 | 47 |
| DHCeA | L929 | 0.1 | 58 |
| c3DHCeA | PRK | 0.7 | 47 |
| c3DHCeA | L929 | 0.03 | 58 |
| DHCaA | L929 | 0.03 | 59 |
| c3DHCaA | L929 | 0.03 | 59 |
| F-C-Ado | L929 | 0.2 | 21 |
| (±)-5′-Noraristeromycin | E6SM | 0.3 | 89 |
| (−)-5′-Noraristeromycin | E6SM | 0.04 | 99 |
| (+)-5′-Noraristeromycin | E6SM | 0.7 | 99 |
| epi(−)-5′-Noraristeromycin | E6SM | 0.1 | 100 |
| (−)-3-Deaza-5′-noraristeromycin | E6SM | 0.4 | 101 |
| (R)-6′-C-Methylneplanocin A | E6SM | 0.04 | 97 |
| 6′-Homoneplanocin A | E6SM | 0.1 | 98 |
| 2-Fluoroneplanocin A | E6SM | 0.1 | 88 |
| 6′-Iodo acetylenic Ado | E6SM | 0.1 | 91 |
| OMP decarboxylase/CTP synthetase inhibitors | |||
| Pyrazofurin | PRK | 0.1 | 51 |
| Pyrazofurin | Vero | 0.7 | 90 |
| 5′-Deoxypyrazofurin | E6SM | 5.5 | 15 |
| Carbodine | PRK | 15 | 48 |
| Ce-Cyd | PRK | 0.02 | 49 |
| Ce-Cyd | Vero | 0.1 | 74 |
| Thymidylate synthase inhibitors | |||
| 5-Fluoro-dUrd | PRK | 0.1 | 23 |
| 5-Chloro-dUrd | PRK | 0.2 | 23 |
| 5-Bromo-dUrd | PRK | 0.1 | 23 |
| 5-Iodo-dUrd | PRK | 0.3 | 23 |
| 5-Trifluoromethyl-dUrd | PRK | 0.2 | 23 |
| 5-Nitro-dUrd | PRK | 0.2 | 23 |
| 5-Formyl-dUrd | PRK | 0.2 | 23 |
| 5-Ethynyl-dUrd | PRK | 0.2 | 23 |
| 5-Vinyl-dUrd | PRK | 0.4 | 23 |
| 5-(1-Chlorovinyl)-dUrd | PRK | 0.7 | 23 |
| 5-Ethyl-dUrd | PRK | 1 | 23 |
| 5-Amino-dUrd | PRK | 1 | 23 |
| 5-Cyano-dUrd | PRK | 4 | 23 |
| 5-Hydroxymethyl-dUrd | PRK | 4 | 23 |
| 5-Thiocyano-dUrd | PRK | 4 | 23 |
| (E)-5-(2-Bromovinyl)-dUrd | PRK | 7 | 23 |
| Nucleoside analogues targeted at viral DNA synthesis | |||
| Adenine arabinoside | PRK | 0.4 | 37 |
| 3′-C-Methyl Ado | PRK | 7c | E. De Clercq and S. N. Mikhailov (unpublished data) |
| 8-Methyl Ado | E6SM | 0.2 | 110 |
| S2242 | E6SM | 0.4 | 83 |
| Acyclic nucleoside phosphonates | |||
| (S)-HPMPA | PRK | 0.3–0.7 | 27, 43 |
| (S)-HPMPC | PRK | 4 | 27 |
| (S)-HPMPC | Vero | 18 | 9 |
| (S)-HPMPC | BSC | 5 | 92 |
| 8-Aza-(S)-HPMPA | E6SM | 0.7–2 | 62 |
| Polyanionic substances | |||
| Dextran sulfate | PRK | 5–20 | 2, 113 |
| Pentosan polysulfate | PRK | 10–20 | 2, 113 |
| PVAS | PRK | 9–14 | 95 |
| PAVAS | PRK | 4–20 | 95 |
| Polyacrylic acid | REF | 0.1–1 | 52 |
| Thiosemicarbazones | |||
| Isatin 3-thiosemicarbazone | HeLa | <1 | 7 |
Abbreviations: PRK, primary rabbit kidney cells; Vero, African green monkey kidney cells; E6SM, embryonic skin-muscle fibroblasts; REF, rat embryo fibroblasts; L929, murine fibroblast cells; BSC, African green monkey kidney cells; HeLa, human cervical epithelial cells.
Concentration required to reduce virus replication (as monitored mostly by virus-induced cytopathogenicity or plaque formation) by 50%.
Minimum cytotoxic concentration, 40 μg/ml.
SAH Hydrolase Inhibitors
SAH hydrolase has been considered an attractive target for antiviral chemotherapy (114), and various (acyclic and, primarily, carbocyclic) analogues of adenosine that block the enzyme have proved to be active against a characteristic spectrum of viruses, encompassing, in particular, poxviruses (VV), negative-strand RNA viruses (bunyaviruses, arenaviruses [e.g., Junin and Tacaribe viruses], rhabdoviruses [e.g., vesicular stomatitis virus (VSV)], paramyxoviruses [e.g., parainfluenza virus, measles virus, and respiratory syncytial virus]), and double-stranded RNA viruses (reoviruses) (25). In fact, SAH hydrolase inhibitors can be recognized by their unique antiviral activity spectrum.
Subsequent to the acyclic adenosine analogues (S)-DHPA [(S)-9-(2,3-dihydroxypropyl)adenine] (35) and (RS)-AHPA [(RS)-3-adenin-9-yl-2-hydroxypropanoic acid] (41), which have rather weak activity against VV (Table 1), a wide variety of carbocyclic adenosine analogues (Fig. 4) have been found to be potent inhibitors of SAH hydrolase and to exhibit marked activity against VV. Thus, carbocyclic 3-deaza-adenosine (C-c3 Ado) (39), neplanocins A and C (24), 3-deazaneplanocin A (47, 109), 9-(trans-2′,trans-3′-dihydroxycyclopent-4′-enyl)adenine (DHCeA) and 9-(trans-2′,trans-3′-dihydroxycyclopent-4′-enyl)-3-deazaadenine (c3DHCeA) (47, 58) and the saturated derivatives thereof (DHCaA, c3DHCaA) (30), (±)-6′β-fluoroaristeromycin (F-C-Ado) (21), (±)-5′-noraristeromycin (89), (+)-5′-noraristeromycin and (−)-5′-noraristeromycin (99) and an epimer thereof (100), (−)-3-deaza-5′-noraristeromycin (101), (R)-6′-C-methylneplanocin A (97), 6′-homoneplanocin A (98), 2-fluoroneplanocin A (88), and 6′-iodo acetylenic Ado (91) all inhibit VV replication in cell culture at an IC50 of less than 1 μg/ml (Table 1). Of note, 3-deazaneplanocin A, DHCaA and c3DHCaA, (−)-5′-noraristeromycin, and (R)-6′-C-methylneplanocin A are among the most potent carbocyclic adenosine analogs, with an IC50 of about 0.1 μM (∼0.03 μg/ml). As a rule, these compounds were not toxic to the host cells (primary human embryonic skin-muscle fibroblasts in stationary culture) at the antivirally active concentrations: 6′-homoneplanocin A (98) and (R)-6′-C-methylneplanocin A (97) were not even cytotoxic at the highest concentration tested (400 μg/ml).
FIG. 4.
SAH hydrolase inhibitors.
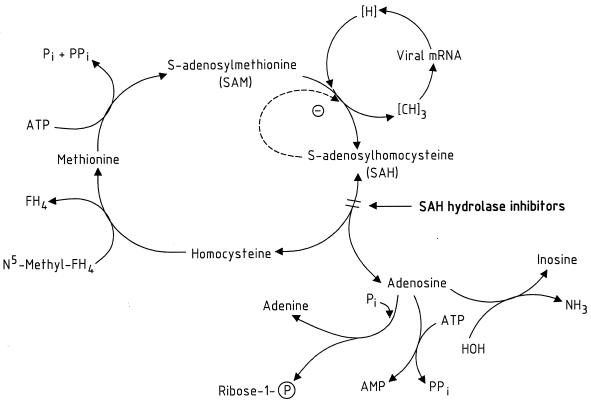
VV and the other viruses that are strongly inhibited by SAH hydrolase inhibitors must correspond to those that depend strongly on methylations (e.g., 5′-capping) requiring S-adenosylmethionine (AdoMet, SAM) as the methyl donor. Borchardt (10) pointed out that SAM-dependent methyltransferases play an important role in the 5′-cap formation and hence the maturation of VV mRNA; following this premise, Borchardt et al. ascertained that neplanocin A, a cyclopentenyl derivative of adenine originally isolated from the actinomycete Ampullariella regularis, potently inhibited both SAH hydrolase and VV replication (11). SAH is a product and inhibitor of the SAM-dependent methyltransferase reactions; it should be removed by SAH hydrolase for the methylations to proceed unabated. SAH hydrolase inhibitors interfere with this process. As shown in Fig. 5, SAH is normally cleaved by the hydrolase into its two components, homocysteine and adenosine (the latter is then further converted to inosine, adenine, or AMP). If this hydrolysis is suppressed by the SAH hydrolase inhibitors, SAH would accumulate and thus negatively affect the methyltransfer from SAM (Fig. 5).
FIG. 5.
Mechanism of action of SAH hydrolase inhibitors. Pi, inorganic phosphate; PPi, pyrophosphate.
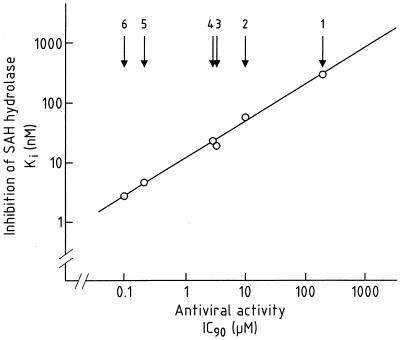
For a series of acyclic and carbocyclic adenosine analogues, a close correlation was found between their inhibitory effect on murine L929-cell SAH hydrolase and their inhibitory effect on the replication of VV (r = 0.993) (Fig. 6); thus, in order of increasing inhibitory action against both virus replication and SAH hydrolase activity, the compounds could be ranked as follows: (S)-DHPA < (RS)-AHPA < 3-deazaneplanocin A ≤ C-c3 Ado < Ado dialdehyde < neplanocin A (18, 42). These findings strongly point to SAH hydrolase as the target for the antiviral action of these compounds. SAH and SAM pool levels have been measured in VV-infected L929 cells exposed to the SAH hydrolase inhibitors (19), and, again, a close correlation (r = 0.972) was found between the SAH/SAM ratio and virus yield reduction, corroborating the role of SAH hydrolase in the anti-VV activity of these compounds.
FIG. 6.
Linear regression for Ki values of (S)-DHPA (point 1), (RS)-AHPA (point 2), 3-deazaneplanocin A (point 3), C-c3 Ado (point 4), Ado aldehyde (point 5), and neplanocin A (point 6) for L929 cell SAH hydrolase as a function of their IC90s for VV replication (yield) in L929 cells. Data taken from reference 18.
The antiviral (i.e., anti-VV) activity of SAH hydrolase inhibitors such as (S)-DHPA, (RS)-AHPA, C-c3 Ado, and neplanocin A can be significantly enhanced by addition of homocysteine: while homocysteine (at 10−3 M) by itself had no effect on VV replication, it lowered the IC50 of (S)-DHPA, (RS)-AHPA, C-c3 Ado, and neplanocin A from 150 to 10, from 2 to 0.07, from 2 to 0.2 and from 0.07 to 0.002 μg/ml, respectively (46). This synergistic activity between homocysteine and SAH hydrolase inhibitors could be attributed to increased SAH levels generated from homocysteine through residual SAH hydrolase activity in the synthetic direction (Fig. 5) while SAH hydrolase remains inhibited in the hydrolytic direction by the SAH hydrolase inhibitors (20). Thus, the class of SAH hydrolase inhibitors offers a unique variety of compounds and strategies that should be further explored as therapeutic modalities against poxvirus infections.
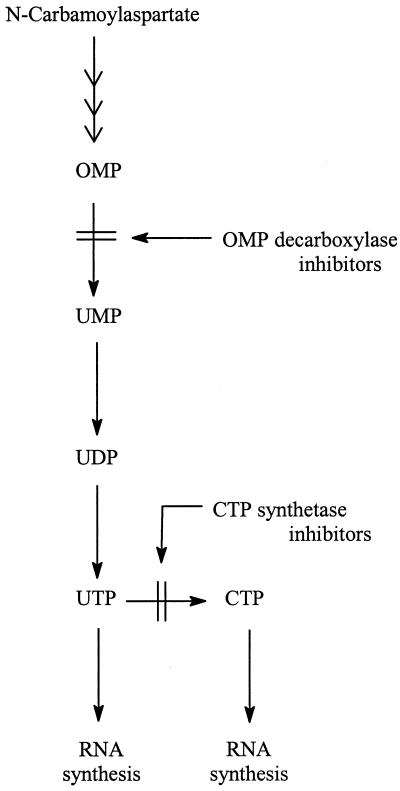
OMP Decarboxylase/CTP Synthetase Inhibitors
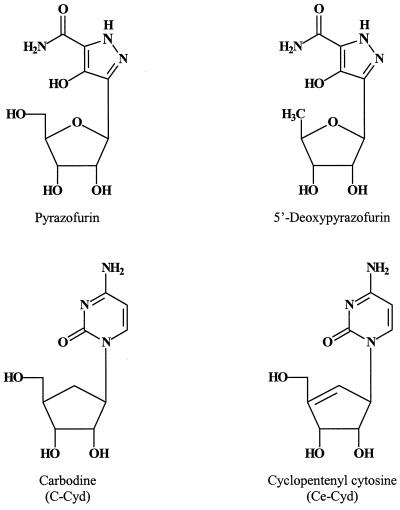
OMP decarboxylase and CTP synthetase have received little attention as potential target enzymes for antiviral agents, possibly because compounds that interact at these levels may be expected to shut off the pyrimidine mononucleotide biosynthetic pathway and hence impair normal cell growth and metabolism. Also, these enzymes are not associated with virus-specific enzymes. This contrasts with SAH hydrolase, which, at least for the poxviruses (such as VV), closely cooperates with a virus-encoded methyltransferase (mRNA-capping enzyme) (77). However, pyrazofurin, the prototype of the OMP decarboxylase inhibitors (Fig. 7), has long been known to inhibit the replication of a large variety of viruses, including VV (Table 1), at a concentration that is apparently not toxic to the host cells (51). With an IC50 of 0.1 μg/ml, and no apparent host cell toxicity up to a 1,000-fold-higher concentration, pyrazofurin exceeds ribavirin, and several other antiviral compounds, in both potency and selectivity. A few derivatives of pyrazofurin have been synthesized (90), including 5′-deoxypyrazofurin (15), but these compounds did not prove superior to pyrazofurin in potency.
FIG. 7.
OMP decarboxylase and CTP synthetase inhibitors.
Among the CTP synthetase inhibitors, carbodine (cyclopentyl cytosine [C-Cyd]) and especially cyclopentenyl cytosine (Ce-Cyd) (Fig. 7) have a prominent place as antiviral agents (48, 49). With an IC50 of 0.02 μg/ml and no host cell toxicity at a concentration of 400 μg/ml, Ce-Cyd may even be accredited with a selectivity index of 20,000 in (stationary-phase) cell cultures (49). Based on virus rating, which is a weighted measurement of antiviral activity that takes into account both the degree of inhibition of virus-induced cytopathicity and the degree of cytotoxicity produced by the test compound, Ce-Cyd can be considered an equally promising inhibitor of VV replication as acyclovir is for herpes simplex virus (HSV) replication (74).
As could be readily deduced from their target of action (Fig. 8), OMP decarboxylase inhibitors and CTP synthetase inhibitors should suppress RNA synthesis, which in stationary-phase (nondividing) cells would be reflected by a specific antiviral activity (e.g., against VV) and in proliferating (rapidly dividing) tumor cells would lead to significant cytotoxicity. While the potent cytotoxicity of Ce-Cyd made the development of the antitumor aspects of the compound the more attractive initially (74), the potential of Ce-Cyd, and pyrazofurin, following topical or systemic application, in the treatment of severe poxvirus infections should not be overlooked.
FIG. 8.
Mechanism of action of OMP decarboxylase inhibitors (e.g., pyrazofurin via its 5′-monophosphate) and CTP synthetase inhibitors (e.g., Ce-Cyd via its triphosphate).
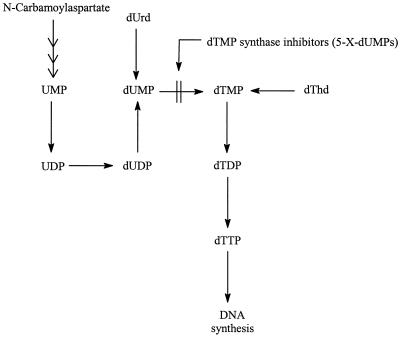
Thymidylate Synthase Inhibitors (5-Substituted 2′-Deoxyuridines)
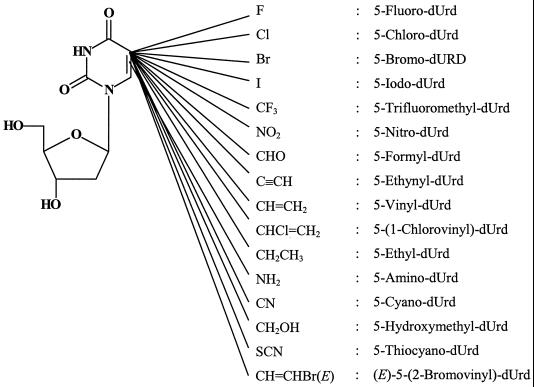
5-Substituted 2′-deoxyuridines (Fig. 9), e.g., 5-iodo-2′-deoxyuridine (5-iodo-dUrd), 5-trifluoromethyl-2′-deoxyuridine (5-trifluoromethyl-dUrd), and (E)-5-(2-bromovinyl)-2′-deoxyuridine (5-bromo-dUrd), have long been recognized as antiviral agents (30). They have been studied particularly intensively in the treatment of HSV infections. However, most of these 5-substituted 2′-deoxyuridines are also active against VV, and some dUrd derivatives, namely, 5-trifluoromethyl-dUrd, 5-nitro-dUrd, 5-formyl-dUrd, 5-ethynyl-dUrd, 5-amino-dUrd, and 5-cyano-dUrd, are even more active against VV than against HSV (23, 36) (Table 1). With IC50s of 0.1 to 0.2 μg/ml (Table 1), 5-fluoro-dUrd, 5-trifluoromethyl-dUrd, 5-formyl-dUrd, 5-ethynyl-dUrd, and 5-nitro-dUrd can be considered the most potent anti-VV agents among the 5-substituted dUrd derivatives (23). These were also the compounds that proved the most inhibitory to tumor (i.e., murine leukemia L1210) cell proliferation (38). For these compounds, thymidylate synthase (Fig. 10) was identified as the target enzyme for their inhibitory effect on tumor cell growth (38): as could be expected from specific thymidylate synthase inhibitors, these compounds were far more inhibitory to [2-14C]dUrd incorporation into host cell DNA than to [methyl-3H]dThd incorporation, and their inhibitory effects on tumor cell proliferation were more readily reversed by dThd than by dUrd. In PRK (primary rabbit kidney) cells, the host cells used to assess anti-VV activity, 5-fluoro-dUrd, 5-trifluoromethyl-dUrd, 5-formyl-dUrd, 5-ethynyl-dUrd, and 5-nitro-dUrd likewise inhibited dUrd incorporation to a much greater extent than they inhibited dThd incorporation (23). It can be inferred, therefore, that the antitumor activity, as well as the anti-VV activity, of these compounds is mediated by an inhibitory effect on the thymidylate synthase step that converts dUMP to dTMP (Fig. 10).
FIG. 9.
5-Substituted 2′-deoxyuridines. [Thymidylate synthase inhibitors: 5-fluoro-dUrd, 5-trifluoromethyl-dUrd, 5-nitro-dUrd, 5-formyl-dUrd, 5-ethynyl-dUrd, 5-(1-chlorovinyl)-dUrd.]
FIG. 10.
Mechanism of action of thymidylate synthase inhibitors. 5-X-dUMPs, 5′-monophosphates of 5-substituted 2′-deoxyuridines.
Nucleoside Analogues (Presumably) Targeted at Viral DNA Synthesis
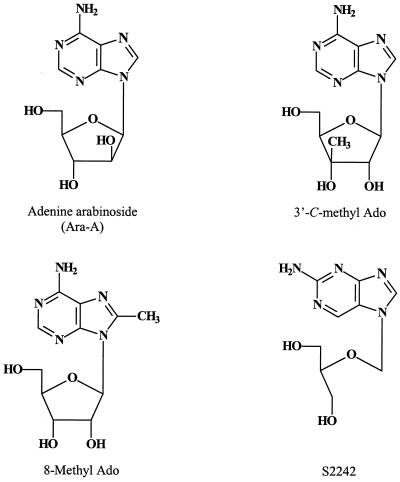
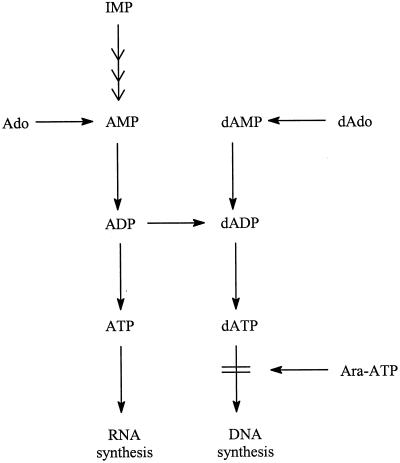
The 5-substituted dUrd derivatives that do not preferentially inhibit dUrd over dThd incorporation (e.g., 5-chloro-dUrd, 5-bromo-dUrd, and 5-iodo-dUrd) may be assumed not to owe their antiviral activity (Table 1) to a specific interaction with thymidylate synthase. They probably interfere with viral DNA synthesis on their conversion to the 5′-triphosphate form. There are a number of other nucleoside analogues that may be postulated to target viral DNA synthesis, e.g., adenine arabinoside (Ara-A) (Fig. 11). Ara-A is about 10 times more potent against VV than against either HSV-1 or HSV-2 (58). Its IC50 for VV is 0.4 μg/ml (Table 1), and its mode of action can be ascribed to an inhibitory effect at the viral DNA polymerase level (Fig. 12), where the 5′-triphosphate of Ara-A (Ara-ATP) enters into competition with dATP, the natural substrate for DNA synthesis. Ara-ATP also inhibits host cell DNA synthesis but only at a concentration that is substantially (i.e., 20-fold) higher than the concentration required to inhibit VV replication (37). In contrast, cytosine arabinoside (Ara-C) inhibits VV replication and host cell DNA synthesis at roughly the same concentrations (0.02 to 0.04 μg/ml); therefore, it cannot be considered a selective antiviral agent (37).
FIG. 11.
Nucleoside analogues (presumably) targeted at viral DNA synthesis.
FIG. 12.
Mechanism of action of adenine arabinoside 5′-triphosphate (Ara-ATP).
Some branched-chain sugar nucleosides (87), such as 3′-C-methyladenosine (Fig. 11) and 3′-C-methylcytidine, have been accredited with activity against VV: at a dose of 2 mg, they completely suppressed vaccinia tail lesion formation in mice (112). However, no attempts were ever made to further explore the therapeutic potential or elucidate the mechanism of action of these branched-chain sugar nucleosides.
Of a series of 2-, 6-, and 8-alkylated adenosine analogues (110), 8-methyladenosine (Fig. 11) emerged as a potent and selective inhibitor of VV (IC50, 0.2 μg/ml [Table 1]; minimum cytotoxic concentration, ≥200 μg/ml). The compound was inactive against VSV, which excludes an action targeted at SAH hydrolase (since VSV falls within the activity spectrum of SAH hydrolase inhibitors). Because of its remarkable anti-VV activity, 8-methyladenosine (110) should be further pursued from both mechanistic and therapeutic viewpoints. A somewhat related series of 8-alkynyl-, 8-alkenyl-, and 8-alkyl-2′-deoxyadenosine analogues have also been evaluated for their antiviral properties, and here the 8-heptynyl derivative emerged as the most active anti-VV agent, albeit with a modest potency (IC50, 7 μg/ml) (93).
Of particular interest is S2242, or 2-amino-7-(1,3-dihydroxy-2-propoxymethyl)purine (Fig. 11), the only acyclic nucleoside analogue with the side chain substituted at the N-7 position of the purine ring that has proved to be antivirally active (83). This compound is a potent and selective inhibitor of virtually all herpesviruses (83) and inhibits VV replication at an IC50 of 0.4 μg/ml (Table 1). In vivo, S2242 was more effective than acyclovir against HSV infection and far more effective than ganciclovir against cytomegalovirus (CMV) infection (84). Recently, this agent was shown to be fully protective against VV infection when given as its diacetate ester prodrug (H961) in both immunocompetent and SCID (severe combined immuno- deficient) mice (86) (see Table 2). Although the mode of anti-VV action of S2242 has not been established, it can be surmised that the compound needs to be phosphorylated intracellularly to its triphosphate (85) before the latter is able to block viral DNA synthesis.
TABLE 2.
Experimental animal models to demonstrate the efficacy of selected antiviral compounds against poxvirus infectionsa
| Animal model infection | Antiviral compound | Efficacious treatment regimen | Reference |
|---|---|---|---|
| Vaccinia keratitis in rabbits | Ribavirin | Ophthalmic ointment (5%) hourly from 8 a.m. to 7 p.m. daily for 7 days starting 1 day after infection | 103 |
| Vaccinia tail lesion formation in mice (following i.v. injection of VV) | Interferon | Five repeated i.p. doses of 10,000 U within 24 h before infection | 32 |
| Polyacrylic acid | Single i.p. dose of 0.5 mg/mouse (33 mg/kg) up to 4 wk before infection | 32 | |
| Ara-C, ribavirin, 5-iodo-dUrd, 5-ethyl-dUrd, 5-thiocyano-dUrd | Repeated i.p. doses of 4, 20, or 100 mg/kg daily for 7 days starting immediately after infection | 33 | |
| C-c3 Ado | Repeated i.p. doses of 5 mg/mouse daily for 4 days starting immediately after infection | 40 | |
| 3-Deazaneplanocin A, Ara-A | Repeated s.c. doses of 300 mg/kg/day (Ara-A) or 4 or 8 mg/kg/day (3-deazaneplanocin A) daily for 7 days starting 1 day before infection | 109 | |
| (S)-HPMPA | Repeated i.p. or s.c. doses of 5 mg/kg/day daily for 5 days starting immediately after infection | 45 | |
| Vaccinia tail lesion formation and death in SCID mice (following i.v. injection of VV) | (S)-HPMPC | Single s.c. dose of 100 mg/kg up to 7 days before infection, or repeated s.c. doses of 1, 5 or 20 mg/kg/day daily for 5 days starting immediately after infection | 82 |
| H961 (diacetate ester prodrug of S2242) | Repeated oral or s.c. doses of 100 mg/kg/day for 10 days, starting immediately after infection | 86 | |
| Intranasal cowpox virus infection in mice | (S)-HPMPC | Single s.c. dose of 100 mg/kg up to 16 days before or 4 days after infection, or repeated s.c. doses of 100 mg/kg every 3 or 6 days (in SCID mice) beginning on day 0 | 13 |
| (S)-HPMPC | Single intranasal dose of 2.5, 5, 10, 20 or 40 mg/kg at 24 h after infection | 104 | |
| Ribavirin followed by (S)-HPMPC | Repeated s.c. injections of ribavirin at 100 mg/kg/day on days 1–5 after infection, followed by single s.c. injection of (S)-HPMPC at 75 mg/kg on day 6, 7, 8, or 9 after infection | 105 |
i.p., intraperitoneal; s.c., subcutaneous; i.v., intravenous.
Acyclic Nucleoside Phosphonates
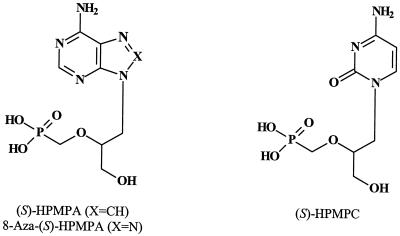
(S) - 9 - (3 - Hydroxy - 2 - phosphonylmethoxypropyl)adenine [(S)- HPMPA] (Fig. 13), the prototype of the acyclic nucleoside phosphonates (43), can be regarded as a hybrid molecule between (S)-DHPA (Fig. 4) and phosphonoacetic acid (PAA) (26). (S)-DHPA and PAA inhibit the replication of VV in PRK cell cultures at IC50s of 20 μg/ml (35) and 30 μg/ml (37), respectively. The “hybrid” molecule (S)-HPMPA inhibits the virus at a 100-fold-lower concentration, i.e., 0.3 μg/ml (43) (Table 1). (S)-HPMPA was found to exhibit a broad-spectrum activity against a wide variety of DNA viruses, including adenoviruses, herpesviruses, hepadnaviruses and iridoviruses as well as poxviruses (43). In contrast to the classical antiherpetic agents (acyclovir, 5-bromo-dUrd, etc.), (S)-HPMPA does not depend on a virus-specific thymidine kinase to exert its antiviral action. In fact, the compound is phosphorylated by cellular enzymes (such as AMP kinase and PRPP [5-phosphoribosyl-1-pyrophosphate] synthetase) to its active diphosphate form, (S)-HPMPApp (111), which then acts as an inhibitor of viral DNA synthesis (Fig. 14).
FIG. 13.
Acyclic nucleoside phosphonates.
FIG. 14.
Mechanism of action of acyclic nucleoside phosphonates.
(S)-1-(3-Hydroxy-2-phosphonylmethoxypropyl)cytosine [(S)-HPMPC, cidofovir], the cytosine counterpart of (S)-HPMPA (Fig. 13), has an activity spectrum quite similar to that of (S)-HPMPA (44); since (S)-HPMPC proved less toxic than (S)-HPMPA in vitro and in vivo (i.e., in mice), it was chosen as the antiviral drug candidate for further development (27). Its activity spectrum encompasses all DNA viruses, in particular papillomaviruses, polyomaviruses, adenoviruses, herpesviruses, and poxviruses. It has been licensed (as Vistide) for the treatment of CMV retinitis in AIDS patients, but it also has therapeutic potential, on either systemic or topical administration, in the treatment of various other herpesvirus, polyomavirus, papillomavirus, adenovirus, and poxvirus infections, as reviewed previously (29, 31). (S)-HPMPC confers a pronounced and prolonged inhibition of viral replication, lasting at least 7 days, after an exposure time as short as 6 h postinfection (78). This long-lasting antiviral action is a unique property of (S)-HPMPC that allows prophylactic and infrequent dosing of the drug (i.e., only once a week or every other week).
The long-lasting antiviral action of (S)-HPMPC may be attributed to the long half-life of its metabolites [i.e., (S)-HPMPCp, (S)-HPMPCpp, and (S)-HPMPCp-choline]; in particular, (S)-HPMPCp-choline may serve as the intracellular depot or reservoir form of (S)-HPMPC, since its intracellular half-life is extremely long (48 h) (16, 61). (S)-HPMPCpp represents the antivirally active metabolite of (S)-HPMPC (Fig. 14); it is formed intracellularly by two consecutive phosphorylation steps, catalyzed by a pyrimidine nucleoside monophosphate kinase and nucleoside diphosphate kinase, pyruvate kinase, or creatine kinase, respectively (17). HPMPCpp can function as a competitive inhibitor or alternate substrate (with regard to dCTP) in the viral DNA polymerase reaction. In human CMV DNA synthesis, two consecutive (S)-HPMPC molecules have to be incorporated into the viral DNA chain to completely arrest viral DNA synthesis (116).
Poxviruses fall within the activity spectrum of cidofovir (Table 1). In a comparative study of cidofovir activity against different poxviruses (92), the following IC50s were noted (in Vero cells): VV, 18 μg/ml; cowpox virus, 16 μg/ml; camelpox virus, 5 μg/ml; monkeypox virus, 19 μg/ml; variola virus, 1.5 μg/ml (data from J. Huggins, mentioned in reference 92). This study indicated that the smallpox virus variola virus was even more sensitive to the inhibitory effects of cidofovir than VV was.
In addition to (S)-HPMPA and (S)-HPMPC (cidofovir), a large variety of acyclic nucleotide analogues, e.g., 2′-aminomethyl derivatives (54), 8-azapurine derivatives (62), and various base-substituted derivatives (63), were synthesized and evaluated for their antiviral activity. Marked anti-VV activity was noted for the 8-aza-adenine counterpart of (S)-HPMPA, 8-aza-(S)-HPMPA (Fig. 13) (IC50, 0.7 to 2 μg/ml [Table 1]). Thus, the antiviral activity of 8-aza-(S)-HPMPA was comparable to that of the parent compound, (S)-HPMPA. Also, the 8-azaguanine counterpart of PMEG [9-(2-phosphonylmethoxyethyl)guanine] showed marked activity against VV (IC50, 0.7 μg/ml) as well as other viruses (HSV, varicella-zoster virus, and CMV) (62).
Polyanionic Substances
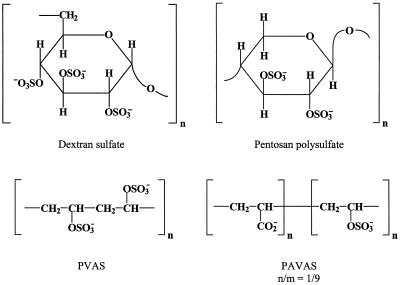
Polyanionic substances such as sulfated polysaccharides (dextran sulfate, pentosan polysulfate, carrageenans, and other polysaccharides extracted from seaweeds) are known for their inhibitory effects on enveloped viruses, including human immunodeficiency virus (HIV), HSV, and CMV, as well as VSV, respiratory syncytial virus, togaviruses, and arenaviruses (2, 113). The antiviral activity spectrum of these sulfated polymers (Fig. 15) also extends to VV, although, as a rule, VV is less sensitive to the sulfated polymers than are HIV, HSV, CMV, VSV, and respiratory syncytial virus. The IC50 of VV is in the range of 4 to 20 μg/ml (Table 1), which is about 10- to 100-fold higher than the IC50 of HIV (2, 113). The anti-VV activity of the sulfated polymers clearly depends on the molecular size of the polymers and their degree of sulfation, as has been determined for dextran sulfate (optimal molecular weight, 40,000) (113) and polyvinyl alcohol sulfate (PVAS) and its copolymer with polyacrylic acid (PAVAS) (95).
FIG. 15.
Sulfated polymers.
As originally demonstrated with HIV as the model virus, the sulfated polysaccharides owe their antiviral action to an inhibition of virion binding to the cells (1, 76). It can be surmised that the inhibitory effects of the sulfated polymers and other polyanions (such as polyacrylic acid [52]) on VV replication are likewise due to interference with the virus adsorption process. Polyanionic substances, such as polyoxometalates (117), telomerized anionic surfactants derived from amino acids (72), and micelle-like polyanionic compounds (73), that had proved active against HIV were also evaluated for, but found to be inactive against, VV. Of the polycationic substances studied for their effect on VV replication, some weak activity (IC50, 40 μg/ml) was noted with poly-l-lysine (molecular weight, 20,000) (65).
Thiosemicarbazones
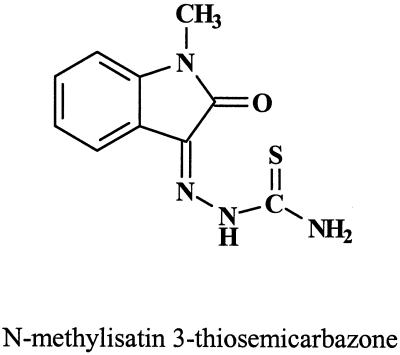
As mentioned in the Introduction, the first true antiviral agents to be discovered were the thiosemicarbazones (6). Their antiviral properties were reviewed by Bauer (7). That review includes clinical accounts of the use of methisazone (Fig. 16), the prototype of the thiosemicarbazones, in the treatment of smallpox, prophylaxis of smallpox, prophylaxis of smallpox vaccination, treatment of eczema vaccinatum, and treatment of vaccinia gangrenosa and also mentions the rather severe side effects of the compound (7). Also, methisazone was credited with activity against a number of viruses other than poxviruses (VV, smallpox virus, and cowpox virus), i.e., adenoviruses, herpesviruses, picornaviruses, reoviruses, arboviruses, and myxoviruses (7). According to the data presented in Bauer's review (7), the IC50 of isatin 3-thiosemicarbazone for VV multiplication in chicken embryo kidney cells or HeLa cells would be <1 μg/ml. From Huggins' data presented by Safrin et al. (92), however, it appears that the IC50 of methisazone for poxviruses is much higher: 33 μg/ml for monkeypox virus and 60 μg/ml for variola virus as measured in Vero and BSC cells. These concentrations are also much higher than the IC50s obtained for cidofovir under similar conditions (92). Mechanism-of-action studies have indicated that isatin 3-thiosemicarbazone interferes with viral maturation, consequently to the inhibition of viral protein synthesis from “late” viral mRNA (115).
FIG. 16.
Methisazone (Marboran).
Miscellaneous Compounds
Various analogues of adenosine-N1-oxide and 1-(benzyloxy)adenosine have been described as inhibitors of VV; 1-(3-methoxybenzyloxy)adenosine is the most potent in cell culture, and 1-(4-methoxybenzyloxy)adenosine is the most active in the mouse tailpox lesion model (71). The mechanism (or target) of action of these compounds was not addressed, however; also, issues on stability and pharmacology need to be addressed for a compound from this series to be carried forward.
VV replication can also be inhibited by antimetabolites that disturb cellular processes required for virus replication, such as N-(phosphonoacetyl)-l-aspartate, a potent inhibitor of aspartate carbamoyl transferase which markedly reduces the intracellular UTP and CTP pools (67).
Of the many compounds that inhibit VV replication, rifampin (mentioned above) and N1-isonicotinoyl-N2-3-methyl-4-chlorobenzoylhydrazine (IMCBH) specifically block virus assembly, rifampin through interacting with a 65-kDa polypeptide encoded by the D13 gene (106) and IMCBH through targeting a virus-encoded 37-kDa protein component of the Golgi-derived viral envelope (94).
In addition to rifampin and IMCBH, novobiocin, a well-known inhibitor of bacterial DNA gyrase and VV type 1 DNA topoisomerase, has also been reported as an inhibitor of VV assembly (96). Although it was doubted that the topoisomerase could act as the target of the antipoxvirus activity of novobiocin (96), other studies have raised the hope that small molecules may be found that may inhibit the replication of poxviruses, e.g., molluscum contagiosum virus, through interaction with its topoisomerase (66).
IN VIVO EFFICACY AGAINST EXPERIMENTAL POXVIRUS INFECTIONS
The tail lesion model (Table 2), as originally described by Boyle et al. (12), has proved to be a highly sensitive and reliable method to assess the in vivo efficacy of antiviral compounds against VV infection. Interferon was shown to be effective in this model if administered within 24 h prior to VV infection (32). Most remarkable was the prophylactic protection afforded by a single injection of polyacrylic acid, which persisted for at least 4 weeks (32). The protection engendered by polyacrylic acid could be attributed partially to interferon induction, but since it persisted for several weeks, long after interferon had ceased to be detectable in the bloodstream, other factors, such as a direct effect of the (long-lasting) compound on virus adsorption (52), must have been involved as well. Ribavirin was shown to be effective in the topical treatment of VV keratitis in rabbits (103) (Table 2). A number of nucleoside analogues were found to inhibit the formation of vaccinia tail lesions in mice infected intravenously with VV, with the order of (decreasing) activity being Ara-C > 5-iodo-dUrd > 5-thiocyano-dUrd > ribavirin > 5-ethyl-dUrd (33) (Table 2). Of the SAH hydrolase inhibitors (Fig. 4), C-c3 Ado (40) and 3-deazaneplanocin A (109) were found to inhibit vaccinia tail lesion formation; in the latter study, 3-deazaneplanocin A at 4 mg/kg/day proved as efficacious as Ara-A at 300 mg/kg/day.
In agreement with its potent in vitro activity against VV (Table 1), (S)-HPMPA strongly suppressed vaccinia tail lesion formation when given at a dose ranging from 5 to 100 mg/kg/day (45). Similarly, (S)-HPMPC suppressed tail lesion formation in both immunocompetent and immunosuppressed mice infected with VV; in SCID mice, (S)-HPMPC also suppressed VV replication in different organs (lungs, kidneys, liver, and brain) and prevented VV-induced death (82). Even if given as a single dose 7 days before infection, (S)-HPMPC was effective in suppressing tail lesion formation and delaying the mean day of death in VV-infected SCID mice (82) (Table 2).
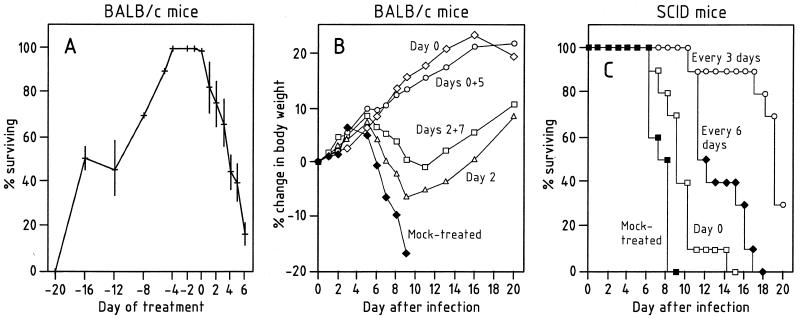
Taking advantage of the long-lasting antiviral efficacy of (S)-HPMPC (81, 82), Bray et al. (13) then assessed whether the compound may be prophylactically effective in their model of cowpox, whereby the cowpox virus was inoculated by aerosol or intranasally in mice (Table 2): they found that a single dose of 100 mg of cidofovir per kg (given subcutaneously) on day 0, 2, or 4 postinfection resulted in 90 to 100% survival after intranasal infection (Fig. 17A) (13). Even if given as long as 16 days before virus challenge, a single dose of 100 mg of cidofovir per kg still protected half of the mice (Fig. 17A). The efficacy displayed by cidofovir was also reflected by the increase in body weight (due to suppression of virus replication) (Fig. 17B). In SCID mice infected intranasally with cowpox virus, cidofovir caused a significant delay of death (Fig. 17C).
FIG. 17.
Intranasal cowpox virus infection. (A) Effect of a single subcutaneous injection of 100 mg of cidofovir per kg on survival after intranasal infection. Crosses, mean percent survival in two to six experiments, except for days −6 and −4, when one experiment was done. Vertical bars indicate standard deviations. (B) Effect on mean body weight of groups of 10 mice treated with 100 mg of cidofovir per kg on indicated days or mock treated with phosphate-buffered saline on day 0. (C) Effect of drug treatment on the course of intranasal cowpox virus infection in SCID mice. Mice were treated with 100 mg of cidofovir per kg on day 0 only or every 3 or 6 days, beginning on day 0, or were mock treated with phosphate-buffered saline on day 0. Reprinted from reference 13 with permission of the publisher.
Infection of mice with cowpox virus by the aerosol or intranasal route simulates the type of exposure that would occur during close contact of a patient with smallpox virus or monkeypox virus or during a hostile attack with an aerosolized biological warfare agent. Aerosol exposure of mice to a virulent orthopoxvirus represents a worst-case scenario in that it replicates a disease that does not occur in nature but that would inevitably result if a nonimmune human were unwittingly exposed to a small-particle aerosol of virulent virus (13). It is encouraging, therefore, that a single dose of cidofovir when given during an interval from a few days before till a few days after cowpox virus infection could enable the mice to survive this severe challenge.
Additional studies (104, 105) have confirmed the utility of cidofovir in the treatment of orthopoxvirus infections by the aerosol route, i.e., in mice infected intranasally with cowpox virus, initially treated with ribavirin (subcutaneously for 5 days at 100 mg/kg/day) and then given a single (subcutaneous) injection of cidofovir at 75 mg/kg on day 6, 7, 8, or 9 after infection (105). Also, a single intranasal application of cidofovir (at 10, 20, or 40 mg/kg) 24 h after intranasal challenge of mice with cowpox virus protected 90 to 100% of the animals against mortality (104).
CLINICAL EFFICACY
Based on the results obtained with cidofovir in the aerosol cowpox mouse model (13) and similar results obtained with nonhuman primates exposed to aerosolized monkeypox (J. W. Huggins, unpublished data, mentioned in reference 13), the drug may be assumed to be effective in the treatment of at least the early stages of human smallpox or monkeypox. There are (as yet) no data on the potential usefulness of cidofovir in the prophylaxis or therapy of smallpox, monkeypox, or vaccinia in humans. However, it has been reported that molluscum contagiosum, a skin disease that is caused by a poxvirus (molluscum contagiosum virus) and that can be quite severe in HIV-infected patients, resolved completely under intravenous and/or topical cidofovir treatment (after other therapies failed) (75). Similarly, topical cidofovir used in a boy with a primary immunodeficiency syndrome and severe molluscum contagiosum caused a dramatic resolution of all treated lesions (22). Furthermore, we have recently seen a particularly severe case of orf, also known as ecthyma contagiosum (and caused by a member of the poxvirus family), that completely regressed under local cidofovir treatment (56). The dramatic clinical responses observed following cidofovir treatment of molluscum contagiosum and orf, even if anecdotal, together with the conclusive evidence for the efficacy of cidofovir against experimental cowpox virus and VV infections, strongly point to the effectiveness of this compound against the poxvirus family at large.
CONCLUSION
Several strategies could be envisaged in the treatment of poxvirus infections: IMP dehydrogenase inhibitors, SAH hydrolase inhibitors, OMP decarboxylase inhibitors, CTP synthetase inhibitors, thymidylate synthase inhibitors, nucleoside analogues targeted at viral DNA synthesis, acyclic nucleoside phosphonates, polyanionic substances, thiosemicarbazones, and various other (miscellaneous) compounds. All these compounds inhibit VV replication in vitro, and some of the SAH hydrolase inhibitors (3-deazaneplanocin A), acyclic nucleoside phosphonates [(S)-HPMPA and (S)-HPMPC (cidofovir)], and polyanionic substances (polyacrylic acid) were also found to suppress the consequences of a VV infection in vivo. There exist a wealth of anti-VV agents which are potentially effective against other poxvirus infections (smallpox, monkeypox, etc.) and should be further pursued for this purpose.
Of the licensed antiviral compounds, cidofovir offers the greatest potential for the chemoprophylaxis and chemotherapy of poxvirus infections including smallpox. Its in vivo efficacy against VV infection has been demonstrated in both immunocompetent and immunodeficient hosts (82). In vivo efficacy of cidofovir has also been demonstrated against aerosolized or intranasal cowpox virus infection, transmitted by the route which may be used during an inadvertent exposure to smallpox virus or monkeypox virus (i.e., if these viruses are used as aerosolized biological warfare agents) (13). The efficacy noted with cidofovir against VV and cowpox virus in vivo allows us to predict that this compound should be effective in vivo against variola virus, which in vitro is more sensitive to cidofovir than are all the other poxviruses (92). In view of the long-lasting antiviral efficacy engendered by cidofovir (81), a single dose suffices to completely suppress virus infection in vitro. This has now also been demonstrated in vivo, in mice inoculated intranasally with cowpox virus (13). Finally, cidofovir has already been successfully used in the treatment of poxvirus infections (molluscum contagiosum [22, 75] and orf [ecthyma contagiosum] [56]) in (immunocompromised) patients.
The disadvantages of cidofovir are its poor oral bioavailability and dose-limiting nephrotoxicity on intravenous administration. The latter can be minimized through concomitant hydration and administration of probenecid. Its oral bioavailability may be increased and its nephrotoxicity decreased by the proper derivatizations, as shown for 1-[((S)-2-hydroxy-2-oxo-1,4,2-dioxaphosphorinan-5-yl)methyl]cytosine, an intracellular prodrug of cidofovir (9), and the bis(pivaloyloxy)methyl and bis[(isopropoxycarbonyl)oxy]methyl esters of the related acyclic nucleoside phosphonates adefovir (PMEA) and tenofovir (PMPA) (80).
In addition to cidofovir, various other compounds have been identified as potent anti-VV agents (Table 1): EICAR, neplanocin A, 3-deazaneplanocin A, DHCaA, c3DHCaA, 5′- noraristeromycin, 6′-homoneplanocin A, 2-fluoroneplanocin A, (R)-6′-C-methylneplanocin A, pyrazofurin, Ce-Cyd, 5-substituted 2′-deoxyuridines, Ara-A, 8-methyl-Ado, S2242, (S)-HPMPA, 8-aza-(S)-HPMPA, and polyacrylic acid. Based on their potency, virtually all these compounds could be envisaged as excellent candidates for further development as drugs to poxvirus infections. If they could be made available in sufficient quantities, and provided that they are not unduly toxic in vivo, these compounds should be further explored for their in vivo efficacy against poxvirus infections. If the drugs are intended for use against an aerosolized poxvirus infection, it should also be assessed whether they could be used prophylactically or therapeutically in aerosolized form. Certainly, for cidofovir, aerosol delivery would seem a viable alternative to intravenous dosing (104).
From a prospective viewpoint for the future design, synthesis, and development of new poxvirus inhibitors, it is gratifying to know that poxvirus infections can be approached by such a multitude of strategies, which, if combined, would most probably lead to a synergistic, or at least additive, antiviral activity as these different strategies are targeted at different steps in the replicative cycle of the virus.
ACKNOWLEDGMENT
I thank Christiane Callebaut for her dedicated editorial assistance.
REFERENCES
- 1.Baba M, Pauwels R, Balzarini J, Arnout J, Desmyter J, De Clercq E. Mechanism of inhibitory effect of dextran sulfate and heparin on replication of human immunodeficiency virus in vitro. Proc Natl Acad Sci USA. 1988;85:6132–6136. doi: 10.1073/pnas.85.16.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baba M, Snoeck R, Pauwels R, De Clercq E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob Agents Chemother. 1988;32:1742–1745. doi: 10.1128/aac.32.11.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balzarini J, De Clercq E. Assay method for monitoring the inhibitory effects of antimetabolites on the activity of inosinate dehydrogenase in intact human CEM lymphocytes. Biochem J. 1992;287:785–790. doi: 10.1042/bj2870785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balzarini J, Karlsson A, Wang L, Bohman C, Horská K, Votruba I, Fridland A, Van Aerschot A, Herdewijn P, De Clercq E. Eicar (5-ethynyl-1-β-d-ribofuranosylimidazole-4-carboxamide) J Biol Chem. 1993;268:24591–24598. [PubMed] [Google Scholar]
- 5.Bauer D J. The antiviral and synergistic actions of isathin thiosemicarbazone and certain phenoxypyrimidines in vaccinia infection in mice. Br J Exp Pathol. 1955;36:105–114. [PMC free article] [PubMed] [Google Scholar]
- 6.Bauer D J. International encyclopedia of pharmacology and therapeutics. 1. Chemotherapy of viral diseases. Oxford, United Kingdom: Pergamon Press Ltd.; 1972. Introduction to antiviral chemotherapy; pp. 1–33. [Google Scholar]
- 7.Bauer D J. International encyclopedia of pharmacology and therapeutics. 1. Chemotherapy of viral diseases. Oxford, United Kingdom: Pergamon Press Ltd.; 1972. Thiosemicarbazones; pp. 35–113. [Google Scholar]
- 8.Bauer D J, St. Vincent L, Kempe C H, Downie A W. Prophylactic treatment of smallpox contacts with N-methylisatin β-thiosemicarbazone. Lancet. 1963;ii:494–496. doi: 10.1016/s0140-6736(63)90230-7. [DOI] [PubMed] [Google Scholar]
- 9.Bischofberger N, Hitchcock M J M, Chen M S, Barkhimer D B, Cundy K C, Kent K M, Lacy S A, Lee W A, Li Z-H, Mendel D B, Smee D F, Smith J L. 1-[((S)-2-hydroxy-2-oxo-1,4,2-dioxaphosphorinan-5-yl)methyl]cytosine, an intracellular prodrug for (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine with improved therapeutic index in vivo. Antimicrob Agents Chemother. 1994;38:2387–2391. doi: 10.1128/aac.38.10.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borchardt R T. S-Adenosyl-l-methionine-dependent macromolecule methyltransferases: potential targets for the design of chemotherapeutic agents. J Med Chem. 1980;23:347–357. doi: 10.1021/jm00178a001. [DOI] [PubMed] [Google Scholar]
- 11.Borchardt R T, Keller B T, Patel-Thombre U. Neplanocin A. A potent inhibitor of S-adenosylhomocysteine hydrolase and of vaccina virus multiplication in mouse L929 cells. J Biol Chem. 1984;259:4353–4358. [PubMed] [Google Scholar]
- 12.Boyle J J, Haff R F, Stewart R C. Evaluation of antiviral compounds by suppression of tail lesions in vaccinia-infected mice. 1967. pp. 536–539. . Antimicrob. Agents Chemother. 1966. [PubMed] [Google Scholar]
- 13.Bray M, Martinez M, Smee D F, Kefauver D, Thompson E, Huggins J W. Cidofovir protects mice against lethal aerosol or intranasal cowpox virus challenge. J Infect Dis. 2000;181:10–19. doi: 10.1086/315190. [DOI] [PubMed] [Google Scholar]
- 14.Breman J G, Henderson D A. Poxvirus dilemmas—monkeypox, smallpox, and biologic terrorism. N Engl J Med. 1998;339:556–559. doi: 10.1056/NEJM199808203390811. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Schneller S W, Ikeda S, Snoeck R, Andrei G, Balzarini J, De Clercq E. Synthesis and antiviral activity of 5′-deoxypyrazofurin. J Med Chem. 1993;36:3727–3730. doi: 10.1021/jm00075a030. [DOI] [PubMed] [Google Scholar]
- 16.Cihlar T, Votruba I, Horská K, Liboska R, Rosenberg I, Holý A. Metabolism of 1-(S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine (HPMPC) in human embryonic lung cells. Collect Czech Chem Commun. 1992;57:661–672. [Google Scholar]
- 17.Cihlar T, Chen M S. Identification of enzymes catalyzing two-step phosphorylation of cidofovir and the effect of cytomegalovirus infection on their activities in host cells. Mol Pharmacol. 1996;50:1502–1510. [PubMed] [Google Scholar]
- 18.Cools M, De Clercq E. Correlation between the antiviral activity of acyclic and carbocyclic adenosine analogues in murine L929 cells and their inhibitory effect on L929 cell S-adenosylhomocysteine hydrolase. Biochem Pharmacol. 1989;38:1061–1067. doi: 10.1016/0006-2952(89)90249-9. [DOI] [PubMed] [Google Scholar]
- 19.Cools M, De Clercq E. Influence of S-adenosylhomocysteine hydrolase inhibitors on S-adenosylhomocysteine and S-adenosylmethionine pool levels in L929 cells. Biochem Pharmacol. 1990;40:2259–2264. doi: 10.1016/0006-2952(90)90720-6. [DOI] [PubMed] [Google Scholar]
- 20.Cools M, Hasobe M, De Clercq E, Borchardt R T. Mechanism of the synergistic antiviral and cytostatic activity of (RS)-3-(adenin-9-yl)-2-hydroxy-propanoic acid isobutyl ester and d,l-homocysteine. Biochem Pharmacol. 1990;39:195–202. doi: 10.1016/0006-2952(90)90665-8. [DOI] [PubMed] [Google Scholar]
- 21.Cools M, Balzarini J, De Clercq E. Mechanism of antiviral and cytotoxic action of (±)-6′ β-fluoroaristeromycin, a potent inhibitor of S-adenosylhomocysteine hydrolase. Mol Pharmacol. 1991;39:718–724. [PubMed] [Google Scholar]
- 22.Davies E G, Thrasher A, Lacey K, Harper J. Topical cidofovir for severe molluscum contagiosum. Lancet. 1999;353:2042. doi: 10.1016/s0140-6736(99)01782-1. [DOI] [PubMed] [Google Scholar]
- 23.De Clercq E. Antiviral and antitumor activities of 5-substituted 2′-deoxyuridines. Methods Find Exp Clin Pharmacol. 1980;2:253–267. [PubMed] [Google Scholar]
- 24.De Clercq E. Antiviral and antimetabolic activities of neplanocins. Antimicrob Agents Chemother. 1985;28:84–89. doi: 10.1128/aac.28.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Clercq E. S-Adenosylhomocysteine hydrolase inhibitors as broad-spectrum antiviral agents. Biochem Pharmacol. 1987;36:2567–2575. doi: 10.1016/0006-2952(87)90533-8. [DOI] [PubMed] [Google Scholar]
- 26.De Clercq E. Broad-spectrum anti-DNA virus and anti-retrovirus activity of phosphonylmethoxyalkylpurines and -pyrimidines. Biochem Pharmacol. 1991;42:963–972. doi: 10.1016/0006-2952(91)90276-b. [DOI] [PubMed] [Google Scholar]
- 27.De Clercq E. Therapeutic potential of HPMPC as an antiviral drug. Rev Med Virol. 1993;3:85–96. [Google Scholar]
- 28.De Clercq E. Antiviral activity spectrum and target of action of different classes of nucleoside analogues. Nucleosides Nucleotides. 1994;13:1271–1295. [Google Scholar]
- 29.De Clercq E. Therapeutic potential of cidofovir (HPMPC, VistideTM) for the treatment of DNA virus (i.e. herpes-, papova-, pox- and adenovirus) Verh K Acad Geneesk Belg. 1996;58:19–49. [PubMed] [Google Scholar]
- 30.De Clercq E. In search of a selective antiviral chemotherapy. Clin Microbiol Rev. 1997;10:674–693. doi: 10.1128/cmr.10.4.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Clercq E. Antiviral agents that are active against CMV: potential of cidofovir for the treatment of CMV and other virus infections. Monogr Virol. 1998;21:193–214. [Google Scholar]
- 32.De Clercq E, De Somer P. Effects of interferon, polyacrylic acid, and polymethacrylic acid on tail lesions in mice infected with vaccinia virus. Appl Microbiol. 1968;16:1314–1319. doi: 10.1128/am.16.9.1314-1319.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Clercq E, Luczak M, Shugar D, Torrence P F, Waters J A, Witkop B. Effect of cytosine arabinoside, iododeoxyuridine, ethyldeoxyuridine, thiocyanatodeoxyuridine, and ribavirin on tail lesion formation in mice infected with vaccinia virus. Proc Soc Exp Biol Med. 1975;151:487–490. doi: 10.3181/00379727-151-39241. [DOI] [PubMed] [Google Scholar]
- 34.De Clercq E, Luczak M, Reepmeyer J C, Kirk K L, Cohen L A. Fluoroimidazoles as antiviral agents and inhibitors of polynucleotide biosynthesis. Life Sci. 1975;17:187–194. doi: 10.1016/0024-3205(75)90502-0. [DOI] [PubMed] [Google Scholar]
- 35.De Clercq E, Descamps J, De Somer P, Holý A. (S)-9-(2,3-Dihydroxypropyl)adenine: an aliphatic nucleoside analog with broad-spectrum antiviral activity. Science. 1978;200:563–565. doi: 10.1126/science.200.4341.563. [DOI] [PubMed] [Google Scholar]
- 36.De Clercq E, Descamps J, Torrence P F, Krajewska E, Shugar D. Antiviral activity of novel deoxyuridine derivatives. In: Siegenthaler W, Lüthy R, editors. Current chemotherapy. Proceedings of the 10th International Congress of Chemotherapy, Zurich, Switzerland, 18–23 September 1977. Washington, D.C.: American Society for Microbiology; 1978. pp. 352–354. [Google Scholar]
- 37.De Clercq E, Descamps J, Verhelst G, Walker R T, Jones A S, Torrence P F, Shugar D. Comparative efficacy of antiherpes drugs against different strains of herpes simplex virus. J Infect Dis. 1980;141:563–574. doi: 10.1093/infdis/141.5.563. [DOI] [PubMed] [Google Scholar]
- 38.De Clercq E, Balzarini J, Torrence P F, Mertes M P, Schmidt C L, Shugar D, Barr P J, Jones A S, Verhelst G, Walker R T. Thymidylate synthetase as target enzyme for the inhibitory activity of 5-substituted 2′-deoxyuridines on mouse leukemia L1210 cell growth. Mol Pharmacol. 1981;19:321–330. [PubMed] [Google Scholar]
- 39.De Clercq E, Montgomery J A. Broad-spectrum antiviral activity of the carbocyclic analog of 3-deazaadenosine. Antivir Res. 1983;3:17–24. doi: 10.1016/0166-3542(83)90011-6. [DOI] [PubMed] [Google Scholar]
- 40.De Clercq E, Bergstrom D E, Holý A, Montgomery J A. Broad-spectrum antiviral activity of adenosine analogues. Antivir Res. 1984;4:119–133. doi: 10.1016/0166-3542(84)90012-3. [DOI] [PubMed] [Google Scholar]
- 41.De Clercq E, Holý A. Alkyl esters of 3-adenin-9-yl-2-hydroxypropanoic acid: a new class of broad-spectrum antiviral agents. J Med Chem. 1985;28:282–287. doi: 10.1021/jm00381a004. [DOI] [PubMed] [Google Scholar]
- 42.De Clercq E, Cools M. Antiviral potency of adenosine analogues: correlation with inhibition of S-adenosylhomocysteine hydrolase. Biochem Biophys Res Commun. 1985;129:306–311. doi: 10.1016/0006-291x(85)91438-x. [DOI] [PubMed] [Google Scholar]
- 43.De Clercq E, Holý A, Rosenberg I, Sakuma T, Balzarini J, Maudgal P C. A novel selective broad-spectrum anti-DNA virus agent. Nature. 1986;323:464–467. doi: 10.1038/323464a0. [DOI] [PubMed] [Google Scholar]
- 44.De Clercq E, Sakuma T, Baba M, Pauwels R, Balzarini J, Rosenberg I, Holý A. Antiviral activity of phosphonylmethoxyalkyl derivatives of purine and pyrimidines. Antivir Res. 1987;8:261–272. doi: 10.1016/s0166-3542(87)80004-9. [DOI] [PubMed] [Google Scholar]
- 45.De Clercq E, Holý A, Rosenberg I. Efficacy of phosphonylmethoxyalkyl derivatives of adenine in experimental herpes simplex virus and vaccinia virus infections in vivo. Antimicrob Agents Chemother. 1989;33:185–191. doi: 10.1128/aac.33.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Clercq E, Cools M, Balzarini J. Homocysteine potentiates the antiviral and cytostatic activity of those nucleoside analogues that are targeted at S-adenosylhomocysteine hydrolase. Biochem Pharmacol. 1989;38:1771–1778. doi: 10.1016/0006-2952(89)90411-5. [DOI] [PubMed] [Google Scholar]
- 47.De Clercq E, Cools M, Balzarini J, Marquez V E, Borcherding D R, Borchardt R T, Drach J C, Kitaoka S, Konno T. Broad-spectrum antiviral activities of neplanocin A, 3-deazaneplanocin A, and their 5′-nor derivatives. Antimicrob Agents Chemother. 1989;33:1291–1297. doi: 10.1128/aac.33.8.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Clercq E, Bernaerts R, Shealy Y F, Montgomery J A. Broad-spectrum antiviral activity of carbodine, the carbocyclic analogue of cytidine. Biochem Pharmacol. 1990;39:319–325. doi: 10.1016/0006-2952(90)90031-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Clercq E, Murase J, Marquez V E. Broad-spectrum antiviral and cytocidal activity of cyclopentenylcytosine, a carbocyclic nucleoside targeted at CTP synthetase. Biochem Pharmacol. 1991;41:1821–1829. doi: 10.1016/0006-2952(91)90120-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Clercq E, Cools M, Balzarini J, Snoeck R, Andrei G, Hosoya M, Shigeta S, Ueda T, Minakawa N, Matsuda A. Antiviral activities of 5-ethynyl-1-β-d-ribofuranosylimidazole-4-carboxamide and related compounds. Antimicrob Agents Chemother. 1991;35:679–684. doi: 10.1128/aac.35.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Descamps J, De Clercq E. Broad-spectrum antiviral activity of pyrazofurin (pyrazomycin) In: Siegenthaler W, Lüthy R, editors. Current chemotherapy. Proceedings of the 10th International Congress of Chemotherapy, Zurich, Switzerland, 18–23 September 1977. Washington, D.C.: American Society for Microbiology; 1978. pp. 354–357. [Google Scholar]
- 52.De Somer P, De Clercq E, Billiau A, Schonne E, Claesen M. Antiviral activity of polyacrylic and polymethacrylic acids. I. Mode of action in vitro. J Virol. 1968;2:878–885. doi: 10.1128/jvi.2.9.878-885.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Domagk G, Behnisch R, Mietzch F, Schmidt H. Über eine neue, gegen Tuberkelbazillen in vitro wirksame Verbindungsklasse. Naturwissenschaften. 1946;10:315. [Google Scholar]
- 54.Dvoraková H, Masojidková M, Holý A, Balzarini J, Andrei G, Snoeck R, De Clercq E. Synthesis of 2′-aminomethyl derivatives of N-(2-(phosphonomethoxy)ethyl) nucleotide analogues as potential antiviral agents. J Med Chem. 1996;39:3263–3268. doi: 10.1021/jm9601314. [DOI] [PubMed] [Google Scholar]
- 55.Fenner F J, White D O. Medical virology. New York, N.Y: Academic Press, Inc.; 1970. p. 190. [Google Scholar]
- 56.Geerinck, K., R. Snoeck, E. De Clercq, and H. Degreef. A case of human orf in an immunocompromised patient, successfully treated with cidofovir cream. J. Med. Virol., in press. [DOI] [PubMed]
- 57.Hamre D, Brownlee K A, Donovick R. Studies on the chemotherapy of vaccinia virus. II. The activity of some thiosemicarbazones. J Immunol. 1951;67:305–312. [PubMed] [Google Scholar]
- 58.Hasobe M, McKee J G, Borcherding D R, Borchardt R T. 9-(trans-2′,trans-3′-Dihydroxycyclopent-4′-enyl)-adenine and -3-deazaadenine: analogs of neplanocin A which retain potent antiviral activity but exhibit reduced cytotoxicity. Antimicrob Agents Chemother. 1987;31:1849–1851. doi: 10.1128/aac.31.11.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hasobe M, Liang H, Ault-Riche D B, Borcherding D R, Wolfe M S, Borchardt R T. (1′R,2′S,3′R)-9-(2′,3′-dihydroxycyclopentan- 1′-yl)-adenine and −3-deaza-adenine: analogues of aristeromycin which exhibit potent antiviral activity with reduced cytotoxicity. Antivir Chem Chemother. 1993;4:245–248. [Google Scholar]
- 60.Henderson D A. Bioterrorism as a public health threat. Emerg Infect Dis. 1998;4:488–492. doi: 10.3201/eid0403.980340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ho H-T, Woods K L, Bronson J J, De Boeck H, Martin J C, Hitchcock M J M. Intracellular metabolism of the antiherpes agent (S)-1-[3-hydroxy-2-(phosphonylmethoxy)propyl]cytosine. Mol Pharmacol. 1992;41:197–202. [PubMed] [Google Scholar]
- 62.Holý A, Dvoraková H, Jindrich J, Masojídková M, Budesinský M, Balzarini J, Andrei G, De Clercq E. Acyclic nucleotide analogs derived from 8-azapurines: synthesis and antiviral activity. J Med Chem. 1996;39:4073–4088. doi: 10.1021/jm960314q. [DOI] [PubMed] [Google Scholar]
- 63.Holý A, Günter J, Dvoráková H, Masojídková M, Andrei G, Snoeck R, Balzarini J, De Clercq E. Structure-antiviral activity relationship in the series of pyrimidine and purine N-[2-(2-phosphonomethoxy)ethyl] nucleotide analogues. 1. Derivatives substituted at the carbon atoms of the base. J Med Chem. 1999;42:2064–2086. doi: 10.1021/jm9811256. [DOI] [PubMed] [Google Scholar]
- 64.Hooper J W, Custer D M, Schmaljohn C S, Schmaljohn A L. DNA vaccination with vaccinia virus L1R and A33R genes protects mice against a lethal poxvirus challenge. Virology. 2000;266:329–339. doi: 10.1006/viro.1999.0096. [DOI] [PubMed] [Google Scholar]
- 65.Hosoya M, Neyts J, Yamamoto N, Schols D, Snoeck R, Pauwels R, De Clercq E. Inhibitory effects of polycations on the replication of enveloped viruses (HIV, HSV, CMV, RSV, influenza A virus and togaviruses) Antivir Chem Chemother. 1991;2:243–248. [Google Scholar]
- 66.Hwang Y, Wang B, Bushman F D. Molluscum contagiosum virus topoisomerase: purification, activities and response to inhibitors. J Virol. 1998;72:3401–3406. doi: 10.1128/jvi.72.4.3401-3406.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Katsafanas G C, Grem J L, Blough H A, Moss B. Inhibition of vaccinia virus replication by N-(phosphonoacetyl)-l-aspartate: differential effects on viral gene expression result from a reduced pyrimidine nucleotide pool. Virology. 1997;236:177–187. doi: 10.1006/viro.1997.8735. [DOI] [PubMed] [Google Scholar]
- 68.Katz E, Margalith E, Winer B. Inhibition of vaccinia virus growth by the nucleoside analogue 1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide (Virazole, Ribavirin) J Gen Virol. 1976;32:327–330. doi: 10.1099/0022-1317-32-2-327. [DOI] [PubMed] [Google Scholar]
- 69.Katz E, Moss B. Formation of a vaccinia virus structural polypeptide from a higher molecular weight precursor: inhibition by rifampicin. Proc Natl Acad Sci USA. 1970;66:677–684. doi: 10.1073/pnas.66.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kirsi J J, North J A, McKernan P A, Murray B K, Canonico P G, Huggins J W, Srivastava P C, Robins R K. Broad-spectrum antiviral activity of 2-β-d-ribofuranosylselenazole-4-carboxamide, a new antiviral agent. Antimicrob Agents Chemother. 1983;24:353–361. doi: 10.1128/aac.24.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kwong C D, Krauth C A, Shortnacy-Fowler A T, Arnett G, Hollingshead M G, Shannon W M, Montgomery J A, Secrist J A., III Synthesis and antiviral evaluation of analogs of adenosine-N1-oxide and 1-(benzyloxy)adenosine. Nucleosides Nucleotides. 1998;17:1409–1443. doi: 10.1080/07328319808003478. [DOI] [PubMed] [Google Scholar]
- 72.Leydet A, Barragan V, Boyer B, Montéro J L, Roque J P, Witvrouw M, Esté J, Snoeck R, Andrei G, De Clercq E. Polyanion inhibitors of human immunodeficiency virus and other viruses. 5. Telomerized anionic surfactants derived from amino acids. J Med Chem. 1997;40:342–349. doi: 10.1021/jm960493b. [DOI] [PubMed] [Google Scholar]
- 73.Leydet A, Jeantet-Segonds C, Bouchitté C, Moullet C, Boyer B, Roque J P, Witvrouw M, Esté J, Snoeck R, Andrei G, De Clercq E. Polyanion inhibitors of human immunodeficiency virus and other viruses. 6. Micelle-like anti-HIV polyanionic compounds based on a carbohydrate core. J Med Chem. 1997;40:350–356. doi: 10.1021/jm960348y. [DOI] [PubMed] [Google Scholar]
- 74.Marquez V E, Lim M-I, Treanor S P, Plowman J, Priest M A, Markovac A, Sami Khan M, Kaskar B, Driscoll J S. Cyclopentenylcytosine. A carbocyclic nucleoside with antitumor and antiviral properties. J Med Chem. 1988;31:1687–1694. doi: 10.1021/jm00117a004. [DOI] [PubMed] [Google Scholar]
- 75.Meadows K P, Tyring S K, Pavia A T, Rallis T M. Resolution of recalcitrant molluscum contagiosum virus lesions in human immunodeficiency virus-infected patients treated with cidofovir. Arch Dermatol. 1997;133:987–990. [PubMed] [Google Scholar]
- 76.Mitsuya H, Looney D J, Kuno S, Ueno R, Wong-Staal F, Broder S. Dextran sulfate suppression of viruses in the HIV family: inhibition of virion binding to CD4+ cells. Science. 1988;240:646–649. doi: 10.1126/science.2452480. [DOI] [PubMed] [Google Scholar]
- 77.Morgan J R, Cohen L K, Roberts B E. Identification of the DNA sequences encoding the large subunit of the mRNA-capping enzyme of vaccinia virus. J Virol. 1984;52:206–214. doi: 10.1128/jvi.52.1.206-214.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moss B. Poxviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2637–2671. [Google Scholar]
- 79.Müller W E G. Mechanism of action and pharmacology: chemical agents. In: Galasso G J, Merigan T C, Buchanan R A, editors. Antiviral agents and viral diseases of man. New York, N.Y: Raven Press; 1979. pp. 77–149. [Google Scholar]
- 80.Naesens L, Snoeck R, Andrei G, Balzarini J, Neyts J, De Clercq E. HPMPC (cidofovir), PMEA (adefovir) and related acyclic nucleoside phosphonate analogues: a review of their pharmacology and clinical potential in the treatment of viral infections. Antivir Chem Chemother. 1997;8:1–23. [Google Scholar]
- 81.Neyts J, Snoeck R, Balzarini J, De Clercq E. Particular characteristics of the anti-human cytomegalovirus activity of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine (HPMPC) in vitro. Antivir Res. 1991;16:41–52. doi: 10.1016/0166-3542(91)90057-x. [DOI] [PubMed] [Google Scholar]
- 82.Neyts J, De Clercq E. Efficacy of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine for the treatment of lethal vaccinia virus infections in severe combined immune deficiency (SCID) mice. J Med Virol. 1993;41:242–246. doi: 10.1002/jmv.1890410312. [DOI] [PubMed] [Google Scholar]
- 83.Neyts J, Andrei G, Snoeck R, Jähne G, Winkler I, Helsberg M, Balzarini J, De Clercq E. The N-7-substituted acyclic nucleoside analog 2-amino-7-[(1,3-dihydroxy-2-propoxy)methyl]purine is a potent and selective inhibitor of herpesvirus replication. Antimicrob Agents Chemother. 1994;38:2710–2716. doi: 10.1128/aac.38.12.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Neyts J, Jähne G, Andrei G, Snoeck R, Winkler I, De Clercq E. In vivo antiherpesvirus activity of N-7-substituted acyclic nucleoside analog 2-amino-7-[(1,3-dihydroxy-2-propoxy)methyl]purine. Antimicrob Agents Chemother. 1995;39:56–60. doi: 10.1128/aac.39.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Neyts J, Balzarini J, Andrei G, Chaoyong Z, Snoeck R, Zimmerman A, Mertens T, Karlsson A, De Clercq E. Intracellular metabolism of the N7-substituted acyclic nucleoside analog 2-amino-7-(1,3-dihydroxy-2-propoxymethyl)purine, a potent inhibitor of herpesvirus replication. Mol Pharmacol. 1998;53:157–165. doi: 10.1124/mol.53.1.157. [DOI] [PubMed] [Google Scholar]
- 86.Neyts J, De Clercq E. Efficacy of 2-amino-7-[(1,3-dihydroxy-2-propoxy)methyl]purine for the treatment of vaccinia (orthopox-) virus infections in mice. Antimicrob Agents Chemother. 2001;45:84–87. doi: 10.1128/AAC.45.1.84-87.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nutt R F, Dickinson M J, Holly F W, Walton E. Branched-chain sugar nucleosides. III. 3′-C-methyladenosine. J Org Chem. 1968;33:1789–1795. doi: 10.1021/jo01269a019. [DOI] [PubMed] [Google Scholar]
- 88.Obara T, Shuto S, Saito Y, Snoeck R, Andrei G, Balzarini J, De Clercq E, Matsuda A. New neplanocin analogues. 7. Synthesis and antiviral activity of 2-halo derivatives of neplanocin A. J Med Chem. 1996;39:3847–3852. doi: 10.1021/jm960145+. [DOI] [PubMed] [Google Scholar]
- 89.Patil S D, Schneller S W, Hosoya M, Snoeck R, Andrei G, Balzarini J, De Clercq E. Synthesis and antiviral properties of (±)-5′-noraristeromycin and related purine carbocyclic nucleosides. A new lead for anti-human cytomegalovirus agent design. J Med Chem. 1992;35:3372–3377. doi: 10.1021/jm00096a012. [DOI] [PubMed] [Google Scholar]
- 90.Petrie C R, III, Revankar G R, Dalley N K, George R D, McKernan P A, Hamill R L, Robins R K. Synthesis and biological activity of certain nucleoside and nucleotide derivatives of pyrazofurin. J Med Chem. 1986;29:268–278. doi: 10.1021/jm00152a016. [DOI] [PubMed] [Google Scholar]
- 91.Robins M J, Wnuk S F, Yang X, Yuan C-S, Borchardt R T, Balzarini J, De Clercq E. Inactivation of S-adenosyl-l-homocysteine hydrolase and antiviral activity with 5′,5′,6′,6′-tetradehydro-6′-deoxy-6′-halohomoadenosine analogues (4′-haloacetylene analogues derived from adenosine) J Med Chem. 1998;41:3857–3864. doi: 10.1021/jm980163m. [DOI] [PubMed] [Google Scholar]
- 92.Safrin S, Cherrington J, Jaffe H S. Clinical uses of cidofovir. Rev Med Virol. 1997;7:145–156. doi: 10.1002/(sici)1099-1654(199709)7:3<145::aid-rmv196>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 93.Sági G, Ötvös L, Ikeda S, Andrei G, Snoeck R, De Clercq E. Synthesis and antiviral activities of 8-alkynyl-, 8-alkenyl-, and 8-alkyl-2′-deoxyadenosine analogues. J Med Chem. 1994;37:1307–1311. doi: 10.1021/jm00035a010. [DOI] [PubMed] [Google Scholar]
- 94.Schmutz C, Payne L G, Gubser J, Wittek R. A mutation in the gene encoding the vaccinia virus 37,000-Mr protein confers resistance to an inhibitor of virus envelopment and release. J Virol. 1991;65:3435–3442. doi: 10.1128/jvi.65.7.3435-3442.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schols D, De Clercq E, Balzarini J, Baba M, Witvrouw M, Hosoya M, Andrei G, Snoeck R, Neyts J, Pauwels R, Nagy M, Györgyi-Edelényi J, Machovich R, Horváth I, Löw M, Görög S. Sulphated polymers are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, respiratory syncytial virus, and toga-, arena- and retroviruses. Antivir Chem Chemother. 1990;1:233–240. [Google Scholar]
- 96.Sekiguchi J A, Shuman S. Novobiocin inhibits vaccinia virus replication by blocking virus assembly. J Virol. 1997;235:129–137. doi: 10.1006/viro.1997.8684. [DOI] [PubMed] [Google Scholar]
- 97.Shuto S, Obara T, Toriya M, Hosoya M, Snoeck R, Andrei G, Balzarini J, De Clercq E. New neplanocin analogues. 1. Synthesis of 6′-modified neplanocin A derivatives as broad-spectrum antiviral agents. J Med Chem. 1992;35:324–331. doi: 10.1021/jm00080a018. [DOI] [PubMed] [Google Scholar]
- 98.Shuto S, Obara T, Saito Y, Andrei G, Snoeck R, De Clercq E, Matsuda A. New neplanocin analogues. 6. Synthesis and potent antiviral activity of 6′-homoneplanocin A. J Med Chem. 1996;39:2392–2399. doi: 10.1021/jm950853f. [DOI] [PubMed] [Google Scholar]
- 99.Siddiqi S M, Chen X, Schneller S W, Ikeda S, Snoeck R, Andrei G, Balzarini J, De Clercq E. Antiviral enantiomeric preference for 5′-noraristeromycin. J Med Chem. 1994;37:551–554. doi: 10.1021/jm00030a014. [DOI] [PubMed] [Google Scholar]
- 100.Siddiqi S M, Chen X, Schneller S W, Ikeda S, Snoeck R, Andrei G, Balzarini J, De Clercq E. An epimer of 5′-noraristeromycin and its antiviral properties. J Med Chem. 1994;37:1382–1384. doi: 10.1021/jm00035a020. [DOI] [PubMed] [Google Scholar]
- 101.Siddiqi S M, Chen X, Rao J, Schneller S W, Ikeda S, Snoeck R, Andrei G, Balzarini J, De Clercq E. 3-Deaza- and 7-deaza-5′-noraristeromycin and their antiviral properties. J Med Chem. 1995;38:1035–1038. doi: 10.1021/jm00006a023. [DOI] [PubMed] [Google Scholar]
- 102.Sidwell R W, Huffman J H, Khare G P, Allen L B, Witkowski J T, Robins R K. Broad-spectrum antiviral activity of virazole: 1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science. 1972;177:705–706. doi: 10.1126/science.177.4050.705. [DOI] [PubMed] [Google Scholar]
- 103.Sidwell R W, Allen L B, Khare G P, Huffman J H, Witkowski J T, Simon L N, Robins R K. Effect of 1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide (Virazole, ICN 1229) on herpes and vaccinia keratitis and encephalitis in laboratory animals. Antimicrob Agents Chemother. 1973;3:242–246. doi: 10.1128/aac.3.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Smee D F, Bailey K W, Wong M-H, Sidwell R W. Intranasal treatment of cowpox virus respiratory infections in mice with cidofovir. Antivir Res. 2000;47:171–177. doi: 10.1016/s0166-3542(00)00105-4. [DOI] [PubMed] [Google Scholar]
- 105.Smee D F, Bailey K W, Sidwell R W. Treatment of cowpox virus respiratory infections in mice with ribavirin as a single agent or followed sequentially by cidofovir. Antivir Chem Chemother. 2000;11:303–309. doi: 10.1177/095632020001100406. [DOI] [PubMed] [Google Scholar]
- 106.Sodeik B, Griffiths G, Ericsson M, Moss B, Doms R W. Assembly of vaccinia virus: effects of rifampin on the intracellular distribution of viral protein p65. J Virol. 1994;68:1103–1114. doi: 10.1128/jvi.68.2.1103-1114.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Streeter D G, Witkowski J T, Khare G P, Sidwell R W, Bauer R J, Robins R K, Simon L N. Mechanism of action of 1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide (Virazole), a new broad-spectrum antiviral agent. Proc Natl Acad Sci USA. 1973;70:1174–1178. doi: 10.1073/pnas.70.4.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Subak-Sharpe J H, Timbury M C, Williams J F. Rifampicin inhibits the growth of some mammalian viruses. Nature. 1969;222:341–345. doi: 10.1038/222341a0. [DOI] [PubMed] [Google Scholar]
- 109.Tseng C K H, Marquez V E, Fuller R W, Goldstein B M, Haines D R, McPherson H, Parsons J L, Shannon W M, Arnett G, Hollingshead M, Driscoll J S. Synthesis of 3-deazaneplanocin A, a powerful inhibitor of S-adenosylhomocysteine hydrolase with potent and selective in vitro and in vivo antiviral activities. J Med Chem. 1989;32:1442–1446. doi: 10.1021/jm00127a007. [DOI] [PubMed] [Google Scholar]
- 110.Van Aerschot A, Mamos P, Weyns N J, Ikeda S, De Clercq E, Herdewijn P. Antiviral activity of C-alkylated purine nucleosides obtained by cross-coupling with tetraalkyltin reagents. J Med Chem. 1993;36:2938–2942. doi: 10.1021/jm00072a013. [DOI] [PubMed] [Google Scholar]
- 111.Votruba I, Bernaerts R, Sakuma T, De Clercq E, Merta A, Rosenberg I, Holý A. Intracellular phosphorylation of broad-spectrum anti-DNA virus agent (S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine and inhibition of viral DNA synthesis. Mol Pharmacol. 1987;32:524–529. [PubMed] [Google Scholar]
- 112.Walton E, Jenkins S R, Nutt R F, Holly F W, Nemes M. Branched-chain sugar nucleosides. V. Synthesis and antiviral properties of several branched-chain sugar nucleosides. J Med Chem. 1969;12:306–309. doi: 10.1021/jm00302a025. [DOI] [PubMed] [Google Scholar]
- 113.Witvrouw M, Desmyter J, De Clercq E. Antiviral portrait series. 4. Polysulfates as inhibitors of HIV and other enveloped viruses. Antivir Chem Chemother. 1994;5:345–359. [Google Scholar]
- 114.Wolfe M S, Borchardt R T. S-adenosyl-l-homocysteine hydrolase as a target for antiviral chemotherapy. J Med Chem. 1991;34:1521–1530. doi: 10.1021/jm00109a001. [DOI] [PubMed] [Google Scholar]
- 115.Woodson B, Joklik W K. The inhibition of vaccinia virus multiplication by isatin-β-thiosemicarbazone. Proc Natl Acad Sci USA. 1965;54:946–953. doi: 10.1073/pnas.54.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xiong X, Smith J L, Chen M S. Effect of incorporation of cidofovir into DNA by human cytomegalovirus DNA polymerase on DNA elongation. Antimicrob Agents Chemother. 1997;41:594–599. doi: 10.1128/aac.41.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yamamoto N, Schols D, De Clercq E, Debyser Z, Pauwels R, Balzarini J, Nakashima H, Baba M, Hosoya M, Snoeck R, Neyts J, Andrei G, Murrer B A, Theobald B, Bossard G, Henson G, Abrams M, Picker D. Mechanism of anti-human immunodeficiency virus action of polyoxometalates, a class of broad-spectrum antiviral agents. Mol Pharmacol. 1992;42:1109–1117. [PubMed] [Google Scholar]