Abstract
Purpose
We aimed to develop and to validate a novel nomogram based on inflammatory markers to preoperatively predict microvascular invasion (MVI) in patients with solitary primary hepatocellular carcinoma (HCC).
Patients and Methods
Data from 658 patients with solitary primary HCC who underwent hepatectomy at the First Affiliated Hospital of Zhengzhou University from June 2018 to October 2021 were retrospectively analyzed. Patients were divided into training (n=441) and validation (n=217) cohorts according to surgical data. Independent risk factors for MVI were identified via univariate and multivariate logistic regression analyses in the training cohort. A novel nomogram was developed based on the independent risk factors identified. Its accuracy was evaluated using a calibration curve and concordance index (C-index). The predictive value was evaluated using the receiver operating characteristic (ROC) curve and decision curve analysis (DCA).
Results
Preoperative alpha-fetoprotein >969 µg/L (P<0.001), tumor size (P=0.002), neutrophil >1.8×109/L (P=0.002), gamma-glutamyl transpeptidase-to-platelet ratio (GPR) >0.32 (P=0.001), aspartate aminotransferase-to-platelet ratio (APR) >0.18 (P<0.001), gamma-glutamyl transpeptidase-to-albumin ratio (GAR) >2.30 (P=0.001), and gamma-glutamyl transpeptidase-to-lymphocyte ratio >29.58 (P<0.001) were identified as preoperative independent risk factors for MVI and were used to establish the nomogram. The C-index of the training and validation cohorts were 0.788 (95% confidence interval [CI]: 0.744–0.831) and 0.735 (95% CI: 0.668–0.802), respectively. The calibration curve analysis revealed that the standard curve fit well with the predicted curve. ROC curve analysis demonstrated high efficiency of the nomogram. DCA verified that the nomogram had notable clinical value.
Conclusion
Preoperative GPR >0.32, APR >0.18, and GAR >2.30 were independent risk factors for MVI in patients with solitary primary HCC, suggesting their utility as preoperative predictors of MVI. The novel nomogram developed and validated in this study may aid in determining optimal therapeutic approaches for patients with solitary HCC at risk for MVI.
Keywords: hepatocellular carcinoma, microvascular invasion, preoperative prediction, nomogram
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers and is the third leading cause of cancer-related death worldwide.1 In China, HCC accounts for 85–90% of primary liver cancers.2 Surgical resection is the first choice for the treatment of HCC, although the recurrence rate 5 years after resection is greater than 50% due to generally high rates of recurrence and metastasis.3 Microvascular invasion (MVI) refers to the presence of cancer cell clusters in blood vessels lined with endothelial cells, which are most pronounced in the branch of the portal vein (including the intracapsular blood vessels) under microscopic observation.2 Many studies have shown that MVI is an independent risk factor for postoperative recurrence and death in patients with HCC.4,5 Preoperative diagnosis of MVI is of great significance to surgeons given that it may aid them in selecting more appropriate therapies and improving patient prognosis.
MVI is a histopathological diagnosis that depends on the examination of surgically resected specimens and is difficult to determine before surgery. Studies have shown that the tumor microenvironment plays an important role in the growth, invasion, and metastasis of HCC cells.6 Inflammatory cytokines are key factors in the regulation of the tumor microenvironment, as they can promote tumor cell proliferation and enhance invasion, metastasis, and angiogenesis.7 In recent years, several studies have used a combination of different routine laboratory inflammatory markers to predict MVI before surgery. Previous studies have demonstrated that some preoperative inflammatory markers can be used to predict MVI in patients with HCC, such as the neutrophil-to-lymphocyte ratio (NLR),8 platelet-to-lymphocyte ratio (PLR),9 alkaline phosphatase (ALT)-to-lymphocyte ratio (ALR),10 gamma-glutamyl transpeptidase (GGT)-to-lymphocyte ratio (GLR),11 and lymphocyte-to-monocyte ratio (LMR).12 Recently, some studies have reported that the GGT-to-platelet ratio (GPR),13 aspartate aminotransferase (AST)-to-platelet ratio (APR),14 and GGT-to-albumin ratio (GAR)15 are independent risk factors that influence the prognosis of patients with HCC after surgery. However, no studies have explored whether preoperative GPR, APR, and GAR levels can be used to predict MVI. Some imaging features are also used to predict MVI, such as peritumoral enhancement,16,17 peritumoral hypointensity in the hepatobiliary phase18,19 and 18F-FDG-Uptake on PET.20 Inflammatory markers are derived from routine blood tests, which are simpler, faster and cheaper than imaging examination. Although many researchers have explored the preoperative diagnosis of MVI using imaging features and serum biomarkers, there is no unified method for predicting MVI before surgery. Before the identification of molecular targets, it may be valuable to develop a model for predicting MVI before surgery based on comprehensive clinical indicators. Presently, nomograms that integrate different prognostic variables are widely used to predict the probability of clinical events. However, few studies have used nomograms to predict the probability of MVI in patients with solitary primary HCC before surgery, and only one or two inflammatory markers have been included; thus, their predictive values have yet to be evaluated. To date, no study has developed a nomogram that includes all existing inflammatory markers.
In this study, we focused on inflammatory markers and aimed to investigate the value of preoperative GPR, APR, and GAR levels for the prediction of MVI in patients with solitary primary HCC and to develop a nomogram for predicting MVI by combining the most significant inflammatory markers with other potential factors.
Materials and Methods
Patients
We retrospectively analyzed the clinical data of patients with solitary primary HCC who underwent hepatectomy at the First Affiliated Hospital of Zhengzhou University from June 2018 to October 2021. This study was conducted in compliance with the Declaration of Helsinki and was approved by the Research Ethics Committee of the First Affiliated Hospital of Zhengzhou University, and informed consent was obtained from all patients.
The inclusion criteria were (1) solitary tumor, (2) liver function of Child-Pugh A or B, (3) completion of surgical resection, and (4) postoperative pathological diagnosis of HCC. The exclusion criteria were as follows: (1) positive surgical margin and postoperative pathological diagnosis of non-HCC, (2) trans-arterial chemoembolization, radiofrequency ablation, radiotherapy, chemotherapy or any anti-cancer-treatment performed preoperatively, (3) extrahepatic metastasis, (4) complications such as infectious diseases, immune system diseases, or blood system diseases, (5) HCC with macrovascular invasion (MAI), and (6) incomplete clinical data. The diagnostic criteria for MVI included the presence of cancer cell clusters in blood vessels lined with endothelial cells, which are most pronounced in the branch of the portal vein (including the intracapsular blood vessels) under microscopic observation.2
Determination of Cut-off Values for the Variables
We analyzed the results of routine, preoperative blood biochemical tests performed closest the date of surgery, and the pathological indices were recorded for the training cohort based on postoperative pathological reports. The cut-off values of continuous variables in this study were determined by the reference ranges. Considering the significant variation of alpha-fetoprotein (AFP) value in patients, the optimal cut-off values of AFP and other inflammatory markers were determined by the receiver operating characteristic (ROC) curve and maximum Youden index. GPR was defined as GGT (U/L)/platelet (109/L). APR was defined as AST (U/L)/platelet (109/L). GAR was defined as GGT (U/L)/albumin (g/L). NLR was defined as neutrophils (109/L)/lymphocyte (109/L). GLR was defined as GGT (U/L)/lymphocyte (109/L). PLR was defined as platelet (109/L)/lymphocyte (109/L). ALR was defined as alkaline phosphatase (U/L)/lymphocyte (109/L), and LMR was defined as lymphocyte (109/L)/monocyte (109/L). The cut-off values of them were also determined by the receiver operating characteristic (ROC) curve and maximum Youden index. Differences were considered statistically significant at P<0.05.
Statistical Analyses
Continuous variables are reported as means±standard deviations (SDs) or medians (first quartile [Q1], third quartile [Q3]), and categorical variables are reported as numbers. The original data were analyzed using SPSS 26.0 (IBM, Armonk, NY). Continuous variables were transformed to categorical variables according to the cut-off values. Univariate logistic regression analyses were used to identify the risk factors for MVI in patients with solitary HCC. The risk factors (P<0.10) and other factors that were considered clinically relevant were selected to be input into a multivariate logistic regression model using the stepwise method. The variance inflation factor (VIF) was calculated to measure the multicollinearity of the regression model. Based on the results, R 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria) was used to establish the nomogram. The concordance index (C-index) with 1000 bootstrap samples was used to quantify the discrimination of the nomogram. The calibration curve and ROC curve were used to measure the accuracy of the nomogram. In addition, we used decision curve analysis (DCA) to evaluate the clinical value and net benefits of the novel nomogram. Differences were considered statistically significant at P<0.05.
Results
Basic Characteristics
A total of 658 patients who underwent hepatectomy at the First Affiliated Hospital of Zhengzhou University were enrolled during the study period. They comprised 436 males and 222 females with an average age of 55 (range, 27–81) years. The patients were divided into two cohorts: 441 patients from June 2018 to June 2020 were classified into the training cohort, and 217 from June 2020 to October 2021 were classified into the validation cohort. The clinical characteristics of the two cohorts are shown in Table 1. The imaging data, including tumor size, tumor number and Hepatocirrhosis, derived from the contrast-enhanced CT report before surgery. Among the 411 patients in the training cohort, 208 had MVI and 233 did not. Among the 217 patients in the validation cohort, 103 had MVI and 114 did not.
Table 1.
Clinical Characteristics of the Two Cohorts
| Variables | Training Cohort | Validation Cohort |
|---|---|---|
| (n=441) | (n=217) | |
| Age | 55.02±10.55 | 54.71±10.05 |
| Sex | ||
| Male | 187 (42.4%) | 35 (16.1%) |
| Female | 254 (57.6%) | 182 (83.9%) |
| Child-Pugh | ||
| A | 235 (53.3%) | 119 (54.8%) |
| B | 206 (46.7%) | 98 (45.2%) |
| HBsAg | ||
| Negative | 400 (46.7%) | 198 (91.2%) |
| Positive | 41 (9.3%) | 19 (8.8%) |
| Hepatocirrhosis | ||
| No | 316 (71.7%) | 159 (73.3%) |
| Yes | 125 (28.3%) | 58 (26.7%) |
| WBC (×109/L) | 5.29 (4.40, 7.20) | 5.15 (4.22, 5.95) |
| GGT (U/L) | 48.50 (28.75, 89.25) | 63.00 (24.75, 104.75) |
| ALP (U/L) | 82.50 (68.00, 102.25) | 91.00 (71.00, 116.5) |
| AST (U/L) | 32.00 (23.00, 54.50) | 29.00 (20.00, 47.25 |
| ALT (U/L) | 31.00 (21.00, 57.25) | 28.50 (19.00, 49.50 |
| AFP (µg/L) | 317.50 (28.00, 2454.00) | 308.80 (16.54, 1794.50) |
| Platelet (×109/L) | 153.00 (112.00, 183.00) | 138.00 (91.00, 165.50) |
| Albumin (g/L) | 40.85 (37.93, 43.25) | 39.00 (35.98, 43.33) |
| Prothrombin time (s) | 11.30 (10.60, 12.20) | 11.110 (10.57, 12.18) |
| Tumor size (cm) | 5.55 (3.50, 7.50) | 5.00 (3.00, 8.00) |
| Monocyte (×109/L) | 0.44 (0.36, 0.63) | 0.46 (0.35, 0.54) |
| Neutrophil (×109/L) | 3.16 (2.45, 4.45) | 2.92 (2.20, 3.77) |
| Lymphocyte (×109/L) | 1.40 (0.99, 1.78) | 1.46 (1.12, 1.81) |
| Total bilirubin (µmol/L) | 12.60 (8.35, 16.44) | 10.60 (7.60, 14.23) |
| GPR | 0.35 (0.20, 0.68) | 0.52 (0.17, 1.02) |
| APR | 0.23 (0.14, 0.42) | 0.24 (0.15, 0.41) |
| GAR | 1.17 (0.68, 2.29) | 1.72 (0.61, 2.64) |
| NLR | 2.17 (1.63, 3.12) | 1.96 (1.43, 3.15) |
| GLR | 37.38 (21.41, 81.31) | 42.94 (16.40, 92.28) |
| ALR | 62.65 (42.72, 95.65) | 58.86 (43.83, 104.60) |
| PLR | 105.44 (77.12, 157.15) | 96.63 (64.72, 123.61) |
| LMR | 3.09 (2.11, 4.17) | 3.25 (2.39, 4.00) |
Abbreviations: WBC, White blood cell; GGT, gamma-glutamyl transpeptidase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; AFP, alpha-fetoprotein; GPR, GGT-to-platelet ratio; APR, AST-to-platelet ratio; GAR, GGT-to-albumin ratio; NLR, neutrophil-to-lymphocyte ratio; GLR, GGT-to-lymphocyte ratio; ALR, ALP-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio.
Cut-off Values of the Variables in the Training Cohort
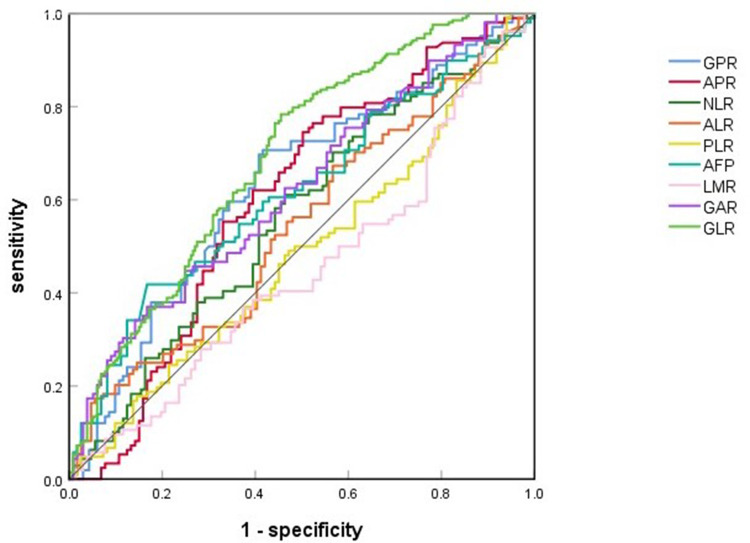
The ROC curve showed that the cut-off values for predicting MVI for AFP, GPR, APR, GAR, NLR, and GLR in patients with solitary HCC before surgery were 969, 0.32, 0.18, 2.30, 1.98, and 29.58, respectively; the areas under the curve (AUCs) were 0.614 (95% confidence interval [CI]: 0.562–0.667, P<0.001), 0.632 (95% CI: 0.580–0.684, P<0.001), 0.604 (95% CI: 0.551–0.657, P<0.001), 0.622 (95% CI: 0.570–0.674, P<0.001), 0.565 (95% CI: 0.511–0.618, P=0.019) and 0.692 (95% CI: 0.643–0.741, P<0.001), respectively (Figure 1, Table 2).
Figure 1.
The receiver operating characteristic (ROC) curve for GPR, APR, NLR, ALR, PLR, AFP, LMR, GAR, and GLR for predicting MVI before surgery.
Abbreviations: GPR, gamma-glutamyl transpeptidase-to-platelet ratio; APR, aspartate aminotransferase-to-platelet ratio; NLR, neutrophil-to-lymphocyte ratio; ALR, alkaline phosphatase-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; AFP, alpha-fetoprotein; LMR, lymphocyte-to-monocyte ratio; GAR, gamma-glutamyl transpeptidase-to-albumin ratio; GLR, gamma-glutamyl transpeptidase-to-lymphocyte ratio; MVI, microvascular invasion.
Table 2.
Value of Preoperative AFP, GPR, APR, and GAR in the Diagnosis of MVI
| Indicator | AUC | 95% CI | Sensitivity | Specificity | Cut-off value | P-value |
|---|---|---|---|---|---|---|
| AFP | 0.614 | 0.562–0.667 | 0.418 | 0.567 | 969.00 | <0.001 |
| GPR | 0.632 | 0.580–0.684 | 0.707 | 0.584 | 0.32 | <0.001 |
| APR | 0.604 | 0.551–0.657 | 0.745 | 0.498 | 0.18 | <0.001 |
| GAR | 0.622 | 0.570–0.674 | 0.370 | 0.839 | 2.30 | <0.001 |
| NLR | 0.565 | 0.511–0.618 | 0.611 | 0.536 | 1.98 | 0.019 |
| GLR | 0.692 | 0.643–0.741 | 0.784 | 0.545 | 29.58 | <0.001 |
| ALR | 0.544 | 0.498–0.598 | / | / | / | 0.110 |
| PLR | 0.485 | 0.431–0.539 | / | / | / | 0.587 |
| LMR | 0.451 | 0.397–0.505 | / | / | / | 0.074 |
Abbreviations: AUC, area under the receiver operating characteristic curve; AFP, alpha-fetoprotein; GPR, gamma-glutamyl transpeptidase-to-platelet ratio; APR, aspartate aminotransferase-to-platelet ratio; GAR, gamma-glutamyl transpeptidase-to-albumin ratio; NLR, neutrophil-to-lymphocyte ratio; GLR, GGT-to-lymphocyte ratio; ALR, ALP-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio.
Preoperative Independent Risk Factors for MVI in the Training Cohort
In the univariate logistic regression analysis, white blood cell (WBC) count >3.5×109/L, GGT >58 U/L, AST >40 U/L, AFP >969 µg/L; platelet >125×109/L; albumin >35 g/L, tumor size (cm), neutrophil >1.8×109/L, GPR >0.32, APR >0.18, GAR >2.30, NLR >1.98, and GLR >29.58 were identified as preoperative risk factors for MVI in patients with solitary HCC. After multivariate logistic regression analysis, only AFP >969 µg/L (odds ratio [OR]=3.147, 95% CI: 1.905–5.199, P<0.001), tumor size (OR=1.143, 95% CI: 1.052–1.240, P=0.002), neutrophil >1.8×109/L (OR=3.761, 95% CI: 1.627–8.696, P=0.002), GPR >0.32 (OR=2.637, 95% CI: 1.487–4.675, P=0.001), APR >0.18 (OR=2.576, 95% CI: 1.514–4.381, P<0.001), GAR >2.30 (OR=3.400, 95% CI: 1.600–7.226, P=0.001), and GLR >29.58 (OR=7.037, 95% CI: 3.403–14.549, P<0.001) were identified as preoperative independent risk factors for MVI in patients with solitary HCC (Table 3). The VIF for each risk factor was less than 2.5; therefore, the regression model did not exist multicollinearity.
Table 3.
Univariate and Multivariate Logistic Regression Analysis of MVI in Patients with Solitary Hepatocellular Carcinoma in the Training Cohort
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age | ||||
| ≤60 | Reference | |||
| >60 | 0.924 (0.617–1.385) | 0.702 | ||
| Sex | ||||
| Female | Reference | |||
| Male | 1.373 (0.815–2.313) | 0.234 | ||
| Child-Pugh | ||||
| A | Reference | |||
| B | 0.865 (0.593–1.262) | 0.451 | ||
| HBsAg | ||||
| Negative | Reference | |||
| Positive | 1.074 (0.565–1.043) | 0.828 | ||
| Hepatocirrhosis | ||||
| NO | Reference | |||
| YES | 0.954 (0.630–1.445) | 0.825 | ||
| WBC | ||||
| ≤3.5×109/L | Reference | |||
| >3.5×109/L | 2.274 (1.060–4.876) | 0.035 | ||
| GGT | ||||
| ≤58 U/L | Reference | |||
| >58 U/L | 1.913 (1.304–2.805) | 0.001 | ||
| ALP | ||||
| ≤40 U/L | Reference | |||
| >40 U/L | 2.259 (0.434–11.769) | 0.333 | ||
| AST | ||||
| ≤40 U/L | Reference | |||
| >40 U/L | 1.706 (1.154–2.520) | 0.007 | ||
| ALT | ||||
| ≤40 U/L | Reference | |||
| >40 U/L | 1.286 (0.863–1.916) | 0.216 | ||
| AFP | ||||
| ≤969 µg/L | Reference | |||
| >969 µg/L | 3.577 (2.302–5.557) | <0.001 | 3.147 (1.905–5.199) | <0.001 |
| Platelet | ||||
| ≤125×109/L | Reference | |||
| >125×109/L | 0.673 (0.452–1.002) | 0.051 | ||
| Albumin | ||||
| ≤35 g/L | Reference | |||
| >35 g/L | 0.586 (0.344–1.000) | 0.050 | ||
| Prothrombin time | ||||
| ≤14 s | Reference | |||
| >14 s | 1.796 (0.821–3.929) | 0.142 | ||
| Tumor size (cm) | 1.194 (1.114–1.279) | <0.001 | 1.143 (1.052–1.240) | 0.002 |
| Monocyte | ||||
| ≤0.1×109/L | Reference | |||
| >0.1×109/L | 1.220 (0.781–1.906) | 0.383 | ||
| Neutrophil | ||||
| ≤1.8×109/L | Reference | |||
| >1.8×109/L | 2.704 (1.280–5.174) | 0.009 | 3.761 (1.627–8.696) | 0.002 |
| Lymphocyte | ||||
| ≤1.1×109/L | Reference | |||
| >1.1×109/L | 0.898 (0.588–1.373) | 0.620 | ||
| Total bilirubin | ||||
| ≤25 µmol/L | Reference | |||
| >25 µmol/L | 1.722 (0.690–4.299) | 0.244 | ||
| GPR | ||||
| ≤0.32 | Reference | |||
| >0.32 | 3.227 (2.176–4.786) | <0.001 | 2.637 (1.487–4.675) | 0.001 |
| APR | ||||
| ≤0.18 | Reference | |||
| >0.18 | 2.939 (1.938–4.459) | <0.001 | 2.576 (1.514–4.381) | 0.001 |
| GAR | ||||
| ≤2.30 | Reference | |||
| >2.30 | 2.924 (1.875–4.560) | <0.001 | 3.400 (1.600–7.226) | 0.001 |
| NLR | ||||
| ≤1.98 | Reference | |||
| >1.98 | 1.815 (1.242–2.652) | 0.002 | ||
| GLR | ||||
| ≤29.58 | Reference | |||
| >29.58 | 2.664 (1.800–3.943) | <0.001 | 7.037 (3.403–14.549) | <0.001 |
Abbreviations: MVI, microvascular invasion; OR, odds ratio; CI, confidence interval; WBC, White blood cell; GGT, gamma-glutamyl transpeptidase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; AFP, alpha-fetoprotein; GPR, GGT-to-platelet ratio; APR, AST-to-platelet ratio; GAR, GGT-to-albumin ratio; NLR, neutrophil-to-lymphocyte ratio; GLR, GGT-to-lymphocyte ratio; ALR, ALP-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio.
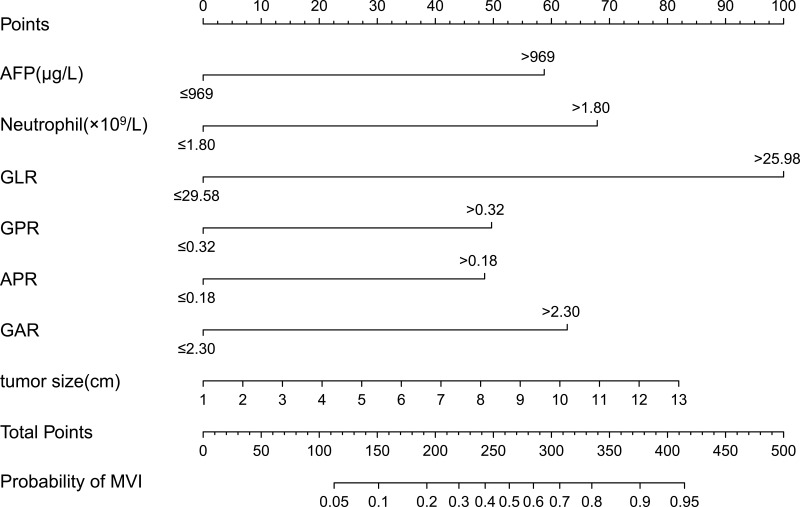
Development and Validation of the Novel Nomogram for the Preoperative Prediction of MVI
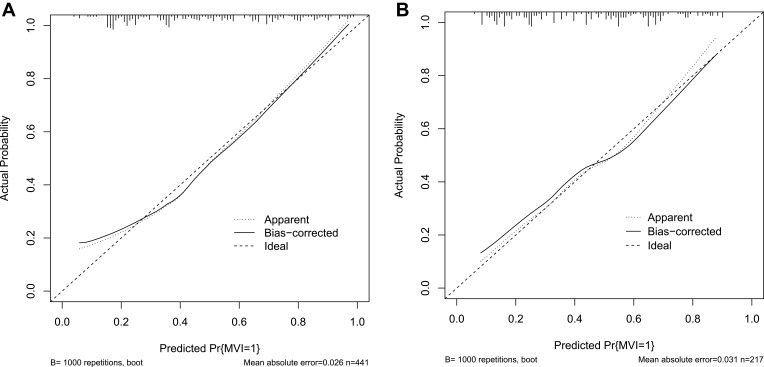
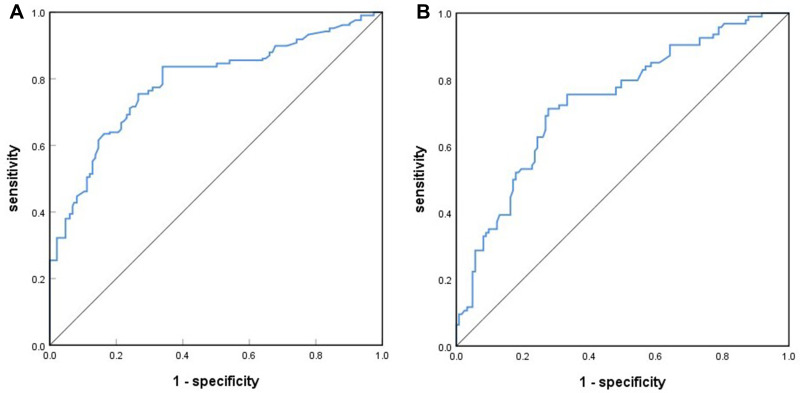
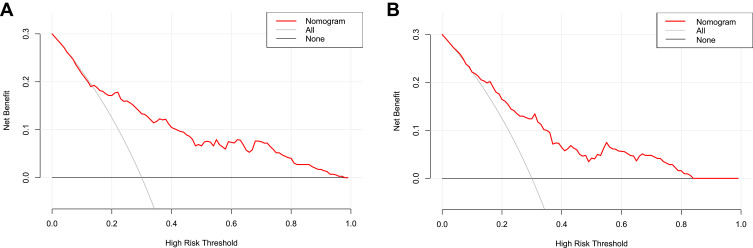
Based on the results of the multivariate logistic regression analysis in the training cohort, we selected neutrophil, AFP, GPR, APR, GAR, GLR, and tumor size to develop a nomogram for the preoperative prediction of MVI in patients with solitary HCC. The nomogram is presented in Figure 2. The total points for the nomogram can be calculated by summing the points for each factor, and the probability of MVI can be estimated from the total number of points. We found that the predictive model of the nomogram had good predictive ability. The C-index of the training and validation cohorts were 0.788 (95% CI: 0.744–0.831) and 0.735 (95% CI: 0.668–0.802), respectively. The analysis of the calibration curves for the training and validation cohorts showed that the standard curve fit well with the predicted curve, indicating agreement between the observed frequency and predicted probability of MVI (Figure 3). The ROC curve analysis of the total points in the training cohort indicated that the cut-off value of the nomogram was 240 points when the Youden index was at its maximum, and the corresponding probability of MVI was 39%. In the training cohort, the sensitivity, specificity, positive predictive value, and negative predictive value were 83.7%, 66.1%, 68.8%, and 81.9%, respectively, when the nomogram was used to distinguish between the presence and absence of MVI. These values were 74.5%, 66.7%, 63.1%, and 77.4%, respectively, in the validation cohort, indicating that the nomogram had a high prediction accuracy for MVI (Figure 4, Table 4). DCA revealed that the prediction model had a high net benefit in almost the entire range of threshold probability, indicating that the novel nomogram has notable clinical application (Figure 5).
Figure 2.
Nomogram for preoperative prediction of MVI in patients with solitary hepatocellular carcinoma. Neutrophil, AFP, GPR, APR, GAR, GLR, and tumor size for predicting MVI before surgery.
Abbreviations: MVI, microvascular invasion; AFP, alpha-fetoprotein; GPR, gamma-glutamyl transpeptidase-to-platelet ratio; APR, aspartate aminotransferase-to-platelet ratio; GAR, gamma-glutamyl transpeptidase-to-albumin ratio; GLR, gamma-glutamyl transpeptidase-to-lymphocyte ratio.
Figure 3.
Calibration curves for the training (A) and validation cohorts (B).
Abbreviation: MVI, microvascular invasion.
Figure 4.
Receiver operating characteristic (ROC) curves of the nomogram total points for predicting MVI in the training (A) and validation cohorts (B).
Abbreviation: MVI, microvascular invasion.
Table 4.
Accuracy of the Nomogram Prediction Score in Predicting the Risk of Microvascular Invasion
| Variable | Training Cohort | Validation Cohort |
|---|---|---|
| Area under the ROC curve | 0.788 | 0.735 |
| 95% CI | 0.744–0.831 | 0.668–0.802 |
| Cut-off score | 240 | 240 |
| Sensitivity (%) | 83.7% | 74.5% |
| Specificity (%) | 66.1% | 66.7% |
| Positive predictive value (%) | 68.8% | 63.1% |
| Negative predictive value (%) | 81.9% | 77.4% |
| Positive likelihood ratio | 2.5 | 2.2 |
| Negative likelihood ratio | 0.2 | 0.4 |
Abbreviations: ROC, receiver operating characteristic curve; CI, confidence interval.
Figure 5.
Decision curve analysis (DCA) for the training (A) and validation cohorts (B).
Discussion
In this study, we developed and validated a novel nomogram that involved GLR, GPR, APR, and GAR for the preoperative prediction of MVI in patients with solitary primary HCC. Our findings indicated that 47.3% cases of solitary primary HCC were complicated by MVI. Furthermore, the risk of MVI was 2.637 times higher in patients with GPR >0.32 than in patients with GPR ≤0.32, 2.576 times higher in patients with APR >0.18 than in patients with APR ≤0.18, and 3.400 times higher in patients with GAR >2.30 than in patients with GAR ≤2.30. The specific biological mechanisms underlying elevated levels of GPR, APR, and GAR in patients with solitary primary HCC with MVI remain unclear, they may be related to the following: (1) GGT plays an important role in the occurrence, vascular invasion, and metastasis of HCC,21 (2) platelets are involved in almost every step in the development of cancer,22 (3) the proliferation of tumor cells requires energy through glutamine metabolism, which requires the participation of AST,23 and (4) albumin reflects the nutritional status and immune function of the human body, and it can inhibit the development of liver cancer through its impact on growth-controlling kinases.24 Thus, increases in GPR, APR, and GAR in patients with HCC may accelerate tumor growth and increase its invasiveness while simultaneously decreasing the body’s defenses, thereby increasing the probability of developing MVI.
Neutrophil count, as a routine laboratory marker, has been proved to be a valuable predictor for the prognosis of HCC.25 We found that neutrophil count was an independent risk factor for the preoperative prediction of MVI, which is consistent with the results of Wang et al’s study.26 The multivariate logistic regression analysis indicated that the risk of MVI was 3.147 times higher in patients with AFP >969 µg/L than in patients with lower AFP levels. For every 1-cm increase in tumor size, the risk of MVI increased by 13.8%. AFP and tumor size were independent risk factors for MVI in patients with solitary HCC. Cho et al27 found that larger tumor sizes in patients with solitary HCC were associated with a higher probability of MVI, which is consistent with the results of this study. Kaibori et al28 reported that an increase in the AFP level was an independent risk factor for solitary HCC with MVI, which is also consistent with our findings. However, the cut-off value of AFP for predicting MVI remains controversial.29,30 Liver cirrhosis and other malignant diseases can also cause an increase in AFP; thus, further studies are required to determine the appropriate cut-off value of AFP in predicting MVI.
Wang et al26 developed a nomogram for predicting MVI in patients with HCC based on the following four preoperative indices: tumor size, tumor number, AFP level, and neutrophil level, without involving a combination of routine laboratory inflammatory markers. Deng et al31 first used NLR to develop a nomogram for predicting MVI in patients with HCC. In their study, the cut-off value of the predicted probability was 44%, although NLR was the only inflammatory marker used in their study. Lei et al32 developed a nomogram for the preoperative estimation of MVI risk in patients with hepatitis B virus-related HCC within the Milan criteria, which included seven preoperative clinical indicators and had good accuracy; however, their study was limited to these patients alone.
Our study had some limitations. Recently, with the development of computer-aided diagnosis technology, radiomics, as a novel form of imaging analysis, can use advanced image processing methods to extract texture features and describe tumor phenotypes objectively and quantitatively, which has been used to predict MVI.17,33 A new diagnostic method called “fluid biopsy” has received great attention recently,34,35 circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) are the basis of fluid biopsy, ctDNA has been widely used in the research of many cancers including liver cancer36 and has been used to predict MVI.37 In this study, we mainly focus on inflammatory markers, so further research is needed to combine inflammatory markers with radiomics and “fluid biopsy”. And all clinical data were obtained from a single hospital, and the results must be validated using data from other centers. In addition, our study was a retrospective analysis, and our findings should be confirmed in future prospective studies.
Conclusion
The nomogram was developed successfully and could effectively distinguish patients with or without MVI before surgery, and the predicted probability of MVI was in agreement with the actual frequency of MVI. This novel nomogram combines four inflammatory biomarkers (GPR, APR, GAR, and GLR) with three other important clinical indicators (neutrophil level, AFP level, and tumor size) with proven credibility and clinical value in predicting MVI.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Sharing Statement
The datasets used in this study are available from the corresponding author on reasonable request.
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Zhou J, Sun H, Wang Z, et al. Guidelines for diagnosis and treatment of primary liver cancer in China (2017 edition). Liver Cancer. 2018;7(3):235–260. doi: 10.1159/000488035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabrizian P, Jibara G, Shrager B, et al. Recurrence of hepatocellular cancer after resection patterns, treatments, and prognosis. Ann Surg. 2015;261(5):947–955. doi: 10.1097/SLA.0000000000000710 [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Wu MC, Cong W. Microvascular invasion predicts a poor prognosis of solitary hepatocellular carcinoma up to 2 cm based on propensity score matching analysis. Hepatol Res. 2019;49(3):344–354. doi: 10.1111/hepr.13241 [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Li J, Shen F, et al. Significance of presence of microvascular invasion in specimens obtained after surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 2017;33(2):347–354. doi: 10.1111/jgh.13843 [DOI] [PubMed] [Google Scholar]
- 6.Wu SD, Ma YS, Fang Y, et al. Role of the microenvironment in hepatocellular carcinoma development and progression. Cancer Treat Rev. 2012;38(3):218–225. doi: 10.1016/j.ctrv.2011.06.010 [DOI] [PubMed] [Google Scholar]
- 7.Comen EA, Bowman RL, Kleppe M. Underlying causes and therapeutic targeting of the inflammatory tumor microenvironment. Front Cell Dev Biol. 2018;6:56. doi: 10.3389/fcell.2018.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu Y, Song J, Zhang R, et al. Preoperative neutrophil-to-lymphocyte ratio and tumor-related factors to predict microvascular invasion in patients with hepatocellular carcinoma. Oncotarget. 2017;8(45):79722–79730. doi: 10.18632/oncotarget.19178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rungsakulkij N, Mingphruedhi S, Suragul W, et al. Platelet-to-lymphocyte ratio and large tumor size predict microvascular invasion after resection for hepatocellular carcinoma. Asian Pac J Cancer Prev. 2018;19(12):3435–3441. doi: 10.31557/APJCP.2018.19.12.3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu Y, Xu D, Zhang Z, et al. A new laboratory-based algorithm to predict microvascular invasion and survival in patients with hepatocellular carcinoma. Int J Surg. 2018;57:45–53. doi: 10.1016/j.ijsu.2018.07.011 [DOI] [PubMed] [Google Scholar]
- 11.Zhang H, Zhou Y, Li Y, et al. Predictive value of gamma-glutamyl transpeptidase to lymphocyte count ratio in hepatocellular carcinoma patients with microvascular invasion. BMC Cancer. 2020;20(1):132. doi: 10.1186/s12885-020-6628-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li P, Huang W, Wang F, et al. Nomograms based on inflammatory biomarkers for predicting tumor grade and micro-vascular invasion in stage i/ii hepatocellular carcinoma. Biosci Rep. 2018;38(6):BSR20180464. doi: 10.1042/BSR20180464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ke MY, Zhang M, Su Q, et al. Gamma-glutamyl transpeptidase to platelet ratio predicts short-term outcomes in hepatocellular carcinoma patients undergoing minor liver resection. J Surg Res. 2018;231:403–410. doi: 10.1016/j.jss.2018.05.049 [DOI] [PubMed] [Google Scholar]
- 14.Shen S, Fu S, Chen B, et al. Preoperative aspartate aminotransferase to platelet ratio is an independent prognostic factor for hepatitis b-induced hepatocellular carcinoma after hepatic resection. Ann Surg Oncol. 2014;21(12):3802–3809. doi: 10.1245/s10434-014-3771-x [DOI] [PubMed] [Google Scholar]
- 15.Liu K, Lv Y, Niu Y, et al. Prognostic value of γ-glutamyl transpeptidase to albumin ratio combined with aspartate aminotransferase to lymphocyte ratio in patients with hepatocellular carcinoma after hepatectomy. Medicine. 2020;99(48):e23339. doi: 10.1097/md.0000000000023339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W, Liu L, Wang P, et al. Preoperative computed tomography and serum α-fetoprotein to predict microvascular invasion in hepatocellular carcinoma. Medicine. 2018;97(27):e11402. doi: 10.1097/MD.0000000000011402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu X, Zhang HL, Liu QP, et al. Radiomic analysis of contrast-enhanced ct predicts microvascular invasion and outcome in hepatocellular carcinoma. J Hepatol. 2019;70(6):1133–1144. doi: 10.1016/j.jhep.2019.02.023 [DOI] [PubMed] [Google Scholar]
- 18.Nishie A, Asayama Y, Ishigami K, et al. Clinicopathological significance of the peritumoral decreased uptake area of gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid in hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29(3):561–567. doi: 10.1111/jgh.12423 [DOI] [PubMed] [Google Scholar]
- 19.Server S, Sabet S, Yaghouti K, et al. Value of imaging findings in the prediction of microvascular invasion in hepatocellular carcinoma. Transplant Proc. 2019;51(7):2403–2407. doi: 10.1016/j.transproceed.2019.01.178 [DOI] [PubMed] [Google Scholar]
- 20.Kornberg A, Freesmeyer M, Bärthel E, et al. 18f-fdg-uptake of hepatocellular carcinoma on pet predicts microvascular tumor invasion in liver transplant patients. Am J Transplant. 2009;9(3):592–600. doi: 10.1111/j.1600-6143.2008.02516.x [DOI] [PubMed] [Google Scholar]
- 21.Zhao W, Fan L, Yang N, et al. Preoperative predictors of microvascular invasion in multinodular hepatocellular carcinoma. Eur J Surg Oncol. 2013;39(8):858–864. doi: 10.1016/j.ejso.2013.04.003 [DOI] [PubMed] [Google Scholar]
- 22.Gay L, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123–134. doi: 10.1038/nrc3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu P, Sabatini D. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134(5):703–707. doi: 10.1016/j.cell.2008.08.021 [DOI] [PubMed] [Google Scholar]
- 24.Bağırsakçı E, Şahin E, Atabey N, et al. Role of albumin in growth inhibition in hepatocellular carcinoma. Oncology. 2017;93(2):136–142. doi: 10.1159/000471807 [DOI] [PubMed] [Google Scholar]
- 25.Sanghera C, Teh JJ. The systemic inflammatory response as a source of biomarkers and therapeutic targets in hepatocellular carcinoma. Liver Int. 2019;39(11):2008–2023. doi: 10.1111/liv.14220 [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Jin Y, Ji Y, et al. Development and validation of a prediction model for microvascular invasion in hepatocellular carcinoma. World J Gastroenterol. 2020;26(14):1647–1659. doi: 10.3748/wjg.v26.i14.1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho E, Choi J. Mri features of hepatocellular carcinoma related to biologic behavior. Korean J Radiol. 2015;16(3):449–464. doi: 10.3348/kjr.2015.16.3.449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaibori M, Ishizaki M, Matsui K, et al. Predictors of microvascular invasion before hepatectomy for hepatocellular carcinoma. J Surg Oncol. 2010;102(5):462–468. doi: 10.1002/jso.21631 [DOI] [PubMed] [Google Scholar]
- 29.McHugh P, Gilbert J, Vera S, et al. Alpha-fetoprotein and tumour size are associated with microvascular invasion in explanted livers of patients undergoing transplantation with hepatocellular carcinoma. HPB. 2010;12(1):56–61. doi: 10.1111/j.1477-2574.2009.00128.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.You Z, Chen L, Ye H. Predictors of microvascular invasion in patients with solitary small hepatitis b related hepatocellular carcinoma. Pak J Med Sci. 2014;30(2):331–334. doi: 10.12669/pjms.302.4652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng G, Yao L, Zeng F, et al. Nomogram for preoperative prediction of microvascular invasion risk in hepatocellular carcinoma. Cancer Manag Res. 2019;11:9037–9045. doi: 10.2147/CMAR.S216178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lei Z, Li J, Wu D, et al. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis b virus-related hepatocellular carcinoma within the milan criteria. JAMA Surg. 2016;151(4):356–363. doi: 10.1001/jamasurg.2015.4257 [DOI] [PubMed] [Google Scholar]
- 33.Ma X, Wei J, Gu D, et al. Preoperative radiomics nomogram for microvascular invasion prediction in hepatocellular carcinoma using contrast-enhanced ct. Eur Radiol. 2019;29(7):3595–3605. doi: 10.1007/s00330-018-5985-y [DOI] [PubMed] [Google Scholar]
- 34.Diaz LA, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32(6):579–586. doi: 10.1200/jco.2012.45.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alix-Panabières C, Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016;6(5):479–491. doi: 10.1158/2159-8290.Cd-15-1483 [DOI] [PubMed] [Google Scholar]
- 36.Xu RH, Wei W, Krawczyk M, et al. Circulating tumour DNA methylation markers for diagnosis and prognosis of hepatocellular carcinoma. Nat Mater. 2017;16(11):1155–1161. doi: 10.1038/nmat4997 [DOI] [PubMed] [Google Scholar]
- 37.Wang D, Xu Y, Goldstein JB, et al. Preoperative evaluation of microvascular invasion with circulating tumour DNA in operable hepatocellular carcinoma. Liver Int. 2020;40(8):1997–2007. doi: 10.1111/liv.14463 [DOI] [PMC free article] [PubMed] [Google Scholar]