INTRODUCTION
The modified Social Ecological Model situates explaining health outcomes among people living with HIV (PLWH) within the complex interplay of individual characteristics, social and sexual networks, neighborhood determinants, and public policies (1, 2). Neighborhood contexts have been increasingly recognized as drivers of health outcomes among PLWH (2–5). Neighborhood characteristics have been associated with a decreased frequency of HIV testing, late entry into care, lower CD4+ T cell (CD4) counts, lower adherence to antiretroviral therapy (ART) and engagement in care, increased HIV mortality, and more frequent risk behavior (6–12). Residing in a poor neighborhood is associated with increased risk of HIV mortality, more frequent behavior associated with HIV transmission (e.g., injection drug use and condomless sex), and lower CD4 counts (6, 8, 12).
Viral suppression, the ultimate goal of the HIV care cascade, provides individual and community-level benefits, including decreases in morbidity, mortality, and HIV transmission risk (13–16). Previous research has predominantly focused on the impact of individual-level variables on viral suppression, such as age, education, and socioeconomic status. However, neighborhood factors should also be considered to better understand outcomes and potential areas of intervention. Latkin et al. suggests that neighborhoods impact HIV-related outcomes through differing risk environments, neighborhood disorder, collective efficacy, and social capital (1). Neighborhood economic disadvantage may increase risk of food insecurity, housing instability, and lack of social support, which have previously been linked to impaired viral suppression (17–19). These factors create structural vulnerabilities that may impede engagement in medical care and ART adherence, leading to poor viral suppression.
Studies on neighborhood characteristics and viral suppression have reached inconsistent conclusions and have largely been conducted in high-income countries (e.g., Canada, Italy, Switzerland, and the United States). Some studies, including in settings of universal healthcare, observed that achieving viral suppression was influenced by neighborhood factors, such as average education level, concentration of poverty, economic advantage, healthcare access, and unemployment (20–24). Others did not find neighborhood socioeconomic indicators as significant predictors of viral suppression (7, 12, 25, 26). The differing sociopolitical contexts, study populations, and neighborhood indicators nuance the interpretation of these studies as well as highlight the need to build on previous research.
Limited literature exists on the influence of neighborhood socioeconomic indicators on viral suppression in Brazil, a middle-income country, and other low- and middle-income countries (LMICs). Brazil has been a pioneer in universal access to HIV care and ART through enaction of its National AIDS Program in 1996 (27). Despite universal coverage, disparities still prevail in HIV outcomes, as being non-white and having a lower education status are associated with increased AIDS mortality, late presentation to care, not being on ART, and non-viral suppression (28–30). Pascom et al (2018) showed that municipality-level social vulnerability was related to late presentation to care and being on ART but not achieving viral suppression among PLWH followed by Brazil’s Unified Health System (Sistema Único de Saúde- SUS) in 2016 (29). However, municipal-level measures may conceal intra-metropolitan inequalities (31). A geospatial analysis of data from Rio de Janeiro, Brazil, observed that poorer census-tract socioeconomic indicators, such as illiteracy and low income, correlate with higher HIV mortality rates (32).
While the impact of neighborhood context on HIV outcomes is understudied in Rio de Janeiro, previous research has demonstrated that economically disadvantaged neighborhoods endure worse health and carry a disproportionate burden of disease (32–34). Embedded in an intricate history of structural violence and systemic marginalization as well as progressive movements and resilience, Rio de Janeiro is marked by vast social, health, and economic inequities. With a unique urban landscape, it contains Brazil’s largest comunidade (“community”, also known as favelas/morros/slums) population, which are informal settlements (35). Nearly a quarter of the population (22%) resides in over 750 comunidades distributed throughout the city, including in high-income and affluent neighborhoods (36). While there is heterogeneity among the comunidades, as a whole, they experience larger infrastructural inequities, concentrated poverty, education gaps, and higher unemployment in comparison to other neighborhoods.
The municipal human development index (MHDI) and social vulnerability index (SVI) are multidimensional scores of population-level development and socioeconomic indicators. The indices quantify neighborhood characteristics, such as education, unemployment, and poverty, which have previously been linked to viral suppression in other settings (20–24). Applying a socioecological lens and understanding the impact of neighborhood context on viral suppression may help to explain disparities and identify opportunities to support PLWH. As such, we sought to examine the association between individual characteristics, neighborhood socioeconomic indicators (MHDI and SVI), and viral suppression among PLWH initiating ART between 2000 and 2017 in the metropolitan region of Rio de Janeiro.
METHODS
Study Population
The HIV Clinical Cohort of the National Institute of Infectious Diseases (INI), at the Oswaldo Cruz Foundation (FIOCRUZ) was established in 1998 and includes all individuals who receive HIV care at the site. For this analysis, eligibility criteria included >18 years of age at the time of ART initiation, ART initiation between January 1, 2000 and December 31, 2017, residence in the metropolitan region of Rio de Janeiro, and no report of prior injection drug use (IDU) (Supplementary Figure I). Exclusion criteria included: suppressed viral load (defined as HIV-1 RNA <400 copies/mL [to allow for assay variance over time] within the window of 365 days before and up to 7 days) at ART initiation and death within 90 days after ART initiation date.
Study Definitions
Outcome
Our outcome of interest was viral suppression at six months (or the closest result within a window of 90 to 270 days) after ART initiation date.
Individual Level Variables
Age at ART initiation was determined by calculating the time difference in years between the date of birth and date of ART initiation. Race classifications included White, Black and Mixed/Other (including unknown). Education was categorized into either less than/equal to or greater than eight years, which is equivalent to secondary school completion in Brazilian education system. Information on gender, sexual orientation and probable mode of HIV acquisition were jointly used to group the study population into: cisgender women; transgender women (TGW); cisgender MSM (cisgender men who have sex with men); heterosexual cisgender men (cisgender men who have sex with women); cisgender men with unknown mode of HIV acquisition (n= 153); and cisgender men with other modes of HIV acquisition (vertical transmission n=2, work accidents n=2, blood-borne n=6). Pre-treatment HIV viral load and CD4 count were defined as values recorded closest to the participant’s ART initiation date (with a window period of 365 days before and up to 30 days after). Pre-treatment opportunistic illnesses and tuberculosis (TB) were defined using the CDC AIDS Surveillance Case Definition 1993.
Geospatial Distribution and Neighborhood Level Socioeconomic Indicators
The MHDI and SVI were employed as the neighborhood socioeconomic indicators. Studied in international and Brazilian settings, the MHDI is an adaptation of the global HDI and a composite score of development with equal weights given to education, income, and life expectancy (31, 37–40). MHDI allows for comparison of development between local and national contexts. It ranges between zero and one, with values closer to one signifying higher development. The Atlas of Human Development in Brazil (ADHB) MHDI classification was adopted: very low to low (MHDI of 0– 0.599), medium (MHDI 0.600– 0.699), high (MHDI of 0.700– 0.799), and very high (MHDI of 0.800–1.0). Provided by the Atlas of Social Vulnerability (ASV), the SVI was created to complement the MHDI. The SVI measures social vulnerability multidimensionally and contextualizes the Brazilian sociopolitical reality (36). The SVI is an index of 16 measures grouped into: human capital, urban infrastructure, and work and income. The index ranges between zero and one, with values closer to zero signifying the lowest social vulnerability. The ASV SVI classification was adopted: high- very high (SVI of 0.4– 1.0), medium (SVI of 0.3– 0.399), low (SVI of 0.200– 0.299), and very low (SVI of 0–0.199). The MHDI and SVI are publicly accessible datasets downloaded from the ADBD and the Brazilian Institute of Geography and Statistics (Instituto Brasileiro de Geografia e Estatística; or IBGE) platforms, respectively. The territorial areas, population size, and population densities of the subdistricts were similarly obtained from the IBGE 2010 census (41).
We obtained the MHDI and SVI of participants’ neighborhoods. Participants’ neighborhoods were defined by the human developmental unit (HDU) of their residential addresses. The HDU is a sociodemographic homogeneous areas created by from the census sectors (31). The small spatial unit of analysis allows for capturing inequalities in intra-metropolitan spaces. For example, the SVI and MHDI of slums (or comunidades) within high-income neighborhoods may by masked by aggregate municipal averages. Participants’ subdistricts were used as the area-level variable for our multilevel analysis. Subdistricts are administrative regions within the metropolitan area of Rio de Janeiro.
We used the freely accessible, geographic datasets developed by the IBGE to geocode participants’ residential addresses and obtain the latitude and longitude coordinates. The coordinates were then matched with their respective HDUs and subdistricts. For participants that started ART before and after 2005, the MHDI from the years 2000 and 2010 were used, respectively. We used R Software (version 3.6.2) to obtain the longitude and latitude coordinates from participants addresses and QGIS Coruna 3.10 to map coordinates and linking them to HDU-level data.
Statistical Analysis
Descriptive statistics of individual-level sociodemographic and clinical factors, and neighborhood-level socioeconomic indicators was performed for all participants and according to viral suppression at six months. Logistic regression and multilevel logistic regression models were used to assess factors associated with viral suppression (odds ratios [OR] and 95% confidence intervals [CI]). Multilevel analysis allows for intracorrelation coefficient (ICC) and accounts for contextual-level factors that may influence health outcomes (42–45). In our multilevel model, participants (level I) were nested within 50 subdistricts (level II). We constructed two multilevel logistic models: one with MHDI and another with SVI. Individual-level variables with p values ≤ 0.20 were used in the adjusted models and included: age, education, gender/sexual orientation/probable mode of HIV acquisition, pre-treatment CD4 count and HIV viral load, and ART initiation year; race and pre-treatment TB diagnosis were forced into the models.
Four additional analyses were performed. First, given that six subdistricts had less than ten participants, we also estimated the multilevel logistic model excluding these subdistricts with model results remaining largely the same (Supplementary Table I). Second, we tested for interactions between neighborhood socioeconomic variables and participants’ age, education, race, and gender/exposure variables in the final multilevel models. However, all interactions were non-significant and results are not shown. Third, as a sensitivity analysis aiming to explore the impact of missing HIV viral load measurements at six months on viral suppression estimates, two alternative scenarios were constructed: a worst-case scenario where we classified all missing data as viral non-suppression and a best-case scenario where we classified all missing data as viral suppression. Finally, given that 27.3% (n=992) of all participants (n=3361) eligible for the baseline analysis were excluded due to a missing viral load at six months, we performed descriptive analysis of MHDI and SVI according to inclusion category, i.e. if included or not in the present analysis. All statistical analyses were performed using R Software (version 3.6.2) with the “dplyr”, “data.table”, “EpiDisplay”, “lme4”, “ggmap” and “sjstats” packages.
Ethics
The study was approved by the Institutional Review Board (IRB) of INI and received IRB exemption from UCLA. Written consent was obtained from all participants, and all personal information was de-identified.
RESULTS
Study Population and Individual Level Variables
Between January 1, 2000 and December 31, 2017, 3895 PLWH who initiated ART at INI and resided in the metropolitan region of Rio de Janeiro. Of these participants, 1256 were excluded from the base analysis: 174 had a suppressed viral load at baseline, 90 died within 90 days of ART initiation, and 992 were missing viral load within 90 to 270 days of ART initiation (Supplementary Figure I). The final study population consisted of 2639 participants.
Table I describes the individual- and neighborhood-level variables, stratified by viral suppression at six months. The participants had a median age of 34.9 years (interquartile range [IQR]: 28.4–42.8), 56.3% completed more than eight years of education, and 45.4% identified as White. The stratification according to gender, sexual orientation and probable mode of HIV acquisition were as follows: 29.2% cisgender women, 3.0% TGW, 38.6% cisgender MSM, 23.0% heterosexual cisgender men, and 6.1% cisgender men with other or unknown exposure risk. Approximately one third of participants (36.5%, n=963) had a pre-treatment CD4 <200 cells/mm3, and 33.7% (n=890) had a viral load >100,000 copies/mL. The prevalence of pre-treatment opportunistic illnesses and TB were 29.3% (n= 772) and 15.6% (n= 411), respectively.
Table I.
Sociodemographics, HIV clinical characteristics, and neighborhood socioeconomic variables, stratified by virologic and non-virologic suppression
| Virologic suppression | Non-virologic suppression | Total | |
|---|---|---|---|
| n= 2214 (83.9%) | n= 425 (16.1%) | n= 2639 (100%) | |
| Age at time of ART initiation (IQR) | 35.0 (28.6, 43) | 34.0 (27.4, 42.4) | 34.9 (28.4, 42.8) |
| Age (years) | |||
| <30 | 674 (30.4) | 150 (35.3) | 824 (31.2) |
| 30–40 | 790 (35.7) | 148 (34.8) | 938 (35.5) |
| 40–50 | 508 (22.9) | 92 (21.6) | 600 (22.7) |
| >50 | 242 (10.9) | 35 (8.2) | 277 (10.5) |
| Race | |||
| White | 1011 (45.7) | 188 (44.2) | 1199 (45.4) |
| Black | 424 (19.2) | 89 (20.9) | 513 (19.4) |
| Mixed/Unknown | 779 (35.2) | 148 (34.8) | 927 (35.1) |
| Education level | |||
| Less than 8 Years | 922 (41.6) | 212 (49.9) | 1134 (43.0) |
| More than 8 Years | 1278 (57.7) | 207 (48.7) | 1485 (56.3) |
| Unknown | 14 (0.6) | 6 (1.4) | 20 (0.8) |
| Gender and exposure risk | |||
| Cisgender women | 616 (27.8) | 155 (36.5) | 771 (29.2) |
| TGW | 66 (3.0) | 14 (3.3) | 80 (3.0) |
| Cis-MSM | 889 (40.2) | 130 (30.6) | 1019 (38.6) |
| Heterosexual cisgender men | 515 (23.3) | 93 (21.9) | 608 (23.0) |
| Cisgender men: other and unknown | 128 (5.8) | 33 (7.8) | 161 (6.1) |
| Pre-treatment CD4 count (cells/mm3) | |||
| <200 | 803 (36.3) | 160 (37.6) | 963 (36.5) |
| 200–350 | 608 (27.5) | 88 (20.7) | 696 (26.4) |
| 350–500 | 321 (14.5) | 45 (10.6) | 366 (13.8) |
| >500 | 267 (12.1) | 37 (8.7) | 304 (11.5) |
| Unknown | 215 (9.7) | 95 (22.4) | 310 (11.7) |
| Pre-treatment log of viral load | 11 (9.6, 12.2) | 11.5 (10.1, 12.8) | 11.1 (9.6, 12.3) |
| Pre-treatment viral load copies (copies/mL) | |||
| <100,000 | 1123 (50.7) | 157 (36.9) | 1280 (48.5) |
| ≥100,000 | 740 (33.4) | 150 (35.3) | 890 (33.7) |
| Unknown | 351 (15.9) | 118 (27.8) | 469 (17.8) |
| Opportunistic illness | |||
| No | 1567 (70.8) | 300 (70.6) | 1867 (70.7) |
| Yes | 647 (29.2) | 125 (29.4) | 772 (29.3) |
| TB | |||
| No | 1867 (84.3) | 361 (84.9) | 2278 (84.4) |
| Yes | 347 (15.7) | 64 (15.1) | 411 (15.6) |
| ART initiation year | |||
| 2000–2004 | 226 (10.2) | 98 (23.1) | 324 (12.3) |
| 2005– 2008 | 521 (23.5) | 105 (24.7) | 626 (23.7) |
| 2009– 2012 | 728 (32.9) | 92 (21.6) | 820 (31.1) |
| 2013– 2017 | 652 (33.4) | 130 (30.6) | 869 (32.9) |
| MHDI (IQR) | 0.684 (0.587, 0.754) | 0.683 (0.589, 0.766) | 0.683 (0.587, 0.758) |
| MHDI category | |||
| High- Very High | 958 (43.3) | 180 (42.4) | 1138 (43.1) |
| Medium | 635 (28.7) | 126 (29.6) | 761 (28.8) |
| Very low- low | 621 (28.0) | 119 (28.0) | 740 (28.0) |
| SVI median (IQR) | 0.301 (0.226,0.363) | 0.298 (0.223, 0.362) | 0.301 (0.226, 0.362) |
| SVI category | |||
| Low- Very Low | 1101 (49.9) | 213 (50.1) | 1314 (49.9) |
| Medium | 785 (35.6) | 153 (36.0) | 938 (35.6) |
| High- Very High | 59 (13.9) | 59 (13.9) | 380 (14.4) |
Overall, 83.9% (n=2214) of the participants achieved viral suppression at six months (Table I). Prevalence of viral suppression in the cohort increased over time from 69.7% among participants that initiated ART in 2000–2004 to 83.2%, 88.8%, and 85.0% among participants initiating ART in 2005–2008, 2009–2012, and 2013–2017, respectively. Viral suppression by ART initiation year is shown in Supplementary Figure II. In a sensitivity analysis exploring the impact of missing viral load values, viral suppression was 88.3% and 61.0% when missing viral loads were counted as success and failures, respectively.
Geospatial Distribution and Neighborhood Level Socioeconomic Indicators
Participants lived in 50 subdistricts of the metropolitan region of Rio de Janeiro (Figure IA), with an average of 52.8 participants per subdistrict (Supplementary Table I). The metropolitan region of Rio de Janeiro occupies about 1,200 km2 with a population density of 5,326.8 people/km2. The mean territorial area per subdistrict is 140.33 km2, ranging from a minimum of 0.94 to a maximum of 956 km2. According to the 2010 census, the mean and median population of subdistricts are 221,680 and 158,230 people, respectively. The standard deviation is 21,410, with the maximum and minimum population sizes ranging from 3,359 to 997,941 people. Neighborhood socioeconomic indices varied among HDUs in Rio de Janeiro (Figures IB and IC). There were statistically significant demographic differences by neighborhood socioeconomic status: participants residing in neighborhoods with higher levels of development (high MHDIs) and low social vulnerability (low SVI) tended to identify as White, more educated, and cis-MSM (Table II). In our supplementary descriptive analysis, we found no statistically significant difference in MHDI (X2 statistic = 2.56, 2df, p= 0.58) and SVI (X2 statistic= 1,17, 1df, p= 0.36) category distribution among participants included in the analysis and those who were excluded due to a missing viral load at six months (Supplementary Table II).
Figure I.
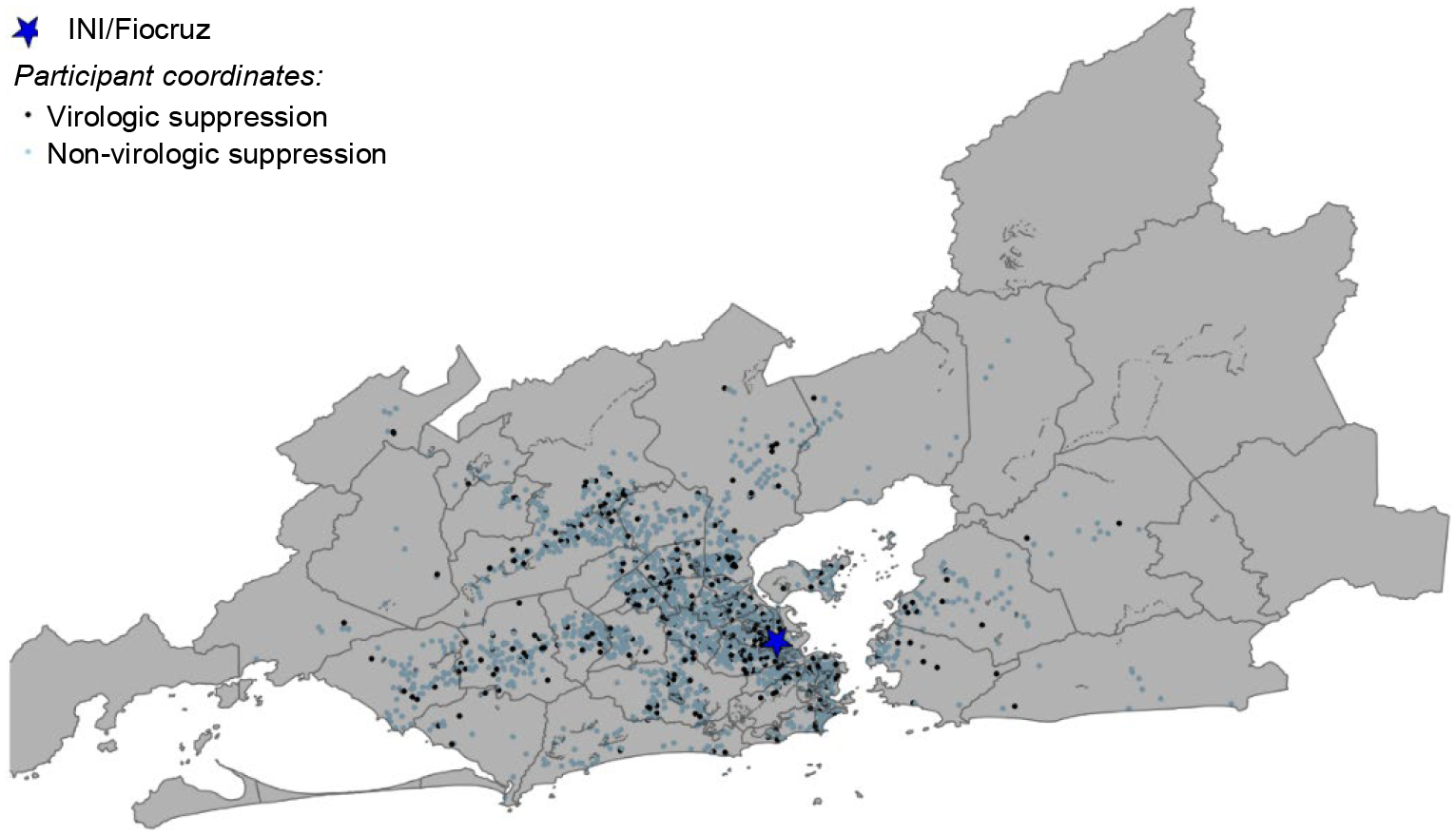
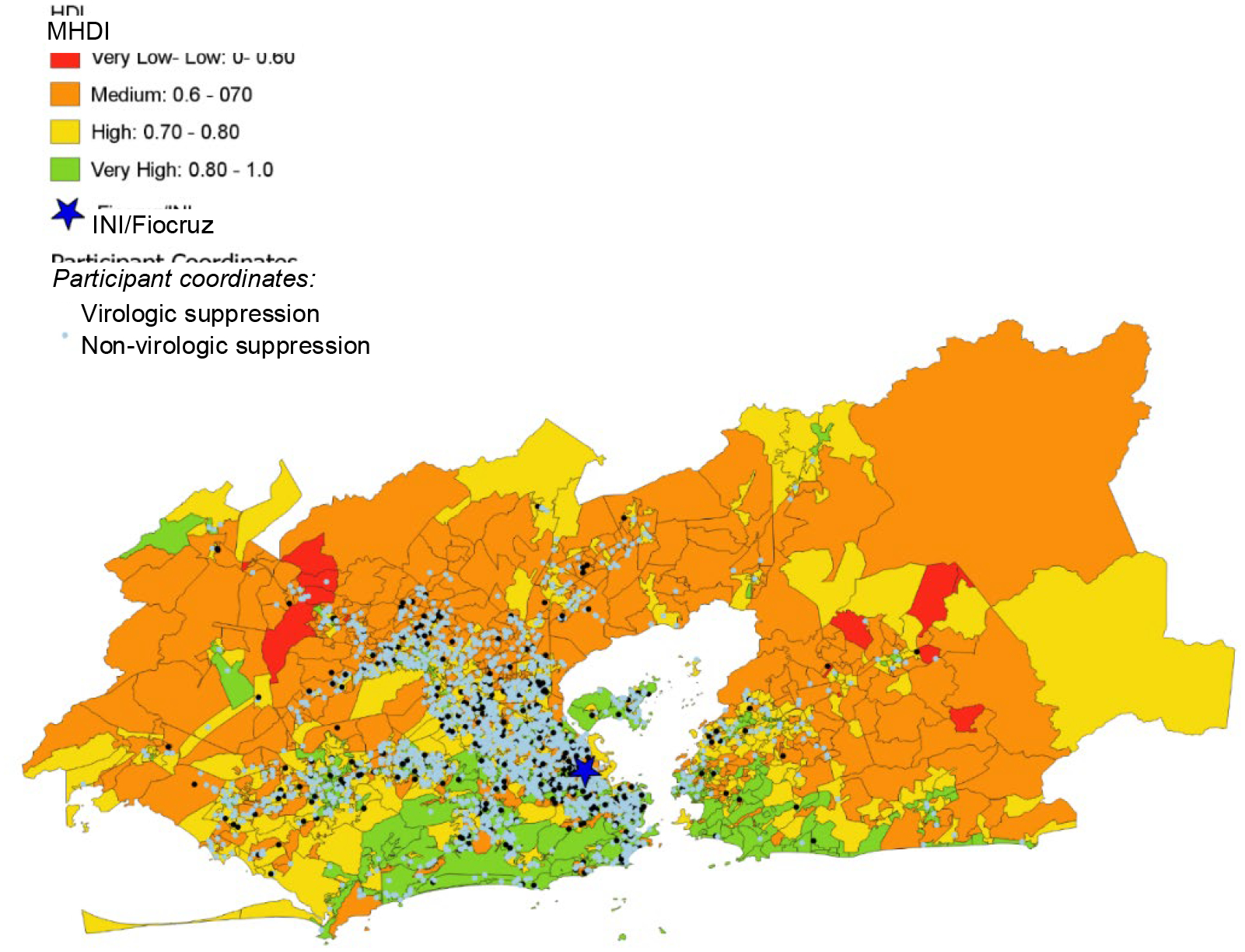
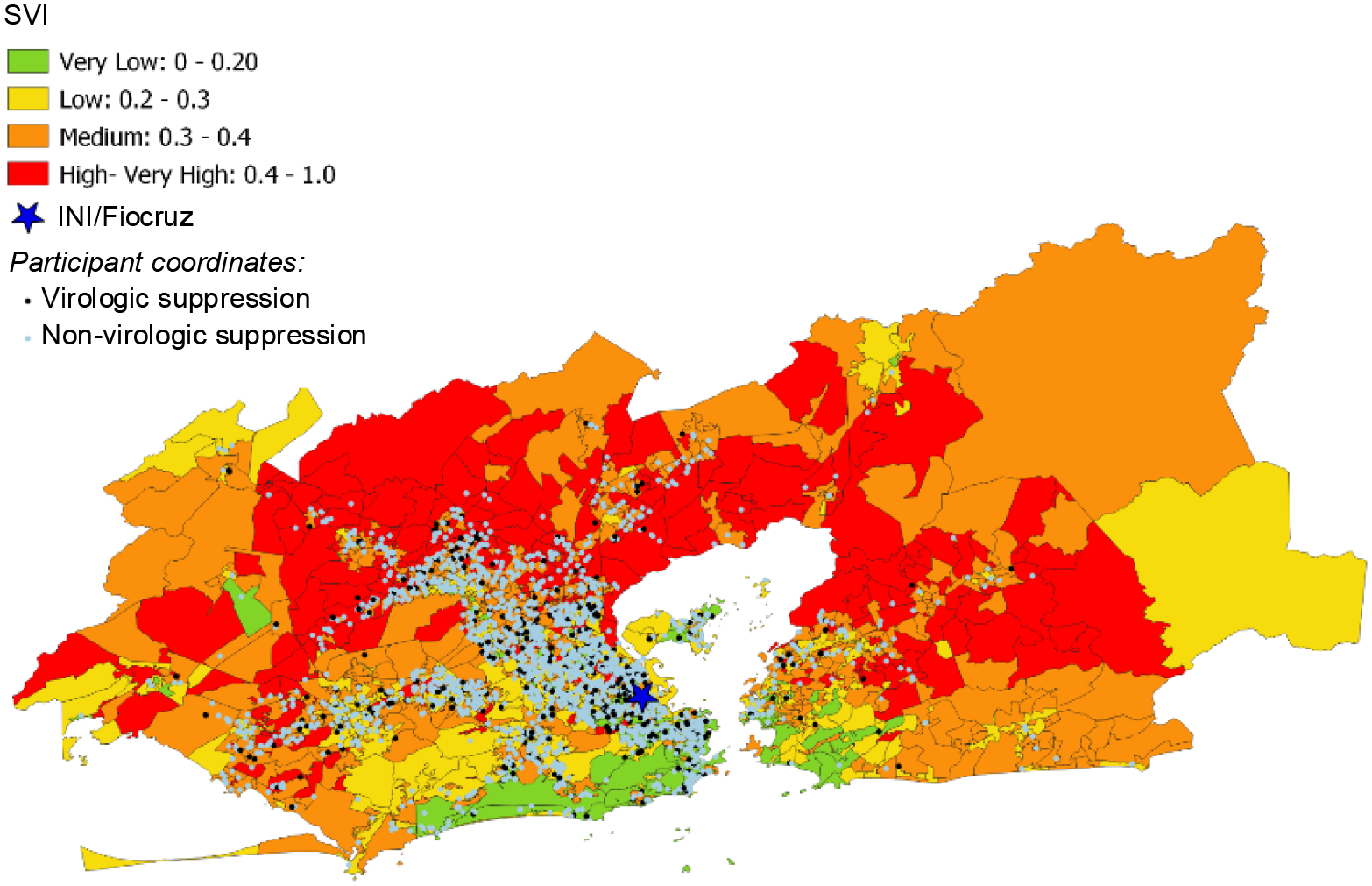
Geospatial distribution of participants and neighborhood socioeconomic context in the metropolitan region of Rio de Janeiro
Figure IA Distribution of participants with virologic and non-virologic suppression in the subdistricts of the metropolitan region of Rio de Janeiro
Figure IB Distribution of Municipal Human Development Index (MHDI) in the metropolitan region of Rio de Janeiro
Figure IC Distribution of Social Vulnerability Index (SVI) in the metropolitan region of Rio de
Table II.
Participant sociodemographics stratified by two neighborhood socioeconomic indices: municipal human development index (MHDI) and social vulnerability index (SVI)
| HDI | SVI | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | High-Very High (HDI 0.7–1.0) | Medium (HDI 0.7–0.8) | Very Low- Low (HDI <0.6) | Test statistic | P value* | Total | Very Low- Low (SVI <0.3) | Medium (SVI 0.3– 0.4) | High-Very High (SVI 0.4–1.0) | Test statistic | P value* | |
| n= 2639 (100%) | n= 1138 (43.1%) | n= 761 (28.8%) | n= 740 (28.0%) | n= 2632 (100%) | n= 1314 (49.9%) | n= 938 (35.4) | n= 380 (14.4%) | |||||
| Age at ART initiation (IQR) | 34.9 (28.4, 42.8) | 35.2 (28.9,44.0} | 34.3 (27.6,42.4} | 35.0 (28.4,42.2) | 5.37 (2 df) | 0.068 | 34.9 (28.4,42.8) | 35 (28.9,43.7) | 34.8 (27.6,42 .4) | 34.7 (28.8,41.5) | 3.79 (2 df) | 0.15 |
| Age Category (years) | 10 (6 df0 | |||||||||||
| <30 | 824 (31.2) | 339 (29.8) | 255 (33.5) | 230 (31.1) | 7.31 (2 df) |
0.293 | 821 (31.2) | 387 (29.5) | 318 (33.9) | 116 (30.5} | 0.125 | |
| 30–40 | 938 (35.5) | 398 (35) | 258 (33.9) | 282 (38.1) | 937 (35.6) | 470 (35.8) | 317 (33.8) | 150 (39.5) | ||||
| 40–50 | 600 (22.7) | 270 (23.7) | 173 (22.7) | 157 (21.2) | 598 (22.7) | 309 (23.5) | 215 (22.9) | 74 (19.5) | ||||
| >50 | 277 (10.5) | 131 (11.5) | 75 (9.9) | 71 (9.6) | 276 (10.5) | 148 (11.3) | 88 (9.4) | 40 (10.5) | ||||
| Race | ||||||||||||
| White | 1199 (45.4) | 625 (54.9) | 304 (39.9) | 270 (36.5) | 76.84 (4 df) | < 0.001 | 1196 (45.4) | 698 (53.1) | 373 (39.8) | 125 (32.9} | 74.55 (4df) | < 0.001 |
| Black | 513 (19.4) | 174 (15.3) | 159 (20.9) | 180 (24.3) | 511 (19.4) | 204 (15.5) | 200 (21.3) | 107 (28.2) | ||||
| Mixed/Unknown | 927 (35.1) | 339 (29.8) | 298 (39.2) | 290 (39.2) | 925 (35.1) | 412 (31.4) | 365 (38.9) | 148 (38.9) | ||||
| Education level | ||||||||||||
| Less than 8 years | 1134 (43) | 329 (28.9) | 367 (48.2) | 438 (59.2) | 191.12 (4 df) | < 0.001 | 1130 (42.9) | 421 (32) | 470 (50.1) | 239 (62.9) | 150.19 (4df) | < 0.001 |
| More than 9 years | 1485 (56.3) | 802 (70.5) | 392 (51.5) | 291 (39.3) | 1482 (56.3) | 885 (67.4) | 461 (49.1) | 136 (35.8) | ||||
| Unknown | 20 (0.8) | 7 (0.6) | 2 (0.3) | 11 (1.5) | 20 (0.8) | 8 (0.6) | 7 (0.7) | 5 (1.3) | ||||
| Gender and exposure risk | < 0.001 | 768 (29.2) | 318 (24.2) | 317 (33.8) | 133 (35) | 90.58 (8 df) | < 0.001 | |||||
| C is-women | 771 (29.2) | 266 (23.4) | 250 (32.9) | 255 (34.5) | 100.27 (8 df) | 80 (3) | 38 (2.9) | 32 (3.4) | 10 (2.6) | |||
| TGW | 80 (3) | 32 (2.8) | 23 (3) | 25 (3.4) | 1016 (38.6) | 613 (46.7) | 305 (32.5) | 98 (25.8) | ||||
| MSM | 1019 (38.6) | 553 (48.6) | 251 (33) | 215 (29.1) | 608 (23.1) | 258 (19.6) | 231 (24.6) | 119 (31.3} | ||||
| Heterosexual Cis-men | 608 (23) | 209 (18.4) | 191 (25.1) | 208 (28.1) | 160 (6.1) | 87 (6.6) | 53 (5.7) | 20 (5.3) | ||||
| Cis-men: Other & Unknown | 161 (6.1) | 78 (6.9) | 46 (6) | 37 (5) |
Participants residing in the city (66.5%, n= 1755) and greater metropolitan area (33.5%, n= 884) had similar viral suppression levels of 83.5% and 84.9%, respectively. Participants with suppressed and detectable viral loads shared similar geospatial clustering patterns, with the greatest density around the INI site (Figure IA).
Viral Suppression and Associated Factors
Table III shows results from unadjusted logistic regression and adjusted multilevel logistic regression models of factors associated with viral suppression. In the unadjusted analysis, neighborhood MHDI and SVI did not impact participant viral suppression. Older age, >8 years of education, and both cisgender MSM and heterosexual cisgender men, compared to cisgender women, were at increased odds of viral suppression at six months. Similarly, initiating ART after 2004 also increased the odds of viral suppression, while pre-treatment viral load ≥100,000 copies/mL was associated with decreased odds of viral suppression. Race, pre-treatment opportunistic illnesses, and pre-treatment TB were not significantly associated with viral suppression.
Table III.
Unadjusted logistic regression models and adjusted multilevel adjusted logistic regression model for VS
| Model 1: MHDI | Model 2: SVI | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) | Wald test z value | P-value | Adjusted OR (95% CI) | Wald test z value | P-value | Adjusted OR (95% CI) | Wald test z value | P-value | |
| Age at time of ART initiation (IQR) | 1.14 (1.02, 1.26) | 2.41 | 0.02 | 1.20 (1.07, 1.34) | 3.19 | 0.001 | 1.20 (1.08, 1.34) | 3.22 | 0.001 |
| Age Category (years) | |||||||||
| <30 | Ref | Ref | Ref | ||||||
| 30–40 | 1.19 (0.93, 1.52) | 1.35 | 0.18 | ||||||
| 40–50 | 1.23 (0.92, 1.63) | 1.42 | 0.16 | ||||||
| >50 | 1.54 (1.04, 2.29) | 2.13 | 0.03 | ||||||
| Race | |||||||||
| White | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Black | 0.87 (0.67, 1.17) | −0.86 | 0.39 | 0.90 (0.67, 1.20) | −0.72 | 0.47 | 0.89 (0.67, 1.20) | −0.75 | 0.45 |
| Mixed/Unknown | 0.98 (0.77, 1.24) | −0.17 | 0.86 | 0.96 (0.74, 1.23) | −0.36 | 0.72 | 0.96 (0.74, 1.23) | −0.35 | 0.73 |
| Education | |||||||||
| Less than 8 Years | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| More than 8 Years | 1.42 (1.15, 1.75) | 3.28 | 0.001 | 1.30 (1.03, 1.65) | 2.21 | 0.03 | 1.31 (1.04, 1.66) | 2.27 | 0.02 |
| Unknown | -- | -- | -- | ||||||
| Gender and exposure risk | |||||||||
| Cisgender women | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| TGW | 1.19 (0.65, 2.17) | 0.55 | 0.58 | 1.24 (0.66, 2.33) | 0.66 | 0.51 | 1.26 (0.67, 2.37) | 0.70 | 0.48 |
| Cis-MSM | 1.72 (1.33, 2.21) | 4.18 | <0.0001 | 1.57 (1.18, 2.08) | 3.12 | 0.002 | 1.58 (1.19, 2.09) | 3.16 | 0.002 |
| Heterosexual cisgender men | 1.39 (1.05, 1.85) | 2.30 | 0.02 | 1.36 (1.01, 1.83) | 2.02 | 0.04 | 1.37 (1.01, 1.84) | 2.05 | 0.04 |
| Cisgender men: other and unknown | 0.98 (0.64, 1.49) | −0.11 | 0.91 | 1.08 (0.68, 1.71) | 0.33 | 0.74 | 1.07 (0.68, 1.70) | 0.30 | 0.76 |
| Pre-treatment CD4 count (cells/mm3) | |||||||||
| <200 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| 200–350 | 1.38 (1.04, 1.82) | 2.32 | 0.03 | 1.23 (0.91, 1.67) | 1.34 | 0.18 | 1.23 (0.91, 1.67) | 1.35 | 0.18 |
| 350–500 | 1.42 (1.00, 2.03) | 1.94 | 0.05 | 1.28 (0.86, 1.90) | 1.23 | 0.22 | 1.27 (0.85, 1.88) | 1.17 | 0.24 |
| >500 | 1.44 (0.98, 2.11) | 1.86 | 0.06 | 1.33 (0.86, 2.05) | 1.29 | 0.20 | 1.34 (0.87, 2.06) | 1.31 | 0.19 |
| Unknown | 0.45 (0.34, 0.61) | −5.29 | <0.0001 | 0.55 (0.35, 0.84) | −2.76 | 0.006 | 0.54 (0.35, 0.84) | −2.77 | 0.006 |
| Pre-treatment viral load (copies/mL) | |||||||||
| <100,000 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥100,000 | 0.69 (0.54, 0.88) | −3.00 | 0.003 | 0.74 (0.56, 0.97) | −2.19 | 0.03 | 0.74 (0.56, 0.97) | −2.17 | 0.03 |
| Unknown | 0.42 (0.32, 0.54) | −6.44 | <0.0001 | 0.78 (0.52, 1.18) | −1.18 | 0.24 | 0.78 (0.52 1.17) | −1.18 | 0.24 |
| Opportunistic illness | |||||||||
| No | Ref | Ref | Ref | ||||||
| Yes | 0.99 (0.79, 1.25) | −0.08 | 0.94 | ||||||
| TB | |||||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 1.05 (0.79, 1.40) | 0.32 | 0.75 | 1.20 (0.87, 1.65) | 1.13 | 0.26 | 1.20 (0.87, 1.64) | 1.18 | 0.26 |
| ART initiation year | |||||||||
| 2000–2004 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| 2005– 2008 | 2.15 (1.57, 2.95) | 4.75 | <0.0001 | 1.90 (1.36, 2.64) | 3.79 | <0.0001 | 1.89 (1.36, 2.63) | 3.75 | <0.001 |
| 2009– 2012 | 3.43 (2.49, 4.73) | 7.52 | <0.0001 | 2.89 (2.06, 4.04) | 6.17 | <0.0001 | 2.87 (2.05, 4.02) | 6.13 | <0.001 |
| 2013– 2016 | 2.47 (1.82, 3.33) | 5.86 | <0.0001 | 2.05 (1.47, 2.86) | 4.20 | <0.0001 | 2.03 (1.45, 2.84) | 4.15 | <0.001 |
| MHDI (IQR) | 0.72 (0.27, 1.87) | −0.68 | 0.49 | ||||||
| MHDI category | |||||||||
| High- Very High | Ref | Ref | Ref | Ref | Ref | Ref | |||
| Medium | 0.95 (0.74, 1.21) | −0.43 | 0.67 | 1.06 (0.81, 1.39) | 0.41 | 0.68 | |||
| Very low- low | 0.98 (0.76, 1.27) | −0.15 | 0.88 | 1.12 (0.84, 1.50) | 0.77 | 0.44 | |||
| SVI median (IQR) | 1.71 (0.62, 4.61) | 1.06 | 0.29 | ||||||
| SVI category | |||||||||
| Very Low- Low | Ref | Ref | Ref | Ref | Ref | Ref | |||
| Medium | 0.99 (0.79, 1.25) | −0.06 | 0.95 | 1.12 (0.87, 1.43) | 0.85 | 0.39 | |||
| High- Very High | 1.05 (0.77, 1.44) | 0.32 | 0.75 | 1.21 (0.85, 1.71) | 1.05 | 0.29 | |||
| Intraclass Correlation Coefficient (ICC) | 0.005 | 0.004 | |||||||
In the adjusted multilevel regression models that considered MHDI and SVI as covariates, neighborhood socioeconomic indicators again did not predict viral suppression. In the model with MHDI, participants residing in neighborhoods with medium (aOR 1.06, 95% CI 0.81–1.39) and very low-to-low (aOR 1.12, 95% CI 0.84–1.50) development did not have significantly different odds of viral suppression compared to participants in high-to-very high MHDI neighborhoods. Similarly, with very low-to-low SVI as the reference group, residing in neighborhoods with medium (aOR 1.12, 95% CI 0.87–1.43) and high-to-very high (aOR 1.21, 95% CI 0.85–1.71) vulnerability did not have statistically different odds of viral suppression.
Initiating ART after 2004 had the strongest association with viral suppression in both multilevel models. In the MHDI model, participants that initiated ART after 2004 were significantly more likely to achieve viral suppression compared to participants initiating ART between the years 2000 and 2004: 2005–2008 (aOR 1.90, 95% CI 1.36–1.64), 2009–2012 (aOR 2.89, 95% CI 2.06–4.04), and 2013–2016 (aOR 2.05, 95% CI 1.47–2.86). In the model with MHDI as a covariate, individual variables of older age (aOR 1.20, 95% CI 1.07–1.34), >8 years of education (aOR 1.30, 95% CI 1.03–1.65), and cis-MSM (aOR 1.57, 95% CI 1.18–2.08) and heterosexual cisgender men (aOR 1.36, 95% CI 1.01–1.83), compared to cisgender women, were associated with greater odds of viral suppression. In contrast, unknown pre-treatment CD4 count (aOR 0.55, 95% CI 0.35–0.84) and pre-treatment viral load ≥100,000 copies/mL (aOR 0.74, 95% CI 0.56–0.97) were associated with decreased odds of viral suppression. The adjusted model with SVI demonstrated similar results for all variables.
Consistent with the findings listed above, the ICC in the multilevel analysis with MHDI and SVI as covariates were 0.004 and 0.005, respectively. These results suggest that participant characteristics were largely homogenous among the subdistricts, and that <1% of differences among participants are attributable to subdistrict-contextual factors.
DISCUSSION
In this study of PLWH in Rio de Janeiro, Brazil, we explored the impact of individual- and neighborhood-level characteristics on achieving viral suppression six months after initiating ART. Together, the multidimensional neighborhood indicators employed in the analysis encompassed education, life expectancy, income, urban infrastructure, and employment status. Our cohort achieved high levels of viral suppression in a setting with universal access to HIV care and ART. After adjusting for individual sociodemographic and HIV clinical variables, neighborhood disadvantage did not contribute to viral suppression at six months. Similar to other studies, individual characteristics such as age, education, gender/probable mode of HIV exposure, in addition to ART initiation year predicted viral suppression.
Of note, we observed sociodemographic differences by neighborhood socioeconomic status. Participants identifying as White, with higher levels of education, and as cis-MSM tended to reside in neighborhoods characterized by higher development and lower social vulnerability. However, achieving viral suppression remained uniform in neighborhoods of different socioeconomic statuses. Additionally, participants residing in the city and greater metropolitan area of Rio de Janeiro had similar viral suppression levels, which may indicate the benefit of geographic accessibility to the INI site. Participants with and without detectable viral loads were also similarly geospatially distributed, suggesting that geographic unit was not a determinant of viral suppression for the study population included in this analysis. The small ICC values in our multilevel models suggest that the participants nested in each subdistrict had little variability in comparison to other subdistricts. The 369 neighborhoods (barrios) comprising the 52 subdistricts of the metropolitan region of Rio de Janeiro have variability in their sociodemographic composition, infrastructure, and resource access, which may limit the ability to detect differences between the subdistricts. The neighborhood socioeconomic indices were linked to the census tracts participants resided in, which is the smallest geographic scale. However, though no significant differences were observed at the subdistrict level in our multilevel analysis, it is important to note that other spatial scales, either at higher or lower resolution, could lead to different findings.
The socioecological model proposes dynamic, interrelated levels of risk, including network, community, and policy factors, to characterize drivers of HIV-related outcomes. Our analysis focused on community-level economic disadvantage and included only participants receiving ART. The lack of association between viral suppression and neighborhood context in our study aligns with results from previous studies: Burke-Miller et al. (2018) employed a neighborhood risk-score (comprising of income, education, and unemployment) similar to our study and did not detect differences in viral suppression by adverse neighborhood socioeconomic context (7). In a study of HIV continuum of care among adults cared for within the Brazilian Unified Health System, Pascom et al. (2018) also did not observe a significant relationship between viral suppression and SVI of participants’ municipality, a larger geographic area of analysis. However, patients living in municipalities with high/very high SVI had significantly increased odds of late presentation to care and not being on ART (29). Additionally, neighborhood poverty decreased odds of receiving ART but did not impact viral suppression in PLWH receiving ART through the Ryan White HIV/AIDS Program in the United States (12). Positioning these results within a socioecological model, community-level economic disadvantage may impact presentation to care. Thus, participants’ engagement in care may have negated detection of differences between viral suppression and neighborhood economic disadvantage in our cohort. Future studies should evaluate the impact of neighborhood contextual factors on other endpoints of the care continuum such as HIV diagnosis, access and timely initiation of ART or clinical outcomes over longer follow-up. Additionally, cis-MSM had the greatest odds of achieving viral suppression in our gender and exposure variable, consistent with previous research (46). The neighborhood indices in our analyses do not capture social network data, such as social support, shared behavioral norms, and attitudes, which have been previously linked to health behaviors (47). Within the socioecological model, variations in social networks across different communities may drive attitudes and behaviors leading to medication adherence and better viral suppression outcomes (48).
Policy-level factors specific to the Brazilian context and likely to have substantially affected our analysis are universal access to HIV care and ART. While not directly measured in our analysis, larger structural policy factors may diminish the impact of neighborhood disadvantage. For example, in other low-income settings (e.g., Botswana, Zambia, Uganda, Kenya and South Africa), implementation of universal test and treat significantly augmented population-level suppression rates (49). Healthcare systems that provide integrated HIV care, such as the U.S. Department of Veteran Affairs Healthcare System (which largely serves a marginalized population) and Kaiser Permanente, have shown that their patients experience better outcomes along the HIV care cascade, including higher levels of viral suppression, compared to the general U.S. population (50, 51).
In a separate analysis of the INI cohort, De Boni et al. found that SVI negatively impacted the odds of viral suppression at 12 months. The contrasting results may be explained by methodological differences: shorter timeframe 2014–2017, definition of viral suppression as <50 copies/mL compared to <400 copies/mL in our study population, and viral suppression outcome at 12 months compared to 6 in our study population. In other studies conducted in settings of universal HIV care, neighborhood disadvantage was associated with viral suppression only in the poorest-quintile neighborhoods, indicating a non-linear relationship of neighborhood poverty and viral suppression (7, 21). These studies also reported lower viral suppression levels of 74–76%, compared to 84% in our cohort. Higher levels of viral suppression in our cohort may have decreased the ability to detect neighborhood socioeconomic differences between participants with and without viral suppression.
Overall, the prevalence of viral suppression in our cohort was 84% and matches pooled estimate of 84% viral suppression at six months described in a meta-analysis of low-to-middle-income countries (52). Reassuringly, viral suppression outcomes are improving, as an older analysis of the INI cohort demonstrated overall ART effectiveness of 77% among participants initiating ART between 2000 and 2010 (53). The improving results over time are promising and demonstrate the feasibility of reaching at least one of the UN 90-90-90 targets of eliminating AIDS with 90% of people on ART achieving viral suppression (54). Integrated services may also positively facilitate care engagement and viral suppression in our cohort. In the present analysis, all participants received care at the INI site, the largest HIV care provider in Rio de Janeiro State. At INI, all participants receive primary, specialty, and tertiary care for HIV and, if present, for related co-morbidities. Furthermore, INI has its own laboratory infrastructure, facilitating laboratory monitoring, as well as a public pharmacy onsite. The quality-of-care echoes that of university hospitals and research-based health services of Brazil. In addition, it is located in the Southeast part of Brazil which is the region with most resources that has faced the HIV epidemic since the 1980s, so health professionals may be better informed and equipped to care for PLWH. All these factors may have acted synergistically to improve health outcomes in our cohort population.
Among individual-level variables, calendar year of ART initiation had the strongest association with viral suppression. Viral suppression improved from 69.8% to 90% among participants initiating ART in the years 2000 to 2004 and 2017 to 2018, respectively. This change is reflective of the simpler, more tolerable, and increasingly effective first-line ART regimens that have been introduced over time (53, 55). In 2014, Brazil adopted the universal test-and-treat policy, in which ART was prescribed to all PLWH regardless of CD4 count, and changed its first-line regimen to a single pill of tenofovir (TDF) + lamivudine (3TC) + efavirenz, potentially improving adherence (27). Of note, although INSTIs like Dolutegravir are currently available in Brazil, our cohort initiated ART before dolutegravir became available to ART-naïve patients.
We also identified individual-level disparities associated with viral suppression based on age, education, and gender/probable mode of HIV exposure. Higher odds of viral suppression were observed among cisgender MSM and heterosexual cisgender men compared to cisgender women. This pattern was also shown in older analyses of the INI cohort, suggesting that despite changes and improvements to first line regimens, women still lag behind men in virologic control (53, 56). Other studies in Brazil have observed that women reported lower ART adherence than men(57, 58). Some research suggests that women experience more ART-related toxicities, which may increase ART discontinuation and thus lead to poor viral suppression rates (59, 60). However, there have been mixed results in other contexts, with some studies showing women have increased likelihood of viral suppression and others reporting the opposite (61–63). These differing conclusions emphasize the complexity of gender dynamics, discrimination, access to HIV care in different cultural contexts, and heterogeneity in cohort demographics and ART regimens, making the findings challenging to generalize. In this situation, it is paramount to consider the complex psychosocial needs and social vulnerabilities of the women included in this cohort. Women living with HIV in the INI cohort have a high prevalence of lifetime history of physical violence (31%), unemployment (52%), and no secondary education (54%) (64). Women receiving HIV care at INI site also reported a lower health-related quality of life compared to heterosexual men (65). These factors may impair opportunities for engagement in care, ART adherence, and viral suppression.
Older age, particularly age >50 years, and higher levels of education were found to be protective in achieving viral suppression in this cohort of people living with HIV and in other contexts (29, 46, 66–69). Younger age has been shown to be associated with decreased adherence and retention in care, leading to lower likelihood of achieving viral suppression (70, 71). For Brazil, these results are particularly concerning given the concurrent rise of AIDS cases by 53.2% among 15–19 and 10.3% among 20–24 year-olds between the years of 2004 and 2013(54). Additionally, higher education level may serve as a protective factor by increasing individual effective agency, financial resources, health literacy, access to information, and social support (72–74). All in all, results from our cohort and other studies underscore the need to support younger, less educated populations and women in closing treatment gaps. A socioecological approach in research and interventions should identify and address health system, institutional, and contextual barriers.
Some limitations should be considered in the interpretations of this study. Nearly a quarter of participants eligible for the present analysis were excluded due to a missing viral load. However, our supplementary analysis did not reveal statistically significant differences of neighborhood socioeconomic status among included and excluded participants. Although high levels of viral suppression are encouraging, they do not account for participants with early mortality and missing viral loads. Participants with missing viral loads may also experience higher structural barriers to accessing care and ART, which has previously been linked to neighborhood disadvantage, and may have affected our results. When classifying participants with missing viral loads as non-suppressed, the estimated prevalence of viral suppression in our cohort decreases to 61.0%. Intention-to-treat analyses of virologic outcomes in low-to-middle-income countries, which counted loss to follow-up, mortality, and stopping ART as non-suppression, demonstrated a similar drop in viral suppression from 84.0% to 74.7% (52). Results from our cohort and systematic reviews of LMICs emphasize that individuals temporarily or permanently lost to follow up should be prioritized in future research (52).
Furthermore, it is important to consider the representativeness of our study population to that of adults living with HIV in Rio de Janeiro State. Our study is based on a single-center and individuals participating in the HIV Clinical cohort tended to have their residences clustered around the INI site, which may limit generalizability of our findings. As an academic research center, INI provides comprehensive primary, secondary, and tertiary HIV care to patients. Our outcomes may be more comparable to other populations who receive care in research-based treatment centers. Nonetheless, Brazil’s universal treatment policy coupled and free access to HIV care has resulted in high percentages of viral suppression with 84% and 85% of those living with HIV on ART achieving viral suppression in the city and state of Rio de Janeiro, respectively, similar to the viral suppression outcomes in our cohort. However, as seen in the MHDI and SVI geospatial distributions in our cohort, there was variability and diversity in our participant pool. Additionally, the median neighborhood SVI of 0.301 and MHDI of 0.683 in our cohort, are comparable to that of the metropolitan region of Rio de Janeiro, which had an SVI of 0.319 and MHDI of 0.771 in 2010 (31, 36). Moreover, we employed objective neighborhood indicators. Participants’ lived experiences and perspectives of neighborhood disorder, stigma, social cohesion, and safety may drive healthcare access and outcomes not captured by MHDI and SVI. Additionally, research suggests that neighborhood socioeconomic disadvantage negatively impacts sustained but not initial viral suppression after HIV diagnosis (75). Thus, our cohort analysis might not fully portray the longitudinal impact of neighborhood context on viral suppression and opportunities to engage in care. Future analyses should consider longer follow-up periods for longitudinal outcomes as well as other neighborhood contextual factors.
CONCLUSIONS
In summary, high levels of viral suppression are achieved in a middle-income country with universal access to HIV care and treatment. Among this cohort of adults living with HIV, neighborhood socioeconomic indicators, as measured by level of development and vulnerability, did not impact viral suppression six months after ART initiation. Individual variables of older age, higher education, identifying as cis-MSM, and later calendar year of ART initiation were associated with improved viral suppression outcomes. Future studies should explore the impact of neighborhood variables on HIV outcomes longitudinally as well as along the HIV care continuum.
Supplementary Material
Funding:
All funding sources and recipients are listed below.
Instituto Nacional de Ciência e Tecnologia (310541/2017-4): Dr. Raquel B. De Boni
National Institute of Mental Health (MH087222): Miss Lyolya Hovhannisyan, Dr. Jesse Clark
Instituto Nacional de Ciência e Tecnologia (306196/2017-4): Dr. Beatriz Grinsztejn, Dr. Paula M. Luz
Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (BR) (E-26/203.287/2016): Dr. Paula M. Luz
Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (BR) (310541/2017-4) : Dr. Beatriz Grinsztejn
Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (E-26/203.154/2017): Dr. Raquel B. De Boni
Footnotes
Conflicts of interest/competing interests: Not applicable
Ethics approval: This study was approved by the Ethics Committee of the Evandro Chagas Clinical Research Institute of the Oswaldo Cruz Foundation (INI/FIOCRUZ, CAAE 0032.0.009.000–10) and was conducted according to the principles expressed in the Declaration of Helsinki. All patient records/information were anonymized prior to analysis.
Consent to participate: Written consent obtained from all participants.
Consent for publication: Provided by all authors.
Code availability: Code is available from the corresponding author on reasonable request
Availability of data and material:
Data is available from the corresponding author on reasonable request.
REFERENCES
- 1).Latkin CA, German D, Vlahov D, Galea S. Neighborhoods and HIV: a social ecological approach to prevention and care. Am Psychol. 2013;68(4):210–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Baral S, Logie CH, Grosso A, Wirtz AL, Beyrer C. Modified social ecological model: a tool to guide the assessment of the risks and risk contexts of HIV epidemics. BMC Public Health. 2013;13(1):482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Auerbach JD, Parkhurst JO, Caceres CF. Addressing social drivers of HIV/AIDS for the long-term response: conceptual and methodological considerations. Global Public Health. 2011;6 Suppl 3:S293–309. [DOI] [PubMed] [Google Scholar]
- 4).Poundstone KE, Strathdee SA, Celentano DD. The social epidemiology of human immunodeficiency virus/acquired immunodeficiency syndrome. Epidemiologic Reviews. 2004;26:22–35. [DOI] [PubMed] [Google Scholar]
- 5).Farmer PE, Nizeye B, Stulac S, Keshavjee S. Structural violence and clinical medicine. PLoS Med. 2006;3(10):e449–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Joy R, Druyts EF, Brandson EK, Lima VD, Rustad CA, Zhang W, et al. Impact of neighborhood-level socioeconomic status on HIV disease progression in a universal health care setting. Journal of AIDS. 2008;47(4):500–5. [DOI] [PubMed] [Google Scholar]
- 7).Burke-Miller JK, Weber K, Cohn SE, Hershow RC, Sha BE, French AL, et al. Neighborhood community characteristics associated with HIV disease outcomes in a cohort of urban women living with HIV. AIDS Care. 2016;28(10):1274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Arnold M, Hsu L, Pipkin S, McFarland W, Rutherford GW. Race, place and AIDS: the role of socioeconomic context on racial disparities in treatment and survival in San Francisco. Social Science & Medicine. 2009;69(1):121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Ransome Y, Kawachi I, Dean LT. Neighborhood Social Capital in Relation to Late HIV Diagnosis, Linkage to HIV Care, and HIV Care Engagement. AIDS Behav. 2017;21(3):891–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Johns MM, Bauermeister JA, Zimmerman MA. Individual and Neighborhood Correlates of HIV testing among african american youth transitioning from adolescence into young adulthood. AIDS Educ Prev. 2010;22(6):509–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Wand H, Reddy T, Ramjee G. Investigating spatial disparities in high-risk women and HIV infections using generalized additive models: Results from a cohort of South African women. Spatial and Spatio-temporal epidemiology. 2019;30:100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Shacham E, Lian M, Onen NF, Donovan M, Overton ET. Are neighborhood conditions associated with HIV management? HIV Medicine. 2013;14(10):624–32. [DOI] [PubMed] [Google Scholar]
- 13).Montaner JSG, Lima VD, Barrios R, Yip B, Wood E, Kerr T, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376(9740):532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. NEJM. 2011;365(6):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Tanser F, Barnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science (New York, NY). 2013;339(6122):966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Das M, Chu PL, Santos GM, Scheer S, Vittinghoff E, McFarland W, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5(6):e11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Friedman MR, Coulter RW, Silvestre AJ, Stall R, Teplin L, Shoptaw S, et al. Someone to count on: social support as an effect modifier of viral load suppression in a prospective cohort study. AIDS Care. 2017;29(4):469–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Weiser SD, Frongillo EA, Ragland K, Hogg RS, Riley ED, Bangsberg DR. Food insecurity is associated with incomplete HIV RNA suppression among homeless and marginally housed HIV-infected individuals in San Francisco. AIDS Behav. 2009;24(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Riley ED, Vittinghoff E, Koss CA, Christopoulos KA, Clemenzi-Allen A, Dilworth SE, et al. Housing First: Unsuppressed Viral Load Among Women Living with HIV in San Francisco. AIDS Behav. 2019;23(9):2326–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Beattie C, Osorio G, Kim S, Anderson N, Pati R. Structural barrier to HIV viral load suppression at an urban HIV/AIDS care center. American Public Health Association Annual Conference; October 31- November 04, 2015; Chicago, IL 2015. [Google Scholar]
- 21).Gueler A, Schoeni-Affolter F, Moser A, Bertisch B, Bucher HC, Calmy A, et al. Neighbourhood socio-economic position, late presentation and outcomes in people living with HIV in Switzerland. AIDS (London, England). 2015;29(2):231–8. [DOI] [PubMed] [Google Scholar]
- 22).Rebeiro PF, Howe CJ, Rogers WB, Bebawy SS, Turner M, Kheshti A, et al. The relationship between adverse neighborhood socioeconomic context and HIV continuum of care outcomes in a diverse HIV clinic cohort in the Southern United States. AIDS Care. 2018;30(11):1426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Chandran A, Edmonds A, Benning L, Wentz E, Adedimeji A, Wilson TE, et al. Longitudinal Associations Between Neighborhood Factors and HIV Care Outcomes in the WIHS. AIDS Behav. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Eberhart MG, Yehia BR, Hillier A, Voytek CD, Fiore DJ, Blank M, et al. Individual and community factors associated with geographic clusters of poor HIV care retention and poor viral suppression. JAIDS. 2015;69 Suppl 1:S37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Kahana SY, Jenkins RA, Bruce D, Fernandez MI, Hightow-Weidman LB, Bauermeister JA, et al. Structural Determinants of Antiretroviral Therapy Use, HIV Care Attendance, and Viral Suppression among Adolescents and Young Adults Living with HIV. PLoS One. 2016;11(4):e0151106–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Sheehan DM, Fennie KP, Mauck DE, Maddox LM, Lieb S, Trepka MJ. Retention in HIV Care and Viral Suppression: Individual- and Neighborhood-Level Predictors of Racial/Ethnic Differences, Florida, 2015. AIDS Patient Care STDS. 2017;31(4):167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Greco DB. Thirty years of confronting the Aids epidemic in Brazil, 1985–2015. Ciencia & Saude coletiva. 2016;21(5):1553–64. [DOI] [PubMed] [Google Scholar]
- 28).Lima TA, Beyrer C, Golub JE, Mota JCD, Malta MS, Silva C, et al. Inequalities in HAART uptake and differential survival according to exposure category in Rio de Janeiro, Brazil. Cadernos de Saude Publica. 2018;34(8):e00009617. [DOI] [PubMed] [Google Scholar]
- 29).Pascom ARP, Meireles MV, Benzaken AS. Sociodemographic determinants of attrition in the HIV continuum of care in Brazil, in 2016. Medicine (Baltimore). 2018;97(1S Suppl 1):S69–S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Tancredi MV, Waldman EA. Predictors of progression to AIDS after HIV infection diagnosis in the pre- and post-HAART eras in a Brazilian AIDS-free cohort. Trans R Soc Trop Med Hyg 2014;108(7):408–14. [DOI] [PubMed] [Google Scholar]
- 31).United Nations Development Programme, João Pinheiro Foundation. The Human Development Atlas in Brazil http://www.atlasbrasil.org.br/2013/en/2010.
- 32).Bortz M, Kano M, Ramroth H, Barcellos C, Weaver SR, Rothenberg R, et al. Disaggregating health inequalities within Rio de Janeiro, Brazil, 2002–2010, by applying an urban health inequality index. Cadernos de Saude Publica. 2015;31 Suppl 1(0 1):107–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Szwarcwald CL, da Mota JC, Damacena GN, Pereira TGS. Health inequalities in Rio de Janeiro, Brazil: lower healthy life expectancy in socioeconomically disadvantaged areas. Am J Public Health. 2011;101(3):517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Szwarcwald CL, Andrade CL, Bastos FI. Income inequality, residential poverty clustering and infant mortality: a study in Rio de Janeiro, Brazil. Social Science & Medicine. 2002;55(12):2083–92. [DOI] [PubMed] [Google Scholar]
- 35).Perlman J The Myth of Marginality Revisited: The Case of Favelas In Rio de Janeiro, 1969– 2003: Washington DC: Woodrow Wilson International Center for Scholars.; 2005. [Google Scholar]
- 36).Instituto de Pesquisa Economica Aplicada. Atlas of Social Vulnerability. http://ivs.ipea.gov.br/index.php/pt/2010.
- 37).de Melo Lucena DM, Dos Santos Figueiredo FW, de Alcantara Sousa LV, da Silva Paiva L, do Carmo Almeida TC, Galego SJ, et al. Correlation between municipal human development index and stroke mortality: a study of Brazilian capitals. BMC. 2018;11(1):540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Costa RFA, Longatto-Filho A, de Lima Vazquez F, Pinheiro C, Zeferino LC, Fregnani J. The Quality of Pap Smears from the Brazilian Cervical Cancer Screening Program According to the Human Development Index. Cancer Prevention Research. 2020;13(3):299–308. [DOI] [PubMed] [Google Scholar]
- 39).Pinho-França JDR, Chein MBDC, Thuler LCS. Patterns of cervical cytological abnormalities according to the Human Development Index in the northeast region of Brazil. BMC Womens Health. 2016;16:54-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Costa JdM, Costa JdM, Coelho LE, Luz PM. Adherence to antiretroviral therapy for HIV/AIDS in Latin America and the Caribbean: Systematic review and meta-analysis. J Int AIDS Soc. 2018;21(1):e25066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Institue of Brazilian Geography and Statistics. Population and demographic density. https://cidades.ibge.gov.br/2010.
- 42).Merlo J, Yang M, Chaix B, Lynch J, Råstam L. A brief conceptual tutorial on multilevel analysis in social epidemiology: investigating contextual phenomena in different groups of people. Journal of Epidemiology and Community Health. 2005;59(9):729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Bauermeister JA, Connochie D, Eaton L, Demers M, Stephenson R. Geospatial Indicators of Space and Place: A Review of Multilevel Studies of HIV Prevention and Care Outcomes Among Young Men Who Have Sex With Men in the United States. Journal of Sex Research. 2017;54(4–5):446–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Ward-Peterson M, Fennie K, Mauck D, Shakir M, Cosner C, Bhoite P, et al. Using multilevel models to evaluate the influence of contextual factors on HIV/AIDS, sexually transmitted infections, and risky sexual behavior in sub-Saharan Africa: a systematic review. Annals of Epidemiology. 2018;28(2):119–34. [DOI] [PubMed] [Google Scholar]
- 45).Vincens N, Emmelin M, Stafström M. The interplay of contextual layers: A multilevel analysis of income distribution, neighborhood infrastructure, socioeconomic position and self-rated health in Brazil. Health & Place. 2018;52:155–62. [DOI] [PubMed] [Google Scholar]
- 46).Meireles MV, Pascom ARP, Duarte EC, McFarland W. Comparative effectiveness of first-line antiretroviral therapy: results from a large real-world cohort after the implementation of dolutegravir. AIDS. 2019;33(10):1663–8. [DOI] [PubMed] [Google Scholar]
- 47).Amirkhanian YA. Social networks, sexual networks and HIV risk in men who have sex with men. Curr HIV/AIDS Rep. 2014;11(1):81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Langebeek N, Gisolf EH, Reiss P, Vervoort SC, Hafsteinsdóttir TB, Richter C, et al. Predictors and correlates of adherence to combination antiretroviral therapy (ART) for chronic HIV infection: a meta-analysis. BMC Medicine. 2014;12:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).Havlir D, Lockman S, Ayles H, Larmarange J, Chamie G, Gaolathe T, et al. What do the Universal Test and Treat trials tell us about the path to HIV epidemic control? J Int AIDS Soc. 2020;23(2):e25455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50).Backus L, Czarnogorski M, Yip G, Thomas BP, Torres M, Bell T, et al. HIV Care Continuum Applied to the US Department of Veterans Affairs: HIV Virologic Outcomes in an Integrated Health Care System. JAIDS. 2015;69(4):474–80. [DOI] [PubMed] [Google Scholar]
- 51).Raymond B, Wheatley B. Toward Better HIV Care: A Thought Leader Interview. Perm J. 2018;22:17–075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).Boender TS, Sigaloff KC, McMahon JH, Kiertiburanakul S, Jordan MR, Barcarolo J, et al. Long-term Virological Outcomes of First-Line Antiretroviral Therapy for HIV-1 in Low- and Middle-Income Countries: A Systematic Review and Meta-analysis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;61(9):1453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Cardoso SW, Luz PM, Velasque L, Torres T, Coelho L, Freedberg KA, et al. Effectiveness of first-line antiretroviral therapy in the IPEC cohort, Rio de Janeiro, Brazil. AIDS Research and Therapy. 2014;11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Brazilian Minsitry of Health. AIDS Response Programme Report. 2015.
- 55).Al-Dakkak I, Patel S, McCann E, Gadkari A, Prajapati G, Maiese EM. The impact of specific HIV treatment-related adverse events on adherence to antiretroviral therapy: a systematic review and meta-analysis. AIDS Care. 2013;25(4):400–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Martin DA, Luz PM, Lake JE, Clark JL, Veloso VG, Moreira RI, et al. Improved virologic outcomes over time for HIV-infected patients on antiretroviral therapy in a cohort from Rio de Janeiro, 1997–2011. BMC Infectious Diseases. 2014;14:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57).de Fatima Bonolo P, Ceccato M, Rocha GM, de Assis Acurcio F, Campos LN, Guimaraes MD. Gender differences in non-adherence among Brazilian patients initiating antiretroviral therapy. Clinics (Sao Paulo, Brazil). 2013;68(5):612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58).Hanif H, Bastos FI, Malta M, Bertoni N, Surkan PJ, Winch PJ, et al. Individual and contextual factors of influence on adherence to antiretrovirals among people attending public clinics in Rio de Janeiro, Brazil. BMC Public Health. 2013;13:574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Cardoso TS, Costa JO, Reis EA, Silveira MR, Bonolo PF, Santos SFD, et al. Which antiretroviral regimen is associated with higher adherence in Brazil? A comparison of single, multi, and dolutegravir-based regimens. Cadernos de Saude Publica. 2019;35(9):e00115518. [DOI] [PubMed] [Google Scholar]
- 60).Godfrey C, Hughes MD, Ritz J, Coelho L, Gross R, Salata R, et al. Brief Report: Sex Differences in Outcomes for Individuals Presenting for Third-Line Antiretroviral Therapy. JAIDS. 2020;84(2):203–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Abah IO, Ncube NBQ, Bradley HA, AgbaJi OO, Kanki P. Antiretroviral Therapy-associated Adverse Drug Reactions and their Effects on Virologic Failure- A Retrospective Cohort Study in Nigeria. Current HIV Research. 2018;16(6):436–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62).Cescon A, Patterson S, Chan K, Palmer AK, Margolese S, Burchell AN, et al. Gender differences in clinical outcomes among HIV-positive individuals on antiretroviral therapy in Canada: a multisite cohort study. PLoS One. 2013;8(12):e83649–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63).Ortego C, Huedo-Medina TB, Santos P, Rodríguez E, Sevilla L, Warren M, et al. Sex differences in adherence to highly active antiretroviral therapy: a meta-analysis. AIDS Care. 2012;24(12):1519–34. [DOI] [PubMed] [Google Scholar]
- 64).Zachek C, Coelho L, Domingues R, Clark J, De Boni R, Luz P, et al. The Intersection of HIV, Social Vulnerability, and Reproductive Health: Analysis of Women Living with HIV in Rio de Janeiro, Brazil from 1996 to 2016. AIDS Behav. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65).Castro R, De Boni RB, Luz PM, Velasque L, Lopes LV, Medina-Lara A, et al. Health-related quality of life assessment among people living with HIV in Rio de Janeiro, Brazil: a cross-sectional study. Quality of Life Research. 2019;28(4):1035–45. [DOI] [PubMed] [Google Scholar]
- 66).de Melo MG, Varella I, Gorbach PM, Sprinz E, Santos B, de Melo Rocha T, et al. Antiretroviral adherence and virologic suppression in partnered and unpartnered HIV-positive individuals in southern Brazil. PLoS One. 2019;14(2):e0212744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67).Ghidei L, Simone MJ, Salow MJ, Zimmerman KM, Paquin AM, Skarf LM, et al. Aging, antiretrovirals, and adherence: a meta analysis of adherence among older HIV-infected individuals. Drugs & Aging. 2013;30(10):809–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68).de Carvalho CV, Merchan-Hamann E, Matsushita R. Determinants of Antiretroviral Treatment Adherence in Brasilia, Federal District: a case-control study. Revista da Sociedade Brasileira de Medicina Tropical. 2007;40(5):555–65. [DOI] [PubMed] [Google Scholar]
- 69).Sabin CA, Smith CJ, d’Arminio Monforte A, Battegay M, Gabiano C, Galli L, et al. Response to combination antiretroviral therapy: variation by age. AIDS. 2008;22(12):1463–73. [DOI] [PubMed] [Google Scholar]
- 70).Kim SH, Gerver SM, Fidler S, Ward H. Adherence to antiretroviral therapy in adolescents living with HIV: systematic review and meta-analysis. AIDS. 2014;28(13):1945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71).Kiweewa F, Esber A, Musingye E, Reed D, Crowell TA, Cham F, et al. HIV virologic failure and its predictors among HIV-infected adults on antiretroviral therapy in the African Cohort Study. PLoS One. 2019;14(2):e0211344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72).Peltzer K, Pengpid S. Socioeconomic factors in adherence to HIV therapy in low- and middle-income countries. Journal of Health, Population, and Nutrition. 2013;31(2):150–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73).Hahn RA, Truman BI. Education Improves Public Health and Promotes Health Equity. International Journal of Health Srvices. 2015;45(4):657–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74).Stopa SR, Malta DC, Monteiro CN, Szwarcwald CL, Goldbaum M, Cesar CLG. Use of and access to health services in Brazil, 2013 National Health Survey. Revista de Saude Publica. 2017;51(suppl 1):3s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75).Brazilian Ministry of Health. Indicators and Data of HIV Clinic Monitoring. http://indicadoresclinicos.aids.gov.br/2020.
- 76).Wiewel EW, Borrell LN, Jones HE, Maroko AR, Torian LV. Neighborhood Characteristics Associated with Achievement and Maintenance of HIV Viral Suppression Among Persons Newly Diagnosed with HIV in New York City. AIDS Behav. 2017;21(12):3557–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available from the corresponding author on reasonable request.


