Abstract
Background:
Diabetes mellitus (DM) is a major comorbidity in people living with HIV (PWH). Hyperglycemia below diabetic range defines pre-diabetes (pre-DM). We compared the progression from pre-DM to DM in PWH and people without HIV (PWOH).
Methods:
Fasting glucose (FG) was measured semi-annually in the MACS since 1999. Men with pre-DM (FG between 100–125 mg/dL, confirmed within a year by FG in the pre-DM range or HbA1c between 5.7–6.4%) were included. The first visit with pre-DM was the baseline visit. Incident DM was defined as FG ≥126 mg/dL, confirmed at a subsequent visit, or self-reported DM, or use of anti-DM medication. We used binomial transition models to compare the progression from pre-DM to DM by HIV serostatus, adjusted for age, number of previous pre-DM to DM transitions, ethnicity, body mass index (BMI), family history of DM, and hepatitis C virus (HCV) infection.
Results:
Between 1999 and 2019, 1584 men (793 PWH; 791 PWOH) with pre-DM were included. At baseline, PWH were younger (48 vs 51 years, p<0.01), had lower BMI (26 vs 27), were more frequently non-white (47% vs 30%), and HCV-infected as per last measure (8% vs 4%) than PWOH (all p<0.01). Over a median 12-year follow-up, 23% of participants developed DM. In adjusted analyses, the risk for incident DM was 40% [95% CI: 0% to 80%] higher among PWH than PWOH (p=0.04).
Conclusion:
Among men with pre-DM, PWH had an increased risk of incident DM adjusted for competing risk factors, warranting the evaluation of DM prevention strategies.
Keywords: Pre diabetes, Incident diabetes, HIV infected patients, MACS cohort study
Introduction
Use of combination antiretroviral therapy (cART) has dramatically improved survival and life expectancy among people living with HIV (PWH). However, in the context of life-long cART use, the management of chronic non-infectious comorbidities, including diabetes mellitus (DM), has become a major issue in the care of aging PWH. The incidence of DM is increasing among PWH and has been linked to the effects of cART, as well as to the effects of chronic HIV infection[1–3]. There is also conflicting evidence on whether HIV infection is an independent risk factor for DM[4]. Similar to the general population, DM in PWH has been associated with hypertension, dyslipidemia, and overall increased risk for cardiovascular disease and renal impairment[5].
Both fasting glucose (FG) and hemoglobin A1c (HbA1c) are now widely used for the diagnosis of DM, supported by current guidelines[6]. The Expert Committee on the Diagnosis and Classification of DM[6] recognizes a group of persons whose glucose levels do not meet the criteria for DM but are not within the normal range. They termed this condition pre-diabetes (pre-DM), defined as a FG level between 100 and 125 mg/dL (5.6 and 6.9 mmol/L), or a HbA1c level between 5.7 and 6.4%. In the MESA study, individuals with pre-DM had an increased incidence of DM during the seven years of follow-up (HR: 10.5 [95% CI: 8.4–13.1], p<0.0001) compared to those with normal FG[7].
As cART-treated PWH age, the prevalence of DM is expected to increase. In a modelling study, Smit et al.[8] predicted a marked increase in the prevalence of DM (from 4% to 17%) between 2010 and 2030 within the ATHENA cohort in the Netherlands. Among PWH, specific factors have been clearly associated with DM, including certain antiretroviral therapies[9], systemic levels of inflammation[10], aging, and genetic factors. However, the clinical significance of pre-DM in PWH has not been established.
In previous Multicenter AIDS Cohort Study (MACS) analyses, we found that PWH have a higher incidence of DM and a higher prevalence of pre-DM, defined by FG criteria[11, 12], compared to people without HIV (PWOH). Using data from the MACS, we sought to determine whether the progression to DM among pre-DM PWH differs from the pre-DM PWOH, and to identify factors associated with the transition from pre-DM to DM.
Methods
Study population
The MACS is an ongoing prospective cohort study of HIV infection among men who have sex with men (MSM) at four centers in the USA (Baltimore, MD/Washington DC; Pittsburgh, PA/Columbus, OH; Chicago, IL; and Los Angeles, CA). Institutional review boards at each site approved the MACS protocol and forms, and each participant gave written informed consent. Details of the study design and follow-up methods have been published[13]. Briefly, participants attend semi-annual study visits, which include a detailed interview featuring longitudinal data capture of all medications received (such as antiretroviral therapy), physical examination, and collection of biological specimens. For the present study, we included all MACS participants with confirmed pre-DM by study definitions. The study baseline was defined as the first visit with pre-DM. Men with prevalent DM at baseline were excluded from our analysis.
Laboratory methods
Since April 1999, glucose and HbA1c were measured from participant serum samples from each semi-annual visit. Glucose levels were measured by the combined hexokinase/glucose-6-phosphate dehydrogenase method at a central laboratory (Heinz Laboratory, Pittsburgh, PA; coefficient of variation 1.8%). HbA1c was measured by Quest Diagnostics (Baltimore, MD) using standard immunoassay assays (Roche Cobas Integra 800 analytical system, Indianapolis, IN; coefficient of variation < 3.3%). Standardized protocols were used to measure T lymphocyte subsets. Plasma HIV-RNA levels were assessed using either, the Roche standard assay, the Roche ultrasensitive assay, the Roche COBAS TaqMan, or the Roche COBAS 6800/8800.
Diabetes and pre-diabetes assessment
We restricted the glucose measures to serum samples that were taken while the participant was fasting (self-reported ≥6 hours without food). Pre-DM was defined as a FG between 100–125 mg/dL (i.e baseline visit), confirmed within a year by either a FG in the pre-DM range (between 100–125 mg/dL) or HbA1c between 5.7–6.4%.
Incident DM was defined as: FG ≥126 mg/dL confirmed with self-reported use of anti-DM medication or a second FG ≥126 mg/dL at a subsequent visit, self-reported DM confirmed with self-reported use of anti-DM medication or FG ≥126 mg/dL confirmed at a subsequent visit(s) or self-reported anti-DM medication use.
Statistical analysis
Baseline characteristics were compared by HIV serostatus using Wilcoxon rank sum and chi-squared tests. Binomial transition models were used to determine whether the progression from pre-DM to DM at the next observed MACS visit differed by HIV serostatus. Briefly, transition models estimate the odds of moving from one health state to another at a future point in time. Individuals can move between any two states unless the probability is assumed to be zero. In the present study, we were focused on the odds that an individual had DM at a MACS visit, given that they were classified as pre-DM at their previous MACS visit. Owing to the time-varying nature of pre-DM and DM, we allowed for an individual to transition from pre-DM to DM multiple times over follow-up, and also examined the odds of transition from DM back to pre-DM.
Our multivariate transition models were adjusted for age (years), number of previous pre-DM to DM transitions, race/ethnicity (non-Latino white and non-white), body mass index (BMI; kg/m2), family history of diabetes, and hepatitis C virus (HCV) infection (last measured negative, and last measured positive antibody or HCV RNA). HCV infection was based on a summary measure that accounted for HCV RNA and antibody status. HCV RNA and antibodies were not measured at every MACS visit – among men who were classified as HCV positive at study baseline, the median (IQR) year of their last HCV RNA measure over follow-up was 2008 (2006–2009), and the median (IQR) year of their last HCV antibody measure over follow-up was 2011 (2009–2013). In between (and after the latest) HCV RNA and antibody measurements, HCV status was assumed to be constant and carried forward. As a sensitivity analysis, we added waist-hip circumference ratio (waist and hip circumferences were recorded to the nearest centimeter by tape measure as previously described[14]) to the multivariate model (including BMI), in order to evaluate the contribution of regional fat distribution to DM transition.
We also performed analysis stratified by HIV serostatus to better estimate differences in the association between DM risk factors and the odds of transition from pre-DM to DM. Models including only PWH adjusted for the variables listed above, plus examiner-assessed lipoatrophy at a visit (moderate/severe vs mild/none measured in the buttocks, legs and face: yes/no), years of known HIV exposure, history of clinical AIDS (yes/no), cumulative HIV viral copy-years after baseline, cumulative years of stavudine use, cumulative years of nucleoside reverse transcriptase inhibitor (NRTI) use, cumulative years of protease inhibitor (PI) use, and thymidine analogue use at a visit.
Finally, we performed several supplemental analyses. Although our main focus was on the transition from a pre-DM state to a DM state, we also modelled the odds of transition from DM to pre-DM, and the probability of transition from pre-DM to normal. The multivariate model for the transition from DM to pre-DM was adjusted for HIV serostatus, age, number of previous DM to pre-DM transitions, race/ethnicity, BMI, weight change between visits (kg), family history of diabetes, HCV infection, and smoking status (never smoked, former smoker, and current smoker). The multivariate model for the transition from pre-DM to normal was adjusted for HIV serostatus, age, number of previous pre-DM to normal transitions, race/ethnicity, BMI, weight change between visits, HCV infection, and smoking status.
Results
Baseline characteristics
Of the 3572 men with a visit between 1 April 1999 and 30 September 2019, 1584 participants (791 PWOH (22%) and 793 (22%) PWH) had observed confirmed pre-DM at baseline (Table 1). The median (IQR) year of the baseline visit was 2005 (2004, 2008).
Table 1.
Baseline participant characteristics.
| Characteristic | A Pre-DM PWOH N = 791 |
B Pre-DM PWH N = 793 |
C MACSa PWOH N = 1718 |
D MACSa PWH N = 1854 |
P A vs. B |
P A+B vs. C+D |
|---|---|---|---|---|---|---|
| Age (years), median (IQR) | 51.3 (45.1–57.9) | 47.7 (41.5–53.6) | 45.1 (37.9–52.4) | 41.7 (35.7–47.5) | <0.01 | <0.01 |
| Calendar year, median (IQR) | 2005 (2004–2008) | 2006 (2004–2009) | 2002 (2000–2003) | 2002 (2000–2003) | <0.01 | <0.01 |
| Non-white race/ethnicity, n (%) | 236 (30) | 372 (47) | 616 (36) | 885 (48) | <0.01 | 0.02 |
| Smoking status, n (%) | 0.048 | <0.01 | ||||
| Never smoked | 236 (30) | 221 (29) | 545 (33) | 544 (30) | ||
| Former smoker | 330 (42) | 298 (39) | 547 (33) | 543 (30) | ||
| Current smoker | 213 (27) | 256 (33) | 582 (35) | 719 (40) | ||
| HCV co-infection per last measure, n (%) | 35 (4) | 66 (8) | 91 (5) | 162 (9) | <0.01 | 0.31 |
| BMI (kg/m2), median (IQR) | 27.0 (24.4–30.3) | 25.5 (23.1–28.3) | 25.7 (23.2–28.7) | 24.8 (22.7–27.5) | <0.01 | <0.01 |
| Waist-hip ratio, median (IQR) | 1.0 (0.9–1.0) | 1.0 (0.9–1.0) | 0.9 (0.9–1.0) | 0.9 (0.9–1.0) | 0.09 | <0.01 |
| Weight change since last visit (kg), median (IQR) | 0.7 (−0.8–2.4) | 0.3 (−1.2–2.2) | 0.9 (−1.74–3.6) | 0.0 (−1.8–1.8) | 0.01 | 0.22 |
| Moderate/severe lipoatrophy, n (%) | 14 (2) | 44 (6) | 44 (3) | 259 (14) | <0.01 | <0.01 |
| Family history of diabetes, n (%) | 431 (55) | 458 (59) | 851 (51) | 985 (55) | 0.14 | 0.02 |
| Duration of HIV infection (years), median (IQR) | - | 18 (14.1–21.9) | - | 12.7 (12.2–18.0) | - | <0.01b |
| History of AIDS, n (%) | - | 74 (9) | - | 196 (11) | - | 0.36b |
| HIV viral load < 50copies/mL, n (%)c | - | 488 (62) | - | 691 (41) | - | <0.01b |
| Cumulative years of NRTI use, median (IQR) | - | 5.5 (2.0–9.5) | - | 2.2 (0.1–4.9) | - | <0.01b |
| Current thymidine analog use, n (%) | - | 252 (32) | - | 823 (46) | - | <0.01b |
| Cumulative years of stavudine use, median (IQR) | - | 0.0 (0.0–2.9) | - | 0.0 (0.0–1.5) | - | <0.01b |
| Cumulative years of PI use, median (IQR) | - | 2.0 (0.0–5.1) | - | 0.3 (0.0–2.5) | - | <0.01b |
: Baseline characteristics for all men measured at the earliest visit after April 1, 1999 (when fasting glucose began to be collected).
: PWH only.
: Excluding the three MACS men with an HIV viral load measured at the lower limit of detection of 400 copies/mL, via the Roche 2nd generation assay.
AIDS = acquired immunodeficiency syndrome; BMI = body mass index; HCV = hepatitis C virus; HIV = human immunodeficiency virus; IQR = interquartile range; NRTI = nucleoside reverse transcriptase inhibitor; PI = protease inhibitor; Pre-DM = pre-diabetes mellitus; PWH = persons with HIV; PWOH = persons without HIV.
When comparing baseline characteristics of our selected pre-DM population (n=1584) to the overall MACS population (n=3572), pre-DM participants were older, had a lower percentage of non-white participants, a smaller rate of current smokers, a higher average BMI and waist-hip ratio, a lower prevalence of lipoatrophy and a greater proportion of individuals with a family history of DM (all p≤0.02). Further, pre-DM PWH had a longer duration of known HIV exposure, had a higher prevalence of HIV virologic control (HIV viral load < 50 copies/mL), a longer use of NRTIs and PIs medications, and less thymidine analog use (all p<0.01) compared to MACS PWH with normal FG (Table 1).
Comparing pre-DM PWOH (n=791) and pre-DM PWH (n=793), PWH were younger, more likely to be non-white, had a lower BMI (all p<0.01), and were more likely to be current smokers (p=0.048) and co-infected with HCV, as per last measure (p<0.01). As expected, lipoatrophy was more common among PWH compared to PWOH (p<0.01) (Table 1).
Among both pre-DM PWOH and PWH groups, 23% of each group of participants had at least one visit with incident DM over a median of 12 years of follow-up. Men with incident DM during follow-up were more likely to have a family history of DM (p<0.01), and at baseline to have a higher average BMI and waist-hip ratio (both p<0.01), compared to those who did not have incident DM during follow-up. In addition, PWH who had an incident DM were more likely to have a history of AIDS (p=0.04), a shorter duration of known HIV infection (p=0.01), and longer use of NRTIs (p=0.02) – stavudine in particular (p=0.03) – compared to PWH who did not have incident DM over follow-up (Table 2).
Table 2.
Baseline characteristics of pre-DM PWOH and PWH who did and did not develop incident DM.
| Characteristic | A PWOH No Incident DM N=611 |
B PWOH Incident DM N=180 |
C PWH No Incident DM N=613 |
D PWH Incident DM N=180 |
A vs B P |
C vs D P |
|---|---|---|---|---|---|---|
| Age (years), median (IQR) | 51.5 (45.2–58.1) | 50.3 (45.1–57.1) | 47.7 (41.2–53.7) | 48.0 (42.5–53.0) | 0.5 | 0.68 |
| Calendar year, median (IQR) | 2006 (2004–2008) | 2004 (2003–2006) | 2006 (2004–2010) | 2004 (2002–2007) | <0.01 | <0.01 |
| Non-white race/ethnicity, n (%) | 175 (29) | 61 (34) | 287 (47) | 85 (47) | 0.19 | 0.93 |
| Smoking status, n (%) | 0.02 | 0.85 | ||||
| Never smoked | 188 (31) | 48 (27) | 170 (28) | 51 (29) | ||
| Former smoker | 264 (44) | 66 (37) | 228 (38) | 70 (40) | ||
| Current smoker | 149(25) | 64 (36) | 201 (34) | 55 (31) | ||
| HCV co-infection per last measure, n (%) | 23 (4) | 12 (7) | 48 (8) | 18 (10) | 0.10 | 0.36 |
| BMI (kg/m2), median (IQR) | 26.5 (23.9–29.5) | 29.4 (26.1–33.8.0) | 25.2 (23.0–28.0) | 26.2 (23.8–29.3) | <0.01 | <0.01 |
| Waist-hip ratio, median (IQR) | 1.0 (0.9–1.0) | 1.0 (0.9–1.0) | 1.0 (0.9–1.0) | 1.0 (0.9–1.0) | <0.01 | <0.01 |
| Weight change since last visit (kg), median (IQR) | 0.6 (−0.9–2.2) | 0.9 (−0.7–3.4) | 0.3 (−1.2–2.1) | 0.5 (−1.2–2.3) | 0.051 | 0.40 |
| Moderate/severe lipoatrophy, n (%) | 8 (1) | 6 (3) | 30 (5) | 14 (8) | 0.10 | 0.14 |
| Family history of diabetes, n (%) | 310 (51) | 121 (67) | 338 (56) | 120 (68) | <0.01 | <0.01 |
| Duration of HIV infection (years), median (IQR) | - | - | 18.0 (14.2–22.4) | 16.8 (13.5–20.1) | - | 0.01 |
| History of AIDS, n (%) | - | - | 50 (8) | 24 (13) | - | 0.04 |
| HIV viral load < 50 copies/mL, n (%) | - | - | 386 (64) | 102 (57) | - | 0.12 |
| Cumulative years of NRTI use, median (IQR) | - | - | 5.3 (1.8–9.4) | 6.2 (3.5–9.9) | - | 0.02 |
| Current thymidine analog use, n (%) | - | - | 163 (27) | 89 (51) | - | <0.01 |
| Cumulative years of stavudine use, median (IQR) | - | - | 0.0 (0.0–2.8) | 0.6 (0.0–3.2) | - | 0.03 |
| Cumulative years of PI use, median (IQR) | - | - | 1.8 (0.0–5.2) | 2.9 (0.0–4.8) | - | 0.12 |
AIDS = acquired immunodeficiency syndrome; BMI = body mass index; HCV = hepatitis C virus; HIV = human immunodeficiency virus; IQR = interquartile range; NRTI = nucleoside reverse transcriptase inhibitor; PI = protease inhibitor; Pre-DM = pre-diabetes mellitus; PWH = persons with HIV; PWOH = persons without HIV.
Multivariate transition models: pre-DM to DM
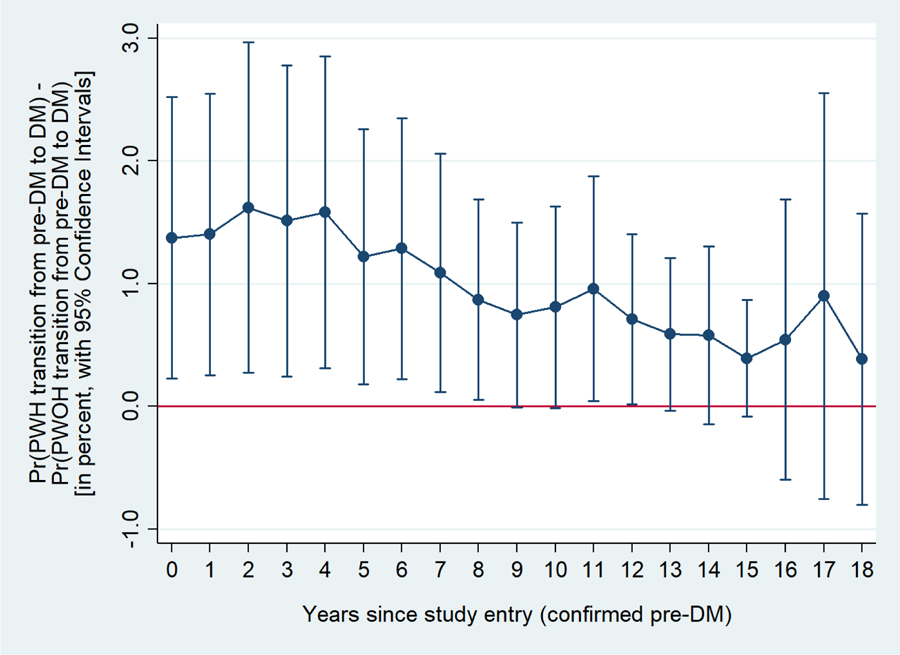
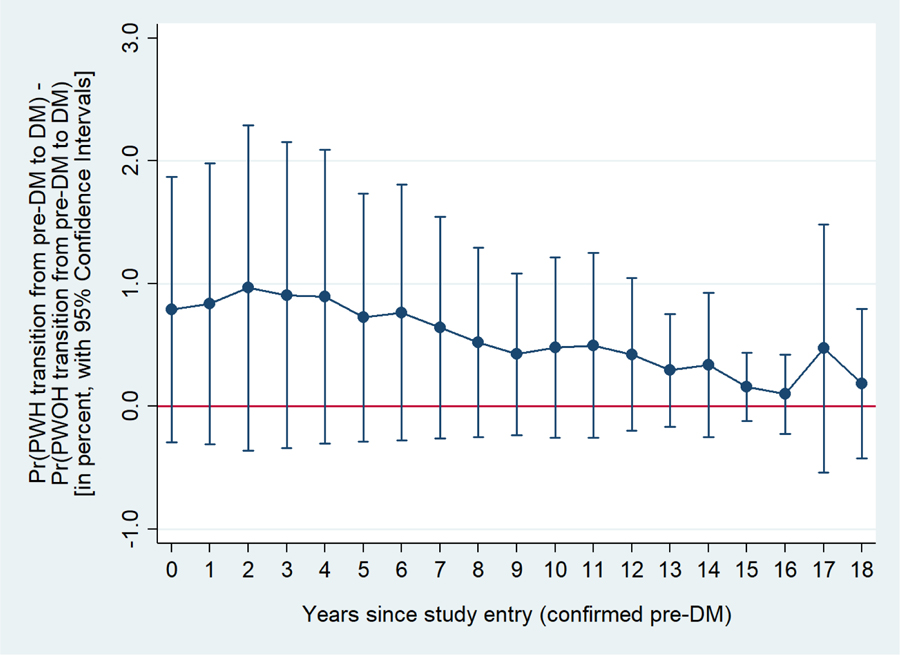
In multivariate transition models adjusted for age, number of previous pre-DM to DM transition, race/ethnicity, HCV infection (ie. last visit measurement), BMI and family history of DM the odds of transition from pre-DM to DM were 40% [95% CI: 0% to 80%] higher among PWH than PWOH (p=0.04; model A, Table 3) whereas it was not significant in univariate models (additionally controlling for age and number of previous transition from pre-DM to DM, Table 3 supplementary file). The greater risk among PWH of transitioning from pre-DM to DM can be observed in Figure 1a, where PWH had a significantly higher probability of transitioning compared to PWOH during up to eight years of follow-up after the first visit with confirmed pre-DM. When waist-hip ratio was added into the multivariate model (Table 3 model B, Figure 1b) there was no longer a statistically significant association between HIV serostatus and the transition from pre-DM to DM (p=0.22).
Table 3.
Multivariate odds of transition from pre-DM to DM among the 1584 pre-DM men
| MODEL A | MODEL B | |||||
|---|---|---|---|---|---|---|
| Variable | PWOH+PWH OR (95% CI), P |
PWOH OR (95% CI), P |
PWH OR (95% CI), P |
PWOH+PWH OR (95% CI), P |
PWOH OR (95% CI), P |
PWH OR (95% CI), P |
| Seropositive for HIV [ref = Seronegative] | 1.4 (1.0, 1.8), 0.04 | - | - | 1.2 (0.9, 1.6), 0.22 | - | - |
| Age, per 10 years | 1.1 (0.9, 1.3), 0.38 | 1.0 (0.8, 1.2), 0.73 | 1.3 (1.0, 1.7), 0.051 | 1.0 (0.8, 1.2), 0.87 | 0.9 (0.7, 1.1), 0.47 | 1.2 (0.9, 1.5), 0.22 |
| Number of previous pre-DM to DM transitions | 3.9 (2.9, 5.1), <0.01 | 3.4 (2.6, 4.5), <0.01 | 7.0 (3.4, 14.2), <0.01 | 3.8 (2.8, 5.1), <0.01 | 3.4 (2.6, 4.4), <0.01 | 7.5 (3.3, 17.2), <0.01 |
| Non-white [ref = Non-Latino white] | 1.2 (0.9, 1.6), 0.29 | 1.3 (0.8, 2.1), 0.27 | 1.5 (1.0, 2.3), 0.048 | 1.2 (0.9, 1.7), 0.18 | 1.4 (0.8, 2.2), 0.19 | 1.6 (1.1, 2.5), 0.03 |
| Last measure seropositive for HCV [ref = Last measure seronegative] | 1.3 (0.8, 2.1), 0.36 | 1.4 (0.8, 2.8), 0.27 | 1.0 (0.4, 2.3), 0.98 | 1.2 (0.7, 2.0), 0.45 | 1.3 (0.7, 2.7), 0.43 | 0.9 (0.4, 2.0), 0.83 |
| BMI, per five kg/m2 | 1.5 (1.3, 1.7), <0.01 | 1.5 (1.3, 1.7), <0.01 | 1.6 (1.4, 2.0), <0.01 | 1.3 (1.2, 1.5), <0.01 | 1.4 (1.2, 1.7), <0.01 | 1.4 (1.1, 1.8), <0.01 |
| Family history of diabetes [ref = No family history] | 1.7 (1.3, 2.3), <0.01 | 2.0 (1.3, 2.9), <0.01 | 1.3 (0.9, 1.9), 0.20 | 1.7 (1.3, 2.3), <0.01 | 2.0 (1.3, 3.0), <0.01 | 1.3 (0.9, 2.0), 0.18 |
| Waist-hip ratio, standardized | - | - | - | 1.3 (1.1, 1.5), <0.01 | 1.2 (0.9, 1.4), 0.19 | 1.4 (1.1, 1.6), <0.01 |
| Examiner-assessed lipoatrophy [ref = No lipoatrophy] | - | - | 2.2 (1.1, 4.4), 0.02 | - | - | 2.3 (1.2, 4.5), 0.02 |
| Duration of HIV infection, per five years | - | - | 0.8 (0.7, 1.0), 0.07 | - | - | 0.8 (0.7, 1.0), 0.03 |
| History of AIDS [ref = No history] | - | - | 2.9 (1.7, 5.0), <0.01 | - | - | 2.8 (1.6, 4.8), <0.01 |
| Cumulative HIV viral copy-years after baseline, per 1,000,000 copy-years | - | - | 0.2 (0.0, 1.5), 0.10 | - | - | 0.2 (0.0, 1.7), 0.13 |
| Cumulative years of stavudine use, per five years | - | - | 1.3 (1.0, 1.7), 0.09 | - | - | 1.3 (1.0, 1.7), 0.10 |
| Cumulative years of NRTI use, per five years | - | - | 1.2 (0.9, 1.5), 0.17 | - | - | 1.2 (0.9, 1.5), 0.26 |
| Cumulative years of PI use, per five years | - | - | 1.1 (0.9, 1.3), 0.34 | - | - | 1.1 (0.9, 1.4), 0.16 |
| Thymidine analog use [ref = No thymidine analog use] | - | - | 1.6 (1.0, 2.5), 0.03 | - | - | 1.7 (1.1, 2.6), 0.02 |
Model A: multivariate transition model with odds of transition from pre-DM to DM among pre-DM PWOH and PWH adjusted for traditional risk factors. Model B: multivariate transition model with odds of transition from pre-DM to DM among pre-DM PWOH and PWH adjusted for traditional risk factors plus waist-hip ratio.
Figure 1a.
Difference in the probability of transitioning from pre-DM to DM between PWH and PWOH at each year in time since the first visit with confirmed pre-DM.
Estimates adjusted for age, number of previous transitions from pre-DM to DM, race/ethnicity, HCV infection as per last measurement, BMI, and family history of DM.
BMI = body mass index; DM = diabetes mellitus; HCV = hepatitis C virus; HIV = human immunodeficiency virus; Pre-DM = pre-diabetes mellitus; PWH = persons with HIV; PWOH = persons without HIV.
Figure 1b.
Difference in the probability of transitioning from pre-DM to DM between PWH and PWOH at each year in time since the first visit with confirmed pre-DM.
Estimates adjusted for age, number of previous transitions from pre-DM to DM, race/ethnicity, HCV infection as per last measurement, BMI, family history of DM, and waist-hip ratio.
BMI = body mass index; DM = diabetes mellitus; HCV = hepatitis C virus; HIV = human immunodeficiency virus; Pre-DM = pre-diabetes mellitus; PWH = persons with HIV; PWOH = persons without HIV
An increased risk of transitioning from pre-DM to DM in PWH was associated with non-white race/ethnicity, more previous pre-DM to DM transitions, and higher BMI (all p≤0.05) (Table 3). However, a family history of DM in PWH was not significantly associated with an increased odds of transitioning from pre-DM to DM, while it was significant among PWOH (all p<0.01). A higher standardized waist-hip ratio was also significantly associated with an increased risk for transitioning among PWH, but not among PWOH (Table 3).
Among PWH, after adjustment for traditional risk factors, men with pre-DM were more likely to develop incident DM if they had lipoatrophy (p=0.02), a history of AIDS (p<0.01), or were using thymidine analogs (p<0.03; Table 3). After adding waist-hip ratio to the model, longer duration of known HIV infection became significantly associated with a reduced risk of transitioning from pre-DM to DM (p=0.03; Table 3).
Other transition models: DM to pre-DM and pre-DM to normal
HIV infection was significantly associated with 30% [95% CI: 0% to 50%] lower odds of transitioning from DM to pre-DM compared to PWOH (p=0.045). HIV infection was not significantly associated with the probability of the transition from pre-DM to normal (aRR 0.9 [95% CI: 0.9, 1.0], p=0.08).
Discussion
This analysis of MACS data that spanned a median 12-year period is the largest study to date that has evaluated the risk of DM transition in pre-DM PWH and PWOH. We found that risk for incident DM was 40% higher among PWH than PWOH after adjustment for competing DM risk factors.
Pre-DM, including impaired fasting glucose (IFG), has been widely studied as a predictor for incident DM[6, 15–19], with ten year incidence ranging from 3 to 33% in European cohorts[15, 16, 19]. Other studies reported a higher proportion of incident DM – up to 72.7% in South America[17] – when impaired glucose tolerance (IGT) was added to IFG as a risk factor. In our study we found that 23% of pre-DM participants developed incident DM over a median of 12 years of follow-up using IFG alone. These rates are similar to other published data in Western countries that recognized pre-DM as a major risk factor for DM[6]. However, we were unable to identify persons with IGT (defined as a 2 hour glucose between 140 and 199 mg/dl after 75 g oral glucose load), as oral glucose tolerance tests have not been routinely performed in the MACS [20].
In the general population, incident DM has been associated with traditional risk factors such as ethnicity, family history of DM, gender, age, BMI, and waist circumference[21]. As expected, in our study, pre-DM participants were older with a higher average BMI and a greater likelihood of family history of DM, which is similar to people with DM from the general population[22–24]. For example, compared to people without a family history of DM, individuals who have a first degree relative with DM have a two to three times increased risk of developing DM[23, 25]. In our study, 67% of pre-DM PWOH who developed incident DM had a family history of DM, compared with a 51% prevalence of family history of DM among pre-DM PWOH who did not develop incident DM. Similarly, the prevalence of obesity – well-known as a risk factor for DM[24, 26] – was greater and the median BMI higher in pre-DM PWOH who developed incident DM than pre-DM PWOH who did not develop incident DM (median BMI 29.4 vs 26.5, respectively).
Among PWH with pre-DM, traditional risk factors were also associated with incident DM. However, compared to pre-DM PWOH, pre-DM PWH were younger, had a lower BMI, and were more likely to be non-white. Our results are in accordance with other published data showing that the prevalence of DM at an earlier age is likely to be higher in PWH[8, 27, 28], and is higher among African Americans in the general population[29]. In our study, history of AIDS was linked to incident DM in PWH after adjustment for traditional risk factors, which may reflect continued immune system dysregulation and inflammation, which are considered to be important drivers for comorbidities, including DM[10, 30]. We also found that longer use of NRTIs, especially stavudine, and lipoatrophy, were related to incident DM among PWH, highlighting the metabolic toxicity of these medications.
In adjusted models, PWH had a 40% greater risk of transitioning from pre-DM to DM compared to PWOH, but after additional adjustment for waist-hip ratio, the magnitude of the effect diminished and statistical significance was no longer present. This suggests that the difference by HIV serostatus in the transition from pre-DM to DM may be related to regional body composition. In the general population, fat distribution is an important determinant of the risk of insulin resistance and DM, and may be explained by genetic and environmental factors[31, 32]. Among PWH, ART-associated lipoatrophy or lipohypertrophy are recognized as DM risk factors[33, 34]. Our findings suggest that waist-hip ratio is a potential mediator in the pathway between HIV infection and incident DM among pre-DM men and should be explored in future studies.
Our results also demonstrated that while non-white race/ethnicity was significantly associated with increased odds of transition from pre-DM to DM in univariate models, after controlling for the effects of HIV and HCV serostatus, BMI, and family history of DM, the effect of race/ethnicity was no longer significant. This is an important finding, as it suggests that it is not race/ethnicity that is specifically associated with DM risk among pre-DM men in our cohort, but rather downstream factors that differ by race/ethnicity – potentially stemming from structural inequalities – that are associated with an elevated DM risk.
Current guidelines emphasize a patient-centered approach in the management of people with DM[6]. Based on the results of the Diabetes Prevention Program, metformin use should be considered to prevent DM in persons with pre-DM who are <60 years old, have a BMI >35 kg/m2, or have a history of gestational diabetes. It is unclear whether HIV serostatus should also be considered a risk factor which may prompt the use of metformin among persons with pre-DM. Given the increased odds of DM transition among pre-DM PWH compared to pre-DM PWOH, it would be prudent to monitor pre-DM PWH closely to avoid the development of DM, and institute lifestyle modifications, including diet and physical activity changes, to prevent DM conversion[26, 35]. Even modest weight loss can have an impact on long term glycemic control[36, 37]. Finally, ART medications that are associated with weight gain, including integrase inhibitors and tenofovir alafenamide[38, 39], should be used carefully in PWH with pre-DM. Future studies should examine treatment strategies to avoid or switch from these medications to evaluate effects on glucose metabolism in persons with pre-DM.
Our study has several limitations. First, we did not perform oral glucose tolerance tests so we were unable to examine conversion of IGT to DM. This may have underestimated the pre-DM population. We were unable to examine the role of genetics, physical activity, or diet on DM development, but these are important factors to examine in future studies.
As previously mentioned, HCV status after the latest HCV RNA and/or antibody measure was assumed to be constant. Some of the men in our study who had a positive HCV status held constant initiated direct acting antivirals (DAA) while under follow-up. However, we did not have updated HCV RNA or antibody measures for these men following their DAA initiation, so could not assess the presence of a sustained virologic response. Since HCV is associated with abnormalities in glucose metabolism, it could be hypothesized that HCV cure may decrease the risk of transition to DM in those with pre-DM. However, when we attempted to examine the impact of time before and after DAA initiation on the transition from pre-DM to DM for men with HCV infection, we found no transitions from pre-DM to DM in these individuals and were unable to address this important question. Finally, our study included only men and whether findings can be extrapolated to women is unknown.
Conclusion
Among men with pre-DM, HIV infection was associated with a greater risk of incident DM, which was related to regional fat distribution. In addition to controlling for classic risk factors associated with DM, such as diet or physical inactivity, additional strategies to prevent DM among PWH with pre-DM should be investigated, including ART strategies that minimize weight gain and use of metformin or other medications that have been shown to prevent DM.
Supplementary Material
Acknowledgments
Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS), now the MACS/WIHS Combined Cohort Study (MWCCS), The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). MWCCS (Principal Investigators): Atlanta CRS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-HL146241; Baltimore CRS (Todd Brown and Joseph Margolick), U01-HL146201; Bronx CRS (Kathryn Anastos and Anjali Sharma), U01-HL146204; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202; Data Analysis and Coordination Center (Gypsyamber D’Souza, Stephen Gange and Elizabeth Golub), U01-HL146193; Chicago-Cook County CRS (Mardge Cohen and Audrey French), U01-HL146245; Chicago-Northwestern CRS (Steven Wolinsky), U01-HL146240; Northern California CRS (Bradley Aouizerat, Jennifer Price, and Phyllis Tien), U01-HL146242; Los Angeles CRS (Roger Detels and Matthew Mimiaga), U01-HL146333; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205; Miami CRS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-HL146203; Pittsburgh CRS (Jeremy Martinson and Charles Rinaldo), U01-HL146208; UAB-MS CRS (Mirjam-Colette Kempf, Jodie Dionne-Odom, and Deborah Konkle-Parker), U01-HL146192; UNC CRS (Adaora Adimora), U01-HL146194. The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute (NHLBI), with additional co-funding from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Institute On Aging (NIA), National Institute Of Dental & Craniofacial Research (NIDCR), National Institute Of Allergy And Infectious Diseases (NIAID), National Institute Of Neurological Disorders And Stroke (NINDS), National Institute Of Mental Health (NIMH), National Institute On Drug Abuse (NIDA), National Institute Of Nursing Research (NINR), National Cancer Institute (NCI), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute on Minority Health and Health Disparities (NIMHD), and in coordination and alignment with the research priorities of the National Institutes of Health, Office of AIDS Research (OAR). MWCCS data collection is also supported by UL1-TR000004 (UCSF CTSA), P30-AI-050409 (Atlanta CFAR), P30-AI-050410 (UNC CFAR), and P30-AI-027767 (UAB CFAR). TTB is supported in part by K24 AI120834.
Footnotes
Conflicts of Interest
LS has served as a consultant to Gilead Sciences, Merck, ViiV. TTB has served as a consultant to ViiV Healthcare, Gilead Sciences, Merck, Theratechnologies, and Janssen. FJP has served on Advisory Boards and has done speaker programs for Gilead Sciences, Janssen, ViiV, Merck. JL has served as a consultant for Merck and Theratechnologies. JPV, JEL, AGA and BWB declared no conflict of interest.
References
- 1.Capeau J, Bouteloup V, Katlama C, Bastard JP, Guiyedi V, Salmon-Ceron D, et al. Ten-year diabetes incidence in 1046 HIV-infected patients started on a combination antiretroviral treatment. AIDS 2012; 26(3):303–314. [DOI] [PubMed] [Google Scholar]
- 2.Erlandson KM, Kitch D, Tierney C, Sax PE, Daar ES, Melbourne KM, et al. Impact of randomized antiretroviral therapy initiation on glucose metabolism. AIDS 2014; 28(10):1451–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tebas P Insulin resistance and diabetes mellitus associated with antiretroviral use in HIV-infected patients: pathogenesis, prevention, and treatment options. J Acquir Immune Defic Syndr 2008; 49 Suppl 2:S86–92. [DOI] [PubMed] [Google Scholar]
- 4.Monroe AK, Glesby MJ, Brown TT. Diagnosing and managing diabetes in HIV-infected patients: current concepts. Clin Infect Dis 2015; 60(3):453–462. [DOI] [PubMed] [Google Scholar]
- 5.Ghehi C, Gabillard D, Moh R, Badje A, Kouame GM, Oouttara E, et al. High correlation between Framingham equations with BMI and with lipids to estimate cardiovascular risks score at baseline in HIV-infected adults in the Temprano trial, ANRS 12136 in Cote d’Ivoire. PLoS One 2017; 12(6):e0177440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Association AD. Diagnosis and Classification of Diabetes Mellitus Diabetes care 2020; 43(Supplement 1). [Google Scholar]
- 7.Yeboah J, Bertoni AG, Herrington DM, Post WS, Burke GL. Impaired fasting glucose and the risk of incident diabetes mellitus and cardiovascular events in an adult population: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2011; 58(2):140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smit M, Brinkman K, Geerlings S, Smit C, Thyagarajan K, Sighem A, et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis 2015; 15(7):810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isa SE, Oche AO, Kang’ombe AR, Okopi JA, Idoko JA, Cuevas LE, et al. Human Immunodeficiency Virus and Risk of Type 2 Diabetes in a Large Adult Cohort in Jos, Nigeria. Clin Infect Dis 2016; 63(6):830–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown TT, Tassiopoulos K, Bosch RJ, Shikuma C, McComsey GA. Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care 2010; 33(10):2244–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med 2005; 165(10):1179–1184. [DOI] [PubMed] [Google Scholar]
- 12.Yang J, Jacobson LP, Becker JT, Levine A, Martin EM, Munro CA, et al. Impact of glycemic status on longitudinal cognitive performance in men with and without HIV Infection. AIDS 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR Jr. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol 1987; 126(2):310–318. [DOI] [PubMed] [Google Scholar]
- 14.Brown T, Wang Z, Chu H, Palella FJ, Kingsley L, Witt MD, et al. Longitudinal anthropometric changes in HIV-infected and HIV-uninfected men. J Acquir Immune Defic Syndr 2006; 43(3):356–362. [DOI] [PubMed] [Google Scholar]
- 15.de Vegt F, Dekker JM, Jager A, Hienkens E, Kostense PJ, Stehouwer CD, et al. Relation of impaired fasting and postload glucose with incident type 2 diabetes in a Dutch population: The Hoorn Study. JAMA 2001; 285(16):2109–2113. [DOI] [PubMed] [Google Scholar]
- 16.Eschwege E, Charles MA, Simon D, Thibult N, Balkau B, Paris Prospective S. Reproducibility of the diagnosis of diabetes over a 30-month follow-up: the Paris Prospective Study. Diabetes Care 2001; 24(11):1941–1944. [DOI] [PubMed] [Google Scholar]
- 17.Gimeno SG, Ferreira SR, Franco LJ, Iunes M. Comparison of glucose tolerance categories according to World Health Organization and American Diabetes Association diagnostic criteria in a population-based study in Brazil. The Japanese-Brazilian Diabetes Study Group. Diabetes Care 1998; 21(11):1889–1892. [DOI] [PubMed] [Google Scholar]
- 18.Nichols GA, Hillier TA, Brown JB. Progression from newly acquired impaired fasting glusose to type 2 diabetes. Diabetes Care 2007; 30(2):228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaccaro O, Ruffa G, Imperatore G, Iovino V, Rivellese AA, Riccardi G. Risk of diabetes in the new diagnostic category of impaired fasting glucose: a prospective analysis. Diabetes Care 1999; 22(9):1490–1493. [DOI] [PubMed] [Google Scholar]
- 20.Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med 2002; 19(9):708–723. [DOI] [PubMed] [Google Scholar]
- 21.Robertson RP. Risk factors for type 2 diabetes mellitus. 2019.
- 22.Biggs ML, Mukamal KJ, Luchsinger JA, Ix JH, Carnethon MR, Newman AB, et al. Association between adiposity in midlife and older age and risk of diabetes in older adults. JAMA 2010; 303(24):2504–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meigs JB, Cupples LA, Wilson PW. Parental transmission of type 2 diabetes: the Framingham Offspring Study. Diabetes 2000; 49(12):2201–2207. [DOI] [PubMed] [Google Scholar]
- 24.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 2003; 289(1):76–79. [DOI] [PubMed] [Google Scholar]
- 25.InterAct C, Scott RA, Langenberg C, Sharp SJ, Franks PW, Rolandsson O, et al. The link between family history and risk of type 2 diabetes is not explained by anthropometric, lifestyle or genetic risk factors: the EPIC-InterAct study. Diabetologia 2013; 56(1):60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helmrich SP, Ragland DR, Leung RW, Paffenbarger RS Jr. Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. N Engl J Med 1991; 325(3):147–152. [DOI] [PubMed] [Google Scholar]
- 27.Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011; 53(11):1120–1126. [DOI] [PubMed] [Google Scholar]
- 28.Schouten J, Wit FW, Stolte IG, Kootstra NA, van der Valk M, Geerlings SE, et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis 2014; 59(12):1787–1797. [DOI] [PubMed] [Google Scholar]
- 29.Cheng YJ, Kanaya AM, Araneta MRG, Saydah SH, Kahn HS, Gregg EW, et al. Prevalence of Diabetes by Race and Ethnicity in the United States, 2011–2016. JAMA 2019; 322(24):2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armah KA, McGinnis K, Baker J, Gibert C, Butt AA, Bryant KJ, et al. HIV status, burden of comorbid disease, and biomarkers of inflammation, altered coagulation, and monocyte activation. Clin Infect Dis 2012; 55(1):126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 1994; 17(9):961–969. [DOI] [PubMed] [Google Scholar]
- 32.Chen KW, Boyko EJ, Bergstrom RW, Leonetti DL, Newell-Morris L, Wahl PW, et al. Earlier appearance of impaired insulin secretion than of visceral adiposity in the pathogenesis of NIDDM. 5-Year follow-up of initially nondiabetic Japanese-American men. Diabetes Care 1995; 18(6):747–753. [DOI] [PubMed] [Google Scholar]
- 33.De Wit S, Sabin CA, Weber R, Worm SW, Reiss P, Cazanave C, et al. Incidence and risk factors for new-onset diabetes in HIV-infected patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. Diabetes Care 2008; 31(6):1224–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ledergerber B, Furrer H, Rickenbach M, Lehmann R, Elzi L, Hirschel B, et al. Factors associated with the incidence of type 2 diabetes mellitus in HIV-infected participants in the Swiss HIV Cohort Study. Clin Infect Dis 2007; 45(1):111–119. [DOI] [PubMed] [Google Scholar]
- 35.Mozaffarian D Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation 2016; 133(2):187–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Esposito K, Maiorino MI, Ciotola M, Di Palo C, Scognamiglio P, Gicchino M, et al. Effects of a Mediterranean-style diet on the need for antihyperglycemic drug therapy in patients with newly diagnosed type 2 diabetes: a randomized trial. Ann Intern Med 2009; 151(5):306–314. [DOI] [PubMed] [Google Scholar]
- 37.Look ARG, Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care 2007; 30(6):1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eckard AR, McComsey GA. Weight gain and integrase inhibitors. Curr Opin Infect Dis 2020; 33(1):10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norwood J, Turner M, Bofill C, Rebeiro P, Shepherd B, Bebawy S, et al. Brief Report: Weight Gain in Persons With HIV Switched From Efavirenz-Based to Integrase Strand Transfer Inhibitor-Based Regimens. J Acquir Immune Defic Syndr 2017; 76(5):527–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.