Abstract
Objectives:
To learn if quantitative ultrasound (QUS) distinguishes the tongues of healthy participants and amyotrophic lateral sclerosis (ALS) patients by echo intensity (EI) and to evaluate if EI correlates with measures of bulbar function.
Methods:
Ultrasound was performed along the midline of the anterior tongue surface in 16 ALS patients and 16 age-matched controls using a linear hockey stick 16–7 MHz transducer. A region of interest was manually drawn and then EI was determined for the upper 1/3 of the muscle. For patients, the ALS functional rating scale – revised (ALSFRS-R) was used to calculate bulbar sub-scores and the Iowa Oral Performance Instrument (IOPI) was used to measure tongue strength.
Results:
EI was significantly higher in ALS patients than in healthy participants (49.8 versus 37.8 arbitrary units, p< 0.01). In the patient group, EI was negatively correlated with ALSFRS-R bulbar sub-score (RS = −0.65, p<0.01). An inverse correlation between EI and tongue strength did not reach significance (RS = −0.34, p = 0.28).
Conclusions:
This study suggests that EI can differentiate healthy from diseased tongue muscle, and correlates with a standard functional measure in ALS patients.
Significance:
Tongue EImay represent a novel biomarker for bulbar dysfunction in ALS.
Keywords: amyotrophic lateral sclerosis, biomarker, bulbar, echo intensity, ultrasound, tongue
1. INTRODUCTION
Speech and swallowing abnormalities commonly develop in the course of amyotrophic lateral sclerosis (ALS), and approximately 25% of patients have predominantly bulbar symptoms at disease onset (Haverkamp et al., 1995). Needle electromyography (EMG) and video fluoroscopy can provide valuable information about oropharyngeal status. Notably, factors including discomfort, patient participation, and the subjectivity inherent in analysis can influence the results of these tests (Costa, 2010, Gans and Kraft, 1977, Jan et al., 1999, Wilcox et al., 1996). ALS care and clinical trials could benefit from more objective measures of bulbar status (Wagner, 2009).
Neuromuscular ultrasound could help address this need. Building on qualitative interpretation techniques, approaches have been developed to quantify sonographic findings from nerve and muscle (Heckmatt et al., 1982, Pillen et al., 2006). Quantitative ultrasound (QUS) is an objective, painless imaging method independent of patient effort that has increasingly been used to evaluate neuromuscular disorders including ALS (Hobson-Webb and Simmons, 2019, Walker et al., 2004). ALS patients have been found to have neurogenic changes including smaller cervical root and peripheral nerve dimensions (Cartwright et al., 2011, Nodera et al., 2014, Noto et al., 2018, Schreiber et al., 2015). However, muscle abnormalities have more consistently provided diagnostic and prognostic information; fasciculations, decreased bulk, and increased echo intensity (EI) have helped characterize ALS status when evaluating neck, diaphragm, core, and limb muscles (Arts et al., 2012, Arts et al., 2011, Grimm et al., 2015, Hensiek et al., 2020, Hiwatani et al., 2013, Misawa et al., 2011, O’Gorman et al., 2017, Pinto et al., 2016, Tsuji et al., 2017).
Studies of the tongue using QUS have generally been dedicated to the assessment of spontaneous activity and muscle thickness in ALS patients (Grimm et al., 2015, Misawa et al., 2011, Nakamori et al., 2016, O’Gorman et al., 2017, Tamburrini et al., 2010, Tsuji et al., 2017, Umemoto et al., 2017). QUS is equal or superior to electromyography (EMG) in the ability to detect tongue fasciculations – and, particularly when combined with EMG, can enhance the ability to achieve more a definitive diagnosis (Grimm et al., 2015, Misawa et al., 2011, O’Gorman et al., 2017, Tsuji et al., 2017). QUS has also shown that ALS patients tend to have diminished tongue thickness, a finding associated with functional abnormalities (Nakamori et al., 2016, Tamburrini et al., 2010, Umemoto et al., 2017).
Tongue EI has not routinely been assessed in ALS patients. However, it would be worthwhile. EI is a numeric correlate of muscle brightness that increases due to the infiltration of fat, development of fibrous tissue, and the disruption of normal architecture (Pillen et al., 2009a). Therefore, EI could build on information about spontaneous activity and muscle quantity by providing insight into muscle quality. Indeed, EI may be emerging as a better prognostic factor than fasciculations and atrophy (Arts et al., 2011, Arts et al., 2008).
Thus, the goals of this study were to: 1) learn if tongue EI differs between healthy participants and individuals with ALS and 2) determine if tongue EI values correlate with more standard measures of bulbar function in an ALS cohort. Consistent with prior ALS work, we expected tongue EI values to be higher in the ALS group and to negatively correlate with both amyotrophic lateral sclerosis functional rating scale – revised (ALSFRS-R) bulbar sub-score and tongue strength.
2. METHODS
The Beth Israel Deaconess Medical Center Institutional Review Board approved the study protocol. All subjects provided written consent prior to participation. All procedures were performed in accord with the Helsinki Declaration of 1975.
2.1. Participants
Healthy adults between the ages of 20 and 79 years were recruited to the study through an online advertisement between 2014 and 2018. In a screening interview, potential participants were excluded if they had conditions that could influence tongue structure (e.g. lingual piercings) or had disorders that could affect tongue function. A medical history was taken, detailed tongue assessment, and a neurological examination was performed for each participant to ensure there was no evidence of neurological abnormality. Age-matching (+1/−4 years) was ultimately performed with the ALS cohort; from a larger pool of healthy controls, 16 were chosen on the basis of age since tongue parameters change over time with this variable (Todd et al., 2013).
A convenience sample of adult patients with ALS was referred to the study from the Beth Israel Deaconess Medical Center Neurology clinic and other on-site clinical trials between 2014 and 2018. Potential participants were excluded if they reported a history of conditions beyond motor neuron disease that could affect tongue function or structure. In addition to a neurological history and exam, results of the most recently performed electromyography (EMG) of the tongue and video swallow studies were reviewed. Site of disease onset was noted. The presence or absence of bulbar symptoms was recorded, as was the use of medications including riluzole, dextromethorphan HBr 20 mg/quinidine sulfate 10 mg, and edaravone.
2.2. Quantitative Ultrasound
To conduct the QUS evaluation, a Terason portable laptop ultrasound system and a linear 16–7 MHz (“hockey stick”) transducer were used. All ultrasound settings were held constant. A single-use sterile cover including gel was placed over the hockey stick transducer. Participants were asked to open the mouth with the jaw as relaxed as possible and the tongue resting. The transducer was placed over the middle of the anterior surface of the tongue (Figure 1) (van den Engel-Hoek et al., 2012). The performance of intra-oral QUS was well-tolerated by all participants. The biggest challenge was ensuring that the transducer was in contact with the tongue surface when there was more pronounced atrophy. Image acquisition took under 10 seconds.
Figure 1.
Placement of the hockey stick transducer on the surface of the tongue.
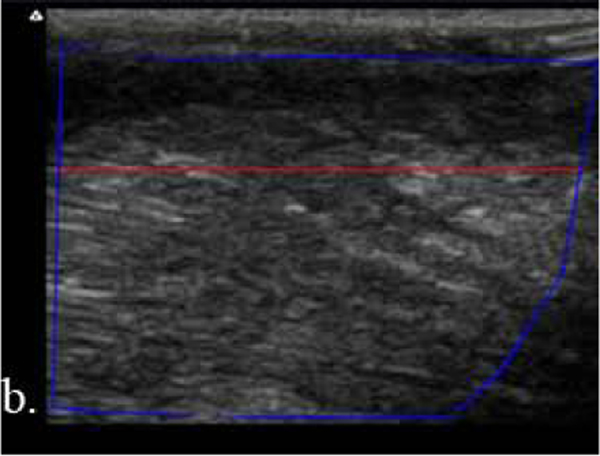
Resultant images were stored as JPEG files with a resolution of approximately 291 dots per image. Without any processing, the ‘raw’ JPEGs were exported from the ultrasound system to a computer. Image analysis was performed using MATLAB® (Mathworks, Natick, MA). A region of interest (ROI) was drawn to include all of the tongue muscle bulk imaged, including the superior longitudinal and transverse muscles. The upper boundary of the ROI was delineated by the lower border of tongue mucosa, which marked the superior border of the longitudinal muscle (van den Engel-Hoek et al., 2012). A change in muscle fiber direction confirmed inclusion of the underlying transverse muscle (van den Engel-Hoek et al., 2012). The lower boundary of the ROI was defined by the deepest point of the transverse muscle imaged; as previously noted, no fascial, bone, or other landmarks fell within the image window for use (van den Engel-Hoek et al., 2012). To avoid depth-related attenuation, which is more common both with the use of high-frequency transducers and in the setting of neuromuscular disease, we calculated average EI for the upper 1/3 of the imaged muscle for all participants (Figure 2) (Jansen et al., 2012, Walker et al., 2004). We looked at EI derived from both gray scale level (GSL) and quantitative backscatter analysis (QBA). Because GSL has effectively become the standard for QUS image analyses and reports, we emphasize those results herein (Hobson-Webb and Simmons, 2019).
Figure 2.
a. Schematic of the (1) tongue surface and mucosa, (2) superior longitudinal muscle, and (3) transverse muscle; adapted from van den Engel-Hoek et al., 2012; b. tongue ultrasound of a healthy participant; and c. tongue ultrasound of an amyotrophic lateral sclerosis patient with the blue line encompassing the region of interest and the red line distinguishing the upper 1/3 and lower 2/3 of the muscle bulk. The depth was 2 cm.
2.3. Functional Measures in ALS Patients
ALSFRS-R bulbar sub-scores were calculated for each patient at the time of the study visit. Within this framework, symptoms related to speech, salivation, and swallowing were scored on a scale of 0 (most impaired) to 4 (normal) (Cedarbaum et al., 1999). A minimal score of 0 reflected prominent bulbar dysfunction while a maximum score of 12 represented no appreciable bulbar dysfunction by the self-report survey.
The Iowa Oral Performance Instrument (PN 1–2300, IOPI System, IOPI Medical LLC) was used to measure tongue strength in ALS patients. Care was taken to place the air-filled bulb on the anterior tongue surface. Participants were encouraged to use the tongue to press the bulb up to the roof of the mouth without clenching the teeth. To reflect the maximum pressure that could be generated in kilopascals (kPa), the peak value from three trials was used in the final analysis. Tongue strength was not measured in the healthy cohort because characterizing tongue function in the controls was not considered a primary aim of this study.
2.4. Statistical Analysis
Data were analyzed using GraphPad Prism 8.3 (GraphPad Software, La Jolla, CA). Normality was confirmed using the Shapiro-Wilk test. Unpaired t-tests were completed to compare the healthy and patient EI data sets. Spearman correlations were performed to assess the relationship between tongue EI and functional measures given the small sample size, and to avoid the influence of outliers.
3. RESULTS
3.1. Participant Characteristics
There was no significant difference in the age, gender composition, or body mass index of participants in the healthy and diseased groups (Table 1). The healthy participants consisted of 16 adults (7 men and 9 women) ranging in age from 26 to 70 years (mean 59 years). The patient group was comprised of 16 adults (6 men and 10 women) aged 25 to 74 years (mean 60 years) who had been diagnosed with laboratory-supported probable, clinically probable, or clinically definite ALS according to the revised El Escorial criteria (Brooks et al., 2000). In some cases, needle electromyography (EMG) of the tongue had been performed months before the study visit, so was not considered a valuable comparator. Of the 11 available, the most recent video fluoroscopy was interpreted as abnormal for all but two of the patients. The majority of patients were taking riluzole, dextromethorphan HBr/quinidine sulfate, edaravone, or combinations of the therapies; 3 patients were on none of these treatments. The population included patients with sporadic and familial forms of ALS; three patients had C9ORF72 positive results and one patient had an SOD1 mutation. Thirty-one percent of patients had bulbar-onset disease and 75% of patients had oropharyngeal symptoms at the time of the study (Table 2).
Table 1.
Participant Characteristics
| Demographic Variable | Amyotrophic Lateral Sclerosis Patients (n=16) | Healthy Participants (n = 16) | Between Group Difference |
|---|---|---|---|
|
| |||
| Average Age (years) | 61 | 59 | p = 0.70 |
| Gender (# men) | 6 | 7 | p = 0.73 |
| Average Body Mass Index | 28.2 | 25.3 | p = 0.26 |
Table 2.
Patient Characteristics and Clinical Status
| Variable | Value | n |
|---|---|---|
|
| ||
| Genetically-confirmed form of amyotrophic lateral sclerosis | 25% | 16 |
| Bulbar-onset amyotrophic lateral sclerosis | 31% | 16 |
| Active bulbar symptoms at time of study | 75% | 16 |
| Abnormal video fluoroscopy when most recently performed | 82% | 11 |
| Riluzole, edaravone, and/or dextromethorphan HBr/quinidine sulfate use | 81% | 16 |
| Average peak tongue strength by Iowa Oral Performance Instrument in kPa, (range) | 33, (5–77) | 12 |
| Average amyotrophic lateral sclerosis functional rating scale-revised bulbar sub-score, (range) | 9, (4–12) | 16 |
kPA = kilopascals
3.2. Tongue EI, ALSFRS-R bulbar sub-scores, and tongue strength
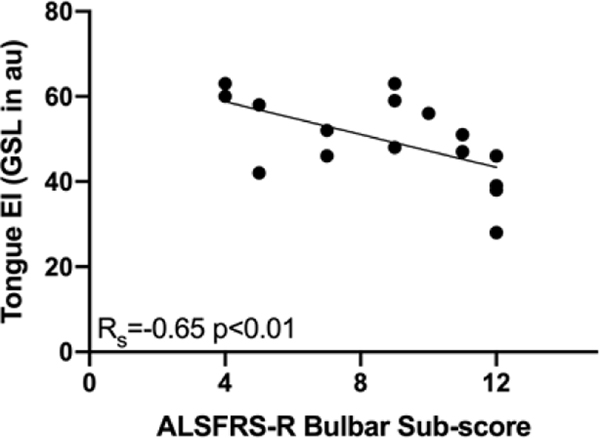
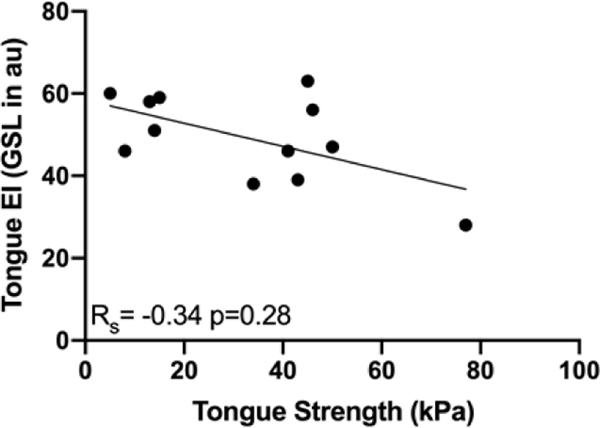
Tongue EI values were assessed for all participants using GSL and QBA. The average GSL was 37.8 arbitrary units (au) with a standard deviation (SD) of 5.8 in healthy participants and 49.8 au (SD 9.9) in ALS patients (p< 0.01, Figure 3a, Table 3). ALSFRS-R bulbar sub-scores ranged from 4 to 12 with a mean of 9 (Table 2). In the 12 ALS patients for whom IOPI testing was performed, maximum tongue elevation strength ranged from 5–77 kPa with an average of peak of 33 (SD 21.7) (Table 2). GSL was inversely correlated with ALSFRS-R bulbar sub-score (RS = −0.65, p<0.01, Figure 3b, Table 3). An inverse correlation was also found between GSL and tongue strength but did not reach significance (RS = −0.34, p = 0.28, Figure 3c, Table 3). There was no relationship between tongue GSL and time since the onset of ALS symptoms (Table 3). All patients with GSL values above the highest recorded value for healthy participants reported oropharyngeal symptoms. The same trends were found when tongue EI was determined by the QBA method (Table 3).
Figure 3.
a. Echo intensity (EI) was significantly higher in tongues of amyotrophic lateral sclerosis (ALS) patients compared to healthy participants (gray scale level (GSL) 49.8 ± standard deviation (SD) 9.9 versus 37.8 ± SD 5.8 arbitrary units (au), p < 0.01); b. Negative correlation between tongue EI and ALS functional rating scale-revised bulbar sub-score in ALS patients; c. Negative correlation between tongue EI and tongue strength measured by the Iowa Oral Performance Instrument (IOPI) in ALS patients.
Table 3.
Tongue Health and Function: A Comparison of Gray Scale Level and Quantitative Backscatter Analysis
| Measures of Tongue Health and Function | Echo Intensity (EI) Mode of Analysis | |||
|---|---|---|---|---|
|
| ||||
| GSL | QBA | |||
|
| ||||
| Healthy EI | 37.8, SD 5.8 | 34.8, SD 5.6 | ||
| Amyotrophic lateral sclerosis EI | 49.8, SD 9.9 | (p < 0.01) | 39.8, SD 5.1 | (p < 0.01) |
| Correlation between EI and ALSFRS-R bulbar sub-score | RS = −0.65 | (p< 0.01) | RS = −0.59 | (p = 0.02) |
| Correlation between EI and tongue strength by IOPI | RS = −0.34 | (p = 0.28) | RS = −0.41 | (p = 0.19) |
| Correlation between EI and time since ALS onset | RS = 0.02 | (p= 0.93) | RS = −0.01 | (p = 0.98) |
ALSFRS-R = amyotrophic lateral sclerosis functional rating scale – revised; EI = echo intensity; GSL = gray scale level; IOPI = Iowa Oral Performance Instrument; QBA = quantitative backscatter analysis; RS =Spearman rho; SD = standard deviation
4. DISCUSSION
Quantitative ultrasound is emerging as a tool to provide biomarker data in ALS. To date, the majority of EI work in ALS has been performed with muscles representing lumbosacral, thoracic, and cervical body segments (Hobson-Webb and Simmons, 2019). In this preliminary study, we examined EI of the tongue as a reflection of the bulbar region. As hypothesized, tongue EI was higher in ALS patients than in age-matched controls and was negatively correlated with ALSFRS-R bulbar sub-score. While it did not reach statistical significance, a negative correlation was also present for EI and tongue strength as measured by the IOPI. Overall, these outcomes add to a growing body of support for employing QUS to study the tongue in ALS.
Focus on the tongue muscle as a representative of the bulbar system makes sense. First, it is easily accessible. Further, ALS has been shown to disproportionately affect the tongue compared to other orofacial muscles - even before the development of appreciable dysarthria (DePaul and Brooks, 1993). Related subclinical changes can be identified by tongue QUS (Grimm et al., 2015, Misawa et al., 2011, O’Gorman et al., 2017). While this research highlighted disease-related changes in EI, complementary information could be gained by expanding the protocol to include assessments of abnormal spontaneous activity, tongue thickness, and other QUS metrics. However, more work is needed to refine measurement parameters and ultrasound approaches given factors including the complexity of tongue structure.
The tongue is comprised of multiple elements. There are four extrinsic muscles, including genioglossus, which have attachments to bony surfaces (Gaige et al., 2007). The four intrinsic muscles lack a skeletal anchor and consist of the superior longitudinal, transverse, inferior longitudinal, and vertical muscles (Gaige et al., 2007). Extrinsic and intrinsic muscles function together as a three-dimensional, interlinking matrix of heterogeneously oriented fibers (Gaige et al., 2007, Gilbert and Napadow, 2005, Sakamoto, 2018). This configuration translates into variable anisotropy (Gilbert and Napadow, 2005), a feature which distinguishes the tongue from other skeletal muscles and helps account for the relatively increased EI of the tongue (Walker et al., 2004).
The unique myoarchitecture of the tongue may be one reason EI has not routinely been assessed. It’s also possible that the submental ultrasound technique most commonly employed in ALS is better suited to quantifying tongue fasciculations and thickness because it allows visualization from the base to the dorsum of the tongue in the coronal plane (Gervasio et al., 2011, Grimm et al., 2015, Gritzmann and Fruhwald, 1988, Hensiek et al., 2020, Nakamori et al., 2016, O’Gorman et al., 2017, Tamburrini et al., 2010, Umemoto et al., 2017). The submental approach captures muscles that form the floor of the mouth and those which constitute the root of the tongue including genioglossus (Gervasio et al., 2011, Gritzmann and Fruhwald, 1988). However, ALS can affect the entire tongue, and sometimes with preferential involvement of the intrinsic muscles not well-distinguished or characterized by the submental view (Cha and Patten, 1989, Fox and Cohen, 2012, Gritzmann and Fruhwald, 1988, Hensiek et al., 2020, Lee et al., 2018).
To evaluate intrinsic tongue muscles, an intra-oral sonographic technique can be employed. Perhaps most often implemented in the care of oral malignancies, this approach has also been applied to evaluate the effects of neuromuscular disease on the tongue (O’Gorman et al., 2017, Tarabichi et al., 2019, van den Engel-Hoek et al., 2012). While O’Gorman et al. (2017) used intra-oral ultrasound to detect fasciculations in ALS patients, van den Engel-Hoek and colleagues (2012) assessed tongue EI in the setting of Duchenne muscular dystrophy.
The intra-oral technique allowed us to evaluate the EI of intrinsic tongue muscles in this ALS study. We further focused analysis on the upper 1/3 of the tongue, which likely includes the portion of muscle examined by needle EMG performed from an intra-oral approach. Going forward, it would be valuable to specifically compare factors including fasciculations and EI in this part of the tongue with those in genioglossus, the target of needle EMG from the percutaneous submental approach (Preston and Shapiro, 2013). Results could optimize the diagnostic yield of needle examinations of the tongue in ALS patients.
Interestingly, duration of ALS symptoms was not strongly associated with tongue EI in this investigation. In contrast, prior work has shown that higher pre-slope EI, which incorporates disease duration and baseline value, predicts shorter survival in ALS (Arts et al., 2011). However, this earlier study used the composite EI of five muscle pairs from different body segments. It may be that we did not find a similar trend due to a smaller dataset and focus on a single muscle.
However, we did examine EI data from the solitary muscle in more than one way: with both GSL and QBA. GSL and QBA are equally reliable measures in neuromuscular disease but the respective processes have strengths and weaknesses (Shklyar et al., 2015). While there are ways to convert data for generalizability across equipment, GSL data are specific to a given ultrasound system – and this may be yet another reason tongue EI has not been more frequently assessed (Arts et al., 2012, Pillen et al., 2009b, Zaidman et al., 2012). Unlike GSL, QBA data are more applicable from one ultrasound system to another since they are relatively free from proprietary image-processing, but they are not commonly available to users (Shklyar et al., 2015).
Consistent with prior studies comparing data from GSL and QBA in neuromuscular disease, in this analysis the two methods generated concordant results when quantifying muscle abnormalities in ALS (Shklyar et al., 2015). From a broader perspective, this finding suggests that the overall trends in work exploring the use of QUS in ALS have merit - even if techniques are not entirely standardized. This is an important point because the studies to date vary not just in imaging analysis technique but also in the realms of equipment, software, choice of representative muscles, and method for defining the ROI.
In this study, the use of individually-drawn ROIs was favored over a template shape or standard surface depth to better account for the atrophy and alterations in tongue configuration that take place in disease course (Cha and Patten, 1989, Hensiek et al., 2020). While all ultrasound settings were held constant across participants, there were natural variations in the relative bulk of the individual muscles comprising the tongue captured in images. Because EI is greater in the transverse muscle than the longitudinal muscle, disproportionate atrophy of the longitudinal muscle may have translated into higher EI in the patients (van den Engel-Hoek et al., 2012).
Elevated tongue EI was expected to correlate with decreased tongue strength, in line with prior work linking tongue QUS parameters with performance on functional tasks (Nakamori et al., 2016, Umemoto et al., 2017). There are a few possible explanations for why the negative trend did not reach statistical significance in this cohort. With the exception of one value, all tongue strength results were lower than age-stratified means – and actually below the 5th percentile (IOPIMedical). The result could therefore reflect a floor effect. Alternatively, the intrinsic tongue muscles for which EI was calculated may not be primarily responsible for exerting maximal pressure with the IOPI device (Palmer et al., 2008). It is also possible that the sample size was too small.
Indeed, the limited number of participants is one of several study drawbacks worth consideration, as is the absence of formal reliability measures and gender-matching. Further, the majority of patients did have subjective and objective evidence of bulbar dysfunction so it remains unclear how generalizable results are to patients with less pronounced oropharyngeal involvement. Additional work is necessary to establish if similar trends are appreciable in patients with upper motor neuron predominant disease such as primary lateral sclerosis. Finally, the cross-sectional design does not provide any insight into changes over time. Given support for the benefit of dextromethorphan HBr/quinidine sulfate on bulbar function, it would be particularly interesting to learn if, and how quickly, corresponding changes in tongue EI occur (Smith et al., 2017). Future studies are necessary to build on this work and better characterize the role of tongue QUS as a biomarker of bulbar dysfunction in motor neuron disease – and other neuromuscular conditions with tongue involvement.
Highlights.
Echo intensity (EI) was significantly higher in the tongues of amyotrophic lateral sclerosis (ALS) patients than in age-matched controls.
Tongue EI was negatively correlated with ALS functional rating scale-revised bulbar sub-score.
Tongue EI holds promise as a novel biomarker of bulbar dysfunction in ALS.
ACKNOWLEDGEMENTS
We are grateful for the funding provided by F32DC014382 and K24NS060951.
Footnotes
CONFLICT OF INTEREST STATEMENT
Dr. Rutkove has equity in, and serves as a consultant and scientific advisor to, Myolex, Inc. a company that designs impedance devices for clinical and research use; he is also a member of the Company’s Board of Directors. The company also has an option to license patented impedance technology of which Dr. Rutkove is named an inventor. None of the other authors have potential conflicts of interest to be disclosed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arts IM, Overeem S, Pillen S, Kleine BU, Boekestein WA, Zwarts MJ, et al. Muscle ultrasonography: a diagnostic tool for amyotrophic lateral sclerosis. Clin Neurophysiol 2012;123(8):1662–7. [DOI] [PubMed] [Google Scholar]
- Arts IM, Overeem S, Pillen S, Schelhaas HJ, Zwarts MJ. Muscle ultrasonography to predict survival in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2011;82(5):552–4. [DOI] [PubMed] [Google Scholar]
- Arts IM, van Rooij FG, Overeem S, Pillen S, Janssen HM, Schelhaas HJ, et al. Quantitative muscle ultrasonography in amyotrophic lateral sclerosis. Ultrasound Med Biol 2008;34(3):354–61. [DOI] [PubMed] [Google Scholar]
- Cartwright MS, Walker FO, Griffin LP, Caress JB. Peripheral nerve and muscle ultrasound in amyotrophic lateral sclerosis. Muscle Nerve 2011;44(3):346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci 1999;169(1–2):13–21. [DOI] [PubMed] [Google Scholar]
- Cha CH, Patten BM. Amyotrophic lateral sclerosis: abnormalities of the tongue on magnetic resonance imaging. Ann Neurol 1989;25(5):468–72. [DOI] [PubMed] [Google Scholar]
- Costa MM. Videofluoroscopy: the gold standard exam for studying swallowing and its dysfunction. Arq Gastroenterol 2010;47(4):327–8. [DOI] [PubMed] [Google Scholar]
- DePaul R, Brooks BR. Multiple orofacial indices in amyotrophic lateral sclerosis. J Speech Hear Res 1993;36(6):1158–67. [DOI] [PubMed] [Google Scholar]
- Fox MD, Cohen AB. “Bright tongue sign” in ALS. Neurology 2012;79(14):1520. [DOI] [PubMed] [Google Scholar]
- Gaige TA, Benner T, Wang R, Wedeen VJ, Gilbert RJ. Three dimensional myoarchitecture of the human tongue determined in vivo by diffusion tensor imaging with tractography. J Magn Reson Imaging 2007;26(3):654–61. [DOI] [PubMed] [Google Scholar]
- Gans BM, Kraft GH. Pain perception in clinical electromyography. Arch Phys Med Rehabil 1977;58(1):13–6. [PubMed] [Google Scholar]
- Gervasio A, D’Orta G, Mujahed I, Biasio A. Sonographic anatomy of the neck: The suprahyoid region. J Ultrasound 2011;14(3):130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert RJ, Napadow VJ. Three-dimensional muscular architecture of the human tongue determined in vivo with diffusion tensor magnetic resonance imaging. Dysphagia 2005;20(1):1–7. [DOI] [PubMed] [Google Scholar]
- Grimm A, Prell T, Decard BF, Schumacher U, Witte OW, Axer H, et al. Muscle ultrasonography as an additional diagnostic tool for the diagnosis of amyotrophic lateral sclerosis. Clin Neurophysiol 2015;126(4):820–7. [DOI] [PubMed] [Google Scholar]
- Gritzmann N, Fruhwald F. Sonographic anatomy of tongue and floor of the mouth. Dysphagia 1988;2(4):196–202. [DOI] [PubMed] [Google Scholar]
- Haverkamp LJ, Appel V, Appel SH. Natural history of amyotrophic lateral sclerosis in a database population. Validation of a scoring system and a model for survival prediction. Brain 1995;118:707–19. [DOI] [PubMed] [Google Scholar]
- Heckmatt JZ, Leeman S, Dubowitz V. Ultrasound imaging in the diagnosis of muscle disease. J Pediatr 1982;101(5):656–60. [DOI] [PubMed] [Google Scholar]
- Hensiek N, Schreiber F, Wimmer T, Kaufmann J, Machts J, Fahlbusch L, et al. Sonographic and 3T-MRI-based evaluation of the tongue in ALS. Neuroimage Clin 2020;26:102233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiwatani Y, Sakata M, Miwa H. Ultrasonography of the diaphragm in amyotrophic lateral sclerosis: clinical significance in assessment of respiratory functions. Amyotroph Lateral Scler Frontotemporal Degener 2013;14(2):127–31. [DOI] [PubMed] [Google Scholar]
- Hobson-Webb LD, Simmons Z. Ultrasound in the Diagnosis and Monitoring of Amyotrophic Lateral Sclerosis: A Review. Muscle Nerve 2019;60(2):114–123. [DOI] [PubMed] [Google Scholar]
- IOPIMedical. Normal Values; Available from:https://iopimedical.com/normal-values/. [Accessed March 23 2020].
- Jan MM, Schwartz M, Benstead TJ. EMG related anxiety and pain: a prospective study. Can J Neurol Sci 1999;26(4):294–7. [DOI] [PubMed] [Google Scholar]
- Jansen M, van Alfen N, Nijhuis van der Sanden MW, van Dijk JP, Pillen S, de Groot IJ. Quantitative muscle ultrasound is a promising longitudinal follow-up tool in Duchenne muscular dystrophy. Neuromuscul Disord 2012;22(4):306–17. [DOI] [PubMed] [Google Scholar]
- Lee E, Xing F, Ahn S, Reese TG, Wang R, Green JR, et al. Magnetic resonance imaging based anatomical assessment of tongue impairment due to amyotrophic lateral sclerosis: A preliminary study. J Acoust Soc Am 2018;143(4):EL248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misawa S, Noto Y, Shibuya K, Isose S, Sekiguchi Y, Nasu S, et al. Ultrasonographic detection of fasciculations markedly increases diagnostic sensitivity of ALS. Neurology 2011;77(16):1532–7. [DOI] [PubMed] [Google Scholar]
- Nakamori M, Hosomi N, Takaki S, Oda M, Hiraoka A, Yoshikawa M, et al. Tongue thickness evaluation using ultrasonography can predict swallowing function in amyotrophic lateral sclerosis patients. Clin Neurophysiol 2016;127(2):1669–74. [DOI] [PubMed] [Google Scholar]
- Nodera H, Takamatsu N, Shimatani Y, Mori A, Sato K, Oda M, et al. Thinning of cervical nerve roots and peripheral nerves in ALS as measured by sonography. Clin Neurophysiol 2014;125(9):1906–11. [DOI] [PubMed] [Google Scholar]
- Noto YI, Garg N, Li T, Timmins HC, Park SB, Shibuya K, et al. Comparison of cross-sectional areas and distal-proximal nerve ratios in amyotrophic lateral sclerosis. Muscle Nerve 2018;58(6):777–83. [DOI] [PubMed] [Google Scholar]
- O’Gorman CM, Weikamp JG, Baria M, Van Den Engel-Hoek L, Kassardjian C, Van Alfen N, et al. Detecting fasciculations in cranial nerve innervated muscles with ultrasound in amyotrophic lateral sclerosis. Muscle Nerve 2017;56(6):1072–6. [DOI] [PubMed] [Google Scholar]
- Palmer PM, Jaffe DM, McCulloch TM, Finnegan EM, Van Daele DJ, Luschei ES. Quantitative contributions of the muscles of the tongue, floor-of-mouth, jaw, and velum to tongue-to-palate pressure generation. J Speech Lang Hear Res 2008;51(4):828–35. [DOI] [PubMed] [Google Scholar]
- Pillen S, Tak RO, Zwarts MJ, Lammens MM, Verrijp KN, Arts IM, et al. Skeletal muscle ultrasound: correlation between fibrous tissue and echo intensity. Ultrasound Med Biol 2009a;35(3):443–6. [DOI] [PubMed] [Google Scholar]
- Pillen S, van Dijk JP, Weijers G, Raijmann W, de Korte CL, Zwarts MJ. Quantitative gray-scale analysis in skeletal muscle ultrasound: a comparison study of two ultrasound devices. Muscle Nerve 2009b;39(6):781–6. [DOI] [PubMed] [Google Scholar]
- Pillen S, van Keimpema M, Nievelstein RA, Verrips A, van Kruijsbergen-Raijmann W, Zwarts MJ. Skeletal muscle ultrasonography: Visual versus quantitative evaluation. Ultrasound Med Biol 2006;32(9):1315–21. [DOI] [PubMed] [Google Scholar]
- Pinto S, Alves P, Pimentel B, Swash M, de Carvalho M. Ultrasound for assessment of diaphragm in ALS. Clin Neurophysiol 2016;127(1):892–7. [DOI] [PubMed] [Google Scholar]
- Preston DC, Shapiro BE. Electromypgraphy and Neuromuscular Disorders: Clinical-Electrophysiologic Correlations: Elsevier Inc., 2013. [Google Scholar]
- Sakamoto Y. Structural arrangement of the intrinsic muscles of the tongue and their relationships with the extrinsic muscles. Surg Radiol Anat 2018;40(6):681–8. [DOI] [PubMed] [Google Scholar]
- Schreiber S, Abdulla S, Debska-Vielhaber G, Machts J, Dannhardt-Stieger V, Feistner H, et al. Peripheral nerve ultrasound in amyotrophic lateral sclerosis phenotypes. Muscle Nerve 2015;51(5):669–75. [DOI] [PubMed] [Google Scholar]
- Shklyar I, Geisbush TR, Mijialovic AS, Pasternak A, Darras BT, Wu JS, et al. Quantitative muscle ultrasound in Duchenne muscular dystrophy: a comparison of techniques. Muscle Nerve 2015;51(2):207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, Pioro E, Myers K, Sirdofsky M, Goslin K, Meekins G, et al. Enhanced Bulbar Function in Amyotrophic Lateral Sclerosis: The Nuedexta Treatment Trial. Neurotherapeutics 2017;14(3):762–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamburrini S, Solazzo A, Sagnelli A, Del Vecchio L, Reginelli A, Monsorro M, et al. Amyotrophic lateral sclerosis: sonographic evaluation of dysphagia. Radiol Med 2010;115(5):784–93. [DOI] [PubMed] [Google Scholar]
- Tarabichi O, Bulbul MG, Kanumuri VV, Faquin WC, Juliano AF, Cunnane ME, et al. Utility of intraoral ultrasound in managing oral tongue squamous cell carcinoma: Systematic review. Laryngoscope 2019;129(3):662–70. [DOI] [PubMed] [Google Scholar]
- Todd JT, Lintzenich CR, Butler SG. Isometric and swallowing tongue strength in healthy adults. Laryngoscope 2013;123(10):2469–73. [DOI] [PubMed] [Google Scholar]
- Tsuji Y, Noto YI, Shiga K, Teramukai S, Nakagawa M, Mizuno T. A muscle ultrasound score in the diagnosis of amyotrophic lateral sclerosis. Clin Neurophysiol 2017;128(6):1069–74. [DOI] [PubMed] [Google Scholar]
- Umemoto G, Furuya H, Tsuboi Y, Fujioka S, Arahata H, Sugahara M, et al. Characteristics of tongue and pharyngeal pressure in patients with neuromuscular diseases. Degener Neurol Neuromuscul Dis 2017;7:71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Engel-Hoek L, Van Alfen N, De Swart BJ, De Groot IJ, Pillen S. Quantitative ultrasound of the tongue and submental muscles in children and young adults. Muscle Nerve 2012;46(1):31–7. [DOI] [PubMed] [Google Scholar]
- Wagner KR. The need for biomarkers in amyotrophic lateral sclerosis drug development. Neurology 2009;72(1):11–2. [DOI] [PubMed] [Google Scholar]
- Walker FO, Cartwright MS, Wiesler ER, Caress J. Ultrasound of nerve and muscle. Clin Neurophysiol 2004;115(3):495–507. [DOI] [PubMed] [Google Scholar]
- Wilcox F, Liss JM, Siegel GM. Interjudge agreement in videofluoroscopic studies of swallowing. J Speech Hear Res 1996;39(1):144–52. [DOI] [PubMed] [Google Scholar]
- Zaidman CM, Holland MR, Hughes MS. Quantitative ultrasound of skeletal muscle: reliable measurements of calibrated muscle backscatter from different ultrasound systems. Ultrasound Med Biol 2012;38(9):1618–25. [DOI] [PMC free article] [PubMed] [Google Scholar]