Abstract
Alterations in polyunsaturated fatty acids (PUFAs), including omega-3 and omega-6, have been implicated in the pathophysiology of psychotic disorders, but little is known about their associations with neuropsychological functioning. The present study includes 46 recent-onset psychosis patients who participated in a larger (n=50) double blind, placebo-controlled randomized clinical trial comparing 16 weeks of treatment with either risperidone+fish oil (FO) (EPA 740mg and DHA 400mg daily) or risperidone+placebo and completed neuropsychological assessments at the baseline timepoint. We investigated the relationship between baseline omega-3 (i.e., eicosapentaenoic acid, EPA; docosapentaenoic acid, DPA and docosahexaenoic acid, DHA) and omega-6 (i.e., arachidonic acid, AA) PUFA with baseline MATRICS Consensus Cognitive Battery (MCCB) and Brief Psychiatric Rating Scale (BPRS) scores. Twenty-five patients had neuropsychological data available at 16 weeks following participation in the clinical trial, which included 12 patients assigned to risperidone+FO and 13 patients assigned to risperidone+placebo. At baseline both higher DHA and EPA correlated significantly with better social cognition after controlling for functioning on other neuropsychological domains, total BPRS score, AA level and substance use. Also, at baseline higher AA correlated significantly with hostility/uncooperativeness after controlling for DHA+EPA+DPA, overall neuropsychological functioning and substance use. Patients treated with risperidone+FO demonstrated a significant longitudinal increase in social cognition that was significantly higher at 16 weeks compared to patients treated with risperidone+placebo. DHA also correlated significantly with social cognition at the 16-week timepoint. This study provides novel evidence for a differential role of omega-3 vs. omega-6 PUFA in neuropsychological deficits and symptoms in recent-onset psychosis and its treatment.
Introduction
Polyunsaturated fatty acids (PUFA) are a broad class of bioactive lipids that are considered essential because they are not produced de novo and must be obtained from the diet. These essential fatty acids include alpha-linolenic acid (ALA) and linoleic acid (LA), which are short-chain precursors of omega-3 PUFA such as eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA) and docosahexaenoic acid (DHA), and omega-6 PUFA such as arachidonic acid (AA), respectively. These fatty acids cross the blood-brain barrier and influence cellular and inflammatory signaling and contribute to healthy brain development, aging, and neuronal resilience (Janssen and Kiliaan, 2014; McCusker and Grant-Kels, 2010; Rapoport, 2013). Omega-3 and omega-6 PUFA have a complex synergistic and antagonistic relationship (Lin et al., 2015; Luxwolda et al., 2011), and extant evidence indicates that an optimal ratio of omega-6 to omega-3 PUFA is important for mental health and cognitive functioning (Nelson and Raskin, 2019).
Several lines of evidence suggest that a deficiency in omega-3 PUFA may be relevant to the pathoetiology of schizophrenia. Animal studies have demonstrated that developmental deficits in brain DHA accrual can recapitulate behavioral and neurobiological features observed in schizophrenia (Li et al., 2015; Maekawa et al., 2017; McNamara et al., 2017; Zimmer et al., 2002). Meta-analyses have indicated that DPA, DHA and AA are lower in patients with schizophrenia compared to healthy controls (Hoen et al., 2013; van der Kemp et al., 2012), and several studies suggest that omega-3 and omega-6 PUFA are associated with clinical symptoms in psychosis (Berger et al., 2019; Kim et al., 2016; Kim et al., 2014; Medema et al., 2016; Pawełczyk et al., 2016; Sethom et al., 2010; Watari et al., 2010). Several clinical trials investigating omega-3 PUFA supplementation on symptoms in patients with multi-episode schizophrenia have reported negative findings (Emsley et al., 2006; Fenton et al., 2001; Peet and Horrobin, 2002), although improvement in overall symptom severity (Emsley et al., 2002; Jamilian et al., 2014; Peet et al., 2001) and positive symptoms (Peet et al., 2001) has been observed. Consistent with clinical stage specific effects (Chen et al., 2014), more consistent evidence for reductions in overall symptom severity following omega-3 PUFA supplementation has been observed in first-episode psychosis patients (Pawełczyk et al., 2016; Peet et al., 2001; Robinson et al., 2019).
Neurocognitive impairment in schizophrenia is difficult to treat and these deficits portend a less favorable clinical outcome (Reynolds et al., 2018). There is therefore a need for alternative treatments that might enhance cognition in schizophrenia and associated psychotic disorders. Cross-sectional and intervention studies reported an association between omega-3 PUFA and cognitive functioning in healthy children and adults (Adjepong et al., 2018; Montgomery et al., 2013; Stonehouse et al., 2013) and healthy elderly subjects (Witte et al., 2014; Yuan et al., 2016; Yurko-Mauro et al., 2015; Zamroziewicz et al., 2017). Few studies, however, examined the relationship between either omega-3 or omega-6 PUFA and neurocognitive functioning in patients with psychosis. One cross-sectional study reported that the Brief Assessment of Cognition (BACS) composite score correlated significantly with EPA and DHA levels, which then predicted social functioning outcome in schizophrenia (Satogami et al., 2017). Another study revealed a significant inverse relationship between AA and semantic memory and general intelligence in unmedicated patients with chronic schizophrenia (Condray and Yao, 2011). Other data indicate that EPA treatment was associated with worse sustained attention among patients with low baseline PUFA levels (Bentsen and Landrø, 2018). In addition, general cognitive performance correlated significantly with the ratios of both omega-3 and DHA to total fatty acids among individuals at risk for psychosis (Ramsay et al., 2018).
The present study examined associations between omega-3 (i.e., EPA, DPA, DHA) and omega-6 (i.e., AA) PUFA and neuropsychological and symptom measures in a cohort of recent-onset psychosis patients. In addition, 25 patients completed both baseline and follow-up neuropsychological assessments following participation in a double-blind randomized placebo controlled 16-week clinical trial comparing the efficacy of risperidone + placebo vs. risperidone + fish oil (FO) on overall clinical functioning (Robinson et al., 2019). We used erythrocyte membrane fatty acid composition (mg fatty acid/100 mg fatty acids) in this study because it is a more reliable biomarker of habitual dietary fatty acid intake compared to plasma (Harris & Thompson, 2010). We predicted that lower total omega-3 PUFA (i.e., EPA+DHA+DPA), lower omega-3 index (i.e., EPA+DHA), a higher level of AA and a higher ratio of AA to total omega-3 PUFA (i.e., AA/(EPA+DHA+DPA) would be associated with greater symptom severity and worse neuropsychological functioning. We further tested the hypothesis that adjuvant treatment of risperidone with FO would be associated with better neuropsychological functioning compared to patients treated with risperidone + placebo.
Methods and Materials
Subjects
The present study includes 46 (35M/11F) recent-onset psychosis patients (mean age = 21.5 years, SD = 5.3) who participated in a larger (n=50) double-blind placebo-controlled randomized clinical trial comparing 16 weeks of treatment with either risperidone + FO (EPA 740 mg and DHA 400 mg daily) or risperidone + placebo and completed neuropsychological assessments at baseline. Patients were recruited from the Zucker Hillside Hospital, a large acute care non-for-profit psychiatric facility in New York between June, 2013 to September, 2015. Twenty-five patients had neuropsychological data available at 16 weeks following participation in the clinical trial, which included 12 patients assigned to risperidone + FO and 13 patients assigned to risperidone + placebo.
Patient diagnoses were based on the Structured Clinical Interview for Axis I DSM-IV Disorders (SCID-I/P) (First et al., 1994) supplemented by information from clinicians and, when available, family members. Patients met DSM-IV criteria for schizophrenia (n = 31), schizophreniform disorder (n = 9), psychotic disorder NOS (n = 1), schizoaffective disorder (n = 1), or bipolar disorder with psychosis (n = 4). Eight patients met DSM-IV criteria for an alcohol use disorder and 18 patients were diagnosed with a cannabis use disorder. All patients received a physical exam and laboratory screening to rule out medical/neurologic causes for their psychotic episode. Ten patients were antipsychotic drug-naïve at the time of consent and the remaining 36 patients had a median exposure of 4.5 days of antipsychotic treatment (range = 1 to 240 days). Duration of untreated psychotic symptoms was 93.7 weeks (range = 2 to 574). Total Brief Psychiatric Rating Scale score (BPRS) at the time of study entry was 41.6 (SD = 6.4). The study was approved by the Northwell Health Institutional Review Board. Written informed consent was obtained from all individuals, and from a parent or legal guardian in the case of minors. Written assent was obtained from all minors.
Inclusion criteria were: (1) current DSM-IV-defined diagnosis of schizophrenia, schizophreniform, schizoaffective disorder or bipolar disorder assessed using the Structured Clinical Interview for Axis I DSM-IV Disorders (SCID-I/P) (First et al., 1994); (2) does not meet DSM-IV criteria for a current substance-induced psychotic disorder, a psychotic disorder due to a general medical condition, delusional disorder, brief psychotic disorder, shared psychotic disorder, or major depressive disorder with psychotic features; (3) current positive symptoms rated ≥4 (moderate) on one or more of these BPRS items: conceptual disorganization, grandiosity, hallucinatory behavior, unusual thought content; (4) in an early phase of illness as defined by having taken antipsychotic medications for a cumulative lifetime period of 2 years or less, (5) age 15 to 40 years; (6) competent and willing to sign informed consent; and (7) for women, negative pregnancy test.
Exclusion criteria were: (1) serious neurological or endocrine disorder or any medical condition or treatment known to affect the brain; (2) any medical condition requiring treatment with a medication with psychotropic effects; (3) significant risk of suicidal or homicidal behavior; and (4) cognitive or language limitations, or any other factor that would preclude participants providing informed consent.
Treatment Trial
A detailed description of the overall treatment trial (registration ID: NCT01786239) and study results using the primary clinical outcome measure, total BPRS score, is provided in Robinson et al (2019). Briefly, all participants received open-label risperidone for 16 weeks with half assigned randomly to receive FO and the other half to placebo in a double-blind manner. Randomization was conducted on a 1:1 basis stratified by sex and length of antipsychotic exposure (< versus ≥16 weeks) by the study biostatistician using a computer generated randomization list. Patients and all staff providing treatment and conducting clinical and neuropsychological assessments were blind to randomization.
The FO and matching placebo (a soybean/corn blend) capsules were manufactured by Ocean Nutrition Canada. Each FO capsule contained 370 mg EPA and 200 mg DHA as well as 2 mg/g tocopherol. Participants took one randomized capsule in the morning and one capsule in the evening so that the total daily dose was 740 mg of EPA and 400 mg of DHA. If participants were receiving an antipsychotic prior to study entry, it was terminated and risperidone initiated and titrated according to the following flexible dosing schedule: 1 mg qhs days 1–3; 2 mg qhs on day 4 and 3 mg on day 7 with the possibility of an increase up to 6 mg over the subsequent weeks, if needed. Risperidone dosage was increased until participants responded or until side effects precluded further increases.
Allowed concomitant medications included benztropine mesylate; lorazepam, propranolol, and if lorazepam was contraindicated for insomnia treatment, zolpidem or rozerem. We dichotomized individuals who received at least one dose of benztropine mesylate or lorazepam vs. those who did not receive these medications during the trial in subsequent analyses.
Neuropsychological and Clinical Assessments
Patients received the MATRICS Cognitive Consensus Battery (MCCB) (Kern et al., 2008; Nuechterlein and Green, 2006) and the Brief Psychiatric Rating Scale – Anchored version (BPRS-A) (Woerner et al., 1988). The MCCB includes the following neuropsychological domains: speed of processing, verbal learning, visual learning, working memory, attention/vigilance, reasoning/problem solving, and social cognition as well as a composite measure of overall functioning. There were missing data for three patients on the social cognition domain and three patients on the attention/vigilance domain at baseline. Twenty-five patients had neuropsychological data available at 16 weeks following participation in the clinical trial, which included 12 patients assigned to risperidone+FO and 13 patients assigned to risperidone+placebo. For the symptom measures we formed BPRS factor scores as defined in prior studies (Robinson et al., 2019; Ventura et al., 2000), which included negative symptoms, positive symptoms, depression/anxiety, and hostility/uncooperativeness. A total BPRS score was formed by summing the individual items.
Handedness
Handedness was assessed using a modified version of the Edinburgh Inventory (Oldfield, 1971) consisting of twenty items. The total number of right and left hand items were scored, and the laterality quotient was computed yielding a total laterality quotient for each participant that ranged from +1.00 (totally dextral) to −1.00 (totally nondextral). Participants with a laterality quotient greater than .70 were classified as dextral (n=34), and the rest as nondextral (n=12).
Erythrocyte fatty acid composition
Whole venous blood was collected into EDTA-coated BD Vacutainer tubes and centrifuged for 20 min (1,500 xg, 4°C). Plasma and the platelet rich interface were removed, and erythrocytes washed three times with 0.9% saline and stored at −80°C. Erythrocyte total fatty acid composition was determined using the saponification and methylation methods described previously (McNamara et al., 2010; Metcalfe et al., 1966). Briefly, the sample was saponified with methanolic sodium hydroxide and fatty acids methylated with BF3 methanol to yield fatty acid methyl esters that were extracted in hexane and used for analysis. Samples were analyzed with a Shimadzu GC-2014 equipped with an auto-injector (Shimadzu Scientific Instruments Inc., Columbia MD). The column was a DB-23 (123–2332): 30m (length), I.D. 0.32 mm wide bore, film thickness of 0.25 μM (J&W Scientific, Folsom CA). Fatty acid identification was determined using retention times of authenticated fatty acid methyl ester standards (Matreya LLC Inc., Pleasant Gap PA). Analysis of fatty acid methyl esters was based on areas calculated with Shimadzu Class VP 4.3 software. Fatty acid composition (mg fatty acid/100 mg fatty acids) was computed by a technician blind to group assignment. Two cases were determined to have outlying values on AA (> 3SD from the mean) at baseline and were excluded from analyses. Twenty-five patients had PUFA data available at 16 weeks following participation in the clinical trial, which included 10 patients assigned to risperidone+FO and 10 patients assigned to risperidone+placebo.
Statistical Analysis
Independent groups t-tests or chi-square analysis was used to compare the 2 treatment groups on distributions of demographic variables. We used Pearson product moment correlations to investigate the relationship between total omega-3 PUFA and neuropsychological functioning on the MCCB and BPRS scores. To minimize Type-I error we limited our initial analyses to investigation of (1) the ratio of AA/(EPA+DHA+DPA), (2) total omega-3 PUFA (i.e., DHA+DPA+EPA) for consistency with another study investigating magnetic resonance imaging correlates of fatty acids (Lyall et al submitted), (3) omega-3 index (EPA+DHA) and (4) AA. We further restricted initial analyses to overall neuropsychological functioning and total BPRS score. Only significant correlations were followed by post-hoc correlations investigating individual omega-3 PUFA or neuropsychological domains.
We used the method of Meng and colleagues (Meng et al., 1992) as implemented in the statistical online program, Cocor (Diedenhofen and Musch, 2015), (http://comparingcorrelations.org) to test the null hypothesis that (following r to z transformations) two correlation coefficients were not significantly different from each other in the same sample of individuals. Correlations were computed between predictor variables (X1 or X2) and a single common dependent variable (Y). In these analyses predictor variables were either neuropsychological domains or symptom scores whereas an individual PUFA (EPA, DPA, DHA or AA) served as the dependent measure.
Neuropsychological domains that correlated significantly with PUFA at the baseline time point were subsequently investigated in the clinical trial using a linear mixed model in an intention-to-treat analysis. Specifically, we focused on social cognition as the outcome measure with group, age, sex, and group*time interaction included as covariates. The correlation between time points was modeled using compound symmetry. We then compared adjusted least square means for the group x time interaction between groups at baseline and follow-up using two-sample t-tests. We also investigated the potential impact of allowed concomitant medications on neuropsychological functioning.
Results
Baseline demographic, clinical, and PUFA data are provided for the entire sample in Table 1. None of the variables in Table 1 differed significantly between treatment groups at baseline. There were no significant differences in the number of weeks patients were treated with risperidone + FO (mean =15.75, SD=1.08) vs. risperidone + placebo (mean = 15.90, SD=.89). There was no significant interaction between treatment group and dropout at 16 weeks in baseline distributions of age, sex, handedness, age at first psychotic symptoms, total BPRS score or neuropsychological domains.
Table 1.
Baseline and Follow-up Sample Characteristics.
| Demographics | Risperidone + Fish Oil | Risperidone + Placebo | ||
|---|---|---|---|---|
| Age (years) | 22.7 (5.4) | 21.3 (5.3) | ||
| Sex (male, female) | 17/6 | 18/5 | ||
| Education class b | 3.6 (1.0) | 3.8 (1.0) | ||
| Laterality Quotient c | .49 (.77) | .62 (.60) | ||
| Baseline | Follow-up | Baseline | Follow-up | |
| Clinical Data | ||||
| Depression/Anxiety | 7.4 (2.8) | 4.1 (1.6) | 7.3 (3.1) | 5.3 (2.8) |
| Hostility/Uncooperativeness | 5.8 (1.4) | 4.7 (1.0) | 6.1 (1.8) | 5.0 (1.6) |
| Negative Symptoms | 4.9 (2.4) | 4.6 (1.5) | 4.9 (2.4) | 5.8 (2.3) |
| Positive Symptoms | 12.3 (2.1) | 5.8 (2.1) | 12.9 (2.2) | 7.5 (3.4) |
| Total Score | 41.0 (5.9) | 24.4 (5.7) | 42.2 (6.9) | 30.0 (9.1) |
| Omega-3 PUFA d | ||||
| Eicosapentaenoic Acid (EPA) | .39 (.14) | 1.3 (.61) e | .43 (.12) | .46 (.08) |
| Docosapentaenoic Acid (DPA) | 2.2 (.51) | 3.3 (.70) e | 2.4 (.37) | 2.4 (.29) |
| Docosahexaenoic Acid (DHA) | 3.8 (1.3) | 5.3 (1.6) e | 4.0 (1.2) | 4.0 (1.1) |
| Omega-6 | ||||
| Arachidonic Acid (AA) | 16.8 (1.2) | 14.5 (2.4) e | 17.3 (1.1) | 17.0 (.81) |
| Omega-3 PUFA ratio | ||||
| AA/(EPA+DPA+DHA) | 2.7 (.57) | 1.6 (.47) e | 2.6 (.51) | 2.5 (.44) |
Notes:
Values are mean (SD) except sex;
Education coded as follows: 5=less than high school education; 4=high school graduate; 3=some college, no degree, 2=college graduate, and 1=some graduate or professional schooling;
handedness was assessed using a modified version of the Edinburgh Inventory (Oldfield, 1971) and ranged from +1.00 (totally dextral) to - 1.00 (totally nondextral);
Fatty acid data expressed as weight percent of total fatty acids ((mg fatty acid/100 mg fatty acids) or ratio;
Values are significantly (p < .05) different compared to baseline (fish oil + risperidone) and follow-up (risperidone + placebo) timepoints (see text for details).
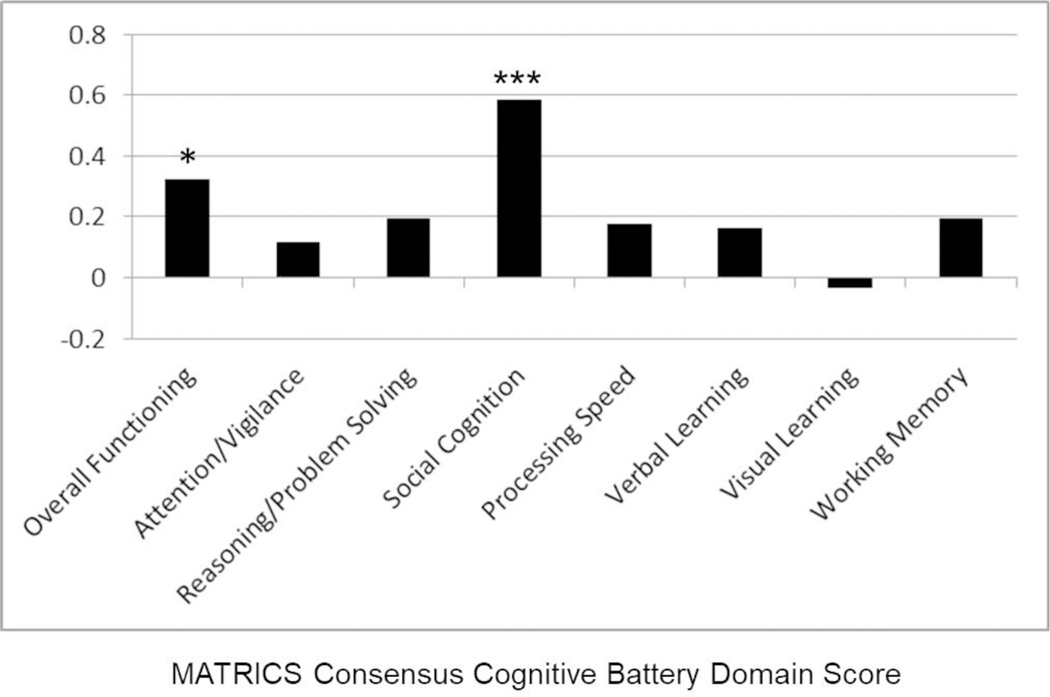
The ratio of AA/(EPA+DHA+DPA) correlated significantly with worse overall neuropsychological functioning (r=−.33, df=38, p=0.045). Post-hoc Pearson correlations revealed a significant negative correlation between this ratio and social cognition (r=−.54, df=39, p<.001) that was statistically stronger compared to the correlation between this ratio and the other 6 domains of NP functioning. The examination of EPA+DHA+DPA versus AA levels separately revealed a significant positive correlation between overall neuropsychological functioning and EPA+DHA+DPA (r = .32, df=39, p=0.04), but not with AA. Post-hoc tests indicated that EPA+DHA+DPA correlated significantly and positively with social cognition (r=.58, df=42, p<0.001; figure 1) and that this correlation was statistically significantly stronger compared to the correlations between EPA+DHA+DPA and the other 6 domains of NP functioning. In addition, the omega-3 index (i.e., EPA+DHA) was significantly correlated with social cognition (r-.63, df=42, p < .001) and this correlation was statistically significantly stronger compared to the correlations between this index and the other 6 domains of NP functioning.
Figure 1.
Correlation Coefficients between MATRICs Cognitive Consensus Battery and DHA + EPA + DPA.
Notes: * p < .05; *** p < .001
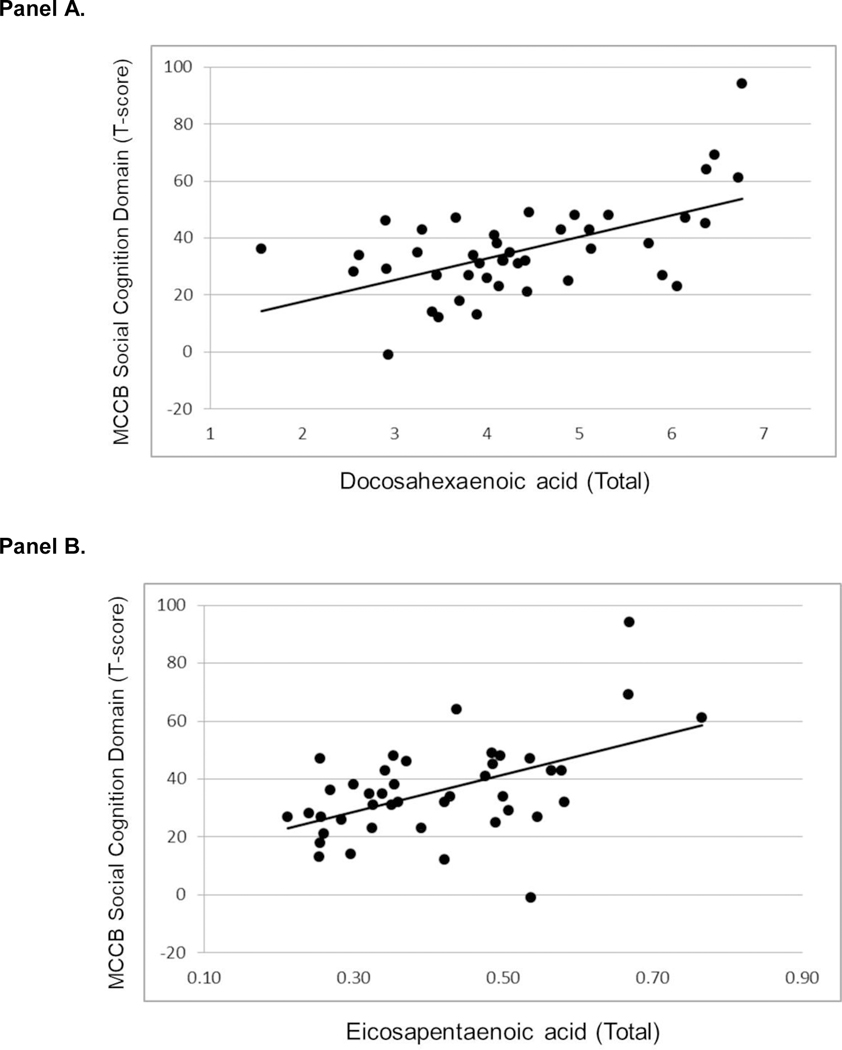
We investigated which individual omega-3 PUFA contributed to the significant correlation between EPA+DHA+DPA and social cognition. There were significant positive correlations between social cognition and DHA (r=0.57, df=43, p<0.001; figure 2A) and EPA (r=0.51, df=42, p<0.001; figure 2B), but not with DPA. The correlations between social cognition and DHA and EPA were statistically significantly stronger compared to the correlation of social cognition with DPA. The correlations between social cognition and DHA (r=0.57, df=32, p<0.001) and EPA (r=0.39, df=32, p=0.025) remained statistically significant when we controlled for functioning on the other 6 neuropsychological domains, total BPRS score, AA level and substance use diagnosis using partial correlation analysis.
Figure 2.
Scatterplots of MATRICS Social Cognition Domain Score and Docosahexaenoic Acid (Panel A) and Eicosapentaenoic Acid (Panel B).
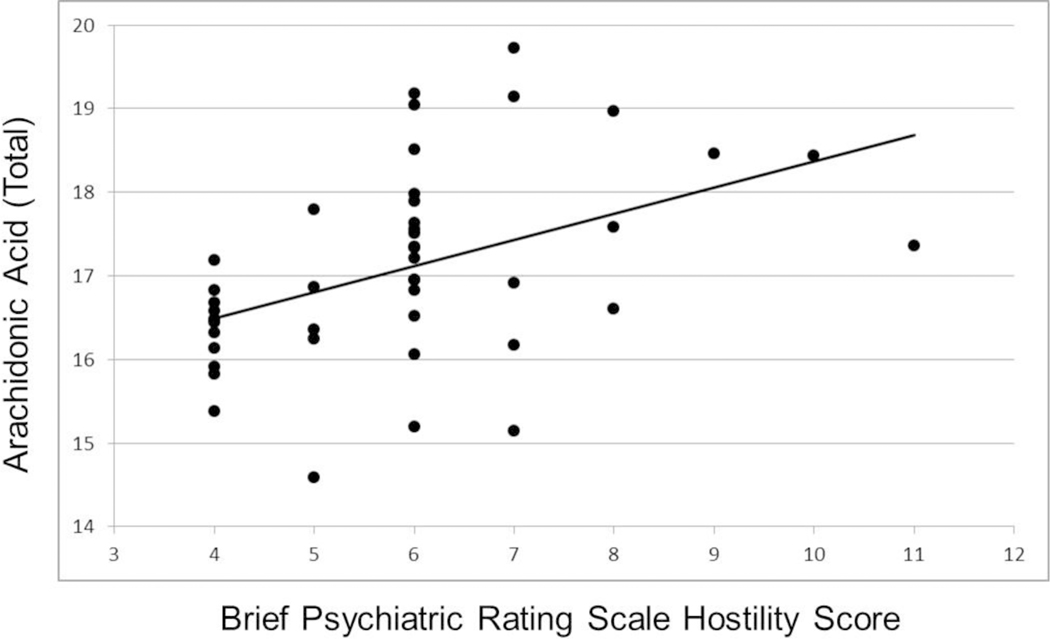
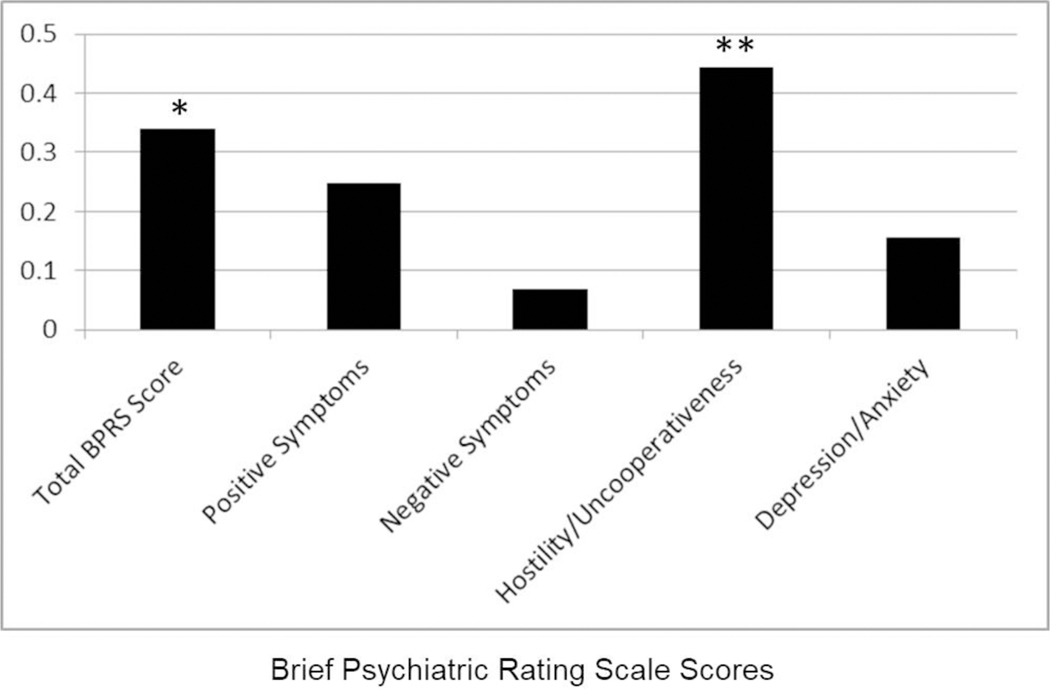
Investigation of clinical measures indicated that total BPRS score did not correlate significantly with either AA/(EPA+DHA+DPA), EPA+DHA+DPA or EPA+DHA, but correlated significantly with AA (r=0.34, df=44, p=0.024). Post-hoc analyses indicated that AA correlated significantly only with hostility/uncooperativeness (r=0.44, df=44, p=0.003; figures 3 and 4), and none of the other factor scores. The correlation between AA and hostility was significantly stronger compared to the correlation of AA with negative symptoms. Furthermore, the correlation between AA and hostility remained statistically significant after controlling for EPA+DHA+DPA, overall neuropsychological functioning and substance use diagnosis using partial correlation analysis (r=.42, df=34, p=.01).
Figure 3.
Scatterplot of Brief Psychiatric Rating Scale Hostility/Uncooperativeness Score and Arachidonic Acid.
Figure 4.
Correlation Coefficients between Brief Psychiatric Rating Scale Scores and Arachidonic Acid.
Notes: * p < .05; ** p < .005
Omega-3 PUFA Changes
There were significant increases in omega-3 PUFA and reductions in AA among patients treated with risperidone + FO (Table 1): DPA (+72.6%; t=−6.99, df=9, p<.001), DHA (+56.2%; t=−4.48, df=9, p=.002), EPA (+281.7%; t = −4.91, df=9, p=.001) and AA (−0.9%; t=2.75; df=9, p=.022;). There were no significant changes (all ps > .05) in omega-3 PUFA in patients treated with risperidone + placebo (Table 1). In addition, at week 16, patients treated with risperidone + FO had significantly higher levels of DHA (t=−2.15, df=18, p=.046), EPA (t = −4.51, df=18, p<.001), DPA (t = −3.64, df=18, p=.002) and lower levels of AA (t = 3.01, df=18, p=.007) compared to patients treated with risperidone + placebo.
Neuropsychological Changes
Examination of within group changes of the adjusted least squares means from the linear mixed model analysis with intention-to-treat revealed a significant increase over time in social cognition among patients treated with risperidone + FO (t=−2.41, df=21.8, p=.025), but no significant change over time in social cognition among patients treated with risperidone + placebo (t=−.50, df=21.3, p=.62). Furthermore, patients treated with risperidone + FO had significantly (t = −2.27, df=58.8, p=.027) higher social cognition scores compared to individuals treated with risperidone + placebo at the 16 week follow-up timepoint. Group differences in social cognition at the follow-up timepoint were associated with a large effect size (Cohen’s d > .90). There was no significant interaction between use of either lorazepam or benztropine with treatment group assignment for week 16 social cognition scores. Exploratory analyses did not reveal any significant group differences at follow-up for the other neuropsychological domains (ps>.05). At the 16-week timepoint there was a significant positive correlation between DHA and social cognition across both cohorts while controlling for functioning on the other 6 NP domains, total BPRS score and AA level (r=.71, df=5, p=.038; one-tailed).
Discussion
This study provides novel evidence for a differential role of omega-3 vs. omega-6 PUFA in recent-onset psychosis that may have implications for better understanding their roles in the pathophysiology of neurocognitive dysfunction in psychosis and its treatment. We found that EPA+DHA+DPA correlated significantly with social cognition and that the magnitude of this correlation was significantly stronger compared to the correlations of EPA+DHA+DPA with other neuropsychological domains. Lower levels of DHA and EPA were associated with worse social cognition even after controlling for functioning on other neuropsychological domains, overall symptom severity, AA level and substance use. In contrast, we found that AA correlated positively with hostility/uncooperativeness even after controlling for overall neuropsychological functioning, EPA+DHA+DPA level and substance use. We further demonstrate significant longitudinal increases in social cognition among patients treated with risperidone + FO and that week 16 social cognition domain scores were significantly higher among patients treated with risperidone + FO compared to those patients treated with risperidone + placebo. At the 16 week follow-up timepoint we identified a significant positive correlation between EPA+DHA+DPA and social cognition performance across all participants, thus replicating findings at baseline.
Few studies have examined the relationship between omega-3 PUFA level and neuropsychological functioning at any stage of psychosis, although some evidence indicates that dietary intake of omega-3 PUFA may have beneficial effects on cognition in patients with affective disorders and schizophrenia (Knochel et al., 2015). Our finding that higher EPA and DHA correlated with better social cognition is broadly consistent with Satogami et al (2017) who reported that EPA and DHA levels were positively correlated with the composite score from the Brief Assessment of Cognition. In 2 cohorts of individuals at risk for psychosis the ratios of both omega-3 and DHA to total fatty acids was associated with cognitive performance (Ramsay et al., 2018). Clinical trials conducted in patients with schizophrenia reported either no effect when 3g ethyl EPA was administered daily on cognition (Fenton et al., 2001) and impairment in sustained attention when ethyl-EPA was administered alone to patients with low baseline PUFA (Bentsen and Landrø, 2018).
Although an initial study by Amminger et al (2010) reported that long-chain omega-3 PUFA reduced the risk of progression to psychotic disorder among individuals at ultra-high risk for psychosis, the subsequent NEURAPRO study failed to show benefits of n-3 PUFAs over placebo (McGorry et al., 2017). In this latter study, however, an increase in omega-3 PUFA was associated with better functioning and less severe psychopathology among these individuals at follow-up timepoints in the clinical trial (Amminger et al., 2020). Other studies reported lower EPA, DHA and AA in individuals at ultra-high risk for psychosis compared to age-matched healthy controls, which was associated with BMI in the patient group only (Alqarni et al., 2019) and that metabolic parameters in combination with a diet low in omega-3 predicted prodromal symptoms and poor role functioning among antipsychotic-free individuals at clinical high risk for psychosis (Cadenhead et al., 2019). Given differences in the consumption of omega-6 and omega-3 PUFA among individuals at ultra-high risk of psychosis and patients with first-episode psychosis compared to healthy controls (Pawełczyk et al., 2017), the examination of modifiable dietary factors and augmentation with omega-3 PUFA in relationship to clinical and neuropsychological functioning may be an important area for subsequent studies.
Our data further converge with animal studies reporting that omega-3 biostatus is associated with neuropsychological deficits targeting social interactions. Animal studies indicate that an omega-3 deficient diet across consecutive generations produce impairments on cognitive tasks (Bondi et al., 2014) that are improved following omega-3 PUFA administration (Chung et al., 2008; Fedorova et al., 2007), and omega-3 PUFA mitigate against the detrimental effects of chronic stress on cognitive functions (Trofimiuk and Braszko, 2013). Our data on social cognition are also consistent with animal studies indicating that dietary changes in PUFA ratios predicted developmental delays in a mouse model of autism spectrum disorder (van Elst et al., 2019) and that an EPA enriched diet was associated with less anxiety-like behavior in socially isolated rats (Oshima et al., 2018). Furthermore, maternal DHA positively affected social behavior in offspring following weaning (Clouard et al., 2015) and prenatal exposure to lipopolysaccharides significantly decreased social interaction in the offspring of rats, which was reversed following omega-3 PUFA supplementation (Fortunato et al., 2017).
There may be several possible neural mechanisms through which omega-3 PUFA may exert beneficial effects on social cognition. It is known that omega-3 PUFA are abundant throughout the brain (Bentsen, 2017) with DHA being the most prominent and accounting for approximately 50% of neuronal membranes (Singh, 2005). Cell membranes of oligodendrocytes contain myelin and the phospholipid bilayers of this myelin sheath contain omega-3 PUFA that may play a role in their regulation (Chen et al., 2014; McNamara et al., 2017). It is conceivable that omega-3 PUFA could play a role in regulating brain functions associated with myelin integrity by enhancing brain plasticity and synaptic connections as well as facilitating anti-inflammatory effects. Prior studies (Peters et al., 2009; Peters et al., 2013), which have been replicated in an independent sample (Lyall et al., Submitted) are consistent with the hypothesis that omega-3 PUFA biostatus predicts putative white matter microstructural integrity as inferred from diffusion tensor imaging. Moreover, a controlled clinical trial found that omega-3 PUFA supplementation significantly improved executive functioning and regional white matter microstructural integrity and gray matter volume compared to placebo in healthy older adults (Witte et al., 2014). It should be acknowledged that despite the importance of DPA in brain regulation (Drouin et al., 2019) and its unique and shared overlap with DHA and EPA (Dyall, 2015), we did not find evidence to support an association between DPA and cognition in recent-onset psychosis. Despite growing evidence for a physiological role of DPA (Drouin et al., 2019), little is currently known, however, regarding the individual role of DPA in brain and cognitive functioning and thus, further studies are needed.
Alterations in AA associated with phospholipid metabolism have been hypothesized to play a key role in the neurobiology of schizophrenia (Skosnik and Yao, 2003; Yao and Reddy, 2002), but few studies have investigated their functional correlates. The finding that higher AA was associated with greater hostility/uncooperativeness in our cohort converges strongly with a prior study reporting that AA levels correlated positively with the hostility score from the Positive and Negative Syndrome Scale in 75 drug-free inpatients with schizophrenia (Watari et al., 2010). Another study provides partial support for the current findings in reporting that prior to interferon-alpha (IFN-α) therapy the ratio of AA/EPA+DHA correlated with subsequent increases in anger during treatment (Lotrich et al., 2013). Our data are consistent with the hypothesis that increased metabolism of AA early in the course of psychotic illness may contribute to hostility/uncooperativeness. The lack of association at follow-up suggests that AA levels may be affected by risperidone and/or omega-3 PUFA treatment.
The direction of our correlations suggests that lower omega-3 PUFA and higher AA in patients may be associated with less favorable neuropsychological and clinical outcomes, respectively. In this regard it should be acknowledged that prior studies investigating group differences in these PUFA have yielded inconsistent findings that further complicate interpretation. In a meta-analysis (Hoen et al., 2013) less AA was most robust in antipsychotic-naive patients compared to healthy controls. However, in one of the largest studies to date (Medema et al., 2016), which was not included in this meta-analysis, higher DHA, DPA and AA were observed in 215 patients with psychosis and 187 siblings compared to 98 controls. The direction of our correlations is consistent with studies demonstrating that erythrocyte EPA+DHA is lower and that either AA or AA/EPA+DHA was higher in patients with schizophrenia compared to healthy controls (Arvindakshan et al., 2003; McNamara, 2013).
It is important to acknowledge that the relationship between omega-3 and omega-6 PUFA is complex and both synergistic and antagonistic effects have been identified (Lin et al., 2015; Luxwolda et al., 2011). For example, omega-6 PUFA deprivation was found to increase DHA loss within brain phospholipids and synergism between omega-3 PUFA and AA was evident at low omega-3 PUFA levels whereas AA could be suppressed at high (i.e., >8 g%) omega-3 levels (Luxwolda et al., 2011). We addressed this issue by examining a ratio incorporating these measures (i.e., AA/(EPA+DPA+DHA)) as well as examining absolute PUFA values. Although we found that lower AA/(EPA+DHA+DPA) correlated significantly with overall neuropsychological functioning, this relationship was driven primarily by EPA+DHA+DPA suggesting that omega-3 PUFA may be important in the identification of functional correlates in patients with recent-onset psychosis.
There are several study limitations that should be acknowledged. The sample was relatively small, especially in the follow-up groups and heterogeneous in terms of diagnosis, although all patients had a psychotic disorder. The social cognition domain from the MCCB was limited by a single measure, the Mayer-Salovey-Caruso Emotional Intelligence Test, although better performance on this task was associated with better psychosocial functioning on the Quality of Life Scale in a large cohort of patients in the schizophrenia spectrum (DeTore et al., 2018). Other measures of social cognition, including those assessing real world situations, are warranted in future studies (Pinkham et al., 2016). Because this study did not include a healthy comparison group we could not determine whether PUFA levels were abnormal at baseline in patients. We also acknowledge that a possible reason for the increase in DPA observed in this study is that EPA is a precursor of DPA and is converted via the enzyme elongase-5 (Drouin et al., 2019). Study strengths, however, include the randomized double-blind placebo-controlled design, well-characterized cohort of recent-onset psychosis patients, demographically and clinically similar treatment groups, and use of erythrocytes, which provide a better index of biostatus compared to plasma measures that can be highly variable and more prone to exogenous influences such as diet (Harris and Thomas, 2010).
In conclusion, we report that omega-3 and omega-6 PUFA are associated with social cognition and hostility/uncooperativeness, respectively, in patients with recent-onset psychosis. We further present preliminary evidence for improved social cognition in these patients following FO treatment compared with placebo. Taken together, omega-3 PUFA supplementation may represent a safe adjunctive treatment for social cognition deficits in psychosis and encourage additional investigation in a larger cohort.
Acknowledgments
This work was supported by grants R21MH101746 (Dr. Robinson and Dr. Szeszko) and R01DK097599 (Dr. McNamara) from the National Institutes of Health and the Empire Clinical Research Investigator Program award Targeting Omega-3 Treatment in First-episode Psychosis from the New York State Department of Health to Dr. Malhotra.
Footnotes
Conflicts of Interest
Dr. Robinson has been a consultant to Costello Medical Consulting, Innovative Science Solutions, Janssen, Lundbeck, Otsuka and US World Meds and has received research support from Otsuka. Dr. McNamara has received research support from Royal DSM Nutritional Products, LLC, and Kyowa Hakko Bio Co., LTD, and served as a consultant for VAYA Pharma Inc., and Vifor Pharma Inc. Dr. Malhotra has been a consultant to Genomind, Concert Pharma and Biogen. Dr. Gallego has served as a consultant to Alkermes. Dr. Peters, Dr. Govindarajulu and Dr. Szeszko have no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adjepong M, Yakah W, Harris WS, Annan RA, Pontifex MB, Fenton JI, 2018. Whole blood n-3 fatty acids are associated with executive function in 2–6-year-old Northern Ghanaian children. The Journal of nutritional biochemistry 57, 287–293. [DOI] [PubMed] [Google Scholar]
- Alqarni A, Mitchell TW, McGorry PD, Nelson B, Markulev C, Yuen HP, Schäfer MR, Berger M, Mossaheb N, Schlögelhofer M, 2019. Comparison of erythrocyte omega-3 index, fatty acids and molecular phospholipid species in people at ultra-high risk of developing psychosis and healthy people. Schizophrenia Research. [DOI] [PubMed] [Google Scholar]
- Amminger GP, Nelson B, Markulev C, Yuen HP, Schäfer MR, Berger M, Mossaheb N, Schlögelhofer M, Smesny S, Hickie IB, 2020. The NEURAPRO biomarker analysis: long-chain Omega-3 fatty acids improve 6-month and 12-month outcomes in youths at ultra-high risk for psychosis. Biological psychiatry 87(3), 243–252. [DOI] [PubMed] [Google Scholar]
- Amminger GP, Schäfer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, Mackinnon A, McGorry PD, Berger GE, 2010. Long-chain ω−3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Archives of general psychiatry 67(2), 146–154. [DOI] [PubMed] [Google Scholar]
- Arvindakshan M, Sitasawad S, Debsikdar V, Ghate M, Evans D, Horrobin DF, Bennett C, Ranjekar PK, Mahadik SP, 2003. Essential polyunsaturated fatty acid and lipid peroxide levels in never-medicated and medicated schizophrenia patients. Biological psychiatry 53(1), 56–64. [DOI] [PubMed] [Google Scholar]
- Bentsen H, 2017. Dietary polyunsaturated fatty acids, brain function and mental health. Microbial ecology in health and disease 28(sup1), 1281916. [Google Scholar]
- Bentsen H, Landrø N, 2018. Neurocognitive effects of an omega-3 fatty acid and vitamins E+ C in schizophrenia: a randomised controlled trial. Prostaglandins, Leukotrienes and Essential Fatty Acids 136, 57–66. [DOI] [PubMed] [Google Scholar]
- Berger M, Nelson B, Markulev C, Yuen H-P, Schaefer M, Mossaheb N, Schlögelhofer M, Smesny S, Hickie I, Berger GE, Chen EYH, de Haan L, Nieman DH, Nordentoft M, Riecher-Rössler A, Verma S, Mitchell TW, Meyer BJ, Thompson A, Yung AR, McGorry PD, Amminger GP, 2019. Relationship between polyunsaturated fatty acids and psychopathology in the NEURAPRO clinical trial. Frontiers in psychiatry 10, 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Taha AY, Tock JL, Totah NK, Cheon Y, Torres GE, Rapoport SI, Moghaddam B, 2014. Adolescent behavior and dopamine availability are uniquely sensitive to dietary omega-3 fatty acid deficiency. Biological psychiatry 75(1), 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenhead KS, Minichino A, Kelsven S, Addington J, Bearden C, Cannon TD, Cornblatt BA, Mathalon D, McGlashan TH, Perkins DO, 2019. Metabolic abnormalities and low dietary Omega 3 are associated with symptom severity and worse functioning prior to the onset of psychosis: Findings from the North American Prodrome Longitudinal Studies Consortium. Schizophrenia research 204, 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Zhang H, Pu H, Wang G, Li W, Leak RK, Chen J, Liou AK, Hu X, 2014. n-3 PUFA supplementation benefits microglial responses to myelin pathology. Scientific reports 4, 7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YS, Kang D-H, Shin NY, Yoo SY, Kwon JS, 2008. Deficit of theory of mind in individuals at ultra-high-risk for schizophrenia. Schizophrenia research 99(1–3), 111–118. [DOI] [PubMed] [Google Scholar]
- Clouard C, Souza AS, Gerrits WJ, Hovenier R, Lammers A, Bolhuis JE, 2015. Maternal fish oil supplementation affects the social behavior, brain fatty acid profile, and sickness response of piglets. The Journal of nutrition 145(9), 2176–2184. [DOI] [PubMed] [Google Scholar]
- Condray R, Yao JK, 2011. Cognition, dopamine and bioactive lipids in schizophrenia. Frontiers in bioscience (Scholar edition) 3, 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeTore NR, Mueser KT, McGurk SR, 2018. What does the Managing Emotions branch of the MSCEIT add to the MATRICS consensus cognitive battery? Schizophrenia Research 197, 414–420. [DOI] [PubMed] [Google Scholar]
- Diedenhofen B, Musch J, 2015. cocor: A comprehensive solution for the statistical comparison of correlations. PloS one 10(4), e0121945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin G, Rioux V, Legrand P, 2019. The n-3 docosapentaenoic acid (DPA): A new player in the n-3 long chain polyunsaturated fatty acid family. Biochimie 159, 36–48. [DOI] [PubMed] [Google Scholar]
- Dyall SC, 2015. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Frontiers in aging neuroscience 7, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley R, Myburgh C, Oosthuizen P, van Rensburg SJ, 2002. Randomized, placebo-controlled study of ethyl-eicosapentaenoic acid as supplemental treatment in schizophrenia. American Journal of Psychiatry 159(9), 1596–1598. [DOI] [PubMed] [Google Scholar]
- Emsley R, Niehaus DJ, Koen L, Oosthuizen PP, Turner HJ, Carey P, van Rensburg SJ, Maritz JS, Murck H, 2006. The effects of eicosapentaenoic acid in tardive dyskinesia: a randomized, placebo-controlled trial. Schizophrenia research 84(1), 112–120. [DOI] [PubMed] [Google Scholar]
- Fedorova I, Hussein N, Di Martino C, Moriguchi T, Hoshiba J, Majchrzak S, Salem N Jr, 2007. An n-3 fatty acid deficient diet affects mouse spatial learning in the Barnes circular maze. Prostaglandins, leukotrienes and essential fatty acids 77(5–6), 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton WS, Dickerson F, Boronow J, Hibbeln JR, Knable M, 2001. A placebo-controlled trial of omega-3 fatty acid (ethyl eicosapentaenoic acid) supplementation for residual symptoms and cognitive impairment in schizophrenia. American Journal of Psychiatry 158(12), 2071–2074. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB, 1994. Structured clinical interview for Axis I DSM-IV disorders. New York: Biometrics Research. [Google Scholar]
- Fortunato JJ, da Rosa N, Laurentino AOM, Goulart M, Michalak C, Borges LP, Soares E.d.C.C., Reis PA, Neto H.C.d.C.F., Petronilho F, 2017. Effects of ω−3 fatty acids on stereotypical behavior and social interactions in Wistar rats prenatally exposed to lipopolysaccarides. Nutrition 35, 119–127. [DOI] [PubMed] [Google Scholar]
- Harris WS, Thomas RM, 2010. Biological variability of blood omega-3 biomarkers. Clinical biochemistry 43(3), 338–340. [DOI] [PubMed] [Google Scholar]
- Hoen WP, Lijmer JG, Duran M, Wanders RJ, van Beveren NJ, de Haan L, 2013. Red blood cell polyunsaturated fatty acids measured in red blood cells and schizophrenia: a meta-analysis. Psychiatry research 207(1–2), 1–12. [DOI] [PubMed] [Google Scholar]
- Jamilian H, Solhi H, Jamilian M, 2014. Randomized, placebo-controlled clinical trial of omega-3 as supplemental treatment in schizophrenia. Global journal of health science 6(7), 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen CI, Kiliaan AJ, 2014. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: the influence of LCPUFA on neural development, aging, and neurodegeneration. Progress in lipid research 53, 1–17. [DOI] [PubMed] [Google Scholar]
- Kern RS, Nuechterlein KH, Green MF, Baade LE, Fenton WS, Gold JM, Keefe RS, Mesholam-Gately R, Mintz J, Seidman LJ, 2008. The MATRICS Consensus Cognitive Battery, part 2: co-norming and standardization. American Journal of Psychiatry 165(2), 214–220. [DOI] [PubMed] [Google Scholar]
- Kim S-W, Jhon M, Kim J-M, Smesny S, Rice S, Berk M, Klier CM, McGorry PD, Schäfer MR, Amminger GP, 2016. Relationship between erythrocyte fatty acid composition and psychopathology in the vienna omega-3 study. PLoS One 11(3), e0151417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-W, Schäfer MR, Klier CM, Berk M, Rice S, Allott K, Bartholomeusz CF, Whittle SL, Pilioussis E, Pantelis C, 2014. Relationship between membrane fatty acids and cognitive symptoms and information processing in individuals at ultra-high risk for psychosis. Schizophrenia research 158(1–3), 39–44. [DOI] [PubMed] [Google Scholar]
- Knochel C, Voss M, Gruter F, S Alves G, Matura S, Sepanski B, Stablein M, Wenzler S, Prvulovic D, F Carvalho A, 2015. Omega 3 fatty acids: novel neurotherapeutic targets for cognitive dysfunction in mood disorders and schizophrenia? Current neuropharmacology 13(5), 663–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Leung Y, Zhou I, Ho L, Kong W, Basil P, Wei R, Lam S, Zhang X, Law A, 2015. Dietary supplementation with n-3 fatty acids from weaning limits brain biochemistry and behavioural changes elicited by prenatal exposure to maternal inflammation in the mouse model. Transl Psychiatry 5(9), e641–e641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LE, Chen CT, Hildebrand KD, Liu Z, Hopperton KE, Bazinet RP, 2015. Chronic dietary n-6 PUFA deprivation leads to conservation of arachidonic acid and more rapid loss of DHA in rat brain phospholipids. Journal of lipid research 56(2), 390–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotrich FE, Sears B, McNamara RK, 2013. Anger induced by interferon-alpha is moderated by ratio of arachidonic acid to omega-3 fatty acids. Journal of psychosomatic research 75(5), 475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxwolda MF, Kuipers RS, Smit EN, Velzing-Aarts FV, Dijck-Brouwer DJ, Muskiet FA, 2011. The relation between the omega-3 index and arachidonic acid is bell shaped: synergistic at low EPA+ DHA status and antagonistic at high EPA+ DHA status. Prostaglandins, leukotrienes and essential fatty acids 85(3–4), 171–178. [DOI] [PubMed] [Google Scholar]
- Lyall AE, Nägele FL, Pasternak O, Gallego JA, Malhotra AK, McNamara RK, Kubicki M, Peters BD, Robinson DG, Szeszko PR, Submitted. Effects of Omega-3 Polyunsaturated Fatty Acid Treatment on White Matter Microstructure in Individuals with Recent-onset Psychosis Concurrently Treated with Risperidone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa M, Watanabe A, Iwayama Y, Kimura T, Hamazaki K, Balan S, Ohba H, Hisano Y, Nozaki Y, Ohnishi T, 2017. Polyunsaturated fatty acid deficiency during neurodevelopment in mice models the prodromal state of schizophrenia through epigenetic changes in nuclear receptor genes. Transl Psychiatry 7(9), e1229–e1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker MM, Grant-Kels JM, 2010. Healing fats of the skin: the structural and immunologic roles of the ω−6 and ω−3 fatty acids. Clinics in Dermatology 28(4), 440–451. [DOI] [PubMed] [Google Scholar]
- McGorry PD, Nelson B, Markulev C, Yuen HP, Schäfer MR, Mossaheb N, Schlögelhofer M, Smesny S, Hickie IB, Berger GE, 2017. Effect of ω−3 polyunsaturated fatty acids in young people at ultrahigh risk for psychotic disorders: the NEURAPRO randomized clinical trial. JAMA psychiatry 74(1), 19–27. [DOI] [PubMed] [Google Scholar]
- McNamara RK, 2013. Deciphering the role of docosahexaenoic acid in brain maturation and pathology with magnetic resonance imaging. Prostaglandins, Leukotrienes and Essential Fatty Acids 88(1), 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Asch RH, Schurdak JD, Lindquist DM, 2017. Glutamate homeostasis in the adult rat prefrontal cortex is altered by cortical docosahexaenoic acid accrual during adolescence: An in vivo 1H MRS study. Psychiatry Research: Neuroimaging 270, 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Rider T, Tso P, Dwivedi Y, Pandey GN, 2010. Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. Journal of affective disorders 126(1–2), 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema S, Mocking RJ, Koeter MW, Vaz FM, Meijer C, de Haan L, van Beveren NJ, GROUPa, Risk, a., investigators:, O.o.P., Kahn R, de Haan L, 2016. Levels of red blood cell fatty acids in patients with psychosis, their unaffected siblings, and healthy controls. Schizophrenia bulletin 42(2), 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X-L, Rosenthal R, Rubin DB, 1992. Comparing correlated correlation coefficients. Psychological bulletin 111(1), 172. [Google Scholar]
- Metcalfe L, Schmitz A, Pelka J, 1966. Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Analytical chemistry 38(3), 514–515. [Google Scholar]
- Montgomery P, Burton JR, Sewell RP, Spreckelsen TF, Richardson AJ, 2013. Low blood long chain omega-3 fatty acids in UK children are associated with poor cognitive performance and behavior: a cross-sectional analysis from the DOLAB study. PloS one 8(6), e66697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J, Raskin S, 2019. The eicosapentaenoic acid: arachidonic acid ratio and its clinical utility in cardiovascular disease. Postgraduate medicine 131(4), 268–277. [DOI] [PubMed] [Google Scholar]
- Nuechterlein K, Green M, 2006. MCCB–MATRICS Consensus Cognitive Battery–Manual. Matrics Assessment Inc. MATRICS assessment battery with permission of Barbara Cornblatt, Biobehavioral Technologies, Inc. [Google Scholar]
- Oldfield RC, 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Oshima Y, Watanabe T, Endo S, Hata S, Watanabe T, Osada K, Takenaka A, 2018. Effects of eicosapentaenoic acid and docosahexaenoic acid on anxiety-like behavior in socially isolated rats. Bioscience, biotechnology, and biochemistry 82(4), 716–723. [DOI] [PubMed] [Google Scholar]
- Pawełczyk T, Grancow-Grabka M, Kotlicka-Antczak M, Trafalska E, Pawełczyk A, 2016. A randomized controlled study of the efficacy of six-month supplementation with concentrated fish oil rich in omega-3 polyunsaturated fatty acids in first episode schizophrenia. Journal of Psychiatric Research 73, 34–44. [DOI] [PubMed] [Google Scholar]
- Pawełczyk T, Trafalska E, Pawełczyk A, Kotlicka-Antczak M, 2017. Differences in omega-3 and omega-6 polyunsaturated fatty acid consumption in people at ultra-high risk of psychosis, first-episode schizophrenia, and in healthy controls. Early intervention in psychiatry 11(6), 498–508. [DOI] [PubMed] [Google Scholar]
- Peet M, Brind J, Ramchand C, Shah S, Vankar G, 2001. Two double-blind placebo-controlled pilot studies of eicosapentaenoic acid in the treatment of schizophrenia. Schizophrenia research 49(3), 243–251. [DOI] [PubMed] [Google Scholar]
- Peet M, Horrobin D, 2002. Study Group E-EM. A dose-ranging exploratory study of the effects of ethyl-eicosapentaenoate in patients with persistent schizophrenic symptoms. J Psychiatr Res 36(1), 7–18. [DOI] [PubMed] [Google Scholar]
- Peters B, Duran M, Vlieger E, Majoie C, Den Heeten G, Linszen D, de Haan L, 2009. Polyunsaturated fatty acids and brain white matter anisotropy in recent-onset schizophrenia: a preliminary study. Prostaglandins, leukotrienes and essential fatty acids 81(1), 61–63. [DOI] [PubMed] [Google Scholar]
- Peters BD, Machielsen MW, Hoen WP, Caan MW, Malhotra AK, Szeszko PR, Duran M, Olabarriaga SD, de Haan L, 2013. Polyunsaturated fatty acid concentration predicts myelin integrity in early-phase psychosis. Schizophrenia bulletin 39(4), 830–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkham AE, Penn DL, Green MF, Harvey PD, 2016. Social cognition psychometric evaluation: Results of the initial psychometric study. Schizophrenia bulletin 42(2), 494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay H, Barnett JH, Murray GK, Miettunen J, Mäki P, Järvelin M-R, Smith GD, Ala-Korpela M, Veijola J, 2018. Cognition, psychosis risk and metabolic measures in two adolescent birth cohorts. Psychological medicine 48(15), 2609–2623. [DOI] [PubMed] [Google Scholar]
- Rapoport SI, 2013. Translational studies on regulation of brain docosahexaenoic acid (DHA) metabolism in vivo. Prostaglandins, Leukotrienes and Essential Fatty Acids 88(1), 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds G, Portillo C, Serper MR, 2018. Predictors of residency status in chronically institutionalized and community dwelling schizophrenia patients. Comprehensive psychiatry 86, 102–106. [DOI] [PubMed] [Google Scholar]
- Rhindress K, Robinson D, Gallego J, Wellington R, Malhotra A, Szeszko P, 2017. Hippocampal subregion volume changes associated with antipsychotic treatment in first-episode psychosis. Psychological Medicine 47(10), 1706. [DOI] [PubMed] [Google Scholar]
- Robinson DG, Gallego JA, John M, Hanna LA, Zhang J-P, Birnbaum ML, Greenberg J, Naraine M, Peters BD, McNamara RK, 2019. A potential role for adjunctive omega-3 polyunsaturated fatty acids for depression and anxiety symptoms in recent onset psychosis: results from a 16 week randomized placebo-controlled trial for participants concurrently treated with risperidone. Schizophrenia research 204, 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satogami K, Takahashi S, Yamada S, Ukai S, Shinosaki K, 2017. Omega-3 fatty acids related to cognitive impairment in patients with schizophrenia. Schizophrenia research: cognition 9, 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethom M, Fares S, Bouaziz N, Melki W, Jemaa R, Feki M, Hechmi Z, Kaabachi N, 2010. Polyunsaturated fatty acids deficits are associated with psychotic state and negative symptoms in patients with schizophrenia. Prostaglandins, leukotrienes and essential fatty acids 83(3), 131–136. [DOI] [PubMed] [Google Scholar]
- Singh M, 2005. Essential fatty acids, DHA and human brain. The Indian Journal of Pediatrics 72(3), 239–242. [PubMed] [Google Scholar]
- Skosnik P, Yao J, 2003. From membrane phospholipid defects to altered neurotransmission: is arachidonic acid a nexus in the pathophysiology of schizophrenia? Prostaglandins, leukotrienes and essential fatty acids 69(6), 367–384. [DOI] [PubMed] [Google Scholar]
- Stonehouse W, Conlon CA, Podd J, Hill SR, Minihane AM, Haskell C, Kennedy D, 2013. DHA supplementation improved both memory and reaction time in healthy young adults: a randomized controlled trial. The American of Clinical Nutrition 97(5), 1134–1143. [DOI] [PubMed] [Google Scholar]
- Trofimiuk E, Braszko JJ, 2013. Concomitant docosahexaenoic acid administration ameliorates stress-induced cognitive impairment in rats. Physiology & behavior 118, 171–177. [DOI] [PubMed] [Google Scholar]
- van der Kemp W, Klomp D, Kahn R, Luijten P, Pol HH, 2012. A meta-analysis of the polyunsaturated fatty acid composition of erythrocyte membranes in schizophrenia. Schizophrenia research 141(2–3), 153–161. [DOI] [PubMed] [Google Scholar]
- van Elst K, Brouwers JF, Merkens JE, Broekhoven MH, Birtoli B, Helms JB, Kas MJ, 2019. Chronic dietary changes in n-6/n-3 polyunsaturated fatty acid ratios cause developmental delay and reduce social interest in mice. European Neuropsychopharmacology 29(1), 16–31. [DOI] [PubMed] [Google Scholar]
- Ventura J, Nuechterlein KH, Subotnik KL, Gutkind D, Gilbert EA, 2000. Symptom dimensions in recent-onset schizophrenia and mania: a principal components analysis of the 24-item Brief Psychiatric Rating Scale. Psychiatry research 97(2–3), 129–135. [DOI] [PubMed] [Google Scholar]
- Watari M, Hamazaki K, Hirata T, Hamazaki T, Okubo Y, 2010. Hostility of drug-free patients with schizophrenia and n− 3 polyunsaturated fatty acid levels in red blood cells. Psychiatry Research 177(1–2), 22–26. [DOI] [PubMed] [Google Scholar]
- Witte AV, Kerti L, Hermannstädter HM, Fiebach JB, Schreiber SJ, Schuchardt JP, Hahn A, Flöel A, 2014. Long-chain omega-3 fatty acids improve brain function and structure in older adults. Cerebral cortex 24(11), 3059–3068. [DOI] [PubMed] [Google Scholar]
- Woerner M, Mannuzza S, Kane J, 1988. Anchoring the BPRS: an aid to improved reliability. Psychopharmacology bulletin 24(1), 112. [PubMed] [Google Scholar]
- Yao JK, Reddy RK, 2002. Membrane pathology in schizophrenia: implication for arachidonic acid signaling. TheScientificWorldJOURNAL 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Zhen J, Ma W, Cai C, Huang X, Xiao R, 2016. The erythrocyte fatty acid profile and cognitive function in old Chinese adults. Nutrients 8(7), 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurko-Mauro K, Alexander DD, Van Elswyk ME, 2015. Docosahexaenoic acid and adult memory: a systematic review and meta-analysis. PloS one 10(3), e0120391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamroziewicz MK, Paul EJ, Zwilling CE, Barbey AK, 2017. Predictors of memory in healthy aging: polyunsaturated fatty acid balance and fornix white matter integrity. Aging and disease 8(4), 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer L, Vancassel S, Cantagrel S, Breton P, Delamanche S, Guilloteau D, Durand G, Chalon S, 2002. The dopamine mesocorticolimbic pathway is affected by deficiency in n− 3 polyunsaturated fatty acids. The American journal of clinical nutrition 75(4), 662–667. [DOI] [PubMed] [Google Scholar]