Abstract
Objective
Compare 30‐day mortality among patients receiving the specific reversal agent andexanet alfa versus replacement prothrombin complex concentrate (PCC) in the management of direct‐acting oral anticoagulant (DOAC)–related bleeds.
Methods
Two patient‐level datasets were used: ANNEXA‐4, a prospective, single‐arm trial of patients taking apixaban or rivaroxaban who received andexanet alfa and ORANGE, a prospective, observational study of anticoagulated patients in UK hospitals, some of whom received PCC. Patients were propensity score matched based on demographic and clinical characteristics. Subgroup analyses were performed by bleed type (intracranial hemorrhage [ICH], gastrointestinal [GI], other). Relative risk (RR) of all‐cause 30‐day mortality was calculated.
Results
322 ANNEXA‐4 patients treated with andexanet alfa (mean age = 77.7 years; 64.9% ICH) were matched with 88 ORANGE patients treated with PCC (mean age = 74.9 years, 67.1% ICH). Adjusted 30‐day mortality for patients treated with andexanet alfa (14.6%) was lower than patients treated with PCC (34.1%; RR, 0.43; 95% CI, 0.29–0.63). In the ICH subgroup, patients treated with andexanet alfa had lower mortality (15.3%) than patients treated with PCC (48.9%; RR, 0.31; 95% CI, 0.20–0.48). Mortality risk was lowest for patients in the GI subgroup but did not differ significantly by treatment (12.2% for andexanet alfa vs 25.0% for PCC; RR, 0.49; 95% CI, 0.21–1.16).
Conclusions
In this propensity score–matched comparison across 2 independent datasets, adjusted 30‐day mortality rates were lower for patients treated with andexanet alfa than in matched patients receiving PCC. This indirect comparison was limited in that it could not account for several highly predictive variables including GCS score, hematoma volume, and expected survival. Further research is warranted to confirm the mortality differences between reversal/replacement agents for DOAC‐related bleeding.
1. INTRODUCTION
1.1. Background
Direct oral anticoagulants (DOACs), including the factor Xa (FXa) inhibitors rivaroxaban and apixaban, are used in stroke prevention and the treatment of venous thromboembolism. All anticoagulants, including DOACs, carry a risk of serious and life‐threatening major bleeding events. 1 , 2 , 3 Major bleeding events related to DOAC treatment are associated with high mortality 4 , 5 , 6 in addition to significant clinical burden and high morbidity. 7 , 8 , 9
Treatment strategies for DOAC‐related bleeds have changed substantially over the past decade. Despite limited evidence and no regulatory approval, 3‐ and 4‐factor prothrombin complex concentrates (PCCs) have often been used off‐label in an effort to reverse the anticoagulant effect of FXa inhibitors in patients with major bleeding events. 10 , 11 In recent years andexanet alfa, a recombinant modified human FXa protein was approved by the US Food and Drug Administration and the European Medicines Agency as a reversal agent for patients treated with the FXa inhibitors apixaban or rivaroxaban when reversal of anticoagulation is needed due to life‐threatening or uncontrollable major bleeding. 12 , 13 , 14 Andexanet alfa sequesters FXa inhibitors away from endogenous FXa by binding reversibly to FXa inhibitors. This results in a reduction in anti‐FXa activity and a restoration of FXa‐dependent thrombin generation. 15 In the single‐arm study Andexanet Alfa, a Novel Antidote to the Anticoagulation Effects of Factor Xa Inhibitors (ANNEXA‐4), treatment with andexanet alfa was associated with a marked reduction in anti‐FXa activity and a hemostatic efficacy rate of 82%. 12 Guidelines now recommend the use of the specific reversal agent andexanet alfa for FXa inhibitor–associated bleeds. 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23
1.2. Importance
Few studies have reported mortality outcomes after DOAC reversal by andexanet alfa 24 , 25 , 26 and only 1 small real‐world study has compared outcomes of treatment with andexanet alfa and other agents, such as PCCs. 26 This lack of available mortality data impedes evidence‐based decision making. Retrospective comparisons, despite their limitations, can provide important insights to help address this knowledge gap.
1.3. Goals of this investigation
The goal of this analysis was to evaluate 30‐day mortality outcomes associated with the management of FXa inhibitor–related major bleeds by comparing data from patients treated with andexanet alfa and PCCs.
2. METHODS
2.1. Study design and setting
This investigation was a retrospective analysis that used propensity score matching (PSM) to compare data from 2 prospective studies enrolling patients with FXa inhibitor–related bleeding: the ANNEXA‐4 trial, 12 a single‐arm study that enrolled patients treated with andexanet alfa and the Oral Anticoagulant Agent‐associated Bleeding Events Reporting System (ORANGE) observational study, 27 which enrolled patients receiving a range of treatments, including PCCs. Individual data were extracted from the ANNEXA‐4 study for the andexanet alfa–treated group and from the ORANGE study for the PCC‐treated group. Because this was a retrospective analysis of de‐identified data, no ethics approval was needed; as previously published, ANNEXA‐4 12 and ORANGE 27 both received appropriate ethics approvals.
ANNEXA‐4 (ClinicalTrials.gov Identifier: NCT02329327) was a multicenter, prospective, open‐label, single‐arm clinical study of patients who were treated with andexanet alfa on experiencing acute major bleeding associated with enoxaparin or the direct FXa inhibitors apixaban, rivaroxaban, or edoxaban. 12 ANNEXA‐4 included 352 patients who were recruited from 63 sites across North America and Europe between April 2015 and May 2018. Patients were enrolled in ANNEXA‐4 if they were ≥18 years of age and if they presented with acute major bleeding within 18 hours of taking apixaban, rivaroxaban, edoxaban, or enoxaparin (at a dose of ≥1 mg/kg of body weight). 12 Patients were excluded from ANNEXA‐4 for any of the following reasons: planned surgery within 12 hours; Glasgow Coma Scale (GCS) score <7; estimated hematoma volume of >60 cc (intracranial hemorrhage [ICH] only); expected survival <1 month; occurrence of a thrombotic event within the 2 weeks before enrollment; or use of vitamin K antagonist, dabigatran, PCC, recombinant factor VIIa, whole blood, or plasma within the prior 7 days.
The ORANGE study was an observational, prospective study of 2192 patients with major bleeds associated with the use of the oral anticoagulants warfarin, dabigatran, rivaroxaban, apixaban, or edoxaban. 27 Patient data were prospectively and consecutively collected from 32 specialist and teaching hospitals across the United Kingdom from 2013 to 2016. Patients were included in the ORANGE study if they were ≥18 years of age and presented with acute major bleeding while taking oral anticoagulants (warfarin, dabigatran, rivaroxaban, apixaban, or edoxaban). There were no exclusion criteria.
ORANGE was chosen as the source of data for the PCC patients because (1) the distribution of bleed types was similar to that of ANNEXA‐4, (2) enrollment in the 2 studies was temporally concordant and (3) the standard of care for anticoagulation treatment and reversal/replacement was similar in the United Kingdom and in the countries involved in the ANNEXA‐4 study. ORANGE was also chosen because it included the highest number of tertiary centers and patients requiring the reversal of oral anticoagulants of any UK study identified from a systematic literature review. 28 , 29
The Bottom Line.
Direct oral anticoagulants are widely prescribed and the optimal approach to reversal is unknown. This propensity‐matched study combining 2 datasets found that patients with direct‐acting oral anticoagulants (DOAC)‐related hemorrhage treated with andexanet alfa had 15% lower mortality than those treated with prothrombin complex concentrate.
2.2. Selection of participants
To promote comparability across the 2 studies and reduce potential heterogeneity, before matching, patient populations of ANNEXA‐4 and ORANGE were refined as shown in Figure 1. Only patients treated with rivaroxaban or apixaban were included (both studies) to be consistent with the prescribing information for andexanet alfa. Only patients treated with PCC were included from the ORANGE study. In addition, patients were only included if data for all baseline characteristics of interest and 30‐day mortality were available. Thus, only 322 of the 352 patients from the ANNEXA‐4 trial and 145 of the 2192 patients from the ORANGE study were included in our analysis.
FIGURE 1.
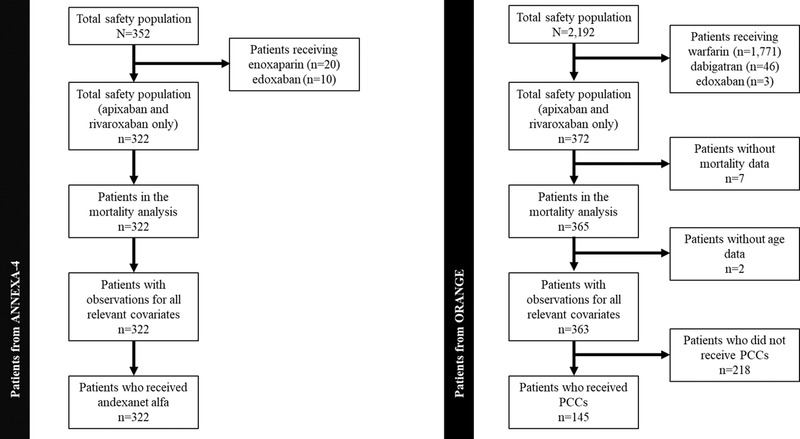
Patient numbers used in PSM analysis for ANNEXA‐4 and ORANGE. Individual data were extracted from the ANNEXA‐4 study for the andexanet alfa–treated group and from the ORANGE study for the PCC‐treated group. PCC, prothrombin complex concentrate; PSM, propensity score matching
2.3. Measurements
Individual data were extracted from both datasets.
2.4. Outcomes
All‐cause 30‐day mortality was analyzed in the whole cohort and by type of bleed: ICH, gastrointestinal (GI) bleed, and other major bleed.
2.5. Analysis
PSM was performed to reduce bias. The PSM methodology was informed by the UK National Institute for Health and Care Excellence methodology 30 and the Caliendo and Kopeinig guidance 31 and was conducted using the MatchIt package version 3.0.2 in R software version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). A feasibility assessment was first performed among patients in the whole patient cohort and in the ICH, GI bleed and other major bleeds subgroups to determine if PSM would generate robust results (Supporting Information Appendices). A logit model was used to reflect binary treatment assignment (treatment with andexanet alfa or PCC). 31 Age (years), bleed site (ICH, GI bleed, and other major bleeds), and medical history of atrial fibrillation, hypertension, diabetes, cancer, renal dysfunction, stroke, coronary artery disease, and transient ischemic attack were the model covariates that fulfilled the pre‐specified criteria of (1) potentially impacting 30‐day mortality and (2) being likely to differ between the 2 treatment groups in the whole cohort. All available covariates with the potential to impact the outcome were included in this analysis.
Model specification and estimation of each individual's propensity score were then performed. Patients in the andexanet alfa group were matched to patients in the PCC group using nearest‐neighbor matching. This matched each andexanet alfa–treated patient with the patient in the PCC‐treated control group who had the most similar propensity score. Matching with replacement (ie, all matches were drawn from the full set of PCC patients, so PCC patients could be matched more than once) was undertaken to minimize bias. Thus, patients in the PCC arm could match with multiple patients in the treatment arm if they were the best match. One‐to‐one matching, where each member of the andexanet alfa treatment group was matched to exactly 1 member of the PCC treatment group, was also used to avoid increasing bias by making poorer matches with second‐best matches. The magnitude of the difference between the baseline characteristics of the 2 groups was calculated before and after matching to see if matching improved similarity. Balance between groups was considered successful if the absolute differences between groups after matching were <10%. 32 Propensity scores were not trimmed as there was overlap in all regions of the propensity score range (see before and after matching propensity score distributions in Supporting Information Figure S3).
Thirty‐day mortality rates for patients receiving andexanet alfa or PCCs were calculated before and after PSM for each treatment group and each bleed type subgroup (ICH, GI bleed, and other major bleeds). Relative risk (RR) of 30‐day mortality and 95% confidence intervals (CIs) were calculated for the 2 treatment groups after PSM adjustment. For the other major bleed subgroup, due to the low number of patients and matches, it was not possible to match patients by specific bleed site.
Last, because of potential differences in the severity of bleeds within the ICH subgroup, a sensitivity analysis was conducted where such patients were further matched by intracranial compartment: intracerebral, subarachnoid and subdural in ANNEXA‐4 (no patients had epidural intracranial bleeds) and intracerebral, subarachnoid, and subdural/epidural in ORANGE.
3. RESULTS
3.1. Characteristics of study subjects
Baseline characteristics for the 2 cohorts before and after matching are shown in Tables 1 and 2. Before matching, the sample included 322 ANNEXA‐4 patients treated with andexanet alfa (mean age of 77.7 years; 64.9% with ICH) and 145 ORANGE patients treated with PCC (mean age of 81.0 years; 50.3% with ICH). Patients treated with andexanet alfa had a higher prevalence of atrial fibrillation, hypertension, diabetes, cancer, renal dysfunction, and stroke, whereas patients treated with PCC had a higher prevalence of coronary artery disease and transient ischemic attack.
TABLE 1.
Baseline characteristics for the whole cohort, before matching
| Before matching | ||||
|---|---|---|---|---|
| Characteristic | Andexanet alfa | PCC | Abs dif | P value a |
| Total (N) | 322 | 145 | – | – |
| Age, mean ± SD, y | 77.7 ± 10.79 | 81.0 ± 9.47 | –3.3 | 0.001 |
| Type of bleed (%) | – | |||
| ICH | 64.9 | 50.3 | 14.6 | – |
| GI bleed | 25.5 | 37.9 | –12.5 | – |
| Other major bleed b | 9.6 | 11.7 | –2.1 | – |
| Medical history (%) | ||||
| Atrial fibrillation | 83.9 | 77.9 | 5.9 | 0.158 |
| Hypertension | 78.3 | 55.9 | 22.4 | <0.001 |
| Diabetes | 30.4 | 22.1 | 8.4 | 0.079 |
| Cancer | 26.7 | 16.6 | 10.2 | 0.023 |
| Renal dysfunction | 23.3 | 15.2 | 8.1 | 0.060 |
| Stroke | 18.9 | 6.2 | 12.7 | 0.001 |
| CAD | 13.0 | 22.8 | −9.7 | 0.012 |
| TIA | 7.5 | 24.1 | −16.7 | <0.001 |
Abbreviations: Abs dif, absolute difference; CAD, coronary artery disease; GI, gastrointestinal; ICH, intracranial hemorrhage; PCC, prothrombin complex concentrate; SD, standard deviation; TIA, transient ischemic attack. Individual data were extracted from the ANNEXA‐4 study for the andexanet alfa–treated group and from the ORANGE study for the PCC‐treated group.
aCalculated using a t test for age (a continuous variable) and a χ2 test for binary variables.
bnon‐ICH/GI.
TABLE 2.
Baseline characteristics for the whole cohort after matching
| After matching | ||||
|---|---|---|---|---|
| Characteristic | Andexanet alfa | PCC | Abs dif. | P value a |
| Total, No. | 322 | 88 | – | – |
| Age, mean ± SD, y | 77.7 ± 10.79 | 74.9 ± 9.96 | 2.9 | 0.768 |
| Type of bleed (%) | – | |||
| ICH | 64.9 | 67.1 | –2.2 | – |
| GI bleed | 25.5 | 28.6 | –3.1 | – |
| Other major bleedb | 9.6 | 4.4 | 5.3 | – |
| Medical history (%) | ||||
| Atrial fibrillation | 83.9 | 78.9 | 5.0 | 0.201 |
| Hypertension | 78.3 | 72.7 | 5.6 | 0.004 |
| Diabetes | 30.4 | 26.7 | 3.7 | 0.389 |
| Cancer | 26.7 | 17.7 | 9.0 | 0.688 |
| Renal dysfunction | 23.3 | 24.5 | –1.2 | 0.380 |
| Stroke | 18.9 | 15.2 | 3.7 | 0.005 |
| CAD | 13.0 | 7.5 | 5.6 | 0.604 |
| TIA | 7.5 | 7.1 | 0.3 | 0.012 |
Abbreviations: abs dif, absolute difference; CAD, coronary artery disease; GI, gastrointestinal; ICH, intracranial hemorrhage; PCC, prothrombin complex concentrate; SD, standard deviation; TIA, transient ischemic attack. Individual data were extracted from the ANNEXA‐4 study for the andexanet alfa–treated group and from the ORANGE study for the PCC‐treated group. Matches were considered successful if the after‐match absolute difference was <10%.
aCalculated using a t test for age (a continuous variable) and a χ2 test for binary variables.
bNon‐ICH/GI.
During matching, all 322 ANNEXA‐4 patients receiving andexanet alfa were matched with 88 ORANGE patients receiving PCCs (53 PCC patients were matched multiple times). After matching, in the whole cohort, baseline characteristics and comorbidity rates in andexanet alfa–treated patients and PCC‐treated patients were similar (Table 2). The absolute differences between the andexanet alfa–treated group and the PCC‐treated group were < 10% for atrial fibrillation (83.9% vs 78.9%), hypertension (78.3% vs 72.7%), diabetes (30.4% vs 26.7%), cancer (26.7% vs 17.7%), renal dysfunction (23.3% vs 24.5%), stroke (18.9% vs 15.2%), coronary artery disease (13.0% vs 7.5%), and transient ischemic attack (7.5% vs 7.1%), respectively.
Before matching, the ICH, GI bleed, and other major bleeds subgroups included 282, 137, and 48 patients, respectively (Table 3). After matching, the ICH, GI bleed, and other major bleeds subgroups included 256, 110, and 39 patients, respectively. In the ICH and GI bleed subgroups, after matching, baseline characteristics of patients in the andexanet alfa and PCC treatment groups were similar (data not shown). Patient characteristics in the other major bleeds subgroup did not align as well due to the low number of patients and matches; types of bleeds included in this subgroup were heterogeneous (data not shown) (Table 4).
TABLE 3.
Unadjusted (before matching) all‐cause 30‐day mortality for andexanet alfa and PCC
| Unadjusted 30‐day mortality | ||||
|---|---|---|---|---|
| Population | No. of patients before matching | Andexanet alfa, % (95% CI) | PCC, % (95% CI) | Unadjusted relative reduction, % |
| Whole cohort (n = 467) |
Andexanet alfa = 322 PCC = 145 |
14.60 (10.72–18.47) | 31.72 (24.06–39.39) | −53.97 |
| ICH subgroup (n = 282) |
Andexanet alfa = 209 PCC = 73 |
15.31 (10.39–20.23) | 42.47 (30.85–54.08) | −63.95 |
| GI bleed subgroup (n = 137) |
Andexanet alfa = 82 PCC = 55 |
12.20 (4.96–19.43) | 21.82 (10.55–33.09) | −44.09 |
| Other major bleeds subgroup (non‐ICH/GI; n = 48) |
Andexanet alfa = 31 PCC = 17 |
16.13 (2.42–29.84) | 17.65 (−2.56 to 37.85) | −8.61 |
Abbreviations: CI, confidence interval; GI, gastrointestinal; ICH, intracranial hemorrhage; PCC, prothrombin complex concentrate; PSM, propensity score matching. Individual data were extracted from the ANNEXA‐4 study for the andexanet alfa–treated group and from the ORANGE study for the PCC‐treated group.
TABLE 4.
Adjusted (after matching) all‐cause 30‐day mortality for andexanet alfa and PCC
| Adjusted 30‐day mortality | ||||
|---|---|---|---|---|
| Population | No. of matched patients a | Andexanet alfa, % (95% CI) | PCC, % (95% CI) | Adjusted relative reduction, % |
|
Whole cohort (n = 410) PCC = 88 |
Andexanet alfa = 322 | 14.60 (10.72–18.47) | 34.09 (23.99–44.19) | −57.17 |
|
ICH subgroup (n = 256) PCC = 47 |
Andexanet alfa = 209 | 15.31 (10.39–20.23) | 48.94 (34.10–63.77) | −68.72 |
| GI bleeds subgroup (n = 110) |
Andexanet alfa = 82 PCC = 28 |
12.20 (4.96–19.43) | 25.00 (7.90–42.10) | −51.20 |
|
Other major bleeds (non‐ICH/GI) subgroup b (n = 39) |
Andexanet alfa = 31 PCC = 8 |
16.13 (2.42–29.84) | 12.50 (−17.06–42.06) | 29.04 |
Abbreviations: CI, confidence interval; GI, gastrointestinal; ICH, intracranial hemorrhage; PCC, prothrombin complex concentrate; PSM, propensity score matching. Individual data were extracted from the ANNEXA‐4 study for the andexanet alfa–treated group and from the ORANGE study for the PCC‐treated group.
aNumber of matched patients in the subgroups does not add up to 88 as in the whole cohort due to the PSM adjustment. Patients in the whole cohort were matched based on bleed type in addition to other covariates.
bIn the other major bleeds subgroup, fewer than 10 matches were found.
Assessments of the PSM using quantile‐quantile plots, jitter plots and histograms are shown in the Supporting Information Appendices.
3.2. Main results
The unadjusted pre‐matching and PSM‐adjusted 30‐day mortality estimates for the whole cohort and the ICH, GI bleeds, and other major bleeds subgroups are presented in Table 3. After matching, the rate of 30‐day mortality in andexanet alfa–treated patients and PCC‐treated patients was 14.6% and 34.1% in the whole cohort, 15.3% and 48.9% in the ICH subgroup, 12.2% and 25.0% in the GI bleed subgroup and 16.1% and 12.5% in the other major bleeds subgroup, respectively (Table 4).
Propensity score–matched RR for 30‐day mortality of patients treated with andexanet alfa compared to patients treated with PCC was 0.43 (95% CI, 0.29–0.63) for the whole cohort, 0.31 (95% CI, 0.20–0.48) for the ICH subgroup, and 0.49 (95% CI, 0.21–1.16) for the GI bleed subgroup (Figure 2). In the other major bleeds subgroup, the RR for 30‐day mortality was 1.29 (95% CI, 0.17–9.55).
FIGURE 2.

Forest plot showing RR of all‐cause 30‐day mortality. RR of all‐cause 30‐day mortality and 95% CI were calculated after PSM for the 2 treatment groups in the whole cohort and in the subgroups. Individual data were extracted from the ANNEXA‐4 study for the andexanet alfa–treated group and from the ORANGE study for the PCC‐treated group. Because of the low number of matches for the other major bleeds subgroup, the CI was large (RR, 1.29; 95% CI, 0.17‐9.55) and was not included in the forest plot. CI, confidence interval; GI, gastrointestinal; ICH, intracranial hemorrhage; PCC, prothrombin complex concentrate; PSM, propensity score matching; RR, relative risk
The sensitivity analyses, which included further matching by ICH bleed compartments (intracerebral, subarachnoid and subdural/epidural hemorrhage), are included in Supporting Information Table S1. Results were similar to the base case results, with 322 patients matched from ANNEXA‐4 and 81 matched from ORANGE, among whom 53 were matched more than once. Results were consistent across the whole cohort, ICH subgroup, and GI bleed subgroup. Propensity score–matched 30‐day mortality after matching in the sensitivity analysis was 14.6% for andexanet alfa–treated patients compared to 33.3% for PCC‐treated patients in the whole cohort and 15.3% for andexanet alfa–treated patients compared to 50.0% for PCC‐treated patients for the ICH subgroup (Supporting Information Table S2).
4. LIMITATIONS
The main limitation of indirect retrospective analyses is the potential for bias due to baseline differences in the patient populations. The PSM we performed reduced the risk of bias, but 2 potential sources of bias, identified in the feasibility assessment, remained: (1) differences in eligibility criteria across the 2 studies, and (2) differences in the variables reported and how they were measured. This latter point limited the number of covariates that could be included. The degree of bias associated with each of these sources is inherently immeasurable in PSM. 33
Specifically, this analysis was limited by different inclusion/exclusion criteria across the 2 studies, with the ANNEXA‐4 trial excluding patients with a GCS <7, ICH with hematoma >60 cc, and expected survival less than 1 month, which are highly predictive variables. A GCS threshold was used as an exclusion criterion for ICH in ANNEXA‐412 but not in ORANGE. 27 Baseline size or volume of bleeds, 34 blood pressure, ventricular involvement for ICH, and time from ICH symptoms to computed tomography, which are all major determinants of mortality risk, could not be included in the propensity score regression model because these were not measured in the ORANGE study. Hematoma volume, for instance, was reported in ANNEXA‐412 but not in ORANGE 27 and is known to influence mortality risk. 34 Similarly, location of GI bleed, which is known to influence severity, 35 could not be used in the propensity score model. In both ANNEXA‐4 and ORANGE, more patients had upper GI bleeds (56% and 62% of known GI bleed sites, respectively) than lower GI bleeds; the site of GI bleed was unknown in 47% of the patients in ANNEXA‐4. 12
Last, this analysis was able to account for most differences in medical history variables; however, although a history of coronary artery disease was included, histories of myocardial infarction and ischemic heart disease were excluded because of differences in definitions between the 2 studies. Additionally even after matching, there were still some significant differences between the 2 treatment groups regarding a previous medical history of TIA P = 0.012, stroke P = 0.005, and hypertension, P = 0.04 (see Table 2). The lack of matching for potentially confounding and highly predictive variables could have led to bias in the 30‐day mortality results. Future larger prospective studies are needed to explore this limitation.
5. DISCUSSION
Successful reversal of anticoagulation in major bleeds related to DOAC treatment has the potential to improve morbidity and mortality. In this PSM analysis of a broad population of patients with life‐threatening bleeding after treatment with rivaroxaban or apixaban, we found that treatment with andexanet alfa was associated with lower RR of all‐cause 30‐day mortality than treatment with PCC (57% reduction). It is quite possible that this difference is due to differences in baseline characteristics between the 2 cohorts for which no adjustment could be made, as hematoma volume was not measured in ORANGE but was measured in ANNEXA. In ANNEXA‐4, ICH patients with larger (estimated hematoma volume of >60 cc) volume were excluded. Subgroup analyses by type of bleed showed that 30‐day mortality in the ICH subgroup was 69% lower in andexanet alfa–treated patients than in PCC‐treated patients, whereas differences in 30‐day mortality in the GI and other bleeds subgroups were not statistically significant.
Reversal of anticoagulation caused by FXa inhibitors is not indicated for PCCs, and the ability of PCCs to reverse major bleeds caused by rivaroxaban or apixaban has not been established. Nonetheless, PCCs have been increasingly used for this purpose due to a lack of specific reversal agents until the approval of andexanet alfa in 2018. As PCCs continue to be used, there is a need for comparative data. However, to date, only 1 small retrospective study (N = 29) has compared andexanet alfa and PCC with respect to 30‐day mortality. 26 Our data, which included 322 andexanet alfa–treated patients matched to 88 PCC‐treated patients, describe the largest cohort to date.
Because this was a real‐world analysis derived from 2 datasets, a power analysis was not calculated and all patients who met inclusion/exclusion criteria were included. Based on published literature, there is little consistency in recommended minimum sample sizes for observational studies, with recommended sample sizes ranging between 3 and 20 times the number of covariates or between 100 to 1000 individuals. Further, certain factors necessitate use of larger sample sizes, like low communality and low numbers of factors and variables per factor. In our analysis, communality was assessed in each patient level dataset. 1 The high baseline rate for the study outcome (mortality) among patients with anticoagulant‐related bleeds decreased the risk of inadequate sample size among the overall population in this study, but due to the uncertainty in estimating the potential effect size due to limited data assessing these outcomes and related predictors, it is critical to replicate these analyses in larger databases, particularly for each bleed type.
5.1. ICH mortality
ICH is associated with particularly high mortality rates. In pivotal DOAC studies, 30‐day ICH mortality rates of 45% in patients treated with apixaban in the randomized controlled trial ARISTOTLE 36 and of 48% in patients treated with rivaroxaban in the randomized controlled trial ROCKET‐AF 37 have been reported. In a real‐world cohort of patients with intracerebral hemorrhage in the Get With the Guidelines Stroke Registry, rates of in‐hospital mortality were 27% among patients with major bleeds with evidence of FXa inhibitor use. 38 In studies of patients treated with PCC, mortality rates associated with ICH vary significantly 26 , 39 , 40 and can be as high as 64%. 26 Here, we show that with andexanet alfa treatment, the rate of 30‐day mortality was 15.3%, whereas with PCC treatment, it was 42% before matching and 49% after matching. These findings for ICH are consistent with data from recent small real‐world studies in which the in‐hospital ICH mortality rate after treatment with andexanet alfa was 10% (N = 39) 24 and 22.2% (vs 63.6% for 4 factor‐PCC) in a comparative case series of patients with ICH (N = 29). 26
5.2. GI bleed mortality
In clinical practice, GI bleeds are the most common major bleeds and account for > 50% of all DOAC‐related major bleeds, thus presenting a substantial clinical and economic burden, even though ICH is associated with higher mortality than GI bleeding. 41 Currently, reported mortality rates in patients admitted to hospitals for GI bleeds vary greatly from study to study. They range from 1.4% in a MarketScan database analysis of 1500 patients hospitalized for major GI bleeds, 41 to 7% in a US single‐center study, 42 to 14% in a 30‐day mortality analysis of 29 FXa inhibitor–treated patients with major bleeding. 43 After matching, we found that the 30‐day mortality rate was 12.2% in patients treated with andexanet alfa and 25.0% in patients treated with PCC (RR 0.49 [95% CI 0.21 to 1.16]). The mortality rate we report is thus at the higher range of the previously published mortality rates. 41 , 42 , 43 These data suggest that the severity of bleeds in ANNEXA‐412 and ORANGE 27 may be greater than those previously reported, but, more importantly, they underscore the importance of assessing the severity of GI bleeds using proxies for severity such as GI bleed site or units of blood transfused.
5.3. Mortality with other bleeds
In the other bleeds subgroup, 30‐day mortality results were inconclusive due to the low number of matches (n < 10) and the heterogeneity of bleed types included in “other major bleeds.” The comparability of the 2 populations limits confidence in the results and it is critical for future research to assess the impact of reversal or replacement treatment among patients with non‐GI, non‐ICH bleeds.
In summary, the data presented herein are consistent with the fact that major bleeds are associated with substantial risk of mortality and further underscore the importance of understanding how to best support clinicians in patient management. In our propensity score–matched study comparing 322 patients treated with andexanet alfa versus 88 treated with PCC for the management of FXa inhibitor‐related bleeds, 30‐day mortality was lower among patients treated with andexanet alfa, particularly for the ICH subgroup. These findings suggest differences may exist between reversal/replacement agents for DOAC‐related major bleeding. Because PSM comparison studies may be subject to bias related to differences in selection criteria and confounding, further research is needed to compare the safety and efficacy of PCCs and andexanet alfa in patients with DOAC‐related bleeds. The ANNEXA‐I study (ClinicalTrials.gov Identifier: NCT03661528) is currently enrolling patients with ICH in a randomized controlled trial of andexanet alfa versus usual care, which includes PCC.
CONFLICTS OF INTERESTS
ATC has received fees for serving on an adjudication committee from Boehringer Ingelheim and AbbVie; grant support and fees for serving on committees from Bristol Myers Squibb, Daiichi Sankyo and Pfizer; consulting fees from Janssen, Portola Pharmaceuticals and Ono Pharmaceuticals; fees for serving on a steering committee and consulting fees from Bayer, and travel support to present this work at the American College of Cardiology annual meeting. ML and AC are employed by FIECON, which performed this analysis at Portola's request and received payment for their contributions to the statistical analysis and drafting the manuscript. SJC has received grant support and consulting fees from Portola Pharmaceuticals, Bristol Myers Squibb, Bayer, and Daiichi Sankyo. PY and JC were employed by and held stock options in Portola Pharmaceuticals at the time of this work. RA has received grant support and consulting fees from Portola Pharmaceuticals, Bristol Myers Squibb, Bayer, Daiichi Sankyo, and Pfizer. PMC has received fees for serving on a committee from Portola Pharmaceuticals. JT and LG report no conflicts of interest.
AUTHOR CONTRIBUTIONS
ATC had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: ATC, AC, PY, and PG. Acquisition, analysis, or interpretation of data: ATC, AC, SJC, PY, JC, RA, PMC, JT, and LG. Drafting of the manuscript: all authors. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: ML and AC. Administrative, technical, or material support: ATC, ML, AC, SJC, PY, JC, RA, PMC, JT, and LG. Supervision: ATC, AC, SJC, PY, JC, RA, PMC, JT, and LG.
Supporting information
SUPPORTING INFORMATION
ACKNOWLEDGMENTS
The authors thank the ANNEXA‐4 and ORANGE investigators, staff, and participants. We also acknowledge Hélène Dassule, PhD (Lexington, MA, USA), a consultant for Alexion Pharmaceuticals, Inc., for providing medical writing and editorial assistance, funded by Alexion Pharmaceuticals, Inc. This study was sponsored by Portola Pharmaceuticals, Inc., South San Francisco, CA, USA, now Alexion Pharmaceuticals, Inc., Boston, MA, USA, following acquisition by Alexion.
Biography
Alexander T. Cohen, MD, MSc, is a vascular physician and epidemiologist at Guy's and St Thomas’ Hospital, King's College, London, UK.

Cohen AT, Lewis M, Connor A, et al. Thirty‐day mortality with andexanet alfa compared with prothrombin complex concentrate therapy for life‐threatening direct oral anticoagulant‐related bleeding. JACEP Open. 2022;3:e12655. 10.1002/emp2.12655
Meetings: This work was presented virtually at the American College of Cardiology 2020 Annual Meeting (March 28–30, 2020).
Trial Registration: ANNEXA‐4 ClinicalTrials.gov Identifier: NCT02329327.
Supervising Editor: Nicholas Johnson, MD.
DATA AVAILABILITY STATEMENT
Alexion will consider requests for disclosure of clinical study participant‐level data provided that participant privacy is assured through methods like data de‐identification, pseudonymization, or anonymization (as required by applicable law), and if such disclosure was included in the relevant study informed consent form or similar documentation. Qualified academic investigators may request participant‐level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion‐sponsored studies. Further details regarding data availability and instructions for requesting information are available in the Alexion Clinical Trials Disclosure and Transparency Policy at https://alexion.com/our‐research/research‐and‐development. Link to Data Request Form: https://alexion.com/contact‐alexion/medical‐information.
REFERENCES
- 1. National Institute for Health and Care Excellence . Atrial fibrillation: medicines to help reduce your risk of a stroke—what are the options?. Accessed March 29, 2021 http://guidance.nice.org.uk/CG180/PatientDecisionAid/pdf/English..
- 2. van Es N, Coppens M, Schulman S, et al. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124:1968–1975. [DOI] [PubMed] [Google Scholar]
- 3. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 4. Chai‐Adisaksopha C, Hillis C, Isayama T, et al. Mortality outcomes in patients receiving direct oral anticoagulants: a systematic review and meta‐analysis of randomized controlled trials. J Thromb Haemost. 2015;13:2012–2020. [DOI] [PubMed] [Google Scholar]
- 5. Wilson D, Seiffge DJ, Traenka C, et al. Outcome of intracerebral hemorrhage associated with different oral anticoagulants. Neurology. 2017;88:1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Inohara T, Xian Y, Liang L, et al. Association of intracerebral hemorrhage among patients taking non–vitamin K antagonist vs vitamin K antagonist oral anticoagulants with in‐hospital mortality. JAMA. 2018;319:463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kuramatsu JB, Gerner ST, Schellinger PD, et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation‐related intracerebral hemorrhage. JAMA. 2015;313:824–836. [DOI] [PubMed] [Google Scholar]
- 8. Kuramatsu JB, Sembill JA, Huttner HB. Reversal of oral anticoagulation in patients with acute intracerebral hemorrhage. Crit Care. 2019;23:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Øie LR, Madsbu MA, Solheim O, et al. Functional outcome and survival following spontaneous intracerebral hemorrhage: a retrospective population‐based study. Brain Behav. 2018;8:e01113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scottish Intercollegiate Guidelines Network . Antithrombotics: indications and management. Accessed March 29, 2021 https://www.sign.ac.uk/media/1067/sign129.pdf..
- 11. Makris M, Van Veen JJ, Tait CR, et al. Guideline on the management of bleeding in patients on antithrombotic agents. Br J Haematol. 2013;160:35–46. [DOI] [PubMed] [Google Scholar]
- 12. Connolly SJ, Crowther M, Eikelboom JW, et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019;380:1326–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. ANDEXXA® (coagulation factor Xa [recombinant], inactivated‐zhzo) lyophilized powder for solution for intravenous injection [package insert]. Alexion Pharmaceuticals, Inc.; 2021. [Google Scholar]
- 14. ONDEXXYA® 200 mg powder for solution for infusion [Summary of Product Characteristics]. Alexion Pharmaceuticals, Inc.; 2021. [Google Scholar]
- 15. Siegal DM, Curnutte JT, Connolly SJ, et al. Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med. 2015;373:2413–2424. [DOI] [PubMed] [Google Scholar]
- 16. Oakland K, Chadwick G, East JE, et al. Diagnosis and management of acute lower gastrointestinal bleeding: guidelines from the British Society of Gastroenterology. Gut. 2019;68:776–789. [DOI] [PubMed] [Google Scholar]
- 17. Christensen H, Cordonnier C, Kõrv J, et al. European Stroke Organisation guideline on reversal of oral anticoagulants in acute intracerebral haemorrhage. Eur Stroke J. 2019;4:294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Steffel J, Verhamme P, Potpara TS, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non‐vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39:1330–1393. [DOI] [PubMed] [Google Scholar]
- 19. Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018;2:3257–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lip GY, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest. 2018;154:1121–1201. [DOI] [PubMed] [Google Scholar]
- 21. Tomaselli GF, Mahaffey KW, Cuker A, et al. 2020 ACC expert consensus decision pathway on management of bleeding in patients on oral anticoagulants. a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76:594–622. [DOI] [PubMed] [Google Scholar]
- 22. Baugh CW, Levine M, Cornutt D, et al. Anticoagulant reversal strategies in the emergency department setting: recommendations of a multidisciplinary expert panel. Ann Emerg Med. 2020;76:470–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. The Joint Commission . Managing the risks of direct oral anticoagulants. In: Sentinel Event Alert. 2019. Accessed March 29, 2021. https://www.jointcommission.org/‐/media/tjc/documents/resources/patient‐safety‐topics/sentinel‐event/sea‐61‐doacs‐final.pdf.
- 24. Giovino A, Shomo E, Busey KV, et al. An 18‐month single‐center observational study of real‐world use of andexanet alfa in patients with factor Xa inhibitor associated intracranial hemorrhage. Clin Neurol Neurosurg. 2020;195:106070. [DOI] [PubMed] [Google Scholar]
- 25. Brown CS, Scott RA, Sridharan M, et al. Real‐world utilization of andexanet alfa. Am J Emerg Med. 2020;38:810–814. [DOI] [PubMed] [Google Scholar]
- 26. Barra ME, Das AS, Hayes BD, et al. Evaluation of andexanet alfa and four‐factor prothrombin complex concentrate (4F‐PCC) for reversal of rivaroxaban‐ and apixaban‐associated intracranial hemorrhages. J Thromb Haemost. 2020;18:1637–1647. [DOI] [PubMed] [Google Scholar]
- 27. Green L, Tan J, Morris JK, et al. A three‐year prospective study of the presentation and clinical outcomes of major bleeding episodes associated with oral anticoagulant use in the UK (ORANGE study). Haematologica. 2018;103:738–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arachchillage DR, Alavian S, Griffin J, et al. Efficacy and safety of prothrombin complex concentrate in patients treated with rivaroxaban or apixaban compared to warfarin presenting with major bleeding. Br J Haematol. 2019;184:808–816. [DOI] [PubMed] [Google Scholar]
- 29. Green L, Tan J, Antoniou S, et al. Haematological management of major bleeding associated with direct oral anticoagulants – UK experience. Br J Haematol. 2019;185:514–522. [DOI] [PubMed] [Google Scholar]
- 30. Faria R, Alava MH, Manca A, et al. NICE DSU Technical Support Document 17: the use of observational data to inform estimates of treatment effectiveness in technology appraisal: methods for comparative individual patient data. 2015. Accessed March 29, 2021. http://nicedsu.org.uk/wp‐content/uploads/2016/03/TSD17‐DSU‐Observational‐data‐FINAL.pdf.
- 31. Caliendo M, Kopeinig S. Some practical guidance for the implementation of propensity score matching. J Econ Surv. 2008;22:31–72. [Google Scholar]
- 32. Mebazaa A, Parissis J, Porcher R, et al. Short‐term survival by treatment among patients hospitalized with acute heart failure: the global ALARM‐HF registry using propensity scoring methods. Intensive Care Med. 2011;37:290–301. [DOI] [PubMed] [Google Scholar]
- 33. Keele LJ, An overview of rbounds: an R package for Rosenbaum bounds sensitivity analysis with matched data. 2009.
- 34. Broderick JP, Brott TG, Duldner JE, et al. Volume of intracerebral hemorrhage. A powerful and easy‐to‐use predictor of 30‐day mortality. Stroke. 1993;24:987–993. [DOI] [PubMed] [Google Scholar]
- 35. Lanas A, García‐Rodríguez LA, Polo‐Tomás M, et al. Time trends and impact of upper and lower gastrointestinal bleeding and perforation in clinical practice. Am J Gastroenterol. 2009;104:1633–1641. [DOI] [PubMed] [Google Scholar]
- 36. Held C, Hylek EM, Alexander JH, et al. Clinical outcomes and management associated with major bleeding in patients with atrial fibrillation treated with apixaban or warfarin: insights from the ARISTOTLE trial. Eur Heart J. 2015;36:1264–1272. [DOI] [PubMed] [Google Scholar]
- 37. Hankey GJ, Stevens SR, Piccini JP, et al. Intracranial hemorrhage among patients with atrial fibrillation anticoagulated with warfarin or rivaroxaban: the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation. Stroke. 2014;45:1304–1312. [DOI] [PubMed] [Google Scholar]
- 38. Xian Y, Zhang S, Inohara T, et al. Clinical characteristics and outcomes associated with oral anticoagulant use among patients hospitalized with intracerebral hemorrhage. JAMA Netw Open. 2021;4:e2037438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Majeed A, Ågren A, Holmström M, et al. Management of rivaroxaban‐ or apixaban‐associated major bleeding with prothrombin complex concentrates: a cohort study. Blood. 2017;130:1706–1712. [DOI] [PubMed] [Google Scholar]
- 40. Panos NG, Cook AM, Sayona J, et al. Factor Xa inhibitor‐related intracranial hemorrhage: results from a multicenter, observational cohort receiving prothrombin complex concentrates. Circulation. 2020;141:1681–1689. [DOI] [PubMed] [Google Scholar]
- 41. Deitelzweig S, Neuman WR, Lingohr‐Smith M, et al. Incremental economic burden associated with major bleeding among atrial fibrillation patients treated with factor Xa inhibitors. J Med Econ. 2017;20:1217–1223. [DOI] [PubMed] [Google Scholar]
- 42. Singer AJ, Quinn A, Dasgupta N, et al. Management and outcomes of bleeding events in patients in the emergency department taking warfarin or a non–vitamin K antagonist oral anticoagulant. J Emerg Med. 2017;52:1–7. [DOI] [PubMed] [Google Scholar]
- 43. Milling TJ Jr, Clark CL, Feronti C, et al. Management of factor Xa inhibitor‐associated life‐threatening major hemorrhage: a retrospective multi‐center analysis. Am J Emerg Med. 2018;36:396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION
Data Availability Statement
Alexion will consider requests for disclosure of clinical study participant‐level data provided that participant privacy is assured through methods like data de‐identification, pseudonymization, or anonymization (as required by applicable law), and if such disclosure was included in the relevant study informed consent form or similar documentation. Qualified academic investigators may request participant‐level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion‐sponsored studies. Further details regarding data availability and instructions for requesting information are available in the Alexion Clinical Trials Disclosure and Transparency Policy at https://alexion.com/our‐research/research‐and‐development. Link to Data Request Form: https://alexion.com/contact‐alexion/medical‐information.


