Abstract
Papillary thyroid cancer (PTC) is a type of epithelial-derived differentiated TC that reportedly accounts for a majority of TCs. Curcumin, a polyphenolic compound and a member of the Zingiberaceae (ginger) family derived from turmeric plants, can exhibit anticancer effects. Herein, we aimed to investigate the effect of curcumin on PTC and elucidate underlying mechanisms. Accordingly, PTC B-CPAP cells were treated with curcumin, in combination with/without long noncoding RNA LINC00691 inhibition, to determine the effect of curcumin and its relationship with LINC00691 in PTC cells. We observed that curcumin treatment decreased B-CPAP cell proliferation and promoted apoptosis. Curcumin inhibited LINC00691 expression in B-CPAP cells. Curcumin administration or si-LINC00691 transfection alone promoted ATP levels, inhibited glucose uptake and lactic acid levels, and inhibited lactate dehydrogenase A and hexokinase 2 protein expression in B-CPAP cells, which were further enhanced by combination treatment. Moreover, curcumin administration or si-LINC00691 transfection alone inhibited p-Akt activity, further suppressed by combination treatment. Akt inhibition promoted apoptosis and suppressed the Warburg effect in B-CPAP cells. In conclusion, our findings indicate that curcumin promotes apoptosis and suppresses proliferation and the Warburg effect by inhibiting LINC00691 in B-CPAP cells. The precise molecular mechanism might be mediated through the Akt signaling pathway, providing a theoretical basis for the treatment of PTC with curcumin.
1. Introduction
Current strategies to treat cancer remain limited. Thyroid cancer (TC) is a prevalent endocrine malignancy worldwide, accounting for approximately 300,000 new cases in 2012 and 40,000 deaths [1–3]. TC is commonly classified into anaplastic TC, medullary TC, and differentiated TC [4]. Papillary TC (PTC) is an epithelial-derived differentiated TC that accounts for most TC cases [3]. Surgery, radioactive iodine treatment, and chemotherapy are primary options for treating PTC. However, these strategies minimally impact the prognosis of recurrent PTC [5]. Thus, a better understanding of underlying molecular mechanisms and identification of novel agents remains crucial to improve therapeutic strategies for patients with PTC. The use of dietary phytochemicals during the early stages of cancer development could be considered an ideal strategy for cancer prevention. Ginger extract reportedly promotes apoptosis, inhibits cell proliferation, and prevents oxidative stress in liver cancer cells [6]. Two promising peptide sequences of pepsin, present in anticancer [7] phytochemicals, were shown to mitigate cancer progression by inducing apoptosis or intracellular oxidative stress [8]. El-Dakhly et al. have shown that combining aescin and diosmin affords a robust hepatoprotective effect [9], and Amin et al. have found that saffron and its main compounds saffron aldehyde and saffronin regulate the cell cycle and DNA damage repair system [10]. Therefore, comprehensively understanding molecular mechanisms and identifying novel therapeutics are essential to improve treatment strategies for patients with cancer.
Long noncoding RNAs (lncRNAs) are noncoding transcripts that are more than 200 nucleotides long and participate in chromosome X inactivation, maintenance of pluripotency, genomic imprinting, various cellular processes, and the formation of different organs by modulating chromatin, transcription, and translation [11, 12]. Accumulating evidence has demonstrated that lncRNAs play a markedly diverse role in the process of tumor occurrence and development by sponging microRNAs (miRNAs), participating in the regulation of cell proliferation, angiogenesis, migration, and apoptosis [13–16]. Zhou et al. have reported that lncRNA ABHD11-AS1 plays a role in regulating PTC progression through the miR-199a-5p/SLC1A5 axis [16]. In addition, Zhang et al. have suggested that lncRNA NEAT1 regulates PTC progression by regulating miR-129-5p expression [17]. Previous studies have demonstrated the upregulation of miR-451a in PTC, which could be characterized as a potential biomarker for PTC [18, 19]. Using bioinformatic analysis, we noted an interactive relationship between miR-451a and LINC00691. However, the effect of LINC00691 on PTC has not been comprehensively explored.
Numerous natural products are employed to manufacture therapeutic nanomedicines, such as albumin, dendrimers, liposomes, micelles, ceramics, and metal nanoparticles, which can be employed as drug delivery agents and exhibit potential in cancer therapy [20]. One study attempted to develop an effective treatment for liver cancer using magnetite nanoparticles (MNPs) coated with saffronin, the main active ingredient in saffron [21]. In addition, several studies have identified the role of certain natural products in treating and preventing various diseases. For example, camel whey protein hydrolysate, produced by gastric and pancreatic enzymes, acts as an antidiabetic and anti-inflammatory agent [22], and vitamin C and vitamin E can improve metabolic biochemical parameters in diabetic rats [23, 24]. Consequently, research in this field has gained considerable momentum. Curcumin is a polyphenolic compound and a member of the Zingiberaceae (ginger) family derived from turmeric plants [25]. It has been extensively used in Chinese medicine to treat various diseases, including inflammation and cancer [26, 27]. Curcumin was found to participate in the process of pancreatic cancer [28], colorectal cancer [29], and hepatocellular cancer [30] by regulating specific lncRNAs and miRNAs. In addition, curcumin can suppress PTC cell metastasis by downregulating the TGF-beta/Smad2/3 signaling pathway [31]. Curcumin was shown to enhance the anticancer activity of cisplatin in PTC cells and cancer stem-like cells by regulating the JAK/STAT3 signaling [32]. However, the effect of curcumin on PTC and its underlying mechanisms needs to be clarified. We have previously reported that curcumin can inhibit proliferation and invasion by regulating miR-451a (data not shown). However, whether the inhibitory effect of curcumin on PTC is associated with LINC00691 needs to be further elucidated.
In the present study, we detected the relative expression of LINC00691 in PTC cells. Subsequently, PTC B-CPAP cells were treated with curcumin, combined with or without LINC00691 inhibition, to detect the effect of curcumin and its relationship with LINC00691 in PTC cells.
2. Materials and Methods
2.1. Cell Culture
PTC cell lines B-CPAP, BHT-101, and KTC-1 were supplied by the Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, and cultured in RPMI-1640 (Hyclone) supplemented with 10% fetal bovine serum (FBS; Gibco), Dulbecco's modified Eagle medium (Hyclone) supplemented with 20% FBS, and RPMI-1640 supplemented with 10% FBS, 1% nonessential amino acids (Invitrogen), 1% Glutamax (Invitrogen), and 1% sodium pyruvate (Sigma), respectively. Thyroid epithelial cells were cultured in RPMI-1640 supplemented with 10% FBS and 1% Glutamax. All cells were maintained at 37°C with 5% CO2. The cells were harvested after reaching 80-90% confluency, and LINC00691 expression was detected using quantitative reverse transcription-polymerase chain reaction (qRT-PCR).
2.2. qRT-PCR
LINC00691 expression in small interfering- (si-) LINC00691 transfected to different curcumin concentration (2.5, 5, 10, and 20 μM)-treated B-CPAP cells was detected using qRT-PCR. The different concentrations were based on the results from previous studies [33–35]. Whole RNA was extracted from B-CPAP cells using TRIzol (Ambion) and reverse-transfected into cDNA. Subsequently, cDNA was amplified under the following thermocycling conditions: 95°C for 3 min; 40 cycles of denaturation at 95°C for 5 s, annealing at 56°C for 10 s, and extension at 72°C for 25 s; final extension was performed at the rate of 5°C/5 s from 65°C to 95°C. The primary sequences were as follows: LINC00691: forward, 5′-GATGAGAAACGGAAACCC C-3′, reverse, 5′-TGACAGGACGCCGACATT-3′ and GAPDH (internal reference): forward, 5′-CCACTCCTCCACCTTTG-3′, reverse, 5′-CCACTCCTCCACCTTTG-3′. The 2-ΔΔCt method was used to calculate the relative lncRNA expression levels [36].
2.3. Cell Counting Kit-8
The cell counting kit-8 (CCK-8) assay was performed to detect B-CPAP cell viability after si-LINC00691 transfection or treatment with different curcumin concentrations. Briefly, 3 × 103 cells in each group were seeded into 96-well plates (100 μL per well) and maintained at 37°C with 5% CO2 overnight. After transfection or curcumin treatment for 24 h, the cells were cultured for 4 h with the addition of 10 μL CCK-8 solution (Solarbio) and subjected to a microplate reader (Allsheng) to detect absorbance at 450 nm.
2.4. Flow Cytometry
Briefly, 1 × 106 resuspended cells in each group were centrifuged for 5 min at 400 × g and 4°C (repeated twice). Next, the cells were resuspended in 200 μL PBS and stained for 30 min with 10 μL Annexin V-fluorescein isothiocyanate (FITC, BD) and 10 μL propidium iodide (PI, BD) in the dark at 4°C. After adding 300 μL PBS, the cells were subjected to flow cytometry (ACEA Biosciences).
2.5. Western Blot
Total proteins were extracted from B-CPAP cells using radioimmunoprecipitation assay and quantified using a bicinchoninic acid assay kit (Solarbio). In brief, 20 μg of harvested proteins from each group was separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes. The membranes were then blocked with 5% skim milk at 4°C overnight and cultured for 1 h with primary antibodies against B cell lymphoma-2 (Bcl-2), Bcl-2/Bcl-XL-associated death promoter (Bad), lactate dehydrogenase A (LDHA), hexokinase 2 (HK2), Akt (all supplied by Bioswamp), cleaved caspase-3, phosphorylated (p)-Akt, and GAPDH (an endogenous control) (supplied by Abcam). Subsequently, the membranes were incubated with goat anti-rabbit IgG secondary antibody for 1 h.
2.6. Biochemical Assay
Levels of adenosine triphosphate (ATP) (A095) and lactic acid (A019-02), as well as glucose uptake (cat. KA4086) in B-CPAP cells, were evaluated using corresponding commercial kits. The ATP and lactic acid assay kits were supplied by the Nanjing Jiancheng Bioengineering Institute. The glucose uptake assay kit was supplied by Abnova.
2.7. Statistical Analysis
Data are presented as the mean ± standard deviation (SD). Statistical differences were analyzed using one-way analysis of variance, followed by Tukey's test. Statistical significance was set at p < 0.05.
3. Results
3.1. LINC00691 Was Overexpressed in B-CPAP Cells and LINC00691 Inhibition Attenuated B-CPAP Cell Proliferation
We examined the expression of LINC00691 in B-CPAP, BHT-101, and KTC-1 cells. Compared with thyroid epithelial cells, B-CPAP, BHT-101, and KTC-1 cells showed increased LINC00691 expression. B-CPAP cells were chosen for subsequent investigations owing to the high LINC00691 expression (Figure 1(a)). LINC00691 expression in B-CPAP cells was then inhibited by si-LINC00691 transfection (si-LINC00691-1 was selected for subsequent experiments) (Figure 1(b)). The CCK-8 assay revealed that si-LINC00691 transfection suppressed the viability of B-CPAP cells.
Figure 1.
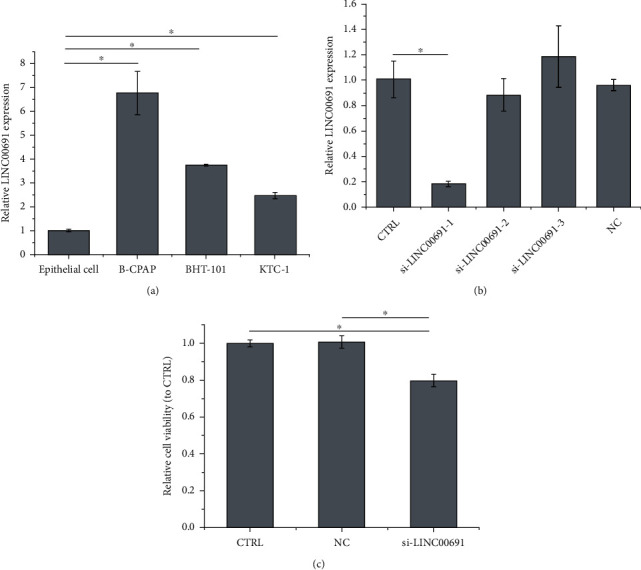
LINC00691 is overexpressed in B-CPAP cells, and LINC00691 inhibition attenuates B-CPAP cell proliferation. (a) qRT-PCR was performed to detect the expression of LINC00691 in PTC cells. (b) qRT-PCR was performed to evaluate the transfection efficiency in B-CPAP cells. (c) CCK-8 assay was performed to examine cell viability. Data are presented as the mean ± standard deviation (SD), n = 3, ∗p < 0.05. CTRL: control; NC: negative control of si-LINC00691; qRT-PCR: quantitative reverse transcription-polymerase chain reaction; PTC: papillary thyroid cancer.
3.2. Curcumin Attenuated Proliferation and LINC00691 Expression in B-CPAP Cells
As shown in Figure 2(a), curcumin administration inhibited B-CPAP cell viability in a concentration-dependent manner. The IC50 of curcumin in B-CPAP cells was approximately 16 μM, which was selected for subsequent experiments. In addition, curcumin suppressed LINC00691 expression in a concentration-dependent manner (Figure 2(b)).
Figure 2.
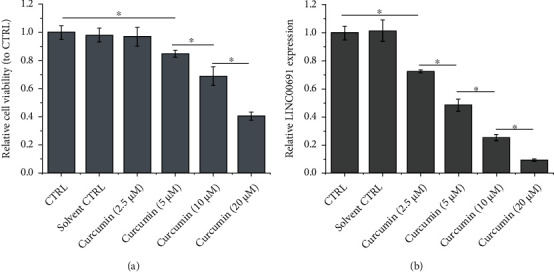
Curcumin attenuates proliferation and LINC00691 expression in B-CPAP cells. (a) CCK-8 assay was performed to detect B-CPAP cell viability. (b) qRT-PCR was performed to detect the expression of LINC00691 in B-CPAP cells. Data are presented as the mean ± standard deviation (SD), n = 3, ∗p < 0.05. CTRL: control; qRT-PCR: quantitative reverse transcription-polymerase chain reaction.
3.3. Curcumin Attenuated Apoptosis by Regulating LINC00691 Expression in B-CPAP Cells
Based on flow cytometric analysis, curcumin administration or si-LINC00691 transfection alone promoted apoptosis in B-CPAP cells, which was further enhanced following combination treatment (Figure 3(a)). Western blot assay results revealed that curcumin administration or si-LINC00691 transfection alone enhanced Bad and cleaved caspase-3 expression and suppressed Bcl-2 expression; combination treatment further strengthened these phenomena (Figure 3(b)).
Figure 3.
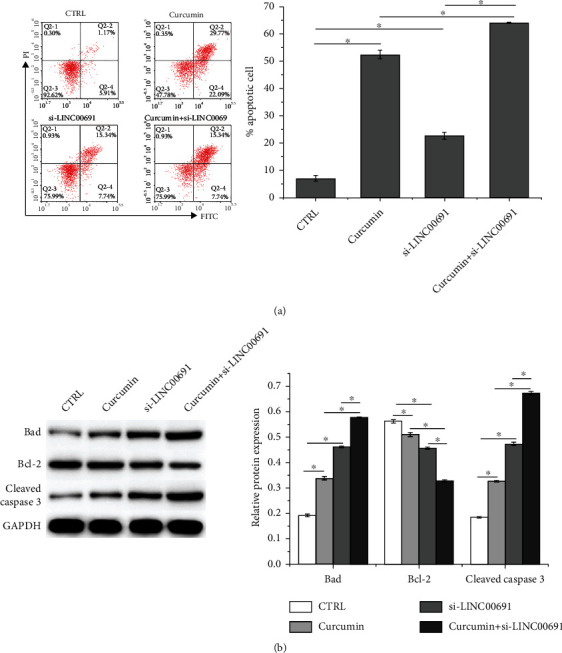
Curcumin attenuates apoptosis by regulating LINC00691 expression in B-CPAP cells. (a) Flow cytometry was performed to detect apoptosis in B-CPAP cells. (b) Western blot was performed to detect the expression of apoptosis-related proteins in B-CPAP cells. Data are presented as the mean ± standard deviation (SD), n = 3, ∗p < 0.05. CTRL: control.
3.4. Curcumin Inhibited the Warburg Effect by Regulating LINC00691 Expression in B-CPAP Cells
As shown in Figures 4(a)–4(c), curcumin administration or si-LINC00691 transfection alone enhanced ATP levels and inhibited glucose uptake and lactic acid levels in B-CPAP cells; combination treatment further strengthened these effects. In addition, curcumin administration or si-LINC00691 transfection alone inhibited LDHA and HK2 protein expressions, which were further inhibited following combination treatment with curcumin administration and si-LINC00691 transfection (Figure 4(d)). The effect of curcumin treatment with/without si-LINC00691 transfection was similar to that of 2-deoxy-D-glucose (2-DG, 10 mM), a glucose inhibitor.
Figure 4.
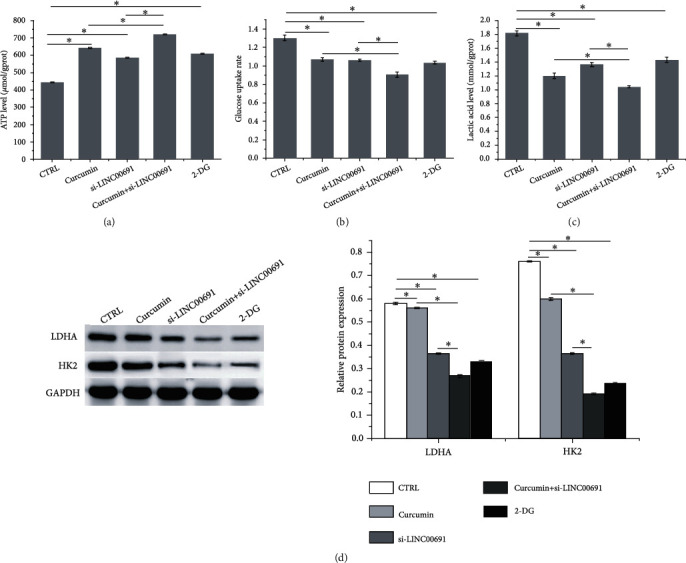
Curcumin inhibits the Warburg effect by regulating LINC00691 expression in B-CPAP cells. The (a) ATP, (b) glucose, and (c) lactic acid levels in B-CPAP cells. (d) Western blot was performed to detect the expression of enzymes involved in the Warburg effect. Data are presented as the mean ± standard deviation (SD), n = 3, ∗p < 0.05. CTRL: control; 2-DG: 2-deoxy-D-glucose.
3.5. Curcumin Attenuated Apoptosis and the Warburg Effect by Regulating the Akt Signaling in B-CPAP Cells
Based on western blotting analysis, curcumin administration or si-LINC00691 transfection alone inhibited p-Akt activity, which was further inhibited following combination treatment (Figure 5(a)). Furthermore, Akt inhibitor, AZD5363, enhanced apoptosis (Figure 5(b)) and suppressed the Warburg effect in B-CPAP cells, as indicated by increased ATP levels (Figure 5(c)) and decreased lactic acid (Figure 5(d)), glucose uptake (Figure 5(e)), and LDHA and HK2 protein expression (Figure 5(f)). The AZD5363-mediated effect was similar to that of curcumin treatment with or without si-LINC00691 transfection.
Figure 5.
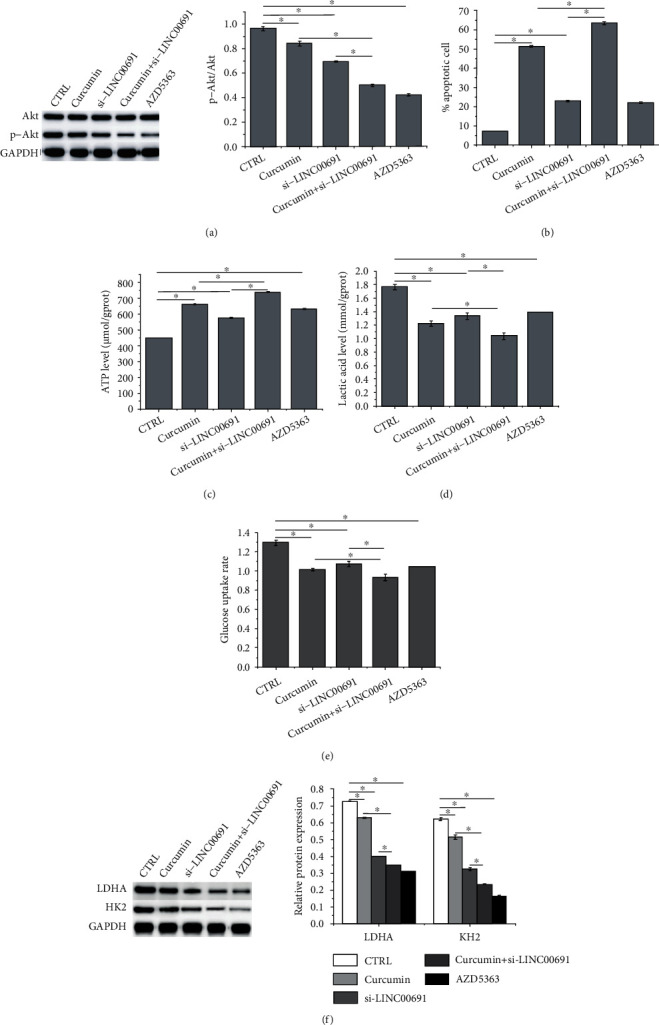
Curcumin attenuates apoptosis and the Warburg effect by regulating the Akt signaling in B-CPAP cells. (a) Western blotting was performed to detect the expression of Akt and p-Akt in B-CPAP cells. (b) Flow cytometry was performed to examine apoptosis in B-CPAP cells. The (c) ATP, (d) glucose, and (e) lactic acid levels in B-CPAP cells. (f) Western blot was performed to detect the expression of enzymes involved in the Warburg effect. Data are presented as the mean ± standard deviation (SD), n = 3, ∗p < 0.05. CTRL: control; AZD: Akt inhibitor.
4. Discussion
The present study revealed that LINC00691 is highly expressed in B-CPAP cells. LINC00691 inhibition suppressed proliferation and promoted apoptosis by enhancing the expression of proapoptosis-related proteins Bad and cleaved caspase-3 and suppressed the expression of the antiapoptotic protein Bcl-2 in B-CPAP cells. Curcumin treatment decreased proliferation and increased apoptosis in B-CPAP cells. In addition, curcumin inhibited LINC00691 expression in B-CPAP cells. Numerous studies have reported the anticancer effect of curcumin by regulating different mechanisms, including lncRNA regulation. Shao et al. have shown that curcumin inhibits proliferation and induces apoptosis in hepatocellular carcinoma cells by downregulating lincROR [37]. Wang et al. have suggested that curcumin enhances apoptosis and suppresses proliferation in lung cancer cells by inhibiting lncRNA UCA1 expression [38]. In the present study, our findings indicate that curcumin suppresses proliferation and enhances apoptosis in B-CPAP cells by inhibiting LINC00691. A previous study has shown that LINC00691 overexpression promotes invasion and proliferation in gastric cancer cells [39]. In contrast, Wan et al. have demonstrated that LINC00691 overexpression inhibited the migration, invasion, and proliferation of osteosarcoma cells [40]. The distinct LINC00691 functions in various cancer cells may be associated with their specific characteristics.
Tumor cells preferentially use glucose for energy via glycolysis, which produces substantial lactic acid via a phenomenon known as the “Warburg effect,” which is an essential form of metabolic reprogramming related to cancer occurrence and development [41, 42]. LDHA and HK2 are important enzymes involved in the Warburg effect. Inhibition of LDHA and HK2 expression suppresses the Warburg effect in cancer cells [43]. The Warburg effect prevents mitochondrial ATP production, thereby resisting apoptosis and promoting tumor aggressiveness [44–46]. In the present study, curcumin treatment with/without si-LINC00691 transfection enhanced ATP levels and suppressed glucose uptake, lactic acid level, and LDHA and HK2 protein expression in B-CPAP cells, indicating that curcumin might inhibit the Warburg effect by regulating LINC00691 expression in B-CPAP cells.
Akt is a downstream protein of phosphatidylinositol 3-kinase, known to participate in the regulation of numerous cellular biological processes [47]. Inhibition of Akt phosphorylation by zinc finger protein 677 suppresses proliferation, colony formation, and tumorigenic potential and induces apoptosis and cell cycle arrest in thyroid cancer cells [48]. Accumulating evidence has demonstrated the regulatory effect of curcumin on the Akt-associated pathway. Wang et al. have reported that curcumin suppressed proliferation and enhanced apoptosis in glioblastoma cells by inhibiting Akt phosphorylation [49]. In addition, curcumin was found to attenuate the epithelial-mesenchymal transition in non-small-cell lung cancer cells by inactivating the Akt-associated signaling pathway [50]. Inactivation of the Akt-related signaling pathway suppresses the Warburg effect in various cancer cells, including osteosarcoma cells [13], hepatocellular carcinoma cells [51], and breast cancer cells [52]. Our present study showed that curcumin administration with/without si-LINC00691 transfection could inhibit p-Akt activity. Akt inhibition promoted apoptosis and suppressed the Warburg effect in B-CPAP cells.
In conclusion, the present study provides evidence that curcumin promotes apoptosis and inhibits proliferation and the Warburg effect by inhibiting LINC00691 in B-CPAP cells. Moreover, the specific molecular mechanism might be mediated through the Akt signaling pathway. This study provides a theoretical basis for the treatment of PTC with curcumin. Additional in vivo and clinical investigations are needed to verify these findings.
Acknowledgments
This study was funded by the Talent Introduction Fund of the Hubei Hospital of Traditional Chinese Medicine.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that there are no conflicts of interest related to the contents of this article.
References
- 1.Siegel R. L., Miller K. D., Jemal A. Cancer statistics, 2020. CA: A Cancer Journal for Clinicians . 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.La Vecchia C., Malvezzi M., Bosetti C., et al. Thyroid cancer mortality and incidence: a global overview. International Journal of Cancer . 2015;136(9):2187–2195. doi: 10.1002/ijc.29251. [DOI] [PubMed] [Google Scholar]
- 3.Bunevicius A., Fribance S., Pikis S., et al. Stereotactic radiosurgery for differentiated thyroid cancer brain metastases: an international, multicenter study. Thyroid . 2021;31(8):1244–1252. doi: 10.1089/thy.2020.0947. [DOI] [PubMed] [Google Scholar]
- 4.Ouyang X., Feng L., Yao L., et al. Testicular orphan receptor 4 (TR4) promotes papillary thyroid cancer invasion via activating circ-FNLA/miR-149-5p/MMP9 signaling. Molecular Therapy-Nucleic Acids . 2021;24:755–767. doi: 10.1016/j.omtn.2021.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aashiq M., Silverman D. A., Na'ara S., Takahashi H., Amit M. Radioiodine-refractory thyroid cancer: molecular basis of redifferentiation therapies, management, and novel therapies. Cancers . 2019;11(9):p. 1382. doi: 10.3390/cancers11091382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamza A. A., Heeba G. H., Hamza S., Abdalla A., Amin A. NC-ND license standardized extract of ginger ameliorates liver cancer by reducing proliferation and inducing apoptosis through inhibition oxidative stress/inflammation pathway. Biomedicine & Pharmacotherapy . 2021;134, article 111102 doi: 10.1016/j.biopha.2020.111102. [DOI] [PubMed] [Google Scholar]
- 7.Murali C., Mudgil P., Gan C. Y., et al. Camel whey protein hydrolysates induced G2/M cellcycle arrest in human colorectal carcinoma. Scientific Reports . 2021;11(1):1–14. doi: 10.1038/s41598-021-86391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juaid N., Amin A., Abdalla A., et al. Anti-hepatocellular carcinoma biomolecules: molecular targets insights. International Journal of Molecular Sciences . 2021;22(19, article 10774) doi: 10.3390/ijms221910774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Dakhly S. M., Salama A., Hassanin S., Yassen N. N., Hamza A. A., Amin A. Aescin and diosmin each alone or in low dose-combination ameliorate liver damage induced by carbon tetrachloride in rats. BMC Research Notes . 2020;13:p. 259. doi: 10.1186/s13104-020-05094-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amin A., Farrukh A., Murali C., et al. Saffron and its major ingredients’ effect on colon cancer cells with mismatch repair deficiency and microsatellite instability. Molecules . 2021;26(13):p. 3855. doi: 10.3390/molecules26133855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J. T., Bartolomei M. S. X-inactivation, imprinting, and long noncoding RNAs in health and disease. Cell . 2013;152(6):1308–1323. doi: 10.1016/j.cell.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Quinn J. J., Chang H. Y. Unique features of long non-coding RNA biogenesis and function. Nature Reviews. Genetics . 2016;17(1):47–62. doi: 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- 13.Dong X., Yang Z., Yang H., Li D., Qiu X. Long non-coding RNA MIR4435-2HG promotes colorectal cancer proliferation and metastasis through miR-206/YAP1 axis. Frontiers in Oncology . 2020;10(160) doi: 10.3389/fonc.2020.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan J. J., Tay Y. Noncoding RNA: RNA regulatory networks in cancer. International Journal of Molecular Sciences . 2018;19(5):p. 1310. doi: 10.3390/ijms19051310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L., Wei Z., Wu K., et al. Long noncoding RNA B3GALT5-AS1 suppresses colon cancer liver metastasis via repressing microRNA-203. Aging (Albany NY) . 2018;10(12):3662–3682. doi: 10.18632/aging.101628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou W., Gong J., Chen Y., et al. Long noncoding RNA LINC00899 suppresses breast cancer progression by inhibiting miR-425. Aging (Albany NY) . 2019;11(22):10144–10153. doi: 10.18632/aging.102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H., Cai Y., Zheng L., Zhang Z., Lin X., Jiang N. Long noncoding RNA NEAT1 regulate papillary thyroid cancer progression by modulating miR-129-5p/KLK7 expression. Journal of Cellular Physiology . 2018;233(10):6638–6648. doi: 10.1002/jcp.26425. [DOI] [PubMed] [Google Scholar]
- 18.Minna E., Romeo P., Dugo M., et al. miR-451a is underexpressed and targets AKT/mTOR pathway in papillary thyroid carcinoma. Oncotarget . 2016;7(11):12731–12747. doi: 10.18632/oncotarget.7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li M., Song Q., Li H., Lou Y., Wang L. Circulating miR-25-3p and miR-451a may be potential biomarkers for the diagnosis of papillary thyroid carcinoma. PLoS One . 2015;10(7, article e0132403) doi: 10.1371/journal.pone.0132403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baig B., Halim S. A., Farrukh A., Greish Y., Amin A. Current status of nanomaterial-based treatment for hepatocellular carcinoma. Biomedicine & Pharmacotherapy . 2019;116, article 108852 doi: 10.1016/j.biopha.2019.108852. [DOI] [PubMed] [Google Scholar]
- 21.El-Kharrag R., Amin A., Hisaindee S., Greish Y., Karam S. M. Development of a therapeutic model of precancerous liver using crocin-coated magnetite nanoparticles. International Journal of Oncology . 2017;50(1):212–222. doi: 10.3892/ijo.2016.3769. [DOI] [PubMed] [Google Scholar]
- 22.Kamal H., Jafar S., Mudgil P., Murali C., Amin A., Maqsood S. Inhibitory properties of camel whey protein hydrolysates toward liver cancer cells, dipeptidyl peptidase-IV, and inflammation. Journal of Dairy Science . 2018;101(10):8711–8720. doi: 10.3168/jds.2018-14586. [DOI] [PubMed] [Google Scholar]
- 23.Al-Shamsi M., Amin A., Adeghate EJAotNYAoS Effect of vitamin C on liver and kidney functions in normal and diabetic rats. Annals of the New York Academy of Sciences . 2010;1084(1):371–390. doi: 10.1196/annals.1372.031. [DOI] [PubMed] [Google Scholar]
- 24.Al-Shamsi M., Amin A., Adeghate EJAotNYAoS Vitamin E ameliorates some biochemical parameters in normal and diabetic rats. Annals of the New York Academy of Sciences . 2006;1084(1):411–431. doi: 10.1196/annals.1372.033. [DOI] [PubMed] [Google Scholar]
- 25.Rahmani A. H., Al Zohairy M. A., Aly S. M., Khan M. A. Curcumin: a potential candidate in prevention of cancer via modulation of molecular pathways. BioMed Research International . 2014;2014 doi: 10.1155/2014/761608.761608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He Y., Yue Y., Zheng X., Zhang K., Chen S., Du Z. Curcumin, inflammation, and chronic diseases: how are they linked? Molecules . 2015;20(5):9183–9213. doi: 10.3390/molecules20059183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giordano A., Tommonaro G. Curcumin and cancer. Nutrients . 2019;11(10):p. 2376. doi: 10.3390/nu11102376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida K., Toden S., Ravindranathan P., Han H., Goel A. Curcumin sensitizes pancreatic cancer cells to gemcitabine by attenuating PRC2 subunit EZH2, and the lncRNA PVT1 expression. Carcinogenesis . 2017;38(10):1036–1046. doi: 10.1093/carcin/bgx065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng Z. H., You H. Y., Feng Y. J., Zhang Z. T. lncRNA KCNQ1OT1 is a key factor in the reversal effect of curcumin on cisplatin resistance in the colorectal cancer cells. Molecular and Cellular Biochemistry . 2021;476(7):2575–2585. doi: 10.1007/s11010-020-03856-x. [DOI] [PubMed] [Google Scholar]
- 30.Zamani M., Sadeghizadeh M., Behmanesh M., Najafi F. Dendrosomal curcumin increases expression of the long non-coding RNA gene MEG3 via up-regulation of epi-miRs in hepatocellular cancer. Phytomedicine . 2015;22(10):961–967. doi: 10.1016/j.phymed.2015.05.071. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L., Cheng X., Gao Y., et al. Curcumin inhibits metastasis in human papillary thyroid carcinoma BCPAP cells via down-regulation of the TGF-beta/Smad2/3 signaling pathway. Experimental Cell Research . 2016;341(2):157–165. doi: 10.1016/j.yexcr.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Khan A. Q., Ahmed E. I., Elareer N., et al. Curcumin-mediated apoptotic cell death in papillary thyroid cancer and cancer stem-like cells through targeting of the JAK/STAT3 signaling pathway. International Journal of Molecular Sciences . 2020;21(2):p. 438. doi: 10.3390/ijms21020438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L., Cheng X., Xu S., Bao J., Yu H. Curcumin induces endoplasmic reticulum stress-associated apoptosis in human papillary thyroid carcinoma BCPAP cells via disruption of intracellular calcium homeostasis. Medicine . 2018;97(24, article e11095) doi: 10.1097/MD.0000000000011095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L., Cheng X., Gao Y., et al. Curcumin inhibits metastasis in human papillary thyroid carcinoma BCPAP cells via down-regulation of the TGF-β/Smad2/3 signaling pathway. Experimental Cell Research . 2016;341(2):157–165. doi: 10.1016/j.yexcr.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Perna A., De Luca A., Adelfi L., Pasquale T., Varriale B., Esposito T. Effects of different extracts of curcumin on TPC1 papillary thyroid cancer cell line. BMC Complementary and Alternative Medicine . 2018;18(1):p. 63. doi: 10.1186/s12906-018-2125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Livak K. J., Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods . 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 37.Shao J., Shi C. J., Li Y., et al. Lincror mediates the suppressive effects of curcumin on hepatocellular carcinoma through inactivating Wnt/beta-catenin signaling. Frontiers in Pharmacology . 2020;11:p. 847. doi: 10.3389/fphar.2020.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang W. H., Chen J., Zhang B. R., et al. Curcumin inhibits proliferation and enhances apoptosis in A549 cells by downregulating lncRNA UCA1. Die Pharmazie . 2018;73(7):402–407. doi: 10.1691/ph.2018.8402. [DOI] [PubMed] [Google Scholar]
- 39.Liang W., Xia B., He C., Zhai G., Li M., Zhou J. Overexpression of LINC00691 promotes the proliferation and invasion of gastric cancer cells via the Janus kinase/signal transducer and activator of transcription signalling pathway. The International Journal of Biochemistry & Cell Biology . 2020;123, article 105751 doi: 10.1016/j.biocel.2020.105751. [DOI] [PubMed] [Google Scholar]
- 40.Wan D., Qu Y., Zhang L., Ai S., Cheng L. The lncRNA LINC00691 functions as a ceRNA for miRNA-1256 to suppress osteosarcoma by regulating the expression of ST5. OncoTargets and Therapy . 2020;13:13171–13181. doi: 10.2147/OTT.S266435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun L., Suo C., Li S. T., Zhang H., Gao P. Metabolic reprogramming for cancer cells and their microenvironment: beyond the Warburg effect. Biochimica et Biophysica Acta. Reviews on Cancer . 2018;1870(1):51–66. doi: 10.1016/j.bbcan.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Du Y., Wei N., Ma R., Jiang S., Song D. A miR-210-3p regulon that controls the Warburg effect by modulating HIF-1α and p53 activity in triple-negative breast cancer. Cell Death & Disease . 2020;11(9):p. 731. doi: 10.1038/s41419-020-02952-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Du P., Liao Y., Zhao H., Zhang J., Mu K. ANXA2P2/miR-9/LDHA axis regulates Warburg effect and affects glioblastoma proliferation and apoptosis. Cellular Signalling . 2020;74, article 109718 doi: 10.1016/j.cellsig.2020.109718. [DOI] [PubMed] [Google Scholar]
- 44.Icard P., Shulman S., Farhat D., Steyaert J. M., Alifano M., Lincet H. How the Warburg effect supports aggressiveness and drug resistance of cancer cells? Drug Resistance Updates . 2018;38:1–11. doi: 10.1016/j.drup.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 45.Hanai J. I., Doro N., Seth P., Sukhatme V. P. ATP citrate lyase knockdown impacts cancer stem cells in vitro. Cell Death & Disease . 2013;4:p. e696. doi: 10.1038/cddis.2013.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou Y., Tozzi F., Chen J., et al. Intracellular ATP levels are a pivotal determinant of chemoresistance in colon cancer cells. Cancer Research . 2012;72(1):304–314. doi: 10.1158/0008-5472.CAN-11-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lien E. C., Dibble C. C., Toker A. PI3K signaling in cancer: beyond AKT. Current Opinion in Cell Biology . 2017;45:62–71. doi: 10.1016/j.ceb.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y., Yang Q., Guan H., Shi B., Ji M., Hou P. ZNF677 suppresses Akt phosphorylation and tumorigenesis in thyroid cancer. Cancer Research . 2018;78(18):5216–5228. doi: 10.1158/0008-5472.CAN-18-0003. [DOI] [PubMed] [Google Scholar]
- 49.Wang Z., Liu F., Liao W., et al. Curcumin suppresses glioblastoma cell proliferation by p-AKT/mTOR pathway and increases the PTEN expression. Archives of Biochemistry and Biophysics . 2020;689, article 108412 doi: 10.1016/j.abb.2020.108412. [DOI] [PubMed] [Google Scholar]
- 50.Wang N., Feng T., Liu X., Liu Q. Curcumin inhibits migration and invasion of non-small cell lung cancer cells through up-regulation of miR-206 and suppression of PI3K/AKT/mTOR signaling pathway. Acta Pharmaceutica . 2020;70(3):399–409. doi: 10.2478/acph-2020-0029. [DOI] [PubMed] [Google Scholar]
- 51.Ye G., Qin Y., Wang S., et al. Lamc1 promotes the Warburg effect in hepatocellular carcinoma cells by regulating PKM2 expression through AKT pathway. Cancer Biology & Therapy . 2019;20(5):711–719. doi: 10.1080/15384047.2018.1564558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yurasakpong L., Apisawetakan S., Pranweerapaiboon K., Sobhon P., Chaithirayanon K. Holothuria scabra extract induces cell apoptosis and suppresses Warburg effect by down-regulating Akt/mTOR/HIF-1 axis in MDA-MB-231 breast cancer cells. Nutrition and Cancer . 2020;73(10):1964–1975. doi: 10.1080/01635581.2020.1814825. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


