Abstract
Free tissue transfer is a cornerstone of complex reconstruction. In many cases, it represents the last option available for a patient and their reconstruction. At high-volume centers, the risk of free flap failure is low but its occurrence can be devastating. Currently, the mainstay for flap monitoring is the clinical examination. Though reliable when performed by experienced clinicians, the flap exam is largely subjective, is performed discontinuously, and often results in significant time delay between detection of flap compromise and intervention. Among emerging flap monitoring technologies, the most promising appear to be those that rely on noninvasive transcutaneous oxygen and carbon dioxide measurements, which provide information regarding flap perfusion. In this article, we review and summarize the literature on various techniques but primarily emphasizing those technologies that rely on transcutaneous gas measurements. We also define characteristics for the ideal flap monitoring tool and discuss critical barriers, predominantly cost, preventing more widespread utilization of adjunct monitoring technologies, and their implications.
Keywords: flap monitoring, transcutaneous oxygen, carbon dioxide, systematic review
Introduction
Free tissue transfer is a cornerstone of complex reconstruction. In many cases, it represents the last option available for a patient and their reconstruction. At the highest volume centers, the risk of free flap failure is low but if it occurs, it may be a devastating result. Currently, the mainstay of flap monitoring includes clinical flap examination with possible adjunct monitoring techniques that vary by facility. Though reliable when performed by experienced clinicians, the flap examination is largely subjective, not done continuously, and often results in significant time delay between detection of flap compromise and intervention. Ideal postoperative surveillance of free flaps would consist of a monitoring system that is accurate, reliable, easily reproducible with a shallow learning curve, non- or minimally invasive, a real-time assessment, affordable, and provides an ability to differentiate arterial from venous compromise, in addition to vessel spasm from occlusion. 1 Flap failure is relatively uncommon with success or flap survival rates of 97 to 99% 2 ; however, flap failure can lead to significant morbidity and, in some cases, mortality risk. Flap compromise generally occurs as a result of arterial or venous occlusion by thrombosis, external compression, vessel kinking, or hematoma. 2 Current flap monitoring mechanisms have led to a highly variable salvage rate of 40 to 80% with the greater salvage rates in instances of venous thrombosis than in arterial thrombosis. 2 Flap tissue oxygen saturation measurement has been found to significantly correlate with blood oxygenation readings without being confounded by blood pressure, supplemental oxygen (O 2 ), flap type, perforator number, or vessel caliber. 3 The literature shows flap success rates and salvage rates are generally equivalent, regardless of the monitoring system used, although transcutaneous oxygen and carbon dioxide monitoring have demonstrated the most promise in its resurgence with studies showcasing their versatility and ability to identify early flap compromise. In this article, we performed a systematic review adherent to preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines and summarize the literature on flap monitoring techniques, with particular attention to those using transcutaneous gas measurements.
Systematic Review of the Literature
Methods
A PubMed, Cochrane Database, and MEDLINE search adherent to PRISMA was performed for articles containing the terms: “flap,” “monitoring,” and/or “transcutaneous,” “oxygen,” “carbon dioxide.” Search terms were stringed using PubMed’s Automatic Term Mapping algorithm. Articles were limited to English with humans as the subjects for this study.
Retrospective studies and prospective analyses were included; case reports, case series fewer than five patients, commentaries, and editorials were excluded. The lead author (S.H.H.) reviewed the articles and two authors (T.M.S., A.S.H.) double-screened articles to decide which to include or exclude; discrepancies or indecisions were resolved via discussion with the other authors. Individual study bias was mitigated by reviewing and confirming the appropriate sources indicated. Studies were limited to those published since 2000.
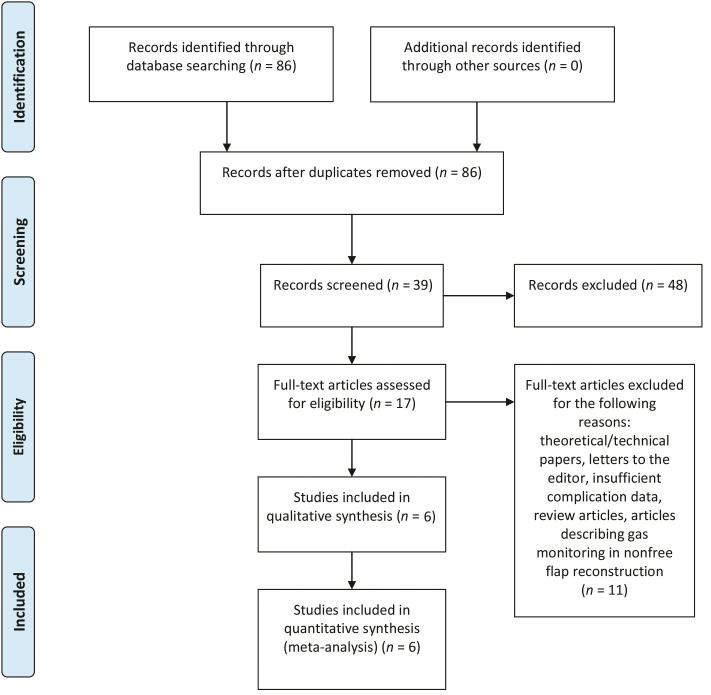
Title and abstract reviews were performed once the initial list of studies was generated ( Fig. 1 ). The reviewers met prior to commencing study selection to ensure consistency in the application of the inclusion criteria. Studies were selected if they met the following inclusion criteria: (1) utilized transcutaneous gas measurements for flap monitoring, (2) included the details of flap survival and outcomes, and (3) detailed the measuring modality used for flap monitoring.
Fig. 1.
PRISMA flow diagram for transcutaneous oxygen and/or carbon dioxide measurements for free flap monitoring61. PRISMA, preferred reporting items for systematic reviews and meta-analyses.
A full-text review was performed on all remaining studies following title and abstract review. Additional studies that did not meet inclusion criteria were excluded. The last search was performed on August 17, 2020.
Results
The initial PubMed/MEDLINE/Cochrane search revealed 17 articles eligible for full-text review; of these, 6 were included in both the qualitative and quantitative analysis. 4 5 6 7 8 9 All studies included were prospective in nature ( Fig. 1 ).
The studies collectively included 162 flaps, each with varying measurement modalities and devices. The overall flap failure rate was 4.3% ( n = 7); the overall consensus from these studies demonstrated transcutaneous oxygen partial pressure ( p O 2 ) monitoring in conjunction with physical exam findings of flap compromise were critical to avoiding flap failure and improving flap salvage rates. Notably, drops in p O 2 levels acutely (i.e., within 4–24 hours postoperatively) were indicative of a failing flap. Additionally, severely congested flaps requiring intervention demonstrated markedly increased levels of carbon dioxide partial pressure ( p CO 2 ) levels. These studies are summarized in Table 1 .
Table 1. Summary of studies identified from a PRISMA-guided search of transcutaneous gas monitoring in free flaps.
| Study reference | No. of flaps | Monitoring modality | Value measured | Summary | Flap loss n (%) |
|---|---|---|---|---|---|
|
Abbreviations:
p
CO
2
, carbon dioxide partial pressure;
p
O
2
, oxygen partial pressure.
a Free as well as pedicled flaps. | |||||
| Schiltz et al 4 | 32 | VisiSens | Transcutaneous p O 2 based on tissue flux of O 2 using ratiometric fluorescence imaging | Increased O 2 flux was associated with increased venous congestion and flap failure | 2 (6.7) |
| Trignano et al 5 | 21 a | Licox Recon | Measures tissue p O 2 and temperature through a Clark-type polarographic micro-catheter positioned in the tip of a flexible probe with an oxygen sensing area | Low p O 2 combined with exam findings were indicative of venous congestion and flap failure | 1 (5.5) |
| Geis et al 6 | 9 | Luminescent lifetime imaging | Transcutaneous p O 2 measurements using dynamic phosphorescence imaging and sensors | Drops in p O 2 below 10 mmHg acutely (within 4 hours) required surgical intervention for flap salvage | 0 (0) |
| Schrey et a. 7 | 13 | Licox Polarographic Probe | Tissue p O 2 was measured using a needle probe in Hartrampf perfusion zone 1 of the flap | Needle probes monitoring p O 2 are reliable to evaluate a single portion of the flap but are inadequate at measuring blood flow throughout the entirety of a flap | 1 (7.7) |
| Hashimoto et al 8 | 27 | Polarographic probes | Transcutaneous p O 2 and p CO 2 were measured using probe contains a Clark-type electrode and a pH-sensitive glass electrode, respectively | Physical exam is a necessary adjunct. p O 2 alone is inadequate to determine venous congestion; a congested flap with a p CO 2 more than 90 mm Hg requires further treatment | 1 (3.7) |
| Kamolz et al 9 | 60 | Licox micropolarographic probe | p O 2 was measured with a microcatheter in some centralized portion of the flap | Failing flaps demonstrate a tissue p O 2 decrease of 10 mm Hg within a half-hour, or a tissue p O 2 drop below 10 mm Hg | 2 (3.3) |
| Total | 162 | 7 (4.3) | |||
Study Quality and Risk of Bias
Given the paucity of well-designed studies on flap monitoring in the past 20 years, the studies included in our quantitative meta-analysis scored 7 or greater on the Newcastle–Ottawa scale (NOS), 10 signifying studies were of sound quality. The major biases present in our included studies were selection bias, given the institutions conducting this high-level work are filled with well-experienced microsurgeons with likely hundreds to thousands of free flaps under their belt, with a team of clinicians well versed in flap monitoring. Additionally, the operative indications/criteria used in individual studies was seldom highlighted and thus suggests the patient population in the combined cohort varies considerably.
Current Flap Monitoring Methods
Creech and Miller first described the ideal characteristics for flap monitoring in 1975 and since then, authors have continued to detail optimal flap monitoring techniques. 4 Ideal qualities for any such device may include but are not limited to accurate, reproducible, real time with continuous acquisition of data, quantitative, easy to use or train, operator independent, safe, and low cost. Additionally, in an era of rapidly evolving technological advances, it would ideally be able to communicate encrypted data wirelessly to an electronic medical record or a mobile device, as this has shown promise in increasing flap survival and decreasing delay in return to the operating room with flap compromise. 5 6 However, no such device currently exists, thus understanding current techniques is critical for optimizing flap monitoring and survival.
While clinical examination (color, turgor, temperature, capillary refill, and bleeding to pin prick) remains the gold standard for flap assessment, there exists a plethora of flap monitoring devices. 11 We provide a brief overview of the available options, along with their advantages and disadvantages based on literature review ( Table 2 ). 3 4 Laser Doppler flowmetry (LDF) and near-infrared spectroscopy are not well-suited for buried flaps. Conversely, implantable Doppler, flow coupler, and microdialysis are invasive monitoring methods suitable for buried flaps. 7
Table 2. Overview of free flap monitoring technologies.
| Technology | Mechanism | Advantages | Disadvantages |
|---|---|---|---|
| Abbreviations: Hb, hemoglobin; LSI, laser speckle imaging, NIRS, near-infrared spectroscopy; SFDI, spatial frequency domain imaging; US, ultrasound; VLS, visible light spectroscopy. | |||
| Handheld Doppler US | Detects sound proportional to flap blood flow | Inexpensive Easy to use Noninvasive Rapid assessment With experience, can differentiate venous from arterial signal |
Operator dependent Not quantitative Intermittent monitoring Cannot surveil buried flaps |
| Color duplex US | Visualizes blood flow using US | Relatively inexpensive Noninvasive Rapid assessment Can differentiate venous from arterial blood flow |
Operator dependent Not quantitative Intermittent monitoring Typically requires technician Cannot surveil buried flaps |
| Implantable Doppler probe | Detects sound proportional to flap blood flow, near anastomosis on pedicle | Relatively easy to use Continuous monitoring Rapid assessment Can monitor buried flaps |
Has to be placed intraoperatively Not quantitative High cost |
| Flow coupler | Implantable Doppler incorporated into venous coupler | Same as “implantable Doppler probe” | Same as “implantable Doppler probe” |
| Laser Doppler flowmetry | Blood flow velocity is estimated by illuminating a tissue sample with laser light and then analyzing the frequency distribution of the backscattered light | Continuous monitoring Semiquantitative Very accurate Rapid assessment |
Operator dependent Typically requires technician Cannot surveil buried flaps Very high cost |
| NIRS or VLS | Light of specific wavelength is emitted toward tissue and changes in intensity attributable to relative changes in hemoglobin concentrations, which correlate with tissue oxygenation | Continuous monitoring Quantitative Rapid assessment |
Operator dependent Cannot surveil buried flaps Extremely high cost |
| Microdialysis | Catheter is placed into tissue, sample extracted and presence of metabolites like lactate analyzed | Quantitative Accurate Used in buried flaps |
Sample variability Intermittent monitoring Invasive Not real time Requires technician High cost |
| SFDI | Near infrared light projected and collected in real time, converted into spatial maps of O 2 Hb and Hb concentrations | Real time Quantitative Continuous Can distinguish arterial from venous complications |
High cost Cannot surveil buried flaps |
| LSI | Laser light reflected into video camera produces speckles that correlate with tissue blood flow | Continuous Real time Noninvasive Can distinguish venous from arterial complications |
Cannot surveil buried flaps |
| Oxygen-sensing bandage | Molecule within paint detected by camera creates 2-map of oxygenation | Real time Noninvasive Can be applied to high topography regions Low cost |
Intermittent monitoring Not for acute use Sample variability |
| Thermal imaging | Temperature of skin measured by infrared sensor on handheld device | Real time Noninvasive Low cost |
Operator dependent Intermittent monitoring Only useful for flaps with cutaneous island |
Transcutaneous Oxygen Monitoring
Although various methods exist that detect a change in blood flow to the flap, none are direct indicators of cellular metabolism or oxygen availability to the tissue. An absolute measurement of arterial oxygen for noninvasive flap monitoring was not described until Achauer et al studied the use of the polarographic Clark-type electrode in measuring transcutaneous oxygen. This was accomplished by measuring the electrical current flowing into the electrode, which is dependent on the number of oxygen molecules available to be reduced as they diffuse through the tissue and out from the skin. 8
Early studies evaluating the use of a transcutaneous oxygen monitor showed it to be a quick and reliable indicator of flap perfusion. 9 In evaluating free flaps and digital replants, Smith et al found transcutaneous oxygen monitoring to be an overall accurate indicator of flap viability and able to detect impending flap failure before clinical signs and temperature changes. Moreover, they showed an increase in the success rate of reintervention using these measurements: a metric largely dependent on the speed of detecting vascular compromise. 10 Although Achauer et al had noted a significant variability between individuals partly due to differences in systemic arterial p O 2 , 8 Serafin et al found that variation in core temperature, systemic blood pressure, and pulse had negligible effects on the transcutaneous oxygen measurements. 9 The residual interobserver variability could be resolved by comparing the flap site measurements with measurements taken from a nonoperated site and then expressing the results as a percent change from “normal.” 12
Despite these promising early findings, there were still numerous flaws with using the Clark electrode. Although some of the interindividual variability could be accounted for, eschar formation, edema, and the measurement site being over bone decreased the reliability of measurement. 8 Several studies also found that while transcutaneous O 2 ( ptc O 2 ) may be a rapid indicator of vascular compromise, it may be too sensitive, so that during low-flow conditions in the early postoperative period, even flaps that go on to survive show low ptc O 2 values indistinguishable from those of failing flaps. 13 14 15 16 Moreover, some have noted that measurement accuracy is highly dependent on probe placement and user expertise 1 12 15 17 18 19 and the device may be difficult to use for continuous monitoring. 1 20 Because normally very few oxygen molecules reach the skin surface by diffusion, this method may require inducing local hyperemia by heating the skin and increasing the amount of oxygen that can be measured. This is more cumbersome and may place the patient at risk for burns with long-term monitoring.
Other than the Clark electrode, luminescence lifetime imaging (LLI), and luminescence ratiometric oxygen imaging (LROI) can also provide a direct measure of tissue oxygen by using a concept of oxygen-dependent fluorescent decay. LLI was found to be better able to produce accurate readings than the Clark electrode, as well as more responsive to microcirculatory changes. 8 21 LROI could also differentiate between healthy and failing flaps, but its accuracy is dependent on a long acquisition period, examiner skill, and experience with the device. Furthermore, the probe cannot reach less accessible flaps such as intraoral and noncutaneous flaps. 22 23 While the monitoring depth of LROI can be increased by performing measurement alongside contrast-enhanced ultrasound (CEUS) to monitor noncutaneous flaps and flap portions, CEUS is a more invasive form of monitoring requiring the intravenous administration of contrast. 24
Since then, other measurement modalities have been developed to monitor tissue oxygen. Notably, near-infrared spectroscopy (NIRS) evaluates the relative concentrations of oxygenated and deoxygenated hemoglobin and oxidized cytochrome aa3 as an indicator of tissue oxygen supply and intracellular oxygen availability. Irwin et al first investigated the use of NIRS to monitor flap viability and found numerous advantages over the polarographic Clark-type electrode. 25 NIRS can monitor deeper depths, possibly even buried flaps, and is immutable to seroma or hematoma formation. 6 26 27 Although measurement accuracy can be affected by probe movement, the movement must be dramatic. However, NIRS technology cannot provide absolute values and numerous other investigators have found relative trend or index values were better predictors of vascular compromise. 25 28 29 30 31 Stranc et al confirmed these findings in a rat model by using an O 2 saturation index that increased the sensitivity of detecting tissue hypoxia and correlated with necrosis onset. 32 They found that using this index gave highly reproducible results allowing for early prediction of flap survival. 32 In the clinical setting, many studies demonstrated that NIRS technology could not only predict flap compromise before clinical manifestation without false positives or negatives, but could also detect and distinguish between arterial and venous occlusion. 6 27 29 33 34 35 In addition, NIRS monitoring was found to have greater sensitivity and specificity than clinical examination alone 27 and led to lower flap failure rates for one investigator. 34 Moreover, it may decrease the amount of time spent in postoperative recovery, the length of intensive care unit (ICU) stays, and the amount of nursing care required for monitoring. 33 Another advantage of NIRS technology include ease-of-use requiring minimal training. 6 33 34 36
Flaws with the NIRS monitoring system have also been reported. Contrary to earlier reports, Scheufler et al found hematoma formation affected the accuracy of NIRS monitoring. 28 35 In addition, they found nonviable tissue may still be perfused even after prolonged ischemia. 28 Serosanguinous buildup, systemic oxygen saturation, and systemic blood pressure variations may affect measurement accuracy and careful interpretation of measurements is needed. 37 Salgarello et al described a negative relationship between regional oxygen saturation and body mass index, as well as flap size, and suggested the current NIRS thresholds may need to be reevaluated in patients with high body fat content. 38 Perhaps the most prohibitive flaw to NIRS monitoring is expense as reported costs range from $8,000 to 50,000 per unit. 3 33 34 36 It has yet to bet determined whether the hospital cost savings can offset the expense associated with currently available NIRS flap monitoring technology. 33
To offset these flaws, some have proposed concurrent use of LDF, even though it has been found to be inferior to NIRS. 28 35 In response, a system combining the two technologies was developed, which Hölzle et al used with some success. 39 40 They found that the combined NIRS/LDF detected all vascular complications with no false positives or negatives and was able to establish a threshold indicating vascular compromise for different flap types. In addition to detecting impending flap failure before clinical signs were present, the combined modalities prevented unnecessary return to the operating room when clinical signs would suggest otherwise. However, continuous monitoring was difficult, and the system was unable to survey buried flaps. Other proposed improvements to measuring tissue oxygenation, such as broadband measurements to detect cytochrome aa3 and Erlangen microlightguide spectrophotometry, failed to show significant superiority to existing technology. 41 42 However, Olenczak et al noted that a more reliable measurement of cytochrome aa3 may be a better indicator of flap compromise than measures of hemoglobin oxygenation and concentration. 41
One the newer technologies for flap monitoring involving transcutaneous oxygen monitoring is the oxygen-sensing paint-on bandage. The oxygen-sensing molecule embedded in the bandage allows for smart-phone cameras to acquire images that can be converted into a two-dimensional map of tissue oxygenation. Early studies indicate the oxygen-sensing bandage is comparable to NIRS in detecting tissue oxygenation during vascular compromise. 43 Despite ease of use, this technology remains costly and not widely available.
Other modalities of note include spatial frequency domain imaging (SFDI), visible light spectroscopy (VLS), laser speckle imaging (LSI), and thermal imaging. SFDI provides similar measurements as NIRS but surveys a larger area of interest and does not require cutaneous contact. While it could reliably distinguish between arterial and venous occlusion with low variability in measurements, it may be difficult to use as it requires correct calibration and relies on patients remaining still for a long period of time. In addition, results may be confounded by thinner flaps, room illumination, and skin pigmentation. 44 45 While SFDI has demonstrated potential usefulness for intraoperative planning, it has not yet been evaluated for postoperative flap monitoring in human subjects. Visible light spectroscopy also allows similar measurements and may be more sensitive to smaller changes in tissue oxygenation. 11 46 47 48 It is more applicable for smaller flaps, such as those used in the upper extremity, including digital replantation. 11 Kagaya et al found that NIRS was better for evaluating fat perfusion or the perfusion of buried flaps, while visible light spectroscopy is more suited to thinner flaps. 46 Despite promising results, high interobserver variation precluded establishment of a threshold for flap failure. 47 Laser speckle imaging involves capturing reflected laser light of a tissue as speckles on a video-camera detector array which correlates with blood flow as the speckles move in relation to the red blood cells moving. 49 This technology is fast, easy to use, noninvasive, and may be a valuable adjunct for use intra- and postoperatively for evaluating tissue perfusion of free flaps. 7 Another relatively new technology for monitoring free flaps is use of thermal imaging cameras. Cruz-Segura et al found that a difference of more than 2°C between flap and surrounding skin as detected by thermal imaging may allow for diagnosis of flap vascular compromise up to 12 hours earlier than clinical examination. 50 Of note, Ricci et al combined the transcutaneous p O 2 monitoring with contemporary communications modalities to notify the surgeon of flap compromise via text message alert to expedite surgical intervention as needed, which highlights how modern technology can continue to improve flap care.
Transcutaneous Carbon Dioxide Monitoring
Minimal work has been done regarding clinical efficacy evaluation of transcutaneous carbon dioxide partial pressure ( p CO 2 or ptc O 2 ) measurement in flap monitoring. Early uses of this technology include detection of skin necrosis in necrotizing fasciitis, bullous pemphigoid arteriosclerosis obliterans, distal ischemia of canine pedicle flaps, and pressure ulcer monitoring. 51 52 53 54 55 One modality recently developed involves a bifunctional probe taped to the skin and which measures both transcutaneous ptc O 2 and ptc CO 2 . 19 While this technology is at least semiquantitative and can detect total venous occlusion, it does not appear sensitive enough to detect partial venous occlusion. Other drawbacks of current technology include the need for substantial training in calibration and placement of the probe for accurate measurement. 19 Similar to ptc O 2 monitoring, ptc CO 2 monitoring may ideally be conducted at supraphysiologic temperatures (~44°C) to increase the gas permeability of skin. 14 However, other studies argue that flaps can be monitored effectively at 37°C. 20 Without having to heat the skin, the probe does not require constant repositioning to spare the underlying skin, thereby improving the accuracy of ptc CO 2 measurements. 20 The ability to attach the probe for long durations raises the possibility of ptc O 2 and ptc CO 2 continuous flap monitoring. 20 56 57 58 59 Combined ptc O 2 and ptc CO 2 monitoring has also shown promise in selecting the number and duration of leech therapy. 60 While ptc O 2 and ptc CO 2 have been measured together, each has its own advantages and disadvantages. Measurements of ptc O 2 respond more rapidly to occlusion than ptc CO 2 , but ptc CO 2 is influenced far less by external factors than ptc O 2 . 14
Future Directions
Flap monitoring is a necessary part of postoperative care following free tissue transfer. In addition to a conventional flap clinical examination, which relies on an experienced clinician and is done intermittently, there is a critical need to develop a method or device that can provide accurate, reliable, rapid, easy-to-use, and continuous assessment of flap perfusion. As discussed earlier, techniques that leverage measurements of ptc O 2 and ptc CO 2 measurements may be the most promising as tissue oximetry is associated with higher salvage rates compared with clinical examination alone. Transcutaneous gas measurements in flaps are not susceptible to core temperature, blood pressure, or pulse rates. If these technologies can be reduced in cost and made more widely available, it may result in systematic improved protocol for flap salvage.
With free flap reconstruction currently being a generally uncommon procedure and flap loss rates generally less than 5%, cost may be the most critical barrier for most institutions investing in newer, promising technology. If a device could be designed and manufactured for much less cost, more microsurgical teams would have access to it, leading to more use and ultimately data collection, more technological refinements to optimize its utility, and thus more universal adoption of a newer adjunct technique for flap monitoring. Some of the newer technologies, such as thermal imaging and oxygen-sensing bandages, require little more than a digital camera or smartphone add on which could prove invaluable for widely distributing these devices. More work needs to be done to reduce the cost of acquiring high fidelity ptc O 2 and ptc CO 2 data as an adjunct to the clinical examination to potentially improve free flap salvage rates.
Footnotes
Conflict of Interest None declared.
References
- 1.Jones B M. Monitors for the cutaneous microcirculation. Plast Reconstr Surg. 1984;73(05):843–850. doi: 10.1097/00006534-198405000-00025. [DOI] [PubMed] [Google Scholar]
- 2.Chae M P, Rozen W M, Whitaker I S. Current evidence for postoperative monitoring of microvascular free flaps: a systematic review. Ann Plast Surg. 2015;74(05):621–632. doi: 10.1097/SAP.0b013e3181f8cb32. [DOI] [PubMed] [Google Scholar]
- 3.Scheufler O, Exner K, Andresen R. Investigation of TRAM flap oxygenation and perfusion by near-infrared reflection spectroscopy and color-coded duplex sonography. Plast Reconstr Surg. 2004;113(01):141–152. doi: 10.1097/01.PRS.0000095940.96294.A5. [DOI] [PubMed] [Google Scholar]
- 4.Schiltz D, Taeger C D, Biermann N. Transcutaneous oxygen measurement using ratiometric fluorescence imaging as a valid method for monitoring free flap transplants. Clin Hemorheol Microcirc. 2019;73(01):113–123. doi: 10.3233/CH-199225. [DOI] [PubMed] [Google Scholar]
- 5.Trignano E, Fallico N, Fiorot L. Flap monitoring with continuous oxygen partial tension measurement in breast reconstructive surgery: a preliminary report. Microsurgery. 2018;38(04):402–406. doi: 10.1002/micr.30256. [DOI] [PubMed] [Google Scholar]
- 6.Geis S, Schreml S, Lamby P.Postoperative assessment of free skin flap viability by transcutaneous pO2 measurement using dynamic phosphorescence imaging Clin Hemorheol Microcirc 200943(1,2)11–18. [DOI] [PubMed] [Google Scholar]
- 7.Schrey A, Niemi T, Kinnunen I. The limitations of tissue-oxygen measurement and positron emission tomography as additional methods for postoperative breast reconstruction free-flap monitoring. J Plast Reconstr Aesthet Surg. 2010;63(02):314–321. doi: 10.1016/j.bjps.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto I, Nakanishi H, Takiwaki H, Takase M T, Yamano M, Sedo H. Flap monitoring by transcutaneous PO2 and PCO2: importance of transcutaneous PCO2 in determining follow-up treatment for compromised free flaps. J Reconstr Microsurg. 2007;23(05):269–274. doi: 10.1055/s-2007-985208. [DOI] [PubMed] [Google Scholar]
- 9.Kamolz L-P, Giovanoli P, Haslik W, Koller R, Frey M. Continuous free-flap monitoring with tissue-oxygen measurements: three-year experience. J Reconstr Microsurg. 2002;18(06):487–491. doi: 10.1055/s-2002-33319. [DOI] [PubMed] [Google Scholar]
- 10.Lo CK-L, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hölzle F, Loeffelbein D J, Nolte D, Wolff K D. Free flap monitoring using simultaneous non-invasive laser Doppler flowmetry and tissue spectrophotometry. J Craniomaxillofac Surg. 2006;34(01):25–33. doi: 10.1016/j.jcms.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Karinja S J, Lee B T. Advances in flap monitoring and impact of enhanced recovery protocols. J Surg Oncol. 2018;118(05):758–767. doi: 10.1002/jso.25179. [DOI] [PubMed] [Google Scholar]
- 13.Hwang J H, Mun G H. An evolution of communication in postoperative free flap monitoring: using a smartphone and mobile messenger application. Plast Reconstr Surg. 2012;130(01):125–129. doi: 10.1097/PRS.0b013e318254b202. [DOI] [PubMed] [Google Scholar]
- 14.Ricci J A, Vargas C R, Lin S J, Tobias A M, Taghinia A H, Lee B T. A novel free flap monitoring system using tissue oximetry with text message alerts. J Reconstr Microsurg. 2016;32(05):415–420. doi: 10.1055/s-0036-1582264. [DOI] [PubMed] [Google Scholar]
- 15.To C, Rees-Lee J E, Gush R J. Intraoperative tissue perfusion measurement by laser speckle imaging: a potential aid for reducing postoperative complications in free flap breast reconstruction. Plast Reconstr Surg. 2019;143(02):287e–292e. doi: 10.1097/PRS.0000000000005223. [DOI] [PubMed] [Google Scholar]
- 16.Achauer B M, Black K S, Litke D K. Transcutaneous PO2 in flaps: a new method of survival prediction. Plast Reconstr Surg. 1980;65(06):738–745. doi: 10.1097/00006534-198006000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Serafin D, Lesesne C B, Mullen R Y, Georgiade N G. Transcutaneous PO2 monitoring for assessing viability and predicting survival of skin flaps: experimental and clinical correlations. J Microsurg. 1981;2(03):165–178. doi: 10.1002/micr.1920020303. [DOI] [PubMed] [Google Scholar]
- 18.Smith A R, Sonneveld G J, Kort W J, van der Meulen J C. Clinical application of transcutaneous oxygen measurements in replantation surgery and free tissue transfer. J Hand Surg Am. 1983;8(02):139–145. doi: 10.1016/s0363-5023(83)80004-5. [DOI] [PubMed] [Google Scholar]
- 19.Achauer B M, Black K S. Transcutaneous oxygen and flaps. Plast Reconstr Surg. 1984;74(05):721–722. doi: 10.1097/00006534-198411000-00025. [DOI] [PubMed] [Google Scholar]
- 20.Raskin D J, Nathan R, Erk Y, Spira M. Critical comparison of transcutaneous PO2 and tissue pH as indices of perfusion. Microsurgery. 1983;4(01):29–33. doi: 10.1002/micr.1920040110. [DOI] [PubMed] [Google Scholar]
- 21.Tuominen H P, Asko-Seljavaara S, Svartling N E, Härmä M A. Cutaneous blood flow in the TRAM flap. Br J Plast Surg. 1992;45(04):261–269. doi: 10.1016/0007-1226(92)90049-4. [DOI] [PubMed] [Google Scholar]
- 22.Lantsberg L, Goldman M. Laser Doppler flowmetry, transcutaneous oxygen tension measurements and Doppler pressure compared in patients undergoing amputation. Eur J Vasc Surg. 1991;5(02):195–197. doi: 10.1016/s0950-821x(05)80687-5. [DOI] [PubMed] [Google Scholar]
- 23.Rochat M C, Pope E R, Payne J T, Pace L W, Wagner-Mann C C. Transcutaneous oxygen monitoring for predicting skin viability in dogs. Am J Vet Res. 1993;54(03):468–475. [PubMed] [Google Scholar]
- 24.Bradford C R. Flap monitoring. Facial Plast Surg. 1996;12(01):19–21. doi: 10.1055/s-2008-1064488. [DOI] [PubMed] [Google Scholar]
- 25.Gimbel M L, Rollins M D, Fukaya E, Hopf H W. Monitoring partial and full venous outflow compromise in a rabbit skin flap model. Plast Reconstr Surg. 2009;124(03):796–803. doi: 10.1097/PRS.0b013e3181b03768. [DOI] [PubMed] [Google Scholar]
- 26.Abe Y, Hashimoto I, Goishi K, Kashiwagi K, Yamano M, Nakanishi H. Transcutaneous PCO2 measurement at low temperature for reliable and continuous free flap monitoring: experimental and clinical study. Plast Reconstr Surg Glob Open. 2013;1(02):1–8. doi: 10.1097/GOX.0b013e3182936cd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geis S, Babilas P, Schreml S. Transcutaneous pO2 measurement during tourniquet-induced venous occlusion using dynamic phosphorescence imaging. Clin Hemorheol Microcirc. 2008;40(04):249–258. [PubMed] [Google Scholar]
- 28.Meier J K, Prantl L, Müller S, Moralis A, Liebsch G, Gosau M.Simple, fast and reliable perfusion monitoring of microvascular flaps Clin Hemorheol Microcirc 201250(1-2)13–24. [DOI] [PubMed] [Google Scholar]
- 29.Meier J K, Prantl L, Geis S. Luminescence ratiometric oxygen imaging (LROI) in microvascular anastomosed fibular and radial forearm flaps. Clin Hemorheol Microcirc. 2013;55(01):169–182. doi: 10.3233/CH-131700. [DOI] [PubMed] [Google Scholar]
- 30.Mueller S, Meier J K, Wendl C M, Jung E M, Prantl L, Gosau M.Mandibular reconstruction with microvascular re-anastomosed fibular free flaps - two complementary methods of postoperative transplant monitoring Clin Hemorheol Microcirc 201252(2,4)141–151. [DOI] [PubMed] [Google Scholar]
- 31.Irwin M S, Thorniley M S, Doré C J, Green C J. Near infra-red spectroscopy: a non-invasive monitor of perfusion and oxygenation within the microcirculation of limbs and flaps. Br J Plast Surg. 1995;48(01):14–22. doi: 10.1016/0007-1226(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 32.Cai Z G, Zhang J, Zhang J G. Evaluation of near infrared spectroscopy in monitoring postoperative regional tissue oxygen saturation for fibular flaps. J Plast Reconstr Aesthet Surg. 2008;61(03):289–296. doi: 10.1016/j.bjps.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 33.McKenna J, Pabbies A, Friesen J R, Sowa M G, Hayakawa T, Kerr P D. Assessing flap perfusion: optical spectroscopy versus venous Doppler ultrasonography. J Otolaryngol Head Neck Surg. 2009;38(05):587–594. [PubMed] [Google Scholar]
- 34.Whitaker I S, Pratt G F, Rozen W M. Near infrared spectroscopy for monitoring flap viability following breast reconstruction. J Reconstr Microsurg. 2012;28(03):149–154. doi: 10.1055/s-0031-1296030. [DOI] [PubMed] [Google Scholar]
- 35.Scheufler O, Andresen R. Tissue oxygenation and perfusion in inferior pedicle reduction mammaplasty by near-infrared reflection spectroscopy and color-coded duplex sonography. Plast Reconstr Surg. 2003;111(03):1131–1146. doi: 10.1097/01.PRS.0000046615.36917.3E. [DOI] [PubMed] [Google Scholar]
- 36.Keller A. Noninvasive tissue oximetry for flap monitoring: an initial study. J Reconstr Microsurg. 2007;23(04):189–197. doi: 10.1055/s-2007-974655. [DOI] [PubMed] [Google Scholar]
- 37.Keller A. A new diagnostic algorithm for early prediction of vascular compromise in 208 microsurgical flaps using tissue oxygen saturation measurements. Ann Plast Surg. 2009;62(05):538–543. doi: 10.1097/SAP.0b013e3181a47ce8. [DOI] [PubMed] [Google Scholar]
- 38.Akita S, Mitsukawa N, Tokumoto H. Regional oxygen saturation index: a novel criterion for free flap assessment using tissue oximetry. Plast Reconstr Surg. 2016;138(03):510e–518e. doi: 10.1097/PRS.0000000000002498. [DOI] [PubMed] [Google Scholar]
- 39.Stranc M F, Sowa M G, Abdulrauf B, Mantsch H H. Assessment of tissue viability using near-infrared spectroscopy. Br J Plast Surg. 1998;51(03):210–217. doi: 10.1054/bjps.1997.0088. [DOI] [PubMed] [Google Scholar]
- 40.Repez A, Oroszy D, Arnez Z M. Continuous postoperative monitoring of cutaneous free flaps using near infrared spectroscopy. J Plast Reconstr Aesthet Surg. 2008;61(01):71–77. doi: 10.1016/j.bjps.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Lin S J, Nguyen M D, Chen C. Tissue oximetry monitoring in microsurgical breast reconstruction decreases flap loss and improves rate of flap salvage. Plast Reconstr Surg. 2011;127(03):1080–1085. doi: 10.1097/PRS.0b013e31820436cb. [DOI] [PubMed] [Google Scholar]
- 42.Payette J R, Kohlenberg E, Leonardi L. Assessment of skin flaps using optically based methods for measuring blood flow and oxygenation. Plast Reconstr Surg. 2005;115(02):539–546. doi: 10.1097/01.prs.0000148415.54546.ca. [DOI] [PubMed] [Google Scholar]
- 43.Colwell A S, Wright L, Karanas Y. Near-infrared spectroscopy measures tissue oxygenation in free flaps for breast reconstruction. Plast Reconstr Surg. 2008;121(05):344e–345e. doi: 10.1097/PRS.0b013e31816b11e5. [DOI] [PubMed] [Google Scholar]
- 44.Ozturk C N, Ozturk C, Ledinh W. Variables affecting postoperative tissue perfusion monitoring in free flap breast reconstruction. Microsurgery. 2015;35(02):123–128. doi: 10.1002/micr.22276. [DOI] [PubMed] [Google Scholar]
- 45.Salgarello M, Pagliara D, Rossi M, Visconti G, Barone-Adesi L. Postoperative monitoring of free DIEP Flap in breast reconstruction with near-infrared spectroscopy: variables affecting the regional oxygen saturation. J Reconstr Microsurg. 2018;34(06):383–388. doi: 10.1055/s-0038-1636527. [DOI] [PubMed] [Google Scholar]
- 46.Hölzle F, Rau A, Loeffelbein D J, Mücke T, Kesting M R, Wolff K D. Results of monitoring fasciocutaneous, myocutaneous, osteocutaneous and perforator flaps: 4-year experience with 166 cases. Int J Oral Maxillofac Surg. 2010;39(01):21–28. doi: 10.1016/j.ijom.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 47.Olenczak J B, Murariu D, Ikeda K, Thiele R H, Campbell C A. Tissue monitoring with three-wavelength light emitting diode-based near-infrared spectroscopy. J Reconstr Microsurg. 2016;32(09):712–718. doi: 10.1055/s-0036-1586256. [DOI] [PubMed] [Google Scholar]
- 48.Cornejo A, Rodriguez T, Steigelman M. The use of visible light spectroscopy to measure tissue oxygenation in free flap reconstruction. J Reconstr Microsurg. 2011;27(07):397–402. doi: 10.1055/s-0031-1281521. [DOI] [PubMed] [Google Scholar]
- 49.Rauh A, Henn D, Nagel S S, Bigdeli A K, Kneser U, Hirche C. Continuous video-rate laser speckle imaging for intra- and postoperative cutaneous perfusion imaging of free flaps. J Reconstr Microsurg. 2019;35(07):489–498. doi: 10.1055/s-0039-1681076. [DOI] [PubMed] [Google Scholar]
- 50.Cruz-Segura A, Cruz-Domínguez M P, Jara L J. Early detection of vascular obstruction in microvascular flaps using a thermographic camera. J Reconstr Microsurg. 2019;35(07):541–548. doi: 10.1055/s-0039-1688749. [DOI] [PubMed] [Google Scholar]
- 51.Wolff K D, Marks C, Uekermann B, Specht M, Frank K H. Monitoring of flaps by measurement of intracapillary haemoglobin oxygenation with EMPHO II: experimental and clinical study. Br J Oral Maxillofac Surg. 1996;34(06):524–529. doi: 10.1016/s0266-4356(96)90250-8. [DOI] [PubMed] [Google Scholar]
- 52.Koolen P GL, Li Z, Roussakis E. Oxygen-sensing paint-on bandage: calibration of a novel approach in tissue perfusion assessment. Plast Reconstr Surg. 2017;140(01):89–96. doi: 10.1097/PRS.0000000000003421. [DOI] [PubMed] [Google Scholar]
- 53.Pharaon M R, Scholz T, Bogdanoff S. Early detection of complete vascular occlusion in a pedicle flap model using quantitative [corrected] spectral imaging. Plast Reconstr Surg. 2010;126(06):1924–1935. doi: 10.1097/PRS.0b013e3181f447ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yafi A, Vetter T S, Scholz T. Postoperative quantitative assessment of reconstructive tissue status in a cutaneous flap model using spatial frequency domain imaging. Plast Reconstr Surg. 2011;127(01):117–130. doi: 10.1097/PRS.0b013e3181f959cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mericli A F, Wren J, Garvey P B, Liu J, Butler C E, Selber J C. A prospective clinical trial comparing visible light spectroscopy to handheld doppler for postoperative free tissue transfer monitoring. Plast Reconstr Surg. 2017;140(03):604–613. doi: 10.1097/PRS.0000000000003600. [DOI] [PubMed] [Google Scholar]
- 56.Kagaya Y, Miyamoto S. A systematic review of near-infrared spectroscopy in flap monitoring: Current basic and clinical evidence and prospects. J Plast Reconstr Aesthet Surg. 2018;71(02):246–257. doi: 10.1016/j.bjps.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 57.Takiwaki H, Arase S, Nakanishi H, Takeda K. Transcutaneous PO2 and PCO2 measurements in various skin lesions. J Dermatol. 1991;18(06):311–313. doi: 10.1111/j.1346-8138.1991.tb03090.x. [DOI] [PubMed] [Google Scholar]
- 58.Rochat M C, Payne J T, Pope E R, Wagner-Mann C C, Pace L W. Evaluation of skin viability in dogs, using transcutaneous carbon dioxide and sensor current monitoring. Am J Vet Res. 1993;54(03):476–480. [PubMed] [Google Scholar]
- 59.Liu L Q, Deegan R, Gall A. Non-invasive technologies of tissue viability measurement for pressure ulcer prevention in spinal cord injury. J Phys Med Rehabil Disabil. 2015;1:2. [Google Scholar]
- 60.Kashiwagi K, Hashimoto I, Abe Y, Kotsu K, Yamano M, Nakanishi H.Quantitative analysis of hemodynamics of congested island flaps under leech therapy J Med Invest 201360(3,4)213–220. [DOI] [PubMed] [Google Scholar]
- 61.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. 2009;6(07):e1000097. [PMC free article] [PubMed] [Google Scholar]