Abstract
Cannabis e-cigarettes containing Δ8-tetrahydrocannabinol (Δ8-THC) produced synthetically from hemp-derived cannabidiol (CBD) have recently risen in popularity as legal means of cannabis consumption, but questions surrounding purity and unlabeled additives have created doubts of their safety. Herein, NMR, GC-MS, and ICP-MS were used to analyze major components of 27 products from 10 brands, and it was determined none of these had accurate Δ8-THC labelling, 11 had unlabeled cutting agents, and all contained reaction side-products including olivetol, Δ4(8)-iso-tetrahydrocannabinol, 9-ethoxyhexahydrocannabinol, Δ9-tetrahydrocannabinol (Δ9-THC), heavy metals, and a previously unidentified cannabinoid iso-tetrahydrocannabifuran.
Keywords: Cannabis, e-cigarette, vaporizer, delta-8-THC, THC, olivetol, heavy metal, EVALI
Graphical Abstract
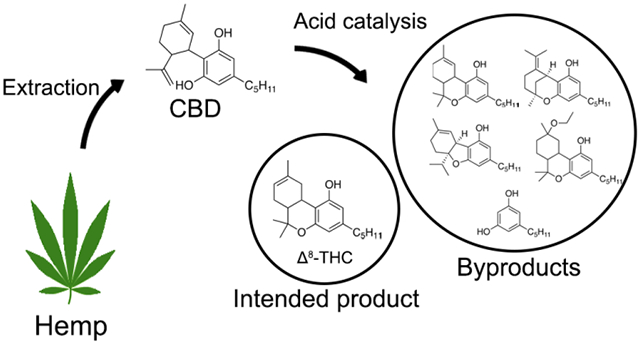
Cannabis e-cigarettes (CECs) are a non-combustion inhalation method which uses technology adapted from electronic nicotine delivery systems. CECs vaporize an oil rich in Δ9-THC, the psychoactive principle of Cannabis sativa, and release hundreds of chemical breakdown products including carcinogenic and irritating gases such as isoprene, benzene, methacrolein, and methyl vinyl ketone.1,2 CECs are popular with teens and young adults in the United States, with 23.7% of 12th graders having reported lifetime cannabis vaping in 2019.3 CEC use was shown to be independently associated with higher odds of respiratory symptoms such as wheezing.4 The 2019 outbreak of e-cigarette or vaping product use-associated lung injury (EVALI) was centered around CECs, and though vitamin E acetate was identified as a potential causative agent, other ingredients or aerosol components were not ruled out.5 Some EVALI CECs were later found to contain unnatural cannabinoid distributions suggesting these were of synthetic origin.6,7 Niche online communities that recount intoxication experiences by minor and/or synthetic cannabinoids have existed likely for decades,8 but it is only recently that cannabinoids other than those naturally-occurring have reached broad commercial availability.9 At the forefront of this trend is Δ8-THC (Chart 1).
Chart 1.
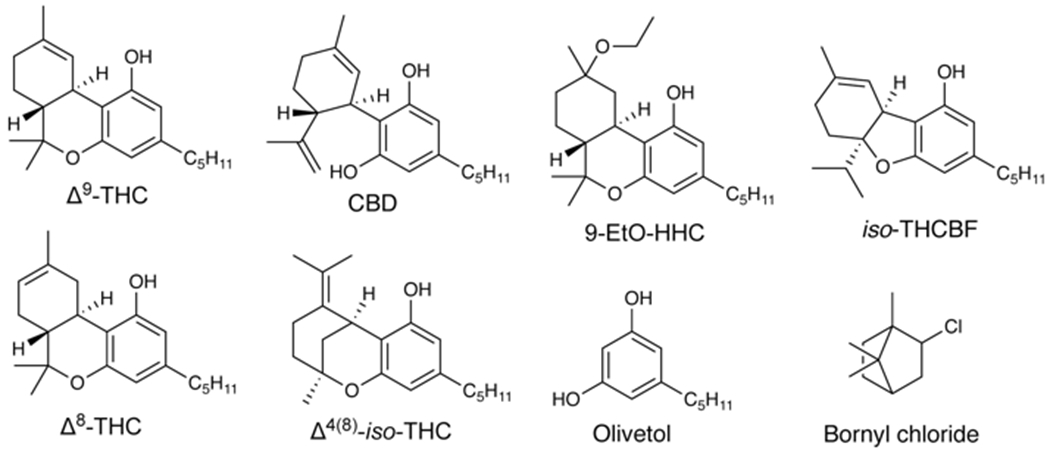
Relevant structures
Δ8-THC is an isomer of Δ9-THC not produced biosynthetically10 but present at low levels in most cannabis products as a result of spontaneous isomerization given its higher thermodynamic stability and resistance to oxidative degradation than Δ9-THC.10 Recent federal regulations that are permissive of hemp-derived products11 have resulted in a rapid growth in usage of Δ8-THC CECs that are abundantly available to consumers through brick-and-mortar and online sources. Extensive hospitalizations involving suspected Δ8-THC consumption have been recently documented.12 Δ8-THC is synthesized via acid-catalyzed cyclization of CBD.13,14 Though Δ9-THC is the direct product of CBD cyclization (Scheme 1), Δ8-THC is favored as a major product at longer reaction times.15,16
Scheme 1.
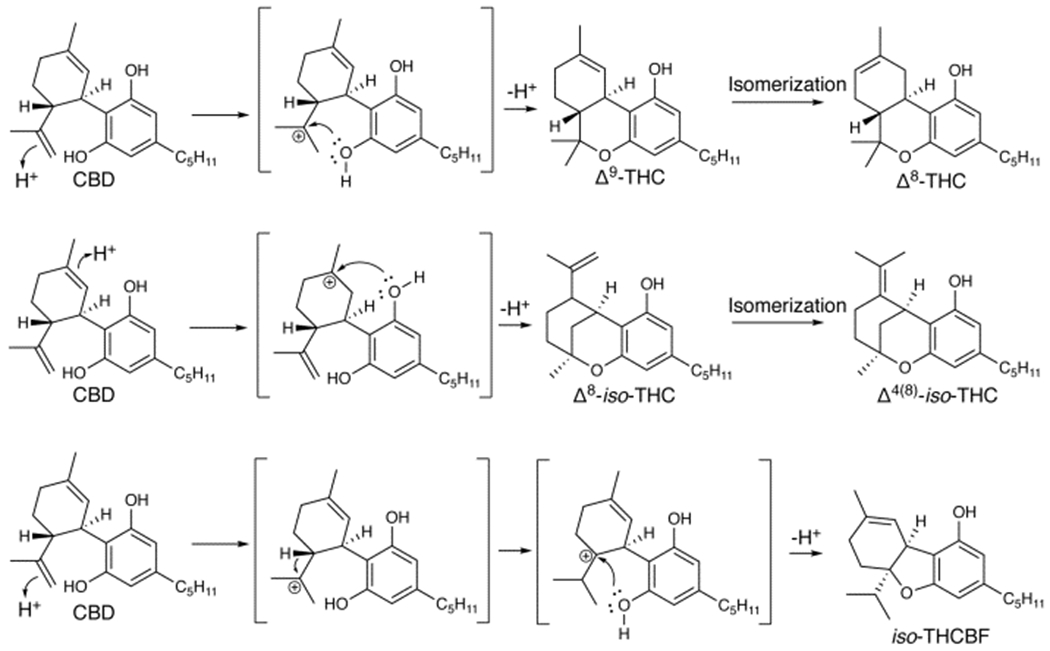
Routes of formation of Δ8-THC (upper), Δ4(8)-iso-THC (middle), and iso-THCBF (lower) from CBD via acid catalysis.
Hydrochloric acid (HCl), sulfuric acid, p-toluenesulfonic acid, boron trifluoride,and camphorsulfonic acid, among others, are viable catalysts, but the acids, solvents, and purification steps used by manufacturers are not known.15,16 In order to address this emerging class of products, available flavor formulations from different Δ8-THC brands were obtained. Proton nuclear magnetic resonance spectroscopy (1H NMR) was chosen as the primary analytical tool given this instrument’s capability for characterizing analytically-challenging vaporizer adulterants17 without the need for derivatization or developing dedicated chromatographic methods which may be necessary complex samples. Quantitative 1H NMR (QNMR) was used to report component levels in these products, a facile but direct quantitative method whose limitations include the fact that some components cannot be identified or quantified due to spectral overlap of their resonances, and its inherently low sensitivity precludes identification of ultra-trace impurities.
Medium chain triglyceride oil was identified in B5 (3.71 ± 0.06%, ± SEM), B6 (3.48 ± 0.06%), B8 (2.94 ± 0.05%), and B9 (5.6±0.1%). Triethyl citrate (TEC) was identified in F20 (6.3 ± 0.06%), F21 (6.27 ± 0.03%), F22 (6.5 ± 0.1%), G23 (7.28 ± 0.05%), G24 (6.2 ± 0.1%), I26 (11.1 ± 0.1%), and J27 (5.34 ± 0.06%). Δ4(8)-iso-Tetrahydrocannabinol (Δ4(8)-iso-THC) is a previously described byproduct of acid-catalyzed CBD cyclization (Scheme 1)18 and was detected in all 27 samples ranging from 2.36 ± 0.05% to 12.79 ± 0.06% with ± SD of 5.4 ± 3.5 % in n=16 where quantification was possible (see SI). Olivetol (5-pentyl-1,3-benzenediol, Chart 1) was identified in 22/27 products, but its quantification was not possible (see SI). Olivetol has been previously shown in EVALI-associated CECs that also contain unnatural cannabinoid distributions7 and is likely a byproduct of chemical synthesis. Olivetol is a synthetic precursor to tetrahydrocannabinols,19,20 and its presence could indicate the use of these pathways for production. 9-Ethoxyhexahydrocannabinol (9-EtO-HHC) is a known byproduct of CBD cyclization in ethanol21 and was detected in D13 and D14. 9-EtO-HHC presence is correlated with lower levels of Δ8-THC (p<0.01) and higher levels of Δ4(8)-iso-THC (p<0.01) than in D15 and D16, suggesting ethanol may favor Δ4(8)-iso-THC formation as opposed to Δ8-THC. Bornyl chloride (Chart 1), a known reaction product of HCl and β-pinene,22 was tentatively identified by GC-MS (Figure S23) in A2 and A3, and is indicative of HCl as a cyclization catalyst. However, its absence in other products does not rule out the use of HCl, as its presence in A2 and A3 may simply be evidence of starting material impure with β-pinene. The potential for bornyl chloride to generate HCl gas when pyrolyzed23 could present a significant inhalation hazard.
In addition to the above, a molecule which, to the best of the authors’ knowledge, has never been previously described was also identified. The cannabinoid (5aR,9aS)-5a-isopropyl-8-methyl-3-pentyl-5a,6,7,9a-tetrahydrodibenzo[b,d]furan-1-ol or iso-tetrahydrocannabifuran (iso-THCBF, Chart 1) is likely the result of a hydride shift in the carbocation intermediate (Scheme 1). iso-THCBF was isolated from Δ8-THC CEC products and characterized by mass spectrometry and 1D and 2D NMR (see SI). iso-THCBF was present in nearly all products tested but was not quantifiable in products containing TEC due to spectral overlap.
CEC screening by inductively coupled plasma-mass spectrometry (ICP-MS) shows the existence of metals such as magnesium (599 ± 391 ppb ±SD n=10), chromium (446 ± 758 ppb), nickel (380 ± 364 ppb), copper (509 ± 1143 ppb), zinc (1.8 ± 2.1 ppm), mercury (160 ± 162 ppb), lead (42 ± 28 ppb), and others (see SI). These metals are likely leachates from vaporizer components or production materials, and their inhalation could cause deleterious effects on the respiratory tract that stem from the generation of reactive oxygen species.24,25 ICP-MS identified elevated levels of silicon (205 ± 108 ppm), a finding that has been previously shown for EVALI-associated CECs.26 Silica gel may be used as a purification medium or decolorizing agent, and its potential delivery to the respiratory tract from these products are subject of further investigation.
QNMR indicates that Δ8-THC levels can vary as much 40% from the labelled value (Table 1), suggestive of poor testing capabilities and falsified results. For brand A, the average of the sums of Δ8-THC and Δ4(8)-iso-THC for each product is not significantly different from the average reported Δ8-THC content (p<0.01), suggesting the analysis method (HPLC-UV as stated in the certificate of analysis) cannot discriminate the two. Brands B-E appear to use one lab result for all their products when these not only have variable levels of Δ8-THC, but also contain distinct levels of byproducts indicating different manufacturing methods in products that otherwise appear identical except for flavor formulation. Significant levels of understudied (Δ4(8)-iso-THC, 9-EtO-HHC) and novel (iso-THCBF) cannabinoids present a danger to users as these compounds are not well characterized pharmacologically and could cause unexpected levels of intoxication. High levels of unlabeled cutting agents present a further complication given the little safety information available. Further chemical, pharmacological, and toxicological testing of these and similar products is necessary.
Table 1.
Major components of 27 products (P) from 10 brands (B). Levels are mass% ± standard error of the mean (SEM).
| B | P | Δ8-THC Reported | Δ8-THC Measured | Δ4(8)-iso-THC | iso-THCBF |
|---|---|---|---|---|---|
|
A |
1 | 83.2 | 76±1 | 4.24±0.07 | 1.22±0.02 |
| 2 | 83.2 | 79.5±0.1 | 3.45±0.03 | 1.25±0.05 | |
| 3 | 86.15 | 81.2±0.6 | 3.48±0.03 | 1.31±0.04 | |
| 4 | 84.66 | 79.5±0.8 | 3.9±0.1 | <LOD | |
|
| |||||
|
B |
5 | 93.0821 | 75±2 | NQ | 0.88±0.05 |
| 6 | 93.0821 | 77±1 | NQ | <LOQ | |
| 7 | 93.0821 | 62.7±0.7 | 7.96±0.09 | <LOD | |
| 8 | 93.0821 | 77±1 | NQ | <LOQ | |
| 9 | 93.0821 | 78±2 | NQ | 0.63±0.03 | |
|
| |||||
|
C |
10 | 90 | 74.8±0.2 | 4.79±0.03 | 0.957±0.004 |
| 11 | 90 | 77.4±0.4 | 4.23±0.04 | 0.9±0.02 | |
| 12 | 90 | 79.4±0.4 | 3.74±0.05 | 0.9±0.02 | |
|
| |||||
|
D |
13 | 90 | 54.8±0.2 | 12.79±0.06 | 1.67±0.02 |
| 14 | 90 | 53.6±0.5 | 12.51±0.05 | 1.65±0.04 | |
| 15 | 90 | 78.0±0.7 | 4.13±0.04 | 1.6±0.02 | |
| 16 | 90 | 79.9±0.7 | 2.36±0.05 | 0.73±0.02 | |
|
| |||||
|
E |
17 | 77.71 | 78.8±0.4 | 3.01±0.03 | 0.61±0.02 |
| 18 | 77.71 | 80.3±0.7 | 2.851±0.005 | 0.415±0.008 | |
| 19 | 77.71 | 79.7±0.2 | 2.75±0.05 | 0.472±0.005 | |
|
| |||||
|
F |
20 | 80.85 | 76.8±0.3 | NQ | NQ |
| 21 | 84.02 | 77.1±0.7 | NQ | NQ | |
| 22 | 81.87 | 77±1 | NQ | NQ | |
|
| |||||
| G | 23 | 85.000 | 70.9±0.7 | NQ | NQ |
| 24 | 81.240 | 78.9±0.9 | NQ | NQ | |
|
| |||||
| H | 25 | 78.43 | 61.6±0.3 | 10.79±0.05 | 1.54±0.01 |
|
| |||||
| I | 26 | NA | 72.2±0.3 | NQ | NQ |
|
| |||||
| J | 27 | NA | 73.4±0.2 | NQ | NQ |
NQ: identified but not quantifiable. ND: not detected; NA: non-available data; LOD: limit of detection (signal-to-noise ≤ 3); LOQ: limit of quantification (signal-to-noise ≤ 12).
Supplementary Material
ACKNOWLEDGMENT
The National Institutes of Health (NIH) 1R01HL135613 and Toxicology Training Grant 5T32ES007026-43 supported this study. We thank Thomas Scrimale at the metal analysis core at the University of Rochester for performing the ICP-MS experiments.
ABBREVIATIONS
- CEC
cannabis e-cigarette
- EVALI
e-cigarette or vaping product-use associated lung injury
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Analytical methodology, structural identifications, relevant spectra, and full ICP-MS data (PDF)
The authors declare no competing financial interests.
REFERENCES
- (1).Meehan-Atrash J; Luo W; McWhirter KJ; Dennis DG; Sarlah D; Jensen RP; Afreh I; Jiang J; Barsanti KC; Ortiz A; Strongin RM The influence of terpenes on the release of volatile organic compounds and active ingredients to cannabis vaping aerosols. RSC Advances 2021, 11 (19), 11714–11723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Meehan-Atrash J; Luo W; McWhirter KJ; Strongin RM Aerosol Gas-Phase Components from Cannabis E-Cigarettes and Dabbing: Mechanistic Insight and Quantitative Risk Analysis. ACS Omega 2019, 4 (14), 16111–16120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Miech RA; Patrick ME; O’Malley PM; Johnston LD; Bachman JG Trends in Reported Marijuana Vaping Among US Adolescents, 2017-2019. JAMA 2020, 323 (5), 475–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Boyd CJ; McCabe SE; Evans-Polce RJ; Veliz PT Cannabis, Vaping, and Respiratory Symptoms in a Probability Sample of U.S. Youth. J. Adolesc .Health 2021, 69 (1), 149–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Krishnasamy VP; Hallowell BD; Ko JY; Board A; Hartnett KP; Salvatore PP; Danielson M; Kite-Powell A; Twentyman E; Kim L; Cyrus A; Wallace M; Melstrom P; Haag B; King BA; Briss P; Jones CM; Pollack LA; Ellington S Update: Characteristics of a Nationwide Outbreak of E-cigarette, or Vaping, Product Use-Associated Lung Injury - United States, August 2019-January 2020. Morb. Mortal. Wkly. Rep 2020, 69 (3), 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Duffy B; Li L; Lu S; Durocher L; Dittmar M; Delaney-Baldwin E; Panawennage D; LeMaster D; Navarette K; Spink D Analysis of Cannabinoid-Containing Fluids in Illicit Vaping Cartridges Recovered from Pulmonary Injury Patients: Identification of Vitamin E Acetate as a Major Diluent. Toxics 2020, 8 (1), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Ciolino LA; Ranieri TL; Brueggemeyer JL; Taylor AM; Mohrhaus AS EVALI Vaping Liquids Part 1: GC-MS Cannabinoids Profiles and Identification of Unnatural THC Isomers. Front. Chem 2021, 9, 746479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Zangani C; Schifano F; Napoletano F; Arillotta D; Gilgar L; Guirguis A; Corkery JM; Gambini O; Vento A The e-Psychonauts’ ‘Spiced’ World; Assessment of the Synthetic Cannabinoids’ Information Available Online. Curr. Neuropharmacol 2020, 18 (10), 966–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Johnson-Arbor K; Smolinske S The current state of delta-8 THC. Am. J. Emerg. Med 2021.In press. DOI: 10.1016/j.ajem.2021.06.066 [DOI] [PubMed] [Google Scholar]
- (10).Hanuš LO; Meyer SM; Muñoz E; Taglialatela-Scafati O; Appendino G Phytocannabinoids: a unified critical inventory. Nat. Prod. Rep 2016, 33 (12), 1347–1448. [DOI] [PubMed] [Google Scholar]
- (11).Implementation of the Agriculture Improvement Act of 2018. In 21 CFR §1308 and §1312, Drug Enforcement Administration, Department of Justice. US Government Publishing Office: 2020; Vol. RIN 1117–AB53. [Google Scholar]
- (12).Increases in Availability of Cannabis Products Containing Delta-8 THC and Reported Cases of Adverse Events. https://emergency.cdc.gov/han/2021/han00451.asp (accessed October 7, 2021).
- (13).Adams R; Pease DC; Cain CK; Baker BR; Clark JH; Wolff H; Wearn RB Conversion of Cannabidiol to a Product with Marihuana Activity. A Type Reaction for Synthesis of Analogous Substances. Conversion of Cannabidiol to Cannabinol. J. Am. Chem. Soc 1940, 62 (8), 2245–2246. [Google Scholar]
- (14).Golombek P; Müller M; Barthlott I; Sproll C; Lachenmeier DW Conversion of Cannabidiol (CBD) into Psychotropic Cannabinoids Including Tetrahydrocannabinol (THC): A Controversy in the Scientific Literature. Toxics 2020, 8 (2), 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Kiselak TD; Koerber R; Verbeck GF Synthetic route sourcing of illicit at home cannabidiol (CBD) isomerization to psychoactive cannabinoids using ion mobility-coupled-LC-MS/MS. Forensic Sci. Int 2020, 308, 110173. [DOI] [PubMed] [Google Scholar]
- (16).Marzullo P; Foschi F; Coppini DA; Fanchini F; Magnani L; Rusconi S; Luzzani M; Passarella D Cannabidiol as the Substrate in Acid-Catalyzed Intramolecular Cyclization. J. Nat. Prod 2020, 83 (10), 2894–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Meehan-Atrash J; Strongin RM Pine rosin identified as a toxic cannabis extract adulterant. Forensic Sci. Int 2020, 312, 110301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Gaoni Y; Mechoulam R Concerning the Isomerization of Δ1-to Δ1(6)-Tetrahydrocannabinol1. J. Am. Chem. Soc 1966, 88 (23), 5674–5675. [Google Scholar]
- (19).Malkov AV; Kočovský P Tetrahydrocannabinol Revisited: Synthetic Approaches Utilizing Molybdenum Catalysts. Collect. Czechoslov. Chem. Commun 2001, 66 (8), 1257–1268. [Google Scholar]
- (20).Razdan RK; Dalzell HC; Handrick GR Hashish.1 A Simple One-Step Synthesis of (–)-Δ1-Tetrahydrocannabinol (THC) from p-Mentha-2,8-dien-1-ol and Olivetol. J. Am. Soc 1974, 96 (18), 5860–5865. [DOI] [PubMed] [Google Scholar]
- (21).Gaoni Y; Mechoulam R Hashish—VII: The isomerization of cannabidiol to tetrahydrocannabinols. Tetrahedron 1966, 22 (4), 1481–1488. [Google Scholar]
- (22).Trukhin A; Kruchkov F; Hansen LK; Kallenborn R; Kiprianova A; Nikiforov V Toxaphene chemistry: Separation and characterisation of selected enantiomers of the Polychloropinene mixtures. Chemosphere 2007, 67 (9), 1695–1700. [DOI] [PubMed] [Google Scholar]
- (23).Bicknell RC; Maccoll A The pyrolysis of bornyl chloride. Chem. Ind 1961, 190, 715. [Google Scholar]
- (24).Collin F Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci 2019, 20 (10), 2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Ball JC; Straccia AM; Young WC; Aust AE The Formation of Reactive Oxygen Species Catalyzed by Neutral, Aqueous Extracts of NIST Ambient Particulate Matter and Diesel Engine Particles. J. Air & Waste Manage. Assoc 2000, 50 (11), 1897–1903. [DOI] [PubMed] [Google Scholar]
- (26).Muthumalage T; Friedman MR; McGraw MD; Ginsberg G; Friedman AE; Rahman I Chemical Constituents Involved in E-Cigarette, or Vaping Product Use-Associated Lung Injury (EVALI). Toxics 2020, 8 (2), 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


