Abstract
Watercress is an excellent source of phenethyl isothiocyanate (PEITC), shown in many studies to enhance carcinogen and toxicant detoxification and to inhibit carcinogenesis. Based on a previous observation that PEITC can enhance the detoxification of common environmental pollutants such as acrolein, crotonaldehyde, benzene, and 1,3-butadiene, we designed a clinical trial testing the hypothesis that daily consumption of a drink containing freeze-dried watercress, an abundant source of PEITC, would have a similar effect, particularly observed in subjects who were null in certain glutathione S-transferase genes. This manuscript describes the preparation of nearly 100 pounds of freeze-dried watercress for this trial, starting with laboratory scale pilot studies and proceeding to industrial scale production of the fully validated product in compliance with all food safety requirements. Initial results validating subject compliance in the clinical trial are also presented.
Keywords: freeze-dried watercress, chemoprevention clinical trial, phenethyl isothiocyanate, carcinogen detoxification, industrial scale freeze-drying
Introduction
This paper describes a partnership between scientists in academia and the food industry to prepare a beverage containing freeze-dried watercress for use in a chemoprevention study. Watercress (Nasturtium officinale) is an excellent source of gluconasturtiin, the precursor to phenethyl isothiocyanate (PEITC), demonstrated in numerous studies to have potential cancer protective effects (1-6) (see Figure 1). Encouraged by the compelling arguments regarding “green cancer chemoprevention” by foods such as broccoli sprouts and berries, as opposed to individual compounds, and having gained insights into the challenges and regulatory hurdles associated with drug development in an academic setting (7-9), we were convinced that freeze-dried watercress would be a practical source of PEITC for a clinical trial of its ability to enhance the detoxification of environmental toxicants and carcinogens such as acrolein, crotonaldehyde, benzene, and 1,3-butadiene (10,11). Yet, scaling up from the laboratory setting to a trial involving 400 subjects who would consume a beverage containing freeze-dried watercress daily for two weeks presented numerous research and practical problems, which are presented and discussed here.
Figure 1.
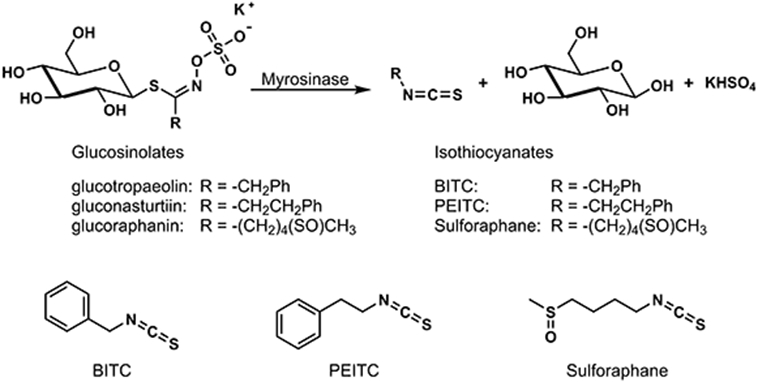
Conversion of glucosinolates to isothiocyanates catalyzed by plant myrosinase, and structures of BITC, PEITC, and sulforaphane. Glucotropaeolin, gluconasturtiin, and glucoraphanin are the characteristic major glucosinolates in garden cress, watercress, and broccoli sprouts, respectively. Minor products in addition to the isothiocyanates are also observed.
Cruciferous vegetables such as broccoli, cabbage, Brussels sprouts, garden cress, and watercress contain characteristic plant defense compounds - glucosinolates. They also have the enzyme myrosinase which is separated cellularly from the glucosinolates. When these vegetables are mechanically disrupted by a predator’s bite or similar occurrence, the glucosinolate and the myrosinase enzyme mix resulting in hydrolysis of the glucosinolate with formation of glucose and an unstable intermediate that undergoes a spontaneous rearrangement producing an isothiocyanate as the major product as illustrated in Figure 1 (12-14). Additional minor products are also formed.
The isothiocyanates formed in this reaction have a bitter or sharp taste. This adverse taste is intended to discourage predators and is responsible for the characteristic flavor profile associated with this family of vegetables. Isothiocyanates and related degradation products characteristic of cruciferous vegetables have numerous potential cancer protective and therapeutic effects and have been studied extensively. PEITC, benzyl isothiocyanate (BITC), and sulforaphane in particular have been investigated in detail, and their potential cancer preventive and therapeutic effects including inhibition of carcinogen metabolic activation, enhancement of detoxification of environmental toxicants, and other beneficial properties as well as some potential toxic effects at high concentrations have been reviewed recently (1,2,4,6). The study reported here focuses on PEITC, the hydrolysis product of gluconasturtiin, the major glucosinolate of watercress.
Our interest in PEITC began in 1985 when Chung et al demonstrated that it inhibited the metabolic activation of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) (15), a powerful tobacco-specific lung carcinogen. NNK and the related compound N’-nitrosonornicotine are considered “carcinogenic to humans” by the International Agency for Research on Cancer (16). Chung’s results led to multiple studies in laboratory animals demonstrating the ability of PEITC as well as its N-acetylcysteine conjugate to inhibit lung carcinogenesis by NNK (17). In one study, we observed virtually complete inhibition of NNK-induced rat lung carcinogenesis by PEITC given chronically in the diet (18). Mechanistic studies clearly demonstrated that PEITC inhibited cytochrome P450 enzymes involved in the metabolic activation and DNA adduct formation by NNK, as reviewed previously (8).
These encouraging results led to a clinical trial of PEITC as an inhibitor of the metabolic activation of NNK in cigarette smokers. Numerous challenges were presented by this trial including the necessity for preparing specially labelled cigarettes containing [pyridine-D4]NNK, the recruiting sufficient numbers of smokers, and perhaps most importantly, the dosage form of PEITC to be employed in the trial. Softgels or a related encapsulated form would have been ideal because of the disagreeable taste of pure PEITC, which was given at a total daily dose of 40 mg, divided into four 10 mg doses. The preparation of softgels in compliance with FDA regulations was prohibitively expensive for an academic laboratory. Ultimately, PEITC was purified under GMP conditions and prepared as a dosage form by dissolving it in olive oil at a concentration of 10 mg/ml, with FDA approval. In a within-subject, cross over design clinical trial, one ml of this solution was then self-administered by the cigarette smoking participants 4 times per day, every 4 hr, using a disposable plastic syringe. Olive oil served as the placebo. The results of this trial showed a modest but significant 7.7% inhibition of NNK metabolic activation when cigarette smokers were dosed with PEITC versus olive oil alone (8). However, we found much more encouraging results when we analyzed the effects of PEITC on mercapturic acid detoxification products of benzene, acrolein, crotonaldehyde, and 1,3-butadiene. Significant increases in levels of urinary mercapturic acids of these compounds were observed, particularly in subjects who were null for either glutathione-S-transferase M1 (GSTM1) or GSTT1, or both. For example, the detoxification of benzene, assessed by its urinary metabolite S-phenyl mercapturic acid, increased by 95% (P<0.001) in the double nulls (10,11). Of interest, a meta-analysis of greater than 8000 lung cancer cases and 684,000 non-cancer subjects showed an overall 20% lower risk of lung cancer associated with cruciferous vegetable intake, with the strongest risk reduction in subjects who were GSTM1 or GSTT1 null, and particularly in the double nulls (odds ratio = 0.4, 95% CI, 0.20 – 0.68) (19).
The results of our study were exciting and inspired further examination of this phenomenon in a new trial focused on detoxification of these common pollutants, but using watercress as a source of PEITC rather than the pure compound. In contrast to our challenging experiences with the formulation of an appropriate dosing form for PEITC, watercress is an inexpensive and readily available food item that delivers milligram amounts of PEITC upon consumption of normal size portions. Thus, we envisioned the formulation of a drink containing freeze-dried watercress that would provide ample doses of PEITC to our study subjects. The new trial includes both non-smokers and cigarette smokers in their natural proportions in Minnesota, and is powered to include sufficient numbers of participants who are null in both GSTM1 and GSTT1, where the greatest effects of PEITC were observed.
The choice of a drink formulated from freeze-dried watercress was based on a number of practical considerations. Freeze-dried watercress has the potential to deliver a reproducible amount of PEITC, as discussed below. This stable form of watercress could be packaged and stored during the conduct of the trial, and the delivery of PEITC could be monitored. The supply chain for fresh watercress, in amounts exceeding 1 ton for a study of this size conducted over at least two years in Minnesota, would likely be variable as would the amounts of gluconasturtiin in fresh watercress harvested at different times of the year. An alternate choice, capsules containing freeze-dried watercress, was not judged to be feasible because of the relatively low density of the freeze-dried material which would have required capsules that were too large or too numerous for practical daily dosing. Furthermore, it was not clear if the release of PEITC from gluconasturtiin in the human stomach would be similar to that observed in H2O.
As described recently by Stoner (9), chemoprevention with food as opposed to food constituents taken as drugs has a number of attractive features including relatively low and stable costs, low likelihood of overdosing or toxicity, and potential increased efficacy due to interaction with other food components. Properly conducted clinical trials of food for chemoprevention will assess the uptake of the presumed active constituents and in this way are equally as rigorous as trials of pure drug substances.
Materials and Methods
Commercial scale freeze-drying of watercress
Pilot study 1 (October 2019)
In order to develop the optimal freeze-drying parameters for a high potency product, two pilot studies were completed at Van Drunen Farms, Momence, IL. The first was in the fall of 2019. Three boxes of fresh watercress (24 bunches/~8 lb per box) were shipped to a Van Drunen Farms pilot facility overnight in iced boxes kept in a cooler. The watercress was stored refrigerated at ~3 °C and then frozen to −18 °C prior to drying. The initial tray temperature was −18 °C. The trays were loaded on the temperature-controlled plates in the freeze-dryer chamber, then full vacuum was applied. Three drying tray/plate temperatures were tested: in one chamber the tray was 4 °C, the second was 16 °C and the third was 23 °C. Then the dried material was ground to a 20 mesh powder and sent to the University of Minnesota for analysis.
Pilot study 2 (February 2020)
A second pilot study was conducted in order to finalize procedures and processing parameters prior to full scale freeze-drying. The processing facility that offered the necessary pre-chilling and low temperature capabilities also required sizing of the whole leaf watercress prior to drying in order to prevent the watercress from touching the heat plates of the drying chambers and to allow more watercress to be loaded on each tray. The sizing machine and watercress were kept at ~−20 °C and the material was chopped to pieces which were ~1/4 – 3/8" in size.
Three boxes of fresh watercress (24 bunches per box) were shipped overnight to a Van Drunen Farms pilot facility under refrigerated conditions. The fresh watercress was frozen and then chopped with the product held at steady temperatures well below the freezing point in order to preserve potency. After drying with the initial tray temperature of 16 °C, the product was ground and sent to the University of Minnesota for analysis.
Large scale production of freeze-dried watercress
Green leaf watercress was donated by B&W Quality Growers (Fellsmere, FL), the largest producer of watercress in the U.S. It was harvested in the summer of 2020 at a growing location in Tennessee. Following harvest, the watercress was hydro-cooled and washed before being packed loose leaf into polyethylene-lined, waxed corrugate cases. It was shipped via refrigerated truck with a temperature of ~−20 °C to Van Drunen Farms for freeze-drying. During all operations, from shipping to loading into the drying chamber, the cold chain of ~−20 °C was maintained.
Upon receipt, the watercress was kept frozen and stored until being sized and loaded into the freeze-drying chamber. One thousand lbs of sized frozen watercress filled one freeze-drying chamber. It was dried at 16 °C and remained at this temperature throughout drying, which took 4.75 days, 24 h longer than anticipated based on the pilot studies. Following freeze-drying, the watercress was ground and sifted to −20 mesh.
The freeze-dried watercress (375 g) was submitted for process control testing to ensure food safety. Tests were conducted by the IEH Laboratories & Consulting Group (Sparta, WI). Microbiological analysis for Salmonella, E. Coli 0157-H7 and Listeria were negative. A certificate of analysis was prepared.
Analysis of PEITC released from freeze-dried watercress.
Powdered freeze-dried watercress (250 mg) was placed in a 50 mL polyethylene container. Water (25 ml) was added and the mixture was shaken for about 10 sec, then allowed to stand with occasional shaking at room temperature for at least 5 min. A mixture of 50:50 (V:V) acetonitrile:methanol (35 ml) was added and the mixture was shaken for about 10 sec. then immediately centrifuged at 3,000 g at 20 °C for 15 min, and 1.5 ml was removed for analysis of PEITC by HPLC. The HPLC system used a Waters XSelect HSS T3 column (3.0 x 50 mm, 2.5 μm particle size C18) with an XP Van Guard cartridge system guard column with the same stationary phase. The solvent system was isocratic with 60% aqueous acetonitrile at a flow rate of 0.5 ml/min. Detection was at 254 nm. The retention time of PEITC was about 2.6 – 2.8 min.
Analysis of PEITC-NAC in urine.
This study was approved by the University of Minnesota Institutional Review Board. All subjects gave written informed consent. The study is being conducted in accordance with U.S. Common Rule. Urine samples were thawed and warmed to 37 °C, then 0.5 ml was centrifuged for 15 min at 10,000 rpm at room temperature, followed by further centrifugation of 0.1 ml at 2000 rpm for 5 – 10 min. The sample (10 μL) was analyzed by HPLC using the column described in the previous paragraph with UV detection at 254 nm. The eluting solvents were 50% A, 10 mM sodium phosphate monobasic, pH 3.0 (with phosphoric acid), and 50% B, CH3OH, under isocratic conditions at a flow rate of 0.40 ml/min. The column temperature was maintained at 35 °C.
Data Availability Statement.
Data described in this study can be obtained by contacting the corresponding author.
Results
Initial Laboratory Scale Experiments.
We purchased watercress at a local market and analyzed it for gluconasturtiin, the concentration of which was about 20 μmol/g dry weight (14). This watercress was freeze-dried in the laboratory. Gluconasturtiin was not lost during freeze-drying due to its relatively high molecular weight of 423 g/mol and was stable in this freeze-dried watercress when stored at −20 °C for at least 9 months. In an important experiment, we found that the freeze-dried watercress, when added to H2O for 5 min, released 20 μmol (3.2 mg) PEITC/g dry weight of watercress, an essentially quantitative conversion. Apparently, cells are disrupted during freeze-drying, but myrosinase and gluconasturtiin can mix only when this freeze-dried material is added to H2O, releasing an equivalent amount of PEITC. This laboratory scale study demonstrated that freeze-drying was a practical approach for preparation of a watercress beverage because gluconasturtiin and myrosinase were not lost during freeze-drying and were converted in high yield to PEITC upon addition of the freeze-dried watercress to H2O.
Pilot Studies with Van Drunen Farms.
In subsequent studies of larger scale freeze-drying, we worked with Van Drunen Farms, a commercial freeze-drying company. Fresh watercress from B&W Growers was shipped overnight in iced boxes to Van Drunen Farms. The dried watercress was powdered to 20 mesh which is suitable for incorporation into a beverage. The 4 °C and 16 °C tray temperatures yielded material with similar potency at levels suitable for the study; approximately 3.2 mg PEITC per g freeze-dried watercress was released within 5 min of addition of the material to H2O. This yield of PEITC was suitable for the study because 3 drinks per day, each containing about 13 mg of PEITC, could be prepared with about 4 g of freeze-dried watercress per drink. We then studied the effects of storage conditions, temperature, and time on the potency of the freeze-dried watercress. We found no decrease in the amount of PEITC released when this freeze-dried watercress was stored at room temperature or at −20 °C for 130 days. We also studied the effect of wilting on PEITC potency of the watercress. Van Drunen Farms would not accept watercress packed on ice at their major freeze-drying facility and we were concerned that the watercress could wilt during shipment from Tennessee to Illinois. To test this, we stored fresh watercress in a refrigerator for 4 days until it was visibly wilted. The result was loss of only about 15% of the PEITC amount in the freeze-dried watercress. Collectively, these results indicated that the process of shipping and freeze-drying watercress would produce a product with acceptable levels of PEITC for the study, so we then moved on to larger scale pilot studies.
Based on these pilot trials, it was determined that a drying cycle took approximately 75 h and with the expected weight ratio of 22:1 before versus after drying, a one chamber load of 1,040 pounds of fresh watercress would yield approximately 47 pounds of freeze-dried material. The actual yield was 95.9 pounds, an approximate ratio of 10:1 of fresh watercress to freeze-dried product. This was higher than anticipated; this type of variability is not uncommon when freeze-drying leafy products. This variability could be due to a variety of factors including use of full scale vs. pilot scale processing equipment, growing conditions, fertilization or time of year. A sample of this freeze-dried watercress yielded 4.4 mg (27 μmol) PEITC per g dry weight when hydrolyzed for 15 min and 3.8 mg (23 μmol) PEITC per g dry weight when hydrolyzed for 2 min.
The powder was shipped in polyethylene bags, each of which contained 20 lbs, via refrigerated truck to a Hormel Foods facility located in the Midwest which specializes in the packing of nutritional powders. One-week supplies (98 g) of freeze-dried watercress were filled into 46 oz. jars along with a 5 g card for moisture absorption; the jars were induction-sealed and covered with screw top lids. Study subjects were provided with a 32 cc scoop to deliver a one-dose serving. Jars were labeled with instructions to keep in a cool and dry place. The product was shipped to the University of Minnesota in a refrigerated truck and stored at −20 °C until distribution.
Each facility in this supply chain completed a thorough vendor approval process and operated under a food safety program, which included a third-party audit, good manufacturing practices and a hazard analysis critical control point (HACCP) plan. Microbiological testing was performed on the watercress to ensure food safety.
Preparation of the Watercress-Containing Beverage
Each subject received 2 canisters of freeze-dried watercress powder with a pre-measured scoop and packets of “Crystal Light” naturally sweetened drink mix. They were instructed to add a level scoop of freeze-dried watercress to approximately 8 oz. of room temperature H2O. They stirred or shook the mixture for about 20 sec, then let it stand at room temperature for 5 min. Then they added the Crystal Light packet (containing 4 g of flavor material), mixed the contents, and drank. They were specifically instructed not to add the flavor packet before the 5 min period because that could lower the pH and prevent enzymatic hydrolysis of gluconasturtiin. Typical flavor packets contained “tangerine mango, raspberry lemonade, and tropical blend.”
Maltodextrin
Maltodextrin served as the control product for the clinical study. It was chosen because it is a bland powder that blends readily with H2O and adds little or no flavor. A two-week supply (125 g) of maltodextrin was filled into 32 oz. jars along with an induction seal and a screw top lid. Study subjects were provided with a 9 cc scoop sized to deliver a one-dose serving. They were instructed to add the exact same type and amount of flavor packets to the control product as they added to the watercress drink. Jars containing maltodextrin were labeled with instructions to keep in a cool and dry place. The product was shipped to the University of Minnesota campus where it was stored at ambient temperature until distribution.
Discussion
Overview of the Clinical Trial Study Design
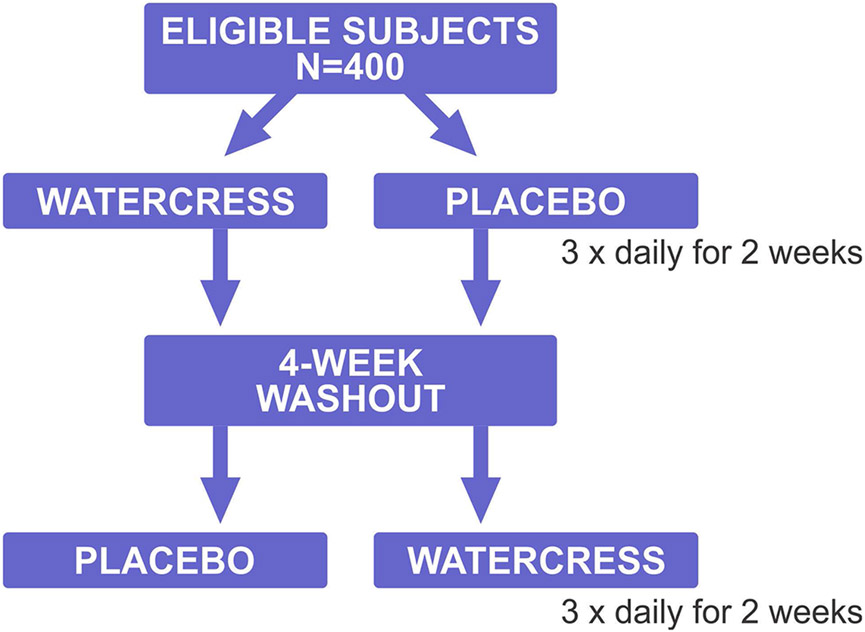
The study is planned for 400 subjects, both non-smokers and smokers (14.6% in Minnesota in 2019). An overview is presented in Figure 2. With the GSTM1 and GSTT1 double nulls comprising about 15% of the population, GSTM1 single nulls (45%) and GSTT1 single nulls (23%), we expect to see 60 double nulls, 180 GSTM1 single nulls, and 92 GSTT1 single nulls. The participants are being randomized in a single-blind manner (blind to participants regarding which drink is beneficial) to either the watercress containing drink or the maltodextrin drink. They consume this drink 3 times daily for 2 weeks; the participants consuming the watercress drink are exposed to approximately 40 mg of PEITC per day. The rationale for the 3 times daily dosing is based on the results of the PEITC trial in which a total dose of 40 mg was given in four 10 mg doses daily (11). After this 2 week period, there is a 4 week washout period and then each participant switches to the other condition (either watercress or maltodextrin drink) for a second 2 week period. The 4 week washout period was chosen based on our completed PEITC trial, the results of which suggested that the 2 week washout period used in that trial may not have been sufficient (8). Participants are asked to attend clinic visits on days 0 (the day of product distribution), 4, 7 and 14 for each of the periods they are receiving product and weekly during the 4 week washout between periods. At each clinic visit, first morning void urine samples are collected and participants are asked to submit saliva and oral cells. The primary endpoints in this study are urinary levels of the mercapturic acids of benzene, acrolein, crotonaldehyde, and 1,3-butadiene, which will test the main hypothesis of the study that watercress consumption increases the detoxification of these volatile toxicants and carcinogens via their conjugation with glutathione, and that this is most clearly observed in the GSTM1 and GSTT1 null individuals.
Figure 2.
Overview of the clinical trial design. Eligible subjects are randomized to the groups shown.
Initial Data on PEITC-NAC in Urine of Study Subjects
After delays due to COVID-19, recruitment for the study began in March, 2021. We analyzed PEITC-NAC in the urine samples of the first 19 subjects who completed the study protocol. HPLC chromatograms of a urine sample from a subject who consumed the watercress-containing drink, and a sample from the washout period, are shown in Figure 3. A clear peak for PEITC-NAC was observed in the chromatogram from the urine sample of the subject who consumed the watercress-containing drink while no such peak was observed from a sample collected during the washout period. Assuming an average urine volume of 1.4 liters and compliance with the study protocol [finishing all three 16 oz. drinks each day, containing a total of about 40 mg of PEITC (from 10 g of freeze-dried watercress, or about 100 g of fresh watercress)] average excretion of the PEITC dose as PEITC-NAC was about 39% (31 mg) among these 19 subjects.
Figure 3.
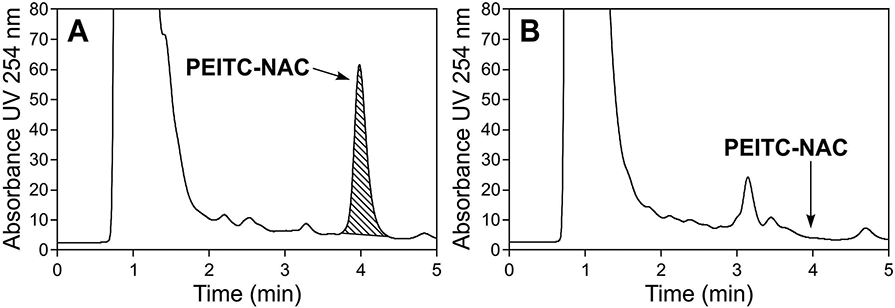
HPLC chromatograms of urine samples. The samples were obtained from a subject who consumed the watercress-containing drink (A), and the same subject during the washout period (B). The arrows indicate the peak for the PEITC metabolite PEITC-NAC in panel A and the lack of this peak in panel B.
Chung et al were the first to quantify PEITC-NAC as a urinary metabolite of PEITC (20). They detected and quantified PEITC-NAC (4.6 – 30 mg) in the 24h urine of 4 subjects who consumed meals containing 30 – 57 g of watercress. In our previous study of watercress consumption, 11 subjects consumed 56 g of watercress at each meal for 3 days and excreted 28.9 – 75.3 mg of PEITC-NAC in their 24h urine (21). These results are roughly consistent with those reported here.
In summary, we have developed a well-characterized method for the industrial scale production of freeze-dried watercress, which can be used to prepare a beverage which contains the potential cancer preventive agent PEITC. This watercress preparation is being used in a clinical trial of its ability to increase the detoxification of environmental toxicants and carcinogens such as acrolein, benzene, and 1,3-butadiene. The methods and results reported here will be useful in future studies of the possible role of watercress in cancer prevention.
Prevention Relevance.
This study describes the preparation of a beverage containing freeze-dried watercress suitable for consumption in a clinical trial to determine whether a constituent of this beverage – PEITC, which has cancer prevention properties – can enhance detoxification of common environmental carcinogens and toxicants such as benzene, which may have a role in environmentally induced cancer.
Acknowledgements.
This study was supported by grant R01 CA-222005 to S.S. Hecht, S.G. Carmella, H. Vanderloo and D.K. Hatsukami from the U.S. National Cancer Institute. This grant supported all expenses except the fresh watercress, which was kindly donated by B&W Quality Growers, Fellsmere, FL. We thank Viviana Paiano, M.S., for expert technical assistance.
Footnotes
Authors’ Disclosures: The authors are employees of the relevant companies or the University of Minnesota and declare no potential conflicts of interest, nor do they have financial interests related to the trial results.
References
- 1.Dayalan Naidu S, Suzuki T, Yamamoto M, Fahey JW, Dinkova-Kostova AT. Phenethyl isothiocyanate, a dual activator of transcription factors NRF2 and HSF1. Mol Nutr Food Res 2018;62:e1700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soundararajan P, Kim JS. Anti-carcinogenic glucosinolates in cruciferous vegetables and their antagonistic effects on prevention of cancers. Molecules 2018;23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta P, Wright SE, Kim SH, Srivastava SK. Phenethyl isothiocyanate: a comprehensive review of anti-cancer mechanisms. Biochim Biophys Acta 2014;1846:405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palliyaguru DL, Yuan JM, Kensler TW, Fahey JW. Isothiocyanates: Translating the power of plants to people. Mol Nutr Food Res 2018;62:e1700965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hecht SS. Inhibition of carcinogenesis by isothiocyanates. Drug Metabol Rev 2000;32:395–411. [DOI] [PubMed] [Google Scholar]
- 6.Abdull Razis AF, Konsue N, Ioannides C. Isothiocyanates and xenobiotic detoxification. Mol Nutr Food Res 2018;62:e1700916. [DOI] [PubMed] [Google Scholar]
- 7.Fahey JW, Talalay P, Kensler TW. Notes from the field: "Green" chemoprevention as frugal medicine. Cancer Prev Res 2012;5:179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan J-M, Stepanov I, Murphy SE, Wang R, Allen S, Jensen J, et al. Clinical trial of 2-phenethyl isothiocyanate as an inhibitor of metabolic activation of a tobacco-specific lung carcinogen in cigarette smokers. Cancer Prev Res 2016;9:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoner GD. Food-based approach to cancer prevention. Food Frontiers 2020;1:6–8. [Google Scholar]
- 10.Boldry EJ, Yuan JM, Carmella SG, Wang R, Tessier K, Hatsukami DK, et al. Effects of 2-phenethyl isothiocyanate on metabolism of 1,3-butadiene in smokers. Cancer Prev Res 2020;13:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan JM, Murphy SE, Stepanov I, Wang R, Carmella SG, Nelson HH, et al. 2-Phenethyl isothiocyanate, glutathione S-transferase M1 and T1 polymorphisms, and detoxification of volatile organic carcinogens and toxicants in tobacco smoke. Cancer Prev Res 2016;9:598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fenwick GR, Heaney RK, Mawson R. Glucosinolates. In: Cheeke PR, editor. Toxicants of Plant Origin, Volume II Glycosides. Boca Raton, FL: CRC Press, Inc.; 1989. p 2–41. [Google Scholar]
- 13.Tookey HL, VanEtten CH, Daxenbichler ME. Glucosinolates. In: Liener IE, editor. Toxic Constituents of Plant Stuffs. New York: Academic Press; 1980. p 103–42. [Google Scholar]
- 14.Hecht SS, Carmella SG, Kenney PMJ, Low S-H, Arakawa K, Yu MC. Effects of cruciferous vegetable consumption on urinary metabolites of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in Singapore Chinese. Cancer Epidemiol Biomarkers Prev 2004;13:997–1004. [PubMed] [Google Scholar]
- 15.Chung FL, Wang M, Hecht SS. Effects of dietary indoles and isothiocyanates on N-nitrosodimethylamine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone alpha-hydroxylation and DNA methylation in rat liver. Carcinogenesis 1985;6:539–43. [DOI] [PubMed] [Google Scholar]
- 16.International Agency for Research on Cancer. Smokeless Tobacco and Some Tobacco-specific N-Nitrosamines. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, v 89. Lyon, FR: IARC; 2007. [PMC free article] [PubMed] [Google Scholar]
- 17.International Agency for Research on Cancer. Cruciferous Vegetables, Isothiocyanates, and Indoles. IARC Handbooks of Cancer Prevention, v 9. Lyon, FR: IARC; 2004. [Google Scholar]
- 18.Hecht SS, Trushin N, Rigotty J, Carmella SG, Borukhova A, Akerkar SA, et al. Complete inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induced rat lung tumorigenesis and favorable modification of biomarkers by phenethyl isothiocyanate. Cancer Epidemiol Biomarkers Prev 1996;5:645–52. [PubMed] [Google Scholar]
- 19.Lam TK, Gallicchio L, Lindsley K, Shiels M, Hammond E, Tao XG, et al. Cruciferous vegetable consumption and lung cancer risk: a systematic review. Cancer Epidemiol Biomarkers Prev 2009;18:184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung FL, Morse MA, Eklind KI, Lewis J. Quantitation of human uptake of the anticarcinogen phenethyl isothiocyanate after a watercress meal. Cancer Epidemiol Biomarkers Prev 1992;1:383–8. [PubMed] [Google Scholar]
- 21.Hecht SS, Chung FL, Richie JP Jr, Akerkar SA, Borukhova A, Skowronski L, et al. Effects of watercress consumption on metabolism of a tobacco-specific lung carcinogen in smokers. Cancer Epidemiol Biomarkers Prev 1995;4:877–84. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data described in this study can be obtained by contacting the corresponding author.