Abstract
Purpose:
We hypothesize that the addition of the phosphodiesterase-5 inhibitor tadalafil to the PD-1 inhibitor nivolumab, is safe and will augment immune-mediated antitumor responses in previously untreated squamous cell carcinoma of the head and neck (HNSCC).
Patients and Methods:
We conducted a two-arm multi-institutional neoadjuvant randomized trial in any-stage resectable HNSCC (NCT03238365). Patients were stratified at randomization by human papillomavirus (HPV) status. Patients in both arms received nivolumab 240 mg intravenously on days 1 and 15 followed by surgery on day 28. Those in the combination therapy arm also received tadalafil 10 mg orally once daily for 4 weeks. Imaging, blood, and tumor were obtained pretreatment and posttreatment for correlative analysis.
Results:
Neoadjuvant therapy was well-tolerated with no grade 3 to 5 adverse events and no surgical delays. Twenty-five of 46 (54%) evaluable patients had a pathologic treatment response of ≥20%, including three (7%) patients with a complete pathologic response. Regardless of HPV status, tumor proliferation rate was a negative predictor of response. A strong pretreatment T-cell signature in the HPV-negative cohort was a predictor of response. Tadalafil altered the immune microenvironment, as evidenced by transcriptome data identifying enriched B- and natural killer cell gene sets in the tumor and augmented effector T cells in the periphery.
Conclusions:
Preoperative nivolumab ± tadalafil is safe in HNSCC and results in more than 50% of the patients having a pathologic treatment response of at least 20% after 4 weeks of treatment. Pretreatment specimens identified HPV status-dependent signatures that predicted response to immunotherapy while posttreatment specimens showed augmentation of the immune microenvironment with the addition of tadalafil.
Translational Relevance.
Neoadjuvant immune checkpoint inhibition is an attractive approach for the treatment of squamous cell carcinoma of the head and neck. In this setting, the addition of immune modulators to immune checkpoint inhibitors has the potential to enhance antitumor effects. We evaluated the PD-1 inhibitor nivolumab alone or in combination with the phosphodiesterase-5 inhibitor tadalafil administered preoperatively in a randomized clinical trial. We demonstrated safety and antitumor effect in support of neoadjuvant use of immune checkpoint inhibitors. While we did not show enhanced clinical response with the addition of tadalafil to nivolumab after 4 weeks, we demonstrated enhancement of B-cell– and natural killer (NK)-cell–associated gene expression signatures in tumors that respond to the combination. Our study provides a comprehensive analysis of response to immune checkpoint inhibition in both human papillomavirus–positive and human papillomavirus–negative squamous cell carcinoma of the head and neck and advocates for further trials of phosphodiesterase-5 inhibition in this context.
Introduction
Immunotherapy with immune checkpoint inhibitors (ICIs) has been incorporated in the standard-of-care management of recurrent or metastatic squamous cell carcinoma of the head and neck (HNSCC), and it is now being evaluated as promising treatment in less advanced disease. Two ICIs, the anti–PD-1 mAbs pembrolizumab and nivolumab, have shown safety and improved overall survival in patients with platinum-refractory recurrent or metastatic HNSCC as compared with investigator's choice standard therapies (1, 2). A subsequent phase III trial (KEYNOTE-048) demonstrated pembrolizumab plus platinum and 5-fluorouracil is an appropriate first-line treatment for recurrent or metastatic HNSCC, and pembrolizumab monotherapy is an appropriate first-line treatment for patients with PD-L1–positive recurrent or metastatic HNSCC (3). These results have solidified the role of immunotherapy in recurrent or metastatic HNSCC and prompted further clinical investigations, including the use of combination immunotherapies to enhance response rates. Previously, PD-1 inhibitors were used in the neoadjuvant therapy setting in untreated patients with advanced human papillomavirus (HPV)–negative (HPV−) tumors with promising results (4–6).
Despite encouraging results with ICIs in HNSCC, only 20% to 30% of patients with metastatic/recurrent cancer are disease-free at 2 years (1, 7) pointing to a long-term benefit from anti–PD-1 monotherapy in HNSCC. Immunosuppressive cells, including Th2/M2 phenotype, anergic T cells, inhibitory costimulatory molecules (e.g., TIM 3, LAG3, TIGIT), and factors in the tumor microenvironment (e.g., kynurenine, glycolysis) contribute to resistance to ICIs (8). This has prompted investigation into combinatorial approaches to augment the immune and clinical responses seen with PD-1 inhibitors. The use of immunotherapy in the neoadjuvant setting is a particularly appealing investigational approach, as it allows for the evaluation of biologic specimens before and after treatment and can contribute to the assessment of the treatment effect of novel combinations (9). Preclinical models and clinical trials in solid tumors have indicated the potential value of neoadjuvant immunotherapy in the preoperative setting (10–12). For example, a phase I/II neoadjuvant trial (CheckMate 358) demonstrated safety in patients with HPV-positive (HPV+) and HPV− HNSCC using preoperative nivolumab (13). A clinical trial that enrolled patients with HNSCC treated with neoadjuvant and adjuvant pembrolizumab showed a promising 1-year relapse rate of 16.7% in high-risk patients (5). Additionally, this group demonstrated a twofold increase in pathologic treatment response (pTR) with the addition of a second cycle of ICIs in the neoadjuvant setting (14).
Phosphodiesterase-5 (PDE-5) inhibitors, such as tadalafil, increase smooth muscle relaxation through the breakdown of cyclic guanosine monophosphate (cGMP). Originally, this class of agents was used as a treatment for pulmonary hypertension and was then applied in the management of erectile dysfunction and benign prostatic hypertrophy (15). In a chance discovery, a patient being treated for multiple myeloma who was placed on treatment for erectile dysfunction with tadalafil was noted to have remarkable effect on downregulation of the immunosuppressive mechanisms (16). Further research to recapitulate this response in a mouse model and clinical trials found that PDE-5 inhibition alters antitumor immune responses by reprogramming myeloid-derived suppressor cells (MDSCs) and enhancing T-cell responses. Specifically, PDE-5 inhibitors downmodulate arginase 1 and nitric oxide synthase-2 expression in tumor-associated myeloid cells (17–19). Furthermore, in mice lacking critical elements of the adaptive immune system (BALB/c-Rag-2−/−), PDE-5 inhibitors were ineffective at altering the immune microenvironment. In aggregate, these data support the hypothesis that PDE-5 inhibitors affect adaptive immune responses by modulating innate immune cells. The observation that PDE-5 inhibition increased tumor infiltrating CD8+ T cells associated with increased IL2 production in mouse models and clinical trials further underscored the importance of T cells in PDE-5 inhibitor effects (16, 20).
Here, we used a neoadjuvant (window of opportunity) paradigm in the management of resectable HNSCC to test our hypothesis that the addition of tadalafil to nivolumab augments the antitumor effect of nivolumab through modulation of the tumor microenvironment. We report our correlative and clinical findings in patients with HPV+ and HPV− status.
Patients and Methods
Study population and design
We conducted a two-arm multi-institutional randomized trial (NCT03238365). Eligibility included newly diagnosed and resectable oral cavity, hypopharynx, larynx, or oropharynx HNSCC of any stage [American Joint Committee on Cancer (AJCC) 8th edition]. Adequate organ function was required as determined by hematologic and biochemical parameters. Subjects could not have a history of an autoimmune disorder, use of systemic steroids ≥ the equivalent of 10 mg of prednisone daily, human immunodeficiency virus, hepatitis B/C, concurrent malignancies, angina, concurrent use of nitrates, current use of PDE-5 inhibitors, or sickle cell anemia.
Upon enrollment, subjects underwent a biopsy of the primary site as well as blood sample collection. CT or MRI was performed within 28 days of enrollment. Subjects were randomized 1:1 to receive nivolumab (Bristol Myers Squibb) alone or nivolumab + tadalafil (Eli Lilly and Company) with stratification for HPV status. Subjects in both cohorts received nivolumab 240 mg intravenously on days 1 and 15 followed by surgery on day 28. Subjects in the combination cohort also received tadalafil 10 mg orally once daily for 4 weeks initiated concurrently with nivolumab. At the 4 weeks ±3 days timepoint, patients were reimaged and taken for definitive surgical resection of the primary site and regional lymph nodes, as clinically indicated. Adjuvant treatment was decided by a multidisciplinary tumor board and based on pathologic risk factors and pretreatment staging. At the time of surgical resection, biopsies of the primary tumor, involved lymph nodes, and blood were obtained and processed for correlative analysis. Patients were followed for 3 months after surgery and evaluated for surgical complications and adverse events (AEs). All AEs were collected prospectively using the Common Terminology Criteria for Adverse Events (CTCAEs) version 4.03. Stopping rules were incorporated into the trial protocol for grade 3 to 5 AEs secondary to treatment utilizing a Bayesian method of Thall and Simon (21). Our expected rate of toxicity was 10%, and we set a limit of 15% toxicity as unacceptable. This clinical trial was approved by Thomas Jefferson University and Vanderbilt University Internal Review Board and all patients signed informed consent. This trial was conducted in accordance with the Declaration of Helsinki and the guidelines for Good Clinical Practice.
Assessment of pTR
Pathologic specimens were independently graded by two pathologists using scanned digital slides. For cases with a discrete mass lesion at the primary site, the entire mass was submitted for histologic examination; for cases without a discrete mass lesion, the entire specimen was examined histologically. All lymph nodes with metastatic disease (either viable or nonviable tumor cells) or evidence of treatment response were also included in the analysis. pTR was scored as follows: pTR % = areas of treatment response/total tumor surface area (5). The histologic criteria constituting pTR included areas of macrophage reaction, multinucleated giant cells and granulomas (with or without associated keratin debris), fibrosis, and chronic inflammation adjacent to residual tumor nests. In cases of complete response, distortion of the normal architecture of the organ at the primary site or the lymph node architecture was included as an indicator of treatment response. pTR on the posttreatment specimen was used as our classification of whether a patient was a complete responder, responder, minimal responder, or nonresponder. A priori, tumors with pTR of ≥20% were defined as responders (Rs), whereas those with pTR <20% were defined as “minimal” responders. Specimens with 0% were defined as pathologic “nonresponders” (NRs) and specimens with 100% pTR were “complete responders” (CRs). For purposes of analysis, we prospectively designated Rs as those with ≥20% pTR and NRs as less than 20% pTR.
Clinical staging was determined at time of enrollment using the AJCC 8th edition clinical exam and CT and F-fluorodeoxyglucose-PET/CT scans. Correlation of imaging to pathologic response has previously been published (22). Two head and neck pathologists determined pathologic staging after resection of primary tumor and lymph nodes. We elected to look at changes in staging from pretreatment clinical stage to posttreatment pathologic stage as this comparison resolves the anticipated background noise that many result from differing states of fluctuation and inflammation, especially if fine-needle biopsy was involved in the diagnostic work-up making radiographic staging less reliable as compared with pathologic staging. In addition, pathologic changes represent the clinical paradigm more accurately in patients treated with upfront surgery where clinical staging is superseded by pathologic staging.
Specimen processing and analysis
The primary endpoints were changes in IFNγ, IL2, IL10, IL12 (p70), and TNFβ from the Luminex panel of inflammatory markers, representing a shift in immune-cell polarization (Th1/Th2; M1/M2). Secondary endpoints included safety, efficacy, and collative analysis.
Details of specimen processing, RNA sequencing (RNA-seq), gene set enrichment analysis (GSEA) analysis, flow cytometric phenotyping, Luminex analysis, extracellular vesicle analysis, and IHC are all provided in Supplementary Methods.
Statistical methods
Patients were randomized based on random permuted blocks within strata defined by location of tumor [oropharyngeal (HPV+) vs. nonoropharyngeal (HPV−)]. The primary endpoints were changes in IFNγ, IL2, IL10, IL12 (p70), and TNFβ from the Luminex panel of inflammatory markers. A sample of 40 subjects with complete data (20 per arm) was estimated to provide 80% power to detect a difference in mean change between groups of 1.1 SDs at the α = 0.01 level.
Descriptive statistics were used for demographic analysis. Adverse events and pathologic response patterns were summarized by treatment arm. Fisher exact test was used to compare randomization groups with respect to AE incidence and pattern of pathologic change. Mixed effect regression analysis was used to model change in markers from pretreatment to posttreatment. The markers were highly skewed, so the outcome variables were log-transformed prior to modeling. From the model, we estimated the geometric mean of response, the change of estimated geometric means of primary markers between pretreatment and posttreatment by treatment group, and the multiplicative difference of the changes in the two groups. The significance level of all tests for the five primary outcomes was set a priori to the 0.01 level and to the 0.05 level for secondary outcomes. P values for secondary outcomes were not adjusted for multiple comparisons. Similar models were used to compare Rs to NRs with respect to change in marker values by HPV status. Mixed effects regression was performed with SAS 9.4 (SAS Institute Inc., Cary, NC).
For flow cytometry analyses, all statistical analysis was performed using JMP software (SAS Institute). ANOVA followed by Dunnett posttest analysis were used to determine relationships between pretreatment and drug-treated response cohorts. Two-way Student t test was used to analyze differences between pretreatment response cohorts.
Results
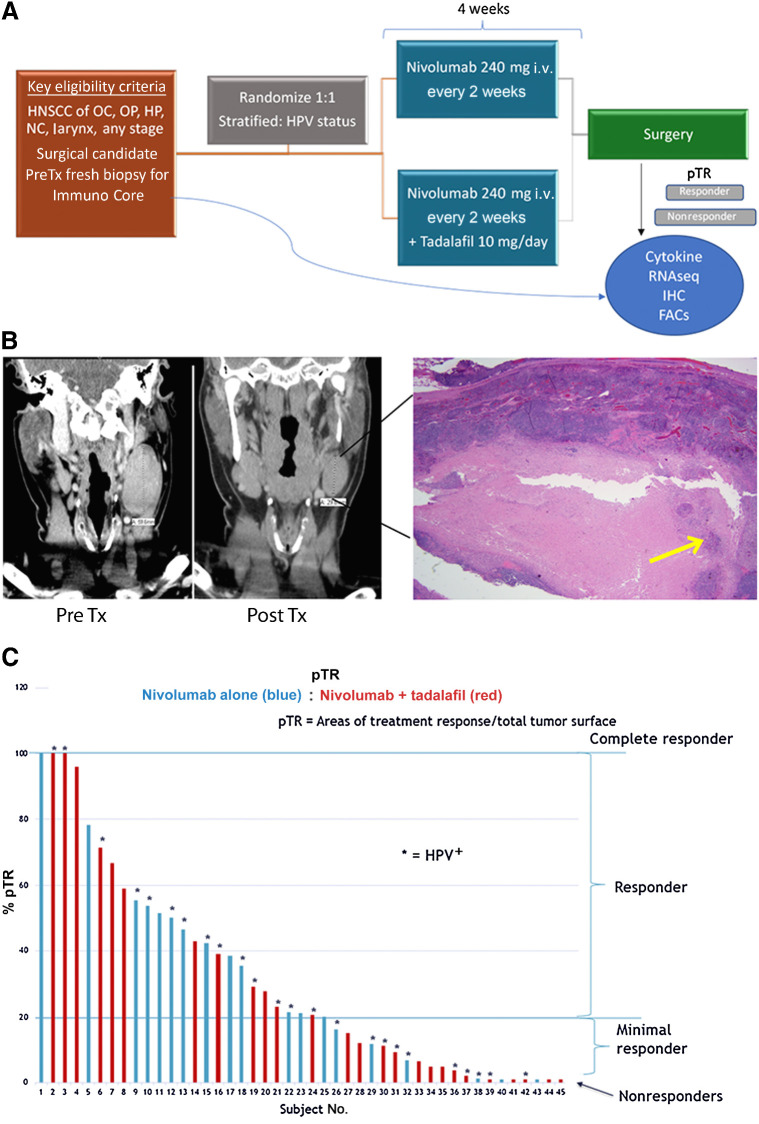
Between August 2017 and July 2019, 50 patients provided consent and were randomized. Of these, 45 enrolled and completed treatment at two institutions. Demographic and tumor characteristics were similar between those receiving nivolumab alone (n = 20) versus nivolumab + tadalafil (n = 25; Table 1). Trial schematic, example of response, and waterfall plot of pathologic response are presented in Fig. 1.
Table 1.
Patient demographics.
| All data | Nivolumab alone | Nivolumab + tadalafil | |
|---|---|---|---|
| (n = 45) | (n = 20) | (n = 25) | |
| Mean age (SD) | 63 (11) | 61 (12) | 65 (10) |
| Male:female | 41:4 | 19:1 | 22:3 |
| Primary site, n (%) | |||
| Larynx/hypopharynx | 6 (13) | 2 (10) | 4 (16) |
| Oral cavity/nasal cavity | 15 (33) | 7 (35) | 8 (32) |
| Oropharynx | 24 (53) | 11 (55) | 13 (52) |
| HPV, n (%) | |||
| Positive | 22 (49) | 10 (50) | 12 (48) |
| Negative | 23 (51) | 10 (50) | 13 (52) |
| cT stage (8th edition), n (%) | |||
| T1 | 11 (24) | 5 (25) | 6 (24) |
| T2 | 19 (42) | 7 (40) | 11 (44) |
| T3 | 4 (9) | 2 (10) | 2 (8) |
| T4 | 11 (24) | 5 (25) | 6 (24) |
| cN stage (8th edition), n (%) | |||
| N0 | 11 (24) | 5 (25) | 6 (24) |
| N1 | 25 (56) | 12 (60) | 13 (52) |
| N2 (HPV oropharynx) | 4 (9) | 0 (0) | 4 (16) |
| N2a | 3 (7) | 1 (5) | 2 (8) |
| N2b | 1 (2) | 1 (5) | 0 (0) |
| N3 | 1 (2) | 1 (5) | 0 (0) |
| Smoking status, n (%) | |||
| Never | 16 (36) | 6 (30) | 10 (40) |
| Current smoker | 7 (15) | 2 (10) | 5 (20) |
| Former smoker | 22 (49) | 12 (60) | 10 (40) |
| Alcohol status, n (%) | |||
| None/light | 31 (69) | 15 (75) | 16 (64) |
| Moderate/heavy | 14 (31) | 5 (25) | 9 (36) |
Note: Former smoker: quit more than 6 months earlier with greater than 10 pack-years. Moderate/heavy alcohol: more than two drinks/night on average.
Figure 1.
Trial schema and overall response. A, Trial schema. B, Example of radiographic and pathologic representation of treatment response. A 6-cm pretreatment lymph node decreased to 3.4 cm posttreatment and on final pathology had 95% pTR with only 5% viable tumor in the lymph node (yellow arrow). C, Waterfall plot of overall pTR by treatment group and HPV status at time of surgery. Threshold of ≥20% was considered a “responder” in the correlative analysis. Analysis of tumor tissue used pTR at the primary site only. Analysis of PBMCs used overall pTR including primary and lymph nodes. OC, oral cavity; HP, hypopharynx; NC, nasal cavity.
Nivolumab alone and the combination with tadalafil were well-tolerated with no grade 3 to 5 treatment-related AEs or grade 3 to 5 immune-related AEs (IRAEs; Table 2). There were no treatment-related delays in surgical intervention. There were no appreciable wound-healing delays after surgery. Unrelated to investigational treatment, one patient in the nivolumab-alone group was newly diagnosed with asymptomatic coronary artery disease on the preoperative risk stratification workup, resulting in cardiac revascularization that delayed the surgery intervention by 6 weeks. This patient was found to have no evidence of tumor in his final specimen. Eleven patients in the combined nivolumab + tadalafil arm developed grade 1 or 2 headaches as compared with three in the nivolumab-alone group (P = 0.06).
Table 2.
Adverse events.
| All | Nivolumab alone | Nivolumab + tadalafil | ||
|---|---|---|---|---|
| (N = 45) | (n = 20) | (n = 25) | P value | |
| Immune-related adverse events | ||||
| (Grade 1 or 2), n (%) | ||||
| Pneumonitis | (0) | 0 (0) | 0 (0) | — |
| Dermatitis | 6 (13) | 4 (20) | 2 (8) | 0.240 |
| Hepatitis | 0 (0) | 0 (0) | 0 (0) | — |
| Thyroiditis | 4 (9) | 3 (15) | 1 (4) | 0.198 |
| Gastroenteritis | 0 (0) | 0 (0) | 0 (0) | — |
| Adverse events (Grade 1 or 2), n (%) | ||||
| Nonimmune related | ||||
| Headache | 14 (31) | 3 (15) | 11 (44) | 0.054 |
| Fatigue | 15 (33) | 5 (25) | 10 (40) | 0.352 |
| Myalgia | 9 (20) | 4 (20) | 5 (20) | 1.000 |
| Presyncope | 1 (2) | 1 (5) | 0 (0) | 0.444 |
| Arthralgia/fever/chills | 6 (13) | 3 (15) | 3 (12) | 0.768 |
| Wound complications | 3 (7) | 2 (10) | 1 (4) | 0.192 |
| Nausea/vomiting | 7 (16) | 2 (10) | 5 (20) | 0.437 |
| Diarrhea | 9 (20) | 4 (20) | 5 (20) | 1.000 |
Note: All immune-related adverse events and adverse events were grade 1 or 2.
In the analysis of our primary outcomes, we did not find a difference in pretreatment peripheral cytokines in nivolumab alone versus nivolumab + tadalafil or in Rs versus NRs. When the analysis was subdivided to look at patients with HPV− and HPV+ status independently, those with HPV− status had three cytokines that demonstrated a significant change after treatment in Rs although they are not connected to a discrete immune pathway (granulocyte colony-stimulating factor, IL9, IL1A; Supplementary Fig. S1; Table 1).
Neoadjuvant nivolumab yields greater than 50% of patients with pathologic response regardless of tadalafil or HPV status
Of 45 patients evaluable for pTR, 26 (58%) had an overall pTR ≥ 20% at the primary and lymph node sites: three patients (7%) demonstrated complete response (CR) with a pTR of 100%, and 23 (51%) were Rs with a pTR 20% to 99%. Nine (20%) patients were minimal Rs with a pTR of 1% to 19%, and 10 (22%) had pTR of 0% and were thus categorized as NRs. When comparing Rs (CRs + Rs) with poor or NRs (MRs + NRs), there was a significant association between the presence of IRAEs and pTR. During the study period, 10 (22%) patients had grade 1 or 2 IRAEs: six with dermatitis, four with thyroiditis, and one with both (Table 2). Almost all of the patients who presented with IRAEs (90%) were pathologic Rs at the primary site and lymph nodes by pTR criteria (P = 0.0132).
The addition of tadalafil to nivolumab did not result in a greater pTR overall: 32.7% nivolumab alone versus 28.8% nivolumab + tadalafil (P = 0.676; Fig. 1C). There was no difference in treatment response by HPV status with mean pTR of 30% in the HPV− group and 31% in the HPV+ group (P = 0.845). Half of the subjects with involved lymph nodes had discordance of treatment response at the primary site versus lymph nodes (22). Radiographic volumetric response correlated with pTR as previously published, demonstrating significant correlation at the primary tumor site (P < 0.001) as well as at metastatic lymph nodes (P < 0.05; ref. 22). Patients with HPV+ status had significantly better disease-free survival (DFS), disease-specific survival (DSS), and overall survival [overall survival (OS); P = 0.018 for DFS, P = 0.031, and P = 0.017 for OS; Supplementary Fig. S6A] compared with patients with HPV− status. Among patients with HPV− status, overall survival is trending toward improvement in those who had evidence of pathologic response (Supplementary Fig. S6D). However, we did not observe any significant difference in DFS, DSS, and OS by treatment response status (P = 0.281 for OS; Supplementary Fig. S6D).
For all patients completing the trial, the T-stage was downstaged in nine patients from the pretreatment clinical stage to the final pathologic stage and upstaged in one patient. Most remarkable were three patients downstaged from T2 to T0 (Table 3). Nodal status was downstaged in three patients. Two patients had significant progression of disease after initiating study treatment. Both patients were HPV− oral cavity primaries and had progression only at the primary site as evident by repeat CT scans. These patients received only one dose of immunotherapy before being taken off the study due to progression. The first patient who received nivolumab alone had hyperprogression (3.1 × 1.5 cm increasing to 5.5 × 2.5 cm on CT scan, representing a >twofold tumor growth rate before treatment to after treatment) at 2 weeks (23). This patient was taken at week 2 to the operating room for resection. There was no pTR at the primary site, although there was 95% pTR in one lymph node without any evidence of disease or pTR in other nodes. The second patient who received nivolumab + tadalafil was taken off the trial after the first dose of nivolumab due to clinical evidence of progression and placed on induction cisplatin + docetaxel followed by surgical resection. Final pathology revealed 95% pTR secondary to induction chemotherapy. Three additional patients were upstaged from clinical to pathologic staging based on nodal status (from N0 to N1) with deposits of carcinoma ranging from 1 mm to 12 mm within a lymph node consistent with occult disease and not secondary to progression. Each of the patients that had findings of occult metastasis on pathologic review, upstaging from N0 to N1, were in the nivolumab + tadalafil cohort. We do not have any biologic explanation for this being found only in the cohort with nivolumab + tadalafil but do not find it to be clinically significant, as adjuvant treatment recommendations were not changed.
Table 3.
Change of staging after 4 weeks of treatment.
| Pretreatment clinical stage change to pathologic stage | All | Nivolumab alone | Nivolumab + tadalafil | |
|---|---|---|---|---|
| (N = 45) | (n = 20) | (n = 25) | P value | |
| T1→T0 | 1 (2) | 1 (5) | 0 (0) | 0.792 |
| T2→T0 | 3 (7) | 1 (5) | 2 (8) | |
| T2→T1 | 4 (9) | 1 (5) | 3 (12) | |
| T3→T4a | 1 (2) | 0 (0) | 1 (4) | |
| No change in T stage | 36 (80) | 17 (85) | 19 (76) | |
| N1→N0 | 3 (7) | 0 (0) | 3 (12) | 0.038 |
| N0→N1 | 4 (9) | 0 (0) | 4 (16) | |
| No change in N stage | 38 (84) | 20 (100) | 18 (72) |
T cells are predictive of response in HPV− but not HPV+ tumors, whereas tumor proliferation index is predictive in all tumors
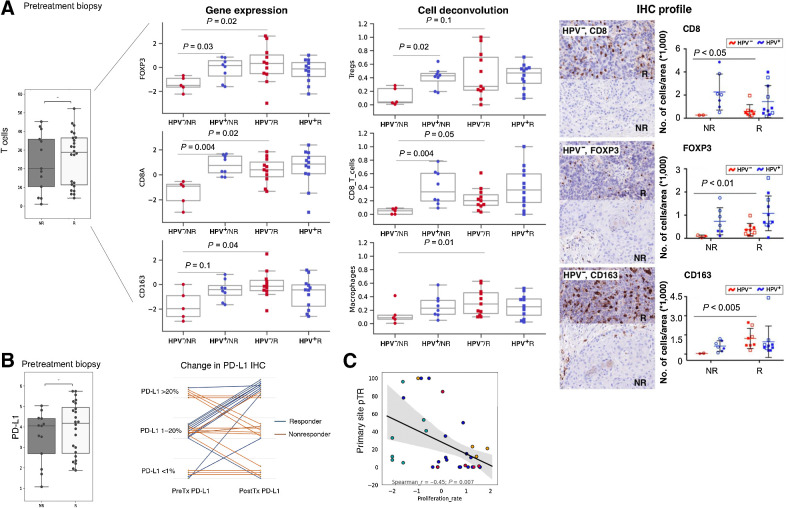
Comparison of biomarker changes in Rs and NRs was performed in pretreatment samples in both arms combined using pTR definitions provided (see Supplementary Data for details of biomarker analysis). Analysis of combined pretreatment tumor RNA-seq showed that T-cell signature and PD-L1 expression agnostic of HPV status were not significantly correlated with response (Fig. 2A and B). On subanalysis of the T-cell signatures between HPV+ and HPV− tumors, we found overall T cells, CD4+, CD8+, and regulatory T cells (Tregs) were predictive of response in the HPV− cohort alone (Fig. 2A). IHC confirmed this finding: HPV− Rs had significantly more CD8, FoxP3, and CD163 cells while the NR group presented as an immune cell desert, void of all immunoreactivity for immune cells. HPV+ tumors had a higher abundance of immune cells compared with HPV− tumors but did not demonstrate a different profile in the pretreatment samples (Fig. 2A). Pretreatment PD-L1 RNA signature and IHC was not predictive of pathologic response (Fig. 2B). Interestingly, tumor proliferation rate signatures (24) were significantly associated with NRs regardless of HPV status (P = 0.007; Fig. 2C).
Figure 2.
HPV− Rs have increased pretreatment T-cell abundance. A, Pretreatment biopsy CD8+ T-cell RNA signature did not demonstrate significance between Rs and NRs. Subgroup analysis with respect to HPV status reveals the HPV− cohort having a significant difference in overall T cells, Tregs, CD8, and CD4 that is not identified in the HPV+ cohort. Pretreatment CD8, FoxP3, and CD163 IHC confirm a significant difference for HPV−but not positive tumors pointing to an immune desert as a significant correlate of nonresponse. B, Quantitation by RNA-seq of PD-L1 and semiquantification by PD-L1 IHC staining demonstrating no predictive significance for response (left graph, y-axis shows log2 expression). In pre- to posttreatment samples PD-L1 staining remained stable in the NRs and trended upward in the Rs (right graph). C, High tumor proliferation rate signatures in the pretreatment samples are associated with nonresponse. The tumor proliferation signature consists of cell cycle– and tumor progression–associated genes.
ICI Rs reveal higher peripheral B-cell count and CD8+:CD4+ ratio, and lower peripheral PDL1 and CD163 pretreatment
The peripheral blood mononuclear cell (PBMC) analysis revealed that Rs have a greater number of B cells (P = 0.007) (Fig. 3). The R group had a lower absolute peripheral T-cell count (P = 0.001), but the ratio of CD8+:CD4+ T cells was far greater in the responder group as compared with NRs (P = 0.03), pointing to a greater effector population. Interestingly, nivolumab + tadalafil showed a greater CD8+:CD4+ ratio after treatment regardless of response (Fig. 3). From a biomarker standpoint, pretreatment peripheral PD-L1 and CD163 were both significantly downregulated in the R group as compared with the NR group, suggesting a less suppressive peripheral environment at the start of treatment (Fig. 3).
Figure 3.
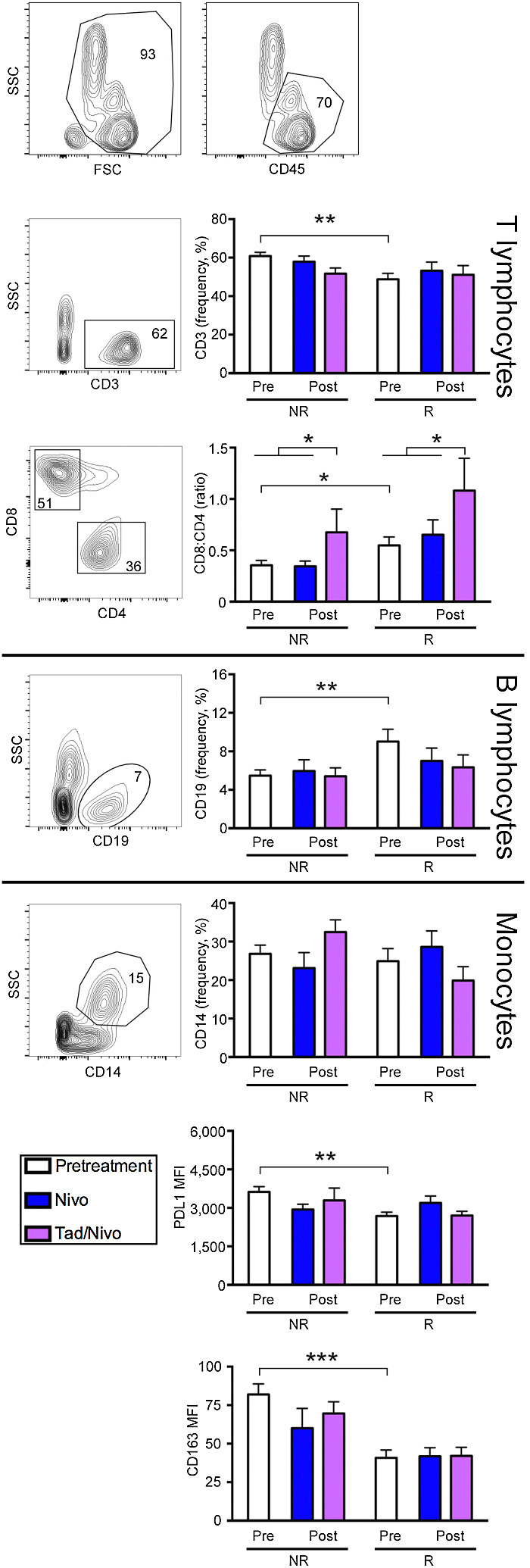
Flow cytometric analysis of peripheral blood cell subsets shows that Rs have different pretreatment peripheral compartments that support cytotoxic T cells. Scatter graphs depict representative subjects with numbers indicating the percentage of cells within a given gate. Graphs show NRs and Rs baseline pretreatment values compared with posttreatment nivolumab versus nivolumab + tadalafil. Rs demonstrated a greater CD8:CD4 ratio in the periphery with the tadalafil cohort significantly augmenting this ratio after treatment regardless of response. In pretreatment samples, B lymphocytes are increased. Peripheral PD-L1 levels and CD163 macrophages are significantly decreased in Rs compared with the NRs, suggesting the peripheral compartment plays role in treatment response. Statistical significance was assessed using ANOVA (*P < 0.05, **P < 0.001, ***P < 0.0005). Nivo, nivolumab; Tad, tadalafil.
Tadalafil amplifies B and NK cells present in Rs, posttreatment
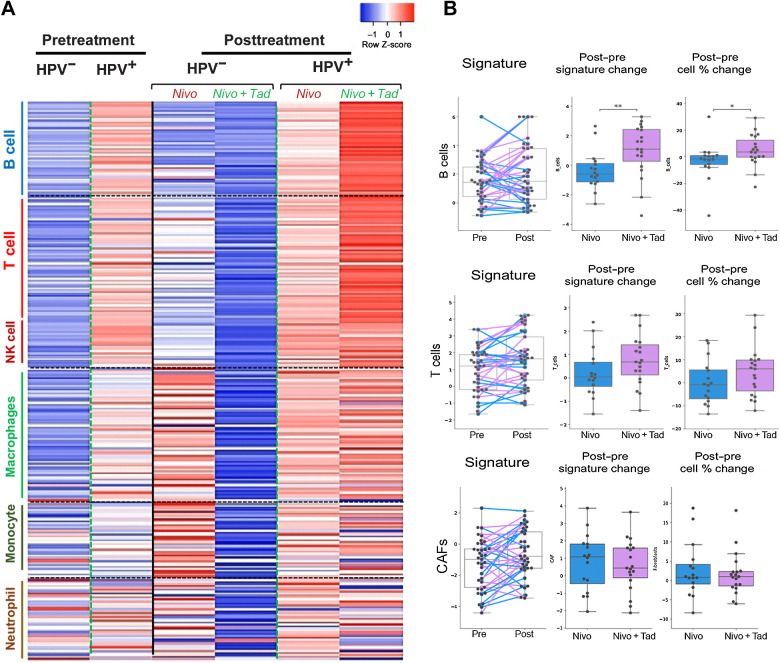
RNA-seq revealed that HPV+ tumors had an increased gene expression associated with B- and T-cell immune signatures with nivolumab plus tadalafil when compared with nivolumab alone, regardless of response (Fig. 4A). In contrast, HPV− tumors had lower expression of all immune signatures (B, T, NK etc.) after receiving tadalafil when compared with nivolumab alone, indicating a clear differential response in HPV+ versus HPV− tumors at the transcriptomic level when analyzed in aggregate and regardless of response. Parallel deconvolution of cell types by Kassandra algorithm found confirmatory results in the B-cell population (25). Dynamic changes caused by tadalafil in both a signature change and percent cell change included a significant increase in B-cell population and upward trends in the T-cell population when analyzed agnostic to HPV status (Fig. 4B). Signatures associated with cancer-associated fibroblasts did not show any change between the two cohorts (Fig. 4B). Although there was no correlation to response, HPV+ tumors demonstrated a higher level of RNA expression of 292 immune genes as compared with the HPV− cohort (Supplementary Fig. S2).
Figure 4.
HPV+ tumors demonstrate the greatest overall immune-related transcriptomic change with the addition of tadalafil. A, Heatmap showing expression of a set of 292 immune signature genes comparing pre- and posttreatment separated out by HPV status and treatment cohort regardless of response. Individual genes (demarcated by rows) are subdivided based on immune cell association (vertical legend). Each column represents a cohort prior to treatment or following treatment. B, Gene signatures demonstrate dynamic changes caused by tadalafil in the B-cell population, with upward trend in the T-cell population and no effect on CAFs. *P < 0.05, ** P < 0.01. Nivo, nivolumab; Tad, tadalafil.
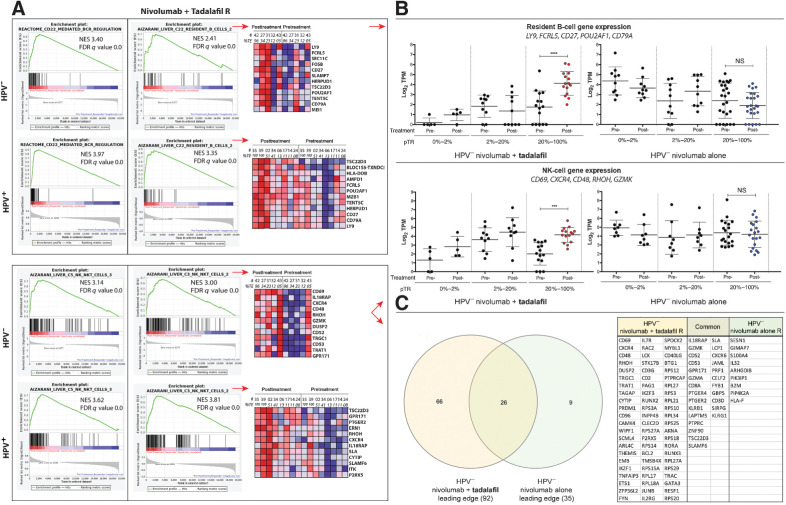
To further look at differences in response, we performed GSEA of bulk RNA sequences from post- versus pretreatment samples. Unbiased query with gene set collections from the Molecular Signatures Database (MSigDB) 7.2 (26, 27) revealed the most significant enrichments in the C2 canonical pathway, C5 bioprocesses, and C8 cell-type signature collections were B-cell– and NK-cell–related gene sets. In the canonical pathway collection, B-cell–related gene sets included those for B-cell receptor regulation, Fc gamma receptor activation, and complement triggering (Fig. 5A; Supplementary Fig. S3A and S3B). For instance, CD22-mediated B-cell receptor regulation elevated to normalized enrichment scores (NES) of 3.97 (FDR q value 0.000) and 3.4 (FDR q value 0.000) in HPV+ and HPV− nivolumab + tadalafil Rs, respectively, posttreatment (Fig. 5A, top left). In contrast, treatment with nivolumab alone did not result in the posttreatment enrichment of these gene sets (Supplementary Fig. S3B). Unbiased query with the bioprocesses component of the C5 collection confirmed posttreatment B-cell pathway enrichments in HPV+ and HPV− nivolumab + tadalafil Rs (not shown).
Figure 5.
Unbiased GSEA of bulk RNA sequences, indicating enrichment of B- and NK-cell gene sets in Rs to nivolumab and tadalafil. A, Enrichment plots and heat maps. Top, left: Result from a canonical pathway collection (C2 cp) query showing enrichment of CD22-mediated B-cell antigen receptor regulation in HPV− and HPV+ Rs, posttreatment. Top, right: Cell-type signature (C8) query revealing enrichment of B cells. Heatmaps show the 12-most upregulated genes, posttreatment. Bottom, left: Cell-type signature collection (C8) query illustrating enrichment of an NK-cell gene set in HPV− and HPV+ Rs. Bottom, right: Enrichment of a second NK-cell gene set, with portions of heatmaps. B, Scatter plots of normalized transcript counts from bulk RNA sequence data. Top: Expression of B-cell genes, selected from heat maps in panel A. Enrichment, posttreatment, manifested in the 20% to 100% pTR group that received nivolumab + tadalafil (left; one-way ANOVA; P < 0.0001), but not nivolumab-alone (right). Bottom: Similarly, a subset of five NK-cell genes were found to be enriched by tadalafil in the 20% to 100% pTR group, posttreatment (one-way ANOVA; P = 0.0003). C, Venn diagram of the GSEA leading edges and table of the 66 genes of an NK-cell signature unique to the tadalafil group. NS, not significant.
Next, an unbiased GSEA query was performed with the cell-type signature collection C8, which sources single-cell RNA-seq studies. GSEA revealed that B cells and NK cells were among those most enriched in Rs with the addition of tadalafil. The pattern of B-cell enrichment seen in C8 was consistent with the enrichments in B-cell canonical pathways observed in C2 and C5. NK-cell gene sets described by Aizarani and colleagues (2019) accounted for four of the top five hits in HPV+ nivolumab + tadalafil Rs posttreatment (Supplementary Table S1; ref. 28). The highest NES was 3.81; FDR q value 0.000 for HPV+ and NES 3.00; FDR q value 0.000 for HPV− (Fig. 5A, bottom, middle). In contrast, nivolumab-alone Rs showed no enrichment (HPV+ NES 0.97, FDR q value 0.606; HPV− NES 1.28, FDR q value 0.162).
Normalized transcript counts for subsets of coordinately expressed B-cell or NK-cell genes were illustrated in scatter plots and again in line plots to detail the full spectrum of response (% pTR) from NR to complete response. Upregulation in the posttreatment group was observed in the presence of nivolumab + tadalafil, but not in the nivolumab-alone group, beginning at 20% pTR for B-cell genes (Fig. 5B, top; Supplementary Fig. S4A and S4B) and NK-cell genes (Fig. 5B, bottom; Supplementary Fig. S5A and S5B).
To further characterize the association between the nivolumab + tadalafil combination and NK cells, we performed supervised GSEA and qualitative Venn diagram analysis of leading edges (Fig. 5C). To identify quantitative changes in the nivolumab + tadalafil versus nivolumab-alone leading edges, a side-by-side comparison of rank-in-gene-list was performed for each gene (Supplementary Table S2). As detailed in the Supplemental Results, an extensive set of genes was found to be (i) enriched only with nivolumab + tadalafil and (ii) upregulated compared to nivolumab alone, with gene rankings that were vastly different between the groups (e.g., CD69, CXCR4, GMZK, DUSP2).
Overall, the addition of tadalafil to patients with HPV+ status resulted in the greatest increase in B- and T-cell gene signatures regardless of response. When stratified by response, GSEA identified consistent upregulation of B- and NK-cell gene expression in all Rs with the addition of tadalafil.
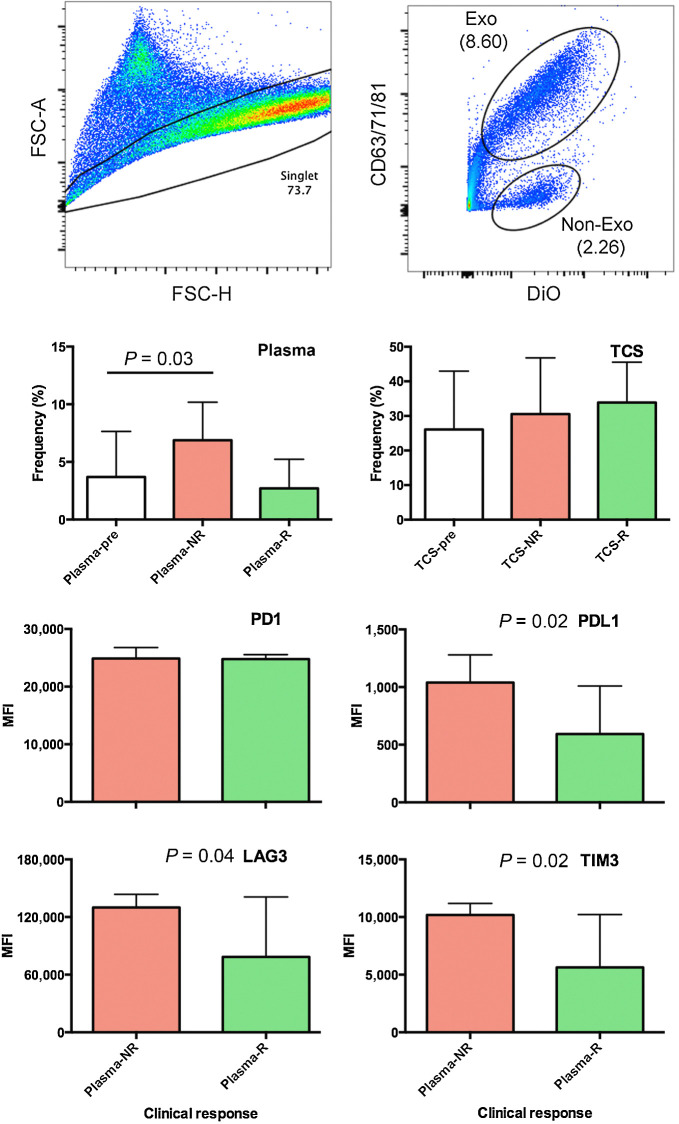
Increased circulating exosomes and increased immune checkpoint receptors observed in exosomes isolated from NRs
Flow cytometric analysis of exosomes in pre- and posttreatment plasma (n = 41) and supernatants (n = 23) from short-term ex vivo tumor cell cultures showed a significant increase in circulating exosomes in the NRs as compared with Rs. Expression of immune checkpoint receptors on exosomes present in posttreatment tumor culture supernatant point to an increase in checkpoints that inhibit an immune response (PD-L1, LAG3, and TIM3) in the tumor supernatants of subjects who did not have a pathologic response (Fig. 6).
Figure 6.
Flow cytometric analysis of exosomes in pre- and posttreatment plasma and supernatants from short-term ex vivo tumor cell cultures identified increase exosomes in NRs. A, Gating strategies for single events (left) and exosomes (right). B, The frequency of circulating exosomes in pretreatment plasma (n = 41; left) and overnight biopsy cultures (n = 23; right) as they relate to clinical response. Statistical significance was assessed using ANOVA (*P = 0.03). C, Bar charts show expression of immune checkpoint receptors on exosomes present in posttreatment tumor culture supernatant as they relate to clinical response (n = 23). Statistical significance was assessed using Student t test (*P < 0.05). Exo, exomes; MFI, mean fluorescence intensity; FSC-A, Forward scatter area; FSC-H, forward scatter height; DiO, 3,3′-dioctadecyloxacarbocyanine; TCS, tumor culture supernatant.
Discussion
Neoadjuvant preoperative treatment with nivolumab with or without tadalafil was safe and demonstrated a wide variety of pTRs ranging from no response to CR. There was evidence that tadalafil triggered an adaptive immune response, however, the 4-week window-of-opportunity treatment with tadalafil did not produce a statistically significant increase in pTR. A drug exposure of 4 weeks may not have been long enough to result in a therapeutic effect of priming with tadalafil to improve the immune checkpoint response. However, despite the small sample size, findings within the pretreatment samples point to predictive modeling of response in an HPV-dependent manner.
This trial contributes to emerging neoadjuvant therapy data supporting safe use of immune checkpoint inhibitors in previously untreated early-stage HNSCC, including HPV+ tumors. Other trials have also demonstrated the safety of nivolumab in the neoadjuvant setting (4, 5). Our cohort of 45 patients did not experience any grade 3 or greater IRAE or severe AEs nor were any surgical cases delayed due to AEs or postoperative complications related to immunotherapy or tadalafil treatment observed. Our rate of IRAEs is consistent with reported data from ICI monotherapy trials in recurrent or metastatic HNSCC, however, our period of observation is short (1, 3, 7). Of clinical interest was the association we found between the presence of grade 1 or 2 IRAEs (dermatitis/thyroiditis) and pTR. This is an intriguing observation in the neoadjuvant setting of short drug exposure. Previous studies in various advanced solid tumors have shown an association between the emergence of IRAEs and ICI efficacy (29–32).
Another point of interest is our analysis based on HPV status. Overall, 63% of the HPV+ tumors had a pTR greater than 20% with nivolumab +/− tadalafil. However, HPV status was not a predictor of pTR response to PD-1 blockade, and our interrogation did not reveal an immune-related pretreatment profile that could predict response in patients with HPV+ tumors as gauged by tumor proliferation. HPV-associated tumors develop within the tonsillar lymphoid tissue, an immune-rich host environment. Therefore, escape mechanisms may develop early in tumor evolution in HPV+ HNSCC (33). Aggarwal and colleagues primed an active immune response in patients with HPV+ tumors by using DNA immunotherapy targeting HPV 16/18 E6/E7 with IL12 encoding plasmids and showed a durable antigen-specific peripheral tumor immune response, but with a concomitant increase in PD-1/PD-L1 activation (34). In our cohort of patients, tadalafil likewise increased the immune-associated gene expression profile of an active adaptive immune response (Fig. 5; Supplementary Fig. S3).
Our data set did not demonstrate that PD-L1 IHC and HPV status were predictive of response to immunotherapy. In the pooled clinical trial data analysis by Wang and colleagues, patients with higher PD-L1 expression who were treated with PD1– or PD-L1–directed immune checkpoint inhibitors had higher response rates compared with those with low PD-L1 expression (1, 3, 7, 35, 36). Similarly, in the previously untreated patient population of the KEYNOTE-048 trial, higher PD-L1 expression correlated with higher response rates to pembrolizumab monotherapy (7). Nevertheless, PD-L1 expression was not useful in predicting survival benefit from nivolumab in the phase III CheckMate 141 trial (1). In a neoadjuvant trial, PD-L1 combined positive score ≥ 1 was not independently associated with 1-year DFS but was highly associated with pTR (P = 0.0007), demonstrating the difficulty with PD-L1 acting as the only prognosticator of outcomes (37). It is unclear whether HPV status results in a differential response to ICIs in HNSCC. In the analysis by Wang and colleagues, the authors proposed that the HPV+ inflamed immune microenvironment would result in an improved immunotherapy response (36). However, in our dataset, this was not the case. To supplement this question, we looked at IFNγ signatures in the bulk RNA-seq and did not identify any predictive findings (Supplementary Fig. S7).
We were, however, able to identify a pretreatment profile that predicted pTR in the HPV− cohort. Quantitative IHC evaluation revealed an immune cell desert in HPV− NRs, with a significantly higher number of CD8+, FoxP3+, and CD163+ cells in the stroma, tumor, and stromal/tumor interface in HPV− Rs. In our RNA-seq analysis, HPV− tumors demonstrated a significant correlation between pretreatment expression of T-cell–specific genes (Treg, CD8+, CD4+) and response. This same pretreatment profile was not found in the HPV+ cohort. In a comprehensive proteo-genomic study of 108 HPV− tumors, investigators found that widespread deletion of immune modulatory genes (through somatic mutation or genomic loss) accounted for the low immune infiltration in immune cold-tumors. Conversely, tumors with some immune infiltrate also have upregulation of checkpoints (38). It is important to note that, in our cohort, neither the PD-L1 IHC nor the absolute level of T-cell expression was predictive of response, regardless of HPV status. Outside the scope of this research, but certainly informative, would be a multiplex platform adding important geographic cell–cell dimension to this analysis.
The observation that exosome levels were greater in the periphery of NRs than in the Rs has a number of potential implications. The role of circulating vesicles has yet to be established in tumor patients, and one significant risk is the potential to inhibit antitumor immunity in the tumor microenvironment thus reducing the efficacy of an ICI. This is exemplified in Schroeder and colleagues' work, noting the concentration of circulating exosomes was increased in patients with HNSCC as compared with healthy donors resulting in an inhibitory effect on the function of B cells (39). Our data showing elevated PD-L1, TIM3, and LAG 3 in NRs, in addition to others, demonstrate that exosomes can have an inhibitory function (40). The complexity of immunotherapy success or failure demands a holistic understanding, including periphery and local tumor microenvironment, to ultimately predict response to ICIs (28).
The addition of tadalafil to nivolumab resulted in a significant immune-related transcriptomic change that points to B-cell–associated genes as a prime target. Additionally, we demonstrated that tadalafil increased the peripheral complement of effector T cells, as demonstrated by a significant augmentation of the CD8:CD4 ratio. Further, GSEA suggests a role for the nivolumab and tadalafil combination in NK-cell reprogramming. After treatment, a 92-gene leading edge of an enriched NK-cell gene set was shown to contain activation-related markers in the nivolumab + tadalafil group, relative to the nivolumab-alone group. Conceivably, these genes represent an expression signature for reactivation of tumor-induced NK-cell exhaustion. At present, however, it is important to recognize that this is a GSEA-based hypothesis. Given the effects of the nivolumab and tadalafil combination treatment on both B- and NK-cell gene sets, it may also be hypothesized that tumor cell killing is occurring via antibody-dependent cell-mediated cytotoxicity. With recent data demonstrating a NKTR-255, a congregated IL15 molecule, having an antitumor effect through activation of NK cells supports the further investigation into NK strategies. Tadalafil, with its strong history of safety in a longer-term strategy, may have therapeutic benefit (41). A pilot trial utilizing tadalafil in patients with melanoma demonstrated stability of disease in three of 12 patients (25% of patients and in this group), and there was a significantly higher number of CD8+ tumor-infiltrating lymphocytes (TILs) in the center of the metastases before treatment as compared with patients who progressed. After treatment, there was an increase in expression of γ-chain (marker of T-cell activation) in CD8 and CD4 TILs and CD8+ T cells in the peripheral blood as compared with baseline (42). In HNSCC trials using PDE-5 inhibitors, correlative findings demonstrated reduction in MDSCs, Tregs, and augmented effector response with CD8+ TILs (17–19). A Veterans Affairs study with 221,538 participants demonstrated patients taking PDE-5 inhibitors for erectile dysfunction had a lower hazard of colorectal cancer as compared with those not on PDE-5 inhibitors (43). Additional trials in hepatocellular carcinoma and other abdominal malignancies are currently enrolling subjects.
Our randomized clinical trial adds to the body of literature that tadalafil is active at augmenting the immune microenvironment and raises the novel possibility that tadalafil can reprogram NK cells. The short duration of this trial is a limitation; follow-up studies are needed to explore the extended use of tadalafil in the setting of PD-1 checkpoint inhibition in individuals who respond to treatment. Additionally, our results provide further support for the exploration of the PD-1 blockade in the previously untreated HNSCC. Finally, the data presented here also points to the importance of understanding treatment response and predictors of response that differentiate tumors according to their HPV status.
Supplementary Material
Acknowledgments
We thank all patients and their families for participating in this study. We thank the Sidney Kimmel Cancer Center at Sidney Kimmel Medical College and Thomas Jefferson University and Vanderbilt University for the efforts put forward by the clinical trials office, which provided protocol development and clinical trial support (including Dawn Poller, Dorit Falk, Kathy Taylor). Y.J. Kim would like to thank the Barry and Amy Baker Endowment and the Rowen Fund for their generous contributions to this study. Financial support: Investigator Initiated Trial funded by Bristol Myers Squibb. The funders had no role in design of the trial, administration of the trial, collection of data, or analysis of data. This project utilized the Biostatistics, Genomics, and Flow Cytometry Shared Resources at the Sidney Kimmel Cancer Center, supported by the NCI, grant 5P30CA056036-17. This work was supported by the NIH (R01DE027749, R01CA178613, to Y.J. Kim and R01CA244522 to A.P. South).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
A.J. Luginbuhl reports grants from Bristol Myers Squibb during the conduct of the study. S.K. Shukla reports grants from Bristol Myers Squibb during the conduct of the study. D.M. Cognetti reports grants from Bristol Myers Squibb during the conduct of the study and personal fees from Rakuten Medical Inc., Intuitive, and Cortexyme outside the submitted work. J.M. Curry reports nonfinancial support from AstraZeneca and personal fees from Rakuten Medical Inc. outside the submitted work. N. Kotlov reports other support from BostonGene Corporation during the conduct of the study and has a patent for BostonGene pending and issued to BostonGene Corporation. Z. Antysheva reports other support from BostonGene Corporation outside the submitted work. S. Degryse reports other support from BostonGene Corporation outside the submitted work. J. Netterville reports grants from Bristol Myers Squibb during the conduct of the study. S. Gargano reports grants from Bristol Myers Squibb during the conduct of the study. B.E. Leiby reports grants from Bristol Myers Squibb and the NCI during the conduct of the study. U. Martinez-Outschoorn reports grants from Otsuka and personal fees from Seagen, AstraZeneca, EUSA outside the submitted work. Y.J. Kim reports grants from the National Institute of Dental and Craniofacial Research outside the submitted work; in addition, Y.J. Kim is coinventor of patents licensed to Aduro Biotech; has served as paid scientific advisor to AZ Medimmune, Sanofi, Takeda, and Mersanna; and is supported by the Barry Baker Biorepository Fund, Jim Rowen Fund, NIH R01 CA178613, R01 DE027749, and the Department of Defense Congressionally Directed Medical Research Programs Breakthrough Award. A.P. South reports owning stock in Krystal Biotech Inc. and consulting for, and ownership interests in Zikani Therapeutics. A. Argiris reports grants and personal fees from Bristol Myers Squibb outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
A.J. Luginbuhl: Conceptualization, resources, data curation, formal analysis, supervision, funding acquisition, validation, investigation, visualization, methodology, writing–original draft, project administration, writing–review and editing. J.M. Johnson: Conceptualization, formal analysis, supervision, funding acquisition, investigation, writing–original draft, project administration, writing–review and editing. L.A. Harshyne: Formal analysis, validation, investigation, methodology, writing–original draft, writing–review and editing. A.J. Linnenbach: Data curation, formal analysis, validation, methodology, writing–original draft, writing–review and editing. S.K. Shukla: Formal analysis, validation, methodology, writing–review and editing. A. Alnemri: Data curation, formal analysis, validation, writing–review and editing. G. Kumar: Formal analysis, methodology, writing–review and editing. D.M. Cognetti: Conceptualization, resources, supervision, project administration, writing–review and editing. J.M. Curry: Conceptualization, data curation, funding acquisition, validation, project administration, writing–review and editing. N. Kotlov: Software, formal analysis, validation, writing–review and editing. Z. Antysheva: Software, formal analysis, validation, writing–review and editing. S. Degryse: Software, formal analysis, validation, investigation, writing–review and editing. K. Mannion: Data curation, supervision, project administration, writing–review and editing. M.K. Gibson: Data curation, supervision, project administration, writing–review and editing. J. Netterville: Data curation, supervision, project administration, writing–review and editing. B. Brown: Data curation, formal analysis, investigation, writing–review and editing. R. Axelrod: Conceptualization, data curation, investigation, writing–review and editing. R. Zinner: Investigation, writing–review and editing. M. Tuluc: Data curation, formal analysis, supervision, validation, investigation, methodology, writing–original draft, writing–review and editing. S. Gargano: Formal analysis, investigation, methodology, writing–review and editing. B.E. Leiby: Conceptualization, data curation, software, formal analysis, supervision, investigation, writing–review and editing. A. Shimada: Data curation, validation, investigation, writing–review and editing. M.G. Mahoney: Formal analysis, validation, project administration, writing–review and editing. U. Martinez-Outschoorn: Conceptualization, resources, formal analysis, methodology, writing–review and editing. U. Rodeck: Conceptualization, resources, data curation, formal analysis, supervision, validation, investigation, visualization, methodology, project administration. Y.J. Kim: Conceptualization, resources, formal analysis, supervision, methodology, project administration, writing–review and editing. A.P. South: Conceptualization, resources, formal analysis, supervision, validation, investigation, methodology, writing–review and editing. A. Argiris: Conceptualization, supervision, funding acquisition, investigation, methodology, project administration, writing–review and editing.
References
- 1. Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol 2016;17:956–65. [DOI] [PubMed] [Google Scholar]
- 3. Cohen EEW, Soulières D, Tourneau CL, Dinis J, Ahn M-J, Soria A, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet North Am Ed 2019;393:156–67. [DOI] [PubMed] [Google Scholar]
- 4. Schoenfeld JD, Hanna GJ, Jo VY, Rawal B, Chen Y-H, Catalano PS, et al. Neoadjuvant Nivolumab or Nivolumab Plus Ipilimumab in untreated oral cavity squamous cell carcinoma: a phase 2 open-label randomized clinical trial. JAMA Oncol 2020;6:1563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Uppaluri R, Campbell KM, Egloff AM, Zolkind P, Skidmore ZL, Nussenbaum B, et al. Neoadjuvant and adjuvant pembrolizumab in resectable locally advanced, human papillomavirus-unrelated head and neck cancer: a multicenter, phase 2 trial. Clin Cancer Res 2020;26:5140–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferrarotto R, Bell D, Rubin ML, Hutcheson KA, Johnson JM, Goepfert RP, et al. Impact of Neoadjuvant Durvalumab with or without Tremelimumab on CD8+ tumor lymphocyte density, safety, and efficacy in patients with oropharynx cancer: CIAO trial results. Clin Cancer Res 2020;26:3211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 2019;394:1915–28. [DOI] [PubMed] [Google Scholar]
- 8. Dos Santos LV, Abrahão CM, William WN Jr. Overcoming resistance to immune checkpoint inhibitors in head and neck squamous cell carcinomas. Front Oncol 2021;11:596290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 2020;367:eaax0182–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu J, Blake SJ, Yong MCR, Harjunpää H, Ngiow SF, Takeda K, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov 2016;6:1382–99. [DOI] [PubMed] [Google Scholar]
- 11. Jia X-H, Xu H, Geng L-Y, Jiao M, Wang W-J, Jiang L-L, et al. Efficacy and safety of neoadjuvant immunotherapy in resectable nonsmall cell lung cancer: a meta-analysis. Lung Cancer 2020;147:143–53. [DOI] [PubMed] [Google Scholar]
- 12. Chalabi M, Fanchi LF, Dijkstra KK, Van den Berg JG, Aalbers AG, Sikorska K, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med 2020;26:566–76. [DOI] [PubMed] [Google Scholar]
- 13. Ferris RL, Spanos WC, Leidner R, Gonçalves A, Martens UM, Kyi C, et al. Neoadjuvant nivolumab for patients with resectable HPV-positive and HPV-negative squamous cell carcinomas of the head and neck in the CheckMate 358 trial. J Immunother Cancer 2021;9:e002568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Uppaluri R, Chernock R, Mansour M, Jackson R, Rich J, Pipkorn P, et al. Enhanced pathologic tumor response with two cycles of neoadjuvant pembrolizumab in surgically resectable, locally advanced HPV-negative head and neck squamous cell carcinoma (HNSCC). J Clin Oncol 2021;39:6008. [Google Scholar]
- 15. Terrett NK, Bell AS, Brown D, Ellis P. Sildenafil (VIAGRATM), a potent and selective inhibitor of type 5 cGMP phosphodiesterase with utility for the treatment of male erectile dysfunction. Bioorg Med Chem Lett 1996;6:1819–24. [Google Scholar]
- 16. Noonan KA, Ghosh N, Rudraraju L, Bui M, Borrello I. Targeting immune suppression with PDE5 inhibition in end-stage multiple myeloma. Cancer Immunol Res 2014;2:725–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Califano JA, Khan Z, Noonan KA, Rudraraju L, Zhang Z, Wang H, et al. Tadalafil augments tumor specific immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weed DT, Vella JL, Reis IM, De la Fuente AC, Gomez C, Sargi Z, et al. Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weed DT, Zilio S, Reis IM, Sargi Z, Abouyared M, Gomez-Fernandez CR, et al. The reversal of immune exclusion mediated by tadalafil and an anti-tumor vaccine also induces PDL1 upregulation in recurrent head and neck squamous cell carcinoma: interim analysis of a phase I clinical trial. Front Immunol 2019;10:1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med 2006;203:2691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thall PF, Simon R. Practical Bayesian guidelines for phase IIB clinical trials. Biometrics 1994;50:337–49. [PubMed] [Google Scholar]
- 22. Merlino DJ, Johnson JM, Tuluc M, Gargano S, Stapp R, Harshyne L, et al. Discordant responses between primary head and neck tumors and nodal metastases treated with neoadjuvant nivolumab: correlation of radiographic and pathologic treatment effect. Front Oncol 2020;10:566315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frelaut M, Tourneau CL, Borcoman E. Hyperprogression under immunotherapy. Int J Mol Sci 2019;20:2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bagaev A, Kotlov N, Nomie K, Svekolkin V, Gafurov A, Isaeva O, et al. Conserved pan-cancer microenvironment subtypes predict response to immunotherapy. Cancer Cell 2021;39:845–65. [DOI] [PubMed] [Google Scholar]
- 25. Zaytcev A, Chelushkin M, Nuzhdina K, Bagaev A, Dyykanov D, Zyrin V, et al. Abstract 853: Novel machine learning based deconvolution algorithm results in accurate description of tumor microenvironment from bulk RNAseq. Clin Res Exclud Clin Trials 2020;853. [Google Scholar]
- 26. Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 2005;102:15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liberzon A, Birger C, Thorvaldsdóttir H, Ghandi M, Mesirov JP, Tamayo P. The molecular signatures database hallmark gene set collection. Cell Syst 2015;1:417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aizarani N, Saviano A, Sagar, Mailly L, Durand S, Herman JS, et al. A human liver cell atlas revealsng heterogeneity and epithelial progenitors. Nature 2019;572:199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou X, Yao Z, Yang H, Liang N, Zhang X, Zhang F. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. Bmc Med 2020;18:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cortellini A, Chiari R, Ricciuti B, Metro G, Perrone F, Tiseo M, et al. Correlations between the immune-related adverse events spectrum and efficacy of anti-PD1 immunotherapy in NSCLC patients. Clin Lung Cancer 2019;20:237–47. [DOI] [PubMed] [Google Scholar]
- 31. Foster CC, Kochanny S, Khattri A, Acharya R, Dekker A, Carol Tan Y-H, et al. Association of immune-related adverse events (irAEs) with improved response, progression-free survival, and overall survival for patients with metastatic head and neck cancer receviing anti-PD-1 therapy. J Clin Oncol 36:15s, 2018(suppl; abstr 6014). [Google Scholar]
- 32. Matsuo M, Yasumatsu R, Masuda M, Toh S, Wakasaki T, Hashimoto K, et al. Relationship between immune-related adverse events and the long-term outcomes in recurrent/metastatic head and neck squamous cell carcinoma treated with nivolumab. Oral Oncol 2020;101:104525. [DOI] [PubMed] [Google Scholar]
- 33. Chen DS, Mellman I. Elements of cancer immunity and the cancer–immune set point. Nature 2017;541:321–30. [DOI] [PubMed] [Google Scholar]
- 34. Aggarwal C, Cohen RB, Morrow MP, Kraynak KA, Sylvester AJ, Knoblock DM, et al. Immunotherapy targeting HPV16/18 generates potent immune responses in HPV-associated head and neck cancer. Clin Cancer Res 2019;25:110–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li W, Matakidou A, Ghazoui Z, Si H, Wildsmith S, Morsli N, et al. Molecular biomarkers to identify patients (pts) who may benefit from durvalumab (D; anti-PD-L1) ± tremelimumab (T; anti-CTLA-4) in recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC) from HAWK and CONDOR studies. J Clin Oncol 2020;38:6548. [Google Scholar]
- 36. Wang J, Sun H, Zeng Q, Guo X-J, Wang H, Liu H-H, et al. HPV-positive status associated with inflamed immune microenvironment and improved response to anti-PD-1 therapy in head and neck squamous cell carcinoma. Sci Rep 2019;9:13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wise-Draper TM, Takiar V, Mierzwa ML, Casper K, Palackdharry S, Worden FP, et al. Association of pathological response to neoadjuvant pembrolizumab with tumor PD-L1 expression and high disease-free survival (DFS) in patients with resectable, local-regionally advanced, head and neck squamous cell carcinoma (HNSCC). J Clin Oncol 2021;39:6006. [Google Scholar]
- 38. Huang C, Chen L, Savage SR, Eguez RV, Dou Y, Li Y, et al. Proteogenomic insights into the biology and treatment of HPV-negative head and neck squamous cell carcinoma. Cancer Cell 2021;20:1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schroeder JC, Puntigam L, Hofmann L, Jeske SS, Beccard IJ, Doescher J, et al. Circulating exosomes inhibit B cell proliferation and activity. Cancers. 2020;12:2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harshyne LA, Nasca BJ, Kenyon LC, Andrews DW, Hooper DC. Serum exosomes and cytokines promote a T-helper cell type 2 environment in the peripheral blood of glioblastoma patients. Neuro-oncol 2015;nov107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miyazaki T, Maiti M, Hennessy M, Chang T, Kuo P, Addepalli M, et al. NKTR-255, a novel polymer-conjugated rhIL-15 with potent antitumor efficacy. J Immunother Cancer 2021;9:e002024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hassel JC, Jiang H, Bender C, Winkler J, Sevko A, Shevchenko I, et al. Tadalafil has biologic activity in human melanoma. Results of a pilot trial with Tadalafil in patients with metastatic Melanoma (TaMe). Oncoimmunology 2017;6:e1326440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sutton SS, Magagnoli J, Cummings TH, Hardin JW. The association between phosphodiesterase-5 inhibitors and colorectal cancer in a national cohort of patients. Clin Transl Gastroenterol 2020;11:e00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.