Abstract
Objective:
To prospectively assess whether sleep patterns modified the lifestyle associated cardiovascular disease (CVD) risk.
Patients and Methods:
This study included 393690 participants without CVD at baseline measurements between March 13, 2006, and October 1, 2010, from UK Biobank. A lifestyle score was calculated on basis of the four lifestyle factors (smoking, alcohol consumption, physical activity, and diet), and the sleep patterns were constructed based on sleep duration, chronotype, insomnia, snoring, and daytime dozing.
Results:
During a median follow-up of 9 years, we observed 10218 incident CVD events, including 6595 myocardial infarction (MI) and 3906 stroke. We found sleep patterns significantly modified the relations of the lifestyle score with incident CVD (P for interaction=.007) and MI (P for interaction=.004). Among participants with a poor sleep pattern, the unfavorable lifestyle (per score increase) was associated with 25% (95% CI, 13%–39%) and 29% (13%–47%) increased risks of CVD and MI; while among participants with a healthy sleep pattern, the unfavorable lifestyle was associated with 18% (15%–21%) and 17% (13%–21%) increased risks of CVD and MI.
Conclusions:
Our results indicate that adherence to a healthy sleep pattern may attenuate the CVD risk associated with an unfavorable lifestyle.
Keywords: Cardiovascular disease, Lifestyle, Sleep pattern, Interaction, Prospective cohort study
Introduction
Cardiovascular disease (CVD) is one of the leading causes of mortality and morbidity worldwide.1 In epidemiological studies, smoking, high alcohol consumption, physical inactivity, unhealthy dietary intake, and an unfavorable lifestyle score combining these lifestyle factors, have been consistently related to an increase of CVD events.2–5
Intriguingly, emerging evidence has shown that lifestyle factors are closely related to sleep behaviors, which are key modulators of daily circadian rhythmicity and chronobiological homeostasis.6–8 For example, poor sleep behaviors such as short sleep duration or sleep disturbance have been associated with intakes of unhealthy foods, physical inactivity, more alcohol use, frequent smoking, and high adiposity.7–10 Given their intertwined relationship, we hypothesized that sleep behaviors might modify the lifestyle-CVD relation. To date, however, no study has assessed such modification effects in prospective cohorts.
We recently developed a healthy sleep score by integrating five sleep behaviors, and associated the sleep patterns defined on basis of this score with cardiometabolic diseases.11–13 In the present prospective cohort study, we investigated the modification effects of the newly developed sleep patterns on the associations between lifestyle and incident CVD outcomes among 393690 adults from the UK Biobank. We also assessed the modification effects of the genetically determined sleep patterns.
Methods
Study population
The UK Biobank is a large population-based prospective cohort study, recruiting about 0.5 million participants aged 37–73 years old in 2006–2010 from 22 assessment centers across the United Kingdom.14–16 In the present study, we excluded participants with self-reported or diagnosed CVD (composite of myocardial infarction (MI) and stroke) at baseline (n=20096), as well as those without complete sleep (n=91818) or lifestyle (n=2992) data. Finally, a total of 393690 participants were included in the preliminary analyses. For genetic analyses, we restricted our sample to a subgroup of 303210 genetically unrelated European individuals with available genotyping data. Supplemental Figure 1 detailed the selection of study participants.
Electronic informed consents were obtained from all participants, and the study was approved by the NHS National Research Ethics Service (Ref: 11/NW/0382) and Institutional Review Board of Tulane University Health Sciences Center (Study number: 2018–1872).
Lifestyle score
A lifestyle score was constructed on the basis of 4 traditional modifiable cardiovascular risk factors, including smoking status,2,3,17 alcohol consumption,2,18,19 physical activity,2,3,20 and diet.2,3 These lifestyle factors were assessed at baseline from a standardized touchscreen questionnaire.21 The detailed assessment of the four lifestyle factors are provided in the Supplementary Method.
Participants scored 1 point for each unfavorable behavior (current smoking, high alcohol consumption, physical inactivity, and unhealthy diet). The lifestyle score ranged from 0 to 4, with a higher score indicating a more unfavorable lifestyle, which was subsequently categorized into favorable (0~1 points), intermediate (2 points), and unfavorable (3~4 point) lifestyle. The unfavorable lifestyle was significantly associated with an increased risk of CVD (P<.001, Supplemental Table 1). We also constructed a weighted lifestyle score based on β coefficients of each lifestyle factor as sensitivity analyses, using the following equation: weighted lifestyle score = (β1×factor1 + β2×factor2 + β3×factor3 + β4×factor4) × (4/sum of the β-coefficients). Each smoking behavior was weighted by its β-coefficient on CVD (see Supplemental Table 1).
Sleep pattern
The sleep pattern was constructed using five sleep behaviors, including sleep duration, chronotype, insomnia, snoring, and daytime dozing, which was the same as Fan et al.11 Questions to assess these sleep behaviors are listed in the Supplemental Method. Low-risk sleep behaviors were defined as sleep duration of 7–8 hours/day, early chronotype (“morning” or “morning than evening”), never/rarely or sometimes insomnia symptoms, no self-reported snoring, and no frequent daytime sleepiness (“never/rarely” or “sometimes”).11 One point was given for each low-risk sleep behavior. All component scores were added up to get the sleep score ranging from 0 to 5, with a higher point suggesting a healthier sleep pattern. Then, we defined three sleep patterns: ‘healthy sleep pattern’ (4~5 points), ‘intermediate sleep pattern’ (2~3 points), and ‘poor sleep pattern’ (0~1 point).11 Supplemental Table 2 showed the associations between each sleep behavior and CVD outcomes. The healthy sleep pattern was significantly associated with reduced risk of CVD, MI, and stroke (P<.05, Supplemental Table 2). We also constructed a weighted sleep score based on β coefficients of the five sleep behaviors as sensitivity analyses.11
Genotyping and polygenic risk scores
Detailed information about genotyping and imputation in the UK Biobank has been previously published.22 Five weighted polygenic risk scores (PRS) for sleep behaviors were constructed base on GWAS-derived single nucleotide polymorphisms (SNPs): (1) PRS of sleep duration (PRS-DUR), calculated with 78 SNPs reported by Dashti et al.;23 (2) PRS of chronotype (PRS-CHR), calculated with 351 SNPs reported by Jones et al.;24 (3) PRS of insomnia (PRS-INS), calculated with 248 SNPs reported by Jansen et al.;25 (4) PRS of snoring (PRS-SNO), calculated with 42 SNPs reported by Jansen et al.;25 (5) PRS of daytime dozing (PRS-DOZ), based on 1 SNP reported by Jansen et al.25 A higher PRS of sleep duration, chronotype, insomnia, snoring, and daytime dozing indicated a higher genetic predisposition to longer sleep duration, being a morning person, insomnia, snoring, and daytime dozing.
Given that intermediate sleep duration was low-risk, we divided PRS-DUR into five groups according to quintiles and considered intermediate PRS-DUR (quintiles 2 to 4) as the low-risk group. Except for PRS-DUR, the other four PRS were subsequently categorized into two groups according to the median of each PRS. Participants scored 1 point for each of the 5 high-risk groups of PRS. In accord with Fan et al.,11 a genetically determined sleep pattern was constructed based on the above 5 PRS, ranging from 0 to 5 (Supplemental Figure 2), and then categorized as low (0~1 point), intermediate (2~3 points), and high (4~5 points) genetic risk. The genetically determined sleep pattern was significantly correlated with observational sleep pattern (β (SE) = −0.06 (0.002), P<.001, Supplemental Figure 2), with low genetic risk indicating healthy sleep pattern and high genetic risk indicating poor sleep pattern.
Ascertainment of outcomes
The primary outcome of our study was CVD, and the secondary outcomes were MI and stroke. Information on the date and cause of hospital admissions was identified through record linkage to the Hospital Episode Statistics for England, Scottish Morbidity Record for Scotland, and Patient Episode Database for Wales. Detailed information about the linkage procedure is available online (http://biobank.ctsu.ox.ac.uk/crystal/label.cgi?id=2000). The International Classification of Diseases, Tenth Revision and Ninth Revision (ICD-10 and ICD-9) were used in medical records. We used the algorithmically derived definitions of MI and stroke as coded by UK Biobank. MI was defined as ICD-10 codes I21-I23, I24.1, I25.2, and ICD-9 codes 410–412, 429.79 (Detailed information can be found at http://biobank.ndph.ox.ac.uk/showcase/docs/alg_outcome_mi.pdf). Stroke was defined as ICD-10 codes I60, I61, I63, I64, and ICD-9 codes 430, 431, 434, and 436 (Detailed information can be found at http://biobank.ndph.ox.ac.uk/showcase/docs/alg_outcome_stroke.pdf).
Covariates
Information about sociodemographic characteristics, medical history and treatments was obtained from a standardized questionnaire.21 Participants were also asked to complete a range of physical measurements and biological sample collection at baseline according to a standard protocol.21
Sociodemographic characteristics at recruitment included sex, age, race (European, mixed, Asian, black, others, or missing), Townsend deprivation index (a greater Townsend deprivation index implies a greater degree of deprivation), and income average total annual household income (<£18 000, £18 000–£30 999, £31 000–£51 999, £52 000–£100 000, >£100 000, or missing). Self-reported family history of CVDs (yes or no) and medical history of cancer (yes or no), diabetes (yes or no), hypertension (yes or no), angina (yes or no), symptom of depression/anxiety (yes or no), insulin treatment (yes or no), antihypertensive drugs (yes or no), lipid treatment (yes or no), aspirin use (yes or no), and hypnotics/antidepressants/anti-anxiety drugs (yes or no), were obtained from the touchscreen questionnaire and supplemented by a verbal interview.
Body mass index (BMI) was calculated using weight in kilograms (BC-418MA body composition analyzer) divided by the square of standing height in meters (Seca 202 stadiometer). Total cholesterol levels were measured by CHO-POD analysis on a Beckman Coulter AU5800. High cholesterol was defined as total cholesterol ≥5.18 mmol/L, based on the recommendations from the American Heart Association.3
Statistical analysis
Participants contributed person-years from the baseline recruitment until the date of diagnosis of CVD, death, loss to follow-up, or the last date of hospital admission (31 January 2018), whichever came first. First, we explored the associations between lifestyle and CVD outcomes among different sleep pattern categories, using Cox proportional hazards regression with years of follow-up as the time metric, reporting the results as hazard ratios (HRs) together with 95% confidence intervals (CIs). Second, interactions between lifestyle and sleep patterns on CVD outcomes were tested by adding their cross-product term into the model. We also tested the interactions between lifestyle and genetically determined sleep patterns on CVD outcomes, as well as their joint associations. In the preliminary analyses, multivariate-adjusted models included covariates of sex, age, race, assessment center, Townsend deprivation index, income, family history of CVDs, medical history of cancer, diabetes, hypertension, angina, symptom of depression/anxiety, high cholesterol, insulin treatment, antihypertensive drugs, lipid treatment, aspirin use, hypnotics/antidepressants/anti-anxiety drugs, and body mass index; in the genetic analyses, batch effects (106 batches) and the first 10 genetic principal components were also included as covariates. Except for Townsend deprivation index, income, high cholesterol, and BMI, there were no missing cases for other covariates. We used missing indicators for high cholesterol (missing rate =5.67%, i.e., yes, no, or missing) and income (missing rate =13.22%), and imputed mean values for Townsend deprivation index and BMI (missing rate <1%).
To reduce the chance of reverse causality, we performed sensitivity analyses by excluding participants who developed CVD events within the first two years of follow-up.
We used SAS version 9.4 (SAS Institute, Cary, NC) to perform all statistical analyses. A statistically significant level was set at a two-tailed P<.05.
Results
Baseline characteristics of participants
Table 1 shows the baseline characteristics of the study participants. Of the 393690 participants (age: 56.3±8.1 years; men: 44.0%), 53.5%, 32.4%, and 14.2% had a favorable, intermediate, or unfavorable lifestyle, respectively. Over half of the participants (56.0%) reported a family history of CVDs, and 8.7%, 4.9%, 54.3%, 2.6%, and 34.3% of the participants had a history of cancer, diabetes, hypertension, angina, and symptom of depression/anxiety. Of note, participants with an unfavorable lifestyle showed a lower percentage of healthy sleep behaviors. About 25% had all five healthy sleep behaviors among participants with a favorable lifestyle, while only 15% had all five healthy sleep behaviors among those with an unfavorable lifestyle.
Table 1.
Baseline characteristics of UK Biobank participants (N=393690)
| Baseline characteristics | All participants | Lifestyle | ||
|---|---|---|---|---|
| Favorable | Intermediate | Unfavorable | ||
| No. of participants | 393690 | 210443 (53.5) | 127452 (32.4) | 55795 (14.2) |
| Age, mean (SD), years | 56.3 (8.1) | 56.7 (8.1) | 56.0 (8.1) | 55.3 (8.1) |
| Sex, male (%) | 173103 (44.0) | 89812 (42.7) | 57593 (45.2) | 25698 (46.1) |
| Race, White European (%) | 373053 (94.8) | 199914 (95) | 120614 (94.6) | 52525 (94.1) |
| Townsend deprivation index, mean (SD) | −1.4 (3.0) | −1.7 (2.8) | −1.3 (3.1) | −0.6 (3.4) |
| Body mass index, mean (SD), kg/m2 | 27.3 (4.7) | 26.8 (4.4) | 27.8 (4.9) | 28.2 (5.4) |
| Total cholesterol level, mean (SD), mmol/L | 5.7 (1.1) | 5.7 (1.1) | 5.7 (1.1) | 5.7 (1.1) |
| Family history of CVDs | 220492 (56.0) | 118968 (56.5) | 70901 (55.6) | 30623 (54.9) |
| Disease history | ||||
| Cancer | 34397 (8.7) | 18580 (8.8) | 10956 (8.6) | 4861 (8.7) |
| Diabetes | 19139 (4.9) | 8709 (4.1) | 6787 (5.3) | 3643 (6.5) |
| Hypertension | 213679 (54.3) | 110375 (52.4) | 71548 (56.1) | 31756 (56.9) |
| Angina | 10158 (2.6) | 4929 (2.3) | 3358 (2.6) | 1871 (3.4) |
| Symptom of depression/anxiety | 134860 (34.3) | 68331 (32.5) | 44117 (34.6) | 22412 (40.2) |
| Treatment/medication | ||||
| Insulin treatment | 3781 (1.0) | 1630 (0.8) | 1344 (1.1) | 807 (1.4) |
| Antihypertensive drugs | 77882 (19.8) | 38576 (18.3) | 26780 (21) | 12526 (22.5) |
| Lipid treatment | 60029 (15.3) | 29755 (14.1) | 20360 (16) | 9914 (17.8) |
| Aspirin use | 46277 (11.8) | 23202 (11) | 15653 (12.3) | 7422 (13.3) |
| Hypnotics/antidepressants/anti-anxiety drugs | 25768 (6.6) | 11210 (5.3) | 8849 (6.9) | 5709 (10.2) |
| Healthy sleep behaviors a | ||||
| Sleep 7–8 h/day | 269885 (68.6) | 149056 (70.8) | 85900 (67.4) | 34929 (62.6) |
| Early chronotype | 246924 (62.7) | 138992 (66) | 78042 (61.2) | 29890 (53.6) |
| Never/rarely/sometimes insomnia | 285448 (72.5) | 156586 (74.4) | 90941 (71.4) | 37921 (68) |
| No self-reported snoring | 248246 (63.1) | 138620 (65.9) | 77656 (60.9) | 31970 (57.3) |
| No frequent daytime sleepiness | 383406 (97.4) | 205745 (97.8) | 123863 (97.2) | 53798 (96.4) |
| All five healthy sleep behaviors | 85165 (21.6) | 52028 (24.7) | 24965 (19.6) | 8172 (14.6) |
CVDs, cardiovascular diseases; SD, standard deviation. Data are expressed as N (%) unless stated otherwise.
Healthy sleep behaviors were defined as follows: sleep 7–8 hours per day; early chronotype (“morning” or “morning than evening”); reported never/rarely or sometimes insomnia symptoms; no self-reported snoring; and no frequent daytime sleepiness (“never/rarely” or “sometimes”).
Modification effect of the sleep patterns on the association of the lifestyle with CVD outcomes
During a median of 8.93 years (interquartile range (IQR): 8.21 to 9.63) of follow-up, we documented 10218 incident CVD events, including 6595 MI, and 3906 stroke. We conducted stratified analyses according to the sleep patterns on the associations of the lifestyle score with the risks of CVD outcomes and tested the interactions. As shown in Figure 1, we found significant interactions between the sleep patterns and lifestyle score on the risks of CVD (P for interaction =.007) and MI (P for interaction =.004). The associations between the unfavorable lifestyle and increased risks of CVD and MI were attenuated in people with a healthy sleep pattern. Among participants with a poor sleep pattern, the unfavorable lifestyle (per score increase) was associated with 25% (95% CI, 13%–39%) and 29% (95% CI, 13%–47%) increased risks of CVD and MI respectively; while among participants with a healthy sleep pattern, the unfavorable lifestyle was associated with 18% (95% CI, 15%–21%) and 17% (95% CI, 13%–21%) increased risks of CVD and MI respectively. No significant interaction between the sleep patterns and lifestyle score on stroke was observed (P for interaction =.72).
Figure 1. Associations of unfavorable lifestyle with risks of cardiovascular diseases stratified by sleep pattern.
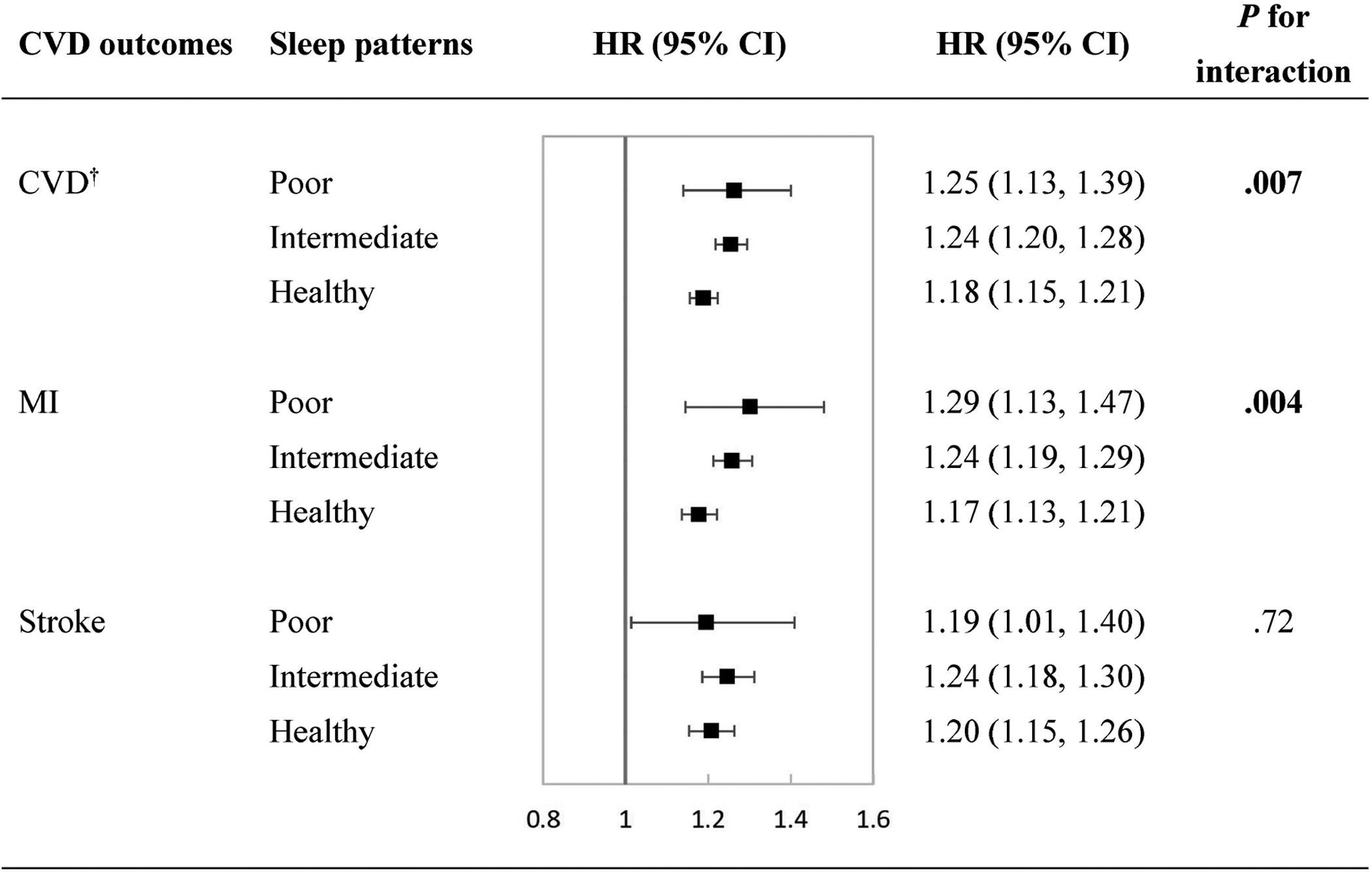
Multivariate adjusted hazard ratios and 95% confidence intervals of cardiovascular diseases were estimated from Cox proportional hazards models, adjusted for sex, age, race, assessment center, Townsend deprivation index, income, family history of CVDs, medical history of cancer, diabetes, hypertension, angina, symptom of depression/anxiety, high cholesterol, insulin treatment, antihypertensive drugs, lipid treatment, aspirin use, hypnotics/antidepressants/anti-anxiety drugs, and body mass index. CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; MI, myocardial infarction.
† Composite of MI and stroke.
The interactions of the lifestyle score with each sleep behavior are shown in Supplemental Table 3. Sleep duration and chronotype significantly interacted with the lifestyle score on CVD and MI (all P for interaction <.05). Similarly, compared with those with unhealthy sleep behaviors, participants with healthy sleep behaviors had attenuated risks of CVD and MI associated with the unfavorable lifestyle.
We further classified the participants according to the joint categories of the lifestyle and sleep patterns and assessed their joint associations with the CVD outcomes. The joint associations of the lifestyle and sleep patterns were significant with the CVD, MI, and stroke (all P for trend<.001, Figure 2). With the increase of the number of unfavorable lifestyle factors and poor sleep behaviors, the risks of CVD outcomes increased in a gradient manner. Compared with the favorable reference group, participants having four unfavorable lifestyle factors and five poor sleep behaviors had the highest risk of developing CVD (HR, 2.28 [95% CI, 1.97–2.63], P <.001), MI (HR, 2.30 [95% CI, 1.92–2.76], P <.001), and stroke (HR, 2.10 [95% CI, 1.65–2.66], P <.001).
Figure 2. Multivariate hazard ratios of cardiovascular diseases according to joint categories of lifestyle and sleep pattern.
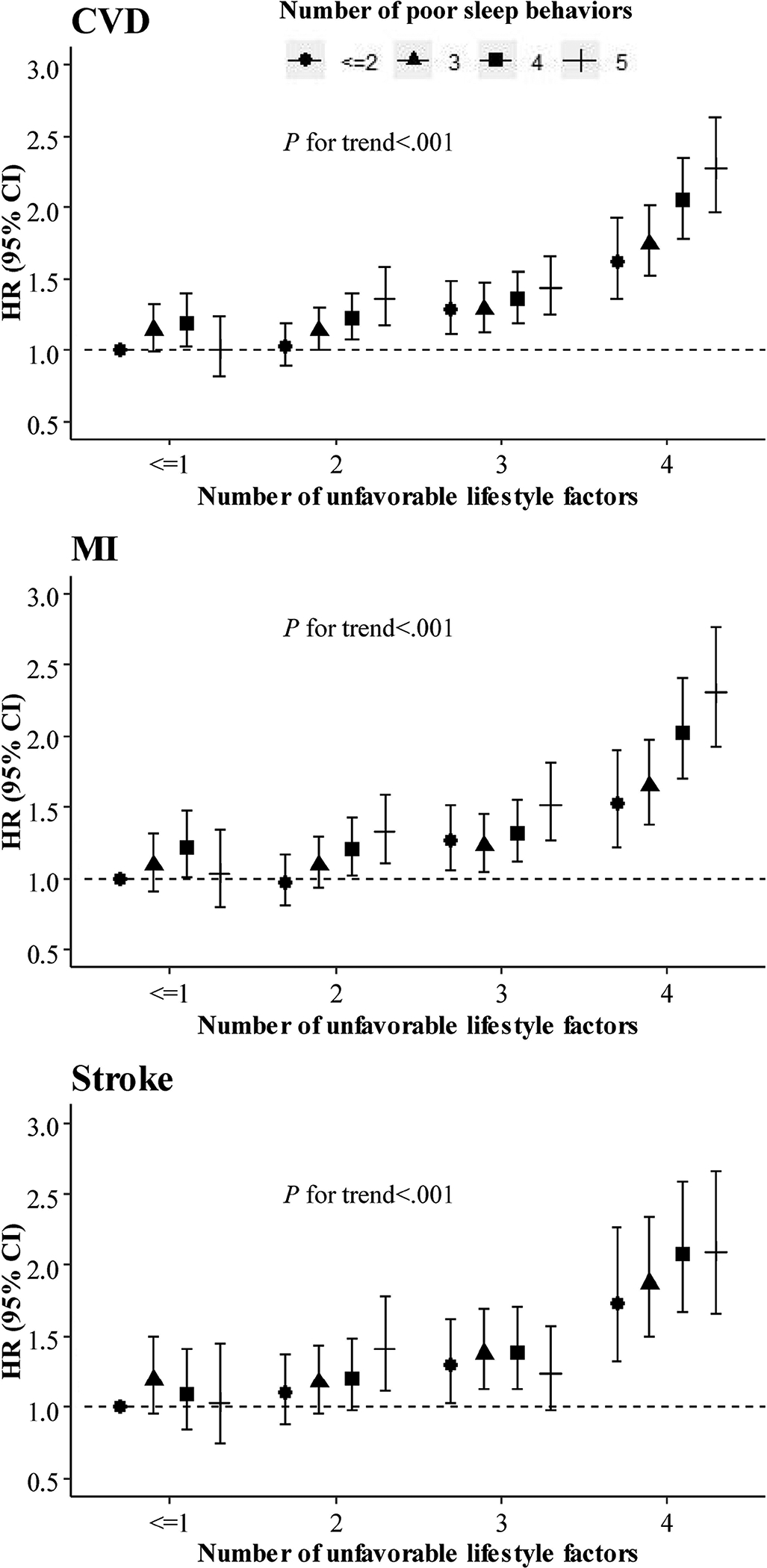
Multivariate adjusted hazard ratios and 95% confidence intervals of cardiovascular diseases were estimated from Cox proportional hazards models, adjusted for sex, age, race, assessment center, Townsend deprivation index, income, family history of CVDs, medical history of cancer, diabetes, hypertension, angina, symptom of depression/anxiety, high cholesterol, insulin treatment, antihypertensive drugs, lipid treatment, aspirin use, hypnotics/antidepressants/anti-anxiety drugs, and body mass index. CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; MI, myocardial infarction.
Modification effect of the genetically determined sleep pattern on the association of the lifestyle with CVD
The interactions between the lifestyle score and the genetically determined sleep pattern on CVD outcomes were also tested. Although no significant interactions were detected, we observed joint associations between the lifestyle score and the genetically determined sleep pattern (all P for trend <.001, Figure 3 and Supplemental Figure 3). Participants with an unfavorable lifestyle and high genetic risk of poor sleep pattern showed an 76% (95% CI, 43%–117%, Figure 3) increased risk of CVD, compared with participants with a favorable lifestyle and low genetic risk of poor sleep pattern. Similar patterns of joint associations were observed for MI and stroke (Supplemental Figure 3).
Figure 3. Multivariate hazard ratios of cardiovascular disease according to joint categories of lifestyle and genetically determined sleep pattern.
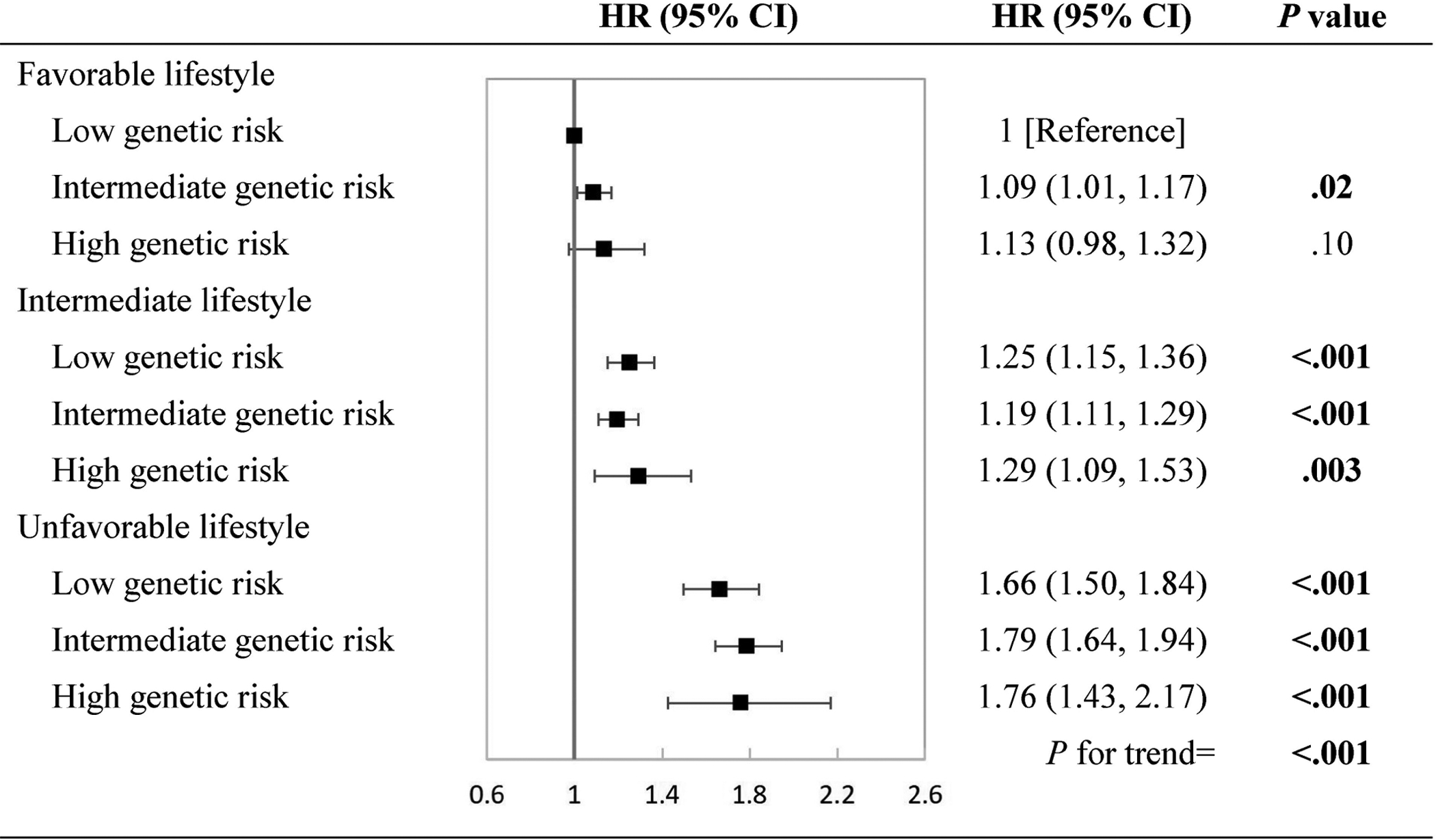
Low, intermediate, and high genetic risk indicated healthy, intermediate, and poor sleep pattern, respectively. Multivariate adjusted hazard ratios and 95% confidence intervals of cardiovascular disease were estimated from Cox proportional hazards models, adjusted for sex, age, assessment center, batch effects (106 batches), the first 10 genetic principal components, Townsend deprivation index, income, family history of CVDs, medical history of cancer, diabetes, hypertension, angina, symptom of depression/anxiety, high cholesterol, insulin treatment, antihypertensive drugs, lipid treatment, aspirin use, hypnotics/antidepressants/anti-anxiety drugs, and body mass index. CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio.
Sensitivity analyses
In the sensitivity analyses, when we excluded participants who developed CVD events within the first two years of follow-up, the modification effect of sleep pattern on the associations of lifestyle with CVD and MI did not change materially (Supplemental Figure 4). Furthermore, the interactions between the weighted lifestyle score and the weighted sleep pattern remained significant on CVD and MI (Supplemental Figure 5).
Discussion
In this large prospective cohort of 393690 participants, we found a significant modification effect of our newly developed sleep patterns combining five sleep behaviors on the associations between the lifestyle score and CVD events. The associations between the unfavorable lifestyle and increased risks of CVD were attenuated in people with a healthy sleep pattern. Similar interaction patterns were observed for MI.
To the best of our knowledge, our study is the first to assess the interaction between the lifestyle score and the newly developed sleep patterns on incident CVD risk. Our findings are supported by previous evidence showing the interactions of the individual lifestyle risk factors with sleep duration or sleep quality on cardiovascular risk.26–28 We observed that the associations between the unfavorable lifestyle and increased CVD risk were consistently attenuated among people with a healthy sleep pattern, indicating significant modification effect of sleep pattern on the associations between lifestyle and CVD events. These findings could be partly explained by the close relationship between sleep behaviors and other lifestyle factors.6,7,29–32 For example, people with short sleep duration or late chronotypes were more likely to report insufficient physical activity,7,33 while those who were physically active usually had a better quality of sleep.29,30 Some studies showed that short sleep duration might increase dietary energy and fat intakes, and late chronotypes consumed fewer fruit and vegetables;8,32,33 on the other hand, diet quality and dietary patterns were also associated with sleep duration.31,34 In addition, both smoking and alcohol consumption have been related to sleep problems.6,9,10
Similar lifestyle-sleep interaction patterns were observed for MI. When we excluded incident CVD cases that occurred during the first 2 years of follow-up, the exclusion did not affect the interactions between lifestyle and sleep patterns on CVD and MI, suggesting that our results were not likely to be reverse causality. Furthermore, the weighted lifestyle score and weighted sleep pattern were also derived, and their interactions remained significant on CVD and MI, indicating that our results were robust and convincing. The lifestyle-sleep interaction was not significant on stroke, probably due to the relatively small number of stroke cases.
Among the individual sleep behaviors, we found that sleep duration and chronotype showed stronger interaction with the lifestyle on CVD and MI than other sleep behaviors. Even though, the interactions between the sleep patterns and lifestyle score were likely to be driven by all the sleep behaviors included, because exclusion of any individual sleep behaviors did not appreciably change the results.
The potential mechanisms underpinning the observed interactions between the lifestyle score and sleep patterns remain to be investigated. However, our findings are biologically plausible. Smoking initiates cardiovascular dysfunction through increasing oxidative stress.35 Moderate alcohol consumption has beneficial effects on cardiovascular risk reduction through the anti-atherogenic effects of ethanol.36 Physical activity is associated with reduced risk of CVD by improving vascular, endocrine, and metabolic function.37,38 In addition, a healthy diet, characterized by higher intakes of fruit, vegetables, and fish, may influence the process of inflammation and endothelial dysfunction, which is the early step in the development of atherosclerosis.39 On the other hand, sleep behaviors may also affect these mechanistic pathways. For example, shortened sleep is associated with endocrine and metabolic disruption, as well as vascular damage.40 Individuals with late chronotype would result in circadian misalignment, increasing cardiometabolic risk.41 Insomnia, especially when accompanied by short sleep duration, has been related to increased systemic inflammation and increased atherogenesis.42 Snoring vibrations might lead to carotid endothelial dysfunction and atherosclerosis and stroke.43 Taken together, we assumed that the interactions between the sleep patterns and lifestyle score might be through multiple mechanisms, including endocrine or metabolic disruption, vascular damage, circadian misalignment, increased inflammation, and endothelial dysfunction, etc.
We also analyzed the interactions between the lifestyle score and the genetically determined sleep pattern on CVD outcomes. In the present study, data on sleep genetics were included to serve as proxy measures for self-reported sleep information and potential way to replicate the results. In addition, the genetic results are less likely to be influenced by reverse causation and confounding factors. The null-interaction might be due to that the included genetic variants could only explain a small percentage of variance in sleep behaviors.23–25 Even so, we found the risks of CVD associated with the unfavorable lifestyle were higher among participants with high genetic predisposition to poor sleep pattern, and these observations were consistent with the results from the analyses of the observed sleep patterns. Our findings have important implications for the development of new public health interventions to reduce cardiovascular risk. Previous efforts to lower the risk of CVD events mainly focused on modifications of lifestyle factors including smoking, alcohol consumption, physical activity, and diet, while the sleep behaviors have been ignored. Our study indicates that adherence to a healthy sleep pattern may attenuate the adverse associations between unfavorable lifestyles and CVD risk, highlighting the importance to consider the sleep patterns in the prevention of CVD.
Strengths and limitations
The main strengths of our study include its prospective study design with an up to 9 years’ follow-up, and large sample size which enabled us to perform stratified analyses and sensitivity analyses. More importantly, our study for the first time analyzed the interactions between the sleep patterns, which integrated five sleep behaviors, and the lifestyle score, which was consisted of smoking status, alcohol consumption, physical activity, and diet, in relation to the risk of CVD.
Despite the strengths of the study, there are several potential limitations. First, most of the variables used in the present study, including information on sleep behaviors, were self-reported, which might introduce recall bias. However, it is not practicable to obtain these data through objective measurements in such a large population-based prospective study. Two previous studies have shown correlations between subjectively and objectively measured sleep duration, and both studies reported subjects tended to overestimate their sleep duration by about 0.5 h compared with actigraphy-assessed sleep duration.44,45 However, additional analyses using self-reported sleep duration after a 0.5 hour reduction did not change the results appreciably. Second, although we adjusted for a wide range of sociodemographic and clinical factors, residual confounding from unknown or unmeasured factors might still exist. Third, the present study was conducted in the UK Biobank, where most of the participants were of European descent. Thus the generalizability of our results to other populations should be cautious. Forth, since this is an observational study, we cannot make conclusions about causality among the lifestyle score, sleep pattern, and CVD outcomes.
Conclusion
In summary, our results indicate that the sleep patterns significantly modify the association between lifestyle and CVD risk. A healthy sleep pattern may attenuate the adverse associations between the unfavorable lifestyles and CVD risk, suggesting that adherence to not only a favorable lifestyle but also a healthy sleep pattern would better prevent CVD. Further research is needed to confirm our findings and to understand the mechanisms underpinning the interaction between the lifestyle and sleep pattern.
Supplementary Material
Acknowledgments
We thank all the participants in the study and the members of the survey teams, as well as the project development and management teams. LQ is the guarantor and has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Support.
This work was supported by grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, HL126024), the National Institute of Diabetes and Digestive and Kidney Diseases (DK091718, DK100383, DK078616). LQ is also supported by National Institute of General Medical Sciences (P20GM109036). Qiying Song is a recipient of a scholarship under the China Scholarship Council to pursue her study in the United States (201806010383).
Abbreviations
- BMI
Body Mass Index
- CI
confidence interval
- CVD
cardiovascular disease
- HR
hazard ratios
- MI
myocardial infarction
- PRS
polygenic risk score
- SNP
single nucleotide polymorphism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Competing Interest. No potential conflicts of interest relevant to this article were reported
Data sharing:
The genetic and phenotypic UK Biobank data are available on application to the UK Biobank (www.ukbiobank.ac.uk/).
References
- 1.GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England). 2018;392(10159):1736–1788. doi: 10.1016/S0140-6736(18)32203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yusuf S, Joseph P, Rangarajan S, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet (London, England). 2020;395(10226):795–808. doi: 10.1016/S0140-6736(19)32008-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 4.Carlsson AC, Wändell PE, Gigante B, Leander K, Hellenius M-L, de Faire U. Seven modifiable lifestyle factors predict reduced risk for ischemic cardiovascular disease and all-cause mortality regardless of body mass index: a cohort study. Int J Cardiol. 2013;168(2):946–952. doi: 10.1016/j.ijcard.2012.10.045 [DOI] [PubMed] [Google Scholar]
- 5.Myint PK, Luben RN, Wareham NJ, Bingham SA, Khaw K-T. Combined effect of health behaviours and risk of first ever stroke in 20,040 men and women over 11 years’ follow-up in Norfolk cohort of European Prospective Investigation of Cancer (EPIC Norfolk): prospective population study. BMJ. 2009;338:b349. doi: 10.1136/bmj.b349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Short MA, Weber N. Sleep duration and risk-taking in adolescents: A systematic review and meta-analysis. Sleep Med Rev. 2018;41:185–196. doi: 10.1016/j.smrv.2018.03.006 [DOI] [PubMed] [Google Scholar]
- 7.Stefan L, Vrgoc G, Rupcic T, Sporis G, Sekulic D. Sleep Duration and Sleep Quality Are Associated with Physical Activity in Elderly People Living in Nursing Homes. Int J Environ Res Public Health. 2018;15(11). doi: 10.3390/ijerph15112512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.St-Onge M-P, Roberts AL, Chen J, et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;94(2):410–416. doi: 10.3945/ajcn.111.013904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Reen E, Roane BM, Barker DH, McGeary JE, Borsari B, Carskadon MA. Current Alcohol Use is Associated with Sleep Patterns in First-Year College Students. Sleep. 2016;39(6):1321–1326. doi: 10.5665/sleep.5862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellatorre A, Choi K, Lewin D, Haynie D, Simons-Morton B. Relationships Between Smoking and Sleep Problems in Black and White Adolescents. Sleep. 2017;40(1). doi: 10.1093/sleep/zsw031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan M, Sun D, Zhou T, et al. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: a prospective study of 385 292 UK biobank participants. Eur Heart J. 2020;41(11):1182–1189. doi: 10.1093/eurheartj/ehz849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M, Zhou T, Li X, et al. Baseline Vitamin D Status, Sleep Patterns, and the Risk of Incident Type 2 Diabetes in Data From the UK Biobank Study. Diabetes Care. 2020;43(11):2776–2784. doi: 10.2337/dc20-1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Xue Q, Wang M, et al. Adherence to a Healthy Sleep Pattern and Incident Heart Failure: A Prospective Study of 408 802 UK Biobank Participants. Circulation. 2021;143(1):97–99. doi: 10.1161/CIRCULATIONAHA.120.050792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fry A, Littlejohns TJ, Sudlow C, et al. Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants With Those of the General Population. Am J Epidemiol. 2017;186(9):1026–1034. doi: 10.1093/aje/kwx246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen NE, Sudlow C, Peakman T, Collins R. UK biobank data: come and get it. Sci Transl Med. 2014;6(224):224ed4. doi: 10.1126/scitranslmed.3008601 [DOI] [PubMed] [Google Scholar]
- 17.Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet (London, England). 2011;378(9799):1297–1305. doi: 10.1016/S0140-6736(11)60781-2 [DOI] [PubMed] [Google Scholar]
- 18.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671. doi: 10.1136/bmj.d671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez-Sola J Cardiovascular risks and benefits of moderate and heavy alcohol consumption. Nat Rev Cardiol. 2015;12(10):576–587. doi: 10.1038/nrcardio.2015.91 [DOI] [PubMed] [Google Scholar]
- 20.Lear SA, Hu W, Rangarajan S, et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet (London, England). 2017;390(10113):2643–2654. doi: 10.1016/S0140-6736(17)31634-3 [DOI] [PubMed] [Google Scholar]
- 21.UK Biobank Coordinating Centre. UK Biobank: Protocol for a large-scale prospective epidemiological resource. Protocol No: UKBB-PROT-09–06 (Main Phase). March 21, 2007. (AMENDMENT ONE FINAL).
- 22.Bycroft C, Freeman C, Petkova D, et al. Genome-wide genetic data on ~500,000 UK Biobank participants. bioRxiv. Published online January 1, 2017:166298. doi: 10.1101/166298 [DOI] [Google Scholar]
- 23.Dashti HS, Jones SE, Wood AR, et al. Genome-wide association study identifies genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. Nat Commun. 2019;10(1):1100. doi: 10.1038/s41467-019-08917-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones SE, Lane JM, Wood AR, et al. Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms. Nat Commun. 2019;10(1):343. doi: 10.1038/s41467-018-08259-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jansen PR, Watanabe K, Stringer S, et al. Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat Genet. 2019;51(3):394–403. doi: 10.1038/s41588-018-0333-3 [DOI] [PubMed] [Google Scholar]
- 26.Donovan LM, Feemster LC, Billings ME, et al. Risk of Cardiovascular Disease Related to Smoking Is Greater Among Women With Sleep-Disordered Breathing. J Clin sleep Med. 2018;14(11):1929–1935. doi: 10.5664/jcsm.7496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavie L, Lavie P. Smoking interacts with sleep apnea to increase cardiovascular risk. Sleep Med. 2008;9(3):247–253. doi: 10.1016/j.sleep.2007.03.018 [DOI] [PubMed] [Google Scholar]
- 28.Xiao Q, Keadle SK, Hollenbeck AR, Matthews CE. Sleep duration and total and cause-specific mortality in a large US cohort: interrelationships with physical activity, sedentary behavior, and body mass index. Am J Epidemiol. 2014;180(10):997–1006. doi: 10.1093/aje/kwu222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King AC, Oman RF, Brassington GS, Bliwise DL, Haskell WL. Moderate-intensity exercise and self-rated quality of sleep in older adults. A randomized controlled trial. JAMA. 1997;277(1):32–37. [PubMed] [Google Scholar]
- 30.de Castro Toledo Guimaraes LH, de Carvalho LBC, Yanaguibashi G, do Prado GF. Physically active elderly women sleep more and better than sedentary women. Sleep Med. 2008;9(5):488–493. doi: 10.1016/j.sleep.2007.06.009 [DOI] [PubMed] [Google Scholar]
- 31.Mondin TC, Stuart AL, Williams LJ, Jacka FN, Pasco JA, Ruusunen A. Diet quality, dietary patterns and short sleep duration: a cross-sectional population-based study. Eur J Nutr. 2019;58(2):641–651. doi: 10.1007/s00394-018-1655-8 [DOI] [PubMed] [Google Scholar]
- 32.Rangan A, Zheng M, Olsen NJ, Rohde JF, Heitmann BL. Shorter sleep duration is associated with higher energy intake and an increase in BMI z-score in young children predisposed to overweight. Int J Obes (Lond). 2018;42(1):59–64. doi: 10.1038/ijo.2017.216 [DOI] [PubMed] [Google Scholar]
- 33.Patterson F, Malone SK, Lozano A, Grandner MA, Hanlon AL. Smoking, Screen-Based Sedentary Behavior, and Diet Associated with Habitual Sleep Duration and Chronotype: Data from the UK Biobank. Ann Behav Med. 2016;50(5):715–726. doi: 10.1007/s12160-016-9797-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castro-Diehl C, Wood AC, Redline S, et al. Mediterranean diet pattern and sleep duration and insomnia symptoms in the Multi-Ethnic Study of Atherosclerosis. Sleep. 2018;41(11). doi: 10.1093/sleep/zsy158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43(10):1731–1737. doi: 10.1016/j.jacc.2003.12.047 [DOI] [PubMed] [Google Scholar]
- 36.Li JM, Mukamal KJ. An update on alcohol and atherosclerosis. Curr Opin Lipidol. 2004;15(6):673–680. doi: 10.1097/00041433-200412000-00008 [DOI] [PubMed] [Google Scholar]
- 37.Metabolic Ball D. and endocrine response to exercise: sympathoadrenal integration with skeletal muscle. J Endocrinol. 2015;224(2):R79–95. doi: 10.1530/JOE-14-0408 [DOI] [PubMed] [Google Scholar]
- 38.Cangemi R, Loffredo L, Battaglia S, et al. Does Regular Physical Exercise Preserve Artery Dilation by Lowering Nox2-Related Oxidative Stress? Antioxid Redox Signal. 2018;28(17):1576–1581. doi: 10.1089/ars.2017.7296 [DOI] [PubMed] [Google Scholar]
- 39.Lopez-Garcia E, Schulze MB, Fung TT, et al. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2004;80(4):1029–1035. doi: 10.1093/ajcn/80.4.1029 [DOI] [PubMed] [Google Scholar]
- 40.Yin J, Jin X, Shan Z, et al. Relationship of Sleep Duration With All-Cause Mortality and Cardiovascular Events: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. J Am Heart Assoc. 2017;6(9). doi: 10.1161/JAHA.117.005947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morris CJ, Purvis TE, Hu K, Scheer FAJL. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci U S A. 2016;113(10):E1402–11. doi: 10.1073/pnas.1516953113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Javaheri S, Redline S. Insomnia and Risk of Cardiovascular Disease. Chest. 2017;152(2):435–444. doi: 10.1016/j.chest.2017.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho J-G, Witting PK, Verma M, et al. Tissue vibration induces carotid artery endothelial dysfunction: a mechanism linking snoring and carotid atherosclerosis? Sleep. 2011;34(6):751–757. doi: 10.5665/SLEEP.1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matthews KA, Patel SR, Pantesco EJ, et al. Similarities and differences in estimates of sleep duration by polysomnography, actigraphy, diary, and self-reported habitual sleep in a community sample. Sleep Heal. 2018;4(1):96–103. doi: 10.1016/j.sleh.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19(6):838–845. doi: 10.1097/EDE.0b013e318187a7b0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


