Abstract
Background:
The associations of geographic atrophy (GA) progression with systemic health status and medication use are unclear.
Methods:
We manually delineated GA in 318 eyes in the Age-Related Eye Disease Study. We calculated GA perimeter-adjusted growth rate as the ratio between GA area growth rate and mean GA perimeter between the first and last visit for each eye (mean follow-up = 5.3 years). Patients’ history of systemic health and medications were collected through questionnaires administered at study enrollment. We evaluated associations between GA perimeter-adjusted growth rate and 27 systemic health factors using univariable and multivariable linear mixed-effects regression models.
Results:
In the univariable model, GA perimeter-adjusted growth rate was associated with GA in the fellow eye at any visit (p=0.002), hypertension history (p=0.03), cholesterol-lowering medication use (p<0.001), beta-blocker use (p=0.02), diuretics use (p<0.001), and thyroid hormones use (p=0.03). Among the 6 factors, GA in the fellow eye at any visit (p=0.008), cholesterol medication use (p=0.002), and diuretic use (p<0.001) were independently associated with higher GA perimeter-adjusted growth rate in the multivariable model. GA perimeter-adjusted growth rate was 51.1% higher in patients with versus without cholesterol-lowering medication use history and was 37.8% higher in patients with versus without diuretics use history.
Conclusions:
GA growth rate may be associated with the fellow-eye status, cholesterol-lowering medication use, and diuretics use. These possible associations do not infer causal relationships and future prospective studies are required to investigate the relationships further.
INTRODUCTION
Age-related macular degeneration (AMD) is one of the leading causes of visual impairment in the elderly worldwide.1 AMD is classified as neovascular or non-neovascular based on the presence or absence of choroidal neovascularization. Geographic atrophy (GA) is the advanced stage of non-neovascular AMD and is characterized by the presence of atrophic lesions in the macula, with progressive degeneration of photoreceptors, retinal pigment epithelium, Bruch’s membrane, and choriocapillaris in the setting of extracellular deposits.2 GA affects over 5 million people worldwide.1 Currently, the underlying mechanism of GA enlargement is unclear and there are no approved therapies for slowing GA progression. However, many clinical trials are ongoing, and several early phase trials demonstrate promising results in slowing GA progression.3
Systemic diseases (e.g., hypertension, hypercholesterolemia, and cardiovascular diseases)4 and the use of systemic medications (e.g., aspirin, calcium channel blockers, beta-blockers, and diuretics)4 5 have been associated with increased risks of developing early and late AMD. However, evidence for the impact of systemic factors on GA progression rate is sparse.6 Recently, Song et al. investigated the association between systemic medication use and the incidence and growth of GA in patients enrolled in the Comparison of Age-related Macular Degeneration Treatments Trial (CATT).7 They found that the use of calcium channel blockers was associated with a higher GA growth rate.7 However, since all CATT patients had choroidal neovascularization, it is unknown if their findings apply to patients with GA secondary to non-neovascular AMD. Because GA occurs in the elderly who often have comorbidities and take systemic medications, understanding the associations of systemic diseases and medications with GA progression is essential to patient counseling and the design of clinical trials.
In the present study, we used data from the Age-Related Eye Disease Study (AREDS) to explore the associations of systemic health and medication use with the progression of GA secondary to non-neovascular AMD. We employed GA perimeter-adjusted growth rate as the primary outcome measure in the present study because we recently showed that this growth rate measure was not confounded by baseline GA morphological factors, including baselines GA size (r2=0.005), lesion number (r2=0.00009), and circularity index (r2=0.007).8 Also, using GA perimeter-adjusted growth rate as the outcome measure reduces the sample size required to achieve the same statistical power as using the growth rate of GA area or square root of area as the outcome measure.8
METHODS
Study participants and procedure
The AREDS was a prospective, multicenter, randomized controlled trial aiming to evaluate the effects of oral supplements on the progression of AMD and cataracts. Previous AREDS reports described the study design extensively.9 In brief, 11 retinal specialty clinics recruited 4757 participants aged 55 to 80 years from 1992 to 1998. The AREDS group obtained informed consent from all participants before enrollment. At the time of study enrollment, the AREDS research group administered detailed questionnaires to obtain demographic information, history of smoking and sunlight exposure, medical history, and history of specific prescription drug and nonprescription medication use.9 10 The AREDS group also performed physical examinations to obtain height and weight of participants. Standard color fundus photographs (CFPs) were taken by the AREDS at the time of enrollment, the 2-year follow-up visit, and annually after that.9 The AREDS group previously investigated the associations of systemic factors with the risk of developing AMD10 but not with GA progression rate (except for age, gender, smoking, and body mass index).11 We obtained raw data (including color fundus photographs and clinical data) from the AREDS via the database of Genotypes and Phenotypes (dbGaP Study Accession: phs000001.v3.p1)12 after receiving approval for authorized access. The Yale University Institutional Review Board reviewed our study protocol and exempted the analyses from the need for approval. This study adhered to the tenets of the Declaration of Helsinki.
The University of Wisconsin fundus photograph reading center previously graded all CFPs from the AREDS for the presence of GA, the status of AMD, and other AMD-related fundus abnormalities. However, delineations of GA lesions were not available in the AREDS data files, so we used ImageJ software (version 1.52p; National Institutes of Health, Bethesda, Maryland, USA)13 to delineate GA on 1654 CFPs of 365 eye manually.14 We reported the image grading methods and intergrader reproducibility results in previous papers.8 14 We excluded images with poor quality, GA lesions extending beyond the imaging field, or GA lesions contiguous with peripapillary atrophy.14 Eyes with neovascular AMD at any visit were also excluded from our study. We termed gradable GA as GA lesions whose borders we were able to delineate on CFPs. As reported previously, we achieved excellent intergrader reproducibility of GA area (intraclass correlation coefficient (ICC)=0.997), GA perimeter (ICC=0.977), and GA perimeter-adjusted growth rate (ICC=0.950).8 In the present study, we included all eyes with GA delineations for at least 2 follow-up visits.8
Based on our gradings of CFPs, we calculated the total area and perimeter of GA lesions. We calculated GA circularity index as .15 A GA circularity index of 1 would indicate a circular GA lesion. We used the original AREDS gradings to determine GA presence in the fellow eyes that we were unable to delineate GA lesions.8 We extracted factors on demographics, medical history, and medication use for each participant from the AREDS dataset.12 Medical history and medication use were defined as having a history of a disease or medication use during their lifetime when the patients completed the questionnaries at the time of enrollment, regardless of the current status of the disease or medication use.
Statistical analysis
The statistical analysis was performed in R 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria). We presented the data as mean ± standard deviation (SD) unless specified otherwise in the manuscript. As proposed by our previous paper, we calculated GA perimeter-adjusted growth rate as the ratio between GA area growth rate and mean GA perimeter between the first and last visit for each eye.8
We performed univariable linear mixed-effects models with the eye as the unit of analysis (‘lme4’ package in R)16 to investigate the associations of GA perimeter-adjusted growth rate with 27 factors (Table 2 and 3). Factors with <10 eyes in a subgroup of a factor were excluded from our analysis. To identify independent factors associated with GA perimeter-adjusted growth rate, we performed a multivariable linear mixed-effects model with a backward elimination procedure (using “step” function in R) based on the Akaike information criterion. We considered only significant variables (p<0.05) in the univariable model in this backward elimination process. We included GA in the fellow eye at any visit in the multivariable model since it was associated with GA perimeter-adjusted growth rate in our previous study of the same cohort (p=0.002).8 In that previous paper, GA perimeter-adjusted growth rate was not significantly associated with baseline GA area, circularity index, number of lesions, center point involvement status, GA in the fellow eye at baseline, and oral AREDS supplements,8 so we did not include these factors in the multivariable analysis.
Table 2.
Association of Demographic Factors and Medical History with GA Perimeter-adjusted Growth Rate Based on Univariate Linear Mixed-effects Model
| Category | Number of Eyes | Mean GA Perimeter-adjusted Growth Rate (SD), mm/year | Coefficient Estimate (95% CI), mm/yr | Overall p | |
|---|---|---|---|---|---|
| Age at enrollment, years | - | 318 | - | 0.001 (−0.000, 0.003) | 0.14 |
| Age at first gradable GA, years | - | 318 | - | 0.001 (−0.001, 0.002) | 0.28 |
| Follow-up duration of gradable GA, years | - | 318 | - | −0.002 (−0.004, 0.001) | 0.14 |
| BMI | - | 318 | - | 0.000 (−0.001, 0.002) | 0.82 |
| Annual daily sunlight exposure, hrs. | - | 317 | - | 0.002 (−0.006, 0.009) | 0.68 |
| Sex | Female | 170 | 0.100 (0.052) | −0.009 (−0.025, 0.006) | 0.24 |
| Male | 148 | 0.095 (0.071) | Reference | - | |
| Highest education | Some college | 149 | 0.095 (0.063) | −0.005 (−0.022, 0.013) | 0.87 |
| Postgraduate | 52 | 0.099 (0.055) | −0.003 (−0.026, 0.020) | ||
| HS or less | 117 | 0.100 (0.063) | Reference | - | |
| Marital status | Not married* | 74 | 0.105 (0.055) | 0.012 (−0.007, 0.031) | 0.22 |
| Married | 244 | 0.095 (0.063) | Reference | - | |
| Cancer history | Yes | 74 | 0.105 (0.067) | 0.008 (−0.010, 0.026) | 0.39 |
| No | 244 | 0.095 (0.060) | Reference | - | |
| Hypertension history | Yes | 120 | 0.109 (0.064) | 0.018 (0.002, 0.034) | 0.03 |
| No | 198 | 0.091 (0.059) | Reference | - | |
| Angina history | Yes | 29 | 0.091 (0.050) | −0.009 (−0.036, 0.018) | 0.51 |
| No | 289 | 0.098 (0.063) | Reference | - | |
| Diabetes history | Yes | 16 | 0.083 | −0.018 (−0.053, 0.017) | 0.32 |
| No | 302 | 0.098 (0.062) | Reference | - | |
| Arthritis history | Yes | 146 | 0.102 (0.054) | 0.009 (−0.007, 0.024) | 0.26 |
| No | 172 | 0.094 (0.067) | Reference | - | |
| Smoking history | Yes | 192 | 0.095 (0.065) | −0.006 (−0.022, 0.010) | 0.48 |
| No | 126 | 0.101 (0.055) | Reference | - |
BMI = body mass index; CI = confidence interval; GA = geographic atrophy; HS = high school; SD = standard deviation.
Not married category includes never married (11), widowed (50), and divorced/separated (13).
Table 3.
Association of Medication Use History with GA Perimeter-adjusted Growth Rate Based on Univariable Linear Mixed-effects Model
| Systemic Medication Use History | Category | Number of Eyes | Mean GA Perimeter-adjusted Growth Rate (SD), mm/year | Coefficient Estimate (95% CI), mm/yr | p |
|---|---|---|---|---|---|
| Acetaminophen | Yes | 26 | 0.126 (0.060) | 0.028 (−0.001, 0.056) | 0.06 |
| No | 292 | 0.096 (0.061) | Reference | - | |
| NSAIDs | Yes | 102 | 0.095 (0.054) | −0.003 (−0.019, 0.014) | 0.75 |
| No | 216 | 0.099 (0.065) | Reference | - | |
| Antacids | Yes | 65 | 0.097 (0.056) | −0.001 (−0.020, 0.018) | 0.91 |
| No | 253 | 0.098 (0.063) | Reference | - | |
| Aspirin | Yes | 96 | 0.095 (0.067) | −0.003 (−0.020, 0.014) | 0.71 |
| No | 222 | 0.099 (0.059) | Reference | - | |
| Cholesterol-lowering medication | Yes | 23 | 0.142 (0.093) | 0.051 (0.022, 0.080) | < 0.001 |
| No | 295 | 0.094 (0.057) | Reference | - | |
| Beta-blocker | Yes | 17 | 0.133 (0.106) | 0.044 (0.009, 0.080) | 0.02 |
| No | 301 | 0.096 (0.058) | Reference | - | |
| ACE inhibitor | Yes | 30 | 0.111 (0.054) | 0.012 (−0.014, 0.039) | 0.37 |
| No | 288 | 0.096 (0.062) | Reference | - | |
| Diuretics | Yes | 74 | 0.124 (0.068) | 0.038 (0.020, 0.056) | < 0.001 |
| No | 244 | 0.090 (0.057) | Reference | - | |
| Calcium channel blocker | Yes | 38 | 0.113 (0.083) | 0.021 (−0.003, 0.045) | 0.09 |
| No | 280 | 0.096 (0.058) | Reference | - | |
| Digoxin | Yes | 12 | 0.104 (0.021) | 0.006 (−0.033, 0.046) | 0.76 |
| No | 306 | 0.097 (0.063) | Reference | - | |
| Potassium chloride | Yes | 12 | 0.135 (0.067) | 0.038 (−0.002, 0.079) | 0.07 |
| No | 306 | 0.096 (0.061) | Reference | - | |
| Thyroid hormones | Yes | 35 | 0.121 (0.089) | 0.027 (0.003, 0.052) | 0.03 |
| No | 285 | 0.095 (0.057) | Reference | - | |
| Tranquilizers | Yes | 66 | 0.109 (0.057) | 0.015 (−0.005, 0.034) | 0.14 |
| No | 252 | 0.095 (0.063) | Reference | - |
ACE = angiotensin converting enzyme; CI = confidence interval; GA = geographic atrophy; NSAIDs = Nonsteroidal anti-inflammatory drugs; SD = standard deviation.
RESULTS
Study cohort
We included 318 eyes from 213 patients (52.1% were females) with GA in the present study. The mean ± SD age was 70.6±5.4 years at the time of study enrollment and was 74.1±5.5 years at first gradable GA. The follow-up duration was 5.1±3.0 years for gradable GA. The baseline GA area was 5.2±7.2 mm2. The remaining characteristics of included eyes were listed in Table 1.
Table 1.
Characteristics of Eyes included in the Study
| Eyes (patients) | 318 (213) |
| Age at enrollment, years, mean (SD) | 70.6 (5.4) |
| Eyes with GA at enrollment, n (%) | 90 (28.3) |
| Duration between enrollment and first gradable GA, years, mean (SD) | 3.5 (2.5) |
| Follow-up duration of gradable GA, years, mean (SD) | 5.1 (3.0) |
| Baseline GA area, mm2, mean (SD) | 5.2 (7.2) |
| Baseline GA perimeter, mm, mean (SD) | 10.8 (9.9) |
| Baseline circularity index, mean (SD) | 0.62 (0.30) |
| Baseline unifocal GA lesion in the eye, n (%) | 211 (66.4) |
| Baseline number of lesions, mean (SD) | 1.8 (1.7) |
| GA in the fellow eye at any visit, n (%) | 280 (88.1) |
| GA area growth rate, mm2 /year, mean (SD) | 1.46 (1.37) |
| GA perimeter-adjusted growth rate, mm/year, mean (SD) | 0.098 (0.062) |
GA = geographic atrophy; SD = standard deviation.
Univariable associations of GA perimeter-adjusted growth rate with prognostic factors
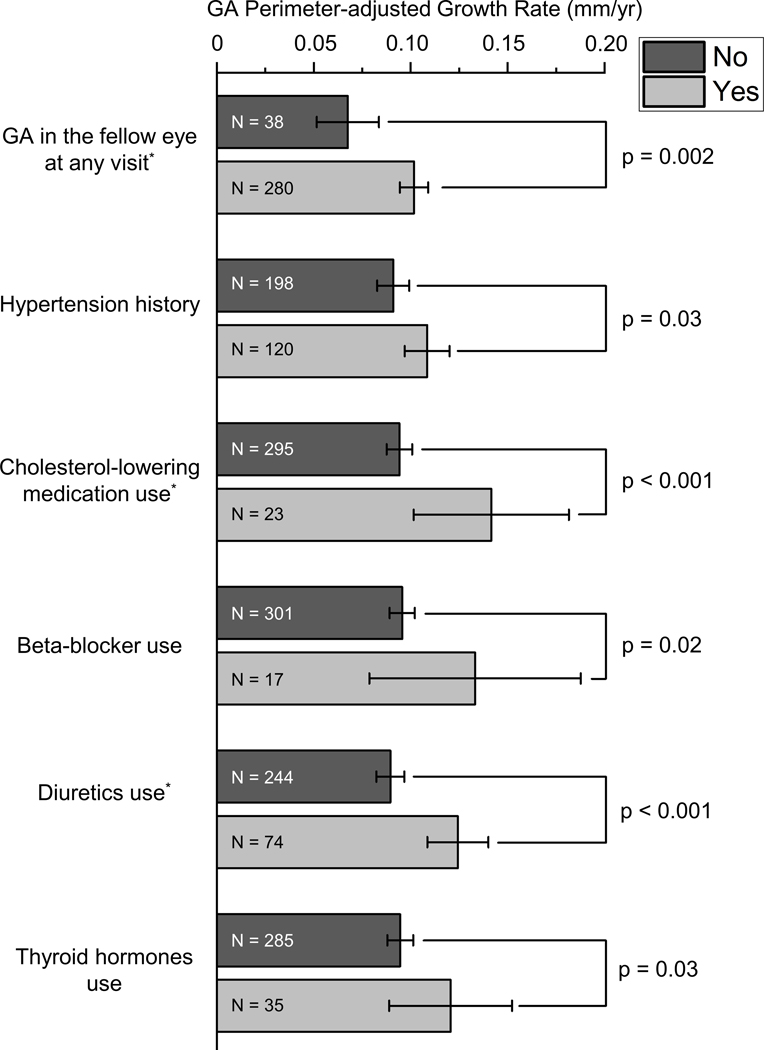
In the univariable analysis, a history of hypertension was associated with a higher GA perimeter-adjusted growth rate (0.109±0.064 vs. 0.091±0.059 mm/year; percent increase=19.8%; p=0.03). In comparison, GA perimeter-adjusted growth rate was not significantly associated with age at enrollment,8 age at first gradable GA, sex,8 the highest education level, marital status, follow-up duration of gradable GA, body mass index, annual daily sunlight exposure, history of any type of cancer, history of angina, history of diabetes, history of arthritis, and smoking history8 (Table 2).
GA perimeter-adjusted growth rate was higher in patients with a history of cholesterol-lowering medication use (0.142±0.093 vs. 0.094±0.057 mm/year; percent increase=51.1%; p<0.001), beta-blocker use (0.133±0.106 vs. 0.096±0.058 mm/year; percent increase=38.5%; p=0.02), diuretics use (0.124±0.068 vs. 0.090±0.057 mm/year; percent increase=37.8%; p<0.001), and thyroid hormones use (0.121±0.089 vs. 0.095±0.057 mm/year; percent increase=27.4%; p=0.03) (Table 3). By contrast, GA perimeter-adjusted growth rate was not significantly associated with a history of using any of the remaining 9 systemic medications: acetaminophen, nonsteroidal anti-inflammatory drugs, antacids, aspirin, angiotensin-converting enzyme inhibitors, calcium channel blocker, digoxin, potassium chloride, and tranquilizers (Table 3). As reported in our previous paper,8 GA perimeter-adjusted growth rate was significantly higher in eyes with versus without GA in the fellow eye at any visit (p=0.002; Supplementary Table 1).
Multivariable associations of GA perimeter-adjusted growth rate with prognostic factors
Six factors were significantly associated with GA perimeter-adjusted growth rate in the univariable model (Figure 1). We included these factors in a multivariable linear mixed-effects model with a backward elimination procedure to identify independent prognostic factors for GA growth rate. We found that GA in the fellow eye at any visit, cholesterol-lowering medication use, and diuretics use were independently associated with higher GA perimeter-adjusted growth rate. In this multivariable model (Table 4), GA in the fellow eye at any visit was associated with 0.028 mm/year (95% confidence interval (CI) = 0.008–0.048; p=0.008) higher GA growth rate; cholesterol-lowering medication use was associated with 0.045 mm/year (95% CI=0.018–0.073; p=0.002) higher GA growth rate; diuretics use was associated with 0.033 mm/year (95% CI=0.015–0.050; p<0.001) higher GA growth rate. After adding baseline systolic and diastolic blood pressures in the multivariable model, GA perimeter-adjusted growth rate remained significantly associated with GA in the fellow eye at any visit (p=0.01), cholesterol-lowering medication use (p=0.001), and diuretics use (p<0.001).
Figure 1.
Six factors were significantly associated with the perimeter-adjusted growth rate of geographic atrophy based on the univariable linear mixed-effects model (p<0.05). The number within each bar represents the number of eyes within the group. The error bar represents the 95% CI of the mean. Among the six factors, three factors (marked with *) remained statistically significant in the multivariable model. GA, geographic atrophy.
Table 4.
Multivariate Linear Mixed-effects Model of GA Perimeter-adjusted Growth Rate
| Coefficient Estimate (95% CI), mm/yr | p | |
|---|---|---|
| GA in the fellow eye at any visit, yes | 0.028 (0.008, 0.048) | 0.008 |
| Cholesterol-lowering medication use, yes | 0.045 (0.018, 0.073) | 0.002 |
| Diuretics use, yes | 0.033 (0.015, 0.050) | < 0.001 |
|
| ||
| CI, confidence interval; GA, geographic atrophy | ||
DISCUSSION
To date, no systemic factors have been associated with GA growth rate consistently in the literature.6 Herein, we explored the associations of systemic health and medication use with GA perimeter-adjusted growth rate in 318 eyes of 213 patients in the AREDS. We employed GA perimeter-adjusted growth rate as the outcome measure because it eliminates the confounding effects of GA lesion morphology and improves the statistical power.8 Based on the univariable model, GA perimeter-adjusted growth rate was significantly associated with GA in the fellow eye at any visit, hypertension history, cholesterol-lowering medication use, beta-blocker use, diuretics use, and thyroid hormones use (Figure 1). The multivariable analysis showed that GA in the fellow eye at any visit (p=0.008), cholesterol medication use (p=0.002), and diuretic use (p<0.001) were independently associated with higher GA perimeter-adjusted growth rate (Table 4). GA perimeter-adjusted growth rate was 51.1% higher in patients with than without a history of cholesterol-lowering medication use, and was 37.8% higher in patients with than without a history of diuretics use. To our knowledge, the study is the first to suggest a significant association of GA progression rate with systemic medication use in patients with non-neovascular AMD. Given the exploratory nature of the study, future prospective studies are required to validate our findings.
Similar to previous reports,6 we did not find significant associations of GA growth rate with patients’ age, sex, body mass index, marital status, education level, sunlight exposure and smoking history. Smoking history was also not associated with GA growth rate in many previous studies11 17–19, although it was significantly associated with a higher GA growth rate in the AREDS220 and the Blue Mountains Eye Study21. Song et al investigated the associations of GA growth rate with systemic medication use in eyes with neovascular AMD.7 Although their patient population (neovascular AMD) differs from our study cohort (non-neovascular AMD), both studies showed that GA growth rate was not significantly associated with the use of antacids, aspirin, and ACE inhibitors. Song et al showed that calcium channel blocker use was associated with a higher GA growth rate in the multivariable analysis (0.40 vs. 0.30 mm/year, p=0.02) even though this association was not statistically significant in the univariable model (p=0.06).7 Our data showed a similar trend in GA perimeter-adjusted growth rate but without statistical significance (0.113 vs. 0.096 mm/year, p=0.09). Our study has a relatively small number of patients with a history of calcium channel blocker use (38 eyes), limiting our statistical power for this analysis.
We found that GA perimeter-adjusted growth rate was significantly associated with diuretics use and cholesterol-lowering medication use. However, Song et al did not find a significant relationship of the growth rate of square root of GA area with diuretics use or statin use in patients with neovascular AMD.7 The discrepancy between our results and the findings by Song et al may be due to differences in the patient population (non-neovascular versus neovascular AMD) and outcome measure (GA perimeter-adjusted growth rate versus growth rate of square root of GA area). Since choroidal neovascularization may affect GA progression,22 patients with neovascular AMD may be affected differently by systemic factors from patients with non-neovascular AMD. Also, the growth rate of square root of GA area is significantly associated with baseline lesion number and circularity index,6 8 15 19 20 23 which were not accounted for by Song et al and might confound their results.7 In comparison, we employed GA perimeter-adjusted growth rate as the outcome measure, uncorrelated with these GA morphological factors.8 Additionally, the cholesterol-lowering medication category recorded in the AREDS includes medications (e.g., niacin, bile acid sequesters) other than statins.
The observed higher GA growth rate in patients with diuretics use history may be related to either the presence of hypertension or the pharmacologic effects of these medications. Hypertension may increase the risk of developing AMD by augmenting age-related retinal and choroidal vascular dysfunction and remodeling.5 24 Given the association between GA growth rate and choriocapillaris flow impairment,25 it is possible that hypertension-induced vascular change may affect GA progression. A few small studies have not detected a significant association between hypertension and GA growth rate.17 18 However, multiple large cross-sectional and longitudinal studies have reported that patients with hypertension, especially those requiring antihypertensive treatments, have an increased risk of developing AMD.4 5 26 For example, Xu et al reported that patients treated for hypertension at baseline had 15% higher risk of AMD compared to patients with untreated hypertension (hazard ratio=1.15, 95% CI=1.03–1.30).23 Interestingly, in their subgroup analysis, daily diuretics use alone was associated with a 45% increased risk of AMD compared to patients using other antihypertensive agents as monotherapy.23 This result corresponds to our finding that GA growth rate was independently associated with the use of the diuretic in the multivariable model, but not with hypertension history, beta-blocker use, ACE inhibitor use, or calcium channel blocker. Although there is no widely accepted biological mechanism for the detrimental effect of diuretics on the retina, hyperlipidemia is a widely acknowledged side effect of diuretics27 and has been associated with an increased risk of AMD in a few studies.28 29 To disentangle the underlying mechanisms of diseases and medications, future prospective studies are required to investigate the association between GA growth rate and diuretics use after adjustment for time-varying blood pressure measures. Such studies should also investigate whether the severity and duration of hypertension affect GA growth rate.
Cholesterol has been hypothesized to play a role in AMD’s pathogenesis,28 but the associations of AMD risk with the cholesterol level and cholesterol-lowering medication use are inconsistent. For example, a population-based study of 3654 participants showed that elevated total/high-density lipoprotein cholesterol ratio increased the risk of developing late AMD (risk ratio=1.35, 95% CI=1.07–1.70) and GA (risk ratio=1.63, 95% CI=1.18–2.25).28 But these associations were not observed in a few other studies.30 31 Additionally, previous authors reported weak associations, no associations, or inverse associations of cholesterol-lowering medication use (e.g., statins) with the risk of AMD.30 32–34 To our knowledge, only 2 previous studies investigated the association of GA growth rate with hyperlipidemia or cholesterol-lowering medication use.7 17 The association was not significant in either study. Given the lack of cholesterol level data in the AREDS and the small number of patients with a history of cholesterol-lowering medication use in our study (23 eyes), we cannot conclude definitively that the cholesterol or cholesterol-lowering medication plays a role in GA expansion. Future cross-sectional and longitudinal studies with a large cohort should investigate this relationship further.
Our study has several limitations. First, although we used data from a prospective study, we retrospectively designed this analysis. Future well-designed prospective studies are required to validate our findings and disentangle the underlying effects of diseases versus medication use. Second, the number of patients with certain systemic diseases or medication use history (e.g., calcium channel block, beta-blocker) was relatively small, limiting our ability to detect the influence of certain systemic factors on GA progression. Additionally, due to the limited number of patients with a history of cholesterol-lowering medication use or diuretics use, we did not perform subgroup analyses to investigate the impact of medication use duration on GA growth. Third, the observational nature of our study precludes an interpretation of causality. Fourth, the history of systemic diseases and medication use was self-reported by patients in questionnaires in the AREDS. The medical history’s accuracy may be affected by patients’ age and imperfect recollection. Fifth, due to the lack of fundus autofluorescence and optical coherence tomography imaging in the AREDS, we were unable to include several previously proposed biomarkers (e.g., hyperfluorescence signals,17 35 hyperreflective foci,36 choriocapillaris flow deficits37) in the present analysis. Lastly, the AREDS dataset does not contain several variables (e.g., cholesterol level, severity and duration of systemic diseases, and dose and compliance of systemic medications), so we could not include these factors in our analysis. Future prospective studies should attempt to obtain expanded details of medication use and investigate their associations with GA progression.
In conclusion, we explored the associations of GA perimeter-adjusted growth rate with a comprehensive list of systemic health factors and medications in 318 eyes of 213 patients with non-neovascular AMD in the AREDS. GA in the fellow eye at any visit, cholesterol-lowering medication use, and diuretic use were independently associated with GA perimeter-adjusted growth rate in the multivariable model. To our knowledge, the study is the first to demonstrate a significant association of GA progression rate with systemic medication use in patients with non-neovascular AMD. Future prospective studies are needed to validate our findings, which may shed light on the underlying mechanism of GA progression and assist clinical trial design and patient counseling.
Supplementary Material
SYNOPSIS.
Our analysis of 318 eyes in the Age-Related Eye Disease study showed that geographic atrophy in the fellow eye, cholesterol-lowering medication use, and diuretic use were independently associated with a higher geographic atrophy growth rate.
ACKNOWLEDGMENTS
The authors thank the AREDS group for gathering the data and making the data available through dbGaP.12 This publication was made possible by the James G. Hirsch Endowed Medical Student Research Fellowship (Recipient: Shen) and P30 EY026878 from the National Eye Institute (NEI) (Recipient: Yale Vision Science Core). The sponsor or funding organization had no role in the design or conduct of this research. We disclose the following conflicts of interest: LLS, Consultant - Boehringer Ingelheim. LVDP, Consultant - Astellas Institute for Regenerative Medicine, LambdaVision, Boehringer Ingelheim; Scientific advisory board - Tissue Regeneration Sciences; Scientific and clinical advisors - CavTheRx.
Financial Support: This publication was made possible by the James G. Hirsch Endowed Medical Student Research Fellowship from Yale School of Medicine (Grant number: None; Recipient: Shen), and P30 EY026878 from the National Eye Institute (NEI) (Recipient: Yale Vision Science Core).
Disclaimers: The sponsor or funding organization had no role in the design or conduct of this research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the institution or funder.
Footnotes
Competing Interests: LLS, Consultant - Boehringer Ingelheim. LVDP, Consultant - Astellas Institute for Regenerative Medicine, LambdaVision, Boehringer Ingelheim; Scientific advisory board - Tissue Regeneration Sciences; Scientific and clinical advisors – CavTheRx.
REFERENCES
- 1.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. The Lancet Global Health 2014;2(2):e106–e16. [DOI] [PubMed] [Google Scholar]
- 2.Li M, Huisingh C, Messinger J, et al. Histology of geographic atrophy secondary to age-related macular degeneration: a multilayer approach. Retina 2018;38(10):1937–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaffe GJ, Westby K, Csaky KG, et al. C5 inhibitor avacincaptad pegol for geographic atrophy due to-age-related macular degeneration: A randomized pivotal phase 2/3 trial. Ophthalmology 2020. [DOI] [PubMed] [Google Scholar]
- 4.Cheung CM, Wong TY. Is age-related macular degeneration a manifestation of systemic disease? New prospects for early intervention and treatment. J. Intern. Med. 2014;276(2):140–53. [DOI] [PubMed] [Google Scholar]
- 5.Xu X, Ritz B, Coleman A, et al. Hypertension, antihypertensive medications use and risk of age-related macular degeneration in California Teachers Cohort. J. Hum. Hypertens. 2020;34(8):568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleckenstein M, Mitchell P, Freund KB, et al. The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology 2018;125(3):369–90. [DOI] [PubMed] [Google Scholar]
- 7.Song D PH, Vanderbeek BL, et al. Systemic Medication Use and the Incidence and Growth of Geographic Atrophy in the Comparison of Age-related Macular Degeneration Treatments Trials (CATT). Retina 2020;Publish Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen LL, Sun M, Ahluwalia A, Young BK, Park MM, Del Priore LV. Geographic Atrophy Growth is Strongly Related to Lesion Perimeter: Unifying Effects of Lesion Area, Number, and Circularity on Growth. Ophthalmology Retina 2020. [DOI] [PubMed] [Google Scholar]
- 9.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study (AREDS): Design implications AREDS report no. 1. Control. Clin. Trials 1999;20(6):573–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Age-Related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration: A case-control study in the age-related eye disease study: age-related eye disease study report number 3. Ophthalmology 2000;107(12):2224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindblad AS, Lloyd PC, Clemons TE, et al. Change in area of geographic atrophy in the age-related eye disease study: AREDS report number 26. Arch. Ophthalmol. 2009;127(9):1168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mailman MD, Feolo M, Jin Y, et al. The NCBI dbGaP database of genotypes and phenotypes. Nat. Genet. 2007;39(10):1181–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rueden CT, Schindelin J, Hiner MC, et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 2017;18(1):529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen LL, Sun M, Ahluwalia A, et al. Relationship of Topographic Distribution of Geographic Atrophy to Visual Acuity in Nonexudative Age-Related Macular Degeneration. Ophthalmology Retina 2020. [DOI] [PubMed] [Google Scholar]
- 15.Domalpally A, Danis RP, White J, et al. Circularity index as a risk factor for progression of geographic atrophy. Ophthalmology 2013;120(12):2666–71. [DOI] [PubMed] [Google Scholar]
- 16.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 2015;67(1):1–48. [Google Scholar]
- 17.Holz FG, Bindewald-Wittich A, Fleckenstein M, Dreyhaupt J, Scholl HPN, Schmitz-Valckenberg S. Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am. J. Ophthalmol. 2007;143(3):463–72.e2. [DOI] [PubMed] [Google Scholar]
- 18.Biarnes M, Arias L, Alonso J, et al. Increased fundus autofluorescence and progression of geographic atrophy secondary to age-related macular degeneration: The GAIN Study. Am. J. Ophthalmol. 2015;160(2):345–53.e5. [DOI] [PubMed] [Google Scholar]
- 19.Rosenfeld PJ, Dugel PU, Holz FG, et al. Emixustat hydrochloride for geographic atrophy secondary to age-related macular degeneration: a randomized clinical trial. Ophthalmology 2018;125(10):1556–67. [DOI] [PubMed] [Google Scholar]
- 20.Keenan TD, Agron E, Domalpally A, et al. Progression of geographic atrophy in age-related macular degeneration: AREDS2 report number 16. Ophthalmology 2018;125(12):1913–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joachim N, Mitchell P, Kifley A, Rochtchina E, Hong T, Wang JJ. Incidence and progression of geographic atrophy: observations from a population-based cohort. Ophthalmology 2013;120(10):2042–50. [DOI] [PubMed] [Google Scholar]
- 22.Hwang CK, Agrón E, Domalpally A, et al. Progression of Geographic Atrophy with Subsequent Exudative Neovascular Disease in Age-Related Macular Degeneration: AREDS2 Report 24. Ophthalmology Retina 2021;5(2):108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen LL, Sun M, Grossetta Nardini HK, Del Priore LV. Progression of unifocal vs. multifocal geographic atrophy in age-related macular degeneration: a systematic review and meta-analysis. Ophthalmol Retina 2020;4(9):899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yun C, Ahn J, Kim M, Hwang S-Y, Kim S-W, Oh J. Ocular Perfusion Pressure and Choroidal Thickness in Early Age-Related Macular Degeneration Patients With Reticular Pseudodrusen. Invest. Ophthalmol. Vis. Sci. 2016;57(15):6604–09. [DOI] [PubMed] [Google Scholar]
- 25.Sacconi R, Corbelli E, Borrelli E, et al. Choriocapillaris flow impairment could predict the enlargement of geographic atrophy lesion. Br. J. Ophthalmol. 2021;105(1):97. [DOI] [PubMed] [Google Scholar]
- 26.Fisher DE, Klein BEK, Wong TY, et al. Incidence of Age-Related Macular Degeneration in a Multi Ethnic United States Population: The Multi-Ethnic Study of Atherosclerosis. Ophthalmology 2016;123(6):1297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ames RP, Hill P. Elevation of serum lipid levels during diuretic therapy of hypertension. The American journal of medicine 1976;61(5):748–57. [DOI] [PubMed] [Google Scholar]
- 28.Tan JS, Mitchell P, Smith W, Wang JJ. Cardiovascular risk factors and the long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Ophthalmology 2007;114(6):1143–50. [DOI] [PubMed] [Google Scholar]
- 29.van Leeuwen R, Klaver CCW, Vingerling JR, et al. Cholesterol and age-related macular degeneration: is there a link? Am. J. Ophthalmol. 2004;137(4):750–52. [DOI] [PubMed] [Google Scholar]
- 30.Klein R, Myers CE, Buitendijk GHS, et al. Lipids, Lipid Genes, and Incident Age-Related Macular Degeneration: The Three Continent Age-Related Macular Degeneration Consortium. Am. J. Ophthalmol. 2014;158(3):513–24.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith W, Assink J, Klein R, et al. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology 2001;108(4):697–704. [DOI] [PubMed] [Google Scholar]
- 32.Hall NF, Gale CR, Syddall H, Phillips DI, Martyn CN. Risk of macular degeneration in users of statins: cross sectional study. BMJ 2001;323(7309):375–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Etminan M, Brophy JM, Maberley D. Use of statins and angiotensin converting enzyme inhibitors (ACE-Is) and the risk of age-related macular degeneration: nested case-control study. Current drug safety 2008;3(1):24–26. [DOI] [PubMed] [Google Scholar]
- 34.Tsao SW, Fong DS. Do statins have a role in the prevention of age-related macular degeneration? Drugs Aging 2013;30(4):205–13. [DOI] [PubMed] [Google Scholar]
- 35.Shen LL, Liu F, Nardini HG, Del Priore LV. Reclassification of fundus autofluorescence patterns surrounding geographic atrophy based on progression rate: a systematic review and meta-analysis. Retina 2019;39(10):1829–39. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt-Erfurth U, Bogunovic H, Grechenig C, et al. Role of Deep Learning–Quantified Hyperreflective Foci for the Prediction of Geographic Atrophy Progression. Am. J. Ophthalmol. 2020;216:257–70. [DOI] [PubMed] [Google Scholar]
- 37.Moult EM, Alibhai AY, Lee B, et al. A framework for multiscale quantitation of relationships between choriocapillaris flow impairment and geographic atrophy growth. Am. J. Ophthalmol. 2020;214:172–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.