Abstract
Background
The effect of concurrent diabetes on the outcome of sepsis is not conclusively known. A meta-analysis published in 2017 indicated that diabetes did not influence the mortality of patients with sepsis but increased the risk of acute renal injury. In view of publication of several new studies in recent years, there is a need for updated evidence.
Methods
A systematic search was conducted using the PubMed, Scopus, Embase, and Google Scholar databases. Studies that were done in patients with sepsis, were observational in design- either cohort or case–control or analysed retrospective data were considered for inclusion. Statistical analysis was performed using STATA software.
Results
A total of 21 studies were included. The risk of in-hospital mortality (RR 0.98, 95% CI 0.93, 1.04) and mortality at latest follow up i.e., within 90 days of discharge (RR 0.94, 95% CI 0.86, 1.04) among diabetic and non-diabetic subjects was statistically similar. There was an increased risk of in-hospital mortality among those with high blood glucose level at admission (RR 1.45, 95% CI 1.01, 2.09). Among those who were diabetic, the risk of acute renal failure (RR 1.54, 95% CI 1.34, 1.78) was higher than non-diabetics. The risk of respiratory failure, adverse cardiac events, need for additional hospitalization post-discharge and length of hospital stay was similar among diabetics and non-diabetics.
Conclusions
Diabetes is not associated with poor survival outcomes in patients with sepsis but is associated with increased risk of acute renal failure. High blood glucose levels, irrespective of the diabetes status, are associated with increased risk of in-hospital mortality. Findings underscore the need for better evaluation of renal function in diabetic patients with concurrent sepsis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13098-022-00803-2.
Keywords: Sepsis, Septic shock, Diabetes, Mortality, Acute renal failure, Outcomes, Meta-analysis
Introduction
Sepsis is defined as a life-threatening organ dysfunction owing to the dysregulated host response to an infection [1]. Sepsis is associated with more than a tenth of the mortality within hospital [1, 2]. An extension to this is a condition known as septic shock that is defined as sepsis in association with circulatory and metabolic abnormalities [3]. In a recent meta-analysis that included 15 studies, the mortality rates due to sepsis and severe sepsis were documented to be 17% and 26%, respectively [4].
It is interesting to note that around one-fifth of the patients with sepsis have associated diabetes mellitus [5]. Diabetes is a metabolic disorder with rising incidence globally. With changing lifestyle and wide acceptance of Western diets that include consumption of processed foods, the incidence of diabetes is nearing pandemic proportions [6]. Patients with diabetes tend to have an increasing predisposition to develop infection and consequent sepsis [7]. In both type 1 and 2 diabetes, there is an increased blood glucose levels and glycemia-dependent immune response alterations that might influence the pathogenesis and outcome of sepsis. Preclinical studies indicate that presence of diabetes influences several components of the innate immune system and exerts an inhibitory effect on the adaptive immune system [8–10]. Diabetes, particularly type 2, results in protracted inflammation, suppression of immune response, and significant morbidity due to infections. In diabetes, there is an activation of inflammatory pathway through activation of toll like receptors such as TLR2 and TLR4 as well as indirect activation through TLR signalling [11, 12].
There has been immense reduction in mortality due to sepsis owing to the advancement in medical treatment and nursing. However, the co-association of sepsis with diabetes is still a considerable medical problem. It is still not conclusively known in what ways the presence of diabetes influences the outcomes of sepsis. In a meta-analysis published in 2017, Wang et al. [13] assessed the impact of diabetes on outcomes of sepsis and included 10 studies. This review concluded that presence of diabetes did not influence the outcome of patients with sepsis; however, the risk of acute renal injury is sufficiently increased in patients with diabetes. In view of publication of several new studies in recent years there is a need for updated evidence. Hence, the purpose of this review was to conduct a thorough literature search and present updated pooled evidence on the impact of diabetes on outcomes of sepsis. The primary outcome of interest was mortality. Other secondary outcomes of interest were complication rates, length of hospital stay and additional need for hospitalization post-discharge.
Materials and methods
Search strategy
The study processes were in compliance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines [14]. A systematic search of English-language publications was conducted via PubMed, Scopus, Embase and Google academic databases for studies published prior to 20th August 2021. Both medical subject heading (MeSH) terminology and free text words were employed (Additional file 1: Table S1). The literature search aimed at identifying studies that examined the association between diabetes status and/or blood glucose levels upon hospital admission with outcomes of interest in patients with sepsis. We registered the study on PROSPERO (CRD42021273785).
Selection criteria and methods
Search results were listed, duplicates removed, and then two subject experts screened titles and abstracts for study suitability. After this, remaining studies underwent full-text review. Any disagreements regarding inclusion status were resolved through group discussions. Only those studies were included in the meta-analysis that fulfilled the inclusion criteria. In order to identify additional literature, the reference list of the included studies was also reviewed.
Inclusion criteria
Studies that were done in patients with sepsis, were observational in design- either cohort or case–control or analysed retrospective data were considered for inclusion. Studies should have examined the outcomes among patients with sepsis based on diabetes status.
Exclusion criteria
Case reports and reviews were excluded. Furthermore, studies that did not provide data on outcomes of interest or did not provide comparative findings based on diabetic status were excluded.
Data extraction and quality assessment
Relevant data was extracted from studies that met inclusion criteria using a pre-determined guide sheet. Extracted data included study identifiers (author names, study year), study setting, study design, subject characteristics, overall sample size, and main findings. Study quality was assessed via the Newcastle–Ottawa Quality Assessment Scale [15].
Statistical analysis
This meta-analysis was conducted using STATA version 16.0 and reported effect sizes as pooled relative risk (RR) for categorical outcomes and weighted mean difference (WMD) for continuous outcomes. A subgroup analysis was conducted in order to document the effect of diabetes on patients with all stages of sepsis and in those with severe sepsis and/or septic shock. We also analysed and documented the association of blood glucose levels, irrespective of the diabetes status, at the time of hospital admission with the outcomes. All effect sizes were reported along with 95% confidence intervals (CI). I2 was used to indicate heterogeneity. If I2 exceeded 40%, a random effects model was used [16]. P values under 0.05 indicated statistical significance. Egger’s test was employed to examine publication bias.
Results
Study selection, characteristics, and quality
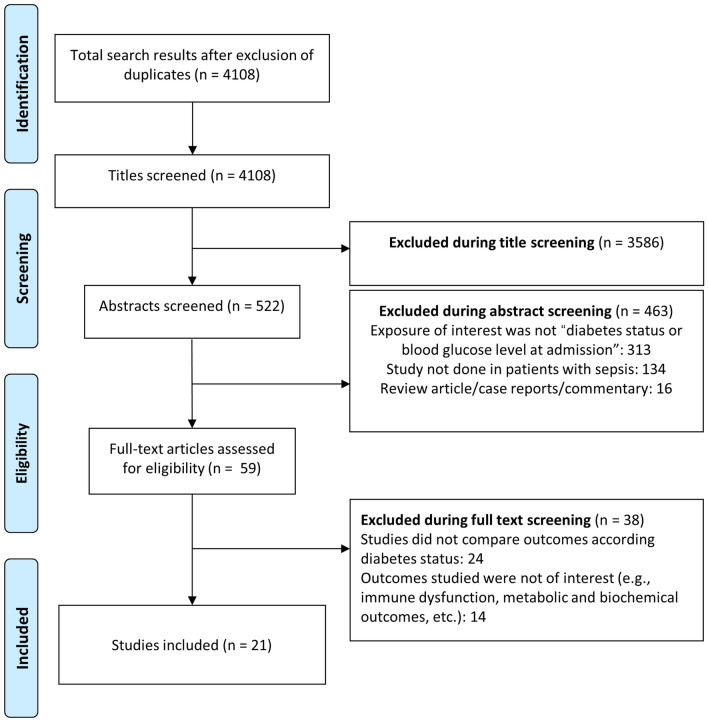
Database screening yielded 4108 unique citations (Fig. 1). Title screening resulted in the removal of 3586 papers. Of the remaining 522, 463 were excluded after abstract review, with a further 38 excluded after full-text review, leaving 21 for inclusion in the final meta-analysis ([17–37], Table 1). Of these, 14 studies were based on analysis of retrospective data whereas seven studies were prospective in design. Five studies were conducted in USA and three in Greece. Two studies each were done in Spain and Taiwan. There were two multicentric studies and one study each was done in China, Israel, Netherlands, Japan, Singapore, France and South Korea. In 10 studies, the patient population had severe sepsis and/or septic shock and in the remaining 11 studies, the included patient population had varied stages of sepsis. The included studies majorly reported on in-hospital mortality and some studies, additionally reported mortality at around 30- and 90-days post-hospital discharge. The included studies were of good quality (Additional file 1: Tables S2 and S3).
Fig. 1.
Selection process of the studies included in the review
Table 1.
Characteristics of the studies included in the meta-analysis
| Author (year of publication) | Study design | Country | Participant characteristics | Sample size | Key outcomes |
|---|---|---|---|---|---|
| Zohar et al. (2021) [17] | Analysis of retrospective data | Israel | Patients with community onset sepsis; median age of 67 years; 56% females; majority with respiratory (36%) or urinary tract (24%) infections; 29% with severe sepsis/septic shock | 1527 (diabetes, DM: 469; non-diabetes, non-DM: 1058) | Diabetes vs. no diabetes |
| Mortality (in-hospital): RR 1.21 (95% CI 0.80, 1.71) | |||||
| Mortality at 30 days post-discharge: RR 1.10 (95% CI 0.79, 1.54) | |||||
| Mortality at 90 days post-discharge: RR 1.13 (95% CI 0.86, 1.49) | |||||
| Functional deterioration: RR 1.10 (95% CI 0.76, 1.67) | |||||
| Discharge to home: RR 1.15 (95% CI 0.65, 2.0) | |||||
| Additional need for hospitalization: RR 1.14 (95% CI 0.84, 1.54) | |||||
| Acute renal failure: RR 2.48 (95% CI 1.93, 3.19) | |||||
| Respiratory failure: RR 1.78 (95% CI 1.21, 2.60) | |||||
| Blood glucose level at admission (> 200 mg/dl vs. < 200 mg/dl) | |||||
| Mortality (in-hospital): RR 1.48 (95% CI 1.02, 2.16) | |||||
| Mortality at 30 days post-discharge: RR 1.80 (95% CI 1.12, 2.58) | |||||
| Mortality at 90 days post-discharge: RR 1.68 (95% CI 1.24, 2.27) | |||||
| Vught et al. (2017) [18] | Analysis of retrospective data | Netherlands | Patients with sepsis; median age of 70 years; 55% females; majority with respiratory (40%), gastrointestinal (25%) infections | 41,492 (DM: 8085; Non-DM: 33,407) | Diabetes vs. no diabetes |
| Mortality (in-hospital): RR 1.14 (95% CI 1.07, 1.21) | |||||
| Mortality at 90 days post-discharge: RR 1.09 (95% CI 0.72, 1.66) | |||||
| Acute renal failure: RR 1.32 (95% CI 1.23, 1.42) | |||||
| Respiratory failure: RR 1.41 (95% CI 1.33, 1.49) | |||||
| Adverse cardiac event: RR 1.11 (95% CI 1.03, 1.19) | |||||
| Additional need for hospitalization: RR 1.33 (95% CI 1.19, 1.48) | |||||
| Length of hospitalization (days; Mean): 15 (3.3) vs. 15 (3.1) | |||||
| Blood glucose level at admission (> 200 mg/dl vs. < 200 mg/dl) | |||||
| Mortality at 90 days post-discharge: RR 0.97 (95% CI 0.90, 1.05) | |||||
| Chao et al. (2017) [19] | Analysis of retrospective data | Taiwan | Patients with median age of 66 years; > 50% males; majority with septic shock (70%); bacteraemia (20%) | 6156 (DM: 3594; Non-DM: 2562) | Diabetes vs. no diabetes |
| Mortality (in-hospital): RR 0.83 (95% CI 0.65, 0.99) | |||||
| Additional need for hospitalization: RR 0.83 (95% CI 0.68, 1.02) | |||||
| Acute renal failure: RR 1.78 (95% CI 1.52, 2.10) | |||||
| Blood glucose level at admission (> 200 mg/dl vs. < 200 mg/dl) | |||||
| Mortality (in-hospital): RR 1.83 (95% CI 1.30, 2.80) | |||||
| Sathananthan et al. (2019) [20] | Analysis of retrospective cohort data | USA | Majority with age > 60 years; > 55% males; majority with severe sepsis (> 80%) | 1698 (DM: 508; non-DM: 1190) | Diabetes vs. no diabetes |
| Mortality at 30 days post-admission: RR 1.00 (95% CI 0.81, 1.25) | |||||
| Acute renal failure: RR 1.53 (95% CI 1.23, 1.90) | |||||
| Respiratory failure: RR 1.16 (95% CI 0.92, 1.48) | |||||
| Adverse cardiac event: RR 1.09 (95% CI 0.88, 1.34) | |||||
| Length of hospitalization (days; Mean): 13 (3.2) vs. 13 (2.8) | |||||
| Discharge to home: RR 0.97 (95% CI 0.76, 1.19) | |||||
| Blood glucose level at admission (> 180 mg/dl vs. < 180 mg/dl) | |||||
| Mortality at 30 days post-admission: RR 0.82 (95% CI 0.62, 1.07) | |||||
| Kushimoto et al. (2020) [21] | Retrospective analysis of prospectively collective data | Japan | Mean age of 73 years; 60% males; Majority with pulmonary (31%) and gastrointestinal infection (26%); 63% with septic shock | 1127 (DM: 261; non-DM: 866) | Diabetes vs. no diabetes |
| In-hospital mortality: RR 1.32 (95% CI 0.96, 1.81) | |||||
| Blood glucose level at admission (> 180 mg/dl vs. < 180 mg/dl) | |||||
| Mortality (in-hospital): RR 0.72 (95% CI 0.51, 1.00) | |||||
| Mortality at 30 days post-discharge: RR 0.90 (95% CI 0.64, 1.27) | |||||
| Discharge to home: RR 0.86 (95% CI 0.62, 1.17) | |||||
| Length of hospitalization (days; Mean): 24 (6.3) vs. 23.8 (5.7) | |||||
| Lin et al. (2021) [22] | Retrospective analysis data | China | Mean age of 66.7 years; 51% males; Majority with bloodstream (44%) and urinary tract infection (21%) | 5774 (2887 in each of the DM and non-DM groups) | Diabetes vs. no diabetes |
| Mortality (in-hospital): RR 0.73 (95% CI 0.62, 0.87) | |||||
| Mortality at 28 days post-discharge: RR 0.86 (95% CI 0.77, 0.97) | |||||
| Length of hospitalization (days; Mean): 10.82 (2.1) vs. 10.62 (2.3) | |||||
| Acute renal failure: RR 0.97 (95% CI 0.77, 1.22) | |||||
| Respiratory failure: RR 0.98 (95% CI 0.88, 1.08) | |||||
| Blood glucose level at admission (> 200 mg/dl vs. < 200 mg/dl) | |||||
| Mortality at 28 days post-discharge: RR 0.49 (95% CI 0.38, 0.64) | |||||
| Akinosoglou et al. (2021) [23] | Retrospective analysis of data | Greece | Mean age of 76.2 years; 45% males; majority (76%) with either frank sepsis and/or septic shock | 812 (406 in each of the DM and non-DM groups) | Diabetes vs. no diabetes |
| Mortality at 28 days post-discharge: RR 1.04 (95% CI 0.75, 1.44) | |||||
| Adverse cardiac event: RR 1.16 (95% CI 0.55, 2.47) | |||||
| Moss et al. (2000) [24] | Prospective cohort study | USA | Mean age of around 54 years; majority of the diabetics were females (62%); Majority with respiratory or urinary tract infections; majority had septic shock | 113 (DM: 32; non-DM: 81) | Diabetes vs. no diabetes |
| Mortality (in-hospital): RR 0.67 (95% CI 0.36, 1.23) | |||||
| Respiratory failure: RR 0.53 (95% CI 0.28, 1.01) | |||||
| Moutzouri et al. (2008) [25] | Prospective cohort study | Greece | Mean age of around 60 years; around 50% were females; majority with urinary tract infections; most had severe sepsis/septic shock | 64 (DM: 24; non-DM: 40) | Diabetes vs. no diabetes |
| Mortality (in-hospital): RR 1.30 (95% CI 0.56, 3.03) | |||||
| Stegenga et al. (2010) [26] | Retrospective study | Multicentric study | Mean age of 60.6 years; around 58% were males; Most had severe sepsis/septic shock | 830 (DM: 188; non-DM: 642) | Diabetes vs. no diabetes |
| Mortality at 28 days post-discharge: RR 1.03 (95% CI 0.81, 1.31) | |||||
| Mortality at 90 days post-discharge: RR 1.00 (95% CI 0.71, 1.41) | |||||
| Blood glucose level at admission (> 200 mg/dl vs. < 200 mg/dl) | |||||
| Mortality at 28 days post-discharge: RR 2.02 (95% CI 1.28, 3.18) | |||||
| Mortality at 90 days post-discharge: RR 2.08 (95% CI 1.31, 3.28) | |||||
| Schuetz et al. (2011) [27] | Retrospective study | USA | Mean age of 59 years; around 49% were males; around one-third (37%) had severe sepsis/septic shock | 7754 (DM: 1844; non-DM: 5910) | Diabetes vs. no diabetes |
| Mortality (in-hospital): RR 0.85 (95% CI 0.71, 1.01) | |||||
| Blood glucose level at admission (> 200 mg/dl vs. < 200 mg/dl) | |||||
| Mortality (in-hospital): RR 2.05 (95% CI 1.40, 2.99) | |||||
| Yang et al. (2011) [28] | Retrospective study | Singapore | Mean age of 60 years; around 50% were males; majority with respiratory, urinary tract or gastrointestinal infections | 9221 (DM: 2943; non-DM: 6278) | Diabetes vs. no diabetes |
| Mortality (in-hospital): RR 0.96 (95% CI 0.88, 1.05) | |||||
| Length of hospitalization (days; Mean): 12.1 (11.1) vs. 12.2 (14.2) | |||||
| Acute renal failure: RR 1.91 (95% CI 1.80, 2.02) | |||||
| Respiratory failure: RR 0.81 (95% CI 0.71, 0.93) | |||||
| Adverse cardiac event: RR 0.94 (95% CI 0.84, 1.05) | |||||
| Schuetz et al. (2012) [29] | Prospective cohort | USA | Mean age of 60 years; around 48% were females; majority with pneumonia (22%) or skin/soft tissue infection (27%) or urinary tract infections (11%) | 1849 (DM: 539; non-DM: 1310) | Diabetes vs. no diabetes |
| Mortality (in-hospital): RR 0.95 (95% CI 0.48, 1.90) | |||||
| Length of hospitalization (days; Mean): 6.28 (6.93) vs. 5.67 (6.91) | |||||
| Blood glucose level at admission (> 180 mg/dl vs. < 180 mg/dl) | |||||
| Mortality (in-hospital): RR 1.48 (95% CI 0.86, 3.83) | |||||
| Chang et al. (2012) [30] | Prospective cohort | Taiwan | Mean age of 67 years; > 50% males; majority with pneumonia (43%) or gastrointestinal infection (34%) or urinary tract infections (26%); majority with severe sepsis/septic shock | 16,497 (DM: 4573; non-DM: 11,924) | Diabetes vs. no diabetes |
| Mortality (in-hospital): RR 1.00 (95% CI 0.94, 1.07) | |||||
| Length of hospitalization (days; Mean): 23.85 (33.52) vs. 23.72 (44.93) | |||||
| Acute renal failure: RR 1.54 (95% CI 1.44, 1.63) | |||||
| Respiratory failure: RR 0.96 (95% CI 0.94, 0.97) | |||||
| Adverse cardiac event: RR 0.98 (95% CI 0.93, 1.03) | |||||
| Al-Dorzi et al. (2012) [31] | Retrospective cohort | Multicentric (Canada, USA and Saudi Arabia) | Subject age > 60 years; around 45% females; Majority with pneumonia or gastrointestinal infection or soft tissue infections; majority with severe sepsis/septic shock | 8670 (DM: 2289; non-DM: 6389) | Diabetes vs. no diabetes |
| Mortality (in-hospital): RR 0.96 (95% CI 0.92, 1.01) | |||||
| Venot et al. (2015) [32] | Prospective cohort | France | Mean age of 67 years; > 60% males; majority with severe sepsis/septic shock | 1064 (DM: 318; non-DM: 746) | Diabetes vs. no diabetes |
| Mortality (in-hospital): RR 1.32 (95% CI 1.00, 1.74) | |||||
| Length of hospitalization (days; Mean): 9.1 (8.5) vs. 16.8 (17.5) | |||||
| Acute renal failure: RR 1.30 (95% CI 1.22, 1.38) | |||||
| De Miguel et al. (2015) [33] | Retrospective cohort | Spain | Mean age of 72 years; > 55% males; majority one or more organ failure | 217,280 (DM: 50,611; non-DM: 166,669) | Diabetes vs. no diabetes |
| Mortality (in-hospital): RR 0.97 (95% CI 0.96, 0.98) | |||||
| Length of hospitalization (days; Mean): 10 (13) vs. 12 (18) | |||||
| Kim et al. (2014) [34] | Prospective cohort | South Korea | Median age of 69 years; all subjects were females; around 50% had bacteraemia; all had complicated urinary tract infection/pyelonephritis | 775 (DM: 246; non-DM: 529) | Diabetes vs. no diabetes |
| Mortality (in-hospital): RR 1.19 (95% CI 0.40, 3.62) | |||||
| Length of hospitalization (days; Mean): 9 (1.2) vs. 7 (1.67) | |||||
| Kofteridis et al. (2009) [35] | Retrospective review of records | Greece | Majority with age above 65 years; 35% males; around 20% had bacteraemia; all had pyelonephritis | 206 (DM: 88; non-DM: 118) | Diabetes vs. no diabetes |
| Mortality (in-hospital): RR 5.47 (95% CI 1.48, 20.1) | |||||
| Length of hospitalization (days; Mean): 10 (4.1) vs. 7 (3.8) | |||||
| Acute renal failure: RR 1.63 (95% CI 0.84, 3.19) | |||||
| Peralta et al. (2009) [36] | Retrospective cohort | Spain | Majority with age above 70 years; 50% males; around 15% had septic shock; foci of infection was majorly urinary tract (50%) and gastrointestinal tract (20%) | 1112 (DM: 181; non-DM: 931) | Diabetes vs. no diabetes |
| Mortality (in-hospital): RR 1.13 (95% CI 0.67, 1.90) | |||||
| Length of hospitalization (days; Mean): 13.3 (12) vs. 13.9 (15) | |||||
| McAlister et al. (2005) [37] | Prospective cohort | USA | Majority with age above 70 years; around 50% males; 15% were nursing home residents, 49% were taking at least four prescribed medications | 2471 (DM: 824; non-DM: 1647) | Diabetes vs. no diabetes |
| Mortality (in-hospital): RR 1.00 (95% CI 0.69, 1.45) | |||||
| Length of hospitalization (days; Mean): 8 (2) vs. 6.67 (1.33) | |||||
| Blood glucose level at admission (> 200 mg/dl vs. < 200 mg/dl) | |||||
| Mortality (in-hospital): RR 1.69 (95% CI 0.97, 2.94) |
Diabetes status, hyperglycaemia and mortality in patients with sepsis
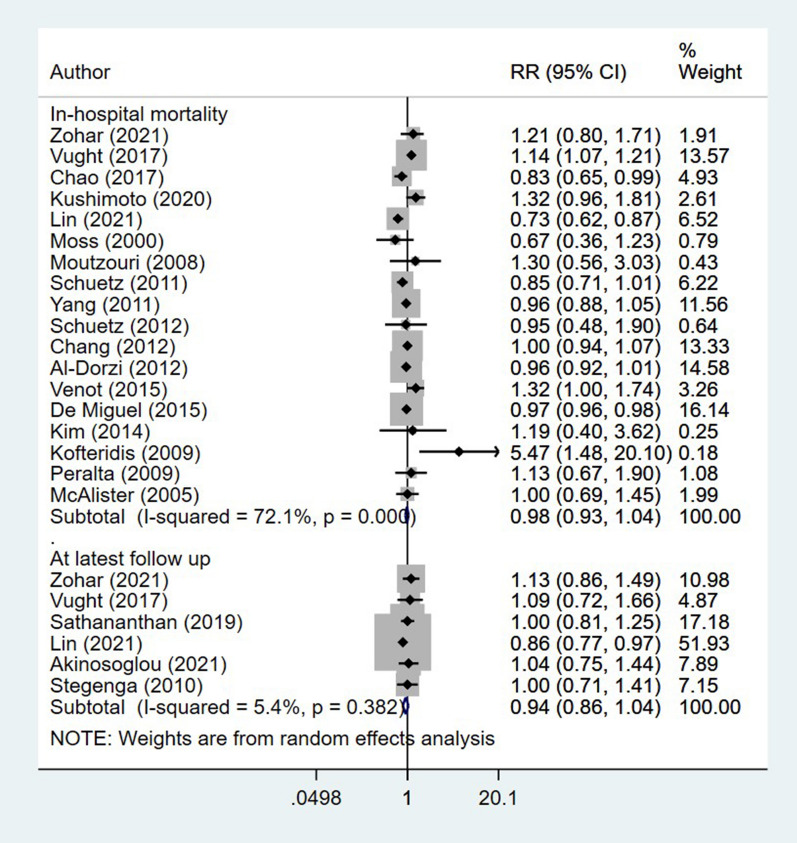
The risk of in-hospital mortality among diabetic and non-diabetic subjects was statistically similar (RR 0.98, 95% CI 0.93, 1.04; I2 = 72.1%, N = 18) (Fig. 2). There were no differences in the risk of mortality at the latest follow up among both the diabetics and non-diabetics (RR 0.94, 95% CI 0.86, 1.04; I2 = 5.4%, N = 6) (Fig. 2). Egger’s test did not indicate the presence of publication bias (P = 0.23 for in-hospital mortality; P = 0.49 for mortality at latest follow up).
Fig. 2.
Association of diabetes status with mortality in patients with sepsis
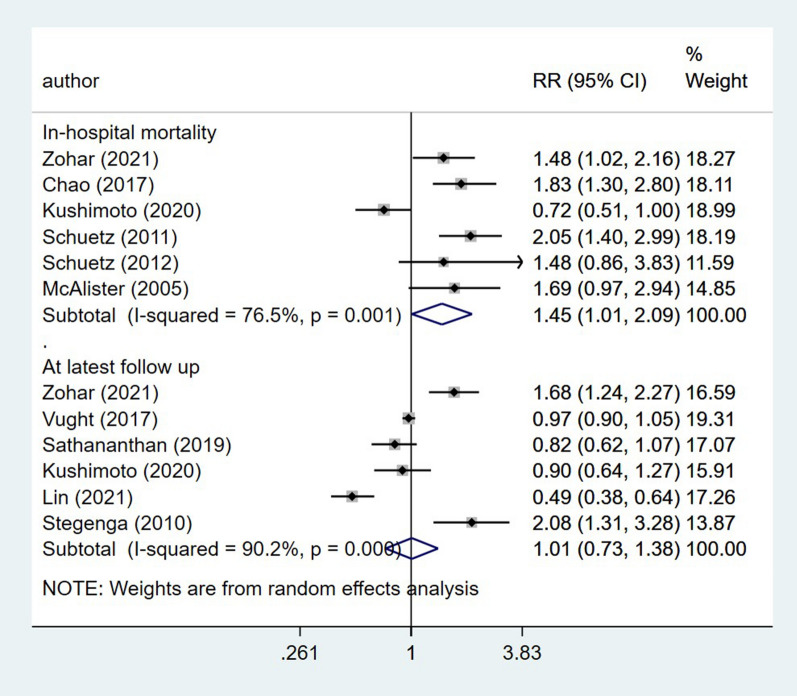
In the subgroup analysis, the risk of in-hospital mortality and mortality at latest follow up was similar among patients with severe sepsis and those with sepsis of all stages (Table 2). There was an increased risk of in-hospital mortality among those with high blood glucose level at admission (> 180 or > 200 mg/dl) (RR 1.45, 95% CI 1.01, 2.09; I2 = 76.5%, N = 6) (Fig. 3). However, at latest follow up, this association was not significant (RR 1.01, 95% CI 0.73, 1.38; I2 = 90.2%, N = 6).
Table 2.
Findings of the subgroup analysis
| Outcomes of interest | Severe sepsis | Sepsis of all stage |
|---|---|---|
| Pooled relative risk (RR) with 95% CI | ||
| In-hospital mortality | 1.00 (0.91, 1.09) | 0.97 (0.89, 1.07) |
| N = 7; I2 = 54.6% | N = 11; I2 = 79.0% | |
| Mortality at latest follow up | 1.01 (0.86, 1.18) | 0.97 (0.79, 1.19) |
| N = 3; I2 = 0.0% | N = 3; I2 = 50.2% | |
| Acute renal failure | 1.51 (1.32, 1.72) | 1.57 (1.20, 2.07) |
| N = 4; I2 = 86.4% | N = 5; I2 = 95.7% | |
| Respiratory failure | 0.96 (0.77, 1.21) | 1.16 (0.85, 1.57) |
| N = 3; I2 = 65.1% | N = 4; I2 = 96.3% | |
| Adverse cardiac event | 0.99 (0.94, 1.04) | 1.03 (0.87, 1.21) |
| N = 3; I2 = 0.0% | N = 2; I2 = 83.4% | |
Fig. 3.
Association of blood glucose level at admission with mortality in patients with sepsis
Diabetes status and risk of complications in patients with sepsis
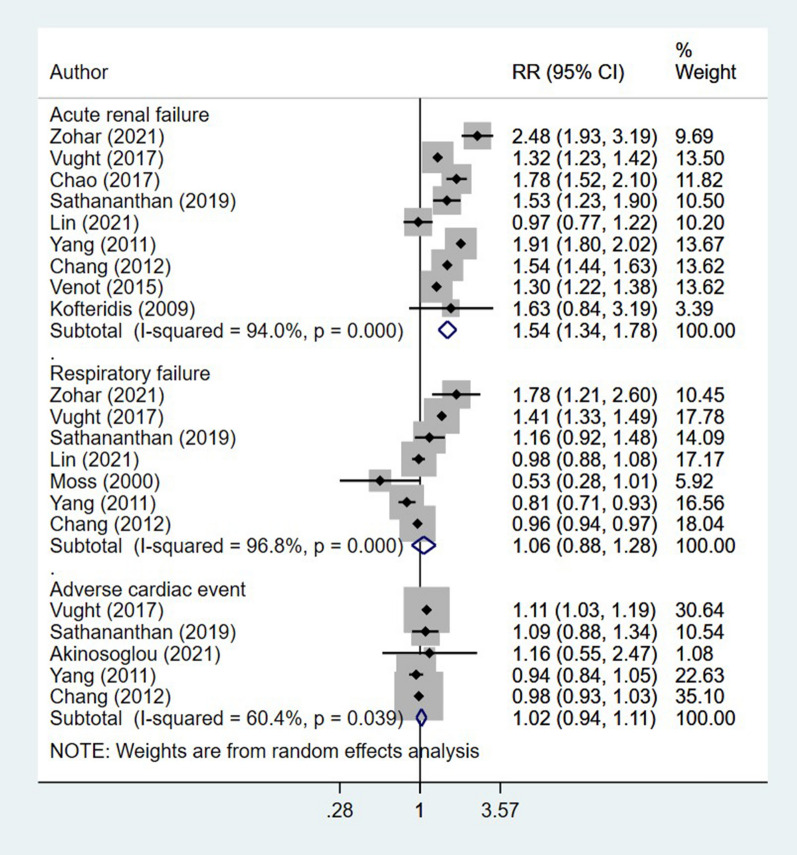
Among those who were diabetic, the risk of acute renal failure (RR 1.54, 95% CI 1.34, 1.78; I2 = 94.0%, N = 9) was higher than non-diabetics (Fig. 4). For other complications such as respiratory failure (RR 1.06, 95% CI 0.88, 1.28; I2 = 96.8%, N = 7) and adverse cardiac events (RR 1.02, 95% CI 0.94, 1.11; I2 = 60.4%, N = 5), the risk was similar among diabetics and non-diabetics (Fig. 4). Egger’s test did not indicate the presence of publication bias (P = 0.81 for acute renal failure; P = 0.34 for respiratory failure and P = 0.64 for adverse cardiac event).
Fig. 4.
Association of diabetic status with risk of complications in patients with sepsis
In the subgroup analysis, the increased risk of acute renal failure was observed in both patients with severe sepsis (RR 1.51, 95% CI 1.32, 1.72; I2 = 86.4%, N = 4) and sepsis of all stages (RR 1.57, 95% CI 1.20, 2.07; I2 = 95.7%, N = 5) (Table 2). The risk of respiratory failure and adverse cardiac event with diabetic status was insignificant among the patients in both groups (i.e., severe sepsis and sepsis of all stages).
Diabetes status and other outcomes of interest in patients with sepsis
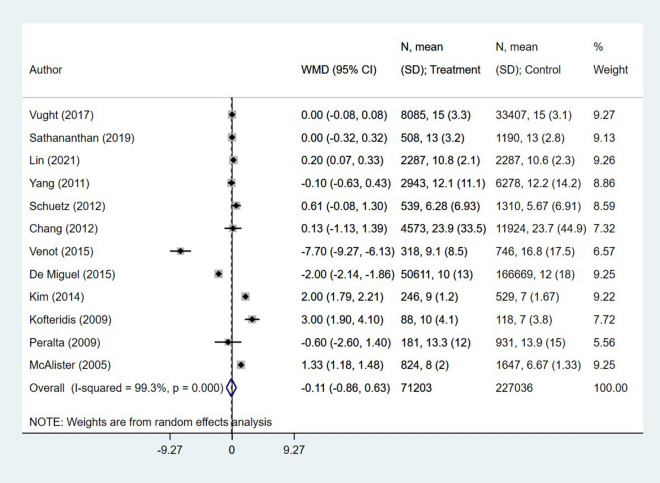
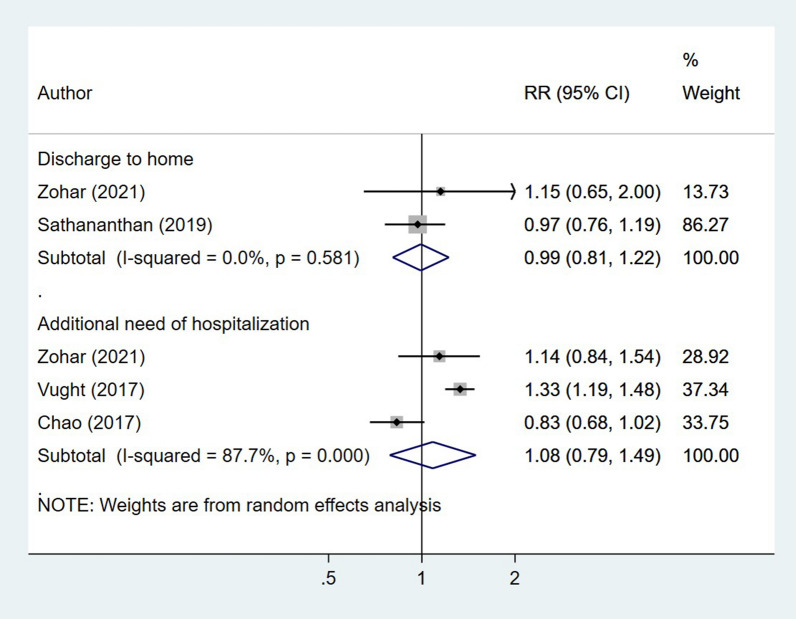
The length of hospital stay (in days) was similar among diabetic and non-diabetics (WMD − 0.11, 95% CI − 0.86, 0.63; I2 = 99.3%, N = 12) (Fig. 5). Similarly, the relative risk of additional need for hospitalization (RR 1.57, 95% CI 1.20, 2.07; I2 = 95.7%, N = 5) and discharge to home (RR 1.57, 95% CI 1.20, 2.07; I2 = 95.7%, N = 5) was similar in both patient groups (Fig. 6). Egger’s test did not indicate the presence of publication bias (P = 0.18 for length of hospital stay; P = 0.39 for additional need for hospitalization and P = 0.48 for discharge to home).
Fig. 5.
Association of diabetic status with length of hospitalization in patients with sepsis
Fig. 6.
Association of diabetic status with risk of discharge to home and additional need for hospitalization in patients with sepsis
Discussion
Diabetes adversely impacts the immunological responses of the host. Increased blood glucose levels tend to hamper the function of polymorphonuclear leukocytes (PMN) by lowering the membrane fluidity, thereby resulting in reduced phagocytosis, intracellular killing and sub-optimal migration and chemotaxis [38–40]. Studies have indicated that those with diabetes have multiple downregulated miRNAs encompassing diverse signalling pathways, including MAPK signalling pathway, hematopoietic cell lineage and Fc gamma R-mediated phagocytosis [2, 3, 41]. The present meta-analysis was conducted to present an updated pooled evidence on the association of diabetes mellitus with outcomes in patients with sepsis. The review, through pooling of findings from 21 studies, found that the risk of in-hospital mortality and mortality until 3 months post-discharge among diabetic and non-diabetic subjects was statistically similar. There was an increased risk of in-hospital mortality among those with high blood glucose level at admission, irrespective of the diabetes status. Among those who were diabetic, the risk of acute renal failure was higher than non-diabetics whereas the risk were similar for respiratory failure and adverse cardiac events. The length of hospital stays (in days) and the risk for additional need for hospitalization, post-discharge was similar among diabetic and non-diabetics. Overall, the findings of this meta-analysis do not suggest an increased risk of short-term mortality among patients with sepsis and concurrent diabetes. However, they do point towards the need for maintaining optimal blood glucose levels.
The findings of this meta-analysis are similar to the earlier meta-analysis on this issue by Wang et al. [13]. Both the meta-analysis suggest that presence of diabetes does not increase the risk of mortality in patients with sepsis. In the earlier review, presence of diabetes was associated with slightly reduced risk of in-hospital mortality (pooled relative risk of 0.97, 95% confidence intervals of 0.96–0.98) but in the present review, there were no significant differences in the risk of mortality. This could be more of an analysis issue. In the earlier review, majority of the decrease in mortality estimate was driven by one study (De Miguel et al. [33]; 88.2% weightage) [32]. The increased risk of acute renal failure amongst diabetics has been documented in both the current and the previous meta-analysis. It is well known that diabetes impairs renal function but the underlying pathophysiology is not conclusively known. It is unclear whether the damage is because of the consistent hyperglycaemia milieu, or it results from the end organ damage due to atherosclerosis. There are suggestions that hyperglycaemia can lead to increased activation of NF-kappa B, TGF-β and oxidant levels and this in turn, leads to renal damage [42, 43].
The existing evidence is mixed with regards to the effect of acute hyperglycaemia on risk of mortality during an event of sepsis. Acute hyperglycaemia has been documented to be an independent risk factor for mortality in critically ill patients with sepsis [44, 45]. There are also studies that indicate that the effect of hyperglycaemia is modified by the concurrent presence or absence of diabetes [46, 47]. Usually in diabetics, the blood glucose levels remain high and therefore, they are better able to tolerate effects of short-term hyperglycaemia during sepsis. On the other hand, increased blood glucose levels during sepsis in a non-diabetic patient leads to massive increase in inflammatory cytokine level and organ damage. In our meta-analysis, we found that hyperglycaemia was associated with increased risk of in-hospital mortality but not with mortality at latest follow up post-discharge. More research is required to conclusively understand the effect of short-term increased blood glucose levels on the outcomes of sepsis. Research is also warranted on the role of glycosylated hemoglobin (HbA1c) and glycated albumin in predicting the outcomes of sepsis. There is evidence to suggest that the predictive ability of glycated hemoglobin for complications in patients with chronic renal failure is reduced [48, 49]. In such circumstances, glycated albumin could prove reliable as a biomarker for predicting and stratifying the risk linked to multiorgan metabolic alterations [48, 49]. Despite the findings of this review that presence of diabetes in patients with sepsis does not significantly increase the risk of adverse cardiac events, it should be noted that diabetes in itself is a strong risk factor for cardiac complication and a comprehensive assessment of indicators for diabetes control and early markers of cardiac injury is warranted. There have been recent studies that have explored a variety of biomarkers such as galectin-3, troponin-I and heart-type fatty acid binding protein (H-FABP) [50–52]. More such important and groundbreaking studies are required to provide a comprehensive scientific knowledge in this respect.
This is the most updated evidence documenting the effect of diabetes on mortality and other outcomes among patients with sepsis. The included studies are from a wide geography and therefore, the findings are applicable to a large setting. There are some limitations as well. First, majority of the included studies were retrospective data based and therefore, the possibility of important confounders not being adjusted in the analysis cannot be ruled out. Due to the lack of studies reporting on the management protocols for the sepsis, the meta-analysis was not able to ascertain the treatment effects on the outcomes considered. It would have been preferable if the analysis would have looked at the association of glycaemic control (using HbA1c) with outcomes, but such an analysis could not be undertaken as the studies did not report on this.
Conclusion
Based on pooling of observational studies, our meta-analysis shows that diabetes is not associated with poor survival outcomes in patients with sepsis but is associated with increased risk of acute renal failure. High blood glucose levels (those > 180 mg/dl), irrespective of the diabetes status, are associated with increased risk of in-hospital mortality. More well-designed studies, that take into account the adjustment for confounders, are required to confirm these associations further. The findings also underscore the need for better evaluation of renal function in diabetic patients with concurrent sepsis.
Supplementary Information
Additional file 1: Table S1. Search strategy for identification of studies to be included in the review. Table S2. Author’s judgements about study quality using the adapted Ottawa-Newcastle Risk of Bias Assessment tool. Table S3. Author’s judgements about study quality using the adapted Ottawa-Newcastle Risk of Bias Assessment tool.
Acknowledgements
Not applicable.
Abbreviations
- MeSH
Medical subject heading
- RR
Relative risk
- WMD
Weighted mean difference
- CI
Confidence intervals
- PMN
Polymorphonuclear
Authors’ contributions
LJ and MC designed the project collected the data, analyzed and wrote the manuscript. Both authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frydrych LM, Fattahi F, He K, Ward PA, Delano MJ. Diabetes and sepsis: risk, recurrence, and ruination. Front Endocrinol. 2017;8:271. doi: 10.3389/fendo.2017.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent J-L. Sepsis and septic shock. Nat Rev Dis Primers. 2016;2:16045. doi: 10.1038/nrdp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleischmann C, Scherag A, Adhikari NKJ, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193:259–72. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 5.Trevelin SC, Carlos D, Beretta M, da Silva JS, Cunha FQ. Diabetes mellitus and sepsis: a challenging association. Shock. 2017;47:276–287. doi: 10.1097/SHK.0000000000000778. [DOI] [PubMed] [Google Scholar]
- 6.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;2016(387):1513–30. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koh GCKW, Peacock SJ, van der Poll T, Wiersinga WJ. The impact of diabetes on the pathogenesis of sepsis. Eur J Clin Microbiol Infect Dis. 2012;31:379–388. doi: 10.1007/s10096-011-1337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehuen A, Diana J, Zaccone P, Cooke A. Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol. 2010;10:501–513. doi: 10.1038/nri2787. [DOI] [PubMed] [Google Scholar]
- 9.Carlos D, Spiller F, Souto FO, Trevelin SC, Borges VF, de Freitas A, et al. Histamine h2 receptor signaling in the pathogenesis of sepsis: studies in a murine diabetes model. J Immunol. 2013;191:1373–1382. doi: 10.4049/jimmunol.1202907. [DOI] [PubMed] [Google Scholar]
- 10.Frolov A, Blüher M, Hoffmann R. Glycation sites of human plasma proteins are affected to different extents by hyperglycemic conditions in type 2 diabetes mellitus. Anal Bioanal Chem. 2014;406:5755–5763. doi: 10.1007/s00216-014-8018-y. [DOI] [PubMed] [Google Scholar]
- 11.Jialal I, Kaur H. The role of toll-like receptors in diabetes-induced inflammation: implications for vascular complications. Curr Diab Rep. 2012 doi: 10.1007/s11892-012-0258-7. [DOI] [PubMed] [Google Scholar]
- 12.Sepehri Z, Kiani Z, Nasiri AA, Kohan F. Toll-like receptor 2 and type 2 diabetes. Cell Mol Biol Lett. 2016;21:2. doi: 10.1186/s11658-016-0002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Ren J, Wang G, Liu Q, Guo K, Li J. Association between diabetes mellitus and outcomes of patients with sepsis: a meta-analysis. Med Sci Monit. 2017;23:3546–3555. doi: 10.12659/MSM.903144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.PRISMA. http://www.prisma-statement.org/PRISMAStatement/. Accessed 11 Sep 2021.
- 15.Wells G, Shea B, O’Connell D, Robertson J, Peterson J, Welch V, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. p. 21.
- 16.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions: Cochrane book series. Hoboken: Wiley; 2019. p. 674. [Google Scholar]
- 17.Zohar Y, Zilberman Itskovich S, Koren S, Zaidenstein R, Marchaim D, Koren R. The association of diabetes and hyperglycemia with sepsis outcomes: a population-based cohort analysis. Intern Emerg Med. 2021;16:719–728. doi: 10.1007/s11739-020-02507-9. [DOI] [PubMed] [Google Scholar]
- 18.van Vught LA, Holman R, de Jonge E, de Keizer NF, van der Poll T. Diabetes is not associated with increased 90-day mortality risk in critically ill patients with sepsis. Crit Care Med. 2017;45:e1026–e1035. doi: 10.1097/CCM.0000000000002590. [DOI] [PubMed] [Google Scholar]
- 19.Chao H-Y, Liu P-H, Lin S-C, Chen C-K, Chen J-C, Chan Y-L, et al. Association of in-hospital mortality and dysglycemia in septic patients. PLoS ONE. 2017;12:e0170408. doi: 10.1371/journal.pone.0170408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sathananthan M, Sathananthan A, Jeganathan N. Characteristics and outcomes of patients with and without type 2 diabetes mellitus and pulmonary sepsis. J Intensive Care Med. 2020;35:836–843. doi: 10.1177/0885066619833910. [DOI] [PubMed] [Google Scholar]
- 21.Kushimoto S, Abe T, Ogura H, Shiraishi A, Saitoh D, Fujishima S, et al. Impact of blood glucose abnormalities on outcomes and disease severity in patients with severe sepsis: an analysis from a multicenter, prospective survey of severe sepsis. PLoS ONE. 2020;15:e0229919. doi: 10.1371/journal.pone.0229919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin S, Ge S, He W, Zeng M. Association between comorbid diabetes mellitus and prognosis of patients with sepsis in the intensive care unit: a retrospective cohort study. Ann Transl Med. 2021;9:22. doi: 10.21037/atm-20-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akinosoglou K, Kapsokosta G, Mouktaroudi M, Rovina N, Kaldis V, Stefos A, et al. Diabetes on sepsis outcomes in non-ICU patients: a cohort study and review of the literature. J Diabetes Complications. 2021;35:107765. doi: 10.1016/j.jdiacomp.2020.107765. [DOI] [PubMed] [Google Scholar]
- 24.Moss M, Guidot DM, Steinberg KP, Duhon GF, Treece P, Wolken R, et al. Diabetic patients have a decreased incidence of acute respiratory distress syndrome. Crit Care Med. 2000;28:2187–2192. doi: 10.1097/00003246-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Moutzouri AG, Athanassiou GA, Dimitropoulou D, Skoutelis AT, Gogos CA. Severe sepsis and diabetes mellitus have additive effects on red blood cell deformability. J Infect. 2008;57:147–151. doi: 10.1016/j.jinf.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Stegenga ME, Vincent J-L, Vail GM, Xie J, Haney DJ, Williams MD, et al. Diabetes does not alter mortality or hemostatic and inflammatory responses in patients with severe sepsis. Crit Care Med. 2010;38:539–545. doi: 10.1097/CCM.0b013e3181c02726. [DOI] [PubMed] [Google Scholar]
- 27.Schuetz P, Jones AE, Howell MD, Trzeciak S, Ngo L, Younger JG, et al. Diabetes is not associated with increased mortality in emergency department patients with sepsis. Ann Emerg Med. 2011;58:438–444. doi: 10.1016/j.annemergmed.2011.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Y, Salam Z-HA, Ong BC, Yang KS. Respiratory dysfunction in patients with sepsis: protective effect of diabetes mellitus. Am J Crit Care. 2011;20:e41–47. doi: 10.4037/ajcc2011391. [DOI] [PubMed] [Google Scholar]
- 29.Schuetz P, Kennedy M, Lucas JM, Howell MD, Aird WC, Yealy DM, et al. Initial management of septic patients with hyperglycemia in the noncritical care inpatient setting. Am J Med. 2012;125:670–678. doi: 10.1016/j.amjmed.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Chang C-W, Kok VC, Tseng T-C, Horng J-T, Liu C-E. Diabetic patients with severe sepsis admitted to intensive care unit do not fare worse than non-diabetic patients: a nationwide population-based cohort study. PLoS ONE. 2012;7:e50729. doi: 10.1371/journal.pone.0050729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Dorzi HM, Tamim H, Bouchama A, Kumar A, Arabi YM. Presentation and outcome of diabetic patients admitted with septic shock. In: B23. outcomes, health services and patient-centered research in the intensive care unit. American Thoracic Society; 2012. p. A2552–A2552.
- 32.Venot M, Weis L, Clec’h C, Darmon M, Allaouchiche B, Goldgran-Tolédano D, et al. Acute kidney injury in severe sepsis and septic shock in patients with and without diabetes mellitus: a multicenter study. PLoS ONE. 2015;10:e0127411. doi: 10.1371/journal.pone.0127411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Miguel-Yanes JM, Méndez-Bailón M, Jiménez-García R, Hernández-Barrera V, Pérez-Farinós N, López-de-Andrés A. Trends in sepsis incidence and outcomes among people with or without type 2 diabetes mellitus in Spain (2008–2012) Diabetes Res Clin Pract. 2015;110:266–275. doi: 10.1016/j.diabres.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Kim Y, Wie S-H, Chang U-I, Kim J, Ki M, Cho YK, et al. Comparison of the clinical characteristics of diabetic and non-diabetic women with community-acquired acute pyelonephritis: a multicenter study. J Infect. 2014;69:244–251. doi: 10.1016/j.jinf.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Kofteridis DP, Papadimitraki E, Mantadakis E, Maraki S, Papadakis JA, Tzifa G, et al. Effect of diabetes mellitus on the clinical and microbiological features of hospitalized elderly patients with acute pyelonephritis. J Am Geriatr Soc. 2009;57:2125–2128. doi: 10.1111/j.1532-5415.2009.02550.x. [DOI] [PubMed] [Google Scholar]
- 36.Peralta G, Sánchez MB, Roiz MP, Garrido JC, Teira R, Mateos F. Diabetes does not affect outcome in patients with Enterobacteriaceae bacteremia. BMC Infect Dis. 2009;9:94. doi: 10.1186/1471-2334-9-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McAlister FA, Majumdar SR, Blitz S, Rowe BH, Romney J, Marrie TJ. The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community-acquired pneumonia. Diabetes Care. 2005;28:810–815. doi: 10.2337/diacare.28.4.810. [DOI] [PubMed] [Google Scholar]
- 38.Alba-Loureiro TC, Munhoz CD, Martins JO, Cerchiaro GA, Scavone C, Curi R, et al. Neutrophil function and metabolism in individuals with diabetes mellitus. Braz J Med Biol Res. 2007;40:1037–1044. doi: 10.1590/s0100-879x2006005000143. [DOI] [PubMed] [Google Scholar]
- 39.Masuda M, Murakami T, Egawa H, Murata K. Decreased fluidity of polymorphonuclear leukocyte membrane in streptozocin-induced diabetic rats. Diabetes. 1990;39:466–470. doi: 10.2337/diab.39.4.466. [DOI] [PubMed] [Google Scholar]
- 40.Cui Y, Chen W, Chi J, Wang L. Comparison of transcriptome between type 2 diabetes mellitus and impaired fasting glucose. Med Sci Monit. 2016;22:4699–4706. doi: 10.12659/MSM.896772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 42.Vallon V. Do tubular changes in the diabetic kidney affect the susceptibility to acute kidney injury? Nephron Clin Pract. 2014;127:133–138. doi: 10.1159/000363554. [DOI] [PubMed] [Google Scholar]
- 43.Patschan D, Müller GA. Acute kidney injury in diabetes mellitus. Int J Nephrol. 2016;2016:6232909. doi: 10.1155/2016/6232909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leonidou L, Mouzaki A, Michalaki M, DeLastic AL, Kyriazopoulou V, Bassaris HP, et al. Cytokine production and hospital mortality in patients with sepsis-induced stress hyperglycemia. J Infect. 2007;55:340–346. doi: 10.1016/j.jinf.2007.05.177. [DOI] [PubMed] [Google Scholar]
- 45.Jiménez-Ibáñez EO, Castillejos-López M, Hernández A, Gorocica P, Alvarado-Vásquez N. High mortality associated with hyperglycemia, neutrophilia, and lymphopenia in critically ill patients. Tohoku J Exp Med. 2012;226:213–220. doi: 10.1620/tjem.226.213. [DOI] [PubMed] [Google Scholar]
- 46.Yu W-K, Li W-Q, Li N, Li J-S. Influence of acute hyperglycemia in human sepsis on inflammatory cytokine and counterregulatory hormone concentrations. World J Gastroenterol. 2003;9:1824–1827. doi: 10.3748/wjg.v9.i8.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nugent K, Edriss H, Selvan K. Hyperglycemia and outcomes in patients with sepsis. J Thorac Dis. 2016;8:E575–E577. doi: 10.21037/jtd.2016.05.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bellia C, Cosma C, Lo Sasso B, Bivona G, Agnello L, Zaninotto M, et al. Glycated albumin as a glycaemic marker in patients with advanced chronic kidney disease and anaemia: a preliminary report. Scand J Clin Lab Invest. 2019;79:293–297. doi: 10.1080/00365513.2019.1613673. [DOI] [PubMed] [Google Scholar]
- 49.Giglio RV, Lo Sasso B, Agnello L, Bivona G, Maniscalco R, Ligi D, et al. Recent updates and advances in the use of glycated albumin for the diagnosis and monitoring of diabetes and renal, cerebro- and cardio-metabolic diseases. J Clin Med. 2020;9:3634. doi: 10.3390/jcm9113634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agnello L, Bellia C, Lo Sasso B, Pivetti A, Muratore M, Scazzone C, et al. Establishing the upper reference limit of galectin-3 in healthy blood donors. Biochem Med. 2017;27:030709. doi: 10.11613/BM.2017.030709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agnello L, Bellia C, Scazzone C, Bivona G, Iacolino G, Gambino CM, et al. Establishing the 99th percentile for high sensitivity cardiac troponin I in healthy blood donors from Southern Italy. Biochem Med. 2019;29:020901. doi: 10.11613/BM.2019.020901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bivona G, Agnello L, Bellia C, Lo Sasso B, Ciaccio M. Diagnostic and prognostic value of H-FABP in acute coronary syndrome: still evidence to bring. Clin Biochem. 2018;58:1–4. doi: 10.1016/j.clinbiochem.2018.04.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Search strategy for identification of studies to be included in the review. Table S2. Author’s judgements about study quality using the adapted Ottawa-Newcastle Risk of Bias Assessment tool. Table S3. Author’s judgements about study quality using the adapted Ottawa-Newcastle Risk of Bias Assessment tool.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.