Abstract
Numerous epidemiological and clinical studies have noted differences in the incidence and severity of parasitic diseases between males and females. Although in some instances this may be due to gender-associated differences in behavior, there is overwhelming evidence that sex-associated hormones can also modulate immune responses and consequently directly influence the outcome of parasitic infection. Animal models of disease can often recreate the gender-dependent differences observed in humans, and the role of sex-associated hormones can be confirmed by experimentally altering their levels. Under normal circumstances, levels of sex hormones not only differ between males and females but vary according to age. Furthermore, not only are females of reproductive age subject to the regular hormonal cycles which control ovulation, they are also exposed to dramatically altered levels during pregnancy. It is thus not surprising that the severity of many diseases, including those caused by parasites, has been shown to be affected by one or more of these circumstances. In addition, infection with many pathogens has been shown to have an adverse influence on pregnancy. In this article we review the impact of sex-associated hormones on the immune system and the development and maintenance of immunity to the intracellular protozoan parasites Toxoplasma gondii, Plasmodium spp., and Leishmania spp.
The literature is full of observations that both the incidence and severity of natural parasitic infections are different between males and females of many species, including humans. These differences are undoubtedly due to many factors, including the different exposure of the sexes to various parasite infective stages. However, under controlled laboratory conditions, a clear dichotomy in the susceptibility of males and females can also be observed. Such experiments demonstrate that physiological differences between males and females play an important role in determining susceptibility to parasitic infection. Moreover, a dichotomy in the incidence and severity of many diseases of noninfectious eitiology is a further indication that the physiology of males and females is an important factor in determining disease susceptibility. These studies have prompted investigation into the ability of sex-associated hormones to influence the immune system. It is now widely accepted that many hormones, including the sex-associated hormones, directly influence the immune system and thus susceptibility to disease. Herein, we review the effects of sex- and pregnancy-associated hormones on the immune system in general and in particular immunity to selective protozoan parasitic diseases.
HORMONAL VARIATION IN LIFE
In humans, levels of sex hormones not only vary between males and females, but also alter with age. In addition, females are subject to regular cyclic changes in hormone levels. Progesterone levels are relatively low in the peripheral blood of children of either sex from 1 year up to the age of 2 years (0.1 nmol/liter) and remain low throughout life in adult males (0.3 to 0.6 nmol/liter). However, the level of progesterone increases considerably in females and fluctuates between 1.8 ± 0.34 and 43 ± 13 nmol/liter during the follicular phase and luteal phases of the menstrual cycle, respectively. Testosterone again is found at very low levels in children between 1 and 2 years of age (0.22 ± 0.1 nmol/liter) but rises to relatively high levels in adult males (19.9 ± 4.7 nmol/liter). Testosterone is found at 0.75 ± 0.2 nmol/liter at the follicular phase and 1.28 ± 0.3 nmol/liter at the luteal phase of the menstrual cycle. Estradiol-17β is low or absent in 1- to 2-year-old children (0.03 nmol/liter), but rises in adult women to 0.35 ± 0.18 and 0.64 ± 0.38 nmol/liter during the follicular and luteal phases, respectively. Progesterone, estrogen, and testosterone levels are raised substantially during pregnancy to 500 to 610 nmol/liter, 3.6 ± 1.5 nmol/liter, and 64.5 ± 33.7 nmol/liter, respectively (41).
Although fluctuation in hormone levels may be regarded primarily as physiological, these hormones also have profound effects on cells associated with the immune system. Communication between the endocrine and immune systems may have a number of evolutionary advantages. For example, the local immunological changes which occur during the menstrual cycle in humans favor the maintenance of an infection-free environment in the uterus prior to ovulation, followed by an immunologically permissive environment that does not kill sperm or inhibit implantation postovulation (92).
Significantly, it has been reported that female susceptibility to pathogens, especially those that infect their reproductive organs, varies according to the stage of the menstrual cycle (137). Another likely evolutionary advantage of hormonal modulation of the immune system would be to facilitate pregnancy. Indeed, the immunomodulatory effects of hormones are most evident during gestation, when they appear to be essential to a successful pregnancy (110). While almost every cell associated with the immune system, including macrophages, natural killer (NK) cells, mast cells, eosinophils, neutrophils, and T cells, is present in the decidua, their functions are tightly regulated (29). This control is largely achieved through the production of estrogen and progesterone, initially by the uterus and then by the placenta. Without this level of control, immunological recognition of the trophoplast could result in tissue damage and termination of pregnancy.
During pregnancy there is a local T-helper 2 (Th2) bias, with murine fetoplacental tissues spontaneously producing the Th2-associated cytokines interleukin (IL)-4, IL-5, and IL-10 (79, 110). Current evidence suggests that the main mediator of this response is progesterone, which has been shown to promote Th2 cell expansion and the production of IL-4 by established Th1 cell clones (105). IL-4 will also primarily favor the production of antibody isotypes that cannot fix complement, ensuring that antibodies produced against paternal antigens are not harmful to the trophoblast (29). The importance of this Th2 bias during pregnancy is further illustrated by studies that show that administration of Th1-associated cytokines such as gamma interferon (IFN-γ) and IL-2 causes abortion in pregnant mice (79, 110). Similarly, substances such as lipopolysaccharide (LPS) and infectious agents which induce inflammatory mediators such as tumor necrosis factor alpha (TNF-α) and nitric oxide also induce abortion in mice (48). Further studies suggest that progesterone may also mediate its affects by inducing an NK cell function-inhibitory protein from CD8+ T cells (140–142).
HORMONES AND IMMUNE RESPONSE
The effects of sex- and pregnancy-associated hormones on the immune response are not limited to reproductive tissues but are clearly evident in the systemic immune system. Sex- and pregnancy-associated hormones influence the function of virtually all immune cells types. For example, hormones can profoundly influence cells of the innate immune system, such as mast cells, eosinophils, macrophages, dendritic cells, and NK cells. These cells not only form the first line of defense against many organisms, but also play an important role in directing the developing adaptive immune response. The adaptive immune response involving T cells and B cells is also directly affected by these hormones. Some of the specific actions of hormones on the various cells of the systemic immune system are discussed below.
Natural Killer Cells
NK cells are important cellular mediators of innate resistance to many pathogens and cancer (147). The principal effector mechanisms that NK cells utilize include the cytolytic destruction of virally infected cells (21) and the secretion of the proinflammatory cytokine IFN-γ (9). In addition to activating cellular killing (155), this early production of IFN-γ by NK cells has been shown to be of crucial importance during parasitic infections, when its production promotes development of a subsequent Th1-type adaptive response (122). Indeed, given the importance of NK cells in resistance to parasitic infections, it is perhaps not surprising that studies investigating the influence of sex hormones on disease outcome have correlated this with NK cell function. Similarly, given the importance of NK cells in immune surveillance against tumor development, many studies have investigated sex hormone-associated effects on the anticancer activities of NK cells.
There are numerous studies describing the effects of female sex hormones on NK cell activity, with the vast majority concentrating on the modulatory role of the steroid hormones estrogen and progesterone. It is well established that sustained estrogen (β-estradiol) treatment of mice leads to a reduction in in vivo NK cell activity, whereas androgen treatment (5α-dihydrotestosterone) has no effect (124, 125). This suppressive effect of estrogen was confirmed in other mouse models; treatment with β-estradiol suppressed NK cell killing of transplanted tumors, leading to enhanced metastasis (57), and other studies have confirmed these effects (11, 40, 59, 97). However, one study concluded that suppression of NK cell activity in vivo by estradiol treatment depended on long-term administration (60 days), with initial treatment actually leading to enhancement of NK cell-killing activity (123). Furthermore, another in vitro study showed that β-estradiol enhanced NK cell proliferation (134), although this study used a human “NK-like” cell line which may not be representative of NK cells in vivo. Other studies supporting the concept that estrogen suppresses NK activity include those correlating NK activity with elevated estrogen levels produced during pathological disease. For example, patients with ovarian tumors (116), endometriosis (108), and mastopathy (117) all have elevated estrogen levels and low NK cell cytotoxicity. Although some studies have demonstrated that estrogen can suppress general IFN-γ production (54, 78), the ability of estrogen to directly modulate this function of NK cells has not been investigated.
There are also numerous studies characterizing the effects of progesterone on NK cell activity. It has been shown that progesterone directly depolarizes NK cell membranes (84), suggesting that NK cells express receptors for this hormone. However, more recent studies have demonstrated that progesterone-induced blocking factor (PIBF) (140) modulates NK cell activity. In particular, this protein decreases NK cell cytotoxic activity (141), and the mechanism of suppression appears to be via inhibition of NK cell degranulation (39). Interestingly, progesterone, both by itself and via PIBF, has been shown recently to suppresses perforin expression in cytolytic cells (77), suggesting that this is another mechanism of progesterone-induced suppression of NK cell activity. Progesterone is highly expressed during pregnancy, and many studies have demonstrated suppression of NK cell cytotoxic activity during this period (8, 44, 145, 146). Indeed, this progesterone-dependent immunomodulation of NK cell activity is thought to be one of the mechanisms that enables pregnancy to proceed to term, as spontaneous pregnancy termination is associated with increased systemic NK activity (32, 142) and failure of the fetoplacental unit to suppress uterine NK cell activity (49, 50). Interestingly, a recent study of uterine NK cells during pregnancy showed that these cells express both TNF-α and inducible nitric oxide synthase (iNOS) in vivo, and this is maintained in the presence of progesterone (61), suggesting that in addition to influencing cytotoxic ability, pregnancy-associated hormones regulate proinflammatory molecule expression by NK cells.
The suppressive effects of pregnancy-associated hormones on NK cell function are supported by studies of pregnant mice showing that these animals are more susceptible to pathogens such as Listeria monocytogenes and Toxoplasma gondii (82). Further studies showed that pregnancy reduced the cytotoxic ability of peritoneal NK cells harvested from animals infected with Corynebacterium parvum or T. gondii (81). Subsequent studies showed that the increased susceptibility of pregnant mice to T. gondii correlated with decreased serum IFN-γ production (130), consistent with our findings that female SCID mice produce less innate IFN-γ early during infection with this parasite (152). These findings are further supported by recent findings that pregnant women who transmit T. gondii to their unborn fetus have low levels of circulating NK cells (96).
Macrophages
Macrophages have in recent years been shown to possess estrogen receptors, explaining previous observations that a number of their functions are modulated by estrogens. Macrophage effector functions influenced by estrogen include phagocytosis, which has been shown to be upregulated by this hormone, as is the production of reactive oxygen intermediates, but not reactive nitrogen intermediates, which are downregulated (28).
Numerous macrophage afferent functions are also influenced by estrogen. Thus, following LPS stimulation and treatment with 17β-estradiol, murine macrophages have been shown to produce less IL-1α, IL-6, and TNF-α, whereas IL-10, IL-12, and macrophage inflammatory protein production was unaffected. These affects were associated with decreased LPS-induced NFκB binding (36). Similarly, studies which used the human monocyte-macrophage cell line THP-1 found that estrogen reduced expression of TNF-α mRNA in response to phorbol ester stimulation (127). However, there is contradictory evidence about the role of estrogen in modulating TNF-α production. For example, recent studies found that estrogen did not affect TNF-α mRNA expression (87) or TNF-α production (157) by murine macrophages in vitro. In contrast, a murine model of endotoxemia estrogen treatment prior to endotoxin challenge was found to increase serum TNF-α. Again, this action was selective, as IL-6 levels were reduced in these mice relative to control animals and appeared to be operative at the transcription level, as mRNA transcript levels for TNF-α were also lower in estrogen-treated animals (157).
The effects of progesterone on TNF-α production are more clearly defined. Progesterone has been shown to decrease levels of TNF-α mRNA and TNF-α production by murine macrophage lines (RAW 264.7 and ANA-1 cells) treated with LPS. Progesterone treatment was shown to elevate mRNA transcripts and protein levels of IκB, suggesting that progesterone exerts its affects on TNF-α production and macrophage activation through inhibition of NFκB by IκB (87).
Progesterone has also been shown to inhibit NO production by murine macrophage cell lines (RAW 264.7 and J774) and bone marrow-derived macrophages, as measured by nitrite accumulation in culture medium. This effect has been shown to be due to a reduction in transcription, as progesterone-treated cells stimulated with IFN-γ and LPS had reduced levels of iNOS mRNA transcripts compared with nontreated cells. The influence of progesterone on iNOS expression was also evident in RAW 264.7 cells transfected with an iNOS gene promoter-luciferase reporter gene construct. Luciferase activity was reduced in these cells when stimulated with IFN-γ and LPS in the presence of progesterone (86)
Mast Cells
Mast cells have high-affinity estrogen receptors (102). Estrogen (17β-estradiol) can augment histamine and serotonin release from rat peritoneally derived mast cells following stimulation by the mast cell secretagogue compound 48/80 or the neuropeptide substance P. This effect is specific, as estrogen had no effect on mast cell degranulation following cross-linking of the Fcɛ receptor (FcɛR). In addition, the ability of estrogen to augment mast cell release of histamine and serotonin in response to 48/80 was abrogated by addition of the estrogen antagonist tamoxifen (151). In contrast to the effects of estrogen, testosterone inhibited mast cell secretion of histamine and serotonin following stimulation by compound 48/80 or neuropeptide substance P (151). Similarly, estrogen increases serotonin release in response to carbachol in rat bladder mast cell cultures (135).
Eosinophils
Eosinophils have both estrogen and progesterone receptors (24). Estrogen has been shown to increase the degranulation of human blood eosinophils at high doses both in vivo and in vitro. (132). Similarly, a combination of estrogen and progesterone was able to significantly enhance human eosinophil degranulation (56). Other studies have found that estrogen treatment of eosinophils was capable of enhancing their adhesion to human mucosal microvascular endothelial cells, whereas testosterone reduced this effect (56).
Dendritic Cells
No information is available on the effects of estrogens on dendritic cell function. However, the antiestrogens toremifene and tamoxifen have been shown to inhibit the IL-4- and granulocyte-macrophage colony-stimulating factor-dependent differentiation of monocytes into dendritic cells. CD14 expression is lost following incubation with antiestrogens, and these cells have reduced ability both to produce IL-12 in response to CD40 ligation and to induce proliferation of T cells (72). As tamoxifen is a partial agonist of estrogen, the effects of estrogen itself on dendritic cells warrant investigation.
Topical application of testosterone propionate can reduce the sensitivity of some humans to type IV hypersensitivity reactions to nickel as measured by patch testing. A reduction in the density of CD1+ dendritic cells in the epidermis was noted in patients responsive to this treatment but not in unresponsive patients (45).
Lymphocytes
Sex hormones have been widely reported to influence not only the development and maturation of T and B cells but also their state of activation (reviewed in references 34, 126, and 133). However, many of the properties attributable to sex hormone influences are not a result of their acting directly on lymphocytes, but arise because of their effects on cells of the innate immune response and on antigen-presenting cells (APC). Thus, for example, gonadal androgens at physiological levels act by inhibiting macrophage IL-1, IL-6, and TNF-α production and thus affect T cells indirectly, while adrenal androgens increase IL-2 and IFN-γ production by T cells directly (33). Also, while it is well documented that testosterone inhibits immunoglobulin M (IgM) and IgG production, a recent study using human peripheral blood mononuclear cells (PBMC) has demonstrated that this effect may be mediated indirectly by testosterone's inhibiting monocyte-derived IL-6 production (64). Furthermore, in a recent study, Taube et al. (143), using T-cell-reconstituted SCID mice, have demonstrated that APC and not T cells are the target for estrogen in suppressing oxazalone-induced T-cell-dependent delayed-type hypersensitivity. However, that sex hormones can influence lymphocytes directly is unquestionable, and while cytosolic and nuclear receptors have been demonstrated, recent elegant studies using fluorescein isothiocyanate- and bovine serum albumin-labeled estrogen and testosterone, confocal laser scanning microscopy, and fluorescence-activated cell sorting analysis have revealed functional testosterone and estrogen receptors not only intracellularly, but also in the plasma membrane of CD4+ and CD8+ T cells (17, 18).
Sex hormones have long been known to affect the structure and function of the thymus as well as lymphopoeisis in this and other tissues. However, many of the observations often appear to be contradictory. Thus, castration of young adult rats before maturity, though not afterwards, resulted in an increase in CD4+ and CD8+ cells as well as CD4+ CD8+ populations (65). Castration of adult male C57BL/6 mice, however, resulted in an increase in thymus weight but a reduction in CD8+ T cells (100, 101). Testosterone replacement reduced thymus weight and increased CD4+ and CD8+ populations, but primarily CD8+ T cells. These effects have been shown to be mediated by fluctuations in transforming growth factor beta (TGF-β). Pregnancy is also associated with a drop in thymus weight and a reduction in the number of CD4+ and CD8+ cells as well as B-cell lymphopoeisis (126). A role for estrogen but not progesterone in these events was initially suggested by the studies of Rijsinghani et al. (111). These workers found that estrogen but not progesterone could reduce thymus weight and deplete the CD4+ and CD8+ populations. While the earlier population of CD44+ CD25− cells was present following estrogen treatment, mature populations of CD44+ CD25+, CD44− CD25+, and CD44− CD25− cells were severely depleted or absent. Oopherectomy, on the other hand, did not alter the thymic T-cell population, suggesting that while estrogen may inhibit T-cell maturation, its absence does not promote this process. Nevertheless, further studies using progesterone receptor null mice question these findings, as these studies demonstrated that local expression of these receptors in the thymic stromal cells was necessary to block T-cell development at the early CD3− CD44+ CD25+ stage (144). The use of these mice as well as estrogen receptor-deficient mice (136) will prove invaluable in coming years in characterizing the effects of these hormones in the development, maturation, and function of the immune response.
In humans, the manifestations of hormone-induced lymphocyte modulation of the immune response are most clearly highlighted during either pregnancy or disease states, such as those associated with autoimmunity, malignancies, and infectious diseases. The various reported effects of androgens, estrogens, and progesterones associated with these conditions on lymphocytes and their functions are summarized below.
Estrogen has been shown to increase B-cell activity and antibody production (97) and also to promote IL-2-driven B-cell production of IgM and IgG in spleen cells while downregulating IgG production by normal blood B cells (38). The downregulatory effects of estrogen on T-cell development in the thymus have been discussed above, but in addition this hormone has also been shown to deplete T cells, particularly CD8+ T cells, from a number of tissues, including the genital mucous membranes and lymphoid tissues of the gut (23). The ability of this hormone to induce T-cell apoptosis may be significant in some of these properties (53). However, in direct contrast to its reported ability to inhibit T-cell development in the thymus, estrogen promotes the extrathymic development of “forbidden” T-cell clones in the liver associated with autoimmune disease (94, 95).
In studies involving T-cell-dependent inflammatory processes, estrogen was found to be regulatory and not only to reduce the number of cells at the site of inflammation and draining lymph nodes but also to reduce the expression of the activation markers CD40, CD44, CD69, and IL-2 receptor alpha (118). Furthermore, the levels of IL-2 and IFN-γ expression were downregulated and those of IL-4 and IL-10 were upregulated (119). While others have also shown upregulation of Th2 cytokines such as IL-5 and downregulation of Th1 cytokines such as IL-2 (1, 153), further studies have suggested that estrogen could, under certain circumstances, promote a type 1 response. For example, the promoter region of the IFN-γ gene has an estrogen response element, and increased levels of mRNA transcripts for this gene have been found in estrogen-stimulated cells (42). Furthermore, estrogen treatment of antigen- and anti-CD3-stimulated CD4+ T cells has been demonstrated to promote IFN-γ as well as IL-10 production, with variable effects on TNF-α and TNF-β but no effect on IL-4 or TGF-β (31, 52).
The general consensus is that progesterone is generally anti-inflammatory, inhibiting the development of a type 1 response while promoting a Th2 response. T-cell development in the thymus is blocked by progesterone (144), which has been shown to inhibit T-cell activity by blocking calcium-activated potassium channels (37). Progesterone not only promotes antigen- and anti-CD3-induced CD4+ T-cell IL-4 production while having no effect on IFN-γ, TGF-β, or TNF-α (31), it can also promote the expression of CD30 (a surface marker consistently found on Th1 but not Th2 cells) as well as the production of IL-4 and IL-5 by established Th1 clones (105). IL-4 and progesterone together also promote the production of the Th2-associated leukemia inhibitory factor, which is essential for embryo implantation (106). Progesterone has also been shown to induce an NK cell-inhibitory factor from T cells (139). Furthermore, progesterone induces a significant increase in the γ1.4δ1 T-cell population during pregnancy (10, 107). Through IL-10 production, these cells are thought to induce suppression of NK cell activity and IL-12 production. Finally, Vassiliadou et al. (149) demonstrated that progesterone does not affect the expression of the chemokine receptors CCR5 and CXCR4 on resting T cells but downregulates their expression on activated cells.
Studies involving gonadectomy and hormone replacement have shown androgens to generally deplete CD4+ cells while promoting the production of CD8+ cells (66, 100). Androgens affect the generation of the mature T-cell repertoire in the thymus (99) while downregulating IgM and IgG production by PBMC (64). Testosterone reduces the number of T-cell receptor-positive extrathymic “forbidden” T cells (67) and promotes the production of γδ type 1-inducing T cells (60). With regard to cytokine production, androgens or their precursors have been shown to downregulate IL-4, IL-5, and IFN-γ (7, 12) and upregulate IL-2, IL-10, and TGF-β (12, 101, 138).
INFLUENCE OF HORMONES ON PROTOZOAN PARASITIC DISEASES
Toxoplasma gondii
T. gondii infection is generally initiated by ingestion of either the tissue cyst stage, found in the meat of infected animals, or the oocyst stage, released in the feces of infected cats (30). Adult-acquired toxoplasmosis is normally mild to asymptomatic, but desease can be severe in the immunosuppressed (25, 80). In addition, the ability of sex- and pregnancy-associated hormones to influence the severity of T. gondii infection is of particular public health interest due to the ability of this parasite to cause congenital disease if infection occurs during pregnancy (25).
There is currently considerable evidence that steroid hormones affect the course of toxoplasmosis in humans and mice. Henry and Beverley (58) were the first to demonstrate differences in the immune and inflammatory responses of male and female mice following infection with T. gondii. In these studies, female mice developed more severe brain inflammation than male mice following infection. Moreover, a direct role for sex hormones was demonstrated in experiments which found that gonadectomy increased resistance, whereas estrogen administration exacerbated disease in mice (68, 70). Similarly, simultaneous gonadectomy and estrogen treatment predisposed guinea pigs to increased parasite burdens compared with nontreated control animals (69).
Most cases of noncongenitally acquired toxoplasmosis are asymptomatic in healthy humans, but lymphadenopathy is a possible clinical symptom. One study, carried out some time ago, found that although the incidence of T. gondii infection was similar in males and females, disease manifestations varied according to gender and age. In those under 15 years of age, lymphadenopathy was more frequently observed in males than in females. However, in sexually mature adults (over 25 years of age), lymphadenopathy was more frequently observed in females (20). T. gondii infection can also be an opportunistic infection of immunocompromised individuals, such as those infected with human immunodeficiency virus (HIV). A frequent manifestation of disease is encephalitis. In a study of nonhomosexual, HIV-positive Europeans, toxoplasmic encephalitis was found to be a more frequent AIDS-defining disease in females than in males (104). These observations support a detrimental role for female hormones during the course of T. gondii infection.
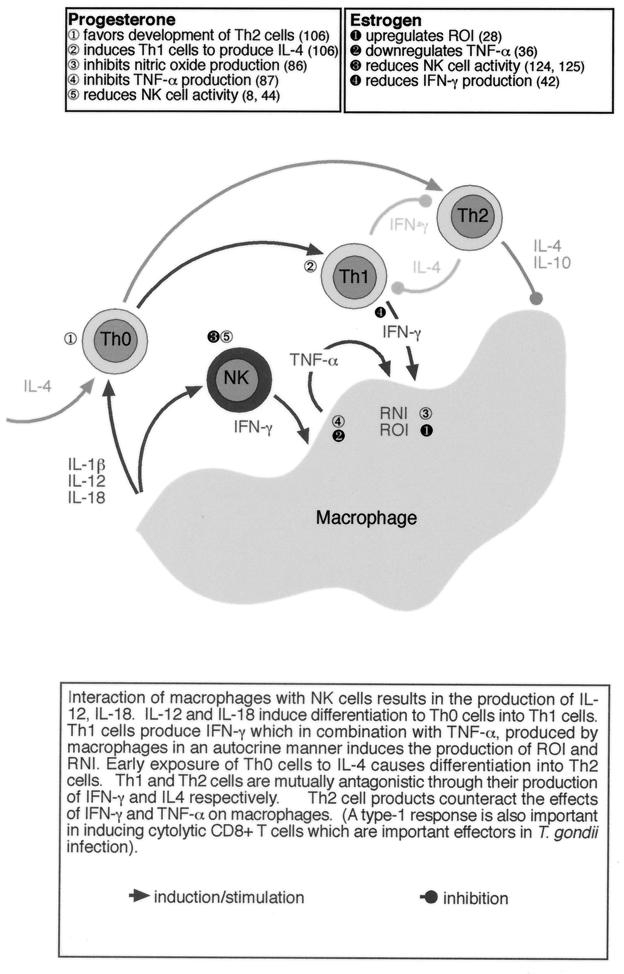
In pursuit of an immunological basis for the sexual dichotomy in the severity of T. gondii infection, we have studied this disease in the murine model (112, 152). Immunological control of T. gondii is complex and involves elements of both the innate and adaptive responses (4). Figure 1 summarizes the development of the immune response to an intracellular parasite such as T. gondii and the possible interaction of sex-and pregnancy-associated hormones.
FIG. 1.
Influence of sex- and pregnancy-associated hormones on the development of an immune response against intracellular protozoan parasites such as T. gondii and L. mexicana. ROI, reactive oxygen intermediates; RNI, reactive nitrogen intermediates.
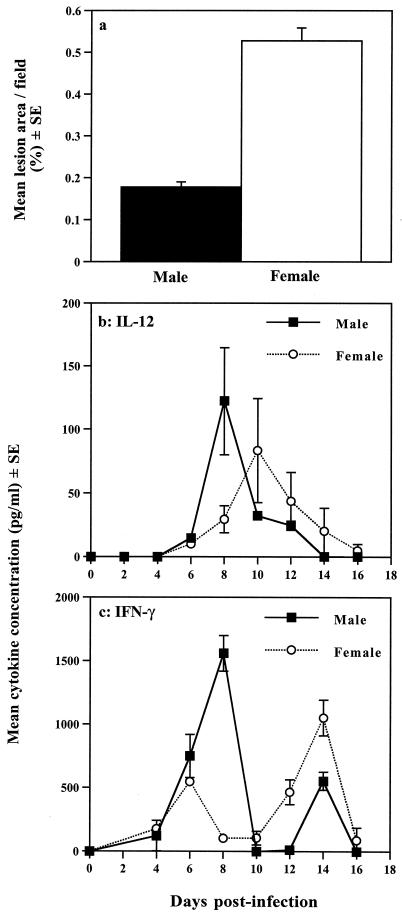
Early immunity with T. gondii has been shown to be dependent on macrophages and NK cells, although neutrophils and dendritic cells may perform similar roles to macrophages (4, 62, 129). Following sensitization with T. gondii, macrophages produce IL-12, which, together with IL-1β, IL-18, and TNF-α, stimulates NK cells to produce IFN-γ. IFN-γ acts in synergy with the TNF-α to induce the expression of iNOS, which results in the production of nitric oxide, which has been shown to kill intracellular T. gondii (Fig. 1). The importance of this system is evident from studies of T. gondii infection in SCID mice, which lack functional T and B cells but survive for as long as 12 to 20 days following oral infection (152). We have found that female SCID mice are more susceptible to T. gondii infection than male mice, as demonstrated by reduced time to death and increased brain pathology (Fig. 2a) (152).
FIG. 2.
Innate resistance to T. gondii is influenced by gender. (a) Determination of the area of brain lesions in T. gondii-infected male and female SCID mice. Chalkley counting (a microscpic method of determining area) of histological sections of brain tissue derived from animals at day 15 postinfection revealed that brains from female animals contained significantly greater lesion areas than those of their male counterparts. Female lesion area, 0.527%; male lesion area, 0.177%; P < 0.01. (b and c) Comparison of innate immunity-associated cytokines in male and female SCID mice infected with T. gondii. (b) Plasma samples from infected animals analyzed for IL-12 expression revealed significant differences between males and females. IL-12 plasma concentrations peaked at day 8 postinfection in males (122.3 ± 42.2 pg/ml) and day 10 in females (83.3 ± 40.8 pg/ml). The level of IL-12 in male samples was significantly higher at day 8 postinfection compared with females (P < 0.03). (c) Similar measurements of IFN-γ levels revealed that male SCID mice produced significantly higher amounts of this cytokine early after infection than females. At day 8, male IFN-γ plasma levels were 1,560 ± 140 pg/ml, compared with 104 ± 10.4 pg/ml in females (P < 0.001). This pattern of cytokine expression was consistently observed in two subsequent experiments. Statistical analyses were performed by the Mann-Whitney U test. For full details of experimental procedures, see reference 152. Reproduced with permission from reference 152.
These differences in susceptibility correlate with functional differences in the immune response, with male mice producing IL-12 and IFN-γ earlier and in greater quantities than female mice (Fig. 2b and c) (152). While the effect of hormones on IL-12 has not been widely examined, estrogen has been shown to affect IFN-γ production through an estrogen response element present on the gene's promoter (42). In addition, the generally inhibitory effects of estrogen and progesterone on macrophage function, most notably nitric oxide production, could also contribute to the comparative susceptibility of female SCID mice to T. gondii infection (28, 36, 61, 86, 87).
Long-term protection is mediated primarily by CD8+ T cells, with CD4+ Th1 cells playing a critical role through production of IFN-γ and IL-2, required for the expansion of CD8+ cytotoxic T lymphocyte cells (26, 46, 47, 103). Studies by us with the immunologically intact BALB/K mouse demonstrated that differences in mortality between the sexes corresponded to differences in both the innate and adaptive immune responses against T. gondii (113). Male mice produced more TNF-α during the early stages of infection than female mice. In addition, IFN-γ production was delayed in female compared with male mice. Moreover, the delayed production of IFN-γ in female mice was simultaneous with the production of IL-10, a cytokine that would antagonize the effects of IFN-γ (113). Together, these studies demonstrate that not only does gender influence the course of T. gondii infection in mice, but this influence involves gender-dependent differences in both the innate and adaptive immune responses.
Levels of many sex hormones, most notably estrogens and progesterone, are vastly increased during pregnancy, and consequently their effects on the immune system can be profound. As mentioned previously, the normal physiological role of these changes would appear to be to protect the developing fetus from the mother's immune response. Although this hormonal manipulation of the immune system serves to prevent the fetus from being rejected, it also has consequences for parasitic infection.
The ability of pregnancy to affect the immune system and indeed of the immune system to affect pregnancy has two important consequences for parasitic infection. First, pregnancy will favor the survival of many parasites that require a type 1 response to control them. Second, parasitic infections that induce a strong type 1 response will adversely affect pregnancy. Both of these scenarios have been demonstrated with the protozoan parasites T. gondii and Leishmania major.
Consequently, it has been demonstrated that pregnant mice are more susceptible to infection with T. gondii and show higher mortality than similarly infected nonpregnant female mice (130). This increased susceptibility of pregnant mice to T. gondii is associated with a reduced type 1 capacity, as demonstrated by a decreased capability to produce IFN-γ (82, 130). Moreover, administration of cytokines associated with type 1, cells such as IFN-γ and IL-2, to pregnant mice infected with T. gondii increases their survival (131). In addition to changes in the Th1-Th2 balance, many other immunological changes that occur during gestation would generally favor parasite survival: notably, reduced NK cell activity, suppression of macrophage function, and inhibition of CD8+ cytotoxic T-cell activity (see previous sections).
The extent to which the immune response to T. gondii plays an adverse role during pregnancy is controversial. Indeed, the ability of the immune response to prevent congenital transmission is well documented in mice, sheep, and humans (25, 27, 112). Chronically infected pregnant humans, although harboring tissue cysts throughout their body, generally do not transmit disease to their offspring. Similarly, chronically infected BALB/c mice do not transmit disease to their pups even if they are rechallenged with T. gondii during pregnancy (112). However, at which period during pregnancy a woman is infected will determine the fate of the pregnancy in terms of both abortion and congenital transmission (25). If infection occurs in the first trimester, when hormone levels are low and there is little Th2 bias, the chance of transmission to the fetus is low, although the chance of abortion is high. Conversely, infection during the third trimester, when there is a strong Th2 bias, is unlikely to induce abortion but more frequently results in congenital transmission. There is every likelihood that the Th1 response induced early during T. gondii infection will induce abortion early in pregnancy. In contrast, during the late stages of pregnancy, the strong Th2 bias and the diminished NK cell, macrophage, and CD8+ T-cell function may facilitate parasite survival and increase the likelihood of congenital transmission.
Plasmodium
The influence of sex- and pregnancy-associated hormones on the incidence and severity of human Plasmodium infection has not been extensively studied. The incidence of infection appears to be similar in males and females (150), although females have been reported to have higher mortality rates (71). The influence of sex- and pregnancy-associated hormones is, however, clearly evident during pregnancy. Plasmodium infection, most notably Plasmodium falciparum, has been shown to have an adverse effect on the outcome of pregnancy in humans. Congenital transmission of Plasmodium is rare, in contrast to the relatively high rate of congenital T. gondii transmission (91). However, fetus loss and infant mortality due to low birth weight are frequent consequences of Plasmodium infection (85). The reason for this is likely to be multifactorial and include the ability of the parasite to bind to the placenta and the immunosuppressive effects of maternal cortisol produced during the later stages of pregnancy (85). However, there is now evidence that infection with P. falciparum during pregnancy induces a type 1 response in the placenta, as opposed to the type 2 response found in normal placentas (43). Specifically, malaria was found to elicit IFN-γ, IL-2, and TNF-α production while decreasing IL-10 production in the placentas of malaria-infected individuals. However, a further study that examined in vitro cytokine production by intervillous blood mononuclear cells found that the ability of these cells to produce IFN-γ in response to malarial antigen was associated with a better prognosis (90). The apparent contradictions between these studies may reflect the different methods used in each study. Notably, the latter study was performed in vitro and, as a consequence, in the absence of hormones during antigen stimulation. The effects of Plasmodium infection on pregnancy are also observed in rodent models, where infection of pregnant mice with Plasmodium berghei is associated with premature delivery, reduced fetus weight, and slower growth (2).
Pregnant women are also at greater risk from malaria, with a higher incidence of infection and more severe disease manifestations, including mortality (71, 85). The increased susceptibility of pregnant women to malaria is more evident in primaparae than multiparae. A possible explanation for this may be the more pronounced reduction in placental and peripheral lymphocyte proliferative responses in primaparid compared with multiparid women. This difference may be due to greater levels of circulating corticosteroids in this group of women (109). Nonhormonal mechanisms may also account for the decreased susceptibility of multiparid women and include the presence of cytoadherence-blocking antibodies in this group of women (36a). These observations are again confirmed in murine models, as spleen cells from pregnant P. berghei-infected mice are less responsive to malarial antigens than those from nonpregnant mice, an effect that has again been attributed to steroid hormones (148).
While the influence of gender itself on the severity of malaria in humans remains a matter of contention, the impact is clearly evident in certain rodent models of disease. Following infection with Plasmodium chabaudi, female C57BL/10 mice self-eure, whereas male mice do not (16). The susceptibility of male mice is dependent on testosterone, as castration prior to infection makes male mice resistant, whereas testosterone administration to females impairs their ability to self-cure (16, 19). However, additional studies have demonstrated that estrogen can also cause immunosuppression and impair resistance in the same model (14). Furthermore, testosterone-induced susceptibility appears to be due to hormonal imprinting, as testosterone treatment of female mice for 3 weeks followed by 9 weeks without treatment, during which time testosterone falls to pretreatment levels, continues to make female mice susceptibile to challenge with P. chabaudi (19). Recently, a novel putative transmembrane protein (IAP30) which is induced on murine spleen cells during P. chabaudi infection in the presence of testosterone has been identified (75, 76). The importance of gender-influenced susceptibility to P. chabaudi is emphasized in experiments that demonstrate that the efficacy of vaccination against this parasite is as high as 90% in female mice but only 55% in males. Moreover, testosterone treatment of females before vaccination reduces the efficacy of vaccination to approximately 34% (154).
The mode of action of testosterone in this disease model appears not to be through classical androgen receptors, as androgen receptor antagonists were unable to prevent testosterone-induced susceptibility of female mice (16). Testosterone-induced susceptibility to P. chabaudi can be adoptively transferred by splenic T cells, which do not possess classical androgen receptors (13). In contrast, estrogen-induced immunosuppression cannot be transferred with spleen cells (15). T cells have recently been shown to have nonclassical but nonetheless functional testosterone receptors on their surface, providing a likely explanation for their implied modulation with testosterone (17). This type of receptor would appear not to be limited to T cells, as similar receptors have been identified on a murine macrophage cell line and their action was found to be dependent on ATP and G proteins (17).
Together, these results demonstrate a role for sex- and pregnancy-associated hormones in modulating resistance to Plasmodium infection and provide a possible explanation for the increased susceptibility of pregnant humans to malaria.
Leishmania
The leishmaniases comprise a group of diseases that display a wide range of clinical manifestations in humans, depending in part on the parasite species initiating infection and on various factors relating to the general health and genetic makeup of the host (reviewed in reference 5). Consequently, there are numerous observations demonstrating that age as well as gender can influence the course of infection with Leishmania spp. and that both male and female hormones can mediate sex-determined resistance and susceptibility to infection (reviewed in references 6 and 114). As studies on Leishmania have played a major role in defining the Th1-Th2 paradigm of resistance and susceptibility to intracellular infection, these observation are particularly intriguing, as they indicate that male and female sex hormones influence the Th1-Th2 balance.
The general consensus is that protective immunity against Leishmania spp. is dependent on IL-12 from macrophages or dendritic cells driving IFN-γ production by elements of both the innate (NK cell) and acquired (CD4+ Th1 cell) immune responses (reviewed in reference 5). IFN-γ mediates protection by upregulating macrophage inducible nitric oxide synthase (NOS2) expression and NO production, which is microbicidal for the parasite. Conversely, the immunological pathways leading to nonhealing disease are less well understood and at present the subject of some debate. While some studies suggest a crucial role for the Th2 response and IL-4 in downregulating IFN-γ production and activity, other studies suggest that it is the failure to produce IL-12 and thus the inability to generate a Th1 response that results in disease susceptibility. Nevertheless, whatever the specific processes involved in developing a nonhealing Leishmania infection, the traditional observation that males develop stronger cell-mediated (Th1) responses than females while females develop stronger humoral (Th2) responses than males suggests that males would be on the whole more resistant to infection with this organism. Indeed, some experimental as well as some epidemiological studies have observed more severe disease in females than males. Male B10/129 and DBA/2 mice are more resistant than females to cutaneous infection with Leishmania major (3, 51), while Leishmania tropica infections have been shown to be more persistent in the human female population (55). That sex hormones may influence such gender-associated responses is well documented (6, 88). Comparatively recent studies, for example, have shown that pregnancy, which is associated with increased estrogen, particularly increased progesterone levels, biases the maternal immune response towards a Th2 type and increases the susceptibility of female C57BL/6 mice to L. major (73). Conversely, a Th1 response in these mice against L. major increased implantation failure and fetal resorption (74). These studies emphasize not only that sex- and pregnancy-associated hormones influence the course of parasitic infection, but that parasitic infection can also have profound effects on pregnancy.
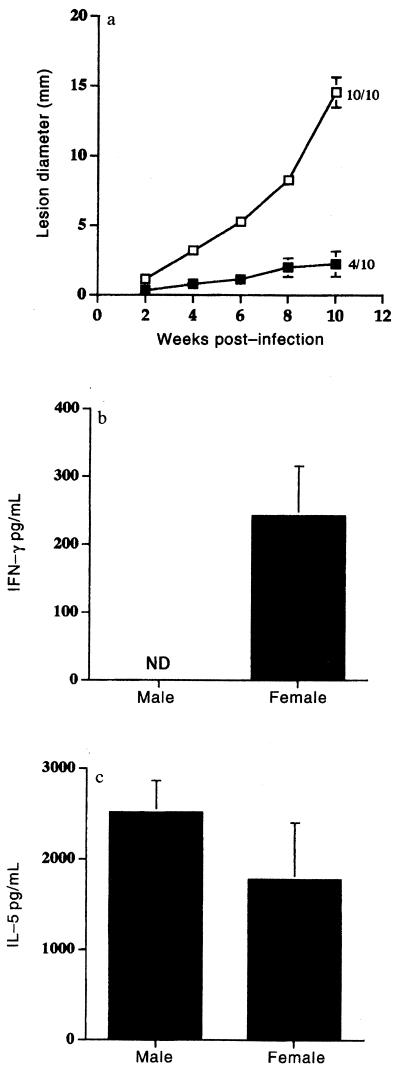
Despite the number of examples quoted above in which females are found to be more susceptible than males to infection with Leishmania spp., the incidence of visceral leishmaniasis, whether caused by Leishmania donovani in humans (35, 63) or Leishmania infantum in dogs (156) is higher in males than females. Similarly, male BALB/c and DBA/2 mice are more susceptible to systemic infection with L. major than females (88). In these studies, orchidectomy was found to increase the resistance of males, while testosterone increased the susceptibility of females. In addition, we have also demonstrated that female DBA/2 mice are more resistant than males to cutaneous infection with L. mexicana (3). Furthermore, gonadectomy and hormone transplant experiments (6) have shown that this resistance is at least in part estrogen dependent and associated with the ability of females but not males to produce IFN-γ and a Th1 response (Fig. 3) (3, 120, 121). Interestingly, a human study on L. mexicana has also shown a clear dichotomy in the responses of males and females. Delayed-type hypersensitivity responses and thus Th1-like responses were greater in females, while Th2-associated IgE levels were greater in males (83). Of significance, resolution of infection was more rapid in females. Nevertheless, these observations certainly do not follow the general supposition that males are capable of mounting stronger cell-mediated responses than females. However, what may be significant is that while estrogen has been shown to upregulate the expression of IFN-γ mRNA and IFN-γ production (54), testosterone has been shown to downregulate T-cell production of this cytokine (7).
FIG. 3.
(a) Lesion growth in the shaven rumps of male (open squares) and female (solid squares) DBA/2 mice infected with L. mexicana. The values represent mean number of animals with lesions at week 10 postinfection/number in group. Similar results were obtained in three independent experiments. Error bars show standard error. (b) IFN-γ and (c) IL-5 production by inguinal lymph node cells from L. mexicana-infected male and female DBA/2 mice at week 10 postinfection following stimulation with Leishmania soluble antigen (20 μgml). ND, not detectable. Cytokine production from lymph node cells from individual mice was assayed separately, and four to five mice were used in each group. For full details of experimental procedures, see reference 120. Reproduced with permission from reference 120.
Resistance of female DBA/2 mice to L. mexicana has been demonstrated to be under the control of a single gene, designated Scl-2 and mapped to a region on mouse chromosome 4. This region shows homology with human 9p (115), for which Jak1 and Jak2 kinase are human candidates (22). These observations are of particular interest, as IFN-γ and IL-4 signaling through Jak1 and Jak2 is an important factor in regulating the immune response, including macrophage activation for the control of intracellular parasites (93). The molecular basis underlying the action of Scl-2 awaits clarification, and it is also a particularly intriguing problem how the expression of the resistance allele is influenced by the presence or absence of sex hormones. A better understanding of these mechanisms would not only clarify how various gender-dependent differences in the immune response to Leishmania arise, but help predict likely disease outcomes and the potential success of vaccination programs between the sexes. A recent vaccine trial carried out over a 2-year period using a vaccine comprising a combination of autoclaved L. major, Mycobacterium bovis BCG, and alum has shown boys to be better protected than girls of the same age (6 to 15 years of age at initiation of vaccination) (89, 128). As the normal incidence of cutaneous leishmaniasis in the trial region is comparable between boys and girls (128), it is unlikely, as the authors suggest, that gender-dependent social behavior influences the success of the vaccine. What may be more significant is that the causative agent of the vast majority of the infections in the region is L. tropica (128), which has been shown to be more persistent in females (55). This suggests an underlying greater susceptibility in this sex, and consequently, successfully vaccinating females could prove more difficult than males.
CONCLUSIONS
Given the reported importance of sex- and pregnancy-associated hormones in influencing the outcome of infectious disease as well as autoimmunity and malignancies, this is an area of research that is likely to grow. Undoubtedly the currently available sex hormone receptor-deficient mice (98) will prove invaluable tools in not only promoting such studies, but also elucidating and characterizing some of the networks linking the hormonal to the immune response. Current developments in molecular biology will shortly provide tissue-specific mutants that will allow specific hormonal effects to be examined in individual cell populations free of other pleiotrophic hormonal activities. On a more immediate practical note, it is evident that successful vaccination can vary with age and sex, and careful consideration should be given to these factors when designing and operating the most effective vaccine program for a particular disease.
ACKNOWLEDGMENTS
W.W. is a Wellcome Trust Temporary Lecturer. J.A. is currently on Wellcome Trust-sponsored research leave.
REFERENCES
- 1.Ahmed S A, Gogal R M, Walsh J E, Saunders G. Estrogen induces defects in T cell functions of mice. FASEB J. 1996;10:961. [Google Scholar]
- 2.Akingbade O A. Embryotoxic and growth retarding effects of malaria on pregnant mice. J Reprod Med. 1992;37:273–276. [PubMed] [Google Scholar]
- 3.Alexander J. Sex differences and cross-immunity in DBA/2 mice infected with L. mexicana and L. major. Parasitology. 1988;96:297–302. doi: 10.1017/s0031182000058303. [DOI] [PubMed] [Google Scholar]
- 4.Alexander J, Hunter C A. Immunoregulation during toxoplasmosis. Chem Immunol. 1998;70:81–102. doi: 10.1159/000058701. [DOI] [PubMed] [Google Scholar]
- 5.Alexander J, Satoskar A R, Russell D G. Leishmania species: models of intracellular parasitism. J Cell Sci. 1999;112:2993–3002. doi: 10.1242/jcs.112.18.2993. [DOI] [PubMed] [Google Scholar]
- 6.Alexander J, Stimson W H. Sex hormones and the course of parasitic infection. Parasitol Today. 1988;4:189–193. [Google Scholar]
- 7.Araneo B A, Dowell T, Diegel M, Daynes R A. Dihydrotestosterone exerts a depressive influence on the production of interleukin-4 (IL-4), IL-5, and gamma-interferon, but not IL-2 by activated murine T-cells. Blood. 1991;78:688–699. [PubMed] [Google Scholar]
- 8.Baley J E, Schacter B Z. Mechanisms of diminished natural killer cell activity in pregnant women and neonates. J Immunol. 1985;134:3042–3048. [PubMed] [Google Scholar]
- 9.Bancroft G J. The role of natural killer cells in innate resistance to infection. Curr Opin Immunol. 1993;5:503–510. doi: 10.1016/0952-7915(93)90030-v. [DOI] [PubMed] [Google Scholar]
- 10.Barakonyi A, Polgar B, SzekeresBartho J. The role of gamma/delta T-cell receptor-positive cells in pregnancy, part II. Am J Reprod Immunol. 1999;42:83–87. [PubMed] [Google Scholar]
- 11.Baral E, Nagy E, Berczi I. Modulation of natural killer cell mediated cytotoxicity by tamoxifen and estradiol. Cancer. 1995;75:591–599. doi: 10.1002/1097-0142(19950115)75:2<591::aid-cncr2820750224>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 12.Bebo B F, Schuster J C, Vandenbark A A, Offner H. Androgens alter the cytokine profile and reduce encephalitogenicity of myelin-reactive T cells. J Immunol. 1999;162:35–40. [PubMed] [Google Scholar]
- 13.Benten W P, Bettenhaeuser U, Wunderlich F, Van Vliet E, Mossman H. Testosterone-induced abrogation of self-healing of Plasmodium chabaudi malaria in B10 mice:mediation by spleen cells. Infect Immun. 1991;1991:4486–4490. doi: 10.1128/iai.59.12.4486-4490.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benten W P, Wunderlich F, Mossman H. Plasmodium chabaudi: estradiol suppresses acquiring, but not once acquired immunity. Exp Parasitol. 1992;75:240–247. doi: 10.1016/0014-4894(92)90184-c. [DOI] [PubMed] [Google Scholar]
- 15.Benten W P, Wunderlich F, Hermann R, Kuhn-Velten W N. Testosterone-induced compared with oestradiol-induced immunosuppression against Plasmodium chabaudi malaria. J Endocrinol. 1993;139:487–494. doi: 10.1677/joe.0.1390487. [DOI] [PubMed] [Google Scholar]
- 16.Benten W P, Wunderlich F, Mossman H. Testosterone-induced suppression of self-healing Plasmodium chabaudi: and effect not mediated by androgen receptors? J Endocrinol. 1992;135:407–413. doi: 10.1677/joe.0.1350407. [DOI] [PubMed] [Google Scholar]
- 17.Benten W P M, Lieberherr M, Giese G, Wrehlke C, Stamm O, Sekeris C E, Mossmann H, Wunderlich F. Functional testosterone receptors in plasma membranes of T cells. FASEB J. 1999;13:123–133. doi: 10.1096/fasebj.13.1.123. [DOI] [PubMed] [Google Scholar]
- 18.Benten W P M, Lieberherr M, Giese G, Wunderlich F. Estradiol binding to cell surface raises cytosolic free calcium in T cells. FEBS Lett. 1998;422:349–353. doi: 10.1016/s0014-5793(98)00039-8. [DOI] [PubMed] [Google Scholar]
- 19.Benten W P M, Ulrich P, KuhnVelten W N, Vohr H W, Wunderlich F. Testosterone-induced susceptibility to Plasmodium chabaudi malaria: persistence after withdrawal of testosterone. J Endocrinol. 1997;153:275–281. doi: 10.1677/joe.0.1530275. [DOI] [PubMed] [Google Scholar]
- 20.Beverley J K A, et al. Age-sex distribution of various diseases with particular reference to toxoplasmic lymphadenopathy. J Hyg. 1976;76:215–228. doi: 10.1017/s002217240005511x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biron C A. Activation and function of natural killer cell responses during viral infections. Curr Opin Immunol. 1997;9:24–34. doi: 10.1016/s0952-7915(97)80155-0. [DOI] [PubMed] [Google Scholar]
- 22.Blackwell J M, Barton C H, White J K, Roach T I, Shaw M A, Whitehead S H, Mock B A, Searle S, Williams H, Baker A M. Genetic regulation of leishmanial and mycobacterial infections: the Lsh/Ity/Bcg gene story continues. Immunol Lett. 1994;43:99–107. doi: 10.1016/0165-2478(94)00161-8. [DOI] [PubMed] [Google Scholar]
- 23.Boll G, Reimann J. Oestrogen treatment depletes extrathymic T cells from intestinal lymphoid tissues. Scan J Immunol. 1996;43:345–350. doi: 10.1046/j.1365-3083.1996.d01-41.x. [DOI] [PubMed] [Google Scholar]
- 24.Bonini S, Lambiase A, Schiavone M, Centofanti M, Palma L A. Estrogen and progesterone receptors in vernal keratoconjunctivitis. Ophthalmology. 1995;102:1374–1379. doi: 10.1016/s0161-6420(95)30861-5. [DOI] [PubMed] [Google Scholar]
- 25.Boyer K, McLeod R. Toxoplasmosis. 1st ed. Vol. 286. New York, N.Y: Churchill Livingstone; 1998. [Google Scholar]
- 26.Brown C R, Hunter C A, Estes R G, Beckmann E, Forman J, David C, Remington J S, McLeod R. Definitive identification of a gene that confers resistance against Toxoplasma cyst burden and encephalitis. Immunology. 1995;85:419–428. [PMC free article] [PubMed] [Google Scholar]
- 27.Buxton D. Ovine toxoplasmosis: a review. J Roy Soc Med. 1990;83:509–511. doi: 10.1177/014107689008300813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chao T C, Vanalten P J, Walter R J. Steroid sex-hormones and macrophage function — modulation of reactive oxygen intermediates and nitrite release. Am J Reprod Immunol. 1994;32:43–52. doi: 10.1111/j.1600-0897.1994.tb00877.x. [DOI] [PubMed] [Google Scholar]
- 29.Clark D A. Controversies in reproductive immunology. Clin Rev Immunol. 1991;11:215–247. [PubMed] [Google Scholar]
- 30.Cook G C. Toxoplasma gondii infection: a potential danger to the unborn fetus and AIDS sufferer. Q J Med. 1990;74:3–19. [PubMed] [Google Scholar]
- 31.Correale J, Arias M, Gilmore W. Steroid hormone regulation of cytokine secretion by proteolipid protein-specific CD4(+) T cell clones isolated from multiple sclerosis patients and normal control subjects. J Immunol. 1998;161:3365–3374. [PubMed] [Google Scholar]
- 32.Coulam C B, Goodman C, Roussev R G, Thomason E J, Beaman K D. Systemic CD56+ cells can predict pregnancy outcome. Am J Reprod Immunol. 1995;33:40–46. doi: 10.1111/j.1600-0897.1995.tb01136.x. [DOI] [PubMed] [Google Scholar]
- 33.Cutolo M, Sulli A, Barone A, Seriolo B, Accardo S. The role of androgens in the pathophysiology of rheumatoid-arthritis. Fundamental Clin Immunol. 1995;3:9–18. [Google Scholar]
- 34.DaSilva J A P. Sex hormones and glucocorticoids: Interactions with the immune system. Ann N Y Acad Sci. 1999;876:102–118. doi: 10.1111/j.1749-6632.1999.tb07628.x. [DOI] [PubMed] [Google Scholar]
- 35.de Beer P, el Harith A, Deng L L, Semiao-Santos S J, Chantal B, van Grootheest M. A killing disease epidemic among displaced Sudanese population identified as visceral leishmaniasis. Am J Trop Med Hyg. 1991;44:283–289. doi: 10.4269/ajtmh.1991.44.283. [DOI] [PubMed] [Google Scholar]
- 36.Deshpande R, Khalili H, Pergolizzi R G, Michael S D, Chang M D Y. Estradiol down-regulates LPS-induced cytokine production and NPκB activation in murine macrophages. Am J Reprod Immunol. 1997;38:46–54. doi: 10.1111/j.1600-0897.1997.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 36a.Duffy P E, Fried M. Malaria during pregnancy: parasites, antibodies and chondroitin sulphate A. Biochem Soc Trans. 1999;27:478–482. doi: 10.1042/bst0270478. [DOI] [PubMed] [Google Scholar]
- 37.Ehring G R, Kerschbaum H H, Eder C, Neben A L, Fanger C M, Khoury R M, Negulescu P, Cahalan M D. A nongenomic mechanism for progesterone-mediated immunosuppression: inhibition of K+ channels, Ca2+ signaling, and gene expression in T lymphocytes. J Exp Med. 1998;188:1593–1602. doi: 10.1084/jem.188.9.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evagelatou M, Farrant J. Effect of 17-beta-estradiol on immunoglobulin secretion by human tonsillar lymphocytes in-vitro. J Steroid Biochem Mol Biol. 1994;48:171–177. doi: 10.1016/0960-0760(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 39.Faust Z, Laskarin G, Rukavina D, Szekeres-Bartho J. Progesterone-induced blocking factor inhibits degranulation of natural killer cells. Am J Reprod Immunol. 1999;42:71–75. [PubMed] [Google Scholar]
- 40.Ferguson M M, McDonald F G. Oestrogen as an inhibitor of human NK cell cytolyis. FEBS Lett. 1985;191:145–148. doi: 10.1016/0014-5793(85)81011-5. [DOI] [PubMed] [Google Scholar]
- 41.Forest M G. Pituitary gonadotropin and sex steroid secretion during the first two years of life. In: Grumbach M M, Sizonenko P C, Aubert M L, editors. Control of the onset of puberty. Baltimore, Md: Williams and Wilkins; 1990. pp. 451–477. [Google Scholar]
- 42.Fox H S, Bond B L, Parslow T G. Estrogen regulates the IFN-gamma promoter. J Immunol. 1991;146:4362–4367. [PubMed] [Google Scholar]
- 43.Fried M, Muga R O, Misore A O, Duffy P E. Malaria elicits type 1 cytokines in the human placenta: IFN-gamma and TNF-alpha associated with pregnancy outcomes. J Immunol. 1998;160:2523–2530. [PubMed] [Google Scholar]
- 44.Furukawa K, Itoh K, Okamura K, Kumagai K, Suzuki M. changes in NK cell activity during the oestrous cycle and pregnancy in mice. J Reprod Immunol. 1984;6:353–363. doi: 10.1016/0165-0378(84)90045-7. [DOI] [PubMed] [Google Scholar]
- 45.Galasso F, Altamura V, Sbano E. Effects of topical testosterone propionate on the positive nickel patch test. J Dermatol Sci. 1996;13:76–82. doi: 10.1016/0923-1811(95)00501-3. [DOI] [PubMed] [Google Scholar]
- 46.Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol. 1992;149:175–180. [PubMed] [Google Scholar]
- 47.Gazzinelli R T, Hakim F T, Hieny S, Shearer G M, Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991;146:286–292. [PubMed] [Google Scholar]
- 48.Gendron R L. Lipopolysaccharide-induced fetal resorption in mice is associated with intrauterine production of tumour necrosis factor-alpha. J Reprod Fertil. 1990;90:395–402. doi: 10.1530/jrf.0.0900395. [DOI] [PubMed] [Google Scholar]
- 49.Gendron R L, Baines M G. Infiltrating decidual natural killer cells are associated with spontaneous abortion in mice. Cell Immunol. 1988;113:261–267. doi: 10.1016/0008-8749(88)90025-1. [DOI] [PubMed] [Google Scholar]
- 50.Gendron R L, Farookhi R, Baines M G. Resorption of CBA/J × DBA/2 mouse conceptuses in CBA/J uteri correlates with failure if the feto-placental unit to suppress natural killer cell activity. J Reprod Fertil. 1990;89:277–284. doi: 10.1530/jrf.0.0890277. [DOI] [PubMed] [Google Scholar]
- 51.Giannini M S. Sex-influenced response in the pathogenesis of cutaneous leishmaniasis in mice. Parasite Immunol. 1986;8:31–7. doi: 10.1111/j.1365-3024.1986.tb00831.x. [DOI] [PubMed] [Google Scholar]
- 52.Gilmore W, Weiner L P, Correale J. Effect of estradiol on cytokine secretion by proteolipid protein-specific T cell clones isolated from multiple sclerosis patients and normal control subjects. J Immunol. 1997;158:446–451. [PubMed] [Google Scholar]
- 53.Gold R, Schmied M, Tontsch U, Hartung H P, Wekerle H, Toyka K V, Lassmann H. Antigen presentation by astrocytes primes rat T lymphocytes for apoptotic cell death — a model for T-cell apoptosis in vivo. Brain. 1996;119:651–659. doi: 10.1093/brain/119.2.651. [DOI] [PubMed] [Google Scholar]
- 54.Grasso G, Muscettola M. The influence of beta-estradiol and progesterone on interferon gamma production in vitro. Int J Neurosci. 1990;51:315–317. doi: 10.3109/00207459008999730. [DOI] [PubMed] [Google Scholar]
- 55.Greenblatt C L. The present and future of vaccination for cutaneous leishmaniasis. Prog Clin Biol Res. 1980;47:259–285. [PubMed] [Google Scholar]
- 56.Hamano N, Terada N, Maesako K, Numata T, Konno A. Effect of sex hormones on eosinophilic inflammation in nasal mucosa. Allergy Asthma Proc. 1998;19:263–269. doi: 10.2500/108854198778557773. [DOI] [PubMed] [Google Scholar]
- 57.Hanna N, Schneider M. Enhancement of tumour metastasis and suppression of natural killer cell activity by β-estradiol treatment. J Immunol. 1983;130:974–980. [PubMed] [Google Scholar]
- 58.Henry L, Beverley J K A. Age and sex differences in the response of lymph node post-capillary venules in mice infected with Toxoplasma gondii. J Exp Pathol. 1976;57:274–281. [PMC free article] [PubMed] [Google Scholar]
- 59.Hou J, Zheng W F. Effect of sex hormones on NK and ADCC activity of mice. Int J Immunopharmacol. 1988;10:15–22. doi: 10.1016/0192-0561(88)90145-2. [DOI] [PubMed] [Google Scholar]
- 60.Huber S A, Kupperman J, Newell M K. Hormonal regulation of CD4+ T-cell responses in coxsackievirus B3-induced myocarditis in mice. J Virol. 1999;73:4689–4695. doi: 10.1128/jvi.73.6.4689-4695.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hunt J S, Miller L, Roby K F, Huamg J, Platt J S, DeBrot B L. Female steroid hormones regulate production of pro-inflammatory molecules in uterine leukocytes. J Reprod Immunol. 1997;3:87–99. doi: 10.1016/s0165-0378(97)00060-0. [DOI] [PubMed] [Google Scholar]
- 62.Hunter C A, Chizzonite R, Remington J S. IL-1beta is required for IL-12 to induce production of IFN-gamma by NK cells: a role for IL-1beta in the T cell-independent mechanism of resistance against intracellular pathogens. J Immunol. 1995;155:4347–4354. [PubMed] [Google Scholar]
- 63.Jahn A, Lelmett J M, Diesfeld H J. Seroepidemiological study on kala-azar in Baringo District, Kenya. J Trop Med Hyg. 1986;89:91–96. [PubMed] [Google Scholar]
- 64.Kanda N, Tsuchida T, Tamaki K. Testosterone inhibits immunoglobulin production by human peripheral blood mononuclear cells. Clin Exp Immunol. 1996;106:410–415. doi: 10.1046/j.1365-2249.1996.d01-842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karapetrovic B, Kosec D, Leposavic G. Differential effects of castration in sexually immature and mature rats on the phenotypic profile of thymocytes. Acta Vet Beograd. 1996;46:271–275. [Google Scholar]
- 66.Kiess W, Liu L L, Hall N R. Lymphocyte subset distribution and natural-killer-cell activity in men with idiopathic hypogonadotropic hypogonadism. Acta Endocrinol. 1991;124:399–404. doi: 10.1530/acta.0.1240399. [DOI] [PubMed] [Google Scholar]
- 67.Kimura M, Tomita Y, Watanabe H, Sato S, Abo T. Androgen regulation of intra-thymic and extra-thymic T-cells and its effect on sex-differences in the immune-system. Int J Androl. 1995;18:127–136. doi: 10.1111/j.1365-2605.1995.tb00398.x. [DOI] [PubMed] [Google Scholar]
- 68.Kittas C, Henry L. Effect of gonadectomy and oestrogen administration on the response of lymph-node post-sapillary venules to infection with Toxoplasma gondii. J Pathol. 1978;127:129–136. doi: 10.1002/path.1711270305. [DOI] [PubMed] [Google Scholar]
- 69.Kittas C, Henry L. Effect of sex hormones on the immune system of guinea-pigs and on the development of toxoplasmic lesionsin non-lymphoid organs. Clin Exp Immunol. 1979;36:16–23. [PMC free article] [PubMed] [Google Scholar]
- 70.Kittas C, Henry L. Effect of sex hormones on the response of mice to infection with Toxoplasma gondii. B J Exp Pathol. 1980;61:590–600. [PMC free article] [PubMed] [Google Scholar]
- 71.Kochar D K, Thanvi I, Joshi A, Shubhakaran A, Agarwal N, Jain N. Mortality trends in falciparum malaria-effect of gender difference and pregnancy. J Assoc Physicians India. 1999;47:774–778. [PubMed] [Google Scholar]
- 72.Komi J, Lassila O. Nonsteroidal anti-estrogens inhibit the functional differentiation of human monocyte-derived dendritic cells. Blood. 2000;95:2875–2882. [PubMed] [Google Scholar]
- 73.Krishnan L, Guilbert L J, Russell A S, Wegmann T G, Mosmann T R, Belosevic M. Pregnancy impairs resistance of C57BL/6 mice to Leishmania major infection and causes decreased antigen-specific IFN-gamma response and increased production of T helper 2 cytokines. J Immunol. 1996;156:644–652. [PubMed] [Google Scholar]
- 74.Krishnan L, Guilbert L J, Wegmann T G, Belosevic M, Mosmann T R. T helper 1 response against Leishmania major in pregnant C57BL/6 mice increases implantation failure and fetal resorptions: correlation with increased IFN-gamma and TNF and reduced IL-10 production by placental cells. J Immunol. 1996;156:653–662. [PubMed] [Google Scholar]
- 75.Krucken J, SchmittWrede H P, MarkmannMulisch U, Wunderlich F. Novel gene expressed in spleen cells mediating acquired testosterone-resistant immunity to Plasmodium chabaudi malaria. Biochem Biophys Res Commun. 1997;230:167–170. doi: 10.1006/bbrc.1996.5876. [DOI] [PubMed] [Google Scholar]
- 76.Krucken J, Stamm O, Schmitt-Wrede H-P, Mincheva A, Lichter P, Wunderlich F. Spleen-specific expression of the malaria-inducible intronless mouse gene imap38. J Biol Chem. 1999;274:24382–24391. doi: 10.1074/jbc.274.34.24383. [DOI] [PubMed] [Google Scholar]
- 77.Laskarin G, Strbo N, Sotosek V, Rukavina D, Faust Z, Szekeres-Bartho J, Podack E R. Progesterone directly and indirectly affects perforin expression in cytolytic cells. Am J Reprod Immunol. 1999;42:312–320. doi: 10.1111/j.1600-0897.1999.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 78.Le N, Yousefi S, Vaziri N, Charandang G, Ocariz J, Cesario T. The effect of beta-estradiol, progesterone and testosterone on the production of human leukocyte derived interferons. J Biol Regul Homeost Agents. 1988;2:199–204. [PubMed] [Google Scholar]
- 79.Lin H, Mosmann T R, Guilbert L, Tuntipopipat S, Wegmann T G. Synthesis of T helper 2-type cytokines at the maternal fetal interface. J Immunol. 1993;151:4562–4573. [PubMed] [Google Scholar]
- 80.Luft B J, Hafner R, Korzun A H, Leport C, Antoniskis D, Bosler E M, Bourland D D, III, Uttamchandani R, Fuhrer J, Jacobson J, Morlat P, Vilde J L, Remington J S, Phillips C, Glauser M, Chave J, Clifford D, Powderly W, Klebert M, et al. Toxoplasmic encephalitis in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1993;329:995–1000. doi: 10.1056/NEJM199309303291403. [DOI] [PubMed] [Google Scholar]
- 81.Luft B J, Remington J S. Effect of pregnancy on augmentation of natural killer cell activity by Corynebacterium parvum and Toxoplasma gondii. J Immunol. 1984;132:2375–2380. [PubMed] [Google Scholar]
- 82.Luft B J, Remington J S. Effect of pregnancy on resistance to Listeria monocytogenes and Toxoplasma gondii infection in mice. Infect Immun. 1982;38:1164–1171. doi: 10.1128/iai.38.3.1164-1171.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lynch N R, Yarzabal L, Verde O, Avila J L, Monzon H, Convit J. Delayed-type hypersensitivity and immunoglobulin E in American cutaneous leishmaniasis. Infect Immun. 1982;38:877–881. doi: 10.1128/iai.38.3.877-881.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mandler R N, Seamer L C, Domalewski M D, Bankhurst A D. Progesterone but not estrogen depolarizes natural killer cells. Nat Immun. 1993;12:128–135. [PubMed] [Google Scholar]
- 85.Menendez C. Malaria during pregnancy: a priority area of malaria research and control. Parasitol Today. 1995;5:178–183. doi: 10.1016/0169-4758(95)80151-0. [DOI] [PubMed] [Google Scholar]
- 86.Miller L, Alley E W, Murphy W J, Russell S W, Hunt J S. Progesterone inhibits inducible nitric oxide synthase gene expression and nitric oxide production in murine macrophages. J Leukocyte Biol. 1996;59:442–450. doi: 10.1002/jlb.59.3.442. [DOI] [PubMed] [Google Scholar]
- 87.Miller L, Hunt J S. Regulation of TNF-alpha production in activated mouse macrophages by progesterone. J Immunol. 1998;160:5098–5104. [PubMed] [Google Scholar]
- 88.Mock B A, Nacy C A. Hormonal modulation of sex differences in resistance to Leishmania major systemic infections. Infect Immun. 1988;56:3316–3319. doi: 10.1128/iai.56.12.3316-3319.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Modabber F. EC Euroleish/WHO-TDR Workshop. Second generation vaccines against leishmaniasis: from antigen discovery to clinical trial. Geneva, Switzerland: World Health Organization; 2000. First generation leishmaniasis vaccines in clinical development: moving but what next? pp. 35–40. [Google Scholar]
- 90.Moore J M, Bernard L, Nahlen B L, Misore A, Lal A A, Udhayakumar V. Immunity to placental malaria. I. Elevated production of Interferon-γ by placental blood mononuclear cells is asscociated with protection in an area with high transmission of malaria. J Infect Dis. 1999;179:1218–1225. doi: 10.1086/314737. [DOI] [PubMed] [Google Scholar]
- 91.Moran N F, Couper I D. Congenital malaria in South Africa—A report of four cases. S Afr Med J. 1999;89:943–946. [PubMed] [Google Scholar]
- 92.Morrell V. Zeroing in on how hormones affect the immune system. Science. 1995;269:773–775. doi: 10.1126/science.7638587. [DOI] [PubMed] [Google Scholar]
- 93.Muller M, Briscoe J, Laxton C, Guschin D, Ziemiecki A, Silvennoinen O, Harpur A, Barbieri G, Witthuhn B A, Schindler C, et al. The protein tyrosine kinase JAK1 complements defects in interferon-alpha/beta and -gamma signal transduction. Nature. 1993;366:129–135. doi: 10.1038/366129a0. [DOI] [PubMed] [Google Scholar]
- 94.Nakayama M, Otsuka K, Sato K, Hasegawa K, Osman Y, Kawamura T, Abo T. Activation by estrogen of the number and function of forbidden T-cell clones in intermediate T-cell receptor cells. Cell Immunol. 1996;172:163–171. doi: 10.1006/cimm.1996.0229. [DOI] [PubMed] [Google Scholar]
- 95.Narita J, Miyaji C, Watanabe H, Honda S, Koya T, Umezu H, Ushiki T, Sugahara S, Kawamura T, Arakawa M, Abo T. Differentiation of forbidden T cell clones and granulocytes in the parenchymal space of the liver in mice treated with estrogen. Cell Immunol. 1998;185:1–13. doi: 10.1006/cimm.1998.1245. [DOI] [PubMed] [Google Scholar]
- 96.Nigro G, Piazze J, Paesano R, Mango T, Provvedi S, Capuano O, Pollastrini L. Low levels of natural killer cells in pregnant women transmitting Toxoplasma gondii. Prenat Diagn. 1999;19:401–404. [PubMed] [Google Scholar]
- 97.Nilsson N, Carlstein H. Estrogen induces suppression of natural killer cell cytotoxicity and augmentation of polyclonal B cell activation. Cell Immunol. 1994;158:131–139. doi: 10.1006/cimm.1994.1262. [DOI] [PubMed] [Google Scholar]
- 98.Ogawa S, Washburn T F, Taylor J, Lubahn D B, Korach K S, Pfaff D W. Modifications of testosterone-dependent behaviors by estrogen receptor-alpha gene disruption in male mice. Endocrinology. 1998;139:5058–5069. doi: 10.1210/endo.139.12.6358. [DOI] [PubMed] [Google Scholar]
- 99.Olsen N J, Viselli S M, Shults K, Stelzer G, Kovacs W J. Induction of immature thymocyte proliferation after castration of normal-male mice. Endocrinology. 1994;134:107–113. doi: 10.1210/endo.134.1.8275924. [DOI] [PubMed] [Google Scholar]
- 100.Olsen N J, Watson M B, Henderson G S, Kovacs W J. Androgen deprivation induces phenotypic and functional-changes in the thymus of adult male-mice. Endocrinology. 1991;129:2471–2476. doi: 10.1210/endo-129-5-2471. [DOI] [PubMed] [Google Scholar]
- 101.Olsen N J, Zhou P, Ong H, Kovacs W J. Testosterone induces expression of transforming growth factor-beta(I) in the murine thymus. J Steroid Biochem Mol Biol. 1993;45:327–332. doi: 10.1016/0960-0760(93)90001-d. [DOI] [PubMed] [Google Scholar]
- 102.Pang X, Cotreaubibbo M M, Sant G R, Theoharides T C. Bladder mast-cell expression of high-affinity estrogen-receptors in patients with interstitial cystitis. Br J Urol. 1995;75:154–161. doi: 10.1111/j.1464-410x.1995.tb07303.x. [DOI] [PubMed] [Google Scholar]
- 103.Parker S J, Roberts C W, Alexander J. CD8+ T cells are the major lymphocyte subpopulation involved in the protective immune response to Toxoplasma gondii in mice. Clin Exp Immunol. 1991;84:207–212. doi: 10.1111/j.1365-2249.1991.tb08150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Phillips A N, Antunes F, Stergious G, Ranki A, Jensen G F, Bentwich Z, Sacks T, Pedersen C, Lundgren J D, Johnson A M. A sex comparison of rates of new AIDS-defining disease and death in 2554 AIDS cases. AIDS. 1994;8:831–835. doi: 10.1097/00002030-199406000-00017. [DOI] [PubMed] [Google Scholar]
- 105.Piccinni M P, Giudizi M G, Biagiotti R, Beloni L, Giannarini L, Sampognaro S, Parronchi P, Manetti R, Annunziato F, Livi C, Romagnani S, Maggi E. Progesterone favors the development of human T-helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane cd30 expression in established Th1 cell clones. J Immunol. 1995;155:128–133. [PubMed] [Google Scholar]
- 106.Piccinni M P, Maggi E, Romagnani S. Role of hormone controlled T-cell cytokines in the maintenance of pregnancy. Biochem Soc Trans. 2000;28:212–215. doi: 10.1042/bst0280212. [DOI] [PubMed] [Google Scholar]
- 107.Polgar B, Barakonyi A, Xynos I, SzekeresBartho J. The role of gamma/delta T cell receptor positive cells in pregnancy. Am J Reprod Immunol. 1999;41:239–244. doi: 10.1111/j.1600-0897.1999.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 108.Provinciali M, DiStefano G, Muzzioli M, Garzetti G G, Clavattini A, Fabris N. Relationship between 17-β-estradiol and prolactin in the regulation of natural killer cell activity during progression of endometriosis. J Endocrinol Investig. 1995;18:645–652. doi: 10.1007/BF03349783. [DOI] [PubMed] [Google Scholar]
- 109.Rasheed F N, Bulmer J N, Dunn D T, Menendez C, Jawla M F, Jepson A, Jakobsen P H, Greenwood B M. Suppressed peripheral and placental blood lymphoproliferative responses in first pregnancies: relevance to malaria. Am J Trop Med Hyg. 1993;48:154–160. doi: 10.4269/ajtmh.1993.48.154. [DOI] [PubMed] [Google Scholar]
- 110.Reghupathy R. Th1-type immunity is incompatible with successful pregnancy. Immunol Today. 1997;18:478–482. doi: 10.1016/s0167-5699(97)01127-4. [DOI] [PubMed] [Google Scholar]
- 111.Rijhsinghani A G, Thompson K, Bhatia S K, Waldschmidt T J. Estrogen blocks early T cell development in the thymus. Am J Reprod Immunol. 1996;36:269–277. doi: 10.1111/j.1600-0897.1996.tb00176.x. [DOI] [PubMed] [Google Scholar]
- 112.Roberts C W, Alexander J. Studies on a murine model of congenital toxoplasmosis: vertical disease transmission only occurs in BALB/c mice infected for the first time during pregnancy. Parasitology. 1992;104:19–23. doi: 10.1017/s0031182000060753. [DOI] [PubMed] [Google Scholar]
- 113.Roberts C W, Cruickshank S M, Alexander J. Sex-determined resistance to Toxoplasma gondii is associated with temporal differences in cytokine production. Infect Immun. 1995;63:2549–2555. doi: 10.1128/iai.63.7.2549-2555.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Roberts C W, Satoskar A, Alexander J. Sex steroids, pregnancy-associated hormones and immunity to parasitic infection. Parasitol Today. 1996;12:382–388. doi: 10.1016/0169-4758(96)10060-0. [DOI] [PubMed] [Google Scholar]
- 115.Roberts M J, Alexander J, Blackwell J M. Genetic analysis of Leishmania mexicana infection in mice: single gene (Scl-2) controlled predisposition to cutaneous lesion development. J Immunogenet. 1990;17:89–100. doi: 10.1111/j.1744-313x.1990.tb00862.x. [DOI] [PubMed] [Google Scholar]
- 116.Roszkowski P I, Hyc A, Malejcyk J. Natural killer cell activity in patients with ovarian tumours and uterine myomas. Eur J Gynaecol Oncol. 1993;14:S114–S117. [PubMed] [Google Scholar]
- 117.Roszkowski P I, Hyc A, Stopinska-Gluszak U, Malejczyk J. Natural killer cell activity and sex hormone levels in mastopathy. Gynecol Endocrinol. 1997;11:399–404. doi: 10.3109/09513599709152567. [DOI] [PubMed] [Google Scholar]
- 118.Salem M L, Hossain M S, Nomoto K. Mediation of the immunomodulatory effect of beta-estradiol on inflammatory responses by inhibition of recruitment and activation of inflammatory cells and their gene expression of TNF-alpha and IFN-gamma. Int Arch Allergy Immunol. 2000;121:235–245. doi: 10.1159/000024323. [DOI] [PubMed] [Google Scholar]
- 119.Salem M L, Matsuzaki G, Kishihara K, Madkour G A, Nomoto K. beta-estradiol suppresses T cell-mediated delayed-type hypersensitivity through suppression of antigen-presenting cell function and Th1 induction. Int Arch Allergy Immunol. 2000;121:161–169. doi: 10.1159/000024312. [DOI] [PubMed] [Google Scholar]
- 120.Satoskar A, Al-Quassi H H, Alexander J. Sex-determined resistance against Leishmania mexicana is associated with the preferential induction of a Th1-like response and IFN-gamma production by female but not male DBA/2 mice. Immunol Cell Biol. 1998;76:159–166. doi: 10.1046/j.1440-1711.1998.00730.x. [DOI] [PubMed] [Google Scholar]
- 121.Satoskar A, Alexander J. Sex-determined susceptibility and differential IFN-gamma and TNF-alpha mRNA expression in DBA/2 mice infected with Leishmania mexicana. Immunology. 1995;84:1–4. [PMC free article] [PubMed] [Google Scholar]
- 122.Scott P, Trinchieri G. The role of natural killer cells in host-parasite interactions. Curr Opin Immunol. 1995;2:34. doi: 10.1016/0952-7915(95)80026-3. [DOI] [PubMed] [Google Scholar]
- 123.Screpanti I, Santoni A, Gulino A, Herberman R B, Frati L. Estrogen and antiestrogen modulation of the levels of mouse natural killer activity and large granular lymphocytes. Cell Immunol. 1987;106:191–202. doi: 10.1016/0008-8749(87)90163-8. [DOI] [PubMed] [Google Scholar]
- 124.Seaman W E, Blackman M A, Gindhart T D, Roubinian J R, Loeb J M, Talal N. beta-Estradiol reduces natural killer cells in mice. J Immunol. 1978;121:2193–2198. [PubMed] [Google Scholar]
- 125.Seaman W E, Gindhart T D. Effect of estrogen on matural killer cells. Arthritis Rheum. 1979;22:1234–1240. doi: 10.1002/art.1780221110. [DOI] [PubMed] [Google Scholar]
- 126.Seiki K, Sakabe K. Sex hormones and the thymus in relation to thymocyte proliferation and maturation. Arch Histol Cytol. 1997;60:29–38. doi: 10.1679/aohc.60.29. [DOI] [PubMed] [Google Scholar]
- 127.Shanker G, Sorcithomas M, Adams M R. Estrogen modulates the expression of tumor-necrosis-factor-alpha messenger-RNA in phorbol ester-stimulated human monocytic THP-1 cells. Lymphokine Cytokine Res. 1994;13:377–382. [PubMed] [Google Scholar]
- 128.Sharifi I, FeKri A, Aflatonian M R, Khamesipour A, Nadim A, Mousavi M R, Momeni A Z, Dowlati Y, Godal T, Zicker F, Smith P G, Modabber F. Randomised vaccine trial of single dose of killed Leishmania major plus BCG against anthroponotic cutaneous leishmaniasis in Bam, Iran. Lancet. 1998;351:1540–1543. doi: 10.1016/S0140-6736(98)09552-X. [DOI] [PubMed] [Google Scholar]
- 129.Sher A, Sousa C R. Ignition of the type I response to intracellular infection by dendritic cell-derived interleukin-12. Eur Cytokine Network. 1998;9:65–68. [PubMed] [Google Scholar]
- 130.Shirahata T, Muroya N, Ohta C, Goto H, Nakane A. Correlation between increased susceptibility to primary Toxoplasma gondii infection and depressed production of gamma interferon in pregnant mice. Microbiol Immunol. 1992;36:81–91. doi: 10.1111/j.1348-0421.1992.tb01644.x. [DOI] [PubMed] [Google Scholar]
- 131.Shirahata T, Muroya N, Ohta C, Goto H, Nakane A. Enhancement by recombinant human interleukin 2 of host resistance to Toxoplasma gondii infection in pregnant mice. Microbiol Immunol. 1993;37:583–590. doi: 10.1111/j.1348-0421.1993.tb01680.x. [DOI] [PubMed] [Google Scholar]
- 132.Silva H, Tchernitchin A N, Tchernitchin N N. Low doses of estradiol-17 alpha degranulate blood eosinophil leucocytes and high doses alter homeostatic mechanisms. Med Sci Res. 1997;25:201–204. [Google Scholar]
- 133.Silver R M, Branch D W. Autoimmune disease in pregnancy—Systemic lupus erythematosus and antiphospholipid syndrome. Clin Perinatol. 1997;24:291–322. [PubMed] [Google Scholar]
- 134.Sorachi K I, Kumagai S, Sugita M, Yodoi J, Imura H. Enhancing effects of 17 beta-estadiol on human NK cell activity. Immunol Lett. 1993;1:31–36. doi: 10.1016/0165-2478(93)90065-a. [DOI] [PubMed] [Google Scholar]
- 135.Spanos C, ElMansoury M, Letourneau R, Minogiannis P, Greenwood J, Siri P, Sant G R, Theoharides T C. Carbachol-induced bladder mast cell activation: Augmentation by estradiol and implications for interstitial cystitis. Urology. 1996;48:809–816. doi: 10.1016/S0090-4295(96)00239-7. [DOI] [PubMed] [Google Scholar]
- 136.Staples J E, Gasiewicz T A, Fiore N C, Lubahn D B, Korach K S, Silverstone A E. Estrogen receptor alpha is necessary in thymic development and estradiol-induced thymic alterations. J Immunol. 1999;163:4168–4174. [PubMed] [Google Scholar]
- 137.Styrt B, Sugarman B. Estrogens and infection. Rev Infect Dise. 1990;13:1139–1148. doi: 10.1093/clinids/13.6.1139. [DOI] [PubMed] [Google Scholar]
- 138.Suzuki N, Suzuki T, Sakane T. Hormones and lupus defective dehydroepiandrosterone activity induces impaired interleukin-2 activity of T lymphocytes in patients with systemic lupus erythematosus. Ann Med Intern. 1996;147:248–252. [PubMed] [Google Scholar]
- 139.Szekeres-Bartho J. Progesterone-treated lymphocytes of healthy pregnant women release a factor inhibiting cytotoxicity and prostoglandin synthesis. Cell Immunol. 1989;122:281–294. doi: 10.1016/0008-8749(89)90077-4. [DOI] [PubMed] [Google Scholar]
- 140.Szekeres-Bartho J, Faust Z, Varga P. The expression of a progesterone-induced immunomodulatory protein in pregnancy lymphocytes. Am J Reprod Immunol. 1995;34:342–348. doi: 10.1111/j.1600-0897.1995.tb00962.x. [DOI] [PubMed] [Google Scholar]
- 141.Szekeres-Bartho J, Par G, Szereday L, Smart C Y, Achtaz I. Progesterone and non-specific immunologic mechanisms in pregnancy. Am J Reprod Immunol. 1997;38:176–182. doi: 10.1111/j.1600-0897.1997.tb00295.x. [DOI] [PubMed] [Google Scholar]
- 142.Szekeres-Bartho J, Varga P, Pacsa A S. Immunologic factors contributing to the initiation of labor-lymphocyte reactivity in term labor and threatened preterm delivery. Am J Obstet Gynecol. 1986;155:108–112. doi: 10.1016/0002-9378(86)90090-6. [DOI] [PubMed] [Google Scholar]
- 143.Taube M, Svensson L, Carlsten H. T lymphocytes are not the target for estradiol-mediated suppression of DTH in reconstituted female severe combined immunodeficient (SCID) mice. Clin Exp Immunol. 1998;114:147–153. doi: 10.1046/j.1365-2249.1998.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tibbetts T A, DeMayo F, Rich S, Conneely O M, Omalley B W. Progesterone receptors in the thymus are required for thymic involution during pregnancy and for normal fertility. Proc Natl Acad Sci USA. 1999;96:12021–12026. doi: 10.1073/pnas.96.21.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Toder V, Nebel L, Elrad H, Blank M, Durdana A, Gleicher N. Studies of natural killer cells in pregnancy. II. The immunoregulatory effect of pregnancy substances. J Clin Lab Immunol. 1984;14:129–133. [PubMed] [Google Scholar]
- 146.Toder V, Nebel L, Gleicher N. Studies of natural killer cells in pregnancy. I. Analysis at the single cell level. J Clin Lab Immunol. 1984;14:123–127. [PubMed] [Google Scholar]
- 147.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Van Zon A A, Termaat R M, Schetters T P, Eling W M. Plasmodium berghei: reduction of the mouse's specific lymphoproliferative response in relation to corticosterone and pregnancy. Exp Parasitol. 1986;62:71–78. doi: 10.1016/0014-4894(86)90009-3. [DOI] [PubMed] [Google Scholar]
- 149.Vassiliadou N, Tucker L, Anderson D J. Progesterone-induced inhibition of chemokine receptor expression on peripheral blood mononuclear cells correlates with reduced HIV-1 infectability in vitro. J Immunol. 1999;162:7510–7518. [PubMed] [Google Scholar]
- 150.Venugopalan P P, Shenoy D U, Kamath A, Rajeev A. Distribution of malarial parasites:effect of gender of construction workers. Indian J Med Sci. 1997;51:89–92. [PubMed] [Google Scholar]
- 151.Vliagoftis H, Dimitriadou V, Boucher W, Rozniecki J J, Correia I, Raam S, Theoharides T C. Estradiol augments while tamoxifen inhibits rat mast-cell secretion. Int Arch Allergy Immunol. 1992;98:398–409. doi: 10.1159/000236217. [DOI] [PubMed] [Google Scholar]
- 152.Walker W, Roberts C W, Ferguson D J P, Jebbari H, Alexander J. Innate immunity to Toxoplasma gondii is influenced by gender and is associated with differences in interleukin-12 and gamma interferon production. Infect Immun. 1997;65:1119–1121. doi: 10.1128/iai.65.3.1119-1121.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang Y, Campbell H D, Young I G. Sex-hormones and dexamethasone modulate interleukin-5 gene-expression in T-lymphocytes. J Steroid Biochem Mol Biol. 1993;44:203–210. doi: 10.1016/0960-0760(93)90080-g. [DOI] [PubMed] [Google Scholar]
- 154.Wunderlich F, Maurin W, Benten W P, Schmitt-Wrede H-P. Testosterone impairs efficacy of protective vaccination against P. chabaudi malaria. Vaccine. 1993;11:1097–1099. doi: 10.1016/0264-410x(93)90068-9. [DOI] [PubMed] [Google Scholar]
- 155.Young H A, Hardy K J. Role of interferon-gamma in immune cell regulation. J Leukocyte Biol. 1995;58:373–381. [PubMed] [Google Scholar]
- 156.Zaffaroni E, Rubaudo L, Lanfranchi P, Mignone W. Epidemiological patterns of canine leishmaniosis in Western Liguria (Italy) Vet Parasitol. 1999;81:11–19. doi: 10.1016/s0304-4017(98)00237-4. [DOI] [PubMed] [Google Scholar]
- 157.Zuckerman S H, Bryanpoole N, Evans G F, Short L, Glasebrook A L. In-vivo modulation of murine serum tumor-necrosis-factor and interleukin-6 levels during endotoxemia by estrogen agonists and antagonists. Immunology. 1995;86:18–24. [PMC free article] [PubMed] [Google Scholar]