Abstract
Acute lymphoblastic leukemia with chromosomal rearrangements involving the mixed-lineage leukemia (MLL) gene (MLL-r ALL) remains an incurable disease. Thus, development of a safe and effective therapeutic agent to treat this disease is crucial to address this unmet medical need. BRD4, a member of the bromodomain and extra-terminal domain (BET) protein family, and cyclic AMP response element binding protein binding protein (CBP) and p300, two paralogous histone acetyltransferases, are all considered cancer drug targets and simultaneous targeting of these proteins may have therapeutic advantages. Here, we demonstrate that a BET/CBP/p300 multi-bromodomain inhibitor, CN470, has anti-tumor activity against MLL-r ALL in vitro and in vivo. CN470, potently inhibited ligand binding to the bromodomains of BRD4, CBP, and p300 and suppressed the growth of MLL-r ALL cell lines and patient-derived cells with MLL rearrangements. CN470 suppressed mRNA and protein expression of MYC and induced apoptosis in MLL-r ALL cells, following a cell cycle arrest in the G1 phase. Moreover, CN470 reduced BRD4 binding to acetylated histone H3. The in vivo effects of CN470 were investigated using SEMLuc/GFP cells expressing luminescent markers in an orthotopic mouse model. Mice administered CN470 daily had prolonged survival compared to the vehicle group. Further, CN470 also showed anti-tumor effects against an MLL-r ALL patient-derived xenograft model. These findings suggest that inhibition of BET/CBP/p300 by the multi-bromodomain inhibitor, CN470, represents a promising therapeutic approach against MLL-r ALL.
Keywords: Bromodomain, MLL, ALL, BET, CBP/p300, PDX
1. Introduction
Acute lymphoblastic leukemia (ALL) is the most common form of childhood leukemia. While the prognosis of patients with ALL has improved significantly due to recent advances in anti-leukemic agents, ALL with chromosomal rearrangements involving the mixed-lineage leukemia (MLL) gene (MLL-r ALL) remains incurable. Hence, development of safe and effective therapeutic agents is of great importance because treatment-related late effects are now recognized as the health problems in childhood cancer survivors [1].
Currently, drugs that target bromodomain and extra-terminal domain (BET) proteins are under investigation as potential clinical therapeutics against hematological malignancies. Many BET inhibitors, including OTX015 [2], I-BET762 [3,4], and ABBV-075 [5] have been developed and are undergoing preclinical and clinical studies. The BET family comprises four proteins, BRD2, BRD3, BRD4, and BRDT, all containing two highly conserved bromodomains, BD1 and BD2, both of which bind to acetylated lysines on proteins including histones. BET inhibitors exhibit anti-tumor effects in many malignancies [6] and their mechanism of action is thought to be based on their ability to disrupt the formation of transcriptional machineries at Super-Enhancers [7,8]. In cancer cells, BRD4 can enhance expression of key oncogenic genes that drive the growth of solid and hematological cancers, such as MYC, CDK6, and BCL2 [9].
Cyclic AMP response element binding protein binding protein (CBP) and p300 are paralogous histone acetyltransferases consisting of nine functional domains, among which the bromodomain and acetyltransferase catalytic domains are being investigated as potential druggable target domains for cancer [10,11]. At least one CBP/p300 bromodomain inhibitor, CCS1477 [12], is undergoing clinical trials for treatment of hematological malignancies and advanced solid tumors. Hence, the bromodomains of BET and CBP/p300 proteins are considered targets for anti-tumor drugs.
We previously developed a novel BET inhibitor, CN470 [13]. In this current study, we showed that CN470 also inhibited the bromodomains of CBP and p300, and investigated its inhibitory effects on MLL-r ALL cell proliferation and evaluated its anti-tumor activities in vivo.
2. Materials and methods
2.1. Cell lines, cell culture, reagents, and animals
The human MLL-AF4 ALL cell line, SEM, was purchased from the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany). KOCL-45, KOCL-58 and KOCL-69 are human MLL-AF4 cell lines; KOPB-26 and YACL-95 are human MLL-AF9 cell lines; and KOCL-44, KOCL-50 and KOPN-1 are human MLL-ENL ALL cell lines. K103, K110, and K114 cells are MLL-AF4 ALL patient-derived cells and K107 cells are MLL-ENL ALL patient-derived cells (Supplementary Table S1). For in vivo experiments, we established SEMLuc/GFP cells. The BET inhibitor, OTX015, was purchased from Cayman Chemical (Ann Arbor, MI). In each assay, SEM and KOPN-1 cells were incubated with CN470 or OTX015 at the doses of 0.75 × and 1.5 × their respective half-maximal inhibition concentration (IC50) values. Specific pathogen-free 11-week-old female BALB/c Rag2−/−/Jak3−/− (BRJ) mice and 11-week-old female NOD/SCID/γcnull (NOG) mice were used for the in vivo experiments.
Approval for the studies was obtained from the Committee on Animal Research of Kyoto Pharmaceutical University (#18–15-001, CTPH-20–004) and Institute of Laboratory Animals of Graduate School of Medicine, Kyoto University (Med Kyo 21291–2). Informed consent for the use of bone marrow cells was obtained from MLL-r ALL patients or their guardians, in accordance with the Declaration of Helsinki and under the approval of the Kyoto Pharmaceutical University Review Board (#20–19-06) and the Kyoto University Review Board (G1030–6). See Supplemental Materials and methods for more details.
2.2. BROMOScan
Inhibition of ligand binding to BRD4, CBP, and p300 bromodomains by CN470 was investigated using BROMOScan, as previously reported [14]. For BRD4, a target containing both bromodomains, BRD4 (BD1-BD2), was used.
2.3. Cell proliferation assay
Proliferation of MLL-r ALL cell lines was evaluated using the WST-8 assay as previously described [15]. MLL-r ALL patient-derived cell proliferation was evaluated using the CellTiter-Glo® 2.0 reagent (Promega, Madison, WI) following the manufacturer’s protocol. The IC50 value for each compound was determined with CalcuSyn software (BIOSOFT, Cambridge, UK). See Supplemental Materials and methods for more details.
2.4. Cell cycle and apoptosis analysis
Cell cycle analysis was performed using propidium iodide (PI) and flow cytometry, as previously described [15]. See Supplemental Materials and methods for more details.
2.5. Quantitative reverse transcription-PCR (qRT-PCR)
Human MYC, CDK6, BCL2, and 18S ribosomal RNA (18S rRNA) mRNA expression levels were measured by real-time PCR. Primers and probes used in this study are shown in Supplementary Table S2. See Supplemental Materials and methods for more details.
2.6. Western blots
Following the incubation with OTX015 or CN470, expression levels of MYC, CDK6, BCL2, caspase-3, cleaved caspase-3, poly (ADP-ribose) polymerase-1 (PARP-1), and cleaved PARP-1 were examined by western blotting. Primary antibodies used in this study are listed in Supplementary Table S3. See Supplemental Materials and methods for more details.
2.7. Co-immunoprecipitation (Co-IP) assay
See Supplemental Materials and methods for details.
2.8. Effects of CN470 on an MLL-r ALL bearing mouse model
The in vivo effects of CN470 on SEMLuc/GFP cells were investigated using an orthotopic mouse model of MLL-r ALL. BRJ mice were inoculated with 2 × 106 SEMLuc/GFP cells through the tail vein. Mice were orally administered with vehicle or CN470 (10 mg/kg) once daily; treatments were initiated when SEMLuc/GFP cells’ luminescence was detected. MLL-r ALL growth was investigated every week using the IVIS Lumina IIIXR imaging system (PerkinElmer, Waltham, MA). See Supplemental Materials and methods for details.
2.9. Effect of CN470 on patient-derived xenograft (PDX) model mice
Xenotransplantation and harvest of MLL-r ALL patient-derived cells were performed as previously described [16]. PDX model mice were generated by inoculating NOG mice with 0.5 × 106 K110 cells via the tail vein. Mice were orally administered with vehicle or CN470 (10 mg/kg) once daily from the day after transplantation. White blood cell (WBC) and platelet (PLT) counts were performed using CellTac α (NIHON KOHDEN, Tokyo, Japan). See Supplemental Materials and methods for more details.
2.10. Statistical analysis
Statistical tests were conducted using R version 4.0.3 software (R Foundation for Statistical Computing, Vienna, Austria). See Supplemental Materials and methods for more details.
3. Results
3.1. CN470 binds to BET/CBP/p300 bromodomains with high affinity and suppresses MLL-r ALL cell proliferation
CN470 (Supplementary Fig. S1A) potently bound to the bromodomains of BRD4 (BD1-BD2), CBP, and p300 with KD values of 23, 32, and 20 nM, respectively, when tested with a competition binding assay (Fig. 1A). However, OTX015, a reference BET inhibitor, did not bind to the bromodomains of CBP or p300, in contrast to its high inhibitory effects on the bromodomain of BRD4. CN470 suppressed the proliferation of MLL-r ALL cell lines in a dose-dependent manner (Fig. 1B, 1C and Supplementary Fig. S1B). CN470 also dose-dependently inhibited the growth of MLL-r ALL patient-derived cells (Fig. 1D and Supplementary Fig. S1C).
Fig. 1.
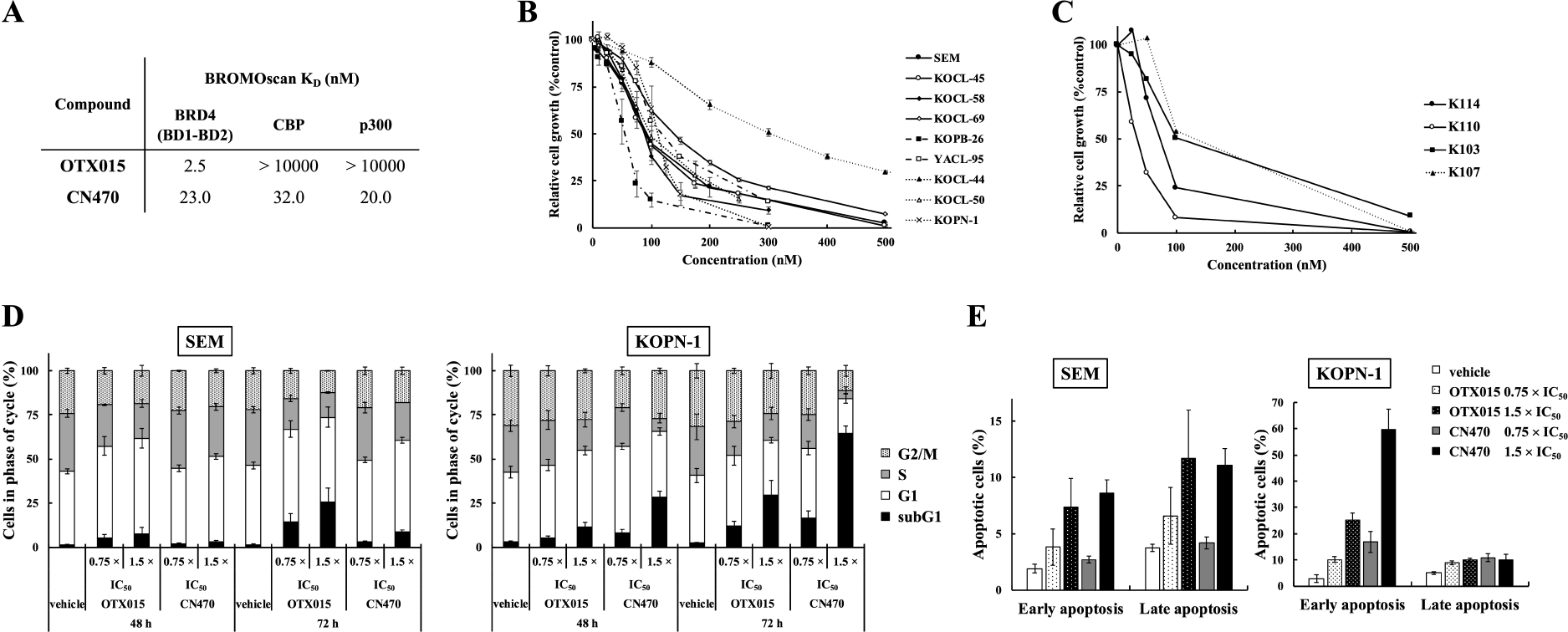
Effects of CN470 on MLL-r ALL cell proliferation and induction of apoptosis. (A) BROMOscan™ assay for CN470. For BRD4, a target containing both bromodomains, BRD4 (BD1-BD2), was used. (B, C, D) Growth inhibitory effects of CN470 on MLL-r ALL (B, C) and patient-derived (D) cells. (B) WST-8 assay data are represented as mean ± standard error (SE) of three independent experiments, each with four replicates experiments. (C) IC50 values for cell proliferation analyses in this study. (D) Cell-Titer Glo® 2.0 viability assay results are presented as mean ± standard deviation (SD) of four replicates for each concentration. (E) CN470 induced changes in SEM and KOPN-1 cell cycle status. (F) Induction of SEM and KOPN-1 cell apoptosis was assessed. Cell cycle and apoptosis assay data are presented as mean ± SD of three independent experiments.
3.2. CN470 induces apoptosis of MLL-r ALL cells following cell cycle arrest
Next, we analyzed the effects of CN470 on the cell cycle in two MLL-r ALL cell lines, SEM and KOPN-1, by flow cytometry. Both CN470 and OTX015 induced G1 arrest, leading to increases in subG1 fraction in a time- and dose-dependent manner (Fig. 1E and Supplementary Fig. S1D). Further evaluation of apoptosis in CN470-treated cells using Annexin-V/PI staining showed that the proportions of early (Annexin-V+/PI−) and late (Annexin-V+/PI+) apoptotic cells both increased dose-dependently in SEM cells. KOPN-1 cells also showed markedly increased early apoptosis in a dose-dependent manner, whereas late apoptosis in KOPN-1 cells did not increase in a dose-dependent manner despite the observation that there were as many late apoptotic cells in KOPN-1 as compared to SEM (Fig. 1F and Supplementary Fig. S1E). Despite a slight difference in apoptotic profiles, these results suggested that both OTX015 and CN470 cause cell cycle arrest and induce apoptosis of MLL-r ALL cells.
3.3. CN470 influences MYC and apoptotic marker expression and inhibits BRD4 binding to acetylated histone H3
We next evaluated mRNA transcript and protein expression levels in MLL-r ALL cells treated with CN470. qRT-PCR analyses demonstrated that 24 h incubation with CN470 reduced MYC mRNA transcripts in SEM cells. CDK6 and BCL2 mRNA transcripts in both SEM and KOPN-1 cells were also decreased by varying degrees (Fig. 2A). In western blot analysis, MYC protein levels were decreased in response to CN470 treatment (Fig. 2B). Furthermore, CDK6 and BCL2 protein levels were also dose-dependently decreased. Additionally, CN470 treatment led to the cleavage of the caspase-3 and PARP-1 proteins into their active forms.
Fig. 2.
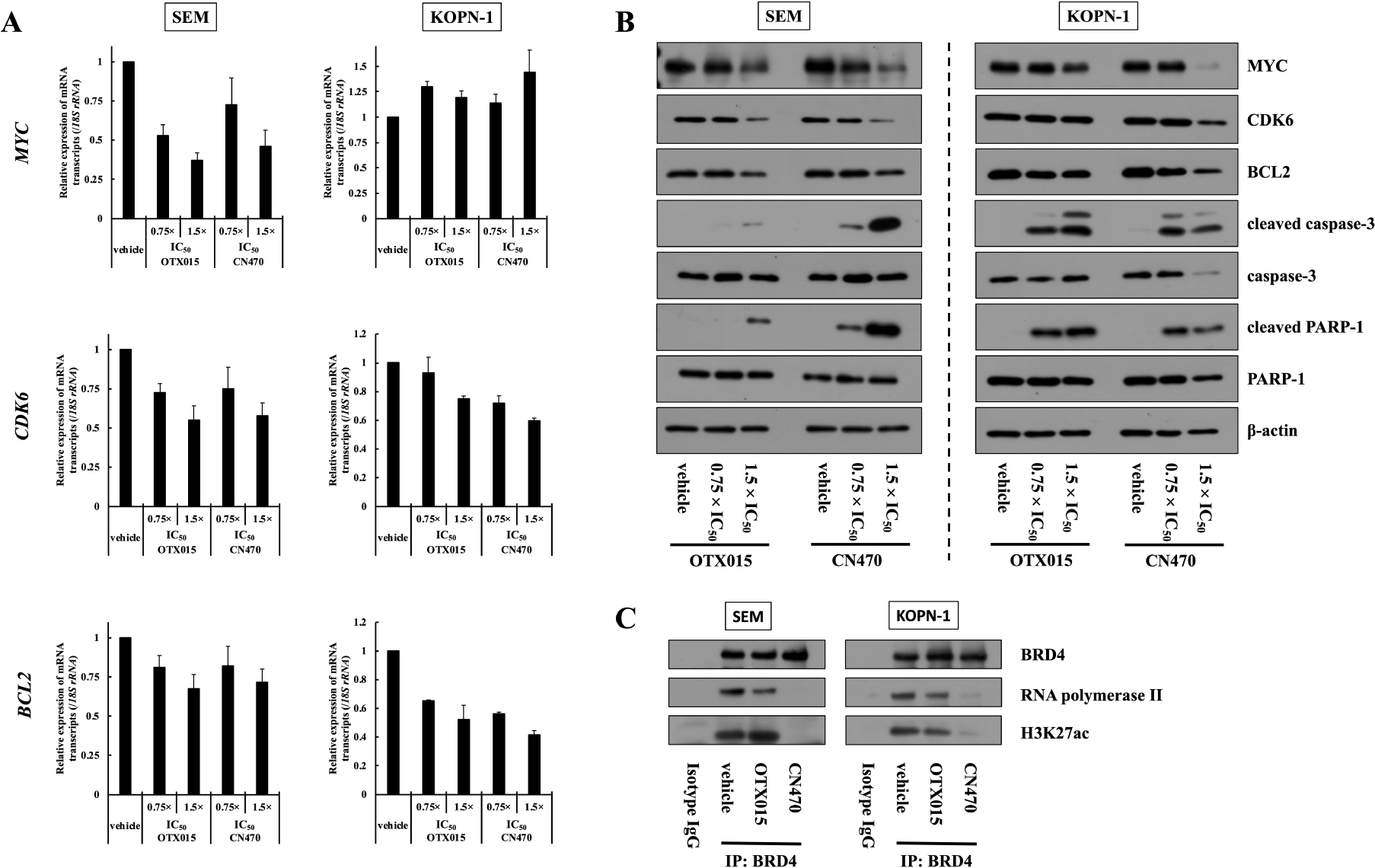
Evaluation of mRNA transcript and protein expression levels in MLL-r ALL cells treated with CN470. (A) SEM and KOPN-1 cells were incubated with CN470 or OTX015 at the doses of 0.75 × and 1.5 × their respective IC50 values for 24 h, and qRT-PCR then performed. Results are presented as mean ± SE of three independent experiments, each with three replicates. (B) SEM and KOPN-1 cells were incubated with CN470 or OTX015 at the doses of 0.75 × and 1.5 × their respective IC50 values for 72 h, and western blotting was then performed. Data are representative of two independent experiments. (C) Co-IP assay using an anti-BRD4 antibody. The IC50 values of CN470 and OTX015 against SEM were 71.2 and 141.1 nM, respectively, and those against KOPN-1 were 106.1 and 194.4 nM, respectively.
Co-IP assay demonstrated that CN470 clearly reduced BRD4 binding to acetylated histone H3 (H3K27ac), as well as RNA polymerase II recruitment, an effect largely absent with OTX015 (Fig. 2C). Taken together, our data suggest that CN470 suppressed MYC mRNA transcription in MLL-r ALL cells with a slightly different mechanism as compared to OTX015, i.e., at least in part by inhibiting BRD4 binding to H3K27ac, resulting in the induction of cell cycle arrest and apoptosis by activating caspase-3 and PARP-1.
3.4. CN470 prolongs the survival of MLL-AF4 ALL-bearing mouse
We also investigated the in vivo effects of CN470 by using SEMLuc/GFP cells in an orthotopic mouse model of MLL-AF4 leukemia. The treatment was initiated when the first luminescence signal of the SEMLuc/GFP cells was detected (day 1), approximately two weeks after the inoculation (Fig. 3A). Oral administration of CN470 (10 mg/kg) once daily resulted in prolonged survival of mice and reduced SEMLuc/GFP cells’ luminescence intensity on days 14 and 21 after the initiation of CN470 treatment (Fig. 3B and 3C). Additionally, there were no statistical differences in body weights or PLT counts between the CN470 and the vehicle group during the treatment period (data not shown).
Fig. 3.
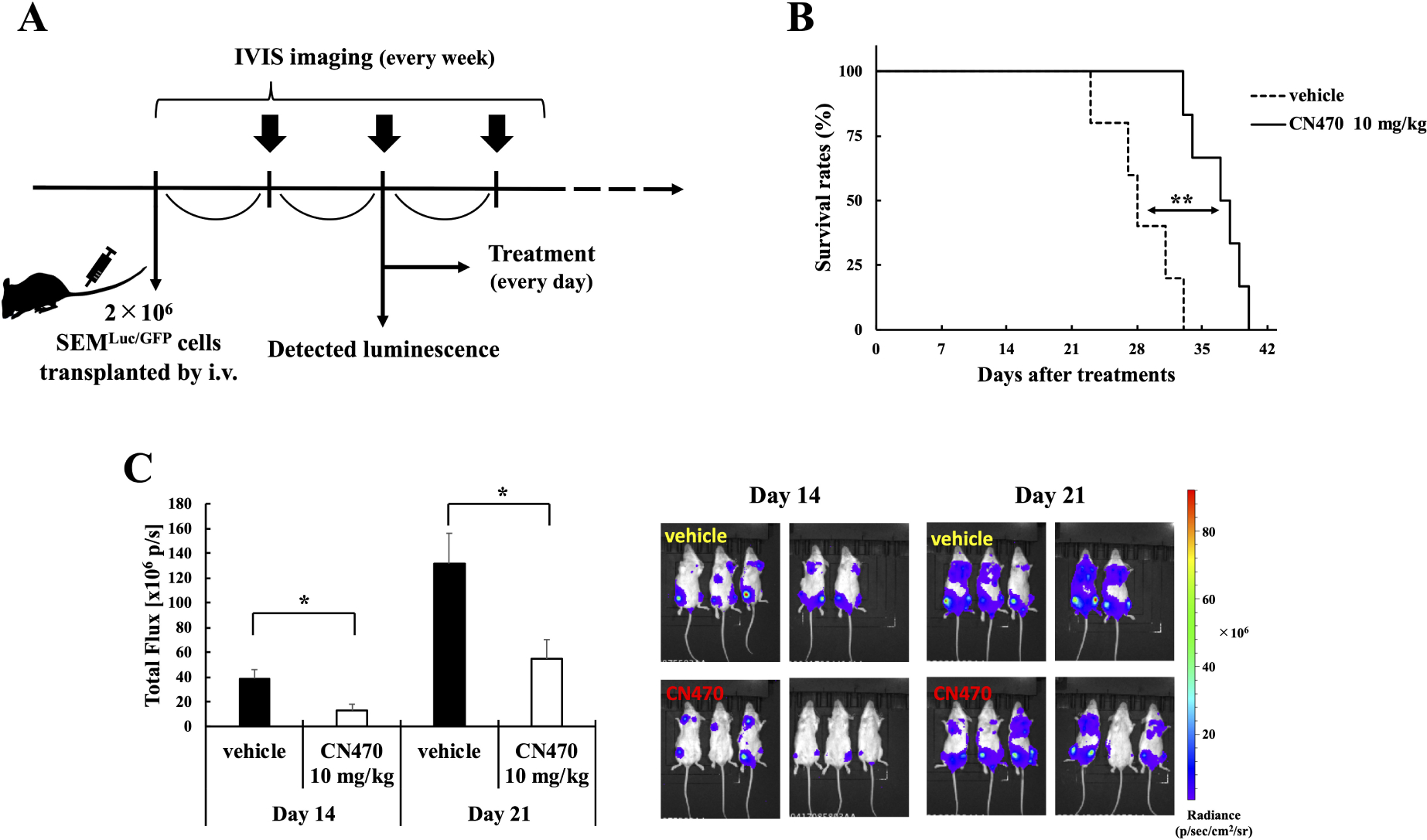
Effects of in vivo CN470 administration. (A) Schedule of the in vivo study. BRJ mice were transplanted intravenously with SEMLuc/GFP cells. Treatment was initiated when bioluminescence was detected in mice using IVIS system. (B) Survival rates of SEMLuc/GFP-xenografted mice. Solid and dashed lines represent the survival rates of the CN470-treated (n = 6 mice) and the vehicle-treated (n = 5 mice) groups, respectively (**p = 0.003). (C) Bioluminescent images generated using the IVIS system and quantification of ventral bioluminescent intensity of SEMLuc/GFP-xenografted mice on 14 and 21 days after treatment. Data are shown as mean + SE ventral total flux for each group (day 14, *p = 0.015; day21, *p = 0.022).
3.5. Survival of PDX model mice was prolonged by CN470 treatment
Finally, we assessed the in vivo effects of CN470 on a PDX mouse model using K110 cells. Mice were administered CN470 (10 mg/kg) orally, once daily, on the day after transplantation (day 1) and complete blood cell counts were evaluated weekly. Investigation of peripheral blood (PB) chimerism on day 42 after treatment showed that CN470-treduced mice had reduced the proportion of patient-derived cells (human CD19-positive) in PB relative to the vehicle group (68.8 ± 2.3% for CN470 group vs. 79.8 ± 1.7% for vehicle group, *p = 0.02), although the WBC count did not differ between the two groups (Fig. 4A and Supplementary Fig. S2A). While all mice eventually died by day 50, CN470-treated mice had significantly prolonged survival rates compared with the vehicle-group (**p = 0.002) (Fig. 4B). There was no difference in PLT count between vehicle- and CN470-treatment groups (Supplementary Fig. S2B). Additionally, to assess the safety of CN470, the healthy BRJ mice were orally administered for six weeks. Thrombocytopenia, a commonly reported toxicity associated with the BET inhibitors [2,5], hematopoietic cells, or body weight loss was not observed following the CN470 treatment (Supplementary Fig. S2C–F).
Fig. 4.
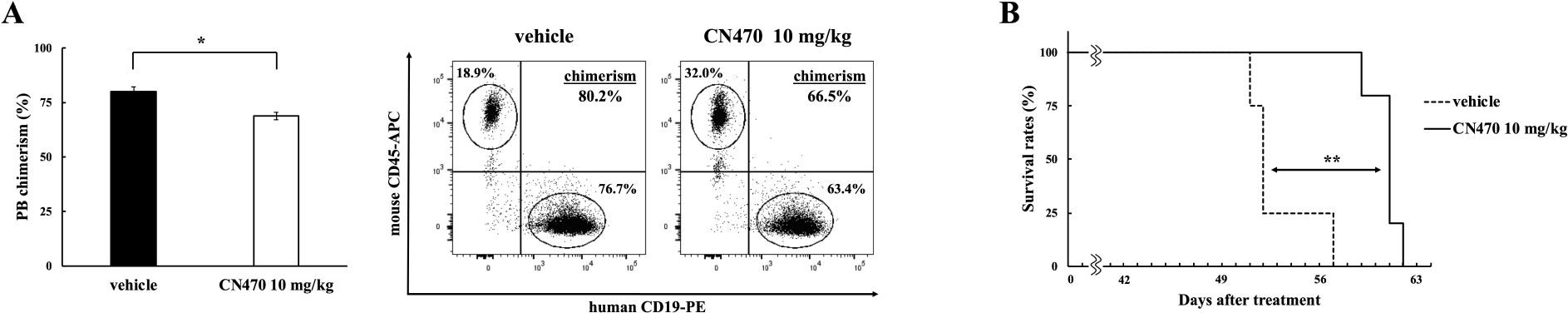
Effect of CN470 on a PDX mouse model using K110 cells. (A) CN470 reduced chimerism in PB. Data are shown as mean + SE of chimerism in PB for each group (day 42, *p = 0.02). The dot plots shown are from representative experiments. (B) CN470 prolonged the survival of PDX model mice (**p = 0.002). Solid and dashed lines represent the survival rates of the CN470-treated (n = 5 mice) and the vehicle-treated (n = 4 mice) groups, respectively.
4. Discussion
Since the late 20th century, anti-cancer drugs have been developed to act against specific targets with high potency and selectivity, under the ‘one target and one drug’ model; however, such targeting strategies can have a high probability of leading to drug resistance. To prevent such resistance, multi-targeting therapeutics are currently being designed. One approach is combination drug therapy, which generates additive or synergistic effects using multiple drugs. One step further in this strategy is to develop a single agent that can act on two or more targets simultaneously. These multi-specific drugs are attracting attention as a fourth revolutionary wave of biopharmaceutical innovation [17]. To be considered multi-specific, drugs must bind to two or more targets, and have potential to be innovative therapeutic agents that are less prone to drug resistance with less toxicity, if lower therapeutic dose can be achieved due to synergistic effects.
We previously reported a series of quinazoline-based BET inhibitors [13]. In this study, we further characterized one of these compounds, CN470, and found that, in addition to its binding to BRD4 with a KD value of 23 nM, it can also bind to the bromodomains of CBP and p300 with KD values of 32 and 20 nM, respectively. Our data demonstrated that CN470 inhibited the proliferation of all MLL-r ALL cell lines. Western blot and qRT-PCR analyses revealed that CN470 suppressed the expression of oncogenic molecule MYC, and that the expression levels of the G1/S transition driver molecule, CDK6, were decreased by CN470. Moreover, the levels of the anti-apoptotic molecule, BCL2, were also decreased, while the apoptotic molecules, caspase-3 and PARP-1, were cleaved into their active forms in response to the CN470 exposure. Similarly, flow cytometric analyses showed that CN470 induced early and late apoptosis following cell cycle arrest in G1 phase. Both CN470 and OTX015 were applied at concentrations of 0.75 × and 1.5 × of their respective IC50 values. CN470 acted at much lower concentrations than OTX015 exposure, suggesting that CN470 may have an advantage over the inhibition of BRD4 alone. Our findings are consistent with the observations from the experiments using NEO2734, a previously developed BET/CBP/p300 multi-bromodomain inhibitor [18,19].
To elucidate the mechanism underlying the effects of CN470 in more detail, we performed Co-IP assays and found that CN470 reduced the binding of BRD4 to H3K27ac and the recruitment of RNA polymerase II more than OTX015. Because BRD4 and CBP/p300 can co-exist in the same protein complex [20,21], it may be possible that CN470’s inhibitory effects on the bromodomains of CBP and p300 indirectly affected the association of BRD4 to H3K27ac. Alternatively, the inhibition of the bromodomains of CBP and p300 by CN470 may have affected the acetylation of H3 histones thereby reducing the amount of the apparent H3K27ac in association with BRD4. The possibility of such allosteric modulatory effects of the bromodomains of CBP and p300 on the proximal acetyltransferase catalytic domain was recently suggested [22].
Lastly, we examined the in vivo anti-tumor effects of CN470 using a mouse xenograft model with SEMLuc/GFP cells and a PDX model using K110 cells containing the MLL-AF4 fusion gene, the most common type of MLL gene arrangement. Analysis of PDX mice is an important approach in new drug development, as PDX models more accurately reflect the patient’s disease conditions. The most appropriate to clarify the potential of CN470 is using K110 cells, derived from the patient with the worst prognosis among four patients. Daily administration of 10 mg/kg CN470 significantly prolonged the survival of both SEMLuc/GFP-xenografted mice and PDX mice. Bioluminescence study of SEMLuc/GFP-xenografted mice showed that the CN470-treated group showed significantly lower luminescent signal than the luminescence of the vehicle group. We also evaluated PB chimerism in PDX model mice, and found that the levels of patient-derived cells were lower after the CN470 treatment than the levels in the vehicle group on day 42. While BET inhibitors were reported to cause thrombocytopenia [2,5], there was no difference in the PLT count between the CN470-treated and vehicle groups of the PDX mice. In addition, oral dosing of CN470 (10 mg/kg) for six weeks did not affect myeloid or erythroid lineage cells, or body weight loss in healthy BRJ mice. We speculate that CN470 could be a safe inhibitor. But it is essential to confirm adverse effects of CN470 in the future. Taken together, multi-bromodomain inhibition of BRD4, CBP, and p300 afforded by CN470 may provide a safe and effective therapeutic option to treat MLL-r ALL.
Supplementary Material
Highlights.
CN470 inhibits the ligand binding to three bromodomain proteins: BRD4, CBP and p300
CN470 suppresses MLL-rearranged ALL cell proliferation
CN470 reduces MYC, inducing MLL-rearranged ALL apoptosis
CN470 prolongs the survivals of MLL-rearranged ALL-bearing mice and PDX model mice
Fundings
This work was partly supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan [grant numbers 19K08826 and S1511024L to E.A.], JSPS Research Fellowships for Young Scientists [grant number 20J22374 to N.I.], and Takeda Science Foundation (to I.K.). This research was supported in part by the Intramural research program of the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) (S.-M.Y. and D.J.M.).
Abbreviations:
- ALL
acute lymphoblastic leukemia
- BET
bromodomain and extra-terminal domain
- BRJ
BALB/c Rag2−/−/Jak3−/−
- CBP
cyclic AMP response element binding protein binding protein
- Co-IP
Co-immunoprecipitation
- IC50
half-maximal inhibition concentration
- MLL
mixed-lineage leukemia
- MLL-r ALL
ALL with chromosomal rearrangements involving MLL
- NOG
NOD/SCID/γcnull
- PARP-1
poly (ADP-ribose) polymerase-1
- PB
peripheral blood
- PDX
patient-derived xenograft
- PI
propidium iodide
- PLT
platelet
- qRT-PCR
quantitative reverse transcription-polymerase chain reaction
- WBC
white blood cell
- 18S rRNA
18S ribosomal RNA
Footnotes
Declaration of competing interest
N. Imayoshi, K. Tanaka, S.-M. Yang, K. Akahane, Y. Toda, S. Hosogi, T. Inukai, S, Okada, D. J. Maloney, I. Kato and E. Ashihara have no financial conflict of interest to disclose. M. Yoshioka is an employee of ConverGene LLC and holds equity in the company.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Armstrong GT, Kawashima T, Leisenring W, Stratton K, Stovall M, Hudson MM, Sklar CA, Robison LL, Oeffinger KC, Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study, J. Clin. Oncol. 32 (2014) 1218–1227. 10.1200/JCO.2013.51.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Berthon C, Raffoux E, Thomas X, Vey N, Gomez-Roca C, Yee K, Taussig DC, Rezai K, Roumier C, Herait P, Kahatt C, Quesnel B, Michallet M, Recher C, Lokiec F, Preudhomme C, Dombret H, Bromodomain inhibitor OTX015 in patients with acute leukaemia: A dose-escalation, phase 1 study, Lancet Haematol. 3 (2016) e186–e195. 10.1016/S2352-3026(15)00247-1. [DOI] [PubMed] [Google Scholar]
- [3].Dawson MA, Prinjha RK, Dittmann A, Giotopoulos G, Bantscheff M, Chan WI, Robson SC, Chung CW, Hopf C, Savitski MM, Huthmacher C, Gudgin E, Lugo D, Beinke S, Chapman TD, Roberts EJ, Soden PE, Auger KR, Mirguet O, Doehner K, Delwel R, Burnett AK, Jeffrey P, Drewes G, Lee K, Huntly BJP, Kouzarides T, Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia, Nature. 478 (2011) 529–533. 10.1038/nature10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Piha-Paul SA, Hann CL, French CA, Cousin S, Braña I, Cassier PA, Moreno V, de Bono JS, Harward SD, Ferron-Brady G, Barbash O, Wyce A, Wu Y, Horner T, Annan M, Parr NJ, Prinjha RK, Carpenter CL, Hilton J, Hong DS, Haas NB, Markowski MC, Dhar A, O’Dwyer PJ, Shapiro GI, Phase 1 study of molibresib (GSK525762), a bromodomain and extra-terminal domain protein inhibitor, in NUT carcinoma and other solid tumors, JNCI Cancer Spectr. 4 (2020) 1–9. 10.1093/JNCICS/PKZ093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Piha-Paul SA, Sachdev JC, Barve M, LoRusso P, Szmulewitz R, Patel SP, Lara PN, Chen X, Hu B, Freise KJ, Modi D, Sood A, Hutti JE, Wolff J, O’Neil BH, First-in-human study of mivebresib (ABBV-075), an oral pan-inhibitor of bromodomain and extra terminal proteins, in patients with relapsed/ refractory solid tumors, Clin. Cancer Res. 25 (2019) 6309–6319. 10.1158/1078-0432.CCR-19-0578. [DOI] [PubMed] [Google Scholar]
- [6].Filippakopoulos P, Knapp S, Targeting bromodomains: Epigenetic readers of lysine acetylation, Nat. Rev. Drug Discov. 13 (2014) 337–356. 10.1038/nrd4286. [DOI] [PubMed] [Google Scholar]
- [7].Hnisz D, Abraham BJ, Lee TI, Lau A, Saint-André V, Sigova AA, Hoke HA, Young RA, XSuper-enhancers in the control of cell identity and disease, Cell. 155 (2013) 934. 10.1016/j.cell.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lovén J, Hoke HA, Lin CY, Lau A, Orlando DA, Vakoc CR, Bradner JE, Lee TI, Young RA, Selective inhibition of tumor oncogenes by disruption of super-enhancers, Cell. 153 (2013) 320–334. 10.1016/j.cell.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bechter O, Schöffski P, Make your best BET: The emerging role of BET inhibitor treatment in malignant tumors, Pharmacol. Ther. 208 (2020) 107479. 10.1016/j.pharmthera.2020.107479. [DOI] [PubMed] [Google Scholar]
- [10].Lasko LM, Jakob CG, Edalji RP, Qiu W, Montgomery D, Digiammarino EL, Hansen TM, Risi RM, Frey R, Manaves V, Shaw B, Algire M, Hessler P, Lam LT, Uziel T, Faivre E, Ferguson D, Buchanan FG, Martin RL, Torrent M, Chiang GG, Karukurichi K, Langston JW, Weinert BT, Choudhary C, De Vries P, Van Drie JH, McElligott D, Kesicki E, Marmorstein R, Sun C, Cole PA, Rosenberg SH, Michaelides MR, Lai A, Bromberg KD, Discovery of a selective catalytic p300/CBP inhibitor that targets lineage-specific tumours, Nature. 550 (2017) 128–132. 10.1038/nature24028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mastracchio A, Lai C, Digiammarino E, Ready DB, Lasko LM, Bromberg KD, McClellan WJ, Montgomery D, Manaves V, Shaw B, Algire M, Patterson MJ, Sun CC, Rosenberg S, Lai A, Michaelides MR, Discovery of a Potent and Selective Covalent p300/CBP Inhibitor, ACS Med. Chem. Lett. 12 (2021) 726–731. 10.1021/acsmedchemlett.0c00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Welti J, Sharp A, Brooks N, Yuan W, McNair C, Chand SN, Pal A, Figueiredo I, Riisnaes R, Gurel B, Rekowski J, Bogdan D, West W, Young B, Raja M, Prosser A, Lane J, Thomson S, Worthington J, Onions S, Shannon J, Paoletta S, Brown R, Smyth D, Harbottle GW, Gil VS, Miranda S, Crespo M, Ferreira A, Pereira R, Tunariu N, Carreira S, Neeb AJ, Ning J, Swain A, Taddei D, Schiewer MJ, Knudsen KE, Pegg N, de Bono JS, Targeting the p300/cbp axis in lethal prostate cancer, Cancer Discov. 11 (2021) 1118–1137. 10.1158/2159-8290.CD-20-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yang SM, Yoshioka M, Strovel JW, Urban DJ, Hu X, Hall MD, Jadhav A, Maloney DJ, Lead optimization and efficacy evaluation of quinazoline-based BET family inhibitors for potential treatment of cancer and inflammatory diseases, Bioorganic Med. Chem. Lett. 29 (2019) 1220–1226. 10.1016/j.bmcl.2019.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yang SM, Urban DJ, Yoshioka M, Strovel JW, Fletcher S, Wang AQ, Xu X, Shah P, Hu X, Hall MD, Jadhav A, Maloney DJ, Discovery and lead identification of quinazoline-based BRD4 inhibitors, Bioorganic Med. Chem. Lett. 28 (2018) 3483–3488. 10.1016/j.bmcl.2018.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Imayoshi N, Yoshioka M, Chauhan J, Nakata S, Toda Y, Fletcher S, Strovel JW, Takata K, Ashihara E, CG13250, a novel bromodomain inhibitor, suppresses proliferation of multiple myeloma cells in an orthotopic mouse model, Biochem. Biophys. Res. Commun. 484 (2017) 262–268. 10.1016/j.bbrc.2017.01.088. [DOI] [PubMed] [Google Scholar]
- [16].Tanaka K, Kato I, Tanaka M, Morita D, Matsuda K, Takahashi Y, Nakahata T, Umeda K, Hiramatsu H, Adachi S, Takita J, Nakazawa Y, Direct Delivery of piggyBac CD19 CAR T Cells Has Potent Anti-tumor Activity against ALL Cells in CNS in a Xenograft Mouse Model, Mol. Ther. - Oncolytics. 18 (2020) 37–46. 10.1016/j.omto.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Deshaies RJ, Multispecific drugs herald a new era of biopharmaceutical innovation, Nature. 580 (2020) 329–338. 10.1038/s41586-020-2168-1. [DOI] [PubMed] [Google Scholar]
- [18].Spriano F, Gaudio E, Cascione L, Tarantelli C, Melle F, Motta G, Priebe V, Rinaldi A, Golino G, Mensah AA, Aresu L, Zucca E, Pileri S, Witcher M, Brown B, Wahlestedt C, Giles F, Stathis A, Bertoni F, Antitumor activity of the dual BET and CBP/EP300 inhibitor NEO2734, Blood Adv. 4 (2020) 4124–4135. 10.1182/bloodadvances.2020001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ryan KR, Giles F, Morgan GJ, Targeting both BET and CBP/EP300 proteins with the novel dual inhibitors NEO2734 and NEO1132 leads to anti-tumor activity in multiple myeloma, Eur. J. Haematol. 106 (2021) 90–99. 10.1111/ejh.13525. [DOI] [PubMed] [Google Scholar]
- [20].Wu T, Kamikawa YF, Donohoe ME, Brd4’s Bromodomains Mediate Histone H3 Acetylation and Chromatin Remodeling in Pluripotent Cells through P300 and Brg1, Cell Rep. 25 (2018) 1756–1771. 10.1016/j.celrep.2018.10.003. [DOI] [PubMed] [Google Scholar]
- [21].Roe JS, Mercan F, Rivera K, Pappin DJ, Vakoc CR, BET Bromodomain Inhibition Suppresses the Function of Hematopoietic Transcription Factors in Acute Myeloid Leukemia, Mol. Cell. 58 (2015) 1028–1039. 10.1016/j.molcel.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Brand M, Clayton J, Moroglu M, Schiedel M, Picaud S, Bluck JP, Skwarska A, Bolland H, Chan AKN, Laurin CMC, Scorah AR, See L, Rooney TPC, Andrews KH, Fedorov O, Perell G, Kalra P, Vinh KB, Cortopassi WA, Heitel P, Christensen KE, Cooper RI, Paton RS, Pomerantz WCK, Biggin PC, Hammond EM, Filippakopoulos P, Conway SJ, Controlling Intramolecular Interactions in the Design of Selective, High-Affinity Ligands for the CREBBP Bromodomain, J. Med. Chem. 64 (2021) 10102–10123. 10.1021/acs.jmedchem.1c00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


