Abstract
Objective:
To investigate the association between white matter diffuse excessive high signal intensity (DEHSI) on neonatal magnetic resonance imaging (MRI) in very preterm infants and neurobehavioral outcomes at the age of 13 years.
Study design:
MR images of very preterm children (<30 weeks’ gestational age or <1250 g birth weight) were evaluated at term-equivalent age with DEHSI classified into 5 grades. Additionally, visibility of the posterior periventricular crossroads was assessed. General intelligence, memory, attention, executive function, motor abilities and behavior were examined in 125 children at age 13 and related to DEHSI grades using linear regression.
Results:
DEHSI was detected in 93% of infants; 21% grade 1, 22% grade 2. 32% grade 3, and 18% grade 4. Neurobehavioral outcomes were similar for all DEHSI groups. There was weak evidence that higher DEHSI grades related to higher verbal IQ and attention; and that lower DEHSI grades related to better planning ability. Adjustment for gestational age, birth weight standard score and sex further weakened these effects. Only 12 children had invisible posterior crossroads and showed slightly poorer outcomes at 13 years.
Conclusion:
There was little evidence that neonatal DEHSI serves as a sensitive biomarker for later impairment. Further investigation on the importance of invisible posterior periventricular crossroads in larger samples is needed.
Keywords: prematurity, neurobehavior, magnet resonance imaging, cognition
Although survival rates of very preterm (< 32 weeks’ gestation) infants have increased significantly due to enhanced neonatal care, preterm birth is associated with neonatal brain injury, in particular to the white matter1. Although focal cystic periventricular lesions are relatively rare, diffuse white matter abnormalities are the most common neuropathology found in very preterm infants1. Furthermore, diffuse excessive high signal intensity (DEHSI) with increased signal intensity in the white matter on T2-weighted magnetic resonance imaging (MRI) around term-equivalent age has been reported in approximately 55–75% of infants born very preterm2 and 75–80% of infants born extremely preterm3. DEHSI can occur in isolation or in addition to other white matter changes, including reduced volume, cystic lesions and delayed myelination4. There has been debate as to whether DEHSI reflects brain pathology serving as biomarker for later neurodevelopment5 or alterations in maturational characteristics reflecting a developmental phenomenon2,6–8.
White matter alterations on neonatal MRI have been associated with consequences on cognition, motor development and behavior in very preterm children.9,10 Cognitive, motor and behavioral problems following preterm birth remain a significant burden11. It is therefore important to determine precisely which findings on neonatal MR serve as predictors of long-term outcome in very preterm children, in order to provide prognostic information for caregivers and families. Although some studies have reported that DEHSI is associated with worse outcome4,12–14 the balance of evidence suggests no relationship with outcome2,7,8,15–17. To date, no study has reported associations between DEHSI and outcome beyond early school-age and there is a lack of studies assessing specific cognitive domains including memory, attention and executive function. Thus, the aim of this study was to investigate the associations between neonatal DEHSI and neurobehavioral outcomes in VPT 13-year olds. We hypothesized that DEHSI would not be strongly related to neurobehavioral outcome.
Methods
Participants were VPT infants (gestational age <30 weeks and/or birth weight <1250 g) admitted to the Royal Women’s Hospital, Melbourne between July 2001 and December 2003 and recruited into a prospective longitudinal cohort study called the Victorian Infant Brain Study (VIBeS). Of 348 eligible VPT infants admitted to the neonatal nursery, 224 children without genetic or congenital abnormalities likely to interfere with development (e.g. craniosynostosis, septo-optic dysplasia) were recruited. Two hundred and nine infants underwent MR imaging at term-equivalent age (40 weeks gestational age ± 2 weeks). Of those, 49 infants were excluded due to limited quality of the T2-weighted sequences (n=24), major cerebral injury seen on term-equivalent MRI (n = 15; 3 with cystic periventricular leukomalacia, 2 with extensive cerebral hemorrhagic lesions, 9 with periventricular hemorrhagic infarction, and 1 with treated posthemorrhagic ventricular dilation) and congenital anomalies (n = 10), resulting in usable neonatal MRI data in 160 infants. Children in the VIBeS cohort have been followed-up at 2, 5, 7, and 13 years. The present study included all children who had usable neonatal MRI data and who were assessed at the 13-year follow-up. This study was approved by the Human Research and Ethics Committees of the Royal Women’s Hospital and the Royal Children’s Hospital, and informed written consent was obtained from the parents at all time points.
MR Imaging
MR images were obtained on a 1.5T Signa LX EchoSpeed system (GE Healthcare Milwaukee, Wisconsin) without any sedation, including 1) a 3D spoiled gradient-recalled echo sequence (0.8 – 1.6-mm coronal sections; flip angle, 45°; TR, 35 ms; TE, 9 ms; FOV, 210 × 157.5 mm; matrix, 256 × 192, interpolated 256 × 256) and 2) a double-echo (proton-density and T2-weighted) spin-echo sequence (1.7 – 3-mm axial sections; flip angle, 90°; TR, 4000 ms; TE, 60 and 160 ms; FOV, 220 × 165 mm; matrix, 256 × 192, interpolated 512 × 512; interleaved acquisition).
MR Imaging Analysis
Grading of DEHSI was conducted by 1 investigator trained in fetal and preterm MR imaging and blinded to the clinical data. Grading was based on severity and extent of signal intensity and classified into 5 grades: 0 = no DEHSI throughout the white matter; 1 = DEHSI visible only within the crossroads; 2 = DEHSI visible in one region additional to the crossroads; 3 = DEHSI visible in two additional regions; 4 = DEHSI visible in three additional regions (extensive white matter) (For an illustration of the grades refer to Kidokoro et al, 201117).
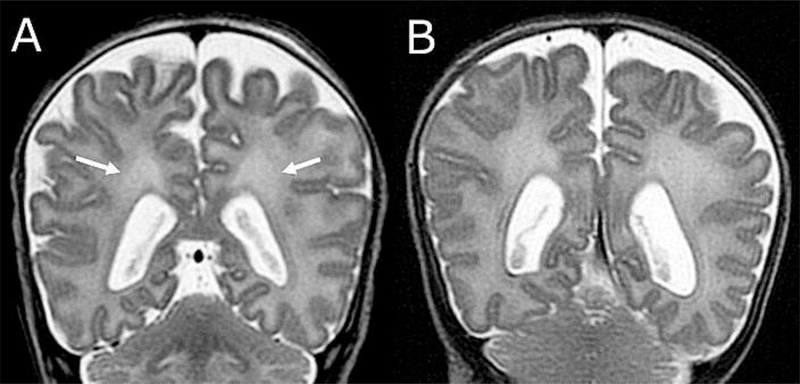
For infants with DEHSI grades 2–4, we additionally assessed whether the margins of the posterior periventricular crossroads were visible. Healthy variations of immature white matter typical for this age of assessment are visible as high signal intensity in the white matter of the posterior periventricular crossroads18 (An example is shown in Figure 1; available at www.jpeds.com). If crossroads were visible, this indicated typical signal intensity; widespread signal intensity such that the periventricular crossroads were invisible indicated signal intensity beyond the healthy maturational variations.
Figure 1.
(online). Samples of visible (typical) and invisible (atypical) posterior periventricular crossroads. A. High signal intensity is visible within the crossroads (arrows). B. Posterior periventricular crossroads are invisible because of widespread homogeneous high signal intensity of the entire cerebral white matter surrounding the crossroads.
The inter-observer agreement on the DEHSI grade was assessed in 15 infants by 2 authors using Kappa statistics. The observers showed complete agreement in 10 infants, a difference of 1 grade in 4, and a difference of 2 grades in 1 infant (κ = 0.58). Test-retest agreement in the 15 infants was perfect (κ = 1).
Neurobehavioral Outcome Measures
At the 13-year follow-up, general intelligence was estimated with the Kaufman Brief Intelligence Test, Second Edition (KBIT-2)19, of which the KBIT IQ Composite, Verbal and Nonverbal standard scores (M = 100, SD = 15) are reported. Short term memory was evaluated using the Digit Recall subtest and working memory was investigated using the Backward Digit Recall subtest (M = 100, SD = 15) of the Working Memory Test Battery for Children (WMTB-C)20. Attention was assessed using subtests from the Test of Everyday Attention for Children (TEA-Ch)21 including Score! (sustained attention) and Map Mission (selective attention; M = 10, SD = 3). Executive Function was evaluated using the subtests from the Behavioural Assessment of the Dysexecutive System for Children (BADS-C)22. The Zoo Map test captures the ability to plan and execute a specific eight-location sequence in accordance with several rules (Zoo Map total score, M = 10, SD = 3) and the Six Part Test examines planning, task scheduling and performance monitoring skills (Six Part total score, M = 10, SD = 3). The behavioral manifestations of children’s executive control functions were assessed with the Behavior Rating Inventory of Executive Function (BRIEF)23, a parent-completed rating scale including a Global Executive Composite (GEC), a Behavioral Regulation Index (BRI) and a Metacognition Index (MI) (M = 50, SD = 10; high scores reflect worse outcome). Motor function was evaluated with the Movement Assessment Battery for Children 2 (M-ABC-2)24. A total composite score, derived from the summarized subtest standard scores, and ‘manual dexterity’, ‘aiming and catching’ as well as ‘balance’ component scores are reported (M = 10, SD = 3). Higher scores reflected better functional outcome in all of the abovementioned measures except the BRIEF parent rating, where elevated scores reflected worse outcome.
Statistical Analyses
Baseline characteristics were described for participants with differing degrees of DEHSI as proportions (categorical data) and means (SDs) (continuous data).
Because our sample included a number of multiple births, regression models were fitted by using generalized estimating equations with an exchangeable correlation structure and are reported with robust standard errors to allow for nonindependence of multiples25. Associations between DEHSI and neurobehavioral outcome were examined using linear regression with separate models for each predictor-outcome combination. Analyses were repeated adjusting for gestational age, female sex and birth weight SD score, variables known to be associated with improvements in most of the outcomes studied.
Further, for infants with DEHSI grades 2–4, linear regression models were fitted to examine differences in neurobehavioral outcomes between those children who had visible and those who had invisible posterior crossroads in their neonatal scan. Analyses were conducted by using SPSS version 24 (IBM SPSS Statistics, IBM Corporation) and Stata 14.2 (Stata Corp, College Station, TX). Interpretation of the findings was based on overall patterns and magnitude of differences rather than individual P values26.
Results
Patient Characteristics
Of 160 preterm infants who were eligible and had usable neonatal MRI data, 125 children had usable MRI data and follow-up data at 13 years (mean age 13.3, 0.4 SD). Thirty-five children were not assessed at the 13-year follow-up (unable to contact n=8; withdrawn n=8; declined to participate n=18; died n=1). Participants’ neonatal characteristics are summarized in Table 1 (available at www.jpeds.com). Participants and non-participants had similar perinatal/neonatal characteristics (Table 2; available at www.jpeds.com).
Table 1.
Participant characteristics
| Grade 0 n=9 | Grade 1 n=26 | Grade 2 n=28 | Grade 3 n=40 | Grade 4 n=22 | Total N = 125 | |
|---|---|---|---|---|---|---|
| Gestational age at birth - weeks (SD) | 26.1 (1.9) | 26.6 (1.4) | 27.8 (2.2) | 27.9 (2.0) | 27.7 (1.7) | 27.5 (1.9) |
| Postmenstrual age at MRI - weeks (SD) | 40.4 (1.5) | 40.1 (1.9) | 39.9 (1.0) | 40.5 (1.5) | 40.5 (1.2) | 40.3 (1.5) |
| Birthweight - g (SD) | 789 (183) | 875 (236) | 967 (228) | 1007 (209) | 985 (214) | 951 (225) |
| Birthweight z-score (SD) | −0.5 (1.3) | −0.5 (0.9) | −0.8 (1.0) | −0.6 (0.9) | −0.6 (0.9) | −0.6 (0.9) |
| Exposure to antenatal corticosteroids No. (%) | 8 (89) | 25 (96) | 26 (93) | 34 (85) | 19 (86) | 112 (90) |
| Exposure to postnatal corticosteroids No. (%) | 2 (22) | 3 (12) | 2 (7) | 3 (8) | 2 (9) | 12 (10) |
| Proven sepsis No. (%) | 4 (44) | 11 (42) | 16 (57) | 14 (35) | 11 (50) | 56 (45) |
| Proven necrotizing enterocolitis No. (%) | 1 (11) | 2 (8) | 0 | 3 (8) | 2 (9) | 8 (6) |
| Bronchopulmonary dysplasia No. (%) | 4 (44) | 10 (39) | 10 (36) | 14 (35) | 6 (27) | 44 (35) |
| Grade III-IV intraventricular hemorrhage No. (%)a | 0 | 1 (4) | 0 | 1 (3) | 0 | 2 (2) |
| Cystic periventricular leucomalacia No. (%)a | 0 | 1 (4) | 0 | 1 (3) | 1 (5) | 3 (2) |
| Patent ductus arteriosus No. (%) | 5 (56) | 17 (65) | 10 (36) | 18 (45) | 12 (54) | 62 (50) |
| Female sex No. (%) | 6 (67) | 13 (50) | 10 (36) | 19 (48) | 10 (46) | 58 (47) |
| Singleton No. (%) | 6 (67) | 9 (35) | 16 (57) | 22 (55) | 16 (73) | 69 (55) |
Note. SD=standard deviation. Bronchopulmonary dysplasia defined as oxygen dependency at 36 weeks of gestation.
Detected on neonatal ultrasound.
Table 2.
Neonatal characteristics in participants and non-participants
| Participants N = 125 | Non-participants N = 99 | Mean difference (95% CI) | P-value | |
|---|---|---|---|---|
| Gestational age at birth - weeks (SD) | 27.5 (1.9) | 27.5 (1.8) | 0 (−0.5, 0.4) | 0.90 |
| Postmenstrual age at MRI - weeks (SD) | 40.3 (1.5) | 40.2 (2.0) | 0.1 (−0.4, 0.5) | 0.82 |
| Birthweight - g (SD) | 951 (225) | 973 (226) | −30.5 (−85.3, 24.3) | 0.27 |
| Birthweight z-score (SD) | −0.6 (0.9) | −0.5 (0.9) | −0.1 (−0.4, 0.1) | 0.28 |
| Exposure to antenatal corticosteroids No. (%) | 112 (90) | 86 (90) | 0.1 (0, 0.2) | 0.21 |
| Exposure to postnatal corticosteroids No. (%) | 12 (10) | 9 (9) | 0 (−0.1, 0.1) | 0.75 |
| Proven sepsis No. (%) | 56 (45) | 42 (42) | 0.4 (−0.1, 0.2) | 0.53 |
| Proven necrotizing enterocolitis No. (%) | 8 (6) | 2 (2) | 0.1 (−0.1, 0.2) | 0.28 |
| Bronchopulmonary dysplasia No. (%) | 44 (35) | 31 (31) | 0 (−0.3, 0.2) | 0.69 |
| Grade III-IV intraventricular hemorrhage No. (%)a | 2 (2) | 6 (6) | 0 (0, 0.1) | 0.09 |
| Cystic periventricular leucomalacia No. (%)a | 3 (2) | 6 (6) | 0 (−0.1, 0) | 0.19 |
| Patent ductus arteriosus No. (%) | 62 (50) | 48 (49) | 0.1 (−0.1, 0.2) | 0.37 |
| Female sex No. (%) | 58 (47) | 52 (53) | 0 (−0.2, 0.1) | 0.47 |
| Singleton No. (%) | 69 (55) | 61 (62) | 0.1 (−0.1, 0.2) | 0.40 |
Note. Estimates of regression coefficients from separate linear regression models fitted using generalized estimating equations to allow for clustering of twins. SD=standard deviation. Bronchopulmonary dysplasia defined as oxygen dependency at 36 weeks of gestation.
Detected on neonatal ultrasound. 35 of the non-participating children had DEHSI scoring with 11% showing grade 0, 40% grade 1, 11% grade 2, 37% grade 3 and none grade 4.
Most infants had some grade of DEHSI visible on their term-equivalent MRI (Table 1). The neonatal characteristics of the DEHSI groups were generally comparable but the DEHSI grade 0 group was small (n=9) and tended to have a lower mean birth weight.
Neurobehavioral Outcomes after DEHSI
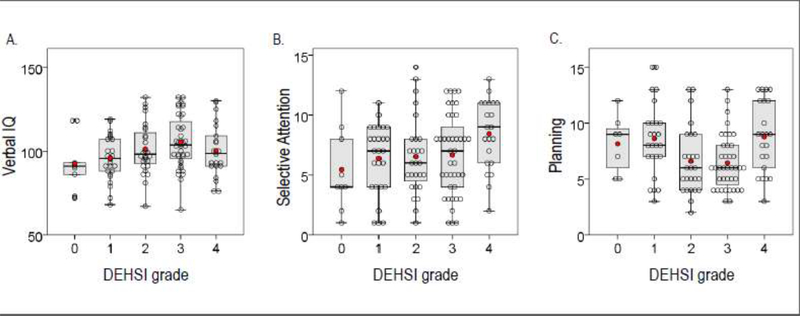
Neurobehavioral outcomes for each DEHSI grade are summarized in Table 3. Outcomes were broadly similar between groups. Regression analysis yielded weak evidence for linear relationships between DEHSI and verbal IQ, selective attention and planning ability (Table 4), but Figure 2 demonstrates that the relationships were more complex, and not clinically meaningful. Adjusting for gestational age, birth weight SD score and sex reduced the strength of the evidence for the linear associations between DEHSI grade and most outcomes; only the linear relationship with planning ability retained some evidence to support it.
Table 3.
Neurobehavioral outcomes at 13 years for children in each grade of DEHSI
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Neurobehavioral domain | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | N | Mean (SD) |
| General intelligence | ||||||||||||
| IQ composite | 9 | 91.9 (20.7) | 26 | 97.9 (14.5) | 28 | 101.1 (16.7) | 40 | 104.8 (18.1) | 22 | 101.3 (16.6) | 125 | 101.0 (17.1) |
| Verbal IQ | 9 | 92.6 (16.4) | 26 | 95.7 (13.2) | 28 | 101.2 (15.0) | 40 | 105.2 (15.7) | 22 | 100.2 (15.7) | 125 | 100.5 (15.4) |
| Nonverbal IQ | 9 | 92.4 (24.1) | 26 | 100.2 (15.7) | 28 | 100.1 (17.7) | 40 | 102.6 (18.3) | 22 | 101.9 (16.9) | 125 | 100.7 (17.8) |
| Memory | ||||||||||||
| Short term memory -Digit span forward | 9 | 95.9 (21.1) | 26 | 97.4 (15.4) | 28 | 98.6 (20.1) | 40 | 92.4 (14.5) | 22 | 93.3 (16.5) | 125 | 95.3 (16.8) |
| Working memory- Digit span backwards | 9 | 89.3 (14.1) | 26 | 94.2 (15.4) | 28 | 95.0 (13.0) | 40 | 89.1 (12.0) | 22 | 94.6 (17.4) | 125 | 92.5 (14.2) |
| Attention | ||||||||||||
| Sustained attention – Score! | 9 | 7.4 (4.3) | 26 | 8.9 (3.5) | 28 | 8.4 (3.7) | 40 | 7.8 (3.6) | 22 | 7.6 (3.6) | 125 | 8.1 (3.6) |
| Selective attention – Map mission | 9 | 5.4 (3.5) | 26 | 6.4 (2.9) | 27 | 6.5 (3.3) | 40 | 6.7 (3.4) | 21 | 8.4 (2.9) | 123 | 6.8 (3.2) |
| Executive function | ||||||||||||
| Planning – Zoo Map 1 | 7 | 8.0 (3.3) | 25 | 7.7 (3.6) | 26 | 8.8 (3.6) | 39 | 7.7 (3.3) | 22 | 8.2 (3.6) | 119 | 8.0 (3.5) |
| Planning & Monitoring – 6 parts test | 7 | 8.1 (2.6) | 25 | 8.6 (3.3) | 26 | 6.6 (3.0) | 39 | 6.4 (2.4) | 22 | 8.8 (3.2) | 119 | 7.5 (3.0) |
| Motor function | ||||||||||||
| Global Composite | 7 | 8.1 (3.6) | 25 | 8.6 (3.0) | 26 | 8.4 (2.2) | 35 | 9.0 (3.0) | 21 | 9.1 (3.0) | 114 | 8.8 (2.9) |
| Manual dexterity | 7 | 7.7 (2.6) | 25 | 7.6 (2.9) | 26 | 7.8 (2.7) | 37 | 8.7 (3.2) | 21 | 8.5 (3.4) | 116 | 8.2 (3.0) |
| Aiming and Catching | 8 | 7.9 (3.4) | 26 | 9.6 (3.7) | 27 | 10.6 (3.4) | 35 | 9.6 (3.2) | 21 | 11.2 (2.9) | 117 | 10.0 (3.4) |
| Balance | 7 | 9.6 (4.2) | 25 | 9.5 (2.4) | 26 | 8.6 (2.4) | 35 | 9.4 (3.1) | 21 | 8.9 (2.9) | 114 | 9.2 (2.8) |
| Parent Rating Behaviora | ||||||||||||
| Global executive Composite | 7 | 59.0 (11.9) | 23 | 56.9 (12.3) | 26 | 55.3 (12.8) | 36 | 52.7 (11.2) | 20 | 55.5 (15.2) | 112 | 55.0 (12.5) |
| Behavioral Regulation Index | 7 | 58.0 (13.3) | 23 | 53.7 (10.2) | 26 | 54.5 (14.8) | 36 | 51.6 (11.1) | 20 | 56.2 (18.7) | 112 | 53.9 (13.5) |
| Metacognition Index | 7 | 58.7 (13.3) | 23 | 58.1 (13.0) | 26 | 55.1 (11.9) | 36 | 52.9 (11.4) | 20 | 54.4 (12.7) | 112 | 55.1 (12.2) |
Note. IQ=Intelligence quotient.
Higher scores reflective of more behavioral difficulties.
Table 4.
Association between DEHSI and neurobehavioral outcomes in very preterm children
| Neurobehavioral outcome | n | Estimate (95%CI) | p-value | Adjusted estimate(95% CI)* | Adjusted p-value* |
|---|---|---|---|---|---|
| General intelligence | |||||
| IQ composite | 125 | 2.5 (0.0, 4.9) | 0.05 | 1.8 (−0.9, 4.4) | 0.19 |
| Verbal IQ | 125 | 2.6 (0.4, 4.9) | 0.022 | 2.2 (−0.2, 4.6) | 0.07 |
| Nonverbal IQ | 125 | 1.7 (−1.1, 4.5) | 0.24 | 1.0 (−1.8, 3.7) | 0.49 |
| Memory | |||||
| Short term memory - Digit span forward | 125 | −1.3 (−3.8, 1.2) | 0.30 | −2.2 (−4.4, 0.1) | 0.06 |
| Working memory- Digit span backwards | 125 | −0.1 (−2.4, 2.2) | 0.94 | −0.8 (−2.9, 1.3) | 0.46 |
| Attention | |||||
| Sustained attention – Score! | 125 | −0.3 (−0.8, 0.3) | 0.35 | −0.4 (−1.0, 0.2) | 0.16 |
| Selective attention – Map mission | 123 | 0.6 (0.1, 1.0) | 0.020 | 0.5 (−0.0, 1.00 | 0.06 |
| Executive function | |||||
| Planning – Zoo Map 1 | 119 | 0.0 (−0.5, 0.6) | 0.93 | −0.1 (−0.6, 0.5) | 0.75 |
| Planning & Monitoring – 6 parts test | 119 | −0.7 (−1.2, -0.1) | 0.023 | −0.6 (−1.1, -0.1) | 0.027 |
| Motor function | |||||
| Global Composite | 114 | 0.2 (−0.3, 0.7) | 0.38 | 0.1 (−0.4, 0.6) | 0.77 |
| Manual dexterity | 116 | 0.4 (−0.1, 0.9) | 0.10 | 0.3 (−0.2, 0.8) | 0.29 |
| Aiming and Catching | 117 | 0.5 (−0.5, 1.0) | 0.08 | 0.3 (−0.3, 0.8) | 0.31 |
| Balance | 114 | −0.1 (−0.6, 0.4) | 0.64 | −0.2 (−0.6, 0.3) | 0.49 |
| Behavioral executive functiona | |||||
| Global Executive Composite | 112 | −1.1 (−3.5, 1.3) | 0.37 | −0.9 (−3.2, 1.4) | 0.45 |
| Behavioral Regulation Index | 112 | 0.4 (−1.7, 2.5) | 0.70 | 0.7 (−1.7, 2.9) | 0.57 |
| Metacognition Index | 112 | −1.5 (−3.8, 0.8) | 0.21 | −1.5 (−4.0, 1.0) | 0.24 |
Note. Estimates of regression coefficients from separate linear regression models fitted using generalized estimating equations to allow for clustering of twins. CI=confidence interval, IQ=Intelligence quotient. Estimates and 95% CI from linear regression models fitted using generalized estimating equations with an exchangeable correlation structure to account for clustering due to multiple births.
Higher scores reflective of higher amount of reported behavioral difficulties.
adjusted for gestational age, female sex and birthweight SD score.
Figure 2.
Neurobehavioral outcomes at 13 years for children in each DEHSI grade. Scatterplots showing individual data points (empty circles), group mean (red dots), overlaid with boxplots indicating first and third quartiles (bottom and top of box), the median (band inside the box) and maximum 1.5 interquartile range (end of whiskers). Results are shown for A. Verbal IQ, B. selective attention and C. planning ability.
Neurodevelopmental Outcomes in “invisible” Posterior Crossroads
Among the 90 children with grade 2–4 DEHSI, 12 had invisible posterior crossroads (5 in grade 3 and 7 in grade 4 DEHSI), and 78 had visible posterior crossroads (28/28 children who had grade 2 DEHSI had visible crossroads, 35/40 children in grade 3 DEHSI and 15/22 in grade 4 DEHSI). There was weak evidence that invisibility of posterior crossroads was related to worse neurobehavioral outcome, with lower scores in tests of intelligence, memory, attention, and executive function, and higher scores in the behavioral questionnaire compared with children with visible crossroads in 15 of the 16 variables (Table 5).
Table 5.
Neurobehavioral outcome in children with invisible vs. visible posterior crossroads
| Neurobehavioral outcome | Invisible Mean | SD | n | Visible Mean | SD | n | Mean Difference (95% CI) | p |
|---|---|---|---|---|---|---|---|---|
| General intelligence | ||||||||
| IQ Composite | 98.8 | 15.2 | 12 | 103.4 | 17.5 | 78 | −3.5 (−12.2, 5.1) | 0.42 |
| Verbal IQ | 96.3 | 14.1 | 12 | 103.7 | 15.5 | 78 | −6.5 (−14.8, 1.8) | 0.13 |
| Nonverbal IQ | 101.6 | 16.6 | 12 | 101.6 | 17.9 | 78 | −1.0 (−9.9, 7.9) | 0.83 |
| Memory | ||||||||
| Short term memory -Digit span forward | 85.8 | 19.3 | 12 | 95.9 | 16.2 | 78 | −9.8 (−20.6, 1.1) | 0.08 |
| Working memory- Digit span backwards | 87.5 | 12.9 | 12 | 93.0 | 14.0 | 78 | −6.4 (−14.4, 1.7) | 0.12 |
| Attention | ||||||||
| Sustained attention – Score! | 7.6 | 4.3 | 12 | 8.0 | 3.5 | 78 | −0.4 (−2.8, 2.0) | 0.75 |
| Selective attention – Map mission | 6.6 | 3.2 | 11 | 7.1 | 3.3 | 77 | −0.7 (−2.7, 1.3) | 0.50 |
| Executive function | ||||||||
| Planning – Zoo Map 1 | 7.3 | 3.6 | 12 | 8.3 | 3.4 | 75 | −1.1 (−3.1, 1.0) | 0.31 |
| Planning & Monitoring – 6 parts test | 6.8 | 2.9 | 12 | 7.1 | 3.0 | 75 | −0.5 (−1.9, 1.1) | 0.56 |
| Motor function | ||||||||
| Global Composite | 8.9 | 3.6 | 11 | 8.9 | 2.7 | 71 | −0.2 (−2.3, 1.9) | 0.86 |
| Manual dexterity | 8.5 | 3.8 | 11 | 8.4 | 3.0 | 73 | −0.5 (−2.8, 1.8) | 0.70 |
| Aiming and Catching | 10.6 | 3.8 | 11 | 10.3 | 3.2 | 72 | 0.3 (−2.0, 2.5) | 0.92 |
| Balance | 8.4 | 3.0 | 11 | 9.1 | 2.8 | 71 | −0.8 (−2.7, 1.0) | 0.37 |
| Behavioral executive functiona | ||||||||
| Global Executive Composite | 58.5 | 13.6 | 10 | 53.4 | 12.6 | 72 | 2.8 (−5.0, 10.5) | 0.49 |
| Behavioral Regulation Index | 55.1 | 15.2 | 10 | 53.2 | 14.3 | 72 | 1.5 (−6.5, 9.5) | 0.71 |
| Metacognition Index | 59.2 | 12.3 | 10 | 53.6 | 11.6 | 72 | 4.5 (−3.0, 12.0) | 0.24 |
Note. Estimates of regression coefficients from separate linear regression models fitted using generalized estimating equations to allow for clustering of twins. CI = confidence interval, IQ=Intelligence quotient.
Higher scores reflective of more behavioral difficulties.
Discussion
It is established that DEHSI is visible on neonatal MRI in a high proportion of VPT children. There is ongoing debate as to whether DEHSI serves as a biomarker for later neurodevelopmental outcome. To date, results are inconsistent and there are no reports on long-term outcome in VPT adolescents, which allow for a reliable examination of specific areas of cognitive function, such as executive function27. In our sample of 125 VPT children without severe cerebral injuries at term-equivalent age, there was little evidence that the extent of DEHSI was systematically related to poorer neurobehavioral outcome including intelligence, memory, attention, executive function, motor abilities and behavior at 13 years of age.
Our findings are in line with studies examining associations between DEHSI and outcome on developmental tests in preschoolers2,6–8,15 and on a broader range of tests in early school-age children16. Nevertheless, some studies have found DEHSI to be related to early development in VPT and extremely low birth weight (<1000g) preschoolers4,12. Further, a sample of high-risk preterm infants with DEHSI performed nearly ten points lower (0.67 SD) on Full-Scale IQ at six years of age compared with those without DEHSI13. Additional follow-up of these groups into late childhood is important to determine whether these group differences persist.
The lack of systematic associations between DEHSI assessed based on the presence and severity of signal intensity in our relatively large cohort followed-up until 13 years suggests that neonatal DEHSI has little predictive value for neurodevelopmental outcome. Thus, our findings suggest that DEHSI reflect a transient developmental phenomenon, or alternatively, that the immature brain has the capacity for compensation. In order to predict functional outcome, a more comprehensive scoring system of neonatal MRI combining assessments of the nature and extent of white-matter signal abnormality, loss of volume of periventricular white matter, the extent of cystic abnormalities, ventricular dilatation and the thinning of the corpus callosum, may be more useful9,28. Such a scoring system has been used to predict poorer cognitive, motor and neurosensory impairment in VPT 2-year-olds28 and poorer IQ, academic and motor outcome at 7 years of age beyond the effect of other perinatal and neonatal variables9.
Children with grade 0 DEHSI had lower means on some of the reported measures. However, this group comprised only 9 children, which limits the generalizability of this finding and limits speculation about whether DEHSI might just be a normal variant in very preterm children at term-equivalent age.
We also investigated the presence of high signal intensity within the periventricular posterior crossroads, which form important intersections for projection and corticocortical fiber pathways29. In the same sample of children assessed for the present study, invisible posterior crossroads were associated with a reduction in cognitive development and an increased risk of cognitive delay at age two years17. In the present study, there was some evidence that 13 year-old children with invisible crossroads showed worse outcome across a range of neurobehavioral measures, although mean differences were non-significant and smaller than at the two-year follow-up. Although our results may be suggestive of invisible periventricular posterior crossroads being a possible biomarker of later neurodevelopmental outcome, our study sample with invisible crossroads was small (n=12) and further studies in other cohorts, with larger samples are required to further investigate this relationship.
Methodological limitations of the DEHSI rating limit the interpretation of our findings. It has been suggested that T2-weighted sequences should be optimized using fluid-attenuated inverse recovery (FLAIR) for a better distinction between DEHSI and CSF, as they may appear in the same intensity distribution in conventional T2 images30. Furthermore, signal intensity of T2-weighted images is susceptible to magnetic field inhomogeneity. Increased signal intensity caused by field inhomogeneity may be confused with DEHSI31. Hence, T2 relaxometry is suggested as it is thought to provide improved distinction between CSF and DEHSI over that of conventional T2-weighted imaging31.
Scoring DEHSI is potentially variable and subjective in nature16. In the present study, scoring was done by one physician only. In a subset of the present study, however, inter- and intra-rater agreement were moderate. This may have contributed to the lack of associations between DEHSI and neurobehavioral outcomes in the present study. Further studies should examine the predictive value of automated DEHSI scoring for long-term neurobehavioral outcomes.
Although we had some attrition, compared with other studies, our sample is large and to the best of our knowledge, no other study has reported relationships between DEHSI and such a broad range of cognitive and motor outcomes in early adolescence.
The presence of qualitatively defined DEHSI on term-equivalent MRI does not appear useful as a predictor of long-term neurobehavioral outcome in VPT children. The predictive value of the less common finding of a lack of visibility of the posterior periventricular crossroads with DEHSI needs further investigation.
Acknowledgements
We thank Merilyn Bear for her assistance in the recruitment of the infants and we are indebted to the families of the Victorian Infant Brain Study for their participation in the study.
Supported by Australia’s National Health and Medical Research Council, including Centre for Clinical Research Excellence (546519), Center for Research Excellence (1060733), Project Grants (237117 and 491209), Senior Research Fellowship (1081288 [to P.A.]), Career Development Fellowship (1085754 [to D.T.] and 1141354 [to J.C.]), and Early Career Fellowship (1012236 [to D.T.] and 1053787 [to J.C.]). Also supported by the Victorian Government’s Operational Infrastructure Support Program and the US National Institutes of Health (HD058056). The authors have no conflicts of interest to disclose.
Abbreviations:
- MRI
Magnetic resonance imaging
- DEHSI
Diffuse Excessive High Signal Intensity
- SD
standard deviation
Footnotes
Portions of this study were presented as an abstract at the Perinatal Society of Australia and New Zealand Meeting, March 25–28, 2018, Auckland, New Zealand, and at the Pediatric Academic Societies annual meeting, May 5–8, 2018, Toronto, Ontario.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Volpe J Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 2009;8:110–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeon TY, Kim JH, Yoo S-Y, Eo H, Kwon J-Y, Lee J, et al. Neurodevelopmental Outcomes in Preterm Infants: Comparison of Infants with and without Diffuse Excessive High Signal Intensity on MR Images at Near–term-equivalent Age. Radiology 2012;263:518–26. [DOI] [PubMed] [Google Scholar]
- 3.Skiöld B, Horsch S, Hallberg B, Engström M, Nagy Z, Mosskin M, et al. White matter changes in extremely preterm infants, a population-based diffusion tensor imaging study. Acta Paediatr 2010;99:842–9. [DOI] [PubMed] [Google Scholar]
- 4.Dyet LE, Kennea N, Counsell SJ, Maalouf EF, Ajayi-Obe M, Duggan PJ, et al. Natural History of Brain Lesions in Extremely Preterm Infants Studied With Serial Magnetic Resonance Imaging From Birth and Neurodevelopmental Assessment. Pediatrics 2006;118:536–48. [DOI] [PubMed] [Google Scholar]
- 5.Cheong JLY, Thompson DK, Wang HX, Hunt RW, Anderson PJ, Inder TE, et al. Abnormal white matter signal on MR imaging is related to abnormal tissue microstructure. Am J Neuroradiol 2009;30:623–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leitner Y, Weinstein M, Myers V, Uliel S, Geva K, Berger I, et al. Diffuse excessive high signal intensity in low-risk preterm infants at term-equivalent age does not predict outcome at 1 year: A prospective study. Neuroradiology 2014;56:669–78. [DOI] [PubMed] [Google Scholar]
- 7.de Bruïne FT, van den Berg-Huysmans AA, Leijser LM, Rijken M, Steggerda SJ, van der Grond J, et al. Clinical Implications of MR Imaging Findings in the White Matter in Very Preterm Infants: A 2-year Follow-up Study. Radiology 2011;261:899–906. [DOI] [PubMed] [Google Scholar]
- 8.Calloni SF, Cinnante CM, Bassi L, Avignone S, Fumagalli M, Bonello L, et al. Neurodevelopmental outcome at 36 months in very low birth weight premature infants with MR diffuse excessive high signal intensity (DEHSI) of cerebral white matter. Radiol Med 2015;120:1056–63. [DOI] [PubMed] [Google Scholar]
- 9.Anderson PJ, Treyvaud K, Neil JJ, Cheong JLY, Hunt RW, Thompson DK, et al. Associations of Newborn Brain Magnetic Resonance Imaging with Long-Term Neurodevelopmental Impairments in Very Preterm Children. J Pediatr 2017;187:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheong JLY, Thompson DK, Spittle AJ, Potter CR, Walsh JM, Burnett AC, et al. Brain Volumes at Term-Equivalent Age Are Associated with 2-Year Neurodevelopment in Moderate and Late Preterm Children. J Pediatr 2016;174:91–97.e1. [DOI] [PubMed] [Google Scholar]
- 11.Hack M Adult Outcomes of Preterm Children. J Dev Behav Pediatr 2009;30:460–70. [DOI] [PubMed] [Google Scholar]
- 12.Parikh NA, He L, Bonfante-Mejia E, Hochhauser L, Wilder PE, Burson K, et al. Automatically quantified diffuse excessive high signal intensity on MRI predicts cognitive development in preterm infants. Pediatr Neurol 2013;49:424–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwata S, Iwata O, Bainbridge A, Nakamura T, Kihara H, Hizume E, et al. Abnormal white matter appearance on term FLAIR predicts neuro-developmental outcome at 6 years old following preterm birth. Int J Dev Neurosci 2007;25:523–30. [DOI] [PubMed] [Google Scholar]
- 14.Iwata S, Nakamura T, Hizume E, Kihara H, Takashima S, Matsuishi T, et al. Qualitative brain MRI at term and cognitive outcomes at 9 years after very preterm birth. Pediatrics 2012;129:e1138–47. [DOI] [PubMed] [Google Scholar]
- 15.Hart A, Whitby E, Wilkinson S, Alladi S, Paley M, Smith M. Neuro-developmental outcome at 18 months in premature infants with diffuse excessive high signal intensity on MR imaging of the brain. Pediatr Radiol 2011;41:1284–92. [DOI] [PubMed] [Google Scholar]
- 16.Broström L, Bolk J, Padilla N, Skiöld B, Eklöf E, Mårtensson G, et al. Clinical Implications of diffuse excessive high signal intensity (DEHSI) on neonatal MRI in school age children born extremely preterm. PLoS One 2016;11:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kidokoro H, Anderson PJ, Doyle LW, Neil JJ, Inder TE. High signal intensity on T2-weighted MR imaging at term-equivalent age in preterm infants does not predict 2-year neurodevelopmental outcomes. Am J Neuroradiol 2011;32:2005–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Judas M, Rados M, Jovanov-Milosevic N, Hrabac P, Stern-Padovan R, Kostovic I. Structural, immunocytochemical, and mr imaging properties of periventricular crossroads of growing cortical pathways in preterm infants. Am J Neuroradiol 2005;26:2671–84. [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test, Second Edition (KBIT-2). Bloomington, MN: Pearson; 2004. [Google Scholar]
- 20.Pickering SJ, Gathercole SE. Working Memory Test Battery for Children (WMTB-C) manual. London: The Psychological Corporation; 2001. [Google Scholar]
- 21.Manly T, Robertson IH, Anderson V, Nimmo-Smith I. TEA-Ch: The Test of Everyday Attention for Children Manual. Bury, St. Edmunds, UK: Thames Valley Test Company Limited; 1999. [Google Scholar]
- 22.Emslie HF, Wilson C, Burden V, Nimmo-Smith I, Wilson BA. Behavioural Assessment of the Dysexecutive Syndrome in Children (BADS-C). London, UK: Harcourt Assessment/The Psychological Corporation; 2003. [Google Scholar]
- 23.Gioia GG, Isquith PK, Retzlaff PD, Espy KA. Confirmatory factor analysis of the Behavior Rating Inventory of Executive Function (BRIEF) in a clinical sample. Child Neuropsychol 2002;8:249–57. [DOI] [PubMed] [Google Scholar]
- 24.Henderson SE, Sugden DA, Barnett AL. Movement assessment battery for children-2 second edition. London, UK: The Psychological Corporation; 2007. [Google Scholar]
- 25.Carlin JB, Gurrin LC, Sterne JA, Morley R, Dwyer T. Regression models for twin studies: a critical review. Int J Epidemiol 2005. Oct 1;34:1089–99. [DOI] [PubMed] [Google Scholar]
- 26.Sterne JA, Davey Smith G. Sifting the evidence-what’s wrong with significance tests? BMJ 2001;322:226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson PJ. Neuropsychological outcomes of children born very preterm. Semin Fetal Neonatal Med 2014;19:90–6. [DOI] [PubMed] [Google Scholar]
- 28.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to Predict Neurodevelopmental Outcomes in Preterm Infants. N Engl J Med 2006;355:685–94. [DOI] [PubMed] [Google Scholar]
- 29.Kostović I, Judaš M. Prolonged coexistence of transient and permanent circuitry elements in the developing cerebral cortex of fetuses and preterm infants. Dev Med Child Neurol 2006;48:388–93. [DOI] [PubMed] [Google Scholar]
- 30.Yu X, Zhang Y, Lasky RE, Datta S, Parikh NA, Narayana PA. Comprehensive Brain MRI Segmentation in High Risk Preterm Newborns. PLoS One 2010;5:e13874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He L, Parikh NA. Automated detection of white matter signal abnormality using T2 relaxometry: Application to brain segmentation on term MRI in very preterm infants. Neuroimage 2013;64:328–40. [DOI] [PMC free article] [PubMed] [Google Scholar]