Abstract
Background:
The International Multiple Sclerosis Visual System Consortium (IMSVISUAL) was formed in November 2014 with the primary goal of improving research, care, and education regarding the role of the visual system in multiple sclerosis (MS) and related disorders.
Methods:
In this review, we describe the formation, goals, activities, and structure of IMSVISUAL, as well as the relationship of IMSVISUAL with the Americas Committee for Treatment and Research in MS (ACTRIMS). Finally, we provide an overview of the work IMSVISUAL has completed to date, as well as an outline of research projects ongoing under the auspices of IMSVISUAL.
Results:
IMSVISUAL has 140 members worldwide and continues to grow. Through IMSVISUAL-related research, optical coherence tomography (OCT)-derived peripapillary retinal nerve fiber layer (pRNFL) thinning has been established as a predictor of future disability in MS. IMSVISUAL has also developed guidelines for reporting OCT studies in MS. Moreover, a systematic review performed by IMSVISUAL found that not only are pRNFL and ganglion cell + inner plexiform layer (GCIPL) thicknesses reduced in patients with MS (particularly in eyes with prior optic neuritis [ON]), but that inner nuclear layer measures may be higher among MS ON eyes, relative to healthy control eyes. Currently, there are several ongoing IMSVISUAL projects that will establish a role for visual outcomes in diagnosing MS and quantifying the effects of emerging therapies in clinical trials.
Conclusions:
The development of IMSVISUAL represents a major collaborative commitment to defining the role of visual outcomes in high-quality, large-scale studies that generate definitive and instructive findings in the field of MS. As a consortium, IMSVISUAL has completed several international collaborative projects, is actively engaged in numerous ongoing research studies, and is committed to expanding the role of vision research in MS and related disorders.
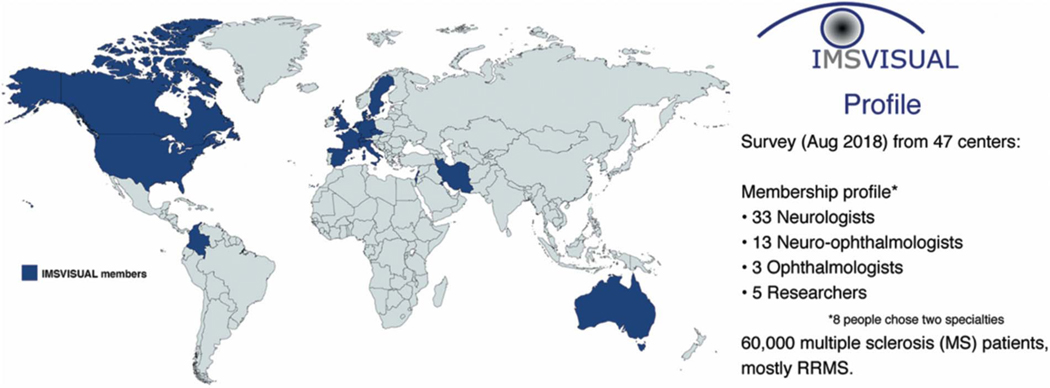
The International Multiple Sclerosis Visual System consortium (IMSVISUAL) was formed in November 2014 (www.imsvisual.org) after a series of international meetings recognizing the need for a platform to ensure high quality research, share data, provide clinical guidance, and promote research and teaching in the field (1,2). Currently, IMSVISUAL has 140 members located worldwide, including the United States, Canada, Europe, Middle East, South America, and Australia, and continues to grow. A membership survey is provided to all IMSVISUAL members on joining. As of August, 12, 2018, of the 47 members who responded to the survey (each are from unique/distinct sites), 33 were neurologists, 13 neuro-ophthalmologists, 5 researchers, and 3 ophthalmologists (of these respondents, 8 indicated having 2 specialties). Collectively, across just this small subset of IMSVISUAL members, there are approximately 60,000 patients with multiple sclerosis (MS) being actively followed, the majority of whom have relapsing remitting MS (RRMS), followed by progressive MS, clinically isolated syndrome (CIS), and finally neuromyelitis optica spectrum disorder (NMOSD) (Fig. 1). Almost all IMSVISUAL members have published at least one article in the past 3 years pertaining to the visual system in MS (or a related disorder), and are participating in ongoing studies. Areas of research include, but are not limited to, the expanding roles of optical coherence tomography (OCT), other imaging techniques including MRI, high- and low-contrast visual function, and electrophysiology (visual evoked potentials [VEPs]) in patients with MS. A large proportion of IMSVISUAL investigators also report routinely using OCT in considering the diagnosis of MS, as well as for monitoring disease progression and drug efficacy.
FIG. 1.
Overview of IMSVISUAL membership. RRMS, relapsing remitting multiple sclerosis.
GOALS AND ACTIVITIES OF IMSVISUAL
The overarching goal of IMSVISUAL is to improve care, research, and education in MS and related neuroinflammatory disorders by advancing our understanding of how the visual system is affected by these conditions. Specifically, IMSVISUAL’s goals are:
To promote and foster vision research, with the purpose of furthering our understanding of disease mechanisms in MS and other neuroinflammatory disorders. The consortium is interested in all stages and types of MS, including progressive MS and pediatric MS. The consortium aims to determine how structural, clinical, and functional measures of the visual system over time are related to other surrogate endpoints, including cognition, biomarkers of inflammation, and neurodegeneration, conventional and nonconventional MRI, as well as genomic, proteomic, and metabolomic profiles, in MS, and related disorders, and healthy controls of all ages.
To establish all aspects of the visual system as a model within which to monitor neurodegeneration and disease progression, as well as neuroregeneration and neuroplasticity in MS and related disorders, both for the purpose of tracking patients clinically, as well as outcomes in clinical trials. Although the consortium is committed to all aspects of the visual system in MS and its related conditions, IMSVISUAL is particularly interested in ocular imaging, progressive MS, pediatric MS, relating the visual system to the global MS disease process, and optic neuritis (ON). The consortium aims to achieve this through the creation of a shared repository of data, including, but not limited to, demographic, OCT, MRI, visual electrophysiology, genetic, diagnostic, therapeutic, immunologic, and cerebrospinal fluid studies. All members can access central repositories by submitting formal project proposals to the consortium.
To improve on existing, as well as develop new, techniques for assessing the visual system in MS and related disorders. The consortium has a particular interest in ocular imaging techniques, including, but not limited, to OCT.
To promote education of all facets of the visual system in MS and related disorders. This objective will be achieved by holding IMSVISUAL meetings at least twice per year, to coincide with the annual Americas Committee for Treatment and Research in MS (ACTRIMS) and European Committee for Treatment and Research in MS (ECTRIMS) Forum meetings, as well as teaching. In 2018, the IMSVISUAL steering committee and numerous IMSVISUAL members participated heavily in teaching at the ECTRIMS summer school on OCT held in Budapest, Hungary. This ECTRIMS summer school provided a detailed overview of the use of OCT in clinical neuroimmunology and included participants from around the world at different stages of their careers with an interest in advancing their knowledge of OCT. Didactic lectures addressed numerous aspects of OCT in MS, ON, and related diseases. In addition, practical workshops allowed for intensive hands-on training performing retinal OCT examinations, OCT quality control, and conducting retinal segmentation. School participants were also trained in how to implement OCT into routine clinical practice.
STRUCTURE OF IMSVISUAL
Membership to IMSVISUAL is free and open to anyone with an interest in the aims of the consortium. Currently, this only includes noncommercial members with an academic, educational, clinical, or personal interest. Membership can be requested through the consortium’s web site (http://imsvisual.org).
IMSVISUAL is governed by an executive committee and a working committee. Together they form the steering committee. The executive committee supervises the overall strategy and activities of IMSVISUAL. The working committee is responsible for coordinating and managing all IMSVISUAL operations. Both committees hold monthly phone conferences, to jointly decide over ongoing proceedings, and currently consist of the founding members of IMSVISUAL.
It is envisaged that IMSVISUAL will become incorporated as a not-for-profit organization in the foreseeable future. In addition, restructuring of IMSVISUAL governance will likely take place, which also will include elected committees.
IMSVISUAL AND ACTRIMS
ACTRIMS is a not-for-profit organization dedicated to providing leadership in the field of MS and related disorders. One of the core missions of ACTRIMS is to focus on knowledge dissemination, education, and collaboration (www.ACTRIMS.org). Over the past 2 years, ACTRIMS has taken steps in this regard by supporting IMSVISUAL. One avenue in which ACTRIMS fulfills its mission is by holding an annual conference. In 2016, ACTRIMS hosted its first annual stand-alone forum, “Progressive MS: Bench to Bedside and Back Again,” which surpassed expectations regarding attendance and abstract submissions. Subsequent ACTRIMS Forums held have built on this success with nearly 1,000 attendees in 2018. The ACTRIMS Forum offers a single track of scientific and clinical presentations in an interactive environment, and includes platform presentations by young investigators and poster sessions. Junior faculty, fellows, and students are eligible for educational grants to support their attendance at the meeting. Moreover, trainees (including residents and medical students) learn about career paths by participating in a preconference resident summit. In accordance with the commitment of ACTRIMS to providing opportunities to other MS-related groups, ACTRIMS partnered with IMSVISUAL in 2017 and 2018. This support has provided IMSVISUAL with programming opportunities, including a dedicated IMSVISUAL symposium during each forum as part of the ACTRIMS program, in addition to supporting separate IMSVISUAL member meetings during the forums. ACTRIMS and IMSVISUAL aim to continue their collaboration and are interested in finding synergies and establishing consensus procedures to conduct exceptional education opportunities and other jointly supported activities. Currently, there is an affiliate understanding in place between ACTRIMS and IMSVISUAL, such that IMSVISUAL will continue to have an ongoing symposium at the annual ACTRIMS Forum until 2021. More- over, ACTRIMS and IMSVISUAL leadership have been discussing additional ways to partner outside the ACTRIMS annual forum. This relationship has been critical to helping advance and promote IMSVISUAL, provide support to IMSVISUAL, and give IMSVISUAL the unique opportunity to disseminate visual system research findings in MS, consistent with one of the central goals of IMSVISUAL.
SUMMARY OF KEY FINDINGS FROM IMSVISUAL STUDIES
Assessing the Role of a Single Measurement of Retinal Thickness by Spectral Domain Optical Coherence Tomography as a Marker of worsening disability in Multiple Sclerosis
By 2010, several studies suggested an inverse association between disability and retinal thickness in MS (3). However, most of these studies used time-domain (TD) OCT and were cross-sectional. To definitively assess the utility of retinal thickness measures as surrogate markers of worsening disability in MS, we conducted a multicenter study of 15 sites in Europe and North America. This study included data prospectively collected between 2008 and 2013 from 879 patients with MS (4). Spectral domain (SD)-OCT was performed with the Spectralis (Heidelberg Engineering, Heidelberg, Germany) or Cirrus HD-OCT (Carl Zeiss, Dublin, CA). We used the mean value of peripapillary retinal nerve fiber layer (pRNFL) thickness and macular volume (MV) in eyes unaffected by prior MS-related ON (MSON), or the value from unaffected eyes in cases of prior unilateral MSON, as predictors of worsening disability, estimated by expanded disability status scale (EDSS) scores. If available, Multiple Sclerosis Functional Composite assessments following standard criteria for worsening disability were also assessed (5–7). Patients with a pRNFL thickness ≤87 or ≤88 μm (measured with Cirrus HD-OCT and Spectralis OCT, respectively) had approximately double the rate of worsening disability at any time after the first and up to the third years of follow-up (HR = 2.06, 95% CI [1.36–3.11]), and the rate was increased by nearly 4 times after the third and up to the fifth years of follow-up (HR = 3.81, 95% CI [1.63–8.91]). We did not find significant associations for MV, and GCIPL thicknesses were not available for inclusion in this study (4).
The Advised Protocol for OCT Study Terminology and Elements (APOSTEL) Recommendations
A critical aspect of OCT research is the underlying quality of imaging data. For example, pRNFL thickness measurements vary according to OCT signal quality (8). Likewise, different segmentation techniques or investigated areas of interest differ greatly in reliability and, thus, utility to investigate specific scientific questions (9,10). In 2012, OCT quality criteria, termed OSCAR-IB criteria (Obvious problem, poor Signal strength, Centration of scan, Algorithm failure, Retinal pathology, Illumination, Beam placement) were developed (1). These criteria were validated in a multicenter approach, with free online training provided by a web site and the need for a regular networked revision recognized (11). Today, the OSCAR-IB criteria are routinely used for assessing OCT image quality in MS studies. However, even with implementation of OSCAR-IB quality criteria, OCT studies in MS still have quality issues. Accordingly, IMSVISUAL aimed to define reporting guidelines for OCT studies, which would not only serve to support the assessment of a study’s quality but also would provide direction for OCT researchers. The recommendations were formed by expert consensus involving the steering committee of IMSVISUAL, several members of IMSVISUAL, as well as collaborating ophthalmologists, and were published in 2016 as the Advised Protocol for OCT Study Terminology and Elements (APOSTEL) recommendations (12). These recommendations include not only reporting image quality analysis procedures, but also OCT relevant elements of the study protocol, acquisition device, acquisition settings, scan protocols, fundus imaging, data analysis, and statistical approaches. The APOSTEL recommendations also made suggestions to harmonize the use of nomenclature and abbreviations among OCT studies.
The initial expert consensus-based APOSTEL recommendations are currently undergoing revision as part of a structured Delphi process, to gain broader input, awareness, and support among colleagues who were not involved in the development of the initial APOSTEL recommendations. A revised version of the APOSTEL recommendations is expected to be available in 2019.
Meta-Analyses of Retinal Layer Thicknesses and the Effect of Optic Neuritis
In 2010, a meta-analysis demonstrated that MSON caused substantial pRNFL atrophy (3). pRNFL atrophy also was present in eyes of patients without MSON. All studies included in this meta-analysis used TD-OCT technology. The reliability of these early OCT measures was assessed by a second meta-analysis based on SD-OCT data from new patient cohorts, performed as part of an IMSVISUAL collaboration (13). A total of 40 studies investigating retinal layer thickness in MS using SD-OCT were included. In addition to peripapillary and macular RNFL, data were obtained for the GCIPL, inner nuclear layer (INL), outer plexiform layer, and outer nuclear layer. Results from the SD-OCT meta-analysis demonstrated that, consistent with pRNFL atrophy, there was inner retinal layer (RNFL, GCIPL) atrophy in the macula, reproducing similar patterns for MSON and non-MSON eyes. In contrast with the reductions in pRNFL and GCIPL thicknesses relative to control eyes, MSON eyes demonstrated INL thickening (13). As previously reported, retrograde trans-synaptic axonal degeneration may halt at the level of the INL (14), with thickening of the INL and deeper retinal layers possibly representing signs of inflammation (see below).
ONGOING IMSVISUAL PROJECTS
Determining Optimal Thresholds for Intereye Differences in Peripapillary Retinal Nerve Fiber Layer and GCIPL Thickness for Predicting a History of Unilateral Optic Neuritis
Despite high prevalence (50%) of acute MSON (15) and nearly ubiquitous optic nerve disease postmortem (16,17) in MS, the optic nerve is not currently considered an imaging lesion site in MS diagnostic criteria (18). Consistent with the data from our meta-analyses, prospective studies have suggested an intereye pRNFL thickness difference of 5–6 μm (19) or a 5% intereye difference in GCIPL thickness (20) as useful thresholds for identifying prior ON. As part of IMSVISUAL, a multicenter international study of 11 sites was conducted to evaluate intereye differences in pRNFL and GCIPL thickness in patients with MS to determine optimal thresholds for predicting a history of unilateral MSON. In addition to SD-OCT, high- and low- contrast acuity and vision-specific quality of life also were performed and correlated with intereye SD-OCT differences. A total of 368 healthy controls and 1,530 patients with MS were recruited. Preliminary results show good agreement with previous studies in a single-center cohort evaluating a 5-μm threshold in pRNFL thickness as optimal for predicting a history of unilateral MSON and that intereye differences above this threshold also correlate with visual dysfunction. Further results from this study will be forthcoming.
Assessing the Relationship of Inner Nuclear Layer Volume Changes With Inflammatory Disease Activity in Multiple Sclerosis
Although the pRNFL and GCIPL consistently demonstrate thinning in MS, thought to predominantly relate to retrograde degeneration caused by either overt or occult optic neuropathy, the INL may not be susceptible to this mechanism or degree of injury. In 2012, Gelfand et al (21) first described the presence of microcystic macular edema in the INL and demonstrated that its presence was associated with increased disability. Also, in 2012, Saidha et al (22) reported that increased INL thickness at baseline in MS was predictive of clinical and radiological disease activity in the future, as well as worsening disability, suggesting that INL thickness may reflect global inflammatory activity in MS. To further investigate the potential of the INL as a biomarker for inflammatory processes, an ongoing IMSVISUAL collaborative project is focusing on the effect of inflammatory disease activity on INL volume changes. Because of the relatively small effect size (13), this has only become possible by pooling data from a large group of well-established centers in the field.
In this longitudinal multicenter study, data were pooled from 11 centers worldwide, resulting in a sample of almost 800 patients with MS. This study demonstrated a significant increase in INL volume in eyes with new episodes of MSON or other clinical relapses. Although the underlying mechanism responsible for thickening of the INL remains unknown, the INL seems to reflect some degree of global disease activity and may play a role in capturing inflammatory disease activity. Indeed, Knier et al (23) have reported the potential for INL measures to capture the antiinflammatory effects of disease-modifying therapies in MS (23).
Measuring Neuroprotection in the Visual Pathway: Identifying Best Outcomes in Randomized Clinical Trials in Patients With Acute Optic Neuritis
The prominent short-term structural and functional changes that can be measured and tracked by SD-OCT and VEPs, respectively, after acute MSON support the use of acute MSON as a model to test drugs that putatively promote neuroprotection and/or myelin repair (24–30). Despite some promising results, the conduction of these studies has raised important methodological concerns regarding inclusion/exclusion criteria, as well as the precise definition and utilization of the SD-OCT, VEP, and other visual outcomes. Through IMSVISUAL and in collaboration with other institutions, we have thus far collected data from 212 patients with acute MSON including SD-OCT, low-contrast visual acuity, and VEPs. Recruitment in this study is ongoing and we expect to generate a highly informative and enriched study population, forming the basis for determining the most accurate outcomes for measuring chronological damage after acute ON using SD-OCT, VEPs, and visual outcomes. This study also will evaluate the role of other demographic and prognostic factors during the course of acute MSON.
Identifying Occult Visual System Involvement in Clinically Isolated Syndrome/Early Multiple Sclerosis
IMSVISUAL currently is collecting OCT and VEP data from patients after their first CIS suggestive of MS to investigate the utility of OCT and VEP for establishing optic nerve involvement in patients with a clinical syndrome other than of the afferent visual system. Smaller-scope studies previously have shown that retinal and electrophysiological changes can be detected even in the earliest stages of MS/CIS (31) and are predictive of future disease activity (32). In this multicenter study, the utility of OCT and VEP in the early disease stages of MS will be further assessed as potential indicators of disease prognosis (33).
Spectral Domain Optical Coherence Tomography in Progressive Multiple Sclerosis
Progressive MS (PMS) is a clinical form of MS characterized by a steady and gradual accumulation of disability over time (7). There are 2 principal subtypes of PMS, primary and secondary. Although some patients with PMS may exhibit superimposed relapses and/or inflammatory activity (on MRI), the majority of patients with PMS do not exhibit any evidence of overt inflammatory activity (clinically or radiologically), yet demonstrate ongoing clinical decline (34).
OCT has emerged as a complementary tool to MRI with particular utility for tracking neurodegeneration (and accordingly neuroprotection) in MS (35). It has been shown that GCIPL thickness correlates with high- and low-contrast letter acuity, as well as EDSS scores (36). It also has been demonstrated that GCIPL atrophy mirrors whole brain atrophy over time, in particular gray matter atrophy, and that rates of GCIPL atrophy are differentially modulated by different disease-modifying therapies in RRMS (37,38). However, the majority of OCT studies in MS have been performed in RRMS, with a paucity performed with PMS patients. Currently, we have an incomplete understanding of the pathobiology of PMS. Moreover, there also is a lack of validated outcomes to identify/distinguish PMS and, therefore, track as well as measure treatment effects in PMS. Although nonconventional MRI techniques, such as whole brain and brain substructure volumetrics, have a role in PMS (39,40), such techniques have not identified MRI signatures specific for PMS. Moreover, nonconventional MRI may be costly, lack sensitivity, and has been challenging to implement into clinical practice (41). Lack of sensitivity to change over time also is a limitation of clinical outcome measures such as the EDSS in PMS (42). An ongoing IMSVISUAL endeavor aims to address these gaps in PMS, helping to elicit if there are distinct differences in retinal changes over time, hopefully leading to development of more specific PMS outcomes and potentially shedding light on the pathophysiology of PMS. Currently, as part of this study, there are 242 people with PMS being tracked with OCT. Preliminary results of this study are expected in early 2019.
CONCLUSIONS
Through IMSVISUAL, neurologists, neuro-ophthalmologists, and ophthalmologists, among other researchers and scientists, have the opportunity to join efforts to develop high-quality scientific evidence to better understand the role of the visual system in MS and related disorders. The development of IMSVISUAL is a testament of the importance and interest in the visual system in MS, and represents a concerted, wide-scale, international, collaborative commitment to its study. IMSVISUAL facilitates extremely powerful, high-quality, large-scale studies of the visual system in MS that generate definitive and instructive findings. As such, IMSVISUAL enables broad-scale studies to be performed in a timely fashion, serving as a driving force for major advancements in visual system research in MS. Several instructive, novel, and important studies have already been completed under the umbrella of IMSVISUAL. Moreover, IMSVISUAL has numerous other ongoing research projects as outlined above. IMSVISUAL has formed an important affiliation with ACTRIMS over the last number of years and is committed to virtually all aspects of visual system–based research in MS and related disorders.
Acknowledgments
F. Paul has received honoraria and research support from Alexion, Bayer, Biogen, Chugai, MerckSerono, Novartis, Genyzme, MedImmune, Shire, and Teva, and serves on scientific advisory boards for Alexion, MedImmune, and Novartis. He has received funding from Deutsche Forschungsgemeinschaft (DFG Exc 257), Bundesministerium für Bildung und Forschung (Competence Network Multiple Sclerosis), Guthy Jackson Charitable Foundation, EU Framework Program 7, and National Multiple Sclerosis Society of the USA. FP serves on advisory boards and steering committees for Novartis and MedImmune and is Associate Editor of Neurology, Neuroimmunology, and Neuroinflammation and Academic Editor of PLoS One. A. Petzod is supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health. He is member of the steering committee for the OCTiMS study (Novartis) and performed OCT QC for the PASSOS study (Novartis) and received consulting fees. S. Saidha has received consulting fees from Medical Logix for the development of CME programs in neurology and has served on scientific advisory boards for Biogen Idec, Genzyme, Genentech Corporation, EMD Serono, and Novartis. He is the PI of investigator-initiated studies funded by Genentech Corporation and Biogen Idec, and received support from the Race to Erase MS foundation. He has received equity compensation for consulting from JuneBrain LLC, a retinal imaging device developer. He is also the site investigator of a trial sponsored by MedDay Pharmaceuticals.
L. J. Balcer has received investigator-initiated research grant funding from Biogen. L. J. Balk has received an institutional research grant from TEVA. A. U. Brandt is a cofounder and shareholder of the startups Motognosis and Nocturne. He is named as inventor on several patent applications description MS serum biomarkers, perceptive visual computing, and retinal image analysis. P. A. Calabresi has received personal honorariums for consulting from Biogen and Disarm Therapeutics. He is PI on research grants to Johns Hopkins from MedImmune, Annexon, Biogen, and Genzyme. E. H. Martinez-Lapiscina has received speaking honoraria from Roche, Biogen, Sanofi-Genzyme, and Novartis, and travel reimbursement from Roche, Sanofi-Genzyme, and Biogen, over the last 3 years. She has received consulting fees from Roche and Sanofi-Genzyme and unrestricted grants from Fundació Privada Cellex. She has received investigator-initiated research grant funding from Sanofi-Genzyme and investigator-initiated educational grant from Novartis. The remaining authors report no conflicts of interest.
L. J. Balcer, L. J. Balk, A. U. Brandt, P. A. Calabresi, E. H. Martinez-Lapiscina, R. Nolan, F. Paul, A. Petzold, and S. Saidha contributed equally and are listed in alphabetical order.
REFERENCES
- 1.Tewarie P, Balk L, Costello F, Green A, Martin R, Schippling S, Petzold A. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS One. 2012;7:e34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balcer LJ, Miller DH, Reingold SC, Cohen JA. Vision and vision-related outcome measures in mul tiple sclerosis. Brain. 2015;138:11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petzold A, de Boer JF, Schippling S, Vermersch P, Kardon R, Green A, Calabresi PA, Polman C. Optical coherence tomography in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 2010;9:921–932. [DOI] [PubMed] [Google Scholar]
- 4.Martinez-Lapiscina EH, Arnow S, Wilson JA, Saidha S, Preiningerova JL, Oberwahrenbrock T, Brandt AU, Pablo LE, Guerrieri S, Gonzalez I, Outteryck O, Mueller AK, Albrecht P, Chan W, Lukas S, Balk LJ, Fraser C, Frederiksen JL, Resto J, Frohman T, Cordano C, Zubizarreta I, Andorra M, Sanchez-Dalmau B, Saiz A, Bermel R, Klistorner A, Petzold A, Schippling S, Costello F, Aktas O, Vermersch P, Oreja-Guevara C, Comi G, Leocani L, Garcia-Martin E, Paul F, Havrdova E, Frohman E, Balcer LJ, Green AJ, Calabresi PA, Villoslada P. Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: a cohort study. Lancet Neurol. 2016;15:574–584. [DOI] [PubMed] [Google Scholar]
- 5.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33:1444–1452. [DOI] [PubMed] [Google Scholar]
- 6.Rudick RA, Polman CH, Cohen JA, Walton MK, Miller AE, Confavreux C, Lublin FD, Hutchinson M, O’Connor PW, Schwid SR, Balcer LJ, Lynn F, Panzara MA, Sandrock AW. Assessing disability progression with the multiple sclerosis functional composite. Mult Scler. 2009;15:984–997. [DOI] [PubMed] [Google Scholar]
- 7.Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sorensen PS, Thompson AJ, Wolinsky JS, Balcer LJ, Banwell B, Barkhof F, Bebo B Jr, Calabresi PA, Clanet M, Comi G, Fox RJ, Freedman MS, Goodman AD, Inglese M, Kappos L, Kieseier BC, Lincoln JA, Lubetzki C, Miller AE, Montalban X, O’Connor PW, Petkau J, Pozzilli C, Rudick RA, Sormani MP, Stuve O, Waubant E, Polman CH. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83:278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Z, Huang J, Dustin L, Sadda SR. Signal strength is an important determinant of accuracy of nerve fiber layer thickness measurement by optical coherence tomography. J Glaucoma. 2009;18:213–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seigo MA, Sotirchos ES, Newsome S, Babiarz A, Eckstein C, Ford E, Oakley JD, Syc SB, Frohman TC, Ratchford JN, Balcer LJ, Frohman EM, Calabresi PA, Saidha S. In vivo assessment of retinal neuronal layers in multiple sclerosis with manual and automated optical coherence tomography segmentation techniques. J Neurol. 2012;259:2119–2130. [DOI] [PubMed] [Google Scholar]
- 10.Oberwahrenbrock T, Weinhold M, Mikolajczak J, Zimmermann H, Paul F, Beckers I, Brandt AU. Reliability of intra-retinal layer thickness estimates. PLoS One. 2015;10:e0137316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schippling S, Balk LJ, Costello F, Albrecht P, Balcer L, Calabresi PA, Frederiksen JL, Frohman E, Green AJ, Klistorner A, Outteryck O, Paul F, Plant GT, Traber G, Vermersch P, Villoslada P, Wolf S, Petzold A. Quality control for retinal OCT in multiple sclerosis: validation of the OSCAR-IB criteria. Mult Scler. 2015;21:163–170. [DOI] [PubMed] [Google Scholar]
- 12.Cruz-Herranz A, Balk LJ, Oberwahrenbrock T, Saidha S, Martinez-Lapiscina EH, Lagreze WA, Schuman JS, Villoslada P, Calabresi P, Balcer L, Petzold A, Green AJ, Paul F, Brandt AU, Albrecht P. The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology. 2016;86:2303–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petzold A, Balcer LJ, Calabresi PA, Costello F, Frohman TC, Frohman EM, Martinez-Lapiscina EH, Green AJ, Kardon R, Outteryck O, Paul F, Schippling S, Vermersch P, Villoslada P, Balk LJ. Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 2017;16:797–812. [DOI] [PubMed] [Google Scholar]
- 14.Balk LJ, Twisk JW, Steenwijk MD, Daams M, Tewarie P, Killestein J, Uitdehaag BM, Polman CH, Petzold A. A dam for retrograde axonal degeneration in multiple sclerosis? J Neurol Neurosurg Psychiatry. 2014;85:782–789. [DOI] [PubMed] [Google Scholar]
- 15.Balcer LJ. Clinical practice. Optic neuritis. N Engl J Med. 2006;354:1273–1280. [DOI] [PubMed] [Google Scholar]
- 16.Toussaint D, Perier O, Verstappen A, Bervoets S. Clinicopathological study of the visual pathways, eyes, and cerebral hemispheres in 32 cases of disseminated sclerosis. J Clin Neuroophthalmol. 1983;3:211–220. [PubMed] [Google Scholar]
- 17.Ikuta F, Zimmerman HM. Distribution of plaques in seventy autopsy cases of multiple sclerosis in the United States. Neurology. 1976;26:26–28. [DOI] [PubMed] [Google Scholar]
- 18.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, Correale J, Fazekas F, Filippi M, Freedman MS, Fujihara K, Galetta SL, Hartung HP, Kappos L, Lublin FD, Marrie RA, Miller AE, Miller DH, Montalban X, Mowry EM, Sorensen PS, Tintore M, Traboulsee AL, Trojano M, U itdehaag BMJ, Vukusic S, Waubant E, Weinshenker BG, Reingold SC, Cohen JA. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–173. [DOI] [PubMed] [Google Scholar]
- 19.Nolan RC, Galetta SL, Frohman TC, Frohman EM, Calabresi PA, Castrillo-Viguera C, Cadavid D, Balcer LJ. Optimal intereye difference thresholds in retinal nerve fiber layer thickness for predicting a unilateral optic nerve lesion in multiple sclerosis. J Neuroophthalmol. 2018;38:451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coric D, Balk LJ, Uitdehaag BMJ, Petzold A. Diagnostic accuracy of optical coherence tomography inter-eye percentage difference for optic neuritis in multiple sclerosis. Eur J Neurol. 2017;24:1479–1484. [DOI] [PubMed] [Google Scholar]
- 21.Gelfand JM, Nolan R, Schwartz DM, Graves J, Green AJ. Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain. 2012;135:1786–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saidha S, Sotirchos ES, Ibrahim MA, Crainiceanu CM, Gelfand JM, Sepah YJ, Ratchford JN, Oh J, Seigo MA, Newsome SD, Balcer LJ, Frohman EM, Green AJ, Nguyen QD, Calabresi PA. Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: a retrospective study. Lancet Neurol. 2012;11:963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knier B, Schmidt P, Aly L, Buck D, Berthele A, Muhlau M, Zimmer C, Hemmer B, Korn T. Retinal inner nuclear layer volume reflects response to immunotherapy in multiple sclerosis. Brain. 2016;139:2855–2863. [DOI] [PubMed] [Google Scholar]
- 24.Tsakiri A, Kallenbach K, Fuglo D, Wanscher B, Larsson H, Frederiksen J. Simvastatin improves final visual outcome in acute optic neuritis: a randomized study. Mult Scler. 2012;18:72–81. [DOI] [PubMed] [Google Scholar]
- 25.Suhs KW, Hein K, Sattler MB, Gorlitz A, Ciupka C, Scholz K, Kasmann-Kellner B, Papanagiotou P, Schaffler N, Restemeyer C, Bittersohl D, Hassenstein A, Seitz B, Reith W, Fassbender K, Hilgers R, Heesen C, Bahr M, Diem R. A randomized, double-blind, phase 2 study of erythropoietin in optic neuritis. Ann Neurol. 2012;72:199–210. [DOI] [PubMed] [Google Scholar]
- 26.Esfahani MR, Harandi ZA, Movasat M, Nikdel M, Adelpour M, Momeni A, Merat H, Fard MA. Memantine for axonal loss of optic neuritis. Graefes Arch Clin Exp Ophthalmol. 2012;250:863–869. [DOI] [PubMed] [Google Scholar]
- 27.McKee JB, Elston J, Evangelou N, Gerry S, Fugger L, Kennard C, Kong Y, Palace J, Craner M. Amiloride Clinical Trial In Optic Neuritis (ACTION) protocol: a randomised, double blind, placebo controlled trial. BMJ Open. 2015;5:e009200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raftopoulos R, Hickman SJ, Toosy A, Sharrack B, Mallik S, Paling D, Altmann DR, Yiannakas MC, Malladi P, Sheridan R, Sarrigiannis PG, Hoggard N, Koltzenburg M, Gandini Wheeler-Kingshott CA, Schmierer K, Giovannoni G, Miller DH, Kapoor R. Phenytoin for neuroprotection in patients with acute optic neuritis: a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15:259–269. [DOI] [PubMed] [Google Scholar]
- 29.Cadavid D, Balcer L, Galetta S, Aktas O, Ziemssen T, Vanopdenbosch L, Frederiksen J, Skeen M, Jaffe GJ, Butzkueven H, Ziemssen F, Massacesi L, Chai Y, Xu L, Freeman S. Safety and efficacy of opicinumab in acute optic neuritis (RENEW): a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16:189–199. [DOI] [PubMed] [Google Scholar]
- 30.Morrow SA, Fraser JA, Day C, Bowman D, Rosehart H, Kremenchutzky M, Nicolle M. Effect of treating acute optic neuritis with bioequivalent oral vs intravenous corticosteroids: a randomized clinical trial. JAMA Neurol. 2018;75:690–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oberwahrenbrock T, Ringelstein M, Jentschke S, Deuschle K, Klumbies K, Bellmann-Strobl J, Harmel J, Ruprecht K, Schippling S, Hartung HP, Aktas O, Brandt AU, Paul F. Retinal ganglion cell and inner plexiform layer thinning in clinically isolated syndrome. Mult Scler. 2013;19:1887–1895. [DOI] [PubMed] [Google Scholar]
- 32.Zimmermann HG, Knier B, Oberwahrenbrock T, Behrens J, Pfuhl C, Aly L, Kaminski M, Hoshi MM, Specovius S, Giess RM, Scheel M, Muhlau M, Bellmann-Strobl J, Ruprecht K, Hemmer B, Korn T, Paul F, Brandt AU. Association of retinal ganglion cell layer thickness with future disease activity in patients with clinically isolated syndrome. JAMA Neurol. 2018;75:1071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Filippi M, Preziosa P, Meani A, Ciccarelli O, Mesaros S, Rovira A, Frederiksen J, Enzinger C, Barkhof F, Gasperini C, Brownlee W, Drulovic J, Montalban X, Cramer SP, Pichler A, Hagens M, Ruggieri S, Martinelli V, Miszkiel K, Tintore M, Comi G, Dekker I, Uitdehaag B, Dujmovic-Basuroski I, Rocca MA. Prediction of a multiple sclerosis diagnosis in patients with clinically isolated syndrome using the 2016 MAGNIMS and 2010 McDonald criteria: a retrospective study. Lancet Neurol. 2018;17:133–142. [DOI] [PubMed] [Google Scholar]
- 34.Krieger SC, Cook K, De Nino S, Fletcher M. The topographical model of multiple sclerosis: a dynamic visualization of disease course. Neurol Neuroimmunol Neuroinflamm. 2016;3:e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saidha S, Calabresi PA. Optical coherence tomography should be part of the routine monitoring of patients with multiple sclerosis: yes. Mult Scler. 2014;20:1296–1298. [DOI] [PubMed] [Google Scholar]
- 36.Saidha S, Syc SB, Durbin MK, Eckstein C, Oakley JD, Meyer SA, Conger A, Frohman TC, Newsome S, Ratchford JN, Frohman EM, Calabresi PA. Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness. Mult Scler. 2011;17:1449–1463. [DOI] [PubMed] [Google Scholar]
- 37.Saidha S, Al-Louzi O, Ratchford JN, Bhargava P, Oh J, Newsome SD, Prince JL, Pham D, Roy S, van Zijl P, Balcer LJ, Frohman EM, Reich DS, Crainiceanu C, Calabresi PA. Optical coherence tomography reflects brain atrophy in multiple sclerosis: a four-year study. Ann Neurol. 2015;78:801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Button J, Al-Louzi O, Lang A, Bhargava P, Newsome SD, Frohman T, Balcer LJ, Frohman EM, Prince J, Calabresi PA, Saidha S. Disease-modifying therapies modulate retinal atrophy in multiple sclerosis: a retrospective study. Neurology. 2017;88:525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rocca MA, Mesaros S, Pagani E, Sormani MP, Comi G, Filippi M. Thalamic damage and long-term progression of disability in multiple sclerosis. Radiology. 2010;257:463–469. [DOI] [PubMed] [Google Scholar]
- 40.Bakshi R, Thompson AJ, Rocca MA, Pelletier D, Dousset V, Barkhof F, Inglese M, Guttmann CR, Horsfield MA, Filippi M. MRI in multiple sclerosis: current status and future prospects. Lancet Neurol. 2008;7:615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sotirchos ES, Saidha S. OCT is an alternative to MRI for monitoring MS - YES. Mult Scler. 2018; 24:701–703. [DOI] [PubMed] [Google Scholar]
- 42.Bermel R, Waldman A, Mowry EM. Outcome measures in multiple sclerosis. Mult Scler Int. 2014;2014:439375. [DOI] [PMC free article] [PubMed] [Google Scholar]