Abstract
It has been proposed that transgenic zebrafish could be designed to detect low levels of chemical contaminants that cause oxidative stress in aquatic environments, such as heavy metals or pesticides. In this paper, we describe such a transgenic zebrafish that produces a luciferase–green fluorescent protein (LUC–GFP) fusion protein under conditions of oxidative stress. The reporter gene expression is under the regulation of the electrophile responsive element (EPRE), which activates gene expression in response to oxidative stressors. The GFP component of this fusion protein allows us to visually detect reporter gene activity in live animals to determine if activity is localized to a particular tissue. The luciferase component is capable of returning a quantitative assessment of reporter gene activity that allows us to determine if reporter gene activity is directly correlated to the concentration of the chemical inducer. We have tested this reporter construct in both transient and stable transgenic fish after exposure to a range of HgCl2 concentrations. GFP expression from the EPRE–LUC–GFP construct was inducible in transient assays but was below the limit of detection in stable lines. In contrast, we observed inducible luciferase activity in both transient assays and stable lines treated with HgCl2. We conclude that the EPRE is capable of driving reporter gene expression in a whole animal assay under conditions of oxidative stress. Furthermore, expression was induced at HgCl2 concentrations that do not result in obvious morphological defects, making this approach useful for the detection of low levels of oxidative contaminants in aquatic environments.
Keywords: Transgenic zebrafish, Oxidative stress, Electrophile response element, Antioxidant response element, EPRE, ARE
Introduction
Fish and mammals share defense mechanisms against toxic environmental chemicals including oxidants and electrophiles, which trigger the oxidative stress response. We are interested in designing transgenic reporter fish that can detect low levels of such stressors by taking advantage of pathways that trigger physiological responses to oxidative stress. One of these stress response systems is regulated by the Keap1-Nrf2 (Kelch ECH associating protein 1–nuclear factor erythroid 2-related factor) pathway, in which the Nrf2 transcription factor is held in the cytoplasm by Keap1 and targeted for degradation under homeostatic conditions (Kensler et al. 2007). In the presence of chemicals that induce oxidative stress, Nrf2 is translocated to the nucleus with the assistance of a nuclear localization sequence where it functions as a strong transcriptional activator of electrophile responsive element (EPRE)-responsive genes (Kensler et al. 2007). EPRE-responsive genes encode proteins with cytoprotective functions including enzymes that directly inactivate oxidants (Hayes et al. 2005), enhance toxin export via the multidrug response transporters (Hayashi et al. 2003), inhibit cytokine-mediated inflammation (Primiano et al. 1996), and enhance the recognition, repair, and removal of damaged proteins (Kwak et al. 2003).
The ability of the Keap1–Nrf2 pathway to up-regulate genes under control of the oxidative stress response is the basis for the EPRE reporter gene constructs. It has been shown that the single EPRE from the mouse Gsta1 (glutathione S-transferase) enhancer region fused to the minimal mouse Mt1 (metalothionein 1) promoter has the ability to up-regulate the luciferase (LUC) reporter gene in transfected cultured zebrafish ZEM2S cells after exposure to environmental pollutants (Carvan et al. 2000). Treatment with electrophiles, reactive oxygenated metabolites, and certain heavy metals are all environmental agents capable of eliciting an intracellular oxidative stress response that up-regulates the LUC gene under control of the EPREmt1 (Carvan et al. 2000). These results suggested that transgenic zebrafish containing an EPRE-driven reporter construct might be capable of detecting chemicals that induce an oxidative stress response. We have generated a reporter gene consisting of a luciferase–green fluorescent protein (LUC–GFP) fusion gene. This construct allows visual confirmation of transgene expression in live fish through fluorescence microscopy and quantitative measurement of gene expression by measuring luciferase activity in embryo extracts (Fig. 1). Our results so far have shown that EPRE-mt1 can promote inducible expression of a LUC–GFP fusion reporter gene in a whole animal assay, using transient transgenic zebrafish. There was no visible GFP expression in transgene-injected fish not treated with HgCl2, indicating that the reporter construct does not have leaky expression. Induced expression was observed in a variety of cell types in the developing zebrafish, upon exposure to HgCl2. Our results demonstrate that the EPRE–LUC–GFP fish have a significant advantage over cultured cell lines with similar reporter constructs in detecting low levels of oxidants present in aquatic environments.
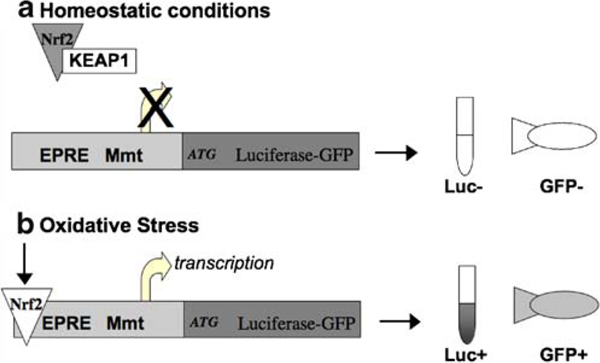
Fig. 1.
Oxidative stress responsive reporter gene. a Under homeostatic conditions, Nrf2 is retained in the cytoplasm and prevented from binding to the EPRE and activating the luciferase–GFP fusion transgene expression. b Under conditions of oxidative stress, Nrf2 is released and translocated into the nucleus where it is able to bind to the EPRE and activate the luciferase–GFP fusion gene, resulting in luciferase (LUC) activity and GFP expression
Materials and Methods
Zebrafish Husbandry
We used the nacre −/− mutant strain of zebrafish (Lister et al. 1999), which fail to produce melanin, giving the fish an albino-like appearance. Zebrafish colonies were maintained as previously described (Westerfield 2000). Juvenile and adult fish were housed in recirculating rack systems (Aquatic Habitats) at 28.5°C on a 14-h light, 10-h dark cycle, and fed twice daily with artemia and/or pellets (Zeigler adult zebrafish diet, Aquatic Habitats). Fertilized eggs were generated by natural spawning in tanks with a mesh bottom that allowed eggs to remain separated from adults before collection. Fry were also raised at 28.5°C on a 14-h light, 10-h dark cycle but were kept in static tanks with daily water changes for 2–3 weeks and fed with dry larval food (GP Reef and Larval food, Brine Shrimp Direct) and artemia before transferring to rack systems. Zebrafish husbandry and all experimental procedures were part of an Institutional Animal Care and Use Committee-approved animal protocol.
Generation and Analysis of Transient and Stable Transgenic Fish
EPRE Reporter Plasmids
A reporter plasmid, pEPRE–LUC–GFP, expressing a green fluorescent protein (GFP)–luciferase fusion protein under the regulation of the electrophile response element (EPRE) was created by modifying the pW1EGFP cloning vector that contains the EGFP coding sequence (Clontech), and the intron and poly-adenylation site from the SV40 small t gene (Linney and Udvadia 2004). The luciferase coding sequence, excluding the stop codon, was polymerase chain reaction (PCR)-amplified from the pMOD–LUCSh plasmid (InvivoGen, San Diego, USA; primers: 5′-GGTGACCTAGGA CAATTGTAG-3′ and 5′-CTCGAGAGTTGTTTGC CACCCTTCTT-3′). The MOD–LUC sequence in the pMOD–LUCSh plasmid contains a number of silent mutations that eliminate CpG motifs that could result in methylation-induced transgene silencing. The EPRE sequence from the mouse Gsta1 gene fused to the minimal promoter from the mouse mt1 gene was PCR-amplified from a previously described EPRE reporter plasmid (Carvan et al. 2001; primers: 5′-GAATTCGGGTGCCAGAACATT-3′ and 5′-CGTCTTCCATGGTGGCTTTCTC-3′) and cloned up-stream of the luciferase–GFP gene. The entire EPRE reporter gene sequence was flanked with I-Sce I meganuclease recognition sites to facilitate the generation of stable transgenic zebrafish lines with this construct (Thermes et al. 2002).
Transgene Injection and HgCl2 Exposure
Initial analysis of transgene expression patterns was carried out using transient transgenic assays of 48-h-old embryos injected at the one-cell stage with purified EPRE–LUC–GFP transgene DNA. Reporter gene sequences were excised from the plasmid with I-Sce I enzyme (New England Biolabs) and gel purified (Qiagen). The DNA–enzyme mixture [20 ng/μl linearized plasmid, 0.5× I-Sce I buffer (NEB), 0.5 IU/μl meganuclease I-Sce I (NEB)] was pressure injected into the yolk-free cytoplasm of one-cell stage embryos (Thermes et al. 2002). Injected embryos were raised for 24 h at 28 C. Embryos displaying morphological abnormalities as compared with uninjected controls were discarded. Embryos displaying normal embryonic development were either raised to maturity and screened for germline transmission of the transgene using PCR (Linney and Udvadia 2004) or treated with HgCl2 to test for transgene induction. At 24 h post fertilization (hpf), EPRE–LUC–GFP-injected fish, fish from EPRE-LUC-GFP stable transgenic lines, or control fish were transferred into six-well plates (ten embryos/well) and exposed to HgCl2 in 30% Danieau buffer [1×=58 mM NaCl, 0.7 mM KCl, 0.4 mM MgSO4 (7H2O), 0.6 mM Ca (NO3)2 (4H2O), 0.5 mM HEPES, pH 7.6]. At least three groups of fish (5–15 embryos/group) were treated at each of the following concentrations of HgCl2: 0.0, 0.1, 0.3, or 1.0 μM. After a 24-h exposure, the embryos were rinsed and transferred to fresh Danieau medium.
Transgene Expression Analysis
Mortality after 24-h exposure was calculated on uninjected embryos. Detailed expression analysis of GFP expression was carried out on F0 and F1 transgenic fish after HgCl2 treatment. Embryos were anesthetized in Tricaine for immobilization during imaging. Images were captured using an inverted fluorescence microscope (Nikon TE 2000) equipped with a cooled charge-coupled device camera (Photometrics Cool Snap ES) using Metamorph image acquisition software (Universal Imaging) and processed using Photoshop (Adobe). GFP expression was scored for relative expression level and axial distribution in embryos. The number of GFP expressing cells in a given embryo were counted. Embryos were scored as low (+), medium (++), or high (+++) for GFP expression levels as follows: + (approximately one to ten cells), ++ (~11–100 cells), or +++ (>100 cells). Each embryo was also assessed for the relative distribution of GFP-expressing cells along the body axis divided into head, trunk, and tail regions.
Luciferase activity induced by HgCl2 exposures was assayed in F0 fish, which were first assessed for GFP expression levels using fluorescence microscopy and grouped as −, +, ++, or +++. Extracts were prepared from fish in each category (two fish/extract). Fish were homogenized in 105 μl Glo lysis buffer (Promega) in 1.5-ml microcentrifuge tubes using disposable plastic pestles. Lysates were stored at −80°C and brought to room temperature before analysis using the Bright Glo kit (Promega) according to manufacturer recommendations. Luciferase assays were carried out in 96-well plates (Nalgene cat. no. 237107) using a plate reader equipped for luminescence readings (Wallac 1420 Victor2, Perkin-Elmer), using purified luciferase to create a standard curve for each plate to calculate the luciferase concentration in each sample.
Luciferase activity was also assessed in stable transgenic fish. Progeny from a cross of two F1 EPRE–LUC–GFP transgenic fish were collected and exposed to HgCl2 in groups of eight at the following concentrations: 0.0, 0.1, 0.2, 0.3, 0.6, or 1.0 μM. All eight fish in a treatment group were homogenized together to make a single extract; three extracts were prepared from each exposure.
Results
Development and Survival of Zebrafish Embryos Exposed to HgCl2
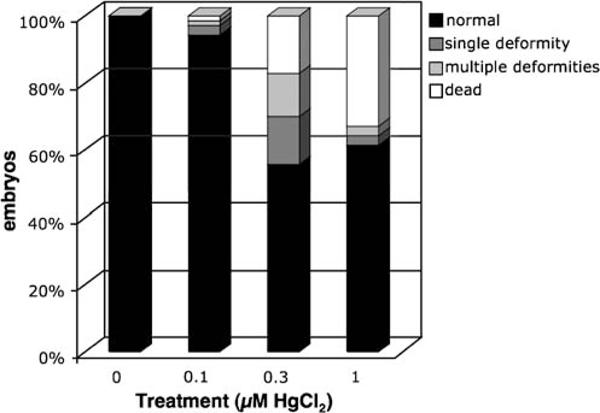
We first exposed non-transgenic fish to a range of concentrations to determine the sensitivity of 24-h-old embryos to a 24-h HgCl2 exposure. We used survival rate and developmental morphology as criteria to assess the effects of exposure (Fig. 2). Embryos were observed 1–3 days after HgCl2 treatment. We found that a 24-h static exposure to 1 μM HgCl2 was highly toxic to the developing embryos, resulting in approximately a 60% survival rate and severe morphological defects in many of the remaining embryos. The survival rate after exposure to 0.3 μM HgCl2 increased to approximately 80%, and fewer embryos exhibited severe morphological defects. These defects were mainly limited to a slight to moderate curvature of the trunk region and mild heart sac edema; however, organ development and circulation appeared normal. At 0.1 μM, treated embryos were virtually indistinguishable from controls in terms of survival and developmental morphology.
Fig. 2.
Twenty-four exposure at ≥0.3 μM HgCl2 leads to significant increases in deformities and death in zebrafish embryos. Embryos were treated in groups of ten at 24 hpf for 24 h, then observed for death/deformities. Results are the average values from seven groups for each treatment
EPRE Reporter Gene can Detect Sub-lethal Doses of HgCl2 in Whole Animal System
To develop a sensitive assay for low-level HgCl2 aquatic contamination, we introduced EPRE reporter genes into single-cell-stage zebrafish embryos. Using EPRE reporter fish, we could detect HgCl2 at sub-lethal concentrations that did not result in morphological abnormalities. Live injected embryos were observed under fluorescence microscopy for GFP expression after a 24-h exposure to HgCl2 at concentrations ranging from 0.1–1.0 μM. We determined the rate of transgene expression by counting the number of live GFP-expressing fish at each exposure level to determine the percentage of surviving embryos expressing GFP. GFP expression was most often observed in skin cells (Fig. 3b,e); however, expression was also observed in muscle, neurons, notochord (Fig. 3c,d, f–h), and blood (not shown). After exposure to 0.1 μM HgCl2, 30% of treated embryos expressed GFP (Fig. 4a). At higher concentrations, 0.3 and 1.0 μM, approximately 80% of embryos surviving treatment expressed GFP (Fig. 4a). The number of GFP-expressing cells/embryo was generally higher (Fig. 4b) and more widespread (Fig. 4c) at higher concentrations. Embryos treated at 0.3 μM had the largest percentage of surviving embryos that expressed GFP in more than ten cells per embryo (++, Fig. 4b). It is likely the lower percentage of highly expressing embryos observed in the 1.0-μM treatment group is due to a lower survival rate of embryos in this treatment group. GFP-expressing cells were observed most frequently in the trunk at 0.1 μM, but at higher concentrations, GFP-expressing cells were more frequently observed throughout the body axis (Fig. 4c). Thus, the frequency and distribution of GFP expressing cells in treated embryos was generally concentration-dependent. We did not observe GFP expression in transgene-injected embryos that were not exposed to HgCl2 (Fig. 4a–d), making “leaky” expression of the transgene or false positive results unlikely. Given that transgene expression could be observed in fish treated with 0.1 μM HgCl2, a concentration that does not result in morphological defects or mortality, our results demonstrate that F0 EPRE–LUC–GFP transgenic fish provide a three- to tenfold more sensitive assay for HgCl2 exposure than morphology or mortality criteria alone.
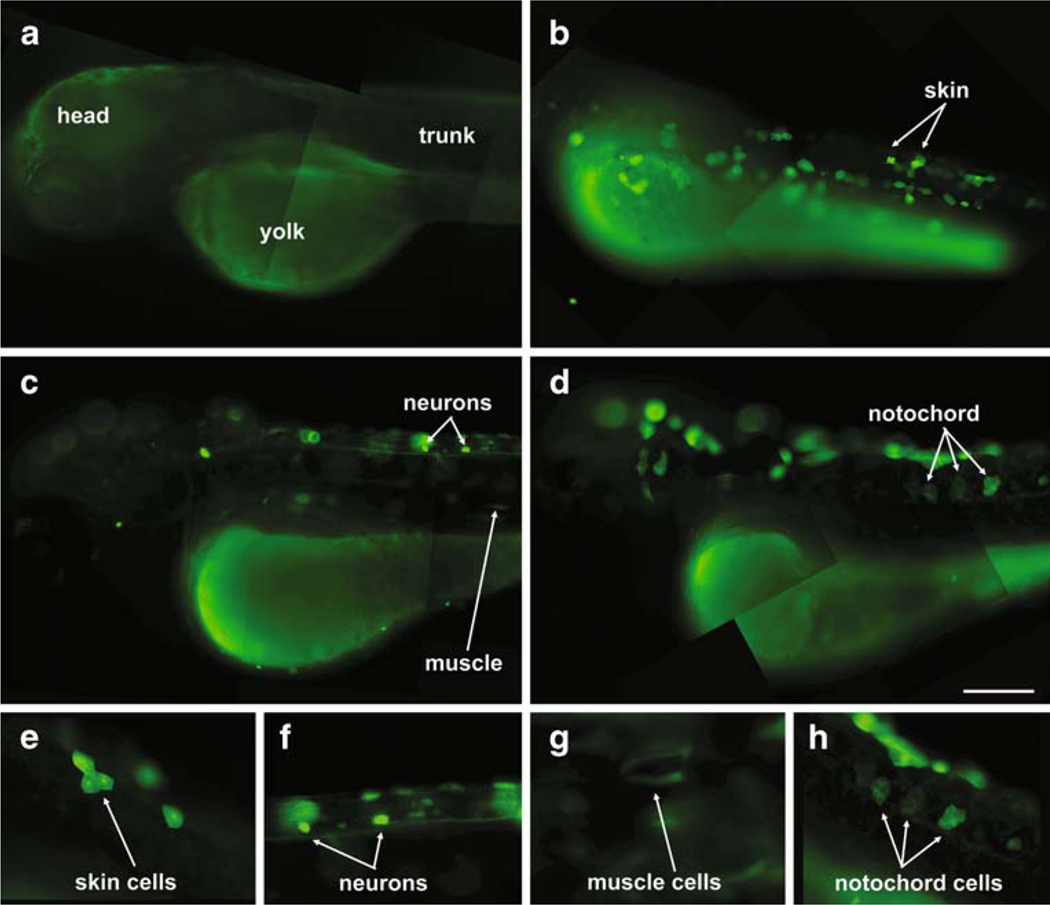
Fig. 3.
Embryos injected with the EPRE–luciferase–GFP reporter plasmid expressed GFP after 24 h exposure to HgCl2. Control embryos that were uninjected or injected but untreated did not express GFP (a). HgCl2-induced GFP expression was most commonly observed in skin cells (b, e), although at treatment, ≥0.3 μM expression was also observed in other cell types including neurons (c, f), muscle cells (c, g), and notochord cells (d, h). Scale bar applies to a–d=200 μm
Fig. 4.
Characterization of transgene expression in injected (F0) embryos. a Embryos that survived 24 h HgCl2 treatment were characterized for the presence or absence of GFP-expressing cells. A greater percentage of embryos expressed GFP at higher concentrations of HgCl2. b Embryos expressing GFP were scored based on the number of cells expressing GFP as + (one to ten cells expressing), ++ (ten to 100 cells expressing), and +++ (more than 100 cells expressing). A greater number of GFP+ cells/embryos were observed at higher concentrations of HgCl2. c Embryos expressing GFP were scored for expression in the head, trunk, and/or tail. GFP expression is more widely distributed at higher concentrations of HgCl2. d Injected embryos treated at 0.3 uM were divided into groups of two based on number of cells expressing GFP as in b. Luciferase activity of these groups was measured. High levels of luciferase activity correlate with high levels of GFP expression. Error bars represent standard error of the mean
We next measured luciferase activity in HgCl2-treated transgenic embryos to determine if levels of luciferase expression were correlated with levels of GFP expression (Fig. 4d). In embryos treated at 0.3 μM, we observed low (1–10 GFP+ cells/embryo), intermediate (10–100 GFP+ cells/embryo), and high (>100 GFP+ cells/embryo) levels of expression. Extracts for luciferase assays were generated from pairs of embryos first scored for GFP expression. Embryos displaying low or intermediate levels of GFP expression had a fourfold increase in luciferase activity over background. Embryos with high levels of GFP expression had a 12-fold increase in luciferase activity over the background. Embryos with no detectable GFP expression also did not have luciferase activity over background levels. Thus, luciferase activity could generally be correlated with GFP expression.
Inducible Expression of Luciferase in Stable Transgenic Lines
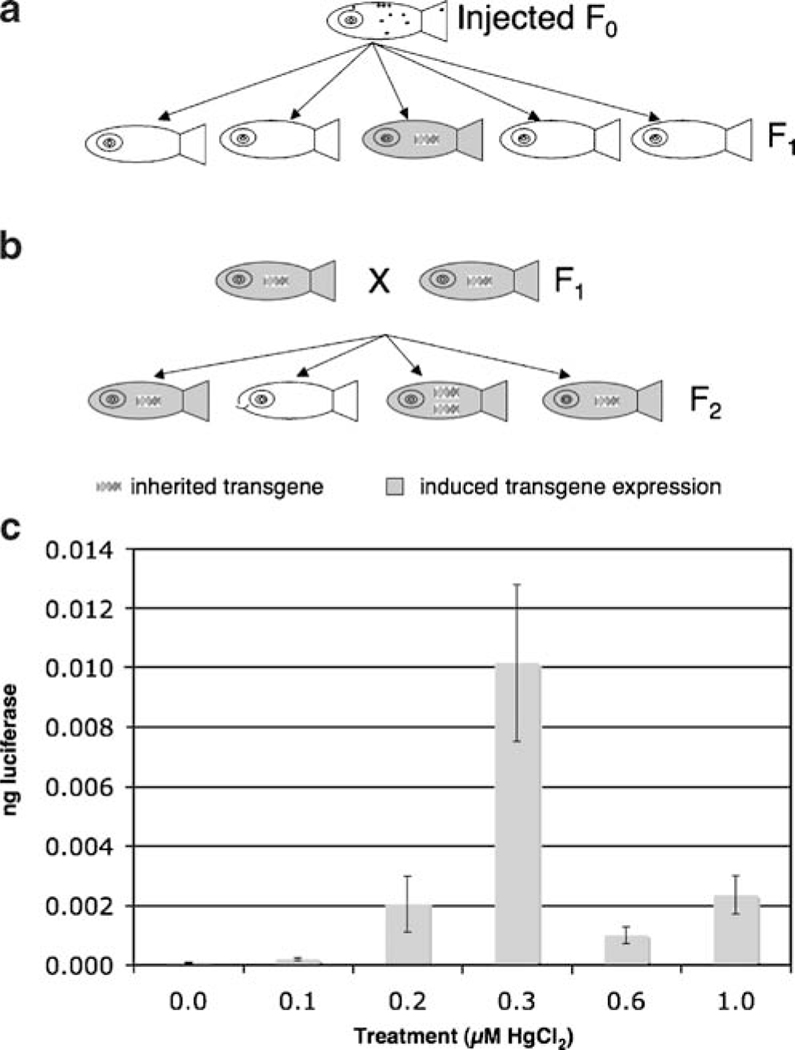
In addition to experiments using transient transgenic fish (fish in which the transgene is injected into single-cell-stage embryos), we also tested for transgene inducibility in stable transgenic fish (fish that inherit the transgene, which is stably integrated into the genome). These experiments were carried out on the progeny of F1 EPRE–LUC–GFP transgenic fish. Three quarters of the progeny from such a cross are expected to carry at least one copy of the transgene, which we confirmed by performing PCR on genomic extracts of single progeny of such crosses. Embryos in groups of eight were exposed to HgCl2 as described above. We observed no GFP expression, using fluorescence microscopy in the control or treated embryos. However, we were able to detect luciferase activity that increased in a dose-dependent manner up to 0.3 μM (Fig. 5). Luciferase activity declined sharply at higher concentrations, which were associated with higher mortality rates, likely due to cellular toxicity or cell death. Absolute luciferase activity was much lower in the stable lines than in the injected fish; however, enzyme activity was still within the linear range of the assay and could be accurately quantified. Thus, although the overall transgene expression levels are lower in the stable lines as has been previously observed for other transgenic lines (Udvadia and Linney 2003), they are still capable of detecting sub-lethal concentrations of HgCl2.
Fig. 5.
Characterization of transgene induction in stable transgenic lines. a Injected F0 fish have mosaic expression of the transgene. F0 founders transmit the transgene through the germ line to a small fraction of F1 progeny. b Crosses of transgene positive F1 fish results in 3/4 of the progeny carrying at least 1 copy of the transgene. c Luciferase activity was detectable in induced stable line embryos, but at lower levels when compared to injected embryos, although levels were still within the linear range of the assay. There was no visible GFP expression in stable line induced embryos. Transgene expression is diminished at concentrations of HgCl2 that are correlated with increased mortality. Results are the average values from 3 groups of 8 for each treatment. Error bars represent standard error of the mean
Discussion
Inorganic mercury released into aquatic environments is subject to biomethylation, eventually reaching animals in higher trophic levels where bioaccumulation can significantly amplify the effective concentration of toxic metals (de Carvalho et al. 2006). In humans, exposure to high levels of mercury is linked to serious neurological disorders and deaths (Goldman and Shannon 2001). Chronic low-level exposure, particularly in children, is linked to more subtle neurodevelopmental disabilities (Guzzi and La Porta 2008). In this study, we describe an approach to detect low levels of inorganic mercury in aquatic environments using transgenic zebrafish.
Previous reports have shown that zebrafish embryos are sensitive to mercury at relatively low concentrations as measured by hatching and survival time (Dave and Xiu 1991). Using these parameters, it was shown that 32 μg/l HgCl2 completely inhibited hatching when administered to embryos at blastula stage (2–4 hpf). In our studies, we began to observe toxic effects at slightly higher concentrations (Fig. 2, 0.3 μM=80 μg/l). This difference is likely due to differences in treatment time and the age of treated embryos. We exposed embryos at a later stage in development (24 hpf) to overcome clutch to clutch variation in early survival. We also exposed for only 24 h rather than the 2- to 4-day exposure required for the hatching time criteria. It is probable that longer exposure times would result in higher sensitivity due to the effect of bioaccumulation. In adult zebrafish, measurable bioaccumulation has been reported after 7 days of exposure either through contaminated water and sediments or through dietary exposure (Gonzalez et al. 2005; de Carvalho et al. 2006). However, similar studies have not been described in zebrafish embryos or larvae.
Our aim was to develop an assay in which mercury could be detected at levels not correlated with outward signs of toxicity. We created EPRE reporter constructs based on those previously tested in zebrafish cell culture (Carvan et al. 2001) to generate transgenic reporter fish that could provide a more sensitive whole animal detection system for agents, such as HgCl2, that cause oxidative stress. As heavy metals, such as mercury, are capable of activating EPRE-regulated gene expression, we hypothesized that transgene expression may be activated at lower concentrations of HgCl2 than those required to cause developmental abnormalities or death in zebrafish embryos.
Although cultured cells from transgenic EPRE reporter mice (Johnson et al., 2002) and cultured zebrafish cells transfected with EPRE reporter plasmids (Carvan et al. 2001) have been previously used to study mechanisms of oxidative stress, to our knowledge, this is the first example of a whole animal reporter gene assay for oxidative stress. There are several advantages to using a transgenic fish reporter over cell culture reporter assays. First, due to the tendency of chemicals from the environment to accumulate in the animal, a whole animal assay is predicted to be more sensitive (Carvan et al. 2001). In our assays, we see an order of magnitude increase in reporter gene sensitivity to HgCl2 in whole fish compared to published results for reporter gene activity in cultured cells exposed to HgCl2 (Carvan et al. 2001). Second, a given toxicant may preferentially elicit responses from particular tissue types or cell lines but not others. With the whole animal reporter assay, it is possible to visualize the response of many tissues at once, thus reducing the chances for false negative readings. Finally, once generated, transgenic fish lines can be propagated and maintained with greater ease and at lower cost than cultured cells. Under laboratory conditions, the fish can be continuously spawned to provide a steady supply of embryos that can be easily used for daily monitoring of water supplies.
In our reporter construct, EPRE–LUC–GFP, expression of a luciferase–GFP fusion protein is regulated by a single copy of the EPRE from murine gsta1 gene and minimal promoter from the murine mt1 gene. Furthermore, we flanked the entire transgene with I-SceI sites, which have previously been demonstrated to reduce transgene mosaicism when co-injected with the I-SceI enzyme in single-cell-stage zebrafish embryos (Thermes et al. 2002). This construct was designed to generate transgenic fish that would display a visual, qualitative measure of exposure with the GFP component but would also be capable of providing a quantitative measure using the luciferase component (Fig. 1). A similar fusion reporter has been previously described for use in cultured cells (Day et al. 1998). The fusion protein we have generated utilizes a modified luciferase coding sequence containing silent mutations that eliminate possible methylation sites that could silence that transgene as it passes through the germline. We have found that in transgenic zebrafish bearing these constructs, the transgene is silent under normal conditions but can be activated with as little as 0.1 μM HgCl2 in fish injected with the transgene (Fig. 4) and 0.2 μM HgCl2 in stable transgenic lines (Fig. 5c). These results demonstrate that the transgenic fish have a five to tenfold greater sensitivity than cultured cells transfected with similar reporter genes (Carvan et al. 2001).
In the range of concentrations that were not toxic to the fish, luciferase activity in the stable lines was dose-dependent (Fig. 5c). At higher concentrations that caused obvious changes in morphology and lead to greater mortality, transgene expression was diminished. This effect does not diminish the usefulness of the whole animal assay because increased morphological defects and mortality themselves are indicators of high concentrations of toxicants. It is at the lower end of the spectrum that increased sensitivity would be beneficial. One way of achieving increased sensitivity would be to employ the bipartite GAL4VP16-UAS system, which has been successfully employed to amplify transgene expression in zebrafish (Koster and Fraser 2001).
Recently, another in vivo assay for mercury using zebrafish has been described (Ko et al. 2006). This assay takes advantage of a rhodamine-based molecular probe that can be rapidly absorbed by zebrafish larvae (20 min) and can fluoresce selectively in response to a 10-min exposure to mercury. Developing selective probes for rapid detection of many different types of heavy metal contaminants could prove extremely useful for monitoring water sources. Given that our EPRE reporter fish are theoretically responsive to conditions of oxidative stress in general, these two systems could be used in tandem. The EPRE reporter fish could be used to detect the presence of heavy metal contaminants, and subsequent testing using a panel of more specific probes could pinpoint the nature of the contaminant.
Interestingly, we found that skin cells were most frequently induced to express the transgene after HgCl2 exposure (Fig. 3b,e). We believe that this result is likely due to the route of exposure rather than a selective effect of mercury on skin because, at higher concentrations, we also observed transgene induction in other cell types, including muscle, neurons, and notochord (Fig. 3c,d, f–h). In humans, it has been shown that the rate of absorption of HgCl2 in the skin is dependent on concentration and exposure time (Baranowska-Dutkiewicz 1982). If exposure to internal organs is dependent on the rate of absorption, then it would be expected that prolonged exposures or higher concentrations of mercury would result in more widespread effects as we have observed in this study (Fig. 3, 4). In studies of adult zebrafish exposed to methyl mercury through diet, significant bioaccumulation was observed in brain, liver, and skeletal muscle (Gonzalez et al. 2005). Although the brain showed the highest accumulation of mercury, changes in protective response-associated gene expression were observed only in liver and skeletal muscle. These results led the authors to conclude that the high neurotoxicity associated with mercury exposure could be attributed to a lack of protective pathways triggered by mercury in other organ systems. In contrast, we did observe EPRE-induced gene expression in neurons after exposures at ≥0.3 μM HgCl2, suggesting that, perhaps, the protective pathways may be active in developing embryos but not adult animals.
In summary, we have successfully created transgenic indicator fish that can be used to screen for the presence of oxidative stressors in aquatic environments. Transgene expression is observed only in exposed animals and displays a dose-dependent pattern of expression at sub-lethal doses. Transgene expression is induced at tenfold lower concentrations of HgCl2 in the whole animal than in cultured cells, bringing the sensitivity of this assay nearer to the range necessary to be useful for environmental monitoring. Modifications to transgene design for future indicator lines may further increase the sensitivity and hence the utility of this assay.
Acknowledgment
This work was supported by pilot project funding to A.J.U. from the UWM Institute of Environmental Health and the UWM Center for WATER Security (DARPA grant no. NBCH1050024).
Contributor Information
Brandon W. Kusik, Department of Biological Sciences, University of Wisconsin–Milwaukee, Milwaukee, WI 53204, USA
Michael J. Carvan, III, Great Lakes WATER Institute, University of Wisconsin–Milwaukee, 600 E. Greenfield Ave.,Milwaukee, WI 53204, USA.
Ava J. Udvadia, Department of Biological Sciences, University of Wisconsin–Milwaukee, Milwaukee, WI 53204, USA Great Lakes WATER Institute, University of Wisconsin–Milwaukee, 600 E. Greenfield Ave.,Milwaukee, WI 53204, USA.
References
- Baranowska-Dutkiewicz B (1982) Evaluation of the skin uptake of mercuric chloride in man. J Appl Toxicol 2:223–225 [DOI] [PubMed] [Google Scholar]
- Carvan MJ III, Solis WA, Gedamu L, Nebert DW (2000) Activation of transcription factors in zebrafish cell cultures by environmental pollutants. Arch Biochem Biophys 376:320–327 [DOI] [PubMed] [Google Scholar]
- Carvan MJ III, Sonntag DM, Cmar CB, Cook RS, Curran MA, Miller GL (2001) Oxidative stress in zebrafish cells: potential utility of transgenic zebrafish as a deployable sentinel for site hazard ranking. Sci Total Environ 274:183–196 [DOI] [PubMed] [Google Scholar]
- Dave G, Xiu RQ (1991) Toxicity of mercury, copper, nickel, lead, and cobalt to embryos and larvae of zebrafish, Brachydanio rerio. Arch Environ Contam Toxicol 21:126–134 [DOI] [PubMed] [Google Scholar]
- Day RN, Kawecki M, Berry D (1998) Dual-function reporter protein for analysis of gene expression in living cells. Biotechniques 25:848–856 [DOI] [PubMed] [Google Scholar]
- de Carvalho S, Lombardi JV, Paiva MJ, de Franca-Monkolski JG, Ferreira JR (2006) Bioaccumulation of mercury in fish exposed to experimentally contaminated water and sediment. Bull Environ Contam Toxicol 77:854–860 [DOI] [PubMed] [Google Scholar]
- Goldman LR, Shannon MW (2001) Technical report: mercury in the environment: implications for pediatricians. Pediatrics 108:197–205 [DOI] [PubMed] [Google Scholar]
- Gonzalez P, Dominique Y, Massabuau JC, Boudou A, Bourdineaud JP (2005) Comparative effects of dietary methylmercury on gene expression in liver, skeletal muscle, and brain of the zebrafish (Danio rerio). Environ Sci Technol 39:3972–3980 [DOI] [PubMed] [Google Scholar]
- Guzzi G, La Porta CA (2008) Molecular mechanisms triggered by mercury. Toxicology 244:1–12 [DOI] [PubMed] [Google Scholar]
- Hayashi A, Suzuki H, Itoh K, Yamamoto M, Sugiyama Y (2003) Transcription factor Nrf2 is required for the constitutive and inducible expression of multidrug resistance-associated protein 1 in mouse embryo fibroblasts. Biochem Biophys Res Commun 310:824–829 [DOI] [PubMed] [Google Scholar]
- Hayes JD, Flanagan JU, Jowsey IR (2005) Glutathione transferases. Annu Rev Pharmacol Toxicol 45:51–88 [DOI] [PubMed] [Google Scholar]
- Johnson DA, Andrews GK, Xu W, Johnson JA (2002) Activation of the antioxidant response element in primary cortical neuronal cultures derived from transgenic reporter mice. J Neurochem 81:1233–1241 [DOI] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N, Biswal S (2007) Cell survival responses to environmental stresses via the Keap1–Nrf2–ARE pathway. Annu Rev Pharmacol Toxicol 47:89–116 [DOI] [PubMed] [Google Scholar]
- Ko SK, Yang YK, Tae J, Shin I (2006) In vivo monitoring of mercury ions using a rhodamine-based molecular probe. J Am Chem Soc 128:14150–14155 [DOI] [PubMed] [Google Scholar]
- Koster RW, Fraser SE (2001) Tracing transgene expression in living zebrafish embryos. Dev Biol 233:329–346 [DOI] [PubMed] [Google Scholar]
- Kwak MK, Kensler TW, Casero RA Jr (2003) Induction of phase 2 enzymes by serum oxidized polyamines through activation of Nrf2: effect of the polyamine metabolite acrolein. Biochem Biophys Res Commun 305:662–670 [DOI] [PubMed] [Google Scholar]
- Linney E, Udvadia AJ (2004) Construction and detection of fluorescent, germline transgenic zebrafish. Methods Mol Biol 254:271–288 [DOI] [PubMed] [Google Scholar]
- Lister JA, Robertson CP, Lepage T, Johnson SL, Raible DW (1999) nacre encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate. Development 126:3757–3767 [DOI] [PubMed] [Google Scholar]
- Primiano T, Kensler TW, Kuppusamy P, Zweier JL, Sutter TR (1996) Induction of hepatic heme oxygenase-1 and ferritin in rats by cancer chemopreventive dithiolethiones. Carcinogenesis 17:2291–2296 [DOI] [PubMed] [Google Scholar]
- Thermes V, Grabher C, Ristoratore F, Bourrat F, Choulika A, Wittbrodt J, Joly JS (2002) I-SceI meganuclease mediates highly efficient transgenesis in fish. Mech Dev 118:91–98 [DOI] [PubMed] [Google Scholar]
- Udvadia AJ, Linney E (2003) Windows into development: historic, current, and future perspectives on transgenic zebrafish. Dev Biol 256:1–17 [DOI] [PubMed] [Google Scholar]
- Westerfield M (2000) The zebrafish book: a guide for the laboratory use of zebrafish (Danio rerio). University of Oregon Press, Eugene, OR [Google Scholar]