Abstract
Objective
Purpose of the study was to identify the relationship among actual plasmatic levels of steroid hormones and behavioral manifestations in boys with autism and to assess the genetic contribution to these manifestations.
Methods
172 boys with autism under 10 years of age and 135 neurotypical boys attended the study. ADI-R and ADOS-2 were used to evaluate the core symptom severities. Problem behavior was assessed using BPI-01 questionnaire. Levels of testosterone, estradiol, dehydroepiandrosterone, dehydroepiandrosterone-sulfate and sex hormone binding globulin (SHBG) were measured in plasma of autistic boys. Three SNPs (in ESR1, SHBG, SRD5A2 genes) and one STR in AR gene (number of CAG repeats in first exon) were assessed. Hormonal levels and number of CAG repeats in AR gene were used for correlation analysis with behavioral measures. Genotype and allelic frequencies were compared among autistic and neurotypical boys.
Results
We found negative relationship among SHBG levels and restricted, repetitive behaviors (measured by ADOS-2) and positive relationship among actual testosterone levels and frequency of stereotyped behavior (measured by BPI-01).
Conclusion
Actual levels of SHBG and testosterone are related to severities of restricted and repetitive behaviors in boys with autism. Mechanisms of action of these hormones in brain require further investigation.
Keywords: Autism, Steroid hormones, Polymorphisms, Repetitive behavior
INTRODUCTION
Autism spectrum disorder (ASD) is a neurodevelopmental disorder of unclear etiology. There are theories depicting the importance of sex steroid hormones in autism, since the prevalence of the disorder is male-biased. What makes boys more vulnerable to achieve the diagnosis of autism remains unclear. One of the theories strengthens the importance of fetal organizational effect of testosterone on brain development. Baron Cohen with coworkers showed that elevated fetal levels of several androgens including testosterone were high in male-fetuses who later in postnatal life achieved the diagnosis of autism and fetal testosterone levels were positively correlated with autistic traits in general population [1-4]. In addition, females with conditions of abnormal prenatal exposure to testosterone and its sex steroid precursors, such as congenital adrenal hyperplasia and polycystic ovary syndrome, were found to have higher rate of autistic traits as well as their children were of higher risk of developing autism [5,6]. However, the exact mechanism by which these hormones influence the manifestation of autistic traits remains undiscovered. Another model explaining higher prevalence of ASD in males is a female protective model which suggests that multiple genetic factors contribute to the development of ASD and that higher threshold of genetic liability is required in females compared to males [7]. Zhang et al. [8] demonstrated genetic evidence of sex differences in ASD confirming female protective model, employing investigation of de novo mutations, common variants of ASD candidate genes and their co-expression in male and female brains. Genetic architecture of ASD involves rare and de novo variants that were identified in studies using whole genome or exome sequencing technologies as well as low-risk common variants. ASD candidates identified by large genome wide association studies belong to three signaling networks including steroidogenesis, neurite outgrowth and synaptic function [9]. This strengthens the importance of studying steroid hormones and their metabolites and their relation to the manifestation of core symptoms of autism.
Peripheral levels of testosterone in young children originate predominantly in adrenal cortex and may reflect brain levels since steroid hormones cross the blood brain barrier [10]. Therefore, it is possible to suppose that peripheral steroid hormonal levels might be related to brain functions, especially to behavioral measures relevant to autism phenotype. Our research group previously reported increased salivary testosterone levels in boys with autism and Asperger syndrome [11]. Moreover, our latest study examined 82 biomarkers of steroidogenesis in plasma of prepubertal boys with autism, and lower levels of metabolites of alternative backdoor pathway of androgen synthesis were identified as well as the correlation of these biological markers with behavioral indices, namely, social interaction, was suggested [12]. Several studies from other laboratories showed alterations in peripheral sex steroid hormones in autism. Ruta et al. [13] showed increased serum androstenedione levels in adults with ASD. Strous found lowered dehydroepiandrosterone-sulfate (DHEA-S) levels in adults with ASD [14]. Increased steroid metabolites were detected in saliva of children with autism [15]. In addition, increased levels of steroid metabolites were detected in urine of boys with autism and Asperger syndrome [16].
Three core domains of autism were described: first, impaired socialization, e.g. delayed peer interactions, absence of seeking to share enjoyment and interests, delayed initiation of interactions, little or no social reciprocity and absence of social judgment, and second, impaired communication, e.g. delay in verbal language without using gestures, impairment in expressive language and conversation, repetitive or idiosyncratic language, delayed imaginative and social imitative play. The third domain is restricted, stereotyped, and repetitive patterns of behavior, which includes preoccupation with stereotyped or restricted interests or topics, adherence to routines, stereotyped, repetitive motor mannerisms, preoccupation and fascination with parts of items with unusual visual or tactile exploration [17]. The severity of these core symptoms can be quantified using the outcomes from the two diagnostic instruments: the Autism Diagnostic Observation Schedule (ADOS-2) [18] and the Autism Diagnostic Interview-Revised (ADI-R) [19].
Apart from core symptom severities children diagnosed with ASD may develop a broad scale of problem behavior that is not necessarily related to ASD symptoms. Problem or challenging behavior is highly prevalent among children diagnosed with ASD [20]. This may include self-isolated ritualistic behavior, self-injurious behavior and various forms of aggressive behavior. It is very likely that various forms of challenging behavior may be influenced by altered steroid hormonal signaling.
There exists a limited number of studies focusing on relationship of steroid hormones and behavioral characteristics in autism. Out of these, elevated repetitive behavior was related to lower cortisol levels in ASD children [21]. More severe restricted and repetitive behaviors were also associated to serum allopregnanolone levels [22]. Another study showed that testosterone administration in healthy women reduced emotion recognition ability, one of the autism features [23].
The aim of our study was to assess the relationship of peripheral steroid hormones, symptom severities and problem behavior in boys diagnosed with autism in Slovakia. In addition to actual hormonal levels, we tested whether selected polymorphisms in genes related to testosterone metabolism may have an influence on these measures. We selected four polymorphisms in genes that were previously described in connection to steroid metabolism and/or linked to autism (Figure 1). We tested whether there exists a difference in genotype distributions among autistic and neurotypical individuals in Slovakia.
Figure 1.
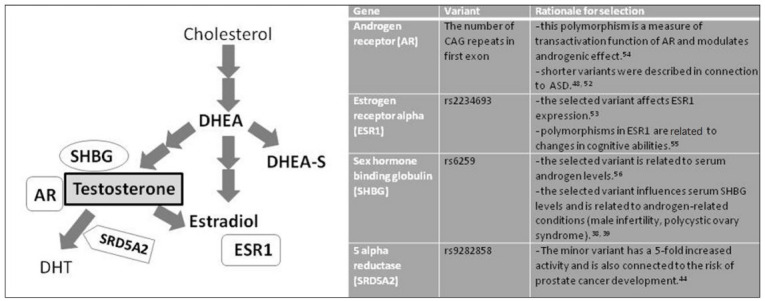
Scheme of steroid hormones, proteins and genes analyzed in ASD boys and rationale for selection of analyzed polymorphisms. DHEA, dehydroepiandrosterone; DHEA-S, dehydroepiandrosterone-sulfate; SHBG, sex hormone binding globulin; AR, androgen receptor; DHT, dihydrotestosterone; SRD5A2, 5-alpha reductase; ESR1, estrogen receptor 1.
METHODS
Presented study was approved by the Ethical committee of the Faculty of Medicine, Comenius University and the University Hospital in Bratislava, Slovakia. It is consistent with the 1964 Helsinki declaration and its later amendments. Parents were aware of the design and the course of the study by signing the informed consent form of the corresponding child.
Inclusion criteria included diagnosis of autism and age under 10 years. Into the analyses, only males were included since females with ASD reached only low sample size due to the lower incidence of ASD in females. Exclusion criteria were other neurodevelopmental, psychiatric, endocrine or genetic disorder.
Cohort description and psychological assessment
172 boys under 10 years of age diagnosed with autism attended the study (mean age 5.19±0.14 years). Control group consisted of 135 neurotypical boys without any of mentioned diagnoses who donated only buccal swabs for DNA isolation and subsequent genetic analyses. The age range of neurotypical boys was 5–18 years (mean age 11±0.55). The diagnosis of ASD was determined by a clinical psychologist or psychiatrist according to ICD 10, and children also underwent behavioral testing by trained specialists at the Academic Research Center for Autism, Institute of Physiology, Faculty of Medicine, Comenius Univeristy. This involved standard protocols of ADI-R and ADOS-2. These instruments were used to assess the core symptom severities.
ADI-R diagnostic tool describes three domains of autism phenotype: Domain A represents the score of qualitative abnormalities in social interaction, Domain B represents the score of qualitative abnormalities in communication and Domain C represents the measure of restrictive, repetitive, and stereotyped patterns of behavior. Because of possible differences in the raw score resulting from the different number of items evaluated according to age, the ADI-R scores were transformed. The final score in each ADI-R domain was calculated as a ratio between the individual’s raw score and maximum possible score that the individual could achieve (i.e. sum of maximal scores of all items evaluated). Thus, the transformed values are expressed in percent (%).
ADOS-2 instrument contains five modules one of which is selected for diagnostic procedure primarily according to level of speech development and secondarily according to age of the child. In our sample, 118 boys were diagnosed using Module 1, 32 boys using Module 2 and 22 boys using Module 3. ADOS-2 scale derived the following scores: social affect score (SA), restricted and repetitive behavior score (RRB), total raw score (RS), overall calibrated severity score (overall-CSS), social affect calibrated severity score (SA-CSS), restricted and repetitive behavior calibrated severity score (RRB-CSS) [24]. Raw scores of ADOS-2 (SA, RRB, and RS) could only be used while evaluating the group of children diagnosed using the same module. Therefore, raw data were mapped onto the 10-point calibrated severity scales based on percentiles of raw totals corresponding to each ADOS diagnostic classification [24,25]. Overall–CSS, SA-CSS, and RRB-CSS thus enable to evaluate the group of children independently on the type of module used and independently on the developmental factors [24]. Use of the calibrated domain scores instead of raw scores increases the probability that biological parameters would be associated specifically to ASD symptoms rather than child characteristics such as developmental level, age, IQ, language development or other non-ASD behavioral abnormalities [24].
Problem behavior was assessed using The Behavior Problems Inventory (BPI-01) questionnaire. BPI-01 is an instrument for the assessment of self-injury, stereotyped behavior, and aggression/destruction in individuals with developmental disabilities [26]. It consists of 52 items, out of which 15 items are related to self-injurious behavior, 25 items are related to stereotyped behavior and 12 items are related to aggressive behavior. Each item was scored on two scales, a five-point frequency scale (never=0, monthly=1, weekly=2, daily=3, hourly=4) and a four-point severity scale (no problem=0, a slight problem=1, a moderate problem=2, a severe problem=1). Separate raw scores were calculated for self-injurious behavior, stereotyped behavior and aggressive behavior as a sum of the points for each item. Raw scores were used for correlation analysis with hormonal levels. Only parents of 90 ASD boys have filled in BPI-01 questionnaire.
Analysis of hormones
Blood samples of ASD boys were collected into the sterile EDTA tubes (Sarstedt, Nümbrecht, Germany) around 10 am immediately after ADOS-2 diagnostic examination. All samples were processed immediately after collection by centrifugation at 3,000×g, 4°C for 10 minutes. Blood plasma and leucocyte mass samples were stored at -80°C until the analysis.
We selected the following peripheral steroid hormones: testosterone (T), estradiol (E), dehydroepiandrosterone (DHEA), dehydroepiandrosterone-sulfate (DHEA-S), and a protein sex hormone binding globulin (SHBG). The concentration of T, E, DHEA, DHEA-S was assessed using ELISA method (DRG Instruments GmbH, Marburg, Germany). Briefly, 25 μL of the samples in the case of detection of E, T and DHEA-S and 20 μL of the samples, standards and controls in the case of detection of DHEA were transferred into the microtiter plate with coated antibody. Thereafter, 200 μL enzyme conjugate was added and whole plate was incubated for 60 minutes (120 minutes for E) at room temperature (RT). After incubation, the content was poured out and the plate was washed with a wash solution for 3 times (400 μL/well). Then, 100 μL of substrate solution was added into each well and the plate was incubated for 145 minutes again. Besides the hormones, the concentration of SHBG was measured (DRG Instruments GmbH). First, the samples and standards were diluted in the ration 1:20 in the assay buffer. Thereafter, 100 μL of the assay buffer and 25 μL of pre-diluted samples and standards were transferred into the microtiter plate and incubated for 30 minutes at RT. Then, plate was washed 3 times with 400 μL of the wash solution per well. After this procedure, 100 μL of enzyme conjugated was added and plated was incubated again for 15 minutes. Then, the plate was washed again and incubated for 12 minutes with 100 μL of the substrate solution. In all cases, the reaction was stopped by adding the 50 μL of stop solution. The absorbance was red at 450+10 nm (Epoch, BioTek Instruments Inc, Winooski, VT, USA).
Genetic analysis
Genomic DNA from leucocytes or buccal swabs (Sarstedt) was extracted using the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions.
Among genes related to steroidogenesis and testosterone metabolism, we have selected androgen receptor (AR), estrogen receptor (ESR1), sex hormone binding globulin (SHBG), and 5-alpha reductase (SRD5A2). We have investigated the differences in distributions of these variants in ASD and healthy individuals and tested the influence of these variants on behavioral indices.
The (CAG)n repeat polymorphism in the gene encoding androgen receptor was amplified using PCR in 20 μL reaction volume with 250 nmoL/L primers: forward: 5'GCGCGAAGTGATCCAGAAC 3' tagged with 6–carboxyfluoresce in and reverse 5'CTCATCCAGGACCAGGTAGC 3', 1×Taq buffer (Fermentas, Vilnius, Lithuania) and 1U of Taq DNA polymerase (Fermentas). The following PCR program was used: initial denaturation step at 94°C for 4 min, followed by 35 cycles each consisting of denaturation at 94°C for 45 s, annealing at 59.5°C for 45 s and polymerization at 72°C for 45 s. The length of the final fragment was 181 bps. The number of repeats was analyzed by capillary electrophoresis. This genotyping process was successful in 120 ASD and 128 control samples. The 3 single nucleotide polymorphisms in genes encoding estrogen alpha receptor ESR1 (rs2234693), sex hormonebinding globulin SHBG (rs6259), steroid 5-alpha reductase 2 (rs9282858) were assessed by Sanger sequencing method in commercial company LGC Genomics Ldt. (Middlesex, London, UK). The rationale for selection of these polymorphisms is explained in Figure 1.
Statistic analysis
First of all, correlation of hormonal levels with behavioral determinants obtained from ADI-R (Domain A, Domain B, Domain C) and ADOS-2 (overall-CSS, SA-CSS, RRB-CSS) was done for the whole cohort (n=172) using Pearson correlation test. Subsequently, since the hormonal parameters correlated with age, we divided the group of boys into three subgroups according to age: 2.5–4.99 years (n=98), 5–6.99 years (n=41), 7–9.99 years (n=33) and correlation analyses were done for all of these groups separately. In addition, raw data obtained from ADOS-2 (RS, SA, RRB) were used for correlation with hormonal levels in the group of boys under five years who underwent ADOS-2 examination using Module 1 (n=80). Finally, hormonal levels were used for correlation with the outcomes from BPI-01 questionnaire in the group of boys where the data were available (n=90). Out of these 90 children, 60 belonged to the youngest age group (2.5–4.99 years), 23 belonged to the middle age group (5–6.99 years) and 7 children belonged to the oldest age group (7–9.99 years).
Genotypes were used to assess the association with behavioral measures. To assess the relationship of a certain genotype in case of SNPs (rs2234693, rs6259, rs9282858), ASD boys were divided into groups according to the genotype and the differences of behavioral measures were tested among groups by T test (in case of two genotypes present) or one-way ANOVA (in case of three genotypes present). To determine the effect size, Cohen’s d value was calculated. Correlations of the number of CAG repeats in AR gene and behavioral parameters were assessed using Pearson correlation test. Differences in genotypic and allelic distributions among autistic and neurotypical individuals were assessed using chi-square test and Fisher’s exact test. Effect sizes were determined by calculation of Cramer’s v and Phi coefficient, respectively.
Level of statistical significance was set to alpha<0.05. For determination of correlations of hormones and SHBG with behavioral parameters, correction for multiple comparisons was used and the level of significance was set to alpha<0.01. For determination of differences in distribution of genotypes and alleles among ASD and control children, the multiple comparison testing derived alpha<0.0125. For statistical analyses Graphpad Prism8 program (GraphPad Software, San Diego, CA, USA) was used.
RESULTS
Levels of steroid hormones and SHBG were assessed in plasma of ASD individuals. Phenotype of ASD individuals was evaluated using ADI-R and ADOS-2 instruments and BPI-01 questionnaire. Genotypes of four polymorphisms in genes related to testosterone metabolism were assessed in ASD and neurotypical individuals. Mean hormonal levels as well as behavioral determinants derived from ADI-R and ADOS-2 instruments are depicted in Table 1. As expected, several parameters (SHBG, DHEA, and DHEA-S) showed significant positive correlation with age (p=0.047, r=0.17; p=0.0001, r=0.35; p=0.0004, r=0.28, respectively). Correlation analyses of steroid hormones with behavioral indices were done for the whole cohort (n=172) and also separately for the three age groups. Results of correlation analysis for the hormonal parameters and behavioral parameters of the whole cohort are displayed in Table 2.
Table 1.
Mean levels of hormones and SHBG in plasma of ASD boys in appropriate age groups and mean scores of ADOS-2 and ADI-R variables
| Age | N | T (ng/mL) | E (pg/mL) | DHEA (ng/mL) | DHEA-S (μg/mL) | SHBG (nmol/L) | ADOS-2 scores |
ADI-R (%) scores |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall- CSS | SA- CSS | RRB- CSS | Domain A | Domain B | Domain C | |||||||
| All boys under 10 years | 172 | 0.32±0.03 | 34.68±2.50 | 1.12±0.12 | 0.25±0.04 | 73.02±5.65 | 7.48±0.14 | 7.13±0.15 | 7.53±0.19 | 0.50±0.02 | 0.56±0.02 | 0.40±0.01 |
| 2.5–4.99 years | 98 | 0.30±0.04 | 41.05±4.21 | 0.64±0.11 | 0.17±0.04 | 67.77±7.86 | 7.52±0.20 | 6.97±0.21 | 7.97±0.20 | 0.52±0.02 | 0.60±0.02 | 0.42±0.02 |
| 5–6.99 years | 41 | 0.27±0.03 | 27.21±2.09 | 1.14±0.18 | 0.30±0.10 | 58.32±8.22 | 7.58±0.27 | 7.23±0.29 | 7.30±0.45 | 0.44±0.03 | 0.48±0.03 | 0.39±0.02 |
| 7–9.99 years | 33 | 0.42±0.10 | 26.92±3.40 | 2.46±0.42 | 0.46±0.08 | 105.1±14.13 | 7.24±0.23 | 7.49±0.33 | 6.55±0.47 | 0.52±0.04 | 0.55±0.04 | 0.36±0.03 |
Values are expressed as mean±SEM. Domain A- score of qualitative abnormalities in social interaction, Domain B- score of qualitative abnormalities in communication and Domain C- restrictive, repetitive and stereotyped patterns of behavior. T, testosterone; E, estradiol; DHEA, dehydroepiandrosterone; DHEA-S, dehydroepiandrosterone sulfate; SHBG, sex hormone binding globulin; Overall CSS, overall calibrated severity score; SA-CSS, social affect calibrated severity score; RRB-CSS, restrictive and repetitive behavior calibrated severity score; ASD, autism spectrum disorders; ADOS-2, autism diagnostic observation schedule, second; ADI-R, autism diagnostic interview, revised; SEM, standard error of mean
Table 2.
Correlation analysis of hormonal levels and behavioral measures derived from ADI-R and ADOS-2 instruments
| Domain A | Domain B | Domain C | SA-CSS | RRB-CSS | Overall-CSS | |
|---|---|---|---|---|---|---|
| T ng/mL | ||||||
| p | 0.045 | 0.046 | 0.679 | 0.308 | 0.333 | 0.122 |
| r | 0.155 | 0.155 | 0.032 | 0.080 | 0.076 | 0.121 |
| E pg/mL | ||||||
| p | 0.039 | 0.093 | 0.689 | 0.295 | 0.175 | 0.208 |
| r | 0.182 | 0.148 | 0.036 | 0.093 | 0.120 | 0.112 |
| DHEA ng/mL | ||||||
| p | 0.090 | 0.157 | 0.539 | 0.709 | 0.352 | 0.721 |
| r | 0.138 | 0.115 | -0.050 | -0.030 | -0.076 | -0.029 |
| DHEA-S µg/mL | ||||||
| p | 0.143 | 0.034 | 0.748 | 0.945 | 0.763 | 0.575 |
| r | 0.118 | 0.171 | -0.026 | 0.006 | 0.024 | 0.045 |
| SHBG nmol/L | ||||||
| p | 0.886 | 0.684 | 0.061 | 0.671 | 0.001* | 0.018 |
| r | 0.013 | -0.035 | -0.160 | -0.037 | -0.274* | -0.203 |
ADI-R variables: Domain A- score of qualitative abnormalities in social interaction, Domain B- score of qualitative abnormalities in communication and Domain C- restrictive, repetitive and stereotyped patterns of behavior. ADOS-2 variables: Overall CSS- overall calibrated severity score, SA-CSS- social affect calibrated severity score, RRB-CSS- restrictive and repetitive behavior calibrated severity score, p-value of significance, r- Pearson correlation coefficient.
p<0.01.
T, testosterone; E, estradiol; DHEA, dehydroepiandrosterone; DHEA-S, dehydroepiandrosterone sulfate; SHBG, sex hormone binding globulin
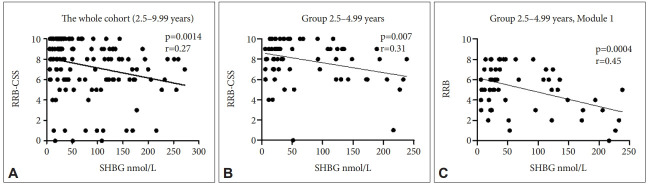
Among all measured parameters we found significant negative relationship among SHBG levels and RRB-CSS ADOS-2 variable which represents the measure of restricted, repetitive behaviors (p=0.001, r=-0.27) in the whole cohort (group of all boys under 10 years of age) (Figure 2A). The same variable negatively correlated with SHBG levels in the youngest age group (2.5–4.99 years) (p=0.007, r=-0.31) (Figure 2B). In addition, in boys of the youngest age group diagnosed using Module 1 (n=80) RRB raw score correlated negatively with SHBG levels (p=0.0004, r=-0.45) (Figure 2C).
Figure 2.
Correlation of variables describing behavioral measures derived from ADOS-2 with SHBG levels. A: The whole cohort of ASD boys, N=172. SHBG levels correlated negatively with RRB-CSS. B: Group of boys 2.5‒4.99 years of age, N=98. SHBG levels correlated negatively with RRB-CSS. C: Group of boys 2.5‒4.99 years of age of Module 1 (ADOS-2) N=80. SHBG levels correlated negatively with RRB. SHBG, sex hormone binding globulin; RRB-CSS, restrictive and repetitive behavior calibrated severity score; RRB, restrictive and repetitive behavior raw score; ADOS-2, autism diagnostic observation schedule, second.
Correlations of steroid hormones and SHBG with scores of problem behavior measured by BPI-01 instrument are shown in Table 3. We found positive relationship among actual testosterone levels and frequency of stereotyped behavior (p=0.006, r=0.30).
Table 3.
Correlation analysis of hormonal levels and behavioral measures derived from BPI-01 questionnaire
| Frequency of SIB | Severity of SIB | Frequency of stereotyped behavior | Severity of stereotyped behavior | Frequency of aggressive behavior | Severity of aggressive behavior | |
|---|---|---|---|---|---|---|
| T ng/mL | ||||||
| p value | 0.911 | 0.778 | 0.005* | 0.076 | 0.998 | 0.935 |
| r | -0.012 | -0.031 | 0.295* | 0.191 | 0.000 | -0.009 |
| E pg/mL | ||||||
| p value | 0.825 | 0.882 | 0.017 | 0.922 | 0.965 | 0.508 |
| r | 0.026 | -0.018 | 0.278 | 0.012 | -0.005 | -0.079 |
| DHEA-S μg/mL | ||||||
| p value | 0.719 | 0.327 | 0.692 | 0.945 | 0.256 | 0.269 |
| r | 0.039 | 0.108 | -0.044 | -0.007 | -0.126 | -0.121 |
| SHBG nmol/L | ||||||
| p value | 0.272 | 0.314 | 0.086 | 0.105 | 0.194 | 0.112 |
| r | 0.133 | 0.122 | 0.207 | 0.196 | 0.157 | 0.192 |
| DHEA ng/mL | ||||||
| p value | 0.991 | 0.535 | 0.758 | 0.941 | 0.251 | 0.215 |
| r | -0.001 | 0.0717 | -0.036 | -0.008 | -0.132 | -0.143 |
p<0.01.
T, testosterone; E, estradiol; DHEA, dehydroepiandrosterone; DHEA-S, dehydroepiandrosterone sulfate; SHBG, sex hormone binding globulin; SIB, self injurious behavior; r, Pearson correlation coefficient; BPI-01, behavior problems inventory
Analysis of genetic background of genes related to testosterone metabolism revealed that ASD boys with GG genotype (rs9282858) in SRD5A2 gene showed higher level of restricted and repetitive behaviors (measured by ADI-R) (p=0.009) (Figure 3A). This genotype was shown to be more frequent in ASD boys compared to neurotypical boys, however without reaching significance level (p=0.053). The allelic distribution in this polymorphism differed significantly among ASD and neurotypical boys (p=0.031), where A allele was more frequently present in neurotypical boys, whereas G allele was more frequently present in ASD boys (Figure 3B). However, the abovementioned difference in genotype and allelic distribution among ASD and neurotypical boys did not survive multiple comparison testing (p>0.0125).
Figure 3.
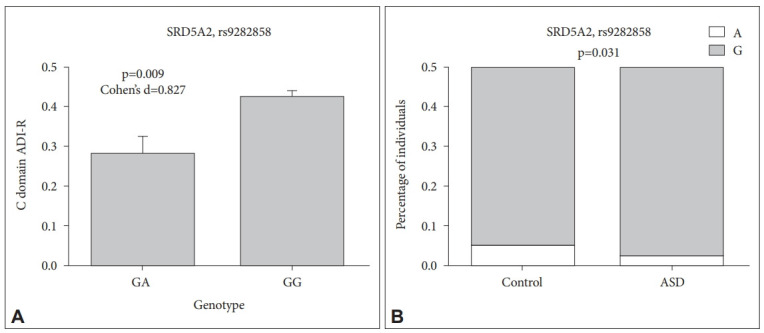
A: Various manifestation of repetitive behavior evaluated by domain C of ADI-R in ASD boys of different genotypes of SRD5A2 polymorphism rs9282858. B: Allelic distribution of SRD5A2 polymorphism rs9282858 among ASD and neurotypical boys. ASD, autism spectrum disorders; ADI-R, autism diagnostic interview, revised; SRD5A2, gene encoding 5-alpha reductase enzyme.
SHBG gene polymorphism also displayed different distribution of genotypes as well as alleles among ASD and neurotypical boys (p=0.045, p=0.019 respectively). Neither did this distribution difference remain significant after the correction for multiple comparisons. Nevertheless, no difference in behavioral parameters among ASD boys of different genotypes (AG, GG) was observed (data not shown).
No significant difference in distribution of genotypes and alleles between ASD and neurotypical boys was found in selected polymorphisms in genes ESR1 and AR. Neither did the genotypes of AR gene (number of CAG repeats) correlate with behavioral parameters. Boys with ASD of different genotypes in ESR1 polymorphism (CC, CT, TT) did not differ in behavioral manifestations (data not shown). Genotypic and allelic frequencies of all tested polymorphisms are shown in Table 4.
Table 4.
Distribution of genotypes in ASD boys and neurotypical boys in Slovakia
| SHBG rs6259 | SRD5A2 rs9282858 | ESR1 rs2234693 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Genotype | AA | AG | GG | AA | AG | GG | CC | CT | TT |
| Control (%) | 2 (1.6) | 35 (28.2) | 87 (70.2) | 1 (0.7) | 23 (17.0) | 111 (82.3) | 22 (20.2) | 56 (51.4) | 31 (28.4) |
| ASD (%) | 0 (0) | 30 (18.1) | 136 (81.9) | 0 (0) | 15 (9.1) | 150 (90.9) | 27 (18.6) | 67 (46.2) | 51 (35.2) |
| Statistics | Fisher’s exact test, p=0.045* | Fisher’s exact test, p=0.053 | Chi-square 1.296, p=0.523 | ||||||
| Phi coefficient=0.125 | Phi coefficient=0.121 | Cramer’s v=0.071 | |||||||
| Allele | A | G | A | G | C | T | |||
| Control (%) | 39 (15.7) | 209 (84.3) | 25 (9.3) | 245 (90.7) | 100 (45.9) | 118 (54.1) | |||
| ASD (%) | 30 (9) | 302 (91) | 15 (4.6) | 315 (95.4) | 121 (41.7) | 169 (58.3) | |||
| Statistics | Fisher’s exact test, p=0.019* | Fisher’s exact test, p=0.031* | Fisher’s exact test, p=0.367 | ||||||
| Phi coefficient=0.102 | Phi coefficient=0.094 | Phi coefficient=0.041 | |||||||
| AR (CAG) repeats | Control | ASD | |||||||
| Number of individuals | 128 | 120 | |||||||
| Mean nr.of CAG repeats±SEM | 21.72±0.25 | 21.73±0.28 | |||||||
| Statistics | Unpaired T test | ||||||||
| p=0.964 | |||||||||
SHBG, sex hormone binding globulin; SRD5A2, 5-alpha reductase; ESR1, estrogen receptor 1; AR, androgen receptor; ASD, autism spectrum disorders
DISCUSSION
Children diagnosed with ASD represent a heterogeneous group of individuals with different manifestation of autism symptoms. We have tested the correlations of actual levels of four steroid hormones and one protein (SHBG) with severity of autism symptoms evaluated by ADI-R and ADOS-2 instruments. The strongest negative correlation of SHBG protein levels was observed with ADOS-2 derived variable RRB. RRB variable describes the presence of restrictive and repetitive forms of behavior that within ADOS-2 classification includes the following items: stereotyped language/intonation of vocalizations in preverbal children, unusual sensory interests directed to person or objects, hand and finger mannerisms and other complex mannerisms (such as spinning in circles), unusual repetitive or stereotyped behaviors (such as lining up toy cars) [27]. These items result in a single RRB total score on the ADOS diagnostic algorithms. RRB-CSS score was used for correlation analyses of hormonal and SHBG levels in the group of children at various developmental levels. Using CSS scores enhances the specificity of the instrument to ASD symptoms regardless of general developmental factors such as age, IQ or language level [24]. In addition, we used RRB raw score for correlation analysis with hormonal and SBHG levels in the group of boys aged 2.5–4.99 years who were diagnosed using Module 1 of ADOS-2 (n=80). These children represent the most developmentally homogenous low functioning ASD group with most severe language impairment. RRB raw score scale offers broader scale of points than 10 point CSS scores and may therefore serve to reveal more accurate correlations with hormonal levels. The analysis showed negative correlation of SHBG plasmatic levels and RRBs and RRB-CSS. It would be beneficial to define RRBs more specifically, since RRBs detected by ADI-R and ADOS-2 comprise both higher and lower order RRBs, where lower order RRBs represent motor repetitive behaviors such as repetitive finger mannerisms whereas higher order RRBs include insistence on sameness or resistance to change phenomena [28]. It is interesting to mention that no hormonal parameters showed significant correlation with overall severity scores of ADOS-2, whereas RRBs correlated significantly with SHBG levels. This finding strengthens the importance to search for the biological factors affecting the particular ASD symptoms rather than the general ASD severity. Nevertheless, the number of studies searching for hormonal biological markers of single autism symptoms is limited. One example presents recent study on adult males with autism that showed decreased serum allopregnanolone levels to be associated with more severe restricted and repetitive behaviors [26]. Plasmatic SHBG binds testosterone in plasma resulting in lower levels of free testosterone biologically available to tissues [29]. Free testosterone passes the cellular membrane of many cells as well as it enters the brain tissue. We showed that lower levels of SHBG were associated with higher presence of RRBs in ASD boys. Our results suggest that optimal SHBG levels could be a protective factor lowering androgenic effect with possible behavioral consequences of lowering RRBs manifestation. The mechanism how SHBG could affect restricted and repetitive behaviors remains to large extent a mystery. The neural circuitry of RRBs has been described by number of neuroimaging studies on ASD and other intellectual disability individuals as well as animal models with overlapping results (reviewed by Wilkes and Lewis) [28]. The neural circuits between frontal and temporal cortices, basal ganglia and cerebellum seem to be involved in RRBs. Volumetric MRI as well as fMRI depicted the differences in volume of these brain areas as well as connectivity among them in subjects with ASD in relation to RRBs [28,30,31]. SHBG influences the levels of free testosterone that is available to tissues for promoting its androgenic effect. No studies explored the relationship between testosterone levels and functional brain connectivity in relation to RRBs. However, fetal testosterone as well as actual plasmatic testosterone was implicated in affecting brain functions in other ASD relevant brain areas, the social brain. Testosterone reduced the connectivity in social brain default mode network of the brain in healthy male adolescents [32]. Fetal testosterone was previously associated with restricted interests in healthy boys [33]. In addition, one study on females explored the effect of testosterone administration on the neural responses in circuits of human social aggression and showed the increased and more persistent neural responses after viewing angry versus happy facial expressions in the areas of reactive aggression involving amygdala and hypothalamus [34]. As mentioned above, there exists some evidence of testosterone involvement in social as well as aggressive behavior in children with ASD. Our study is the first to link the SHBG and testosterone levels and its metabolism to manifestation of restrictive and repetitive behaviors in boys with ASD. In the present study we showed different distribution of genotypes as well as alleles of SHBG gene polymorphism rs6259 among ASD and neurotypical individuals. A allele of this polymorphism is a minor allele and GG genotypes are considered wild type genotype, whereas GA and very rare AA genotypes are a variant genotypes in general population [35]. In our study ASD boys possessed GG genotype more frequently than controls and G allele of this polymorphism was present more frequently in ASD group than control group. Previous studies showed that GG genotypes are connected to lower SHBG levels and variant A allele (AA, GA genotypes) is connected to higher SHBG levels [35,36], however others found no association of this polymorphism with SHBG levels [37]. In addition, variant A allele and consequently lower SHBG levels were described to be associated with polycystic ovary syndrome in women and infertility in men [38,39]. In our study boys with ASD possessed more frequently G allele than controls, so we can predict lower levels of SHBG and thus enhanced androgenic effect in boys with ASD. However, this cannot be confirmed due to the absence of plasma samples from healthy children where plasmatic SHBG and other hormonal levels could be determined. In addition to strengthening the importance of SHBG and testosterone in regulation of RRBs, among all core symptoms of autism the RRBs only showed strong sex differences, where RRBs were strongly reduced in girls with ASD [40]. This study also revealed sex differences in brain morphometry especially in motor system and in social brain areas. This is the only study investigated the correlation of SHBG levels and behavior, where SHBG was positively associated with craving sweet and carbohydrate rich foods in luteal phase of menstrual cycle in healthy premenopausal women [41]. We are not aware of other relevant behavioral studies.
In the present study we wanted to broaden the description of ASD phenotype in terms of problem behavior, since problem behavior is frequently associated with ASD. BPI-01 questionnaire was used to analyze severity and frequency of three types of problem behavior: self-injurious behavior, stereotyped behavior and aggressive behavior. Plasmatic testosterone levels correlated positively with the frequency of stereotyped behavior. Stereotyped behavior is described by BPI-01 as behavior that looks unusual, strange, or inappropriate, that is voluntary and occurs repeatedly in the same way, however it does not cause physical damages described by the questionnaire [26]. Results from the BPI-01 questionnaire add some more information value to RRBs described by ADI-R and ADOS-2 instruments. This outcome strengthens the importance of testosterone and SHBG levels in regulation of RRBs. In our study only 90 parents of ASD children filled the BPI-01 questionnaire. It is interesting to mention that 60 out of these 90 children belonged to the youngest age group (2.5–4.99 years) and only 7 belonged to the oldest age group (7–9.99 years). Taken together with the results of SHBG correlations described above, it seems that restrictive, repetitive and stereotyped behavior may be under regulation of higher testosterone and lower SHBG levels especially in the youngest age group of ASD boys in Slovakia. It is also necessary to mention that these regulations may apply only for this specific population and cannot be generalized to the entire population of children diagnosed with ASD.
Limitations of the present study include the absence of control blood samples which would enable the comparison of measured hormones among ASD and age-matched neurotypical boys as well as the absence of data describing problem behavior in these boys (BPI-01 questionnaire). It seems that this is the limiting line in other laboratories too, since the number of studies comparing plasmatic hormonal levels among ASD and healthy children is very limited. Out of them, Ruta et al. [13] showed elevated androstenedione, a precursor of sex steroids in adults with ASD compared to neurotypical controls. In our previous research we showed that salivary testosterone levels were higher in boys with Asperger syndrome compared to healthy children [11]. Finally, our recent study showed lower levels of metabolites of alternative backdoor pathway of androgen synthesis in ASD boys [12]. Taken together, association among sex steroid hormones and ASD was recognized on the basis of several findings, such as the existence of the differences among steroid hormonal levels among ASD and neurotypical population [12,13], the confirmed association of prenatal sex steroids and autistic traits [2], the presence of androgen related conditions in females with ASD [42], and also the association of several genes involved in steroidogenesis with ASD [43]. In the present study, polymorphism rs9282858 in gene SRD5A2 encoding 5-alpha reductase enzyme was analyzed in ASD boys in comparison with neurotypical boys. ASD boys with GG genotype showed higher incidence of restrictive and repetitive behavior. A allele was more frequently present in neurotypical boys, whereas minor G allele was more frequently present in ASD boys, which confirmed our previous findings on smaller sample size [11]. This minor variant was detected to cause the 5-fold stronger reductase activity [44]. Thus ASD children may be under stronger androgenic activity also due to the carrying minor variant of this polymorphism, since 5-alpha reductase enzyme catalyzes the conversion of testosterone to dihydrotestosterone (DHT), which carries more potent androgenic activity. To separate the effect of testosterone and estradiol on tissues, it is purposeful to study DHT regulated genes, since DHT is non- aromatizable to estradiol. A recent study on human neural cells by Quartier et al. [45] showed that DHT regulates the expression of a number of genes that overlap with ASD candidate genes identified by GWAS. Relationship of GG genotype of SRD5A2 polymorphism with RRBs further strengthens the importance of testosterone metabolism in ASD pathogenesis, esp. in relation to repetitive behaviors. In addition, rs9282858 was also shown to predict serum testosterone and SHBG levels in adult men, where GA carriers had higher serum testosterone and SHBG levels than GG carriers [46]. This result corresponds to our findings in ASD boys, where GG carriers showed stronger RRBs and stronger RRBs were shown to be related to lower SHBG levels.
To study the effect of non-aromatized testosterone on tissues we analyzed the androgen receptor STR polymorphic region (number of CAG repeats) in intron 1 that was earlier described to regulate the androgen receptor transactivation function [47]. Longer variants of this polymorphic region decrease the ability of AR to activate transcription of several different AR-responsive genes [47]. Inversely, the lower number of CAG repeats is responsible for higher transactivational activity of AR enhancing the androgenic effect. Our previous study showed lower number of (CAG)n repeats in the AR gene in boys with Asperger syndrome [11]. Another study showed an association of shorter CAG variants and ASD in females only [48]. The number of CAG triplets ranges from 11–35 repeats, with a mean of 22, where long variants adversely influence AR transcriptional function resulting in e.g. impaired sperm production and male infertility [49]. The length of CAG triplets was associated with mental giftedness [50], performance of mental rotation tasks in healthy population [51] as well as with hyperactivity symptoms in ASD boys [52]. The present study does not add a confirmation to the importance of AR gene polymorphism in ASD pathogenesis. No statistical difference in CAG length among ASD and neurotypical individuals was found, neither did the CAG length correlate with severity of ASD symptoms measured by ADI-R or ADOS-2 instruments. However, it needs to be mentioned that the AR genotyping data were available only in 120 ASD boys, which is another limitation of the study.
In the present study we also examined ESR1 polymorphism that was previously described to affect ESR1 expression [53]. Neither did we find any differences in genotypic distributions in ESR1 gene polymorphism among ASD and neurotypical population, nor did the ESR1 genotypes show association with behavioral measures. In addition, no differences in estradiol levels were found in our sample. This suggests a minor importance of estrogen regulated mechanisms in ASD pathogenesis in our specific ASD population.
In conclusion, we found that SHBG levels negatively correlated with restrictive and repetitive behaviors in boys with autism. Lower levels of SHBG supposedly promote stronger androgenic effect due to higher doses of free testosterone available to tissues. How higher doses of testosterone regulate restrictive repetitive behaviors is not exactly known; however, the mechanism potentially involves motor brain areas and circuitry and involves the tissues with androgen and estrogen receptors that up-or down-regulate the number of androgen and estrogen dependent genes that should be explored in more detail. In fact, the four selected polymorphisms in genes related to testosterone metabolism are presumably not sufficient to elucidate the genetic contribution of this signaling cascade to manifestation of autism symptoms, which is another limitation of the present study. RRBs should also be investigated in more detail and captured more specifically.
Finally, ASD individuals differ from neurotypical population in distribution of genotypes as well as alleles of polymorphisms in SHBG and 5alpha reductase genes. Moreover, ASD individuals carrying specific variants of 5alpha reductase gene tend to express more severe core autism symptoms such as restrictive and repetitive behaviors. This behavior showed negative correlation with plasmatic SHBG levels. In addition, stereotypic behavior was positively correlated with plasmatic testosterone. Further investigation is needed to clarify the exact mechanisms how androgens regulate ASD related behaviors in males and females with autism.
Acknowledgments
Authors thank to Faculty of Natural Sciences, Department of Molecular biology, Comenius University in Bratislava for enabling the Fragment analysis on ABI 3100-Avant Genetic Analyzer.
Footnotes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Silvia Lakatošová, Daniela Ostatníková. Methodology: Katarína Janšáková, Gabriela Repiská, Jaroslava Babková, Ivan Belica. Formal analysis: Silvia Lakatošová. Investigation: Jaroslava Babková, Katarína Janšáková, Gabriela Repiská, Mária Vidošovičová, Ivan Belica. Resources: Daniela Ostatníková. Supervision: Daniela Ostatníková. Writing—original draft: Silvia Lakatošová. Writing—review & editing: Katarína Janšáková, Gabriela Repiská, Ivan Belica.
Funding Statement
This study was supported by grants APVV150045, APVV150085, VEGA1/0068/21.
REFERENCES
- 1.Baron-Cohen S, Auyeung B, Nørgaard-Pedersen B, Hougaard DM, Abdallah MW, Melgaard L, et al. Elevated fetal steroidogenic activity in autism. Mol Psychiatry. 2015;20:369–376. doi: 10.1038/mp.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auyeung B, Taylor K, Hackett G, Baron-Cohen S. Foetal testosterone and autistic traits in 18 to 24-month-old children. Mol Autism. 2010;1:1–11. doi: 10.1186/2040-2392-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman E, Baron-Cohen S, Auyeung B, Knickmeyer R, Taylor K, Hackett G. Fetal testosterone and empathy: evidence from the empathy quotient (EQ) and the “reading the mind in the eyes” test. Soc Neurosci. 2006;1:135–148. doi: 10.1080/17470910600992239. [DOI] [PubMed] [Google Scholar]
- 4.Baron-Cohen S, Knickmeyer RC, Belmonte MK. Sex differences in the brain: implications for explaining autism. Science. 2005;310:819–823. doi: 10.1126/science.1115455. [DOI] [PubMed] [Google Scholar]
- 5.Knickmeyer R, Baron-Cohen S, Fane BA, Wheelwright S, Mathews GA, Conway GS, et al. Androgens and autistic traits: a study of individuals with congenital adrenal hyperplasia. Horm Behav. 2006;50:148–153. doi: 10.1016/j.yhbeh.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Cherskov A, Pohl A, Allison C, Zhang H, Payne RA, Baron-Cohen S. Polycystic ovary syndrome and autism: a test of the prenatal sex steroid theory. Transl Psychiatry. 2018;8:136. doi: 10.1038/s41398-018-0186-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson EB, Lichtenstein P, Anckarsäter H, Happé F, Ronald A. Examining and interpreting the female protective effect against autistic behavior. Proc Natl Acad Sci U S A. 2013;110:5258–5262. doi: 10.1073/pnas.1211070110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Li N, Li C, Zhang Z, Teng H, Wang Y, et al. Genetic evidence of gender difference in autism spectrum disorder supports the femaleprotective effect. Transl Psychiatry. 2020;10:4. doi: 10.1038/s41398-020-0699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poelmans G, Franke B, Pauls DL, Glennon JC, Buitelaar JK. AKAPs integrate genetic findings for autism spectrum disorders. Transl Psychiatry. 2013;3:e270. doi: 10.1038/tp.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pardridge WM, Mietus LJ. Regional blood-brain barrier transport of the steroid hormones. J Neurochem. 1979;33:579–581. doi: 10.1111/j.1471-4159.1979.tb05192.x. [DOI] [PubMed] [Google Scholar]
- 11.Schmidtova E, Kelemenova S, Celec P, Ficek A, Ostatnikova D. Polymorphisms in genes involved in testosterone metabolism in Slovak autistic boys. Endocrinologist. 2010;20:245–249. [Google Scholar]
- 12.Janšáková K, Hill M, Čelárová D, Celušáková H, Repiská G, Bičíková M, et al. Alteration of the steroidogenesis in boys with autism spectrum disorders. Transl Psychiatry. 2020;10:340. doi: 10.1038/s41398-020-01017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruta L, Ingudomnukul E, Taylor K, Chakrabarti B, Baron-Cohen S. Increased serum androstenedione in adults with autism spectrum conditions. Psychoneuroendocrinology. 2011;36:1154–1163. doi: 10.1016/j.psyneuen.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Strous RD, Golubchik P, Maayan R, Mozes T, Tuati-Werner D, Weizman A, et al. Lowered DHEA-S plasma levels in adult individuals with autistic disorder. Eur Neuropsychopharmacol. 2005;15:305–309. doi: 10.1016/j.euroneuro.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Majewska MD, Hill M, Urbanowicz E, Rok-Bujko P, Bieńkowski P, Namysłowska I, et al. Marked elevation of adrenal steroids, especially androgens, in saliva of prepubertal autistic children. Eur Child Adolesc Psychiatry. 2014;23:485–498. doi: 10.1007/s00787-013-0472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gasser BA, Kurz J, Dick B, Mohaupt MG. Steroid metabolites support evidence of autism as a spectrum. Behav Sci (Basel) 2019;9:52. doi: 10.3390/bs9050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy SE, Mandell DS, Schultz RT. Autism. Lancet. 2009;374:1627–1638. doi: 10.1016/S0140-6736(09)61376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lord C, Rutter M, Dilavore PC, Risi S, Gotham K, Bishop S. Autism diagnostic observation schedule. Torrance, CA: Western Psychological Services; 2012. [Google Scholar]
- 19.Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 20.Heyvaert M, Saenen L, Campbell JM, Maes B, Onghena P. Efficacy of behavioral interventions for reducing problem behavior in persons with autism: an updated quantitative synthesis of single-subject research. Res Dev Disabil. 2014;35:2463–2476. doi: 10.1016/j.ridd.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 21.Gabriels RL, Agnew JA, Pan Z, Holt KD, Reynolds A, Laudenslager ML. Elevated repetitive behaviors are associated with lower diurnal salivary cortisol levels in autism spectrum disorder. Biol Psychol. 2013;93:262–268. doi: 10.1016/j.biopsycho.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 22.Chew L, Sun KL, Sun W, Wang Z, Rajadas J, Flores RE, et al. Association of serum allopregnanolone with restricted and repetitive behaviors in adult males with autism. Psychoneuroendocrinology. 2021;123:105039. doi: 10.1016/j.psyneuen.2020.105039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bos PA, Hofman D, Hermans EJ, Montoya ER, Baron-Cohen S, van Honk J. Testosterone reduces functional connectivity during the ‘Reading the Mind in the Eyes’ Test. Psychoneuroendocrinology. 2016;68:194–201. doi: 10.1016/j.psyneuen.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hus V, Gotham K, Lord C. Standardizing ADOS domain scores: separating severity of social affect and restricted and repetitive behaviors. J Autism Dev Disord. 2014;44:2400–2412. doi: 10.1007/s10803-012-1719-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J Autism Dev Disord. 2009;39:693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rojahn J, Matson JL, Lott D, Esbensen AJ, Smalls Y. The Behavior Problems Inventory: an instrument for the assessment of self-injury, stereotyped behavior, and aggression/destruction in individuals with developmental disabilities. J Autism Dev Disord. 2001;31:577–588. doi: 10.1023/a:1013299028321. [DOI] [PubMed] [Google Scholar]
- 27.Kim SH, Lord C. Restricted and repetitive behaviors in toddlers and preschoolers with autism spectrum disorders based on the Autism Diagnostic Observation Schedule (ADOS) Autism Res. 2010;3:162–173. doi: 10.1002/aur.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilkes BJ, Lewis MH. The neural circuitry of restricted repetitive behavior: magnetic resonance imaging in neurodevelopmental disorders and animal models. Neurosci Biobehav Rev. 2018;92:152–171. doi: 10.1016/j.neubiorev.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selby C. Sex hormone binding globulin: origin, function and clinical significance. Ann Clin Biochem. 1990;27:532–541. doi: 10.1177/000456329002700603. [DOI] [PubMed] [Google Scholar]
- 30.Cheung C, Chua SE, Cheung V, Khong PL, Tai KS, Wong TK, et al. White matter fractional anisotrophy differences and correlates of diagnostic symptoms in autism. J Child Psychol Psychiatry. 2009;50:1102–1112. doi: 10.1111/j.1469-7610.2009.02086.x. [DOI] [PubMed] [Google Scholar]
- 31.Milardi D, Arrigo A, Anastasi G, Cacciola A, Marino S, Mormina E, et al. Extensive direct subcortical cerebellum-basal ganglia connections in human brain as revealed by constrained spherical deconvolution tractography. Front Neuroanat. 2016;10:29. doi: 10.3389/fnana.2016.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lombardo MV, Auyeung B, Pramparo T, Quartier A, Courraud J, Holt RJ, et al. Sex-specific impact of prenatal androgens on social brain default mode subsystems. Mol Psychiatry. 2020;25:2175–2188. doi: 10.1038/s41380-018-0198-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knickmeyer R, Baron-Cohen S, Raggatt P, Taylor K. Foetal testosterone, social relationships, and restricted interests in children. J Child Psychol Psychiatry. 2005;46:198–210. doi: 10.1111/j.1469-7610.2004.00349.x. [DOI] [PubMed] [Google Scholar]
- 34.Hermans EJ, Ramsey NF, van Honk J. Exogenous testosterone enhances responsiveness to social threat in the neural circuitry of social aggression in humans. Biol Psychiatry. 2008;63:263–270. doi: 10.1016/j.biopsych.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 35.Cousin P, Calemard-Michel L, Lejeune H, Raverot G, Yessaad N, Emptoz-Bonneton A, et al. Influence of SHBG gene pentanucleotide TAAAA repeat and D327N polymorphism on serum sex hormone-binding globulin concentration in hirsute women. J Clin Endocrinol Metab. 2004;89:917–924. doi: 10.1210/jc.2002-021553. [DOI] [PubMed] [Google Scholar]
- 36.Pang XN, Yuan Y, Sun Y, Shen JP, Zha XY, Hu Y. The relationship of sex hormone-binding globulin (SHBG) gene polymorphisms with serum SHBG level and metabolic syndrome in Chinese Han males. Aging Clin Exp Res. 2014;26:583–589. doi: 10.1007/s40520-014-0215-1. [DOI] [PubMed] [Google Scholar]
- 37.Martínez-García MÁ, Gambineri A, Alpañés M, Sanchón R, Pasquali R, Escobar-Morreale HF. Common variants in the sex hormone-binding globulin gene (SHBG) and polycystic ovary syndrome (PCOS) in Mediterranean women. Hum Reprod. 2012;27:3569–3576. doi: 10.1093/humrep/des335. [DOI] [PubMed] [Google Scholar]
- 38.Abu-Hijleh TM, Gammoh E, Al-Busaidi AS, Malalla ZH, Madan S, Mahmood N, et al. Common variants in the Sex Hormone-Binding Globulin (SHBG) gene influence SHBG levels in women with polycystic ovary syndrome. Ann Nutr Metab. 2016;68:66–74. doi: 10.1159/000441570. [DOI] [PubMed] [Google Scholar]
- 39.Cui YR, Guo YH, Qiao SD, Leng LF, Xie ZH, Chen H, et al. Correlation between SHBG gene polymorphism and male infertility in Han population of Henan province of China: a STROBE-compliant article. Medicine (Baltimore) 2017;96:e7753. doi: 10.1097/MD.0000000000007753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Supekar K, Menon V. Sex differences in structural organization of motor systems and their dissociable links with repetitive/restricted behaviors in children with autism. Mol Autism. 2015;6:50. doi: 10.1186/s13229-015-0042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krishnan S, Tryon RR, Horn WF, Welch L, Keim NL. Estradiol, SHBG and leptin interplay with food craving and intake across the menstrual cycle. Physiol Behav. 2016;165:304–312. doi: 10.1016/j.physbeh.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 42.Ingudomnukul E, Baron-Cohen S, Wheelwright S, Knickmeyer R. Elevated rates of testosterone-related disorders in women with autism spectrum conditions. Horm Behav. 2007;51:597–604. doi: 10.1016/j.yhbeh.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Chakrabarti B, Dudbridge F, Kent L, Wheelwright S, Hill-Cawthorne G, Allison C, et al. Genes related to sex steroids, neural growth, and socialemotional behavior are associated with autistic traits, empathy, and Asperger syndrome. Autism Res. 2009;2:157–177. doi: 10.1002/aur.80. [DOI] [PubMed] [Google Scholar]
- 44.Makridakis NM, Ross RK, Pike MC, Crocitto LE, Kolonel LN, Pearce CL. Association of mis-sense substitution in SRD5A2 gene with prostate cancer in African-American and Hispanic men in Los Angeles, USA. Lancet. 1999;354:975–978. doi: 10.1016/S0140-6736(98)11282-5. [DOI] [PubMed] [Google Scholar]
- 45.Quartier A, Chatrousse L, Redin C, Keime C, Haumesser N, MaglottRoth A, et al. Genes and pathways regulated by androgens in human neural cells, potential candidates for the male excess in autism spectrum disorder. Biol Psychiatry. 2018;84:239–252. doi: 10.1016/j.biopsych.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 46.Yeap BB, Knuiman MW, Handelsman DJ, Ho KKY, Hui J, Divitini ML, et al. A 5α-reductase (SRD5A2) polymorphism is associated with serum testosterone and sex hormone-binding globulin in men, while aromatase (CYP19A1) polymorphisms are associated with oestradiol and luteinizing hormone reciprocally. Clin Endocrinol (Oxf) 2019;90:301–311. doi: 10.1111/cen.13885. [DOI] [PubMed] [Google Scholar]
- 47.Chamberlain NL, Driver ED, Miesfeld RL. The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Res. 1994;22:3181–3186. doi: 10.1093/nar/22.15.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henningsson S, Jonsson L, Ljunggren E, Westberg L, Gillberg C, Råstam M, et al. Possible association between the androgen receptor gene and autism spectrum disorder. Psychoneuroendocrinology. 2009;34:752–761. doi: 10.1016/j.psyneuen.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 49.Xiao F, Lan A, Lin Z, Song J, Zhang Y, Li J, et al. Impact of CAG repeat length in the androgen receptor gene on male infertility - a meta-analysis. Reprod Biomed Online. 2016;33:39–49. doi: 10.1016/j.rbmo.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 50.Celec P, Tretinárová D, Minárik G, Ficek A, Szemes T, Lakatošová S et al. Genetic polymorphisms related to testosterone metabolism in intellectually gifted boys. PLoS One. 2013;8:e54751. doi: 10.1371/journal.pone.0054751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Durdiaková J, Lakatošová S, Kubranská A, Laznibatová J, Ficek A, Ostatníková D, et al. Mental rotation in intellectually gifted boys is affected by the androgen receptor CAG repeat polymorphism. Neuropsychologia. 2013;51:1693–1698. doi: 10.1016/j.neuropsychologia.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 52.Pivovarciova A, Durdiakova J, Babinska K, Kubranska A, Vokalova L, Minarik G, et al. Testosterone and androgen receptor sensitivity in relation to hyperactivity symptoms in boys with autism spectrum disorders. PLoS One. 2016;11:e0149657. doi: 10.1371/journal.pone.0149657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maruyama H, Toji H, Harrington CR, Sasaki K, Izumi Y, Ohnuma T, et al. Lack of an association of estrogen receptor alpha gene polymorphisms and transcriptional activity with Alzheimer disease. Arch Neurol. 2000;57:236–240. doi: 10.1001/archneur.57.2.236. [DOI] [PubMed] [Google Scholar]
- 54.Zitzmann M. The role of the CAG repeat androgen receptor polymorphism in andrology. Front Horm Res. 2009;37:52–61. doi: 10.1159/000175843. [DOI] [PubMed] [Google Scholar]
- 55.Dahlman-Wright K, Cavailles V, Fuqua SA, Jordan VC, Katzenellenbogen JA, Korach KS, et al. International union of pharmacology. LXIV. Estrogen receptors. Pharmacol Rev. 2006;58:773–781. doi: 10.1124/pr.58.4.8. [DOI] [PubMed] [Google Scholar]
- 56.Sato Y, Tajima A, Katsurayama M, Nozawa S, Yoshiike M, Koh E, et al. An independent validation study of three single nucleotide polymorphisms at the sex hormone-binding globulin locus for testosterone levels identified by genome-wide association studies. Hum Reprod Open. 2017;hox002 doi: 10.1093/hropen/hox002. [DOI] [PMC free article] [PubMed] [Google Scholar]