Abstract
Background
Previous studies have already revealed that triggering receptor expressed on myeloid cells-2 (TREM2) plays a significant protective role during the pathogenesis of ischemia injury in both brain and liver. This study aims to investigate the effect of TREM2 in myocardial ischemic injury.
Methods
The mice myocardial infarction (MI) model was established via left anterior descending coronary artery ligation. TREM2 expression was examined with RT-PCR and Western blot. Whereafter, mice were randomly divided into control, sham, MI, Ad.TREM2 transfection group and Ad.Null transfection group. Recombinant adenovirus containing the gene coding full-length mouse TREM2 and EGFP (Ad.TREM2) or control vector containing EGFP gene only (Ad.Null) were immediately intramyocardial injected after left anterior descending ligated. After 7 days of MI, HE, Masson and TUNEL staining were performed to find the myocardial injury, infarcted size and cell apoptosis. Besides, echocardiography was performed to determine cardiac function. In addition, Western blot was performed to check the activity of PI3K/AKT signaling pathway in myocardial tissue. Furthermore, the plasma concentrations of TREM2 in 19 coronary artery disease (CAD) patients and 8 healthy controls were measured.
Results
Compared with the sham group, TREM2 expression was significantly up-regulated in cardiac tissue in mice with MI. Cardiac tissue in mice transfected with Ad.TREM2 was demonstrated with alleviated injury, reduced infarct size, and decreased number of apoptotic cells. Echocardiography revealed that heart function was significantly improved in Ad.TREM2 transfection mice. Also, TREM2 transfection significantly activated the phosphorylation of AKT. At last, the plasma concentration of TREM2 was significantly elevated in patients with CAD and correlated with the severity of CAD.
Conclusions
TREM2 may curb myocardial ischemia injury via activating PI3K/AKT signal pathway. Besides, plasma TREM2 may be treated as a potential biomarker in the diagnosis of CAD to reflect the severity of coronary stenosis.
Keywords: Triggering receptor expressed on myeloid cells-2 (TREM2), myocardial ischemic injury, cardiac function, apoptosis
Introduction
Myocardial infarction (MI) is a major global public health problem (1). Although the development of interventional techniques remarkably decreased the mortality of MI, heart failure after MI is still a big challenge. Currently, researchers focused on the repair of cardiomyocytes and cardiac function recovery. Preventing heart failure after MI is still a problem that needed to be further studied (2).
Triggering receptor expressed on myeloid cells-2 (TREM2) is a classic cell surface receptor expressed on monocyte-derived cells which regulated differentiation of inflammatory cells and immune reaction (3-5). A great number of studies indicated that TREM2 is a key regulator of macrophage function and macrophage polarization (6,7). TREM2 was widely studied in Alzheimer’s disease (AD) that the deficiency of TREM2 in microglia promotes the pathological progression of AD (8). Furthermore, plasma TREM2 could serve as a biomarker of neuronal injury in Parkinson’s disease (9). Recently, the role of TREM2 in the organ protecting effect has been revealed (10). In sepsis, previous studies also revealed that TREM2 was the key molecular that elevated the survival and regulated the clearance of bacteria (11). However, in the circulation system, knowledge about TREM2 is still poor. Researchers have found that TREM2 may participate in atherosclerosis via single-cell RNA-Seq (12). According to The Human Protein Atlas (https://www.proteinatlas.org/), TREM2 was low expressed on cardiomyocytes. If the TREM2 functions in cell apoptosis or cell-protecting in ischemic heart injury was still unknown. Accordingly, we designed this study to identify if TREM2 has a protective effect in MI and preliminary revealed the mechanism using mice MI model and adenovirus transfection. We present the following article in accordance with the ARRIVE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-490/rc).
Methods
Measurement of TREM2 expression in myocardial tissue in MI mice model
C57BL/6 mice were purchased from Qing Long Shan Animal Breeding Farm and fed in SPF animal house in Yi Ji Shan hospital. Mice were housed in individually ventilated cages under a 12:12 light: dark cycle in microisolator cages under static conditions with autoclaved rodent chow and autoclaved tap water ad libitum and bedding. The male C57BL6 mice (weight 20–22 g) were anesthetized with intraperitoneal pentobarbital (35 mg/kg) and intubated. The left anterior descending (LAD) coronary artery was ligated proximally with 7–0 silk suture via a left thoracotomy incision. After 1, 2, 3, 5 and 7 days, mice were received over-dose of intraperitoneal pentobarbital (130 mg/kg) and myocardial tissue was collected. mRNA was extracted using Trizol method and the expression of TREM2 mRNA was determined by RT-PCR (n=5 for each group). Additionally, to rule out the effects of macrophages, we used clodronate liposomes to delete cardiac macrophages after MI. The TREM2 mRNA expression and protein expression were analyzed using RT-PCR and western blot (shown in Appendix 1).
Neonatal mouse cardiomyocytes culture
Neonatal C57BL/6 mice were purchased from Qing Long Shan Animal Breeding Farm, Nanjing, China. Primary neonatal mouse cardiomyocytes were prepared according to the procedure described previously (13,14). After 48 hours of cell culture to achieve subconfluence, cardiomyocytes were used for immunofluorescence and harvested for real-time PCR analysis.
Characterization of cultured mouse cardiomyocytes
Neonatal mouse cardiomyocytes were primarily characterized by immunofluorescence to evaluate the expression of TnI using rabbit anti-mouse TnI antibody (Proteintech Group, USA) and FITC labeled donkey anti-rabbit secondary antibody (Abcam, UK).
RT-PCR
The Mouse cardiomyocytes were lysate in TRIzol at −80 °C. RNA from bone marrow cells was collected as a positive control. Femurs of the adult C57BL/6 mouse were flushed using standard PBS to collect bone marrow (BM) cells. FACSTM-Lysing solution (BD Biosciences, USA) was used to deplete erythrocytes. The RNA extraction, reverse transcriptrion and PCR were performed using Trizol method, PrimeScriptTM RT reagent kit with gDNA Eraser (TAKARA, Japan) and TB Green Premix Ex Taq Kit (TAKARA, Japan) according to the manufacturer’s protocol. TREM2 primers (forward: TATGACGCCTTGAAGCACTG, reverse: AGAGTGATGGTGACGGTTCC) and β-actin primers (forward: AGAGGGAAATCGTGCGTGAC, reverse: AGGAAGAGGATGCGGCAGT) were used.
TREM2 adenovirus
Recombinant adenovirus containing the gene coding full-length mouse TREM2 and enhanced green fluorescent protein (EGFP) and control vector was produced according to previous study (11). The detailed description was shown in Appendix 1.
Transfection of TREM2 adenovirus in cardiomyocytes
The male C57BL6 mice (weight 20–22 g) have received thoracotomy after intraperitoneal pentobarbital (35 mg/kg) and are intubated. Mice have received 50 µL of intramyocardial injections of 5×105 plaque-forming units (pfu) Ad.TREM2 and Ad.Null, respectively (grouping by random number method). The injection point was 1–2 mm above ligation point. After 7 days, the animals were euthanasia by over-dose of intraperitoneal pentobarbital (130 mg/kg) and GFP expression in cardiomyocytes was detected. Additionally, the myocardial tissue was devided into above injection, around injection and below injection according to injection point. The TREM2 expression in these three partes were determined using western blot (shown in Appendix 1).
Mice model of acute MI and transfection of TREM2
The male C57BL6 mice (weight 20–22 g) were anesthetized with intraperitoneal pentobarbital (35 mg/kg) and intubated. The MI moded was performed as described above. Animal models were randomly divided into three groups (n=5 in each group; grouping by random number method): (I) mice were subjected to LAD coronary ligation; (II) mice have received 50 µL of intramyocardial injections of 5×105 Ad.Null; (III) mice have received 50 µL of intramyocardial injections of 5×105 Ad.TREM2. The injection point was 1–2 mm above the ligation site. Non-operated control and sham surgery were performed.
Echocardiography
After 7 days of the MI model being established, 2-dimensional echocardiography was performed on the mice using a transthoracic echocardiogram (Visual Sonic, Canada) as previously described (15). The ejection fraction (EF), left ventricular internal dimension-end-systolic (LVIDs) and left ventricular internal dimension-end-diastolic (LVIDd) were measured and the shortening fraction (FS) was calculated (n=5 for each group).
Immunofluorescence staining
All the mice were received an over-dose of intraperitoneal pentobarbital (130 mg/kg) after echocardiography. Frozen sections of the heart were prepared. The GFP fluorescence was detected by fluorescence microscopy (Olympus, Tokyo, Japan) with an excitation wavelength of 470 nm. Further, for detecting the expression of TREM2 in myocardial tissue, the myocardial tissue paraffin section (2 µm thick) was stained with sheep anti-mouse TREM2 antibody (R&D, USA) and rabbit anti-mouse TnI antibody (Proteintech Group, USA) at 4 °C overnight and then stained with Alexa Flour 488 labeled donkey anti-sheep secondary antibody (Abcam, UK) and Alexa Flour 647 labeled Goat anti-rabbit secondary antibody (Beyotime, China) to measure the expression of TREM2. And TnI. The nucleus was stained by DAPI (Beyotime, China). Fluorescence microscopy (Olympus, Tokyo, Japan) was used to detect the fluorescence.
Cardiac histopathology
All heart samples with infarct site was not in the left ventricle were excluded from the data analysis. The heart tissue was fixed in 10% formaldehyde and then embedded in paraffin. Tissue sections (2 µm thick) were stained with hematoxylin and eosin (HE) and Masson staining using a HE Staining Kit and Masson (Beyotime, China) and Masson stain kit (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer’s protocol. Infarct size was calculated using ImageJ (Calculate the percentage of green-stained myocardium). Injury of myocardial tissue were observed using an optical microscope (Olympus, Japan) (n=5 for each group). The morphological evaluations were performed in a blinded manner by 2 independent pathologists blinded to the treatment group.
TUNEL immunohistochemistry
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) was performed to detect apoptotic nuclei by TUNEL Staining Kit (Abcam, UK) according to the manufacturer’s protocol. The apoptotic cell number was counted by 2 independent observers blinded to the treatment group and expressed as a percentage of the total myocyte population.
Western blot
Protein was extracted from mouse myocardial tissue using a Tissue Protein Extraction Kit (Beyotime, China) according to the protocol provided by the manufacturer. Protein concentrations were measured using the BCA Assay Kit (Beyotime, China). Rabbit monoclonal anti-mouse AKT antibodies, rabbit monoclonal anti-mouse phosphorylated Ser473AKT (P-AKT) antibody (Santa Cruz, USA)was used to detect AKT and P-AKT. GAPDH was used as a loading control (KangCheng Biotech, China). Horseradish peroxidase-conjugated goat anti-rabbit secondary antibody was obtained from Boster, China.
Blood sample collected
Nineteen coronary artery disease (CAD) patients who consecutively underwent coronary angiography (CAG) in the Department of cardiology, Yi Ji Shan Hospital affiliated to Wan Nan Medical College were involved in the study. The CAD was defined as previous described (16). Two experienced interventional cardiologists blinded to the clinical information analyzed the CAG. Three mL peripheral venous blood was collected after CAG in an EDTA-coated tube immediately. After being centrifugated at 500 ×g for 10 min, the plasma was collected and store and −80 °C. The 8 sex and age-matched healthy controls were involved and the plasma sample was collected in the same way. The Gensini score in CAD patients were calculated according to the previous study (17).
Measurement of plasma concentrations of TREM2
The plasma concentrations of TREM2 were measure using Human TREM2 SimpleStep ELISA Kit (Abcam, UK) according to the manufacturer’s protocol.
Statistical analysis
For all experiments, the data were analyzed using either a Student’s t-test or Bonferroni’s test, and values are expressed as the means ± SD. Student’s t-test was used for the two sets of data. Bonferroni’s test was used for more than two groups of data. All statistical analyses were performed using SPSS software (SAS Institute Inc., USA); P value <0.05 was considered to indicate significance (α=0.05, β=0.1). PASS was used to calculate the sample size of each group according to the experimental standard deviation of previous results of mouse MI model.
Ethical statement
Experiments were performed under a project license (No. 2021LSGY No. 31) granted by the Ethics Committee of Yi Ji Shan Hospital affiliated to Wannan Medical College, in compliance with Guidelines for the care and use of laboratory animals in biomedical research (18). The study was conducted ethically in accordance with the World Medical Association Declaration of Helsinki (as revised in 2013). Written informed consents were obtained from all subjects for the use of their urine and biopsy samples for research purposes. The protocol for this study was not registered.
Results
Expression of TREM2 in myocardial tissue after MI in mice model
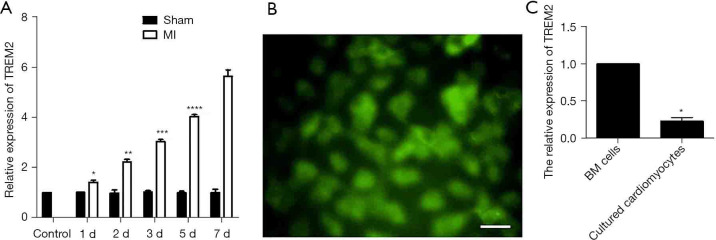
To evaluated the level of TREM2 expression in infarcted myocardial tissue, we detected the TREM2 expression after MI using male C57BL/6 mice MI model. RT-PCR showed that after 1 d, 2 d, 3 d, 5 d and 7 d of MI, the expression of TREM2 mRNA in myocardial tissue gradually increased compared to control. The sham group showed no increased TREM2 expression compared to MI (Figure 1A). After clodronate liposomes treatment (the efficiency of macrophage clearance was shown in Figure S1), the TREM2 mRNA expression and protein expression were also increased after MI (Figure S2). Five heart samples where infarct site was not in the left ventricle were excluded. Adverse events were not observed in mice model.
Figure 1.
The expression of TREM2 mRNA in myocardial tissue was increased after MI and cultured neonatal mouse cardiomyocytes lowly expressed TREM2. (A) RT-PCR showed that after 1 d, 2 d, 3 d, 5 d and 7 d of MI, the expression of TREM2 mRNA in myocardial tissue gradually increased (n=5, *, P<0.05 vs. control; **, P<0.05 vs. control, 1 d, 3 d, 5 d and 7 d; ***, P<0.05 vs. control, 1 d, 2 d, 5 d and 7 d; ****, P<0.05 vs. control, 1 d, 2 d, 3 d and 7 d). The sham group showed no increased expression of TREM2. (B) Immunofluorescence showed that cultured neonatal mouse expressed TnI. (C) RT-PCR showed that cultured neonatal mouse cardiomyocytes lowly expressed TREM2 compared to mice BM cells. Magnification: ×400; bar: 50 µm; n=5, *, P<0.05 vs. BM cells. TREM2, triggering receptor expressed on myeloid cells-2; MI, myocardial infarction; RT-PCR, reverse transcription-polymerase chain reaction; BM, bone marrow.
Characterization of cultured neonatal mouse cardiomyocytes and the expression of TREM2
The cultured male C57BL/6 neonatal mice cardiomyocytes were charactered by detecting TnI expression. Immunofluorescence showed that cultured neonatal mouse cardiomyocytes expressed TnI (Figure 1B). Further, the mouse cardiomyocytes have low mRNA expression of TREM2 compared to mouse bone marrow cells (Figure 1C).
Expression of TREM2 in cardiomyocytes after TREM2 adenovirus transfection
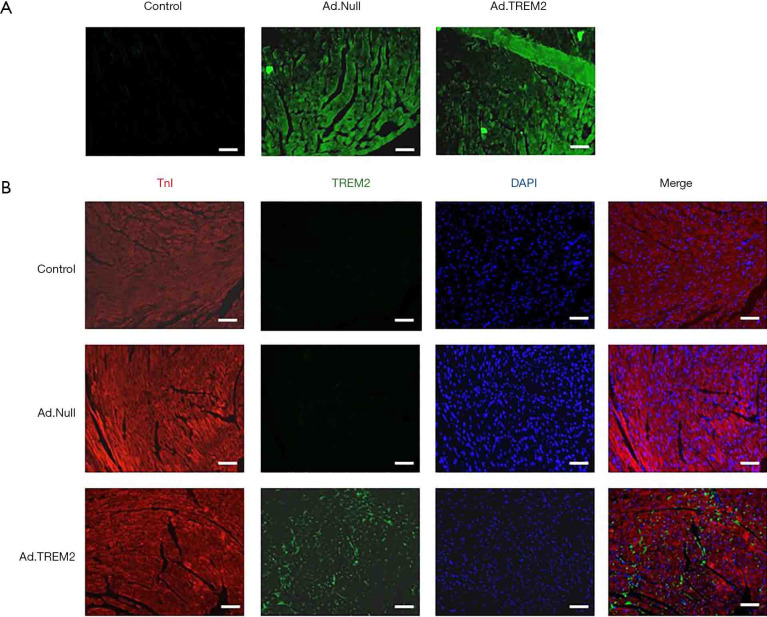
To determine the transfection efficiency, we analyzed the GFP and TREM2 expression on myocardial tissue of male C57BL/6 mice after Ad.TREM2 tansfection. Immunofluorescence showed that both Ad.TREM2 and Ad.Null successfully transfected in cardiomyocytes (Figure 2A). TREM2 expressed in cardiomyocytes after Ad.TREM2 transfection compared to Ad.Null transfection and control (Figure 2B). Additionally, the TREM2 protein expression around the adenovirus injection point and below the adenovirus injection point were analyzed by western blot. Both adenovirus injection point and below adenovirus injection point expressed TREM2 and no difference was found (Figure S3). Adverse events were not observed in mice.
Figure 2.
Transfection of TREM2 adenovirus in the heart tissue of mice. After Ad.TREM2 transfection, myocardial tissue expressed GFP and TREM2. (A) Immunofluorescence showed that TREM2 adenovirus and control adenovirus successfully transfected in heart tissue; (B) immunofluorescence showed that heart tissue expressed TREM2 after TREM2 adenovirus transfection. The control and control adenovirus transfection had no TREM2 expression. Magnification: ×200; bar: 100 µm. TREM2, triggering receptor expressed on myeloid cells-2; GFP, green fluorescent protein. DAPI, 4',6-diamidino-2-phenylindole; TnI, troponin I.
Effects of TREM2 adenovirus transfection on cardiac function after MI
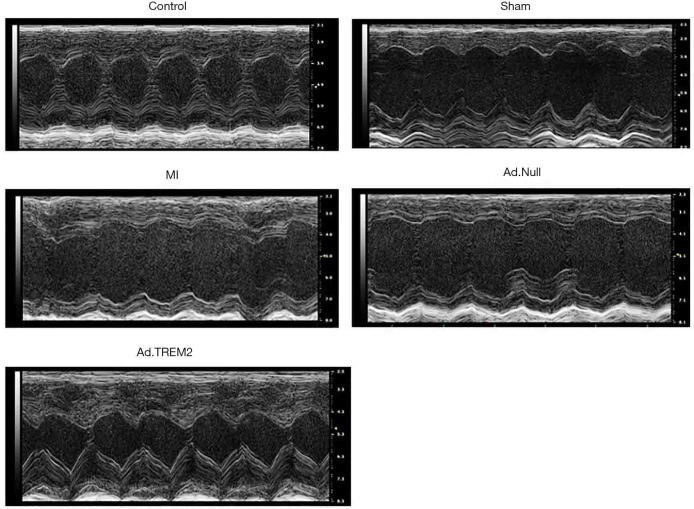
We analyzed the cardiac function to detect the effect of Ad.TREM2 transfection in male C57BL/6 mice MI model. Cardiac function was evaluated at 7 days after MI as shown in Table 1 and Figure 3. MI group had lower LVEF (48.9%±2.1% vs. 68.4%±3.6%, 69.1%±2.3%, P<0.05, respectively) and LVFS (22.7%±3.1% vs. 43.3%±3.2%, 42.4%±1.9%, P<0.05, respectively) than the control and Sham. The LVEF (59.3%±4.4% vs. 48.9%±2.1%, 47.2%±2.1%, P<0.05, respectively) and LVFS (35.3%±3.1% vs. 22.7%±3.1%, 23.2%±3.8%, P<0.05, respectively) were significantly higher in Ad.TREM2 group than MI and Ad.Null group. Further, the LVIDd (4.52±0.28 vs. 3.97±0.18, 3.90±0.23 mm, P<0.05, respectively) and LVIDs (3.20±0.24 vs. 2.40±0.10, 2.31±0.34 mm, P<0.05, respectively) were higher in the MI group than control and sham. Ad.TREM2 group had lower LVIDd (4.12±0.25 vs. 4.52±0.28, 4.51±0.36 mm, P<0.05, respectively) and LVIDs (2.69±0.11 vs. 3.20±0.24, 3.19±0.14 mm, P<0.05, respectively) than MI and Ad.Null. Adverse events were not observed in mice model.
Table 1. Effects of TREM2 adenovirus on physiological parameters and cardiac function in MI.
| Control | Sham | MI | Ad.Null | Ad.TREM2 | |
|---|---|---|---|---|---|
| LVEF, % | 68.4±3.6 | 69.1±2.3 | 48.9±2.1a | 47.2±2.1 | 59.3±4.4b |
| LVFS, % | 43.3±3.2 | 42.4±1.9 | 22.7±3.1a | 23.2±3.8 | 35.3±3.1b |
| LVIDd, mm | 3.97±0.18 | 3.90±0.23 | 4.52±0.28a | 4.51±0.36 | 4.12±0.25b |
| LVIDs, mm | 2.40±0.10 | 2.31±0.34 | 3.20±0.24a | 3.19±0.14 | 2.69±0.11b |
a, P<0.05 vs. control and MI; b, P<0.05 vs. MI and Ad.Null. TREM2, triggering receptor expressed on myeloid cells-2; MI, myocardial infarction. LVEF, left ventricular ejection fraction; LVFS, left ventricular fractional shortening; LVIDd, left ventricular internal dimension-end-diastolic; LVIDs, left ventricular internal dimension-end-systolic.
Figure 3.
Effect of TREM2 on cardiac function after MI. Ad.TREM2 transfection increased cardiac function after myocardial infaction. The impairment of cardiac function occurred in the MI group. The expression of TREM2 prevented cardiac dysfunction after MI. The LVEF and LVFS were significantly higher in the TREM2 transfection group (n=5). TREM2, triggering receptor expressed on myeloid cells-2; MI, myocardial infarction; LVEF, left ventricular ejection fraction; LVFS, left ventricular fractional shortening.
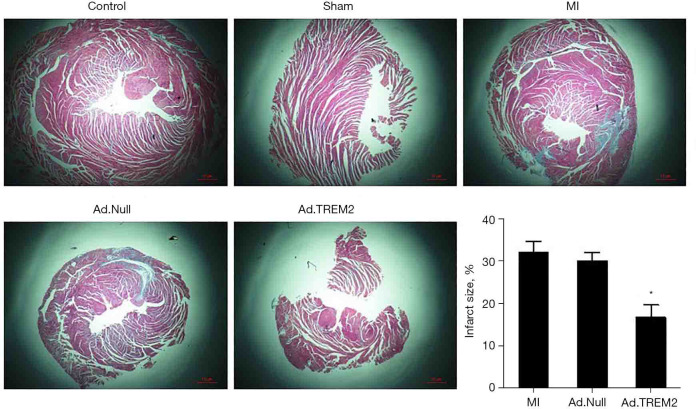
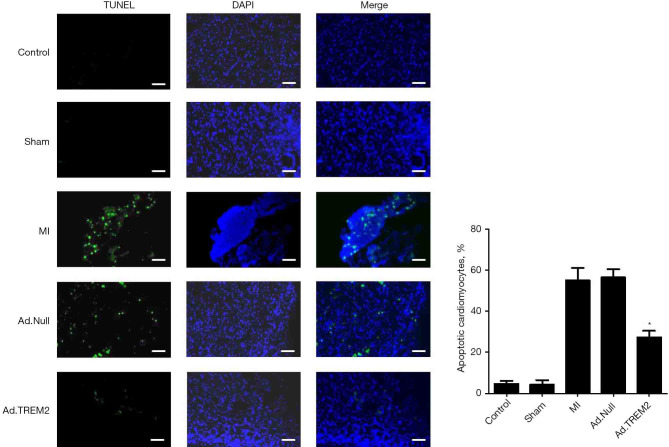
Effects of TREM2 adenovirus transfection on the myocardial injury, infarction size and cell apoptosis
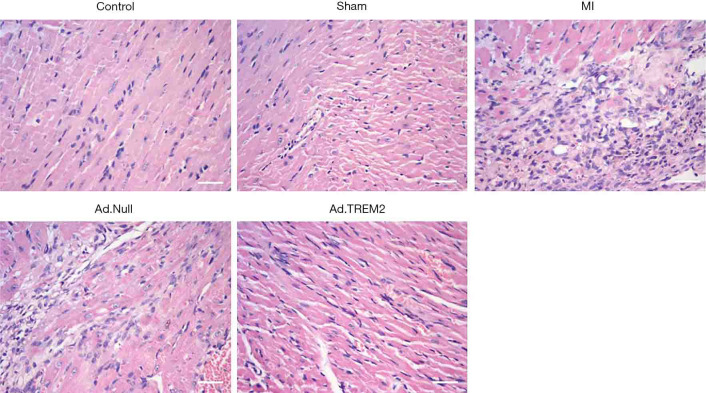
The effect of Ad.TREM2 transfection was determined by myocardial histopathology. The myocardial injury was determined by HE and TUNEL staining. Masson staining was used to detect the infarcted size. HE staining showed numerous inflammatory cells infiltration in infarct site, cardiomyolysis and cell apoptosis in the MI group compared to control and sham. In the Ad.TREM2 group, the myocardial injury was significantly alleviated compared to MI and Ad.Null (Figure 4). Masson staining showed the infarct size was rremarkably reduced after Ad.TREM2 transfection compared to MI and Ad.Null (16.7%±3.0% in Ad.TREM2 vs. 32.0%±2.6% in MI and 30.0%±2.0% in Ad.Null, P<0.05, respectively, Figure 5). TUNEL staining showed that the number of apoptotic cardiomyocytes markedly increased in the MI group compared to the control and sham. Ad.TREM2 transfection decreased the number of apoptotic cells compared to MI and Ad.Null (27±3/field in Ad.TREM2 vs. 55±6/field in MI and 56±4/field in Ad.Null, P<0.05, Figure 6). 3 heart samples where infarct site was not in the left ventricle were excluded. Adverse events were not observed in mice model.
Figure 4.
Effect of TREM2 expression on pathological change in myocardial tissue. Ad.TREM2 transfection alleviated myocardial injury after MI. HE staining showed that in the MI group, HE staining showed myocardial dissolving and numbers of inflammatory cells infiltration. TREM2 expression alleviated the myocardial injury after the MI group compared to MI and Ad.Null. Magnification: ×400; bar: 50 µm; n=5. TREM2, triggering receptor expressed on myeloid cells-2; MI, myocardial infarction.
Figure 5.
Effect of TREM2 expression on infarcted size after MI. The Ad.TREM2 transfection alleviated myocardial injury after MI, Masson staining showed that TREM2 adenovirus transfection significantly reduced the infarcted size after MI compared to Ad.Null transfection and MI group. Histogram showed the infarcted size in all groups. Magnification: 4×; n=5, *, P<0.05 vs. MI and Ad.Null group. TREM2, triggering receptor expressed on myeloid cells-2; MI, myocardial infarction.
Figure 6.
Effect of TREM2 on cardiomyocyte apoptosis after MI. The Ad.TREM2 transfection decreased cell apoptosis after myocardial infection. TUNEL staining showed that TREM2 adenovirus transfection significantly reduced the number of apoptotic cells after MI compared to Ad.Null transfection and MI group. Histogram showed the number of apoptotic cells in all groups. Magnification: ×200; bar: 100 µm; n=5, *, P<0.05 vs. MI and Ad.Null group. TREM2, triggering receptor expressed on myeloid cells-2; MI, myocardial infarction. DAPI, 4',6-diamidino-2-phenylindole; TnI, troponin I.
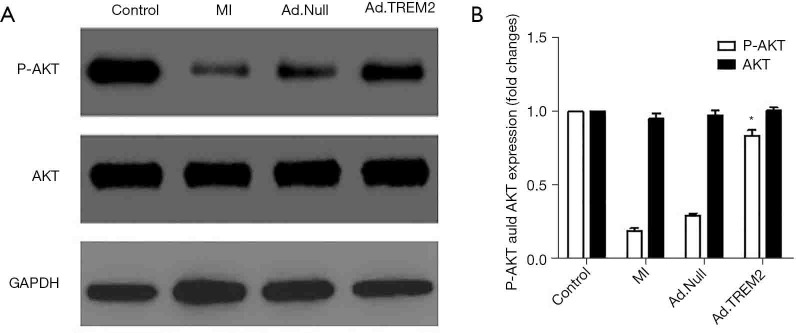
TREM2 adenovirus transfection activated PI3K/AKT signal pathway
We detected the expression of AKT and phosphorylated Akt in myocardial tissue to evaluate the effect of Ad.TREM2 transfection. In MI and Ad.Null group, the expression of phosphorylated AKT was significantly lower than the Ad.TREM2 group. TREM2 adenovirus transfection significantly activated the phosphorylation of AKT (Figure 7).
Figure 7.
Effect of TREM2 expression on the expression of signal protein expression in heart tissue. The Ad.TREM2 transfection activated the phosphorylation of AKT. (A) Representative band showed that in MI, phosphorylation of AKT was inhibited and TREM2 expression increased the expression of phosphorylated AKT. TREM2 promoted the activation of phosphorylation of AKT compared to MI and Ad.Null. (B) Densitometry quantitation of protein expression levels are shown as fold changes in the histogram. n=5, *, P<0.05 vs. MI and Ad.Null group. TREM2, triggering receptor expressed on myeloid cells-2; MI, myocardial infarction. GAPDH, glyceraldehyde-3-phos-phate dehydrogenase.
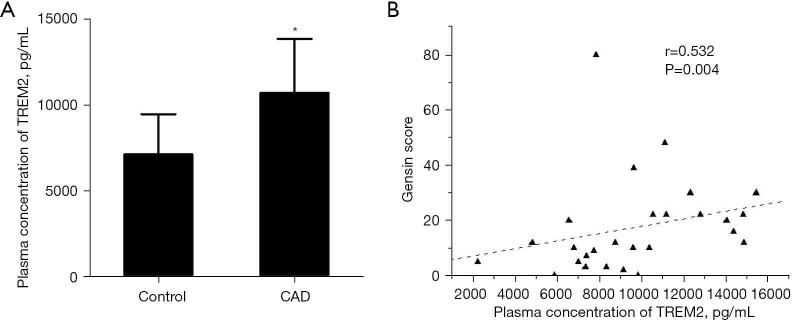
The plasma concentration of TREM2 was increased in CAD and correlated with the severity of coronary stenosis
To assessed the clinical application value of TREM2, we analyzed the plasma concentration of TREM2 in CAD patients and healthy controls. The plasma level of TREM2 was significantly higher in CAD patients compared with healthy control (10,713.6 pg/mL in CAD vs. 7,134.3 pg/mL in controls, P=0.008). Pearson correlation analysis showed that the plasma concentration of TREM2 significantly correlated with the Gensini score (r=0.532, P=0.004) (Figure 8).
Figure 8.
The plasma concentration of TREM2 in CAD patients and controls. The plasma concentration of TREM2 in CAD patients was significantly higher than controls. (A) Plasma concentration of TREM2 were elevated in CAD patients (*, P=0.008 vs. control); (B) plasma concentration of TREM2 were correlated with severity coronary stenosis (r=0.532, P=0.004). TREM2, triggering receptor expressed on myeloid cells-2; CAD, coronary artery disease.
Discussion
In this study, we firstly found that TREM2 protects against ischemic myocardial injury and promotes the survival of cardiomyocytes after MI. P13K/AKT signal pathway may regulate the protecting effect of TREM2. TREM2 has the potential to serve as the biomarker of CAD and the new therapeutic target of myocardial ischemic injury.
Heart failure is the major complication of MI due to a large number of cells apoptosis. The prevention of ischemic myocardial injury is a big change for clinicians and researchers. In nearly ten years, the therapeutic potential of stem cells attracted more attention (19). Clinical trials showed that cardiac progenitor cells have the potential to improve ventricular function (20). However, the credibility of cardiac progenitor cells has been questioned that the mechanism of cardiac progenitor cells protecting effect has not been well demonstrated and the stem cell character of cardiac progenitor cells has not been proved (21). Although other pluripotent stem cells have been found the potential candidate for cardiac cell therapy (22), the clinical application of stem cells in treating ischemic or other myocardial injuries are need to be further demonstrated.
TREM2 is a cell surface receptor and express on monocyte-derived cells, such as macrophages. In some other cells, the expression of TREM2 was not yet determined. So far, there was no research reporting if cardiomyocytes could express TREM2. Our data firstly showed that mouse cardiomyocytes expressed a low level of TREM2. TREM2 was widely studied in the nervous system. In cardiovascular disease, the knowledge of TREM2 islimited. In sepsis, TREM2 had the protecting effect and regulated the immune reaction (23,24). Further, the organ protecting effect has been reported. In immune-mediated hepatocellular damage and acute lung injury, the protecting role of TREM2 has been revealed (25,26). In this study, we found that over-expression of TREM2 in cardiomyocytes reduced the infarcted size and improved cardiomyocytes survival after acute MI in the mice model. These data indicated that TREM2 may directly regulate the anti-apoptosis ability in cardiac ischemic injury independent of the regulation of macrophages. TREM2 has the potential to serve as a target that could reverse cardiac function after MI.
Now, we are known more and more about the ligand of TREM2. It is known that DNAX adaptor protein 12 (DAP12) is the ligand of TREM2 which contains an immunoreceptor tyrosine-based activation motif (3). The previous study has focused on regulating macrophages polarization of TREM2, and auto-phage in neuroinflammation (27). Classic PI3K/AKT signal pathway is also involved in TREM2 signaling. Various studies indicated that PI3K/AKT was the key regulator that mediated the effect of TREM2 in bacterial infection and cancer (28), and also in regulating the microglia in the nervous system (29). In this study, our data firstly showed that the over-expression of TREM2 in cardiomyocytes couldincrease the ability of anti-apoptosis and protected against acute myocardial ischemic injury. TREM2 further activated the PI3K/AKT pathways. However, if other key signal pathway molecules participated in signal transduction is still need further study.
In this study, we further suggested that plasma TREM2 concentration was elevated in CAD patients. The knowledge of the relationship between CAD and TREM2 was limited. Previous study showed that peripheral blood mononuclear cells from CAD patients have high level expression of TREM2 (30). Our data showed that over expression of TREM2 in cardiac myocytes can promote the survival of cardiac myocytes after MI. These data revealed the clinical application value of TREM2 in ischemic heart disease. Plasma TREM2 level may indicate the progression of CAD and elevated expression of TREM2 in cardiac myocytes may have therapeutic effects in MI.
However, this research have several limitations. Firstly, the mechanisms of signaling are need to be further demonstrated through gene knockdown animal models. Secondly, the detailed signal pathways that participated as the downstream of PI3K/AKT were not well studied. Thirdly, Large sample size is required to confirm the clinical value of TREM2 as a biomarker of coronary heart disease.
Conclusions
TREM2 may curb myocardial ischemia injury via activating PI3K/AKT signal pathway. Besides, plasma TREM2 may be treated as a potential biomarker in the diagnosis of CAD to reflect the severity of coronary stenosis.
Acknowledgments
We thank Dr. Cuifeng Zhang in Anesthesia Laboratory & Training Center of Wannan Medical College for his kind support on animal feeding.
Funding: The study was supported by The National Natural Science Foundation of China (81702092 to Y Cao, 81700265 to C Fu) and Research Foundation of Yi Ji Shan hospital, Wannan Medical College (WK2020ZF18 and YR202003 to C Liu). These sources played no role in the preparation of data or the manuscript.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. 2021LSGY No. 31) granted by the Ethics Committee of Yi Ji Shan Hospital affiliated to Wannan Medical College, in compliance with Guidelines for the care and use of laboratory animals in biomedical research for the care and use of animals. The study was conducted ethically in accordance with the World Medical Association Declaration of Helsinki (as revised in 2013). Written informed consents were obtained from all subjects for the use of their urine and biopsy samples for research purposes. The protocol of the study was not registered.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-490/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-490/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-490/coif). The authors have no conflicts of interest to declare.
References
- 1.Anderson JL, Morrow DA. Acute Myocardial Infarction. N Engl J Med 2017;376:2053-64. 10.1056/NEJMra1606915 [DOI] [PubMed] [Google Scholar]
- 2.Ritsinger V, Nyström T, Saleh N, et al. Heart failure is a common complication after acute myocardial infarction in patients with diabetes: A nationwide study in the SWEDEHEART registry. Eur J Prev Cardiol 2020;27:1890-901. 10.1177/2047487319901063 [DOI] [PubMed] [Google Scholar]
- 3.Daws MR, Lanier LL, Seaman WE, et al. Cloning and characterization of a novel mouse myeloid DAP12-associated receptor family. Eur J Immunol 2001;31:783-91. [DOI] [PubMed] [Google Scholar]
- 4.Colonna M. TREMs in the immune system and beyond. Nat Rev Immunol 2003;3:445-53. 10.1038/nri1106 [DOI] [PubMed] [Google Scholar]
- 5.Ford JW, McVicar DW. TREM and TREM-like receptors in inflammation and disease. Curr Opin Immunol 2009;21:38-46. 10.1016/j.coi.2009.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sieber MW, Jaenisch N, Brehm M, et al. Attenuated inflammatory response in triggering receptor expressed on myeloid cells 2 (TREM2) knock-out mice following stroke. PLoS One 2013;8:e52982. 10.1371/journal.pone.0052982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawabori M, Kacimi R, Kauppinen T, et al. Triggering receptor expressed on myeloid cells 2 (TREM2) deficiency attenuates phagocytic activities of microglia and exacerbates ischemic damage in experimental stroke. J Neurosci 2015;35:3384-96. 10.1523/JNEUROSCI.2620-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krasemann S, Madore C, Cialic R, et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 2017;47:566-581.e9. 10.1016/j.immuni.2017.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson EN, Swarovski MS, Linortner P, et al. Soluble TREM2 is elevated in Parkinson's disease subgroups with increased CSF tau. Brain 2020;143:932-43. 10.1093/brain/awaa021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu R, Li X, Xu P, et al. TREM2 protects against cerebral ischemia/reperfusion injury. Mol Brain 2017;10:20. 10.1186/s13041-017-0296-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Q, Zhang K, Jin Y, et al. Triggering receptor expressed on myeloid cells-2 protects against polymicrobial sepsis by enhancing bacterial clearance. Am J Respir Crit Care Med 2013;188:201-12. 10.1164/rccm.201211-1967OC [DOI] [PubMed] [Google Scholar]
- 12.Cochain C, Vafadarnejad E, Arampatzi P, et al. Single-Cell RNA-Seq Reveals the Transcriptional Landscape and Heterogeneity of Aortic Macrophages in Murine Atherosclerosis. Circ Res 2018;122:1661-74. 10.1161/CIRCRESAHA.117.312509 [DOI] [PubMed] [Google Scholar]
- 13.Zhai T, Zhang J, Zhang Y, et al. Cathelicidin deficiency exacerbates cardiac dysfunction in lipopolysaccharide-induced endotoxaemic mice. Clin Exp Pharmacol Physiol 2020;47:677-86. 10.1111/1440-1681.13234 [DOI] [PubMed] [Google Scholar]
- 14.Sheng Z, Yao Y, Li Y, et al. Bradykinin preconditioning improves therapeutic potential of human endothelial progenitor cells in infarcted myocardium. PLoS One 2013;8:e81505. 10.1371/journal.pone.0081505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goradel NH, Hour FG, Negahdari B, et al. Stem Cell Therapy: A New Therapeutic Option for Cardiovascular Diseases. J Cell Biochem 2018;119:95-104. 10.1002/jcb.26169 [DOI] [PubMed] [Google Scholar]
- 16.Yao YY, Fu C, Ma GS, et al. Tissue kallikrein is related to the severity of coronary artery disease. Clin Chim Acta 2013;423:90-8. 10.1016/j.cca.2013.04.017 [DOI] [PubMed] [Google Scholar]
- 17.Wu Y, Lu X, Xiang FL, et al. North American ginseng protects the heart from ischemia and reperfusion injury via upregulation of endothelial nitric oxide synthase. Pharmacol Res 2011;64:195-202. 10.1016/j.phrs.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 18.Jones-Bolin S. Guidelines for the care and use of laboratory animals in biomedical research. Curr Protoc Pharmacol 2012;Appendix 4:Appendix 4B. [DOI] [PubMed]
- 19.Sano T, Ousaka D, Goto T, et al. Impact of Cardiac Progenitor Cells on Heart Failure and Survival in Single Ventricle Congenital Heart Disease. Circ Res 2018;122:994-1005. 10.1161/CIRCRESAHA.117.312311 [DOI] [PubMed] [Google Scholar]
- 20.van Berlo JH, Kanisicak O, Maillet M, et al. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature 2014;509:337-41. 10.1038/nature13309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duran AG, Reidell O, Stachelscheid H, et al. Regenerative Medicine/Cardiac Cell Therapy: Pluripotent Stem Cells. Thorac Cardiovasc Surg 2018;66:53-62. 10.1055/s-0037-1608761 [DOI] [PubMed] [Google Scholar]
- 22.Molloy EJ. Triggering Receptor Expressed on Myeloid Cells (TREM) family and the application of its antagonists. Recent Pat Antiinfect Drug Discov 2009;4:51-6. 10.2174/157489109787236292 [DOI] [PubMed] [Google Scholar]
- 23.Gawish R, Martins R, Böhm B, et al. Triggering receptor expressed on myeloid cells-2 fine-tunes inflammatory responses in murine Gram-negative sepsis. FASEB J 2015;29:1247-57. 10.1096/fj.14-260067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perugorria MJ, Esparza-Baquer A, Oakley F, et al. Non-parenchymal TREM-2 protects the liver from immune-mediated hepatocellular damage. Gut 2019;68:533-46. 10.1136/gutjnl-2017-314107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu D, Dong Y, Liu Z, et al. Impact of TREM-2 gene silencing on inflammatory response of endotoxin-induced acute lung injury in mice. Mol Cell Biochem 2014;394:155-61. 10.1007/s11010-014-2091-6 [DOI] [PubMed] [Google Scholar]
- 26.Zhuang X, Yu Y, Jiang Y, et al. Molecular hydrogen attenuates sepsis-induced neuroinflammation through regulation of microglia polarization through an mTOR-autophagy-dependent pathway. Int Immunopharmacol 2020;81:106287. 10.1016/j.intimp.2020.106287 [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Sheng L, Tao J, et al. Depletion of the triggering receptor expressed on myeloid cells 2 inhibits progression of renal cell carcinoma via regulating related protein expression and PTEN-PI3K/Akt pathway. Int J Oncol 2016;49:2498-506. 10.3892/ijo.2016.3740 [DOI] [PubMed] [Google Scholar]
- 28.Harry GJ. Microglia during development and aging. Pharmacol Ther 2013;139:313-26. 10.1016/j.pharmthera.2013.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selman C, Withers DJ. Mammalian models of extended healthy lifespan. Philos Trans R Soc Lond B Biol Sci 2011;366:99-107. 10.1098/rstb.2010.0243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, He X, Wang J, et al. The Regulation of Exosome-Derived miRNA on Heterogeneity of Macrophages in Atherosclerotic Plaques. Front Immunol 2020;11:2175. 10.3389/fimmu.2020.02175 [DOI] [PMC free article] [PubMed] [Google Scholar]