Abstract
The biology of Borna disease virus (BDV) strongly supports the likelihood of human infection with BDV or a variant of BDV. Thus far, the evidence supporting BDV infection in humans has initiated much controversy among basic and clinical scientists; only time and additional research will support or refute the hypothesis of human BDV infection. Until an assay of acceptable specificity and sensitivity has been developed, validated, and used to document human BDV infection, scientists cannot reasonably begin to associate BDV infection with specific disease syndromes. Clinical studies seeking causal associations between BDV infection and specific diseases must ensure the proper identification of the BDV infection status of patients and control subjects by using a validated, highly sensitive, and highly specific assay (or series of assays). For clinical studies, a highly sensitive “screening” test followed by a highly specific confirmatory test will be of significant benefit. Although it is possible to formulate hypotheses about the clinical outcomes of human BDV infection based on animal model work, to date no human disease has been causally linked to human BDV infection. Scientists all over the world are actively pursuing these issues, and with continuing advances in clinical and basic BDV research, the answers cannot be far away.
For over a century, a fatal encephalitis, Borna disease, has been diagnosed in horses and sheep in Central Europe (120). In 1929, Borna disease was found to be caused by an infectious agent, and in 1990, this agent was determined to be a negative-sense, single-stranded RNA virus (25, 39). Borna disease virus (BDV) persistently infects the nervous system of many animal species, from primate to avian (120). Indeed, by natural or experimental inoculation, the ability of BDV to replicate in the nervous system of virtually every warm-blooded animal strongly suggests that BDV-like viruses are very unlikely to spare the human host.
Over the years, information has also accumulated about unusual features of BDV-induced disease in experimental animals such as rats, mice, and tree shrews. In these animals, BDV can induce behavioral disease (e.g., anxiety, aggression, cognitive defects, and hyperactivity) without obvious physiological signs of viral encephalitis (e.g., fever, neurological signs, and decreased level of consciousness) (7, 48, 58, 66, 68, 99, 111, 112, 114, 115, 122, 136).
Studies of behavioral disease in BDV-infected animals have sparked reasonable speculation that BDV infection in humans might also lead to psychiatric disease. It is tempting to speculate that BDV might be linked to some psychiatric disease syndromes such as affective disorders (e.g., depression) or psychosis (e.g., schizophrenia) or to idiopathic acute or chronic encephalitis. In the 1980s, the first significant serological evidence for BDV infection of humans was reported in the scientific literature (1). However, despite two decades of study and published serological, pathological, or virological evidence of BDV infection in humans, complete medical and scientific acceptance of the human as a natural target of BDV has yet to be achieved. Even more controversial are the specific human disease syndromes for which BDV has been proposed to be an etiologic agent.
Much of the controversy in the study of human BDV infection is linked to technical difficulties in developing and validating a uniform test for diagnosis of BDV infection in humans. Clearly, without a validated test for diagnosing BDV infection in humans, data from a clinical study to identify possible human diseases linked to BDV infection should be evaluated with proper caution.
HISTORY
Borna disease and, later, BDV were named after the town of Borna in Saxony, Germany, where an epidemic of infectious encephalitis caused a large number of equine deaths in 1885 (120). Veterinary scientists spent many years carefully describing the natural history of BDV infection in animals in Central Europe, including infections of horses, sheep, rabbits, and birds, and subsequently started working with the virus in tissue culture (120). BDV research spread from laboratories in Central Europe to the United States in the mid-1980s (99), where projects in disease pathogenesis and animal models were initiated (28). Later, the first cDNA clones were isolated that identified the Borna disease agent as an RNA virus (88, 143) and the sequencing of the BDV genome was reported (26, 39). Since that time, researchers all over the world, including groups in Asia, Scandinavia, and Australia, have joined in the study of BDV.
EPIDEMIOLOGY
BDV infection was originally believed to be limited to farm animals (e.g., horses and sheep) and some wild animals (e.g., rabbits) in areas of endemic infection in Germany and Switzerland (120). With the advent of more modern tools for diagnosis of BDV infection (e.g., in situ hybridization, reverse transcriptase PCR [RT-PCR]) and with the increasing international research interest in BDV, reports of susceptible species and the geographic location of cases of natural infection have expanded (120). Animals at risk for natural or experimental infection include rhesus monkeys, horses, sheep, cattle, goats, rabbits, deer, llamas, alpacas, cats, rats, mice, gerbils, dogs, and ostriches (2, 10, 27, 35, 72, 77, 89, 95, 102, 104, 120; Y. Weisman, D. Huminer, M. Malkinson, R. Meir, S. Kliche, W. I. Lipkin, and S. Pitlik, Letter, Lancet 344:1232–1233, 1994). Evidence for natural BDV infection of animals has now spread beyond the confines of Central Europe to the United Kingdom, Israel, Japan, Sweden, Australia, and the United States (11, 76, 82, 89, 116, 120; Weisman et al., Letter). Since the first subjects studied in the United States and Germany, evidence of human BDV infection has been reported in other countries in the Eurasian continent including Taiwan, Thailand, Iran, and Japan (3, 5, 36; Weisman et al., Letter).
VIROLOGY
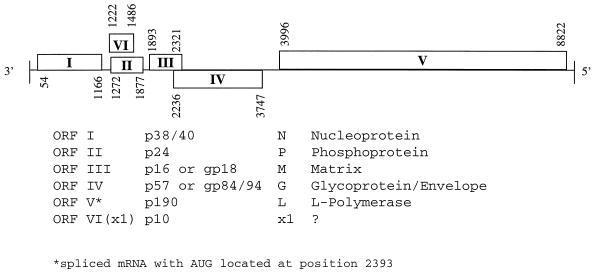
BDV is a nonsegmented, negative-sense RNA virus with at least six identified open reading frames, producing proteins N, P, M, G, L, and p 10 (26, 43). BDV proteins may be glycosylated (M and G) or phosphorylated (P and L), and often associate to form heterocomplexes (e.g., P + N + p10) (90, 144). These BDV protein heterocomplexes may be important in shuttling the viral mRNAs into and out of the nucleus. Although the genome organization and replication strategies resemble those of other viruses in the Mononegavirales order (Fig. 1), BDV is the only nonsegmented negative-sense RNA animal virus known to replicate in the nucleus (23, 30, 38, 45). BDV mRNAs include mono- and polycystronic transcripts, as well as spliced transcripts.
FIG. 1.
BDV genome and gene products. Figure courtesy of Patrick Lai, Salem-Teikyo University, Salem, W.V.
BDV infectious particles are tightly cell associated, and little infectious virus is released into body fluids or infected tissue culture supernatant (41, 42). BDV is known to infect cells of neural origin, such as human oligodendroglial or rat glioma cell lines, with high efficiency after direct application. Other cell types, such s MDCK or Vero cells, are somewhat difficult to infect by direct virus application but can be infected via cocultivation with BDV-infected primary neural cells. Once infected, these nonneural cell lines can replicate BDV to titers equivalent to those in neural cell lines (41, 42) Interestingly, the production of infectious BDV and levels of noninfectious viral subunits (e.g., viral ribonucleoproteins or protein aggregates) can vary among different cell lines (32).
BIOLOGY AND PATHOGENESIS OF BORNA DISEASE
Although it is suspected that BDV may be spread by bodily secretions (e.g., intranasal transmission by nasal discharge) in natural infection (120), there is no formal proof of this assumption. In experimental settings, BDV, in the form of concentrated virus stocks, can reach the brain by the intracranial, intranasal, peripheral (footpad), or peritoneal cavity routes (120).
BDV replicates preferentially and persistently in cells of the nervous system, including neurons, astrocytes, and oligodendrocytes (56). Initially BDV was believed to replicate exclusively in the central nervous system, but later, BDV sites of replication were found to include the peripheral nervous system and nonneural cells in the blood, thymus, and bone marrow (62, 123, 133). The nonneuronal tissue infection with BDV is more commonly seen in settings of BDV-specific or generalized immunosuppression (62, 133, 139).
The specific disease syndromes produced by BDV infection depend on many host factors including the species and strain and the age of the animal at the time of infection; they may also depend on the strain of virus. If humans are indeed hosts for BDV, identifying and understanding the various clinical syndromes in animals will probably provide important information that is applicable to human BDV disease.
Encephalitic Borna Disease
Unlike most viruses, much of the morbidity and mortality following BDV infection stems from the host immune response to the virus and the ensuing immune-mediated death of infected and nearby uninfected cells rather than from direct virus-mediated lysis of infected cells (137). Encephalitic Borna disease (EBD) typically develops following infection of an adult animal and is associated with a massive mononuclear cell immune infiltration into the brain parenchyma (137). In rats, initial immune cell infiltrates in the perivascular spaces are CD8+ and CD4+ T cells, NK cells, and macrophages (12, 60, 119). Over time, B cells, NK cells, and activated microglia dominate in a parenchymal reaction (60). Astrocyte and microglia cell activation is also seen (34, 94, 149). Disease may be expressed initially as behavioral abnormalities including hyperactivity, hyperreactivity, and aggression and subsequently as a rapidly progressive, often fatal, neurological impairment, including seizures, ataxia, and paraplegia. Horses, sheep, many adult-infected rat strains, and, to some degree, cats fall victim to this form of EBD.
For reasons that are not yet clear, some animals survive the acute disease and, after several weeks of EBD, begin to show signs of chronic Borna disease (CBD) (78). CBD is sometimes associated with near resolution of central nervous system (CNS) inflammatory infiltrates (100). Lewis rats surviving with CBD have significant, permanent brain destruction (e.g., cortical thinning and hydrocephalus) and chronic signs of neurological disease, (e.g., chronic apathy, blindness) (28, 100).
Behavioral Borna Disease
Unlike adult Lewis rats, adult black-hooded rats and adult BALB/c and SJL mice have limited susceptibility to EBD (61, 125). While these animals replicate BDV in the nervous system and may even have severe encephalitis, they fail to exhibit the signs of serious neurological disease. Some animals fail to develop fatal encephalitis and exhibit significant behavioral abnormalities in a form of behavioral Borna disease (BBD). BBD is seen following BDV infection of some species or strains of immature animals (e.g., newborn Lewis rats), adult animals with suppressed immune systems (e.g., athymic or thymectomized rats or rats immunosuppressed via drug treatment), and certain species or strains of adult animals (e.g., tupiais glis or MRL strain mice) (7, 48, 65, 66, 99, 125, 136, 140, 141).
Of all the animal models of experimental BBD, the most extensively studied is the neonatally infected Lewis rat, first described in the early 1980s (66, 99). In neonatally BDV-inoculated rats, the lack of significant immune cell infiltration in the brain is believed to stem from infection of the thymus during immune system maturation, leading to BDV-specific “immune tolerance,” although direct proof of this hypothesis is lacking (31).
Although animals with BBD appear normal to the casual observer, they have documented behavioral abnormalities associated with neuroanatomical, neurochemical, and neuroimmune deficits. Some of these behavioral abnormalities have been measured by formal behavioral testing, which revealed hyperactivity, cognitive deficits, social behavior (play) abnormalities, and chronic anxiety (7, 48, 68, 114, 115, 122, 124). Animals with an immature CNS at the time of BDV infection may show evidence of developmental neuroanatomical damage, including dropout of specific neurons in the cerebellum (granule cells and Purkinje cells), dentate gyrus of the hippocampus and cerebral cortex (6, 32, 49, 68), and alterations in synaptic plasticity (55). Interestingly, despite little, if any, cellular inflammation in these animals, abnormal levels of cytokines (e.g., tumor necrosis factor, interleukin-1β, and transforming growth factor β) and chemokines (68, 109, 128, 129) and of serotonin, norepinephrine, dopamine, and other neurotransmitters (68, 113) have been reported.
HUMAN BDV INFECTION
Diagnostic Tests for BDV Infection in Humans
Scientists have been searching for evidence of human BDV infection and associated disease states for over 15 years. Indeed, there are reports of the recovery of anti-BDV antibodies, BDV RNA, BDV proteins, and infectious BDV from human tissues and body fluids from normal humans and humans with a wide variety of psychiatric disorders. Using a variety of testing methods and clinical study designs, many investigators have used evidence for and against human BDV infection to postulate an association, or lack thereof, of BDV infection with specific human diseases. The numerous tests used to diagnose BDV infection have evolved over the years, coincident with the availability of BDV-specific reagents such as cDNA clones (88, 143) and with general scientific technological advancements, such as RT-PCR (133). Therefore, before continuing the discussion of reported evidence of human BDV infection, it is important to understand the advantages and limitations of the numerous different tests used to obtain evidence of BDV infection in humans.
Reliable and accurate diagnosis of human infection with BDV, or a BDV-related virus, is a prerequisite for the confirmation of a BDV-induced human disease. Generally, BDV assays are modeled after tests used in animal studies and include tests for anti-BDV antibody, immunologically based tests for BDV proteins, RT-PCR assays for BDV RNA, and in vitro or in vivo assays for infectious BDV. To validate the ability of an assay to identify subjects with BDV infection as “positive” and subjects without BDV infection as “negative,” results should be provided from experiments using this assay with a sufficient type and number of samples from animals whose BDV infection state is clearly known, e.g., from several subjects in different species of experimentally inoculated animals or naturally infected animals with independent confirmation of BDV infection.
The validity of a diagnostic test can be determined by measuring the rate of sensitivity (true-negative rate) and specificity (true-positive rate), and, in general, the more sensitive the test the lower the specificity and vice versa. In assay development and validation, the weighted emphasis on the specificity or sensitivity of a specific assay also depends on the use for which the assay is intended (101). In cases where false-negative results might cause significant harm (e.g., missing a virus-infected unit of blood intended for transfusion), a highly sensitive test is used, even if specificity is somewhat compromised, i.e., a slightly higher false-positive rate, resulting in the disposal of some good units of blood. These highly sensitive tests are often used as “screening” assays. In clinical settings where false-positive results might lead to unnecessary treatment, a positive result on a sensitive screening assay is often confirmed by a test of higher specificity.
In designing a BDV assay for human samples, one must consider the question to be answered. To answer the question “Does BDV infect humans?” a highly specific test is preferred, even if sensitivity is reduced, to ensure the maximum possible true-positive rate. In this situation, confirmatory testing of the individuals who test BDV positive by the assay in question will uniformly show further evidence of infection (e.g., cultivation of infectious virus). Thus, confidence in the accuracy of the test for identifying a BDV-infected subject will be high. To answer the question “Is disease state ‘X’ caused by BDV infection?” a highly sensitive and specific determination of the BDV infection status is required. To perform a meaningful clinical study to causally associate BDV with a specific disease, it is critical to have a test, or series of tests, to place the subjects in the correct category of BDV-infected and control subjects. This may necessitate the combined use of a highly sensitive screening test, e.g., an enzyme-linked immunosorbent assay (ELISA), followed by a more specific confirmatory test for those testing BDV positive by ELISA, e.g., Western blot.
Anti-BDV antibody detection.
Serological tests evaluate the presence of a humoral immune response to a virus infection, e.g., that antibodies bind to specific virus antigens. When the individual contracts a virus infection, the first serological evidence of virus infection is often the immunoglobulin M (IgM) antibody. As the immune response matures, an antiviral IgG antibody response is detected. When the onset of infection can be identified, typically acute-phase (early in infection) and convalescent-phase (several weeks into the infection) sera are sampled and tested as a pair. A significant increase in the titer of virus-specific IgG from the acute-phase to the convalescent-phase serum sample is indicative of infection.
In natural BDV infections, acute-phase serum is not always available and, as a result, most serological diagnostic tests for natural BDV infection are a single test for anti-BDV IgG from presumed convalescent-phase serum. In many animal species, BDV infection is associated with persistence of anti-BDV antibodies. It has been debated whether the anti-BDV antibody titer in humans is consistent or variable on repeated measures, but in several individuals anti-BDV antibodies have been detected by repeated testing (14). Notably, virus may or may not be present in individuals who test positive by serological assays. In some virus infections, a positive serological result indicates cure or “clearance” of the virus, e.g., mumps virus. In other cases of persistent virus infection, a positive antibody test may indicate latent (e.g., herpes simplex virus) or persistent (e.g., human immunodeficiency virus [HIV]) virus replication. BDV is a persistent virus infection in many species and may persist at low (e.g., detectable in mononuclear white blood cells by RT-PCR) or high (i.e., comparable to acute infection in brain) levels (133). It is unknown whether humans might become persistently infected with BDV or clear the virus infection. Thus, we do not know how the detection of anti-BDV antibody relates to the presence or clearance of BDV in humans.
All serological tests for BDV use natural BDV antigens (from infected cells) or recombinant antigens (from BDV strains recovered from animals) (Table 1). Using BDV derived from nonhuman sources as the antigen in serological studies has caused some investigators to question the validity of low-avidity, low-titer binding of antibodies in human sera. Whether these findings are due to cross-reactivity necessitated by using a nonhuman strain of BDV or suggest the nonspecificity of human antibody binding to BDV antigens is not known.
TABLE 1.
Summary of BDV serological assays
| Assay | Antigen | Relative specificitya | Relative sensitivitya |
|---|---|---|---|
| IFA | Fixed, BDV-infected cells | Poor | Good |
| IB | Natural or recombinant BDV proteins | Good | Moderate |
| ELISA/ECLIAb | Natural or recombinant proteins | Moderate to good | Moderate to low |
For human sera.
ECLIA, electrochemiluminescence immunoassay.
(i) Immunofluorescence assay.
Diagnostic tests for anti-BDV antibodies were the first method by which BDV infection was diagnosed in animals (120). The first serological diagnostic test for BDV infection in humans was the indirect immunofluorescence assay (IFA) (1). In this test, human serum is overlaid on a slide covered with fixed BDV-infected cells to allow anti-BDV antibodies in the serum to bind to the viral antigens expressed by the cells. BDV-infected MDCK (64) or C6 (32) cells are most commonly used in the IFA. After the cells are washed, a fluorescence-labeled anti-human IgG antibody is added to signal the presence of human anti-BDV antibodies. Using a fluorescent light-equipped microscope, infected cells are detected on the basis of brightly lit inclusions in the nucleus, often associated with a more finely patterned signal in the cytoplasm (Fig. 2A). To control for false-positive signals, e.g., the artifactual binding of autoantibodies to the cell antigens, the same human serum should be overlaid on uninfected cells. Positive controls for the IFA include the use of anti-BDV serum from experimentally infected animals on duplicate cells. Some researchers have incorporated a “double-label” technique using a monoclonal mouse anti-BDV antibody mixed with the human serum sample and a second-color fluorescently labeled anti-mouse IgG added to the anti-human IgG, to identify colocalization of the human serum signal with the mouse anti-BDV antibody signal (19, 147). While the double-label technique can identify false-positive signals from human sera (when human serum binds to cell locations that are distant from binding sites of positive-control animal BDV antiserum) at the light microscope level, the technique has insufficient resolution to clearly prove that human sera and animal control sera are binding to identical antigens in the infected cell.
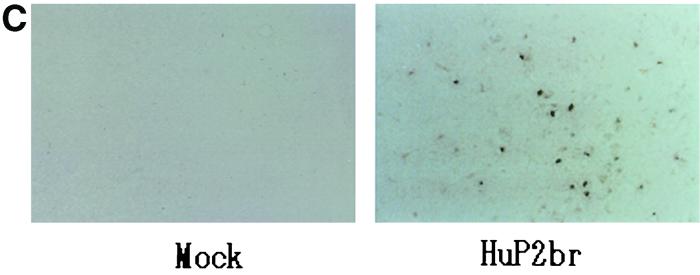
FIG. 2.
(A and B) OL cells mock infected or persistently infected with HuP2br were immunohistochemically stained with polyclonal rabbit anti-BDV p40 and then with fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (A) and were subjected to in situ hybridization using antisense digoxigenin-conjugated riboprobe directed to BDV p40 region and then stained with fluorescent-labeled anti-digoxigenin Ig (B). (C) Newborn gerbils were mock infected or infected with HuP2Br. The sections prepared from the cerebral cortex (frontal lobe) of the gerbils on day 30 postinfection were subjected to in situ hybridization using antisense riboprobe directed to the BDV p40 region. Figure courtesy of Kazuyoshi Ikuta, Osaka University, Osaka, Japan.
The IFA is a rapid, sensitive assay in controlled, experimental BDV infections. However, when screening human sera where exposure or infection with BDV is unknown, the IFA may be insufficiently specific (147). The major technical concerns with the IFA technique are specificity and the variability introduced by reader expertise (i.e., correct recognition of the specific, characteristic pattern of BDV antigens in the infected cell). Many laboratories performing BDV serological testing have a great deal of expertise in IFA techniques; however, for accurate determination of BDV seropositivity by IFA, reader-to-reader variability in testing makes it difficult or impossible to replicate serology results among independent laboratories. Moreover, unless careful use of negative controls is incorporated into the assay (e.g., duplicate serum samples run in parallel using uninfected cells), there is a risk of confusion between signals from binding of cell-specific autoantibodies and anti-BDV antibodies. Sensitivity concerns are raised, for example, when trying to determine if a faint signal is specific for BDV or represents artifactual “background” staining. This latter situation is often a problem in testing human sera, where antibody levels are generally quite low.
(ii) Immunoblot assay.
Like IFA, the immunoblot assay (IB) is a serological technique that also relies on detection of human antibody bound to virus antigens. In IB, virus antigens are separated by electrophoresis through a gel matrix and then transferred to a specialized blotting membrane. Strips of the blot are incubated in human serum and washed, and binding of human antibodies is detected by a secondary, enzyme-labeled anti-human IgG antibody. Binding of human anti-BDV antibodies is indicated by an enzymatic reaction resulting in a visible dye stain or a light signal captured on X-ray film or image equipment. The image can be evaluated qualitatively (by eye) or by more quantitative methods using computer-based imaging systems.
Molecular weight markers are included in the blot to confirm the location of the known sizes of BDV proteins. Positive-control sera from infected animals are applied to duplicate blots to confirm the appropriate technical performance and provide the location of the BDV antigens on the blot. Typically, negative-control lanes with uninfected material are run in duplicate with lanes containing BDV antigens. The sources of BDV antigens in IB include experimentally infected animal brain, lysates of infected neuronal or nonneuronal cell lines, baculovirus recombinant proteins, and prokaryotic recombinant proteins (37, 53, 70, 74, 82, 107, 130, 145).
There are some general aspects of IB that may affect the sensitivity of the technique. While baculovirus and prokaryotic recombinant BDV proteins can be synthesized in large quantities and are easily purified, normal human glycosylation of virus proteins is altered (70). Although not proven for BDV proteins, both nonhuman glycosylation patterns of virus antigens as well as the typically protein-reducing and -denaturing characteristics of the gel can destroy or alter conformational virus epitopes.
Although some reader expertise is involved in interpreting IB results, the specificity of this technique is believed to be excellent. In part, the ability of IB to show which virus antigen(s) is recognized by the serum sample provides increased specificity for the IB compared to the IFA (146). Seropositivity criteria have included the recognition of a single BDV protein (53) or of multiple BDV proteins by the serum (146). While there is a risk that a single virus protein will be recognized by “nonspecific” antibodies from an uninfected subject, there is a reduced probability of nonspecific recognition of multiple virus proteins by antibodies in a single serum. The specificity of antibody binding in the IB may be improved by the use of a second IB run in tandem using soluble BDV antigens to show specific inhibition of antibody binding to BDV antigens. Drawbacks of the IB include the time-consuming and costly nature of this technique and the disadvantage that the high specificity seen with IB may be accompanied by some decrease in sensitivity of the test (146).
(iii) Enzyme-linked immunosorbent assay.
ELISAs are commonly used serological tests designed for high-throughput screening; unlike IFA and IB, they are designed to give non-reader-dependent quantitative results. Several different types of ELISAs have been developed to perform BDV serological assays, using native or recombinant antigens singly or in combinations (24, 67, 81, 150; Weisman et al., Letter). Antigens are bound directly to the ELISA plate or “captured” by BDV-specific antibodies bound to the plate. The human serum to be tested is overlaid on the bound BDV antigens, and a secondary, enzyme-labeled anti-human IgG antibody is added. Following enzymatic reactions that produce a visible pigment or fluorescent product, the amount of bound BDV-specific antibody is measured in a specialized spectrophotometer.
Although ELISA is generally believed to be a highly sensitive serological assay system, BDV-specific ELISAs have been reported to have some difficulty with sensitivity. For example, ELISAs that are capable of reliably detecting anti-BDV antibody in one species (e.g., rats) have been unable to detect anti-BDV antibody reliably in other species (e.g., rabbits and horses) (67, 78). Therefore, it is unclear whether the inability of these ELISAs to detect anti-BDV antibody in humans, where anti-BDV antibody titers are generally low, represents a false-negative due to species-specific variability in the sensitivity of the ELISA or a true-negative result.
Specificity concerns with these ELISAs are demonstrated when human sera shown seronegative by IB give a positive result in ELISA due to nonspecific reactivity (50). As with IB, some ELISA protocols have incorporated a tandem “blocking” step using soluble BDV antigens to confirm the specificity of the antibodies binding to the BDV antigens on the ELISA plate in order to improve BDV specificity (150).
Methods to detect virus protein in tissues or cells.
Methods for detecting virus protein expression in human tissues (immunohistochemistry) are most often used on postmortem brain specimens. Anti-BDV-specific antisera are applied to tissue and bind virus antigens, and enzymatic reactions are used to indicate areas where BDV proteins are recognized by the antisera (Fig. 3). Although flow cytometry has been reported to detect BDV antigens in peripheral blood cells (L. Bode, F. Steinbach, and H. Ludwig, Letter, Lancet 343:297–298, 1994), this technique has not gained wide acceptance.
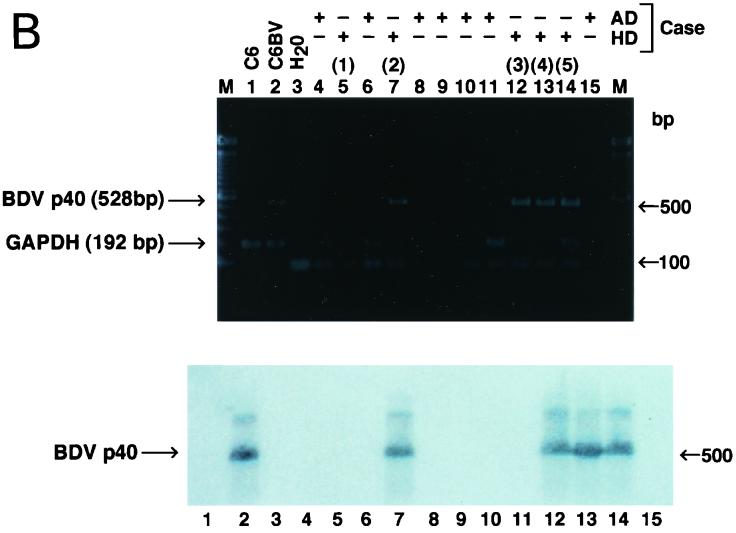
FIG. 3.
Expression of BDV N (p40) antigen and BDV RNA in autopsy tissue from the human brain. (A) For detection of viral antigen, hippocampal sections from brains of representative patients with hippocampal sclerosis (HS) or Alzheimer's disease (AD), immunolabeled with either a rabbit anti-BDV N serum or an anti-GFAP antibody, were used. (B) For detection of viral nucleic acid, RNA was isolated from frozen brain samples of hippocampal sclerosis or Alzheimer's disease patients and analyzed by RT-PCR using specific primers to amplify a 528-nucleotide segment of the BDV N ORF. An aliquot of cDNA from each sample was also used to amplify glyceraldehyde-3-phosphate dehydrogenase (GAPDH) sequences by RT-PCR as a control of RNA quality. The BDV specificity of the PCR products was confirmed by Southern blot hybridization using a BDV N probe internal to the predicted PCR product. Figure courtesy of Juan Carlos de la Torre, Scripps Research Institute, La Jolla, Calif.
Sensitivity in these assays is dependent on the amount of BDV proteins expressed in the examined tissues and cells. In nonhuman, experimentally infected species, BDV antigens can be expressed at relatively high (rat) or low (mouse) levels (28, 125). The sensitivity of the immunohistochemical assay can also be affected by the binding properties of the BDV antibody used to detect BDV antigens. In addition, the specificity of these assays depends on the cross-reactivity with non-BDV proteins of the anti-BDV antibody that is used to detect the BDV antigen in tissues or cells. Artifacts can occur through nonspecific antibody binding or other technical problems, and reader expertise is needed to interpret the results.
Virus nucleic acid detection.
In 1990, with the advent of cDNA clones for BDV, techniques to locate BDV RNA became feasible. In situ hybridization uses a radioactively or enzymatically labeled complementary strand of BDV sequence-specific nucleic acid probe that binds to BDV RNA in infected cells. In situ hybridization can be a sensitive technique, especially if the infected cells are localized or are few and inhomogeneously distributed in the tissue. In situ hybridization provides excellent histological information, especially when combined with immunohistochemistry techniques that identify the infected cell type (34). On the other hand, in situ hybridization can yield false-negative results if low concentrations of BDV RNA are present or if the BDV RNA is degraded (as might occur in human autopsy specimens), or it can yield false-positive results if non-BDV-specific probe binding occurs. BDV RNA has also been detected in RNA extracted from cells in a soluble form via capture by antibodies or by complementary nucleic acid sequences bound to microtiter plates (54, 133). RT-PCR assays have been used extensively to locate BDV RNA in human samples. In RT-PCR, purified RNA is reverse transcribed and the cDNA is amplified repeatedly with specific primers, and subjected to a sequence-specific confirmation method, e.g., Southern blotting.
RT-PCR for BDV was initially developed to identify very small amounts of BDV RNA in circulating peripheral blood mononuclear cells in neonatally infected rats (133). Prior to the report by Sierra-Honigmann et al., it was believed that BDV did not circulate in the peripheral blood. Thus, recovery of BDV from humans was believed to require invasive sampling techniques (e.g., lumbar puncture for cerebrospinal fluid (CSF) or tissue biopsy). After the discovery of BDV in rat blood cells, it became common to use RT-PCR to test samples of human peripheral blood cells for evidence of BDV RNA. Since experimental rat studies showed that the number of infected cells and level of infectious virus per cell are estimated to be very low, the sensitivity was increased by the use of a “nested” RT-PCR technique with two sequential rounds of amplification using two separate sets of BDV primers (127, 133). Using nested RT-PCR, between 10 and 100 copies of BDV genome per sample can be detected.
Although RT-PCR is generally believed to be highly sensitive, the presence of RT-PCR inhibitors, such as heparin or hemoglobin in a blood sample, may result in false-negative results (133). Moreover, the low level of BDV RNA in peripheral blood may fall below the detection sensitivity of RT-PCR and produce a false-negative result. With such a low level of viral RNA in blood samples, poor specimen handling, such as freeze-thawing, can produce false-negative results (A. M. Sierra-Honigmann, unpublished results). Based on the number of BDV-infected white blood cells in rats (around 1 in 105 to 106 cells), avoiding false-negative results in humans due to sampling error may require at least 5 ml of blood to obtain a sufficient number of infected cells for testing (127). Finally, the ability of BDV primers designed from animal virus sequences to recognize a putative human BDV also needs to be taken into account.
In addition to the primers chosen for amplification of the BDV RNA, the specificity of RT-PCR is further documented by Southern blot or sequencing analysis of the amplified DNA arising from the RT-PCR. The specificity of the RT-PCR results is also supported by the use of multiple controls, including the amplification of normal negative control cells in parallel with positive control BDV-infected cells; however, typically the cells chosen are tissue culture lines that are not from the same tissue areas as the test samples from humans (e.g., dog kidney tissue culture cells used as controls for human brain or blood cell samples). A mismatch of sample and control cells can be problematic for RT-PCR assays in which cellular mRNA may vary from cell type to cell type.
Most concerns regarding false-positive results obtained with the RT-PCR technique have revolved around fears of contamination by BDV cDNA or PCR products. This contamination problem was clearly demonstrated in a multiple-laboratory study (117). Several technical adjustments have been used by BDV researchers to reduce the risk of BDV contamination, including running the RT reaction without the RT enzyme (wherein a positive signal identifies contaminating BDV cDNA in the sample or reagents), utilizing a single tube RT-PCR method to minimize opportunities for contamination with BDV cDNA, and “tagging” BDV cDNAs so that contamination of those products can be easily identified in future reactions (92, 93, 127).
Nucleic acid sequencing can be used to confirm that the material is BDV. In many viruses, sequence differences among wild-type and laboratory strains can be used to ensure that laboratory virus strains are not contaminating the human specimens being tested. However, for the most part, the sequence variations of the BDV strains isolated from any source are limited, on the order of 0 to 5% of the genome, and “human-specific” sequence changes are limited and controversial, (13, 44, 63, 80, 131). Thus, using the sequence to confirm the source of the recovered strain, e.g., to distinguish a human BDV strain from a laboratory contaminant, is not feasible at present. However, a recent report of a horse strain of BDV with substantial RNA (more than 15%) and protein (almost 20% in the p10 protein) sequence variation from previous horse strains provides hope that a unique strain might be recovered from humans as well (103). Notably, this new horse strain was not easily detected by some standard BDV RT-PCR primers, intimating that using standard BDV primers designed to detect virus RNA in animals may not be the best approach for screening for the presence of BDV in human tissue.
Infectious-virus isolation.
The “gold standard” for diagnosis of BDV infection is the isolation of infectious virus from the subject. BDV is largely a cell-associated virus, and it is difficult to recover infectious virus from bodily fluids. In general, infectious-BDV isolation is performed by inoculating tissue homogenates into cell culture or by in vivo testing (i.e., animal infectivity assays). Depending on the virus titer in the inoculated test material, evidence of infectious BDV in tissue homogenates can be detected as early as 24 h (e.g., high-titer virus detected by expression of BDV antigens in cell culture) or as late as several months (e.g., low-titer virus detected by expression of Borna disease in inoculated animals). Limitations of this technique include the low level of infectious BDV replication in some species (probably including humans), the relatively restricted replication preference for the virus in specific species or strains in vivo or in specific tissues or cells in vitro. Commonly used cell lines for recovery of infectious BDV include primary neural cells from rat or rabbit and neurological cell lines such as C6 rat glioma or OL human oligodendroglial cells (16, 32). Animals used for bioassay of infection BDV include rabbits and rats (78, 121).
BDV—a Human Pathogen?
Given what is known about the biology of BDV, there is little doubt that humans will be found to be natural hosts for BDV. The question therefore is “Has human infection with BDV been convincingly demonstrated yet?”
The first reports suggesting that BDV was a human pathogen were published in 1985 (1), using the only available BDV serological test at that time, IFA, to identify anti-BDV antibodies in 4.5% of patients with major depressive disorders and in 0% of control subjects. Both patients and control subjects were from a psychiatric research population and had been evaluated for major psychiatric disorders. Notably, all CSF samples available from the population of seropositive patients (five subjects) were negative for anti-BDV antibodies. Subsequently, reports appeared from several laboratories suggesting an association of BDV seropositivity by IFA with several other psychiatric diseases. Most of these reports were in the form of case reports, single-illness serological screening studies, or large, multidiagnosis serological survey studies (19; K. Bechter, R. Schuttler, and S. Herzog, Letter, Psychiatry Res. 42:291–294, 1992; L. Bode, S. Riegel, H. Ludwig, J. D. Amsterdam, W. Large, and H. Koprowski, Letter, Lancet ii:689, 1988). While these studies helped bolster the evidence supporting the hypothesis that BDV infected humans, they provided no definitive evidence of an association between BDV and psychiatric disease.
In 1993, the first publication of BDV serological testing by IB was published by Fu et al. (53), using an IB with p40 and p24 BDV antigens purified from infected cells. These investigators found a significantly increased prevalence of anti-BDV antibodies to either or both antigens in patients with affective disorder compared with that in healthy controls. Notably, the controls were evaluated to rule out major psychiatric illness. The prevalence of recognition of BDV antigens by sera from psychiatric patients was 38% for p40, 12% for p24, and 6.5% for both antigens, while for control subjects the results were 16, 12 and 0.85%, respectively.
Although not explicitly discussed by Fu et al., evidence for an apparently increased specificity of serological tests using a stringent criteria for seropositivity (i.e., recognition of two or more different BDV antigens in the same patient serum) was presented (53). When this criterion is applied to these data, the seropositivity prevalence in these patients remains significant while the seropositive prevalence in control subjects drops to under 1%. In 1995, Waltrip et al. published a serological analysis of patients with schizophrenia by using an IB with p40, p24, and gp18 BDV antigens from lysates of an infected human neuroblastoma cell line (146). In this study, the stringent criterion for seropositivity and blinded IB analysis showed a BDV seropositivity prevalence of 14.4 and 0% in patients and controls, respectively.
Many subjects reported to be seropositive to BDV have not had identified exposures to naturally infected animals. However, in 1994, an ELISA using p40 and gp18 was used to test human sera, and the authors reported that contact with BDV-infected ostriches was associated with a higher BDV-seropositive rate (46%) than that found in non-ostrich-exposed controls (10%) (Weisman et al., Letter). Similar findings were reported of increases in the BDV serological responses and RNA levels in individuals living near thoroughbred-horse farms with respect to controls in 1997 (142).
Some studies have attempted to correlate abnormalities on brain-imaging studies with BDV seropositivity. In 1989, psychiatric patients found to be seropositive and seronegative for BDV by IFA were studied by brain magnetic resonance imaging (MRI) for evidence of CNS lesions. In patients matched for diagnosis, age, sex, and duration of illness (less than 1 year), BDV-seropositive patients were more likely to have focal cerebral lesions in the white matter (60%) than were BDV-seronegative patients (0%) (8). There was no difference in MRI findings in BDV-seropositive and -seronegative patients whose duration of illness was greater than 1 year. In 1995, an MRI study was performed on schizophrenic patients found to be BDV seropositive by stringent IB criteria (146). Significant abnormalities were found in several brain structures of BDV-seropositive patients with schizophrenia, including the putamen and amygdala-hippocampus complex.
In 1991 the first report was published suggesting the possibility of transient isolation of a BDV-like virus from cell cultures inoculated with CSF from a patient with schizophrenia (121). These cocultures were found to have a few foci of BDV antigens by IFA, but the signal disappeared with further passages. Rabbits inoculated with the same CSF sample developed anti-BDV antibodies by IFA, but no virus was recovered. In 1995, two groups published papers reporting the detection of BDV RNA in patients with psychiatric disease from samples of peripheral blood cells using a nested RT-PCR (22, 82). In 1996, BDV RNA was detected by immunohistochemistry and RT-PCR in the brain of a patient with hippocampal sclerosis and dementia (46), and infectious BDV was reported to be isolated from blood cells from a patient with psychiatric disease (16). The type of blood cell infected with BDV has been controversial, ranging from fibroblastic stromal cells in the rat (123) to monocytes (Bode et al., Letter, 1994) or cells located in the granulocyte fraction (107) in humans. In 2000, BDV was isolated from the brain of a patient with schizophrenia by passage in gerbils (Fig. 2C) (96).
Simultaneous testing for BDV RNA and anti-BDV antibodies has yielded interesting but often inconsistent results. For example, Kishi et al. found that anywhere from none to half of the subjects with nested RT-PCR evidence of BDV RNA in blood cells simultaneously had serum anti-BDV antibodies by IB or ELISA (81, 82). Other groups have also reported inconsistencies in the simultaneous recovery of BDV RNA and anti-BDV antibodies from the same subjects (74, 105, 107, 127, 130). Moreover, when both p24 and p40 BDV RNA and/or anti-BDV p24 or p40 antibodies were tested for in human sera, rarely was evidence of antibodies to both BDV proteins detected in the same human serum sample (36, 150). This “mismatch” of BDV serological with respect to RNA and infectious-virus test results may be a explained by one or all of the following scenarios: (i) BDV is cleared from human tissues through an immune response (e.g., anti-BDV antibody positive and BDV RNA negative), (ii) BDV is persistently replicating in the human host with a nonneutralizing immune response (e.g., anti-BDV antibody positive and BDV RNA positive), or (iii) BDV is replicating in the human host without a measurable immune response (e.g., anti-BDV antibody negative and BDV RNA positive).
In contrast to findings in humans, many animals infected with BDV (those that survived acute infection) have antibodies to several BDV proteins coincident with evidence of BDV RNA and/or infectious-virus replication (120). Because much of the human evidence is not consistent with this finding in animals, it is possible that (i) BDV detection in human samples is an artifact and humans are not infected with BDV, (ii) humans are infected with BDV but our current tests are not well designed to detect human BDV strains, or (iii) humans are infected with BDV but the human immune response to BDV may be capable of clearing the virus infection.
Questions continue to be raised about technical artifacts in RT-PCR assays or sequencing that may have introduced inaccuracies in reported BDV sequences (127). For example, there is a report of sequence similarities between human BDV strains and the BDV laboratory strains used in some laboratories, and the authors of the report suggested that most, if not all, “human” BDV strains recovered were contaminated by laboratory strains (M. Schwemmle, C. Jehle, S. Formella, and P. Staeheli, Letter, Lancet 354:1973–1974, 1999). However, it has also been argued that this hypothesis was based on comparison of limited sequences and is inaccurate (O. Planz, H. J. Rziha, and L. Stitz, Letter, Lancet 355:656–657, 2000). Despite the continued controversy, it is encouraging that since the inception of the search for human BDV in 1985, publications have appropriately provided increased technical details and control procedures used in the assays for BDV RNA, e.g., the inclusion of “RT-negative” and water samples to demonstrate the absence of contaminating BDV PCR products.
While the failure to detect BDV in some human samples has been interpreted as evidence of lack of human infection with BDV, these interpretations should be viewed with appropriate caution. As we learn more about BDV infection in different species of animals, information is accumulating to support the hypothesis that evidence of BDV infection may be difficult to obtain for some species, such as cats (104) and experimentally infected ponies (78); it may, therefore also be difficult to obtain for humans. Since BDV is likely to be a relatively rare infection in humans, problems could lie with the clinical design, e.g., the proper selection of appropriate human subjects. For example, based on the findings in the rat model of hippocampal degeneration (31), a more targeted patient selection approach was used by screening samples of hippocampus from 600 non-Alzheimer's dementia patients (46). Of the five patients with evidence of hippocampal degeneration, four were found to have evidence of BDV RNA and protein expression (Fig. 3).
Evidence for BDV-Induced Disease in Humans: Possible Clinical Syndromes of BDV Infection
To date, BDV has not been identified as a clear etiology of any known human disease. However, based on what is known about BDV biology and disease pathogenesis in many nonhuman species, it is tempting to speculate about the clinical outcomes of human BDV infection.
Although BDV might be the etiologic agent of idiopathic acute or chronic inflammatory encephalitis in humans, no large-scale studies have evaluated such a connection and so no data are available to support or refute this hypothesis. Nonetheless, a clinical picture of human EBD might be similar to that of a hospitalized patient with idiopathic acute encephalitis associated with changes in the level of consciousness, along with fever and significant neurological impairment. As with rabies or herpes simplex encephalitis, damage to the neuroanatomical targets of BDV, such as the limbic system, might lead to erratic personality changes or even violent behavior. Individuals who survived EBD and developed CBD might show evidence of chronic neurological damage, even in the face of a receding inflammatory response in the brain. Impaired cognitive function, apathy, and emotional instability would probably be exhibited by the survivors of the infection.
An alternative expression of human BDV infection may more closely resemble BBD as seen in the neonatally BDV-infected rat, with a preponderance of behavioral disease symptoms. Humans with BDV infection might not have fever, changes in mental alertness, or other typical signs of viral encephalitis but instead might express signs of psychiatric disease, such as depression, mania, anxiety, cognitive disorders, tardive dyskinesia, social dysfunction, eating disorders, and idiopathic seizures (7, 48, 114, 115, 124, 135, 136). Moreover, if infection occurred in utero or during the first 3 years of life, BDV infection might result in autistic spectrum disorder, with abnormal social interactions, chronic anxiety, cognitive deficits, and evidence of abnormal development of the cerebellum and hippocampus (6, 31, 48, 49, 110–112, 122).
Some post-virus infection sequelae are not due to direct virus damage but to autoimmune responses stimulated by virus infection (e.g., post-viral encephalitis and Guillain-Barré syndrome). Therefore, some consideration must be given to the role of autoimmunity in BDV-associated disease as well. In some experimental systems, BDV infection induces autoantibodies, e.g., antibodies to cellular proteins seen in post-BDV infection sera but not in preinfection sera (70). As with other virus infections, it is possible that autoantibodies are the result of BDV-associated cell lysis and release of host proteins, to which an autoimmune response develops. Alternatively, autoantibodies might be the result of “molecular mimicry” of host antigens by BDV proteins. Finally, the possibility that antibodies that coincidentally recognize BDV antigens may be present in the serum of individuals who have not been infected with BDV should also be considered. These autoantibodies may or may not be relevant to the clinical disease presentation of the individual but are only coincidentally detected in tests including BDV antigens.
Since there is no agreed upon, validated assay for diagnosing BDV infection in humans, it is not possible to causally associate any disease with BDV infection. Nonetheless, below are data summarized from publications from many international groups supporting and refuting the hypothesis of human BDV infection associated with numerous disease outcomes. The techniques used in these publications are varied and sometimes are not accompanied by the technical information needed to evaluate the assay specificity and sensitivity. Similarly, many clinical studies of BDV do not clearly indicate the incorporation of one or more significant clinical trial design elements required to demonstrate a causal association between BDV infection and specific disease. These missing or unreported clinical design elements include appropriate patient and control screening and selection techniques, blinding of laboratory workers to clinical status, and collection and analysis of associated non-BDV-related factors that may influence the trial outcome (e.g., hospitalizations, pharmacotherapy, age, sex, race, socioeconomic status, and exposure to animals). Thus, when examining the conclusions of BDV association with specific diseases, care should be taken not to overinterpret the presented evidence.
In the past decade, BDV RNA, BDV proteins, anti-BDV antibody, and/or infectious virus have been found in the blood, CSF, and/or brains of patients with chronic fatigue syndrome (83, 97, 98), blood donors (81), HIV-infected patients (3), patients with schizophrenia or with deficit syndrome schizophrenia subtype (36, 37, 73, 74, 96, 145, 146; M. Salvatore, S. Morzunov, M. Schwemmle, and W. I. Lipkin, Letter, Lancet 349:1813–1814, 1997), normal humans (57), patients with multiple sclerosis and/or depression (52; M. Deuschle, L. Bode, I. Heuser, J. Schmider, and H. Ludwig, Letter, Lancet 352:1828–1829, 1998), patients with non-Alzheimer's dementia and hippocampal degeneration (46), and patients with various psychiatric disorders (150).
Reports of the failure to find evidence of BDV infection associated with specific disease syndromes have been increasing. The inability to find evidence of significant BDV infection in blood, CSF, and/or brain has been reported for studies of patients with chronic fatigue syndrome (50; L. Bode, A. L. Komaroff, and H. Ludwig, Letter, Clin. Infect. Dis. 15:1049, 1992), patients with schizophrenia (133), patients with multiple sclerosis (B. Kitze, S. Herzog, P. Rieckmann, S. Poser, and J. Richt, Letter, J. Neurol. 243:660–662, 1996), HIV-infected patients (4), and various psychiatric patients (79, 85, 117; K. Lieb, W. Hallensleben, M. Czygan, L. Stitz, and P. Staeheli, Letter, Lancet 350:1002, 1997)
Notably, the positive and negative studies have been reported from assays performed on samples from the same patient cohorts and sometimes by the same research group. In sum, it is not possible to discern the diseases, if any, that may be caused by BDV infection of humans. Much more work is needed in this area, since many technical factors, as well as our lack of understanding of the biology of BDV infection in the animal and human, probably have contributed to the controversy.
Treatment
Our inability to connect human BDV infection to specific disease states also limits our ability to substantiate the use of unapproved “anti-BDV” treatment in persons in whom unvalidated research assays suggest BDV infection. Nonetheless, proposed therapies for BDV infection have been reported (51; L. Bode, D. E. Dietrich, R. Stoyloff, H. M. Emrich, and H. Ludwig, Letter, Lancet 3:178–179, 1997). As with any virus infection and, perhaps, especially with persistent virus infections, prevention of infection through vaccination is likely to be the preferred approach over treatment following established infection.
Experimental passive transfer of humoral immunity has not been shown to cure established BDV infection or prevent infection of the immunized rat, nor has vaccination with killed virus been shown to be protective against disease (137). There is a report that vaccination with high-titer tissue culture-passaged BDV can offer incomplete protection of rats from Borna disease following challenge with brain-derived virus (106). Transfer of BDV-specific immune T lymphocytes prior to infection can limit or prevent BDV infection and/or Borna disease in Lewis rats (118).
Amantadine has been reported as being effective (51; Bode et al., Letter, 1997) and ineffective (59, 138) at reducing BDV replication in vitro, in vivo (rats), and/or in treating patients. Since there are some reports of amantadine having direct antidepressive effects (71) and since no controlled, blinded clinical trials have been performed, the use of amantadine to treat BDV infection has not been adequately supported. Mizutani et al. reported that ribavirin inhibited BDV transcription in vitro (91), a finding that has been replicated by an independent group (75). There are no reports of ribavirin use in humans with evidence of BDV infection. Notably, ribavirin has significant adverse effects, and its use for treatment of putative BDV infection in patients is not an approved indication.
CONCLUSIONS
The biology of BDV strongly supports the likelihood of human infection with BDV or a variant of BDV. Thus far, the evidence supporting BDV infection in humans has initiated much controversy among basic and clinical scientists; only time and additional research will support or refute the hypothesis of human BDV infection.
What is needed first is a validated assay or series of assays that are capable of reliably identifying BDV infection in humans. Difficulties that must be overcome include species variability in replication of virus and development of anti-BDV antibodies, e.g., the apparent low titer and low affinity of human antibodies against BDV; the use of animal virus components (proteins and RNA sequences) rather than human virus components in these assays; possible differences in the natural history of BDV infection in humans and animals (e.g., persistence versus clearance of virus); and the low infectious-virus recovery from human samples.
Validation of the reproducibility of the assay(s) requires that each assay be performed in several independent laboratories using uniform reagents and reference samples (blinded). In addition, the sensitivity and specificity of the assay should be determined using documented reference samples from a variety of species. For example, for a serological assay, reference samples would include sera from animals from a variety of species of low-avidity (e.g., horse) and high-avidity (e.g., rat) anti-BDV antibodies as well as low- and high-titer samples. These reference sera should be unquestionably from BDV-infected animals, recovered either from animals with experimental infection with BDV or from animals in which the BDV infection has been independently confirmed (e.g., by cultivation of infectious virus). With this approach, reproducible assays of predictable sensitivity and specificity can be developed.
After assays of acceptable specificity and sensitivity have been developed and validated using animal virus and postinfection sera, the next step would be to use the assays on human sera with the intention of identifying possibly BDV-infected individuals. Attempts to document human infection with BDV should precede any attempt to associate BDV positivity with specific clinical diseases. Human BDV infection can be successfully identified using a small number of carefully chosen subjects. It is likely that a series of tests for BDV infection status will perform more reliably than a single test; thus, expectation of a sensitive serological screening test (e.g., ELISA) followed by a confirmatory test (e.g., IB or RT-PCR) would be reasonable. When a cohort of likely BDV-infected individuals is identified, careful evaluation of these individuals by additional methods (e.g., infectious-virus recovery) would be needed to confirm the findings.
Once BDV has been reliably detected in humans, scientists can reasonably begin to study an association of BDV infection with specific disease syndromes. For clinical studies seeking causal associations between BDV infection and specific diseases, a reliable, validated test for BDV infection in humans must be in place in order to ensure proper identification of the BDV infection status of patients and control subjects. Of utmost importance is careful patient and control cohort selection and screening. Control subjects must be carefully matched to the patient population on the basis of social, economic, racial, gender, and other critical features. The control population must be carefully screened by active testing to document the absence of the disease condition being evaluated in the patient population. Sufficient numbers of patients and control subjects should be evaluated to provide a reasonable “power” of the study to detect differences between the two populations in BDV positivity rates. Success of these studies might be improved by wise preselection of patients based on known BDV disease pathogenesis in animals.
Causal association of BDV with human disease will be hindered by the current lack of documentation of the pathogenesis and etiology of many psychiatric syndromes. Many psychiatric diseases are described “syndromes,” i.e., constellations of symptoms and signs of disease that tend to group into a routinely defined syndrome. Thus, it would not be unreasonable to expect that even if BDV is identified in humans with a specific type of disease syndrome, it may be the etiologic agent in only a small proportion of patients with this syndrome. In addition, given the clear effects of genetic background (e.g., strain and species) on BDV replication and expression of disease, it would not be surprising to see a constellation of different disease symptoms, signs, and syndromes all associated with BDV infection. However, over the years, BDV researchers have faced many unique and difficult problems on the way to scientific discovery in BDV research, and with concerted effort and continued work, these thorny issues that surround BDV human infection will be resolved.
REFERENCES
- 1.Amsterdam J D, Winokur A, Dyson W, Herzog S, Gonzalez F, Rott R, Koprowski H. Borna disease virus. A possible etiologic factor in human affective disorders? Arch Gen Psychiatry. 1985;42:1093–1096. doi: 10.1001/archpsyc.1985.01790340077011. [DOI] [PubMed] [Google Scholar]
- 2.Ashash E, Malkinson M, Meir R, Perl S, Weisman Y. Causes of losses including a Borna disease paralytic syndrome affecting young ostriches of one breeding organization over a five-year period (1989–1993) Avian Dis. 1996;40:240–245. [PubMed] [Google Scholar]
- 3.Auwanit W, Ayuthaya P I, Nakaya T, Fujiwara S, Kurata T, Yamanishi K, Ikuta K. Unusually high seroprevalence of Borna disease virus in clade E human immunodeficiency virus type 1-infected patients with sexually transmitted diseases in Thailand. Clin Diagn Lab Immunol. 1996;3:590–593. doi: 10.1128/cdli.3.5.590-593.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachmann S, Caplazi P, Fischer M, Ehrensperger F, Cone R W. Lack of association between Borna disease virus infection and neurological disorders among HIV-infected individuals. J Neurovirol. 1999;5:190–195. doi: 10.3109/13550289909022001. [DOI] [PubMed] [Google Scholar]
- 5.Bahmani M K, Nowrouzian I, Nakaya T, Nakamura Y, Hagiwara K, Takahashi H, Rad M A, Ikuta K. Varied prevalence of Borna disease virus infection in Arabic, thoroughbred and their cross-bred horses in Iran. Virus Res. 1996;45:1–13. doi: 10.1016/0168-1702(96)01355-x. [DOI] [PubMed] [Google Scholar]
- 6.Bautista J R, Rubin S A, Moran T H, Schwartz G J, Carbone K M. Developmental injury to the cerebellum following perinatal Borna disease virus infection. Dev Brain Res. 1995;90:45–53. doi: 10.1016/0165-3806(96)83485-7. [DOI] [PubMed] [Google Scholar]
- 7.Bautista J R, Schwartz G J, de la Torre J C, Moran T H, Carbone K M. Early and persistent abnormalities in rats with neonatally acquired Borna disease virus infection. Brain Res Bull. 1994;34:31–40. doi: 10.1016/0361-9230(94)90183-x. [DOI] [PubMed] [Google Scholar]
- 8.Bechter K, Herzog S, Schuttler R, Rott R. MRI in psychiatric patients with serum antibodies against Borna disease virus. Psychiatry Res. 1989;29:281–282. doi: 10.1016/0165-1781(89)90062-0. [DOI] [PubMed] [Google Scholar]
- 9.Reference deleted.
- 10.Berg A L, Berg M. A variant form of feline Borna disease. J Comp Pathol. 1998;119:323–331. doi: 10.1016/s0021-9975(98)80054-6. [DOI] [PubMed] [Google Scholar]
- 11.Berg A L, Reid-Smith R, Larsson M, Bonnett B. Case control study of feline Borna disease in Sweden. Vet Rec. 1998;142:715–717. doi: 10.1136/vr.142.26.715. [DOI] [PubMed] [Google Scholar]
- 12.Bilzer T, Stitz L. Immune-mediated brain atrophy. CD8+ T cells contribute to tissue destruction during borna disease. J Immunol. 1994;153:818–823. [PubMed] [Google Scholar]
- 13.Binz T, Lebelt J, Niemann H, Hagenau K. Sequence analyses of the p24 gene of Borna disease virus in naturally infected horse, donkey and sheep. Virus Res. 1994;34:281–289. doi: 10.1016/0168-1702(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 14.Bode L. Human infections with Borna disease virus and potential pathogenic implications. Curr Top Microbiol Immunol. 1995;190:103–130. doi: 10.1007/978-3-642-78618-1_7. [DOI] [PubMed] [Google Scholar]
- 15.Reference deleted.
- 16.Bode L, Durrwald R, Rantam F A, Ferszt R, Ludwig H. First isolates of infectious Borna disease virus from patients with mood disorders. Mol Psychiatry. 1996;1:200–212. [PubMed] [Google Scholar]
- 17.Reference deleted.
- 18.Reference deleted.
- 19.Bode L, Riegel S, Lange W, Ludwig H. Human infections with Borna disease virus: seroprevalence in patients with chronic diseases and healthy individuals. J Med Virol. 1992;36:309–315. doi: 10.1002/jmv.1890360414. [DOI] [PubMed] [Google Scholar]
- 20.Reference deleted.
- 21.Reference deleted.
- 22.Bode L, Zimmermann W, Ferszt R, Steinbach F, Ludwig H. Borna disease virus genome transcribed and expressed in psychiatric patients. Nat Med. 1995;1:232–236. doi: 10.1038/nm0395-232. [DOI] [PubMed] [Google Scholar]
- 23.Briese T, de la Torre J C, Lewis A, Ludwig H, Lipkin W I. Borna disease virus, a negative-strand RNA virus, transcribes in the nucleus of infected cells. Proc Natl Acad Sci USA. 1992;89:11486–11489. doi: 10.1073/pnas.89.23.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Briese T, Hatalski C G, Kliche S, Park Y S, Lipkin W I. Enzyme-linked immunosorbent assay for detecting antibodies to Borna disease virus-specific proteins. J Clin Microbiol. 1995;33:348–351. doi: 10.1128/jcm.33.2.348-351.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Briese T, Lipkin W I, de la Torre J C. Molecular biology of Borna disease virus. Curr Top Microbiol Immunol. 1995;190:1–16. doi: 10.1007/978-3-642-78618-1_1. [DOI] [PubMed] [Google Scholar]
- 26.Briese T, Schneemann A, Lewis A J, Park Y S, Kim S, Ludwig H, Lipkin W I. Genomic organization of Borna disease virus. Proc Natl Acad Sci USA. 1994;91:4362–4366. doi: 10.1073/pnas.91.10.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caplazi P, Waldvogel A, Stitz L, Braun U, Ehrensperger F. Borna disease in naturally infected cattle. J Comp Pathol. 1994;111:65–72. doi: 10.1016/s0021-9975(05)80112-4. [DOI] [PubMed] [Google Scholar]
- 28.Carbone K M, Duchala C S, Griffin J W, Kincaid A L, Narayan O. Pathogenesis of Borna disease in rats: evidence that intra-axonal spread is the major route for virus dissemination and the determinant for disease incubation. J Virol. 1987;61:3431–3440. doi: 10.1128/jvi.61.11.3431-3440.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reference deleted.
- 30.Carbone K M, Moench T R, Lipkin W I. Borna disease virus replicates in astrocytes, Schwann cells and ependymal cells in persistently infected rats: location of viral genomic and messenger RNAs by in situ hybridization. J Neuropathol Exp Neurol. 1991;50:205–214. doi: 10.1097/00005072-199105000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Carbone K M, Park S W, Rubin S A, Waltrip R W, Vogelsang G B. Borna disease: association with a maturation defect in the cellular immune response. J Virol. 1991;65:6154–6164. doi: 10.1128/jvi.65.11.6154-6164.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carbone K M, Rubin S A, Sierra-Honigmann A M, Lederman H M. Characterization of a glial cell line persistently infected with Borna disease virus (BDV): influence of neurotrophic factors on BDV protein and RNA expression. J Virol. 1993;67:1453–1460. doi: 10.1128/jvi.67.3.1453-1460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reference deleted.
- 34.Carbone K M, Trapp B D, Griffin J W, Duchala C S, Narayan O. Astrocytes and schwann cells are virus host cells in the nervous system of rats with Borna disease. J Neuropathol Exp Neurol. 1989;48:631–644. doi: 10.1097/00005072-198911000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Cervos-Navarro J, Roggendorf W, Ludwig H, Stitz H. Die borna-Krankheit beim Affen unter besonderer Berucksichtigung der encephalitischen Reaktion. Verh Dtsch Ges Pathol. 1981;65:208–212. [PubMed] [Google Scholar]
- 36.Chen C H, Chiu Y L, Shaw C K, Tsai M T, Hwang A L, Hsiao K J. Detection of Borna disease virus RNA from peripheral blood cells in schizophrenic patients and mental health workers. Mol Psychiatry. 1999;4:566–571. doi: 10.1038/sj.mp.4000568. [DOI] [PubMed] [Google Scholar]
- 37.Chen C H, Chiu Y L, Wei F C, Koong F J, Liu H C, Shaw C K, Hwu H G, Hsiao K J. High seroprevalence of Borna virus infection in schizophrenic patients, family members and mental health workers in Taiwan. Mol Psychiatry. 1999;4:33–38. doi: 10.1038/sj.mp.4000484. [DOI] [PubMed] [Google Scholar]
- 38.Cubitt B, de la Torre J C. Borna disease virus (BDV), a nonsegmented RNA virus, replicates in the nuclei of infected cells where infectious BDV ribonucleoproteins are present. J Virol. 1994;68:1371–1381. doi: 10.1128/jvi.68.3.1371-1381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cubitt B, Oldstone C, de la Torre J C. Sequence and genome organization of Borna disease virus. J Virol. 1994;68:1382–1396. doi: 10.1128/jvi.68.3.1382-1396.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reference deleted.
- 41.Danner K, Heubeck D, Mayr A. In vitro studies on Borna virus. I. The use of cell cultures for the demonstration, titration and production of Borna virus. Arch Virol. 1978;57:63–75. doi: 10.1007/BF01315638. [DOI] [PubMed] [Google Scholar]
- 42.Danner K, Mayr A. In vitro studies on Borna virus. II. Properties of the virus. Arch Virol. 1979;61:261–271. doi: 10.1007/BF01315012. [DOI] [PubMed] [Google Scholar]
- 43.de la Torre J C. Molecular biology of borna disease virus: prototype of a new group of animal viruses. J Virol. 1994;68:7669–7675. doi: 10.1128/jvi.68.12.7669-7675.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de la Torre J C, Bode L, Durrwald R, Cubitt B, Ludwig H. Sequence characterization of human Borna disease virus. Virus Res. 1996;44:33–44. doi: 10.1016/0168-1702(96)01338-x. [DOI] [PubMed] [Google Scholar]
- 45.de la Torre J C, Carbone K M, Lipkin W I. Molecular characterization of the Borna disease agent. Virology. 1990;179:853–856. doi: 10.1016/0042-6822(90)90154-j. [DOI] [PubMed] [Google Scholar]
- 46.de la Torre J C, Gonzalez-Dunia D, Cubitt B, Mallory M, Mueller-Lantzsch N, Grasser F A, Hansen L A, Masliah E. Detection of Borna disease virus antigen and RNA in human autopsy brain samples from neuropsychiatric patients. Virology. 1996;223:272–282. doi: 10.1006/viro.1996.0479. [DOI] [PubMed] [Google Scholar]
- 47.Reference deleted.
- 48.Dittrich W, Bode L, Ludwig H, Kao M, Schneider K. Learning deficiencies in Borna disease virus-infected but clinically healthy rats. Biol Psychiatry. 1989;26:818–828. doi: 10.1016/0006-3223(89)90122-4. [DOI] [PubMed] [Google Scholar]
- 49.Eisenman L M, Brothers R, Tran M H, Kean R B, Dickson G M, Dietzschold B, Hooper D C. Neonatal Borna disease virus infection in the rat causes a loss of Purkinje cells in the cerebellum. J Neurovirol. 1999;5:181–189. doi: 10.3109/13550289909022000. [DOI] [PubMed] [Google Scholar]
- 50.Evengard B, Briese T, Lindh G, Lee S, Lipkin W I. Absence of evidence of Borna disease virus infection in Swedish patients with chronic fatigue syndrome. J Neurovirol. 1999;5:495–499. doi: 10.3109/13550289909045378. [DOI] [PubMed] [Google Scholar]
- 51.Ferszt R, Kuhl K P, Bode L, Severus E W, Winzer B, Berghofer A, Beelitz G, Brodhun B, Muller-Oerlinghausen B, Ludwig H. Amantadine revisited: an open trial of amantadine sulfate treatment in chronically depressed patients with Borna disease virus infection. Pharmacopsychiatry. 1999;32:142–147. doi: 10.1055/s-2007-979220. [DOI] [PubMed] [Google Scholar]
- 52.Ferszt R, Severus E, Bode L, Brehm M, Kuhl K-P, Berzewski H, Ludwig H. Activated Borna disease virus in affective disorders. Pharmacopsychiatry. 1999;32:93–98. doi: 10.1055/s-2007-979201. [DOI] [PubMed] [Google Scholar]
- 53.Fu Z F, Amsterdam J D, Kao M, Shankar V, Koprowski H, Dietzschold B. Detection of Borna disease virus-reactive antibodies from patients with affective disorders by western immunoblot technique. J Affective Disord. 1993;27:61–68. doi: 10.1016/0165-0327(93)90098-5. [DOI] [PubMed] [Google Scholar]
- 54.Fujiwara S, Takahashi H, Nakaya T, Nakamura Y, Nakamura K, Iwahashi K, Kazamatsuri H, Iritani S, Kuroki N, Ikeda K, Ikuta K. Microplate hybridization for Borna disease virus RNA in human peripheral blood mononuclear cells. Clin Diagn Lab Immunol. 1997;4:387–391. doi: 10.1128/cdli.4.3.387-391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gonzalez-Dunia D, Watanabe M, Syan S, Mallory M, Masliah E, de la Torre J C. Synaptic pathology in Borna disease virus persistent infection. J Virol. 2000;74:3441–3448. doi: 10.1128/jvi.74.8.3441-3448.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gosztonyi G, Ludwig H. Borna disease—neuropathology and pathogenesis. Curr Top Microbiol Immunol. 1995;190:39–73. [PubMed] [Google Scholar]
- 57.Haga S, Yoshimura M, Motoi Y, Arima K, Aizawa T, Ikuta K, Tashiro M, Ikeda K. Detection of Borna disease virus genome in normal human brain tissue. Brain Res. 1997;770:307–309. doi: 10.1016/s0006-8993(97)00903-7. [DOI] [PubMed] [Google Scholar]
- 58.Hallensleben W, Schwemmle M, Hausmann J, Stitz L, Volk B, Pagenstecher A, Staeheli P. Borna disease virus-induced neurological disorder in mice: infection of neonates results in immunopathology. J Virol. 1998;72:4379–4386. doi: 10.1128/jvi.72.5.4379-4386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hallensleben W, Zocher M, Staeheli P. Borna disease virus is not sensitive to amantadine. Arch Virol. 1997;142:2043–2048. doi: 10.1007/s007050050221. [DOI] [PubMed] [Google Scholar]
- 60.Hatalski C G, Hickey W F, Lipkin W I. Evolution of the immune response in the central nervous system following infection with Borna disease virus. J Neuroimmunol. 1998;90:137–142. doi: 10.1016/s0165-5728(98)00076-9. [DOI] [PubMed] [Google Scholar]
- 61.Herzog S, Frese K, Rott R. Studies on the genetic control of resistance of black hooded rats to Borna disease. J Gen Virol. 1991;72:535–540. doi: 10.1099/0022-1317-72-3-535. [DOI] [PubMed] [Google Scholar]
- 62.Herzog S, Kompter C, Frese K, Rott R. Replication of Borna disease virus in rats: age-dependent differences in tissue distribution. Med Microbiol Immunol. 1984;173:171–177. doi: 10.1007/BF02122108. [DOI] [PubMed] [Google Scholar]
- 63.Herzog S, Pfeuffer I, Haberzettl K, Feldmann H, Frese K, Bechter K, Richt J A. Molecular characterization of Borna disease virus from naturally infected animals and possible links to human disorders. Arch Virol Suppl. 1997;13:183–190. doi: 10.1007/978-3-7091-6534-8_17. [DOI] [PubMed] [Google Scholar]
- 64.Herzog S, Rott R. Replication of Borna disease virus in cell cultures. Med Microbiol Immunol. 1980;168:153–158. doi: 10.1007/BF02122849. [DOI] [PubMed] [Google Scholar]
- 65.Herzog S, Wonigeit K, Frese K, Hedrich H J, Rott R. Effect of Borna disease virus infection on athymic rats. J Gen Virol. 1985;66:503–508. doi: 10.1099/0022-1317-66-3-503. [DOI] [PubMed] [Google Scholar]
- 66.Hirano N, Kao M, Ludwig H. Persistent, tolerant or subacute infection in Borna disease virus-infected rats. J Gen Virol. 1983;64:1521–1530. doi: 10.1099/0022-1317-64-7-1521. [DOI] [PubMed] [Google Scholar]
- 67.Horimoto T, Takahashi H, Sakaguchi M, Horikoshi K, Iritani S, Kazamatsuri H, Ikeda K, Tashiro M. A reverse-type sandwich enzyme-linked immunosorbent assay for detecting antibodies to Borna disease virus. J Clin Microbiol. 1997;35:1661–1666. doi: 10.1128/jcm.35.7.1661-1666.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hornig M, Weissenbock H, Horscroft N, Lipkin W I. An infection-based model of neurodevelopmental damage. Proc Natl Acad Sci USA. 1999;96:12102–12107. doi: 10.1073/pnas.96.21.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reference deleted.
- 70.Hsu T A, Carbone K M, Rubin S A, Vonderfecht S L, Eiden J J. Borna disease virus p24 and p38/40 synthesized in a baculovirus expression system: virus protein interactions in insect and mammalian cells. Virology. 1994;204:854–859. doi: 10.1006/viro.1994.1608. [DOI] [PubMed] [Google Scholar]
- 71.Huber T J, Dietrich D E, Emrich H M. Possible use of amantadine in depression. Pharmacopsychiatry. 1999;32:47–55. doi: 10.1055/s-2007-979191. [DOI] [PubMed] [Google Scholar]
- 72.Ihlenburg H. Experimentelle Prufung der Empfanglichkeit der Katze fur das Virus der Bornaschen Krankheit. Arch Exp Veterinaermed. 1996;20:859–864. [PubMed] [Google Scholar]
- 73.Iwahashi K, Watanabe M, Nakamura K, Suwaki H, Nakaya T, Nakamura Y, Takahashi H, Ikuta K. Clinical investigation of the relationship between Borna disease virus (BDV) infection and schizophrenia in 67 patients in Japan. Acta Psychiatr Scand. 1997;96:412–415. doi: 10.1111/j.1600-0447.1997.tb09941.x. [DOI] [PubMed] [Google Scholar]
- 74.Iwahashi K, Watanabe M, Nakamura K, Suwaki H, Nakaya T, Nakamura Y, Takahashi H, Ikuta K. Positive and negative syndromes, and Borna disease virus infection in schizophrenia. Neuropsychobiology. 1998;37:59–64. doi: 10.1159/000026477. [DOI] [PubMed] [Google Scholar]
- 75.Jordan I, Briese T, Averett D R, Lipkin W I. Inhibition of Borna disease virus replication by ribavirin. J Virol. 1999;73:7903–7906. doi: 10.1128/jvi.73.9.7903-7906.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kao M, Hamir A N, Rupprecht C E, Fu Z F, Shankar V, Koprowski H, Dietzschold B. Detection of antibodies against Borna disease virus in sera and cerebrospinal fluid of horses in the USA. Vet Rec. 1993;132:241–244. doi: 10.1136/vr.132.10.241. [DOI] [PubMed] [Google Scholar]
- 77.Kao M, Ludwig H, Gosztonyi G. Adaptation of Borna disease virus to the mouse. J Gen Virol. 1984;65:1845–1849. doi: 10.1099/0022-1317-65-10-1845. [DOI] [PubMed] [Google Scholar]
- 78.Katz J B, Alstad D, Jenny A L, Carbone K M, Rubin S A, Waltrip R W. Clinical, serologic, and histopathologic characterization of experimental Borna disease in ponies. J Vet Diagn Investig. 1998;10:338–343. doi: 10.1177/104063879801000405. [DOI] [PubMed] [Google Scholar]
- 79.Kim Y K, Kim S H, Choi S H, Ko Y H, Kim L, Lee M S, Suh K Y, Kwak D I, Song K J, Lee Y J, Yanagihara R, Song J W. Failure to demonstrate Borna disease virus genome in peripheral blood mononuclear cells from psychiatric patients in Korea. J Neurovirol. 1999;5:196–199. doi: 10.3109/13550289909022002. [DOI] [PubMed] [Google Scholar]
- 80.Kishi M, Arimura Y, Ikuta K, Shoya Y, Lai P K, Kakinuma M. Sequence variability of Borna disease virus open reading frame II found in human peripheral blood mononuclear cells. J Virol. 1996;70:635–640. doi: 10.1128/jvi.70.1.635-640.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kishi M, Nakaya T, Nakamura Y, Kakinuma M, Takahashi T A, Sekiguchi S, Uchikawa M, Tadokoro K, Ikeda K, Ikuta K. Prevalence of Borna disease virus RNA in peripheral blood mononuclear cells from blood donors. Med Microbiol Immunol. 1995;184:135–138. doi: 10.1007/BF00224350. [DOI] [PubMed] [Google Scholar]
- 82.Kishi M, Nakaya T, Nakamura Y, Zhong Q, Ikeda K, Senjo M, Kakinuma M, Kato S, Ikuta K. Demonstration of human Borna disease virus RNA in human peripheral blood mononuclear cells. FEBS Lett. 1995;15:293–297. doi: 10.1016/0014-5793(95)00406-y. [DOI] [PubMed] [Google Scholar]
- 83.Kitani T, Kuratsune H, Fuke I, Nakamura Y, Nakaya T, Asahi S, Tobiume M, Yamaguti K, Machii T, Inagi R, Yamanishi K, Ikuta K. Possible correlation between Borna disease virus infection and Japanese patients with chronic fatigue syndrome. Microbiol Immunol. 1996;40:459–462. doi: 10.1111/j.1348-0421.1996.tb01094.x. [DOI] [PubMed] [Google Scholar]
- 84.Reference deleted.
- 85.Kubo K, Fujiyoshi T, Yokoyama M M, Kamei K, Richt J A, Kitze B, Herzog S, Takigawa M, Sonoda S. Lack of association of Borna disease virus and human T-cell leukemia virus type 1 infections with psychiatric disorders among Japanese patients. Clin Diagn Lab Immunol. 1997;4:189–194. doi: 10.1128/cdli.4.2.189-194.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reference deleted.
- 87.Lipkin W I, Carbone K M, Wilson M C, Duchala C S, Narayan O, Oldstone M B. Neurotransmitter abnormalities in Borna disease. Brain Res. 1988;475:366–370. doi: 10.1016/0006-8993(88)90627-0. [DOI] [PubMed] [Google Scholar]
- 88.Lipkin W I, Travis G H, Carbone K M, Wilson M C. Isolation and characterization of Borna disease agent cDNA clones. Proc Natl Acad Sci USA. 1990;87:4184–4188. doi: 10.1073/pnas.87.11.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lundgren A L, Ludwig H. Clinically diseased cats with non-suppurative meningoencephalomyelitis have Borna disease virus-specific antibodies. Acta Vet Scand. 1993;34:101–103. doi: 10.1186/BF03548230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Malik T H, Kishi M, Lai P K. Characterization of the P protein-binding domain on the 10-kilodalton protein of Borna disease virus. J Virol. 2000;74:3413–3417. doi: 10.1128/jvi.74.7.3413-3417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mizutani T, Inagaki H, Araki K, Kariwa H, Arikawa J, Takashima I. Inhibition of Borna disease virus replication by ribavirin in persistently infected cells. Arch Virol. 1998;143:2039–2044. doi: 10.1007/s007050050440. [DOI] [PubMed] [Google Scholar]
- 92.Mizutani T, Nishino Y, Kariwa H, Takashima I. Reverse transcription-nested polymerase chain reaction for detecting p40 RNA of Borna disease virus, without risk of plasmid contamination. J Vet Med Sci. 1999;61:77–80. doi: 10.1292/jvms.61.77. [DOI] [PubMed] [Google Scholar]
- 93.Mizutani T, Ogino M, Nishino Y, Kimura T, Inagaki H, Hayasaka D, Kariwa H, Takashima I. Single-step reverse transcriptase-polymerase chain reaction for detection of Borna disease virus RNA in vitro and in vivo. Jpn J Vet Res. 1999;46:165–169. [PubMed] [Google Scholar]
- 94.Morimoto K, Hooper D, Bornhorst A, Corisdeo M, Bette M, Fu Z F, Schafer M, Koprowski H, Weihe E. Intrinsic responses to Borna disease virus infection of the central nervous system. Proc Natl Acad Sci USA. 1996;93:13345–13350. doi: 10.1073/pnas.93.23.13345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nakamura Y, Nakaya T, Hagiwara K, Momiyama N, Kagawa Y, Taniyama H, Ishihara C, Sata T, Kurata T, Ikuta K. High susceptibility of Mongolian gerbil (Meriones unguiculatus) to Borna disease virus. Vaccine. 1999;17:480–489. doi: 10.1016/s0264-410x(98)00222-9. [DOI] [PubMed] [Google Scholar]
- 96.Nakamura Y, Takahashi H, Shoya Y, Nakaya T, Watanabe M, Tomonaga K, Iwahashi K, Ameno K, Momiyama N, Taniyama H, Sata T, Kurata T, de la Torre J C, Ikuta K. Isolation of Borna disease virus from human brain tissue. J Virol. 2000;74:4601–4611. doi: 10.1128/jvi.74.10.4601-4611.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nakaya T, Takahashi H, Nakamur Y, Kuratsune H, Kitani T, Machii T, Yamanishi K, Ikuta K. Borna disease virus infection in two family clusters of patients with chronic fatigue syndrome. Microbiol Immunol. 1999;43:679–689. doi: 10.1111/j.1348-0421.1999.tb02456.x. [DOI] [PubMed] [Google Scholar]
- 98.Nakaya T, Takahashi H, Nakamura Y, Asahi S, Tobiume M, Kuratsune H, Kitani T, Yamanishi K, Ikuta K. Demonstration of Borna disease virus RNA in peripheral blood mononuclear cells derived from Japanese patients with chronic fatigue syndrome. FEBS Lett. 1996;378:145–149. doi: 10.1016/0014-5793(95)01439-x. [DOI] [PubMed] [Google Scholar]
- 99.Narayan O, Herzog S, Frese K, Scheefers H, Rott R. Behavioral disease in rats caused by immunopathological responses to persistent borna virus in the brain. Science. 1983;220:1401–1403. doi: 10.1126/science.6602380. [DOI] [PubMed] [Google Scholar]
- 100.Narayan O, Herzog S, Frese K, Scheefers H, Rott R. Pathogenesis of Borna disease in rats: immune-mediated viral ophthalmoencephalopathy causing blindness and behavioral abnormalities. J Infect Dis. 1983;148:305–315. doi: 10.1093/infdis/148.2.305. [DOI] [PubMed] [Google Scholar]
- 101.Nielsen K, Bryson Y J. Diagnosis of HIV infection in children. Pediatr Clin North Am. 2000;47:39–63. doi: 10.1016/s0031-3955(05)70194-2. [DOI] [PubMed] [Google Scholar]
- 102.Nishino Y, Funaba M, Fukushima R, Mizutani T, Kimura T, Iizuka R, Hirami H, Hara M. Borna disease virus infection in domestic cats: evaluation by RNA and antibody detection. J Vet Med Sci. 1999;61:1167–1170. doi: 10.1292/jvms.61.1167. [DOI] [PubMed] [Google Scholar]
- 103.Nowotny N, Kolodziejek J, Jehle C O, Suchy A, Staeheli P, Schwemmle M. Isolation and characterization of a new subtype of Borna disease virus. J Virol. 2000;74:5655–5658. doi: 10.1128/jvi.74.12.5655-5658.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nowotny N, Weissenbock H. Description of feline nonsuppurative meningoencephalomyelitis (“staggering disease”) and studies of its etiology. J Clin Microbiol. 1995;33:1668–1669. doi: 10.1128/jcm.33.6.1668-1669.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ogino M, Yoshimatsu K, Tsujimura K, Mizutani T, Arikawa J, Takashima I. Evaluation of serological diagnosis of Borna disease virus infection using recombinant proteins in experimentally infected rats. J Vet Med Sci. 1998;60:531–534. doi: 10.1292/jvms.60.531. [DOI] [PubMed] [Google Scholar]
- 106.Oldach D, Zink M C, Pyper J M, Herzog S, Rott R, Narayan O, Clements J E. Induction of protection against Borna disease by inoculation with high-dose-attenuated Borna disease virus. Virology. 1995;206:426–434. doi: 10.1016/s0042-6822(95)80058-1. [DOI] [PubMed] [Google Scholar]
- 107.Planz O, Rentzsch C, Batra A, Winkler T, Buttner M, Rziha H J, Stitz L. Pathogenesis of borna disease virus: granulocyte fractions of psychiatric patients harbor infectious virus in the absence of antiviral antibodies. J Virol. 1999;73:6251–6256. doi: 10.1128/jvi.73.8.6251-6256.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reference deleted.
- 109.Plata-Salaman C R, Ilyin S E, Gayle D, Romanovitch A, Carbone K M. Persistent Borna disease virus infection of neonatal rats causes brain regional changes of mRNAs for cytokines, cytokine receptor components and neuropeptides. Brain Res Bull. 1999;49:441–451. doi: 10.1016/s0361-9230(99)00081-7. [DOI] [PubMed] [Google Scholar]
- 110.Pletnikov M, Rubin S A, Schwartz G J, Carbone K M, Moran T. Effects of neonatal rat Borna disease virus (BDV) infection on the postnatal development of brain monoaminergic systems. Dev Brain Res. 2000;119:179–185. doi: 10.1016/s0165-3806(99)00168-6. [DOI] [PubMed] [Google Scholar]
- 111.Pletnikov M, Rubin S A, Schwemmle M, Moran T, Sobatka T, Carbone K M. Persistent neonatal Borna disease virus (BDV) infection of the brain causes chronic emotional abnormalities in adult rats. Physiol Behav. 1999;66:823–831. doi: 10.1016/s0031-9384(99)00021-9. [DOI] [PubMed] [Google Scholar]
- 112.Pletnikov M, Rubin S A, Vasudevan K, Moran T, Carbone K M. Developmental brain injury associated with abnormal play behavior in neonatally Borna disease virus (BDV) infected Lewis rats: a model of autism. Behav Brain Res. 1999;100:30–45. doi: 10.1016/s0166-4328(98)00111-9. [DOI] [PubMed] [Google Scholar]
- 113.Pletnikov M V, Rubin S A, Schwartz G J, Carbone K M, Moran T H. Effects of neonatal rat Borna disease virus (BDV) infection on the postnatal development of the brain monoaminergic systems. Dev Brain Res. 2000;119:179–185. doi: 10.1016/s0165-3806(99)00168-6. [DOI] [PubMed] [Google Scholar]
- 114.Pletnikov M V, Rubin S A, Schwartz G J, Moran T H, Sobotka T J, Carbone K M. Persistent neonatal Borna disease virus (BDV) infection of the brain causes chronic emotional abnormalities in adult rats. Physiol Behav. 1999;66:823–831. doi: 10.1016/s0031-9384(99)00021-9. [DOI] [PubMed] [Google Scholar]
- 115.Pletnikov M V, Rubin S A, Vasudevan K, Moran T H, Carbone K M. Developmental brain injury associated with abnormal play behavior in neonatally Borna disease virus-infected Lewis rats: a model of autism. Behav Brain Res. 1999;100:43–50. doi: 10.1016/s0166-4328(98)00111-9. [DOI] [PubMed] [Google Scholar]
- 116.Reeves N A, Helps C R, Gunn-Moore D A, Blundell C, Finnemore P L, Pearson G R, Harbour D A. Natural Borna disease virus infection in cats in the United Kingdom. Vet Rec. 1998;143:523–526. doi: 10.1136/vr.143.19.523. [DOI] [PubMed] [Google Scholar]
- 117.Richt J A, Alexander R C, Herzog S, Hooper D C, Kean R, Spitsin S, Bechter K, Schuttler R, Feldmann H, Heiske A, Fu Z F, Dietzschold B, Rott R, Koprowski H. Failure to detect Borna disease virus infection in peripheral blood leukocytes from humans with psychiatric disorders. J Neurovirol. 1997;3:174–178. doi: 10.3109/13550289709015807. [DOI] [PubMed] [Google Scholar]
- 118.Richt J A, Schmeel A, Frese K, Carbone K M, Narayan O, Rott R. Borna disease virus-specific T cells protect against or cause immunopathological Borna disease. J Exp Med. 1994;179:1467–1473. doi: 10.1084/jem.179.5.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Richt J A, Stitz L, Wekerle H, Rott R. Borna disease, a progressive meningoencephalomyelitis as a model for CD4+ T cell-mediated immunopathology in the brain. J Exp Med. 1989;170:1045–1050. doi: 10.1084/jem.170.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rott R, Becht H. Natural and experimental Borna disease in animals. Curr Top Microbiol Immunol. 1995;190:17–30. doi: 10.1007/978-3-642-78618-1_2. [DOI] [PubMed] [Google Scholar]
- 121.Rott R, Herzog S, Bechter K, Frese K. Borna disease, a possible hazard for man? Arch Virol. 1991;118:143–149. doi: 10.1007/BF01314025. [DOI] [PubMed] [Google Scholar]
- 122.Rubin S A, Bautista J R, Moran T H, Schwartz G J, Carbone K M. Viral teratogenesis: brain developmental damage associated with maturation state at time of infection. Dev Brain Res. 1999;112:237–244. doi: 10.1016/s0165-3806(98)00180-1. [DOI] [PubMed] [Google Scholar]
- 123.Rubin S A, Sierra-Honigmann A M, Lederman H M, Waltrip R W, Eiden J J, Carbone K M. Hematologic consequences of Borna disease virus infection of rat bone marrow and thymus stromal cells. Blood. 1995;85:2762–2769. [PubMed] [Google Scholar]
- 124.Rubin S A, Sylves P, Vogel M, Pletnikov M, Moran T H, Schwartz G J, Carbone K M. Borna disease virus-induced hippocampal dentate gyrus damage is associated with spatial learning and memory deficits. Brain Res Bull. 1999;48:23–30. doi: 10.1016/s0361-9230(98)00133-6. [DOI] [PubMed] [Google Scholar]
- 125.Rubin S A, Waltrip R W, Bautista J R, Carbone K M. Borna disease virus in mice: host-specific differences in disease expression. J Virol. 1993;67:548–552. doi: 10.1128/jvi.67.1.548-552.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Reference deleted.
- 127.Sauder C, de la Torre J C. Sensitivity and reproducibility of RT-PCR to detect Borna disease virus (BDV) RNA in blood: implications for BDV epidemiology. J Virol Methods. 1998;71:229–245. doi: 10.1016/s0166-0934(98)00005-6. [DOI] [PubMed] [Google Scholar]
- 128.Sauder C, de la Torre J C. Cytokine expression in the rat central nervous system following perinatal Borna disease virus infection. J Neuroimmunol. 1999;96:29–45. doi: 10.1016/s0165-5728(98)00272-0. [DOI] [PubMed] [Google Scholar]
- 129.Sauder C, Hallensleben W, Pagenstecher A, Schneckenburger S, Biro L, Pertlik D, Hausmann J, Suter M, Staeheli P. Chemokine gene expression in astrocytes of Borna disease virus-infected rats and mice in the absence of inflammation. J Virol. 2000;74:9267–9280. doi: 10.1128/jvi.74.19.9267-9280.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sauder C, Muller A, Cubitt B, Mayer J, Steinmetz J, Trabert W, Ziegler B, Wanke K, Mueller-Lantzsch N, de la Torre J C, Grasser F A. Detection of Borna disease virus (BDV) antibodies and BDV RNA in psychiatric patients: evidence for high sequence conservation of human blood-derived BDV RNA. J Virol. 1996;70:7713–7724. doi: 10.1128/jvi.70.11.7713-7724.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schneider P A, Briese T, Zimmermann W, Ludwig H, Lipkin W I. Sequence conservation in field and experimental isolates of Borna disease virus. J Virol. 1994;68:63–68. doi: 10.1128/jvi.68.1.63-68.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Reference deleted.
- 133.Sierra-Honigmann A M, Rubin S A, Estafanous M G, Yolken R H, Carbone K M. Borna disease virus in peripheral blood mononuclear and bone marrow cells of neonatally and chronically infected rats. J Neuroimmunol. 1993;45:31–36. doi: 10.1016/0165-5728(93)90160-z. [DOI] [PubMed] [Google Scholar]
- 134.Reference deleted.
- 135.Solbrig M V, Koob G F, Fallon J H, Lipkin W I. Tardive dyskinetic syndrome in rats infected with Borna disease virus. Neurobiol Dis. 1994;1:111–119. doi: 10.1006/nbdi.1994.0014. [DOI] [PubMed] [Google Scholar]
- 136.Sprankel H, Richarz K, Ludwig H, Rott R. Behavior alterations in tree shrews (Tupaia glis, Diard 1820) induced by Borna disease virus. Med Microbiol Immunol. 1978;165:1–18. doi: 10.1007/BF02121228. [DOI] [PubMed] [Google Scholar]
- 137.Stitz L, Dietzchold B, Carbone K M. Immunopathogenesis of Borna disease. Curr Top Microbiol Immunol. 1995;190:75–92. doi: 10.1007/978-3-642-78618-1_5. [DOI] [PubMed] [Google Scholar]
- 138.Stitz L, Planz O, Bilzer T. Lack of antiviral effect of amantadine in Borna disease virus infection. Med Microbiol Immunol. 1998;186:195–200. doi: 10.1007/s004300050064. [DOI] [PubMed] [Google Scholar]
- 139.Stitz L, Schliken D, Frese K. Atypical dissemination of the highly neurotropic Borna disease virus during persistent infection in cyclosporin A-treated, immunosuppressed rats. J Virol. 1990;65:457–460. doi: 10.1128/jvi.65.1.457-460.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Stitz L, Sobbe M, Bilzer T. Preventive effects of early anti-CD4 or anti-CD8 treatment on Borna disease in rats. J Virol. 1992;66:3316–3323. doi: 10.1128/jvi.66.6.3316-3323.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stitz L, Soeder D, Deschl U, Frese K, Rott R. Inhibition of immune-mediated meningoencephalitis in persistently Borna disease virus-infected rats by cyclosporine A. J Immunol. 1989;143:4250–4256. [PubMed] [Google Scholar]
- 142.Takahashi H, Nakaya T, Nakamura Y, Asahi S, Onishi Y, Ikebuchi K, Takahashi T A, Katoh T, Sekiguchi S, Takazawa M, Tanaka H, Ikuta K. Higher prevalence of Borna disease virus infection in blood donors living near thoroughbred horse farms. J Med Virol. 1997;52:330–335. [PubMed] [Google Scholar]
- 143.VandeWoude S, Richt J A, Zink M C, Rott R, Narayan O, Clements J E. A borna virus cDNA encoding a protein recognized by antibodies in humans with behavioral diseases. Science. 1990;250:1278–1281. doi: 10.1126/science.2244211. [DOI] [PubMed] [Google Scholar]
- 144.Walker M P, Jordan I, Briese T, Fischer N, Lipkin W I. Expression and characterization of the Borna disease virus polymerase. J Virol. 2000;74:4425–4428. doi: 10.1128/jvi.74.9.4425-4428.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Waltrip R W, Buchanan R W, Carpenter W T J, Kirkpatrick B, Summerfelt A, Breier A, Rubin S A, Carbone K M. Borna disease virus antibodies and the deficit syndrome of schizophrenia. Schizophr Res. 1997;23:253–257. doi: 10.1016/s0920-9964(96)00114-4. [DOI] [PubMed] [Google Scholar]
- 146.Waltrip R W, Buchanan R W, Summerfelt A, Breier A, Carpenter W T J, Bryant N L, Rubin S A, Carbone K M. Borna disease virus and schizophrenia. Psychiatry Res. 1995;56:33–44. doi: 10.1016/0165-1781(94)02600-n. [DOI] [PubMed] [Google Scholar]
- 147.Waltrip R W, II, Buchanan R W, Summerfelt A, Breier A, Carpenter W T, Bryant N L, Rubin S A, Carbone K M. Borna disease virus and schizophrenia. Psychiatry Res. 1995;56:33–44. doi: 10.1016/0165-1781(94)02600-n. [DOI] [PubMed] [Google Scholar]
- 148.Reference deleted.
- 149.Weissenbock H, Hornig M, Hickey W F, Lipkin W I. Microglial activation and neuronal apoptosis in Bornavirus infected neonatal Lewis rats. Brain Pathol. 2000;10:260–272. doi: 10.1111/j.1750-3639.2000.tb00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yamaguchi K, Sawada T, Naraki T, Igata-Yi R, Shiraki H, Horii Y, Ishii T, Ikeda K, Asou N, Okabe H, Mochizuki M, Takahashi K, Yamada S, Kubo K, Yashiki S, Waltrip R W, Carbone K M. Detection of borna disease virus-reactive antibodies from patients with psychiatric disorders and from horses by electrochemiluminescence immunoassay. Clin Diagn Lab Immunol. 1999;6:696–700. doi: 10.1128/cdli.6.5.696-700.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]